Abstract
The process of measles virus (MV) assembly and subsequent budding is thought to occur in localized regions of the plasma membrane, to favor specific incorporation of viral components, and to facilitate the exclusion of host proteins. We demonstrate that during the course of virus replication, a significant proportion of MV structural proteins were selectively enriched in the detergent-resistant glycosphingolipids and cholesterol-rich membranes (rafts). Isolated rafts could infect the cell through a membrane fusion step and thus contained all of the components required to create a functional virion. However, they could be distinguished from the mature virions with regards to density and Triton X-100 resistance behavior. We further show that raft localization of the viral internal nucleoprotein and matrix protein was independent of the envelope glycoproteins, indicating that raft membranes could provide a platform for MV assembly. Finally, at least part of the raft MV components were included in the viral particle during the budding process. Taken together, these results strongly suggest a role for raft membranes in the processes of MV assembly and budding.
The Mononegavirales Measles virus (MV) is responsible for an acute respiratory disease and causes the death of over one-million children each year, mainly in the third world (28). The principal cause of mortality is thought to result from virus-induced immunosuppression of lymphocyte function, which allows secondary infections (2, 10). A rare persistent infection of the central nervous system causes a subacute sclerosis panencephalitis (SSPE) (3). The MV RNA negative-strand genome codes for six structural proteins: nucleoprotein N, phosphoprotein P, RNA polymerase L, hemagglutinin H, fusion protein F, and matrix protein M (18). The F protein is synthesized as an inactive precursor (F0) that is cleaved by a host cell proteolytic enzyme to form the fusion-competent protein consisting of the disulfide-linked subunits F1 and F2 (18). The P cistron also encodes three nonstructural proteins—C, V, and R (18, 21)—whose functions remain poorly defined. The N, P, and L proteins, in association with the RNA genome, form the transcriptional and replicative unit or ribonucleoparticle. The ribonucleoparticle is packaged into an envelope protein complex composed of the two integral membrane glycoproteins H and F and the inner-membrane-associated M protein. H mediates virus-cell attachment by binding to CD46 receptor on human cells (11, 30), while both H and F are required for virus-host cell membrane fusion (44). After MV transcription and replication, electron microscopic studies suggest that the ribonucleoparticle assembly occurs in the cytoplasm and is then directed to the plasma membrane, where it can contact the envelope protein complex (13, 29). A current model suggests an organizer role for the M protein, which lines the inner surface of the host cell plasma membrane, from which the MV lipid envelope will be derived during the budding process. The M protein appears to act by concentrating the F and H proteins, as well as the ribonucleoparticle, at the sites of virus assembly (8). Thus, final MV assembly would occur at the plasma membrane just prior to the virus budding. Alteration of MV assembly, including abrogation of M protein function, is likely responsible for MV-associated SSPE (8).
Influenza virus has recently been shown to select specialized glycosphingolipids and cholesterol-rich membrane domains during budding (35). These lipid domains were originally characterized by their insolubility in nonionic detergents such as Triton X-100 (TX-100) (6). Although, their existence in vivo has been debated, recent reports favor the concept of the detergent-resistant membrane domains in living cells (15, 42). Glycosphingolipids would preferentially self-associate and associate with cholesterol (17) to constitute membrane domains as a liquid-ordered environment which confers cold nonionic detergent resistance (1, 37). Such nonsolubilized membranes can be easily isolated from low-density fractions after flotation in a sucrose gradient (6). Different acronyms have been attached to these membrane domains, including detergent-insoluble glycolipid-enriched structures, detergent-resistant membranes, or detergent-insoluble lipid rafts (38). Caveolae, which are morphologically distinct invaginations characterized by the presence of the scaffold protein caveolin, would appear to be a subclass within the raft membranes (17). Rafts can incorporate specific proteins, among which are many glycophosphatidylinositol (GPI)-anchored proteins, and function as platforms for intracellular sorting and signal transduction events (5, 38). More recently, a role for lipid rafts in T-cell activation has been demonstrated (26, 27, 43, 45, 47). Lipidation with saturated acyl chains of many of the raft-associated proteins would participate in their preferential raft localization (25).
The present study was undertaken to explore whether MV proteins can associate with rafts.
MATERIALS AND METHODS
Cells and virus.
The human B-lymphoblastoid cell line BJAB was grown in RPMI 1640 supplemented with 10% fetal calf serum (FCS), 50 μg of gentamicin per ml, and 5 mM glutamine. Recombinant MV Tag (derived from the Edmonston strain [32]) and chimeric MGV in which the H and F cistrons have been replaced by a cistron encoding the vesicular stomatitis virus (VSV) G protein (39) were kindly provided by M. Billeter. Supernatant from infected Vero cells was used as the stock virus. BJAB cells were infected at a multiplicity of infection (MOI) of 1 50% tissue culture infective dose (TCID50)/cell for 1 h at 37°C. Unadsorbed virus was washed out once with fresh medium, and the cells were incubated for 20 h at 37°C in a 7% CO2 incubator. Under these conditions, more than 80% of the cells were infected, as indicated by H and F cell surface expression and as detected by flow cytometry analyses (FACScan; Becton Dickinson). BJAB cell-associated virus was recovered by one freezing-thawing cycle of extensively washed cell pellet, followed by centrifugation at 400 × g to remove cell debris. Cell-free viruses were isolated from supernatants of 10 × 106 infected BJAB cells. Supernatants were first clarified by centrifugagtion at 7,000 × g for 20 min and then layered on a cushion of 2 ml each of 30 and 50% sucrose in phosphate-buffered saline (PBS). After centrifugation at 200,000 × g for 2 h, the sucrose interface (∼1 ml) containing MV particles was harvested. The titers of BJAB cell-free or cell-associated viruses were determined by the TCID50 method on a Vero cell monolayer.
Reagents.
Antibodies used in this study include anti-CD46 (Immunotech, Marseille, France), anti-CD55 (a generous gift from B. Loveland, ARI, Melbourne, Australia), anti-CD29 (Transduction Laboratories, Lexington, Ky.), anti-MV-H (14), anti-MV-F (19), anti-MV-M (Chemicon, Temecula, Calif.), anti-MV-N (16), anti-MV-V (22), and anti-VSV-G (Sigma). Peroxidase-coupled secondary antibodies were from Promega (Madison, Wis.). Peroxidase-coupled cholera toxin β subunit, peroxidase-coupled streptavidin, octyl-glucoside, methyl-β-cyclodextrin, and protein G-Sepharose were from Sigma. Sulfo-NHS-LC-Biotin was from Pierce.
