Abstract
Bovine tuberculosis (bTB), caused by Mycobacterium bovis (M. bovis), is a globally prevalent zoonotic infectious disease. World Organization for Animal Health (WOAH) estimates indicate that up to 10% of the total human TB cases in developing countries are attributed to M. bovis. Pakistan ranks 4th in global milk production with a livestock population of over 212 million animals. Over 8 million families are involved in raising these animals as a means of livelihood. To date, there is an absence of national-level data on the prevalence of bTB and an effective control program is still lacking. The multifaceted impacts and substantial economic losses render addressing bTB a daunting, but highly important challenge. In this review, we summarise all the freely available literature on M. bovis infection from Pakistan using Google scholar and PubMed databases. A total of 40 animal studies were identified using search terms: “bovine tuberculosis in Pakistan, bTB, Pakistan, Mycobacterium bovis in Pakistan, M. bovis in Pakistan”; while seven human studies were identified using the terms: zoonotic tuberculosis in Pakistan’, ‘M. bovis in humans Pakistan’, ‘zTB in TB patients in Pakistan”. We have summarized all these studies to identify critical risk factors involved in transmission of bTB among animals and humans. Despite lack of comprehensive and geographically representative studies, the literature suggests a varying prevalence of bTB in animals, ranging from as low as 2% to as high as 19%. Regarding zTB prevalence in humans, estimates range from 1.5% to 13% in high-risk group of farm and abattoir workers, with notably higher percentages in extra-pulmonary TB cases. The review also addresses the challenges that Pakistan faces in formulating an effective policy for the control and eradication of bTB. We conclude with one-health based recommendations as a way forward for controlling TB caused by M. bovis in cattle and humans.
Keywords: Bovine tuberculosis (bTB), Mycobacterium bovis (M. bovis), Pakistan, Public health, Zoonotic tuberculosis (zTB)
Highlights
-
•
Pakistan lacks accurate prevalence information for M. bovis infection in livestock (bovine tuberculosis, bTB) and its impact on humans (zoonotic tuberculosis, zTB).
-
•
This review covers published data until 2023 on M. bovis infection in humans and animals from Pakistan.
-
•
Regional prevalence reports confirm the presence of bTB in livestock species and M. bovis infection in human TB patients (zTB); with no measures in place for reporting and mitigation.
-
•
The review analyzes the challenges and risk factors for bTB and zTB; and proposes One Health-based recommendations for control of M. bovis infection in humans and animals.
1. Bovine tuberculosis and its zoonotic potential
Tuberculosis is a pervasive infectious disease with a history dating back over 3 million years [1]. Mycobacterium tuberculosis complex (MTBC) includes pathogenic mycobacteria such as Mycobacterium tuberculosis (M. tb), M. bovis [2], M. caprae, M. africanum, M. canetti, M. microtti, M. pinnipedii, and M. leprae [3]. MTBC members demonstrate a remarkably broad host range [4]. In humans, the major infections include tuberculosis (M. tb), leprosy (M. leprae), and zoonotic tuberculosis (M. bovis). In cattle, M. bovis is the etiological agent for bovine tuberculosis (bTB) [5].
Although M. tb primarily causes TB in humans; however, it is also occasionally isolated from diseased cattle and buffaloes in several countries of Asia and Africa [[6], [7], [8], [9], [10]]. M. bovis also exhibits an extensive range of hosts, encompassing domestic animals, livestock, wildlife, and humans [11]. Transmission among animals is thought to mostly occur through the respiratory route; however, vertical transmission via the digestive route from an infected mother to calf has also been reported [12,13]. This disease prompts substantial financial losses in animals worldwide. The financial losses are estimated to reach 3 billion USD each year worldwide due to loss of production, culling of animals and trade impediments [14,15].
M. bovis is zoonotic in nature causing zoonotic TB (zTB) in humans. The transmission of the bacteria is linked to human-animal proximity, lack of sanitation, consumption of unpasteurized milk and uncooked meat [[16], [17], [18]]. This makes it an important public health threat for low-and-middle income countries (LMICs). In Pakistan, 63% of the population lives in rural areas [19] and 62% of these people are either directly or indirectly involved with livestock. It has been reported that M. bovis can contribute up to 15% of human TB cases in LMICs, compared to <1% in high income countries [4,18,[20], [21], [22]]. Concurrently, Pakistan is ranked 5th among human TB high-burden countries worldwide and alone accounts for 61% of the TB burden in the WHO Eastern Mediterranean Region [23]. Each year over 500,000 new human TB cases are reported in Pakistan [23]. However, national-level data on the prevalence of bTB and the contribution of M. bovis to the incidence of human TB is unavailable. The symptoms and tissue lesions caused by M. bovis infection in humans cannot be distinguished from those caused by M. tuberculosis [4,24,25]. Moreover, zTB is frequently extra-pulmonary, which complicates accurate diagnosis using conventional human TB diagnostic tests; leading to misdiagnosis or missed diagnosis of zTB [26]. Despite its immense economic and public health importance, there are currently no surveillance or control programs in place against bTB in Pakistan. In this manuscript, we seek to fill this paucity of knowledge by providing an up-to-date overview of bTB disease status in animals and its contribution to human TB cases in Pakistan. The review in addition, highlights the challenges and proposes a way forward for control.
2. Current status of bTB in Pakistan
Bovine TB is endemic in Pakistan and was first reported in 1969 in district Faisalabad with a prevalence of 6.72% in dairy animals [27]. For this review, a literature search was conducted for all the studies described until 2023 on bTB in Pakistan. The strategy (presented as flow chart, Fig. 1) involved selecting all the published literature, in English language, from Google scholar and PubMed databases with the search terms: “bovine tuberculosis in Pakistan, bTB, Pakistan, Mycobacterium bovis in Pakistan, M. bovis in Pakistan”.
Fig. 1.
Flow chart depicting the structure of this review. Aim and the data search strategy has been described with the categories of information summarized from all the identified studies. Comprehensive analysis of risk factors, current challenges and control recommendations have also been included.
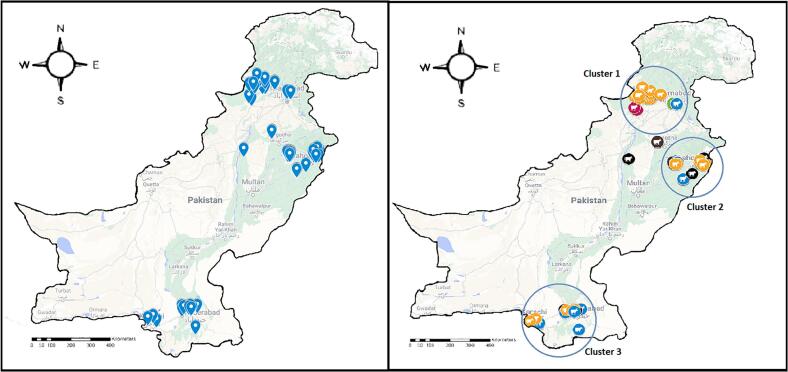
The search led to the identification of 40 studies (all of them were summarized and have been presented in literature review matrix in Table 1). The studies available online were conducted over a period of two decades, even though research on bTB had been conducted earlier but was not available on online search portals. These 40 studies reported data from overlapping district locations accounting to a total of 19 districts, with most studies being conducted in Lahore (n = 11), followed closely by Faisalabad (n = 8), Tando Allahyar (n = 4), Peshawar (n = 4) and Okara (n = 4) (supplementary fig. 1). The 19 districts covered 56 testing sites. On mapping the study sites (Fig. 2, left), three main clusters were formed (Fig. 2, right), one in Khyber Pakhtunkhwa (KPK), the second in Northern Punjab and the third in Sindh, leaving behind Southern Punjab, Baluchistan, Gilgit Baltistan as well as Azad Jammu and Kashmir. The clustering patterns and the affiliations of the corresponding authors in those clusters suggests that the majority of studies were performed near veterinary educational institutes that had academic expertise as well as laboratory facilities to conduct the required research. The data points were further parsed based on species tested in each study (Fig. 3). The data set from Pakistan showed that out of 56 testing sites, 19 sites tested buffaloes only, 13 targeted cattle, 19 sites included cattle as well as buffaloes, two sites tested cattle, buffalo, goat (or) sheep, and three sites tested captive wild animals (including deer, chinkara, antelope, black buck, gazelle, goral, nilgai, urial and zebra) (Fig. 3).
Table 1.
