Abstract
While adenovirus (Ad) gene delivery vectors are useful in many gene therapy applications, their broad tropism means that they cannot be directed to a specific target cell. There are also a number of cell types involved in human disease which are not transducible with standard Ad vectors, such as Epstein-Barr virus (EBV)-transformed B lymphocytes. Adenovirus binds to host cells via the viral fiber protein, and Ad vectors have previously been retargeted by modifying the fiber gene on the viral chromosome. This requires that the modified fiber be able to bind to the cell in which the vector is grown, which prevents truly specific vector targeting. We previously reported a gene delivery system based on a fiber gene-deleted Ad type 5 (Ad5) vector (Ad5.βgal.ΔF) and packaging cells that express the viral fiber protein. Expression of different fibers in packaging cells will allow Ad retargeting without modifying the viral chromosome. Importantly, fiber proteins which can no longer bind to the producer cells can also be used. Using this approach, we generated for the first time pseudotyped Ad5.βgal.ΔF particles containing either the wild-type Ad5 fiber protein or a chimeric fiber with the receptor-binding knob domain of the Ad3 fiber. Particles equipped with the chimeric fiber bound to the Ad3 receptor rather than the coxsackievirus-adenovirus receptor protein used by Ad5. EBV-transformed B lymphocytes were infected efficiently by the Ad3-pseudotyped particles but poorly by virus containing the Ad5 fiber protein. The strategy described here represents a broadly applicable method for targeting gene delivery to specific cell types.
Adenovirus (Ad)-based gene delivery vectors efficiently infect many different cells and tissues, making them promising tools for gene therapy (15, 23, 24, 52). However, this broad tropism means that gene delivery cannot be directed to a specific target cell. For example, a large fraction of intravenously administered Ad is retained by the liver, which may not be a desirable target (18, 44). Ad type 5 (Ad5) also transduces dendritic cells, which present antigens very efficiently (20, 60) and may exacerbate the antivector immune response. Vectors with different targeting preferences might eliminate these problems, allowing use of a lower (and therefore less immunogenic) total particle dose. Altered vector tropism would also extend the use of Ad-mediated gene delivery to cell types not infected by Ad5, such as cells of the hematopoietic system.
Infection by Ad involves two distinct virus-cell interactions (reviewed in reference 2). Attachment to a target cell occurs via high-affinity binding of the viral fiber protein to a specific cell surface receptor (6, 39). Internalization is then mediated by interaction of the viral penton base protein with cellular αv integrins (57). This is distinct from fiber-receptor binding and appears to be conserved among many Ad serotypes (35). Ads (including Ad5) of all subgroups except subgroup B appear to use a fiber receptor termed coxsackievirus-Ad receptor (CAR) (3, 41, 49). Subgroup B includes Ad3, which has been shown to use a different fiber receptor (6, 46). While CAR is widely expressed in vivo (49), low CAR levels or its expression on an inaccessible part of the cell (such as the basolateral surface of lung epithelial cells) can prevent efficient transduction by Ad5 vectors (28, 38, 53). Replacement or alteration of the fiber gene in the Ad chromosome has been shown to alter viral tropism (12, 26, 45). In addition to the use of natural variants, fiber proteins have been engineered to bind different receptors either by genetic modification or by antibody- or ligand-mediated strategies (8, 13, 25, 26, 36, 40, 54, 58). However, these modified vectors can be propagated only if their fiber protein retains the ability to bind to the cells used for virus production. This places a significant constraint on vector retargeting. A method for producing virions that cannot bind their native receptors (e.g., CAR) would be more versatile.
Gene therapy strategies have been proposed for treatment of Epstein-Barr virus (EBV)-induced diseases (11, 17, 21, 22, 42, 56). EBV is associated with such life-threatening disorders as transplant-associated lymphoproliferation (30, 48), Hodgkin's disease (55), and AIDS-associated B-cell malignancies (1, 9, 16, 32). EBV-infected B-lymphoid cell lines (B-LCLs) are infectable only at high particle/cell ratios of Ad5-based vectors. This is likely due to their low level of CAR expression, as these cells express elevated levels of αv integrins on their surfaces as a consequence of EBV infection (17). More efficient transduction of these cells should be possible through manipulation of the Ad fiber protein, facilitating the development of effective therapies.
We recently described a system consisting of fiber-expressing cell lines and a fiber gene-deleted Ad vector (50, 51). Since the fiber incorporated into such a vector during the last round of viral growth need not bind the producing cells, this method will allow the use of a much broader variety of fiber proteins in retargeting. While the cells previously reported could complement a fiber gene-deleted virus, the level of fiber expression varied from cell to cell and was significantly below that seen in a normal infection (51). By improving translational regulation of fiber expression in the packaging cell lines, we have now increased the amount of fiber incorporated into particles to near-wild-type levels. Pseudotyped Ad particles with distinct cell tropisms were produced in packaging cell lines which expressed two different fiber proteins (Fig. 1). In particular, Ad particles containing a chimeric fiber protein with the receptor-binding domain of Ad3 (46) infected EBV-infected B-LCLs much more efficiently than standard Ad5 vectors.
FIG. 1.
Strategy used for production of pseudotyped Ad vectors. A fiber gene-deleted Ad vector such as Ad5.βgal.ΔF is grown in packaging cell lines expressing different fiber proteins. The resulting particles will have distinct cell tropisms.
MATERIALS AND METHODS
Cells and viruses.
THP-1, MRC-5, FaDu, and A-10 cells were purchased from the American Type Culture Collection. 211B is a 293-derived cell line that expresses the wild-type Ad5 fiber protein (51). E1-2a (14), an A549-derived cell line which complements Ad E1 and E2a functions, was obtained from Michael Kadan, Genetic Therapy, Inc. The JR, TO, and TL LCLs were established as described previously (17) by EBV infection of lymphocytes from three healthy donors. B-10 cells are a subclone of the JR LCL and were produced by limiting dilution followed by PCR analysis to determine loss of the EBV genome (S. Huang, unpublished data). THP-1, all LCLs, and B-10 cells were maintained in RPMI 1640 medium (Gibco)–10% fetal calf serum (FCS; Hyclone). 211B, MRC-5, and A-10 cells were grown in Dulbecco modified Eagle medium–10% FCS. E1-2a and its derivatives were grown in Richter's modified medium (Bio Whitaker)–10% FCS. Peripheral blood mononuclear cells (PBMCs) were isolated from normal human blood (General Clinical Research Center, Scripps Clinic) by sedimentation on Ficoll-Paque (Pharmacia) according to the manufacturer's instructions. Wild-type Ad2 and Ad3 were purchased from the American Type Culture Collection. Construction of Ad5.βgal.wt and Ad5.βgal.ΔF (50) has been previously described. Av1LacZ4 (37) is a first- generation Ad5 vector containing a Rous Sarcoma virus-driven β-galactosidase reporter gene. Av9LacZ4 (45) is identical to Av1LacZ4 except that the fiber gene in the vector chromosome was replaced by a recombinant gene encoding a chimeric fiber protein with the receptor-binding domain of the Ad3 fiber (46).
DNA constructs.
