Abstract
Background
Ivermectin (IVM) reduces the lifespan of malaria-transmitting mosquitoes after feeding on humans treated with IVM. If this effect is sufficiently long and strong, IVM could form part of a drug combination that not only treats malaria patients but also reduces onward transmission. Limited data are available on the exact duration of the mosquitocidal effect of IVM; daily mosquito feeding assays are required for this.
Materials and Methods
We determined mortality rates of Anopheles stephensi mosquitoes that took a blood meal on Swiss mice, Wistar rats and Cynomolgus monkeys that received IVM orally at 200-400 μg/kg. Mosquito feeding assays were performed on five consecutive days after IVM administration. Mosquito mortality was determined in the first 72 hours after feeding.
Results
Mosquito mortality was 70-100% when mosquitoes fed on any of the animals 1-2 days after the last IVM administration. After this time-point the mosquitocidal effect was still evident in some animals but became more variable.
Conclusions
Our findings of a pronounced but short-lived mosquitocidal effect makes the timing of IVM administration crucial to form a useful addition to anti-malarial drugs.
1 Introduction
The search for malaria transmission-blocking drugs has so far focused on drugs that clear gametocytes, the sexual stage of Plasmodium parasites that are responsible for the transmission of malaria to mosquitoes. An alternative/supplementary approach to prevent malaria transmission after treatment would be a strategy that kills mosquitoes before they become infectious after feeding on a gametocytaemic blood meal.Ivermectin (IVM) may fulfil a role in such a strategy. IVMis a drug with broad-spectrum activity against nematodes and ectoparasites, and is widely used in mass treatment campaigns against onchocerciasis [1]. It has recently received much attention because of the reduced lifespan of malaria-transmitting mosquitoes after feeding on humans and cattle treated with IVM [2-5]. This makes IVM a potentially attractive component of malaria control efforts, where it could be part of a drug combination that not only treats malaria patients but also reduces onward transmission of the disease. The duration of the mosquitocidal effect of IVM is key to its potential role as an adjunct malaria therapy. Few data are available on the exact duration of this effect.
Fritz and colleagues showed previously that addition of IVM to bovine blood that is directly fed to Anopheles gambiae s.s. and An. arabiensis mosquitoes reduces their survivorship and fecundity [5], and that IVM is already lethal to An. arabiensis at low concentrations (LC50 of 7.9b)[6]. Work in rodents and cattle suggests a long-acting effect of IVM on anophelines; affecting mosquito survival rates as long as 10 days after feeding on an IVM-treated animal [5,7]. Chaccour and colleagues directly fed An. gambiae mosquitoes on healthy human volunteers 1 and 14 days post-IVM administration and observed an increased mortality at only the first time-point [2]. In a study from Papua New Guinea, blood-fed mosquitoes were collected from the huts of people 1-3 days and 28 days after an IVM mass treatment campaign. An. punctulatus or An. koliensis caught 1-3 days post-treatment showed 70% mortality within 24 hours after capture compared to 2% caught 28 days post-treatment [8]. A study from Senegal similarly determined survival in blood-fed An. gambiae s.s. mosquitoes sampled from houses after IVM mass treatment campaigns and found that survival rates were lower for mosquitoes that were caught 1-6 days post treatment; the effect for the individual days was not reported [4]. A study where An. farauti mosquitoes were allowed to feed on the skin of a single Indonesian volunteer 0, 7, 10, 14, 26 and 44 days after administration of 250 μg/kg IVM suggested that the mosquitocidal effect may last for 14 days [3].
In short, precise estimates of the duration of the mosquitocidal effect of IVM are unavailable and studies have been hampered by logistical and ethical challenges in performing frequently repeated mosquito feeding experiments. We aimed to support the discussion on the best strategy of IVM deployment by analysing previously conducted and unpublished experiments on the mortality rate of An. stephensi mosquitoes after feeding on different animals treated with IVM at doses within the range that is recommended for use in humans (200-400 μg/kg).
