Abstract
Aims/hypothesis
Our aim was to characterise the in-depth metabolic response to aerobic exercise and the impact of residual pancreatic beta cell function in type 1 diabetes. We also aimed to use the metabolome to distinguish individuals with type 1 diabetes with reduced maximal aerobic capacity in exercise defined by .
Methods
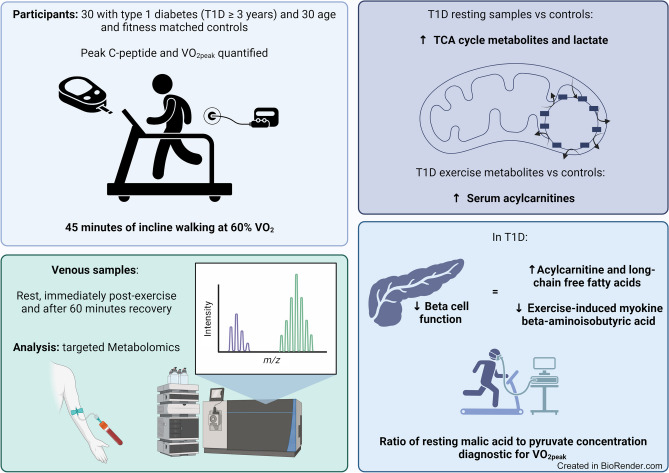
Thirty participants with type 1 diabetes (≥3 years duration) and 30 control participants were recruited. Groups did not differ in age or sex. After quantification of peak stimulated C-peptide, participants were categorised into those with undetectable (<3 pmol/l), low (3–200 pmol/l) or high (>200 pmol/l) residual beta cell function. Maximal aerobic capacity was assessed by test and did not differ between control and type 1 diabetes groups. All participants completed 45 min of incline treadmill walking (60% ) with venous blood taken prior to exercise, immediately post exercise and after 60 min recovery. Serum was analysed using targeted metabolomics. Metabolomic data were analysed by multivariate statistics to define the metabolic phenotype of exercise in type 1 diabetes. Receiver operating characteristic (ROC) curves were used to identify circulating metabolomic markers of maximal aerobic capacity () during exercise in health and type 1 diabetes.
Results
Maximal aerobic capacity () inversely correlated with HbA1c in the type 1 diabetes group (r2=0.17, p=0.024). Higher resting serum tricarboxylic acid cycle metabolites malic acid (fold change 1.4, p=0.001) and lactate (fold change 1.22, p=1.23×10−5) differentiated people with type 1 diabetes. Higher serum acylcarnitines (AC) (AC C14:1, F value=12.25, p=0.001345; AC C12, F value=11.055, p=0.0018) were unique to the metabolic response to exercise in people with type 1 diabetes. C-peptide status differentially affected metabolic responses in serum ACs during exercise (AC C18:1, leverage 0.066; squared prediction error 3.07). The malic acid/pyruvate ratio in rested serum was diagnostic for maximal aerobic capacity () in people with type 1 diabetes (ROC curve AUC 0.867 [95% CI 0.716, 0.956]).
Conclusions/interpretation
The serum metabolome distinguishes high and low maximal aerobic capacity and has diagnostic potential for facilitating personalised medicine approaches to manage aerobic exercise and fitness in type 1 diabetes.
Graphical Abstract
Supplementary Information
The online version contains peer-reviewed but unedited supplementary material available at 10.1007/s00125-024-06153-0.
Keywords: Aerobic exercise, Diagnostic, Metabolism, Targeted metabolomics, Type 1 diabetes
Introduction
Exercise provides health benefits for individuals with type 1 diabetes, through reducing CVD, improving skeletal muscle health and increasing fitness [1, 2]. Despite the benefits of exercise, barriers to its use as a diabetes therapy remain. Exercise tolerance is impaired in type 1 diabetes [3–10] and the metabolic response to exercise can be acutely disrupted, with high inter-individual variability, leading to dysglycaemia [11–14]. This is despite high intra-individual reproducibility under repeated laboratory conditions, suggesting the existence of distinct subpopulations within the type 1 diabetes population exhibiting perturbed metabolic responses to exercise [15].
The variability in metabolic response to exercise in people with type 1 diabetes is not fully explained, is poorly classified and cannot be predicted [15]. A number of physiological contributors to exercise response are disrupted in people with type 1 diabetes, possibly contributing to inter-individual variation [15]. These include perturbations to fuel selection [16] in the form of carbohydrates (glycolysis) and lipids (NEFA, triacylglycerols, acylcarnitines), and anaerobic processes (lactate) and central carbon metabolism (tricarboxylic acid [TCA] cycle) [16].
The phenotype and population heterogeneity of aerobic capacity in exercise and metabolic dysfunction in type 1 diabetes makes effective screening, diagnosis and management a substantial challenge, contributing to lower activity levels within the type 1 diabetes population [16–18]. Omic approaches are a potential solution. Metabolomics has advanced understanding of metabolic diseases and is ripe for application to the heterogeneous pathological metabolic phenotype induced by exercise in people with type 1 diabetes, having become a core tool in personalised medicine [19–21]. Metabolomics is effective in defining ‘silent’ phenotypes [22] (that manifest under specific stressors) and identifying disease biomarkers preceding overt pathology [20, 21]. Therefore, metabolomics is highly appropriate for studying the metabolic perturbation in type 1 diabetes that acutely manifests under stress (increased metabolic rate, carbohydrate oxidation, and insulin sensitivity) in exercise. The study of the metabolome alongside exercise indices, such as maximal aerobic capacity (), may provide novel information regarding an individual’s metabolic phenotype and distinguish those with lower or higher aerobic capacity.
Another unexplored mechanism possibly contributing to the metabolic response to exercise is residual beta cell function (i.e. C-peptide secretion) in people with type 1 diabetes of long duration. Between 35% and 80% of people with type 1 diabetes have detectable beta cell function at >5 years post diagnosis [23, 24], with 5–16% at the threshold for clinical benefits (peak C-peptide 200 pmol/l) found in the DCCT. As beta cell function declines in individuals with type 1 diabetes, glycaemic control deteriorates [25, 26]. Individuals with high stimulated C-peptide (>400 pmol/l) spend greater time in euglycaemia at rest compared with those with undetected, low (17–200 pmol/l) and intermediate (200–400 pmol/l) levels, suggesting an influence on basal metabolism [27]. Individuals with higher beta cell function (>200 pmol/l C-peptide) display lower glycaemic variability than individuals with low and undetectable C-peptide following aerobic exercise [28]. This suggests that residual beta cell function may contribute to variation in acute blood glucose levels following exercise in type 1 diabetes. Since C-peptide status influences metabolism (glycaemic control) post exercise, it may also contribute to the metabolic response to exercise. Application of metabolomics in people with type 1 diabetes during aerobic exercise, alongside characterisation of C-peptide status, may provide valuable insight into the impact of residual beta cell function on metabolic phenotype.
We aimed to use targeted metabolomics to characterise the metabolic signature of type 1 diabetes at rest, exercise and recovery. We explored the contribution made by residual beta cell function to metabolic response to exercise in individuals with type 1 diabetes and sought to identify circulating metabolite markers diagnostic of maximal aerobic capacity to facilitate the personalised medicine approach to diabetes.
Methods
Participants and recruitment
Participants with type 1 diabetes were included if they had a confirmed clinical diagnosis, age 18–65 years with type 1 diabetes duration ≥3 years, HbA1c <86 mmol/mol (10%) and stable multiple daily injections or continuous insulin infusion without changes over the preceding 6 months. A diabetes duration ≥3 years avoided the ~2 year ‘honeymoon’ period [29]. Participants were excluded if they had diabetes-related complications (except non-proliferative retinopathy), other chronic conditions, history of smoking, or BP >140/90 mmHg at study visits. Participants’ clinical notes confirmed normal renal function (absence of albuminuria, GFR >60 ml/min per 1.73 m2). Control participants were aged 18–65 years, non-smokers and free from chronic disease, with confirmed absence of diabetes (HbA1c <40 mmol/mol [5.8%]). Thirty control participants and 30 volunteers with type 1 diabetes, further subdivided into participants with either undetectable (n=11), low (n=9) or high (n=10) C-peptide, were recruited from Newcastle Diabetes Centre. Participant demographics are shown in Tables 1, 2 and electronic supplementary material (ESM) Table 1. The study population is representative of the larger type 1 diabetes population in terms of age and sex. Sex did not differ between control and type 1 diabetes groups and was determined by self-reporting and confirmed from participants’ clinical notes. We previously reported on this study population [28, 30]. Participants provided written informed consent prior to enrolment following approval from the NHS HRA North East Tyne & Wear South Research Ethics and Newcastle University Ethics Committees (16/NE/0192, ISRCTN63739203).
Table 1.
