Abstract
Shiga toxigenic E. coli are important foodborne zoonotic pathogens. The present study was envisaged to standardize loop-mediated isothermal amplification assays targeting stx1 and stx2 genes for rapid and visual detection of STEC and compare its sensitivity with PCR. The study also assessed the effect of short enrichment on the detection limit of LAMP and PCR. The developed LAMP assays were found to be highly specific. Analytical sensitivity of LAMP was 94 fg/µLand 25.8 fg/µL for stx-1 and stx-2 while LOD of 5 CFU/g of carabeef was measured after 6–12 h enrichment. The study highlights the importance of short (6–12 h) enrichment for improving the sensitivity of LAMP. The entire detection protocol could be performed within 9 h yielding results on the same day. The developed LAMP assays proved to be a handy and cost-effective alternative for screening STEC contamination in meat.
Supplementary Information
The online version contains supplementary material available at 10.1007/s42770-024-01335-9.
Keywords: LAMP, STEC, Stx1, Stx2, Carabeef, Isothermal amplification
Introduction
Foodborne diseases caused by pathogenic microbes are a global public health concern and are responsible for causing more than 75% of food poisoning cases [1]. Escherichia coli (E. coli) embraces a diverse set of Gram-negative bacteria and are common inhabitants of the intestinal tracts of humans and animals. Detection and enumeration of E. coli in food and water are used as hygiene or sanitary indicators. Particularly in the beef industry, E. coli enumeration is usually done to check hygienic slaughtering conditions and to assess meat quality [2]. Although E. coli are commensals in the intestine, some can cause clinical disease owing to the presence of virulence factors carried by them. One such pathotype, the Shiga toxigenic E. coli (STEC), encompasses strains producing Shiga-like toxins (Stx1 and Stx2) and is a foodborne zoonotic pathogen of significant public health concern [3]. The capacity of E. coli to produce Shiga toxins is bestowed by the stx1 and stx2 genes. Stx1 is almost identical to the Shiga toxin produced by Shigella dysenteriae type 1, with a single amino acid difference, whereas stx2 shares 55 to 60% homology with Stx1 and is immunologically discrete [4]. Enterohaemorrhagic E. coli (EHEC) is a subset of STEC which possess one or both of the stx genes and an eae gene, encoding the intimin adhesion molecule which is an additional and important virulence feature [5]. STEC infection in humans causes mild to severe diarrhoea; nevertheless, EHEC is explicitly associated with causing haemorrhagic colitis and haemolytic uremic syndrome (HUS) [3].
Worldwide, STEC causes 2,801,000 cases of acute illness, of these 3890 develop HUS, 270 permanent end-stage kidney disease, and 230 deaths, annually [6]. Although E. coli O157:H7 persists as the single most common and virulent STEC strain, the significance of some non-O157 STEC strains is also emerging worldwide [7]. FoodNet data in the United States have steadily exposed more infections caused by non-O157 strains than O157 strains of STEC [8]. The main sources of STEC implicated in previous foodborne outbreaks are foods of cattle origin like raw milk, undercooked ground, or minced beef. Foodborne illnesses by STEC are also reported to be caused by other food sources like salads, unpasteurized fruit juices, and sprouts [5]. Although many food laboratories routinely test for E. coli O157:H7, they often do not test for other STEC serotypes. Considering the high prevalence of STEC-associated (O157 and non-O157) foodborne illnesses in many parts of the world, and to curtail their incidence, it is imperative to detect them at various stages of the food value chain.
Traditional culture-based methods remain the gold standard for the detection of various pathogens, however, they are often laborious, technically demanding, and time-consuming [9]. Most of the rapid screening tests developed for microbial pathogens are nucleic acid-based (e.g. PCR) or antibody-based (e.g. lateral flow assay). There are pros and cons to either of these methods. Antibody-based approaches are simple, specific, and rapid but can lack the sensitivity that food processors need to make instant food product disposition judgments [5], while most molecular methods are rapid, sensitive, and specific but require specific instruments and sophisticated laboratory setup [10]. Thus, there is a need to develop diagnostic tests that are sensitive, specific as well as less time-consuming and should have the potential to be used in resource-limited settings under field conditions.
LAMP (Loop-mediated isothermal amplification) was recently developed by Notomi et al. (2000) [11]. LAMP uses 4/6 primers to amplify 6/8 target sequences under isothermal conditions. LAMP satisfies the “ASSURED” criteria of the diagnostic method development guidelines given by the WHO since it is affordable (inexpensive), sensitive, specific, user-friendly, rapid (robust), equipment-free, and deliverable to the end user [12, 13]. LAMP amplified products can be visualized by the naked eye simply by observing for turbidity or by the addition of SYBR Green I, a DNA intercalating dye that exhibits a fluorescent green colour in positive samples when a suitable light source is used. The principle of LAMP is based on the ability of Bst polymerase to displace the strand as well as synthesize DNA under isothermal conditions at a temperature range of 60–72 °C [14]. LAMP assays have been reported to be used in the detection of many foodborne pathogens including Salmonella [13], Clostridium perfringens [15], Escherichia coli O157 [16], Vibrio parahaemolyticus [17], Staphylococcus aureus [18], etc. Several LAMP assays targeting the stx1 and stx2 genes of STEC have also been developed and deployed in food animals and foods of animal origin [19–22].
In India, due to the prohibition on cattle slaughter, demand has increased for carabeef (buffalo meat). In 2016-17, carabeef contributed 94% (in value terms) to India’s total meat export. India is the second largest carabeef exporter with an 18% share in global exports [23]. Therefore, the increasing demand for carabeef warrants pathogen testing before consumption, processing, and export. To date, no LAMP assay has been optimized for the detection of STEC in carabeef.
