Abstract
Titanium dioxide nanoparticles (TiO2 NPs) have been extensively utilized in various applications. However, the regulatory mechanism behind the reproductive toxicity induced by TiO2 NP exposure remains largely elusive. In this study, we employed a Drosophila model to assess potential testicular injuries during spermatogenesis and conducted bulk RNA-Seq analysis to elucidate the underlying mechanisms. Our results reveal that while prolonged exposure to lower concentrations of TiO2 NPs (0.45 mg/mL) for 30 days did not manifest reproductive toxicity, exposure at concentrations of 0.9 and 1.8 mg/mL significantly impaired spermatid elongation in Drosophila testes. Notably, bulk RNA-seq analysis revealed that TiO2 NP exposure affected multiple metabolic pathways including carbohydrate metabolism and cytochrome P450. Importantly, the intervention of glutathione (GSH) significantly protected against reproductive toxicity induced by TiO2 NP exposure, as it restored the number of Orb-positive spermatid clusters in Drosophila testes. Our study provides novel insights into the specific detrimental effects of TiO2 NP exposure on spermatid elongation through multiple metabolic alterations in Drosophila testes and highlights the protective role of GSH in countering this toxicity.
1. Introduction
Nanoparticles (NPs) are known for their unique physical and chemical properties, which can be attributed to their small size and large surface-to-volume ratio.1 These properties have led to their widespread application in various fields, including optical materials, medical imaging, drug delivery, and material reinforcement.2,3 Titanium dioxide (TiO2) NPs, in particular, are widely used due to their exceptional stability, corrosion resistance, and photocatalytic capabilities. TiO2 NPs constitute a significant portion of environmental contaminants, with approximately 760 tons of TiO2 NPs being discharged into the soil annually via sewage and sludge.4,5 Oral ingestion represents the primary route through which TiO2 NPs enter the human body, with an estimated intake of 15–37.5 mg/kg per day for an adult weighing 75 kg.6 They are found in a variety of products such as sunscreens, food packaging materials, pharmaceuticals, and toothpaste.7,8 Despite their extensive use, the omnipresence of TiO2 NPs in everyday products raises concerns regarding inhalation and skin exposure risks.9−11
Different morphological structures of TiO2 NPs may exhibit distinct toxic mechanisms. The toxicity of TiO2 NPs is influenced by various factors such as size, shape, and crystal structure.12 Particle size impacts the toxicity and accumulation of TiO2 NPs in different organs, with larger particles (80 nm) primarily accumulating in the liver, while smaller particles (25 nm) are found in the spleen, kidneys, and lungs.13 TiO2 NPs exist in various morphologies, including spherical, rod-shaped, and nanoplate forms.14,15 TiO2 NPs exist in three crystalline forms: rutile, anatase, and brookite.16 Due to the higher photocatalytic activity of anatase, it increases the production of reactive oxygen species (ROS) and cellular damage, making it the most toxic form.7,17−19 In summary, different morphological structures of TiO2 NPs may induce toxic effects through various intracellular mechanisms, including oxidative stress, cell apoptosis, and inflammatory responses.
Recent advances in nanoparticle research, including studies of TiO2 NPs, have highlighted significant health and environmental implications. This includes evidence of hepatotoxicity, nephrotoxicity, splenic toxicity, neurotoxicity, and cardiotoxicity associated with TiO2 NP exposure.20−23 For instance, exposure to TiO2 NPs has been shown to induce hydropic degeneration and apoptosis in hepatocytes.24 Moreover, the intragastric administration of TiO2 NPs leads to renal inflammation and cell necrosis.22 Related studies warn about potential risks posed by TiO2 NPs on the reproductive and developmental health of invertebrates and vertebrates.25,26 Considering the significance of the reproductive system in life sciences research, substantial attention has been devoted to investigating the potential toxic effects of TiO2 NPs on this system.3 Experimental evidence has demonstrated that upon exposure, TiO2 NPs accumulate in ovaries and testes, resulting in damage to the reproductive system.27,28 Prolonged exposure of female mice to anatase TiO2 NPs disrupts hormonal balance, alters ovarian gene expression, and reduces fertility.29 Furthermore, changes in key ovarian genes suggest that TiO2 NPs directly affect ovarian function.29 In mammalian testicles, TiO2 NP exposure can cross the blood–testis barrier (BTB), resulting in testicular lesions, sperm malformations, and alterations in serum sex hormone levels.27,28 TiO2 NP exposure also induces ROS production, activating ROS–MAPK(ERK1/2)–StAR pathway, which is necessary for the inhibition of testosterone synthesis.30 Quercetin and rutin, by preserving endogenous antioxidant capacity and scavenging free radicals, have beneficial effects on the reproductive toxicity induced by TiO2 NPs in male rats.31 However, the regulatory mechanism underlying the spermatogenic toxicity induced by TiO2 NPs remains largely unexplored.
Given this backdrop, there is growing interest in using alternative models for toxicological studies. Drosophila, with its short life cycle, small size, genetic tractability, and low maintenance costs, presents a viable alternative to traditional mammalian models.32−35 Importantly, both Drosophila and mammals exhibit highly conserved spermatogenesis processes that are well understood anatomically and histologically.36,37 Furthermore, Drosophila is recommended as an alternative animal model for investigating developmental toxicology related to environmental pollutants.38 Given that one of the primary pathways for NPs to enter the human system is via oral ingestion, it is noteworthy that the Drosophila model can replicate this mode of entry through the intestinal barrier.39−41 Simultaneously, the discernible structure of the Drosophila testes facilitates the precise identification of distinct stages affected by toxic compounds during spermatogenesis.42 As a result, the use of Drosophila as a model organism has significantly advanced our understanding of spermatogenesis and toxicity induced by contaminants.42−44
Therefore, in this study, we utilized a Drosophila model to investigate the impact of TiO2 NP exposure on spermatogenesis. We provide novel insights into the specific disruptions caused by TiO2 NP exposure in spermatid elongation and identify genetic alterations associated with spermatid differentiation through RNA sequencing. Our findings also highlight the role of TiO2 NPs in altering various metabolic pathways and demonstrate the potential of antioxidant drugs in mitigating oxidative stress caused by these nanoparticles. This study aims to deepen our understanding of TiO2 NP-induced reproductive toxicity and identify potential therapeutic targets.
2. Results
2.1. Characterization of TiO2 NPs
The morphological properties and particle size distribution of TiO2 NPs were assessed using scanning electron microscopy (SEM) and dynamic light scattering (DLS) techniques, as depicted in Figure 1A,B. The analyses revealed that the TiO2 NPs predominantly exhibited an average particle size of around 100 nm. Furthermore, the ζ potential of the TiO2 NPs in aqueous suspension was determined, registering at −8.005 mV (Figure 1C). These characterizations provide foundational insights into the physicochemical properties of TiO2 NPs, which are essential for understanding their biological interactions and subsequent functional analyses.
Figure 1.
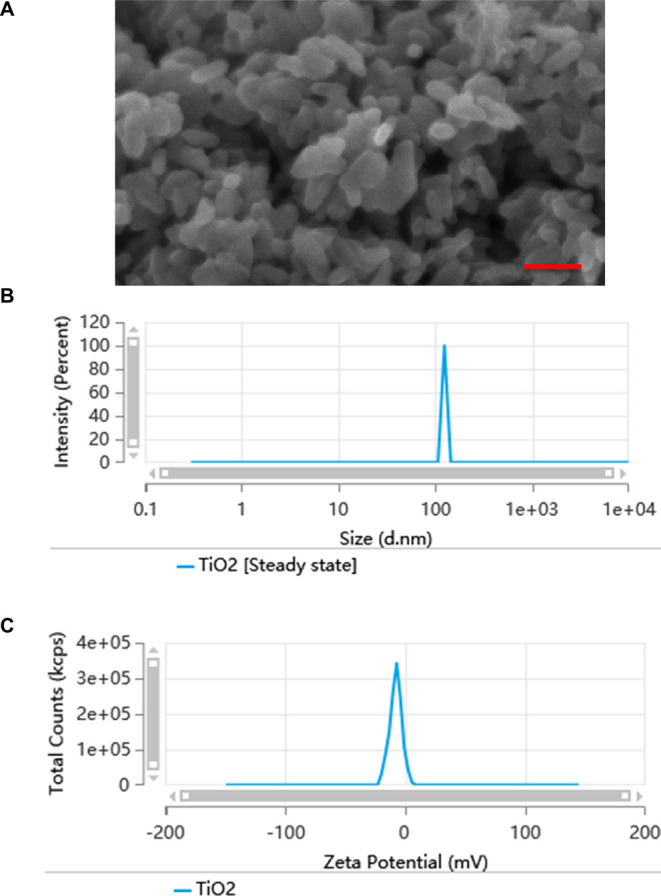
Characteristics of the TiO2 NPs. (A) SEM image of the TiO2 NPs. (B) The diameters of TiO2 NPs. (C) The ζ potential (mV) of TiO2 NPs measured in water. Scale bar: 100 nM.