Participant demographic data
| Characteristic | Type 1 diabetes | Control group | p value |
|---|---|---|---|
| N | 30 | 30 | |
| No. male/female | 16/14 | 16/14 | |
| Age, years | 38.2±12.0 | 37.6±12.1 | 0.84 |
| HbA1c, mmol/mol | 58.5±9.1 | 33.5±2.3 | <0.001 |
| HbA1c, % | 7.5±3.0 | 5.2±2.4 | <0.001 |
| BMI, kg/m2 | 25.2±3.7 | 24.7±4.6 | 0.656 |
| , ml kg−1 min−1 | 38.8±9.5 | 42.4±12.4 | 0.205 |
| Age at diagnosis, years | 18.2±8.6 | _ | |
| Range, years | 8–35 | ||
| Diabetes duration, years | 20.0±13.0 | _ | |
| Range, years | 3–47 | ||
|
Method of control, MDI/CSII |
15/15 | _ | |
| Total cholesterol, mmol/l | 4.6±0.7 | 4.1±0.9 | 0.017712 |
| Triglycerides, mmol/l | 1.0±0.4 | 0.7±0.3 | 0.004876 |
| HDL-cholesterol, mmol/l | 1.6±0.4 | 1.6±0.3 | 0.897524 |
| Total/HDL-cholesterol ratio | 3.1±0.9 | 2.7±0.8 | 0.061241 |
| Non-HDL-cholesterol, mmol/l | 3.0±0.7 | 2.5±0.9 | 0.017246 |
Data are presented as mean ± SD
p value from independent samples t test
CSII, continuous subcutaneous insulin infusion; MDI, multiple daily injection of insulin
Table 2.
Type 1 diabetes participant demographic data by C-peptide status and MMTT results
| Characteristic | Cpepund | Cpeplow | Cpephigh | p value |
|---|---|---|---|---|
| N | 11 | 9 | 10 | |
| No. male / female | 5/6 | 6/3 | 5/5 | |
| Age, years | 40.09±11.18 | 38.67±14.73 | 35.80±10.98 | 0.738 |
| Range, years | 26–58 | 25–61 | 18–53 | |
| Age at diagnosis, years | 13.27±4.5 | 16.56±8.57 | 25.1±8.2* | 0.003 |
| Range, years | 8–24 | 8–32 | 13–35 | |
| Duration of diabetes, years | 26.82±13.24 | 21.89±13.34 | 10.70±6.15* | 0.015 |
| Range, years | 13–47 | 9–44 | 3–20 | |
| HbA1c, mmol/mol | 61.64±10.64 | 58.11±7.11 | 55.40±8.47 | 0.297 |
| Range, mmol/mol | 42–78 | 51–74 | 41–69 | |
| HbA1c, % | 7.8±3.1 | 7.5±2.8 | 7.2±2.9 | |
| Range, % | 6.0–9.3 | 6.8–8.9 | 5.9–8.5 | |
| BMI, kg/m2 | 25.65±3.27 | 24.20±4.13 | 25.67±4.04 | 0.259 |
| Daily insulin dose, U | 39.93±15.15 | 47.88±23.21 | 38.30±31.23 | 0.242 |
| Daily insulin dose, U/kg | 0.54±0.19 | 0.63±0.25 | 0.49±0.29 | 0.332 |
|
Method of control (MDI/CSII) |
5/6 | 4/5 | 6/4 | |
| , ml kg−1 min−1 | 35.61±7.69 | 43.93±9.03 | 35.67±10.77 | |
| Range, ml kg−1 min−1 | 21.05–49 | 31.8–58.25 | 21.25–51.00 | 0.194 |
| Total cholesterol, mmol/l | 4.5±1.1 | 3.7±0.7‡ | 4.0±0.7 | 0.0145 |
| Triglycerides, mmol/l | 0.8±0.2 | 0.7±0.3 | 0.7±0.3 | 0.0485 |
| HDL-cholesterol, mmol/l | 1.7±0.4 | 1.6±0.3 | 1.5±0.3 | 0.5876 |
| Total/HDL-cholesterol ratio | 2.8±0.9 | 2.5±0.7 | 2.8±0.8 | 0.2282 |
| Non-HDL-cholesterol, mmol/l | 2.8±1.1 | 2.2±0.7‡ | 2.5±0.7 | 0.0373 |
| Peak C-peptide, pmol/l | 0±0.00 | 42.00±32.58 | 671.7±435.15*† | <0.001 |
| Range, pmol/l | 0–0 | 4–83 | 221–1640 | |
| Media C-peptide | 0 | 53 | 568.5 |
Data are presented as mean ± SD
*p<0.05, vs Cpepund; †p<0.05 vs Cpeplow; ‡p<0.05 vs control group (one-way ANOVA with Tukey post hoc)
Cpepund, undetectable C-peptide (<3 pmol/l); Cpephigh, high C-peptide (≥200 pmol/l); Cpeplow, low C-peptide (3–200 pmol/l); CSII, continuous subcutaneous insulin infusion; MDI, multiple daily injection of insulin
Visit 1 mixed-meal tolerance test
Participants attended the National Institute for Health Research Newcastle Clinical Research Facility (CRF) at 08:30 hours after an overnight fast, and a cannula was inserted into an antecubital vein. Participants maintained their basal insulin regimen. Peak serum C-peptide response to a mixed-meal tolerance test (MMTT) was measured to determine residual beta cell function, according to published protocols [28]. For details, see ESM Methods. Participants were allocated into three peak C-peptide groupings: undetectable (<3 pmol/l); low (3–200 pmol/l); and high (≥200 pmol/l). For reference, peak serum C-peptide following MMTT in healthy individuals is 1000–3000 pmol/l [31, 32].
Visit 2 maximal aerobic capacity testing
Participants’ height, weight (seca 220 stadiometer/seca 889 scale; seca, Hamburg, Germany) and medical history were recorded. Participants were screened for cardiac anomalies using a modified 12-lead resting/exercising ECG. and peak heart rate were defined using a maximal-graded walking treadmill (Valiant 2 CPET; Lode, Groningen, the Netherlands) test (Bruce protocol) [33]. For details, see ESM Methods.
Visit 3 exercise intervention
The exercise protocol was conducted as previously published [28]. Participants arrived at the CRF at 08:30 hours following an overnight fast, had abstained from exercise for 48 h and maintained their basal insulin regimen. Participants were cannulated and resting (baseline) blood samples (10 ml) were drawn. Participants walked on an incline for 45 min at 60% , then blood was drawn from the cannula immediately and after participants had rested for 60 min. Serum was separated and stored at −80°C. For details, see ESM Methods.
Metabolomic sample preparation
An internal standard spiking solution (10 μmol/l each of palmitoyl-l-carnitine-[N-methyl-d3], palmitic acid-d31, deoxycholic acid-d6 and l-tryptophan-d5, and 5 µmol/l citric acid-d4; all from Sigma) in LC-MS-grade methanol was prepared. Serum (100 µl) was protein-precipitated with 400 µl internal standard spiking solution and centrifuged (3 min; 25,000 g). The supernatant fraction (100 µl) was diluted with 100 µl LC-MS-grade water, vortexed and transferred to LC vials. Sample order was randomised using a random number generator. Experimenters were blinded to experimental groups.
Targeted metabolomic LC-MS analysis of acylcarnitines, NEFA, bile acids, tryptophan metabolism and TCA cycle metabolites
Chromatography
An Acquity UPLC system (Waters, USA) equipped with a CORTECS T3 2.7 μm (2.1×30 mm) column at 60°C was used for acylcarnitine (AC), NEFA, bile acid and tryptophan metabolite analyses and an Acquity CSH Phenyl-Hexyl 2.1×100 mm column at 80°C was used for analysis of TCA cycle intermediates, following published protocols [34]. For details, see ESM Methods.
MS
The Acquity UPLC system was coupled to a Xevo TQ-XS mass spectrometer (Waters). Analyses were performed using multiple reaction monitoring (MRM). For transitions and ionisation conditions, see ESM Tables 2−6. For AC and tryptophan metabolite analyses, positive electrospray ionisation was used. For NEFA, bile acid and TCA cycle analyses, negative electrospray ionisation was used. A cone gas flow rate of 50 ml/h and a desolvation temperature of 650°C were used.
MS data analysis
Data were processed and peak integration performed using Waters TargetLynx Version 4.1 (Waters, USA). Integrated AC, NEFA, bile acid, tryptophan metabolite and TCA cycle metabolite peak areas were normalised to the palmitoyl-l-carnitine-(N-methyl-d3), palmitic acid-d31, deoxycholic acid-d6, l-tryptophan-d5 or citric acid-d4 internal standard, respectively.
Data analysis
Univariate
Pearson’s correlation, performed using Prism (version 6; Graphpad, USA), determined the relationship between both HbA1c and metabolite concentrations and . ANOVA, two-way repeated measures ANOVA with Bonferroni correction, and receiver operating characteristic (ROC) curve analysis were performed in MetaboAnalyst (version. 4.0.14) (https://www.metaboanalyst.ca/) [35]. Nominal significance was p<0.05.
