Abstract
Cancer cachexia (CC) is a devastating metabolic syndrome characterized by skeletal muscle wasting and body weight loss, posing a significant burden on the health and survival of cancer patients. Despite ongoing efforts, effective treatments for CC are still lacking. Metabolomics, an advanced omics technique, offers a comprehensive analysis of small‐molecule metabolites involved in cellular metabolism. In CC research, metabolomics has emerged as a valuable tool for identifying diagnostic biomarkers, unravelling molecular mechanisms and discovering potential therapeutic targets. A comprehensive search strategy was implemented to retrieve relevant articles from primary databases, including Web of Science, Google Scholar, Scopus and PubMed, for CC and metabolomics. Recent advancements in metabolomics have deepened our understanding of CC by uncovering key metabolic signatures and elucidating underlying mechanisms. By targeting crucial metabolic pathways including glucose metabolism, amino acid metabolism, fatty acid metabolism, bile acid metabolism, ketone body metabolism, steroid metabolism and mitochondrial energy metabolism, it becomes possible to restore metabolic balance and alleviate CC symptoms. This review provides a comprehensive summary of metabolomics studies in CC, focusing on the discovery of potential therapeutic targets and the evaluation of modulating specific metabolic pathways for CC treatment. By harnessing the insights derived from metabolomics, novel interventions for CC can be developed, leading to improved patient outcomes and enhanced quality of life.
Keywords: Cancer cachexia, Metabolic pathway, Metabolomics, Therapeutic target
Introduction
Cancer cachexia (CC) is a complex multi‐organ and multifactorial syndrome characterized by metabolic disorders, systemic inflammation and anorexia. 1 , 2 It affects over 50% of cancer patients and is responsible for approximately 30% of cancer‐related deaths. 3 CC is characterized by a prominent loss of body weight, primarily due to progressive skeletal muscle atrophy, accompanied by a possible reduction in adipose tissue mass. 4 Skeletal muscle atrophy leads to debilitating fatigue, compromising the tolerance and efficacy of clinical anti‐cancer interventions, such as chemotherapy and surgery, and significantly impacting the patients' quality of life. 5 Consequently, extensive research has focused on unravelling the molecular mechanisms underlying CC, with specific emphasis on skeletal muscle. The current understanding suggests that systemic inflammation and abnormal metabolism, resulting from complex tumour–host interactions, are major contributors to skeletal muscle loss in CC.
The excessive production of inflammatory cytokines, including interleukin‐6 (IL‐6), interleukin‐1 (IL‐1), tumour necrosis factor alpha (TNF‐α) and transforming growth factor beta (TGF‐β), by cancer cells and host immune cells contributes to oxidative stress, activation of the ubiquitin‐proteasome pathway (UPP) and autophagy‐lysosome pathway (ALP) and a negative nitrogen balance with increased catabolism and decreased anabolism. 6 , 7 These cytokines also activate downstream components, such as atrogin‐1 and muscle RING‐finger protein‐1 (MuRF1), through signalling pathways like insulin‐like growth factor 1 (IGF‐1)–protein kinase B (Akt/PKB)–forkhead box O (FoxO)/mammalian target of rapamycin (mTOR), nuclear factor kappa‐B (NF‐κB) and IL‐6–signal transducer and activator of transcription 3 (STAT3), leading to muscle atrophy and protein metabolism dysregulation. Additionally, cancer cells require a substantial amount of energy derived from peripheral organs, such as skeletal muscle, in the form of glucose, glutamine, ketone bodies and acetate, 8 , 9 , 10 resulting in skeletal muscle hypercatabolism and energy deficiency.
