Abstract
This review aims to explore the current application of Cranial Ultrasound Screening (CUS) in the diagnosis and treatment of brain diseases in extremely preterm infants. It also discusses the potential role of emerging ultrasound-derived technologies such as Super Microvascular Structure Imaging (SMI), Shear Wave Elastography (SWE), Ultrafast Doppler Ultrasound (UfD), and 3D ventricular volume assessment and automated segmentation techniques in clinical practice. A systematic search of medical databases was conducted using the keywords "(preterm OR extremely preterm OR extremely low birth weight) AND (ultrasound OR ultrasound imaging) AND (neurodevelopment OR brain development OR brain diseases OR brain injury OR neuro*)" to identify relevant literature. The titles, abstracts, and full texts of the identified articles were carefully reviewed to determine their relevance to the research topic. CUS offers unique advantages in early screening and monitoring of brain diseases in extremely preterm infants, as it can be performed at the bedside without the need for anesthesia or special monitoring. This technique facilitates early detection and intervention of conditions such as intraventricular hemorrhage, white matter injury, hydrocephalus, and hypoxic-ischemic injury in critically ill preterm infants. Continuous refinement of the screening and follow-up processes provides reliable clinical decision-making support for healthcare professionals and parents. Emerging ultrasound technologies, such as SWE, SMI, and UfD, are being explored to provide more accurate and in-depth understanding of brain diseases in extremely preterm infants. SWE has demonstrated its effectiveness in assessing the elasticity of neonatal brain tissue, aiding in the localization and quantification of potential brain injuries. SMI can successfully identify microvascular structures in the brain, offering a new perspective on neurologic diseases. UfD provides a high-sensitivity and quantitative imaging method for the prevention and treatment of neonatal brain diseases by detecting subtle changes in red blood cell movement and accurately assessing the status and progression of brain diseases. CUS and its emerging technologies have significant applications in the diagnosis and treatment of brain diseases in extremely preterm infants. Future research aims to address current technical challenges, optimize and enhance the clinical decision-making capabilities related to brain development, and improve the prevention and treatment outcomes of brain diseases in extremely preterm infants.
Keywords: Extremely preterm infants, Neurosonography, Cranial ultrasound screening, Microvascular structure imaging, Shear wave elastography, Ultrafast Doppler ultrasound
1. Introduction
The importance of neonatal bedside CUS in neurological examinations of premature infants is increasing day by day. Its main advantages are that it can be performed bedside, without the need for anesthesia or special monitoring. Although the existence of CT and MRI makes ultrasound considered as a preliminary screening measure, in diagnosing and continuously monitoring brain injuries in premature babies, CUS has unique advantages in NICU due to its low cost, powerful diagnostic capabilities, and operational convenience. Premature infants have significantly increased risks of hypoxic-ischemic injury, intraventricular hemorrhage, periventricular leukomalacia, and post-hemorrhagic hydrocephalus [1], but these complications can be assessed by CUS in the early life of newborns for active intervention. Over the past decade, CUS technology has made significant progress, prompting us to review and update this topic. We hope to provide a comprehensive and in-depth perspective by integrating the latest research results and technological advancements, summarizing the application of CUS in the current brain examination of premature infants, and discussing the potential roles of some emerging technologies in future clinical practice, such as ultra-microvascular imaging [[2], [3], [4]], elastography [5,6], fast Doppler ultrasound imaging [7], and 3D ventricular volume evaluation and automatic segmentation technology [[8], [9], [10]], which have opened new perspectives for us to explore the brain functions of premature babies. We also expect this article to guide more professionals to pay attention to this field, explore the potential of CUS and apply it to clinical practice, providing more effective tools for the diagnosis and treatment of brain diseases in premature babies.
2. Literature collection and review
To identify research related to extremely preterm infants, ultrasound technology (ultrasound or sonography), as well as neurodevelopment or brain disorders or injuries, we crafted a precise search strategy using the Boolean operators “AND” and “OR".
We input a combination of the following keywords into various large databases to query: '(preterm OR extremely preterm OR extremely low birth weight) AND (ultrasound OR sonography) AND (neurodevelopment OR brain development OR cerebral diseases OR brain injuries OR neuro*)'. This search yielded a multitude of potential articles, from which we meticulously screened each article's title and abstract to determine its true relevance to our literature topic. Once we obtained all the full texts of the relevant articles, we further examined the referenced literature to ensure no important research was overlooked. We excluded non-English literature, studies that had not undergone peer review, and duplicate or off-topic research. We also noted that some key research about extremely preterm infants might be included in broader pediatric or neurology literature, and we took these articles into account as well. Our detailed search strategy ensured that we obtained a wide and important range of literature, accurately and comprehensively reflecting the current understanding and discoveries in the medical field regarding the application of bedside neurosonography in the prevention and treatment of brain diseases in extremely preterm infants.
3. Screening for brain injury in high-risk infants
Identifying neonatal brain injury early in life is a current focus of clinical attention and research in neonatal medicine, especially for premature infants and high-risk groups. With the rapid development of technologies such as cranial ultrasound screening (CUS) and Magnetic Resonance Imaging (MRI), we now have more tools to identify and prevent these potential health issues [11,12].
Researchers used portable ultrasound to monitor cerebral hemodynamics in critically ill preterm infants with gestational age ≤30 weeks during transport, with ultrasound monitoring performed on average within 3 h after birth. The results found that before transport, there were already 10 cases of Grade I IVH, 2 cases of Grade II IVH, 1 case of Grade IV IVH, and 32 normal scans. After transport, 3 new cases of Grade I IVH were added. Compared with preterm newborns with normal blood flow, those with low systemic circulation had a higher mortality rate: 33 % (4/12) died (OR = 7.2, 95 % CI: 1.1–47, p = 0.022). The study demonstrated that using portable ultrasound during transport can provide early screening [11], which can improve the survival rate of these extremely preterm newborns.
Although there is currently no clear consensus on the timing and frequency of brain injury screening, a retrospective study recommends that in extremely preterm infants who have had early head ultrasound screening with negative results, the routine head ultrasound examination on the 30th day after birth has limited effectiveness in detecting severe intraventricular hemorrhage or cystic periventricular leukomalacia. For infants with abnormal early head ultrasound results, it is recommended that a comprehensive MRI examination should be completed on the 30th day after birth to correct the gestational age [13]. For low-level intraventricular hemorrhage, magnetically sensitive weighted imaging has higher sensitivity [14]. However, cranial ultrasound (CUS) still retains its unique advantages in some areas, such as screening for intracranial hemorrhage in the shortest possible time in critically ill preterm infants [12]. For extremely preterm infants who did not find intraventricular hemorrhage (IVH) or have mild IVH but no other risk factors on initial CUS screening, they can selectively receive CUS subsequently. However, for infants who need long-term mechanical ventilation, further screening is needed even if the initial ultrasound results are negative [15].
Further research has assessed the accuracy of CUS grading in determining brain injury in extremely preterm infants and its relationship with cognitive, psychological, and motor development. Among 129 extremely preterm infants, the possibility of neurodevelopmental impairment in newborns with moderate and severe brain injuries shown by initial CUS screening increased, and the CUS grading had high sensitivity and specificity in predicting cerebral palsy. These results highlight the importance of CUS examination in the early assessment of extremely preterm infants [16]. At the same time, CUS is also used to assess the risk of brain injury in newborns with fetal growth restriction (FGR). Research has found that compared with non-FGR preterm infants, the risk of death, severe intraventricular hemorrhage, or periventricular leukomalacia (PVL) in preterm infants with FGR and abnormal CUS is more than three times higher [17].
The effective application of CUS requires medical staff to have professional skills. Using advanced task trainers and computer simulators that simulate skulls, combined with theoretical teaching courses, can effectively train novices' CUS screening capabilities [18]. After a workshop-style training that includes theoretical and practical sessions, the CUS-related knowledge and scanning level of the neurosonographers participating in the training can be significantly improved. When reassessed in the third and sixth months after the end of the course, the majority of doctors can still guarantee the quality of scanning. This shows that this blended structured training model is effective in training neurosonographers [18].
