Abstract
Vein of Galen aneurysmal malformation (VGAM) is a rare vascular anomaly originating during embryonic development, specifically between the 6th and 11th weeks of gestation. This malformation results from abnormal arteriovenous connections between primitive choroidal arteries and the median prosencephalic vein (MPV) of Markowski. Typically, the MPV regresses by the 11th week, but in VGAM, this regression is hindered, leading to persistent abnormal flow and the formation of arteriovenous shunts. We present a case of successful prenatal detection, as well as a comprehensive literature review that summarizes current knowledge, emphasizes the importance of prenatal detection, detailed imaging techniques, understanding clinical presentations, and outlines treatment options. Prenatal detection, crucial for early intervention, has become feasible through ultrasonography and MRI. Fetal MRI has emerged as the gold standard, offering detailed insights into arterial feeders, nidus presence, fistula position, venous drainage, and potential complications. The clinical presentation of VGAM varies with age, and neonates diagnosed in utero may exhibit signs of high-output cardiac failure. Early detection is critical for timely intervention, as untreated VGAMs often result in high mortality rates. Prognosis depends on the severity of heart failure, the number of arteriovenous shunts, and the presence of accompanying fetal abnormalities. Various imaging modalities, including CT angiography and digital subtraction angiography (DSA), aid in the assessment and treatment of VGAM. DSA remains the gold standard for evaluating angioarchitecture and guiding endovascular interventions. The optimal treatment for VGAM is transarterial embolization, offering significant improvements in prognosis. Surgical interventions are limited due to high morbidity and mortality. Management decisions should consider the balance between minimizing neurological damage and achieving maximum embolization effectiveness.
Keywords: Vein of Galen malformation, VGAM, Colour Doppler, MR angiography, Prenatal diagnosis
Highlights
-
•
Vein of Galen aneurysmal malformation is a rare vascular anomaly during embryonic development.
-
•
Prenatal detection has become feasible through ultrasonography and MRI.
-
•
DSA remains the gold standard for guiding endovascular interventions.
Introduction
The vein of Galen malformation (VGAM) is an intracranial anomaly and a rare form of embryonic arteriovenous shunt located in the midline in the choroidal fissure. It is a multifaceted, particularly complex and high-flow arteriovenous abnormality with complicated vascular architecture. Roughly 29% of VGAMs are diagnosed in utero, normally first identified by fetal ultrasound [1]. Ultrasound imaging plays an imperative role in the diagnosis of VGAMs, typically either at third trimester prenatal ultrasound or in the neonatal period with transcranial Doppler ultrasound. Other imaging modalities include fetal echocardiography and fetal brain MR imaging. A timely diagnosis throughout the prenatal period is of uttermost importance because the large shunting of blood within the fetal brain often results in a substantial steal of blood, which can later lead to perinatal death owing to cardiac failure and hydrops. The vein of Galen malformation has generally a male predominance. Its incidence is about 1 in 3 million population, and it represents less than 1% of the fetal cerebral AV malformations [2], [3]. The first reported case of a VGAM was published in 1937 [4]. In most cases it is isolated, although there are cases in which it is found alongside cardiac anomalies or cystic hygroma [5]. VGAM consists of numerous feeding arteries, predominantly the anterior and posterior choroidal arteries and the anterior cerebral artery which are draining straight into a distended venous sac. Treatment is reliant on the timing of presentation and clinical manifestations. With the improvement of endovascular techniques, treatment standards have changed, and clinical outcomes have improved considerably.