2 Materials and Methods
Experiments were performed at the Radboud University Nijmegen Medical Centre animal facility in the period May 1986-October 1987, where the test animals and mosquitoes were housed according to local and national guidelines. Permission to conduct the experiments in this study was given under approval number DGVGZ/VVP-83262. Swiss mice (Mus musculus; n=10) weighing ∼25g each were given forage containing 2mg/kg IVM. Oral take-up of 5g of forage per day resulted in a total take-up of 10 μg IVM per day per mouse, or a daily dose of 400 μg/kg IVM. Four to five days old An. stephensi mosquitoes were allowed to feed directly on the skin of all animals exactly 1, 2, 3, 4 and 5 days after IVM treatment. After each feeding experiment fully engorged mosquitoes were selected. The median number of fully engorged mosquitoes per mouse per feeding day was 10 (interquartile range 10-13.25, total number of mosquitoes=503). Mosquito mortality for each of the five mosquito feeding days was compared with 15 mosquito feeding experiments on untreated Swiss mice; an entirely separate group of animals. Mice were given IVM treatment for 1-5 days prior to the first feeding experiment. The total dose of IVM therefore differed between animals. However, we observed no effect of longer treatment on mosquito mortality after the last dose of IVM (Table 1; p=0.87) and animals were therefore combined and analysed as a group.
Table 1.
Mosquito feeding experiments after ivermectin (IVM) treatment in different animals.
| Mortality on experiment day #, % (n/N) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Animal species | # of animals | # of treatment days | Daily dose (µg/kg) | 1* | 2* | 3* | 4* | 5* |
| Swiss mouse | 2 | 1 | 400 | 100 (17/17) | 89.5 (17/19) | 0.0 (0/7) | 0.0 (0/11) | 9.1 (1/11) |
| 2 | 2 | 400 | 100 (17/17) | 95.2 (20/21) | 85.7 (18/21) | 37.5 (6/16) | 65.6 (21/32) | |
| 2 | 3 | 400 | 100 (22/22) | 87.5 (21/24) | 0.0 (0/6) | 6.3 (1/16) | 0.0 (0/16) | |
| 2 | 4 | 400 | 100 (20/20) | 100 (24/24) | 66.7 (18/27) | 50.0 (11/22) | 30.4 (7/23) | |
| 2 | 5 | 400 | 100 (25/25) | 88.5 (23/26) | 67.9 (19/28) | 52.0 (13/25) | 0.0 (0/27) | |
| Wistar rat | 1 | 1 | 400 | 100 (22/22) | 100 (24/24) | 65.4 (17/26) | 44.1 (15/34) | 29.4 (10/34) |
| 1 | 2 | 400 | 92.9 (26/28) | 85.0 (17/20) | 96.2 (25/26) | 42.9 (15/35) | 16.7 (3/18) | |
| 1 | 3 | 400 | 85.0 (17/20) | 100 (29/29) | 72.4 (21/29) | 53.8 (21/39) | 45.7 (16/35) | |
| 1 | 4 | 400 | 84.6 (22/26) | 91.7 (22/24) | 66.7 (12/18) | 13.3 (4/30) | 18.5 (5/27) | |
| 1 | 5 | 400 | 100 (16/16) | 100 (19/19) | 77.8 (14/18) | 31.4 (11/35) | 35.7 (10/28) | |
| Cynomolgus monkey | 1 | 1 | 200 | 100 (10/10) | 76.9 (10/13) | 37.5 (3/8) | 14.3 (1/7) | 12.5 (1/8) |
| 1 | 1 | 200 | 100** | 91** | 86** | 0** | 0** | |
| 1 | 1 | 400 | 100** | 92** | 45** | 72** | 8** | |
* Determined in the first 72 hours after feeding; ** Only the proportion of dead mosquitoes was recorded for these experiments
Wistar rats (Rattus norvegicus, n=5) weighing ∼120g each were given Ivomec® solution orally. Ivomec® solution containing 10 μg/ml IVM was diluted to 100 μg/ml IVM using distilled water, and administered orally by syringe to reach a daily dose of 400 μg/kg IVM. Mosquito feeding assays were performed as above. The median number of fully engorged mosquitoes per rat per feeding day was 26 (IQR 20-30, total number of mosquitoes=673). Mosquito mortality for each of the five feeding days was compared with 15 mosquito feeding experiments on a completely separate group of untreated Wistar rats. While IVM was given 1-5 days prior to mosquito feeding, we observed no effect of longer treatment on mosquito mortality after the last dose of IVM (p=0.83) and animals were therefore combined and analysed as a group.