Chronological summary of bTB studies conducted in Pakistan. Geographical locations; study designs as well as major findings/conclusions have been summarized. Abbreviations: bTB, bovine tuberculosis; PPDa and PPDb, purified protein derivatives of M. avium and M. bovis; CITT, comparative intradermal tuberculin test; LJ, Lowenstein Jensen (media); SITT, single intradermal tuberculin test; ZN staining, Ziehl-Neelsen staining; AFB, acid fast bacilli.
| Sr. No. | Geographical location i.e., city/district and (province) | Study design | Major findings | Reference |
|---|---|---|---|---|
| 1. | Lahore (Punjab) |
Type of study: cross sectional Target animal species: dead zoo/captive animals Target location: breeding parks, government and private zoos, captive wild animals Sample size: 185 Sample types for testing: 1–3 pieces of lung tissues (2 × 2 cm) preserved in buffered formalin, lung tissue samples for PCR Diagnostic tests: microscopic examination of HE-stained tissues; ZN staining of impression smears, PCR |
|
[28] |
| 2. | Mirpurkhas and Badin districts (Sindh) |
Type of study: cross sectional Target animal species: Cattle Target location: urban and peri-urban areas Sample size: 200 Sample types for testing: blood, nasal discharge and milk (total 600 samples, 200 of each type) Diagnostic tests: SITT, Lilli rapid Ab test (Lilly dale Diagnostics, England), ELISA, microscopy and culture (growth on LJ media supplemented with 1% sodium pyruvate; followed by niacin and urease tests for specie differentiation) |
|
[29] |
| 3. | 4 public sector livestock experiment stations at Pattoki, Lahore, Bhakkar and Khushab (across Punjab) |
Type of study: cross sectional Target animal species: buffaloes Target location: Livestock/dairy farms Sample size: 627 Diagnostic tests: CITT |
|
[30] |
| 4. | Bahawalnagar (Punjab) |
Type of study: cross sectional Target animal species: cattle and buffalo Target location: Bahawalnagar Sample size: 340 Sample types for testing: blood Diagnostic tests: CITT and ELISA |
|
[31] |
| 5. | Hyderabad, Tando Allahyar (Sindh) |
Type of study: cross sectional Target animal species: cattle Target location: Peri-urban and rural areas Sample size: 160 Sample types for testing: blood, milk, nasal discharge and feces Diagnostic tests: SITT, rapid bovine antibody test and culture (growth on LJ media, followed by nitrate reduction and niacin tests for specie differentiation) |
|
[32] |
| 6. | Khyber Pakhtunkhwa province (KPK) |
Type of study: cross sectional Target animal species: cattle and buffaloes Target location: Peshawar, Nowshera, Charsadda, Mardan and Swabi districts Sample size: 2400 (1225 cattle and 1175 buffaloes) Sample types for testing: milk from 1608 lactating animals Diagnostic tests: CITT, PCR and culture (growth on Stone brink's media, followed ZN staining) |
|
[33] |
| 7. | Hyderabad, Tando Allahyar (Sindh) |
Type of study: cross-sectional study Target animal species: buffaloes Target location: rural and peri-urban farming Sample size: 120 Sample types for testing: blood and milk Diagnostic tests: SITT, ELISA and culture (growth on LJ media, followed by nitrate reduction and niacin tests for specie differentiation) |
|
[34] |
| 8. | Karachi (Sindh) |
Type of study: cross-sectional study Target animal species: cattle Target location: two abattoirs Sample size: 943 (700 males and 243 females) Sample types for testing: blood (collected but not processed) and lungs samples (total 1170 collected including lungs 338, liver 257, lymph nodes 313, spleen 110 and intestines 152) Diagnostic tests: Gross examination of organs after slaughtering |
|
[35] |
| 9. | Lahore (Punjab) |
Type of study: cross-sectional Target animal species: five species of antelopes suspected of having TB Target location: wildlife parks and zoos Sample size: 100 Sample types for testing: blood Diagnostic tests: PCR and cytokine ELISA testing for M. bovis detection. Samples were also tested for M. tuberculosis to detect reverse zoonosis |
|
[36] |
| 10. | Faisalabad (Punjab) |
Type of study: cross-sectional Target animal species: cattle and buffalo Target location: two cattle/buffalo colonies Sample size: 265 (133 cattle and 132 buffaloes) Sample types for testing: milk and nasal swabs Diagnostic tests: SITT, PCR and culture (media not specified) |
|
[37] |
| 11. | Peshawar, Nowshera, Charsadda, Mardan and Swabi districts of Khyber Pakhtunkhwa (KPK) |
Type of study: cross-sectional Target animal species: cattle and buffalo Target location: urban and rural areas Sample size: 2400 asymptomatic large animals, comprising 1225 cattle and 1175 buffaloes Diagnostic tests: CITT *190 dairy farmers were interviewed to gather information on animal management practices and potential risk factors for bTB |
|
[38] |
| 12. | Kohat - Khyber Pakhtunkhwa (KPK) |
Type of study: cross-sectional Target animal species: cattle, buffalo, goat and milk shops Target location: 5 randomly selected union councils of district Kohat Sample size: 200 milk samples [cattle (62), buffaloes (64), goats (47) and milk shops (27)] Sample types for testing: milk Diagnostic tests: ZN staining followed by microscopy and PCR |
|
[39] |
| 13. | Karachi (Sindh) |
Type of study: cross-sectional Target animal species: Target location: two abattoirs Sample types for testing: blood and tissue samples were collected from lymph node of respiratory tract, lung and liver tissue, lymph nodes from gastrointestinal tract Diagnostic tests: lateral flow technique, PCR, ZN staining |
|
[40] |
| 14. | Karachi (Sindh) |
Type of study: cross-sectional Target animal species: cattle and buffalo Target location: small holders dairy farms in 5 towns of Karachi Sample size: 1000 animals (435 cows and 565 buffaloes) Diagnostic tests: CITT |
|
[41] |
| 15. | Lahore (Punjab) |
Type of study: cross-sectional Target animal species: cattle and buffalo Target location: 4 organized dairy farms Sample size: 192 animals suspected of bTB (96 cattle and 96 buffaloes) Sample types for testing: blood Diagnostic tests: SITT, ELISA and PCR |
|
[42] |
| 16. | Hyderabad, Tando Allahyar (Sindh) |
Type of study: cross-sectional Target animal species: cattle Target location: Sample size: 160 cattle (80 from each district) Sample types for testing: 160 nasal secretion and 120 milk samples Diagnostic tests: culture (LJ media), staining (ZN) and biochemical tests (nitrate reduction and niacin tests) |
|
[43] |
| 17. | Peshawar – Khyber Pakhtunkhwa (KPK) |
Type of study: cross-sectional Target animal species: cattle and buffalo Target location: small holdings and commercial farms Sample size: 556 animals (139 from each of four towns) were selected for testing, comprising of 368 cattle and 188 buffaloes. Of these, 143 were from smallholdings, while the remaining 443 were from commercial farms. Diagnostic tests: CITT |
|
[44] |
| 18. | Kohat - Khyber Pakhtunkhwa (KPK) |
Type of study: cross-sectional Target animal species: cattle, buffalo, sheep and goats Target location: abattoir Sample size: 200 Sample types for testing: lungs, lymph nodes and liver Diagnostic tests: NZ staining and PCR |
|
[45] |
| 19. | Public livestock farms (Punjab) |
Type of study: cross-sectional Target animal species: cattle and buffalo Target location: livestock farms Sample size: 215 Sample types for testing: milk and nasal swabs Diagnostic tests: CITT, PCR |
|
[46] |
| 20. | Faisalabad (Punjab) |
Type of study: cross-sectional Target animal species: cattle and buffalo Target location: slaughterhouse Sample size: 400 Sample types for testing: tissues with suspected TB lesions Diagnostic tests: HE staining and microscopy |
|
[47] |
| 21. | Faisalabad (Punjab) |
Type of study: cross-sectional Target animal species: crossbred cattle Target location: Livestock Dairy Farm, University of Agriculture Faisalabad Sample size: 107 Sample types for testing: 8 animals were positive by CITT. Six were female animals from whom milk samples were collected. Tissue samples (lung and liver) from 05 dead animals Diagnostic tests: CITT, ZN staining and PCR |
|
[48] |
| 22. | Lahore (Punjab) |
Type of study: cross-sectional Target animal species: cattle and buffalo Target location: public and private farms Sample size: selected 1031 (517 cattle and 514 buffaloes) animals exhibited signs of emaciation and swollen lymph nodes, suggesting potential bTB infection Diagnostic tests: CITT |
|
[49] |
| 23. | Islamabad (Islamabad Capital Territory) |
Type of study: cross-sectional Target animal species: cattle Target location: dairy farms Sample size: 200 Diagnostic tests: CITT |
|
[50] |
| 24. | Peshawar - Khyber Pakhtunkhwa (KPK) |
Type of study: cross-sectional Target animal species: cattle and buffalo Target location: abattoirs Sample size: 151 Sample types for testing: lung and liver tissues (302 samples were collected from an abattoir: Cattle (30 liver, 30 lung samples) and buffaloes (121 lung, 141 liver samples) Diagnostic tests: ZN staining and PCR |
|
[10] |
| 25. | Faisalabad, Okara (Punjab) |
Type of study: cross-sectional Target animal species: cattle Target location: farm/herd Sample size: 521 Diagnostic tests: CITT |
|
[51] |
| 26. | Lahore (Punjab) |
Type of study: cross-sectional Target animal species: cattle Target location: peri-urban areas Sample size: 1000 Sample types for testing: milk Diagnostic tests: SITT and PCR |
|
[52] |
| 27. | Livestock experiment stations (Punjab) |
Type of study: cross-sectional Target animal species: buffalo Target location: livestock experiment stations Sample size: 965 Diagnostic tests: CITT |
|
[53] |
| 28. | Lahore (Punjab) |
Type of study: cross-sectional Target animal species: buffalo Target location: buffalo/cattle colony Sample size: 100 Sample types for testing: blood Diagnostic tests: CITT and PCR |
|
[54] |
| 29. | Tando Allahyar (Sindh) |
Type of study: cross-sectional Target animal species: cattle and buffalo Target location: rural areas Sample size: 187 Diagnostic tests: SITT |
|
[55] |
| 30. | Faisalabad (Punjab) |
Type of study: cross-sectional Target animal species: buffalo Target location: rural areas around Faisalabad (within 12 km from clock tower) Sample size: 1052 Diagnostic tests: CITT |
|
[56] |
| 31. | Islamabad (Islamabad Capital Territory) |
Type of study: cross-sectional Target animal species: bovidae, cervidae and equidae Target location: zoo animals including members of Bovidae (n = 55), Cervidae (n = 31) and Equidae (n = 4) Sample size: 87 Diagnostic tests: CITT |
|
[57] |
| 32. | 11 Livestock experiment stations (Punjab) |
Type of study: cross-sectional Target animal species: cattle Target location: livestock experiment stations Sample size: 1751 Diagnostic tests: CITT |
|
[58] |
| 33. | 7 Livestock experiment stations (Punjab) |
Type of study: cross-sectional Target animal species: sheep and goats Target location: livestock experiment stations Sample size: 1987 goats (1472 at farms and 515 in two cities, Okara and Faisalabad) and 4987 sheep (4729 at farms and 254 in two cities, Okara and Faisalabad) Diagnostic tests: CITT |
|
[59] |
| 34. | Faisalabad, Okara (Punjab) |
Type of study: cross-sectional Target animal species: buffalo Target location: rural areas/villages Sample size: 1092 (697 from Faisalabad and 395 from Okara) Diagnostic tests: CITT |
|
[60] |
| 35. | Lahore, Faisalabad, Okara (Punjab) |
Type of study: cross-sectional Target animal species: buffalo Target location: dairy farms Sample size: 395 Diagnostic tests: CITT |
|
[61] |
| 36. | Lahore (Punjab) |
Type of study: cross-sectional Target animal species: buffalo Target location: cattle colonies and dairy farms in the peri-urban areas Sample size: 31 Sample types for testing: milk Diagnostic tests: CITT and PCR |
|
[62] |
| 37. | Livestock experiment station, Khushab (Punjab) |
Type of study: cross-sectional Target animal species: buffalo Target location: livestock experiment station Sample size: 159 Sample types for testing: milk and feces Diagnostic tests: CITT, culture, ZN staining and isolation (growth on LJ and Stone brink's media, followed by niacin, nitrate reduction, catalase and urease tests for specie differentiation) |
|
[63] |
| 38. | Lahore, Okara, Faisalabad (Punjab) |
Type of study: cross-sectional Target animal species: buffalo Target location: livestock experiment stations Sample size: 2526 Diagnostic tests: CITT |
|
[64] |
| 39. | Livestock experiment stations (Punjab) |
Type of study: cross-sectional Target animal species: buffalo Target location: livestock experiment stations Sample size: 328 (165 from farm 1 and 163 from farm 2) Diagnostic tests: CITT |
|
[65] |
| 40. | Lahore (Punjab) |
Type of study: cross-sectional Target animal species: cattle and buffalo Target location: cattle colonies Sample size:1000 Sample types for testing: milk, nasal discharge and feces Diagnostic tests: CITT |
|
[66] |
Fig. 2.
