Abstract
A new method was established for the simultaneous analysis of four homologous benzalkonium chlorides (dodecyldimethylbenzyl ammonium chloride, tetradecyldimethylbenzyl ammonium chloride, hexadecyldimethylbenzyl ammonium chloride, and octadecyldimethylbenzyl ammonium chloride) in compound chemical disinfectants using non-aqueous capillary electrophoresis (CE) based on a micellar electrokinetic chromatography mode with direct ultraviolet detection. The separation was performed on an uncoated fused quartz capillary with a total length of 60.2 cm and a diameter of 25 μm. The separation buffer consisted of a mixture of methanol/acetonitrile (60:40, v/v) containing 70 mmol/L sodium acetate, 60 mmol/L trifluoroacetic acid and 20 mmol/L sodium dodecyl sulfate. The sample buffer was a methanol solution containing only 2 mmol/L trifluoroacetic acid. The separation voltage was set at 8 kV with a working current of approximately 2.3 μA. The detection wavelength was 214 nm. Under optimal conditions, the limit of detection and limit of quantification for these four benzalkonium chlorides (BACs) were 1.0 mg/L and 5.0 mg/L, respectively. Good linearities were observed in the concentration ranges from 5.0 to 100.0 mg/L, with correlation coefficients above 0.999 for all compounds. The recoveries of these four BACs ranged from 92.5 % to 109.1 % with relative standard deviations below 4.7 %. With the new method, all four BACs could be analyzed in a single injection. In contrast, the aqueous CE method in the National Standard GB/T 26369-2020 only allowed for the simultaneous analysis of the first three homologous. The new method demonstrated the improved peak shape compared to the aqueous CE method and then was successfully applied to the analysis of 19 commercially available samples, such as object table disinfectants, hand sanitizers, and disinfectant wipes, which claimed to contain quaternary ammonium compound. The results obtained using the new method were compared with those of the aqueous CE of the National Standard Method, and no statistically significant differences were observed. The new method is simple in pre-treatment and provides accurate results, making it highly suitable for routine analysis.
Keywords: Benzalkonium chloride, Compound chemical disinfectants, Non-aqueous capillary electrophoresis, Micellar electrokinetic chromatography, Ultraviolet detection
1. Introduction
According to the information obtained from the National Disinfection Product Filing Information Service Platform [1], it is becoming increasingly common for disinfection products to combine benzalkonium chloride (BAC) with ultraviolet (UV) absorption and those without UV absorption as mentioned in articles published by our laboratory [2] to enhance bactericidal efficacy. BAC, also known as alkyl dimethyl benzyl ammonium chloride, is typically highly soluble in polar and protonic solvents such as water and alcohol. Its solubility sharply decreases with the increasing of carbon chain length. Conversely, BAC with longer chains exhibits significantly increased solubility in non-polar solvents [3]. In 1935, German pathologist and bacteriologist Domagk first observed the disinfectant properties of BAC [4]. Its bactericidal mechanism involves adsorption on the cell membrane, permeation, and reaction with the cytoplasmic membrane (lipid or protein), leading to structural disruption and disintegration of the cell membrane and leakage of intracellular low molecular substances [5]. In fact, BAC is a mixture mainly composed of three homologues: dodecyldimethylbenzyl ammonium chloride (C12-BAC), tetradecyldimethylbenzyl ammonium chloride (C14-BAC) and hexadecyldimethylbenzyl ammonium chloride (C16-BAC). For residue analysis in food, the European Food Safety Authority define BAC as a mixture of compounds ranging from C8-BAC to octadecyldimethylbenzyl ammonium chloride (C18-BAC), as well as mixtures of dialkyl dimethyl ammonium with long alkyl chains such as di-C8, di-C10, and di-C12 [6]. The antibacterial activity of BAC is related to the length of the alkyl chain [7] and pH [8]. The C12-BAC, C14-BAC and C16-BAC homologues are most effective against yeast and fungi, gram-positive bacteria, and gram-negative bacteria, respectively. Their proportions and concentrations determine their bactericidal efficacy. It is widely used as a disinfectant [9] for disinfecting surfaces in contact with both food and non-food items [10], fabric softeners [11], personal hygiene and cosmetic products such as shampoos, conditioners, body lotions, as well as ophthalmic solutions and medications administered through the nasal route [12]. It has even been used illegally as a food preservative.
To strictly regulate and control the quality of related products, it is necessary to simultaneously determine the content of each component in them [13]. In the current existing detection methods, titration is only suitable for determining the content of raw materials or single-component disinfectants [14]; spectrophotometric method determines the total amount of BAC but cannot identify the specific types of BAC and has a narrow linear range [15], making it unsuitable for disinfectants combined with guanidine compounds [16]. The new potentiometric sensor method is not applicable to samples with complex matrices [17]. Ion mobility spectrometry, based on ion migration spectra, cannot be used for disinfectant sample analysis [18]. Paper spray mass spectrometry causes significant matrix effects when detecting complex samples [19]. High-performance liquid chromatography (HPLC) is used for separating BAC components, but the separation efficiency of the chromatographic column makes it challenging to completely separate the three components of BAC from the real sample matrix, making it unsuitable for simultaneously detecting multiple quaternary ammonium components in compound chemical disinfectant. Reverse-phase ion-pair HPLC using ion-pair reagents can alter the column efficiency and has a long equilibration time [20]. Furthermore, the unfavorable strong adsorption capacity of long-chain BAC can lead to peak tailing, affecting the integration of peaks for quantitative analysis [21]. HPLC-tandem mass spectrometry [22], although capable of simultaneously analyzing the three aforementioned components as well as domiphen and two non-UV-absorbing components (dodecyl trimethyl ammonium chloride and didecyldimethylammonium chloride), is generally suitable for trace or ultra-trace analysis. When used for the analysis of high-content disinfectant components, the large dilution factor may result in significant quantitative errors. Moreover, as cationic surfactants, BAC easily contaminates the ion source and needs frequent cleaning, which is a cumbersome process and reduces the ion source's lifespan [2].
Capillary electrophoresis (CE) has been used for the analysis of two, three, or five homologues in wet wipes [23], compound chemical disinfectants [14], medicines [13], and raw materials of BAC [24]. They were all carried out using aqueous buffers containing organic solvents for separation according to the rule of thumb. Namely, for the analysis of water-soluble components, aqueous CE is generally preferred, while for the analysis of poorly soluble components, non-aqueous CE (NACE) is preferred. However, our laboratory's extensive testing of a large number of quaternary ammonium disinfectant products over many years has shown that such a rule of thumb can be misleading, especially in the selection of separation buffer for surfactant quaternary ammonium salts. These salts are water-soluble and prone to forming micelles in aqueous solutions. Additionally, the increase in types of quaternary ammonium in compounded products leads to increased adsorption, resulting in less-than-ideal reproducibility by the National Standard Method [14]. Interference from polyhexamethylene biguanide (PHMB) prevents the testing of some products compounded with PHMB. Furthermore, when the concentration of homologues is high, especially for C18-BAC, serious peak tailing occurs, which leads to less-than-ideal analytical results. Therefore, based on a previously published paper within the same group on NACE methods for single and double long-chain quaternary ammonium salts, we developed a new NACE method using the MEKC mode with UV detection (NAMEKC-UV) for analyzing four components in benzalkonium chloride. This approach distinguishes our work as a novel contribution compared to the previously published research.
