Abstract
Background
Immunosuppression at intensive care unit (ICU) admission has been associated with a higher incidence of ICU-acquired infections, some of them related to opportunistic pathogens. However, the association of immunosuppression with the incidence, microbiology and outcomes of ICU-acquired bacterial bloodstream infections (BSI) has not been thoroughly investigated.
Methods
Retrospective single-centered cohort study in France. All adult patients hospitalized in the ICU of Lille University-affiliated hospital for > 48 h between January 1st and December 31st, 2020, were included, regardless of their immune status. Immunosuppression was defined as active cancer or hematologic malignancy, neutropenia, hematopoietic stem cell and solid organ transplants, use of steroids or immunosuppressive drugs, human immunodeficiency virus infection and genetic immune deficiency. The primary objective was to compare the 28-day cumulative incidence of ICU-acquired bacterial BSI between immunocompromised and non-immunocompromised patients. Secondary objectives were to assess the microbiology and outcomes of ICU-acquired bacterial BSI in the two groups.
Results
A total of 1313 patients (66.9% males, median age 62 years) were included. Among them, 271 (20.6%) were immunocompromised at ICU admission. Severity scores at admission, the use of invasive devices and antibiotic exposure during ICU stay were comparable between groups. Both prior to and after adjustment for pre‐specified baseline confounders, the 28-day cumulative incidence of ICU-acquired bacterial BSI was not statistically different between immunocompromised and non-immunocompromised patients. The distribution of bacteria was comparable between groups, with a majority of Gram-negative bacilli (~ 64.1%). The proportion of multidrug-resistant bacteria was also similar between groups. Occurrence of ICU-acquired bacterial BSI was associated with a longer ICU length-of-stay and a longer duration of invasive mechanical ventilation, with no significant association with mortality. Immune status did not modify the association between occurrence of ICU-acquired bacterial BSI and these outcomes.
Conclusion
The 28-day cumulative incidence of ICU-acquired bacterial BSI was not statistically different between patients with and without immunosuppression at ICU admission.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13613-024-01314-1.
Keywords: Intensive care units, Critical illness, Immunocompromised hosts, Neoplasms, Bloodstream infections, Bacteremia, Cross infections, Antimicrobial resistance
Background
Critically ill patients are at increased risk for intensive care unit (ICU)-acquired infections [1] because of the frequent disruption of anatomic barriers by invasive devices (intravascular catheters, endotracheal tubes, etc.) and impairments in immune defenses present either at admission or acquired during ICU stay [2]. ICU-acquired bloodstream infections (BSI) are among the most prevalent ICU-acquired infections and are predominantly related to bacterial pathogens [3]. They are often classified as primary or secondary, the latter referring to cases where bacteremia occurs in the setting of a primary source of infection, most often hospital- and ventilator-associated pneumonia (HAP and VAP, respectively) and intravascular catheter-related BSI (CRBSI). Occurrence of ICU-acquired BSI is associated with a longer ICU length-of-stay and a higher mortality [4, 5], especially when antimicrobial treatment is postponed or inappropriate [6], or in the absence of appropriate source control [7, 8]. Initial empirical antibiotic treatment is challenging due to the rising prevalence of multidrug-resistant (MDR) bacteria in ICUs [9].
Immunocompromised patients account for an increasing proportion of users of the healthcare system as a result of recent advances in the treatment of cancer, hematologic malignancies and immune-mediated diseases [10]. In the last two decades, the mortality of immunocompromised patients hospitalized in ICUs has decreased substantially [11], and consequently their proportion in the typical ICU case-mix has increased to reach approximately a third of all ICU patients [12, 13]. There is clear evidence of an increased risk of community-acquired infections related to common and opportunistic pathogens in immunocompromised patients, especially those with neutropenia or hematologic malignancies [14–16]. While several recent studies have investigated the epidemiology of hospital- and ICU-acquired BSI [1, 3, 5, 7, 8, 17, 18], there is a paucity of data related specifically to immunocompromised patients. Immunosuppression is often cited as a risk factor for ICU-acquired BSI [3], but has not been confirmed in several recent studies [4, 5, 18], and the association of immune status with the microbiology and outcomes of BSI is unclear.
To investigate this, we conducted the COCONUT study, a retrospective single-center study in the ICU of Lille University-affiliated hospital. The primary objective was to examine the association between immunosuppression at ICU admission and the 28-day cumulative incidence of ICU-acquired bacterial BSI. We reasoned that immunocompromised patients are often exposed to several risk factors for ICU-acquired BSI (including long-term vascular catheters such as implanted ports or peripherally implanted central catheters), thus our hypothesis was that the incidence of ICU-acquired bacterial BSI would be higher in immunocompromised than in non-immunocompromised patients. Secondary objectives included: (1) to describe the microbiology of ICU-acquired bacterial BSI in immunocompromised and non-immunocompromised patients; (2) to examine the association between occurrence of BSI and patient outcomes; (3) to assess whether immune status modifies the association between occurrence of BSI and patient outcomes; and (4) to examine the association between immunosuppression at ICU admission and patient outcomes.
Methods
Population and definitions
The COCONUT study (ICU-acquired blOodstream infeCtiONs in immUnocompromised paTients) was a retrospective single-center observational study at the ICU of Lille University-hospital (France). All adult patients hospitalized for > 48 h between January 1st and December 31st, 2020 were included, regardless of their immune status.
Immunosuppression was defined as solid cancer or hematologic malignancy (active or in remission for less than 5 years), neutropenia (neutrophil count < 1.5 G/L), hematopoietic stem cell transplant (HSCT), solid-organ transplant, long-term (≥ 28 days) use of steroids (at a dose ≥ 10 mg of prednisone per day or equivalent) or other immunosuppressant drugs, human immunodeficiency virus (HIV) infection, and genetic immune deficiency [13]. Immunological studies were not performed to further characterize immune functions among patients recruited to the study.