The present study was envisaged to develop and evaluate simple isothermal amplification assays targeting stx1 and stx2 genes for rapid and visual detection of STEC in meat. The sensitivity and specificity of LAMP assays were compared with conventional end-point PCR. The applicability of the developed LAMP assays was evaluated by spiking studies in carabeef as well as on field samples. The study also evaluated the effect of short enrichment (6–12 h) on the limit of detection of LAMP and PCR.
Materials and methods
Bacterial strains
STEC (n = 7) and non-STEC (n = 17) reference bacterial strains maintained at the Food Microbiology Laboratory, Division of Livestock Products Technology, ICAR-Indian Veterinary Research Institute, Izatnagar were employed in the present study (Table 1). All the bacterial strains were cultured in Brain Heart Infusion broth (HiMedia, India) and incubated at 37 °C. The DNA extracted from reference strains of STEC viz. C338 (stx1+) and C173A (stx2+) were used as a positive control in LAMP and PCR assays. For the spiking study in carabeef, reference STEC strains (C338 and C173A) were cultured and diluted in phosphate-buffered saline (1X PBS).
Table 1.
Bacterial strains used in the study
| S. No. | Genus / Species | Strain |
|---|---|---|
| 1. | Shiga toxigenic E.coli (stx2) | C173 |
| 2. | Shiga toxigenic E. coli (stx2) | 1 A |
| 3. | Shiga toxigenic E. coli (stx2) | 2B |
| 4. | Shiga toxigenic E. coli (stx1) | 23 A |
| 5. | Shiga toxigenic E. coli (stx1) | C338 |
| 6. | Shiga toxigenic E. coli (stx1) | 558 |
| 7. | Escherichia coli | DE 95 |
| 8. | Escherichia coli O157:H7 | ATCC 43,888 |
| 9. | Staphylococcus aureus | ATCC 43,300 |
| 10. | Proteus vulgaris | P-1 |
| 11. | Pseudomonas aeruginosa | MTCC 1688 |
| 12. | Brucella abortus | S19 Vaccine strain |
| 13. | Klebsiella pneumoniae | ATCC 700,603 |
| 14. | Listeria monocytogenes | ATCC 19,115 |
| 15. | Pasteurella multocida | P52 Vaccine strain |
| 16. | Edwardsiellatarda | MTCC 2400 |
| 17. | Campylobacter jejuni | ATCC 29,428 |
| 18. | Citrobacter freundii | MTCC 2956 |
| 19. | Bacillus cereus | MTCC 430 |
| 20. | Mycobacterium tuberculosis | MTCC 300 |
| 21. | Salmonella Typhimurium | MTCC 3224 |
| 22. | Clostridium perfringens | ATCC 13,124 |
Extraction of genomic DNA and template preparation
After cultivating all the bacterial strains for 24 h at 37 °C, genomic DNA was isolated with a bacterial genomic DNA extraction kit (HiMedia, India). All the extractions were done in duplicate. The concentration of the isolated DNA was measured with the Nanodrop system and kept at -20 °C for further use. To analyze analytical sensitivity, ten-fold serial dilutions of genomic DNA were prepared using an elution buffer supplied with a DNA extraction kit.
Primer design
The LAMP primers were designed targeting the stx1 gene of STEC employing PrimerExplorer V5 (http://primerexplorer.jp/elamp5.0.0/index.html) platform. The oligos were designed from the well-conserved area after examining 15 stx1 gene sequences obtained from the National Center for Biotechnology Information (NCBI). Two outer (F3 and B3) and two inner (FIP and BIP) primers were included in the LAMP reaction (Table 1S). The previously published LAMP primers as reported by Zhao et al. (2010) for detecting stx2 gene were used [16]. PCR primer pairs targeting stx1 and stx2 genes were designed and PCR assays were standardized to compare the performance of LAMP with PCR (Table 2S). All the oligos designed in this study were tested in silico using the BLAST-N tool.
Table 2.
Results of analytical sensitivity, limit of detection and field sampling and comparative evaluation of PCR and LAMP
| Strain /Sample ID | PCR Results | LAMP Results |
|---|---|---|
| Analytical sensitivity analysis | ||
| C338 (stx1+) and C173A (stx2+) | stx1- 94 fg/µL; stx2-25.8 fg/µL | stx1- 9.4 pg/µL; stx2- 258 fg/µL |
| Limit of detection determination by artificially spiking carabeef (unenriched conditions) | ||
| C338 (stx1+) and C173A (stx2+) |
stx1- 4.5 × 107CFU/g stx2-5 × 106 CFU/g |
stx1- 4.5 × 107CFU/g stx2-5 × 104 CFU/g |
| Field Applicability analysis using carabeef samples | ||
| ICB1 | - | - |
| ICB2 | - | - |
| ICB3 | - | - |
| ICB4 | + (stx1) | + (stx1) |
| ICB5 | + (stx1) | + (stx1) |
| ICB6 | - | - |
| ICB7 | - | - |
| ICB8 | - | - |
| ICB9 | - | - |
| ICB10 | - | - |
| ICB11 | - | - |
| ICB12 | + (stx2) | + (stx2) |
| ICB13 | - | - |
| ICB14 | - | - |
| ICB15 | - | - |
| ICB16 | - | - |
| ICB17 | + (stx2) | + (stx2) |
| ICB18 | - | - |
| ICB19 | - | - |
| ICB20 | - | - |
| ICB21 | + (stx1) | + (stx1) |
| ICB22 | - | - |
| ICB23 | - | - |
| ICB24 | - | - |
| ICB25 | - | - |
| ICB26 | + (stx1) | + (stx1) |
| ICB27 | + (stx1) | + (stx1) |
| ICB28 | + (stx1) | + (stx1) |
| ICB29 | - | - |
| ICB30 | - | - |
| ICB31 | - | - |
| ICB32 | - | - |
| ICB33 | - | - |
| ICB34 | - | - |
| ICB35 | + (stx1) | + (stx1) |
| ICB36 | - | - |
