Abstract
Mutations that negatively or positively affect the fusion properties of murine leukemia viruses (MLVs) have been found within all subdomains of their SU (surface) and TM (transmembrane) envelope units. Yet, the interrelations between these different regions of the envelope complex during the cell entry process are still elusive. Deletion of the histidine residue of the conserved PHQV motif at the amino terminus of the amphotropic or the ecotropic MLV SU resulted in the AdelH or the MOdelH fusion-defective mutant envelope, respectively. These delH mutant envelopes are incorporated on retroviral particles at normal densities and normally mediate virion binding to cells expressing the retroviral receptors. However, both their cell-cell and virus-cell fusogenicities were fully prevented at an early postbinding stage. We show here that the fusion defect of AdelH or MOdelH envelopes was also almost completely reverted by providing either soluble SU or a polypeptide encompassing the receptor-binding domain (RBD) to the target cells, provided that the integrity of the amino-terminal end of either polypeptide was preserved. Restoration of delH envelope fusogenicity was caused by activation of the target cells via specific interaction of the latter polypeptides with the retrovirus receptor rather than by their association with the delH envelope complexes. Moreover crossactivation of the target cells, leading to fusion activation of AdelH or MOdelH envelopes, was achieved by polypeptides containing various type C mammalian retrovirus RBDs, irrespective of the type of entry-defective glycoprotein that was used for infection. Our results indicate that although they recognize different receptors for binding to the cell surface, type C mammalian retroviruses use a common entry pathway which is activated by a conserved feature of their envelope glycoproteins.
Retroviruses enter cells following their attachment to specific cell surface receptors. This function is mediated by the envelope glycoproteins expressed as trimeric complexes on the viral particles (14). Interaction with the receptor is thought to cause a conformational change of the glycoprotein structure necessary to expose a fusion peptide buried within the native envelope complex and involved in the actual membrane fusion process (10). On the basis of both genetic (2, 24, 26) and structural (8, 9) evidence, the attachment and fusion functions of murine leukemia virus (MLV) envelopes have been determined to be separated on the two subunits of the envelope monomer. Thus, it is generally considered that the amino-terminal half of the MLV SU (surface) subunit is responsible for the binding to the receptor, whereas the ectodomain of the TM (transmembrane) subunit contains the fusion machinery, which is composed of an amino-terminal fusion peptide associated to a coiled-coil structure that is rearranged upon receptor interaction and brings in close vicinity both the viral and the cell membranes.
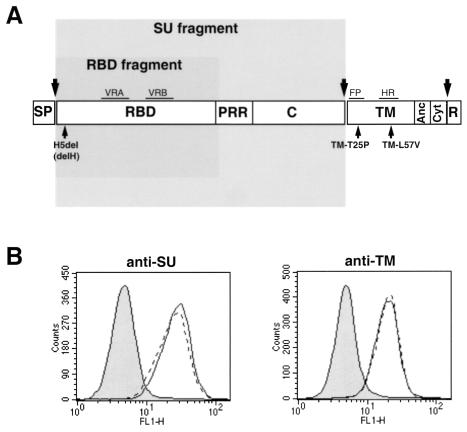
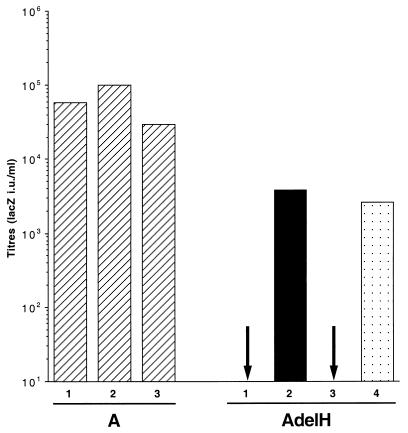
How retrovirus envelope glycoproteins convert binding to the receptor to a signal which activates the fusion machinery is currently unknown (32). Ample evidence from the literature indicates that functional interrelations between different domains of the envelope complex are essential to promote fusion activation (22, 30, 37, 38). Thus, although the SU subunit is not involved in the actual membrane fusion per se, several mutations which negatively (1) or positively (22, 27) affect fusion activation have been located within the different subdomains of the MLV SU envelope subunit. Close to the N terminus of SU, there is a conserved peptide motif (PHQV) centering on a histidine residue that was recently shown to be critical for postbinding events that lead to membrane fusion (1). Deletion (H8del) or mutations (H8A and H8R) of this histidine residue in ecotropic Moloney MLV (MoMLV) envelope glycoproteins have no effect on receptor recognition and virus binding, but they abrogate fusion triggering in both cell-cell and virus-cell fusion assays (1, 36a). Similarly, the deletion of histidine 5 of the SU subunit of the amphotropic MLV glycoprotein (H5del mutation) results in the AdelH fusion-defective envelopes (Fig. 1A). AdelH mutant envelope glycoproteins are incorporated into retroviral particles (D. Lavillette, M. Maurice, S. J. Russell, and F.-L. Cossett, unpublished data) and bind to cells expressing PiT-2 amphotropic receptors as efficiently as wild-type amphotropic envelope glycoproteins (Fig. 1B). However, their infectivity is inhibited at a postbinding level. Unlike the case for other fusion-defective envelope glycoproteins, such as the T461P and L493V mutants (TM-T25P and TM-L57V [Fig. 1A]), which harbor substitutions in the fusion peptide or in the heptad repeat region of the TM envelope subunit (17, 37), respectively, two lines of evidence suggest that the delH mutation affects an early step of the fusion process. First, the fusion-defective phenotype of H5del amphotropic envelopes or H8R ecotropic envelopes can be partially restored by introducing compensatory mutations in the proline-rich region adjacent to the receptor-binding domain (RBD) of the SU envelope subunit (36a; Lavillette et al., unpublished data). This proline-rich region, whose beginning seems to be close to the SU amino terminus in the globular structure of the RBD (8), has recently been proposed by us to participate in initiating and/or modulating the fusion activation of the retroviral envelope as a consequence of receptor binding (22). Second, retroviruses coexpressing AdelH and either TM-T25P and TM-L57V fusion-defective envelope mutants are highly infectious, thus suggesting that these mutations could efficiently complement each other in trans (Lavillette et al., unpublished data). These data therefore confirm that functional interactions occur within the retroviral envelope complex between monomers which are individually defective for retrovirus entry (30, 37, 38). Together, these results suggest that, in contrast to the case for the TM-T25P and TM-L57V mutants, the fusion machinery of the AdelH fusion-defective envelopes is left intact but is not activated or recruited upon receptor binding.
FIG. 1.