Data collection
Data were extracted from healthcare records into an electronic case report form. Data collected at baseline included: age, gender, body mass index (BMI), dates of ICU admission and discharge, Simplified Acute Physiology Score (SAPS) II score [19], Sequential Organ Failure Assessment(SOFA) [20], immune status at ICU admission, comorbidities, recent (i.e., in the 3 months before ICU admission) hospitalization for > 48 h, recent surgery, recent antibiotic treatment or known colonization with MDR bacteria, type of admission (medical vs. surgical), COVID-19 status, location before ICU admission, and reason for ICU admission.
Data collected during ICU stay included: invasive devices (central venous, arterial, dialysis catheters, endotracheal tube and tracheostomy), duration of invasive mechanical ventilation (IMV), prone positioning, extracorporeal membrane oxygenation (ECMO) or extracorporeal life support (ECLS), treatments received during ICU stay (including parenteral nutrition, transfusion, antibiotics and steroids), ICU-acquired colonization with MDR bacteria and ICU-acquired BSI.
Infection control and prevention
All patients enrolled in the study were hospitalized in single-bed ICU rooms. Infection control and prevention (IPC) measures routinely used in our center are in line with European guidelines, including contact precautions and isolation measures (as indicated), specifically with enhanced air filtration and positive room air pressure for high risk patients, and the prompt removal of catheters for all patients. Chlorhexidine bathing and selective digestive decontamination are not used routinely.
Endpoints
The primary endpoint was the 28-day cumulative incidence of ICU-acquired bacterial BSI, and was compared between immunocompromised and non-immunocompromised patients. ICU-acquired BSI related to fungi were not considered. Secondary endpoints included all-cause ICU mortality, ICU length-of-stay and duration of IMV, all censored at day 28 post-ICU admission.
Microbiology
The diagnosis of ICU-acquired bacterial BSI was based on a positive blood culture in the context of hyperthermia (temperature > 38 °C) or elevated blood markers of inflammation (C-reactive protein [CRP] or procalcitonin) occurring at least 48 h after ICU admission [21]. A single positive blood culture was sufficient to diagnose ICU-acquired BSI for most bacteria except for skin commensals (coagulase-negative staphylococci [CNS], Bacillus spp., Corynebacterium spp., and Cutibacterium acnes). In those cases, at least two sets of positive blood cultures collected from different sites or at a different time points were needed to rule out contamination. The time between sampling of different sets of blood cultures was not taken into consideration [22]. Blood cultures positive with fungi were excluded. ICU-acquired BSI was deemed secondary to another infection in cases where patients fulfilled criteria for another infection—including HAP, VAP [23] and CRBSI [21]—with the same microorganisms at the time blood cultures were sampled. Only the first episode of ICU-acquired BSI was considered.
Bacteria were identified by matrix-assisted laser desorption ionization time of flight mass spectrometry (MALDI-TOF-MS) with a Microflex mass spectrometer (Bruker Daltonik S.A., Wissembourg, France) according to the manufacturer’s instructions after extraction using formic acid. Antimicrobial susceptibility testing was performed using the Vitek-2 system (bioMérieux, Marcy-l’Étoile, France), combined with the MASTDISCS ID ESBL detection disc diffusion tests (Mast Diagnostics, Amiens, France) to confirm the presence of an extended spectrum beta-lactamase (ESBL) or the overexpression of a cephalosporinase. In case of carbapenemase, the OKNVI Resist Coris test (CorisBioconcept, Gembloux, Belgium) was used to determine the type of carbapenemase. Clinical breakpoints were interpreted using criteria proposed by the Comité de l’Antibiogramme de la Société Française de Microbiologie (CA-SFM EUCAST 2019) [24]. MDR bacteria were defined as: third generation cephalosporins (3GC)-resistant Enterobacteriaceae, including through expression of an ESBL; carbapenem-resistant Enterobacteriaceae; methicillin-resistant Staphylococcus aureus (MRSA); vancomycin-resistant Enterococcus faecalis and Enterococcus faecium (VRE); Pseudomonas aeruginosa resistant to imipenem and ceftazidime; and carbapenem-resistant Acinetobacter baumannii (CRAB) [25].
Statistical analysis
Patient characteristics at ICU admission and during ICU stay were described according to immune status without statistical comparisons. Categorical variables were reported as number and percentage, whereas quantitative variables were expressed as median (and 25th to 75th percentiles).
We assessed the risk of ICU-acquired bacterial BSI using competing risk survival analysis to take into account the duration of ICU stay by treating ICU discharge (alive or dead) as a competing event. We also used competing risk survival analysis to analyze ICU mortality (considering ‘death in ICU’ as event of interest and ‘ICU discharge alive’ as competing event), duration of IMV (considering ‘successful weaning’ as event of interest and ‘death under IMV’ as competing event) and ICU length-of-stay (considering ‘ICU discharge alive’ as event of interest and ‘death in the ICU’ as competing event). In survival analyses, start time was set at the date of ICU admission for ICU mortality and ICU length-of-stay, and as the date of first intubation for duration of IMV (analysis carried out in a subset of 666 patients under IMV). All analyses were censored at 28 days.
We estimated the cumulative incidence of ICU-acquired bacterial BSI, ICU mortality, successful weaning of IMV and ICU discharge alive according to immune status by using the non-parametric Kalbfleisch and Prentice method to account for competing events [26]. The association of immune status with each outcomes was assessed using cause-specific Cox proportional hazard models regarding the causal research question [27]. We chose to use cause-specific Cox models rather than Fine and Gray models because our aim was to assess etiological associations [27, 28]. Cause-specific hazard ratios (cHR) for immunocompromised vs. non-immunocompromised were derived from Cox regression models with theirs 95% confidence intervals (CI) as effect size, and the proportional hazard assumption was assessed by using the scaled Schoenfeld residuals plots. The association of immune status with the risk of ICU-acquired bacterial BSI was further investigated after adjustment for pre-specified baseline confounders (age, gender, COVID-19, SAPS-II and SOFA scores) and pre-specified time-varying confounders (exposure to central venous catheters, arterial catheters, renal replacement therapy, IMV and antibiotic treatment in ICU). The association of immune status with prognostic outcomes (ICU mortality, duration of IMV and ICU length-of-stay) was further investigated after adjustment for pre-specified baseline confounders (age, gender, COVID-19, SAPS-II, heart failure, chronic respiratory disease, chronic kidney disease). To account for the fact that the proportional hazard assumption was violated for COVID-19 in all Cox models, the effect of COVID-19 status was modeled by including time-dependent coefficients in the multivariable Cox models. For the duration of IMV and ICU length-of-stay, the proportional hazard assumption for SAPS-II was also not satisfied, thus the effect of SAPS-II was also modeled by including time-dependent coefficients. As a secondary analysis, we also estimated and compared the incidence rates of ICU-acquired bacterial BSI (expressed as number of events per 1000 ICU days, and per 1000 catheter days for patients with at least one ICU day with catheter) of ICU-acquired bacterial BSI according to immune status by using a Poisson regression model, using ICU duration (or catheter duration) as offset variable (after applying a log-transformation), before and after adjustment for pre-specified baseline confounders.