| ICB37 | - | - |
| ICB38 | - | - |
| ICB39 | - | - |
| ICB40 | - | - |
| ICB41 | - | - |
| ICB42 | + (stx1) | + (stx1) |
| ICB43 | + (stx2) | + (stx2) |
| ICB44 | - | - |
| ICB45 | - | - |
| ICB46 | - | - |
| ICB47 | - | - |
| ICB48 | - | - |
| ICB49 | - | - |
| ICB50 | - | - |
| ICB51 | - | - |
| ICB52 | - | - |
| ICB53 | - | - |
| ICB54 | - | - |
| ICB55 | - | - |
| ICB56 | + (stx2) | + (stx2) |
| ICB57 | - | - |
| ICB58 | + (stx2) | + (stx2) |
| ICB59 | - | - |
| ICB60 | - | - |
| ICB61 | - | - |
| ICB62 | - | - |
| ICB63 | - | - |
| ICB64 | - | - |
| ICB65 | - | - |
Optimization of LAMP
LAMP assays aiming stx1 and stx2 genes were optimized for concentrations of various reagents (200–1400 µM dNTP; 0.2–0.8 M betaine and 2.0–8.0 mM MgSO4) and amplification conditions including time (45–90 min) and temperature (60–68 °C). The inner (FIP and BIP) and outer (F3 and B3) LAMP primers were added in the 4:1 molar ratio. The amplified products of LAMP were visually interpreted by adding 1 µL of 10,000X SYBR Green I dye (Invitrogen, USA) diluted in 1X PBS (1:10). Production of greenish fluorescent colour was considered as positive and orange colour (no change) was considered as negative outcome in LAMP. For comparison, the PCR reactions were carried out in 25 µL volume and the optimized PCR reaction mixture contained 2.5 µL PCR buffer (10 X), 0.5 µl MgCl2 (50 mM), 0.5 µL dNTP mix (10 mM), 0.5 µL of Taq DNA polymerase (5 U/ µL), 0.5 µl (10 pmol/ µL) of each forward and reverse primers, 2.0 µL template DNA, and nuclease-free water to make up the reaction volume to 25 µL. The thermocycling conditions standardized included initial denaturation at 95 °C for 5 min, followed by 36 cycles of denaturation at 95 °C for 1 min, annealing at 68 °C for 1 min, and extension at 72 °C for 1 min and a final extension step of 72 °C for 10 min. The LAMP and PCR amplifications were performed using the Nexus GX2 thermocycler (Eppendorf, Germany).The amplified products of LAMP and PCR were analyzed using agarose gel electrophoresis on 1.5% gel.
Analytical sensitivity and specificity
To study the analytical sensitivity of the standardized LAMP and PCR assays, DNA extracted from the reference STEC strains C338 (stx1+) and C173A (stx2+) were tenfold serially diluted (10− 1 to 10− 8) with elution buffer supplied in DNA extraction kit (HiMedia, India). The analytical specificity of the assays was demonstrated by examining their reactivity with non-STEC bacterial strains’ DNA.
Limit of detection (LOD) analysis
The LOD was determined by conducting an artificial spiking study in Carabeef (buffalo meat). Carabeef was procured from the local retail meat market of Bareilly and confirmed to be negative for STEC by culture and PCR (stx1 and stx2 gene). Initially, 25 g of carabeef tested free from STEC was homogenized at 6000 rpm for 2 min (ULTRATURRAX, IKA, Germany). Homogenized meat (9 mL) was transferred to 15 mL sterile culture tubes. STEC reference strains carrying stx1 (C338) and stx2 (C173A) were grown overnight in BHI broth at 37 °C and centrifuged for 10 min at 10,000 x g to collect the bacterial pellet. After washing the pellet with PBS twice, tenfold serial dilutions of these bacterial suspensions were prepared in PBS (1X). The initial stock’s bacterial concentration was estimated by spread plate technique by spreading 100 µl of dilutions onto MacConkey agar plates. Different dilutions of bacterial suspension (1 mL) were inoculated into 9 mL carabeef homogenates. Immediately following inoculation (without allowing any enrichment), DNA was isolated from the carabeef homogenates carrying respective dilutions, and LAMP and PCR assays were carried out. Meat homogenate inoculated with 1X PBS was used as a negative control. To assess the impact of enrichment on the sensitivity of the developed assays, an enrichment step (6 h and 12 h) was also included in the spiking experiment. Briefly, 2 mL of such enriched samples collected at two time points (6 h and 12 h) were pelleted and re-suspended in 180 µL lysis buffer and progressed as per the manufacturer’s protocol of the DNA extraction kit. The extracted DNA was used as a template in both LAMP and PCR assay, and the LOD was measured and compared.
Applicability of developed assays
To evaluate the field applicability of the developed assays, they were deployed to screen the carabeef samples (n = 65). The relative accuracy of the developed LAMP for detecting STEC was compared with the end-point PCR.
Results & discussion
The optimized LAMP reaction mixture contained 1X Bst buffer, 1.4 mM dNTP mix, 5 pM of F3 and B3 primers, 20 pM of FIP and BIP primers, 8.0 mM MgSO4, 8.0 U Bst 2.0 WarmStart polymerase, 0.8 M betaine, 1.0 µL of template DNA and nuclease-free water (to make up volume 25.0 µL). The reaction was incubated at 65 °C for 90 min for amplification followed by incubation at 80 °C for 5 min for enzyme inactivation.