The maps demarcate the political borders of Pakistan. Left): The blue location icons represent the cities in which the 40 studies were conducted. Bovine tuberculosis prevalence studies were performed in 19 districts, with most districts targetted multiple times over the course of two decades. Right): Data points of 40 studies. Each location icon is represented by either a cow or a deer icon with different background colours. Blue colour represents studies that tested only cattle, black colour represents studies that only tested buffalos, pink colour represents studies that tested buffaloes, cattle, goat and (or) sheep, orange colour represents studies that tested both buffaloes and cattle. Green colour deer icon represents studies that tested different wildlife species. The large circles represent the three clusters of studies identified in this review paper. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
Fig. 3.
Zoomed images of the three clusters created by the 40 studies performed in Pakistan to evaluate prevalence of bovine tuberculosis. Cluster 1: Top left, Cluster 2: Top right, Cluster 3: Bottom left. Note that the location represented on the maps by each icon is that of the city and not the exact farm where the animals were tested.
Table 1 summarizes all studies conducted on bTB in Pakistan along with their study designs, sampling/approaches used and major conclusions drawn by the authors. Most studies adopt a similar approach, reporting prevalence estimates using a variety of diagnostic approaches. While we understand the funding constraints for the local researchers, many studies lack a proper study design and comparison to gold-standard mycobacterial culture for confirmation. Nonetheless, these studies suggest that the prevalence of bTB is <10% in many areas, while exceeding 10% in other locations, with estimated rates varying from 2% to 19.3%. Differences in bTB prevalence arise from diverse factors, including geographical variations influenced by farm management conditions [67]. Natural susceptibility levels also vary among species and breeds, as highlighted in two studies [46,68]. Additionally, the variation in bTB prevalence is influenced by topography, climate, weather patterns, and the presence of diverse animal species including wildlife. Notably, these estimations often stem from limited studies rather than comprehensive, geographically representative investigations [69]. The listed studies also suggest that the prevalence of bTB is increasing at well-established government production farms, which is alarming [46,53]. Different risk factors identified in studies conducted in Pakistan include the age of the animal, close animal sheltering, number of calving, origin of animals (brought in by purchasing from other locations) and herd size.
Studies conducted in Pakistan have also shown that bTB is present in captive wild animals. A study conducted recently proved that bTB is present in zoo animals with prevalence range of 3.78% to 8.1% depending on diagnostic tool employed [28]. Similarly, results of a 2019 study on captive antelopes, which belong to the Bovidae family, demonstrated an M. bovis infection incidence of around 30%, suggesting a high affinity for bTB in these animals [36]. In 2015, a study conducted on zoo animals belonging to bovidae, cervidae and equidae family indicated an overall prevalence estimate of 3.3% [57]. These studies suggest that bTB is present in captive wildlife as well as zoo animals, which poses immense challenge of spread of bTB not only to animals but also people working at these facilities and visiting as tourists.
The studies reported in the current review used a variety of TB diagnostic tools such as the single intra-dermal tuberculin test (SITT; n = 7), comparative intra-dermal tuberculin test (CITT; n = 23), PCR (n = 14), rapid bovine antibody test (n = 3), ELISA (n = 5), Ziehl-Neelsen (ZN) staining (n = 10), culture (n = 7), and gross examination of visceral organs especially lungs and liver (n = 1) (supplementary table 1). Of the 40 studies, 20 studies employed only one form of test while the remaining 19 studies used a combination of tests to determine the status of bTB in animals. On further exploration of the publications, only seven studies used mycobacterial culture to confirm the presence of live bacteria (supplementary table 1). In addition, molecular tests were only employed in few research studies where sophisticated laboratories were accessible [33]. Therefore, variable prevalence of bTB was reported by different studies: making it difficult to ascertain the true picture of bTB in Pakistan. The situation is further complicated by the fact that most studies utilized convenience sampling that can only provide an estimate rather than the true prevalence of the disease. Nonetheless, the studies provide a starting point for the government agencies to initiate an integrated surveillance study to ascertain the true prevalence of bTB. Overall, it is not possible using the currently available data to accurately assess the impact of bTB on either the bovine or human population (next sections) in Pakistan.
3. Status of zoonotic tuberculosis due to M. bovis infection in Pakistan
Research articles that determined causative contribution of M. bovis in human TB cases were searched using Google scholar and PubMed with the following inputs: ‘zoonotic tuberculosis in Pakistan’, ‘M. bovis in humans Pakistan’, ‘zTB in TB patients in Pakistan’. The information is summarized in Table 2. A total of seven studies were identified from the search that spanned over a decade starting from 2012. No studies performed prior to that were available online. Furthermore, these studies have been conducted in five cities that represent only three of the five provinces of Pakistan. These provinces include Baluchistan [Quetta (n = 1)], KPK [Peshawar (n = 4)] and Punjab [Gujranwala and Hafizabad (n = 1), Lahore (n = 1)]. The studies report variable percentages of zTB ranging from 1.5% to 13% in high risk groups such as farm workers and abattoir workers [70]. The percentage of zTB was also found to be drastically different between pulmonary and extra-pulmonary human TB cases [71]. Ali et al., [71] reported that 5 out of 244 pulmonary TB patients (2.5%) and 19 out of 91 extra-pulmonary TB patients (20.9%) tested positive for M. bovis. The results should have concerned the local TB reporting authorities, since one-fifth of all extra-pulmonary cases in the study were zoonotic that required immediate intervention by the public health authorities. Yet the public health domain to-date remains unconcerned about zTB and the impact it would have on the goal of having a TB-free Pakistan by 2035. With sustained transmission of TB from animals to humans, this goal seems unrealistic.
Table 2.
List of studies conducted in Pakistan to access contribution of M. bovis in human TB cases (zTB). The abbreviations: LJ, Lowenstein Jensen (media); PCR, Polymerase chain reaction; ZN staining, Ziehl-Neelsen staining; zTB, zoonotic tuberculosis.