The results obtained from real sample analysis using the new method are compared with those obtained using the aqueous CE in the National Standard Method [14]. Currently, there are no references on NAMEKC-UV method for the simultaneous separation and quantification of BAC homologues in compound chemical disinfectants has been reported. The establishment of this new method can provide technical support for quality supervision and scientific use of compound chemical disinfectants and related products.
2. Materials and methods
2.1. Instruments and reagents
PA 800 Plus Capillary Electrophoresis (equipped with a PDA detector, Beckman Coulter, USA); uncoated fused silica capillary (inner diameter 25 μm, Hebei Yongnian Ruifeng Co., Ltd., China); Millipore Milli-Elix/RiOs ultrapure water system (Millipore, USA); Vortex-Genie 2 vortex mixer (Scientific Industries, USA); SB25-12DTD ultrasonic cleaner (Ningbo Xinzhi Biotechnology Co., Ltd., China); Mettler Toledo XPE 105 electronic balance (1 mg, Mettler-Toledo, Switzerland); 2003-1 CE constant temperature metal bath (CEB, USA).
Sodium hydroxide (NaOH) was purchased from Beijing Chemical Factory (China); Phosphoric acid and sodium dihydrogen phosphate were purchased from China National Pharmaceutical Group Chemical Reagent Co., Ltd.; Anhydrous sodium acetate (NaAc) was purchased from Wenzhou Chemical Reagent Factory (China); Methanol (MeOH, chromatographic grade), acetonitrile (ACN, chromatographic grade), glacial acetic acid (HAc, ACS reagent), trifluoroacetic acid (TFA, ACS grade), sodium deoxycholate (SD, purity >99.0 %) and sodium dodecyl sulfate (SDS, purity >99.0 %) were all purchased from Sigma-Aldrich (USA).
C12-BAC (purity >99.0 %) was purchased from Sigma-Aldrich (USA); C14-BAC (purity >99.0 %) and C16-BAC (purity >97.0 %) were purchased from Fluka (Switzerland); C18-BAC (purity >97.0 %) was purchased from TRC (Canada).
A total of 19 samples were used. The details were as follows. Samples 1–2 were disinfectant laundry detergent; Sample 3 was disinfectant hand wash; Samples 4–5 were skin disinfectant solution; Samples 6–17 were surface disinfectant for objects, and samples 18–19 were disinfectant wipes. All samples were purchased from an e-commerce platform. Blank sample for disinfectant were provided by the manufacturer. A mixed standard solution of the four BACs with a mass concentration of 1 g/L for each BAC was added to 20 mL of a blank sample to prepare a homemade sample containing four BACs for testing the precision of the method.
2.2. Preparation of solutions
2.2.1. Preparation of standard stock solution and standard working solution
-
a
NAMEKC
A standard stock solution of 10 g/L was first prepared for each of the four BACs in ACN. A mixed standard solution of the four BACs was then prepared by diluting the stock solution with a sample medium (MeOH containing 2 mmol/L TFA) to obtain four mixed standard solutions, each with a mass concentration of 1 g/L for each BAC. Standard working solutions with mass concentrations of 5, 10, 20, 40, 60, 80, and 100 mg/L were prepared by sequentially diluting the mixed standard solution with the sample medium.
-
b
aqueous CE
A standard stock solution of 10 g/L was first prepared for each of the three BACs in MeOH. A mixed standard solution of the three BACs was then prepared by diluting the stock solution with sample medium (a 50 % ACN solution containing 25 mmol/L HAc) to obtain three mixed standard solutions, each with a mass concentration of 1 g/L for each BAC. Standard working solutions with mass concentrations of 10, 20, 40, 60, and 80 mg/L were prepared by a series dilution of the mixed standard solution with the sample medium.
2.2.2. Preparation of sample medium and separation buffer solution
Stock solution of 100 mmol/L TFA was prepared in ACN. Stock solutions of 100 mmol/L TFA, 200 mmol/L NaAc, 100 mmol/L SDS and 100 mmol/L SD were all prepared in MeOH.
Stock solutions of 500 mmol/L NaH2PO4, 1 mol/L H3PO4 and 50 mmol/L HAc stock solution were all prepared in pure water.
A MeOH-ACN solution containing 70 mmol/L NaAc, 60 mmol/L TFA, and 20 mmol/L SDS in a ratio of 60:40 (v/v) was used as separation buffer for NAMEKC. A MeOH solution containing 2 mmol/L TFA was used as sample medium.
Refer to GB/T 26369-2020 ‘Hygienic requirements for quaternary ammonium disinfectant’ [14]. A 40 % ACN solution containing 62.5 mmol/L NaH2PO4 and 62.5 mmol/L H3PO4 was used as the separation buffer. A 50 % ACN solution containing 25 mmol/L HAc was used as sample medium.
2.3. Sample pretreatment
Liquid sample: Pipette approximately 0.2 mL of the liquid sample into a 10 mL plastic centrifuge tube with graduations. Dilute the sample 5 or 10 times with the sample medium and vortex to mix thoroughly. Based on the indicated content of the target substance in the actual sample, dilute it to approximately 10 mg/L for injection.
Wet wipe sample: Squeeze out the liquid component of the wet wipe, then pipette a minimum of 0.2 mL of the liquid into a 10 mL plastic centrifuge tube with graduations. Dilute the sample 5 or 10 times with the sample medium and vortex to mix thoroughly. Based on the indicated content of the target substance in the actual sample, dilute it to approximately 10 mg/L for injection. For samples that require drying, generally take 0.2 mL and place it in a 10 mL round-bottom plastic centrifuge tube. Dry it at 100 °C in a thermostatic metal bath. After drying, re-dissolve the sample in the sample medium and then dilute it for injection.
2.4. Electrophoresis conditions
NAMEKC conditions: Uncoated quartz capillary: 25 μm, 60.2 cm (effective length: 50 cm); detection wavelength: 214 nm; separation voltage: 8 kV; operating current: approximately 2.3 μA; injection pressure and time: 0.5 psi, 80 s; operating temperature: 25 °C. Activation treatment for newly installed capillary: Prior to use, flush with 1 mol/L NaOH at 70 psi for 30 min, followed by a 2 min rinse with ultrapure water. Directly use the capillary. Wash with separation buffer for 3 min before each injection.