We investigated the association of occurrence of ICU-acquired bacterial BSI with prognostic outcomes by using univariable and multivariable cause-specific Cox regression models, treating ICU-acquired BSI as a time-varying covariate. The same confounders included in analyses of the association of immune status with prognostic outcomes were included in these models. In addition, we did a subgroup analysis of the association of ICU-acquired bacterial BSI and patient prognostic outcomes according to immune status by fitting separate cause-specific Cox regressions models. Heterogeneity in the association of occurrence of ICU-acquired BSI with patient outcomes according to immune status was assessed using the chi-square heterogeneity test.
Statistical testing was performed with a two-tailed α level of 0.05. Data were analyzed using the SAS software package, release 9.4 (SAS Institute, Cary, NC).
Results
Patients characteristics
A total of 1313 patients were included between January 1st and December 31st, 2020. Among them, 271 (20.6%) were immunocompromised and 1042 (79.4%) were non-immunocompromised at ICU admission. The main causes of immunosuppression were the use of immunosuppressive therapies (n = 134, 49.4%), cancer (n = 103, 38.0%), steroids (n = 90, 33.2%), hematologic malignancy (n = 78, 28.8%) and neutropenia (n = 48, 17.7%). One hundred and seventy patients (62.7%) had more than one cause of immunosuppression (Supplementary Table 1).
Patients were mostly male (66.9%), with a median age of 62 years (Table 1). Some comorbidities were more common among immunocompromised patients, including chronic cardiovascular disease, chronic lung disease and chronic kidney disease. Immunocompromised patients were more likely than non-immunocompromised patients to have been hospitalized on a ward for > 48 h, to have had surgery and to have received antibiotics for > 48 h in the 3 months prior to ICU admission. The proportion of patients colonized with MDR bacteria at ICU admission was also higher among immunocompromised patients than among non-immunocompromised patients. The type of ICU admission, severity scores, exposure to invasive devices, use and duration of antibiotics during ICU stay were comparable between groups. Transfusion of blood products and corticosteroids exposure during ICU stay were more frequent in immunocompromised patients than in non-immunocompromised patients. However, the doses of steroids received during ICU stay were similar between groups (Table 2).
Table 1.
Patient characteristics at ICU admission
| Characteristics | Overall cohort (n = 1313) | Immuno-compromised patients (n = 271) | Non-immuno-compromised patients (n = 1042) |
|---|---|---|---|
| Age (years) | 62 (50–70) | 65 (54–72) | 61 (50–70) |
| Male gender | 8793 (66.9) | 165 (60.9) | 714 (68.5) |
| Body mass index (kg/m2) | 27.4 (23.7–32.7)1 | 25.6 (22.5–30.1)2 | 27.9 (24.0–33.7)3 |
| Smoking | 366 (27.9) | 72 (26.6) | 294 (28.2) |
| Chronic alcohol use | 216 (16.5) | 25 (9.2) | 191 (18.3) |
| Diabetes mellitus | 390 (29.7) | 71 (26.2) | 319 (30.6) |
| Cardiovascular disease | 719 (54.8) | 164 (60.5) | 555 (53.3) |
| Hypertension | 642 (48.9) | 143 (52.8) | 499 (47.9) |
| Coronary-artery disease | 188 (14.3) | 39 (14.4) | 149 (14.3) |
| Heart failure | 154 (11.7) | 42 (15.5) | 112 (10.7) |
| Venous thromboembolic disease | 86 (6.5) | 28 (10.3) | 58 (5.6) |
| Lung disease | 284 (21.6) | 76 (28.0) | 208 (20.0) |
| Chronic respiratory disease | 250 (19.0) | 70 (25.8) | 180 (17.3) |
| COPD | 177 (13.5) | 37 (13.7) | 140 (13.4) |
| Chronic kidney disease | 128 (9.7) | 40 (14.8) | 88 (8.4) |
| Liver cirrhosis | 56 (4.3) | 16 (5.9) | 40 (3.8) |
| Recent surgery | 111 (8.5) | 39 (14.4) | 72 (6.9) |
| Recent antibiotic treatment for > 48 h | 327 (24.9) | 107 (39.5) | 220 (21.1) |
| Recent hospitalization for > 48 h | 351 (26.7) | 132 (48.7) | 219 (21.0) |
| Known colonization with MDR bacteria | 149 (11.3) | 48 (17.7) | 101 (9.7) |
| COVID-19 | 488 (37.2) | 64 (23.6) | 424 (40.7) |
| SAPS-II | 39 (29–55)4 | 44 (34–57) | 38 (28–54)5 |
| SOFA score | 4 (2–8)6 | 4 (2–7)7 | 4 (2–8)8 |
| Type of ICU admission | |||
| Medical | 1228 (93.5) | 256 (94.5) | 972 (93.5) |
| Surgical | 853 (6.5) | 15 (5.5) | 70 (6.7) |
Values are as number (%), or median (25th to 75th percentiles)
COPD chronic obstructive pulmonary disease, ICU intensive care unit, MDR multidrug-resistant, SAPS-II simplified acute physiology score II, SOFA sequential organ failure assessment
Missing values: 1111, 215, 396, 42, 52, 680, 719, 861
Table 2.