Surface biotinylation, radiolabelling, and methyl-β-cyclodextrin extractions.
A total of 107 BJAB cells infected by MV for 20 h were washed twice with ice-cold PBS and biotinylated for 20 min with 0.5 mg/ml of Sulfo-NHS-LC-Biotin in PBS at 4°C. Cells were then washed with ice-cold PBS, and an excess of Sulfo-NHS-LC-Biotin was quenched with 0.5% bovine serum albumin and 0.1 M glycine in PBS. Cells were subsequently extracted. For radiolabelling, the cells were washed with PBS at 20 h after infection, starved with cysteine- and methionine-free Dulbecco modified Eagle medium (DMEM) for 1 h, and pulse-labeled for 20 min in the same medium containing 100 μCi of Trans35S-Label (ICN Pharmaceuticals, Inc., Irvine, Calif.) per ml. The cells were then washed once with warm PBS and chased in warmed DMEM containing 10% FCS for the indicated times. The chase was terminated by washing the cells in ice-cold PBS, followed by detergent extraction. Methyl-β-cyclodextrin (5 mM) treatment was performed on 107 BJAB cells in serum-free medium containing 50 mM HEPES for 30 min at 37°C with gentle agitation. Cells were then washed twice in PBS and detergent extracted.
Detergent extraction and flotation assay.
Infected cells (107) were lysed in 0.2 ml of ice-cold TNE buffer (25 mM Tris-HCl, pH 7.5; 150 mM NaCl; 5 mM EDTA) containing 1% TX-100 plus a cocktail of protease inhibitors (Complete; Boehringer Mannheim). The ratio of lysis buffer volume to cell number was kept constant throughout the experiments. In some cases, 1% octyl-glucoside was substituted for TX-100 as specified. After a 30-min incubation on ice, the preparation was made 40% with respect to sucrose. Then, 0.8 ml of lysate-sucrose mixture was sequentially overlaid with 2 ml of 30% sucrose and 1 ml of 4% sucrose prepared in TNE, and the mixture was centrifuged at 200,000 × g for 14 to 16 h in an SW50.1 rotor (Beckman). The gradient was fractionated into 0.42-ml fractions from the top of the tube. The pellet at the bottom of the tube was rinsed twice with ice-cold TNE and resuspended in 0.42 ml of sodium dodecyl sulfate (SDS) sample buffer. The protein content of the different fractions was determined as previously described (31), and the sucrose content was determined by using a refractometer (Sopelem).
Immunoprecipitation and immunoblot analyses.
Sucrose fractions were diluted in TNE containing 1% octyl-glucoside (final sucrose concentration, <8%) and incubated on a rocker at 4°C with 10 μg of irrelevant antibodies per ml preabsorbed on protein G-coupled Sepharose beads for 2 h. These precleared sucrose fractions were immunoprecipitated on a rocker at 4°C by adding approximately 10 μg of specific antibodies per ml for 1 h, followed by an hour of incubation with protein G-coupled Sepharose beads. Immunoprecipitates were washed five times with TNE containing 1% octyl-glucoside and dissolved in SDS sample buffer. After SDS-polyacrylamide gel electrophoresis (PAGE), the proteins were transferred onto polyvinylidene difluoride membranes (Boehringer Mannheim). Blots were then incubated with specific antibodies, followed by the appropriate horseradish peroxidase (HRP)-coupled secondary antibodies. GM1, which migrated with the dye front (45), was labeled by reaction with peroxidase-coupled cholera toxin β subunit. Protein and GM1 detection was performed by using the enhanced chemiluminescence (Amersham) system. Quantification of the autoradiograms was performed by using National Institutes of Health Image 1.61 software. When radiolabelled, the proteins were detected by using a PhosphorImager (Molecular Dynamics), and quantification was performed by using ImageQuant software (Molecular Dynamics).
RESULTS
Association of MV proteins with rafts in infected cells.
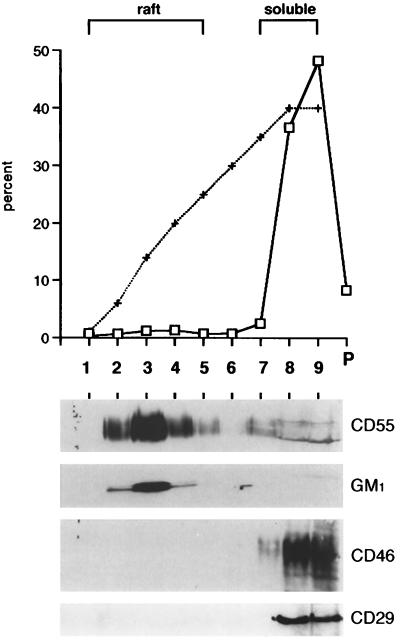
Raft membranes were isolated from MV-infected BJAB cells by using a flotation assay based on resistance to solubilization by TX-100 at 4°C and buoyancy at low-density fractions of a bottom-loaded discontinuous sucrose gradient, with steps of 5, 30, and 40% sucrose. The graph shown in Fig. 1 shows that ∼87% of the proteins remained within the 35 to 40% sucrose region of the gradient, i.e., fractions 7 to 9, referred to as the soluble fractions. These included the MV receptor CD46 and the β1 integrin subunit CD29 (Fig. 1, lower panel). On the contrary, most of the GPI-anchored CD55 proteins migrated to fraction 3 (the ca. 15 to 20% sucrose region) as expected from a resident of rafts. Similarly, the glycosphingolipid GM1, another resident of rafts, was partitioned into fractions identical to those of CD55. Therefore, rafts were mostly recovered in fractions 2 to 5, referred to as the raft fractions. Approximately 8% of the total proteins, which include some insoluble cytoskeleton components and nuclear remnants, were recovered in a pellet at the bottom of the tube (Fig. 1, P).
FIG. 1.