2.2. TiO2 NP Exposure Induces Spermatid Elongation Defects
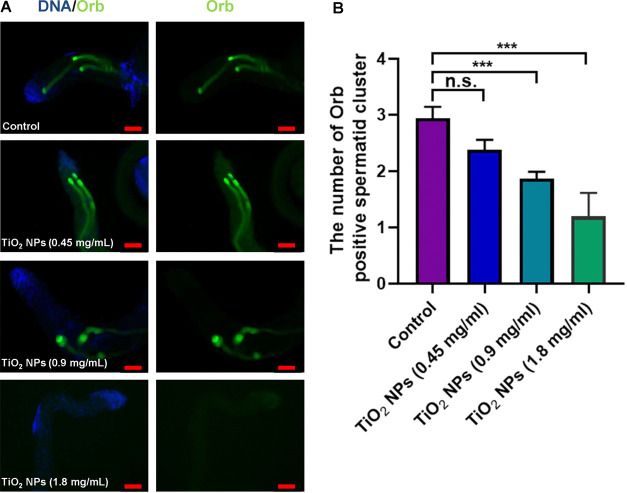
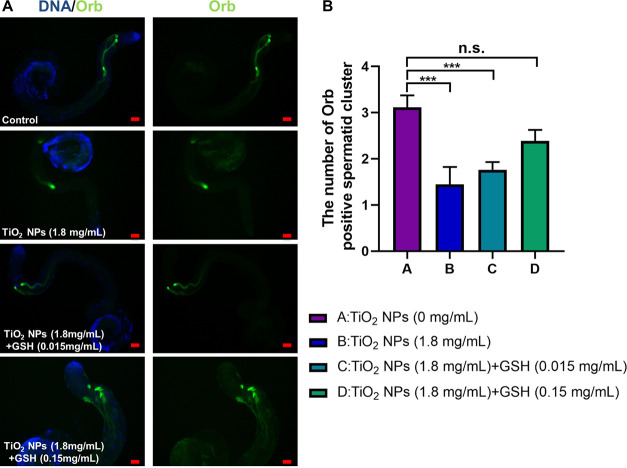
We subsequently examined the reproductive toxicity by subjecting male flies to concentration gradients of 0.45, 0.9, and 1.8 mg/mL of TiO2 NPs for a duration of 30 days. To assess elongated spermatids, we employed immunofluorescence staining with the Orb protein, a marker specific to Drosophila testes. Our findings revealed a reduction in the quantity of elongated spermatid clusters following exposure to TiO2 NPs at concentrations of 0.45 mg/mL in comparison to the control group. However, no statistically significant variance was observed between these two groups (Figure 2). Moreover, exposure to 0.9 and 1.8 mg/mL resulted in marked reductions in the number of elongated spermatid clusters (Figure 2), underscoring the dose-dependent effect of TiO2 NPs on spermatid development. Within our model, we also assessed male fertility and observed that exposure to TiO2 NPs (1.8 mg/mL) in adult males (30 days) significantly impaired male fertility when compared with control (Figure S1).
Figure 2.
Phenotype analysis of spermatid elongation for TiO2 NP exposure. (A) Immunostaining of Orb (green) to label elongated spermatids in control and TiO2 NP exposure at 0.45, 0.9, and 1.8 mg/mL. DNA was stained with Hoechst33342 (blue). (B) The number of Orb-positive spermatid cluster. ***P < 0.001, n.s. represents no statistical difference. Scale bar: 50 μM.
2.3. TiO2 NP Exposure Does Not Induce Toxicity for Early-Stage Germ Cells and Somatic Cells
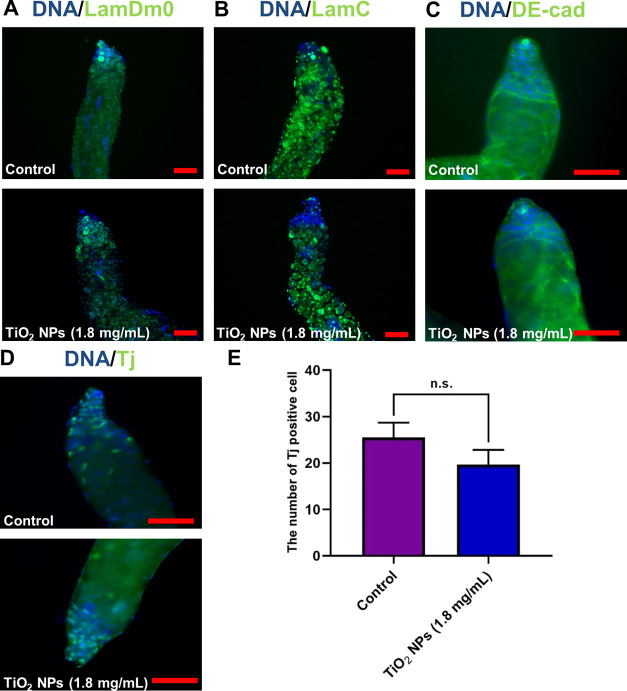
Further exploration was conducted to assess the impact of TiO2 NPs on early-stage germ and somatic cells. We subsequently examined the cellular distributions at the apex of Drosophila testes and did not observe any conspicuous phenotypic evidence of the significant loss of cellular clusters following exposure to TiO2 NPs at concentrations of 0.45, 0.9, and 1.8 mg/mL compared to the control group (Figure S2). Using specific markers, LamDm0 and LamC, for labeling spermatogonia and spermatocytes, respectively, no significant abnormalities were observed in these cells post exposure to 1.8 mg/mL of TiO2 NPs (Figure 3A,B). Similarly, DE-cad, a marker for cyst and hub cells, indicated no notable changes post exposure (Figure 3C). The quantification of early-stage cyst cells, using Tj as a marker, also did not reveal any significant alterations post exposure (Figure 3D,E). These observations collectively suggest that a high-dose TiO2 NP exposure selectively affects spermatid elongation while sparing early-stage germ cells, hub cells, and cyst cells.
Figure 3.
Phenotype analysis for TiO2 NP exposure at the apex of testes. (A) Immunostaining of LamDm0 (green) to label spermatogonia in control and TiO2 NP (1.8 mg/mL)-exposed testes. (B) Immunostaining of LamC (green) to label spermatocytes in the control and TiO2 NP (1.8 mg/mL)-exposed testes. (C) Immunostaining of DE-cad (green) to identify cyst cells and hub cells in control and TiO2 NP (1.8 mg/mL)-exposed testes. (D) Immunostaining of Tj (green) was used to examine the distribution of early-stage cyst cells in control and TiO2 NP (1.8 mg/mL)-exposed testes. (E) The number of Tj positive cells. DNA was stained with Hoechst33342 (blue). n.s. represents no statistical difference. Scale bar: 50 μM.