| Sr. No. | Geographical area | Study design | Major findings | Reference |
|---|---|---|---|---|
| 1. | Quetta (Balochistan) |
Target population: suspected TB patients (presented with chronic signs such as cough, night sweat, fever, weight and appetite loss) Sample size: 200 Sample collected: sputum Tests conducted: fluorescent microscopy and PCR |
|
[72] |
| 2. | Peshawar - Khyber Pakhtunkhwa (KPK) |
Target population: livestock farm workers, abattoir workers, butchers, veterinarians and veterinary assistants Sample size: 390 Sample collected: sputum samples were collected: human TB patients from different hospitals (100); livestock workers (200); abattoir workers (23); butchers (35); veterinarians (10); and veterinary assistants (22) Tests conducted: ZN staining, culture (LJ and stone brink's media followed by morphological characterization) and PCR |
|
[70,73] |
| 3. | Peshawar - Khyber Pakhtunkhwa (KPK) |
Target population: clinically diagnosed TB patients (before start of any medication) and school children Sample size: 300 Sample collected: sputum Tests conducted: culture (on LJ and stone brink's media) and PCR |
|
[74] |
| 4. | Peshawar - Khyber Pakhtunkhwa (KPK) |
Target population: occupationally exposed individuals Sample size: 103 samples were collected from occupationally exposed individuals with clinical signs: abattoir workers (16), butchers (29), livestock farmers (50), veterinarians (3), and veterinary assistants (5) Sample collected: sputum Tests conducted: PCR |
|
[75] |
| 5. | Peshawar - Khyber Pakhtunkhwa (KPK) |
Target population: TB patients admitted in hospitals Sample size: 100 Sample collected: sputum Tests conducted: culture (LJ and stone brink media followed by nitrate reduction test), ZN staining and PCR tests |
|
[76] |
| 6. | Gujranwala and Hafizabad districts (Punjab) |
Target population: suspected TB patients Sample size: 335 Sample collected: sputum, pus, fluid from lymph nodes, peritoneal effusion and pleural effusion while samples could not be collected from 11 patients Tests conducted: ZN staining and PCR |
|
[71] |
| 7. | Lahore (Punjab) |
Target population: suspected TB patients Sample size: 100 Sample collected: 200 samples (100 blood and 100 sputum samples) Tests conducted: culture (LJ and stone brink media; followed by niacin accumulation and nitrate reduction tests), ZN staining and PCR |
|
[77] |
4. Risks factors for zTB in Pakistan
There are many factors which exacerbate the spread of M. bovis to humans and M. tb from human to animals (reverse zoonosis) in LMICs [78]. In their studies, Desta et al., [79] and Kouengoua et al., [80] identified several direct and indirect factors that can influence the spread of tuberculosis between animals and humans. These include demographics like age and gender, along with dietary habits such as consuming unpasteurized milk and meat. Close contact with livestock also plays a role, whether through shared living spaces, common water sources, or direct interactions. The presence of tuberculosis within a household, either human or animal, further increases the risk. Practices like spitting indoors and using manure in animal feed can also contribute to transmission. Furthermore, poor ventilation in homes and a lack of awareness about TB transmission modes can hinder control efforts.
In Pakistan, people in rural areas often have traditional livestock housing systems with close contact between humans and animals, thereby increasing the risk of zTB transmission from infected animals [81]. Similarly, sale and purchase of infected animals in the market is found to be a significant risk factor [31]. Co-sleeping in poorly ventilated areas with animals and consumption of unpasteurized milk and dairy products in rural Pakistani settings is quite common [74,81]. In fact, most people in rural areas who are already at high risk due to close contact with infected animals prefer drinking raw milk, which further increases the risk of M. bovis transmission [56,82]. Yet there is no mandatory pasteurization law in Pakistan to prevent transmission of M. bovis through contaminated milk. Currently only 6% of total milk produced is pasteurized [83]. In 2018, Punjab Food Department enacted a law called ‘Minimum Pasteurization Law’ in Punjab, which to date has not been implemented in the country [84].
Another important risk factor for transmission of M. bovis to humans is through handling of contaminated carcasses at abattoirs [85]. Unfortunately, the awareness level in slaughterhouse workers and animal owners regarding the risk of zoonosis is very low in Pakistan: as demonstrated by a study conducted at abattoirs in Karachi reporting that only 15% workers and herdsmen knew about bTB and only 30% workers were taking some precautions such as wearing of personal protection equipment and washing their hands [81]. These risk factors have been observed globally: in Mexico 11.8% of sputum samples from cattle farm workers were positive for M. bovis [86], while in Argentina, 65% of M. bovis-infected patients were found to have occupational exposure [87].
The role of National Tuberculosis Control Program (NTP) in humans becomes important under current conditions. Yet the NTP in Pakistan has not even acknowledged the existence of zTB and its contribution to the TB burden in a largely agrarian country [88]. The absence of legislation mandating pasteurization, coupled with indoor animal housing practices and insufficient awareness within susceptible populations, has afforded M. bovis unchecked transmission to humans, thereby posing enormous health challenges to public health.
5. Challenges in control of bTB and zTB in Pakistan
Developed nations (global north) have successful programs that have reduced infections in animals to <1% at the herd level. Unfortunately, many LMICs lack similar efforts since it requires knowledge of data on prevalence and associated risk factors in each country. Absence of systematic surveillance and incomplete data on disease incidence and prevalence results in lack of attention and resources from policymakers [24,82,[89], [90], [91]]. Furthermore, compromised field performance of the available diagnostic tests for bTB i.e., low sensitivity and specificity, is also a challenge [[92], [93], [94]]. Bovine TB prevalence is primarily concentrated in specific regions where poverty intersects with high population density, a predictable outcome for an illness that thrives in areas with limited resources to prevent bTB transmission [95,96].
Pakistan is among the LMICs that have yet to develop a surveillance program to effectively determine the prevalence of bTB in animals [30,75]. The veterinary education establishments such as University of Veterinary Animal Science Lahore and University of Agriculture Faisalabad have conducted small scale studies to determine the prevalence of bTB (Table 1). Yet, the Ministry of National Food Security and Research of Pakistan does not have programs to conduct active surveillance of bTB even though they have the required infrastructure in place that was previously developed during the eradication of Rinderpest and maintained for the current control efforts being targeted towards Foot-and-Mouth Disease (FMD) control.
Majority of the bTB studies have been conducted around metropolitan cities like Karachi and Lahore; where animals have been removed from city centres and gathered in the peri-urban areas in dense dairy colony structures. This facilitates the transport of milk directly to consumers within a short frame of time. The forced clustering of animals in small areas has likely led to ideal conditions for the increased transmission of M. bovis in animals. Indeed, two studies have demonstrated significantly high prevalence of bTB in both animals and milk samples [41,52]. This is worrisome since the dairy animals that are brought to the colonies get infected and at the end of the lactation-cycle are either sent to the slaughterhouse or back to their original villages, facilitating transmission of bacteria to different parts of the country.
A significant challenge in control of bTB in animals is the lack of knowledge regarding the dynamics of M. bovis infection in wildlife. Wildlife hosts such as white-tailed deer in Michigan (USA), Eurasian badgers in the UK, brush-tailed possums in New Zealand etc. are considered complicating factors in control of bTB in developed countries [97]. In Pakistan, no study till date has assessed the prevalence of bTB in wildlife species while only a handful studies have reported prevalence of bTB in captive wildlife (Table 1). Lack of bTB data from both captive and wildlife species also makes it difficult to assess their potential role (if any) in transmission of bTB to farm animals.
Another important challenge is the non-existence of government policies for the control of bTB. Policies such as those related to animal movement across borders, insufficient funding for culling, lack of quarantine facilities, shortages of meat inspectors and veterinarians in slaughterhouses; all contribute to a hostile environment for bTB control [98]. Pakistan has porous borders with Afghanistan and Iran leading to frequent cross-border animal and human movement, which is very serious risk factor for spread of infectious diseases like bTB [38,70]. This, combined with lack of a surveillance program and a coordinated effort between veterinary research and educational establishments in the country to conduct a thorough research are major hurdles in initiating control of bTB.
So far, the challenges described above were specific to control of bTB in animals in Pakistan. Yet the zoonotic nature of this disease with ∼ 10% contribution to human TB cases in LMICs [22], makes it imperative that we highlight the challenges faced in preventing transmission of the bacteria from infected animals to the human population, since the clinical presentation of tuberculosis caused by M. bovis and M. tuberculosis is indistinguishable. The African region has the highest cases of zTB (72700), followed by Southeast Asian region (46700); yet they lack sufficient resources, laboratory infrastructure as well as technical expertise for differentiating M. bovis from M. tuberculosis at essentially all healthcare levels [[99], [100], [101]]. Additionally, there is absence of training and lack of knowledge among occupationally exposed groups about the zoonotic potential and spread of M. bovis infection to humans [75]. According to studies conducted to evaluate knowledge and awareness of different exposed high risk groups, it was found that none of the abattoir workers received formal training and majority of them do not practice any precaution such as wearing PPE and washing of their hands [74,75]. In another study, it was established that abattoir workers are more affected by zTB due to direct contact and continuous exposure [74]. The condition is worsened because M. bovis inherently exhibits resistance to pyrazinamide [102]. In a Peshawar-based study, 12 patients were diagnosed with M. bovis infection and 11 of those patients' demonstrated resistance to pyrazinamide treatment. This underscores a significant challenge posed by antimicrobial resistance associated with zTB [70].
It is crucial that bTB in animals is controlled to prevent its transmission to humans. On the human health side, M. bovis is neither considered a risk factor for human TB, nor are there any guidelines for its control in the newly formed “National Guidelines for the Control of Tuberculosis in Pakistan-2019” [88]. This is irrespective of the numerous studies having reported the impact of M. bovis on human TB (zTB) cases in veterinarians, livestock workers, butchers, abattoir workers and other high risk groups Table 2: List of studies conducted in Pakistan to access contribution of M. bovis in human TB cases (zTB) [70]. Lack of concentrated efforts by animal and human health departments so far has led to a precarious situation that needs immediate attention.
6. Future perspectives for bTB and zTB control in Pakistan
The way forward for Pakistan to control bTB is to carry out comprehensive surveillance to determine incidence and prevalence of M. bovis infection in cattle and other hosts, along with risk assessment of zoonosis and reverse zoonosis. The data regarding prevalence of bTB and share of zTB in human TB should be compiled. This will be helpful in efficient allocation and management of resources [69] because the lack of data is the main hurdle in seeking policymakers' attention and provision of funding to fight the problem [103,104].