Isolation of raft membranes from MV-infected BJAB cells. Bottom-loaded sucrose step gradient fractions (fraction 1 represents the top of the gradient) were analyzed for total protein content (□) or sucrose (+) content. The protein concentration was also determined in the insoluble pellet (P). Immunoblots of proteins from each fraction (equal volume loaded) were labelled with anti-CD55, anti-CD46, or anti-CD29 antibodies as described in Materials and Methods. GM1, which migrated with the dye front, was detected by reaction with HRP-coupled cholera toxin.
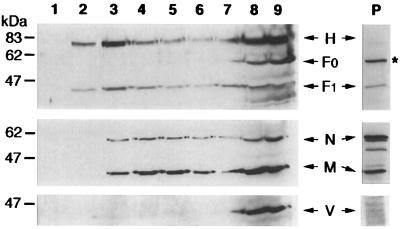
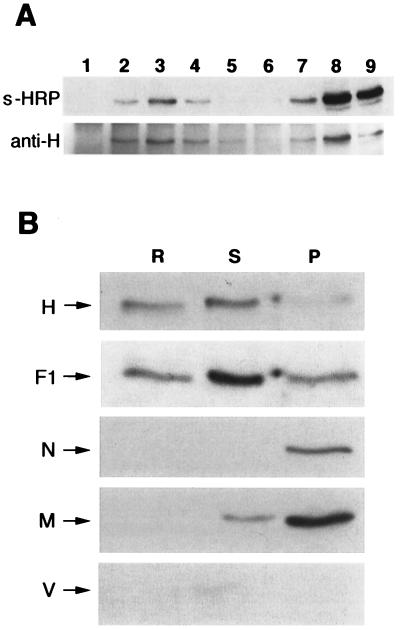
Figure 2 shows that a significant proportion (ranging from ca. 15 to 40% in independent experiments) of H and F1 transmembrane viral proteins colocalize with the raft fractions. Fewer than 1% of the H proteins and 5% of the F proteins were recovered in the insoluble pellet (P). The precursor F0, which is cleaved in the trans-Golgi network to form the disulfide-linked subunits F1 and F2 (4), was recovered from the soluble fractions but was absent from both the raft fractions and the insoluble pellet. The prominent 60-kDa band detected in the insoluble pellet is nonspecifically labelled since it was recognized by the secondary HRP-conjugated antibodies (not shown). We then investigated the presence of M, N, and V intracellular proteins in the different fractions. Approximately 20% of the M proteins and 50% of the N proteins were recovered in the insoluble pellet, likely reflecting, at least in part, the sedimentation through the 40% sucrose of the free ribonucleoparticle and some associated M protein (density of ∼1.3 [40]). The nature of the minor bands migrating between M and N is unknown. However, ∼35 and ∼25% of the total M and N, respectively, were predominantly detected in the raft fractions. In contrast, the nonstructural V protein was essentially recovered from the soluble fractions. Similar flotation behavior for MV proteins was also observed in infected peripheral blood leukocytes, as well as in Chinese hamster ovary cells expressing the CD46 cellular receptor (not shown).
FIG. 2.
Compartmentation of MV proteins into the raft fractions. The distribution of MV proteins into the sucrose gradient fractions and the insoluble pellet (P) was assayed by immunoblotting with specific antibodies. The positions of the H, F1, F0, N, M, and V viral proteins are indicated. The migration positions of size markers are shown to the left of the figure. The asterisk indicates the nonspecifically labelled protein in the insoluble pellet.
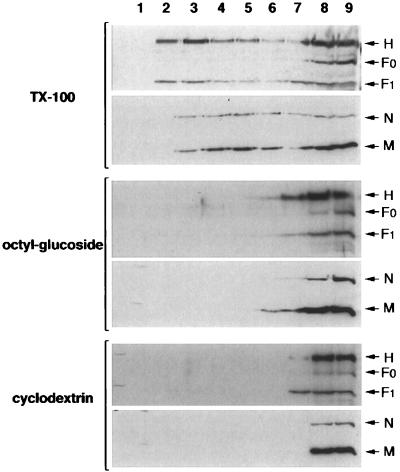
The detergent octyl-glucoside is capable of solubilizing TX-100-insoluble GPI-anchored proteins (6). When octyl-glucoside was substituted for TX-100 during cell extraction, the buoyancy of H, F1, M, and N viral proteins (Fig. 3), as well as the buoyancy of CD55 (not shown), was effectively eliminated. Selective extraction of cholesterol from plasma membrane by cyclodextrin has been shown to abolish the resistance to TX-100 solubilization of raft-associated proteins (46, 36). Treatment of MV-infected BJAB cells with cyclodextrin, prior to TX-100 extraction, greatly reduced the flotation of viral proteins to low-density fractions (Fig. 3), indicating that the buoyancy of H, F1, M, and N proteins is cholesterol dependent. Taken together, these data indicate that, in infected cells, part of the H, F1, M, and N proteins associates with complexes that satisfy raft criteria.
FIG. 3.
Association of MV proteins with rafts is impaired by octyl-glucoside detergent extraction or methyl-β-cyclodextrin pretreatment. MV-infected BJAB cells were extracted with 1% TX-100 or 1% octyl-glucoside or else exposed to 5 mM methyl-β-cyclodextrin before the TX-100 extraction. Sucrose gradient fractions from the three different conditions were performed as indicated, and the distribution of MV proteins in each fraction was determined by immunoblotting. The positions of the H, F1, F0, N, and M proteins are indicated on the right of the figure.
Kinetics of raft attachment and cell surface expression of MV proteins.
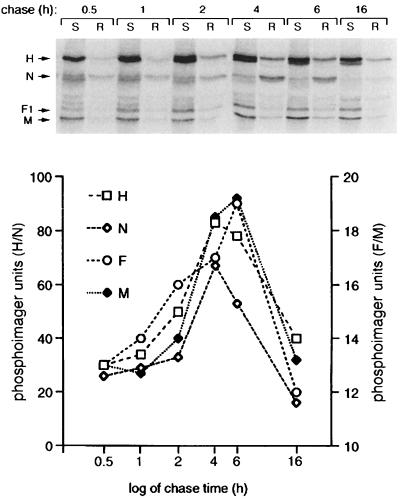
To further characterize the raft attachment of MV proteins, pooled raft or soluble fractions from [35S]methionine[35S]cysteine pulse-chased MV-infected BJAB cells were immunoprecipitated with anti-H, -F, -M, and -N antibodies in a time course experiment. Quantification analysis was performed with a phosphorimager. The fine resolution of the protein bands in this assay showed that, as observed with immunoblotting of F proteins, it was predominantly the mature form of H (highest-migrating band due to full glycosylation) that associated with rafts (Fig. 4, gel). Maximal attachment of viral proteins to rafts occurred within 4 to 6 h of synthesis and declined thereafter (Fig. 4, graph). At 4 h postlabelling, the raft fraction contained, within the sucrose gradient, 17, 66, 28, and 22% of the neosynthesized H, N, F1, and M proteins, respectively.