2.4. Transcriptomic Alterations Induced by TiO2 NPs
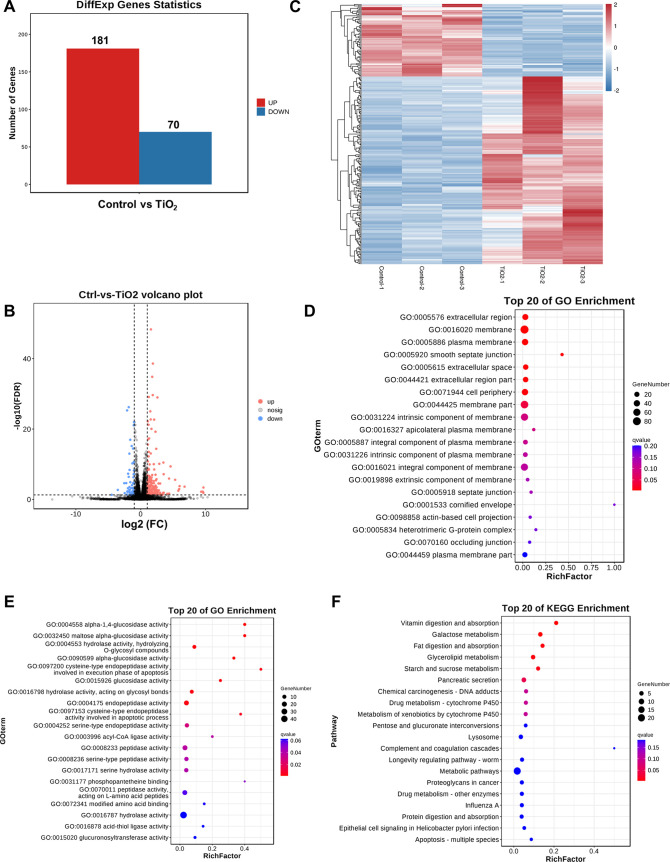
To elucidate the transcriptional changes induced by TiO2 NP exposure, bulk RNA-seq analysis was performed. This analysis identified 251 differentially expressed genes (DEGs), comprising 181 upregulated and 70 downregulated genes (Figure 4A). Visualizations including volcano plots and heatmaps were utilized to present the distribution of these DEGs (Figure 4B,C). Gene Ontology (GO) enrichment analysis pointed to significant changes in cellular components, molecular functions, and biological processes, particularly related to glucosidase activities and extracellular regions (Figures 4D,E and S3). The KEGG pathway analysis highlighted alterations in several metabolic pathways, notably those related to vitamin digestion and glycerolipid metabolism (Figure 4F).
Figure 4.
Transcriptional network of TiO2 NP exposure in Drosophila testes. (A) Statistics of DEGs between the control and TiO2 NP (1.8 mg/mL) groups. (B) Volcano plot based on −log 10(FDR) and log2(FC) from the comparison of control and TiO2 NP (1.8 mg/mL) groups. (C) Clustering heatmap of DEGs for the comparison between the control and TiO2 NP (1.8 mg/mL) groups. (D, E) Top 20 of GO enrichment of cellular components (D) and molecular function (E) for DEGs between the control and TiO2 NP (1.8 mg/mL) groups. (F) KEGG enrichment analysis of DEGs between the control and TiO2 NP (1.8 mg/mL) groups.
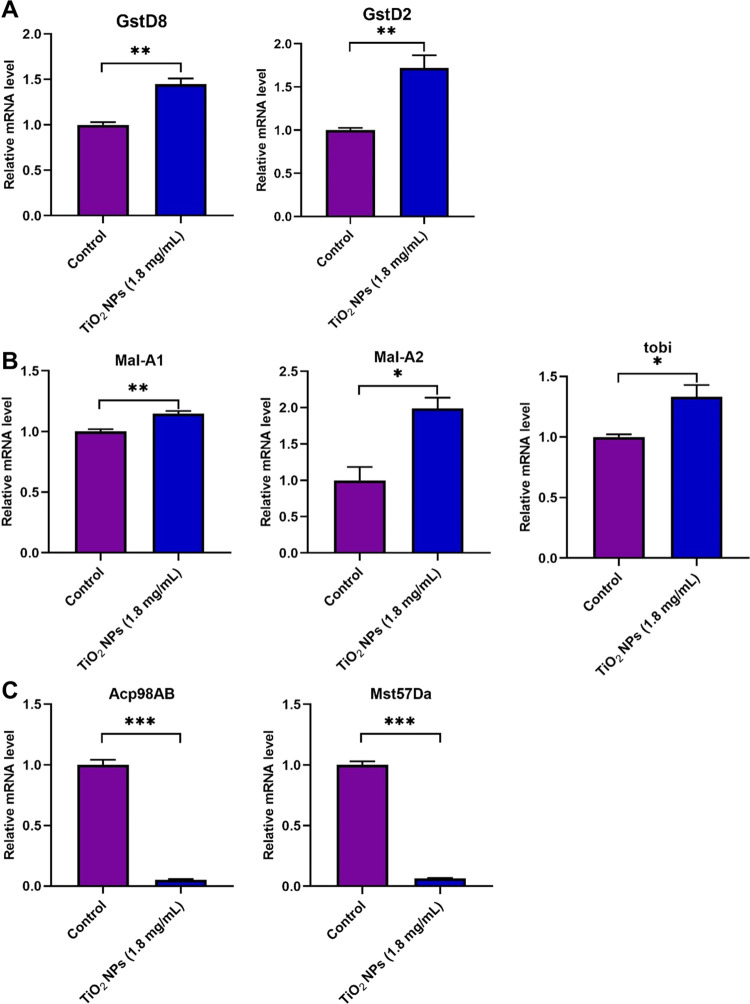
To validate these findings, qRT-PCR was conducted for representative DEGs. Notably, the relative mRNA expressions of GstD8 and GstD2 were upregulated (Figure 5A). To delve deeper into comprehending the modulation of oxidative stress equilibrium following TiO2 exposure in Drosophila testicular tissue, we performed a lipid peroxidation (LPO) assay, a pivotal metric for assessing oxidative harm. Our results demonstrated that TiO2 NP exposure led to an increase in LPO levels in Drosophila testes (Figure S4). Interestingly, Mal-A1, Mal-A2, and tobi exhibited significantly increased expression levels following TiO2 NP exposure (Figure 5B). Furthermore, Acp98AB and Mst57 Da showed a dramatic decrease after TiO2 NP exposure, potentially leading to defects in spermatid elongation (Figure 5C). Our validations of the aforementioned DEGs were consistent with the RNA-seq data, aiding in a deeper comprehension of TiO2 NP exposure-mediated testicular toxicity in Drosophila.
Figure 5.
Verifications of representative DEGs mediated by TiO2 NP exposure. (A) Relative mRNA of GstD8 and GstD2 in the control and TiO2 NP (1.8 mg/mL)-exposed testes. (B) Relative mRNA of Mal-A1, Mal-A2, and tobi in control and TiO2 NP (1.8 mg/mL)-exposed testes. (C) Relative mRNA of Acp98AB and Mst57 Da in control and TiO2 NP (1.8 mg/mL)-exposed testes. *P < 0.05, **P < 0.01, and ***P < 0.001.
2.5. Glutathione (GSH) Restores Spermatid Elongation Defects, Which Result from TiO2 NP Exposure
Previous studies have highlighted the role of oxidative stress in reproductive toxicity induced by metal exposure in Drosophila.45 Given our observation of the upregulated expression of GstD8 and GstD2, which encode enzymes associated with GSH metabolism, subsequent in vivo GSH rescue assays were conducted using varying doses of GSH (0.015 and 0.15 mg/mL) in conjunction with TiO2 NPs (1.8 mg/mL). Our findings unequivocally demonstrate that GSH supplementation effectively mitigates reproductive toxicity induced by TiO2 NP exposure, leading to the restoration of the number of Orb-positive spermatid clusters in Drosophila testes (Figure 6). These results imply that the administration of the GSH antioxidant alone is sufficient to ameliorate testicular damage caused by TiO2 NP exposure.
Figure 6.