Recent research has shown promising results in targeting specific receptors or pathways to mitigate CC, offering potential therapeutic approaches for clinical trials. For example, blocking the activin type 2 receptor (ActRIIB/ACVR2A) pathway reversed skeletal muscle atrophy and extended survival in multiple mouse models. 11 Further research has focused on targeting specific receptors to mitigate the effects of CC. For instance, studies have shown that targeting the tumour necrosis factor receptor superfamily member 12A (Fn14/TNFRSF12A) receptor can effectively prevent inflammation and the loss of adipose and skeletal muscle mass, subsequently extending the lifespan in mouse models of CC. 12 Additionally, targeting the receptor for advanced glycation end‐products (RAGE) has been identified as an effective strategy to reduce skeletal muscle wasting and prolong survival in CC models. 13 More recently, studies have demonstrated the potential of activating the Akt/mTOR signalling pathway to completely reverse muscle wasting associated with CC. 14
However, considering the metabolic abnormalities present in approximately 50% of cancer patients with cachexia, targeting metabolites and metabolic pathways could offer further anti‐cachexia therapeutic strategies. 15 Metabolomics, an increasingly popular ‘omics’ approach, provides a comprehensive analysis of small‐molecule metabolites involved in cellular metabolism. 16 Metabolomics, particularly through metabolic flux analysis (MFA), can capture subtle yet significant alterations in biological systems. Utilizing isotopic labels such as 13C and 2H, MFA enables a dynamic and real‐time depiction of the phenotype, thereby delivering a nuanced and thorough understanding of the intricate metabolic processes and their variations. 17
Metabolomics has been applied to investigate various human diseases, including cancer, 18 , 19 coronavirus disease 2019 (COVID‐19), 20 , 21 sarcopenia, 22 , 23 cardiovascular disease 24 , 25 and diabetes. 26 , 27 In recent years, metabolomics has played a crucial role in identifying biomarkers, elucidating molecular mechanisms and discovering potential therapeutic targets. 28 In the context of CC, metabolomics has contributed to the discovery of novel biofluid biomarkers and systemic metabolic alterations for early diagnosis and understanding the pathogenesis of CC.
In this review, we will provide a comprehensive summary of studies investigating CC using metabolomics approaches. Specifically, we will highlight the results related to the discovery of potential therapeutic targets and the evaluation of targeting specific metabolic pathways for the treatment of CC. By analysing the metabolomics data from these studies, we aim to shed light on novel strategies for managing CC and improving the outcome for cancer patients.
Materials and methods
Search strategy
A comprehensive search strategy was implemented to retrieve relevant articles from primary databases, including Web of Science, Google Scholar, Scopus and PubMed. The search focused on the ‘title/abstract’ field and employed advanced search techniques. The following keywords were used: ‘metabolomic and cachexia’, ‘metabolomic and muscle atrophy’, ‘metabolomics and cachexia’, ‘metabolomics and muscle atrophy’, ‘metabonomic and cachexia’, ‘metabonomic and muscle atrophy’, ‘metabonomics and cachexia’, ‘metabonomics and muscle atrophy’, ‘metabolic and cachexia’ and ‘metabolic and muscle atrophy’. The search was conducted up until May 2023 to ensure a comprehensive exploration of the available literature in this field.
Inclusion criteria
Our review specifically focused on experimental articles investigating potential therapeutic targets for CC using the metabolomics approach. We broadened our analysis to encompass research studies that investigated the impact of metabolites or metabolic pathways on muscle atrophy, extending beyond the scope of metabolomics studies alone. We intentionally excluded articles classified as letters, reviews, editorial comments and conference abstracts from our analysis. Additionally, studies exploring cachexia induced by factors other than cancer, such as heart failure, chemotherapy, aging, chronic diseases, acquired immunodeficiency syndrome (AIDS) and disuse (immobility, bedrest and hindlimb suspension), were deliberately excluded. Furthermore, studies primarily focused on the identification of metabolic biomarkers in CC through the application of the metabolomics approach were not included in our analysis.
Metabolomics approach
Compared with prior ‘omics’ methodologies, such as proteomics, transcriptomics and genomics, metabolomics can accurately and quantitatively detect downstream biological changes (Figure 1 ). The detailed procedures of metabolomics can be found in the supporting information.
Figure 1.
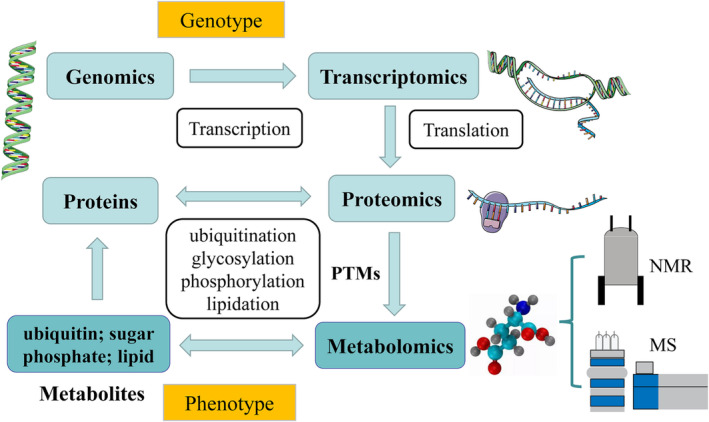
Multiple levels targeted by omics approaches in biological systems. Metabolomics can accurately and quantitatively detect downstream biological changes. MS, mass spectrometry; NMR, nuclear magnetic resonance; PTMs, post‐translational modifications.