Aqueous CE conditions: Referencing the electrophoresis conditions used in the National Standard Method [14]: Uncoated quartz capillary: 50 μm, 30.2 cm (effective length: 20 cm); detection wavelength: 214 nm; injection pressure and time: 0.5 psi, 4 s; separation voltage: 9 kV; operating current: approximately 24.5 μA; operating temperature: 25 °C. Treatment for newly installed capillary: Prior to use, flush with 1 mol/L NaOH at 20 psi for 10 min, followed by a 2 min rinse with ultrapure water. Flush with separation buffer for 3 min, then apply a 25 kV voltage in the separation buffer for 10 min. Wash with 1 mol/L NaOH for 2 min, followed by a 2 min rinse with ultrapure water, and a 2 min flush with separation buffer before each injection.
3. Results and discussion
3.1. Selection of detection wavelength
The ultraviolet absorption spectra of BAC were scanned using a PDA detector, and maximum absorption peaks were observed at 205 nm and 257 nm, respectively. However, the absorption peak at 257 nm and its detection sensitivity were lower than those at 250 nm and significantly lower than at 214 nm. All four disinfectant components exhibited higher sensitivity at 214 nm, which aligns with the detection wavelength used in the National Standard Method [14]. Therefore, 214 nm is chosen as the optimal detection wavelength to achieve simultaneous determination of the four components while avoiding matrix interference.
3.2. Optimization of the separation buffer solution
3.2.1. Organic solvents and their contents in the separation buffer solution
BAC easily forms micelles and readily adsorbs to the capillary wall. To address issues such as poor separation efficiency, peak tailing, and poor reproducibility of migration time, it is recommended to increase the concentration of inorganic salts in the separation buffer while adding a high content of organic solvent to suppress adsorption [25]. Currently, the maximum organic solvent content in the separation buffer is 80 %, and it is necessary to add ammonium acetate at a concentration of 100 mmol/L for good compatibility with the organic solvent [26]. In the initial stage of this study, the buffer was applied to the analysis of composite quaternary ammonium disinfectants, but the results were not satisfactory.
Considering the literature reports on NACE, which minimizes the adsorption between analytes and the capillary wall, providing separation efficiencies with theoretical plate numbers as high as 8.7 × 104 N/m∼3.0 × 105 N/m, the present study adopts the NAMEKC mode. MeOH, ACN, or their mixture are the most commonly used organic solvents in NACE [27]. In this study, MeOH is preferred due to its good compatibility with weak acid salts such as acetates and borates, as well as its strong solvating power to disrupt the SDS micelles present in the sample for pre-concentration and stacking of analytes prior to separation [28].
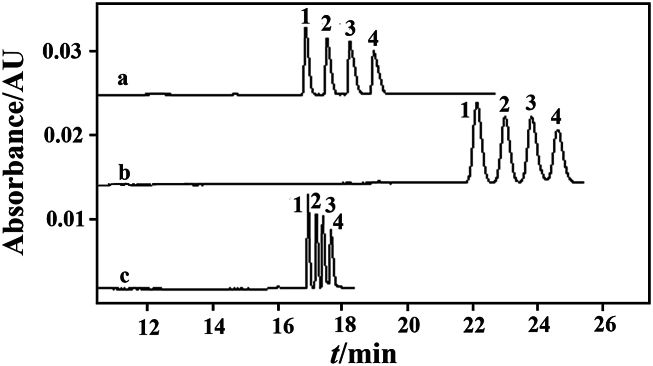
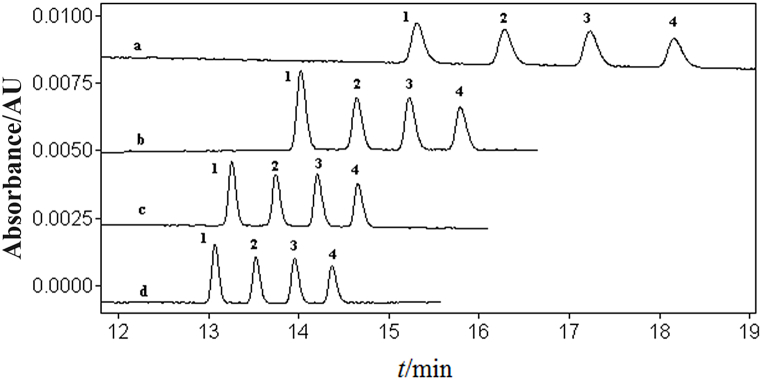
When keeping other conditions unchanged in the NAMEKC separation buffer, the current becomes unstable or even interrupted when using 100 % MeOH as the organic solvent, making it impossible to run the separation program. Attempts were made to add ACN and evaluate the effects of different volume ratios of MeOH and ACN (50:50, 60:40, 70:30, 80:20, 90:10, v/v) on the separation of four disinfectant components (Fig. 1).
Fig. 1.
The effects of organic solvent composition in the separation buffer solution containing 70 mmol/L NaAc, 60 mmol/L TFA and 20 mmol/L SDS unchanged on the separation of four disinfectant active ingredients: a. 60:40; b. 70:30; c. 80:20.20 mg/L of mixed standard solution of BACs in a MeOH solution containing 2 mmol/L TFA. Peaks: 1. C12-BAC; 2. C14-BAC; 3. C16-BAC; 4. C18-BAC. Electrophoresis conditions: uncoated quartz capillary: 25 μm, 60.2 cm (effective length: 50 cm); detection wavelength: 214 nm; separation voltage: 8 kV; operating current: approximately 2.3 μA; injection pressure and time: 0.5 psi, 80 s; operating temperature: 25 °C.
When using a 50:50 ratio of MeOH-ACN (v/v), the current is prone to interruption; attempting to increase the MeOH content, a 60:40 ratio of MeOH-ACN (v/v) achieves good separation of the four components with a stable current. As the MeOH content increases, at a 70:30 ratio of MeOH-ACN (v/v), the peaks of C16-BAC and C18-BAC cannot reach baseline separation, and the migration time is too long; further increasing the MeOH ratio to an 80:20 ratio of MeOH-ACN (v/v) deteriorates the separation. When using a 90:10 ratio of MeOH-ACN (v/v), the working current is interrupted, making it impossible to run the separation program.
Finally, a 60:40 ratio of MeOH-ACN (v/v) is chosen as the organic solvent composition in the separation buffer. The non-aqueous buffer has a low conductivity [28] and requires the addition of inorganic salts to enhance its conductivity.
3.2.2. Salts and their concentrations in the separation buffer solution
The presence of salts in the separation buffer affects factors such as solute adsorption on capillary walls, ionic strength of the buffer solution, and electroosmotic flow [29]. In this experiment, a constant composition of MeOH-ACN 60:40 (v/v), 60 mmol/L TFA, and 20 mmol/L SDS was maintained, while investigating the effects of NaAc, Na2HPO4, NaH2PO4, and NH4Ac. After comparing and referring to the separation conditions of single long-chain and double long-chain quaternary ammonium compounds [2], NaAc was selected and its concentration was optimized.