3.1. Intracranial hemorrhage
Bedside ultrasound can be used to monitor the occurrence of intraventricular hemorrhage (IVH) in critically ill extremely low birth weight infants in real time [19]. Sartori and colleagues compared the brain MRI and CUS examination results of 51 newborns (43 of them were extremely preterm infants) to verify the effectiveness of CUS in diagnosing IVH. The study showed that CUS and MRI have comparable value in identifying brain abnormalities such as germinal matrix hemorrhage, especially CUS's performance in screening and distinguishing between mild and severe hemorrhages in the germinal matrix layer (GMH) is particularly prominent [20]. Especially for preterm infants with III-IV degree cerebral hemorrhage, it can be seen that CUS is an appropriate tool for screening and follow-up of intracranial hemorrhage. Preterm infants with severe intracranial hemorrhage are prone to ventricular enlargement, which can develop into malignant hydrocephalus and cause neurodevelopmental disorders. Beijst et al. included 31 preterm infants with germinal matrix intraventricular hemorrhage (GMH-IVH). Through 2D-CUS image acquisition, the ventricular index, frontal horn width, and thalamus-occipital distance were measured, and head MRI examinations were also completed. The results showed that the ventricular index, frontal horn width, and thalamus-occipital distance were significantly correlated with MRI ventricular volume [21].
However, due to the limited accuracy of manually estimating ventricular volume with 2D-CUS images, 3D-CUS imaging systems have been developed on the market, which can more accurately image preterm infants at high risk of ventricular enlargement based on the realism of 3D reconstruction of ventricular volume [22]. In 2017, researchers first reported an automatic segmentation method based on 3D-CUS images, which achieved more precise monitoring of ventricular volume in infants with IVH [8]. Using a 2D and 3D convolutional neural network (CNN) to automatically segment the ventricles in three-dimensional ultrasound images [9,10], and by introducing a fully convolutional network (FCN) and a Compositional Pattern Producing Network (CPPN), the location of the ventricles can be further precisely located, saving a lot of manual segmentation time, and quantitatively identifying the phenotype of ventricular enlargement in preterm infants.
Kishimoto and colleagues used 3D-CUS to monitor ventricular enlargement and dynamically measured the ventricular volume of 38 preterm infants. The study determined the treatment threshold (45.45 cm3 or 0.31 cm3/d). When the ventricular volume or growth rate is higher than this threshold, ventricular puncture and drainage treatment are required; if it is lower than this threshold, it can be considered for self-recovery and absorption [23]. A prospective cohort study used both 3D-CUS and functional near-infrared spectroscopy (fNIRS) to monitor newborns with GMH-IVH. The results showed a significant correlation between the increase in ventricular volume and the decrease in spontaneous functional connectivity (sFC) in infants with severe GMH-IVH. This indicates that regional enlargement of the ventricular volume may potentially affect the development of brain white matter, and the combined use of 3D-CUS and fNIRS can monitor the progression of GMH-IVH in preterm infants. These new technologies can improve the judgment and monitoring capabilities of neurosonographers on the issue of ventricular enlargement in preterm infants.
3.2. White matter injury and periventricular leukomalacia (PVL)
CUS can also identify cystic white matter injury (WMI) [24]. A study reviewed all live preterm infants born between 22 and 31 weeks gestational age in Nova Scotia, Canada from 1993 to 2013. Among the 1184 eligible preterm infants, 7 % (87/1184) were found to have cystic WMI. With the development of AI technology, it is now possible to simultaneously segment white matter and predict the risk of WMI from ultrasound images. The study segmented images of 158 preterm infants (32 of whom had WMI), and the platform performed well in both diagnostic performance and white matter segmentation. This could have been achieved by MR imaging in the past, but now it can be achieved by AI analysis of ultrasound images, providing a new plan for the diagnosis of WMI in preterm infants [25].
Jung et al. compared the texture parameters of continuous CUS images of PVL and normal periventricular echo (PVE) in extremely preterm infants. The results showed that PVL can be predicted within the first three weeks after birth in extremely preterm infants using texture analysis [26]. A prospective cohort study covered 704 preterm infants born before 30 weeks of gestation between 2014 and 2016, and CUS examinations were performed on days 3–14 after birth, at 36 weeks corrected gestational age, and at discharge [27]. Neurobehavioral evaluations were conducted using the NICU Network Neurobehavioral Scale (NNNS) method. The results showed that preterm infants with WMI found by CUS performed poorly in attention, muscle tone, and motor quality. Newborns who found white matter abnormalities in the first CUS showed more pronounced phenotypes of decreased attention and hypotonia. For these preterm infants who found white matter abnormalities early, some personalized interventions should be used to improve the long-term neurobehavioral outcome.
3.3. Infection monitoring
Due to the limitations of blood culture diagnosis, Liu et al. aimed to monitor the early changes in cerebral hemodynamics in preterm infants through CUS Doppler to timely and objectively detect early-onset sepsis (EOS). The results showed that when EOS occurs, the pulsatility index increases, the resistance index decreases, the average speed increases, and cerebral blood flow increases [28]. This means that early CUS Doppler monitoring of cerebral hemodynamics has certain clinical significance for suspected EOS in preterm infants. Claessens et al. [29] conducted a prospective study on 117 extremly preterm neonates suspected of late-onset sepsis (LOS) with a brain ultrasound examination. In the end, cerebral abnormalities were found in 9 preterm infants. There were 7 in the LOS confirmed group (blood culture positive), including thalamic infarction, abnormal dense signals in the thalamus/basal ganglia, and I-III degree IVH; and 2 in the clinical sepsis group (blood culture negative).
3.4. Hypoxic-ischemic encephalopathy
A study compared the effects of CUS and MRI in assessing suspected hypoxic-ischemic brain injury. The study analyzed 147 preterm infants, and the results showed that ultrasound showed very high sensitivity and specificity in detecting hydrocephalus, ventricular enlargement, and obvious structural abnormalities [30]. However, to identify minor hemorrhage or WMI, CUS still needs to be evaluated in conjunction with MRI. Selective use of CUS or MRI based on specific clinical conditions can reduce medical costs and achieve early detection and prevention of hypoxic-ischemic brain injury.
4. Neurodevelopment evaluation (structural morphology)
With the application of various emerging ultrasound technologies such as 3D-CUS, and derivative ultrasound imaging, the assessment of neonatal brain development has become a reality. These techniques provide us a new window to deeply understand brain development. A study involving 344 ultrasound examinations in 94 preterm infants developed a brain development ultrasound scoring system for evaluating cortical development in preterm infants [31]. In the process of building this system, the research team identified three brain developmental indicators significantly related to gestational age: the opening of the temporal lobe, the cortical height of the insular gyrus, and the depth of the cingulate gyrus. Each of these three indicators was assigned a score from 0 to 2, totaling 6 points. The final results showed that the scores were significantly correlated with gestational age. Notably, another method named B-GREAT for assessing brain growth in preterm infants was proposed by Arena and others [32]. This study involved 80 preterm infants born around 28 weeks of gestation who underwent a total of 528 CUS examinations. The results indicated that B-GREAT demonstrated good consistency and repeatability in assessing brain growth in preterm infants. Based on these data, the researchers created a brain growth assessment chart using the B-GREAT method. Both of the assessment methods can directly monitor brain development status at the bedside of preterm infants. In addition, one study achieved the measurement of brain volume in preterm infants using 2D-CUS (estimated absolute brain volume, EABV), and multiple regression analysis showed that ventricular enlargement, postnatal resuscitation, bronchopulmonary dysplasia, and late-onset sepsis could be independent risk factors leading to reduced brain volume. Follow-up results of Psychomotor Development Index (PDI) showed a mild positive correlation with EABV, that is, preterm infants with lower EABV had a worse PDI, indicating that abnormal changes in brain volume in preterm infants can affect the long-term neurodevelopmental outcomes of preterm infants [33].
2D-CUS has high reliability in assessing the size of the cerebellum, with a consistency of up to 90 % [34] when measuring the transverse cerebellar diameter (TCD-MF) through the mastoid fontanel, thereby reflecting the developmental status of the cerebellum. Further research involved 71 preterm infants of less than 32 weeks without brain structural abnormalities and 58 full-term infants. Their cerebellar dimensions (including transverse cerebellar diameter, cerebellar fissure height, anteroposterior fissure diameter, and cerebellar fissure area) were measured using Image Arena software [35]. The results showed that after adjusting for head shape, there were no significant differences between the two groups, indicating that even the smallest preterm infants could maintain certain stability in cerebellar growth. Muehlbacher and others retrospectively analyzed the ultrasound scan data of 105 preterm infants and found that the diameters of the cerebellar hemispheres obtained through the mastoid fontanel and the foramen magnum were highly correlated, showing a close relationship between corrected gestational age and cerebellar growth [36]. The occipital ultrasound performed through the foramen magnum demonstrated superior image quality and resolution compared to other directions, validating its effectiveness in monitoring postnatal cerebellar growth in preterm infants.