Multivariate
Multivariate analysis was performed using MetaboAnalyst. Study size power calculations were conducted using MetaboAnalyst and published methods [36]. Datasets were mean-centred and auto-scaled before analysis using partial least-squares discriminant analysis (PLS-DA). PLS-DA models were validated using the permutation test (see ESM Methods). Metabolite changes responsible for clustering or regression trends within the pattern recognition models were identified by interrogating the corresponding loadings plot. Metabolites identified in the variable importance in projections (VIP)/coefficients plots were deemed to have changed globally if they contributed to separation in the models with 95% confidence limits and a VIP score >1. For comparisons between two groups, these were verified using univariate Student’s t test (fold change ≥1.2; p<0.05) and displayed as scatterplots or tables. For multiple group comparisons, these were confirmed by two-way ANOVA with Tukey’s or Bonferroni post hoc (as stated) and displayed as scatterplots or tables. ANOVA simultaneous component analysis (ASCA), performed in Metaboanalyst, was used for analysis of multivariate datasets, with both multiple group comparison and a longitudinal component (see ESM Methods) [37].
Results
All participants completed the exercise protocol (mean ± SD 8.06±5.09% incline at 4.30±0.47 km/h), mean 58.8% of , with no between-group differences (p=0.907). No hypoglycaemic incidences occurred during the exercise intervention. Six diabetic participants received additional carbohydrates (10 g) during exercise (when glucose <7 mmol/l): one C-peptide undetectable; two C-peptide low; and three C-peptide high. Pre- and post-exercise, and recovery blood glucose concentrations are shown in ESM Table 7 and were reported previously [28].
HbA1c concentration inversely correlates with maximal aerobic capacity in people with type 1 diabetes
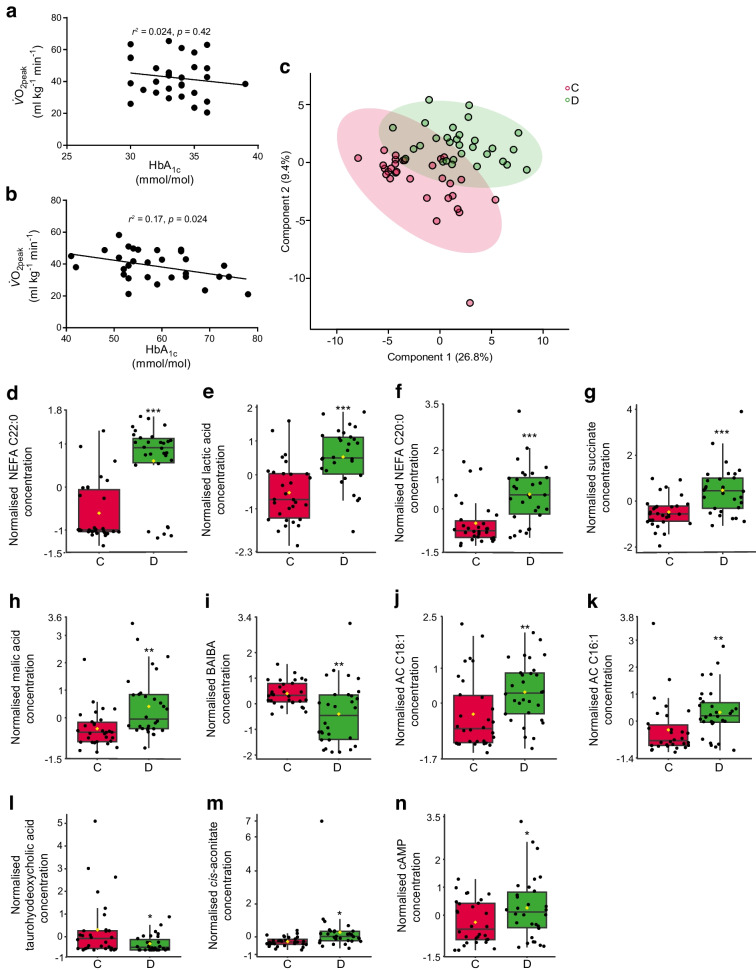
The control and diabetes populations did not differ for (Table 1). We examined the correlation between and HbA1c in control participants (Fig. 1a) (r2=0.024, p=0.42) and participants with type 1 diabetes using Pearson correlation. Perturbed glycaemic control (HbA1c) was significantly inversely correlated with in diabetic participants (Fig. 1b) (r2=0.17, p=0.024).
Fig. 1.
Relationship between long-term glycaemic control (HbA1c mmol/mol) and maximal aerobic capacity () in participants with type 1 diabetes. (a, b) Correlation between HbA1c and in non-diabetic control participants (n=30; r2=0.024, p=0.42) (a) and in participants with type 1 diabetes (n=30; r2=0.17, p=0.024) (b). (c–n) Metabolomics and multivariate statistics identify a metabolic signature for type 1 diabetes. (c) PLS-DA plot of metabolomic data from serum samples at baseline showing separation of control (red) and type 1 diabetes (green) groups. 95% confidence regions are displayed. (d–n) Metabolites distinguishing control participants from participants with type 1 diabetes at baseline as determined by PLS-DA VIP scores >1 and confirmed by Student’s t test. Univariate analysis is given in Table 3. Box and whisker plots show 25th and 75th percentile and the median. Upper whisker is Q3+1.5×IQR; lower whisker is Q1−1.5×IQR. Data are mean-centred and auto-scaled (normalised). Yellow point is median value. ***p<0.001, **p<0.01, *p<0.05. n=30 for control group and type 1 diabetes group. C, non-diabetes control; D, type 1 diabetes
Metabolomics identifies a metabolic signature for type 1 diabetes at baseline
To characterise the metabolic signature of people with type 1 diabetes at rest, we used targeted metabolomics to compare baseline rested serum of control participants and volunteers with type 1 diabetes. Multivariate statistics using PLS-DA identified metabolite concentrations that distinguish control participants from those with diabetes at baseline (Fig. 1c and ESM Fig. 1) (permutation test, p=0.0005). Differentiating metabolites were established if they both contributed to the PLS-DA model with VIP score >1 and had a significant difference between groups by univariate Student’s t test (Fig. 1d–n, Table 3). These included higher concentrations of long-chain NEFA, NEFA C22:0 (p<0.001) (Fig. 1d) and C20:0 (p<0.001) (Fig. 1f) and, TCA cycle intermediates succinate (p<0.001) (Fig. 1g), malate (p<0.01) (Fig. 1h) and cis-aconitate (p<0.05) (Fig. 1m), and lactate (p<0.001) (Fig. 1e) in the serum of diabetic participants, reflecting differential energy metabolism (Fig. 1d–n, Table 3). Serum levels of exercise-regulated myokine β-aminoisobutyric acid (BAIBA) [38, 39] were lower in diabetic participants (p<0.01) (Fig. 1i, Table 3).
Table 3.
Serum metabolites significantly differing between non-diabetes control participants and participants with type 1 diabetes at rest (baseline)
| Metabolite | Fold change | Log2(fold change) | p value |
|---|---|---|---|
| NEFA C22:0 | 1.6834 | 0.75142 | 3.63×10−8 |
| Lactic acid | 1.2215 | 0.28862 | 1.23×10−5 |
| NEFA C20:0 | 1.6568 | 0.72838 | 4.45×10−5 |
| Succinate | 1.3117 | 0.39145 | 0.00011 |
| Malic acid | 1.3939 | 0.47913 | 0.00116 |
| BAIBA | 0.65902 | −0.60161 | 0.001335 |
| AC C18:1 | 1.5043 | 0.58909 | 0.001857 |
| AC C16:1 | 1.724 | 0.78579 | 0.003675 |
| Taurohyodeoxycholic acid | 0.2695 | −1.8917 | 0.020038 |
| cis-Aconitate | 1.5488 | 0.63113 | 0.025681 |
| cAMP | 1.4947 | 0.57985 | 0.044342 |
The table shows the fold change and log2(fold change) of metabolites in the serum of participants with type 1 diabetes compared with control participants at the baseline time point of the aerobic exercise study (n=30 for both groups)
p value was generated by two-tailed Student’s t test
The metabolic profile of aerobic exercise in people with and without type 1 diabetes
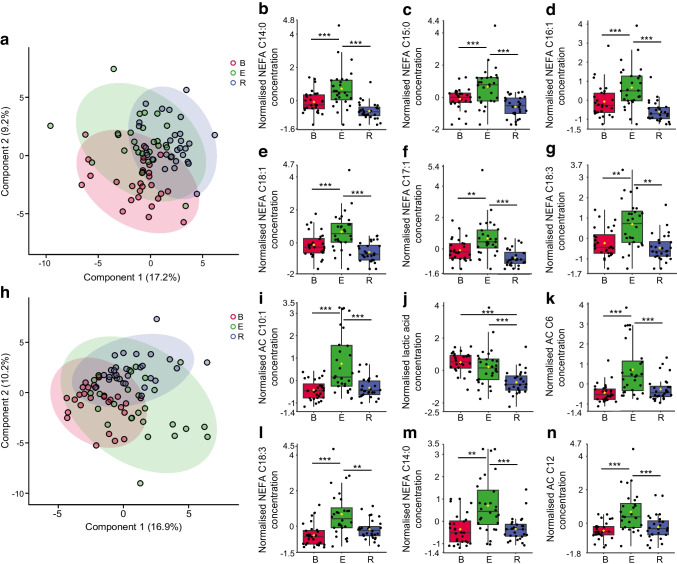
We characterised the metabolic signature of aerobic exercise in diabetic participants. We first used targeted metabolomics of baseline, exercise and post-exercise 1 h recovery serum from control participants to build a PLS-DA model of the metabolic profile of aerobic exercise in health (Fig. 2a and ESM Fig. 2) (permutation test, p<0.0005). Metabolites were determined to change in response to exercise and recovery if they had a VIP score >1 and a significant difference between groups by univariate one-way ANOVA (ESM Table 8). The top six most significant metabolites ranked by univariate p value are shown in Fig. 2b–g. In the control participants the metabolic response to exercise was dominated by an increase in circulating long-chain NEFA, including NEFA C14:0 (p<0.001) (Fig. 1b), C16:1 (p<0.001) (Fig. 1d) and C18:1 (p<0.001) (Fig. 1e), which returned to baseline concentration at the recovery time point (Fig. 2b–g and ESM Table 8).