Cynomolgus monkeys (Macaca fascicularis; n=3) were given an orange containing Ivomec® solution at a single dose of 200 (n=2) or 400 μg/kg bodyweight (n=1). Mosquito feeding assays were performed as above. The median number of fully engorged mosquitoes per monkey per feeding day was 14 (IQR 10.5-16.5) and was not recorded for all experiments. For some experiments only the proportion of surviving mosquitoes was recorded; this did not affect the statistical analysis where we used a single estimate per mosquito feeding experiment (see below) but limited the information that could be given for individual experiments on Cynomolgus monkeys (Table 1). Mosquito mortality for each of the five feeding days was compared with 21 mosquito feeding experiments on a separate group of untreated Cynomolgus monkeys.
For each of the five time-points after IVM, the proportion of mosquitoes that died within 72 hours after their blood meal was compared with control experiments using STATA version 12.0 (StataCorp LP, Texas, US). Because the number of animals was deemed too small to reliably estimate clustering of individual mosquito observations that fed on the same animal, we chose the most conservative analytical approach: each feeding experiment contributed one observation of the proportion of mosquitoes that died within 72 hours after this feeding experiment. This continuous variable was compared between test and control experiments using a non-parametric Wilcoxon rank-sum test; a Bonferroni correction was used to counteract the problem of multiple comparisons.
3 Results
In mice, the proportion of mosquitoes that died within 72 hours after their bloodmeal was strongly elevated when feeding one or two days after IVM (Table 1; Figure 1a). On these days 70-100%of mosquitoes died within 72 hours after feeding on any of the IVM-treated mice compared to 0-10% in control mice (p<0.0001 for both days). Beyond day two, higher mosquito mortality was still observed in some animals but we no longer found evidence for a statistically significantly elevated mosquito mortality compared to control mice.
Figure 1.
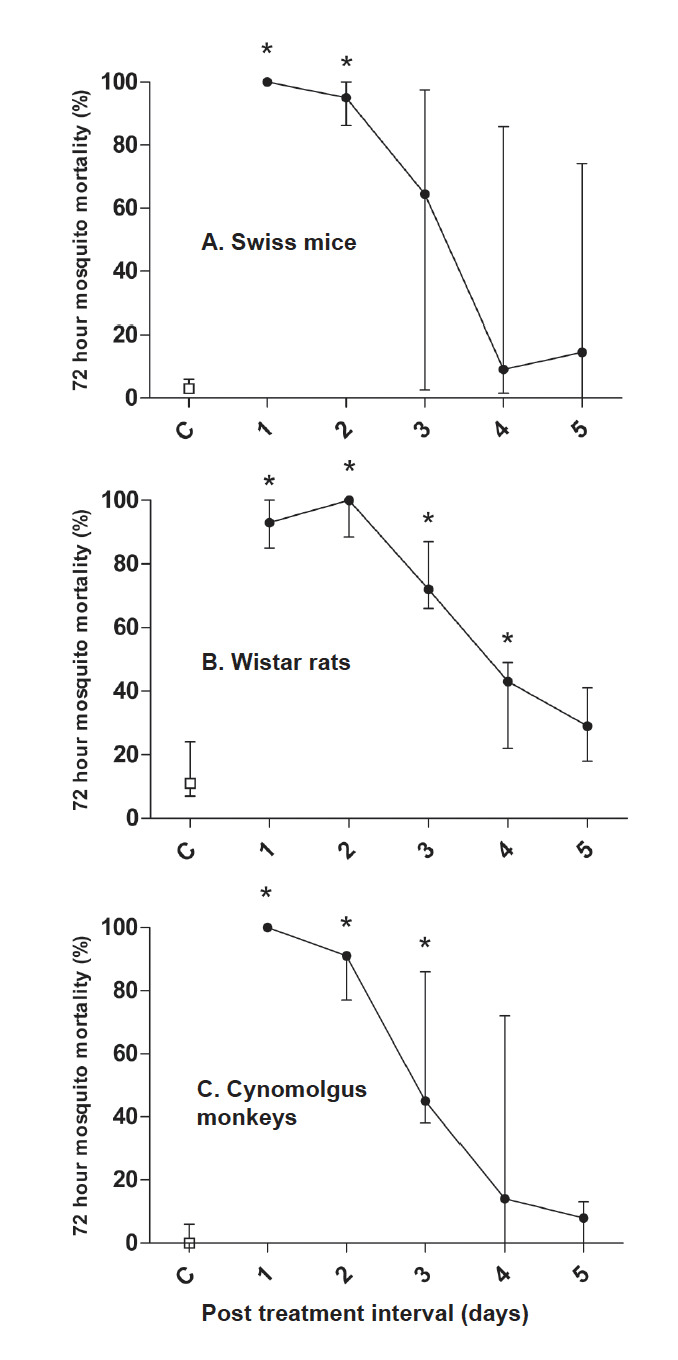
Mosquito mortality within 72 hours after feeding on different animals treated with ivermectin (IVM). Mosquito feeding experiments were conducted on five consecutive days following IVM treatment and mosquito mortality was calculated for the first 72 hours. Plotted on the X-axes is the day since the last IVM dose, plotted on the Y-axes is the proportion of all fully fed mosquitoes that died within 72 hours after their blood meal. Swiss mice (n=10) and Wistar rats (n=5) received IVM 400 μg/kg for 1-5 days (A and B). Cynomolgus monkeys (n=3) received a single dose of IVM at 200 (n=2) or 400 μg/kg (n=1) (C). Dots indicate the median mosquito mortality and error bars indicate the interquartile range; for IVM-treated monkeys this interquartile range equals the total range because only 3 monkeys received IVM. Lines are drawn between the estimates of the median and do not represent fitted or smoothed curves. * = statistically significant after adjusting p-values for multiple comparisons.
For rats, mosquito mortality was also most pronounced for mosquitoes feeding one or two days after IVM: 85-100% of all mosquitoes died within 72 hours after feeding on any of the IVM-treated rats compared to 2-28% in control rats (Table 1; Figure 1b; p=0.001 for day 1 and p=0.0009 for day 2). Mosquito mortality decreased after this time-point but remained significantly elevated up to day 4 after IVM-treatment (p=0.008).
Similar as in rats and mice, mosquito mortality in experiments with Cynomolgus monkeys was highest when feeding one or two days after IVM(Table 1; Figure 1c; 77-100% mosquito mortality within 72 hours after feeding, p=0.002 for both days). On the third day after treatment, mosquito mortality was more variable (38-86%) although still significantly higher than the control experiments (p=0.002).
4 Discussion
We observed a pronounced effect of IVM on mosquito mortality rates in all animal models. The strong but short-lived mosquitocidal effect of IVM that we observed supports several previous observations [2,4,8] with more detailed daily assessments and a larger number of mosquito observations.
A strength of our study is that we have performed daily mosquito feeding experiments in three animal species and thereby are able to add an estimate of mortality on different days post IVM to the available literature. Since the mosquitocidal effect of IVM was previously shown to be highly dependent on the concentration of IVM in plasma [9], we interpret our finding that the mosquitocidal effect becomes more variable after two days, as an indication for inter-individual variation in IVM metabolism. Since we did not determine IVM plasma concentrations in our study animals, we were unable to address this further. This is a shortcoming of the current study. We found no evidence in literature that the plasma disposition of IVM differs between our animal models and humans. Since our findings are consistent between the different animals, we consider it plausible that also in humans the mosquitocidal effect of IVM is limited to the first few days after treatment.
