Abstract
Because mutations in envelope glycoproteins of retroviruses or in their cell surface receptors can eliminate function by multiple mechanisms, it has been difficult to unambiguously identify sites for their interactions by site-directed mutagenesis. Recently, we developed a gain-of-function approach to overcome this problem. Our strategy relies on the fact that feline leukemia virus subgroup B (FeLV-B) and amphotropic murine leukemia virus (A-MLV) have closely related gp70 surface envelope glycoproteins and use related Na+-dependent phosphate symporters, Pit1 and Pit2, respectively, as their receptors. We previously observed that FeLV-B/A-MLV envelope glycoprotein chimeras spliced between the variable regions VRA and VRB were unable to use Pit1 or Pit2 as a receptor but could efficiently use specific Pit1/Pit2 chimeras. The latter study suggested that the VRA of A-MLV and FeLV-B functionally interact with the presumptive extracellular loops 4 and 5 (ECL4 and -5) of their respective receptors, whereas VRB interacts with ECL2. We also found that FeLV-B gp70 residues F60 and P61 and A-MLV residues Y60 and V61 in the first disulfide-bonded loop of VRA were important for functional interaction with the receptor's ECL4 or -5. We have now extended this approach to identify additional VRA and VRB residues that are involved in receptor recognition. Our studies imply that FeLV-B VRA residues F60 and P61 interact with the Pit1 ECL5 region, whereas VRA residues 66 to 78 interact with Pit1 ECL4. Correspondingly, A-MLV VRA residues Y60 and V61 interact with the Pit2 ECL5 region, whereas residues 66 to 78 interact with Pit2 ECL4. Similar studies that focused on the gp70 VRB implicated residues 129 to 139 as contributing to specific interactions with the receptor ECL2. These results identify three regions of gp70 that interact in a specific manner with distinct portions of their receptors, thereby providing a map of the functionally interacting surfaces.
Receptor recognition by murine leukemia viruses (MLVs) and feline leukemia viruses (FeLVs) is determined by the amino-terminal domain of their surface envelope glycoproteins (2, 4, 5, 7, 22, 28, 35). This domain consists of several conserved sequences that are interrupted by variable regions termed VRA and VRB. VRA and VRB are highly divergent between MLVs, FeLVs, and other type C retroviruses, both in length and in sequence, and they have been implicated in receptor specificity (4, 5, 7, 35). Most of the sequence diversities in VRA and VRB occur in regions that are enclosed by cysteine residues that are conserved in all mammalian type C envelope glycoproteins. As confirmed by a recent X-ray crystallographic study of an ecotropic MLV envelope glycoprotein (10), these conserved cysteines form disulfide-bonded loops, with two loops within VRA and one loop in VRB. Consistent with these ideas, specific residues critical for receptor recognition have been identified within the first disulfide-bonded loop of VRA (VRA1), in the envelope glycoproteins of ecotropic MLV (2), amphotropic MLV (A-MLV) (3, 35), and feline leukemia virus subgroups B (FeLV-B) (35) and C (8, 32). Residues outside VRA1 have also been implicated in receptor recognition. Two amino acids located between VRA1 and VRA2 in the envelope glycoprotein of PVC-211 MLV, an ecotropic MLV variant, are responsible for its enhanced ability to infect Chinese hamster ovary cells (21). Residues adjacent to VRA1 in A-MLV and 10A1 MLV envelope glycoproteins have also been implicated in receptor recognition (12, 13).
Surprisingly, the amino-terminal domains of FeLV-B and A-MLV gp70s share substantial sequence identity in VRA and VRB, despite their use of different cell surface receptors. FeLV-B uses the Na+-dependent phosphate symporter Pit1 (15, 26, 27, 37), which was originally identified as a receptor for gibbon ape leukemia virus (26), whereas A-MLV uses a related Na+-dependent phosphate symporter, Pit2 (15, 24, 38). Several studies have suggested that the presumptive extracellular loops 2 and 4 (ECL2 and -4) of Pit1 and Pit2 are essential for receptor function (14, 17–19, 30, 35, 36). Additional studies have implicated Pit1 ECL5 in FeLV-B infections (29, 35), and we have previously reported that Pit2 ECL5 may also be involved in A-MLV infections (35).
A major problem in previous efforts to identify critical sites involved in functional interactions of retroviral envelope glycoproteins and receptors has derived from the possible existence of multiple contact sites in both components. Consequently, identification of an important amino acid in either gp70 or its receptor does not provide evidence concerning its contact site in other component. Moreover, traditional mutagenesis approaches have been complicated because loss-of-function mutations in gp70 or the receptor can be caused by diverse mechanisms including global abnormalities of folding or posttranslational processing. Because of this problem, effects of mutations are often considered to be significant only if they reduce function by several orders of magnitude.
Recently, we initiated studies that can potentially overcome some of these problems. We found that viruses pseudotyped with several chimeric FeLV-B/A-MLV envelope glycoproteins were unable to utilize native Pit1 or Pit2 as a receptor but could efficiently use specific Pit1/Pit2 chimeras (35). This gain-of-function evidence enabled us to identify specific functional interactions of VRA and VRB with particular correspondent sites in the receptors. Specifically, we obtained evidence that FeLV-B and A-MLV VRA functionally interact with the receptor's ECL4/ECL5 region, whereas VRB interact with ECL2 (35). By using an FeLV-B/A-MLV chimeric envelope that was specifically deficient in its VRA interactions with a Pit1/Pit2 chimeric receptor and by substituting specific residues within this chimeric envelope so that it could gain receptor recognition, we determined that residues FeLV-B F60 and P61 and residues A-MLV Y60 and V61 were important for specific recognition of the receptor's ECL4/ECL5 region. As more thoroughly discussed below, subsequent mutagenesis studies of the A-MLV envelope glycoprotein appeared to contradict several of our results. Battini et al. reported that A-MLV VRB is not essential for Pit2 utilization (3). Furthermore, Han et al. suggested that A-MLV residues Y60 and V61 were not individually necessary for interaction with Pit2, whereas a sequence between VRA1 and VRA2 was essential (13).