Fig. 2.
The metabolic profile of aerobic exercise in non-diabetic control participants and participants with type 1 diabetes. (a) Multivariate PLS-DA plot of metabolomic data from baseline (red), exercise (green) and post-exercise 1 h recovery (blue) of non-diabetic control participants identifying metabolic differences between the three states. 95% confidence regions are displayed (n=30). (b–g) The top six metabolites representing the metabolic profile of aerobic exercise in non-diabetic control participants (ranked by univariate p value; ESM Table 8) (n=30). (h) Multivariate PLS-DA plot of metabolomic data from baseline (red), exercise (green) and post-exercise 1 h recovery (blue) of people with type 1 diabetes identifying metabolic differences between the three states. 95% confidence regions are displayed (n=30). (i–n) The top six metabolites representing the metabolic profile of aerobic exercise in people with type 1 diabetes (ranked by univariate p value; Table 4) (n=30). Mean-centred and auto-scaled (normalised) data are shown. Box and whisker plots show 25th and 75th percentile and the median. Upper whisker is Q3+1.5×IQR, lower whisker is Q1−1.5×IQR. Yellow point is median value. ***p<0.001, **p<0.01. B, baseline; E, exercise; R, post-exercise 1 h recovery
Independently, we generated a PLS-DA model to characterise the metabolic phenotype of aerobic exercise and recovery in participants with type 1 diabetes (Fig. 2h and ESM Fig. 3) (permutation test, p<0.0005). Metabolites were determined to change in response to exercise and recovery in diabetes if they had a VIP score >1 and a significant difference between groups by univariate one-way ANOVA (Table 4). The top six most significant metabolites ranked by univariate p value are shown in Fig. 2i–n. The response to exercise in diabetic participants was characterised by an increase in NEFA species, consistent with observations for control participants. However, exercise in diabetic participants was uniquely characterised by increased circulating concentrations of short-, medium- and long-chain AC species including AC C6 (p<0.001), AC C8 (p<0.01), AC C10 (p<0.001), AC C10:1 (p<0.001), AC C12 (p<0.001), AC C14:1 (p<0.01) and AC C14:2 (p<0.01) (Table 4).
Table 4.
ANOVA showing serum metabolites altered following aerobic exercise in participants with type 1 diabetes
| Metabolite | F value | p value | FDR | Significant group differences (Tukey’s post hoc test) |
|---|---|---|---|---|
| AC C10:1 | 16.219 | 1.19×10−6 | 6.61×10−5 | E-B; E-R |
| Lactic acid | 15.83 | 1.57×10−6 | 6.61×10−5 | E-B; E-R |
| AC C6 | 14.51 | 4.11×10−6 | 0.000115 | E-B; E-R |
| NEFA C18:3 | 13.475 | 8.87×10−6 | 0.000161 | E-B; E-R |
| NEFA C14:0 | 13.372 | 9.59×10−6 | 0.000161 | E-B; E-R |
| AC C12 | 12.34 | 2.10×10−5 | 0.000294 | E-B; E-R |
| AC C12:1 | 12.045 | 2.63×10−5 | 0.000316 | E-B; E-R |
| Succinate | 11.746 | 3.32×10−5 | 0.000348 | E-B; E-R |
| NEFA C18:1 | 11.365 | 4.46×10−5 | 0.000384 | E-B; E-R |
| NEFA C17:1 | 11.335 | 4.57×10−5 | 0.000384 | E-B; E-R |
| NEFA C20:4 | 10.868 | 6.59×10−5 | 0.000503 | E-B; E-R |
| AC C14:1 | 9.5497 | 0.000189 | 0.001264 | E-B; E-R |
| AC C10 | 9.5065 | 0.000196 | 0.001264 | E-B; E-R |
| NEFA C16:1 | 9.3802 | 0.000217 | 0.0013 | E-B; E-R |
| NEFA C16:0 | 8.9702 | 0.000303 | 0.001695 | E-B; E-R |
| NEFA C15:0 | 8.3633 | 0.000499 | 0.00262 | E-B; E-R |
| NEFA C16:2 | 8.1612 | 0.00059 | 0.002917 | E-B; E-R |
| AC C14:2 | 7.8697 | 0.000753 | 0.003513 | E-B; E-R |
| NEFA C20:3 | 7.6202 | 0.000928 | 0.004104 | E-B; E-R |
| NEFA C20:5 | 6.578 | 0.002253 | 0.009463 | E-B; E-R |
| AC C8 | 6.4956 | 0.002419 | 0.009676 | E-B; E-R |
| NEFA C22:4 | 5.6039 | 0.005255 | 0.020064 | E-B; E-R |
| Glycolithocholic acid | 5.0903 | 0.008272 | 0.030209 | E-B |
| NEFA C12:0 | 4.8637 | 0.010121 | 0.035424 | E-B; E-R |
| Malic acid | 4.8019 | 0.010696 | 0.035939 | E-B; E-R |
N=30
p value generated by one-way ANOVA
B, baseline; E, aerobic exercise; FDR, false discovery rate; R, post-exercise 1 h recovery
The differential metabolic response to aerobic exercise in people with type 1 diabetes
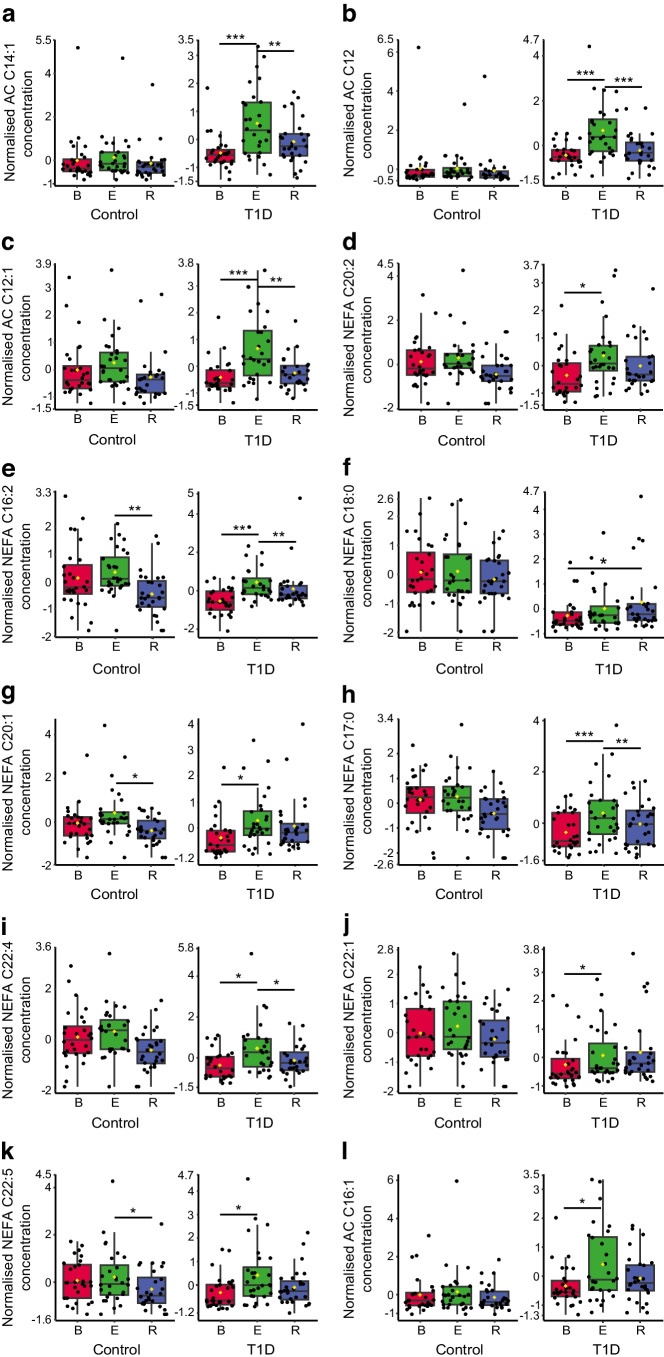
We directly compared the metabolomic data for exercise in control participants vs participants with type 1 diabetes. We used both univariate (two-way repeated measures ANOVA with Bonferroni post hoc) and multivariate (multivariate empirical Bayes ANOVA [MEBA] for time series [40]) approaches to facilitate the analysis of differences between groups (diabetes, control) having two or more within-subject factors (baseline, exercise and recovery). We identified the metabolites that were commonly significant by both multivariate and univariate approaches to define the similarities (ESM Table 9, ESM Fig. 4) and differences (Fig. 3a–l and ESM Table 10) in response to exercise in control participants and those with type 1 diabetes. Common responses to exercise were observed for multiple medium-chain and long-chain NEFA (ESM Table 9; ESM Fig. 4). However, there were distinct differences in the response of several AC species, including AC C14:1 (F value=12.25, p=0.001345) (Fig. 3a), AC C12 (F value=11.055, p=0.0018) (Fig. 3b), AC C12:1 (F value=9.768, p=0.0019) (Fig. 3c) and AC C16:1 (F value=5.511, p=0.029) (Fig. 3l), which increased in response to exercise in the type 1 diabetes group only (Fig. 3 and ESM Table 10).