Case report
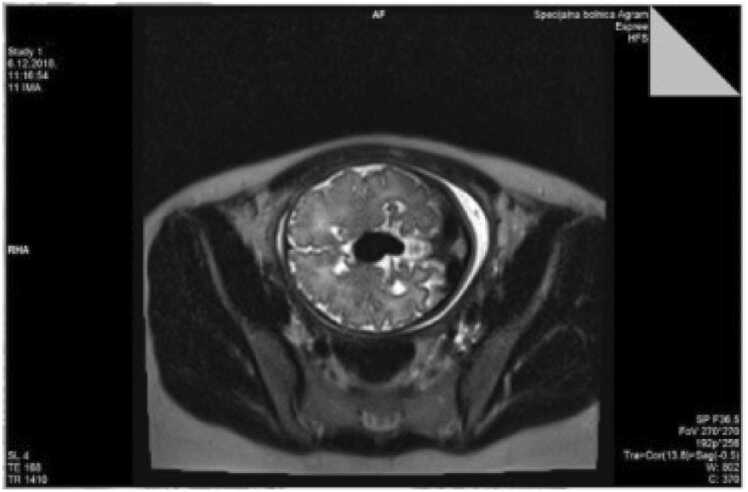
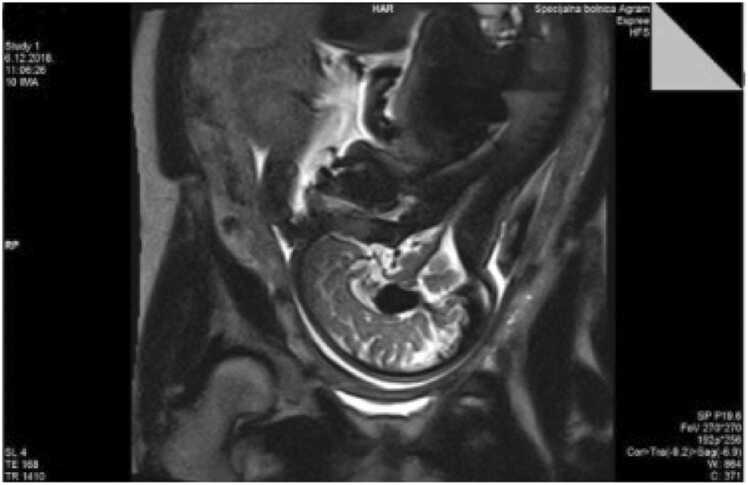
A 28-year-old pregnant Caucasian woman (gravida 2, para 1) underwent serial regular fetal growth check-ups at a primary centre, including anomaly scan, up until 30 weeks of gestational age (GA). Pregnancy was uneventful. She was then transferred to our institution for further regular growth check-ups. Her past medical history was unremarkable, there weren’t any known diseases in her family history. Her first child had Down syndrome, undetected during pregnancy. Regular ultrasound at our institution at 33 weeks of gestation demonstrated a 20 mm anechoic midline structure within the fetal brain containing prominent vascular flow on Doppler examination (Fig. 1.). The brain parenchyma and ventricular size were normal. Fetal ultrasound reconfirmed the anomaly by colour Doppler sonography as vascular in nature. Platelet antigen testing and TORCH titres were negative. Amniocentesis showed a normal male karyotype. Diagnosis was definite by fetal magnetic resonance imaging. Fetal magnetic resonance (MR) images demonstrated a 21 mm mass within the fetuses quadrigeminal plate cistern that matched to the sonographic abnormality (Figs. 2. and 3). The parents were suggested full specialist consultation comprising of a perinatology specialist, paediatric neuroradiologist and an intensivist to improve perinatal management. Serial follow-up examinations were implemented weekly to monitor the cardiovascular status of the fetus, which stayed stable. A vigorous male baby was born by a scheduled C-section at 38 + 5/7 weeks of GA, birth weight and length 2880 g and 47 cm respectively. Immediately after birth the newborn was admitted to the neonatal intensive care unit (NICU) of a tertiary care centre. The identification of VGAM was confirmed by postnatal transfontanelle sonography. Multiple interventional radiology embolization of the feeding vessels was performed successfully. The child was later released from hospital. In the forthcoming months the child underwent a series of treatments, including a one more embolization. His neurological and intellectual development showed no abnormality. Today he is a healthy 5-year-old without sequalae.
Fig. 1.
Colour Doppler ultrasound of transtalamic section thorugh given malformation.
Fig. 2.
Magnetic resonance imaging (transversal section) of transtalamic section thorugh given malformation.