We have attempted to address these issues and to develop a more comprehensive map of the functional gp70-receptor interactions. For this purpose, we generated pseudotype virions bearing mutant FeLV-B envelopes in which specific FeLV-B VRA sequences were substituted with corresponding A-MLV sequences, and we assessed the infectivity of the virions on mouse cells that expressed specific chimeric Pit1/Pit2 receptors. These Pit1/Pit2 chimeras were all active in phosphate transport, confirming their proper folding and processing to the cell surfaces. Moreover, all of the FeLV-B/A-MLV chimeric or mutant envelope glycoproteins used in this investigation were processed and incorporated into virion particles (data not shown). Our results strongly suggest that VRA1 residues 60 and 61 are important for receptor recognition and that they functionally interact in a specific manner with a receptor sequence in the presumptive ECL5 region. In addition, a gp70 sequence at positions 66 to 78, located between VRA1 and VRA2, specifically interacts with ECL4 of the receptor. Finally, we identified a region of A-MLV VRB that functionally interacts with ECL2 of the receptor. These results provide a map of the functionally important virus-receptor interactions and indicate that the virus specifically recognizes multiple sites in the receptor protein.
MATERIALS AND METHODS
Cell lines.
Mus dunni tail fibroblast (MDTF) cells and TELCeB6 packaging cells were maintained in Dulbecco's modified Eagle medium supplemented with 10% fetal bovine serum. TELCeB6 cells (provided by Y. Takeuchi and F. L. Cosset) (9) produce noninfectious virions which contain the nlslacZ retroviral vector (11). MDTF cells expressing chimeric Pit1/Pit2 proteins were generated by transduction with pseudotyped A-MLV containing chimeric Pit1/Pit2 genes. The pseudotype virions were generated by transfecting the Pit1/Pit2 cDNA expression vectors pLGGRSN, pLGRRSN, and pLGGrGSN (provided by A. Dusty Miller) into PA12 amphotropic packaging cells (23). Transfected cells were incubated with G418 selection medium (1.2 mg of G418/ml); resistant cells were pooled, and the viral supernatant was harvested to transduce MDTF cells. Transduced MDTF cells were selected with 1.5 mg of G418 per ml, and individual resistant clones were analyzed for phosphate uptake. Clones showing the best uptake, and therefore representing high Pit1/Pit2 expression levels, were used for infection studies. MDTF cells expressing Pit1 (GGG) were generated as described before (35).
Mutagenesis of FeLV-B and A-MLV envelopes.
The baBB, baBB1, baBB2, and baBB3 mutant FeLV-B envelopes and the ABA envelope (see Fig. 1 and 3) were generated in our previous study (35). Specific FeLV-B VRA residues were mutated by PCR mutagenesis as described before (35). The mutant envelopes were subsequently cloned into the FBsalf retroviral expression vector (9). The ABA1 mutant envelope was generated by mutagenesis of specific A-MLV VRB sequence to the corresponding FeLV-B VRB sequence (see Fig. 4). In addition, the A-MLV VRB disulfide-loop sequence was mutated to a foreign sequence that encoded a 16-amino-acid peptide containing the RGD-integrin recognition motif. This was done by PCR mutagenesis using the specific primer 5′-TGCCAAGCCGGTACCTTTGCGCTCCGCGGCGATAATCCCCAAGGATGC-3′. A BamHI-EcoRI A-MLV PCR fragment which includes the VRB mutations was ligated with an EcoRI-ClaI A-MLV envelope cDNA fragment into a BamHI-ClaI-digested FBsalf expression vector. All envelope cDNAs were sequenced to confirm the mutations by the Microbiology and Molecular Immunology Core Facility on a PE/ABD 377 sequencer by using dye terminator cycle-sequencing chemistry (Applied Biosystems, Foster City, Calif.).
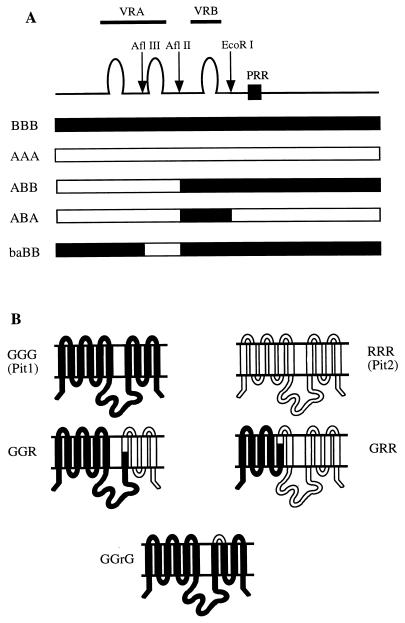
FIG. 1.
Structures of chimeric FeLV-B/A-MLV envelopes and chimeric Pit1/Pit2 proteins. (A) The chimeric FeLV-B/A-MLV envelopes were generated in our previous study (35). The variable regions VRA and VRB, the proline-rich region (PRR), and the restriction sites used to generate the chimeric envelope cDNAs are indicated. The two potential disulfide-bonded loops in VRA and one disulfide-bonded loop in VRB are indicated by the loop structures. BBB and AAA represent wild-type FeLV-B and A-MLV envelopes, respectively. (B) Topological model of wild-type and chimeric Pit1 and Pit2 receptors showing the five presumptive ECLs and the large cytoplasmic loop. The chimeric Pit1/Pit2 cDNAs were generated by Miller and Miller (25).
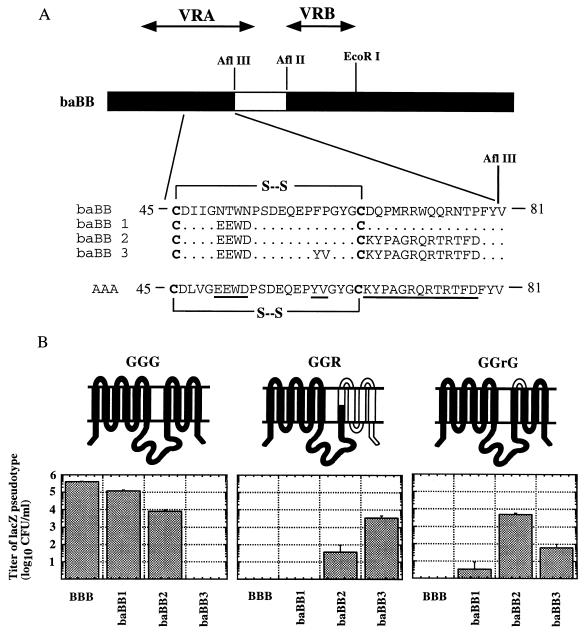
FIG. 3.
Susceptibility of GGG, GGR, and GGrG cells to pseudotype virus bearing mutant FeLV-B envelopes. (A) The chimeric baBB and mutant baBB envelopes were generated in our previous study (35). Divergent residues are underlined in the AAA sequence, and unchanged amino acids are indicated by dots. The conserved cysteines are highlighted in boldface. (B) Infection of MDTF cells expressing the GGG (Pit1), GGR, or GGrG receptor with lacZ pseudotype virus bearing mutant baBB envelopes. Topological diagrams of the chimeric receptors are shown above the histograms. Titers are mean values of three infection studies; error bars are indicated.