Patient characteristics during ICU stay
| Characteristics | Overall cohort (n = 1313) | Immuno-compromised patients (n = 271) | Non-immuno-compromised patients (n = 1042) |
|---|---|---|---|
| Invasive devices and procedures | |||
| Central venous catheter | 804 (61.2) | 177 (65.3) | 627 (60.2) |
| Duration (days) | 10 (6–20) | 9 (5–20) | 11 (6–22) |
| Arterial catheter | 944 (71.9) | 198 (73.1) | 746 (71.6) |
| Duration (days) | 10 (6–18) | 9 (5–14) | 10 (6–20) |
| Renal replacement therapy | 137 (10.4) | 24 (8.9) | 113 (10.8) |
| Invasive mechanical ventilation | 666 (50.7) | 122 (45.0) | 544 (52.2) |
| Duration (days) | 8 (3–18) | 7 (3–14) | 9 (3–19) |
| Treatments | |||
| Antibiotics | 1127 (85.8) | 237 (87.5) | 890 (85.4) |
| Duration (days) | 5 (3–7)1 | 5 (3–8)2 | 5 (3–7)3 |
| Parenteral nutrition | 111 (8.5) | 18 (6.6) | 93 (8.9) |
| Transfusions | 326 (24.8) | 93 (34.3) | 233 (22.4) |
| Corticosteroids | 615 (46.8) | 153 (56.4) | 462 (44.3) |
| Prednisone dose equivalence (mg) | 60 (40–130)4 | 50 (40–100)5 | 62 (40–130)6 |
Values are as number (%) or median (25th to 75th percentiles)
ICU intensive care unit
Missing values: 19, 21, 38, 431, 59, 622
Association between immune status and the incidence of ICU-acquired bacterial BSI
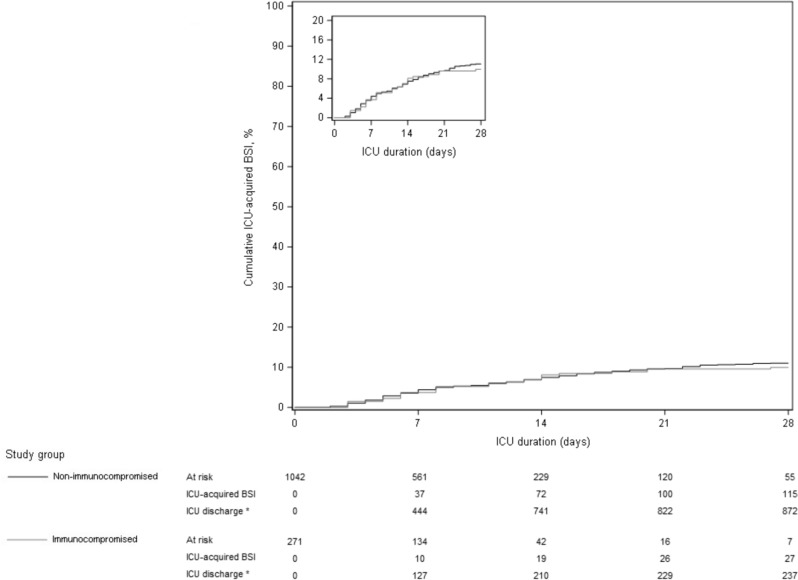
As shown in Fig. 1 and Table 3, 27 immunocompromised patients over 271 (incidence, 10.0%) presented at least one episode of ICU-acquired bacterial BSI in the 28 days following ICU admission, in comparison to 115 over 1042 non-immunocompromised patients (incidence, 11.0%). In cause-specific Cox regression analysis, the occurrence of ICU-acquired bacterial BSI was not associated with immune status, both in univariate analysis (cHR 1.12, 95% CI 0.73–1.70) and after adjustment for pre-specified confounders (adjusted cHR 1.57, 95% CI 0.99–2.49).
Fig. 1.
Cumulative incidence of ICU-acquired BSI according to immune status, considering death as a competing event
Table 3.
Association of immunosuppression at ICU admission with ICU-acquired BSI and main prognostic outcomes
| 28-day outcomes | Non-immuno-compromised patients (n = 1042) | Immuno-compromised patients (n = 271) | Unadjusted | Adjusted | ||
|---|---|---|---|---|---|---|
| cHR (95% CI) | P-Value | cHR (95% CI) | P-Value | |||
| ICU-acquired BSI1 | ||||||
| Cumulative incidence (%) | 115 (11.0) | 27 (10.0) | 1.12 (0.73–1.70) | 0.60 | 1.57 (0.99–2.49)2 | 0.053 |
| Incidence rate (95%CI) per 1000 ICU-days | 20.8 (18.3–23.7) | 22.9 (17.6–30.0) | 1.10 (0.82–1.47)3 | 0.52 | 1.33 (0.97–1.80)3,4 | 0.069 |
| Incidence rate (95%CI) per 1000 catheter-days5 | 25.3 (22.2–28.9) | 29.3 (22.6–37.9) | 1.16 (0.86–1.55)3 | 0.32 | 1.38 (1.01–1.89)3,4 | 0.038 |
| 28-day mortality | 166 (15.9) | 79 (29.2) | 2.10 (1.60–2.75) | < 0.001 | 1.81 (1.37–2.41)6 | < 0.001 |
| ICU discharge alive | 777 (74.6) | 176 (64.9) | 0.97 (0.82–1.15) | 0.75 | 0.88 (0.74–1.04)6 | 0.13 |
| Successful weaning of IMV | 408 (75.9) | 69 (56.6) | 0.82 (0.63–1.07) | 0.14 | 0.68 (0.52–0.89)6 | 0.005 |
Values are number of events (28-day cumulative incidence, in %) otherwise as indicated. IMV analysis was done in the 666 patients treated by IMV during the first 28 days of ICU stay
BSI bloodstream infections, CI confidence interval, cHR cause-specific hazard ratio, IMV invasive mechanical ventilation, ICU intensive care unit, SAPS-II simplified acute physiology Score II, SOFA sequential organ failure assessment
1Pre-specified as primary outcome
2Adjusted for pre-specified baseline confounders (age, gender, COVID-19, SAPS-II and SOFA scores) and pre-specified time-dependent confounders (exposure to central venous catheters, arterial catheters, renal replacement therapy, IMV and antibiotic treatment in ICU)
3Incidence rate ratio
4Adjusted for pre-specified baseline confounders (age, gender, COVID-19, SAPS-II and SOFA scores)
5Calculated in 956 patients with a catheter for at least one day
6Adjusted for pre-specified baseline confounders (age, gender, COVID-19, SAPS-II, heart failure, chronic respiratory disease, chronic kidney disease)
Microbiology
Among the bacteria responsible for ICU-acquired BSI, Gram-negative bacilli were the most frequent organisms identified (64.1%), mainly Klebsiella pneumoniae and Enterobacter spp., followed by Gram-positive cocci (34.5%), mainly coagulase-negative staphylococci (Supplementary Table 2). The distribution of bacteria was comparable between groups. The distribution of BSI sources was also comparable between groups, with a majority of secondary BSI related to VAP, followed by CRBSI.