Figure 3.
Magnetic resonance imaging (sagital section) of transtalamic section thorugh given malformation.
Discussion and literature review
Definition and anatomy
The basic knowledge of embryology underlying the formation of the normal deep cerebral venous system is necessary to understand the formation of Vein if Galen aneurysmal malformation since the first accurate description of VGAM by Raybaud and co-workers [6], [7]. VGAM grows between the 6th and 11th weeks of gestation. It is a consequence of an abnormal arteriovenous connection between primitive choroidal arteries and the median prosencephalic vein (MPV) of Markowski, a predecessor to the vein of Galen [2], [8]. Typically, by the 11th week of gestation the MPV retreats. The perseverance of this connection between those structures has an abnormal persistent flow through the MPV and that obstructs its normal involution [7]. Moreover, anomalous flow prevents the formation of the vein of Galen and leads to the appearance of some abnormal arteriovenous shunts. Consequently, development of VGAMs may be considered as a mistake in the early stage of vasculogenesis [9]. The normal development of the vascular cerebral system is created in 3 phases: stage I prechoroidal, stage II prechoroidal, and stage III choroidal [2]. The cerebral vascularization in the choroidal stage is formed from the choroidal arteries, and the venous drainage is ensured by Markowski median vein. The anterior section of Markowski vein degenerates while the posterior section of the Markowski vein will persevere and is then named the Galen vein. For an unknown reason, the creation of some arteriovenous shunts causes the anterior segment of the Markowski vein not to degenerate. Consequently, that anterior segment dilates and widens so these sutures drain in the vein of Galen [8], [10]. Typically, there are 2 groups of VGAM angioarchitecture. They were first defined by Lasjaunias - he divided them into choroidal and mural subtypes [11]. Choroidal VGAMs have numerous choroidal arterial feeders, which shape a nidus and then connect to the enlarged MPV. The vascular steal occurs because of these multiple arteriovenous shunts amongst the vein of Galen and the choroidal arteries. They cause the rise in blood returning to the heart at the level of cerebral cortex. Later it becomes an overload of the right heart and causes a gradual heart decompensation. If the right heart decompensation occurs, it often leads to a progressive hydrops [5], [8]. The VGAM abnormality also causes some secondary cerebral consequences, because of both the phenomenon of cerebral vascular steal and the mass effect. Secondary consequences provoked by continuous rise of flow can be seen on both the arterial and venous sides of a VGAM. Expanded arteries can display sinuosity and can be related to arterial aneurysm and/or steno-occlusive disease. Similarly, the dural sinuses involved in VGAM drainage can become stenotic or occluded, with redistribution of venous flow into cortical veins. Both of those can lead to cerebral ischemia, hydrocephalus and leukomalacia [12]. Mural subtype VGAM is a direct arteriovenous shunt. This network is usually located in the quadrigeminal cistern and results in a less significant blood flow increase. It has late medical symptomatology in the extrauterine life and is related to a minor level of heart failure [6]. The choroidal VGAM is more frequently seen and correlates with a worse prognosis [13]. The type of shunt existing in a VGAM defines its clinical presentation and has consequently been used to propose several VGAM classifications [3], [11].
Imaging
Nearly 29% of VGAMs are detected prenatally, in utero [1], [14]. Before the introduction of sonography, neonates with VGAM generally had heart failure of “unknown” aetiology, and the accurate diagnosis was made only at the time of fatal cardiac failure or post-mortem. Nowadays, the prenatal identification of this abnormality is made by 2D ultrasonography, pulsed Doppler, colour mapping, 3D Doppler sonography, and most importantly MRI. Usually, it is first discovered by fetal ultrasonography from about 25 weeks' GA (most frequently at the end of the second trimester and the beginning of the third trimester of pregnancy) [14], [15].