Characterization of AdeH envelope glycoproteins. (A) Domain organization of MLV envelope glycoprotein. SP, signal peptide; PRR, proline-rich region; C, SU carboxy terminal domain. Separation between the ectodomain, anchor domain (Anc), cytoplasmic tail (Cyt), and R peptide of the TM subunit is indicated by a vertical bar. The positions of envelope glycoprotein subdomains are shown: VRA and VRB, variable regions A and B; FP, fusion peptide; HR, heptad repeat. The large arrows mark the positions of cleavage of the envelope precursor. The locations of some fusion-defective mutations are shown. The envelope regions expressed as soluble polypeptides, encompassing either the SU or the RBD fragments, are shaded. (B) Binding assays of soluble (left; probed with anti-SU antibodies) versus virion-associated (right; probed with anti-TM antibodies) wild-type amphotropic (solid lines) and AdelH (broken lines) envelope glycoproteins on Cear13 cells. The background of fluorescence (filled histograms) was provided by incubating the cells with supernatants devoid of envelope fragments. The Env glycoprotein contents of the different samples were similar, as checked by immunoblotting with the viral supernatants (left) or the viral pellets (right).
Recent evidence from the literature suggests that although they can bind envelope glycoproteins, not all of the receptors expressed on the surface of a target cell may be competent to mediate virus entry (3, 36); interaction with the viral particles may activate them in order to trigger the fusion process. Furthermore, in addition to inducing the envelope conformational changes necessary to achieve membrane fusion, envelope-receptor interactions may also locally activate the cell membrane surrounding the receptor-bound retroviral particles (34, 36). Several cell cofactors, such as actin or microtubule cytoskeletons, for example, are likely to be recruited upon receptor binding and could probably assist retrovirus entry by allowing internalization, transport, and cytosolic penetration of virions (16, 18, 31). Therefore the effect of certain mutations of the envelope glycoprotein that affect retrovirus entry might be explained by their inability to activate the receptors or, alternatively, the cellular pathways necessary for infection. Thus, because the H5del mutation seems to inactivate an early postbinding entry event, we hypothesized that interaction of retroviruses carrying AdelH envelopes with amphotropic receptors may not be able to activate the target cell receptor or membrane.
Here we report that the infectivity of AdelH retroviruses could be efficiently rescued by providing in trans a soluble form of the retroviral envelope during infection. Thus, the target cells could be activated to a fusion-competent state by soluble receptor-binding fragments of the viral SU glycoprotein, and we demonstrated that this activated state persisted after removal of the initial stimulus and returned slowly to the inactive state. Moreover, we found that target cell activation was achieved by polypeptides containing various type C retrovirus RBDs, irrespective of the type of entry-defective glycoprotein that was used for infection. Our results provide evidence that the target cell participates actively in the process of virus-cell membrane fusion and that type C mammalian retroviruses use a common entry pathway which is activated by a conserved feature of their envelope glycoproteins.
MATERIALS AND METHODS
Cell lines.
TELCeB6 cells (6), derived from TE671 human rhabdomyosarcoma cells (ATCC CRL8805), express MoMLV Gag and Pol proteins and an nlsLacZ reporter MLV retroviral vector. Production of infectious retroviral particles by TELCeB6 cells depends on newly introduced envelope expression vectors.
Cerd9 and Cear13 cells (20) and CHO-PiT-2 cells (31) were derived from Chinese hamster ovary (CHO) cells (ATCC CCL-61) and express either ecotropic MLV receptors alone, both ecotropic and amphotropic MLV receptors, or amphotropic MLV receptors alone, respectively. NIH 3T3 mouse fibroblasts, XC rat sarcoma cells (ATCC CCL-165), and Cerd9, Cear13, CHO-PiT-2, and CHO cells were grown in Dulbecco modified Eagle medium (Life Technologies) supplemented with 10% fetal bovine serum and with proline (Life Technologies).
Construction of envelope expression vectors.
Plasmids FBASALF and FBMOSALF, carrying a phleomycin resistance gene and encoding the MLV 4070A amphotropic (noted as A) and MoMLV ecotropic (noted as MO) envelope glycoproteins, respectively, have been described elsewhere (6) and were used as backbones for construction and expression of envelope mutants. All subsequent constructs were generated by PCR-mediated and oligonucleotide site-directed mutagenesis (details and sequences are available upon request).
The FBASALF plasmid was modified to produce a cell entry-defective form of the amphotropic glycoprotein, designated AdelH envelope, by deleting the 36th codon of the 4070A env gene (25). The resulting mutant envelope glycoprotein (Fig. 1), in which the fifth residue of the SU envelope subunit was removed, was named AdelH (Lavillette et al., unpublished data). The expression plasmid encoding the fusion-defective MOdelH envelope glycoproteins, harboring the equivalent delH mutation, which was obtained by deleting the eighth residue of the SU envelope subunit (corresponding to the 41th codon of the MoMLV env gene) (33), was derived from FBMOSALF.
Plasmids encoding secreted RBDs were derived from FBASALF and FBMOSALF expression vectors. The carboxy-terminal ends of either amphotropic (A-RBD) or ecotropic (E-RBD) RBDs, defined as A32-G244 and A34-G269, were fused in frame with the 11-amino-acid vesicular stomatitis virus G protein (VSV-G) tag (YTDIEMNRLGK) (21). Residues are numbered starting from the initiation methionine deduced from the amino acid sequence of the amphotropic MLV 4070A (25) or MoMLV (33). Expression vectors encoding either A-RBDdelH or E-RBDdelH were generated similarly by using the plasmids expressing the AdelH or MOdelH envelope glycoproteins. The expression vector for E-RBD.D84K was derived from the plasmid encoding the E-RBD envelope fragment by introducing the D84K substitution, a mutation that inactivates MoMLV envelope binding, into the E-RBD (23).
Production of retrovirus vectors.
Envelope glycoprotein expression plasmids were transfected into TELCeB6 cells as reported elsewhere (6). Transfected cells were selected with phleomycin (50 μg/ml), and phleomycin-resistant colonies were pooled. Virus-containing supernatants were collected after overnight production from confluent env-transfected cells, filtered through 0.45-μm-pore-size membranes, and stored at 4°C.
Production of soluble SU or of soluble RBD fragments.
Envelope glycoprotein expression plasmids were transfected into TE671 cells as reported elsewhere (6). RBD expression vectors were transfected by calcium phosphate precipitation in NIH 3T3, XC, or TE671 cells. Transfected cells were selected with phleomycin (50 μg/ml), and individual phleomycin-resistant colonies were isolated. Expression of SUs or of RBDs in each clone was analyzed by immunoblotting of cell lysates, using anti-SU or anti-VSV-G tag antibodies, respectively. Clones that expressed equivalent amounts of SU or RBD polypeptides were retained for production of soluble SU or soluble RBD fragments. SU-containing supernatants (i.e., SU accumulating as soluble material after dissociation from envelope complexes by shedding) or RBD-containing supernatants were collected after overnight production from confluent env- or RBD-transfected cells, filtered through 0.22-μm-pore-size membranes, and stored at 4°C. The expression and receptor specificity of SU or of RBD in these conditioned supernatants were checked in receptor binding assays, and small binding differences, indicating weak variations of polypeptide concentrations, were corrected.
Infection assays.