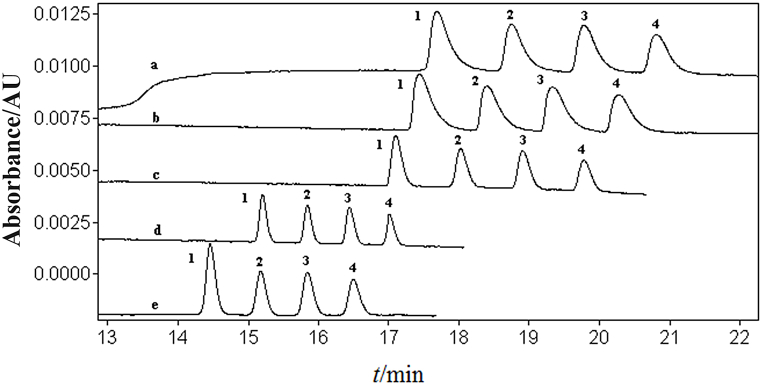
The influence of NaAc concentrations of 10, 30, 50, 60, and 70 mmol/L NaAc on the separation of four disinfectant components in a mixed standard solution was studied (Fig. 2). As the NaAc concentration increased, the four disinfectant components achieved baseline separation, and the resolution and sensitivity gradually increased while migration time shortened. Sensitivity did not significantly change when NaAc reached 70 mmol/L, and there was minimal further increase in resolution. However, the migration time increased, and if NaAc concentration was further increased, the working current would easily be interrupted, making it impossible to run the separation program stably. Therefore, based on the condition of maintaining resolution and stable operation while achieving high sensitivity and minimizing separation time, the NaAc concentration was optimized at 70 mmol/L.
Fig. 2.
The effect of sodium acetate concentration on the separation of four disinfectant active ingredients: a. 10; b. 30; c. 50; d. 60; e. 70.20 mg/L of mixed standard solution of BACs. 1. C12-BAC; 2. C14-BAC; 3. C16-BAC; 4. C18-BAC. Electrophoresis conditions: the same as Fig. 1.
3.2.3. Types and concentrations of acids in the separation buffer solution
Drawing inspiration from the separation of two single long-chain and three double long-chain quaternary ammonium salts by NACE [2], TFA was also selected as the acid in the separation buffer.
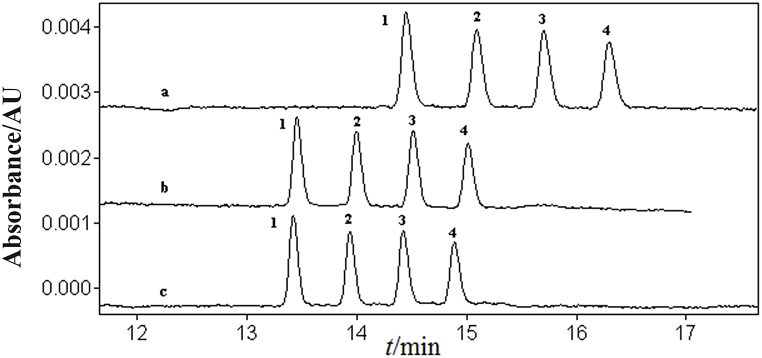
While maintaining a constant composition of MeOH-ACN 60:40 (v/v), 70 mmol/L NaAc, and 20 mmol/L SDS in the separation buffer solution, the effects of 40, 60, and 80 mmol/L TFA on the separation of four disinfectant components in a mixed standard solution were studied (Fig. 3). With an increase in TFA concentration, the separation of the four disinfectant components improved, resulting in increased migration time. However, there was no significant change in sensitivity. When TFA reached 80 mmol/L, although the resolution of peaks increased, the migration time became longer. Considering the requirement for good resolution and stable operation while achieving high sensitivity and minimizing separation time, the optimal TFA concentration was determined to be 60 mmol/L.
Fig. 3.
The effect of TFA concentration (mmol/L) on the separation of four disinfectant active ingredients: a. 80; b. 60; c. 40.20 mg/L of mixed standard solution of BACs. 1. C12-BAC; 2. C14-BAC; 3. C16-BAC; 4. C18-BAC. Electrophoresis conditions: the same as Fig. 1.
3.2.4. Selection of SDS concentration in the separation buffer solution
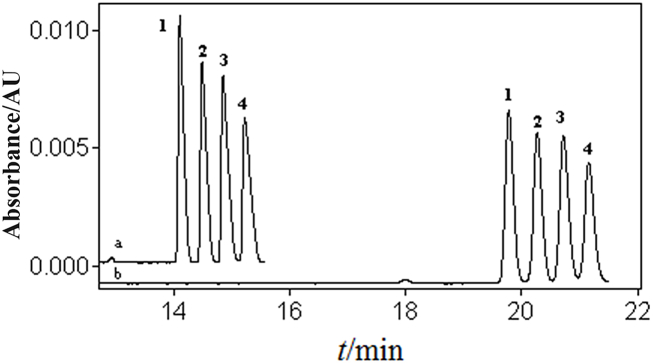
In aqueous CE, SDS is an anionic surfactant that plays a role in inhibiting adsorption, solubilization, and improving separation [30]. Literature reports have indicated that SD can also serve the same purpose and has better separation ability than SDS [31]. To verify if it is similarly applicable in this NAMEKC separation buffer, the composition was kept constant with MeOH-ACN 60:40 (v/v), 70 mmol/L NaAc, and 60 mmol/L TFA. The CMC for SD is 5 mmol/L [32], while the CMC for SDS is 3.92 mmol/L [33]. To compare their separation effects, the same concentration (10 mmol/L) of SDS and SD were separately added (Fig. 4). When SD was added, both sensitivity and resolution decreased, and the migration time increased. Therefore, SDS was still chosen.
Fig. 4.
The effect of SDS and SD on the separation of four disinfectant active ingredients: a. SDS; b. SD. 20 mg/L of mixed standard solution of BACs. 1. C12-BAC; 2. C14-BAC; 3. C16-BAC; 4. C18-BAC. Electrophoresis conditions: the same as Fig. 1.
In this NAMEKC method, micelles are formed using 20 mmol/L of SDS in the background electrolyte. The formation of micelles, rather than the interaction between the negatively charged capillary wall surface and positively charged quaternary ammonium compounds, appears to be the critical factor for the successful separation of alkylbenzyl quaternary ammonium compounds with a long-chain alkyl group as the hydrophobic group. This factor is consistent with the aqueous CE reported by Liu et al. [34]. What differs from Ref. [34] is that in this NAMEKC, the hydrophobic solvent effect of the quaternary ammonium compounds weakens, leading to a decrease in their distribution within the SDS micelle and a change in their electromigration behavior, which facilitates their separation [35,36]. SDS micelles carry a negative charge, and the speed of the electroosmotic flow (EOF) exceeds the migration rate of SDS. Consequently, the EOF propels the SDS micelles containing BAC towards the detection window for analysis.