3D-CUS is a novel ultrasound technology that has emerged in recent years. Early neurodevelopmental status in FGR preterm infants was explored using 3D-CUS. It was found that there were no significant differences in the total ventricular volume between FGR preterm infants and full-term infants under corrected gestational age, but FGR preterm infants performed worse in the Test of Infant Motor Performance (TIMP) assessment at 12–14 weeks postnatal. In addition, there was a significant correlation between large ventricular volume and low TIMP scores. The study showed that early use of 3D-CUS to evaluate ventricular volume could predict early neuro-motor development in infants [37]. Meanwhile, 3D-CUS technology can be used to construct biological reference values for the corpus callosum in extremely preterm infants, including size, length, circumference, and surface area. The size of the corpus callosum at corrected full-term age was related to neurodevelopmental outcomes at 5 years of age, demonstrating the clinical value of accurately measuring corpus callosum data through 3D-CUS in predicting neurodevelopmental outcomes [38].
5. Evaluation of brain function (hemodynamics)
Recently, a novel ultrasound system named NeoDoppler has been utilized in preterm infants [39]. NeoDoppler can be fixed on the anterior fontanel, accurately and continuously measuring blood flow velocity at different depths in the brain, while recording dynamic data. By continuously monitoring cerebral blood flow, a deeper understanding of early cerebral hemodynamics in preterm infants can be obtained, helping to prevent brain damage during the sensitive stage of brain development. Camfferman et al. systematically reviewed the association of CUS measurement of different cerebral arterial blood flow parameters with preterm brain injury and long-term neurodevelopmental outcomes. Infants with PDA showing significant changes in hemodynamics indeed have a significantly increased CUS Resistance Index (RI), but there is no clear evidence yet that RI can predict neurodevelopmental outcomes [40]. A study monitored the effects of Kangaroo Mother Care (KMC) on the cerebral hemodynamics of premature infants using CUS [41]. The results showed that after 60 min of KMC, cerebral blood flow speed in preterm infants increased, the Doppler index decreased, heart rate decreased, and SpO2 and mean pressure increased. These changes were still visible 60 min after stopping KMC, showing the lasting effect of KMC, and also reflecting the flexibility of CUS in monitoring cerebral hemodynamics in preterm infants. In 2022, researchers invented a three-dimensional dynamic blood volume (3D-FMBV) monitoring technology to quantify cerebral perfusion in newborns. The study successfully demonstrated the feasibility and reliability of using 3D-FMBV technology to measure cerebral perfusion in a healthy preterm infant population. This technology can perform a correlational analysis with standardized quantification standards by calculating the perfusion of flowing blood in the region of interest, thereby judging the perfusion situation [42].
6. Long-term neurodevelopmental outcome prediction
Researchers have invented a CUS quantitative scoring system that encompasses forms of brain injury related to prematurity, which is used to examine extremely low birth weight infants when corrected to full-term, to predict neurodevelopmental outcomes at 30 months of corrected age [43]. The system includes ten different scoring items: I. cyst or cavity, II. cortical gray matter abnormalities, III. deep gray matter abnormalities, IV. maturity of gyri and sulci, V. cerebellar abnormalities, VI. anterior horn size, VII. middle ventricle size, VIII. subarachnoid space width, IX. trans-cerebral fissure size, X. corpus callosum thickness. Each item's score is added to obtain a final score. The results showed that the specificity of CUS quantitative scoring in predicting neurodevelopmental outcomes was slightly lower than that of MR (cerebral palsy: 90 % vs.97 %, severe cognitive delay: 90 % vs.95 %), but the sensitivity of the two was the same (cerebral palsy: 75 %, severe cognitive delay: 100 %).
At the end of the first week of life, extremly preterm infants with a diagnosis of Grade II or higher IVH from the initial ultrasound screening had a higher probability of poor neurodevelopmental prognosis [44]. In extremely preterm infants with a gestational age of less than 28 weeks, if CUS examination at term corrected age still shows brain damage of grade III or higher, it can predict 100 % neurodevelopmental abnormalities at 1 year and 3 years [45]. Children with severe CUS result abnormalities have a significantly increased chance of minor motor abnormalities. Compared with children with no motor function abnormalities, children with minor motor abnormalities have evidence of functional impairment and require more rehabilitation resources [46]. These findings have important guiding significance for early follow-up and rehabilitation planning for extremely preterm infants.
Some studies use changes in brain volume or local structures to predict long-term neurodevelopment in patients. Hou et al. performed CUS assessments on 343 extremely preterm infants with birth weights <1250 g at corrected term age, and used Bayley-III to assess the neurodevelopmental outcomes of patients at 2 years corrected age. The study found that the CUS-measured “ventricular to brain volume ratio >0.35″ is the best predictive value for mental developmental delay at 2 years old [47]. In a CUS evaluation of 197 premature infants, it was shown that premature infants (average gestational age at 32.1 weeks) with expanded subarachnoid space (ESS) (>3.5 mm between ventricle and cortex) had significantly higher positive rates in the Korean Infant-Child Development Screening Test (K-DST) at 18–24 months corrected age than infants without ESS [48]. Corpus callosum-fast length (CCF) also plays an important role in predicting the cognitive and behavioral development outcomes of premature infants. Regardless of the stage of CUS examination, the length of CCF was negatively correlated with long-term social, symbolic, and overall scores of the patient [49].
7. Optimization of clinical decision support
We have reviewed the CUS protocols for preterm infants with a gestational age of <32 weeks, building on the work of our predecessors. After a systematic review, we found that there are at least over 10 CUS protocols specifically designed for preterm infants. These protocols differ in their recommended timing of screenings, but generally advise that an “early” scan, i.e., at least one CUS within the first week of life, should be conducted. The timing of the “late” or final ultrasound varies, with options including at the 6th week post-birth, at term corrected age, or at discharge [50]. There is no consensus as to whether continuous CUS should be performed weekly or bi-weekly after the first week of life.
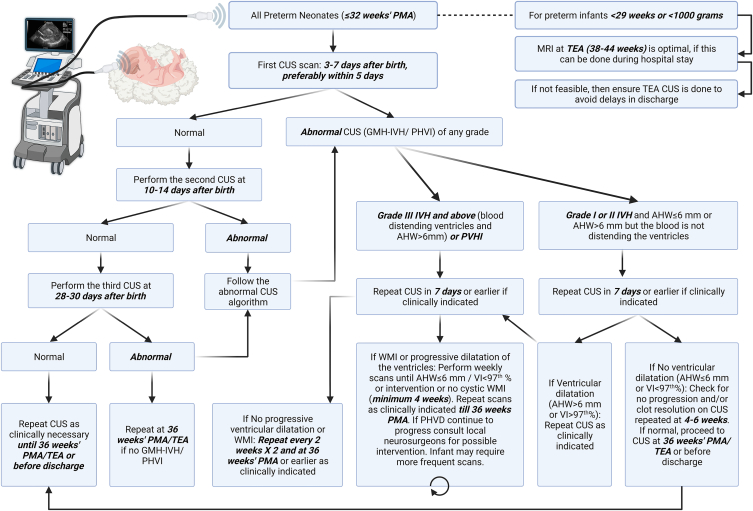
In 2018, Ibrahim and colleagues developed a CUS and MRI plan for preterm infants under 32 weeks of gestational age, based on a review of previous studies. This plan differentiated between asymptomatic preterm infants with gestational ages of 29–32 weeks (birth weight <1500 g) and with gestational ages of <29 weeks (birth weight <1000 g) [51]. The first, second, and third CUS for both groups of preterm infants were scheduled for the 3rd-5th day, the 10th-14th day, and the 28th-30th day after birth. For preterm infants with a gestational age of <29 weeks (birth weight <1000 g), the 4th and 5th screenings are suggested to be conducted monthly or according to clinical needs. It is also recommended that preterm infants with a gestational age of <29 weeks (birth weight <1000 g) should complete an MRI during hospitalization when they reach a corrected gestational age of 38–44 weeks. If this is not achievable, they should ensure to complete another CUS follow-up before discharge. If there are clinical indications, an MRI should be completed before discharge when vital signs are stable.