Fig. 3.
Metabolic responses to aerobic exercise distinguishing non-diabetic control participants from participants with type 1 diabetes. (a–l) Serum metabolite species from baseline (red), exercise (green) and post-exercise 1 h recovery (blue) with distinct responses to exercise in control participants and participants with type 1 diabetes, identified with MEBA for time series, which is designed to compare temporal profiles across different biological conditions. These were confirmed using corresponding univariate two-way within-subject ANOVA with Bonferroni correction (data shown in ESM Table 10). Data are mean-centred and auto-scaled (normalised). Box and whisker plots show 25th and 75th percentile and the median. Upper whisker is Q3+1.5×IQR, lower whisker is Q1−1.5×IQR. Yellow point is median value. ***p<0.001, **p<0.01, *p<0.05. Control, n=30; type 1 diabetes, n=30. B, baseline; E, exercise; R, post-exercise 1 h recovery; T1D, type 1 diabetes
A metabolic pattern distinguishes C-peptide status in people with type 1 diabetes
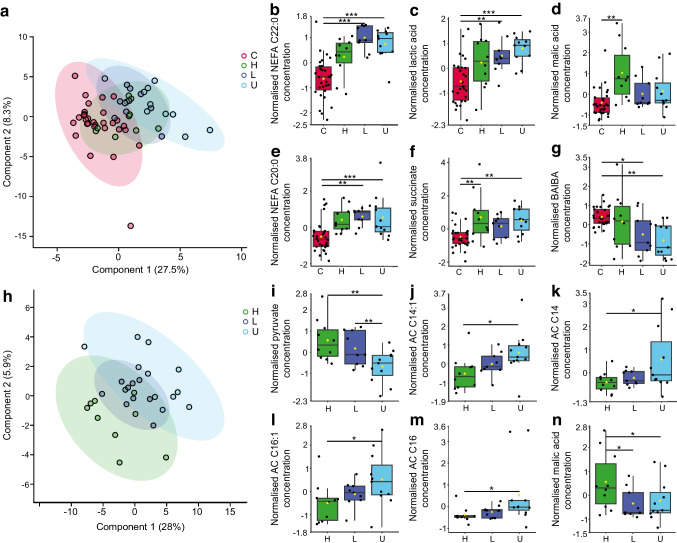
We characterised the contribution made by residual beta cell function to the metabolic phenotype of individuals with type 1 diabetes at rest. We grouped baseline serum metabolomic data according to diabetic participants’ peak C-peptide and compared these with control participant data using PLS-DA (Fig. 4a and ESM Fig. 5) (permutation test, p=0.01). By examining the metabolites with both PLS-DA VIP scores >1 and significance by one-way ANOVA with Tukey’s post hoc, we identified a unique metabolic pattern associated with C-peptide status in diabetic vs control participants (Fig. 4b–g). A residual beta cell function (C-peptide concentration)-associated effect was identified in increasing serum concentrations of lactate (p<0.001) (Fig. 4c) and succinate (p<0.001) (Fig. 4f), while BAIBA (Fig. 4g) concentration decreased (p<0.001) with reduced C-peptide relative to control (Fig. 4b–g and ESM Table 11). Higher concentrations of long-chain NEFA C20:0 (p<0.01) and C22:0 (p<0.001) distinguished low and undetected C-peptide groups from control and high C-peptide groups (Fig. 4b,e and ESM Table 11). Group separation in PLS-DA suggested that the variation between control and C-peptide groups was greater than that between individual C-peptide groups. To explore the metabolic phenotype distinguishing the C-peptide groups we generated a PLS-DA model for the three C-peptide groups only (Fig. 4h and ESM Figure 6) (permutation test, p=0.0245). Interrogation of the metabolite species with VIP scores >1, with confirmed significance by one-way ANOVA with Tukey’s post hoc, identified increases in ACs C14:1 (p<0.05), C14 (p<0.05), C16:1 (p<0.05) and C16 (p<0.05) as C-peptide decreases (Fig. 4j–m and ESM Table 12). The TCA cycle substrate pyruvate (p<0.01) and intermediate malic acid (p<0.05) distinguished the metabolic phenotype of the C-peptide groups (Fig. 4i,n and ESM Table 12).
Fig. 4.
A unique metabolic pattern distinguishes C-peptide status in people with type 1 diabetes. (a) PLS-DA plot of metabolomic data from serum samples at baseline showing separation of control participants (red) from participants with type 1 diabetes and high (green), low (dark blue) and undetectable (light blue) plasma C-peptide. (b–g) Metabolites distinguishing control participants from diabetic participants with high, low and undetectable plasma C-peptide at baseline as determined by PLS-DA VIP scores >1 and confirmed by one-way ANOVA with Tukey’s post hoc. Univariate analysis is shown in ESM Table 11. (h) PLS-DA plot of metabolomic data from serum samples at baseline showing separation of high (green), low (dark blue) and undetectable (light blue) plasma C-peptide groups within the type 1 diabetes group. (i–n) Metabolites distinguishing high, low and undetectable plasma C-peptide groups at baseline as determined by PLS-DA VIP scores >1 and confirmed by one-way ANOVA with Tukey’s post hoc. Data are mean-centred and auto-scaled (normalised). Box and whisker plots show 25th and 75th percentile and the median. Upper whisker is Q3+1.5×IQR, lower whisker is Q1−1.5×IQR. Yellow point is median value. Univariate analysis is shown in ESM Table 12. ***p<0.001, **p<0.01, *p<0.05. C, control; H, high C-peptide (≥200 pmol/l); L, low C-peptide (3–200 pmol/l); U, undetectable C-peptide (<3 pmol/l)
C-peptide status influences the metabolic response to exercise in type 1 diabetes
We defined the contribution that residual beta cell function makes to the metabolic profile of exercise in individuals with type 1 diabetes. As this dataset incorporates multiple group comparison (control, undetectable, low and high C-peptide groups) across longitudinal measures (baseline, exercise, recovery), we applied ASCA, a statistical generalisation of ANOVA, to the multivariate case [37]. We identified metabolites exhibiting a differential effect to exercise dependent on C-peptide status (ESM Table 13, ESM Fig. 7). Distinguishing metabolites included AC C16:1 (leverage=0.05; squared prediction error=5.94), AC C18:1 (leverage=0.066; squared prediction error=3.07), NEFA C20:0 (leverage=0.064; squared prediction error=4.45) and BAIBA (leverage=0.062; squared prediction error=8.14).
The basal metabolome is associated with maximal aerobic capacity in people with type 1 diabetes
We used Pearson correlation to establish the metabolites from the baseline serum samples that significantly correlated with across our total study population (ESM Table 14). Multiple serum long-chain NEFAs demonstrated a significant inverse correlation to .
We then identified the relationship between the baseline serum metabolome and specifically relevant to our type 1 diabetes and control groups to investigate whether distinct metabolites associated with maximal aerobic capacity in type 1 diabetes. The long-chain NEFAs were significantly inversely correlated with in our control group (ESM Table 15). Contrastingly, serum NEFAs did not significantly correlate with in type 1 diabetes (Table 5). The TCA cycle intermediates malic acid (r=−0.5, p<0.01) and cis-aconitate (r=−0.4, p<0.05) inversely correlated with in diabetic participants, while the TCA cycle substrate pyruvate (r=0.4, p<0.05) significantly positively correlated with (Table 5).
Table 5.