Ultrasound
Two-dimensional standard ultrasonography generally visualizes anechoic cystic midline brain lesions with turbulent vascular Doppler flow. Typically, the VGAM is located on the midline of the anterior wall of the third ventricle (the “comet tail” or “keyhole” sign). The addition of three-dimensional (3D) power Doppler ultrasound can differentiate more anatomical particulars. Also, this technique assists in spatial angle-independent visualization of blood vessels [16]. Prenatal detection of VGAM has advanced significantly using colour Doppler sonography, which is essential for discriminating this abnormality from other cystic lesions of the brain because the vein of Galen malformation is the only lesion that unmistakably shows blood flow within it. Fine Doppler examination of a VGAM can be made not only for diagnostic purposes but also to observe the hemodynamic aftermath of the treatment. Ultrasonographic cranial analysis can also reveal jugular vein distention and ventriculomegaly. Also, it can find signs of fetal heart failure (cardiomegaly, ascites) which is caused by high blood flow in the arteriovenous fistula. Associated prenatal sonographic features were present in 76% of the fetuses, including cardiomegaly in 64%, jugular vein distention in 32%, and ventriculomegaly in 24% [16], [17]. Fetal echocardiogram is also an important part of the medical diagnosis, because VGAMs present a risk of high output cardiac failure. Sonographic confirmation of tricuspid valve insufficiency, supraventricular extrasystoles or tachycardia implies poor outcome [17].
Magnetic resonance imaging (MRI)
In recent years fetal magnetic resonance imaging (MRI) has become the golden standard and it is superior to Doppler ultrasonography in the diagnosis of VGAM [18]. The adjoining anatomy can be superiorly analysed with the use of MRI in the fetus. Magnetic resonance imaging can assess the number and type of arterial feeders, it can detect existence of any nidus, the exact fistula position, evaluate venous drainage and identify venous thrombosis [18]. Also, it allows evaluation of any previous impairment due to secondary repercussions to the brain. It detects cerebral ischemic areas, cerebral atrophy, third ventricle or aqueduct compression which is important because damage from venous congestion and anomalous cerebral spinal fluid (CSF) flow may preclude aggressive management [19]. A definite degree of irreversible cerebral damage is essential for both therapeutic decision-making and prognosis evaluation [20]. There are some restrictions of fetal MRI. It can’t evaluate the blood-flow spectrum of arteriovenous fistula. Also, it cannot evaluate the fetal cardiac function which is correlated to the prognosis [20].
Other methods
CT angiography has a vast prospective for the assessment of cerebrovascular pathology affecting neonates and infants. Although not as precise as conventional catheter angiography, CT angiography is non-invasive and offers more comprehensive vascular data than sonographic and MR techniques [21], [22].
Digital subtraction angiography (DSA) remains the gold standard technique for the evaluation and more importantly treatment of the cerebrovascular system, which is described later. Only DSA offers accurate evaluation of the VGAM angio-architecture and provides approach to endovascular management of the malformation [11]. DSA specifics the fine anatomy of the arterial feeders in terms of number, origin and size, and identifies accompanying vascular anomalies.
Fetal imaging
With a study of previous literature, the ultrasonography remains the most common technique of diagnosis, but both ultrasound and MRI were used to diagnose fetal VGAM [23]. The diagnosis of VGAM by ultrasound combined MRI was rarely reported. More frequently VGAMs are identified after birth [18]. Postnatal verification is made by means of transfontanellar ultrasonography, MRI, angiography, computed tomography [14]. The existing literature generally contains only case reports, due to the rarity of the VGAM.