We identified a total of 47 ICU-acquired BSIs related to MDR bacteria (33.1%). The proportion of ICU-acquired BSI related to MDR bacteria was comparable between groups (29.6% in immunocompromised vs. 33.9% in non-immunocompromised patients). Among those MDR bacteria, 3GC-resistant Enterobacteriaceae were the most frequently isolated organisms (63.8%), followed by carbapenem-resistant Enterobacteriaceae, imipenem-resistant Acinetobacter spp. and MRSA (Supplementary Table 2).
Association between ICU-acquired bacterial BSI and prognosis
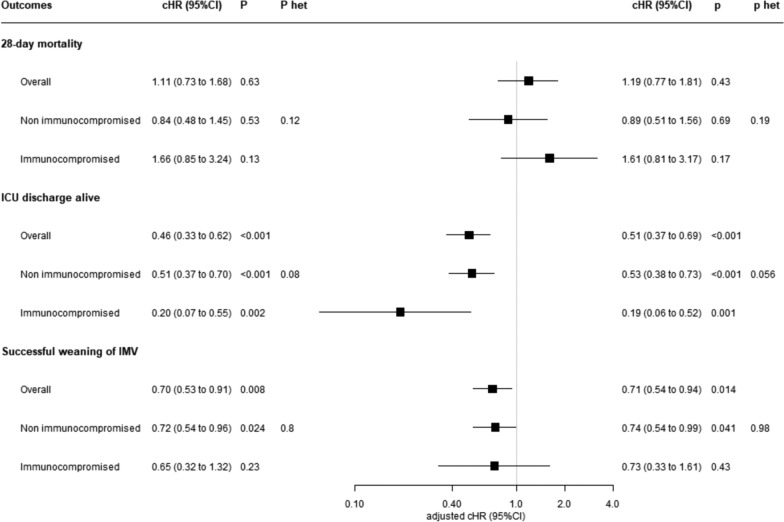
Considering the whole cohort, there was no significant association between occurrence of ICU-acquired bacterial BSI and ICU mortality during the first 28 days following ICU admission (adjusted HR 1.19, 95% CI 0.77–1.81) (Fig. 2). Occurrence of ICU-acquired bacterial BSI was associated with a longer ICU length-of-stay (adjusted cHR 0.51, 95% CI 0.37–0.69 for the event ‘ICU discharge alive’) and a longer duration of IMV (adjusted cHR 0.71, 95% CI 0.54–0.94 for the event ‘successful weaning of IMV’). There was no evidence of heterogeneity in the association between occurrence of ICU-acquired bacterial BSI and prognostic outcomes according to immune status, i.e., comparable associations were found when considering immunocompromised and non-immunocompromised patients separately.
Fig. 2.
Unadjusted and adjusted effect size for occurrence of ICU-acquired BSI on ICU mortality, ICU discharge alive and successful weaning of IMV (censored at day 28). cHRs were calculated using Cox proportional hazard models with a cause-specific hazard approach, by treating ICU-acquired BSI as a time-dependent binary covariate, with adjustment on pre-specified baseline confounders (age, gender, COVID-19, SAPS-II, heart failure, chronic respiratory disease, chronic kidney disease). P-het indicates p-values for heterogeneity (i.e., p-value for comparison in effect size associated with ICU-acquired BSI between non-immunocompromised and immunocompromised patients). A cHR > 1 indicates a decrease in ICU survival (i.e., an increased risk of mortality), duration of IMV (i.e., an increased risk of successful weaning) and ICU length-of-stay (i.e., an increased risk of ICU discharge alive). Conversely, a cHR < 1 indicates an increase in ICU survival (i.e., a decreased risk of mortality), duration of IMV (i.e., a decreased risk of successful weaning) and ICU length-of-stay (i.e., a decreased risk of ICU discharge alive). Note that the event of interest for ICU survival is a pejorative event (death), whereas for duration of IMV and ICU length-of-stay the event of interest is a positive event (successful weaning or ICU discharge alive). BSI bloodstream infections, CI confidence interval, cHR cause-specific hazard ratio, ICU intensive care unit, IMV invasive mechanical ventilation, SAPS-II simplified acute physiology Score II
Association between immune status and prognosis
As shown in Table 3 and Supplementary Fig. 1, immunosuppression was associated with a higher 28-day ICU mortality (adjusted cHR 1.81, 95% CI 1.37–2.41) and a longer duration of IMV (among the 666 patients receiving IMV, adjusted cHR 0.68, 95% CI 0.52–0.89 for the event ‘successful weaning of IMV’). No significant association was found between immune status and ICU length-of-stay.
Discussion
In this observational retrospective single-center cohort study, we found that the incidence of ICU-acquired bacterial BSI was not significantly different between immunocompromised and non-immunocompromised patients. The microbiology of BSI was similar between groups. The occurrence of ICU-acquired bacterial BSI was associated with longer ICU length-of-stay and duration of IMV, but not with an increased mortality.