Target cells were seeded in 24-well plates at a density of 5 × 104 cells per well and incubated overnight at 37°C. Unless otherwise indicated in figure legends, 200 μl of conditioned cell culture medium containing the RBDs was added to the cells after removal of their supernatants. Then, 200 μl of viral supernatant dilutions containing 5 μg of Polybrene per well was added, and cells were incubated for 3 h at 37°C. Cell supernatants were then removed, and cells were incubated in regular medium for 48 h. X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) staining and viral titer determination were performed as previously described, and titers were expressed as LacZ infectious units (IU) per milliliter of viral supernatant (6).
Antibodies.
Antibodies were as follows: anti-SU, a rat monoclonal antibody (83A25) (7) cell culture supernatant against MLV SU used undiluted for fluorescence-activated cell sorter (FACS) analysis; anti-TM, a mouse monoclonal antibody (372) (ATCC CRL-1893) (5) cell culture supernatant against MLV TM used undiluted for FACS analysis; and anti-VSV-G tag, a mouse monoclonal antibody (P5D4) (Sigma-Aldrich) used diluted 1/100 for FACS analysis.
Binding assays and FACS analysis.
For binding assays, target cells were washed in phosphate-buffered saline (PBS) and detached by a 10-min incubation at 37°C with versene (0.02% in PBS). Cells were washed in PBA (PBS with 2% fetal calf serum and 0.1% sodium azide). A total of 5 × 105 cells were incubated with 1 ml of conditioned supernatants containing the viral particles or containing the VSV-G-tagged RBDs for 45 min at 37°C. The cells were then washed with PBA and stained for 45 min at 4°C with anti-SU or anti-TM antibodies to detect binding of soluble SU or of virion-associated SU, respectively (35), or were stained with P5D4 monoclonal antibodies to detect binding of soluble RBDs. Cells were washed twice with PBA and incubated with fluorescein isothiocyanate-conjugated antibodies (DAKO, Trappes, France). At 5 min before the two final washes in PBA, cells were counterstained with 20 μg of propidium iodide per ml. Fluorescence of living cells was analyzed with a fluorescence-activated cell sorter (FACSCalibur; Becton Dickinson).
RESULTS
The H5del phenotype can be efficiently compensated by soluble A-RBD.
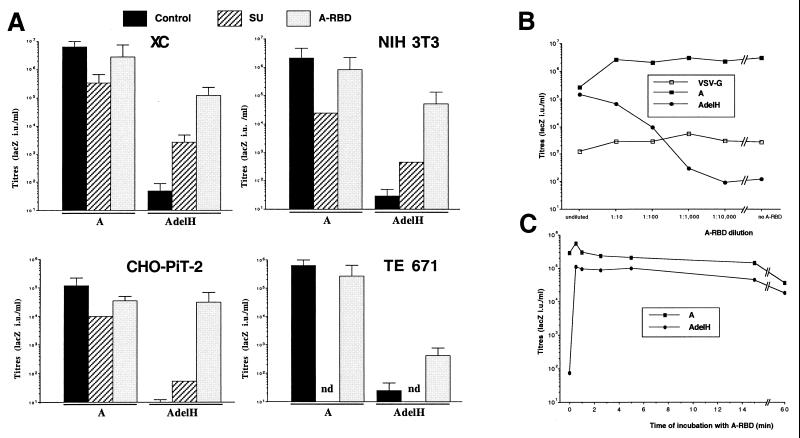
Viral particles were generated with either wild-type amphotropic A or AdelH envelope glycoproteins (Fig. 1A) and used to infect target cells in the presence or absence of soluble SU or soluble A-RBD, a secreted envelope fragment encompassing the amphotropic RBD (Fig. 1A). When SU or A-RBD was provided during infection, the infectivity of retroviruses carrying wild-type amphotropic envelope glycoproteins was decreased by approximately 5- to 100-fold (Fig. 2A), most likely owing to the partial blocking of PiT-2 receptors on the target cell surface. In striking contrast, while in the absence of envelope fragments retroviruses generated with AdelH envelopes were very poorly infectious, with titers in the range of 101 to 102 LacZ IU/ml, the presence of SU or A-RBD in the medium of infected cells could strongly stimulate the infectivity of the former retroviruses by up to 30,000-fold (Fig. 2A). Stimulation of AdelH infection with either soluble envelope fragment was detected on all target cell types tested, including rat XC cells, murine 3T3 fibroblasts, human TE671 cells, and PiT-2-transfected hamster CHO cells, though with different efficacies (Fig. 2A). The RBD envelope fragments were found to work by restoring the fusion defect of the AdelH mutant, since only the presence of A-RBD in cell-cell fusion assays that were performed with AdelH envelope glycoproteins could induce the formation of syncytia (data not shown). Thus, the results of virus-cell (infection) and cell-cell fusion assays confirmed that the delH mutation affects a membrane fusion step of infection (1) rather than a step that follows fusion (e.g., uncoating). Significant differences were detected when soluble SU and A-RBD were used to activate the AdelH retroviruses (Fig. 2A), most probably because in contrast to soluble SU, which is expressed as an integral transmembrane protein before it can dissociate from the envelope complex and accumulate in the cell supernatant, A-RBD is a secreted polypeptide. These results also indicated that the determinants of activation were located in the first half of the SU protein. Therefore, to facilitate the characterization of this activation pathway, subsequent experiments were performed by using the soluble RBD fragments. Compared to infection assays performed with undiluted A-RBD, no significant decrease in the titer of AdelH retroviruses was detected when A-RBD was diluted 1:10, and an only 10-fold reduction of titer was found at a 1:100 dilution, thus suggesting that A-RBD-mediated activation was effective at very low doses (Fig. 2B). In contrast, the infectivity of control retroviruses pseudotyped with either wild-type amphotropic envelopes or VSV-G glycoproteins was weakly increased or unchanged, respectively, when A-RBD was diluted (Fig. 2B). Additionally, the origin of the cell types used to produce the envelope fragments did not influence the stimulation of infectivity, since conditioned medium harvested from 3T3, TE671, or XC cells that were engineered to express the envelope fragments could similarly activate the infectivity of AdelH retroviruses (data not shown). Finally, activation of AdelH retroviruses was found to be the result of a specific interaction of A-RBD with the PiT-2 amphotropic receptor. Indeed, while A-RBD could efficiently rescue the infectivity of AdelH retroviruses on PiT-2-transfected CHO cells, no infection could be detected when parental CHO cells were used as target cells (see below and Fig. 6B). Together these data demonstrated that activation of cell entry of AdelH retroviruses by A-RBD was specifically mediated via interaction with PiT-2.
FIG. 2.