Baseline rested serum metabolites significantly correlated with in the type 1 diabetes study population
| Metabolites | Correlation | t statistic | p value |
|---|---|---|---|
| Malic acid | −0.49834 | −3.0415 | 0.005068 |
| NEFA C12:0 | −0.48092 | −2.9025 | 0.00714 |
| cis-Aconitate | −0.39626 | −2.2837 | 0.030175 |
| Pyruvate | 0.36872 | 2.099 | 0.044959 |
Correlation analysis by Pearson r showing correlation, t statistic and p value; N=30
The metabolome as a diagnostic for maximal aerobic capacity in people with type 1 diabetes
To further investigate the potential of the metabolome as a diagnostic tool in determining lower maximal aerobic capacity in people with type 1 diabetes, we structured our dataset for diagnostic analysis by separating the metabolomic data for control and type 1 diabetes populations. These were classified as either low maximal aerobic capacity (bottom 50% of the study population for ; positive for condition) or high maximal aerobic capacity (top 50% of the study population for ; negative for condition). We used ROC curves to investigate whether metabolites have diagnostic potential in distinguishing individuals with lower maximal aerobic capacity from those with higher maximal aerobic capacity within both the control and type 1 diabetes population. ROC curves plot the sensitivity against specificity of a test [41]. The AUC summarises the test diagnostic efficacy: 0 indicates a wholly inaccurate test; 1 indicates a fully accurate test; and 0.7–0.8 and 0.8–0.9 indicates acceptable and excellent diagnostic efficacy, respectively [41].
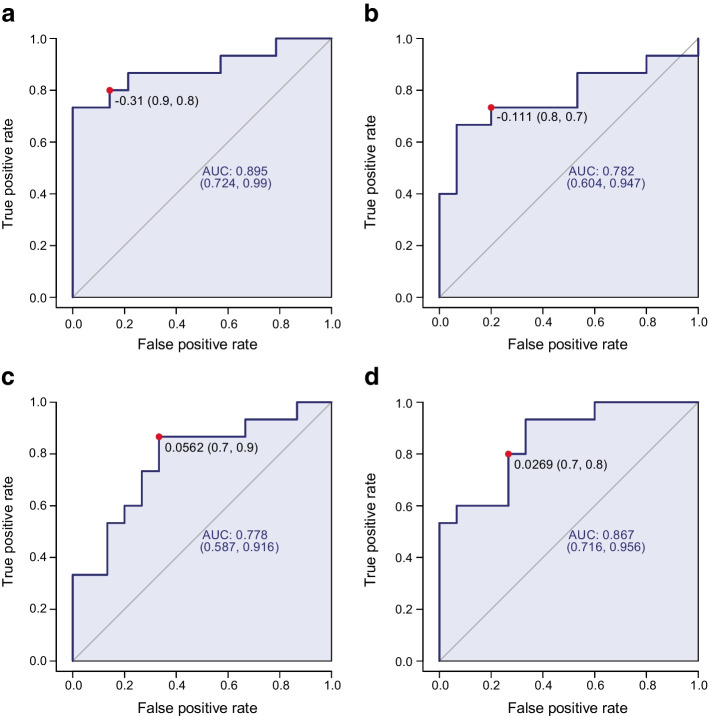
ROC curve analysis of baseline serum metabolites of control participants with low and high maximal aerobic capacity indicated that NEFA C16:1 had the greatest ROC curve AUC (0.895 [95% CI 0.724, 0.99]; p=0.0026, sensitivity=0.9, specificity=0.8) (Fig. 5a). Rested serum NEFA C16:1 exhibited excellent diagnostic efficacy in differentiating low from high maximal aerobic capacity in healthy individuals.
Fig. 5.
ROC curve diagnostic assessment of maximal aerobic capacity (distinguishing top and bottom 50% of population for ) for NEFA 16:1 in baseline serum of healthy control participants (p=0.0026) (a), and pyruvate (p=0.013) (b), malic acid (p=0.013) (c) and the malic acid/pyruvate ratio (p=0.00018) (d) in baseline serum of participants with type 1 diabetes. ROC curves indicate cut offs, sensitivity, specificity, AUC and 95% CI. The diagonal line represents points at which the false positive rates = the true positive rates. The red dot indicates the optimal cutoff with the associated sensitivity and specificity values. Control participants, n=30; participants with type 1 diabetes, n=30
Next, we determined the metabolome’s utility in distinguishing people with low vs high maximal aerobic capacity in our type 1 diabetes population. ROC curve analysis of baseline serum metabolites in low and high maximal aerobic capacity diabetic participants identified that the TCA cycle substrate pyruvate was the most discriminatory metabolite (AUC 0.782 [95% CI 0.604, 0.947]; p=0.013, sensitivity=0.8, specificity=0.7) (Fig. 5b), followed by the TCA cycle metabolite malic acid (AUC 0.778 [95% CI 0.587, 0.916]; p=0.013, sensitivity=0.7, specificity=0.9) (Fig. 5c), with AUCs of 0.7–0.8 indicating these are only adequate as diagnostic indicators of maximal aerobic capacity.
Serum pyruvate and malic acid exhibited reciprocal correlations with maximal aerobic capacity in the type 1 diabetes population: pyruvate was positively and malic acid negatively correlated to (Table 5). Therefore, we examined whether the malic acid/pyruvate ratio is a more specific and sensitive diagnostic marker for maximal aerobic capacity in the type 1 diabetes population. ROC curve analysis identified that the malic acid/pyruvate ratio (AUC 0.867 [95% CI 0.716, 0.956]; p=0.00018, sensitivity=0.7, specificity=0.8) (Fig. 5d), displayed a stronger diagnostic efficiency than either of the metabolites alone, suggesting that this ratio offers potential as a diagnostic marker for maximal aerobic capacity in individuals with type 1 diabetes.
Discussion
We used targeted metabolomics to define the metabolic baseline phenotype and response to exercise in serum of individuals with type 1 diabetes and control individuals. We showed that residual beta cell function contributes to the metabolic phenotype of individuals with type 1 diabetes both at rest and in response to acute aerobic exercise. During aerobic exercise, NEFAs are released from triacylglycerol in adipose tissue via lipolysis and enter the circulation to provide fuel for contracting muscle, where they are converted to ACs to enter the mitochondria and undergo β-oxidation [42–44]. This response is reflected in our data by the increased serum NEFAs common to control and diabetic participants in aerobic exercise. Unique to people with type 1 diabetes was an increase in serum ACs in aerobic exercise, a feature that also distinguishes the metabolic effect of lower residual beta cell function. We identified that the serum metabolome of both control and diabetic participants at rest was related to maximal aerobic capacity. In healthy volunteers, this relationship was characterised by an inverse correlation between medium/long-chain NEFAs and . In diabetic participants this relationship was distinctly described by an inverse correlation with TCA cycle metabolites malic acid and cis-aconitate and a positive correlation with TCA cycle substrate pyruvate. A key driver for reduced physical activity in people with type 1 diabetes is fear of hypoglycaemia [18], although this is multifactorial, with exercise intolerance [3–10] and high inter-individual variation in the metabolic response to exercise [16, 17] contributing. Such barriers limit the use of exercise as a therapy and reduce population-wide uptake, with low maximal aerobic capacity contributing to worse outcomes and risk of diabetes complications [15]. Therefore, we used ROC curves to identify the diagnostic potential of metabolites for maximal aerobic capacity. The malic acid/pyruvate ratio was revealed to be a putative diagnostic marker for differentiating maximal aerobic capacity specifically in individuals with type 1 diabetes. Recently, altered mitochondrial function has been described in the skeletal muscle in type 1 diabetes [2, 45, 46]. Moreover, blood ACs are a marker of mitochondrial (dys)function [44, 47]. Therefore, perturbed serum ACs and TCA cycle intermediates, mitochondrial metabolite species, during exercise may reflect dysfunction of skeletal muscle mitochondria in type 1 diabetes.
Study limitations include participant recruitment from a single cohort and centre. Most of the diabetic participants had good glycaemic control and exercise testing was conducted under laboratory conditions with acute exercise intervention and close monitoring of blood glucose levels. Although our experimental groups did not differ for sex and therefore our findings may be generalisable across sexes in the type 1 diabetes population, we did not directly investigate interacting variables (e.g. age, sex and duration of diabetes, which were not significantly different between groups in our study) that may contribute to both the metabolome and maximal aerobic capacity in the diabetic population.
Exogenous insulin may blunt the metabolic response to exercise in type 1 diabetes [48]. We did not observe this phenomenon, possibly because our volunteers with type 1 diabetes arrived fasted, maintaining only basal exogenous insulin delivery. In addition, although C-peptide is a robust equimolar insulin secretion marker and indicator of beta cell function in response to an MMTT [49], other factors such as insulin resistance and renal impairment can affect serum C-peptide concentration [50]. However, these increase C-peptide concentrations [50] and given our volunteers have normal kidney function and did not exhibit between-group difference for HbA1c, are unlikely to have affected our study. Additionally, C-peptide has direct insulin-independent metabolic effects on several tissues (e.g. sensory nerves, vasculature) [31]. The C-peptide-associated metabolic responses to exercise may be influenced by these direct effects and not residual beta cell insulin secretion alone. This may be pertinent, as the high-C-peptide group had a slightly shorter duration of diabetes than comparator groups. Nevertheless, four volunteers within this group had diabetes for >15 years, and recruitment of volunteers ≥3 years post diagnoses reduces the impact of the early honeymoon phase.