Metabolomics‐driven therapeutic targets for cancer cachexia
Glucose metabolism
The observation of disrupted glucose metabolism in muscle atrophy suggests its significant role in the development and progression of CC. 29 , 30 , 31 , 32 , 33 Previously, we utilized metabolomic analyses to investigate two CC murine models. We found decreased glucose levels in cachectic muscles associated with glioma, 29 as well as reduced glucose abundance in both serum and muscle of gastric CC. 30 Furthermore, we identified significant up‐regulation of gene expression of three key enzymes involved in the rate‐limiting steps of glucose metabolism: hexokinase, phosphofructokinase and phosphoglycerate mutase, in cachectic muscles. 30 These results provide valuable insights into the impaired state of glucose metabolism in CC and highlight potential therapeutic targets that could be explored for intervention.
Wei et al. observed consistent results, showing a significant increase in hexokinase expression in atrophic muscles, coupled with reduced blood glucose in cachexia groups. 33 To further investigate the potential therapeutic implications of these results, Wei et al. explored the effects of 2‐deoxy‐d‐glucose (2‐DG), a glucose analogue known to competitively inhibit hexokinase function and suppress glycolysis. 34 , 35 The administration of 2‐DG specifically resulted in elevated muscular glycogen levels, increased serum ketone bodies and the preservation of lean mass. Significantly, 2‐DG treatment not only stimulated liver ketogenesis and enhanced the utilization of ketone bodies by skeletal muscles but also exhibited a down‐regulatory effect on the UPP signalling and autophagy degradation.
Wei et al. revealed that 2‐DG led to significant improvements in weight loss without causing a notable reduction in tumour growth. 2‐DG has been primarily studied for its ability to disrupt tumour metabolism, specifically targeting glycolysis and adenosine triphosphate (ATP) production. 36 These findings suggest that 2‐DG, by directly targeting muscle metabolism, may have therapeutic potential in mitigating the symptoms of CC. However, it is important to consider that the weight loss attenuation observed with 2‐DG treatment might also be partially attributed to its indirect effects on cancer metabolism. Future research should explore the dual effects of 2‐DG, examining its impact on both muscle metabolism and cancer metabolism, to fully understand its potential in combatting the multifaceted aspects of CC syndrome.
Similarly, Tseng et al. investigated the dysregulation of glucose metabolism in muscle through metabolomics analysis, revealing depleted glycogen levels and reduced abundances of glucose and key glycolytic intermediates. 37 Additionally, they identified elevated levels of 2‐hydroxybutyrate, a biomarker associated with insulin resistance. To explore potential therapeutic strategies, the researchers explored the effects of AR‐42, a novel histone deacetylase (HDAC) inhibitor, on abnormal metabolism in CC. Their results highlighted AR‐42 as a promising therapeutic candidate for CC, emphasizing the need for further investigation into its efficacy and mechanisms of action.
In addition, Ohbuchi et al. conducted plasma metabolomics analyses to explore the effects of rikkunshito (RKT) as a traditional Japanese herbal medicine and address the underlying mechanisms. 32 The data unveiled significant alterations in 23 metabolites following RKT treatment in CC rats. Notably, glucarate, a compound known for its anti‐cancer activity through the inhibition of β‐glucuronidase, exhibited increased levels in both the tumour‐bearing and normal groups after RKT administration. Intriguingly, the researchers investigated the therapeutic potential of glucarate in alleviating CC symptoms using a rat model. Glucarate demonstrated remarkable reductions in ascites volume, decreased levels of plasma inflammatory cytokines, improved gastrocnemius muscle atrophy and inhibited body weight loss. However, the underlying molecular mechanisms and metabolic regulations responsible for the effects of glucarate remain unknown and warrant further investigation.
In various animal models of CC, a marked decrease in glucose and glycogen levels was observed, leading to an energy deficit. This was accompanied by a reduction in metabolic intermediates and an increase in key glycolytic enzymes. Although studies have identified compounds such as 2‐DG, AR‐42 and RKT that target the impaired glucose metabolism to mitigate CC symptoms, there is a need for further research. Both preclinical models and clinical trials are essential to delve deeper into the molecular mechanisms and comprehensively evaluate the effectiveness of these drugs in treating CC.