Clinical presentation
The clinical presentation has a tight relationship between the age of neonates or infants at the time presentation and the angioarchitecture and hemodynamic characteristics of the VGAM [24]. Neonates diagnosed in utero may already exhibit signs of cardiac failure prior to delivery. The volume overload imposed by a VGAM with high-flow shunts is such that it can promptly cause cardiovascular and respiratory failures. Most of the cases (94%) diagnosed in the neonatal period will therefore present with high-output cardiac failure [24]. In the past, before the access to endovascular treatment, the mortality rate for this group was close to 100% [24]. Repeatedly, if the VGAM was not diagnosed in utero, delivery and the first 24 h of neonates are often unremarkable. Larger VGAMs may then show rapid deterioration in clinical status with rapidly deteriorating cardiac failure leading on to multiorgan failure [24]. The decreased resistance and high blood flow in the lesion cause the high-output heart failure in the neonates [24], [25]. Initial investigations seek a cardiac cause for the high output failure, but when all the cardiac examinations are within the normal range, transfontanellar ultrasound will discover the VGAM [19], [26]. Other than cardiac failure, a brain haemorrhage from the malformation can occur. Accompanying findings with VGAM feature cerebral ischemic changes [27]. Also, the VGAM may result in mass effects, initiating progressive neurological damage. Lastly, it may cause obstruction of the cerebrospinal fluid (CSF) drainage and formation of hydrocephalus, resulting in increased head circumference and/or seizures. Other symptoms may incorporate cranial bruit, enlarged scalp veins (most often in the periorbital region) and recurring epistaxis [1], [12]. Older children frequently have a headache that may be concomitant with subarachnoid haemorrhage. Gold et al. found that subarachnoid haemorrhage occurred in 10 of the 13 patients belonging to this age category [28]. Late diagnosis in asymptomatic patients is more often seen because of the widespread use of MR imaging. In such instance, the VGAM is typically small, and the amount of arteriovenous shunt limited.
Prognosis
The prognosis and outcome of VGAMs varies upon two factors. Primary factor is the gravity of heart failure, which is directly associated to the number of arteriovenous shunts [29]. Prenatal ultrasonographic signs such as polyhydramnios, tricuspid insufficiency, pericardial and pleural effusion, cardiomegaly and ascites indicate heart failure and an intractable high-flow anomaly, so the prognosis is worse [25]. The secondary prognostic factor is the number of accompanying anomalous fetal disorders. Additional fetal abnormalities have a poorer prognosis, whereas isolated fetal VGAM frequently demonstrates a better prognosis [26]. There is no evidence on the optimal methods for delivering these neonates [29]. However, it is suggested that delivery ought to take place at a tertiary care centre with specialists in perinatology, pediatric cardiology, pediatric neuroradiology and neurosurgery.
Differential diagnosis
Once abnormal vessels are identified, there is usually little diagnostic uncertainty, with the only two entities to be considered being arteriovenous malformation or vascular intracranial tumour. Arteriovenous malformations (AVMs) are characterized by an abnormal leash of vessels allowing for arteriovenous shunting [1]. They can occur anywhere in the body but are most common in the brain. There is direct arteriovenous communication with no intervening capillary bed. They can be congenital or acquired.
Treatment
Transarterial embolization is the supreme mode and golden standard of management of VGAMs [12], [30]. The endovascular therapy has radically changed the treatment and prognosis of VGAM patients [31], [32]. The objective of embolization is to allow maturation of the vascular system by decreasing flow through the shunt. Surgery plays little role in VGAM management because of its high morbidity and mortality. Evidence suggests that patients who are treated with embolization before substantial neurological damage has occurred have a good prognosis [33]. Lasjaunias et al. have shown that management at 5 months results in the best equilibrium of minimal possibility of cerebral maturation delay and maximum effectiveness of embolization [11]. If there are signs of cardial decompensation, patients are given diuretics to decrease cardiac preload and are closely monitored for clinical decompensation [9], [11]. If cardiac failure is refractory to therapy, embolization may be performed sooner [34]. Transvenous method can be used if transarterial approach is deemed impossible, however it is related to a greater risk of haemorrhagic complications and poorer success rates [35]. Complete angiographic treatment is not necessary; reduction by 30% to 50% of the shunt has been shown to result in substantial systemic effect [30], [36], [37].