Titers of AdelH retroviruses in the presence of soluble envelope fragments. Retroviral vectors carrying VSV-G or wild-type amphotropic (A) envelope glycoproteins were used as controls. (A) Different target cell types were incubated with both lacZ retroviruses and conditioned media containing, or not (control), the indicated polypeptides during the 3 h of infection. Error bars indicate standard deviations. (B) XC target cells were incubated with dilutions of A-RBD and infected with lacZ retroviruses carrying AdelH, A, or VSV-G envelope glycoproteins. (C) Retroviral vectors carrying A or AdelH envelope glycoproteins were incubated with XC target cells for 1 h at 4°C to allow virion binding while preventing cell entry. After being washed with PBS to remove unbound retroviruses, cells were incubated at 37°C with undiluted A-RBD-containing supernatants for the indicated times. A-RBD was then eliminated from the cell supernatant by washing the cells four times with 1 ml of PBS (resulting in dilution of unbound A-RBD by more than 100,000-fold). Cells were then grown in regular medium for 2 days before X-Gal staining.
FIG. 6.
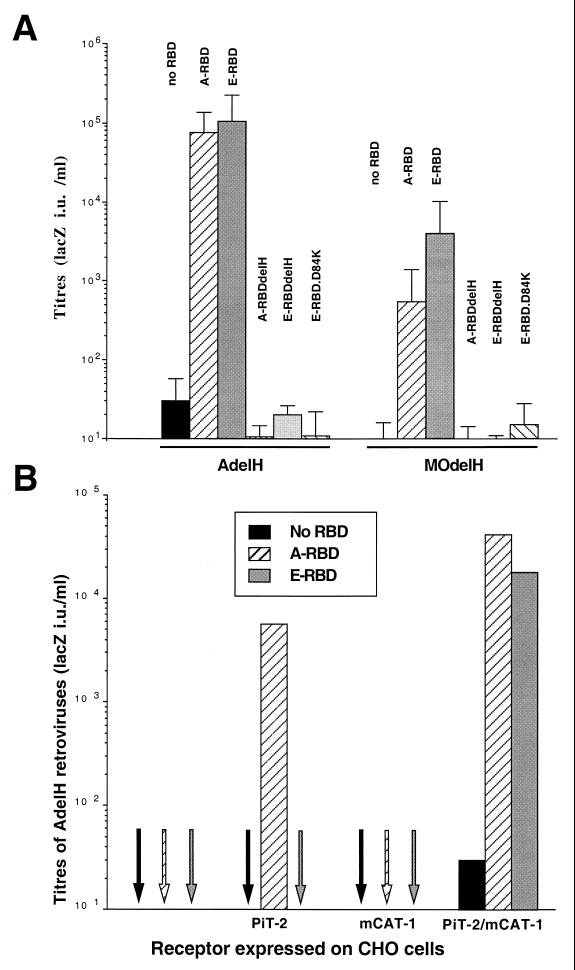
Cross-activation of AdelH and MOdelH retroviruses by MLV RBDs. (A) Titers, determined on XC target cells, of retroviruses carrying AdelH or MOdelH envelope glycoproteins in the presence of the indicated RBDs. Error bars indicate standard deviations. (B) Titers of AdelH retroviruses on CHO cells expressing, or not (control), PiT-2 and/or mCAT-1 MLV receptors in the presence of the indicated RBDs.
The ability of the soluble envelope fragments to rescue the infectivity of AdelH retroviruses was similar whether the target cells (i) constitutively expressed and secreted the envelope fragments, (ii) were coincubated with both AdelH retroviruses and A-RBD, (iii) were preincubated with A-RBD prior to infection, or (iv) were preincubated with the AdelH retroviruses before addition of A-RBD (Table 1). Under the last experimental condition, the kinetics of activation of infection was found to be very rapid. Indeed, a brief incubation (no more than 30 s) of target cells with A-RBD was found to be sufficient to fully stimulate the infectivity of prebound AdelH retroviruses (Fig. 2C).
TABLE 1.
Activation of AdelH retroviruses by A-RBD
| Cells | Prebinding conditiona | Binding and infection conditionb | Titer |
|---|---|---|---|
| XC | lacZ (AdelH) | 5 × 101 | |
| XC | lacZ (AdelH) + A-RBDc | 3.1 × 105 | |
| XC-A-RBDd | lacZ (AdelH) | 9 × 104 | |
| XC | A-RBD | lacZ (AdelH) | 1.1 × 105 |
| XC | lacZ (AdelH) | A-RBD | 2.7 × 105 |
Target cells were preincubated with conditioned supernatants containing A-RBD (used undiluted) or with a lacZ retroviral vector carrying AdelH envelope glycoprotein, as indicated.
Target cells were incubated for 3 h at 37°C.
Both A-RBD (used undiluted) and AdelH retroviruses were added at the same time.
XC cells constitutively expressing A-RBD polypeptides.
Persistent activation of target cells by A-RBD.
In theory, it is possible that the soluble SU or RBD envelope fragments might rescue the infectivity of AdelH retroviruses either by activating the target cells upon receptor binding or, alternatively, by interacting with the fusion-defective envelope complex of the viral particles. Indeed, within the envelope complex, the SU subunits are not tightly attached to either the TM subunits or the other SU units and can easily shed off the viral particles (15). However, the reassociation of shed SU with the viral particle, which has never been reported so far in the literature, is unlikely, since SU subunits are incorporated on virions by virtue of their association with the TM subunits resulting from their synthesis as a common SU-TM polypeptide precursor (15). Nevertheless, the following experiments were performed to address these two possibilities.
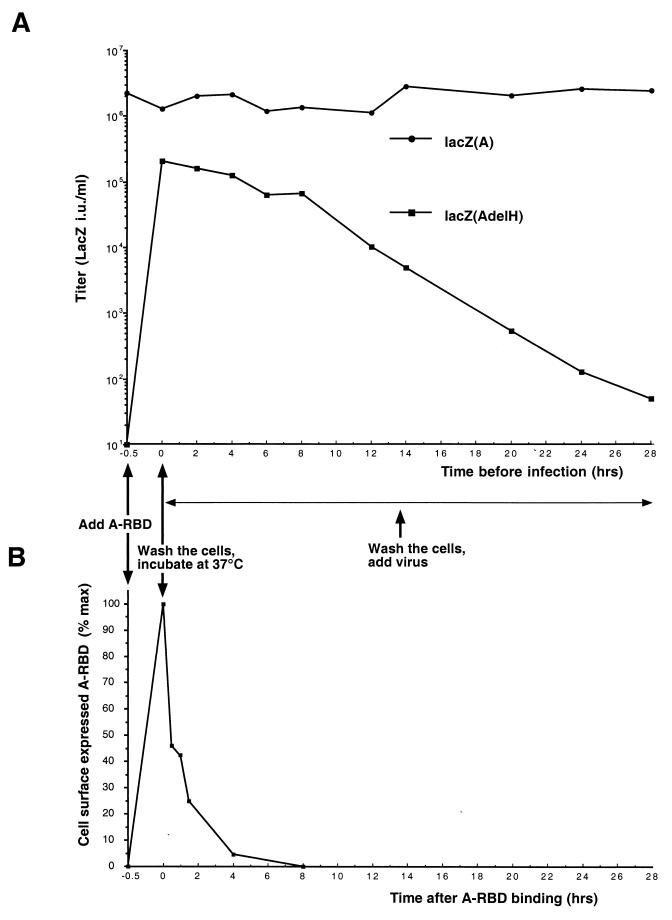
A-RBD was bound to target cells at 37°C for 30 min. After removal of unbound fragments by washing the target cells, binding of A-RBD was verified by FACS analysis (Fig. 3B). Cells were then incubated at 37°C for 0 h (T0) to 28 h (T28) to allow internalization of receptor–A-RBD complexes (31) and A-RBD disappearance from the cell surface, as checked by FACS analysis (Fig. 3B). At different time points, the cells were washed again to remove A-RBD that may have been released by the cells, and retroviruses carrying the wild-type A or the AdelH envelope glycoproteins were then added (Fig. 3A). Titers of retroviruses coated with wild-type amphotropic envelopes were not affected by A-RBD bound onto the target cells (Fig. 3A), suggesting that some binding sites were still available on the cell surface and allowed efficient attachment of viral particles at the various time points. Compared to infection performed at T0, no significant decrease of infectivity could be detected when the AdelH retroviruses were added until 4 h after the initial binding with A-RBD. Likewise, the infectivity of AdelH retroviruses added at T20 was at least 50-fold higher than that before prestimulation of the cells with A-RBD, thus indicating that stimulation of infectivity was durable (Fig. 3A). Additionally, since cell-bound A-RBD was efficiently removed from the cell surface after 8 h of incubation (Fig. 3B), these results suggested that activation of AdelH retroviruses most likely occurred by activation of the target cells rather than by interaction of A-RBD with the AdelH envelope complex itself.