The complete Ad5 tripartite leader (TPL) contained in pDV67 and pDV69 was constructed by assembly of PCR fragments. The third TPL exon (nucleotides [nt] 9644 to 9731 of the Ad5 genome) was amplified by using the primers 5′ CTC AAC AAT TGT TGG ATC CGT ACT CC 3′ and 5′ GTG CTC AGC AGA TCT TGC GAC TGT G 3′. The resulting product was cloned to the BamHI and BglII sites of pΔE1Sp1a (Microbix Biosystems) by using novel sites in the primers (in boldface) to create pDV52. A fragment corresponding to the first TPL exon, the natural first intron, and the second TPL exon (Ad5 nt 6049 to 7182) was amplified by using primers 5′ GGC GCG TTC GGA TCC ACT CTC TTC C 3′ and 5′ CTA CAT GCT AGG CAG ATC TCG TTC GGA G 3′ and cloned into the BamHI site of pDV52, again using novel sites in the primers (in boldface), to create pDV55. This plasmid contains a 1.2-kb BamHI/BglII fragment consisting of the first TPL exon, the natural first intron, and the fused second and third TPL exons. Finally, pDV60 was constructed by inserting this TPL cassette into the BamHI site upstream of the Ad5 fiber gene in pcDNA3/Fiber (51).
To construct pDV61, a 1.9-kb Asp718/NotI fragment containing the partial Ad5 TPL and wild-type Ad5 fiber gene was transferred from pCLF (51) to pcDNA3.1/Zeo(+) (Invitrogen). In an analogous process, pDV67 was constructed by transferring a 2.9-kb Asp718/XbaI fragment from pDV60 to the pcDNA3.1/Zeo(+) backbone.
The chimeric Ad3-Ad5 fiber gene was amplified from pGEM5T3H (46) by using the primers 5′ ATG GGA TCC AAG ATG AAG CGC GCA AGA CCG 3′ and 5′ CAC TAT AGC GGC CGC ATT CTC AGT CAT CTT 3′ and cloned to the BamHI and NotI sites of pcDNA3.1/Zeo(+) via novel BamHI and NotI sites (in boldface) engineered into the primers to create pDV68. Finally, the complete TPL fragment described above was then added to the unique BamHI site of this plasmid to create pDV69.
Construction of stable cell lines.
E1-2a cells were electroporated as previously described (51) with pDV61, pDV67, or pDV69, and stable lines were selected with Zeocin (600 μg/ml; Invitrogen). Candidate clones were evaluated by immunofluorescence (51) using a polyclonal antibody generated against the Ad2 fiber (57). Those lines expressing the highest level of nuclear fiber were further characterized. Lines 601 and 633 were produced by transfection of pDV61 and pDV67, respectively, and therefore express the wild-type Ad5 fiber. Line 644 contains pDV69 and expresses the chimeric 5T3H fiber.
Virus growth and analysis.
Ad stocks were prepared in the indicated cell lines and plaque titered on 633 cells essentially as described elsewhere (50). E1-2a cells (14) and their derivatives contain a dexamethasone-inducible construct for complementation of E1a. 601, 633, and 644 cells were therefore treated with 0.3 μM dexamethasone for 24 h prior to infection, and 0.5 μM dexamethasone was included in the overlay for plaque assays. Protein concentrations of viral preparations were determined by using the Bio-Rad protein assay with purified bovine serum albumin as a standard. Particle number was calculated by using the formula 1 μg of protein = 4 × 109 viral particles. Western blotting was performed as described elsewhere (51), using polyclonal rabbit antibodies raised against either the Ad2 (57) or Ad3 (45) fibers.
Infection and receptor binding assays.
Cells (2 × 105) in a total volume of 200 μl were incubated with the indicated Ad preparation for 3 h at 37°C. Cells were then washed twice with fresh medium and returned to 37°C. Two days later, cells were fixed and stained with 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) and counted by light microscopy as described previously (50). For competition assays, cells were preincubated on ice for 1 h with either recombinant Ad3 fiber (10 μg/ml) purified from baculovirus or with a crude baculovirus lysate (100 μg/ml) containing the recombinant Ad2 fiber protein (57). Expression of αv integrins on cell surfaces was assayed by fluorescence-activated cell sorting assay using monoclonal antibodies (the gift of David Cheresh, Scripps Research Institute) against either αvβ3 (LM609) or αvβ5 (P1F6) as previously described (17). For virus binding assays, CsCl-purified Ad2 or Ad3 was labeled with 125I by using Iodogen tubes (Pierce). Free iodine was removed by filtration with a PD-10 Sephadex column (Pharmacia). Cells (106 cells in a volume of 200 μl either with or without a 100-fold excess of unlabeled virus) were rocked at 4°C for 2 h with 106 cpm of the labeled virus, washed three times with phosphate-buffered saline, and counted.
RESULTS
Increased fiber expression in packaging lines.
We previously reported the development of packaging cell lines that expressed Ad5 fiber at a level considerably below that seen in a normal infection (51). In an attempt to increase fiber production, we explored the use of additional viral regulatory elements. During a normal Ad infection, the translation of host cell proteins is inhibited. Viral proteins continue to be produced due to the action of the TPL, three small exons which are spliced onto the 5′ end of late viral mRNAs. In addition to the leader's role in translational control, sequences in the first TPL intron have been reported to increase transcription from the viral major late promoter in Ad-infected cells (19, 29, 33, 34). Inclusion of a cassette containing a nearly complete TPL cDNA but lacking any TPL introns (43) allowed nuclear accumulation of fiber in packaging cell lines (51). We hypothesized that a more complete version of the TPL might improve fiber expression. A construct (pDV67) containing the entire first TPL intron, as well as complete copies of all three exons, was constructed and incorporated into expression constructs (Fig. 2A). Both versions of the fiber expression construct were electroporated into the E1a/E2a-complementing cell line E1-2a (14), and stable cell lines were isolated. Fiber expression was assayed by indirect immunofluorescence using a polyclonal antibody raised against the Ad2 fiber protein. As the Ad2 and Ad5 fibers are nearly identical, this antibody efficiently recognizes the Ad5 fiber protein. The improved TPL resulted both in a generally higher level of fiber protein expression and in a much smaller number of low-expressing cells (Fig. 2). One fiber-expressing clone carrying each construct (lines 601 and 633 contain the original and improved constructs, respectively) was selected for further evaluation.
FIG. 2.
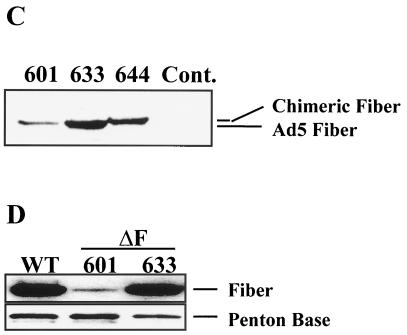
Expression of Ad5 fiber in cell lines. (A) Constructs used for fiber expression. In all plasmids, the fiber cDNA is driven by the cytomegalovirus (CMV) immediate-early promoter. pDV61 contains a partial TPL cDNA with no introns and lacking the first 32 nt of the first leader segment. In pDV67, this fragment was replaced by a TPL cassette (see Materials and Methods) which includes all three complete leader segments as well as the native first intron. (B) Nuclear expression of fiber protein. Cells (approximately 50,000/well) were plated on eight-well chamber slides, fixed, and stained with a polyclonal anti-Ad2 fiber serum (which also detects the Ad5 fiber). Line 601 and 633 were generated by transfection of pDV61 and pDV67, respectively. Note the increased and more consistent fiber expression in nuclei of 633 cells. As a control, non-fiber-expressing E1-2a cells were stained in parallel. (C) Increased synthesis of fiber protein from the complete TPL. Proteins extracted from the indicated cell lines (3 × 105 cells/lane) were electrophoresed and immunoblotted as described previously (50), and fiber was detected by using the anti-Ad2 fiber polyclonal antibody. Cont., control. (D) The complete TPL increases fiber content of Ad5.βgal.ΔF particles. Ad5.βgal.ΔF was CsCl purified from either 601 or 633 cells. The purified particles (10 μg) were electrophoresed on a sodium dodecyl sulfate–8 to 16% polyacrylamide gel (Novex) and immunoblotted. Fiber protein was detected with the anti-Ad2 fiber antibody. As a control, 10 μg of purified Ad5.βgal.wt (WT) was run alongside the mutants. To verify equal loading, the blot was reprobed with an antibody against the Ad2 penton base.