FIG. 4.
Kinetics of raft attachment of MV proteins. At 20 h after infection, BJAB cells were pulse-labeled for 20 min in the presence of Trans35S-Label and chased for the indicated times. Pooled raft (R) or soluble (S) fractions from sucrose gradients of each time point were immunoprecipitated with anti-H, -F, -M, and -N antibodies; subjected to SDS-PAGE; and analyzed with a phosphorimager. The positions of the viral proteins are shown on the left of the gel. The graph shows the quantification of the viral proteins in the raft fractions, with the scale for H and N proteins displayed on the left of the graph and the scale for F and M displayed on the right of the graph.
We next analyzed whether raft association of H and F transmembrane proteins persists after cell surface expression. Immunoprecipitation with the anti-H antibodies of each sucrose fraction from biotin-labelled MV-infected cells indicated that ∼10% of the biotinylated (i.e., cell-surface-expressed) H protein was raft associated (Fig. 5A). The specificity of cell surface protein labelling was confirmed by the absence of biotin labelling of the intracellular N protein immunoprecipitated from the same samples (not shown). Probing of the membrane with anti-H antibodies revealed that, in this experiment, raft fractions contained ∼40% of total immunoprecipitated H proteins (Fig. 5A). Therefore, the majority of the raft-associated H proteins are intracellular, suggesting either that they dissociate from the rafts once they reach the cell surface or that they are released from the cells.
FIG. 5.
Raft-associated MV proteins are present at the plasma membrane and in the envelope of the mature virion. (A) Surface-biotinylated proteins of BJAB cells infected for 20 h with MV were subjected to the flotation assay before immunoprecipitation with anti-H antibody. The immunoprecipitates were resolved by SDS-PAGE and blotted by using peroxidase-coupled streptavidin (s-HRP) to reveal the surface-biotinylated proteins or else blotted by using anti-H antibody (anti-H) to reveal the total cell-associated H proteins. (B) Virus released from infected BJAB was purified as described in Materials and Methods. After extraction by addition of cold TX-100 and flotation assay, pooled raft (R) or soluble (S) fractions, as well as the pellet (P), were analyzed for viral protein content by immunoblotting. The positions of the H, F1, N, M, and V proteins are indicated.
Raft membranes are included in the MV envelope.
MV obtains its lipid envelope from the host cell plasma membrane during the budding process. To investigate whether cell surface raft-associated MV proteins contributed to the MV envelope during budding, released viruses from infected BJAB were isolated and subjected to flotation assay after extraction with cold TX-100. Pooled raft or soluble fractions, as well as the pellet, were then analyzed for viral protein content (Fig. 5B). Approximately 30% of the H and 10% of the F1 glycoproteins were associated with raft membranes within the virus envelope. Thus, at least part of the raft-attached H and F1 proteins in infected cells have been included in the MV envelope during budding. In contrast to the results obtained from infected cells, up to 10% of the F1 protein and the majority of the N and M proteins were now recovered in the insoluble pellet. The nonstructural V protein is not incorporated in the virus particles and therefore was not detected in any of the fractions.
Raft attachment of M and N proteins does not depend on the presence of H and F proteins.
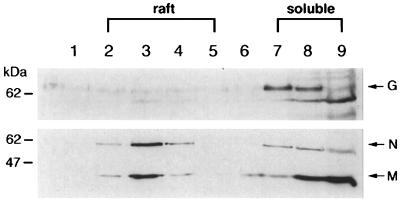
The study of Cathomen et al. strongly suggested an interaction between the cytoplasmic domain of the F protein and the intracellular M protein (9). To investigate whether such an interaction is required to drive the attachment of the M protein to raft membranes, we took advantage of a recombinant chimeric MV (MGV), in which the H and F glycoproteins were substituted by the VSV G glycoprotein (39). The G protein has been shown not to associate with rafts (6). Western blot analysis of chimeric MGV-infected cells (Fig. 6) verified that the G protein remained in the soluble fractions. However, M and N proteins were still able to migrate toward the low-density fractions of the sucrose gradient. These data demonstrate that M and N proteins can attach to rafts independently of an interaction with the cytoplamic tails of H and/or F proteins.
FIG. 6.
Viral internal M and N proteins associate with rafts independently of the presence of H and F transmembrane proteins. BJAB cells were infected for 20 h with chimeric MGV virus and were subjected to the flotation assay. Immunoblots of proteins from sucrose fractions were labeled with either anti-VSV-G, anti-MV-N, or anti-MV-M antibodies. The positions of the viral proteins are indicated on the right of the figure, and the migration positions of size markers are shown to the left of the figure.
Raft fractions from infected cells contain infectious material distinct from mature viral particles.
The results obtained above raise the possibility that some virus assembly could occur in the raft membrane subcompartment. Electron microscopy analysis of raft membranes isolated by flotation assay has revealed a population of vesicles more heterogeneous in size and larger than intact intracellular transport vesicles (6). For this reason, it is believed that a vesiculation step of raft membranes occurs either during cell lysis or during sucrose gradient flotation. We speculated that if the remainder of the viral particle components (i.e., L and P proteins and the RNA genome) were also present in the raft fractions, the vesiculation step might have created some infectious raft-derived virus-like particles.