There is a need to identify and address research gaps in zoonotic and bovine TB to determine the true burden of TB caused by M. bovis and to unravel the epidemiology of disease at the human, animal, and environmental interface [26]. This can be achieved through meaningful collaboration between local researchers and experts from developed world and international organizations working on TB control. This will also offer capacity building opportunity for the local researchers, enabling them to better design future research studies. Additionally, in endemic countries like Pakistan, epidemiological studies to determine which animal species, both domestic and wild, serve as hosts or reservoirs for M. bovis can be very informative. This process can identify areas of high prevalence, enabling the allocation of additional funds and efforts to those specific regions [105,106].
Routine surveillance of bTB in live animals through skin testing and blood tests can play vital role in control of disease. Animal owners should be incentivized for screening their animals for bTB and maintenance of disease free herds [104]. Additionally, inspection of the animals prior to slaughtering and detailed post-mortem inspection (following the protocols implemented in UK and the EU) should be ensured to stop entry of meat of any infected animal into the food chain [107,108]. This should be accompanied by tracing back to the herd to which the diseased animal belonged [101].
The effective control of zoonotic diseases is only possible by adopting multi-sectorial, one health approach [109,110]. There is need to design a national control program with integrated ‘One Health’ approach through collaboration between public health and animal health departments with aim to develop control measures while considering human, animals, wildlife and environmental fronts [104,111]. The success of the TB control program in humans in the USA is commendable, owing to collaborative efforts from states, businesses, and the federal government to control and eradicate the disease [104]. Pakistan can establish a similar program through collaboration between the federal and provincial governments on both animal and human health sector. The program should emphasize surveillance to determine the prevalence of bTB and identify risk factors. Integrated and collective control efforts are imperative: including awareness among masses, necessary legislation, and its implementation along with uplifting socioeconomic status of the people.
The surveillance campaign to detect cases of human TB due to M. bovis should be strengthened by improved laboratory facilities, including polymerase chain reaction (PCR) and gene sequencing after culturing the isolates from patients [77].
Pasteurization and food safety laws should be introduced with strict implementation by adopting WHO food safety measures to break the cycle of transmission of M. bovis to the masses. This will require a public awareness campaign to educate people about the importance of pasteurizing milk and other dairy products [26]. The control strategies can be made successful by educating the masses, especially the farmers about the economic and public health significance.
Field veterinarians and para-veterinary staff should be aware of the mode of transmission, range of hosts, clinical signs, and diagnosis of the disease [29,74]. There is also need for implementation of standard operating procedures (SOPs), creating a national database, training of technical and non-technical staff for ante-mortem and post mortem examination along with educating animal handlers to break the cycle of zTB transmission [75,112].
7. Conclusion
The absence of comprehensive data for bTB and zTB in Pakistan has resulted in failure to develop a structured control and eradication program. Recognizing this critical gap, it is imperative to develop an effective policy that prioritizes the control and eradication of bTB and zTB. A One-Health approach is advocated, calling for collaboration among various departments such as livestock and dairy development, ministry of national health services, public health, agriculture, and food security to collectively achieve targeted goals. Furthermore, there is a pressing need for active surveillance to accurately determine the prevalence of M. bovis and its contribution to the human TB burden. This data is crucial not only for understanding the disease's national status but also for identifying pertinent risk factors. Such insights are integral in the formulation of a comprehensive control program against the disease and for efficient resource allocation. In conclusion, representative data is pivotal for addressing the substantial challenge posed by bTB to both livestock and human population. The establishment of a robust control program, informed by accurate prevalence data and a One-Health approach, is imperative for mitigating the impact of this infectious disease in Pakistan.
CRediT authorship contribution statement
Zahid Fareed: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. Aysha Rana: Conceptualization, Data curation, Methodology, Writing – original draft. Syeda Anum Hadi: Data curation, Formal analysis, Methodology, Software, Writing – original draft. Annemieke Geluk: Supervision, Writing – review & editing. Jayne C. Hope: Supervision, Writing – review & editing. Hamza Khalid: Conceptualization, Formal analysis, Project administration, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.onehlt.2024.100763.
Appendix A. Supplementary data
Details of the districts where bTB studies included in this review were performed and the tests used by each study to detect bTB.
Data availability
No data was used for the research described in the article.
References
- 1.Good M., et al. The history of in vivo tuberculin testing in bovines: tuberculosis, a “one health” issue. Front. Vet. Sci. 2018;5:59. doi: 10.3389/fvets.2018.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li D., et al. Prevalence of Mycobacterium bovis in deer in mainland China: a systematic review and meta-analysis. Front. Vet. Sci. 2024;11:1333975. doi: 10.3389/fvets.2024.1333975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alexander K.A., et al. Novel Mycobacterium tuberculosis complex pathogen, M. mungi. Emerg. Infect. Dis. 2010;16(8):1296. doi: 10.3201/eid1608.100314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuria J.K. Bacterial Cattle Diseases. IntechOpen; 2019. Diseases caused by Bacteria in cattle: tuberculosis. [Google Scholar]
- 5.Khalid H., et al. Development of lateral flow assays to detect host proteins in cattle for improved diagnosis of bovine tuberculosis. Front. Vet. Sci. 2023;10:1193332. doi: 10.3389/fvets.2023.1193332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elsayed M.S.A.E., Amer A. The rapid detection and differentiation of Mycobacterium tuberculosis complex members from cattle and water buffaloes in the delta area of Egypt, using a combination of real-time and conventional PCR. Mol. Biol. Rep. 2019;46(4):3909–3919. doi: 10.1007/s11033-019-04834-3. [DOI] [PubMed] [Google Scholar]
- 7.Romha G., et al. Epidemiology of Mycobacterium bovis and Mycobacterium tuberculosis in animals: transmission dynamics and control challenges of zoonotic TB in Ethiopia. Prev. Vet. Med. 2018;158:1–17. doi: 10.1016/j.prevetmed.2018.06.012. [DOI] [PubMed] [Google Scholar]
- 8.Mittal M., et al. Evidence of presence of Mycobacterium tuberculosis in bovine tissue samples by multiplex PCR: possible relevance to reverse zoonosis. Transbound. Emerg. Dis. 2014;61(2):97–104. doi: 10.1111/tbed.12203. [DOI] [PubMed] [Google Scholar]
- 9.Abdel-Moein K.A., Hamed O., Fouad H. Molecular detection of Mycobacterium tuberculosis in cattle and buffaloes: a cause for public health concern. Trop. Anim. Health Prod. 2016;48:1541–1545. doi: 10.1007/s11250-016-1125-3. [DOI] [PubMed] [Google Scholar]
- 10.Khan J., et al. Prevalence of tuberculosis in buffalo and cattle. J. Pure Appl. Microbiol. 2014;8:721–726. https://www.researchgate.net/profile/Taif-Shah/publication/289156786_Prevalence_of_tuberculosis_in_buffalo_and_cattle/links/60c88d3ea6fdcc8267cf8bbc/Prevalence-of-tuberculosis-in-buffalo-and-cattle.pdf Available from: [Google Scholar]
- 11.Michel A.L., et al. Wildlife tuberculosis in south African conservation areas: implications and challenges. Vet. Microbiol. 2006;112(2–4):91–100. doi: 10.1016/j.vetmic.2005.11.035. [DOI] [PubMed] [Google Scholar]
- 12.Xu F., et al. High prevalence of extrapulmonary tuberculosis in dairy farms: evidence for possible gastrointestinal transmission. PLoS One. 2021;16(3) doi: 10.1371/journal.pone.0249341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Phepa P.B., Chirove F., Govinder K.S. Modelling the role of multi-transmission routes in the epidemiology of bovine tuberculosis in cattle and buffalo populations. Math. Biosci. 2016;277:47–58. doi: 10.1016/j.mbs.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 14.OIE Terrestrial animal health code. chapter 11.5. bovine tuberculosis. article. 2015 [cited 2023 05-11-2023] 2015. http://www.oie.int/index.php?id=169&l=0&htmfile=chapitre_bovine_tuberculosis.htm Available from: oie. terrestrial animal health code. chapter 11.5. bovine tuberculosis. article 11.5.5. available at: (accessed 1 june 2016)