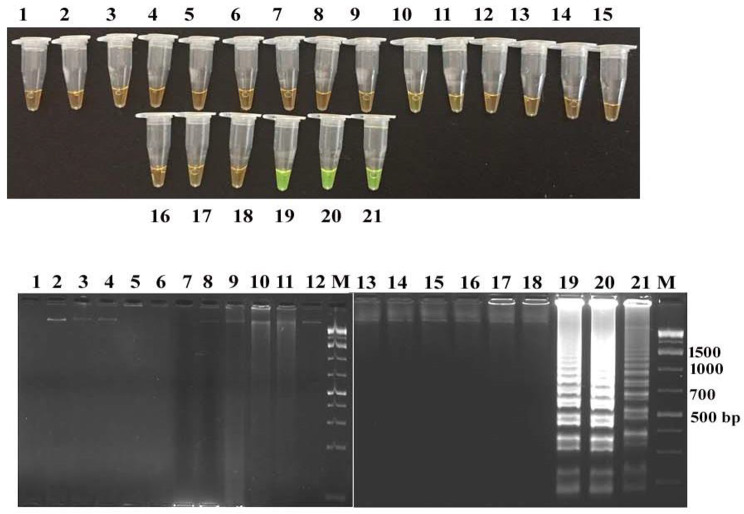
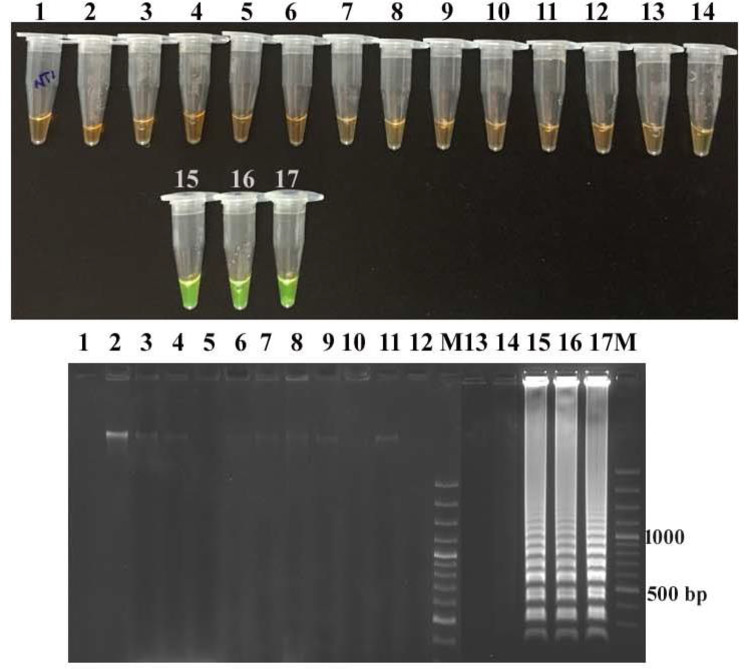
The LAMP assays developed targeting the stx1 and stx2 genes were found to be specific in detecting STEC strains as none of the non-STEC strains yielded positive results (Figs. 1 and 2). Conventional PCR also exhibited similar specificity indicating that stx1 and stx2 genes are specific targets for STEC detection. LAMP amplicons were interpreted with SYBR green I dye and agarose gel-electrophoresis which displayed greenish fluorescence and ladder pattern in positive outcomes, respectively. PCR amplification yielded specific bands of 680 bp and 400 bp for stx1 and stx2, respectively, as indicated by agarose gel electrophoresis (Figs. 1 and 2).
Fig. 1.
Specificity of the stx1-LAMP assay Detection of LAMP products in tubes by addition of SYBR Green I indicating green fluorescence and their corresponding agarose gel electrophoresis indicating ladder pattern in stx-1 positive E. coli strains.Tube (1–21): NTC (No template control), DE 95 Clinical isolate, stx2 positive E. coli strain 1 A, S. aureus ATCC 43,300, E. coli O157:H7 ATCC 43,888, Proteus vulgaris P-1, Klebsiella pneumoniae ATCC 700,603, Pseudomonas aeruginosa MTCC 1688, Pasteurella multocida P52 Vaccine strain, Brucella abortus S19 Vaccine strain, Campylobacter jejuni ATCC 29,428, Edwardsiella tarda MTCC 2400, Citrobacter freundii MTCC 2956, Listeria monocytogenes ATCC 19,115, Bacillus cereus MTCC 430, Mycobacterium tuberculosis MTCC 300, Salmonella Typhimurium MTCC 3224, C. perfringens ATCC 13,124; stx1 positive E. coli 23 A, stx1 positive E. coli 558, stx1 positive E. coli C338 Agarose gel electrophoresis of stx1-LAMP amplified products Lane M: 1 kb plus DNA ladder
Fig. 2.
