Key Clinical Message
Tuberculous peritonitis (TB peritonitis) is one of the most challenging forms of extrapulmonary TB to diagnose. While tumor markers can be elevated in patients with TB peritonitis, FDG‐PET/CT can aid in distinguishing TB peritonitis from malignancies, if an apron‐like omentum pattern is seen. Laparoscopy is crucial for accurate and early diagnosis.
Keywords: laparoscopic, PET/CT, tuberculosis, tuberculous peritonitis
1. CASE ILLUSTRATED
An 82‐year‐old Japanese woman, with known aortic stenosis and cerebral infarction, presented with a 3‐month history of bilateral leg edema and abdominal distension, along with 2 days of fever. Abdominal tenderness was noted, specifically in the left lower quadrant. Laboratory tests showed albumin of 3.3 g/dL, high CA‐125 (266 U/mL), high soluble interleukin‐2 receptors (sIL‐2R) (3784 U/mL), and a positive MTB (Mycobacterium tuberculosis) interferon gamma release assay (IGRA) (T‐SPOT®: Oxford Immunotec Ltd.). Computed tomography (CT) scan depicted cake‐like thickening of the greater omentum, and significant ascites (Figure 1), without any abnormality in lung. Ascitic fluid analysis showed clear yellow fluid with an elevated white blood cell count of 1175 (lymphocytes: 80%), normal serum‐to‐ascites albumin gradient (SAAG) (0.09 g/dL), and increased adenosine deaminase (ADA) of 85.1 U/L (reference range: 8.6–20.5). Microbiological study of the ascitic fluid was negative for acid‐fast bacillus (AFB) smear and culture, as well as Mycobacterium tuberculosis polymerase chain reaction (MTB‐PCR). FDG‐positron emission tomography/computed tomography (PET/CT) scan demonstrated diffuse elevated tracer uptake in ascites, peritoneum, mesentery, and the greater omentum (Figure 2). Laparoscopic examination revealed prevalent white nodules and adhesions on the surface of the intestine, peritoneal wall, and perihepatic area, which were biopsied (Figure 3). The histopathology exam showed noncaseating granulomas were scattered throughout the peritoneal tissue with numerous lymphocytes (Figure 4). Additionally, the Ziel–Nielsen stain and MTB‐PCR of the tissue biopsied were negative. However, the AFB culture of the biopsied specimen grew Mycobacterium tuberculosis 3 weeks later, confirming TB peritonitis.
FIGURE 1.
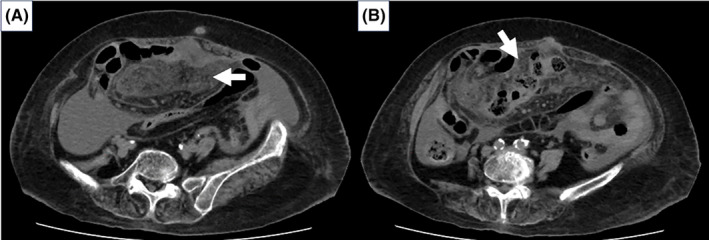
(A) Non‐contrast enhanced computed tomography (CT) in axial view on admission revealed the thickened soft tissue strands in the mesentery alongside significant ascites accumulation without any mass lesions (white arrow). (B) Cake‐like thickening of greater omentum is noted (white arrow).
FIGURE 2.