- 15.Waters W.R., et al. Bovine tuberculosis vaccine research: historical perspectives and recent advances. Vaccine. 2012;30(16):2611–2622. doi: 10.1016/j.vaccine.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 16.Cadmus S.I., Ayanwale F.O. Bovine tuberculosis: epidemiology, zoonotic transmission, activities, and challenges toward its control in Nigeria. Zoonot. Tuberculosis Mycobacterium Bovis Other Pathog. Mycobacteria. 2014:149–158. doi: 10.1002/9781118474310.ch12. [DOI] [Google Scholar]
- 17.de Kantor I.N., et al. Mycobacterium bovis infection in humans and animals with an emphasis on countries in central and South America. Zoonot. Tuberculosis Mycobacterium Bovis Other Pathog. Mycobacteria. 2014:35–49. doi: 10.1002/9781118474310.ch4. [DOI] [Google Scholar]
- 18.Kasir D., et al. Zoonotic tuberculosis: a neglected disease in the Middle East and North Africa (MENA) region. Diseases. 2023;11(1):39. doi: 10.3390/diseases11010039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ullah I. Re-identifying the rural/urban: a case study of Pakistan. Espaço e Economia. Revista brasileira de geografia econômica. 2022 doi: 10.4000/espacoeconomia.22019. [DOI] [Google Scholar]
- 20.Ashford D., et al. Epidemiology of selected mycobacteria that infect humans and other animals. Revue scientifique et technique (International Office of Epizootics) 2001;20(1):325–337. doi: 10.20506/rst.20.1.1266. [DOI] [PubMed] [Google Scholar]
- 21.Srinivasan S., et al. Prevalence of bovine tuberculosis in India: a systematic review and meta-analysis. Transbound. Emerg. Dis. 2018;65(6):1627–1640. doi: 10.1111/tbed.12915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.WOAH Bovine tuberculosis 2023. https://www.woah.org/en/disease/bovine-tuberculosis/ [cited 2024 2 Fabruary]; Available from:
- 23.EMRO-WHO WHO (Eastern Mediterranean Region) Programme Areas Pakistan. 2023. https://www.emro.who.int/pak/programmes/stop-tuberculosis.html [cited 2023 Nov 30]; Available from:
- 24.Pérez-Lago L., Navarro Y., García-de-Viedma D. Current knowledge and pending challenges in zoonosis caused by Mycobacterium bovis: a review. Res. Vet. Sci. 2014;97:S94–S100. doi: 10.1016/j.rvsc.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 25.Jemal A.M. Review on zoonotic importance of bovine tuberculosis and its control. Open Access Lib. J. 2016;3(3):1–13. doi: 10.4236/oalib.1102504. [DOI] [Google Scholar]
- 26.WHO Roadmap for Zoonotic Tuberculosis 2017. https://iris.who.int/bitstream/handle/10665/259229/?sequence=1 [cited 2023 Novermber 30]; Available from:
- 27.Javed M.T. 2014. Status and control of tuberculosis in animals in Pakistan. Zoonotic Tuberculosis: Mycobacterium bovis and Other Pathogenic Mycobacteria; pp. 181–190. Third. [DOI] [Google Scholar]
- 28.Akhtar K., et al. Molecular identification and infection pathology of Mycobacterium spp. in captive wild animals in Pakistan. J. Infect. Dev. Countries. 2023;17(08):1107–1113. doi: 10.3855/jidc.17287. [DOI] [PubMed] [Google Scholar]
- 29.Mazari M.Q., et al. Prevalence and risk factors of bovine tuberculosis in cattle and dairy farm Workers in Mirpurkhas and Badin Districts of Sindh, Pakistan. Pak. J. Zool. 2022;54(3):1115. doi: 10.17582/journal.pjz/20190915060930. [DOI] [Google Scholar]
- 30.Ehtisham-ul-Haque S., et al. Monitoring the health status and herd-level risk factors of tuberculosis in water Buffalo (Bubalus bubalis) dairy farms in Pakistan. Pak. Vet. J. 2021;41(4) doi: 10.29261/pakvetj/2021.051. [DOI] [Google Scholar]
- 31.Zahoor M.Y. A cross-sectional study of bovine tuberculosis and its associated zoonotic risk factors in district Bahawalnagar, Punjab, Pakistan. Board Review. Editors. 2021;51:192–193. doi: 10.5555/20220315533#page=251. Available from: [DOI] [Google Scholar]
- 32.Leghari A., et al. Prevalence and risk factors associated with bovine tuberculosis in cattle in Hyderabad and Tando Allahyar districts, Sindh, Pakistan. Pakistan J. Zool. 2020;52(1) doi: 10.17582/journal.pjz/2020.52.1.207.212. [DOI] [Google Scholar]
- 33.Ullah A., et al. Bovine tuberculosis (bTB)-isolation and species-specific identification of Mycobacterium bovis from bovine raw milk in Pakistan. Sarhad J. Agric. 2020;36(2):489–498. doi: 10.17582/journal.sja/2020/36.2.489.498. [DOI] [Google Scholar]
- 34.Malhi K.K., et al. Prevalence of bovine tuberculosis in buffaloes in Hyderabad and Tando Allahyar districts of Sindh, Pakistan. Ind. J. Anim. Res. 2020;54(1):101–105. doi: 10.18805/ijar.B-931. [DOI] [Google Scholar]
- 35.Bhutto A.L., et al. Prevalence and pathological lesions of bovine tuberculosis assessment through routine procedures of meat inspection in infected cattle in Karachi metropolitan corporation abattoirs. Pure Appl. Biol. 2019;8(3):1909–1918. doi: 10.19045/bspab.2019.80134. [DOI] [Google Scholar]
- 36.Akhtar R., et al. Use of molecular probes for presumptive diagnosis of tuberculosis associated with Mycobacterium tuberculosis and Mycobacterium bovis infection in antelopes in Pakistan. Bovis. 2019;53(52.6):470. doi: 10.29261/pakvetj/2019.067. [DOI] [Google Scholar]
- 37.Aslam M.S., et al. Bacterial and PCR based diagnosis of naturally occurring bovine tuberculosis in cattle and buffaloes. Pak. J. Agric. Sci. 2019;56(2) doi: 10.21162/PAKJAS/19.6608. [DOI] [Google Scholar]
- 38.Ullah A., et al. Bovine tuberculosis (bTB): prevalence and associated risk factors in large ruminants in the central zone of Khyber Pakhtunkhwa, Pakistan. Pakistan J. Zool. 2019;51(1) doi: 10.17582/journal.pjz/2019.51.1.127.133. [DOI] [Google Scholar]
- 39.Basit A., et al. Occurrence and risk factors associated with Mycobacterium tuberculosis and Mycobacterium bovis in Milk samples from north east of Pakistan. Pak. Vet. J. 2018;38(2) doi: 10.29261/pakvetj/2018.038. [DOI] [Google Scholar]
- 40.Memon M., et al. Prevalence of bovine tuberculosis in slaughtering animals at selected muncipal slaughterhouses: its impact on public health: Department of Veterinary Medicine, Sindh agriculture university, Tandojam, Pakistan. Pakistan J. Agricult. Agricult. Eng. Vet. Sci. 2018;34(2):168–175. https://pjaaevs.sau.edu.pk/index.php/ojs/article/view/291 Available from: [Google Scholar]
- 41.Memon M., et al. Prevalence and risk factor analysis of bovine tuberculosis in bovine population in Karachi. Pakistan. J. Anim. Health. Prod. 2017;5(2):44–49. doi: 10.17582/journal.jahp/2017/5.2.44.49. [DOI] [Google Scholar]
- 42.Tariq A., et al. A preliminary study on prevalence of bovine tuberculosis in cattle and buffalo in outskirts of Lahore, Pakistan. Wayamba J. Anim. Sci. 2024:1518–1526. https://wayambajournal.com/paper/a-preliminary-study-on-prevalence-of-bovine-tuberculosis-in-cattle-and-buffalo-in-outskirts-of-lahore-pakistan.pdf Available from: [Google Scholar]
- 43.Leghari A., et al. Isolation of Mycobacterium bovis from milk and nasal discharge samples of cattle from Hyderabad and Tando Allahyar districts. J. Anim. Health Prod. 2016;4(4):105–110. doi: 10.14737/journal.jahp/2016/4.4.105.110. [DOI] [Google Scholar]
- 44.Khattak I., et al. Risk factors associated with Mycobacterium bovis skin positivity in cattle and buffalo in Peshawar, Pakistan. Trop. Anim. Health Prod. 2016;48(3):479–485. doi: 10.1007/s11250-015-0976-3. [DOI] [PubMed] [Google Scholar]
- 45.Basit A., et al. Isolation and identification of Mycobacterium bovis and Mycobacterium tuberculosis from animal tissues by conventional and molecular method. Ind. J. Anim. Res. 2015;49(5):687–693. doi: 10.18805/ijar.5583. [DOI] [Google Scholar]
- 46.Akhtar F., et al. The use of PCR technique in the identification of Mycobacterium species responsible for bovine tuberculosis in cattle and buffaloes in Pakistan. Trop. Anim. Health Prod. 2015;47:1169–1175. doi: 10.1007/s11250-015-0844-1. [DOI] [PubMed] [Google Scholar]
- 47.Waqas A., Javed M., Ashfaque K. Mehwish Q (2015) an Abat-toir based study on brucellosis, bovine tuberculosis and Paratubercu-losis in buffaloes and cattle at Faisalabad, Pakistan. Int. J. Vet. Health Sci. Res. 2015;3(1):34–38. https://d1wqtxts1xzle7.cloudfront.net/40287951/IJVHSR-2332-2748-03-101-libre.pdf?1448272676=&response-content-disposition=inline%3B+filename%3DAn_Abattoir_Based_Study_on_Brucellosis_B.pdf&Expires=1715106248&Signature=PGKj∼JgP0guFdD5mlSkvzqrSBskhkryrr24fY-AnmEYruh15U-qMVSvM0dfL9hJ4yR4xCCKQRREiUYgKgKzznW5OWo--d49N8DdBKFiBR8JLqXJ0YOYsFT∼WpwRyZf0Lgrb-3KFOkxJ5fgczHzeAYFmnlhfzyfDWrfs2KRl-y-QdLyxgcPVFs92ogPHbl∼Qggms68KB82JPSI5HL2CUjKH5A8XTBhb4t6Pi0S36X4tWy2Mk3mAkt8NlDcZm3ur1op5hCeVOuIIhTl5BS811SMJkgdOi8kPpWq-OoK3K7YyLb8igQSUA-LwzcFVSW2yU11cV8twFp68c∼xvNlN6KjOQ__&Key-Pair-Id=APKAJLOHF5GGSLRBV4ZA Available from: [Google Scholar]
- 48.Mahmood, F., et al., Molecular based epidemiology of bovine pulmonary tuberculosis–a mortal foe. Pak. Vet. J., 2014. 34(2): p. 185–188. http://www.pvj.com.pk/pdf-files/34_2/185-188.pdf.