GSH intervention treatment for TiO2 NP exposure. (A) Immunostaining of Orb (green) in control, TiO2 NP- (1.8 mg/mL), TiO2 NP- (1.8 mg/mL) + GSH (0.015 and 0.15 mg/mL)-exposed testes. DNA was stained with Hoechst33342 (blue). (B) The number of Orb-positive spermatid cluster. ***P < 0.001, n.s. represents no statistical difference. Scale bar: 50 μM.
3. Discussion
The escalating deployment of TiO2 NPs has precipitated growing apprehension about their potential deleterious effects on health, as underscored in the recent literature.16 In mammalian males, experimental evidence indicates that exposure to TiO2 NPs leads to damage in the testes and epididymis, resulting in a reduced sperm concentration and motility, as well as an increased proportion of abnormal sperm.46−48 These phenomena are ostensibly linked to perturbations in testicular enzyme activity and augmented levels of oxidative stress.49,50 Nonetheless, a comprehensive understanding of the transcriptional regulatory mechanisms involved remains elusive. Our study explored the effects of prolonged TiO2 NP exposure (30 days) at varying concentrations and found that TiO2 NP exposure resulted in a marked decrease in elongated spermatid clusters within Drosophila testes, signifying the dose-dependent effect of TiO2 NP exposure-induced spermatid elongation defects. Our findings exclusively unveiled a phenotype linked to spermatid elongation. It was possible that the differences in findings were due to variations between the species studied in Drosophila and mammalian testicles. Another explanation could be that the concentrations or durations of exposure to the experimental conditions in our study were lower or shorter than those in the other studies.
To elucidate the regulatory mechanisms underlying these effects, we embarked on comprehensive transcriptome profiling via bulk RNA-Seq, aimed at deciphering the biological processes and pathways affected by TiO2 NP exposure in Drosophila testes. Moreover, our data demonstrated that carbohydrate metabolism and cytochrome P450-associated metabolic pathways were enriched for DEGs between the control and TiO2 NP groups. In testicular germ cells, carbohydrate metabolism-associated pathways were involved in multiple stages during spermatogenesis.51 Evidences have indicated that multiple cytochrome P450-related genes, which encoded key enzymes for steroidogenesis pathway, were essential for the maintenance and differentiation of germ cells.52,53 Significantly, we observed alterations in genes associated with galactose metabolism and glucosidase activity, such as Mal-A1, Mal-A2, and tobi. Moreover, Mal-A1, Mal-A2, and Tobi encoded proteins with maltase α-glucosidase activity, participating in carbohydrate metabolism processes.54,55Mal-A1 and Mal-A2 genes also exhibited significant enrichment in maltose and disaccharide metabolic pathways, as indicated by GO enrichment analysis of biological processes. A recent study indicated that the intestinal carbohydrate metabolism processes mediated by these genes played a crucial role in the male-biased intestinal metabolic state, controlling food intake and sperm production through gut-derived citrate in Drosophila.56 These findings, in conjunction with the data presented, implied that the modulation of glycometabolism or carbohydrate metabolism might have repercussions on spermatogenesis.
Acp98AB was primarily expressed at high levels in the male accessory gland and responsible for physiological and behavioral changes in female Drosophila.57Mst57 Da encoded an antimicrobial peptide that was predominantly expressed in the male accessory glands and transferred to females during mating.58−60 Single-cell RNA-seq results of Drosophila testes also suggested that Sb exposure could mediate changes in the expression of the Acp98AB and Mst57 Da genes in testicular germ cells,42 indicating their crucial roles during spermiogenesis. Our study found that TiO2 NP exposure led to decreases in the expression levels of Acp98AB and Mst57 Da, revealing that TiO2 NP exposure could disrupt the differentiation of elongated spermatids through these genes. Currently, little has been known about their regulatory mechanisms of reproductive toxicity. Our validations of the aforementioned DEGs were consistent with the RNA-seq data, aiding in a deeper comprehension of the TiO2 NP exposure-mediated testicular toxicity in Drosophila.
Previous studies have delved into the amelioration of germ cell quality through the mitigation of oxidative stress. For instance, mitochondrial dysfunction and redox changes induced by 4-methylimidazole (4-MI) have been implicated in oocyte damage.61 Mogroside V (MV), a major extract of Siraitia grosvenorii, has been investigated for its potential to ameliorate oxidative stress-induced meiosis defects by restoring mitochondrial integrity in oocytes.62 The role of ROS signaling in apoptosis and autophagy in methotrexate (MTX)-induced GC2 cells has been documented.63 Besides, puerarin has been shown to reverse oxidative stress and spermatogenesis changes induced by busulfan.64 Our previous study demonstrated that prenatal exposure to metals, such as antimony (Sb), significantly impaired larval growth and development by disrupting oxidative stress homeostasis.45 Sb exposure in male testes could also induce reproductive toxicity during spermatogenesis.42 In models of oligospermia, interventions such as ferroptosis inhibition and peptide administration derived from the croceine croaker have shown promise.65,66 Additionally, oxidative stress damage induced by zinc oxide (ZnO) NPs in meiosis was partially mitigated by antioxidants.67 Notably, TiO2 NP exposure has been linked to excessive ROS production in testes, impeding spermatogenesis.49GstD8 and GstD2 were subunits of the Drosophila glutathione S-transferase superfamily that were involved in the glutathione metabolic process.68,69 Previous studies have shown that exposure to the heavy metal antimony (Sb) in Drosophila led to the upregulation of GstD8 and GstD2.45 In our study, an increased expression of GstD8 and GstD2 following TiO2 NP exposure suggested disrupted oxidative stress homeostasis in Drosophila testes. Morin/rutin have shown enhanced efficacy against TiO2 NP-induced reproductive toxicity, safeguarding endogenous antioxidant mechanisms and neutralizing free radicals.31
GSH acted as a tripeptide thiol antioxidant, engaging in direct or enzymatic interactions with oxidants, leading to the production of glutathione disulfide (GSSG) and thus regulating cellular redox balance to protect cells from damage.70,71 Simultaneously, GSH assumed pivotal functions in a wide array of metabolic and physiological processes, including cell differentiation, proliferation, apoptosis, ferroptosis, and immunity.72 Operating as an antioxidant, GSH scavenged ROS and safeguards against oxidative damage, possessing the ability to influence gene expression through various signaling pathways.45 GSH protected lipid oxidation, neutralized peroxides, and inhibited oxidation, thereby mitigating the impact of oxidative stress reactions during spermatogenesis. Sustaining optimal glutathione levels and its redox equilibrium was imperative for bolstering the structural and functional metamorphoses for spermatid maturation.73,74 Therefore, we employed GSH to rectify the spermatid elongation defects induced by TiO2 NP exposure by mitigating oxidative stress damage. Evidence suggested that metal exposure-induced oxidative stress imbalance could be mitigated by the antioxidant properties of GSH in Drosophila.45 Our study further demonstrated that exposure to TiO2 NPs led to an increase in the LPO levels in Drosophila testes. Quantification of sperm elongation indicated the successful restoration of the compromised state through dietary supplementation with GSH, highlighting the central role of GSH in restoring oxidative stress homeostasis.
4. Conclusions
In conclusion, our findings demonstrate that TiO2 NP exposure specifically induced defects in spermatid elongation through multiple metabolic pathways in Drosophila testes. Furthermore, the intervention of GSH significantly ameliorated testicular damage caused by TiO2 NPs, highlighting the potential of targeting oxidative stress homeostasis as a therapeutic avenue for countering nanoparticle toxicity.
5. Materials and Methods
5.1. Fly Strains and Culture
w1118 line was used for in vivo experiments. With a relative humidity of 40–60% and the same photoperiod as outside, all Drosophila were raised at 25 °C and replicated in vials containing standard cornmeal molasses agar media.
5.2. TiO2 NP Characteristics
TiO2 NPs were purchased from Beijing Deke Daojin Science and Technology Co., Ltd. The morphology of TiO2 NPs was analyzed by SEM. The diameters and ζ potential of TiO2 NPs were measured by DLS using a Malvern Zetasizer Nano ZS 90.