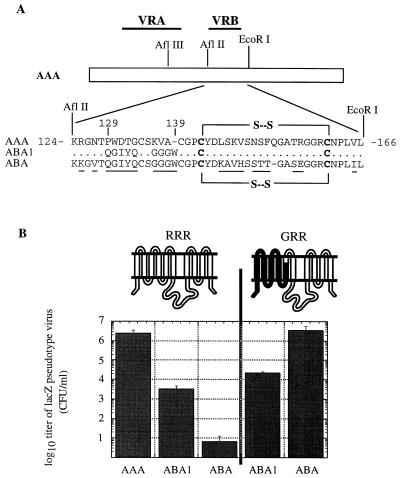
FIG. 4.
Mutagenesis of A-MLV VRB. (A) A-MLV VRB residues were mutated to the corresponding FeLV-B VRB residues by PCR mutagenesis. The ABA envelope contains the full FeLV-B VRB sequence. Unchanged residues are represented by dots; divergent amino acids are underlined in the ABA sequence. The cysteine residues that form the disulfide-bonded loop in VRB are in boldface. (B) Infection of parental MDTF cells, which naturally express RRR, and MDTF cells expressing GRR receptor with pseudotype virions bearing mutant AAA (A-MLV) envelopes. Titers are mean values of three infection studies; error bars are indicated.
Viruses and infection.
The envelope cDNA expression vectors were transfected into TELCeB6 cell lines by calcium phosphate coprecipitation (Stratagene). Transfectants were selected with phleomycin (50 μg/ml), and resistant colonies were pooled 2 weeks after addition of selection. Viral supernatants were harvested and infection assays were carried out as previously described (35). Viral titers were expressed as the number of CFU per milliliter of viral supernatant.
RESULTS
Characteristics of chimeric FeLV-B/A-MLV envelopes and Pit1/Pit2 receptors.
Figure 1 shows the chimeric FeLV-B/A-MLV envelopes (Fig. 1A) and Pit1/Pit2 receptors (Fig. 1B) used in this investigation. The gp70 envelope glycoproteins were separated into three distinct domains, an N-terminal domain containing VRA, a mid-domain containing VRB, and a C-terminal domain. Expression vectors for the chimeric or wild-type envelope genes were transfected into TELCeB6 retroviral packaging cells to produce pseudotype virions that encode β-galactosidase (LacZ), and the viral infectivities were assayed in MDTF cells that expressed the specific Pit1/Pit2 chimeras. In our previous study, we found that the BBB virus (FeLV-B) could use GGG (Pit1) but not GGR as a receptor, whereas the ABB virus used GGR but not GGG (35); we have expanded on that observation in this investigation (see below). In addition, we used the GGrG receptor chimera, which was generated by replacing the critical nine-amino-acid ECL4 region A of Pit1 (14, 36) with the corresponding sequence from Pit2 (25).
Amino acids 66 to 78 of FeLV-B and A-MLV gp70s functionally interact with ECL4 and/or ECL5 of their receptors.
Recent mutagenesis studies by Han et al. suggested that residues Y60 and V61 of A-MLV gp70 are not individually essential for Pit2 recognition (13). Similarly, in our previous study we used mutant FeLV-B viruses in which specific VRA sequences were replaced with corresponding sequences from A-MLV, and we found that Y60 and V61 of A-MLV were individually insufficient to switch the receptor usage from GGG to GGR (35). However, in the context of other A-MLV VRA residues, our results suggested that the presence of the Y60-V61 dipeptide sequence was essential for utilization of the GGR receptor. To more thoroughly investigate this issue, we constructed the FeLV-B VRA substitution mutations shown in Fig. 2 and analyzed viral infectivities in MDTF cells that expressed GGG (Pit1) or GGR receptors. Mutation of either FeLV-B F60 to Y (BBB1 virus) or P61 to V (BBB2 virus) reduced titers of infection on GGG expressing cells only two- to fivefold compared to the BBB virus (Fig. 2), whereas no infection was observed on GGR cells. This confirms that these individual substitutions were insufficient for recognition of Pit2 sequences in the GGR receptor. However, the BBB3 virus that contains both F60Y and P61V mutations only weakly infected GGG cells, suggesting that FeLV-B residues F60 and P61 are important for efficient recognition of the Pit1 ECL4/ECL5 region. In addition, other FeLV-B VRA residues must also contribute to specific recognition of Pit1 ECL4/ECL5 because BBB3 virus weakly utilizes the GGG receptor. Moreover, the BBB3 virus was unable to utilize the GGR receptor, indicating that A-MLV residues Y60 and V61 alone were insufficient for functional recognition of Pit2 ECL4/ECL5. In contrast, the ABB virus that contains the complete A-MLV VRA sequence efficiently utilizes the GGR receptor (Fig. 2), suggesting that additional A-MLV VRA residues are involved in recognition of Pit2 ECL4/ECL5. Evidence described below strongly suggests that A-MLV residues Y60 and V61 are important for Pit2 recognition.
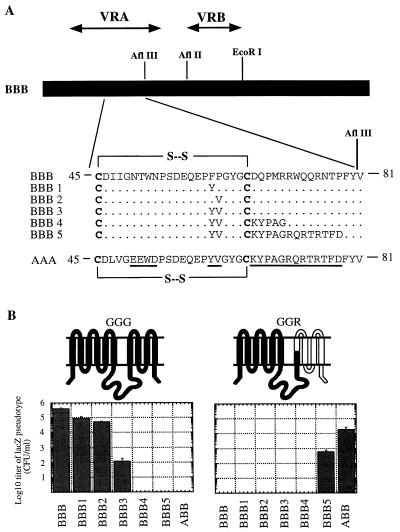
FIG. 2.
Mutagenesis of FeLV-B VRA. (A) Sequences of BBB (FeLV-B) and AAA (A-MLV) VRAs. The VRA residues of FeLV-B were mutated to the corresponding A-MLV VRA residues by PCR mutagenesis. Unchanged amino acids are indicated by dots, and divergent amino acids are underlined in the AAA VRA sequence. The cysteine residues that form the first disulfide-bonded loop in VRA and which are conserved in all mammalian type C retroviruses are in boldface. (B) Infection of MDTF cells expressing GGG (Pit1) or GGR receptor with lacZ pseudotype virus bearing mutant BBB (FeLV-B) envelopes. Topological diagrams of the chimeric receptors expressed by MDTF cells are shown above the histograms. Titers are mean values of three infection studies; error bars are indicated.