In 2021, Canada proposed a CUS consensus for the detection and categorization of brain injury in extremely preterm infants to reduce discrepancies in diagnosis and categorization between neonatologists and radiologists. This algorithm can help grade early GMH-IVH, white matter injury, and ventriculomegaly in extremly preterm infants [52]. The ultimate goal is to achieve consistency in the categorization of brain injuries, timing of brain injury screenings, and frequency of follow-up imaging among different NICUs.
In 2021, Canada proposed a CUS consensus for detecting and classifying brain injuries in extremely preterm infants, aiming to reduce the discrepancy in diagnosis and classification between neonatologists and radiologists. This algorithm can assist in the grading of early GMH-IVH, WMI, and ventriculomegaly in extremely preterm infants [52]. The ultimate goal is to achieve consistency in the classification of brain injuries, the timing of brain injury screenings, and the frequency of follow-up imaging across different NICUs.
In 2023, McLean and colleagues investigated whether extremely preterm infants need to undergo CUS on both the 3rd and 8th days. Their research suggests that low-risk preterm infants of 26–32 weeks' gestation, who have no complications and are clinically stable, might consider having a single CUS on the 8th day after birth. However, preterm infants of 23–26 weeks (high risk), due to a higher incidence of CUS abnormalities, still need to undergo CUSs on both the 3rd and 8th days [53].
A study that reviewed data from 651 preterm infants treated in the NICU over 6 years used logistic regression analysis to find that lower gestational age, the use of vasopressors and mechanical ventilation at admission were high-risk factors for brain injury. The researchers divided the preterm infants into three risk groups based on these three factors (<29 weeks GA with 1 major risk factor; 29 weeks GA with 1 major risk factor or <29 weeks with no major risk factor; 29 weeks GA with no major risk factor) and customized the number and timing of optimized ultrasound scans for each group (high risk group: 1 ultrasound check each at 8–14 days and 28–42 days after birth; medium risk group: 1 ultrasound check at 8–14 days after birth; low risk group: 1 ultrasound check at 28–42 days after birth). The results showed that using this grouping process reduced the number of ultrasound scans by 40 % while still accurately detecting severe brain injury in preterm infants [54]. We have summarized these protocols for early screening and follow-up, forming an integrated version that incorporates recommendations from multiple teams (see Fig. 1). By optimizing the CUS scan process, we can more effectively screen for severe brain injuries in preterm infants with a gestational age of less than 32 weeks, while also saving medical resources.
Fig. 1.
CUS protocol for preterm infants under 32 weeks gestational age.
What we need to realize is that the clinical significance of CUS is not just dependent on the discovery of severe IVH through the Papile grading system (proposed by Antonio R. Papile in 1978), but it also needs to consider various factors to help families make appropriate decisions: such as the severity of the bleeding, the location and scope of the bleeding, etc. Because of the uncertainty of the prognosis of IVH, the significance of early bedside ultrasound is not only in the diagnosis of IVH. The key point is that the preterm infant treatment team needs to combine ultrasound results with comprehensive early discussions with the parents of critically ill infants about possible future outcomes (such as neurodevelopmental disorders and cerebral palsy), goals and family values, in order to make decisions to continue treatment or give up [55]. We believe future strategies will be more scientific and personalized, targeting precise patient groups, making smart choices about screening timing, and refining the follow-up process when high-risk factors are present. All of this is with the aim of minimizing brain injuries in preterm infants as much as possible [50].
8. Future perspectives
8.1. Shear wave elastography (SWE)
In recent years, SWE has shown significant potential in neonatal brain elasticity assessing [56] and disease detection. SWE uses an acoustic radiation force, or high-intensity ultrasound pulses, to deform a tissue and generate laterally propagating shear waves in the region of interest. The speed of these propagating waves can be used to quantify tissue stiffness [6]. Garcés Iñigo et al. used SWE to measure the elasticity of the thalamus and corona radiata in healthy term neonates [57]. They found the average speed of the thalamus was 1.17 m/s (95 % CI: 1.13–1.22 m/s), while the average speed of the corona radiata was 1.60 m/s (95 % CI: 1.57–1.64 m/s). The difference in elasticity between these two areas was statistically significant (P < 0.001), providing baseline data for healthy neonatal brain tissue.
A prospective study focused on the brain tissue elasticity of preterm infants, using shear wave elastography to measure brain tissue elasticity. The results showed no correlation between the weight of preterm infants and brain tissue elasticity, but the shear wave speed of the parietal lobe white matter and thalamus was correlated with corrected gestational age, indicating that elastography is suitable for measuring the brain tissue elasticity of preterm infants [5]. A preliminary study on 21 healthy newborns used strain elastography, finding variability in elasticity among different brain regions: cortical gray matter had significantly higher elasticity scores than periventricular white matter, caudate nucleus, and subcortical white matter [58]. Wang et al. evaluated SWE data from preterm and full-term infants [59], discovering that the average brain hardness was lower in preterm infants than in full-term infants (P < 0.001). There was a positive correlation between body mass index (BMI) and brain hardness, showing that SWE could reflect the relationship between brain development and weight gain in preterm infants, providing strong evidence for early intervention.
In studying the potential of SWE for detecting intracranial lesions, El-Ali et al. evaluated its feasibility and repeatability in head ultrasound exams for 78 children [60]. Their study showed that the SWE technique had a high success rate (79.5 %) and consistency (ICC of 0.91). Additionally, the research found that white matter had less stiffness than deep gray matter, indicating that SWE could reliably detect intracranial abnormalities. Dirrichs et al. [61] assessed 184 infants with SWE and found that the average SWE value in infants with hydrocephalus was 21.8 kPa, significantly higher than the 14.1 kPa in healthy infants (P = 0.0083). Furthermore, SWE positively correlated with invasive intracranial pressure measurements (r = 0.69, P < 0.001), suggesting that SWE could also monitor brain changes due to increased intracranial pressure.
These findings indicate that the SWE technique is feasible and valuable for neonatal brain imaging. It can help detect different types of intracranial lesions and provide scientific evidence for early diagnosis and intervention.
8.2. Super microvascular structure imaging (SMI)
Changes in cerebral microvasculature precede macroscopic blood flow and tissue changes in various neurological diseases. Goeral et al. used new ultrasound technology to achieve SMI in the neonatal brain with good repeatability. The researchers obtained images on the coronal and sagittal planes, using the gyri in the left and right frontal areas as anatomical landmarks, to assess whether the microvasculature outside the striatum (i.e., cortex and medulla) and the striatum could be seen. Without the need for a contrast agent, SMI successfully identified ‘short parallel’ cortical vessels (90–100 %), ‘smoothly curved’ medullary vessels (95–100 %), and deep striatal microvessels (71 %). SMI's performance was better on coronal views than on sagittal ones. Microvascular visibility increased with gestational age, and the vessels of the striatum and thalamus were clearly visible regardless of gestational age [3]. In a newborn ECMO study, researchers used SMI to assess microvascular structure, finding that the most common microvascular manifestation in the basal ganglia-thalamus region under ECMO was tortuous reticulate striatal vessels, and the most common microvascular manifestation in cortical gray matter was enhanced perfusion. Vascular filling in cortical gray matter and white matter was significantly increased in adverse outcomes (such as death, seizures, or stroke) [4]. These conclusions fully demonstrate the significant advantage of the new ultrasound technology in microvascular imaging, providing a new non-invasive imaging tool for refined feedback on brain function and brain microvascular imaging [2,4].
8.3. Ultrafast Doppler ultrasound (UfDU)
In recent years, ultrafast ultrasound technology has made significant progress in the fields of neonatal and brain imaging. Demené et al. [7] found that ufDU imaging can non-invasively detect microvascular changes during epileptic seizures, providing real-time imaging with high sensitivity and resolution at the bedsid. At the same time, El-Ali et al. [62] explored neonatal brains of various gestational ages using ufDU imaging. They observed that white matter is less stiff than deep gray matter, showing that the technology has good detection performance for intracranial lesions, with a success rate of 79.5 % and an intraclass correlation coefficient (ICC) of 0.91.