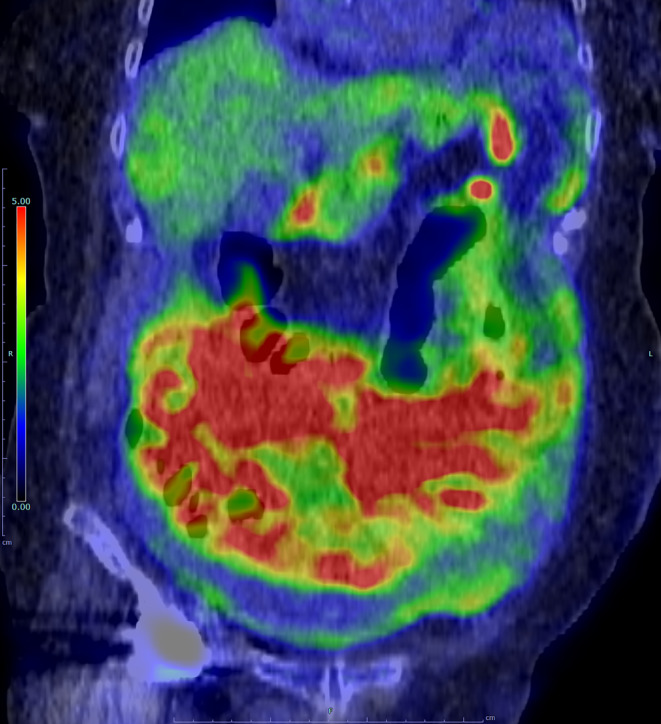
Coronal view of fused positron emission tomography/computed tomography (PET/CT) imaging. Elevated tracer uptake is observed in the ascites, peritoneum, mesentery, and greater omentum, exhibiting an apron‐like distribution. The standardized uptake value (SUV) max was 11.45 in the peritoneum, which is significantly above the normal range, aiding in the suspicion towards tuberculous (TB) peritonitis.
FIGURE 3.
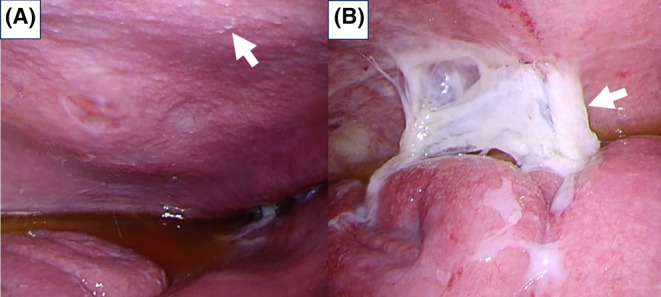
(A) Laparoscopy revealed numerous miliary white nodules on the parietal peritoneum (white arrow) with serous yellow ascites. (B) Adhesions between the peritoneal wall and intestine are noted (white arrow).
FIGURE 4.
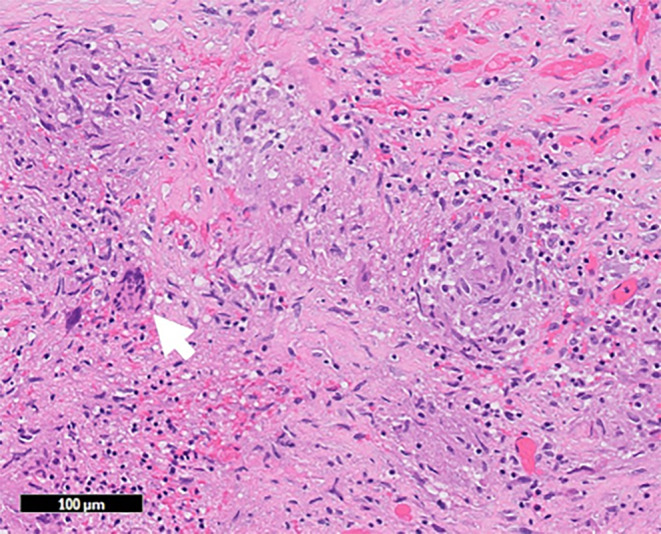
Peritoneal tissue revealed scattered noncaseating granulomas (white arrow) with numerous lymphocytes.