A chimeric fiber protein composed of amino acid residues 1 to 403 of the Ad5 fiber (the N-terminal tail and shaft domains) and 136 to 319 of the Ad3 protein (the receptor-binding C-terminal domain) was previously found to bind the Ad3 receptor rather than CAR (46). Incorporation of the gene encoding this chimeric protein (termed 5T3H) into an Ad5 chromosome produced a vector which infected cells with the tropism expected for Ad3 (45). An expression plasmid (pDV69) encoding this protein in place of the wild-type Ad5 fiber was constructed (Fig. 2A) and transfected into E1-2a cells as described above. The modified fiber protein was expressed at high levels in several of the resulting lines, as seen both by immunofluorescence and by Western blotting (Fig. 2C). Since the N-terminal 403 amino acids of the chimeric protein are derived from Ad5, it is efficiently detected by the anti-Ad2 fiber antibody. One line, clone 644, was selected for further evaluation.
The E1-, E3-, and fiber gene-deleted Ad5 vector Ad5.βgal.ΔF (50) was used to assess fiber complementation by the various cell lines. We prepared stocks of this virus in cells expressing the wild-type Ad5 fiber under the control of either the partial Ad TPL contained in pDV61 (line 601) or the complete TPL plus intron as in pDV67 (line 633). As a control, fiber levels in these preparations were compared to the level in the first-generation vector Ad5.βgal.wt, which is identical to Ad5.βgal.ΔF except for the fiber deletion. Consistent with the increased level of fiber synthesis in line 633, Ad5.βgal.ΔF grown in these cells contained a much larger (nearly normal) amount of fiber protein than those produced in line 601 (Fig. 2D).
Particle yields of Ad5.βgal.ΔF were comparable to those of Ad5.βgal.wt (Table 1). However, the infectious titers of the viral preparations were quite different. Virus produced in cells (line 601) expressing fiber from the incomplete TPL construct was much less infectious than the control, as seen by the increased particle/PFU and particle/β-galactosidase transducing unit (TU) ratios (Table 1). As well as increasing the amount of fiber on the particles, growth of Ad5.βgal.ΔF in cells carrying the improved expression construct (line 633) partially rescued the defect in plaque formation. Interestingly, while the number of particles per PFU was still approximately 70-fold higher than for a first-generation Ad vector, LacZ transduction of 293 cells was now similar to (within fourfold of) normal (Table 1; compare preparations 1 and 2 to preparations 7 and 8). This may indicate that deletion of the fiber gene affects late events in the virus life cycle, rather than early events such as gene delivery to the nucleus (see Discussion). Together, these results demonstrated that the reduced infectivity of Ad5.βgal.ΔF grown in cells (such as 211B or 601) expressing fiber under the control of the partial TPL was at least partly due to a deficit of fiber, as increased fiber content on particles due to the improved expression construct correlated with improved infectivity.
TABLE 1.
Particle titers and infectivities of vector preparationsa
| Prepn | Virus | Cell line | Particles/ml | PFU/ml | Particles/PFU | TU/ml | Particles/TU |
|---|---|---|---|---|---|---|---|
| 1 | Ad5.βgal.wt | 293 | 7.1 × 1011 | 1.8 × 1010 | 39 | 1.3 × 1011 | 5.5 |
| 2 | Ad5.βgal.wt | 211B | 1.6 × 1012 | 1.2 × 1011 | 13 | 6.5 × 1011 | 2.5 |
| 3 | Ad5.βgal.ΔF | 293 | 8.2 × 1011 | 1.9 × 107 | 43,157 | 9.6 × 108 | 854 |
| 4 | Ad5.βgal.ΔF | 293 | 3.2 × 1011 | 1.4 × 107 | 22,857 | 1.5 × 108 | 2133 |
| 5 | Ad5.βgal.ΔF | 601 | 9.3 × 1011 | 1.8 × 108 | 5,166 | 1.5 × 1010 | 62 |
| 6 | Ad5.βgal.ΔF | 601 | 1.6 × 1011 | 6.5 × 107 | 2,461 | 3.1 × 109 | 52 |
| 7 | Ad5.βgal.ΔF | 633 | 2.3 × 1012 | 1.0 × 109 | 2,300 | 1.7 × 1011 | 13.5 |
| 8 | Ad5.βgal.ΔF | 633 | 1.4 × 1012 | 1.0 × 109 | 1,400 | 2.3 × 1011 | 6.1 |
| 9 | Ad5.βgal.ΔF | 644 | 2.0 × 1012 | 2.7 × 109 | 740 | ND | ND |
| 10 | Ad5.βgal.ΔF | 644 | 7.9 × 1011 | 1.0 × 109 | 790 | ND | ND |
Each line represents a separate preparation of virus. Either Ad5.βgal.ΔF or Ad5.βgal.wt was grown in the indicated cell line (typically 10 162-cm2 flasks/preparation), and viral stocks were prepared. Following CsCl purification, particle number was determined by assaying protein concentration and infectious virus titers were determined by plaque assay on 633 cells (PFU per milliliter) and by infection of 293 cells followed by X-Gal staining for the β-galactosidase transgene product (TU per milliliter). ND, not determined.
Pseudotyping of an Ad vector.
In addition to producing vectors with the wild-type Ad5 tropism, the packaging cell technology should allow retargeting by pseudotyping a vector with modified fiber proteins or with fibers from different Ad serotypes. To test this hypothesis, we used the chimeric 5T3H fiber described above (46). Virus particles containing this fiber would be expected to bind and infect cells via the Ad3 receptor rather than the CAR protein used by Ad5.
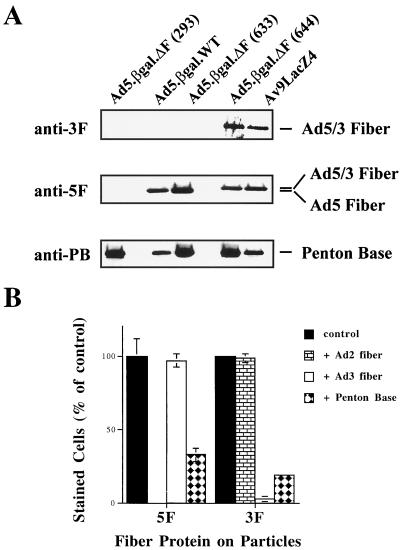
Growth of Ad5.βgal.ΔF in cells (line 644) expressing the chimeric fiber produced yields of viral particles similar to those seen with the other packaging lines (Table 1), and immunoblot analysis (Fig. 3A) demonstrated that they contained high levels of the chimeric fiber protein. Purified particles of Av9LacZ4, which is an E1-deleted virus containing the chimeric 5T3H gene in its chromosome (45), and of Ad5.βgal.wt were analyzed as positive controls. While both the wild-type Ad5 and chimeric 5T3H fibers could be detected by a polyclonal antibody raised against the Ad2 fiber, an anti-Ad3 fiber antibody detected only the chimeric fiber by virtue of its Ad3 knob domain. As a control for protein loading, the membrane was reprobed with a polyclonal antibody directed against the Ad2 penton base protein (which cross-reacts with the Ad5 penton base protein).