Kim and Lee compared ufDU with conventional Doppler technology, finding that the former can quickly and efficiently acquire blood flow velocity data for the anterior cerebral artery (ACA), middle cerebral artery (MCA), and posterior cerebral artery (PCA) (peak systolic velocity, end-diastolic velocity, and resistive index). UfDU can also effectively evaluate the speed of distal ACA branches, with a peak systolic velocity 9.4–36.7 cm/s higher than proximal ACA, while acquisition time is significantly shortened to 6.7 s [63]. In patients' brain imaging, Demené et al. used fast ultrasound localization microscopy to detect hemodynamic parameters of deep brain vasculature in patients. They quantified blood flow vortices in the vasculature with a resolution of up to 25 μm, providing new insights into cerebral hemodynamics and its relationship to neurological pathology [64].
At the same time, Zhang et al. proposed using fractional moving blood volume (FMBV) as a new indicator for quantitatively assessing coronary microcirculation [65]. It improved contrast and accuracy in ultrafast coronary Doppler imaging. In vivo experiments showed that the FMBV estimation error was less than 5 %, significantly improving microcirculation evaluation. Huang et al. proposed a spatial-angular adaptive scaling Wiener postfilter to further optimize ufDU imaging. By combining spatial and angular signals to estimate signal and noise power, they improved contrast by up to 34.7 dB and reduced background noise by 52 dB in human comparison tests, significantly enhancing image quality [66].
For chronic pain, Rahal et al. used fast ultrasound imaging to analyze the functional network states of arthritic animal brains. They found significant changes in their somatomotor (SM) networks. The time distribution in different brain states showed different synchrony levels with the SM network in pain animals [67]. Fakhari et al. also demonstrated that ufDU can assess the association between brain perfusion and coronary bypass surgery. They showed a difference of up to 20 % in cerebral blood volume [68].
Overall, these studies indicate that ultrafast ultrasound technology has significant potential in neonatal and brain imaging, providing accurate evidence for disease diagnosis and intervention.
8.4. Support of ultrasound imaging by artificial intelligence technology
Artificial intelligence technology has been widely applied and researched in neonatal neurosonography and cranial ultrasound. Researchers have utilized deep learning methods to automatically estimate total brain volume from 3D ultrasound images. Jafrasteh et al. [69] proposed a method based on deep convolutional neural networks. By introducing dilated residual connections and a fuzzy C-means clustering layer, it further separates features from different regions, enabling direct estimation of total brain volume without additional image segmentation steps. The method was validated on datasets acquired from two different ultrasound devices, and the prediction results showed a strong correlation with actual brain volume values.
For detecting white matter injury in preterm infants, Zhu et al. [25] employed a multi-task deep learning model to simultaneously perform white matter segmentation and predict white matter injury risk. They included 807 cranial ultrasound images from 158 preterm infants. The results demonstrated that the model achieved good performance in both white matter segmentation (Dice coefficient 0.78) and white matter injury prediction (AUC 0.863). This study showcased the potential of data-driven diagnostic systems for automatic detection of white matter injury in preterm infants. One study focused on using ultrasound imaging for ventricular phenotyping and prognosis prediction. Tabrizi [70] proposed a fully automatic method that combines ventricular quantification, morphological phenotypes, and clinical information to predict whether intraventricular hemorrhage in preterm infants will progress to hydrocephalus requiring intervention. Experiments showed that the method obtained accurate results in both ventricular segmentation (Dice coefficient 0.86) and hydrocephalus prognosis prediction (accuracy 0.91). This provides a new approach for early identification of high-risk infants with severe hydrocephalus and timely intervention.
To further improve the efficiency of neonatal ventricular segmentation, Szentimrey et al. [71] developed an integrated model based on 3D U-Net. It combines U-Net++, attention U-Net, and U-Net with shape priors, using an average voting strategy for ensemble. They conducted five-fold cross-validation on a dataset containing 190 three-dimensional ultrasound images (87 with bilateral ventricles, 103 with unilateral ventricles). The model achieved better segmentation performance than existing 2D methods and significantly faster speed (about 5 s per case). This study has the largest sample size to date for this segmentation task and provides a new tool for rapid and accurate assessment of ventricular volume.
The integration of artificial intelligence technology and medical imaging is expected to enable automatic segmentation of brain structures, volume estimation, and pathology prediction. It provides strong support for timely diagnosis and early intervention of neonatal central nervous system diseases. However, large-sample, multi-center studies are still needed to further validate its clinical application value.
9. Limitation of CUS
Despite the numerous advantages and wide application of cranial ultrasound (CUS) in screening and monitoring brain injury in preterm infants, it is important to acknowledge its limitations compared to magnetic resonance imaging (MRI). MRI provides comprehensive imaging of both structural and functional brain abnormalities, while CUS is limited by lower resolution and the potential for operator dependence, which can lead to variability in interpretation [72,73]. MRI's high contrast resolution and multiplanar imaging capabilities make it superior in identifying subtle white matter injuries [74] and detecting cortical malformations [75] that may be missed on CUS. CUS has lower sensitivity for detecting subtle white matter abnormalities and small hemorrhages, which can be better identified by MRI techniques such as diffusion-weighted imaging (DWI) [76].
Additionally, ultrasound has a limited acoustic window to fully visualize posterior fossa structures, including the cerebellum and brainstem, which may lead to missed posterior fossa malformations that could be detected by MRI [75]. While CUS is highly sensitive in detecting the presence of brain abnormalities, MRI provides greater specificity in defining the exact nature and extent of the injury, such as differentiating hemorrhage from ischemia [77]. Furthermore, the sensitivity of CUS for brain injury decreases at term-equivalent age compared to earlier scans, likely due to increased calcification and decreased fontanelle size, whereas MRI maintains higher sensitivity at term age [78]. Despite these limitations, CUS remains an essential tool for brain injury screening in preterm infants, particularly when MRI is unavailable, impractical, or unsafe. A combination of serial CUS and term-equivalent MRI will provide a more comprehensive assessment for the infant.
10. Conclusion
Significant progress in bedside cranial ultrasound (CUS) has expanded its role in preterm infant brain disease management, providing non-invasive, accurate, and accessible diagnostics. While CUS continues to show promise, challenges remain in improving imaging quality and diagnostic accuracy. Emerging technologies like shear wave elastography and ultrafast Doppler ultrasound, along with artificial intelligence advancements, offer potential solutions. Continued research should focus on refining these technologies, standardizing interpretations, and enhancing diagnostic precision to realize CUS's full potential in early and personalized interventions for preterm infant brain diseases.
Funding source
The Shanghai Committee of Science and Technology, China (Grant No. 21Y11907200) and the Shanghai Municipal Health Commission, China (Grant No. 202140443).
Ethical approval
Not applicable.
CRediT authorship contribution statement
Lukun Tang: Writing – review & editing, Writing – original draft, Conceptualization. Qi Li: Writing – review & editing, Writing – original draft, Conceptualization. Feifan Xiao: Writing – review & editing, Writing – original draft, Visualization, Conceptualization. Yanyan Gao: Writing – review & editing, Writing – original draft, Conceptualization. Peng Zhang: Writing – review & editing, Writing – original draft, Conceptualization. Guoqiang Cheng: Writing – review & editing, Writing – original draft, Conceptualization. Laishuan Wang: Writing – review & editing, Writing – original draft, Conceptualization. Chunmei Lu: Writing – review & editing, Writing – original draft, Conceptualization. Mengmeng Ge: Writing – review & editing, Writing – original draft, Conceptualization. Liyuan Hu: Writing – review & editing, Writing – original draft, Conceptualization. Tiantian Xiao: Writing – review & editing, Writing – original draft, Conceptualization. Zhaoqing Yin: Writing – review & editing, Writing – original draft, Conceptualization. Kai Yan: Writing – review & editing, Writing – original draft, Funding acquisition, Formal analysis, Conceptualization. Wenhao Zhou: Writing – review & editing, Writing – original draft, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the Shanghai Committee of Science and Technology, China (Grant No. 21Y11907200) and the Shanghai Municipal Health Commission, China (Grant No. 202140443) to Kai Yan.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e31742.
Contributor Information
Kai Yan, Email: fhyankai@163.com.