FIG. 3.
Titers of AdelH retroviruses after A-RBD-mediated target cell activation. (A) A-RBD (used undiluted) was bound to XC target cells at 37°C for 30 min. Unbound A-RBD was removed by two PBS washes, and cells were incubated at 37°C to allow internalization of A-RBD–receptor complexes. At the indicated times, cells were further washed two times with PBS and infected with retroviruses carrying A or AdelH envelope glycoproteins. (B) Detection of A-RBD fragments expressed at the surface of the target cells after incubation for the indicated periods of time at 37°C. Mean values of binding expressed as percentages of the maximal fluorescence at T0 are shown, with the background value being the fluorescence of cells before addition of A-RBD.
Further results indicated that A-RBD could not directly reassociate or interact with the envelope complex of the AdelH retroviral particles. First, retroviruses generated with either A or AdelH envelopes and mixed or coexpressed with A-RBD were not found to incorporate A-RBD as shown by Western blotting of purified viral particles with antibodies recognizing A-RBD (data not shown). Second, a mixture of A-RBD and retroviruses carrying either AdelH or wild-type A envelope glycoproteins was separated on two consecutive 700-kDa-cutoff ultrafiltration columns in order to elute out A-RBD (Fig. 4, bars 3). No decrease of infectivity could be detected for retroviruses carrying wild-type amphotropic A envelopes after the two subsequent ultrafiltrations, demonstrating that this process did not affect the viability of the retroviruses. However, while before ultrafiltration the AdelH viral particles mixed with A-RBD could efficiently infect XC target cells (Fig. 4, bars 2), as expected, no infectivity could be detected after A-RBD elution (bars 3), indicating that stimulation of AdelH retroviruses was lost after separation from A-RBD. As a control, the AdelH retroviruses processed through the two columns retained their full capacity to be stimulated by newly added A-RBD (Fig. 4, bar 4). Similar conclusions were drawn when separation of AdelH retroviruses from A-RBD was carried out by Sephacryl chromatography (data not shown).
FIG. 4.
Titers of AdelH retroviruses after ultrafiltration. Titers on XC target cells of retroviruses carrying wild-type (A) or AdelH envelope glycoproteins before (bars 1) or after (bars 2) incubation with A-RBD and following A-RBD elution on two successive 700-kDa-cutoff ultrafiltration cartriges (bars 3). After elution, AdelH retroviruses were subjected to restimulation with fresh A-RBD bar (4).
Together these data strongly suggested that stimulation of infectivity of the AdelH retroviruses proceeded via activation of the target cells, most likely as a consequence of interaction of A-RBD with the PiT-2 amphotropic receptor, rather than via interaction with the AdelH envelopes incorporated on the retrovirus itself. Thus, A-RBD could activate the target cells, which in turn became competent to allow entry of viral particles carrying the fusion-defective AdelH envelope glycoproteins.
The SU amino-terminal end activates a cell entry pathway common to type C mammalian retroviruses.
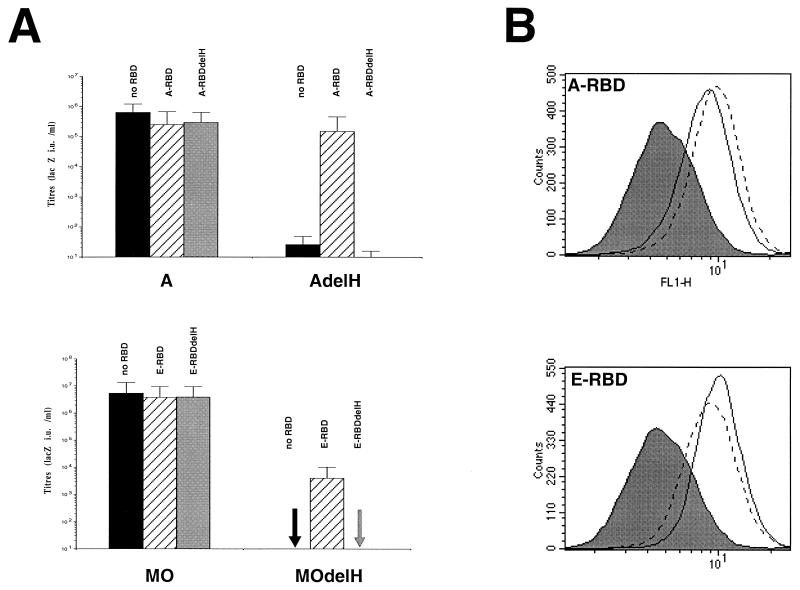
The histidine residue deleted in the H5del mutant glycoprotein belongs to a peptide motif, PHQV, found at the amino termini of the SUs of all type C mammalian retroviruses (Lavillette et al., unpublished data), suggesting a conserved function of this motif. Thus, the H8del mutant ecotropic envelope glycoprotein (MOdelH), harboring the deletion of the histidine in the PHQV peptide motif of the MoMLV SU, is impaired for both cell-cell and virus-cell fusion (1). We then performed further experiments to demonstrate that MOdelH and AdelH (delH) envelopes were phenotypically similar. Indeed, the E-RBD fragment, containing the MoMLV ecotropic RBD, could rescue the infectivity of MOdelH retroviruses by up to 1,000-fold (Fig. 5A). This result indicated that fusion activation by soluble envelope fragments was not particular to AdelH envelopes and suggested that the MLV SU amino terminus may convey a signal common to other MLV types which may be required to achieve early postbinding events.
FIG. 5.