FIG. 3.
Incorporation of fiber proteins into viral particles. (A) Production of particles containing wild-type Ad5 or chimeric fiber proteins. Ten-microgram aliquots of Ad5.βgal.ΔF purified from either 293 (non-fiber-expressing), 633 (wild-type fiber-expressing), or 644 (chimeric 5T3H fiber-expressing) cells were analyzed by immunoblotting. Equal amounts of the first-generation vectors Ad5.βgal.wt (which contains the wild-type Ad5 fiber gene) and Av9LacZ4 (containing the chimeric 5T3H fiber gene) were analyzed as positive controls. An anti-Ad3 fiber antibody detects only the chimeric fiber protein in Av9LacZ4 or 644-grown Ad5.βgal.ΔF, while both fiber proteins are detected by the anti-Ad2 fiber serum. As a control, the blot was reprobed with an anti-penton base antibody, which detects all viral preparations. (B) Receptor usage by the pseudotyped particles. 211B cells were infected with Ad5.βgal.ΔF (1,000 particles/cell) produced in either 633 (5F) or 644 (3F) cells. To assess receptor usage, cells were preincubated with an excess of recombinant Ad2 or Ad3 fiber or of recombinant Ad2 penton base. Twenty-four hours after infection, cells were fixed and stained with X-Gal and the number of infected cells was counted by light microscopy. Values are expressed as the percentage of cells infected in the absence of competitor and represent the mean ± standard deviation of triplicate samples. This experiment was repeated several times with similar results.
We found that Ad5.βgal.ΔF viral particles containing the chimeric fiber protein were slightly more infectious than those equipped with the Ad5 fiber (Table 1). This might reflect either a somewhat higher level of the Ad3 receptor on 633 cells or more efficient complementation of the fiber deletion. Since comparing plaque titers of virions which use different attachment receptors may be misleading, we have given all multiplicities of infection as the number of physical particles per cell. Receptor usage by the pseudotyped Ad5.βgal.ΔF was assessed in competition experiments. 211B cells (which express both CAR and the Ad3 receptor) were infected in the presence or absence of excess recombinant Ad2 or Ad3 fiber proteins (Fig. 3B). Addition of recombinant Ad2 fiber completely blocked infection by virus containing the Ad5 fiber protein but not by Ad3-pseudotyped virus. Conversely, an excess of recombinant Ad3 fiber which abolished infection by the Ad3-pseudotyped particles had no effect on those containing the Ad5 fiber. Consistent with the role of αv integrins in infection and internalization of both Ad3 and Ad5 (35), infection by either preparation of Ad5.βgal.ΔF was blocked by addition of excess recombinant Ad2 penton base protein.
Altered in vitro tropism and infection of B-LCLs.
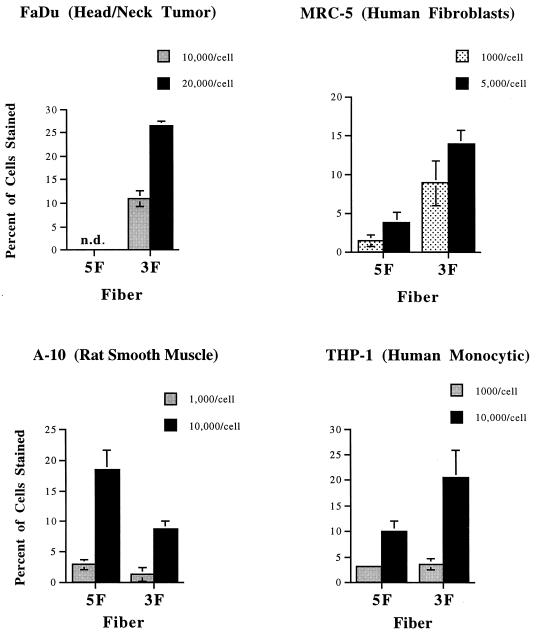
Experiments with genetically modified viruses showed that a number of different cell types are more readily infected through interaction with the Ad3 receptor than by the CAR-dependent pathway used by Ad5 (45). To further evaluate our pseudotyping system, we assayed the ability of Ad5.βgal.ΔF carrying either the Ad5 or chimeric 5T3H fibers to infect several of these: FaDu (a head and neck tumor line), THP-1 monocytic cells, and MRC-5 fibroblasts. Consistent with the previous studies (45), use of the chimeric Ad5-Ad3 fiber protein increased infection of all of these lines at equal particle/cell ratios (Fig. 4). In contrast, the rat smooth muscle cell line A-10 was infected somewhat more readily by Ad5- than by Ad3-pseudotyped particles (Fig. 4), also in agreement with previous results (S. C. Stevenson, unpublished data).
FIG. 4.
Differential infectivity of pseudotyped particles. The cell lines indicated were incubated with Ad5.βgal.ΔF, produced in either 633 (5F) or 644 (3F) cells, at the particle/cell ratios indicated. After 3 h, virus was removed and the cells were washed twice with medium. Forty-eight hours after infection, cells were fixed and stained with X-Gal, and the percentage of cells infected was determined by light microscopy. Values shown are the mean ± standard deviation of triplicate samples and are representative of several experiments. n.d., no infection was detected.
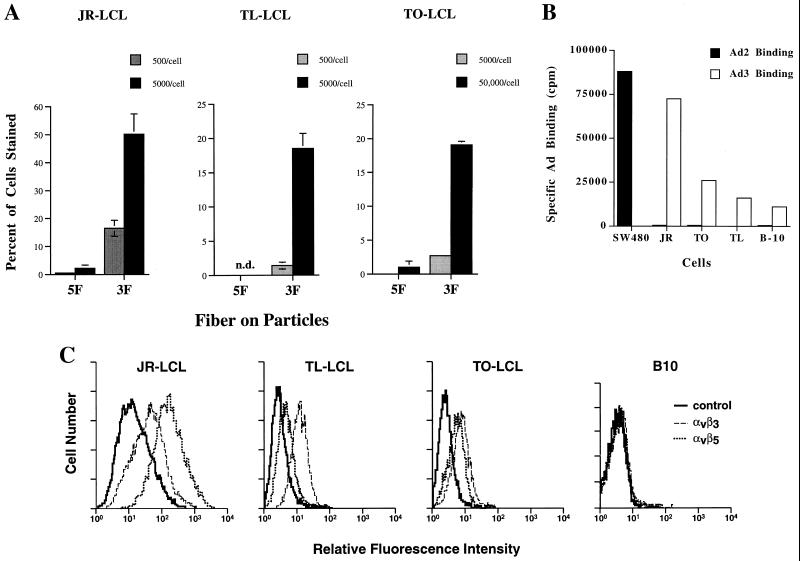
Gene delivery to EBV-infected B cells could allow the development of therapies for a variety of lymphoproliferative disorders. For example, ex vivo purging of donor marrow to eliminate infected cells could reduce the risk of EBV-associated lymphoproliferative disease, and EBV-induced malignancies such as AIDS-associated lymphoma are also potential targets. However, neither B cells nor EBV-transformed LCLs are efficiently infected by Ad5-based vectors. As the tropism of Ad3-pseudotyped particles appeared to be somewhat broader, we examined whether EBV-infected LCLs could be infected by using this system. The ability of Ad3-pseudotyped particles to infect LCLs generated by EBV infection of lymphocytes from three different healthy human donors was tested. In agreement with previous reports, there was little or no infection of these by particles carrying the Ad5 fiber (Fig. 5A). In contrast, virus particles equipped with the chimeric fiber protein were able to efficiently infect all of these lines. At equal particle/cell ratios, all LCLs examined were at least 10-fold more infectable with the Ad3 receptor. We also examined infection of a cell line (B-10) which was derived from the JR LCL by limiting dilution and no longer carries detectable levels of the EBV genome (Huang, unpublished data). Although the parental JR cells are very efficiently infected by the Ad3-pseudotyped particles (Fig. 5A), infection of B-10 was undetectable even at very high (up to 50,000 particles/cell) multiplicities of infection (data not shown).