5.3. TiO2 NP Exposure Methods
TiO2 NP powder was dispersed in 1 × phosphate-buffered saline (PBS), treated ultrasonically for 5 min, and then mechanically vibrated for 10 min. The solutions containing TiO2 NPs were then added to normal foods for flies to achieve the final exposure concentrations (0.45, 0.9, and 1.8 mg/mL). 2–3 day old male flies of the w1118 line were then placed in standard Drosophila food medium containing TiO2 NPs for 30 days. Control males were placed in standard Drosophila food medium without TiO2 NPs for 30 days.
5.4. Immunofluorescence
Immunofluorescence was carried out as described previously.44 Briefly, Drosophila testes were dissected in 1m PBS, fixed for 30 min in 4% paraformaldehyde (PFA), washed three times with 0.3% PBS-Triton X-100 (PBST), and incubated in 5% bovine serum albumin (BSA) for 30 min. Primary antibodies were diluted in 5% BSA, and testes were incubated at 25 °C for 1 h and then washed three times with 0.3% PBST. Secondary antibodies were conjugated with Cy3 or A647 (Jackson ImmunoResearch Laboratories, West Grove, PA), diluted at a ratio of 1:400, and incubated at room temperature for 1 h, avoiding light. The testes were then washed three times with 0.3% PBST and stained with Hoechst33342 (1.0 mg/mL, C0031; Solarbio, Beijing, China), which were diluted with PBS according to the potency of 1:800 for 5 min before finalizing. Detailed information about the primary antibodies is provided in Table S1.
5.5. RNA Extraction and RNA Sequencing (RNA-seq)
Drosophila testes were treated with TRIzol (no. 15596026, Invitrogen), according to the manufacturer’s instructions, to extract the total RNA. The Nanodrop 2000 instrument (Thermo Fisher Scientific) was used to measure the total RNA concentration and purity. Gel electrophoresis was used to determine the integrity of the RNA, and the RNA integrity number (RIN) values were calculated using an Agilent 2100 instrument (Agilent Technologies). Utilizing the TruSeq RNA Library Prep Kit v2 (Illumina) following the manufacturer’s instructions, libraries for indexed RNA-Seq were created from 800 ng of total RNA. In this experiment, poly(A) mRNA was purified using oligo-dT magnetic beads, RNA was fragmented, double-stranded cDNA was produced using SuperScript II Reverse Transcriptase (Invitrogen), indexed Illumina adapters were ligated, and limited-cycle PCR was used to amplify the results. After the libraries underwent qualification testing, the DNA nano ball (DNB) was created and loaded onto the sequencing chip and sequenced using Illumina HiSeq2500 by Gene Denovo Biotechnology Co. (Guangzhou, China).
5.6. Quantitative Reverse Transcription Polymerase Chain Reaction (qRT-PCR) Analysis
The qRT-PCR was carried out by the manufacturer’s instructions. Drosophila testes’ RNA were extracted using the Trizol Reagent (#15596026, Invitrogen), and cDNA was then generated through reverse transcription using the PrimeScript II first Strand cDNA Synthesis Kit (#6210A, Takara). qRT-PCR analysis was carried out using Light Cycler equipment (Roche). The relative expression level of each mRNA was determined and standardized to Gapdh mRNA. Detailed information for primers used in this study is shown in Table S2.
5.7. GSH Prevention and Intervention Treatment
A set of experiments involving cotreatment with TiO2 NPs and GSH (#G4251, Sigma-Aldrich) were initiated, for which the following treatment groups were formed: control (TiO2 NPs, 0 mg/mL), TiO2 NPs (1.8 mg/mL), TiO2 NPs (1.8 mg/mL) + GSH (0.015 and 0.15 mg/mL). TiO2 NP powder was dispersed in 1 × PBS, subjected to ultrasonication for 5 min, and then subjected to mechanical vibration for 10 min. Likewise, GSH powder was dissolved in 1 × PBS and then mechanically vibrated for 10 min. Subsequently, TiO2 NP solution was introduced to the regular diet, followed by the addition of the GSH solution to the diet containing TiO2 NPs.
5.8. LPO Assay
According to the manufacturer’s protocol, LPO level was assessed by using an LPO content assay kit (BC5245, Solarbio). Drosophila testes were extracted and homogenized in ice-cold saline using an ultrasonic homogenizer, followed by centrifugation at 12,000g for 10 min. The supernatants were gathered for quantification of the LPO contents.
5.9. Male Fertility Assay
Male flies aged 2–3 days from the w1118 line were introduced into a nutrient medium with or without TiO2 NPs for a duration of 30 days. In individual male fertility assessment, a single male and three virgin w1118 female flies were cohabited in a tube at ambient temperature. The male was deemed infertile if no larvae were observed within 7 days. Subsequently, data on the male fertility rate was recorded.
5.10. Statistical Analysis
The quantitative results were given as the mean ± standard error of the mean (SEM) and were performed using GraphPad Prism 8.0 (GraphPad Software, CA). Two-tailed Student’s t test was used to determine significant differences between two groups, and one-way analysis of variance (ANOVA) was conducted to examine multiple comparison using Dunnett’s test. The chi-square test was used to evaluate for ratio results. * P < 0.05; ** P < 0.01; *** P < 0.001; n.s. represents no statistical difference.
Acknowledgments
The authors wish to thank all study participants, research staff, and students who assisted with this work. This work was supported partly by the National Key R&D Program of China (2022YFC2703202, 2018YFC1003602), the National Natural Science Foundation of China (81901528), the Natural Science Foundation of Jiangsu Province (BK20221376), the Nantong Project of Science and Technology (MS12022027), the Basic Science Research Program of Nantong City (JC12022006), the Jiangsu Innovation and Entrepreneurship Talent Plan, and the Jiangsu Health Innovation Team Program (2020).
Data Availability Statement
The original contributions presented in the study are included in the article; further inquiries can be directed to the corresponding author/s.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.4c01140.