The composition of the separation buffer solution, including MeOH-ACN (60:40, v/v), 70 mmol/L NaAc, and 60 mmol/L TFA, was kept constant. The effects of 0, 10, 20, and 30 mmol/L SDS on the separation of four disinfectant components in the mixed standard solution were studied (Fig. 5). As the concentration of SDS increased, the resolution and migration time of the four disinfectant components gradually increased, and the peak shapes became broader, indicating that micelles began to take effect. This suggests that the CMC of SDS in MeOH-ACN (60:40, v/v) is less than 10 mmol/L. Since above the CMC, monomers and micelles exist in dynamic equilibrium [37], there may be interactions between SDS monomers and the four disinfectant components. SDS is the most suitable and convenient surfactant not only in aqueous CE but also in NACE.
Fig. 5.
The effect of SDS concentration (mmol/L) on the separation of four disinfectant active ingredients: a. 30; b.20; c. 10; d. 0.20 mg/L of mixed standard solution of BACs. 1. C12-BAC; 2. C14-BAC; 3. C16-BAC; 4. C18-BAC. Electrophoresis conditions: the same as Fig. 1.
When the SDS concentration was below 20 mmol/L, although the migration time was shorter, it could potentially cause current interruption and hinder smooth operation. When it reached 20 mmol/L, the migration time slightly increased, but the current gradually stabilized. Considering the need for stable separation and operation of the separation program, the migration time should be minimized while achieving higher and more stable sensitivity and avoiding in current interruptions. Therefore, the optimized SDS concentration was determined to be 20 mmol/L.
3.3. Selection of capillary inner diameter
The separation efficiency of capillary is inversely proportional to the inner diameter. Capillary with an inner diameter of 50 μm is most commonly used. In the initial experiments, using a capillary with an inner diameter of 50 μm, baseline separation of the four homologues could not be achieved regardless of variations in the concentration of buffer salts and SDS in the separation buffer solution. The main reason is that while pure organic solvents can effectively suppress adsorption, they cannot achieve the separation of four structurally similar benzalkonium homologues that differ by only 2 carbon atoms. However, switching to a capillary with an inner diameter of 25 μm easily achieved the separation and no clogging issues were encountered during actual operation. The reason for no clogging may be due to the good water solubility of disinfectant products containing quaternary ammonium salts and the absence of particulate matter that is prone to clogging the capillary.
3.4. Optimization of separation voltage
In general, higher separation voltage results in a shorter analysis time. However, excessively high current can generate significant Joule heat, leading to peak broadening, decreased sensitivity, and reduced separation efficiency. In NACE method, due to the lower boiling points of organic solvents, high currents can easily cause the solvent to boil, interrupting the flow of current and preventing separation. Therefore, only relatively low voltages can be used to maintain the stability of the current and ensure continuous separation. With all other electrophoretic conditions held constant, the effects of separation voltages (4, 6, 8, 10, 12, and 14 kV) on the separation of four disinfectant components were compared. As the separation voltage increased, sensitivity improved and migration time shortened, but it also caused potential current interruptions. When the voltage was set at 8 kV, the measured current remained relatively stable at 2.3 μA. Considering factors such as stability, repeatability, sensitivity, and separation efficiency, 8 kV was determined as the optimal choice.
3.5. Optimization of sample medium
A suitable sample matrix can increase the solubility and detection sensitivity of the sample, improve the quantitative repeatability, and facilitate the separation and quantification of the four disinfectant components in NAMEKC.
3.5.1. Organic solvents in the sample medium
The composition of organic solvents is a key factor that affects migration time, sensitivity, and peak shape. When using separation buffer as the sample matrix, no conductivity difference exists between the separation buffer and the sample matrix, resulting in peak broadening and inaccurate quantification. When using 100 % MeOH as the sample matrix, the peak shape and sensitivity of the four disinfectant components are optimal, and the migration time is short. However, when using 100 % ACN as the sample matrix, abnormal current or interruption may occur, making it impossible to run the separation program. A mixed solution of MeOH and ACN was attempted, and the effects of four different volume ratios of MeOH and ACN (50:50, 60:40, 80:20, 90:10, v/v) on the separation of the four disinfectant components were tested. At the ratio of 50:50 (v/v), the four components could not achieve baseline separation, and the working current was unstable. It was not until the MeOH content increased to 80 % that the working current gradually stabilized, but the migration time was long and could not reach baseline separation. At the ratio of 90:10 (v/v), although the four components could achieve baseline separation, repeated experiments revealed poor reproducibility of migration time. Ultimately, 100 % MeOH was chosen as the organic solvent composition in the sample matrix (Fig. 6).
Fig. 6.
The effect of the composition of organic solvents (MeOH: ACN, v/v) in the sample matrix on the separation of four disinfectant active ingredients: a. 100; b. 90:10; c. 80:20; d. 50:50.20 mg/L of mixed standard solution of BACs. 1. C12-BAC; 2. C14-BAC; 3. C16-BAC; 4. C18-BAC. Electrophoresis conditions: the same as Fig. 1.
3.5.2. Optimization of trifluoroacetic acid concentration in the sample medium
To increase the sensitivity of the peaks for the four disinfectant components and achieve stable experimental conditions with good quantitative repeatability, 2 mmol/L TFA was added to the sample matrix, inspired by the separation buffer. After optimizing various key factors, the optimal electrophoretic conditions were obtained. Under these conditions, the four disinfectant components in the mixed standard solution were successfully separated (Fig. S1). The theoretical plate numbers calculated according to the USP method were 2.4 × 105 N/m, 2.3 × 105 N/m, 2.9 × 105 N/m, and 2.3 × 105 N/m, for each peak, respectively. These values all fall into the ranges of 8.7 × 104 N/m∼3.0 × 105 N/m reported by NACE literatures. However, for a CE separation, these values can be considered modest. The separation factors were 2.287, 1.978, and 1.839. This method can greatly reduce interference caused by the matrix and facilitate accurate quantification of complex matrix samples. Compared to the three disinfectant components in the aqueous separation buffer with tailing factors of 1.473, 1.624, and 1.445, the tailing factors in NAMEKC method were 1.086, 1.163, 1.136, and 1.189, indicating an improvement in peak shape, suppression of adsorption, and resolution of tailing issues.