Amino acid metabolism
Metabolomics analyses in various animal models have consistently highlighted an imbalance between muscle protein synthesis and proteolysis as a prominent characteristic of CC. 38 , 39 , 40 Notably, branched‐chain amino acids (BCAAs), which are plentiful in dietary components, are both produced and broken down by the gut microbiome. Importantly, the levels of BCAAs circulating in the host's system have been found to be associated with insulin resistance. This relationship underscores the significant impact of gut microbiome activity on host metabolism and health outcomes related to insulin sensitivity. 41 , 42 Der‐Torossian et al. observed a decrease in serum levels of BCAAs in CC patients compared with fasting or aging individuals. 43 Moreover, Yang et al. conducted an integrated evaluation of serum and muscle metabolomics using high‐resolution magic angle spinning nuclear magnetic resonance (HR‐MAS NMR)‐based technology in CC mice. They observed increased ketone bodies and decreased BCAAs as the distinctive metabolic signature of CC. 31 The gut microbiota also plays vital roles in regulating gut hormones, CC‐related cytokines and host metabolism. 44 Furthermore, Ni et al. demonstrated the involvement of the gut microbiota in regulating gut hormones, CC‐related cytokines and host metabolism. They observed significantly reduced levels of BCAAs in the plasma of cancer patients, along with impaired BCAA functional pathways in the gut microbiota. 45 These results collectively highlight the consistent alterations in BCAAs and their association with CC, underscoring the potential role of BCAAs and the gut microbiota in CC pathogenesis.
Individuals with CC often exhibit low levels of BCAAs, particularly leucine. Leucine plays vital roles in muscle metabolism, as it can stimulate protein synthesis through the mTOR pathway and inhibit protein breakdown. 46 , 47 Viana et al. demonstrated that leucine supplementation could reduce muscle loss and alter metabolic profiles without affecting tumour mass. 48 They further found that leucine‐rich diets activated the mTOR pathway, maintained muscle function and mitigated muscle weight loss. 49 Through the integration of metabolomics and proteomics analyses, they uncovered that the leucine‐rich diet exerted improvements in glycolysis, the tricarboxylic acid (TCA) cycle and mitochondrial function, together with the activation of protein anabolism via an increased mTOR signalling pathway. 50 These results suggest that dietary supplementation with leucine may be a potential therapeutic strategy for CC treatment. However, it is important to conduct further research to evaluate the safety of leucine supplementation for tumour growth. A more comprehensive analysis of leucine supplementation for potential preclinical studies is discussed in other reviews. 51 , 52
In CC patients, skeletal muscle often exhibits an imbalance in protein catabolism and anabolism. Despite extensive research on muscle hypercatabolism, therapeutic strategies to prevent muscle atrophy in CC have had limited success. Through untargeted metabolomics analysis, Kunz et al. investigated metabolic pathways associated with muscle anabolism. 53 The CC group showed elevated levels of asymmetric dimethylarginine (ADMA) and NG‐monomethyl‐l‐arginine, which were associated with suppressed protein synthesis. These results suggest that targeting ADMA may hold promise for enhancing muscle protein anabolism in cachexia treatment.
Through a combination of RNA sequencing and untargeted metabolomics on murine myoblasts treated with lung cancer cell‐conditioned media, 54 Arneson‐Wissink and Doles unveiled significantly enriched pathways primarily linked to amino acid metabolism, with arginine metabolism identified as the top enriched pathway. Moreover, they demonstrated that tumour‐secreted factors modulated metabolism via nitric oxide synthase 2 (NOS2), negatively impacting myoblast differentiation. These results suggest that targeting NOS2, which regulates arginine catabolism, could potentially counteract muscle wasting induced by inflammatory cytokines. To validate and further explore these results, future studies incorporating animal models or human cohorts are warranted.
Lipid metabolism
Fatty acid metabolism
Fukawa et al. investigated the underlying causes of hypermetabolism in CC and revealed an abnormality in fatty acid metabolism associated with muscle wasting. 55 To gain insights into the metabolic signatures, they performed metabolomics analysis and demonstrated that inflammatory cytokines could induce excessive fatty acid oxidation. Interestingly, inhibition of fatty acid oxidation not only showed improvements in human myotubes in vitro but also effectively prevented weight loss in vivo. These results highlight the potential of targeting fatty acid oxidation as a promising therapeutic avenue for cachexia treatment.