Male fertility; testicular phenotype; GO enrichment; LPO level; antibodies; primers; and DEGs (PDF)
Author Contributions
# X.C., T.J., and Q.H. contributed equally to this work. J.Y., H.C., and X.W. initiated the project, designed the study, coordinated the experiment, and wrote the manuscript. X.C., T.J., Q.H., X.Z., H.Y., L.J., and J.L. performed the experiments and provided conceptual inputs for the paper. X.K., X.Z., X.H., X.D., T.W., Y.S., X.Z., and L.L. analyzed the data. All authors read and approved the final manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Roduner E. Size matters: why nanomaterials are different. Chem. Soc. Rev. 2006, 35 (7), 583–592. 10.1039/b502142c. [DOI] [PubMed] [Google Scholar]
- Hou J.; Wang L.; Wang C.; Zhang S.; Liu H.; Li S.; Wang X. Toxicity and mechanisms of action of titanium dioxide nanoparticles in living organisms. J. Environ. Sci. 2019, 75, 40–53. 10.1016/j.jes.2018.06.010. [DOI] [PubMed] [Google Scholar]
- Samrot A. V.; Noel Richard Prakash L. X. Nanoparticles Induced Oxidative Damage in Reproductive System and Role of Antioxidants on the Induced Toxicity. Life 2023, 13 (3), 767 10.3390/life13030767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottschalk F.; Sonderer T.; Scholz R. W.; Nowack B. Modeled environmental concentrations of engineered nanomaterials (TiO(2), ZnO, Ag, CNT, Fullerenes) for different regions. Environ. Sci. Technol. 2009, 43 (24), 9216–9222. 10.1021/es9015553. [DOI] [PubMed] [Google Scholar]
- Zhang X.; Song Y.; Gong H.; Wu C.; Wang B.; Chen W.; Hu J.; Xiang H.; Zhang K.; Sun M. Neurotoxicity of Titanium Dioxide Nanoparticles: A Comprehensive Review. Int. J. Nanomed. 2023, 18, 7183–7204. 10.2147/IJN.S442801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimipour M.; Zirak Javanmard M.; Ahmadi A.; Jafari A. Oral administration of titanium dioxide nanoparticle through ovarian tissue alterations impairs mice embryonic development. Int. J. Reprod. Biomed. 2018, 16 (6), 397–404. 10.29252/ijrm.16.6.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir A.; Westerhoff P.; Fabricius L.; Hristovski K.; von Goetz N. Titanium dioxide nanoparticles in food and personal care products. Environ. Sci. Technol. 2012, 46 (4), 2242–2250. 10.1021/es204168d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornu R.; Béduneau A.; Martin H. Ingestion of titanium dioxide nanoparticles: a definite health risk for consumers and their progeny. Arch. Toxicol. 2022, 96 (10), 2655–2686. 10.1007/s00204-022-03334-x. [DOI] [PubMed] [Google Scholar]
- Shi H.; Magaye R.; Castranova V.; Zhao J. Titanium dioxide nanoparticles: a review of current toxicological data. Part. Fibre Toxicol. 2013, 10, 15 10.1186/1743-8977-10-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najahi-Missaoui W.; Arnold R. D.; Cummings B. S. Safe Nanoparticles: Are We There Yet?. Int. J. Mol. Sci. 2021, 22 (1), 385 10.3390/ijms22010385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson T. A.; Sanchez W. Y.; Roberts M. S. Are commercially available nanoparticles safe when applied to the skin?. J. Biomed. Nanotechnol. 2010, 6 (5), 452–468. 10.1166/jbn.2010.1145. [DOI] [PubMed] [Google Scholar]
- Grande F.; Tucci P. Titanium Dioxide Nanoparticles: a Risk for Human Health?. Mini-Rev. Med. Chem. 2016, 16 (9), 762–769. 10.2174/1389557516666160321114341. [DOI] [PubMed] [Google Scholar]
- Wang J.; Zhou G.; Chen C.; Yu H.; Wang T.; Ma Y.; Jia G.; Gao Y.; Li B.; Sun J.; Li Y.; Jiao F.; Zhao Y.; Chai Z. Acute toxicity and biodistribution of different sized titanium dioxide particles in mice after oral administration. Toxicol. Lett. 2007, 168 (2), 176–185. 10.1016/j.toxlet.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Schoelermann J.; Burtey A.; Allouni Z. E.; Gerdes H. H.; Cimpan M. R. Contact-dependent transfer of TiO2 nanoparticles between mammalian cells. Nanotoxicology 2016, 10 (2), 204–215. 10.3109/17435390.2015.1048322. [DOI] [PubMed] [Google Scholar]
- Kose O.; Tomatis M.; Leclerc L.; Belblidia N. B.; Hochepied J. F.; Turci F.; Pourchez J.; Forest V. Impact of the Physicochemical Features of TiO(2) Nanoparticles on Their In Vitro Toxicity. Chem. Res. Toxicol. 2020, 33 (9), 2324–2337. 10.1021/acs.chemrestox.0c00106. [DOI] [PubMed] [Google Scholar]
- Baranowska-Wójcik E.; Szwajgier D.; Oleszczuk P.; Winiarska-Mieczan A. Effects of Titanium Dioxide Nanoparticles Exposure on Human Health-a Review. Biol. Trace Elem. Res. 2020, 193 (1), 118–129. 10.1007/s12011-019-01706-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourikas K.; Kordulis C.; Lycourghiotis A. Titanium dioxide (anatase and rutile): surface chemistry, liquid-solid interface chemistry, and scientific synthesis of supported catalysts. Chem. Rev. 2014, 114 (19), 9754–9823. 10.1021/cr300230q. [DOI] [PubMed] [Google Scholar]
- Wang J.; Li N.; Zheng L.; Wang S.; Wang Y.; Zhao X.; Duan Y.; Cui Y.; Zhou M.; Cai J.; Gong S.; Wang H.; Hong F. P38-Nrf-2 signaling pathway of oxidative stress in mice caused by nanoparticulate TiO2. Biol. Trace Elem. Res. 2011, 140 (2), 186–197. 10.1007/s12011-010-8687-0. [DOI] [PubMed] [Google Scholar]
- Wang J.; Fan Y. Lung injury induced by TiO2 nanoparticles depends on their structural features: size, shape, crystal phases, and surface coating. Int. J. Mol. Sci. 2014, 15 (12), 22258–22278. 10.3390/ijms151222258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S. Q.; Zhu R. R.; Zhu H.; Xue M.; Sun X. Y.; Yao S. D.; Wang S. L. Nanotoxicity of TiO(2) nanoparticles to erythrocyte in vitro. Food Chem. Toxicol. 2008, 46 (12), 3626–3631. 10.1016/j.fct.2008.09.012. [DOI] [PubMed] [Google Scholar]
- Cui Y.; Gong X.; Duan Y.; Li N.; Hu R.; Liu H.; Hong M.; Zhou M.; Wang L.; Wang H.; Hong F. Hepatocyte apoptosis and its molecular mechanisms in mice caused by titanium dioxide nanoparticles. J. Hazard. Mater. 2010, 183 (1–3), 874–880. 10.1016/j.jhazmat.2010.07.109. [DOI] [PubMed] [Google Scholar]
- Gui S.; Zhang Z.; Zheng L.; Cui Y.; Liu X.; Li N.; Sang X.; Sun Q.; Gao G.; Cheng Z.; Cheng J.; Wang L.; Tang M.; Hong F. Molecular mechanism of kidney injury of mice caused by exposure to titanium dioxide nanoparticles. J. Hazard. Mater. 2011, 195, 365–370. 10.1016/j.jhazmat.2011.08.055. [DOI] [PubMed] [Google Scholar]
- Shimizu M.; Tainaka H.; Oba T.; Mizuo K.; Umezawa M.; Takeda K. Maternal exposure to nanoparticulate titanium dioxide during the prenatal period alters gene expression related to brain development in the mouse. Part. Fibre Toxicol. 2009, 6, 20 10.1186/1743-8977-6-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alarifi S.; Ali D.; Al-Doaiss A. A.; Ali B. A.; Ahmed M.; Al-Khedhairy A. A. Histologic and apoptotic changes induced by titanium dioxide nanoparticles in the livers of rats. Int. J. Nanomed. 2013, 8, 3937–3943. 10.2147/IJN.S47174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philbrook N. A.; Winn L. M.; Afrooz A. R.; Saleh N. B.; Walker V. K. The effect of TiO(2) and Ag nanoparticles on reproduction and development of Drosophila melanogaster and CD-1 mice. Toxicol. Appl. Pharmacol. 2011, 257 (3), 429–436. 10.1016/j.taap.2011.09.027. [DOI] [PubMed] [Google Scholar]