Conclusion
Vein of Galen aneurysmal malformation is a rare abnormality of the central nervous system, with an even scarcer rate of prenatal detection and correct diagnosis. With today's possibilities of modern diagnostic imaging used in perinatology (ultrasonography, Doppler, MRI), it should be recognized more often prenatally. Early recognition, along with regular check-ups, planned termination of pregnancy and immediate care of the patient during delivery, reduce potential complications and improve the clinical outcome. With the imaging techniques available today, it is possible to make a diagnosis in the early stages of pregnancy. In the presence of unclear anechoic inclusions in the central nervous system that deviates from the regular anatomical findings, it is necessary to suspect an aneurysm of the vein of Galen in the differential diagnosis. It is from our presentation of the case that we can see how with a timely diagnosis, a planned approach to the termination of pregnancy and immediate adequate multidisciplinary care of the patient, excellent long-term outcomes and results can be obtained.
CRediT authorship contribution statement
Jasminka Stipanović: Conceptualization, Investigation, Methodology, Project administration, Supervision. Jasenka Zmijanac Partl: Conceptualization, Data curation, Supervision. Dejana Lučić: Conceptualization, Data curation. Daria Hadžić: Writing – original draft. Diana Culej Bošnjak: Conceptualization, Formal analysis, Writing – review & editing. Danijel Bursać: Conceptualization, Supervision, Validation, Writing – review & editing. Željko Duić: Supervision, Validation.
Declaration of Competing Interest
none.
Acknowledgments
none.
Grant support
none.
References
- 1.Long D.M., Seljeskog E.L., Chou S.N., French L.A. Giant arteriovenous malformations of infancy and childhood. J Neurosurg. 1974;40(3):304–312. doi: 10.3171/jns.1974.40.3.0304. [DOI] [PubMed] [Google Scholar]
- 2.Yan J., Wen J., Gopaul R., Zhang C.Y., Xiao S. wen. Outcome and complications of endovascular embolization for vein of Galen malformations: a systematic review and meta-analysis. J Neurosurg. 2015;123(4):872–890. doi: 10.3171/2014.12.JNS141249. [DOI] [PubMed] [Google Scholar]
- 3.Mortazavi M.M., Griessenauer C.J., Foreman P., et al. Vein of Galen aneurysmal malformations: critical analysis of the literature with proposal of a new classification system. J Neurosurg Pedia. 2013;12(3):293–306. doi: 10.3171/2013.5.PEDS12587. [DOI] [PubMed] [Google Scholar]
- 4.Jaeger R. Bilateral congenital arteriovenous communications (aneurysm) of the cerebral vessels. Arch Neurol Psychiatry. 1946;55(6):591. doi: 10.1001/archneurpsyc.1946.02300170035005. [DOI] [PubMed] [Google Scholar]
- 5.Hoang S., Choudhri O., Edwards M., Guzman R. Vein of Galen malformation. Neurosurg Focus. 2009;27(5) doi: 10.3171/2009.8.FOCUS09168. [DOI] [PubMed] [Google Scholar]
- 6.Raybaud C.A., Strother C.M. Persisting abnormal embryonic vessels in intracranial arteriovenous malformations. Acta Radio Suppl. 1986;369:136–138. [PubMed] [Google Scholar]
- 7.Raybaud C.A., Strother C.M., Hald J.K. Aneurysms of the vein of Galen: embryonic considerations and anatomical features relating to the pathogenesis of the malformation. Neuroradiology. 1989;31(2):109–128. doi: 10.1007/BF00698838. [DOI] [PubMed] [Google Scholar]