FIG. 5.
Infection of LCLs by pseudotyped Ad vectors. (A) The EBV-transformed lines JR, TO, and TL were infected as described in the legend to Fig. 4 with the indicated particle/cell ratios of Ad5.βgal.ΔF produced in either 633 (5F) or 644 (3F) cells. n.d., no infection was detected. Values shown are the mean ± standard deviation of triplicate samples and are representative of several experiments. (B) Ad binding to LCLs. Purified 125I-labeled wild-type Ad2 or Ad3 was incubated (106 cpm) at 4°C with the indicated cells (106 cells) to assess virus binding. As a positive control, Ad2 binding to SW480 cells (which express high levels of CAR) was also measured. To determine the level of nonspecific binding, identical samples were incubated with 125I-Ad in the presence of 100-fold excess of unlabeled virus. Cells were then washed three times in cold phosphate-buffered saline, and bound radioactivity was determined. Specific binding was determined as (cpm bound in the absence of competitor) − (cpm bound with competitor). Values reported are means of duplicate samples and are representative of several experiments. (C) αv integrin expression on LCLs. Cells were incubated with antibodies directed against either αvβ3 (LM609) or αvβ5 (P1F6) followed by incubation with fluorescein isothiocyanate-labeled goat anti-mouse immunoglobulin. Binding was then analyzed by flow cytometry. Control samples were incubated with the secondary antibody alone.
Further studies were performed to correlate the efficiency of infection with the level of attachment and internalization receptors expressed by the cells. The three LCLs tested all bound very low levels of radiolabeled Ad2 particles, indicating that they expressed little or no CAR (Fig. 5B). In contrast, all three were able to specifically bind labeled Ad3 particles (Fig. 5B). This result suggested that fiber receptor distribution was largely responsible for the increased infection of these cells by Ad3-pseudotyped particles. Although B-10 cells were not infectable by the Ad3-pseudotyped virus, we found that they could nonetheless bind Ad3 at a low level. The inability of Ad to infect these cells might therefore be due to lack of integrin expression or function. To examine this possibility, we analyzed expression of αv integrins on all four cell lines by fluorescence-activated cell sorting using antibodies specific for αvβ5 or αvβ3 integrins. All of the LCLs which supported infection expressed both αvβ5 and αvβ3 integrins, while B-10 cells expressed neither (Fig. 5C). This finding emphasizes that expression of both a fiber receptor and of αv integrins are required for efficient Ad infection.
Selective gene delivery to EBV-infected cells.
The results above suggested that the minority of EBV-infected B cells present in donor marrow or peripheral blood would be preferentially infected by vectors using the Ad3 receptor. To test this hypothesis, we performed a mixing experiment with uninfected PBMCs and EBV-infected cells. JR LCLs were mixed at various ratios with PBMCs isolated from a healthy human donor, and the mixture was then infected with Ad5.βgal.ΔF particles containing the 5T3H fiber protein. No infection of normal PBMCs alone was detected. Moreover, the percent of total cells infected increased with the fraction of JR cells added (Fig. 6). This finding indicates that EBV-infected cells can be selectively infected in vitro by relatively short (3-h) exposure to a retargeted Ad vector.
FIG. 6.
Selective infection of LCLs versus normal lymphocytes. Peripheral blood lymphocytes were isolated from a healthy donor and mixed with increasing amounts of JR LCL cells. The samples were infected for 3 h with Ad5.βgal.ΔF (50,000 particles/cell) produced in line 644 (carrying the chimeric 5T3H fiber protein). The cells were then washed twice and resuspended in fresh medium. After 48 h, cells were fixed and stained, and the percentage of cells infected was calculated. Values shown are the mean ± standard deviation of triplicate samples.
DISCUSSION
We previously reported a fiber gene-deleted Ad5 vector and its growth in fiber-expressing packaging cell lines. The data presented here demonstrate that such a vector can be retargeted simply by production in packaging lines expressing different fiber proteins. A wide variety of fibers, including those which cannot bind to the host cells used for virus production, should be useful in such cell lines. This is especially important since developing viruses targeted to infect a single cell type would involve eliminating their binding to the natural fiber receptor (CAR). Our system will also simplify development of Ad-mediated therapies aimed at different applications. Rather than generating a new viral chromosome each time a transgene is to be delivered to a new target, a single vector could be targeted to different cell populations by pseudotyping with the appropriate fiber proteins. The cell lines described here were derived from the very efficient E1a/E2a-complementing line (14) and should allow the future development of Ad vectors with deletions of E1a, E3, L5 (fiber), and E2a.
Our original generation of packaging cell lines (51) expressed the Ad5 fiber at a relatively low level. They produced Ad particles containing a substoichiometric amount of fiber protein, with reduced infectivity relative to first-generation vector particles (50). This appears to be a property of the fiber expression construct used to generate these lines. While inclusion of an incomplete TPL fragment greatly increased fiber expression over that seen from a construct lacking TPL sequences, there was a large amount of cell-to-cell variation in fiber expression (51). Attempts to isolate cells that expressed fiber more uniformly by recloning line 211B were unsuccessful (data not shown).
In addition to the TPL's role in translational regulation, its natural first intron has been reported to act as a transcriptional enhancer for the Ad major late promoter (19, 29, 33, 34). We found that inclusion of this intron could also greatly increase fiber production from our cytomegalovirus-driven construct. Cell lines (such as line 633) carrying this construct not only expressed more fiber protein but exhibited much less cell-to-cell variability in expression level. Since synthesis of the major Ad structural proteins is coordinately regulated, this type of construct may be useful in developing systems for complementation of other viral proteins such as penton and hexon.
Ad5.βgal.ΔF growth in the improved packaging lines resulted in a near-normal fiber content on the particles and improved infectivity severalfold relative to particles grown in cells carrying the original expression construct. Interestingly, while the ability of Ad5.βgal.ΔF particles produced in the new cell lines to transduce LacZ is very close to the wild-type level (Table 1), the particle/PFU ratio remains significantly (approximately 70-fold) higher. This suggests that there is an additional defect associated with the fiber gene deletion, beyond a simple deficit of fiber protein, which is not efficiently complemented by the packaging system that we are using. The fact that plaque formation but not gene delivery is reduced suggests that this defect might affect a stage in the viral life cycle occurring after delivery of the viral chromosome to the nucleus. In this light, it is interesting that Yeh and coworkers (59) reported similar findings in work with an E4-lacking Ad5 vector.