3.6. Methodological validation
3.6.1. Limit of detection and limit of quantification
The laboratory's existing disinfectant, which contains benzaldehyde, was diluted in the sample matrix. The absence of peaks in the blank sample confirmed that it was free of the four target components. The mixed standard solution of the four BACs was then diluted and added to the blank sample, which was further diluted 100 times with a non-aqueous sample matrix. The limit of detection (LOD, S/N = 3) and limit of quantification (S/N = 10) were determined to be 1.0 mg/L and 5.0 mg/L, respectively.
3.6.2. Linearity
Under optimal conditions, the mixed standard stock solution of the four BAC disinfectant components was diluted in the sample matrix. Mixed standard solutions with mass concentrations of 5, 10, 20, 40, 60, 80, and 100 mg/L were prepared and sequentially analyzed using the external calibration method. The linear relationship between the corrected peak area (y, peak area divided by migration time) and the mass concentration (x, mg/L) was calculated, as shown in Table 1.
Table 1.
Linear regression equations, linearity range and correlation cofficient of four BACs.
| Four BACs | Linear regression equations | Linearity (mg/L) | Correlation Coefficient |
|---|---|---|---|
| C12-BAC | y = 42.676x+82.004 | 5∼100 | 0.9993 |
| C14-BAC | y = 36.61x+72.119 | 5∼100 | 0.9995 |
| C16-BAC | y = 39.239x-8.8982 | 5∼100 | 0.9996 |
| C18-BAC | y = 31.929x-9.8019 | 5∼100 | 0.9992 |
3.6.3. Method precision
A disinfectant sample that does not contain any of the four target substances was selected. 0.2 mL of the mixed standard solution with a mass concentration of 1 g/L for each of the four target components was added, resulting in self-made samples containing all four disinfectant components. Method precision experiments were conducted under optimal electrophoretic conditions. The relative standard deviation (RSD) of the content of the four disinfectant components was calculated for seven parallel preparations to assess the intra-day precision of the method. Using the same method, a home-made sample were measured in triplicate for seven consecutive days, and the RSD of the content of the four disinfectant components was calculated to assess the inter-day precision of the method. The results of intra-day precision and inter-day precision for the home-made sample are shown in Table 2. The intra-day and inter-day precisions were 2.6 %–4.7 % and 0.26 %–4.7 %, respectively, demonstrating good precision. The reasons why the precision of intra-day measurements is not as good as that of inter-day measurements are twofold. First, the precision of intra-day measurements is based on 7 replicates, whereas inter-day precision is based on 3 replicates conducted simultaneously over 7 consecutive days, resulting in a total of 21 replicates for inter-day measurements. This larger sample size contributes to better precision in inter-day measurements. Secondly, the volatility of organic solvents in the separation buffer may affect the precision. Intra-day precision is based on 7 consecutive runs without changing the buffer solution, while inter-day precision is based on only 3 runs without changing the buffer solution. Consequently, more organic solvents tend to volatilize after 7 runs compared to 3 runs, as its challenging to completely prevent solvent volatilization in existing commercial instrument setups. This discrepancy in solvent volatilization contributes to the inferior intra-day precision compared to inter-day precision.
Table 2.
Intra-day and inter-day precisions (n = 7) of four BACs.
| RSD (%) | Contents (%) |
|||
|---|---|---|---|---|
| C12-BAC | C14-BAC | C16-BAC | C18-BAC | |
| Intra-day | 4.7 | 2.6 | 2.6 | 2.7 |
| Inter-day | 4.7 | 2.1 | 0.26 | 1.6 |
3.6.4. Spike recovery
A disinfectant sample that does not contain any of the four target substances was selected as the background for the spike recovery experiment. 0.1 mL of blank disinfectant sample was taken and placed in a 15 mL plastic centrifuge tube with graduation. Then, 0.1, 0.4, and 0.8 mL of the mixed standard solution with a mass concentration of 1 g/L for each of the four target components were added separately to create spike recovery solutions with spiked levels of 10.0, 40.0, and 80.0 mg/L. Each spike recovery sample at different spike levels was prepared in parallel with a total of seven repetitions to assess the spike recovery rate of the method. The results of the measurements are shown in Table 3, and the electropherogram is shown in Fig. S2. The recoveries of the NAMEKC-UV method at the three spiked levels were 92.5 %–109.1 %. Satisfactory recoveries were achieved.
Table 3.
Recoveries of the NAMEKC-UV method (n = 7).
| BACs | Spiked concentrations (mg/L) | Recoveries (%) | RSD(%) |
|---|---|---|---|
| C12-BAC | 10.0 | 101.4 | 4.6 |
| 40.0 | 92.5 | 4.5 | |
| 80.0 | 106.7 | 3.2 | |
| C14-BAC | 10.0 | 106.1 | 2.7 |
| 40.0 | 102.9 | 2.0 | |
| 80.0 | 104.8 | 2.5 | |
| C16-BAC | 10.0 | 104.6 | 2.1 |
| 40.0 | 99.3 | 0.3 | |
| 80.0 | 102.6 | 2.6 | |
| C18-BAC | 10.0 | 109.1 | 2.5 |
| 40.0 | 104.3 | 1.5 | |
| 80.0 | 104.1 | 2.7 |
3.7. Sample analysis
A total of 19 samples of disinfecting laundry detergent, disinfecting hand sanitizer, skin disinfectant, surface disinfectant for environmental objects, and disinfecting wipes were purchased. According to the method in section 2.3, each sample was processed in quadruplicate, and the results were compared with the product labeling values (see Table S1). It was found that some of the measured values did not match the indicated values on the products. This discrepancy may be due to the presence of other commonly used single-long-chain or double-long-chain quaternary ammonium salt components. The product labeling indicates the total amount of quaternary ammonium salt, which is quantitatively combined with the two single-long-chain quaternary ammonium salts (C12-TMA, C14-TMA) and three double-long-chain quaternary ammonium salts (di-C8, di-C9, di-C10) established using the NACE combined with UV detection method [2]. This suggests that there are still issues with the labeling accuracy of commercially available compound quaternary ammonium salt disinfectants.
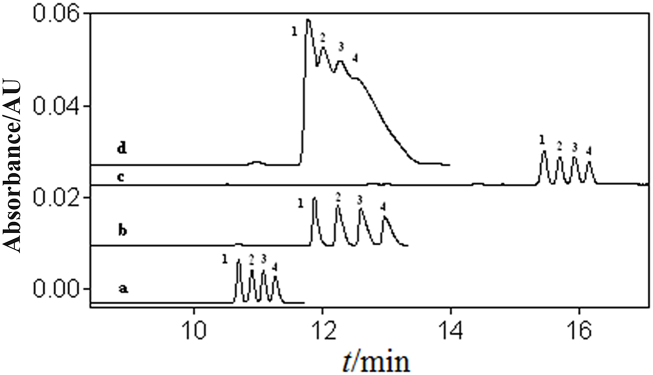
Some samples may have a high water content and need to be dried before being reconstituted in the sample medium and diluted for injection. Sample No. 7 may also contain PHMB, which can cause matrix interference as illustrated in Fig. S3. After drying, the sample can be effectively separated and quantified using NAMEKC, while it cannot be quantified using the aqueous CE method specified by the National Standard Method. Sample No. 11 also exhibits matrix interference with unseparated peaks also shown in Fig. S4. After drying, the sample can be separated and accurately quantified using NAMEKC, whereas the aqueous CE method specified by the National Standard Method cannot achieve quantification. One-way analysis of variance using SPSS 25 software showed no statistically significant difference (p = 0.869 and 0.936) between the results obtained by the two methods, confirming the accuracy of the developed NAMEKC-UV method.