- 8.Alvarez H., Garcia Monaco R., Rodesch G., Sachet M., Krings T., Lasjaunias P. Vein of galen aneurysmal malformations. Neuroimaging Clin N Am. 2007;17(2):189–206. doi: 10.1016/j.nic.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 9.Bhattacharya J.J. Vein of Galen malformations. J Neurol Neurosurg Psychiatry. 2003;74(90001):42i–444. doi: 10.1136/jnnp.74.suppl_1.i42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gailloud P.H., Souei M., Manera L., Brunel H., Raybaud C., Levrier O. Normal galenic drainage of the deep cerebral venous system in two cases of vein of Galen aneurysmal malformation. Childs Nerv Syst. 2004;20(2):91–97. doi: 10.1007/s00381-003-0841-y. [DOI] [PubMed] [Google Scholar]
- 11.Lasjaunias P., Terbrugge K., Piske R., Lopez Ibor L., Manelfe C. Dilatation of the vein of Galen. Anatomoclinical forms and endovascular treatment apropos of 14 cases explored and/or treated between 1983 and 1986. Neurochirurgie. 1987;33(4):315–333. [PubMed] [Google Scholar]
- 12.Gailloud P., O’Riordan D.P., Burger I., et al. Diagnosis and management of vein of galen aneurysmal malformations. J Perinatol. 2005;25(8):542–551. doi: 10.1038/sj.jp.7211349. [DOI] [PubMed] [Google Scholar]
- 13.Sepulveda W., Vanderheyden T., Pather J., Pasquini L. Vein of Galen Malformation. J Ultrasound Med. 2003;22(12):1395–1398. doi: 10.7863/jum.2003.22.12.1395. [DOI] [PubMed] [Google Scholar]
- 14.Taylor G.A. Intracranial venous system in the newborn: evaluation of normal anatomy and flow characteristics with color Doppler US. Radiology. 1992;183(2):449–452. doi: 10.1148/radiology.183.2.1561348. [DOI] [PubMed] [Google Scholar]
- 15.Yukhayev A., Meirowitz N., Madankumar R., Timor‐Tritsch I.E., Monteagudo A. Uncommon second‐trimester presentation of vein of Galen malformation. Ultrasound Obstet Gynecol. 2018;51(3):421–423. doi: 10.1002/uog.17462. [DOI] [PubMed] [Google Scholar]
- 16.Félix L., Souza A.R., Queiroz A.P., et al. Prenatal ultrasonography in the diagnosis of vein of Galen aneurysm. Acta Med Port. 2010;23(3):505–510. [PubMed] [Google Scholar]
- 17.Sepulveda W., Platt C.C., Fisk N.M. Prenatal diagnosis of cerebral arteriovenous malformation using color Doppler ultrasonography: case report and review of the literature. Ultrasound Obstet Gynecol. 1995;6(4):282–286. doi: 10.1046/j.1469-0705.1995.06040282.x. [DOI] [PubMed] [Google Scholar]
- 18.Li T. gang, Zhang Y. yue, Nie F., Peng M. juan, Li Y.Z., Li P. long. Diagnosis of foetal vein of galen aneurysmal malformation by ultrasound combined with magnetic resonance imaging: a case series. BMC Med Imaging. 2020;20(1):63. doi: 10.1186/s12880-020-00463-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paladini D., Deloison B., Rossi A., et al. Vein of Galen aneurysmal malformation (VGAM) in the fetus: retrospective analysis of perinatal prognostic indicators in a two-center series of 49 cases. Ultrasound Obstet Gynecol. 2017;50(2):192–199. doi: 10.1002/uog.17224. [DOI] [PubMed] [Google Scholar]
- 20.Brunelle F. Brain vascular malformations in the fetus: diagnosis and prognosis. Childs Nerv Syst. 2003;19(7-8):524–528. doi: 10.1007/s00381-003-0791-4. [DOI] [PubMed] [Google Scholar]
- 21.Jordan L., Raymond G., Lin D., Gailloud P. CT angiography in a newborn child with hydranencephaly. J Perinatol. 2004;24(9):565–567. doi: 10.1038/sj.jp.7211138. [DOI] [PubMed] [Google Scholar]
- 22.Alberico R.A., Barnes P., Robertson R.L., Burrows P.E. Helical CT angiography: dynamic cerebrovascular imaging in children. AJNR Am J Neuroradiol. 1999;20(2):328–334. [PMC free article] [PubMed] [Google Scholar]