Specificity of the stx2-LAMP assay Detection of LAMP products in tubes by addition of SYBR Green I indicating green fluorescence and their corresponding agarose gel electrophoresis indicating ladder pattern in stx-2 positive E. coli strains. Tube (1–17): NTC (No template control), stx1 positive E. coli C338, S. aureus ATCC 43,300, E. coli O157:H7 ATCC 43,888, Proteus vulgaris P-1, Klebsiella pneumoniae ATCC 700,603, Pseudomonas aeruginosa MTCC 1688, Pasteurella multocida P52 Vaccine strain, Campylobacter jejuni ATCC 29,428, Citrobacter freundii MTCC 2956, Listeria monocytogenes ATCC 19,115, Bacillus cereus MTCC 430, Salmonella Typhimurium MTCC 3224, C. perfringens ATCC 13,124; stx2 positive E. coli 1 A, stx2 positive E. coli 2B, stx2 positive E. coli C173 Agarose gel electrophoresis of stx2-LAMP amplified products; Lane M: 100 bp DNA ladder
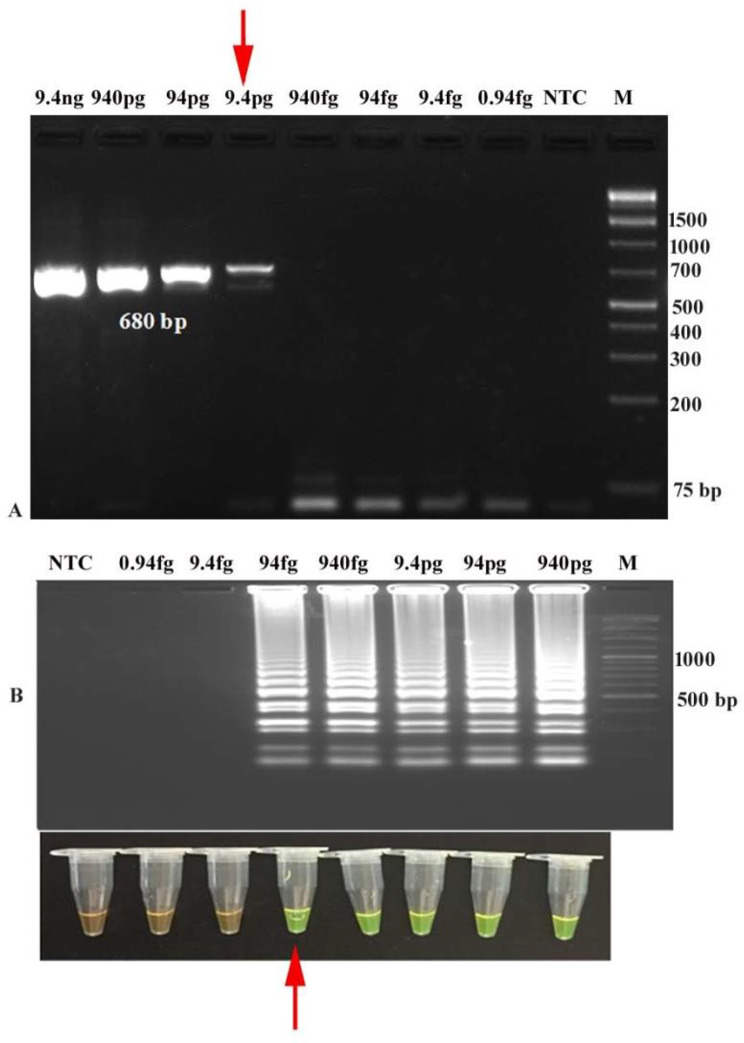
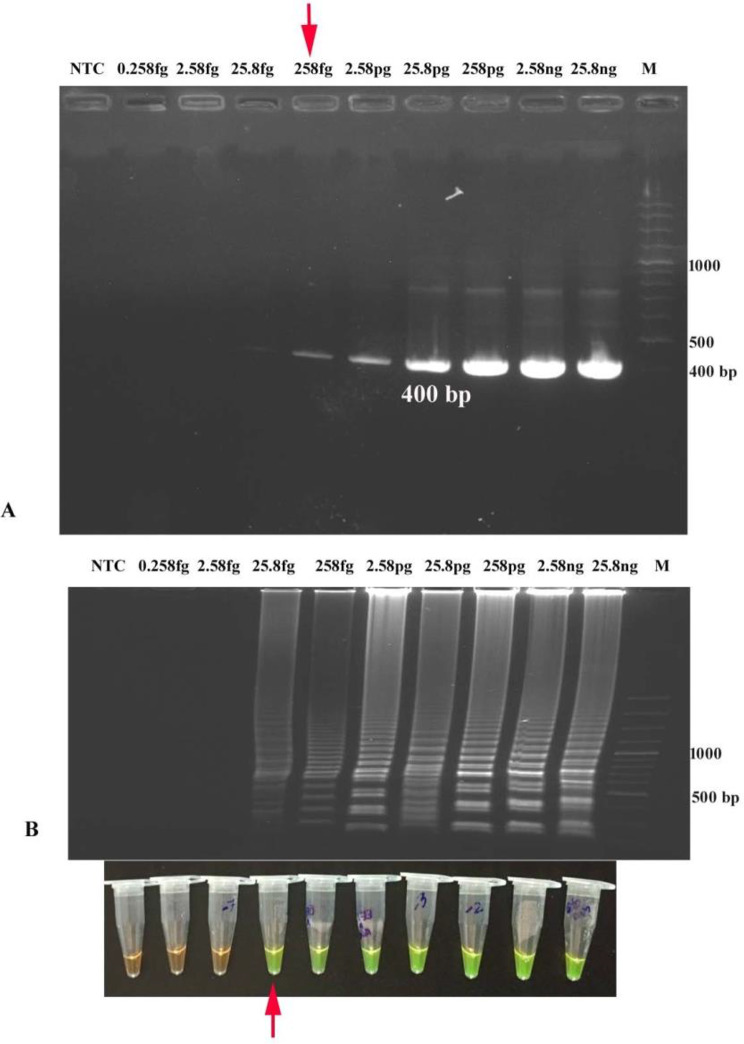
The concentration of DNA extracted from STEC reference strains C338 (stx1+) and C173A (stx2+) was found to be 9.4 ng/µL and 25.8 ng/µL, respectively. The concentration of 10-fold serially diluted DNA ranged from 9.4 ng/µL to 0.94 fg/µL for C338 (stx1+) and 25.8 ng/µl to 0.258 fg/µL for C173A (stx2+). The analytical sensitivity of developed LAMP for detecting stx1 and stx2 genes was calculated to be 94 fg/µL and 25.8 fg/µl, respectively after 90 min amplification at 65 °C, while conventional PCR detected 9.4 pg/µL of stx1 and 258 fg/µL of stx2, indicating that LAMP assays to be about 10-fold more sensitive than endpoint PCR (Figs. 3 and 4; Table 2).
Fig. 3.
Analytical Sensitivity ofstx1-LAMP and PCR (A) Analytical sensitivity of stx1-PCR (680 bp) by agarose gel electrophoresis up to 9.4 pg DNA/µL (M: 1 kb plus DNA ladder; NTC: No Template Control) (B) Analytical sensitivity of stx1-LAMP using SYBR Green I dye and agarose gel electrophoresis showing amplification up to of 94 fg DNA/µL (M: 100 bp DNA ladder; NTC: No Template Control)
Fig. 4.