To identify additional A-MLV VRA residues that may contribute to receptor recognition, we constructed the BBB4 and BBB5 mutants. As shown in Fig. 2, the BBB4 virus was unable to use either GGG or GGR as a receptor. Western blot analysis of viral supernatant harvested from TELCeB6 cells transfected with the expression vector for the BBB4 envelope gene indicated efficient incorporation of the BBB4 gp70 into virions (data not shown). Consequently, the loss of GGG recognition by the BBB4 virus compared to the BBB3 virus suggests that the FeLV-B sequence DQPMR might be involved to some degree in recognition of Pit1 ECL4/ECL5. However, the inability of BBB4 virus to use GGR as a receptor suggests that the corresponding A-MLV sequence KYPAG is insufficient for recognition of Pit2 ECL4/ECL5. Interestingly, however, the BBB5 virus had a switched pattern of receptor recognition that was essentially the same as that of the ABB virus (Fig. 2). This result clearly shows that A-MLV VRA residues 72 to 78 (QRTRTFD) are involved in recognition of Pit2 ECL4/ECL5. This is consistent with our previous observations that substitution of FeLV-B residues 66 to 78 with the corresponding A-MLV sequence caused a 100-fold reduction in the viral titer on GGG cells and an ability to weakly infect GGR cells. Together with our previous data (35), these results imply that two subregions within VRA of FeLV-B and A-MLV (residues 60 and 61 and residues 66 to 78) may functionally interact with ECL4/ECL5 of their receptors. These different VRA subregions appear to contribute to receptor recognition in a cooperative manner because the effects of substitutions in either subregion are affected by the sequence in the other subregion (see also reference 35).
Residues 60 and 61 specifically recognize the ECL5 region, whereas residues 66 to 78 recognize ECL4.
The identification of two subregions within VRA that may functionally interact with the receptor's ECL4/ECL5 region raised the possibility that the gp70 subregions recognize distinct receptor sequences. To test this and to further investigate the above evidence, we used the GGG, GGR, and GGrG receptors (Fig. 1) to analyze virions containing the mutant FeLV-B envelopes, baBB1, baBB2, and baBB3 (Fig. 3A). The baBB and baBB1 viruses have the same host range as the BBB virus (Fig. 3B and reference 35), suggesting that the substituted A-MLV sequences including the VRA sequence EEWD at positions 50 to 53 are unimportant for specific receptor recognition. The baBB virus was not used in this study.
Our infection data revealed a surprising difference in receptor utilization by virions bearing baBB1, baBB2, and baBB3 envelopes. We analyzed the infection of baBB3 virus on our panel of GGG-, GGR-, and GGrG-expressing cells in comparison to the baBB2 virus. Interestingly, the baBB3 virus was weakly infectious for GGrG (Pit1 with Pit2 ECL4) cells (ca. 80 CFU/ml), whereas baBB2 titer was much higher (ca. 7,000 CFU/ml) (Fig. 3B). In contrast, inverse results were obtained on GGR cells, which contains both Pit2 ECL4 and ECL5. The baBB3 titer on these cells was 5,000 CFU/ml, whereas the baBB2 titer was 50 CFU/ml. This result shows that the presence of FeLV-B F60 and P61 in the baBB2 envelope favors recognition of GGrG, whereas the presence of Y60 and V61 in baBB3 favors recognition of GGR. This finding suggests that FeLV-B residues F60 and P61 interact with the Pit1 ECL5 region and that A-MLV Y60 and V61 functionally interacts with Pit2 ECL5. We next analyzed the infection of the baBB1 virus in comparison to baBB2 virus (Fig. 3B). The infection titer of baBB2 virus on GGG cells was 50-fold lower than the titer of wild-type BBB virus and was approximately 20-fold lower than the titer of baBB1 virus, supporting a role for FeLV-B residues 66 to 78 in receptor recognition as described above. However, the presence of the Pit2 ECL4 sequence in the GGrG receptor caused a 10,000-fold reduction in the infection titer of the baBB1 virus, whereas the baBB2 titer was unaffected (compare infections of GGG and GGrG cells in Fig. 3B). This finding confirms previous evidence that the ECL4 region A of Pit1 is critical for FeLV-B receptor function (36). Second, since baBB1 and baBB2 differ only in VRA residues 66 to 78, these results suggest that FeLV-B residues 66 to 78 interact with Pit1 ECL4 and that A-MLV residues 66 to 78 most likely interact with Pit2 ECL4 but do not exclude interaction with Pit1 ECL5. Additional observations indicate that A-MLV residues 66 to 78 interact with Pit2 ECL4 and not Pit1 ECL5. For example, the baBB3 virus, which contains A-MLV envelope residues Y60 and V61 and residues 66 to 78, does not use GGG as a receptor. However, replacement of Pit1 ECL4 with Pit2 ECL4 (GGrG) enhanced baBB3 infection approximately 70-fold. This result suggests (i) that Pit2 ECL4 is involved in the interaction with A-MLV VRA residues, in agreement with previous studies (25, 29), and (ii) that the enhancement of infection is caused by the interaction of A-MLV residues 60 and 61 or residues 66 to 78 with Pit2 ECL4. If A-MLV residues 60 and 61 interact with Pit2 ECL4 and also ECL5, as described above, this would suggest that A-MLV residues 66 to 78 play a redundant role and do not interact with Pit2. However, the data in Fig. 2 strongly imply that A-MLV residues 66 to 78 interact with Pit2 sequences. Thus, we infer from these results that the enhancement of baBB3 infection, and the enhancement of baBB2 infection compared to baBB1, on GGrG cells is most likely caused by the interaction of A-MLV residues 66 to 78 with Pit2 ECL4. The ability of the baBB2 virus to use GGG, which lacks Pit2 ECL4 sequence, as efficiently as GGrG suggests that A-MLV residues 66 to 78 can also interact with Pit1 ECL4, although to a weaker extent than the interaction of FeLV-B residues 66 to 78 with Pit1 ECL4 (Fig. 3; compare infection of baBB1 and baBB2 on GGG cells). Consistent with this interpretation, previous studies have reported that A-MLV can weakly use Pit1 receptor for infection and strongly use Pit2 chimeras that contain Pit1 ECL4 (29). Together, these results confirm that the two subregions in FeLV-B and A-MLV VRA (i.e., residues 60 and 61 and residues 66 to 78) functionally interact with distinct loops on their respective receptors. Specifically, VRA residues 60 and 61 interact with ECL5 whereas VRA residues 66 to 78 interact with ECL4.