- Hong F.; Zhou Y.; Zhao X.; Sheng L.; Wang L. Maternal exposure to nanosized titanium dioxide suppresses embryonic development in mice. Int. J. Nanomed. 2017, 12, 6197–6204. 10.2147/IJN.S143598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassinari R.; Cubadda F.; Moracci G.; Aureli F.; D’Amato M.; Valeri M.; De Berardis B.; Raggi A.; Mantovani A.; Passeri D.; Rossi M.; Maranghi F. Oral, short-term exposure to titanium dioxide nanoparticles in Sprague-Dawley rat: focus on reproductive and endocrine systems and spleen. Nanotoxicology 2014, 8 (6), 654–662. 10.3109/17435390.2013.822114. [DOI] [PubMed] [Google Scholar]
- Gao G.; Ze Y.; Zhao X.; Sang X.; Zheng L.; Ze X.; Gui S.; Sheng L.; Sun Q.; Hong J.; Yu X.; Wang L.; Hong F.; Zhang X. Titanium dioxide nanoparticle-induced testicular damage, spermatogenesis suppression, and gene expression alterations in male mice. J. Hazard. Mater. 2013, 258–259, 133–143. 10.1016/j.jhazmat.2013.04.046. [DOI] [PubMed] [Google Scholar]
- Gao G.; Ze Y.; Li B.; Zhao X.; Zhang T.; Sheng L.; Hu R.; Gui S.; Sang X.; Sun Q.; Cheng J.; Cheng Z.; Wang L.; Tang M.; Hong F. Ovarian dysfunction and gene-expressed characteristics of female mice caused by long-term exposure to titanium dioxide nanoparticles. J. Hazard. Mater. 2012, 243, 19–27. 10.1016/j.jhazmat.2012.08.049. [DOI] [PubMed] [Google Scholar]
- Liu S.; Tang Y.; Chen B.; Zhao Y.; Aguilar Z. P.; Tao X.; Xu H. Inhibition of testosterone synthesis induced by oral TiO(2) NPs is associated with ROS-MAPK(ERK1/2)-StAR signaling pathway in SD rat. Toxicol. Res. 2021, 10 (4), 937–946. 10.1093/toxres/tfab077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussein M. M. A.; Gad E.; Ahmed M. M.; Arisha A. H.; Mahdy H. F.; Swelum A. A.; Tukur H. A.; Saadeldin I. M. Amelioration of titanium dioxide nanoparticle reprotoxicity by the antioxidants morin and rutin. Environ. Sci. Pollut. Res. Int. 2019, 26 (28), 29074–29084. 10.1007/s11356-019-06091-0. [DOI] [PubMed] [Google Scholar]
- Pandey U. B.; Nichols C. D. Human disease models in Drosophila melanogaster and the role of the fly in therapeutic drug discovery. Pharmacol. Rev. 2011, 63 (2), 411–436. 10.1124/pr.110.003293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker H.; Gallant P. Getting started: an overview on raising and handling Drosophila. Methods Mol. Biol. 2008, 420, 27–44. 10.1007/978-1-59745-583-1_2. [DOI] [PubMed] [Google Scholar]
- Ong C.; Lee Q. Y.; Cai Y.; Liu X.; Ding J.; Yung L. Y.; Bay B. H.; Baeg G. H. Silver nanoparticles disrupt germline stem cell maintenance in the Drosophila testis. Sci. Rep 2016, 6, 20632 10.1038/srep20632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellen H. J.; Tong C.; Tsuda H. 100 years of Drosophila research and its impact on vertebrate neuroscience: a history lesson for the future. Nat. Rev. Neurosci. 2010, 11 (7), 514–522. 10.1038/nrn2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White-Cooper H. Molecular mechanisms of gene regulation during Drosophila spermatogenesis. Reproduction 2010, 139 (1), 11–21. 10.1530/REP-09-0083. [DOI] [PubMed] [Google Scholar]
- Barreau C.; Benson E.; Gudmannsdottir E.; Newton F.; White-Cooper H. Post-meiotic transcription in Drosophila testes. Development 2008, 135 (11), 1897–1902. 10.1242/dev.021949. [DOI] [PubMed] [Google Scholar]
- Rand M. D.; Montgomery S. L.; Prince L.; Vorojeikina D. Developmental toxicity assays using the Drosophila model. Curr. Protoc. Toxicol. 2014, 59, 1.12.1–20. 10.1002/0471140856.tx0112s59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey A.; Chandra S.; Chauhan L. K.; Narayan G.; Chowdhuri D. K. Cellular internalization and stress response of ingested amorphous silica nanoparticles in the midgut of Drosophila melanogaster. Biochim. Biophys. Acta 2013, 1830 (1), 2256–2266. 10.1016/j.bbagen.2012.10.001. [DOI] [PubMed] [Google Scholar]
- Alaraby M.; Hernández A.; Annangi B.; Demir E.; Bach J.; Rubio L.; Creus A.; Marcos R. Antioxidant and antigenotoxic properties of CeO2 NPs and cerium sulphate: Studies with Drosophila melanogaster as a promising in vivo model. Nanotoxicology 2015, 9 (6), 749–759. 10.3109/17435390.2014.976284. [DOI] [PubMed] [Google Scholar]
- Siddique Y. H.; Khan W.; Khanam S.; Jyoti S.; Naz F.; Rahul; Singh B. R.; Naqvi A. H. Toxic potential of synthesized graphene zinc oxide nanocomposite in the third instar larvae of transgenic Drosophila melanogaster (hsp70-lacZ)Bg9. Biomed. Res. Int. 2014, 2014, 382124 10.1155/2014/382124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J.; Fu Y.; Li Z.; Huang Q.; Tang J.; Sun C.; Zhou P.; He L.; Sun F.; Cheng X.; Ji L.; Yu H.; Shi Y.; Gu Z.; Sun F.; Zhao X. Single-cell RNA sequencing reveals cell landscape following antimony exposure during spermatogenesis in Drosophila testes. Cell Death Discovery 2023, 9 (1), 86 10.1038/s41420-023-01391-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demir E.; Turna Demir F. Drosophila melanogaster as a dynamic in vivo model organism reveals the hidden effects of interactions between microplastic/nanoplastic and heavy metals. J. Appl. Toxicol. 2023, 43 (2), 212–219. 10.1002/jat.4353. [DOI] [PubMed] [Google Scholar]
- Yu J.; Zheng Q.; Li Z.; Wu Y.; Fu Y.; Wu X.; Lin D.; Shen C.; Zheng B.; Sun F. CG6015 controls spermatogonia transit-amplifying divisions by epidermal growth factor receptor signaling in Drosophila testes. Cell Death Discovery 2021, 12 (5), 491 10.1038/s41419-021-03783-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.; Zhou P.; Zhang Z.; Huang Q.; Chen X.; Ji L.; Cheng X.; Shi Y.; Yu S.; Tang J.; Sun C.; Zhao X.; Yu J. A Drosophila model of gestational antimony exposure uncovers growth and developmental disorders caused by disrupting oxidative stress homeostasis. Free Radical Biol. Med. 2023, 208, 418–429. 10.1016/j.freeradbiomed.2023.09.002. [DOI] [PubMed] [Google Scholar]
- Morgan A. M.; Ibrahim M. A.; Noshy P. A. Reproductive toxicity provoked by titanium dioxide nanoparticles and the ameliorative role of Tiron in adult male rats. Biochem. Biophys. Res. Commun. 2017, 486 (2), 595–600. 10.1016/j.bbrc.2017.03.098. [DOI] [PubMed] [Google Scholar]
- Behairy A.; Hashem M. M. M.; Abo-El-Sooud K.; Soliman A. M.; Mouneir S. M.; El-Metwally A. E.; Ismail S. H.; Hassan B. A.; Abd-Elhakim Y. M. Influence of titanium dioxide nanoparticles and/or cadmium chloride oral exposure on testicular morphology, oxidative stress, and apoptosis in rats: Ameliorative role of co-enzyme Q10. Heliyon 2024, 10 (1), e24049 10.1016/j.heliyon.2024.e24049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X.; Li L.; An H.; Deng Y.; Ling C.; Lu T.; Song G.; Wang Y. Lycopene Alleviates Titanium Dioxide Nanoparticle-Induced Testicular Toxicity by Inhibiting Oxidative Stress and Apoptosis in Mice. Biol. Trace Elem. Res. 2022, 200 (6), 2825–2837. 10.1007/s12011-021-02881-1. [DOI] [PubMed] [Google Scholar]
- Hong F.; Si W.; Zhao X.; Wang L.; Zhou Y.; Chen M.; Ge Y.; Zhang Q.; Wang Y.; Zhang J. TiO2 Nanoparticle Exposure Decreases Spermatogenesis via Biochemical Dysfunctions in the Testis of Male Mice. J. Agric. Food Chem. 2015, 63 (31), 7084–7092. 10.1021/acs.jafc.5b02652. [DOI] [PubMed] [Google Scholar]