There is a wealth of epidemiological evidence demonstrating that immunocompromised patients are at higher risk than non-immunocompromised patients for infections in general [14, 15], and for community-acquired infections in particular, including community-acquired BSI [16] and pneumonia [29]. It is also clear that patients with profound immunosuppression, especially patients with prolonged neutropenia or hematologic malignancies, are at higher risk of hospital-acquired infections, and that these infections carry a higher risk of worse outcomes in this population. However, few studies have specifically investigated the association between immunosuppression—using a broader definition including patients with different types of immunosuppression—and ICU-acquired infections, especially ICU-acquired BSI. Interestingly, immunosuppression of any cause at ICU admission was not a risk factor for the occurrence of ICU-acquired BSI in a retrospective analysis on 571 ICU-acquired BSI episodes among 10,734 patients from the Outcomerea Database (France) [4], nor in a retrospective study on 1306 ICU-acquired BSI episodes among 150,948 ICU admissions in 85 American ICUs [18]. In a retrospective study on 330 ICU-acquired BSI episodes among 6339 patients in Australia, immune deficiency and malignancies were more prevalent in patients with at least one ICU-acquired BSI than in patients without (10.6 vs. 7.0%, p = 0.02 for immunosuppression and 19.1 vs. 14.8%, p = 0.04 for malignancies), but immunosuppression was not an independent risk factor for ICU-acquired BSI in multivariate analysis [5]. Overall, the practical implication of these findings—if they are confirmed in subsequent larger multicenter studies—is that the level of clinical suspicion, the microbiological work-up and the management of ICU-acquired bacterial BSI should not differ between immunocompromised and non-immunocompromised patients.
Several factors could explain these somewhat counter-intuitive findings. First among these is antibiotic exposure in the ICU, which can modulate the risk of ICU-acquired BSI in several ways. On the one hand, exposure to antibiotics (especially if broad-spectrum) could lead to a decreased sensitivity of blood cultures, and consequently induce a bias in our results towards a lower rate of detection of BSI. On the other hand, antibiotics also have an untargeted effect on the normal commensal flora, including that of the skin, which leads to a decreased resistance to colonization by pathogenic strains and can facilitate secondary infections [30]. In the COCONUT study, we did not record antibiotic exposure with enough granularity to characterize this further. However, the number of days on antibiotics was similar in immunocompromised and non-immunocompromised patients, and this variable was accounted for by multivariate analysis when assessing the association of immune status with the incidence of ICU-acquired BSI.
Second, the sources of ICU-acquired bacterial BSI should be considered. In the COCONUT study, the primary sources of ICU-acquired BSI were pulmonary infections, including VAP. Contrary to common assumptions, we have shown in an ancillary analysis of the prospective multinational TAVeM database that the incidence of ventilator-associated lower respiratory tract infection was lower in immunocompromised than non-immunocompromised patients (16.6% vs. 24.2%, respectively, subdistribution HR 0.65, 95% CI 0.53–0.80) [31]. This could explain in part why, in the COCONUT study, the incidence of ICU-acquired bacterial BSI was not higher in immunocompromised than in non-immunocompromised patients.
Third, we adopted a broad definition of immunosuppression, and it is probable that our findings also reflect a substantial heterogeneity in the nature, depth and duration of immune defects in this patient group. A detailed analysis of the risk of ICU-acquired BSI in patients with different types of immunosuppression (e.g., neutropenic patients vs. others) could provide more detailed insight into this, but our limited sample size precluded such analysis in this cohort. Furthermore, it is now well established that a large proportion of patients with an apparently normal immune system at baseline develop features of acquired immunosuppression as a result of the initial insult—sepsis, major surgery or trauma—that precipitated their ICU admission, or because they are exposed to immune-modulating therapies in the ICU [32, 33]. This might explain that the ‘actual net state of immunosuppression’—an ill-defined concept that is for now impossible to quantify precisely at the bedside—could actually be comparable between patients labeled as ‘immunocompromised’ at admission and their apparently non-immunocompromised counterparts.
In the COCONUT study, there was no significant difference in the proportion of ICU-acquired BSI related to MDR bacteria among immunocompromised and non-immunocompromised patients. These findings are in line with a recent observational multicenter study where the incidence of ICU-acquired infections with MDR bacteria (including BSI) were not different between these two patient groups [13]. Whether this relates to differences in IPC strategies or other factors (differential exposure to antimicrobials, immune functions) remains to be explored specifically.
We found that the occurrence of ICU-acquired bacterial BSI was associated with longer ICU length-of-stay and duration of IMV, but was not significantly associated with mortality. This is in contradiction with several studies which have documented an association between occurrence of ICU-acquired BSI and higher mortality, including the study by Adrie et al. (adjusted HR 1.40, 95% CI 1.16–1.69) [4], the study by Prowle et al. (adjusted HR 2.89, 95% CI 2.41–3.46) [5], and a retrospective study on 232 ICU-acquired BSI episodes among 3247 patients in 12 ICUs in France (odds ratio [OR] 3.20 95% CI 2.30–4.43) [6]. This could be explained by a lack of statistical power to detect an impact on mortality, due to a smaller sample size (and subsequently a small number of ICU-acquired BSI) in the COCONUT study.
Little data has been published on the way baseline immunosuppression modifies the association between occurrence of ICU-acquired BSI and outcomes. In the EUROBACT study, Tabah et al. found that among patients with hospital-acquired BSI (76% of which were acquired in the ICU), immunosuppression was associated with an increased mortality risk (OR 2.11, 95% CI 1.40–3.19) [7]. However, in the COCONUT study we found that immunosuppression at ICU admission had no effect on the association between occurrence of BSI and patient outcomes. This could be due to different definitions of immunosuppression, to the evolution of practices between these two studies, or to a lack of power in the COCONUT study. Several studies have documented a clear positive impact of source control to reduce mortality related to BSI [7, 8], but unfortunately the proportion of patients achieving prompt source control in our cohort was not recorded. However, because it is standard practice at our institution to promptly change all central venous and arterial lines in case of ICU-acquired sepsis of unknown origin—independently of immune status—this could also explain why mortality associated with the occurrence with BSI was similar in immunocompromised and non-immunocompromised patients.