Wenhao Zhou, Email: zhouwenhao@fudan.edu.cn.
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- 1.Zhang X.H., Qiu S.J., Chen W.J., Gao X.R., Li Y., Cao J., et al. Predictive value of cranial ultrasound for neurodevelopmental outcomes of very preterm infants with brain injury. Chin. Med. J. 2018;131:920–926. doi: 10.4103/0366-6999.229895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goeral K., Hojreh A., Kasprian G., Klebermass-Schrehof K., Weber M., Mitter C., et al. Microvessel ultrasound of neonatal brain parenchyma: feasibility, reproducibility, and normal imaging features by superb microvascular imaging (SMI) Eur. Radiol. 2019;29:2127–2136. doi: 10.1007/s00330-018-5743-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barletta A., Balbi M., Surace A., Caroli A., Radaelli S., Musto F., et al. Cerebral superb microvascular imaging in preterm neonates: in vivo evaluation of thalamic, striatal, and extrastriatal angioarchitecture. Neuroradiology. 2021;63:1103–1112. doi: 10.1007/s00234-021-02634-w. [DOI] [PubMed] [Google Scholar]
- 4.Tierradentro-Garcia L.O., Stern J.A., Dennis R., Hwang M. Utility of cerebral microvascular imaging in infants undergoing ECMO. Children. 2022;9 doi: 10.3390/children9121827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang H., Li H., Liao J., Yuan X., Shi C., Liang W. Compression elastography and shear wave ultrasound elastography for measurement of brain elasticity in full-term and premature neonates: a prospective study. J. Ultrasound Med. 2023;42:221–231. doi: 10.1002/jum.16075. [DOI] [PubMed] [Google Scholar]
- 6.deCampo D., Hwang M. Characterizing the neonatal brain with ultrasound elastography. Pediatr. Neurol. 2018;86:19–26. doi: 10.1016/j.pediatrneurol.2018.06.005. [DOI] [PubMed] [Google Scholar]
- 7.Demené C., Mairesse J., Baranger J., Tanter M., Baud O. Ultrafast Doppler for neonatal brain imaging. Neuroimage. 2019;185:851–856. doi: 10.1016/j.neuroimage.2018.04.016. [DOI] [PubMed] [Google Scholar]
- 8.Qiu W., Chen Y., Kishimoto J., de Ribaupierre S., Chiu B., Fenster A., et al. Automatic segmentation approach to extracting neonatal cerebral ventricles from 3D ultrasound images. Med. Image Anal. 2017;35:181–191. doi: 10.1016/j.media.2016.06.038. [DOI] [PubMed] [Google Scholar]
- 9.Gontard L.C., Pizarro J., Sanz-Peña B., Lubián López S.P., Benavente-Fernández I. Automatic segmentation of ventricular volume by 3D ultrasonography in post haemorrhagic ventricular dilatation among preterm infants. Sci. Rep. 2021;11:567. doi: 10.1038/s41598-020-80783-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martin M., Sciolla B., Sdika M., Quétin P., Delachartre P. Automatic segmentation and location learning of neonatal cerebral ventricles in 3D ultrasound data combining CNN and CPPN. Comput. Biol. Med. 2021;131 doi: 10.1016/j.compbiomed.2021.104268. [DOI] [PubMed] [Google Scholar]
- 11.Browning Carmo K., Lutz T., Greenhalgh M., Berry A., Kluckow M., Evans N. Feasibility and utility of portable ultrasound during retrieval of sick preterm infants. Acta Paediatr. 2017;106:1296–1301. doi: 10.1111/apa.13881. [DOI] [PubMed] [Google Scholar]
- 12.Guillot M., Sebastianski M., Lemyre B. Comparative performance of head ultrasound and MRI in detecting preterm brain injury and predicting outcomes: a systematic review. Acta Paediatr. 2021;110:1425–1432. doi: 10.1111/apa.15670. [DOI] [PubMed] [Google Scholar]
- 13.Khazanchi R., Lyden E.R., Peeples E.S. Reevaluating 30-day head ultrasound screening for preterm infants in the era of decreasing periventricular leukomalacia. J. Matern. Fetal Neonatal Med. 2022;35:907–913. doi: 10.1080/14767058.2020.1733521. [DOI] [PubMed] [Google Scholar]
- 14.Nataraj P., Svojsik M., Sura L., Curry K., Bliznyuk N., Rajderkar D., et al. Comparing head ultrasounds and susceptibility-weighted imaging for the detection of low-grade hemorrhages in preterm infants. J. Perinatol. 2021;41:736–742. doi: 10.1038/s41372-020-00890-x. [DOI] [PubMed] [Google Scholar]
- 15.Daigneault J., White H., Dube A., Shi Q., Gauguet J.M., Rhein L. Lack of progression of intraventricular hemorrhage in premature infants: implications for head ultrasound screening. Glob Pediatr Health. 2021;8 doi: 10.1177/2333794X211010729. 2333794x211010729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang X.H., Chen W.J., Gao X.R., Li Y., Cao J., Qiu S.J. Predicting the developmental outcomes of very premature infants via ultrasound classification: a CONSORT - clinical study. Medicine (Baltim.) 2021;100 doi: 10.1097/MD.0000000000025421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim F., Bateman D.A., Goldshtrom N., Sheen J.J., Garey D. Intracranial ultrasound abnormalities and mortality in preterm infants with and without fetal growth restriction stratified by fetal Doppler study results. J. Perinatol. 2023;43:560–567. doi: 10.1038/s41372-023-01621-8. [DOI] [PubMed] [Google Scholar]
- 18.Mohammad K., Murthy P., Aguinaga F., Fajardo C., Eguiguren L., Castro Y., et al. Simulation-based structured education supports focused neonatal cranial ultrasound training. J. Ultrasound Med. 2020;39:1195–1201. doi: 10.1002/jum.15207. [DOI] [PubMed] [Google Scholar]
- 19.Rodríguez-Fanjul J. Point of care ultrasound to diagnose real-time intraventricular hemorrhage in a crashing extremely preterm newborn. Med. Intensiva. 2023;47:305–306. doi: 10.1016/j.medine.2021.08.017. [DOI] [PubMed] [Google Scholar]
- 20.Sartori J.T., Ambros L.E., Callegaro G.I.S. Alterations on magnetic resonance imaging of the neonatal brain: correlations with prenatal risk factors and transfontanellar ultrasound findings. Radiol. Bras. 2022;55:280–285. doi: 10.1590/0100-3984.2021.0149-en. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beijst C., Dudink J., Wientjes R., Benavente-Fernandez I., Groenendaal F., Brouwer M.J., et al. Two-dimensional ultrasound measurements vs. magnetic resonance imaging-derived ventricular volume of preterm infants with germinal matrix intraventricular haemorrhage. Pediatr. Radiol. 2020;50:234–241. doi: 10.1007/s00247-019-04542-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kishimoto J., de Ribaupierre S., Lee D.S., Mehta R., St Lawrence K., Fenster A. 3D ultrasound system to investigate intraventricular hemorrhage in preterm neonates. Phys. Med. Biol. 2013;58:7513–7526. doi: 10.1088/0031-9155/58/21/7513. [DOI] [PubMed] [Google Scholar]
- 23.Kishimoto J., Fenster A., Lee D.S.C., de Ribaupierre S. Quantitative 3-D head ultrasound measurements of ventricle volume to determine thresholds for preterm neonates requiring interventional therapies following posthemorrhagic ventricle dilatation. J. Med. Imaging. 2018;5 doi: 10.1117/1.JMI.5.2.026001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ghotra S., Vincer M., Allen V.M., Khan N. A population-based study of cystic white matter injury on ultrasound in very preterm infants born over two decades in Nova Scotia, Canada. J. Perinatol. 2019;39:269–277. doi: 10.1038/s41372-018-0294-5. [DOI] [PubMed] [Google Scholar]
- 25.Zhu J., Yao S., Yao Z., Yu J., Qian Z., Chen P. White matter injury detection based on preterm infant cranial ultrasound images. Front Pediatr. 2023;11 doi: 10.3389/fped.2023.1144952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jung H.N., Suh S.I., Park A., Kim G.H., Ryoo I. Early prediction of periventricular leukomalacia using quantitative texture analysis of serial cranial ultrasound scans in very preterm infants. Ultrasound Med. Biol. 2019;45:2658–2665. doi: 10.1016/j.ultrasmedbio.2019.06.413. [DOI] [PubMed] [Google Scholar]