- Hong F.; Zhao X.; Si W.; Ze Y.; Wang L.; Zhou Y.; Hong J.; Yu X.; Sheng L.; Liu D.; Xu B.; Zhang J. Decreased spermatogenesis led to alterations of testis-specific gene expression in male mice following nano-TiO2 exposure. J. Hazard. Mater. 2015, 300, 718–728. 10.1016/j.jhazmat.2015.08.010. [DOI] [PubMed] [Google Scholar]
- Bajpai M.; Gupta G.; Setty B. S. Changes in carbohydrate metabolism of testicular germ cells during meiosis in the rat. Eur. J. Endocrinol. 1998, 138 (3), 322–327. 10.1530/eje.0.1380322. [DOI] [PubMed] [Google Scholar]
- Park H. J.; Zhang M.; Lee W. Y.; Hong K. H.; Do J. T.; Park C.; Song H. Toxic Effects of Nonylphenol on Neonatal Testicular Development in Mouse Organ Culture. Int. J. Mol. Sci. 2020, 21 (10), 3491 10.3390/ijms21103491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.; Ye D.; Zhang F.; Zhang R.; Zhu J.; Wang H.; He M.; Sun Y. Cyp11a2 Is Essential for Oocyte Development and Spermatogonial Stem Cell Differentiation in Zebrafish. Endocrinology 2022, 163 (2), bqab258 10.1210/endocr/bqab258. [DOI] [PubMed] [Google Scholar]
- Zhou F.; Liu B.; Liu X.; Li Y.; Wang L.; Huang J.; Luo G.; Wang X. The Impact of Microbiome and Microbiota-Derived Sodium Butyrate on Drosophila Transcriptome and Metabolome Revealed by Multi-Omics Analysis. Metabolites 2021, 11 (5), 298 10.3390/metabo11050298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler E. K.; Bradley T.; Moxon S.; Chapman T. Divergence in Transcriptional and Regulatory Responses to Mating in Male and Female Fruitflies. Sci. Rep. 2019, 9 (1), 16100 10.1038/s41598-019-51141-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudry B.; de Goeij E.; Mineo A.; Gaspar P.; Hadjieconomou D.; Studd C.; Mokochinski J. B.; Kramer H. B.; Plaçais P. Y.; Preat T.; Miguel-Aliaga I. Sex Differences in Intestinal Carbohydrate Metabolism Promote Food Intake and Sperm Maturation. Cell 2019, 178 (4), 901–918.e16. 10.1016/j.cell.2019.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfner M. F.; Harada H. A.; Bertram M. J.; Stelick T. J.; Kraus K. W.; Kalb J. M.; Lung Y. O.; Neubaum D. M.; Park M.; Tram U. New genes for male accessory gland proteins in Drosophila melanogaster. Insect Biochem. Mol. Biol. 1997, 27 (10), 825–834. 10.1016/S0965-1748(97)00056-8. [DOI] [PubMed] [Google Scholar]
- Findlay G. D.; MacCoss M. J.; Swanson W. J. Proteomic discovery of previously unannotated, rapidly evolving seminal fluid genes in Drosophila. Genome Res. 2009, 19 (5), 886–896. 10.1101/gr.089391.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findlay G. D.; Yi X.; Maccoss M. J.; Swanson W. J. Proteomics reveals novel Drosophila seminal fluid proteins transferred at mating. PLoS Biol. 2008, 6 (7), e178 10.1371/journal.pbio.0060178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller J. L.; Ravi Ram K.; McGraw L. A.; Bloch Qazi M. C.; Siggia E. D.; Clark A. G.; Aquadro C. F.; Wolfner M. F. Cross-species comparison of Drosophila male accessory gland protein genes. Genetics 2005, 171 (1), 131–143. 10.1534/genetics.105.043844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y.; Tang H.; Xu J.; Sun F. Toxic effects of 4-methylimidazole on the maturation and fertilization of mouse oocytes. Food Chem. Toxicol. 2022, 164, 113051 10.1016/j.fct.2022.113051. [DOI] [PubMed] [Google Scholar]
- Pan C.; Chen J.; Chen Y.; Lu Y.; Liang X.; Xiong B.; Lu Y. Mogroside V ameliorates the oxidative stress-induced meiotic defects in porcine oocytes in vitro. Reprod. Toxicol. 2022, 111, 148–157. 10.1016/j.reprotox.2022.05.008. [DOI] [PubMed] [Google Scholar]
- Xiong S.; Song D.; Xiang Y.; Li Y.; Zhong Y.; Li H.; Zhang P.; Zhou W.; Zeng X.; Zhang X. Reactive oxygen species, not Ca(2+), mediates methotrexate-induced autophagy and apoptosis in spermatocyte cell line. Basic Clin. Pharmacol. Toxicol. 2020, 126 (2), 144–152. 10.1111/bcpt.13306. [DOI] [PubMed] [Google Scholar]
- Li H. T.; Zhong K.; Xia Y. F.; Song J.; Chen X. Q.; Zhao W.; Zeng X. H.; Chen T. X. Puerarin improves busulfan-induced disruption of spermatogenesis by inhibiting MAPK pathways. Biomed. Pharmacother. 2023, 165, 115231 10.1016/j.biopha.2023.115231. [DOI] [PubMed] [Google Scholar]
- Zhao X.; Liu Z.; Gao J.; Li H.; Wang X.; Li Y.; Sun F. Inhibition of ferroptosis attenuates busulfan-induced oligospermia in mice. Toxicology 2020, 440, 152489 10.1016/j.tox.2020.152489. [DOI] [PubMed] [Google Scholar]
- Zhao X.; Sang M.; Han P.; Gao J.; Liu Z.; Li H.; Gu Y.; Wang C.; Sun F. Peptides from the croceine croaker (Larimichthys crocea) swim bladder attenuate busulfan-induced oligoasthenospermia in mice. Pharm. Biol. 2022, 60 (1), 319–325. 10.1080/13880209.2022.2034895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C.; Wu D.; Khan F. A.; Wang Y.; Xu J.; Luo C.; Zhang K.; Sun F.; Huo L. Zinc oxide nanoparticle causes toxicity to the development of mouse oocyte and early embryo. Toxicol. Lett. 2022, 358, 48–58. 10.1016/j.toxlet.2022.01.010. [DOI] [PubMed] [Google Scholar]
- Garmash E. V.; Velegzhaninov I. O.; Ermolina K. V.; Rybak A. V.; Malyshev R. V. Altered levels of AOX1a expression result in changes in metabolic pathways in Arabidopsis thaliana plants acclimated to low dose rates of ultraviolet B radiation. Plant Sci. 2020, 291, 110332 10.1016/j.plantsci.2019.110332. [DOI] [PubMed] [Google Scholar]
- Marelja Z.; Dambowsky M.; Bolis M.; Georgiou M. L.; Garattini E.; Missirlis F.; Leimkühler S. The four aldehyde oxidases of Drosophila melanogaster have different gene expression patterns and enzyme substrate specificities. J. Exp. Biol. 2014, 217 (Pt 12), 2201–2211. 10.1242/jeb.102129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy L.; Sandhu J. K.; Harper M. E.; Cuperlovic-Culf M. Role of Glutathione in Cancer: From Mechanisms to Therapies. Biomolecules 2020, 10 (10), 1429 10.3390/biom10101429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaucher C.; Boudier A.; Bonetti J.; Clarot I.; Leroy P.; Parent M. Glutathione: Antioxidant Properties Dedicated to Nanotechnologies. Antioxidants 2018, 7 (5), 62 10.3390/antiox7050062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu B.; Liao K.; Zhou Y.; Wen T.; Quan G.; Pan X.; Wu C. Application of glutathione depletion in cancer therapy: Enhanced ROS-based therapy, ferroptosis, and chemotherapy. Biomaterials 2021, 277, 121110 10.1016/j.biomaterials.2021.121110. [DOI] [PubMed] [Google Scholar]
- Ricketts P. G.; Minimair M.; Yates R. W.; Klaus A. V. The effects of glutathione, insulin and oxidative stress on cultured spermatogenic cysts. Spermatogenesis 2011, 1 (2), 159–171. 10.4161/spmg.1.2.17031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J.; Li Z.; Fu Y.; Sun F.; Chen X.; Huang Q.; He L.; Yu H.; Ji L.; Cheng X.; Shi Y.; Shen C.; Zheng B.; Sun F. Single-cell RNA-sequencing reveals the transcriptional landscape of ND-42 mediated spermatid elongation via mitochondrial derivative maintenance in Drosophila testes. Redox Biol. 2023, 62, 102671 10.1016/j.redox.2023.102671. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article; further inquiries can be directed to the corresponding author/s.