A-MLV and FeLV-B VRB residues 129 to 139 interact with ECL2.
Previously we reported that FeLV-B and A-MLV VRB functionally interact with ECL2 of their receptors (35). This conclusion was based on studies of a chimeric A-MLV envelope glycoprotein (ABA in Fig. 1), in which the A-MLV VRB sequence had been substituted with the corresponding VRB sequence of FeLV-B. As shown in Fig. 4, the ABA virus only weakly infects normal MDTF cells which contain an endogenous Pit2 receptor (RRR) but strongly infects GRR cells. Because Pit1 and Pit2 contain identical ECL1 sequences, the presumptive extracellular surfaces of GRR and RRR (Pit2) differ only in ECL2.
To identify specific VRB residues involved in this functional interaction with ECL2, we mutated specific A-MLV VRB residues to FeLV-B residues and assayed the infectivities of the resulting viruses in normal MDTF cells, which express RRR (Pit2), or in GRR-expressing MDTF cells. As shown in Fig. 4, the ABA1 virus which contained FeLV-B VRB residues 129 to 139 was 1,000-fold less infectious for MDTF (RRR-expressing) cells compared with the control AAA virus. This result suggests that A-MLV residues 129 to 139 contribute to the interaction with Pit2 ECL2. The presence of Pit1 ECL2 in the GRR protein enhanced the titer of the ABA1 over the background in the MDTF (RRR-expressing) cells approximately 10-fold, supporting the hypothesis that FeLV-B residues 129 to 139 interact with Pit1 ECL2. The differences in titers between ABA1 and ABA viruses on RRR- and GRR-expressing cells suggested that additional sequence difference(s) within VRB such as the disulfide-bonded loops might be involved. We therefore substituted the A-MLV VRB disulfide-bonded loop sequence with a foreign 16-amino-acid peptide containing an integrin recognition motif (see Materials and Methods), but this caused an approximate 10-fold reduction in viral infection on RRR-expressing MDTF cells (results not shown). Together, these results strongly suggest that A-MLV and FeLV-B VRB sequences functionally interact with ECL2 of the receptors and imply that residues 129 to 139 contribute to this specific interaction.
DISCUSSION
Mapping the functionally interacting surfaces of the A-MLV and FeLV-B glycoproteins with their cell surface receptors.
In this investigation, we have further developed a novel strategy for simultaneously mapping the functionally interacting surfaces of a retroviral envelope glycoprotein and its cell surface receptor. Our approach has relied on the close sequence similarities between the gp70 glycoproteins of FeLV-B and A-MLV and also between their Pit1 and Pit2 cell surface receptors. Initially, we anticipated that chimeras of these viral glycoproteins and of these receptors would very likely fold into potentially functional structures that would be properly processed to cell surfaces or into virion particles; these expectations have been, with one exception (35), confirmed by our results. Thus, the Pit1/Pit2 chimeras were all processed to cell surfaces where they functioned as Na+-dependent phosphate symporters, and the envelope glycoprotein chimeras and site-directed mutants used in this study were also all properly processed and incorporated into virion particles (results not shown). Interestingly, many of the chimeric or mutated gp70s that we have analyzed cannot functionally recognize the native Pit1 or Pit2 receptors, although they can interact functionally with specific Pit1/Pit2 chimeras (Fig. 2 to 4). By using this approach, we have obtained evidence that A-MLV VRA1 residues Y60 and V61 specifically interact with the Pit2 presumptive ECL5 region and that the nearby sequence between amino acids 72 and 78 functionally interacts with ECL4. In addition, the VRB sequence appears to functionally interact with ECL2, and at least part of this interaction appears to involve gp70 residues 129 to 139 (Fig. 4). Interestingly, our results suggest that the corresponding sequences of FeLV-B gp70 also participate in functional interactions with the same regions of its Pit1 receptor. Thus, the maps of the functionally interacting surfaces of A-MLV with Pit2 and of FeLV-B with Pit1 are highly homologous and strikingly similar. These gp70-receptor interactions appear to involve multiple functionally important contacts that occur at discrete positions on their surfaces.
In addition to its ability to simultaneously map interacting sites on both an envelope glycoprotein and its receptor, this method has other advantages compared with previously used mutagenesis strategies. Unlike the latter method, which results in only losses of functions, the current approach typically enables us to identify substitutions that weaken the interaction with one receptor but simultaneously increase the use of another (Fig. 2 to 4). Thus, for example, the replacement of FeLV-B residues 60 and 61 and residues 66 to 78 with the corresponding sequences of A-MLV eliminates use of the Pit1 (GGG) receptor but facilitates use of a GGR receptor that contains ECL4 and ECL5 of Pit2 (Fig. 3). This enables us to conclude that the replacement sequence is not merely a poison that interferes with proper gp70 function, but that it makes a positive contribution to specific functional interaction of gp70 and its receptor.
Although this study has provided us with a broadened map of the functionally important gp70-receptor interactions, it is important to also recognize the limitations of this approach. First, it can only identify functionally important sequence differences that distinguish the gp70s and the receptors being compared. For example, because Pit1 and Pit2 receptors have identical ECL1 sequences, our results cannot exclude the possibility that this sequence interacts with the viruses in a manner that is necessary but insufficient for infection. Second, there are indications that the two VRA subregions that we have identified (i.e., at positions 60 and 61 and positions 66 to 78) (Fig. 2 and 3) may interact with the receptors in a manner that is to a degree functionally redundant or cooperative. Thus, exchanging either of these sequences between FeLV-B and A-MLV does not cause a complete switch in utilization of the GGG and GGR receptors. On the contrary, substitutions of both VRA subregions have more pronounced effects. Consequently, when these sites are sequentially substituted in different orders, the final substitutions appear to have the greatest influences. Similar results of Han et al. (13), who used a different experimental approach, were also consistent with the hypothesis that these two nearby regions of VRA cooperatively influence receptor interactions. Such redundant or cooperative interactions, which also occur with xenotropic MLV (20) and human immunodeficiency virus type 1 (1, 6, 16, 31, 34), are very difficult to identify by either the conventional mutagenesis method or by our approach. Nevertheless, we have succeeded in resolving these VRA interactions by using appropriate virus and receptor chimeras (Fig. 3). Third, our approach is feasible only for viruses and receptors that are closely related. On the other hand, if the viruses or receptors are too similar, the first problem described above would be accentuated, with the consequence that only a subset of the functionally important interactions would be revealed.
A possible alternative topology for Pit1 and Pit2.