Furthermore, Gumpper‐Fedus et al. reported a significant elevation in the levels of oleic acid and a corresponding decrease in the levels of linoleic acid among pancreatic cancer patients with cachexia when compared with those without cachexia. 56 Further research is needed to evaluate the therapeutic potential of oleic acid and linoleic acid in clinical trials.
In addition to skeletal muscle and blood, metabolomics studies investigating CC have also shed light on the involvement of other tissues and organs. 57 , 58 , 59 , 60 Through metabolomics and metagenomics analyses of liver and intestinal samples from a colorectal cancer (C26) murine model of CC, Potgens et al. observed marked changes in hepatic fatty acid metabolism, including a significant increase in lipid and triglyceride levels, indicative of hepatic steatosis. Concurrently, they noted a reduction in acetate and butyrate in the intestine. Their study highlighted that decreased hepatic levels of carnitine, vital for fatty acid catabolism, and the down‐regulation of enzymes such as acyl‐CoA dehydrogenase medium chain (Acadm) and acetyl‐CoA acyltransferase 2 (Acaa2) indicated diminished mitochondrial fatty acid β‐oxidation in the liver. The lowered levels of butyrate and acetate suggest adverse effects on the symbiotic relationship between the gut microbiome and host metabolism. These comprehensive findings suggest that such disruptions in fatty acid metabolism contribute to the compromised hepatic and intestinal homeostasis observed in CC mice. 57
Ketone body metabolism
Ketone bodies, including 3‐hydroxybutyrate, acetone and acetoacetate, are end‐products of fatty acid metabolism and are generated through ketogenesis in liver tissue. These molecules have been applied to various diseases due to their anti‐tumour and anti‐inflammatory properties. 61 , 62 , 63 Von Renesse et al. performed serum metabolomics analyses on patients with upper gastrointestinal and pancreatic cancer and revealed that CC led to significant increases in ketone bodies, ketogenic amino acid lysine and metabolites associated with lipid utilization. 64
We previously conducted muscle metabolomics analyses to investigate metabolic impairments using a murine model of glioma cachexia. 29 Our results showed a significant decrease in 3‐hydroxybutyrate, a major component of ketone bodies, in cachectic muscles. Notably, 3‐hydroxybutyrate can serve as an alternative energy source to glucose during periods of energy deficiency, such as fasting. This observation suggests that ketogenic diets might be an effective treatment for CC, as they can spare glucose for tumours while providing fuel in the form of ketone bodies to skeletal muscles.
Several studies have explored the effects of ketone bodies on attenuating muscle atrophy. 63 , 65 , 66 Shukla et al. performed metabolomics analyses and demonstrated that ketogenic diets induced metabolic reprogramming, including increased blood levels of ketone bodies and decreased uptake of glucose and glutamine in tumour cells. Their work showed that ketone bodies reduce tumour growth and subsequently inhibit muscle atrophy through metabolic alterations in pancreatic cancer cells and mice. 63
Koutnik et al. have demonstrated that CC mice treated with a ketone diester exhibit systemic metabolic changes, including elevated levels of ketone bodies and decreased levels of glucose in the blood. 66 These results suggest that ketone diester treatment can reduce muscle atrophy and exert anti‐catabolic effects by modulating the low IGF‐1/insulin signalling pathway in inflammation‐induced atrophy.
Chen et al. conducted muscle metabolomics analyses to investigate the beneficial effects of 3‐hydroxybutyrate on muscle atrophy. 65 Their results revealed reduced purine degradation and decreased levels of uric acid, as well as an increased metabolic ratio of glutamine to glutamate in cachectic muscles. Although the used murine model was not specifically related to cancer, these results provide valuable insights into the potential role of ketone bodies in mitigating muscle wasting associated with CC.
The efficacy of ketogenic diets in cancer treatment, particularly in the context of CC, remains a topic of considerable debate. While numerous preclinical studies and a few clinical trials have shown that ketogenic diets may inhibit tumour growth, increase lifespan and mitigate the effects of CC in various animal models, contradictory findings have also been reported. These include accelerated tumour growth in some models and adverse effects like nausea, hypoglycaemia and fatigue in patients using ketone supplements. The ketogenic diet, as an adjunct cancer therapy, faces challenges due to factors like diverse tumour types, patient demographics (age, sex and general health), diet composition variations and differences in study designs. Although preclinical data often indicate beneficial impacts of ketogenic diets, clinical trial outcomes are not yet conclusive. Thus, refined research designs and specific guidelines are essential to advance the understanding and application of ketogenic diets in cancer therapy. 67 , 68 Considering the availability of ketone supplements, future research should concentrate on diverse cancer types and evaluate the long‐term implications of exogenous ketones in human trials, particularly focusing on cancer progression and risk.