4. Conclusion
In this study, the NAMEKC method combined with UV detection was applied for simultaneous separation and determination of four types of BACs. Compared to the LOD of the aqueous CE method (0.5 mg/L), specified in GB/T 26369-2020, the LOD of the present NAMEKC-UV method was double (1 mg/L). The resolution is greater than 2, far exceeding the baseline resolution of 1.5, greatly mitigating the influence of matrix interference and facilitating accurate quantification of complex sample components. The column efficiency of the two methods is almost equivalent in terms of a theoretical plate number, up to 2.9 × 105 N/m for NAMEKC and 3.8 × 105 N/m for aqueous CE. Additionally, the method featured a straightforward pre-treatment process. For some samples with interference from other components, drying and reconstitution in the sample medium followed by dilution were necessary prior to injection. This method addressed the issues of tailing peaks. It also enabled simultaneous separation and determination of the four BAC congeners. The spiked recoveries ranged from 92.5 % to 109.1 %, and the RSDs of migration time and content were both below 5 %, demonstrating precise and accurate analytical results. The method exhibited good reproducibility and stability.
Although the separation time of this NAMEKC method is longer compared to the National Standard Method, it can be considered as the preferred method for the analysis of the four BAC congeners. It is suitable for the analysis and determination of a wide range of commercially available quaternary ammonium salt disinfectant products, including surface disinfectants, hand sanitizers, laundry disinfectants, and disinfectant wipes. Further optimization of experimental conditions and reduction of separation time could enhance detection efficiency, potentially making it an effective alternative to the National Standard Method.
Funding
This work was funded by Capital's Funds for Health Improvement and Research (CFH 2022-4G-30118), and Beijing Municipal Science and Technology Project (Z211100007021008).
Data availability statement
The data supporting reported results can be obtained from the corresponding author upon request.
CRediT authorship contribution statement
Kai Yao: Writing – original draft, Validation. Ruoke Jiang: Writing – original draft, Validation. Ping Wang: Methodology, Conceptualization. Jing Zhang: Formal analysis. Bing Shao: Conceptualization. Xiaojing Ding: Writing – review & editing, Supervision.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e31797.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.National Disinfection Product Filing Information Service Platform. http://credit.jdzx.net.cn/xdcp/loginpage.do.
- 2.Yao K., Jiang R., Wang P., Zhang J., Shao B., Ding X. Analysis of two single and three double long-chain quaternary ammonium compounds via non-aqueous capillary electrophoresis with indirect ultraviolet detection. Separations. 2023;10:387. doi: 10.3390/separations10070387. [DOI] [Google Scholar]
- 3.Bureš F. Quaternary ammonium compounds: simple in structure, complex in application. Top. Curr. Chem. 2019;377:2–21. doi: 10.1007/s41061-019-0239-2. [DOI] [PubMed] [Google Scholar]
- 4.Domagk G. Eine neue klasse von desinfektionsmitteln. Dtsch. Med. Wochenschr. 1935;61:829–832. [Google Scholar]
- 5.Mcdonnell G., Russell A.D. Antiseptics and disinfectants: activity, action, and resistance. Clin. Microbiol. Rev. 1999;12:147–179. doi: 10.1128/CMR.12.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.EFSA Reasoned opinion on the dietary risk assessment for proposed temporary maximum residue levels (MRLs) of didecyldimethylammonium chloride (DDAC) and benzalkonium chloride (BAC) EFSA J. 2014;12:3675. doi: 10.2903/j.efsa.2014.3675. [DOI] [Google Scholar]
- 7.Hostetler K.A., Fisher L.C., Burruss B.L. Prenatal developmental toxicity of alkyl dimethyl benzyl ammonium chloride and didecyl dimethyl ammonium chloride in CD rats and New Zealand white rabbits. Birth Defects Res. 2021;113:925–944. doi: 10.1002/bdr2.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prieto-Blanco M.C., Argente-García A., Campíns-Falcó P. A capillary liquid chromatography method for benzalkonium chloride determination as a component or contaminant in mixtures of biocides. J. Chromatogr. A. 2016;1431:176–183. doi: 10.1016/j.chroma.2015.12.073. [DOI] [PubMed] [Google Scholar]
- 9.Takeoka G.R., Dao L.T., Wong R.Y., Harden L.A. Identification of benzalkonium chloride in commercial grapefruit seed extracts. J. Agric. Food Chem. 2005;53:7630–7636. doi: 10.1021/jf0514064. [DOI] [PubMed] [Google Scholar]
- 10.Boucher C., Waite-Cusic J., Stone D., Kovacevic J. Relative performance of commercial citric acid and quaternary ammonium sanitizers against listeria monocytogenes under conditions relevant to food industry. Food Microbiol. 2021;97 doi: 10.1016/j.fm.2021.103752. [DOI] [PubMed] [Google Scholar]
- 11.Jiao Y., Niu L., Ma S., Li J., Tay F.R., Chen J. Quaternary ammonium-based biomedical materials: state-of-the-art, toxicological aspects and antimicrobial resistance. Prog. Polym. Sci. 2017;71:53–90. doi: 10.1016/j.progpolymsci.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldstein M.H., Silva F.Q., Blender N., Tran T., Vantipalli S. Ocular benzalkonium chloride exposure: problems and solutions. Eye. 2022;36:361–368. doi: 10.1038/s41433-021-01668-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Para B.V., Núñez O., Moyano E., Galceran M.T. Analysis of benzalkonium chloride by capillary electrophoresis-tandem mass spectrometry. Electrophoresis. 2006;27:2225–2232. doi: 10.1002/elps.200500716. [DOI] [PubMed] [Google Scholar]
- 14.State Administration for Market Regulation; Administration of China Standardization . China Standards Press; Beijing, China: 2020. GB/T 26369-2020 Hygienic Requirement for Quanternary Ammonium Disinfectant. [Google Scholar]