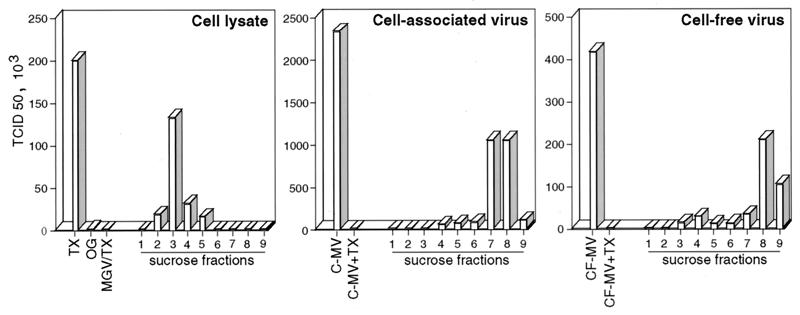
The cell lysate panel in Fig. 7 shows that the TX-100 lysate of infected BJAB cells (TX) contained a significant amount of infectious material. This infectious material was partitioned into the low-density raft fractions and was abolished when octyl-glucoside was substituted for TX-100 during cell extraction (OG). In addition, this infectious material originated from the plasma since the raft-associated infectivity was abolished by pronase treatment of the cells prior to lysis and raft extraction (not shown). It was verified that, under the conditions used (0.8 mg of pronase per ml for 20 min at 37°C), H and F were effectively released from the cell surface, whereas the quantity of the F0 precursor, which is mainly intracellular, was not affected by the treatment. Because the majority of MV infectivity remains closely associated with infected cells and can be recovered by one freezing-thawing cycle (41), the so-called cell-associated virus was also analyzed. The titers of the raft-associated material ranged between 8 and 50%, in independent experiments, of what can be recovered from the cell-associated virus harvested from a duplicate culture. In the cell-associated virus panel of Fig. 7, it can be seen that in the absence of TX-100 treatment more than 85% of the cell-associated virus infectivity (C-MV) was partitioned within the high-density fraction. In addition, TX-100 treatment of the cell-associated virus (C-MV+TX) abolished its infectivity. Therefore, the raft-associated infectious material could be distinguished from the cell-associated virus with regard to TX-100 resistance and density behavior. It can similarly be distinguished from the virus released from the cells (Fig. 7, cell-free virus panel). In order to conserve the membrane/detergent ratio of TX-100-treated infected cells, the viruses were mixed with noninfected cells prior to TX-100 treatment, but again their infectivities were abolished (not shown). The final concentration of TX-100 in all of the plaque assays was identical and cannot account for the observed differences. Infection of Vero cells with the raft-associated infectious material might result from the nonspecific delivery of infectious material, i.e., the ribonucleoparticle, into the cells. However, the TX-100 lysate of MGV-infected cells is not associated with some infectious material (Fig. 7, MGV/TX in the cell lysate panel), although M and N proteins and, presumably, the ribonucleoparticle are associated with the raft fractions (Fig. 6). In addition, preincubation of raft fractions with neutralizing anti-H or anti-F antibodies prior to the titration test prevented their infectivity (not shown). This indicates that raft-associated infectious material requires H and F engagement to infect Vero cells. Taken together, these results suggest that some functional virus assembly occurs in the raft membrane.
FIG. 7.
Raft fractions contain infectious material distinct from mature viral particles. Cell lysate, cell-associated virus, or cell-free virus was prepared from BJAB cells infected for 20 h, as described in Materials and Methods. Cell lysate panel: TX, TX-100 extraction; OG, octyl-glucoside extraction; MGV/TX, MGV-infected cells extracted with TX-100. Cell-associated virus panel: C-MV, cell-associated virus; C-MV+TX, cell-associated virus extracted with TX-100. Cell-free virus panel: CF-MV, cell-free virus; CF-MV+TX, cell-free virus extracted with TX-100. The titers of infectious material present in the total preparation or in the sucrose fractions were determined by the TCID50 method on a Vero cell monolayer.
DISCUSSION
Budding of measles virus is preceded by the assembly of viral components at specific sites of the plasma membrane (13, 29). However, the precise factors determining localization of viral assembly are not known. The data presented here strongly suggest that lipid raft microdomains provide a cellular location for measles virus assembly in infected cells.
Selective association of MV proteins with rafts.
In infected cells, MV proteins attach to low-density detergent-insoluble complexes that are disrupted by octyl-glucoside or cyclodextrin (see Fig. 3) or by TX-100 solubilization at 37°C (not shown), thereby satisfying the biochemical criteria of rafts. We found that although raft membranes account for less than 5% of the total cellular proteins, they contain a significant proportion of total cell-associated structural viral proteins. The specificity of this enrichment is reinforced by the observations that (i) the mature F (F1 plus F2) protein, but not its F0 precursor, is associated with rafts; (ii) the nonstructural V protein remains excluded from raft membranes; and (iii) in cells infected with the chimeric virus MGV, in which MV H and F glycoproteins were substituted by the VSV G glycoprotein, both M and N proteins attach to raft membranes, whereas G protein does not.
Raft association of MV glycoproteins occurs during Golgi maturation.
H and F proteins are synthesized on membrane-bound ribosomes, mature through the endoplasmic reticulum and the Golgi, and become integral plasma membrane proteins (18). The F protein is synthesized as an inactive precursor (F0) that is cleaved in the trans-Golgi network to form the biologically active protein consisting of the disulfide-linked subunits F1 and F2 (4). The heterogeneity of H proteins, resolved as a cohort of discrete bands (Fig. 4A), has been shown to reflect the processing pathway from high-mannose-type to complex-type carbohydrate chains, the latter mature form migrating toward higher molecular weights (20). We observed that the mature forms of both the H and F proteins were preferentially incorporated into rafts, indicating that they become resistant to TX-100 extraction after transport to the Golgi complex. This Golgi-located raft incorporation has been described for many transmembrane raft-associated proteins, including the influenza virus HA protein and GPI-anchored proteins, and it has been proposed that raft assembly occurs in this cell compartment (38). Recent studies indicated that the structural basis for the association of influenza HA with rafts resides in its transmembrane segment (36), although palmitoylation of the membrane-proximal cysteine residues in the cytoplasmic tail might also play a role (25). We found that, like influenza virus HA, in the absence of any other viral proteins, the F glycoprotein, which has also palmitoylated membrane-proximal cysteine residues (7), possesses the intrinsic property to attach to rafts (S. Manié, unpublished observation). However, unlike HA, only ∼50% of transfected F localized with raft membranes, recapitulating what was observed in cells infected with MV. Work is in progress in our laboratory to define the potential raft attachment domain of MV transmembrane proteins.
Internal M and N proteins associate with rafts independently of the envelope glycoproteins.