Bile acid metabolism
Furthermore, Thibaut et al. identified altered metabolites associated with bile acid metabolism and further investigated the role of bile acid pathways in liver inflammation and CC. 58 Similarly, Feng et al. performed metabolomic profiling to elucidate the involvement of the liver and intestinal microbes in impaired bile acid metabolism in the C26 model, revealing up‐regulated bile acid conjugation, down‐regulated bile acid synthesis and microbial bile acid metabolism in CC mice. 59 These studies highlight the importance of investigating metabolic alterations in different organs and their potential contribution to the pathogenesis of CC.
Feng et al. investigated the potential therapeutic effects of tauroursodeoxycholic acid on cachexia by targeting bile acid metabolism. Surprisingly, this agent demonstrated significant efficacy in reducing symptoms associated with CC, including muscle, heart and liver atrophy and weight loss. Based on these results, Thibaut et al. explored the potential of another agent, ursodeoxycholic acid, for CC treatment 60 ; however, their results indicated that this agent neither reduced liver inflammation nor rescued muscle atrophy in CC mice. These contrasting results highlight the complexity of bile acid metabolism in CC and the need for further research in this area.
Takeda G protein‐coupled receptor 5 (TGR5) has also gained attention as a promising therapeutic target for CC due to its involvement in bile acid activation. 60 These studies suggest that interventions targeting bile acid metabolism, such as the use of tauroursodeoxycholic acid or TGR5 agonists, may hold potential as beneficial strategies for CC treatment. Expectedly, interventions aimed at improving treatment outcomes for CC may increasingly focus on understanding and targeting the metabolism of skeletal muscle, liver and gut microbes. By unravelling the intricate interplay between these organs and their metabolic alterations in CC, novel therapeutic avenues may be identified to effectively manage this complex syndrome.
Steroid metabolism
Steroids, synthesized in small amounts from cholesterol, are complex lipophilic biomolecules with chemical properties similar to lipids. 69 These metabolites, including cortisol, aldosterone, oestradiol and testosterone, play critical roles in regulating metabolism, sexual development and reducing inflammation. 70 Steroids have been traditionally used in both preclinical models and human trials to treat cachexia.
Specifically, corticosteroids like dexamethasone can induce myotube atrophy at certain doses by increasing MuRF1 expression. 71 A recent study by Ferrer et al. demonstrated that dexamethasone treatment increased food intake, reduced hepatic fatty acid metabolism, delayed cachexia progression and prolonged lifespan in CC mice on ketogenic diets. 72 While corticosteroids are known to improve appetite and body weight and are beneficial in CC management, 73 their use is typically limited to less than a month due to potential side effects and decreased efficacy over time. 74
Glucocorticoids, another class of steroids, play vital metabolic roles. They have been found in increased concentrations in both murine models and patients with CC, 75 known to up‐regulate protein degradation and down‐regulate synthesis in skeletal muscles. 76 However, a glucocorticoid receptor antagonist did not reverse CC in a murine model, 77 suggesting the need for further studies to understand glucocorticoids' role in CC. Consequently, the activation of glucocorticoid‐responsive genes plays a significant role in skeletal muscle protein catabolism and liver metabolism. 78 This involvement is particularly critical in the context of CC, where these genes contribute to the metabolic alterations observed in affected tissues. However, the precise role of glucocorticoids in the pathophysiology of CC remains to be fully elucidated. Therefore, further research, both in preclinical models and in clinical trials, is essential to deepening our understanding of the impact of glucocorticoids on CC, ultimately leading to more effective therapeutic strategies.
Decreased levels of circulating testosterone, linked to hypogonadism in CC patients, have been observed. 79 Testosterone administration can increase muscle growth and lean body mass. 80 Testosterone analogues like oxandrolone have shown benefits in cachexia treatment but require careful monitoring due to potential side effects. 15 , 81 , 82 In summary, the use of steroid hormones in the treatment of CC shows potential as an effective therapeutic strategy. However, it is crucial to meticulously weigh the advantages and disadvantages of steroid use in clinical trials. This careful consideration is necessary to ensure that the therapeutic benefits of steroids significantly outweigh any potential risks associated with their use. Such an approach is essential for optimizing patient outcomes and minimizing adverse effects in the management of CC.