- 15.Ma W., Ma X., Sha O., Liu Y. Two spectrophotometric methods for the assay of benzalkonium chloride in bandage samples. J. Surfactants Deterg. 2014;17:177–181. doi: 10.1007/s11743-013-1446-4. [DOI] [Google Scholar]
- 16.State Administration for Market Regulation . China Standards Press; Beijing, China: 2020. Administration of China Standardization, GB/T 26367-2020 Hygienic Requirement for Biguanides Disinfectants. [Google Scholar]
- 17.Gaber M., Shawish H.M.A., Khedr A.M., Abed-Almonem K.I. Determination of benzalkonium chloride preservative in pharmaceutical formulation of eye and ear drops using new potentiometric sensors. Mater. Sci. Eng. C. 2012;32:2299–2305. doi: 10.1016/j.msec.2012.06.018. [DOI] [Google Scholar]
- 18.Gallart-Mateu D., Armenta S., Esteve-Turrillas F.A., de la Guardia M. Ion mobility spectrometry as a fast analytical tool in benzalkonium chloride homologs determination. Talanta. 2017;164:110–115. doi: 10.1016/j.talanta.2016.11.024. [DOI] [PubMed] [Google Scholar]
- 19.Liu J., Deng W., Yu M., Wen R., Yao S., Chen B. Rapid analysis of benzalkonium chloride using paper spray mass spectrometry. J. Pharm. Biomed. Anal. 2017;145:151–157. doi: 10.1016/j.jpba.2017.06.030. [DOI] [PubMed] [Google Scholar]
- 20.Kang H.I., Shin H.S. Rapid and sensitive determination of benzalkonium chloride biocide residues in soil using liquid chromatography-tandem mass spectrometry after ultrasonically assisted extraction. Bull. Kor. Chem. Soc. 2016;37:1219–1227. doi: 10.1002/bkcs.10842. [DOI] [Google Scholar]
- 21.Zheng G.J., Lin Y.K., Yang J.Q. Determination of 7 kinds of quaternary ammonium salt in disinfectants by HPLC. Chin. J. Anal. Instrum. 2021;4:49–53. doi: 10.3969/j.issn.1001-232x.2021.04.010. [DOI] [Google Scholar]
- 22.State Administration for Market Regulation . China Standards Press; Beijing, China: 2021. Administration of China Standardization, GB/T 39873-2021 Determination of Quaternary Ammonium Salt in Disinfectant-Liquid Chromatography-Tandem Mass Spectrometry. [Google Scholar]
- 23.Yildirim G., Türköz Acar E. Determination of benzalkonium chloride in wet wipes by using a validated capillary electrophoresis method. J. Cosmet. Sci. 2017;68:1–10. [PubMed] [Google Scholar]
- 24.Turnes-Carou I., Prieto-Blanco C., López-Mahía P., Muniategui-Lorenzo S., Prada-Rodríguez D. Simultaneous separation of amidoamines and benzalkonium chloride surfactants by capillary zone electrophoresis. Chromatographia. 2002;56:605–609. doi: 10.1007/BF02497677. [DOI] [Google Scholar]
- 25.Hou Y.H., Wu C.Y., Ding W.H. Development and validation of a capillary zone electrophoresis method for the determination of benzalkonium chlorides in ophthalmic solutions. J. Chromatogr. A. 2002;976:207–213. doi: 10.1016/S0021-9673(02)00943-3. [DOI] [PubMed] [Google Scholar]
- 26.Para B.V., Nunez O., Moyano E., Galceran M.T. Analysis of benzalkonium chloride by capillary electrophoresis-tandem mass spectrometry. Electrophoresis. 2006;27:2225–2232. doi: 10.1002/elps.200500716. [DOI] [PubMed] [Google Scholar]
- 27.Kenndler E. A critical overview of non-aqueous capillary electrophoresis. Part i: mobility and separation selectivity. J. Chromatogr. A. 2014;1335:16–30. doi: 10.1016/j.chroma.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 28.Zhu H., Lue W., Li H., Ma Y., Hu S., Chen H., Chen X. Micelle to solvent stacking of two alkaloids in nonaqueous capillary electrophoresis. J. Chromatogr. A. 2011;1218:5867–5871. doi: 10.1016/j.chroma.2011.06.106. 0.1016/j.chroma.2011.06.106. [DOI] [PubMed] [Google Scholar]
- 29.Feng Z., Ju L., Yu T., Du Y., Sun X. Imidazolium-based ionic liquid surfactants as pseudostationary in combination with a chiral selector in micellar electrokinetic chromatography. Anal. Bioanal. Chem. 2019;411:3849–3856. doi: 10.1007/s00216-019-01861-8. [DOI] [PubMed] [Google Scholar]
- 30.Evans K., Wang X., Roper M.G. Chiral micellar electrokinetic chromatographic separation for determination of l- and d-primary amines released from murine islets of langerhans. Anal. Methods. 2019;11:1276–1283. doi: 10.1039/c8ay02471e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu Y., Li J., Zhao S., Ding X. Simultaneous determination of 11 food additives by micellar electrokinetic capillary chromatography. Food Anal. Methods. 2016;9:589–595. doi: 10.1007/s12161-015-0225-4. [DOI] [Google Scholar]
- 32.Kuhn R., Hoffstetter-Kuhn S. Springer-Verlag; Berlin, Germany: 1993. Capillary Electrophoresis: Principles and Practice; p. 192. [DOI] [Google Scholar]
- 33.Morelli J.J., Szajer G. Analysis of surfactants: part I. J. Surfactants Deterg. 2000;3:539–552. doi: 10.1007/s11743-000-0154-8. [DOI] [Google Scholar]
- 34.Liu H.Y., Ding W.H. Determination of homologues of quaternary ammonium surfactants by capillary electrophoresis using indirect UV detection. J. Chromatogr. A. 2004;1025:303–312. doi: 10.1016/j.chroma.2003.10.108. [DOI] [PubMed] [Google Scholar]
- 35.Huang R., Mu X.J., Yin Y.G., Wei W.L., Chen Z.T., Xia Z.N. Separation of phthalates in non-aqueous micelle using capillary electrokinetic chromatography. Chin. J. Chromatogr. 2006;24:597–600. doi: 10.3321/j.issn:1000-8713.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 36.Guo X., Wang K., Chen G.H., Shi J., Wu X., Di L.L., Wang Y. Determination of strobilurin fungicide residues in fruits and vegetables by nonaqueous micellar electrokinetic capillary chromatography with indirect laser-induced fluorescence. Electrophoresis. 2017;38:2004–2010. doi: 10.1093/chromsci/bmt001. [DOI] [PubMed] [Google Scholar]
- 37.Yang Y., Zhang Q., Wang T., Yang Y. Determination of the critical micelle concentration of sodium dodecyl sulphate in aqueous solution in the presence of an organic additive of acetonitrile by conductometry and an inorganic additive of phosphate by fluorometry. Asian J. Chem. 2013;25:6657–6660. doi: 10.14233/ajchem.2013.14403. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting reported results can be obtained from the corresponding author upon request.