Recently, we have learned that the presumptive topology of Pit1 and Pit2 that has been assumed by workers in this field, including ourselves, may be incorrect (33; P. Rodrigues, C. Salaun, and J. M. Heard, personal communication). From the perspective of our results, it is relevant that the regions we have termed ECL2 and ECL4 are exposed to the extracellular milieu in both models. However, in the new model the region that we have termed ECL5 is intracellular, whereas the carboxyl-terminal sequence is extracellular. Consequently, the evidence we have presented with reference to ECL5 may, alternatively, pertain to this extreme carboxyl-terminal region of the receptors. If this alternative model is correct, our evidence would suggest that VRA residues 60 and 61 of FeLV-B and A-MLV functionally interact with this carboxyl-terminal region. Interestingly, the carboxyl termini of Pit1 and Pit2 are highly divergent in sequence. Resolution of these issues will require more detailed evaluations of these topological models. Until such resolution is available, studies designed to evaluate our conclusions regarding ECL5 must also investigate the extreme carboxyl-terminal region of the receptors.
Relationship to previous results.
We believe that our results are generally in close agreement with previous evidence. For example, several groups have determined that the presumptive ECL2 and ECL4 of Pit1 and Pit2 are required for receptor function (14, 17–19, 29, 30, 35, 36). In addition, a region encompassing ECL5 and the extreme carboxyl-terminal region of Pit1 has also been implicated in FeLV-B reception (29, 35). Furthermore, the amino-terminal region of gp70 that includes VRA and VRB has been strongly implicated in specific associations with cell surface receptors for diverse type C retroviruses (4, 5, 7, 22, 35). As described above, specific amino acids essential for receptor utilization have been identified within the first disulfide-bonded VRA loop (VRA1) of several retroviruses (2, 3, 8, 32, 35) and between the two disulfide-bonded loops of VRA (12, 13, 21) in the region corresponding to residues 66 to 78 of FeLV-B and A-MLV that we have identified as important for interactions with Pit1 and Pit2 ECL4.
A recent study by Lundorf et al. (18) disagrees with our proposed model that efficient A-MLV infections require the interactions of both VRA with Pit2 ECL4/ECL5 and VRB with ECL2. Their conclusion was based on previous studies which showed that a chimeric receptor containing Pit2 ECL1 to -3 and Pit1 ECL4 and -5 (chimera RRG in reference 25) supported a wild-type level of A-MLV infection despite lacking Pit2 ECL4 and -5 sequences. In addition, a chimera containing Pit1 ECL1 to -3 and Pit2 ECL4 and -5 (GGR in reference 25) and a Pit1 chimera that contains Pit2 ECL4 (pOJ102 in reference 29) also mediates A-MLV infections. Although, we do not dispute these previous results, we have an alternative interpretation. Because Pit1 and Pit2 are closely related (62% identity), it would not be surprising, as discussed above, if A-MLV could weakly interact with certain Pit1 sequences, resulting in utilization of some Pit1/Pit2 chimeras. Indeed, our current results suggest that A-MLV residues 66 to 78 can recognize the Pit1 ECL4 sequence, although this recognition is much weaker than the recognition of the same loop by FeLV-B residues 66 to 78 (Fig. 3). From this perspective, it is not surprising that a Pit2 chimera containing Pit1 ECL4 (pOJ80 in reference 29), a chimera containing Pit2 ECL1 and -2 and Pit1 ECL3 to -5 (RGG in reference 25), and the RRG chimera can all support A-MLV infections to some extent. Similarly, it is not surprising that the GGR and pOJ102 chimeras, which contain Pit1 ECL2 and Pit2 ECL4, can also support A-MLV infections. However, Lundorf et al. (18) do not interpret the substantial evidence that all Pit1/Pit2 chimeras that contain either Pit2 ECL2 or Pit2 ECL4, such as GGR, RGG, and pOJ102, mediate A-MLV infections that are weaker than infections mediated by native Pit2. These quantitative results suggest that interactions of A-MLV with Pit2 ECL2 and -4 are very important for efficient infection. The exceptions are the RRG and pOJ80 chimeras, which support wild-type levels of A-MLV infections. These exceptions have been discussed in our previous report (35). We suggest that the Pit2 ECL3, present in RRG and pOJ80, plays a role in receptor function by influencing overall receptor folding (35). This conclusion has been supported by subsequent studies reported by Leverett et al. (17) and by Lundorf et al. (18). Thus, we do not disagree that A-MLV can recognize ECLs from other related phosphate symporters, such as Pit1 and Pho-4. However, we conclude, based on our previous data and evidence from this study, that within the context of Pit2, A-MLV interacts with Pit2 ECL2, -4, and -5 and that these interactions make important contributions to efficient infection. Substitutions of these ECLs result in decreases in virus infection which can range from a mild to a severe decrease, depending on which ECLs are substituted and the replacement sequences that are used. Similarly, within the context of Pit1, efficient FeLV-B infection appears to require the interaction with Pit1 ECL2, -4, and -5. In this context, we emphasize that a site that is critical for virus-receptor interaction would not be detected in a mutagenesis or chimera study if the replacement sequence were too similar to or only slightly different from the natural sequence. Therefore, the decision about whether a specific sequence is necessary or of only minor importance for infection cannot be based on studies of only a few chimeras or mutants. Based on these considerations, we believe that our proposal that VRA and VRB interactions may both be essential for infections is compatible with the available evidence.
In addition, two other groups have reported evidence that appears to partially disagree with our data on A-MLV envelope domains involved in receptor recognition (3, 13). Results supporting our conclusion that VRA1 of A-MLV is required for interactions with Pit2 were reported by Battini et al. (3). However, they also found that replacement of the VRB disulfide-bonded loop or upstream sequences with a foreign sequence caused only 10-fold reductions in receptor activity, and they inferred that VRB is unimportant for A-MLV infections. On the contrary, our results suggest that replacing the sequence from 129 to 139 of A-MLV with FeLV-B sequences reduced utilization of Pit2 by 3 orders of magnitude (Fig. 4) and that replacement of the A-MLV VRB disulfide-bonded loop with a foreign peptide reduced utilization of Pit2 approximately 10-fold. We believe that this difference in our results is not substantive and may be principally a consequence of methodological differences. Because site-directed mutations in envelope glycoproteins often result in abnormalities in processing or stability, infectivity losses of only 10-fold are generally discounted. In contrast, we are able to interpret relatively small changes in receptor activities because the mutant viruses gain the ability to use certain receptor chimeras while simultaneously losing the ability to use others. Thus, we have an internal positive control that enables us to better exclude nonspecific defects in function.