In various preclinical and clinical models of CC, notable metabolic alterations have been observed, including decreased levels of ketone bodies and circulating testosterone, diminished bile acid synthesis and increased fatty acid oxidation. To address these changes, recent research has focused on molecules like tauroursodeoxycholic acid, TGR5 agonists, ketone esters and steroid hormones that target lipid metabolism, aiming to mitigate the symptoms of CC. Among the potential treatments, ketogenic diets and steroid‐based therapies are emerging as promising approaches. The availability of ketone supplements and steroid analogues on the market underscores the need for future research. This research should concentrate on exploring the efficacy of these treatments across various cancer types, assessing the associated cancer risks and monitoring disease progression during long‐term effects in human trials.
Mitochondrial energy metabolism
In mammalian cells, acetyl‐CoA is typically generated within mitochondria through the decarboxylation of pyruvate, β‐oxidation of fatty acids and catabolism of amino acids. 83 Crucially, disturbances in glucose, amino acid and lipid metabolism might lead to compromised oxidative phosphorylation and mitochondrial dysfunction. 84 CC is a metabolic derangement associated with impaired mitochondrial function and disturbed energy metabolism, which is induced by muscle protein imbalance and an inflammatory response. 85 , 86 , 87 , 88 Pin et al. performed comprehensive metabolomics analyses to characterize the metabolic signatures between chemotherapy‐induced cachexia and CC. 89 Metabolomics analysis revealed a significant decrease in plasma glucose, citrate and succinate. Muscle metabolic profiling further demonstrated a decrease in TCA cycle flux, accompanied by down‐regulated enzymatic activities of pyruvate dehydrogenase (PDH) and succinate dehydrogenase (SDH). Additionally, the levels of succinate and the energy carriers ATP and creatine phosphate were found to be significantly reduced. Thus, cachectic muscles show down‐regulated oxidative phosphorylation, impaired TCA cycle activity and an intracellular energy deficiency, collectively resulting in mitochondrial dysfunction.
Metabolic regulators by compounds
Researchers have used several compounds, such as SS‐31, trimetazidine (TMZ), curcumin and niacin, that can all act as metabolic regulators to regulate mitochondrial function and improve impaired mitochondrial metabolism.
Metabolic alterations induced by SS‐31 treatment
Ballaro et al. undertook a study with the objective of targeting mitochondrial dysfunction as a strategic approach to combat muscle wasting in CC. 90 They utilized SS‐31, a mitochondria‐targeted peptide that binds to cardiolipin on the inner mitochondrial membrane, 91 , 92 , 93 to protect and preserve mitochondrial integrity. Using the C26 model, they examined the impact of SS‐31 on muscle loss. Additionally, they conducted metabolomic analyses of plasma, liver and skeletal muscle to elucidate the metabolic alterations with SS‐31 treatment. The results revealed that SS‐31 had a partial regulatory effect on liver and muscle metabolism. Although SS‐31 did not prevent mitochondrial protein loss or promote protein anabolism, it did improve muscle SDH activity, including increased levels of intracellular ATP, alanine, glucose, glycogen and glutamine. These results underscore the significance of maintaining mitochondrial function and achieving a balance in energy metabolism in muscle and other organs for future CC treatments. The study highlights the interconnectedness of metabolism across multiple organs and emphasizes the importance of targeting mitochondrial dysfunction as a potential therapeutic approach for CC.
Metabolic alterations induced by trimetazidine treatment
In recent research, TMZ, a metabolic modulator, has shown promise in counteracting muscle atrophy by improving mitochondrial function and correcting abnormal metabolism. 94 , 95 , 96 To investigate its potential therapeutic effects, Molinari et al. conducted a study using the C26 model. 97 Although TMZ was unable to delay the loss of body weight or the weight of specific muscles such as the tibialis anterior and gastrocnemius, it did have positive effects on metabolic modulations such as increased grip force, stimulated mitochondrial biogenesis and improved energy metabolism. Specifically, the expressions of key regulators such as peroxisome proliferator‐activated receptor gamma coactivator 1‐alpha (PGC1α), transcription factor A, protein Tom20 and ATP synthase were partially restored, and the phosphoinositide 3‐kinase (PI3K)/AKT signalling pathway was involved in the maintenance of muscle mass. Furthermore, TMZ treatment effectively increased SDH activity in vivo and enhanced mitochondrial membrane potential in vitro.
Conclusively, it is noteworthy that TMZ is a clinically approved drug and possesses exercise‐mimetic effects, which suggests that it could be beneficial for individuals with CC who are unable to engage in physical activity. The results of this study highlight the potential of TMZ as a therapeutic option for improving mitochondrial function and metabolism in the context of CC.