M and N intracellular proteins are synthesized on free cytoplasmic ribosomes (18). The M protein is known to associate with cellular membranes even in the absence of proteins H and F, presumably through hydrophobic bonding (9). The M protein could also interact with the cytoplasmic tail of F protein (9), which thus could be responsible for the attachment of M protein to rafts. However, the substitution of H and F proteins by the G protein of VSV (which is unable to associate with rafts) revealed that the targeting of M and N to rafts is independent of the presence of H and F. Acylation by saturated chains, as in Src family kinases, is believed to drive preferential partitioning into rafts of plasma membrane-associated intracellular proteins (33, 25). Although M can attach to membranes and bind to N, which in turn can bind to P and L (18), L, M, N, and P proteins are not known to be acylated. Therefore, the mechanism(s) that drives raft attachment of MV intracellular proteins would appear to be different.
Is the association of MV proteins with rafts a regulated process?
The finding that only 20 to 40% of MV proteins in infected cells localize to rafts could reflect a saturation by MV proteins of the raft membranes. Despite the fact that the transmembrane proteins and the intracellular proteins are synthesized in different subcellular compartments, similar kinetics of raft association were observed. One could speculate that this reflects a regulated and/or a synchronized process. In support of this, we found that the maximal radioactivity associated with the labelled pool of F1 proteins in the soluble fractions was recovered between 2 and 4 h after the chase (see the gel in Fig. 4A), whereas the maximal raft attachment occurred between 4 and 6 h after the chase. This 2-h lag suggests that the localization of MV proteins into raft membranes is somehow regulated rather than a random process.
Raft microdomains might provide a cellular location for MV assembly.
MV budding at the plasma membrane, as defined by electronic microscopy studies (29, 12, 13), requires an accumulation of the viral components at specific sites, leading to patches of tightly packed material. During the packing step, the ribonucleoparticle remains closely associated with the membrane, and this attachment disappears as soon as viral particles are released from the cell (13). Although the M protein is likely to play an important role by coordinating the interactions of the viral components at the internal cell membranes (8), the precise factors determining localization of viral assembly remain unknown. In that context, independently raft-targeted viral proteins would provide a means for a selective enrichment in a membrane subcompartment and consequently facilitate viral protein-protein interactions.
Some infectious units, which can be distinguished from mature viral particles with regard to TX-100 resistance and density behavior, were recovered from the raft fractions. Pronase treatment of intact cells prior to cell lysis abolished the raft-associated infectivity, indicating that these infectious units were derived from the plasma membrane. In addition, the raft-associated infectious material was dependent upon the engagement of both H and F glycoproteins, i.e., requiring a fusion step between the infectious material membrane and the target cellular membrane, ruling out the nonspecific delivery of free ribonucleoparticle into the cells. In support of this, rafts isolated from MGV-infected cells were not infectious, although they contained the M and N proteins, and thus presumably the ribonucleoparticle, but no longer the envelope G protein (Fig. 6). It is reasonable to assume that the vesiculation step occurring during the raft isolation procedure (6) had generated some vesicles containing H and F proteins and the ribonucleoparticle. Therefore, all of the components required to create a functional virion are present in the raft fractions. We propose that the raft-associated infectious material represents some step of the virus assembly. In support of this is the finding that the virus envelope includes some H- and F-raft-associated proteins, indicating that MV-raft-located proteins contribute to the generation of mature virus. In contrast to the results obtained from isolated rafts, the majority of N and M proteins extracted from the mature virion are recovered in the insoluble pellet. This finding might reflect the fact that the ribonucleoparticle loses its membrane attachment once budding has occurred (13). Not all H and F proteins are associated with rafts in the mature virus released from the cells. Consequently, the integrity of the virion envelope, and therefore its infectivity, was destroyed by cold TX-100 treatment. If it is assumed that the lipid composition of the virion envelope reflects that of the membrane where the budding took place, these results would indicate that MV budding did not occur solely from the raft membranes. The VSV G protein did not associate with rafts (see Fig. 6 and reference 6), and the chimeric MGV can still produce infectious virions in Vero cells, although less efficiently (39). However, comparison of these two viruses with regard to the budding mechanisms is limited because (i) MGV virions did not contain the M protein thought to play a central role in MV assembly and budding (39) and (ii) VSV or rabies virus G proteins are endowed with the intrinsic ability to autonomously form budding vesicles, pinching off from membranes of G-expressing cells (34, 24).
An attractive possibility is that localization of MV components in raft membranes represents a necessary, but intermediary, step during virus assembly. Interestingly, MV budding has been reported to occur preferentially from the apical side of polarized MDCK cells (23), in which rafts can act as an apical sorting device (38). Obviously, an important issue is to evaluate the quantitative contribution of the rafts in the production of mature virions. Our attempts to reduce the cellular cholesterol concentration in order to affect the integrity of the raft lipids, without affecting the cellular viability necessary for virus replication, have proven to be difficult so far. Definitive proof for our proposal thus relies on the ability of being able to interfere with MV protein attachment to rafts. While this work was in progress, Scheiffele et al. published a study showing that the influenza virus selects raft membranes during budding from the plasma membrane (35). Therefore, raft lipid domains might also be involved in influenza virus assembly and subsequent budding.
Regardless of whether raft domains contribute significantly to MV particle production or not, the identification of a subcellular compartment enriched in functional MV particle components may offer new means to analyze the molecular mechanisms of viral protein interactions underlying virus assembly and budding.
ACKNOWLEDGMENTS
This work was supported in part by a grant from the Ministère de l'Education Nationale de la Recherche et de la Technologie (grant PRFMMIP).
We would like to thank M. Billeter, R. Cattaneo, B. Loveland, H. Y. Naim, and C. Muller for providing reagents; L. Roux for stimulating discussions; D. Christiansen for critical reading of the manuscript; and the members of our lab for advice and criticism.