Our study has several limitations. Its retrospective and mono-centric design make it difficult to extend our findings to other ICU settings. Due to its limited sample size and the relatively low incidence of ICU-acquired bacterial BSI, it is possible that a small but substantial difference in the incidence of ICU-acquired BSI between immunocompromised and non-immunocompromised patients could not be detected because of a lack of statistical power. This is also suggested by the fact that the adjusted cause-specific hazard ratio for the incidence of ICU-acquired BSI almost reaches statistical significance (indicating a higher risk in immunocompromised patients) after adjustment for confounders. We did not record detailed data on antibiotic use, a key determinant of the epidemiology of ICU-acquired infections in general, and ICU-acquired BSI in particular. We acknowledge that our definition of immunosuppression is imperfect, as it groups together patients with clearly heterogeneous immune dysfunctions, and fails to capture ICU-acquired immune defects known to be associated with the occurrence of ICU-acquired infections. The limited sample size precluded a more detailed analysis aiming to compare the incidence, microbiology and outcomes of ICU-acquired BSI between patients with different types of immunosuppression (e.g., neutropenic patients vs. others). Finally, we did not collect data on antibiotic therapy initiated after identification of ICU-acquired BSI episodes, and it would have been interesting to assess whether the appropriateness of initial empiric antibiotic treatments had an impact of the association between ICU-acquired BSI and outcomes.
Conclusion
In this monocentric, retrospective observational cohort study, the incidence of ICU-acquired bacterial BSI was not different between immunocompromised and non-immunocompromised patients. This suggests that the clinical management of ICU-acquired bacterial BSI should not differ between immunocompromised and non-immunocompromised patients. Further studies are required to better assess the relationship between immunosuppression—both present at ICU admission or acquired during ICU stay—and the incidence, microbiology and outcomes of ICU-acquired infections in general, and ICU-acquired BSI specifically.
Supplementary Information
Acknowledgements
None.
Abbreviations
- 3GC
Third-generation cephalosporin
- BMI
Body mass index
- BSI
Bloodstream infection
- COPD
Chronic obstructive pulmonary disease
- CRBSI
Intravascular catheter-related bloodstream infection
- ECLS
Extra-corporeal life support
- ECMO
Extra-corporeal membrane oxygenation
- ESBL
Extended spectrum beta-lactamase
- HIV
Human immunodeficiency virus
- HR
Hazard ratio
- cHR
Cause-specific hazard ratio
- HSCT
Hematopoietic stem cell transplant
- ICU
Intensive care unit
- IMV
Invasive mechanical ventilation
- MDR
Multidrug resistant
- MRSA
Methicillin-resistant Staphylococcus aureus
- SAPS-II
Simplified Acute Physiology Score
- SOFA
Sequential Organ Failure Assessment
- VA-LRTI
Ventilator-associated lower respiratory tract infection
- VAP
Ventilator-associated pneumonia
- VAT
Ventilator-associated tracheobronchitis
- VRE
Vancomycin-resistant Enterobacterales
Author contributions
Study conception and design: MH, SN. Data curation: GZ, AP, GP, SRA and BH. Statistical analysis: JL. Manuscript drafting: GZ, LK. Critical revision: all authors.
Funding
There was no specific funding for this study.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study was conducted in accordance with the 1964 Declaration of Helsinki and its later amendments. In accordance with the French law, patient consent was waved due to the retrospective collection of anonymized data. The database was registered to the Commission Nationale l’Informatique et des Libertés.
Consent for publication
All authors consent to the publication of the manuscript in Annals of Intensive Care, should the article be accepted by the Editor-in-Chief upon completion of the review process.
Competing interests
LK has received speaking fees and a research scholarship from BioMérieux, and has been employed by Transgene. SN has received speaking fees from MSD, Pfizer, BioMérieux, Fischer and Paykel, Medtronic, and Mundipharma.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ghadi Zebian and Louis Kreitmann have contributed equally.
References
- 1.Vincent J-L, Sakr Y, Singer M, et al. Prevalence and outcomes of infection among patients in intensive care units in 2017. JAMA. 2020;323:1478–1487. doi: 10.1001/jama.2020.2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kreitmann L, Helms J, Martin-Loeches I, et al. ICU-acquired infections in immunocompromised patients: a narrative review. Intensive Care Med. 2024 doi: 10.1007/s00134-023-07295-2. [DOI] [PubMed] [Google Scholar]
- 3.Timsit J-F, Ruppé E, Barbier F, et al. Bloodstream infections in critically ill patients: an expert statement. Intensive Care Med. 2020;46:266–284. doi: 10.1007/s00134-020-05950-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adrie C, Garrouste-Orgeas M, Ibn Essaied W, et al. Attributable mortality of ICU-acquired bloodstream infections: impact of the source, causative micro-organism, resistance profile and antimicrobial therapy. J Infect. 2017;74:131–141. doi: 10.1016/j.jinf.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 5.Prowle JR, Echeverri JE, Ligabo EV, et al. Acquired bloodstream infection in the intensive care unit: incidence and attributable mortality. Crit Care. 2011;15:R100. doi: 10.1186/cc10114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garrouste-Orgeas M, Timsit JF, Tafflet M, et al. Excess risk of death from intensive care unit-acquired nosocomial bloodstream infections: a reappraisal. Clin Infect Dis. 2006;42:1118–1126. doi: 10.1086/500318. [DOI] [PubMed] [Google Scholar]
- 7.Tabah A, Koulenti D, Laupland K, et al. Characteristics and determinants of outcome of hospital-acquired bloodstream infections in intensive care units: the EUROBACT international cohort study. Intensive Care Med. 2012;38:1930–1945. doi: 10.1007/s00134-012-2695-9. [DOI] [PubMed] [Google Scholar]