Our findings have implications for the next steps in evaluating IVM as a tool for use in malaria control. The strong but short-lived mosquitocidal effect makes IVM less attractive as a stand-alone drug for mass drug administration (MDA) campaigns that aim to reduce malaria transmission. IVM may, however, be a potent addition to antimalarial drugs in MDA campaigns or to prevent transmission shortly after treatment of symptomatic malaria cases. Current therapy for malaria patients is based on treatment with artemisinin-combination therapy (ACT).Whilst ACTs are highly effective against asexual parasite stages and immature gametocytes, mature gametocytes persist for several weeks after treatment [10]. As a result, there is a pronounced but incomplete reduction of malaria transmission in the first 7-14 days after treatment [11-13]. Primaquine (PQ) is the only currently available drug that can play a role in reducing the infectious period after ACT by actively clearing mature gametocytes. Addition of PQ to ACT reduces the duration of gametocyte carriage fourfold compared to ACTs alone [14]. However, a single dose of PQ at the currently recommended concentration (0.75 mg/kg) is associated with haemolysis in glucose-6-phosphate-dehydrogenase (G6PD) deficient individuals [15,16]. This sub-optimal safety profile hinders wide-scale use of PQ in malaria control [17]. There are currently no safe alternatives to PQ available, although dose-finding studies to determine a lower but efficacious dose of PQ are ongoing and the gametocytocidal activity and safety profile of the drug candidate methylene blue are promising [18].
Adding IVM to ACTs may be a promising strategy to further reduce post-treatment malaria transmission. For this, the timing of IVM administration has to be optimised to cover the period when an individual is most infectious, which is plausibly the first week after initiation of treatment with ACTs [11,19]. An attractive element of IVM as a ’targeted vector control tool’ is that it acts against both indoor and outdoor biting vectors and targets different effector molecules compared to indoor residual spraying with insecticides and insecticide treated bednets [20,21]. An additional benefit in terms of integrated disease management would be the curative effect of IVM on intestinal strongyloidiasis, onchocerciasis and scabies [1]. Several important safety and tolerability data of an ACT-IVM drug combination are needed to confirm its potential, as well as pharmacokinetic data to ensure there are no drug interactions that may reduce the efficacy of the ACT or partner drug component.
5 Acknowledgements
We would like to thank Jolanda Klaassen and Jacqueline Kuhnen for rearing mosquitoes and their help with these experiments.
References
- 1.Foy BD, Kobylinski KC, da Silva I, Rasgon JL et al. Endectocides for malaria control. Trends Parasitol. 2011;27:423–428. doi: 10.1016/j.pt.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chaccour C, Lines J, Whitty CJ. Effect of ivermectin on Anopheles gambiae mosquitoes fed on humans: the potential of oral insecticides in malaria control. J. Infect. Dis. 2010;202:113–116. doi: 10.1086/653208. [DOI] [PubMed] [Google Scholar]
- 3.Foley DH, Bryan JH, Lawrence GW. The potential of ivermectin to control the malaria vector Anopheles farauti. Trans. R. Soc. Trop. Med. Hyg. 2000;94:625–628. doi: 10.1016/s0035-9203(00)90211-6. [DOI] [PubMed] [Google Scholar]
- 4.Sylla M, Kobylinski KC, Gray M, Chapman PL et al. Mass drug administration of ivermectin in south-eastern Senegal reduces the survivorship of wild-caught, blood fed malaria vectors. Malar. J. 2010;9:365. doi: 10.1186/1475-2875-9-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fritz ML, Siegert PY, Walker ED, Bayoh MN et al. Toxicity of bloodmeals from ivermectin-treated cattle to Anopheles gambiae s.l. Ann. Trop. Med. Parasitol. 2009;103:539–547. doi: 10.1179/000349809X12459740922138. [DOI] [PubMed] [Google Scholar]