REFERENCES
- 1.Ahmed S N, Brown D A, London E. On the origin of sphingolipid/cholesterol-rich detergent-insoluble cell membranes: physiological concentrations of cholesterol and sphingolipid induce formation of a detergent-insoluble, liquid-ordered lipid phase in model membranes. Biochemistry. 1997;36:10944–10953. doi: 10.1021/bi971167g. [DOI] [PubMed] [Google Scholar]
- 2.Beckford A P, Kaschula R O, Stephen C. Factors associated with fatal cases of measles. A retrospective autopsy study. S Afr Med J. 1985;68:858–863. [PubMed] [Google Scholar]
- 3.Billeter M A, Cattaneo R, Spielhofer P, Kaelin K, Huber M, Schmid A, Baczko K, ter Meulen V. Generation and properties of measles virus mutations typically associated with subacute sclerosing panencephalitis. Ann N Y Acad Sci. 1994;724:367–377. doi: 10.1111/j.1749-6632.1994.tb38934.x. [DOI] [PubMed] [Google Scholar]
- 4.Bolt G, Pedersen I R. The role of subtilisin-like proprotein convertases for cleavage of the measles virus fusion glycoprotein in different cell types. Virology. 1998;252:387–398. doi: 10.1006/viro.1998.9464. [DOI] [PubMed] [Google Scholar]
- 5.Brown D A, London E. Functions of lipid rafts in biological membranes. Annu Rev Cell Dev Biol. 1998;14:111–136. doi: 10.1146/annurev.cellbio.14.1.111. [DOI] [PubMed] [Google Scholar]
- 6.Brown D A, Rose J K. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell. 1992;68:533–544. doi: 10.1016/0092-8674(92)90189-j. [DOI] [PubMed] [Google Scholar]
- 7.Caballero M, Carabana J, Ortego J, Fernandez-Munoz R, Celma M L. Measles virus fusion protein is palmitoylated on transmembrane-intracytoplasmic cysteine residues which participate in cell fusion. J Virol. 1998;72:8198–8204. doi: 10.1128/jvi.72.10.8198-8204.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cathomen T, Mrkic B, Spehner D, Drillien R, Naef R, Pavlovic J, Aguzzi A, Billeter M A, Cattaneo R. A matrix-less measles virus is infectious and elicits extensive cell fusion: consequences for propagation in the brain. EMBO J. 1998;17:3899–3908. doi: 10.1093/emboj/17.14.3899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cathomen T, Naim H Y, Cattaneo R. Measles viruses with altered envelope protein cytoplasmic tails gain cell fusion competence. J Virol. 1998;72:1224–1234. doi: 10.1128/jvi.72.2.1224-1234.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coovadia H M, Wesley A, Brain P. Immunological events in acute measles influencing outcome. Arch Dis Child. 1978;53:861–867. doi: 10.1136/adc.53.11.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dörig R E, Marcil A, Chopra A, Richardson C D. The human CD46 molecule is a receptor for measles virus (Edmonston strain) Cell. 1993;75:295–305. doi: 10.1016/0092-8674(93)80071-l. [DOI] [PubMed] [Google Scholar]
- 12.Dubois-Dalcq M, Barbosa L H. Immunoperoxidase stain of measles antigen in tissue culture. J Virol. 1973;12:909–918. doi: 10.1128/jvi.12.4.909-918.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dubois-Dalcq M, Reese T S. Structural changes in the membrane of Vero cells infected with a paramyxovirus. J Cell Biol. 1975;67:551–565. doi: 10.1083/jcb.67.3.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fournier P, Brons N H, Berbers G A, Wiesmuller K H, Fleckenstein B T, Schneider F, Jung G, Muller C P. Antibodies to a new linear site at the topographical or functional interface between the haemagglutinin and fusion proteins protect against measles encephalitis. J Gen Virol. 1997;78:1295–1302. doi: 10.1099/0022-1317-78-6-1295. [DOI] [PubMed] [Google Scholar]
- 15.Friedrichson T, Kurzchalia T V. Microdomains of GPI-anchored proteins in living cells revealed by crosslinking. Nature. 1998;394:802–805. doi: 10.1038/29570. [DOI] [PubMed] [Google Scholar]
- 16.Giraudon P, Gerald C, Wild T F. A study of measles virus antigens in acutely and persistently infected cells using monoclonal antibodies: differences in the accumulation of certain viral proteins. Intervirology. 1984;21:110–120. doi: 10.1159/000149509. [DOI] [PubMed] [Google Scholar]
- 17.Harder T, Simons K. Caveolae, DIGs, and the dynamics of sphingolipid-cholesterol microdomains. Curr Opin Cell Biol. 1997;9:534–542. doi: 10.1016/s0955-0674(97)80030-0. [DOI] [PubMed] [Google Scholar]
- 18.Horikami S M, Moyer S A. Structure, transcription, and replication of measles virus. Curr Top Microbiol Immunol. 1995;191:35–50. doi: 10.1007/978-3-642-78621-1_3. [DOI] [PubMed] [Google Scholar]
- 19.Hu A, Cathomen T, Cattaneo R, Norrby E. Influence of N-linked oligosaccharide chains on the processing, cell surface expression and function of the measles virus fusion protein. J Gen Virol. 1995;76:705–710. doi: 10.1099/0022-1317-76-3-705. [DOI] [PubMed] [Google Scholar]
- 20.Kohama T, Sato T A, Kobune F, Sugiura A. Maturation of measles virus hemagglutinin glycoprotein. Arch Virol. 1985;85:257–268. doi: 10.1007/BF01314235. [DOI] [PubMed] [Google Scholar]
- 21.Liston P, Briedis D J. Ribosomal frameshifting during translation of measles virus P protein mRNA is capable of directing synthesis of a unique protein. J Virol. 1995;69:6742–6750. doi: 10.1128/jvi.69.11.6742-6750.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liston P, DiFlumeri C, Briedis D J. Protein interactions entered into by the measles virus P, V, and C proteins. Virus Res. 1995;38:241–259. doi: 10.1016/0168-1702(95)00067-z. [DOI] [PubMed] [Google Scholar]
- 23.Maisner A, Klenk H, Herrler G. Polarized budding of measles virus is not determined by viral surface glycoproteins. J Virol. 1998;72:5276–5278. doi: 10.1128/jvi.72.6.5276-5278.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mebatsion T, Konig M, Conzelmann K K. Budding of rabies virus particles in the absence of the spike glycoprotein. Cell. 1996;84:941–951. doi: 10.1016/s0092-8674(00)81072-7. [DOI] [PubMed] [Google Scholar]