- 8.Tabah A, Buetti N, Staiquly Q, et al. Epidemiology and outcomes of hospital-acquired bloodstream infections in intensive care unit patients: the EUROBACT-2 international cohort study. Intensive Care Med. 2023;49:178–190. doi: 10.1007/s00134-022-06944-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Waele JJ, Akova M, Antonelli M, et al. Antimicrobial resistance and antibiotic stewardship programs in the ICU: insistence and persistence in the fight against resistance. A position statement from ESICM/ESCMID/WAAAR round table on multi-drug resistance. Intensive Care Med. 2018;44:189–196. doi: 10.1007/s00134-017-5036-1. [DOI] [PubMed] [Google Scholar]
- 10.Harpaz R, Dahl RM, Dooling KL. Prevalence of immunosuppression among US adults, 2013. JAMA. 2016;316:2547–2548. doi: 10.1001/jama.2016.16477. [DOI] [PubMed] [Google Scholar]
- 11.Pène F, Percheron S, Lemiale V, et al. Temporal changes in management and outcome of septic shock in patients with malignancies in the intensive care unit. Crit Care Med. 2008;36:690–696. doi: 10.1097/CCM.0B013E318165314B. [DOI] [PubMed] [Google Scholar]
- 12.Azoulay E, Russell L, Van de Louw A, et al. Diagnosis of severe respiratory infections in immunocompromised patients. Intensive Care Med. 2020;46:298–314. doi: 10.1007/s00134-019-05906-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kreitmann L, Vasseur M, Jermoumi S, et al. Relationship between immunosuppression and intensive care unit-acquired colonization and infection related to multidrug-resistant bacteria: a prospective multicenter cohort study. Intensive Care Med. 2023 doi: 10.1007/s00134-022-06954-0. [DOI] [PubMed] [Google Scholar]
- 14.Bodey GP, Buckley M, Sathe YS, Freireich EJ. Quantitative relationships between circulating leukocytes and infection in patients with acute leukemia. Ann Intern Med. 1966;64:328–340. doi: 10.7326/0003-4819-64-2-328. [DOI] [PubMed] [Google Scholar]
- 15.van Delden C, Stampf S, Hirsch HH, et al. Burden and timeline of infectious diseases in the first year after solid organ transplantation in the swiss transplant cohort study. Clin Infect Dis. 2020;71:e159–e169. doi: 10.1093/cid/ciz1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laupland KB, Gregson DB, Zygun DA, et al. Severe bloodstream infections: a population-based assessment. Crit Care Med. 2004;32:992–997. doi: 10.1097/01.CCM.0000119424.31648.1E. [DOI] [PubMed] [Google Scholar]
- 17.European Centre for Disease Prevention and Control (2023) Annual Epidemiological Report for 2019 – Healthcareassociated infections acquired in intensive care units.
- 18.Gouel-Cheron A, Swihart BJ, Warner S, et al. Epidemiology of ICU-onset bloodstream infection: prevalence, pathogens, and risk factors among 150,948 ICU patients at 85 U.S. hospitals*. Crit Care Med. 2022;50:1725–1736. doi: 10.1097/CCM.0000000000005662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Le Gall JR, Lemeshow S, Saulnier F. A new simplified acute physiology score (SAPS II) based on a European/North American multicenter study. JAMA. 1993;270:2957–2963. doi: 10.1001/jama.1993.03510240069035. [DOI] [PubMed] [Google Scholar]
- 20.Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ failure assessment) score to describe organ dysfunction/failure. On behalf of the working group on sepsis-related problems of the European society of intensive care medicine. Intensive Care Med. 1996;22:707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 21.Mermel LA, Allon M, Bouza E, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 update by the infectious diseases society of America. Clin Infect Dis. 2009;49:1–45. doi: 10.1086/599376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fabre V, Jones GF, Hsu Y-J, et al. To wait or not to wait: optimal time interval between the first and second blood-culture sets to maximize blood-culture yield. Antimicrob Steward Healthcare Epidemiol. 2022;2:e51. doi: 10.1017/ash.2022.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Torres A, Niederman MS, Chastre J, et al. International ERS/ESICM/ESCMID/ALAT guidelines for the management of hospital-acquired pneumonia and ventilator-associated pneumonia: guidelines for the management of hospital-acquired pneumonia (HAP)/ventilator-associated pneumonia (VAP) of the European Respiratory Society (ERS), European Society of Intensive Care Medicine (ESICM), European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and Asociación Latinoamericana del Tórax (ALAT) Eur Respir J. 2017 doi: 10.1183/13993003.00582-2017. [DOI] [PubMed] [Google Scholar]
- 24.EUCAST (2020) The European Committee on Antimicrobial Susceptibility Testing. Breakpoint tables for interpretation of MICs and zone diameters, version 10.0, 2020
- 25.Magiorakos A-P, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18:268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 26.Prentice RL, Kalbfleisch JD, Peterson AV, et al. The analysis of failure times in the presence of competing risks. Biometrics. 1978;34:541–554. doi: 10.2307/2530374. [DOI] [PubMed] [Google Scholar]
- 27.Andersen PK, Geskus RB, de Witte T, Putter H. Competing risks in epidemiology: possibilities and pitfalls. Int J Epidemiol. 2012;41:861–870. doi: 10.1093/ije/dyr213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Austin PC, Lee DS, Fine JP. Introduction to the analysis of survival data in the presence of competing risks. Circulation. 2016;133:601–609. doi: 10.1161/CIRCULATIONAHA.115.017719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Certan M, Garcia Garrido HM, Wong G, et al. Incidence and predictors of community-acquired pneumonia in patients with hematological cancers between 2016 and 2019. Clin Infect Dis. 2022;75:1046–1053. doi: 10.1093/cid/ciac005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fishbein SRS, Mahmud B, Dantas G. Antibiotic perturbations to the gut microbiome. Nat Rev Microbiol. 2023 doi: 10.1038/s41579-023-00933-y. [DOI] [PubMed] [Google Scholar]
- 31.Moreau A-S, Martin-Loeches I, Povoa P, et al. Impact of immunosuppression on incidence, aetiology and outcome of ventilator-associated lower respiratory tract infections. Eur Respir J. 2018 doi: 10.1183/13993003.01656-2017. [DOI] [PubMed] [Google Scholar]
- 32.Venet F, Monneret G. Advances in the understanding and treatment of sepsis-induced immunosuppression. Nat Rev Nephrol. 2018;14:121–137. doi: 10.1038/nrneph.2017.165. [DOI] [PubMed] [Google Scholar]
- 33.Venet F, Textoris J, Blein S, et al. Immune profiling demonstrates a common immune signature of delayed acquired immunodeficiency in patients with various etiologies of severe injury. Crit Care Med. 2022;50:565–575. doi: 10.1097/CCM.0000000000005270. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.