- 6.Fritz ML, Walker ED, Miller JR. Lethal and sublethal effects of avermectin/milbemycin parasiticides on the African malaria vector, Anopheles arabiensis. J. Med. Entomol. 2012;49:326–331. doi: 10.1603/me11098. [DOI] [PubMed] [Google Scholar]
- 7.Iakubovich VI, Zakharova NF, Alekseev AN, Alekseev EA. Evaluation of the action of ivermectin on blood-sucking mosquitoes. Med. Parazitol. (Mosk) 1989:60–64. [PubMed] [Google Scholar]
- 8.Bockarie MJ, Hii JL, Alexander ND, Bockarie F et al. Mass treatment with ivermectin for filariasis control in Papua New Guinea: impact on mosquito survival. Med. Vet. Entomol. 1999;13:120–123. doi: 10.1046/j.1365-2915.1999.00159.x. [DOI] [PubMed] [Google Scholar]
- 9.Kobylinski KC, Deus KM, Butters MP, Hongyu T et al. The effect of oral anthelmintics on the survivorship and refeeding frequency of anthropophilic mosquito disease vectors. Acta Trop. 2010;116:119–126. doi: 10.1016/j.actatropica.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bousema T, Okell L, Shekalaghe S, Griffin JT et al. Revisiting the circulation time of Plasmodium falciparum gametocytes: molecular detection methods to estimate the duration of gametocyte carriage and the effect of gametocytocidal drugs. Malar. J. 2010;9:136. doi: 10.1186/1475-2875-9-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bousema JT, Schneider P, Gouagna LC, Drakeley CJ et al. Moderate effect of artemisinin-based combination therapy on transmission of Plasmodium falciparum. J. Infect. Dis. 2006;193:1151–1159. doi: 10.1086/503051. [DOI] [PubMed] [Google Scholar]
- 12.Sutherland CJ, Ord R, Dunyo S, Jawara M et al. Reduction of malaria transmission to Anopheles mosquitoes with a six-dose regimen of co-artemether. PLoS Med. 2005;2:e92. doi: 10.1371/journal.pmed.0020092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Targett G, Drakeley C, Jawara M, von SL et al. Artesunate reduces but does not prevent posttreatment transmission of Plasmodium falciparum to Anopheles gambiae. J. Infect. Dis. 2001;183:1254–1259. doi: 10.1086/319689. [DOI] [PubMed] [Google Scholar]
- 14.Shekalaghe S, Drakeley C, Gosling R, Ndaro A et al. Primaquine clears submicroscopic Plasmodium falciparum gametocytes that persist after treatment with sulphadoxine-pyrimethamine and artesunate. PLoS One. 2007;2:e1023. doi: 10.1371/journal.pone.0001023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baird JK, Surjadjaja C. Consideration of ethics in primaquine therapy against malaria transmission. Trends Parasitol. 2011;27:11–16. doi: 10.1016/j.pt.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 16.Shekalaghe SA, ter BR, Daou M, Kavishe R et al. In Tanzania, hemolysis after a single dose of primaquine coadministered with an artemisinin is not restricted to glucose-6-phosphate dehydrogenase-deficient (G6PD A-) individuals. Antimicrob. Agents Chemother. 2010;54:1762–1768. doi: 10.1128/AAC.01135-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moonen B, Cohen JM, Snow RW, Slutsker L et al. Operational strategies to achieve and maintain malaria elimination. Lancet. 2010;376:1592–1603. doi: 10.1016/S0140-6736(10)61269-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coulibaly B, Zoungrana A, Mockenhaupt FP, Schirmer RH et al. Strong gametocytocidal effect of methylene blue-based combination therapy against falciparum malaria: a randomised controlled trial. PLoS One. 2009;4:e5318. doi: 10.1371/journal.pone.0005318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schneider P, Bousema JT, Gouagna LC, Otieno S et al. Submicroscopic Plasmodium falciparum gametocyte densities frequently result in mosquito infection. Am. J. Trop. Med. Hyg. 2007;76:470–474. [PubMed] [Google Scholar]
- 20.Kane NS, Hirschberg B, Qian S, Hunt D et al. Drug-resistant Drosophila indicate glutamate-gated chloride channels are targets for the antiparasitics nodulisporic acid and ivermectin. Proc. Natl. Acad. Sci. USA. 2000;97:13949–13954. doi: 10.1073/pnas.240464697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.World Health Organization. World Malaria Report. 2009. Geneva, Switzerland; 2009.