In agreement with our results, Han et al. recently described evidence that both VRA1 and nearby downstream residues 66 to 78 may collaboratively or redundantly contribute to utilization of the Pit2 receptor (13). Their evidence was based on the observation that substitutions of either VRA1 or residues 66 to 78 with corresponding sequences from polytropic MLV did not eliminate Pit2 utilization whereas replacement of both regions abolished this activity. In apparent contrast with our results, however, they also reported that mutations including Y60 and V61 of A-MLV VRA1 did not abolish viral infectivity for NIH 3T3 fibroblasts although they reduced infectivity by extents ranging from 4- to 20-fold. In correspondence with their data, we also find that substitution of Y60 and V61 alone into the VRA1 region of FeLV-B is insufficient to switch the receptor recognition toward utilization of Pit2 (Fig. 2) and that the effect of the Y60-V61 replacement depends in a cooperative or partially redundant manner with downstream sequences between amino acids 66 and 78 (Fig. 3). However, in the context of the FeLV-B gp70, replacement of F60 and P61 with A-MLV residues Y60 and V61 caused a 1,000-fold reduction of Pit1 utilization (Fig. 2). The effects of these substitutions clearly depend on the overall gp70 context in which they occur. We believe that the available evidence is consistent with our conclusions that the residues at VRA positions 60 and 61 are not critical by themselves although they have a strong partially cooperative effect on specific interactions of these viruses with the Pit1 and Pit2 receptors.
ACKNOWLEDGMENTS
We thank A. Dusty Miller (Fred Hutchinson Cancer Center, Seattle, Wash.) for providing the chimeric Pit1/Pit2 cDNA expression vectors and Yasuhiro Takeuchi for providing the TELCeB6 packaging cell line and FBsalf retroviral expression vector. We are grateful to our coworkers Susan Kozak, Emily Platt, Navid Madani, Mariana Marin, and Shawn Kuhmann for helpful suggestions.
This work was supported by NIH grant CA25810 and by The Wellcome Trust.
REFERENCES
- 1.Atchison R E, Gosling J, Monteclaro F S, Franci C, Digilio L, Charo I F, Goldsmith M A. Multiple extracellular elements of CCR5 and HIV-1 entry: dissociation from response to chemokines. Science. 1996;274:1924–1926. doi: 10.1126/science.274.5294.1924. [DOI] [PubMed] [Google Scholar]
- 2.Bae Y, Kingsman S M, Kingsman A J. Functional dissection of the Moloney murine leukemia virus envelope protein gp70. J Virol. 1997;71:2092–2099. doi: 10.1128/jvi.71.3.2092-2099.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Battini J L, Danos O, Heard J M. Definition of a 14-amino-acid peptide essential for the interaction between the murine leukemia virus amphotropic envelope glycoprotein and its receptor. J Virol. 1998;72:428–435. doi: 10.1128/jvi.72.1.428-435.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Battini J L, Danos O, Heard J M. Receptor binding domain of murine leukemia virus envelope glycoprotein. J Virol. 1995;69:713–719. doi: 10.1128/jvi.69.2.713-719.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Battini J L, Heard J M, Danos O. Receptor choice determinants in the envelope glycoproteins of amphotropic, xenotropic, and polytropic murine leukemia viruses. J Virol. 1992;66:1468–1475. doi: 10.1128/jvi.66.3.1468-1475.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bieniasz P D, Fridell R A, Aramori I, Ferguson S S, Caron M G, Cullen B R. HIV-1-induced cell fusion is mediated by multiple regions within both the viral envelope and the CCR-5 co-receptor. EMBO J. 1997;16:2599–2609. doi: 10.1093/emboj/16.10.2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boomer S, Eiden M V, Burns C C, Overbaugh J. Three distinct envelope domains, variably present in subgroup B feline leukemia virus recombinants, mediate Pit1 and Pit2 receptor recognition. J Virol. 1997;71:8116–8123. doi: 10.1128/jvi.71.11.8116-8123.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brojatsch J, Kristal B S, Viglianti G A, Khiroya R, Hoover E A, Mullins J I. Feline leukemia virus subgroup C phenotype evolves through distinct alterations near the N terminus of the envelope surface glycoprotein. Proc Natl Acad Sci USA. 1992;89:8457–8461. doi: 10.1073/pnas.89.18.8457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cosset F L, Takeuchi Y, Battini J L, Weiss R A, Collins M K L. High-titer packaging cells producing recombinant retroviruses resistant to human serum. J Virol. 1995;69:7430–7436. doi: 10.1128/jvi.69.12.7430-7436.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fass D, Davey R A, Hamson C A, Kim P S, Cunningham J M, Berger J M. Structure of a murine leukemia virus receptor-binding glycoprotein at 2.0 angstrom resolution. Science. 1997;277:1662–1666. doi: 10.1126/science.277.5332.1662. [DOI] [PubMed] [Google Scholar]
- 11.Ferry N, Duplessis O, Houssin D, Danos O, Heard J M. Retroviral-mediated gene transfer into hepatocytes in vivo. Proc Natl Acad Sci USA. 1991;88:8377–8381. doi: 10.1073/pnas.88.19.8377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Han J Y, Cannon P M, Lai K M, Zhao Y, Eiden M V, Anderson W F. Identification of envelope protein residues required for the expanded host range of 10A1 murine leukemia virus. J Virol. 1997;71:8103–8108. doi: 10.1128/jvi.71.11.8103-8108.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Han J Y, Zhao Y, Anderson W F, Cannon P M. Role of variable regions A and B in receptor binding domain of amphotropic murine leukemia virus envelope glycoprotein. J Virol. 1998;72:9101–9108. doi: 10.1128/jvi.72.11.9101-9108.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johann S V, Zeijl M V, Cekleniak J, O'Hara B. Definition of a domain of GLVR1 which is necessary for infection by gibbon ape leukemia virus and which is highly polymorphic between species. J Virol. 1993;67:6733–6736. doi: 10.1128/jvi.67.11.6733-6736.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kavanaugh M P, Miller D G, Zhang W, Law W, Kozak S L, Kabat D, Miller A D. Cell-surface receptors for gibbon ape leukemia virus and amphotropic murine retrovirus are inducible sodium-dependent phosphate symporters. Proc Natl Acad Sci USA. 1994;91:7071–7075. doi: 10.1073/pnas.91.15.7071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuhmann S E, Platt E J, Kozak S L, Kabat D. Polymorphisms in the CCR5 genes of African green monkeys and mice implicate specific amino acids in infections by simian and human immunodeficiency viruses. J Virol. 