To our knowledge, we are the first to suggest a circulating metabolome-derived marker of maximal aerobic capacity in people with type 1 diabetes. This approach may facilitate future studies validating the serum malic acid/pyruvate ratio as a biomarker of maximal aerobic capacity in the wider type 1 diabetes population. Our approach may expedite identification of patients with type 1 diabetes and lower maximal aerobic capacity to target structured support for exercise. Moreover, future, higher-powered, studies will be important to investigate the influence of other interacting variables (e.g. age of diabetes onset, diabetes duration) and could focus on whether metabolomic signatures associate with increased hypoglycaemic risk during and post exercise. This approach could enable the identification of individuals at highest risk and generate personalised medicine strategies to reduce exercise-induced hypoglycaemia in those most in need. It will be important to establish the metabolomic response to exercise in fasting, fed and high and low exogenous insulin conditions, while carefully avoiding hypo- and hyperglycaemia, to determine the contribution of exogenous insulin to the metabolic response to aerobic exercise in type 1 diabetes. Studies of the interaction between the metabolome and exercise capacity in differing exercise modalities such as resistance and high-intensity interval training, with physiological markers distinct from and over longer durations, are needed. The analysis of blood ACs and TCA cycle intermediates in people with type 1 diabetes, with concomitant assessment of muscle mitochondrial function, may inform personalised medicine approaches to target aerobic exercise programmes to improve muscle health.
In summary, people with type 1 diabetes exhibit a unique metabolic phenotype in response to aerobic exercise, characterised by increased circulating ACs. We identify a metabolic signature of perturbed serum TCA cycle intermediates at rest, correlating with maximal aerobic capacity in people with type 1 diabetes. The serum malic acid/pyruvate ratio may have diagnostic potential in determining individuals with type 1 diabetes and lower maximal aerobic capacity. The putative metabolite markers may inform identification, management and therapy of lower maximal aerobic capacity in exercise in people with type 1 diabetes.
Supplementary Information
Below is the link to the electronic supplementary material.
Abbreviations
- AC
Acylcarnitine
- ASCA
ANOVA simultaneous component analysis
- BAIBA
β-Aminoisobutyric acid
- CRF
Clinical research facility
- MEBA
Multivariate Empirical Bayes ANOVA
- MMTT
Mixed-meal tolerance test
- PLS-DA
Partial least-squares discriminant analysis
- ROC
Receiver operating characteristic
- TCA
Tricarboxylic acid
- VIP
Variable importance in projections
Acknowledgements
Data describing the demographics of the study population in this manuscript have been included in the PhD thesis ‘The role of residual beta cell function on glycaemic and vascular outcomes at rest and post exercise in people with type 1 diabetes’ by the study author G. S. Taylor [51]. We acknowledge C. Arden for our Graphical Abstract produced using BioRender.
Data availability
All data are available from the authors on reasonable request.
Funding
LDR acknowledges the support of the Biotechnology and Biological Research Council (BB/R013500/1) and Diabetes UK (19/0006049). The exercise study was funded by the Diabetes Research and Wellness Foundation (SCA/OF/12/15) award to DJW. Funding was also provided by the Francis James Bell Endowment Fund, County Durham Community Foundation to DJW.
Authors’ relationships and activities
The authors declare that there are no relationships or activities that might bias, or be perceived to bias, their work.
Contribution statement
GST and JAS recruited participants. GST, DJW, JAS, LDR, KS and JS designed the study. GST and DJW researched data; KS and JS collected data. LDR performed all metabolomics analysis. TJM designed C-peptide analysis protocols and measured and interpreted C-peptide status. JAS provided clinical cover. All authors contributed to drafting the article and reviewing it critically for intellectual content. All authors approved the final version of the paper. LDR is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Daniel J. West and Lee D. Roberts contributed equally to this article.
Contributor Information
Daniel J. West, Email: Daniel.West@newcastle.ac.uk
Lee D. Roberts, Email: L.D.Roberts@leeds.ac.uk
References
- 1.Colberg SR, Sigal RJ, Yardley JE, et al. Physical activity/exercise and diabetes: a position statement of the American Diabetes Association. Diabetes Care. 2016;39:2065–2079. doi: 10.2337/dc16-1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Minnock D, Annibalini G, Valli G, et al. Altered muscle mitochondrial, inflammatory and trophic markers, and reduced exercise training adaptations in type 1 diabetes. J Physiol. 2022;600:1405–1418. doi: 10.1113/JP282433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gusso S, Hofman P, Lalande S, Cutfield W, Robinson E, Baldi JC. Impaired stroke volume and aerobic capacity in female adolescents with type 1 and type 2 diabetes mellitus. Diabetologia. 2008;51:1317–1320. doi: 10.1007/s00125-008-1012-1. [DOI] [PubMed] [Google Scholar]
- 4.Gusso S, Pinto T, Baldi JC, et al. Exercise training improves but does not normalize left ventricular systolic and diastolic function in adolescents with type 1 diabetes. Diabetes Care. 2017;40:1264–1272. doi: 10.2337/dc16-2347. [DOI] [PubMed] [Google Scholar]
- 5.Gusso S, Pinto TE, Baldi JC, Robinson E, Cutfield WS, Hofman PL. Diastolic function is reduced in adolescents with type 1 diabetes in response to exercise. Diabetes Care. 2012;35:2089–2094. doi: 10.2337/dc11-2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Komatsu WR, Gabbay MA, Castro ML, et al. Aerobic exercise capacity in normal adolescents and those with type 1 diabetes mellitus. Pediatr Diabetes. 2005;6:145–149. doi: 10.1111/j.1399-543X.2005.00120.x. [DOI] [PubMed] [Google Scholar]
- 7.Koponen AS, Peltonen JE, Paivinen MK, et al. Low total haemoglobin mass, blood volume and aerobic capacity in men with type 1 diabetes. Eur J Appl Physiol. 2013;113:1181–1188. doi: 10.1007/s00421-012-2532-4. [DOI] [PubMed] [Google Scholar]
- 8.Peltonen JE, Koponen AS, Pullinen K, et al. Alveolar gas exchange and tissue deoxygenation during exercise in type 1 diabetes patients and healthy controls. Respir Physiol Neurobiol. 2012;181:267–276. doi: 10.1016/j.resp.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 9.Rissanen AP, Tikkanen HO, Koponen AS, Aho JM, Peltonen JE. Central and peripheral cardiovascular impairments limit VO(2peak) in type 1 diabetes. Med Sci Sports Exerc. 2015;47:223–230. doi: 10.1249/MSS.0000000000000419. [DOI] [PubMed] [Google Scholar]
- 10.Goulding RP, Roche DM, Scott SN, Koga S, Weston PJ, Marwood S. Limitations to exercise tolerance in type 1 diabetes: the role of pulmonary oxygen uptake kinetics and priming exercise. J Appl Physiol (1985) 2020;128:1299–1309. doi: 10.1152/japplphysiol.00892.2019. [DOI] [PubMed] [Google Scholar]
- 11.Riddell MC, Gallen IW, Smart CE, et al. Exercise management in type 1 diabetes: a consensus statement. Lancet Diabetes Endocrinol. 2017;5:377–390. doi: 10.1016/S2213-8587(17)30014-1. [DOI] [PubMed] [Google Scholar]
- 12.Temple MY, Bar-Or O, Riddell MC. The reliability and repeatability of the blood glucose response to prolonged exercise in adolescent boys with IDDM. Diabetes Care. 1995;18:326–332. doi: 10.2337/diacare.18.3.326. [DOI] [PubMed] [Google Scholar]
- 13.Abraham MB, Davey RJ, Cooper MN, et al. Reproducibility of the plasma glucose response to moderate-intensity exercise in adolescents with type 1 diabetes. Diabet Med. 2017;34:1291–1295. doi: 10.1111/dme.13395. [DOI] [PubMed] [Google Scholar]
- 14.Tansey MJ, Tsalikian E, Beck RW, et al. The effects of aerobic exercise on glucose and counterregulatory hormone concentrations in children with type 1 diabetes. Diabetes Care. 2006;29:20–25. doi: 10.2337/diacare.29.01.06.dc05-1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Riddell MC, Peters AL. Exercise in adults with type 1 diabetes mellitus. Nat Rev Endocrinol. 2023;19:98–111. doi: 10.1038/s41574-022-00756-6. [DOI] [PubMed] [Google Scholar]
- 16.Yardley JE, Brockman NK, Bracken RM. Could age, sex and physical fitness affect blood glucose responses to exercise in type 1 diabetes? Front Endocrinol (Lausanne) 2018;9:674. doi: 10.3389/fendo.2018.00674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bohn B, Herbst A, Pfeifer M, et al. Impact of physical activity on glycemic control and prevalence of cardiovascular risk factors in adults with type 1 diabetes: a cross-sectional multicenter study of 18,028 patients. Diabetes Care. 2015;38:1536–1543. doi: 10.2337/dc15-0030. [DOI] [PubMed] [Google Scholar]