- 25.Melkonian K A, Ostermeyer A G, Chen J Z, Roth M G, Brown D A. Role of lipid modifications in targeting proteins to detergent-resistant membrane rafts. Many raft proteins are acylated, while few are prenylated. J Biol Chem. 1999;274:3910–3917. doi: 10.1074/jbc.274.6.3910. [DOI] [PubMed] [Google Scholar]
- 26.Montixi C, Langlet C, Bernard A M, Thimonier J, Dubois C, Wurbel M A, Chauvin J P, Pierres M, He H T. Engagement of T cell receptor triggers its recruitment to low-density detergent-insoluble membrane domains. EMBO J. 1998;17:5334–5348. doi: 10.1093/emboj/17.18.5334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moran M, Miceli M C. Engagement of GPI-linked CD48 contributes to TCR signals and cytoskeletal reorganization: a role for lipid rafts in T cell activation. Immunity. 1998;9:787–796. doi: 10.1016/s1074-7613(00)80644-5. [DOI] [PubMed] [Google Scholar]
- 28.Murray C J, Lopez A D. Mortality by cause for eight regions of the world: Global Burden of Disease Study. Lancet. 1997;349:1269–1276. doi: 10.1016/S0140-6736(96)07493-4. [DOI] [PubMed] [Google Scholar]
- 29.Nakai M, Imagawa D T. Electron microscopy of measles virus replication. J Virol. 1969;3:187–197. doi: 10.1128/jvi.3.2.187-197.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Naniche D, Varior-Krishnan G, Cervoni F, Wild T F, Rossi B, Rabourdin-Combe C, Gerlier D. Human membrane cofactor protein (CD46) acts as a cellular receptor for measles virus. J Virol. 1993;67:6025–6032. doi: 10.1128/jvi.67.10.6025-6032.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peterson G L. Determination of total protein. Methods Enzymol. 1983;91:95–119. doi: 10.1016/s0076-6879(83)91014-5. [DOI] [PubMed] [Google Scholar]
- 32.Radecke F, Spielhofer P, Schneider H, Kaelin K, Huber M, Dotsch C, Christiansen G, Billeter M A. Rescue of measles viruses from cloned DNA. EMBO J. 1995;14:5773–5784. doi: 10.1002/j.1460-2075.1995.tb00266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rodgers W, Crise B, Rose J K. Signals determining protein tyrosine kinase and glycosyl-phosphatidylinositol-anchored protein targeting to a glycolipid-enriched membrane fraction. Mol Cell Biol. 1994;14:5384–5391. doi: 10.1128/mcb.14.8.5384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rolls M M, Webster P, Balba N H, Rose J K. Novel infectious particles generated by expression of the vesicular stomatitis virus glycoprotein from a self-replicating RNA. Cell. 1994;79:497–506. doi: 10.1016/0092-8674(94)90258-5. [DOI] [PubMed] [Google Scholar]
- 35.Scheiffele P, Rietveld A, Wilk T, Simons K. Influenza viruses select ordered lipid domains during budding from the plasma membrane. J Biol Chem. 1999;274:2038–2044. doi: 10.1074/jbc.274.4.2038. [DOI] [PubMed] [Google Scholar]
- 36.Scheiffele P, Roth M G, Simons K. Interaction of influenza virus haemagglutinin with sphingolipid-cholesterol membrane domains via its transmembrane domain. EMBO J. 1997;16:5501–5508. doi: 10.1093/emboj/16.18.5501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schroeder R J, Ahmed S N, Zhu Y, London E, Brown D A. Cholesterol and sphingolipid enhance the Triton X-100 insolubility of glycosylphosphatidylinositol-anchored proteins by promoting the formation of detergent-insoluble ordered membrane domains. J Biol Chem. 1998;273:1150–1157. doi: 10.1074/jbc.273.2.1150. [DOI] [PubMed] [Google Scholar]
- 38.Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 39.Spielhofer P, Bachi T, Fehr T, Christiansen G, Cattaneo R, Kaelin K, Billeter M A, Naim H Y. Chimeric measles viruses with a foreign envelope. J Virol. 1998;72:2150–2159. doi: 10.1128/jvi.72.3.2150-2159.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stallcup K C, Wechsler S L, Fields B N. Purification of measles virus and characterization of subviral components. J Virol. 1979;30:166–176. doi: 10.1128/jvi.30.1.166-176.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Udem S A. Measles virus: conditions for the propagation and purification of infectious virus in high yield. J Virol Methods. 1984;8:123–136. doi: 10.1016/0166-0934(84)90046-6. [DOI] [PubMed] [Google Scholar]
- 42.Varma R, Mayor S. GPI-anchored proteins are organized in submicron domains at the cell surface. Nature. 1998;394:798–801. doi: 10.1038/29563. [DOI] [PubMed] [Google Scholar]
- 43.Viola A, Schroeder S, Sakakibara Y, Lanzavecchia A. T lymphocyte costimulation mediated by reorganization of membrane microdomains. Science. 1999;283:680–682. doi: 10.1126/science.283.5402.680. [DOI] [PubMed] [Google Scholar]
- 44.Wild T F, Malvoisin E, Buckland R. Measles virus: both the haemagglutinin and fusion glycoproteins are required for fusion. J Gen Virol. 1991;72:439–442. doi: 10.1099/0022-1317-72-2-439. [DOI] [PubMed] [Google Scholar]
- 45.Xavier R, Brennan T, Li Q, McCormack C, Seed B. Membrane compartmentation is required for efficient T cell activation. Immunity. 1998;8:723–732. doi: 10.1016/s1074-7613(00)80577-4. [DOI] [PubMed] [Google Scholar]
- 46.Yancey P G, Rodrigueza W V, Kilsdonk E P C, Stoudt G W, Johnson W J, Phillips M C, Rothblat G H. Cellular cholesterol efflux mediated by cyclodextrins. Demonstration of kinetic pools and mechanism of efflux. J Biol Chem. 1996;271:16026–16034. doi: 10.1074/jbc.271.27.16026. [DOI] [PubMed] [Google Scholar]
- 47.Zhang W, Trible R P, Samelson L E. LAT palmitoylation: its essential role in membrane microdomain targeting and tyrosine phosphorylation during T cell activation. Immunity. 1998;9:239–246. doi: 10.1016/s1074-7613(00)80606-8. [DOI] [PubMed] [Google Scholar]