- 23.Stone McGuire L., Nikas D. Vein of Galen Malformation. NEJM. 2020;383(15) doi: 10.1056/NEJMicm191365. [DOI] [PubMed] [Google Scholar]
- 24.Rodesch G., Hui F., Alvarez H., Tanaka A., Lasjaunias P. Prognosis of antenatally diagnosed vein of Galen aneurysmal malformations. Childs Nerv Syst. 1994;10(2):79–83. doi: 10.1007/BF00302765. [DOI] [PubMed] [Google Scholar]
- 25.Smith A., Abruzzo T., Mahmoud M. Vein of galen malformation and high-output cardiac failure. Anesthesiology. 2016;125(3) doi: 10.1097/ALN.0000000000001095. [DOI] [PubMed] [Google Scholar]
- 26.Deloison B., Chalouhi G.E., Sonigo P., et al. Hidden mortality of prenatally diagnosed vein of Galen aneurysmal malformation: retrospective study and review of the literature. Ultrasound Obstet Gynecol. 2012;40(6):652–658. doi: 10.1002/uog.11188. [DOI] [PubMed] [Google Scholar]
- 27.Issa R., Barakat A., Salman R., Naffaa L. Vein of galen malformation, a cause of intracranial calcification: case report and review of literature. J Radio Case Rep. 2019;13(3):13–18. doi: 10.3941/jrcr.v13i3.3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gold A., Ranshoff J., Carter S. Vein of Galen malformations. Acta Neurol Scand Suppl. 1964;40(SUPPL 11):1–31. [PubMed] [Google Scholar]
- 29.Dören M., Tercanli S., Holzgreve W. Prenatal sonographic diagnosis of a vein of Galen aneurysm: relevance of associated malformations for timing and mode of delivery. Ultrasound Obstet Gynecol. 1995;6(4):287–289. doi: 10.1046/j.1469-0705.1995.06040287.x. [DOI] [PubMed] [Google Scholar]
- 30.Jones B.V., Ball W.S., Tomsick T.A., Millard J., Crone K.R. Vein of Galen aneurysmal malformation: diagnosis and treatment of 13 children with extended clinical follow-up. AJNR Am J Neuroradiol. 2002;23(10):1717–1724. [PMC free article] [PubMed] [Google Scholar]
- 31.Halbach V.V., Dowd C.F., Higashida R.T., Balousek P.A., Ciricillo S.F., Edwards M.S.B. Endovascular treatment of mural-type vein of Galen malformations. J Neurosurg. 1998;89(1):74–80. doi: 10.3171/jns.1998.89.1.0074. [DOI] [PubMed] [Google Scholar]
- 32.Mitchell P.J., Rosenfeld J.V., Dargaville P., et al. Endovascular management of vein of Galen aneurysmal malformations presenting in the neonatal period. AJNR Am J Neuroradiol. 2001;22(7):1403–1409. [PMC free article] [PubMed] [Google Scholar]
- 33.Casasco A., Lylyk P., Hodes J.E., Kohan G., Aymard A., Merland J.J. Percutaneous transvenous catheterization and embolization of vein of galen aneurysms. Neurosurg Publ Online. February 1991:260. doi: 10.1097/00006123-199102000-00013. [DOI] [PubMed] [Google Scholar]
- 34.Garcia-Monaco R., De Victor D., Mann C., Hannedouche A., Terbrugge K., Lasjaunias P. Congestive cardiac manifestations from cerebrocranial arteriovenous shunts. Childs Nerv Syst. 1991;7(1):48–52. doi: 10.1007/BF00263834. [DOI] [PubMed] [Google Scholar]
- 35.Dowd C.F., Halbach V.V., Barnwell S.L., Higashida R.T., Edwards M.S., Hieshima G.B. Transfemoral venous embolization of vein of Galen malformations. AJNR Am J Neuroradiol. 1990;11(4):643–648. [PMC free article] [PubMed] [Google Scholar]
- 36.Akanni D., Savi De Tove K.M., Yekpe-Ahouansou P., Biaou O., Boco V. Vein of Galen aneurysmal malformation in a Neonate: a case report. Mali Med. 2018;33(2):23–25. [PubMed] [Google Scholar]
- 37.Lasjaunias P.L., Alvarez H., Rodesch G., et al. Aneurysmal malformations of the vein of galen. Inter Neuroradiol. 1996;2(1):15–26. doi: 10.1177/159101999600200102. [DOI] [PubMed] [Google Scholar]