- 27.Helderman J., O'Shea T.M., Dansereau L., Check J., Hofheimer J.A., Smith L.M., et al. Association of abnormal findings on neonatal cranial ultrasound with neurobehavior at neonatal intensive care unit discharge in infants born before 30 Weeks' gestation. JAMA Netw. Open. 2022;5 doi: 10.1001/jamanetworkopen.2022.6561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu C., Fang C., Shang Y., Yao B., He Q. Transcranial ultrasound diagnostic value of hemodynamic cerebral changes in preterm infants for early-onset sepsis. Transl. Pediatr. 2022;11:1149–1155. doi: 10.21037/tp-22-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Claessens L.C., Zonnenberg I.A., van den Dungen F.A., Vermeulen R.J., van Weissenbruch M.M. Cerebral ultrasound abnormalities in preterm infants caused by late-onset sepsis. PLoS One. 2017;12 doi: 10.1371/journal.pone.0173227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ho S.S., Zhou Y., Rajderkar D. Intracranial imaging of preterm infants with suspected hypoxic ischemic encephalopathy: comparing MRI and ultrasound. Curr. Pediatr. Rev. 2023;19:179–186. doi: 10.2174/1573396318666220417233146. [DOI] [PubMed] [Google Scholar]
- 31.Stein A., Sody E., Bruns N., Felderhoff-Müser U. Development of an ultrasound scoring system to describe brain maturation in preterm infants. AJNR Am J Neuroradiol. 2023;44(2023):846–852. doi: 10.3174/ajnr.A7909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arena R., Gallini F., De Rose D.U., Conte F., Giraldi L., Pianini T., et al. Brain growth evaluation assessed with transfontanellar (B-GREAT) ultrasound. Old and new bedside markers to estimate cerebral growth in preterm infants: a pilot study. Am. J. Perinatol. 2022;41(2024):488–497. doi: 10.1055/a-1704-1716. [DOI] [PubMed] [Google Scholar]
- 33.Şimşek G.K., Canpolat F.E., Büyüktiryaki M., Okman E., Keser M., Üstünyurt Z., et al. Developmental outcomes of very low birthweight infants with non-hemorrhagic ventricular dilatations and the relationships thereof with absolute brain volumes measured via two-dimensional ultrasonography. Childs Nerv Syst. 2020;36:1231–1237. doi: 10.1007/s00381-019-04464-x. [DOI] [PubMed] [Google Scholar]
- 34.Bravo M.C., Valverde E. Reliability in cerebellar size assessment by 2D cranial ultrasonography in neonates. Eur. J. Paediatr. Neurol. 2017;21:610–613. doi: 10.1016/j.ejpn.2017.03.010. [DOI] [PubMed] [Google Scholar]
- 35.Graça A.M., Geraldo A.F., Cardoso K., Cowan F.M. Preterm cerebellum at term age: ultrasound measurements are not different from infants born at term. Pediatr. Res. 2013;74:698–704. doi: 10.1038/pr.2013.154. [DOI] [PubMed] [Google Scholar]
- 36.Muehlbacher T., Schaefer R.N., Buss C., Bührer C., Schmitz T. A closer look at a small brain: transnuchal ultrasound facilitates high-resolution imaging of the cerebellum in preterm infants. Ultraschall der Med. 2021;42:395–403. doi: 10.1055/a-1072-5207. [DOI] [PubMed] [Google Scholar]
- 37.McLean G., Hough C., Sehgal A., Ditchfield M., Polglase G.R., Miller S.L. Three-dimensional ultrasound cranial imaging and early neurodevelopment in preterm growth-restricted infants. J. Paediatr. Child Health. 2018;54:420–425. doi: 10.1111/jpc.13808. [DOI] [PubMed] [Google Scholar]
- 38.Klebermass-Schrehof K., Aumüller S., Goeral K., Vergesslich-Rothschild K., Fuiko R., Brandstetter S., et al. Biometry of the corpus callosum assessed by 3D ultrasound and its correlation to neurodevelopmental outcome in very low birth weight infants. J. Perinatol. 2017;37:448–453. doi: 10.1038/jp.2016.231. [DOI] [PubMed] [Google Scholar]
- 39.Vik S.D., Torp H., Follestad T., Støen R., Nyrnes S.A. NeoDoppler: new ultrasound technology for continous cerebral circulation monitoring in neonates. Pediatr. Res. 2020;87:95–103. doi: 10.1038/s41390-019-0535-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Camfferman F.A., de Goederen R., Govaert P., Dudink J., van Bel F., Pellicer A., et al. Diagnostic and predictive value of Doppler ultrasound for evaluation of the brain circulation in preterm infants: a systematic review. Pediatr. Res. 2020;87:50–58. doi: 10.1038/s41390-020-0777-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chaudhari A.J., Nimbalkar S.M., Patel D.V., Phatak A.G. Effect of Kangaroo mother care on cerebral hemodynamics in preterm neonates assessed by transcranial Doppler sonography in middle cerebral artery. Indian Pediatr. 2023;60:27–32. doi: 10.1007/s13312-023-2690-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kuhasri A., Jayatilake S., Stevenson G., Beirne G., Welsh A., Schindler T. Evaluation of neonatal cerebral perfusion using three-dimensional power Doppler ultrasound volumes. Acta Paediatr. 2022;111:511–518. doi: 10.1111/apa.16163. [DOI] [PubMed] [Google Scholar]
- 43.Skiöld B., Hallberg B., Vollmer B., Ådén U., Blennow M., Horsch S. A novel scoring system for term-equivalent-age cranial ultrasound in extremely preterm infants. Ultrasound Med. Biol. 2019;45:786–794. doi: 10.1016/j.ultrasmedbio.2018.11.005. [DOI] [PubMed] [Google Scholar]
- 44.Petrova A., Karatas M., Mehta R. Features of serial cranial ultrasound detected neuropathology in very preterm infants. J. Neonatal Perinat. Med. 2019;12:65–71. doi: 10.3233/NPM-1826. [DOI] [PubMed] [Google Scholar]
- 45.Burkitt K., Kang O., Jyoti R., Mohamed A.L., Chaudhari T. Comparison of cranial ultrasound and MRI for detecting BRAIN injury in extremely preterm infants and correlation with neurological outcomes at 1 and 3 years. Eur. J. Pediatr. 2019;178:1053–1061. doi: 10.1007/s00431-019-03388-7. [DOI] [PubMed] [Google Scholar]
- 46.DeMauro S.B., Bann C., Flibotte J., Adams-Chapman I., Hintz S.R. Cranial ultrasound and minor motor abnormalities at 2 Years in extremely low gestational age infants. J. Dev. Behav. Pediatr. 2020;41:308–315. doi: 10.1097/DBP.0000000000000758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hou W., Tang P.H., Agarwal P. The most useful cranial ultrasound predictor of neurodevelopmental outcome at 2 years for preterm infants. Clin. Radiol. 2020;75:278–286. doi: 10.1016/j.crad.2019.11.009. [DOI] [PubMed] [Google Scholar]
- 48.Yum S.K., Im S.A., Seo Y.M., Sung I.K. Enlarged subarachnoid space on cranial ultrasound in preterm infants: neurodevelopmental implication. Sci. Rep. 2019;9 doi: 10.1038/s41598-019-55604-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yuan J., Cao X., Deng Y. An exploratory study into a new head ultrasound marker for predicting neurodevelopmental outcomes in preterm infants. Ultrasound Q. 2021;38:43–48. doi: 10.1097/RUQ.0000000000000582. [DOI] [PubMed] [Google Scholar]
- 50.McLean G., Malhotra A., Lombardo P., Schneider M. Cranial ultrasound screening protocols for very preterm infants. Ultrasound Med. Biol. 2021;47:1645–1656. doi: 10.1016/j.ultrasmedbio.2021.03.006. [DOI] [PubMed] [Google Scholar]
- 51.Ibrahim J., Mir I., Chalak L. Brain imaging in preterm infants <32 weeks gestation: a clinical review and algorithm for the use of cranial ultrasound and qualitative brain MRI. Pediatr. Res. 2018;84:799–806. doi: 10.1038/s41390-018-0194-6. [DOI] [PubMed] [Google Scholar]