- 18.Brazeau AS, Rabasa-Lhoret R, Strychar I, Mircescu H. Barriers to physical activity among patients with type 1 diabetes. Diabetes Care. 2008;31:2108–2109. doi: 10.2337/dc08-0720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wishart DS. Emerging applications of metabolomics in drug discovery and precision medicine. Nat Rev Drug Discov. 2016;15:473–484. doi: 10.1038/nrd.2016.32. [DOI] [PubMed] [Google Scholar]
- 20.Roberts LD, Gerszten RE. Toward new biomarkers of cardiometabolic diseases. Cell Metab. 2013;18:43–50. doi: 10.1016/j.cmet.2013.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roberts LD, Koulman A, Griffin JL. Towards metabolic biomarkers of insulin resistance and type 2 diabetes: progress from the metabolome. Lancet Diabetes Endocrinol. 2014;2:65–75. doi: 10.1016/S2213-8587(13)70143-8. [DOI] [PubMed] [Google Scholar]
- 22.Griffin JL, Nicholls AW, Keun HC, Mortishire-Smith RJ, Nicholson JK, Kuehn T. Metabolic profiling of rodent biological fluids via 1H NMR spectroscopy using a 1 mm microlitre probe. Analyst. 2002;127:582–584. doi: 10.1039/b201207c. [DOI] [PubMed] [Google Scholar]
- 23.Williams GM, Long AE, Wilson IV, et al. Beta cell function and ongoing autoimmunity in long-standing, childhood onset type 1 diabetes. Diabetologia. 2016;59:2722–2726. doi: 10.1007/s00125-016-4087-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oram RA, McDonald TJ, Shields BM, et al. Most people with long-duration type 1 diabetes in a large population-based study are insulin microsecretors. Diabetes Care. 2015;38:323–328. doi: 10.2337/dc14-0871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buckingham B, Cheng P, Beck RW, et al. CGM-measured glucose values have a strong correlation with C-peptide, HbA1c and IDAAC, but do poorly in predicting C-peptide levels in the two years following onset of diabetes. Diabetologia. 2015;58:1167–1174. doi: 10.1007/s00125-015-3559-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brooks AM, Oram R, Home P, Steen N, Shaw JA. Demonstration of an intrinsic relationship between endogenous C-peptide concentration and determinants of glycemic control in type 1 diabetes following islet transplantation. Diabetes Care. 2015;38:105–112. doi: 10.2337/dc14-1656. [DOI] [PubMed] [Google Scholar]
- 27.Rickels MR, Evans-Molina C, Bahnson HT, et al. High residual C-peptide likely contributes to glycemic control in type 1 diabetes. J Clin Invest. 2020;130:1850–1862. doi: 10.1172/JCI134057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taylor GS, Smith K, Capper TE, et al. Postexercise glycemic control in type 1 diabetes is associated with residual beta-cell function. Diabetes Care. 2020;43:2362–2370. doi: 10.2337/dc20-0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scholin A, Berne C, Schvarcz E, Karlsson FA, Bjork E. Factors predicting clinical remission in adult patients with type 1 diabetes. J Intern Med. 1999;245:155–162. doi: 10.1046/j.1365-2796.1999.00426.x. [DOI] [PubMed] [Google Scholar]
- 30.Taylor GS, Shaw A, Smith K, et al. Type 1 diabetes patients increase CXCR4(+) and CXCR7(+) haematopoietic and endothelial progenitor cells with exercise, but the response is attenuated. Sci Rep. 2021;11:14502. doi: 10.1038/s41598-021-93886-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yosten GL, Maric-Bilkan C, Luppi P, Wahren J. Physiological effects and therapeutic potential of proinsulin C-peptide. Am J Physiol Endocrinol Metab. 2014;307:E955–968. doi: 10.1152/ajpendo.00130.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lundberg R, Beilman GJ, Dunn TB, et al. Early alterations in glycemic control and pancreatic endocrine function in nondiabetic patients with chronic pancreatitis. Pancreas. 2016;45:565–571. doi: 10.1097/MPA.0000000000000491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bruce RA, Kusumi F, Hosmer D. Maximal oxygen intake and nomographic assessment of functional aerobic impairment in cardiovascular disease. Am Heart J. 1973;85:546–562. doi: 10.1016/0002-8703(73)90502-4. [DOI] [PubMed] [Google Scholar]
- 34.Zougman A, Wilson JP, Roberts LD, Banks RE. Detergent-free simultaneous sample preparation method for proteomics and metabolomics. J Proteome Res. 2020;19:2838–2844. doi: 10.1021/acs.jproteome.9b00662. [DOI] [PubMed] [Google Scholar]
- 35.Chong J, Wishart DS, Xia J. Using MetaboAnalyst 4.0 for comprehensive and integrative metabolomics data analysis. Curr Protoc Bioinformatics. 2019;68:e86. doi: 10.1002/cpbi.86. [DOI] [PubMed] [Google Scholar]
- 36.van Iterson M, t Hoen PA, Pedotti P, et al. Relative power and sample size analysis on gene expression profiling data. BMC Genomics. 2009;10:439. doi: 10.1186/1471-2164-10-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smilde AK, Jansen JJ, Hoefsloot HC, Lamers RJ, van der Greef J, Timmerman ME. ANOVA-simultaneous component analysis (ASCA): a new tool for analyzing designed metabolomics data. Bioinformatics. 2005;21:3043–3048. doi: 10.1093/bioinformatics/bti476. [DOI] [PubMed] [Google Scholar]
- 38.Roberts LD, Bostrom P, O'Sullivan JF, et al. Beta-aminoisobutyric acid induces browning of white fat and hepatic beta-oxidation and is inversely correlated with cardiometabolic risk factors. Cell Metab. 2014;19:96–108. doi: 10.1016/j.cmet.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roberts LD, Ashmore T, McNally BD, et al. Inorganic nitrate mimics exercise-stimulated muscular fiber-type switching and myokine and gamma-aminobutyric acid release. Diabetes. 2017;66:674–688. doi: 10.2337/db16-0843. [DOI] [PubMed] [Google Scholar]
- 40.Tai YC, Speed TP. A multivariate empirical bayes statistic for replicated microarray time course data. Ann Stat. 2006;34:2387–2412. doi: 10.1214/009053606000000759. [DOI] [Google Scholar]
- 41.Mandrekar JN. Receiver operating characteristic curve in diagnostic test assessment. J Thorac Oncol. 2010;5:1315–1316. doi: 10.1097/JTO.0b013e3181ec173d. [DOI] [PubMed] [Google Scholar]
- 42.Wolfe RR. Fat metabolism in exercise. Adv Exp Med Biol. 1998;441:147–156. doi: 10.1007/978-1-4899-1928-1_14. [DOI] [PubMed] [Google Scholar]
- 43.Longo N, Frigeni M, Pasquali M. Carnitine transport and fatty acid oxidation. Biochim Biophys Acta. 2016;1863:2422–2435. doi: 10.1016/j.bbamcr.2016.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reuter SE, Evans AM. Carnitine and acylcarnitines: pharmacokinetic, pharmacological and clinical aspects. Clin Pharmacokinet. 2012;51:553–572. doi: 10.1007/BF03261931. [DOI] [PubMed] [Google Scholar]
- 45.Monaco CMF, Hughes MC, Ramos SV, et al. Altered mitochondrial bioenergetics and ultrastructure in the skeletal muscle of young adults with type 1 diabetes. Diabetologia. 2018;61:1411–1423. doi: 10.1007/s00125-018-4602-6. [DOI] [PubMed] [Google Scholar]
- 46.Cree-Green M, Newcomer BR, Brown MS, et al. Delayed skeletal muscle mitochondrial ADP recovery in youth with type 1 diabetes relates to muscle insulin resistance. Diabetes. 2015;64:383–392. doi: 10.2337/db14-0765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peng KY, Watt MJ, Rensen S, et al. Mitochondrial dysfunction-related lipid changes occur in nonalcoholic fatty liver disease progression. J Lipid Res. 2018;59:1977–1986. doi: 10.1194/jlr.M085613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brugnara L, Vinaixa M, Murillo S, et al. Metabolomics approach for analyzing the effects of exercise in subjects with type 1 diabetes mellitus. PLoS One. 2012;7:e40600. doi: 10.1371/journal.pone.0040600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Palmer JP, Fleming GA, Greenbaum CJ, et al. C-peptide is the appropriate outcome measure for type 1 diabetes clinical trials to preserve beta-cell function: report of an ADA workshop, 21–22 October 2001. Diabetes. 2004;53:250–264. doi: 10.2337/diabetes.53.1.250. [DOI] [PubMed] [Google Scholar]
- 50.Wahren J, Shafqat J, Johansson J, Chibalin A, Ekberg K, Jornvall H. Molecular and cellular effects of C-peptide–new perspectives on an old peptide. Exp Diabesity Res. 2004;5:15–23. doi: 10.1080/15438600490424479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Taylor GS (2020) The role of residual beta cell function on glycaemic and vascular outcomes at rest and post exercise in people with type 1 diabetes. [PhD Thesis], Newcastle University. Available from: https://theses.ncl.ac.uk/jspui/bitstream/10443/5226/1/Taylor%20G%20S%202020.pdf
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available from the authors on reasonable request.