Analytical Sensitivity of thestx2-LAMP and PCR (A) Analytical sensitivity of stx2-PCR (400 bp) by agarose gel electrophoresis up to 258 fg DNA/µL (B) Analytical sensitivity and visual detection of stx2-LAMP using SYBR Green I dye and Agarose gel electrophoresis of stx2-LAMP products showing amplification up to 25.8 fg DNA/µL (M: 100 bp DNA ladder; NTC: No Template Control)
In this study, the LAMP assay was optimized to carry out the amplification reaction at 65 °C for 90 min without the use of loop primers (only 4 primers). The assay was specific as it detected only STEC with no cross-reactivity with other non-STEC bacterial genera tested. In addition, the assay time can further be reduced by using loop primers as loop primers accelerate the rate of amplification [24]. However, reducing the number of primers and obtaining comparable results has its advantages. In the developed LAMP assay, the analytical sensitivity of detecting stx1 and stx2 was 94 fg/µL and 25.8 fg/µL, respectively. In a previously published LAMP assay, the analytical sensitivity of 100 fg was estimated for stx1 and stx2 genes [16], however, a superior analytical sensitivity of 10 fg was reported in the LAMP assay developed to detect stx1 and stx2 in STEC strains by Dong et al. 2014 [20].
In the LOD analysis of the developed assay, the STEC reference strains C338 (stx1+) and C173A (stx2+) had bacterial concentrations of 4.5 × 107CFU/mL and 5 × 107CFU/mL, respectively in their stock cultures. 9 mL of homogenized carabeef was added with 1 mL of 10-fold diluted reference strains prepared in 1X PBS. Therefore, inoculated carabeef homogenates were carrying C338 (stx1+) ranging from 4.5 × 107CFU to 4.5 CFU and C173A (stx2+) ranging from 5 × 107CFU to 5 CFU.
Under un-enriched conditions, the LOD for stx1 by both PCR and LAMP assay was 4.5 × 107CFU/g and 4.5 × 107CFU/g of meat, respectively, revealing comparable performance (Table 2; Figure S1). After 6 h enrichment, the LOD of LAMP was 100 times more than PCR with 45 CFU/g and 4.5 × 103CFU/g of meat, respectively (Figure S2), whereas, after 12 h enrichment, the LOD remained similar for LAMP but improved for PCR with 45 CFU/g of meat for both the assays (Figure S3). Under un-enriched conditions, the detection limit for stx2 by PCR and LAMP test was calculated to be 5 × 104 CFU/g and 5 × 106CFU/g of meat respectively, revealing PCR to be 100-fold more sensitive than LAMP (Table 2; Figure S4). Under the conditions enrichment of 6 h and 12 h, the LOD of LAMP and PCR improved significantly with a detection limit of 5 CFU/g of meat for both assays (Figure S5 and S6). Visualization by gel electrophoresis was found to be similar for LAMP assays and PCR.
The spiking studies revealed conventional PCR to be 10 (stx1) to 100 (stx2) fold more sensitive than LAMP if performed without enrichment. This might be due to the inhibitory factors present in complex food matrices leading to inhibition of amplification by Bst polymerase, however, Bst polymerase has been reported to have better resistance against inhibitors than Taq polymerase. The lower detection limit in the developed LAMP assay in comparison to the previous studies conducted in meat may be due to a varied range of biochemical components, such as high content of oleic acid, manganese, sodium, zinc, copper, myoglobin in carabeef and interference of template binding and inhibition of enzyme activity due to non-pathogenic inherent microflora [24, 25]. After a brief enrichment of 6 h, the LOD of LAMP assays was improved significantly yielding 100 times more sensitivity than conventional PCR. A similar kind of observation of improvement in LOD after brief enrichment of 6 h and lesser sensitivity of LAMP assay than conventional PCR was reported earlier for Clostridium perfringens detection in goat meat [15]. Similarly, a previous study on LAMP-based detection of STEC in ground beef and human stool samples also demonstrated rapid detection of STEC after 4 h enrichment, whereas qPCR required 4 to 6 h [26]. The result of the present study also emphasizes the need for a brief enrichment step to overcome the inhibitory effects of ingredients in different complex food matrices and for sensitive detection of target organisms by molecular methods. By including a brief enrichment step, the detection of dead organisms can be avoided in addition to the detection of low-level contamination by recovering stressed or injured microorganisms, thus enhancing the overall efficiency of the molecular diagnostic assay. Such improvement in the LOD has also been reported earlier in the detection of various foodborne pathogens in animal-origin foods [10, 13].
The detection limit of 45 CFU and 5 CFU for stx1 and stx2 carrying STEC, respectively, after 6 h enrichment is noteworthy as less than 100 bacteria (infective dose) of some STEC serotypes can cause human illness [27]. Thus, the developed LAMP may serve as a promising tool for the on-site detection of STEC in food and the prevention of foodborne illness. The LODs remained the same after 12 h enrichment as both PCR and LAMP assays yielded similar results. The developed LAMP assays can be used as a point-of-care diagnostic test as it does not require any thermocycler, post-amplification processing, or visualization equipment. Apart from this, the results can be determined by the naked eye by visualizing for turbidity due to the precipitation of magnesium pyrophosphate or by the addition of SYBR Green I dye which makes the detection much easier [28]. The comparative sensitivity of LAMP assays developed in the present study to the previously reported studies is summarized in Table 2S [26, 29–32]. Out of 65 field meat samples screened, 5 samples (7.7%) were found positive for stx2 and 8 samples (12.3%) were positive for stx1 using LAMP and PCR assays (Table 2). The developed LAMP and PCR assays correctly identified all theSTEC strains (n = 13) used in the study. Therefore, relative specificity, relative sensitivity, PPV andNPV for detection of STEC were 100% for the newly developed LAMP assay. The accuracy was also calculatedto be 100%. The Cohen’s Kappa valueof 1.0 demonstrates a perfect level ofagreement between the two testingmethods.