Infection assays of AdelH and MOdelH retroviruses in the presence of RBD or RBDdelH polypeptides. (A) Retroviruses carrying A or AdelH envelope glycoproteins were mixed with A-RBD or A-RBDdelH during infection of XC cells. Retroviruses carrying MO or MOdelH envelope glycoproteins were mixed with E-RBD or E-RBDdelH during infection of XC cells. Titers are shown as LacZ IU per milliliter. Error bars indicate standard deviations. (B) Top, binding assays of A-RBD (solid lines) and A-RBDdelH (broken lines) fragments on Cear13 cells. Bottom, binding assays of E-RBD (solid lines) and E-RBDdelH (broken lines) fragments on Cear13 cells. The background of fluorescence (filled histograms) was provided by incubating the cells with supernatants devoid of envelope fragments. The RBD contents of the different samples were similar, as checked by immunoblotting.
To address this hypothesis, the delH mutations H5del and H8del were introduced in the A-RBD and E-RBD fragments, respectively. The resultant A-RBDdelH and E-RBDdelH could bind their respective cell surface receptors as efficiently as the parental envelope fragments (Fig. 5B). They could also decrease the infectivity of retroviruses bearing wild-type amphotropic or ecotropic glycoproteins, respectively, by up to 10-fold (Fig. 5A). This effect was most likely a consequence of partial receptor blocking. However, in contrast to A-RBD or E-RBD, neither the A-RBDdelH nor the E-RBDdelH fragments could rescue the infectivity of AdelH or MOdelH retroviruses, respectively (Fig. 5A). These data therefore demonstrated that the integrity of the amino-terminal ends of either RBD fragments was absolutely required to activate postbinding functions and suggested that an essential determinant of target cell activation may reside at the amino-terminal end of the MLV RBD. Thus, the so-called RBD located in the amino-terminal half of the MLV SU may in fact be composed of two different entities, one involved in specific receptor binding and an second, likely to be nonfunctional in AdelH or MOdelH envelopes, involved in transmission of a signal that may activate cell entry upon receptor binding.
Therefore, to test whether the binding function and the activating function of the MLV RBD could be uncoupled, cells “infected” with AdelH or MOdelH retroviruses were cross-incubated with either E-RBD or A-RBD polypeptides, respectively. Interestingly, the infectivity of MOdelH retroviruses could be activated by either A-RBD or E-RBD fragments (Fig. 6A). This cross-activation was reciprocal, since either fragments could activate, with similar efficacies, the infectivity of AdelH retroviruses (Fig. 6A). While these data indicated that the RBD fragments could restore infectivity irrespective of the type of entry-defective glycoprotein that was used for infection, we also found that either RBD fragments could cross-activate delH envelope glycoproteins in cell-cell fusion assays (data not shown). The cross-reactivity of either type of RBD was dependent on the presence of both mCAT-1 ecotropic and PiT-2 amphotropic receptors on the cell surface, since neither PiT-2-transfected CHO cells nor mCAT-1-transfected CHO cells could be infected when E-RBD was used to activate the infectivity of AdelH retroviruses (Fig. 6B) and vice versa (data not shown). The specificity of activation of AdelH and MOdelH retroviruses by ecotropic envelope fragments was further demonstrated by using the E-RBD.D84K envelope fragment, an ecotropic RBD polypeptide harboring the D84K point mutation, which inactivates MoMLV envelope binding (23). This fragment could activate neither AdelH nor MOdelH retroviruses despite the presence of both amphotropic and ecotropic receptors on target cells (Fig. 6A). Cross-activation of AdelH retroviruses could also be obtained by using soluble envelope glycoproteins from gibbon ape leukemia virus (data not shown), thus indicating that infection by delH retroviruses could be activated by a pathway common to type C mammalian retroviruses.
DISCUSSION
Binding of retrovirus envelope glycoproteins to cell surface receptors initiates the early steps of retroviral infection. This primary interaction is thought to trigger a structural rearrangement of the glycoprotein necessary to expose the fusion peptide that associates with the target cell lipid bilayer, leading to fusion between the viral and cellular membranes. In addition to the molecular changes that affect the overall envelope structure, several cellular events are also likely to occur in the time between receptor binding and subsequent membrane fusion and may actively participate in the process of membrane fusion. The dissection of this cascade of early infection events benefits from the availability of envelope mutants that are defective for a particular step of retrovirus entry. Thus, mutations that affect the late steps of the fusion process, i.e., the actual virus-cell membrane fusion, have been found inside the TM envelope subunit (17, 37–39). Although these various mutants were useful to dissect domains of the glycoproteins which are subject to conformational changes, they do not allow easy investigation of the putative cellular pathways that might be involved in retrovirus entry. In contrast, the delH mutation of MLV glycoproteins (1; Lavillette et al., unpublished data) is unique in that it affects an early postbinding stage of the MLV entry process (Lavillette et al., unpublished data). In a manner similar to that for their parental envelopes, ecotropic or amphotropic MLV envelope glycoproteins that harbor the delH mutation are efficiently expressed, maturated as SU-TM subunits, and incorporated on viral particles, and they bind normally to cell surface receptors. However, they fail to mediate membrane fusion in both cell-cell and virus-cell membrane fusion assays (1; Lavillette et al., unpublished data).
Here we report for the first time that soluble forms of type C mammalian retrovirus RBD added to target cells are necessary and sufficient to allow efficient infection by cell entry-defective retroviruses carrying delH-mutated MLV envelope glycoproteins. Importantly, the characterization of this infection-activating mechanism has permitted the identification of an as-yet-unknown pathway of the MLV entry process which is likely to be common to type C mammalian retroviruses. Thus, delH-mutated entry-defective MLVs can be efficiently rescued by homologous or heterologous retrovirus RBDs, via a receptor-mediated mechanism. Although our data cannot formally exclude the possibility that a fusion-active envelope complex is restored in the delH mutant virus by a direct interaction of the RBD fragment, we do not favor this possibility. Indeed, as an alternative possibility, our data suggest that the inability of delH-mutated MLV envelope glycoproteins to promote cell entry might be caused by their incapacity to induce conformational changes on the receptors and/or to recruit cell cofactors required for postbinding events. The cell components which affect retrovirus entry into cells are not known in detail. However, different reports indicate that MLV receptors do not have a passive attachment role in the cell entry process but are likely to play an essential role by activating several steps of the membrane fusion pathway that are probably common to MLVs (34). Previous reports, revealing the heterogeneity of MLV receptors on living cells, have suggested that only a fraction of retrovirus binding sites could function as entry ports and that receptors may positively cooperate to allow infection (3, 36). Other observations revealed the existence of an accessory factor or process which may be limiting for MLV entry into cells and for which the supply might differ between different cell types (34, 36). Several studies have underlined the necessity of receptor clustering for the formation of multivalent complexes with viral particles of different membrane enveloped viruses (12). Efficient receptor clustering is likely to depend on the distribution of receptors in suitable subcellular environments or on particular physiological conditions of the target cells (31, 34, 36). In particular, recent studies have suggested that the actin and microtubule cytoskeletons with which the receptors are associated may play an important part in cell entry of ecotropic or amphotropic MLVs (18, 31), as they may promote receptor aggregation, internalization, and intracellular transport of receptor-bound viral particles (19). In addition, inhibition of PiT-2 conformational changes associated with Pi transport could strongly impair virus internalization and infection (31), and this provided further evidence that the cell is an active participant in these early steps of virus entry.