- 49.Shaukat Ali R.A. Muhammad Younus, Gulbeena Saleem, Qamar un Nisa and Beenish Zahid, comparative trends of bovine tuberculosis in cattle and buffalo population around Lahore, Pakistan. Eur. J. Environ. Ecol. 2014;1:7–11. https://www.researchgate.net/profile/Beenish-Zahid/publication/303370406_COMPARATIVE_TRENDS_OF_BOVINE_TUBERCULOSIS_IN_CATTLE_AND_BUFFALO_POPULATION_AROUND_LAHORE_PAKISTAN/links/573eae7008aea45ee842f262/COMPARATIVE-TRENDS-OF-BOVINE-TUBERCULOSIS-IN-CATTLE-AND-BUFFALO-POPULATION-AROUND-LAHORE-PAKISTAN.pdf Available from: [Google Scholar]
- 50.Azam A., Younas U., Husna A., Ullah N., Ali Q., Akhter S. Hematological studies among bovine tuberculosis suspected herds of cattle in suburb of Islamabad, Pakistan. Wayamba J. Anim. Sci. 2014;6:921–926. [Google Scholar]
- 51.Javed M.T., et al. Brief communication (original). Certain risk factors associated with positive SCCIT test for tuberculosis in cattle at two cities in Pakistan. Asian Biomed. 2013;7(2):267–274. doi: 10.5372/1905-7415.0702.175. [DOI] [Google Scholar]
- 52.Tipu M.Y., et al. A cross sectional study of Mycobacterium bovis in dairy cattle in and around Lahore city, Pakistan. Pakistan J. Zool. 2012;44(2) https://zsp.com.pk/pdf44/393-398%20_14_%20PJZ-658-11%20after%20suggestions%20included%20May%2031%202011.pdf Available from: [Google Scholar]
- 53.Javed M.T., et al. Analysis of some of the epidemiological risk factors affecting the prevalence of tuberculosis in buffalo at seven livestock farms in Punjab Pakistan. Asian Biomed. 2012;6(1):35–42. doi: 10.5372/1905-7415.0506.124. [DOI] [Google Scholar]
- 54.Khan A., et al. Detection of Mycobacterium bovis in buffaloes blood through polymerase chain reaction (PCR) and tuberculin test. JAPS. 2012;22(3 Supplement):237–241. http://www.thejaps.org.pk/docs/Supplementary/03/023.pdf Available from: [Google Scholar]
- 55.Qazi I.H., et al. Prevalence of Bovine Tuberculosis in Rural Areas of District Tando Allahy ar. IJAVMS. 2024;6(5):345–348. doi: 10.5455/ijavms.157. [DOI] [Google Scholar]
- 56.Arshad M., et al. Epidemiological studies on tuberculosis in buffalo population in villages around Faisalabad. J. Anim. Plant Sci. 2012;22(3):246–249. https://thejaps.org.pk/docs/Supplementary/03/025.pdf Available from: [Google Scholar]
- 57.Shahid A., et al. Prevalence of bovine tuberculosis in zoo animals in Pakistan. Iran. J. Vet. Res. 2012;13(1):58–63. https://pesquisa.bvsalud.org/portal/resource/pt/emr-131301 Available from: [Google Scholar]
- 58.Javed M.T., et al. Risk factors identified associated with tuberculosis in cattle at 11 livestock experiment stations of Punjab Pakistan. Acta Trop. 2011;117(2):109–113. doi: 10.1016/j.actatropica.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 59.Javed M.T., et al. Percentage of reactor animals to single comparative cervical intradermal tuberculin (SCCIT) in small ruminants in Punjab Pakistan. Acta Trop. 2010;113(1):88–91. doi: 10.1016/j.actatropica.2009.08.026. [DOI] [PubMed] [Google Scholar]
- 60.Javed M.T., et al. Risk factors associated with the presence of positive reactions in the SCCIT test in water buffalo around two cities in Punjab, Pakistan. Acta Trop. 2010;115(3):242–247. doi: 10.1016/j.actatropica.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 61.Javed M.T., Farooqi A., Ullah H. Epidemiological basis of bovine tuberculosis in buffaloes. Pak. J. Zool. 2009;9:417–420. https://www.researchgate.net/profile/Javed-M/publication/282243536_Epidemiological_Basis_of_Bovine_Tuberculosis_in_Buffaloes/links/573f30ce08ae9f741b3219ba/Epidemiological-Basis-of-Bovine-Tuberculosis-in-Buffaloes.pdf Available from: [Google Scholar]
- 62.Mumtaz N., et al. Reliability of PCR for detection of bovine tuberculosis in Pakistan. Pakistan J. Zool. 2008;40(5) https://zsp.com.pk/pdf2/347-351%20(5).pdf Available from. [Google Scholar]
- 63.Khan Imtiaz A., Mubarak A., Ali S. Factors affecting prevalence of bovine tuberculosis in Nili Ravi buffaloes. Pak. Vet. J. 2008;28(4):155–158. http://www.pvj.com.pk/pdf-files/28_4/155-158.pdf Available from: [Google Scholar]
- 64.Khan I., Khan A. Prevalence and risk factors of bovine tuberculosis in Nili Ravi buffaloes in the Punjab, Pakistan. Ital. J. Anim. Sci. 2007;6(sup2):817–820. doi: 10.4081/ijas.2007.s2.817. [DOI] [Google Scholar]
- 65.Javed M.T., et al. A study on tuberculosis in buffaloes: some epidemiological aspects, along with haematological and serum protein changes. Veterinarski arhiv. 2006;76(3):193–206. https://hrcak.srce.hr/file/8193 Available from: [Google Scholar]
- 66.Jalil H.D., Suleman A., et al. Bovine tuberculosis in Dairy Animals at Lahore, Threat to the Public Health. 2003. https://www.priory.com/vet/bovinetb.htm Available online. Available from:
- 67.Imtiaz A., Khan A.K., Mubarak A., Ali S. Factors affecting prevalence of bovine tuberculosis in Nili Ravi buffaloes. Pak. Vet. J. 2008:155–158. http://www.pvj.com.pk/pdf-files/28_4/155-158.pdf Available from: [Google Scholar]
- 68.Mujeeb-ur-Rahman Memon A.L., et al. Prevalence and pathological lesions of bovine tuberculosis assessment through routine procedures of meat inspection in infected cattle in Karachi metropolitan corporation abattoirs. Pure Appl. Biol. 2019;8(3):1909–1918. doi: 10.19045/bspab.2019.80134. [DOI] [Google Scholar]
- 69.Luciano S.A., Roess A. Human zoonotic tuberculosis and livestock exposure in low-and middle-income countries: a systematic review identifying challenges in laboratory diagnosis. Zoonoses Public Health. 2020;67(2):97–111. doi: 10.1111/zph.12684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ullah A., et al. An emerging zoonosis of bovine tuberculosis-a neglected zoonotic disease (NZD) in work-related occupational groups in Pakistan. J. Microb Pathog. 2018;2:105. https://d1wqtxts1xzle7.cloudfront.net/86640750/an-emerging-zoonosis-of-bovine-tuberculosisa-neglected-zoonotic-disease-nzd-in-workrelated-occupational-groups-in-pakistan-libre.pdf?1653817139=&response-content-disposition=inline%3B+filename%3DAn_Emerging_Zoonosis_of_Bovine_Tuberculo.pdf&Expires=1715108919&Signature=U3kcaX∼u3rXb1YIPHxNjv∼KvMYHZUf5WBC5we59abqkKY∼8g0xZ8r22qDQCPcV5pMDiLj∼FXqTHsFZKS-KYewlzvWqQhm0wVt∼BycOzCoM4pWY∼jkHILZs1ZiPvSqRWEqvI6gjPcMGByETDOnavsR8lJFMSQqgDkc2TRlnGvlHm9fL4zoR7UZlOtBM∼s9zBX1GD1o6VgVFF5E3AFm-2pUnc83HdQjT-jmMletVz5t3f5dvJ8fRuEEXaG1ACNlhLVWgGJEYFnGsK6kRuOTXvS-tTuV352hrbKdSeYZmWnkoN2-KLSTMppUyPxnPoF11zEY42vVIMicvi6Xl3AQMA6kQ__&Key-Pair-Id=APKAJLOHF5GGSLRBV4ZA Available from: [Google Scholar]
- 71.Ali B., et al. Identification of bovine and human tuberculosis in AFB positive and negative patients. Pakistan. J. Zool. 2013;45(1) https://zsp.com.pk/pdf45/255-260%20_34_%20PJZ-1200-13%2016-1-13%20Basharat%20_1_.pdf Available from: [Google Scholar]
- 72.Spozmai Tareen A.R., Tariq Nabeela, Khan Muhammad Ali, Shafee Muhammad. Comparison between fluorescent microscopy and duplex PCR to detect Mycobacterium bovis and Mycobacterium tuberculosis in tuberculosis suspected patients. J. Postgraduate Med. Inst. 2022;35(4) doi: 10.54079/jpmi.35.4.2872. Available from: file:///C:/Users/DELL/Downloads/New+6+JPMI+2872%20(4).pdf. [DOI] [Google Scholar]
- 73.Ullah A., et al. Bovine tuberculosis (bTB): detection of Mycobacterium bovis infection with specie-specific primers in sputum samples in Pakistan. Sarhad J. Agricult. 2022;38(1) doi: 10.17582/journal.sja/2020/36.2.489.498. [DOI] [Google Scholar]
- 74.Khattak I., et al. Incidence and drug resistance of zoonotic Mycobacterium bovis infection in Peshawar, Pakistan. Advan. Microbiol. Infect. Dise. Public Health. 2018;9:111–126. doi: 10.1007/5584_2018_170. [DOI] [PubMed] [Google Scholar]
- 75.Khattak I., et al. Zoonotic tuberculosis in occupationally exposed groups in Pakistan. Occup. Med. 2016;66(5):371–376. doi: 10.1093/occmed/kqw039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jabbar A., et al. Detection of Mycobacterium tuberculosis and Mycobacterium bovis from human sputum samples through multiplex PCR. Pak. J. Pharm. Sci. 2015;28(4):1275–1280. https://www.researchgate.net/profile/Hazir-Rahman/publication/279807420_Detection_of_Mycobacterium_tuberculosis_and_Mycobacterium_bovis_from_human_sputum_samples_through_multiplex_PCR/links/55c44e6c08aebc967df1bf5c/Detection-of-Mycobacterium-tuberculosis-and-Mycobacterium-bovis-from-human-sputum-samples-through-multiplex-PCR.pdf Available from: [PubMed] [Google Scholar]