STEC strains are important foodborne zoonotic pathogens with considerable public health concern as they are frequently encountered in outbreaks and their ability to cause haemorrhagic colitis (HC) and life-threatening complications like haemolytic uremic syndrome (HUS). Considering the importance of carabeef contributing to the Indian economy and delivering quality meat to consumers, the developed LAMP may assist as a tool for on-site STEC detection in food and prevention of foodborne illness. The LAMP method standardized in this study gives comparable results to that of any other routine test like PCR. Further, LAMP assay is more suitable for resource-limited field laboratory settings especially in developing countries as it does not require sophisticated equipment involved in thermocycling, post-amplification processing of amplified products and equipment for visualization [18, 22].
In the present study, LAMP assays for the detection of STEC in carabeef were developed, and evaluated for their applicability for sensitive and specific detection of STEC by spiking studies and in field samples as well as the effect of enrichment on the detection limit of STEC was evaluated. The developed LAMP assays yielded results with the desired accuracy in both artificially spiked and naturally contaminated field samples. The inclusion of a brief enrichment step for LAMP was found to enhance the sensitivity of the molecular assays which can help in detecting low abundant, stressed, or injured bacterial pathogens.
Conclusion
The developed LAMP assays targeting stx1 and stx2 genes for detection of STEC in carabeef were found to be promising diagnostic tests as they were specific, sensitive, less time-consuming and have the potential to be performed in resource-limited settings under field conditions. The whole detection protocol could be performed within 10–12 h including the enrichment step providing results on the same day. Overall, developed LAMP assays proved to be a handy and cost-effective alternative for screening STEC and ensuring food safety.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors are grateful to the Director, ICAR-IVRI, Izatnagar (Bareilly) UP, India for providing necessary infrastructural facilitiesfor conducting the study.
Author contributions
Govindarajan Bhuvana Priya:Investigation, Formal analysis, Writing - original draft; Ravi Kant Agrawal: Conceptualization, Writing - original draft, Review & Editing; A.A.P. Milton: Writing - Review & Editing; Madhu Mishra: Formal analysis, Visualization; S.K. Mendiratta: Visualization; B.R. Singh: Methodology, Writing – Review & Editing; Deepak Kumar: Formal analysis, Investigation; R.K. Gandham: Methodology, Writing – Review & Editing; Z.B. Dubal: Validation, Writing - original draft; S. Rajkhowa: Visualization, Writing – original draft; Ashish Luke: Investigation, Writing – original draft; Girish Patil: Conceptualization, Writing – Review & Editing.
Funding
The authors thankfully acknowledge funding provided by Department of Biotechnology, Ministry of Science and Technology, Govt. of India for conducting the research work under NER-BPMC scheme (Grant no. BT/PR16754/95/275/2015).
Data availability
The data underlying this article are available in the article and in its online supplementary material.
Declarations
Conflict of intrest
No conflict of interest declared by any author.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Yan M, Xu L, Jiang H, et al. PMALAMP for rapid detection of Escherichia coli and shiga toxins from viable but non-culturable state. Microb Pathog. 2017;105:245–250. doi: 10.1016/j.micpath.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 2.Barros MDAF, Nero LA, Monteiro AA, et al. Identification of main contamination points by hygiene indicator microorganisms in beef processing plants. Food Sci Technol (Campinas) 2007;27:856–862. doi: 10.1590/S0101-20612007000400028. [DOI] [Google Scholar]
- 3.Newell DG, La Ragione RM. Enterohaemorrhagic and other Shiga toxin-producing Escherichia coli (STEC): where are we now regarding diagnostics and control strategies? Transbound Emerg Dis. 2018;65(Suppl1):49–71. doi: 10.1111/tbed.12789. [DOI] [PubMed] [Google Scholar]
- 4.Jackson MP, Neill RJ, O’Brien AD, et al. Nucleotide sequence analysis and comparison of the structural genes for Shiga-like toxin I and Shiga-like toxin II encoded by bacteriophages from Escherichia coli 933. FEMS Microbiol Lett. 1987;44:109–114. doi: 10.1111/j.1574-6968.1987.tb02252.x. [DOI] [Google Scholar]
- 5.Singh P, Liu Y, Bosilevac JM, et al. Detection of Shiga toxin-producing Escherichia coli, stx1, stx2 and Salmonella by two high resolution melt curve multiplex real-time PCR. Food Control. 2019;96:251–259. doi: 10.1016/j.foodcont.2018.09.024. [DOI] [Google Scholar]
- 6.Majowicz SE, Scallan E, Jones-Bitton A, et al. Global incidence of human Shiga toxin–producing Escherichia coli infections and deaths: a systematic review and knowledge synthesis. Foodborne Pathog Dis. 2014;11:447–455. doi: 10.1089/fpd.2013.1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scallan E, Hoekstra RM, Angulo FJ, et al. Foodborne illness acquired in the United States-Major pathogens. Emerg Infect Dis. 2011;17:7–15. doi: 10.3201/eid1701.P11101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang F, Yang Q, Qu Y, et al. Rapid, Reliable, and Robust Detection of Shiga Toxin-Producing Escherichia coli in produce. Appl Environmen Microbiol. 2014;80:2516–2525. doi: 10.1128/AEM.04203-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parsons BD, Zelyas N, Berenger BM, et al. Detection, characterization, and typing of Shiga Toxin-Producing Escherichia coli. Front Microbiol. 2016;7:478. doi: 10.3389/fmicb.2016.00478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Momin KM, Milton AAP, Ghatak S, et al. Development of a novel and rapid polymerase spiral reaction (PSR) assay to detect Salmonella in pork and pork products. Mol Cell Probes. 2020;50:101510. doi: 10.1016/j.mcp.2020.101510. [DOI] [PubMed] [Google Scholar]