- 52.Mohammad K., Scott J.N., Leijser L.M., Zein H., Afifi J., Piedboeuf B., et al. Consensus approach for standardizing the screening and classification of preterm brain injury diagnosed with cranial ultrasound: a Canadian perspective. Front Pediatr. 2021;9 doi: 10.3389/fped.2021.618236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McLean G., Ditchfield M., Paul E., Malhotra A., Lombardo P. Evaluation of a cranial ultrasound screening protocol for very preterm infants. J. Ultrasound Med. 2023;42:1081–1091. doi: 10.1002/jum.16121. [DOI] [PubMed] [Google Scholar]
- 54.Patel S., Martel-Bucci A., Wintermark P., Shalish W., Claveau M., Beltempo M. Optimizing timing and frequency of head ultrasound screening for severe brain injury among preterm infants born <32 weeks' gestation. J. Matern. Fetal Neonatal Med. 2022;35:10330–10336. doi: 10.1080/14767058.2022.2128647. [DOI] [PubMed] [Google Scholar]
- 55.Chevallier M., Barrington K.J., Terrien Church P., Luu T.M., Janvier A. Decision-making for extremely preterm infants with severe hemorrhages on head ultrasound: Science, values, and communication skills. Semin. Fetal Neonatal Med. 2023;28 doi: 10.1016/j.siny.2023.101444. [DOI] [PubMed] [Google Scholar]
- 56.Hwang M., Zhang Z., Katz J., Freeman C., Kilbaugh T. Brain contrast-enhanced ultrasonography and elastography in infants. Ultrasonography. 2022;41:633–649. doi: 10.14366/usg.21224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Garcés Iñigo E., Llorens Salvador R., Escrig R., Hervás D., Vento M., Martí-Bonmatí L. Quantitative evaluation of neonatal brain elasticity using shear wave elastography. J. Ultrasound Med. 2021;40:795–804. doi: 10.1002/jum.15464. [DOI] [PubMed] [Google Scholar]
- 58.Kim H.G., Park M.S., Lee J.D., Park S.Y. Ultrasound elastography of the neonatal brain: preliminary study. J. Ultrasound Med. 2017;36:1313–1319. doi: 10.7863/ultra.16.06079. [DOI] [PubMed] [Google Scholar]
- 59.Wang J., Zhang Z., Xu X., Lu X., Wu T., Tong M. Real-time shear wave elastography evaluation of the correlation between brain tissue stiffness and body mass index in premature neonates. Transl. Pediatr. 2021;10:3230–3236. doi: 10.21037/tp-21-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.El-Ali A.M., Subramanian S., Krofchik L.M., Kephart M.C., Squires J.H. Feasibility and reproducibility of shear wave elastography in pediatric cranial ultrasound. Pediatr. Radiol. 2020;50:990–996. doi: 10.1007/s00247-019-04592-1. [DOI] [PubMed] [Google Scholar]
- 61.Dirrichs T., Meiser N., Panek A., Trepels-Kottek S., Orlikowsky T., Kuhl C.K., et al. Transcranial shear wave elastography of neonatal and infant brains for quantitative evaluation of increased intracranial pressure. Invest. Radiol. 2019;54:719–727. doi: 10.1097/RLI.0000000000000602. [DOI] [PubMed] [Google Scholar]
- 62.Xu K., Yan S., Song J. Ultrafast Doppler imaging of brain arteriovenous malformation. World Neurosurg. 2023;177:3–4. doi: 10.1016/j.wneu.2023.05.088. [DOI] [PubMed] [Google Scholar]
- 63.Kim H.G., Lee J.H. Feasibility of Ultrafast Doppler technique for cranial ultrasound in neonates. Med Ultrason. 2019;21:288–293. doi: 10.11152/mu-1901. [DOI] [PubMed] [Google Scholar]
- 64.Demené C., Robin J., Dizeux A., Heiles B., Pernot M., Tanter M., et al. Transcranial ultrafast ultrasound localization microscopy of brain vasculature in patients. Nat. Biomed. Eng. 2021;5:219–228. doi: 10.1038/s41551-021-00697-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang N., Nguyen M.B., Mertens L., Barron D.J., Villemain O., Baranger J. Improving coronary ultrafast Doppler angiography using fractional moving blood volume and motion-adaptive ensemble length. Phys. Med. Biol. 2022;67 doi: 10.1088/1361-6560/ac7430. [DOI] [PubMed] [Google Scholar]
- 66.Huang L., Wang Y., Wang R., Wei X., He Q., Zheng C., et al. High-quality ultrafast power Doppler imaging based on spatial angular coherence factor. IEEE Trans. Ultrason. Ferroelectrics Freq. Control. 2023;70:378–392. doi: 10.1109/TUFFC.2023.3253257. [DOI] [PubMed] [Google Scholar]
- 67.Rahal L., Thibaut M., Rivals I., Claron J., Lenkei Z., Sitt J.D., et al. Ultrafast ultrasound imaging pattern analysis reveals distinctive dynamic brain states and potent sub-network alterations in arthritic animals. Sci. Rep. 2020;10 doi: 10.1038/s41598-020-66967-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Faure F., Baranger J., Alison M., Boutillier B., Frérot A., Lim C., et al. Quantification of brain-wide vascular resistivity via ultrafast Doppler in human neonates helps early detection of white matter injury. J. Cerebr. Blood Flow Metabol. 2024 doi: 10.1177/0271678X241232197. Advance online publication271678x241232197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jafrasteh B., Lubián-López S.P., Benavente-Fernández I. A deep sift convolutional neural networks for total brain volume estimation from 3D ultrasound images. Comput. Methods Progr. Biomed. 2023;242 doi: 10.1016/j.cmpb.2023.107805. [DOI] [PubMed] [Google Scholar]
- 70.Tabrizi P.R., Mansoor A., Obeid R., Cerrolaza J.J., Perez D.A., Zember J., et al. Ultrasound-based phenotyping of lateral ventricles to predict hydrocephalus outcome in premature neonates. IEEE Trans. Biomed. Eng. 2020;67:3026–3034. doi: 10.1109/TBME.2020.2974650. [DOI] [PubMed] [Google Scholar]
- 71.Szentimrey Z., de Ribaupierre S., Fenster A., Ukwatta E. Automated 3D U-net based segmentation of neonatal cerebral ventricles from 3D ultrasound images. Med. Phys. 2022;49:1034–1046. doi: 10.1002/mp.15432. [DOI] [PubMed] [Google Scholar]
- 72.Proceedings of the Seminars in Perinatology. Elsevier; 2010. Cranial ultrasonography in neonates: role and limitations. [DOI] [PubMed] [Google Scholar]
- 73.Meijler G., Steggerda S.J. Springer; 2012. Neonatal Cranial Ultrasonography. [Google Scholar]
- 74.Tusor N., Benders M.J., Counsell S.J., Nongena P., Ederies M.A., Falconer S., et al. Punctate white matter lesions associated with altered brain development and adverse motor outcome in preterm infants. Sci. Rep. 2017;7 doi: 10.1038/s41598-017-13753-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gupta P., Sodhi K.S., Saxena A.K., Khandelwal N., Singhi P. Neonatal cranial sonography: a concise review for clinicians. J. Pediatr. Neurosci. 2016;11:7–13. doi: 10.4103/1817-1745.181261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dyet L.E., Kennea N., Counsell S.J., Maalouf E.F., Ajayi-Obe M., Duggan P.J., et al. Natural history of brain lesions in extremely preterm infants studied with serial magnetic resonance imaging from birth and neurodevelopmental assessment. Pediatrics. 2006;118:536–548. doi: 10.1542/peds.2005-1866. [DOI] [PubMed] [Google Scholar]
- 77.Hintz S.R., Barnes P.D., Bulas D., Slovis T.L., Finer N.N., Wrage L.A., et al. Neuroimaging and neurodevelopmental outcome in extremely preterm infants. Pediatrics. 2015;135:e32–e42. doi: 10.1542/peds.2014-0898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Horsch S., Skiöld B., Hallberg B., Nordell B., Nordell A., Mosskin M., et al. Cranial ultrasound and MRI at term age in extremely preterm infants. Arch. Dis. Child. Fetal Neonatal Ed. 2010;95:F310–F314. doi: 10.1136/adc.2009.161547. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.