There were several previous reports (4, 7, 10) of fiber mutants being associated with defects in viral assembly or maturation, but we did not detect obvious assembly problems with Ad5.βgal.ΔF even in the complete absence of fiber. Indeed, a cryoelectron microscopic analysis of our fiberless particles showed their structure to be essentially normal (50). However, another group recently reported production of a fiber gene-deleted Ad vector and did find differences in several aspects of the viral biology (27). In addition to the expected reduced infectivity, they reported slight differences in maturation of some capsid proteins as determined by pulse-labeling with [35S]methionine, increased cytoplasmic versus nuclear localization of the particles, and altered particle density on CsCl gradients (27). It is possible that while the defects detected by LeGrand et al. (27) are functionally significant, they were not severe enough to have been detected by the methods that we used. We are now examining the phenotype of our vector particles in more detail in order to understand the differences in our results.
Use of the chimeric 5T3H fiber protein generated Ad5 vector particles which infected cells via the Ad3 receptor rather than the CAR protein used by Ad5 (Fig. 3 and 4). This is in agreement with previous data showing that an Ad5 vector with this chimeric fiber gene substituted into its chromosome displayed Ad3 tropism (45). The retargeted virus remained dependent on the integrin-penton base interaction, since infection by particles containing either fiber was blocked by competition with excess recombinant Ad2 penton base. We also found that the LCL-derived B-10 cell line, which lacks detectable αv integrins, was refractory to infection even though the cells could bind labeled Ad3 particles.
EBV-infected B lymphocytes are logical therapeutic targets for a number of diseases such as AIDS-associated central nervous system lymphoma or transplant-associated lymphoproliferation. Although uninfected B cells are not efficiently infected by Ad due to lack of integrin expression, the upregulation of αv integrins induced by EBV infection allows virus internalization and gene delivery (17) if very high particle/cell ratios of an Ad5 vector are used. This inefficient infection is likely due to the low level of CAR expression which we and others (47) have found on these cells. As would be predicted from the binding data shown here, we found that LCLs were much more readily infected by vector particles that contained the Ad3 fiber knob. Our results are in agreement with a previous report (5) showing that LCLs could be transduced by an Ad-polylysine complex (in which the Ad moiety mediated endosome disruption) provided that the complex was able to bind to the cells. Interestingly, recent studies have detected replication of subgroup B (the subgroup which includes Ad3) Ads, notably Ad35, in patients who are immunosuppressed due to transplantation or to AIDS (31). These serotypes are rarely found in healthy individuals. EBV infection is often reactivated in such patients (48), and it is possible that the EBV-induced upregulation of integrins allows productive infection of lymphocytes by these Ad serotypes. In preliminary studies, we have found that wild-type Ad3 is indeed capable of infecting and replicating in B-LCLs (D. J. Von Seggern and S. K. Fleck, unpublished data).
The differential infectivities of normal and EBV-infected B cells may be useful in such gene therapy strategies as purging donor bone marrow of EBV-infected cells before transplantation or the in vivo treatment of EBV-induced lymphomas. Previous work in our group has shown that introduction of a ribozyme targeted against the EBNA-1 transcript can reduce the EBV genome to undetectable levels in LCLs (17). Gene therapy strategies for these diseases based on specific transcription of suicide genes driven by EBV-responsive promoters have also been proposed (11, 21, 22, 42). Combining selective infection of EBV-positive cells by retargeted Ad vectors with such EBV-targeted therapeutic strategies might provide effective and specific treatments.
ACKNOWLEDGMENTS
This work was supported by NIH grants EY11431 and HL54352 to G. R. Nemerow and by GTI/Novartis grant SFP1089 to D. J. Von Seggern and G. R. Nemerow.
We thank Joan Gausepohl for assistance with the manuscript, and we thank Phyllis Frosst and Colleen McKiernan for their comments. We also thank David Cheresh for his gift of monoclonal antibodies. Normal human donor blood was obtained from the GCRC under protocol 95-13.
REFERENCES
- 1.Bashir R, Luka J, Cheloha K, Chamberlain M, Hochberg F. Expression of Epstein-Barr virus proteins in primary CNS lymphoma in AIDS patients. Neurology. 1993;43:2358–2361. doi: 10.1212/wnl.43.11.2358. [DOI] [PubMed] [Google Scholar]
- 2.Bergelson J M. Receptors mediating adenovirus attachment and internalization. Biochem Pharmacol. 1999;57:975–979. doi: 10.1016/s0006-2952(98)00332-3. [DOI] [PubMed] [Google Scholar]
- 3.Bergelson J M, Cunningham J A, Droguett G, Kurt-Jones E A, Krithivas A, Hong J S, Horwitz M S, Crowell R L, Finberg R W. Isolation of a common receptor for coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275:1320–1323. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- 4.Chee-Sheung C C, Ginsberg H S. Characterization of a temperature-sensitive fiber mutant of type 5 adenovirus and effect of the mutation on virion assembly. J Virol. 1982;42:932–950. doi: 10.1128/jvi.42.3.932-950.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Curiel T J, Cook D R, Bogedain C, Jilg W, Harrison G S, Cotten M, Curiel D T, Wagner E. Efficient foreign gene expression in Epstein-Barr virus-transformed human B-cells. Virology. 1999;198:577–585. doi: 10.1006/viro.1994.1069. [DOI] [PubMed] [Google Scholar]
- 6.Defer C, Belin M-T, Caillet-Boudin M-L, Boulanger P. Human adenovirus-host cell interactions: comparative study with members of subgroups B and C. J Virol. 1990;64:3661–3673. doi: 10.1128/jvi.64.8.3661-3673.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.D'Halluin J-C, Milleville M, Martin G R, Boulanger P. Morphogenesis of human adenovirus type 2 studied with fiber- and fiber and penton base-defective temperature-sensitive mutants. J Virol. 1980;33:88–99. doi: 10.1128/jvi.33.1.88-99.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dmitriev I, Krasnykh V, Miller C R, Wang M, Kashentseva E, Mikheeva G, Belousova N, Curiel D T. An adenovirus vector with genetically modified fibers demonstrates expanded tropism via utilization of a coxsackievirus and adenovirus receptor-independent cell entry mechanism. J Virol. 1998;72:9706–9713. doi: 10.1128/jvi.72.12.9706-9713.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ernberg I, Altiok E. The role of Epstein-Barr virus in lymphomas of HIV carriers. APMIS. 1989;97:58–61. [PubMed] [Google Scholar]