1997;71:8642–8656. doi: 10.1128/jvi.71.11.8642-8656.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leverett B, Farrell K B, Eiden M V, Wilson C A. Entry of amphotropic murine leukemia virus is influenced by residues in the putative second extracellular domain of its receptor, Pit2. J Virol. 1998;72:4956–4961. doi: 10.1128/jvi.72.6.4956-4961.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lundorf M D, Pedersen F S, O'Hara B, Pedersen L. Amphotropic murine leukemia virus entry is determined by specific combinations of residues from receptor loops 2 and 4. J Virol. 1999;73:3169–3175. doi: 10.1128/jvi.73.4.3169-3175.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lundorf M D, Pedersen F S, O'Hara B, Pedersen L. Single amino acid insertion in loop 4 confers amphotropic murine leukemia virus receptor function upon murine Pit1. J Virol. 1998;72:4524–4527. doi: 10.1128/jvi.72.5.4524-4527.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marin M, Tailor C S, Nouri A, Kozak S L, Kabat D. Polymorphisms of the cell surface receptor control mouse susceptibilities to xenotropic and polytropic leukemia viruses. J Virol. 1999;73:9362–9368. doi: 10.1128/jvi.73.11.9362-9368.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Masuda M, Masuda M, Hanson C A, Hoffman P M, Ruscetti S K. Analysis of the unique hamster cell tropism of ecotropic murine leukemia virus PVC-211. J Virol. 1996;70:8534–8539. doi: 10.1128/jvi.70.12.8534-8539.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McDougall A S, Terry A, Tzavaras T, Chene C, Rojko J, Neil J C. Defective endogenous proviruses are expressed in feline lymphoid cells: evidence for role in natural resistance to subgroup B feline leukemia viruses. J Virol. 1994;68:2151–2160. doi: 10.1128/jvi.68.4.2151-2160.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Metsikko K, Garoff H. Role of heterologous and homologous glycoproteins in phenotypic mixing between Sendai virus and vesicular stomatitis virus. J Virol. 1989;63:5111–5118. doi: 10.1128/jvi.63.12.5111-5118.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller D G, Edwards R H, Miller A D. Cloning of the cellular receptor for amphotropic murine retroviruses reveals homology to that for gibbon ape leukemia virus. Proc Natl Acad Sci USA. 1994;91:78–82. doi: 10.1073/pnas.91.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller D G, Miller A D. A family of retroviruses that utilize related phosphate transporters for cell entry. J Virol. 1994;68:8270–8276. doi: 10.1128/jvi.68.12.8270-8276.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O'Hara B, Johann S V, Klinger H P, Blair D G, Rubinson H, Dunn K J, Sass P, Vitek S M, Robbins T. Characterisation of the human gene conferring sensitivity to infection by gibbon ape leukemia virus. Cell Growth Differ. 1990;1:119–127. [PubMed] [Google Scholar]
- 27.Olah Z, Lehel C, Anderson W B, Eiden M V, Wilson C A. The cellular receptor for gibbon ape leukemia virus is a novel high affinity sodium-dependent phosphate transporter. J Biol Chem. 1994;269:25426–25431. [PubMed] [Google Scholar]
- 28.Ott D, Rein A. Basis for receptor specificity of nonecotropic murine leukemia virus surface glycoprotein gp70. J Virol. 1992;66:4632–4638. doi: 10.1128/jvi.66.8.4632-4638.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pedersen L, Johann S V, Zeijl M V, Pederson F S, O'Hara B. Chimeras of receptors for gibbon ape leukemia virus/feline leukemia virus B and amphotropic leukemia virus reveal different modes of receptor recognition by retrovirus. J Virol. 1995;69:2401–2405. doi: 10.1128/jvi.69.4.2401-2405.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pedersen L, Zeijl M V, Johann S V, O'Hara B. Fungal phosphate transporter serves as a receptor backbone for gibbon ape leukemia virus. J Virol. 1997;71:7619–7622. doi: 10.1128/jvi.71.10.7619-7622.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Picard L, Simmons G, Power C A, Meyer A, Weiss R A, Clapham P R. Multiple extracellular domains of CCR5 contribute to human immunodeficiency virus type 1 entry and fusion. J Virol. 1997;71:5003–5011. doi: 10.1128/jvi.71.7.5003-5011.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rigby M A, Rojko J L, Stewart M A, Kociba G J, Cheney C M, Rezanka L J, Mathes L E, Hartke J R, Jarrett O, Neil J C. Partial dissociation of subgroup C phenotype and in vivo behaviour in feline leukaemia viruses with chimeric envelope genes. J Gen Virol. 1992;73:2839–2847. doi: 10.1099/0022-1317-73-11-2839. [DOI] [PubMed] [Google Scholar]
- 33.Rodrigues P, Heard J M. Modulation of phosphate uptake and amphotropic murine leukemia virus entry by posttranslational modifications of Pit2. J Virol. 1999;73:3789–3799. doi: 10.1128/jvi.73.5.3789-3799.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rucker J, Samson M, Doranz B J, Libert F, Berson J F, Yi Y, Smyth R J, Collman R G, Broder C C, Vassart G, Doms R W, Parmentier M. Regions in beta-chemokine receptors CCR5 and CCR2b that determine HIV-1 cofactor specificity. Cell. 1996;87:437–446. doi: 10.1016/s0092-8674(00)81364-1. [DOI] [PubMed] [Google Scholar]
- 35.Tailor C S, Kabat D. Variable regions A and B in the envelope glycoproteins of feline leukemia virus subgroup B and amphotropic murine leukemia virus interact with discrete receptor domains. J Virol. 1997;71:9383–9391. doi: 10.1128/jvi.71.12.9383-9391.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tailor C S, Takeuchi Y, O'Hara B, Johann S V, Weiss R A, Collins M K L. Mutation of amino acids within the gibbon ape leukemia virus (GALV) receptor differentially affects feline leukemia virus subgroup B, simian sarcoma associated virus, and GALV infection. J Virol. 1993;67:6737–6741. doi: 10.1128/jvi.67.11.6737-6741.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takeuchi Y, Vile R G, Simpson G, O'Hara B, Collins M K L, Weiss R A. Feline leukemia virus subgroup B uses the same cell surface receptor as gibbon ape leukemia virus. J Virol. 1992;66:1219–1222. doi: 10.1128/jvi.66.2.1219-1222.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zeijl M V, Johann S V, Cross E, Cunningham J, Eddy R, Shows T B, O'Hara B. An amphotropic virus receptor is a second member of the gibbon ape leukemia virus receptor family. Proc Natl Acad Sci USA. 1994;91:1168–1172. doi: 10.1073/pnas.91.3.1168. [DOI] [PMC free article] [PubMed] [Google Scholar]