- 11.Notomi T, Okayama H, Masubuchi H, et al. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000;28:63. doi: 10.1093/nar/28.12.e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mabey D, Peeling RW, Ustianowski A, et al. Diagnostics for the developing world. Nat Rev Microbiol. 2004;2:231–240. doi: 10.1038/nrmicro841. [DOI] [PubMed] [Google Scholar]
- 13.Priya GB, Agrawal RK, Milton AAP, et al. Rapid and visual detection of Salmonella in meat using invasin A (invA) gene-based loop-mediated isothermal amplification assay. LWT - Food Sci Technol. 2020;126:109262. doi: 10.1016/j.lwt.2020.109262. [DOI] [Google Scholar]
- 14.Zhong J. Zhao X isothermal amplification technologies for the detection of Foodborne pathogens. Food Anal Methods. 2018;11:1543–1541. doi: 10.1007/s12161-018-1177-2. [DOI] [Google Scholar]
- 15.Priya GB, Agrawal RK, Milton AAP, et al. Development and evaluation of isothermal amplification assay for the rapid and sensitive detection of Clostridium perfringens from chevon. Anaerobe. 2018;54:178–187. doi: 10.1016/j.anaerobe.2018.09.005. [DOI] [PubMed] [Google Scholar]
- 16.Zhao X, Li Y, Wang L, et al. Development and application of a loop-mediated isothermal amplification method on rapid detection Escherichia coli O157 strains from food samples. Mol Bio Rep. 2010;37:2183–2188. doi: 10.1007/s11033-009-9700-6. [DOI] [PubMed] [Google Scholar]
- 17.Yamazaki W, Kumeda Y, Misawa N, et al. Development of a loop-mediated isothermal amplification assay for sensitive and rapid detection of the tdh and trh genes of Vibrio parahaemolyticus and related Vibrio species. Appl Environ Microbiol. 2010;76:820–828. doi: 10.1128/AEM.02284-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Priya GB, Agrawal RK, Milton AAP, et al. Isothermal amplification assay for visual on-site detection of Staphylococcus aureus in Chevon. Food Biotechnol. 2021;35(3):221–236. doi: 10.1080/08905436.2021.1941078. [DOI] [Google Scholar]
- 19.Hara-Kudo Y, Nemoto J, Ohtsuka K, et al. Sensitive and rapid detection of Vero toxin-producing Escherichia coli using loop-mediated isothermal amplification. J Med Microbiol. 2007;56:398–406. doi: 10.1099/jmm.0.46819-0. [DOI] [PubMed] [Google Scholar]
- 20.Dong HJ, Cho AR, Hahn T, et al. Development of a multiplex loop-mediated isothermal amplification assay to detect shiga toxin-producing Escherichia coli in cattle. J Vet Sci. 2014;15:317–325. doi: 10.4142/jvs.2014.15.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stratakos AC, Linton M, Millington S, et al. A loop-mediated isothermal amplification method for rapid direct detection and differentiation of nonpathogenic and verocytotoxigenic Escherichia coli in beef and bovine faeces. J Appl Microbiol. 2017;122:817–828. doi: 10.1111/jam.13381. [DOI] [PubMed] [Google Scholar]
- 22.Baraily P, Zende RJ, Kshirsagar DP, et al. Rapid Detection of Shiga toxin-producing E. Coli in Animal Origin Foods using Loop-mediated isothermal amplification (LAMP) assay. Agric Res. 2019;8:490–496. doi: 10.1007/s40003-018-0391-x. [DOI] [Google Scholar]
- 23.Bardhan D, Kumar S, Kumar S, et al. Value chain analysis of buffalo meat (carabeef) in India. Agric Econ Res Rev. 2019;32:149–163. doi: 10.5958/0974-0279.2019.00024.7. [DOI] [Google Scholar]
- 24.Dwivedi HP, Jaykus L Detection of pathogens in foods: the current state-of-the-art and future directions. Crit Rev Microbiol 2011:37:40–63 [DOI] [PubMed]
- 25.Tamburrano A, Tavazzi B, Callà CAM, et al. Biochemical and nutritional characteristics of buffalo meat and potential implications on human health for apersonalized nutrition. Italian J Food Saf. 2019;8:8317. doi: 10.4081/ijfs.2019.8317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang W, Jiang L, Ge B (2012) Loop-mediated isothermal amplification assays for detecting Shiga Toxin-Producing Escherichia coli in Ground Beef and Human stools. J Clin Microbiol;91–97 [DOI] [PMC free article] [PubMed]
- 27.Thorpe CM. Shiga toxin-producing Escherichia coli infection. Clin Infect Dis. 2004;38:1298–1303. doi: 10.1086/383473. [DOI] [PubMed] [Google Scholar]
- 28.Xu Z, Li L, Chu J, et al. Development and application of loop-mediated isothermal amplification assays on rapid detection of various types of staphylococci strains. Food Res Int. 2012;47:166–173. doi: 10.1016/j.foodres.2011.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hara-Kudo Y, Konishi N, Ohtsuka K et al (2008) Detection of Verotoxigenic Escherichia coli O157 and O26 in food by plating methods and LAMP method: a collaborative study. Int J Food Microbiol 122:156–161 [DOI] [PubMed]
- 30.Ohtsuka K, Tanaka M, Ohtsuka T et al (2010) Comparison of detection methods for Escherichia coli O157 in beef livers and carcasses. Foodborne Pathog Dis 7:1563–1567 [DOI] [PubMed]
- 31.Wang F, Jiang L, Yang Q et al (2012) Rapid and Specific Detection of Escherichia coli Serogroups O26, O45,O103, O111, O121, O145, and O157 in Ground Beef, Beef Trim, and produce by Loop-mediated isothermal amplification. Appl Environ Microbiol;2727–2736 [DOI] [PMC free article] [PubMed]
- 32.Kim JH, Oh SW (2019) Development of a filtration-based LAMP–LFA method as sensitive and rapid detection of E. Coli O157:H7. J Food Sci Technol 56:2576–2583 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article and in its online supplementary material.