- 10.Falgout B, Ketner G. Characterization of adenovirus particles made by deletion mutants lacking the fiber gene. J Virol. 1988;62:622–625. doi: 10.1128/jvi.62.2.622-625.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Franken M, Estabrooks A, Cavacini L, Sherburne B, Wang F, Scadden D T. Epstein-Barr virus-driven gene therapy for EBV-related lymphomas. Nat Med. 1996;2:1379–1382. doi: 10.1038/nm1296-1379. [DOI] [PubMed] [Google Scholar]
- 12.Gall J, Kass-Eisler A, Leinwand L, Falck-Pedersen E. Adenovirus type 5 and 7 capsid chimera: fiber replacement alters receptor tropism without affecting primary immune neutralization epitopes. J Virol. 1996;70:2116–2123. doi: 10.1128/jvi.70.4.2116-2123.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldman C K, Rogers B E, Douglas J T, Sosnowski B A, Ying W, Siegal G P, Baird A, Campain J A, Curiel D T. Targeted gene delivery to Kaposi's sarcoma cells via the fibroblast growth factor receptor. Cancer Res. 1997;57:1447–1451. [PubMed] [Google Scholar]
- 14.Gorziglia M I, Kadan M J, Yei S, Lim J, Lee G M, Luthra R, Trapnell B C. Elimination of both E1 and E2a from adenovirus vectors further improves prospects for in vivo human gene therapy. J Virol. 1996;70:4173–4178. doi: 10.1128/jvi.70.6.4173-4178.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haddada H, Cordier L, Perricaudet M. Gene therapy using adenovirus vectors. Curr Top Microbiol Immunol. 1995;199:297–306. doi: 10.1007/978-3-642-79586-2_14. [DOI] [PubMed] [Google Scholar]
- 16.Hamilton-Dutoit S J, Raphael M, Audouin J, Diebold J, Lisse I, Pedersen C, Oksenhendler E, Marelle L, Pallesen G. In situ demonstration of Epstein-Barr virus small RNAs (EBER 1) in acquired immunodeficiency syndrome-related lymphomas: correlation with tumor morphology and primary site. Blood. 1993;82:619–625. [PubMed] [Google Scholar]
- 17.Huang S, Stupack D G, Mathias P, Wang Y, Nemerow G. Growth arrest of Epstein-Barr virus immortalized B lymphocytes by adenovirus-delivered ribozymes. Proc Natl Acad Sci USA. 1997;94:8156–8161. doi: 10.1073/pnas.94.15.8156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huard J, Lochmüller H, Acsadi G, Jani A, Massie B, Karpati G. The route of administration is a major determinant of the transduction efficiency of rat tissues by adenoviral recombinants. Gene Ther. 1995;2:107–115. [PubMed] [Google Scholar]
- 19.Jansen-Durr P, Boeuf H, Kédinger C. Replication-induced stimulation of the major late promoter of adenovirus is correlated to the binding of a factor to sequences in the first intron. Nucleic Acids Res. 1988;16:3771–3786. doi: 10.1093/nar/16.9.3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jooss K, Yang Y, Fisher K J, Wilson J M. Transduction of dendritic cells by DNA viral vectors directs the immune response to transgene products in muscle fibers. J Virol. 1998;72:4212–4223. doi: 10.1128/jvi.72.5.4212-4223.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Judde J-G, Spangler G, Magrath I, Bhatia K. Use of Epstein-Barr virus nuclear antigen-1 in targeted therapy of EBV-associated neoplasia. Hum Gene Ther. 1996;7:647–653. doi: 10.1089/hum.1996.7.5-647. [DOI] [PubMed] [Google Scholar]
- 22.Kenney S, Ge J-Q, Westphal E M, Olsen J. Gene therapy strategies for treating Epstein-Barr virus-associated lymphomas: comparison of two different Epstein-Barr virus-based vectors. Hum Gene Ther. 1998;9:1131–1141. doi: 10.1089/hum.1998.9.8-1131. [DOI] [PubMed] [Google Scholar]
- 23.Kovesdi I, Brough D E, Bruder J T, Wickham T J. Adenoviral vectors for gene transfer. Curr Opin Biotechnol. 1997;8:583–589. doi: 10.1016/s0958-1669(97)80033-x. [DOI] [PubMed] [Google Scholar]
- 24.Kozarsky K F, Wilson J M. Gene therapy: adenovirus vectors. Curr Opin Genet Dev. 1993;3:499–503. doi: 10.1016/0959-437x(93)90126-a. [DOI] [PubMed] [Google Scholar]
- 25.Krasnykh V, Dmitriev I, Mikheeva G, Miller C R, Belousova N, Curiel D T. Characterization of an adenovirus vector containing a heterologous peptide epitope in the HI loop of the fiber knob. J Virol. 1998;72:1844–1852. doi: 10.1128/jvi.72.3.1844-1852.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krasnykh V N, Mikheeva G V, Douglas J T, Curiel D T. Generation of recombinant adenovirus vectors with modified fibers for altering viral tropism. J Virol. 1996;70:6839–6846. doi: 10.1128/jvi.70.10.6839-6846.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Legrand V, Spehner D, Schlesinger Y, Settelen N, Pavirani A, Mehtali M. Fiberless recombinant adenoviruses: virus maturation and infectivity in the absence of fiber. J Virol. 1999;73:907–919. doi: 10.1128/jvi.73.2.907-919.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leon R P, Hedlund T, Meech S J, Li S, Schaack J, Hunger S P, Duke R C, DeGregori J. Adenoviral-mediated gene transfer in lymphocytes. Proc Natl Acad Sci USA. 1998;95:13159–13164. doi: 10.1073/pnas.95.22.13159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leong K, Lee W, Berk A J. High-level transcription from the adenovirus major late promoter requires downstream binding sites for late-phase-specific factors. J Virol. 1990;64:51–60. doi: 10.1128/jvi.64.1.51-60.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lucas K G, Pollok K E, Emanuel D J. Post-transplant EBV induced lymphoproliferative disorders. Leuk Lymphoma. 1997;25:1–8. doi: 10.3109/10428199709042491. [DOI] [PubMed] [Google Scholar]
- 31.Lukashok S A, Horwitz M S. New perspectives in adenovirus. Curr Clin Top Infect Dis. 1998;18:286–304. [PubMed] [Google Scholar]
- 32.MacMahon E M E, Glass J D, Hayward S D, Mann R B, Becker P S, Charache P, McArthur J C, Ambinder R F. Epstein-Barr virus in AIDS-related primary central nervous system lymphoma. Lancet. 1991;338:969–973. doi: 10.1016/0140-6736(91)91837-k. [DOI] [PubMed] [Google Scholar]
- 33.Mansour S L, Grodzicker T, Tjian R. Downstream sequences affect transcription initiation from the adenovirus major late promoter. Mol Cell Biol. 1986;6:2684–2694. doi: 10.1128/mcb.6.7.2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mason B B, Davis A R, Bhat B M, Chengalvala M, Lubeck M D, Zandle G, Kostek B, Cholodofsky S, Dheer S, Molnar-Kimber K, Mizutani S, Hung P P. Adenovirus vaccine vectors expressing hepatitis B surface antigen: importance of regulatory elements in the adenovirus major late intron. Virology. 1990;177:452–461. doi: 10.1016/0042-6822(90)90509-p. [DOI] [PubMed] [Google Scholar]
- 35.Mathias P, Wickham T J, Moore M, Nemerow G. Multiple adenovirus serotypes use αv integrins for infection. J Virol. 1994;68:6811–6814. doi: 10.1128/jvi.68.10.6811-6814.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Michael S I, Hong J S, Curiel D T, Engler J A. Addition of a short peptide ligand to the adenovirus fiber protein. Gene Ther. 1995;2:660–668. [PubMed] [Google Scholar]