Tuberculous peritonitis typically arises from the reactivation of latent tuberculous lesions within the peritoneum, initially seeded through hematogenous dissemination from a primary lung infection. Elderly patients with peritoneal tuberculosis may show few constitutional symptoms, yet low‐grade fever, weight loss, or abdominal pain are often observed. 1 Additionally, more than 90% of patients with TB peritonitis have ascites at the time of presentation. Diagnosing TB peritonitis can be challenging when the CA‐125 and sIL‐2R levels are elevated and mimic malignancy. The sensitivity of ascitic AFB cultures ranges from 35% to 83% based on fluid volume. 1 Despite the high validity of ascitic fluid ADA, with a sensitivity of 0.90 and specificity of 0.94, 2 interpretation can be complex where malignancy and infection‐related ascites are more common, as in developed countries and the elderly population. The diagnostic value of FDG‐PET/CT for distinguishing tuberculous peritonitis from other malignancies is acknowledged but needs further validation. Omental findings are notable for guiding the site of abdominal issues. 3 A study showed that tuberculous peritonitis often preserves an apron‐pattern omentum, possibly because these patients seek medical attention earlier and undergo imaging before omentum contracts. 3 In this case, despite a cake‐like CT pattern typical of peritoneal carcinomatosis, FDG‐PET/CT showed an apron‐like pattern, leading us to laparoscopy. Laparoscopy, combining visual and histological analysis, proved crucial for accurate and early diagnosis, showing 93% sensitivity and 98% specificity. 1 This case underscores the value of integrating FDG‐PET/CT and ascitic fluid analysis for timely laparoscopy, leading to a definitive TB peritonitis diagnosis.
AUTHOR CONTRIBUTIONS
Kenji Yamada: Writing – original draft. Kazuaki Aoki: Supervision; writing – review and editing. Rina Tanaka: Writing – review and editing. Seito Matsushima: Writing – review and editing. Akiyuki Sato: Writing – review and editing. Takaaki Kobayashi: Supervision; writing – review and editing. Sandra Moody: Writing – review and editing. Masayuki Nogi: Writing – review and editing.
FUNDING INFORMATION
None.
CONFLICT OF INTEREST STATEMENT
The authors have no conflict of interest to declare.
ETHICAL APPROVAL
The local ethics committee's approval does not apply in this case.
CONSENT
Written informed consent was obtained from the patient to publish this report in accordance with the journal's patient consent policy.
ACKNOWLEDGMENTS
We thank Wang Ziren,1 Yasunori Yoshida,1 Hideki Ueda,1 Yuki Sato,2 Daisuke Ikeda,3 Yuta Ushida4, Wataru Uegami5, and Asami Inoue6 for the patient's care. 1: Department of Infectious Diseases, Kameda Medical Center, Kamogawa, Chiba, Japan. 2: Department of Respiratory Medicine, Kameda Medical Center, Kamogawa, Chiba, Japan. 3: Department of Hematology, Kameda Medical Center, Kamogawa, Chiba, Japan. 4: Department of Gastroenterological Surgery, Kameda Medical Center, Kamogawa, Chiba, Japan. 5: Department of Pathology, Kameda Medical Center, Kamogawa, Chiba, Japan. 6: Department of Radiology, Kameda Medical Center, Kamogawa, Chiba, Japan.
Yamada K, Aoki K, Tanaka R, et al. On the fence: Combining multimodal imaging and laparoscopy for diagnosing tuberculous peritonitis. Clin Case Rep. 2024;12:e8996. doi: 10.1002/ccr3.8996
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Sanai FM, Bzeizi KI. Systematic review: tuberculous peritonitis‐ presenting features, diagnostic strategies and treatment. Aliment Pharmacol Ther. 2005;22:685‐700. [DOI] [PubMed] [Google Scholar]
- 2. Mahajan M, Prasad ML, Kumar P, et al. An updated systematic review and meta‐analysis for the diagnostic test accuracy of ascitic fluid adenosine deaminase in tuberculous peritonitis. Infect Chemother. 2023;55:264‐277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Duan H, Xu D, Lu R, Wang S, Xie R, Wang S. Characterizing omental PET/CT findings for differentiating tuberculous peritonitis from peritoneal carcinomatosis. Abdom Radiol. 2021;46:5574‐5585. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