- 77.Nawaz A., et al. Detection of Mycobacterium tuberculosis and Mycobacterium bovis in sputum and blood samples of human. J. Agric. Sci. 2012;22:117–120. http://www.thejaps.org.pk/docs/Supplementary/02/25.pdf Available from: [Google Scholar]
- 78.Messenger A.M., Barnes A.N., Gray G.C. Reverse zoonotic disease transmission (zooanthroponosis): a systematic review of seldom-documented human biological threats to animals. PLoS One. 2014;9(2) doi: 10.1371/journal.pone.0089055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Desta G.B., et al. Factors associated with zoonosis and reverse zoonosis of Mycobacterium tuberculosis and Mycobacterium bovis in Ethiopia: a systematic review and meta-analysis. Int. J. Health Sci. 2022;V:1630–1653. doi: 10.53730/ijhs.v6nS5.8922. [DOI] [Google Scholar]
- 80.Kouengoua A.P.K., et al. Prevalence and zoonotic risk factors of Mycobacterium bovis tuberculosis in cattle at the cattle-wildlife-human interface in south and East Cameroon. Veterinary World. 2024;17(1):8. doi: 10.14202/2Fvetworld.2024.8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Baloch J.A., et al. Factors influencing knowledge and practices for prevention of bovine tuberculosis amongst workers at abattoir in Karachi. Pure Appl. Biol. 2020;9(2):1497–1503. doi: 10.19045/bspab.2020.90155. [DOI] [Google Scholar]
- 82.Olea-Popelka F., et al. Zoonotic tuberculosis in human beings caused by Mycobacterium bovis—a call for action. Lancet Infect. Dis. 2017;17(1):e21–e25. doi: 10.1016/S1473-3099(16)30139-6. [DOI] [PubMed] [Google Scholar]
- 83.Anjum M.A., et al. Estimating the willingness to pay for the purchase of pasteurized milk in Faisalabad, Pakistan. Pak. J. Agric. Sci. 2021;58(4) doi: 10.21162/PAKJAS/21.1183. [DOI] [Google Scholar]
- 84.PFA PUNJAB PURE FOOD REGULATIONS. 2018. https://food.punjab.gov.pk/system/files/16.%20Punjab%20Pure%20Food%20Regulations%2C%202018%20%28PPFR%2C%202018%29.pdf 2018 [cited 2023 23 December]; Available from:
- 85.Mia M.M., Hasan M., Pory F.S. Occupational exposure to livestock and risk of tuberculosis and brucellosis: a systematic review and meta-analysis. One Health. 2022 doi: 10.1016/j.onehlt.2022.100432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Milián-Suazo F., et al. Molecular epidemiology of human cases of tuberculosis by Mycobacterium bovis in Mexico. Prev. Vet. Med. 2010;97(1):37–44. doi: 10.1016/j.prevetmed.2010.06.015. [DOI] [PubMed] [Google Scholar]
- 87.Cordova E., et al. Human Mycobacterium bovis infection in Buenos Aires: epidemiology, microbiology and clinical presentation. Int. J. Tuberc. Lung Dis. 2012;16(3):415–417. doi: 10.5588/ijtld.10.0605. [DOI] [PubMed] [Google Scholar]
- 88.Pakistan-ntp, N.T.P National guidelines for the control of tuberculosis in Pakistan-revised. 2019. http://ntp.gov.pk/ntp-old/uploads/National_Guidelines_for_TB_Revised_2019.pdf [cited 2023 Nov 30]; ntp Pakistan]. Available from:
- 89.Müller B., et al. Zoonotic Mycobacterium bovis–induced tuberculosis in humans. Emerg. Infect. Dis. 2013;19(6):899. doi: 10.3201/2Feid1906.120543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Drobniewski F., et al. Audit of scope and culture techniques applied to samples for the diagnosis of Mycobacterium bovis by hospital laboratories in England and Wales. Epidemiol. Infect. 2003;130(2):235–237. doi: 10.1017/S0950268802008105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.UNICEF, W.H.O.a Global report for research on infectious diseases of poverty. 2012. https://iris.who.int/bitstream/handle/10665/44850/9789241564489_eng.pdf Available from.
- 92.Singh A.V., et al. Mycobacterium bovis induced human tuberculosis in India: current status, challenges & opportunities. Indian J. Med. Res. 2022;156(1):21. doi: 10.4103/ijmr.IJMR_1161_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Abubakar A. 2007. Epidemiology of human and bovine tuberculosis in the Federal Capital Territory and Kaduna State of Nigeria. [DOI] [Google Scholar]
- 94.Schiller I., et al. Bovine tuberculosis: a review of current and emerging diagnostic techniques in view of their relevance for disease control and eradication. Transbound. Emerg. Dis. 2010;57(4):205–220. doi: 10.1111/j.1865-1682.2010.01148.x. [DOI] [PubMed] [Google Scholar]
- 95.Bapat P.R., et al. Prevalence of zoonotic tuberculosis and associated risk factors in central Indian populations. J. Epidemiol. Global Health. 2017;7(4):277–283. doi: 10.1016/j.jegh.2017.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lönnroth K., et al. Drivers of tuberculosis epidemics: the role of risk factors and social determinants. Soc. Sci. Med. 2009;68(12):2240–2246. doi: 10.1016/j.socscimed.2009.03.041. [DOI] [PubMed] [Google Scholar]
- 97.Pfeiffer D. Epidemiology caught in the causal web of bovine tuberculosis. Transbound. Emerg. Dis. 2013;60:104–110. doi: 10.1111/tbed.12. [DOI] [PubMed] [Google Scholar]
- 98.FAO Bovine tuberculosis at the animal-human-ecosystem Interface. FAO Anim. Prod. Health Div. 2012;40 https://www.fao.org/4/i2811e/i2811e.pdf Available from: [Google Scholar]
- 99.Teppawar R.N., et al. Zoonotic tuberculosis: a concern and strategies to combat. Basic Biol. Appl. Actinobacteria. 2018:23–38. doi: 10.5772/intechopen.76802. [DOI] [Google Scholar]
- 100.WHO, W, FAO Zoonotic TB Factsheet. https://cdn.who.int/media/docs/default-source/hq-tuberculosis/zoonotic-tb-factsheet-2017.pdf?sfvrsn=66fdf3a1_3&download=true 2017 [cited 2024 28 January]; Available from:
- 101.WOAH Roadmap for Zoonotic TB. 2023. https://www.woah.org/app/uploads/2021/03/roadmap-zoonotic-tb.pdf [cited 2023 November]; Available from:
- 102.Vázquez-Chacón C.A., et al. Identification of drug resistance mutations among Mycobacterium bovis lineages in the Americas. PLoS Negl. Trop. Dis. 2021;15(2) doi: 10.1371/journal.pntd.0009145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.de Macedo Couto R., et al. One health and surveillance of zoonotic tuberculosis in selected low-income, middle-income and high-income countries: a systematic review. PLoS Negl. Trop. Dis. 2022;16(6) doi: 10.1371/journal.pntd.0010428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ramanujam H., Palaniyandi K. Bovine tuberculosis in India: the need for one health approach and the way forward. One Health. 2023 doi: 10.1016/j.onehlt.2023.100495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Martínez-Guijosa J., et al. Environmental DNA: a promising factor for tuberculosis risk assessment in multi-host settings. PLoS One. 2020;15(5) doi: 10.1371/journal.pone.0233837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gortázar C., et al. Will we ever eradicate animal tuberculosis? Ir. Vet. J. 2023;76(Suppl. 1):24. doi: 10.1186/s13620-023-00254-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Anaelom N.J., et al. Zoonotic tuberculosis: a review of epidemiology, clinical presentation, prevention and control. J. Public Health Epidemiol. 2010;2(6):118–124. https://academicjournals.org/journal/JPHE/article-full-text-pdf/7C667B2835 Available from: [Google Scholar]
- 108.Woldemariyam F.T., et al. Evaluation of postmortem inspection procedures to diagnose bovine tuberculosis at Debre Birhan municipal abattoir. Animals. 2021;11(9):2620. doi: 10.3390/ani11092620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ghai R.R., et al. A generalizable one health framework for the control of zoonotic diseases. Sci. Rep. 2022;12(1):8588. doi: 10.1038/s41598-022-12619-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yasmeen N., et al. One health paradigm to confront zoonotic health threats: a Pakistan prospective. Front. Microbiol. 2022;12 doi: 10.3389/fmicb.2021.719334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Robinette C., et al. Zoos and public health: a partnership on the one health frontier. One Health. 2017;3:1–4. doi: 10.1016/j.onehlt.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Admassu B., Kebede E., Shite A. Review on bovine tuberculosis. Eur. J. Biol. Sci. 2015 doi: 10.5829/idosi.ejbs.2015.7.04.96114. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Details of the districts where bTB studies included in this review were performed and the tests used by each study to detect bTB.
Data Availability Statement
No data was used for the research described in the article.