- 37.Mittereder N, March K L, Trapnell B C. Evaluation of the concentration and bioactivity of adenovirus vectors for gene therapy. J Virol. 1996;70:7498–7509. doi: 10.1128/jvi.70.11.7498-7509.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nalbantoglu J, Pari G, Karpati G, Holland P C. Expression of the primary coxsackie and adenovirus receptor is downregulated during skeletal muscle maturation and limits the efficacy of adenovirus-mediated gene delivery to muscle cells. Hum Gene Ther. 1999;10:1009–1019. doi: 10.1089/10430349950018409. [DOI] [PubMed] [Google Scholar]
- 39.Philipson L, Lonberg-Holm K, Pettersson U. Virus-receptor interaction in an adenovirus system. J Virol. 1968;2:1064–1075. doi: 10.1128/jvi.2.10.1064-1075.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rancourt C, Rogers B E, Sosnowski B A, Wang M, Piché A, Pierce G F, Alvarez R D, Siegal G P, Douglas J T, Curiel D T. Basic fibroblast growth factor enhancement of adenovirus-mediated delivery of the herpes simplex virus thymidine kinase gene results in augmented therapeutic benefit in a murine model of ovarian cancer. Clin Cancer Res. 1998;4:2455–2461. [PubMed] [Google Scholar]
- 41.Roelvink P W, Lizonova A, Lee J G M, Li Y, Bergelson J M, Finberg R W, Brough D E, Kovesdi I, Wickham T J. The coxsackievirus-adenovirus receptor protein can function as a cellular attachment protein for adenovirus serotypes from subgroups A, C, D, E, and F. J Virol. 1998;72:7909–7915. doi: 10.1128/jvi.72.10.7909-7915.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rogers R P, Ge J-Q, Holley-Guthrie E, Hoganson D K, Comstock K E, Olsen J C, Kenney S. Killing Epstein-Barr virus-positive B lymphocytes by gene therapy: comparing the efficacy of cytosine deaminase and herpes simplex virus thymidine kinase. Hum Gene Ther. 1996;7:2235–2245. doi: 10.1089/hum.1996.7.18-2235. [DOI] [PubMed] [Google Scholar]
- 43.Sheay W, Nelson S, Martinez I, Chu T-H T, Bhatia S, Dornburg R. Downstream insertion of the adenovirus tripartite leader sequence enhances expression in universal eukaryotic vectors. Bio Techniques. 1993;15:856–862. [PubMed] [Google Scholar]
- 44.Smith T A G, Mehaffey M G, Kayda D B, Saunders J M, Yei S, Trapnell B C, McClelland A, Kaleko M. Adenovirus mediated expression of therapeutic plasma levels of human factor IX in mice. Nat Genet. 1993;5:397–402. doi: 10.1038/ng1293-397. [DOI] [PubMed] [Google Scholar]
- 45.Stevenson S C, Rollence M, Marshall-Neff J, McClelland A. Selective targeting of human cells by a chimeric adenovirus vector containing a modified fiber protein. J Virol. 1997;71:4782–4790. doi: 10.1128/jvi.71.6.4782-4790.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stevenson S C, Rollence M, White B, Weaver L, McClelland A. Human adenovirus serotypes 3 and 5 bind to two different cellular receptors via the fiber head domain. J Virol. 1995;69:2850–2857. doi: 10.1128/jvi.69.5.2850-2857.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Teoh G, Chen L, Urashima M, Tai Y-T, Celi L A, Chen D, Chauhan D, Ogata A, Finberg R W, Webb I J, Kufe D W, Anderson K C. Adenovirus vector-based purging of multiple myeloma cells. Blood. 1998;92:4591–4601. [PubMed] [Google Scholar]
- 48.Thomas J A, Allday M J, Crawford D H. Epstein-Barr virus-associated lymphoproliferative disorders in immunocompromised individuals. Adv Cancer Res. 1991;57:329–380. doi: 10.1016/s0065-230x(08)61003-9. [DOI] [PubMed] [Google Scholar]
- 49.Tomko R P, Xu R, Philipson L. HCAR and MAR: the human and mouse cellular receptors for subgroup C adenoviruses and group B coxsackieviruses. Proc Natl Acad Sci USA. 1997;94:3352–3356. doi: 10.1073/pnas.94.7.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Von Seggern D J, Chiu C Y, Fleck S K, Stewart P L, Nemerow G R. A helper-independent adenovirus vector with E1, E3, and fiber deleted: structure and infectivity of fiberless particles. J Virol. 1999;73:1601–1608. doi: 10.1128/jvi.73.2.1601-1608.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Von Seggern D J, Kehler J, Endo R, Nemerow G R. Complementation of a fiber mutant adenovirus by packaging cell lines stably expressing the Ad5 fiber protein. J Gen Virol. 1998;79:1461–1468. doi: 10.1099/0022-1317-79-6-1461. [DOI] [PubMed] [Google Scholar]
- 52.Von Seggern D J, Nemerow G R. Adenoviral vectors for protein expression. In: Fernandez J, Hoeffler J, editors. Gene expression systems: using nature for the art of expression. San Diego, Calif: Academic Press; 1999. pp. 111–156. [Google Scholar]
- 53.Walters R W, Grunst T, Bergelson J M, Finberg R W, Welsh M J, Zabner J. Basolateral localization of fiber receptors limits adenovirus infection from the apical surface of airway epithelia. J Biol Chem. 1999;274:10219–10226. doi: 10.1074/jbc.274.15.10219. [DOI] [PubMed] [Google Scholar]
- 54.Watkins S J, Mesyanzhinov V V, Kurochkina L P, Hawkins R E. The 'adenobody‘ approach to viral targeting: specific and enhanced adenoviral gene delivery. Gene Ther. 1997;4:1004–1012. doi: 10.1038/sj.gt.3300511. [DOI] [PubMed] [Google Scholar]
- 55.Weiss L M, Movahed L A, Warnke R A, Sklar J. Detection of Epstein-Barr viral genomes in Reed-Sternberg cells of Hodgkin’s disease. N Engl J Med. 1989;320:502–506. doi: 10.1056/NEJM198902233200806. [DOI] [PubMed] [Google Scholar]
- 56.Westphal E-M, Mauser A, Swenson J, Davis M G, Talarico C L, Kenney S C. Induction of lytic Epstein-Barr virus (EBV) infection in EBV-associated malignancies using adenovirus vectors in vitro and in vivo. Cancer Res. 1999;59:1485–1491. [PubMed] [Google Scholar]
- 57.Wickham T J, Mathias P, Cheresh D A, Nemerow G R. Integrins αvβ3 and αvβ5 promote adenovirus internalization but not virus attachment. Cell. 1993;73:309–319. doi: 10.1016/0092-8674(93)90231-e. [DOI] [PubMed] [Google Scholar]
- 58.Wickham T J, Tzeng E, Shears II L L, Roelvink P W, Li Y, Lee G M, Brough D E, Lizonova A, Kovesdi I. Increased in vitro and in vivo gene transfer by adenovirus vectors containing chimeric fiber proteins. J Virol. 1997;71:8221–8229. doi: 10.1128/jvi.71.11.8221-8229.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yeh P, Dedieu J-F, Orsini C, Vigne E, Denefle P, Perricaudet M. Efficient dual transcomplementation of adenovirus E1 and E4 regions from a 293-derived cell line expressing a minimal E4 functional unit. J Virol. 1996;70:559–565. doi: 10.1128/jvi.70.1.559-565.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhong L, Granelli-Piperno A, Choi Y, Steinman R M. Recombinant adenovirus is an efficient and non-perturbing genetic vector for human dendritic cells. Eur J Immunol. 1999;29:964–972. doi: 10.1002/(SICI)1521-4141(199903)29:03<964::AID-IMMU964>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]