Our results may therefore bring further understanding of the activation of cell entry triggered by type C mammalian retrovirus binding to receptors. Thus, the infectivity of retroviruses carrying delH mutant envelopes can be rescued by providing in trans a soluble envelope fragment which may supply the missing receptor activation signal. In addition, our data demonstrate that activation of cell entry was dependent on the integrity of the amino-terminal end of the RBD, since delH-mutated MLV RBDs were unable to rescue the fusogenicity of delH MLV envelope glycoproteins. These results therefore underline the critical importance of the SU amino-terminal tail in the fusion process. Of note is that the SU amino-terminal end harbors a conserved peptide motif, PHQV, whose disruption similarly affects the fusion properties of all type C mammalian retrovirus envelope glycoproteins examined to date (Lavillette et al., unpublished data). It is possible that type C mammalian retrovirus envelope glycoproteins have diverged from a common ancestor and differ only by discrete variations of loops that are involved in recognition of related cell surface receptors (13). Thus, the different type C mammalian retroviruses have adapted to a class of different, albeit related, receptors which may share the way they activate virus entry upon binding. Therefore, in addition to allowing binding to the different retrovirus receptor, the type C mammalian retrovirus RBDs govern a second conserved function required for infection: activation of a cell entry pathway which depends on the SU amino-terminal end. It is probable that MLV RBDs interact with receptors via a two-step process, with the first step being the attachment to the receptor and the second one being conformational changes of the bound receptor allowing post-binding events which, in turn, activate the virus-cell fusion process.
While the pathway of receptor activation reported here was deciphered as a result of the characterization of a particular MLV envelope mutant, it is important to return it to the context of infection with wild-type retroviruses. Glycoproteins of membrane-enveloped viruses are expressed as stable envelope precursors which undergo several posttranslational modifications, such as proteolytic cleavage, in order to be displayed on the virion in metastable forms that must be activated to initiate fusion (4). For retroviruses such as MLV, the proteolytically processed SU and TM subunits are held together via a labile disulfide bond whose isomerization is likely to provide more metastability to the envelope complex and increased instability of the SU subunit (28). Instability of the envelope complex, resulting in SU shedding from virions (11), has been proposed to be important for membrane fusion (22). Based on the results in this report, one can envisage SU shedding as an important factor of the retrovirus entry process. Indeed, adsorption of viral particles on the cell surface (29) may increase the local concentration of soluble shed SU, which in turn may activate the target cell membrane, e.g., by recruiting receptors whose clustering may assist the penetration of the forthcoming virion. According to this model, AdelH SU glycoproteins, although they allow virion binding to the cell surface (Lavillette et al., unpublished data), are not able to activate the target cell surface and cannot mediate cell entry. Additionally, one can envisage that SU shedding from envelope complexes expressed at the surface of retrovirus producer cells might sensitize neighboring cells to infection. Thus, the release of SU, from either virus- or cell-associated envelope complexes, is likely to stimulate the propagation of retroviruses.
ACKNOWLEDGMENTS
We are grateful to Mark P. Chadwick for comments on the manuscript.
This work was supported by Agence Nationale pour la Recherche contre le SIDA (ANRS), Association pour la Recherche contre le Cancer (ARC), Centre National de la Recherche Scientifique (CNRS), and Institut National de la Santé Et de la Recherche Médicale (INSERM).
REFERENCES
- 1.Bae Y, Kingsman S M, Kingsman A J. Functional dissection of the Moloney murine leukemia virus envelope protein gp70. J Virol. 1997;71:2092–2099. doi: 10.1128/jvi.71.3.2092-2099.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Battini J L, Heard J M, Danos O. Receptor choice determinants in the envelope glycoproteins of amphotropic, xenotropic, and polytropic murine leukemia viruses. J Virol. 1992;66:1468–1475. doi: 10.1128/jvi.66.3.1468-1475.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Battini J L, Rodrigues P, Müller R, Danos O, Heard J-M. Receptor-binding properties of a purified fragment of the 4070A amphotropic murine leukemia virus envelope glycoprotein. J Virol. 1996;70:4387–4393. doi: 10.1128/jvi.70.7.4387-4393.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carr C M, Chaundhry C, Kim P S. Influenza hemagglutinin is spring-loaded by a metastable native conformation. Proc Natl Acad Sci USA. 1997;23:14306–14313. doi: 10.1073/pnas.94.26.14306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chesebro B, Wehrly K, Cloyd M, Britt W, Portis J, Collins J, Nishio J. Characterization of mouse monoclonal antibodies specific for Friend murine leukemia virus-induced erythroleukemia cells: Friend-specific and FMR-specific antigens. Virology. 1981;112:131–144. doi: 10.1016/0042-6822(81)90619-x. [DOI] [PubMed] [Google Scholar]
- 6.Cosset F-L, Takeuchi Y, Battini J L, Weiss R A, Collins M K L. High-titer packaging cells producing recombinant retroviruses resistant to human serum. J Virol. 1995;69:7430–7436. doi: 10.1128/jvi.69.12.7430-7436.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Evans L H, Morrison R P, Malik F G, Portis J, Britt W J. A neutralizable epitope common to the envelope glycoproteins of ecotropic, polytropic, xenotropic, and amphotropic murine leukemia viruses. J Virol. 1990;64:6176–6183. doi: 10.1128/jvi.64.12.6176-6183.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fass D, Davey R A, Hamson C A, Kim P S, Cunningham J M, Berger J M. Structure of a murine leukemia virus receptor-binding glycoprotein at 2.0 angstrom resolution. Science. 1997;277:1662–1666. doi: 10.1126/science.277.5332.1662. [DOI] [PubMed] [Google Scholar]
- 9.Fass D, Harrison S C, Kim P S. Retrovirus envelope domain at 1.7 A resolution. Nat Struct Biol. 1996;3:465–469. doi: 10.1038/nsb0596-465. [DOI] [PubMed] [Google Scholar]
- 10.Gerlier D. Entrée des virus enveloppés à l'échelle moléculaire. Virologie. 1998;2:215–226. [Google Scholar]
- 11.Gliniak B C, Kozak S L, Jones R T, Kabat D. Disulfide bonding controls the processing of retroviral envelope glycoproteins. J Biol Chem. 1991;266:22991–22997. [PubMed] [Google Scholar]
- 12.Haywood A. Virus receptors: binding, adhesion strengthening, and changes in viral structure. J Virol. 1994;68:1–5. doi: 10.1128/jvi.68.1.1-5.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heard, J.-M., P. Rodrigues, and C. Salaün. Une collaboration étroite et efficace: les enveloppes rétrovirales et leurs récepteurs cellulaires. Med. Sci., in press.
- 14.Hunter E. Viral entry and receptors. In: Coffin J M, Hughes S H, Varmus H E, editors. Retroviruses. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1997. pp. 71–120. [PubMed] [Google Scholar]
- 15.Hunter E, Swanstrom R. Retrovirus envelope glycoproteins. Curr Top Microbiol Immunol. 1990;157:187–253. doi: 10.1007/978-3-642-75218-6_7. [DOI] [PubMed] [Google Scholar]
- 16.Iyengar S, Hildreth J E, Schwartz D H. Actin-dependent receptor colocalization required for human immunodeficiency virus entry into host cells. J Virol. 1998;72:5251–5255. doi: 10.1128/jvi.72.6.5251-5255.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones J S, Risser R. Cell fusion induced by the murine leukaemia virus envelope glycoprotein. J Virol. 1993;67:67–74. doi: 10.1128/jvi.67.1.67-74.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kizhatil K, Albritton L M. Requirements for different components of the host cell cytoskeleton distinguish ecotropic murine leukemia virus entry via endocytosis from entry via surface fusion. J Virol. 1997;71:7145–7156. doi: 10.1128/jvi.71.10.7145-7156.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klasse P J, Bron R, Marsh M. Mechanism of enveloped virus entry into animal cells. Adv Drug Delivery Rev. 1998;34:65–91. doi: 10.1016/S0169-409X(98)00002-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kozak S L, Siess D C, Kavanaugh M P, Miller A D, Kabat D. The envelope glycoprotein of an amphotropic murine retrovirus binds specifically to the cellular receptor/phosphate transporter of susceptible species. J Virol. 1995;69:3433–3440. doi: 10.1128/jvi.69.6.3433-3440.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kreis T E, Lodish H F. Oligomerization is essential for transport of vesicular stomatitis viral glycoprotein to the cell surface. Cell. 1986;46:929–937. doi: 10.1016/0092-8674(86)90075-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lavillette D, Maurice M, Roche C, Russell S J, Sitbon M, Cosset F-L. A proline-rich motif downstream of the receptor binding domain modulates conformation and fusogenicity of murine retroviral envelopes. J Virol. 1998;72:9955–9965. doi: 10.1128/jvi.72.12.9955-9965.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.MacKrell A J, Soong N W, Curtis C M, Anderson W F. Identification of a subdomain in the Moloney murine leukemia virus envelope protein involved in receptor binding. J Virol. 1996;70:1768–1774. doi: 10.1128/jvi.70.3.1768-1774.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morgan R A, Nussbaum O, Muenchau D D, Shu L, Couture L, Anderson W F. Analysis of the functional and host range-determining regions of the murine ecotropic and amphotropic retrovirus envelope proteins. J Virol. 1993;67:4712–4721. doi: 10.1128/jvi.67.8.4712-4721.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ott D, Friedrich R, Rein A. Sequence analysis of amphotropic and 10A1 murine leukemia virus: close relationship to mink cell focus forming viruses. J Virol. 1990;64:757–766. doi: 10.1128/jvi.64.2.757-766.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ott D, Rein A. Basis for receptor specificity of nonecotropic murine leukemia virus surface glycoprotein gp70. J Virol. 1992;66:4632–4638. doi: 10.1128/jvi.66.8.4632-4638.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park B H, Matuschke B, Lavi E, Gaulton G N. A point mutation in the env gene of a murine leukemia virus induces syncytium formation and neurologic disease. J Virol. 1994;68:7516–7524. doi: 10.1128/jvi.68.11.7516-7524.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pinter A, Kopelman R, Li Z, Kayman S C, Sanders D A. Localization of the labile disulfide bond between SU and TM of the murine leukemia virus envelope protein complex to a highly conserved CWLC motif in SU that resembles the active-site sequence of thiol-disulfide exchange enzymes. J Virol. 1997;71:8073–8077. doi: 10.1128/jvi.71.10.8073-8077.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pizzato M, Marlow S A, Blair E D, Takeuchi Y. Initial binding of murine leukemia virus particles to cells does not require specific Env-receptor interaction. J Virol. 1999;73:8599–8611. doi: 10.1128/jvi.73.10.8599-8611.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rein A, Yang C, Haynes J A, Mirro J, Compans R W. Evidence for cooperation between murine leukemia virus Env molecules in mixed oligomers. J Virol. 1998;72:3432–3435. doi: 10.1128/jvi.72.4.3432-3435.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rodrigues P, Heard J M. Modulation of phosphate uptake and amphotropic murine leukemia virus entry by posttranslational modifications of PIT-2. J Virol. 1999;73:3789–3799. doi: 10.1128/jvi.73.5.3789-3799.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Russell, S. J., and F.-L. Cosset. Modifying the host range properties of retroviral vectors. J. Gene Med., in press. [DOI] [PubMed]
- 33.Shinnick T M, Lerner R A, Sutcliffe J G. Nucleotide sequence of Moloney murine leukemia virus. Nature (London) 1981;293:543–548. doi: 10.1038/293543a0. [DOI] [PubMed] [Google Scholar]
- 34.Siess D C, Kozak S L, Kabat D. Exceptional fusogenicity of Chinese hamster ovary cells with murine retroviruses suggests roles for a cellular factor(s) and receptor clusters in the membrane fusion process. J Virol. 1996;70:3432–3439. doi: 10.1128/jvi.70.6.3432-3439.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Valsesia-Wittmann S, Morling F J, Hatziioannou T, Russell S J, Cosset F-L. Receptor co-operation in retrovirus entry: recruitment of an auxilliary entry mechanism after retargeted binding. EMBO J. 1997;16:1214–1223. doi: 10.1093/emboj/16.6.1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang H, Paul R, Burgeson R, Keene D, Kabat D. Plasma membrane receptors for ecotropic murine retroviruses require a limiting accessory factor. J Virol. 1991;65:6468–6477. doi: 10.1128/jvi.65.12.6468-6477.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36a.Zavorotinskaya T, Albritton L M. Supression of a fusion defect by second site mutation in the ecotropic murine leukemia virus surface protein. J Virol. 1999;73:5034–5042. doi: 10.1128/jvi.73.6.5034-5042.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao Y, Lee S, Anderson W F. Functional interactions between monomers of the retroviral envelope protein complex. J Virol. 1997;71:6967–6972. doi: 10.1128/jvi.71.9.6967-6972.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao Y, Zhu L, Benedict C A, Chen D, Anderson W F, Cannon P M. Functional domains in the retroviral transmembrane protein. J Virol. 1998;72:5392–5398. doi: 10.1128/jvi.72.7.5392-5398.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhu N L, Cannon P M, Chen D, Anderson W F. Mutational analysis of the fusion peptide of Moloney murine leukemia virus transmembrane protein p15E. J Virol. 1998;72:1632–1639. doi: 10.1128/jvi.72.2.1632-1639.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]