Metabolic alterations induced by curcumin treatment
Curcumin, a traditional Asian herb with diverse bioactivities, including anti‐tumour and anti‐inflammatory properties, 98 , 99 has attracted attention for mitigating muscle atrophy. Recently, Zhang et al. conducted a study using muscle metabolomic analyses and biochemical experiments to investigate the effects of curcumin on muscle atrophy in a murine model. 100
The results showed that curcumin treatment delayed the loss of body weight and skeletal muscle mass, restored muscle force strength and muscle fibre density and improved mitochondrial morphology and ATP generation by inhibiting the activation of the NF‐κB/UPP signalling pathway. Muscle metabolomics analysis demonstrated that curcumin modulated energy metabolism associated with glucose metabolism, pyruvate metabolism, the TCA cycle and glyoxylate and dicarboxylate metabolism, thus alleviating muscle loss.
Similarly, Penedo‐Vázquez et al. demonstrated that curcumin treatment effectively delayed the signalling pathways associated with muscle atrophy and proteolysis in CC mice. Moreover, curcumin treatment promptly improved the condition of type I and type II limb muscle fibres, surpassing the outcomes observed in untreated cachexia mice. 101 Chaiworramukkul et al. investigated the effects of curcumin in CC patients. 102 Although no statistically significant differences were observed in body composition parameters such as muscle and adipose tissue mass between the curcumin and placebo groups, patients receiving curcumin exhibited diminished handgrip muscle force loss and basal metabolic rate compared with those receiving placebo.
Overall, these studies provide encouraging evidence for the potential benefits of curcumin in mitigating muscle atrophy and improving muscle function in the context of CC. However, further investigations with larger cohorts of cancer patients and optimized clinical assessment of curcumin are necessary to fully understand its therapeutic effects and establish its clinical applications.
Metabolic alterations induced by niacin treatment
Recently, Beltra et al. performed metabolomics analyses in human muscle biopsies and serum based on the expression of the nicotinamide adenine dinucleotide (NAD+) biosynthetic enzyme nicotinamide riboside kinase 2 (NRK2). 103 Compared with healthy and high NRK2 muscles, low NRK2 muscles displayed increased amounts of glycolysis and TCA intermediates, nucleotides and amino acids, representing the outcome of disturbed energy metabolism. To counteract the CC syndromes induced by deficiency of NAD+ and down‐regulation of NRK2, the researchers explored the therapeutic effects of a NAD+ precursor niacin in a CC mouse model. Surprisingly, vitamin B3 niacin significantly restored the NAD+ abundances in muscle and liver, improved mitochondrial biogenesis and attenuated CC symptoms. Overall, their results imply NAD+ and mitochondrial metabolism as promising therapeutic targets and highlight the development of niacin‐based treatments for CC patients in the future.
Conclusions and perspectives
This review provides a comprehensive summary of metabolomics studies focusing on the discovery of potential therapeutic targets for CC and the evaluation of treatment outcomes targeting specific metabolic pathways in the past decade (Table 1 ). Notably, interventions aimed at modulating these pathways, such as glucose, amino acid, fatty acid, bile acid, ketone body, steroid and mitochondrial energy metabolism, have demonstrated promising effects in restoring muscle mass and function, attenuating body weight loss and extending lifespan in CC models. Importantly, these studies have revealed the interplay and cross‐talk among various tissues and organs involved in CC. For example, hepatic gluconeogenesis and the Cori cycle, which involve muscle glycolysis, and the interaction between bile acid metabolism and gut microbial activities play roles in the enterohepatic cycle. Moreover, ketone body metabolism is known to affect hepatic fatty acid oxidation and the TCA cycle in muscle mitochondria. These interconnected metabolic processes emphasize the complex nature of CC and the need for a systemic approach to its treatment.
Table 1.
Summary of metabolomics‐driven discoveries for cancer cachexia
| Reference | Study object | Altered metabolic pathway | Identified targets | Potential strategy |
|---|---|---|---|---|
| Shukla et al. (2014) 63 | Mice | Fatty acid metabolism | Ketone bodies | Ketogenic diet |
| Ohbuchi et al. (2015) 32 | Rats | Glucose metabolism | Glucarate | Rikkunshito |
| Tseng et al. (2015) 37 | Rats | Glucose metabolism | Glycolysis; glycogen synthesis | AR‐42 |
| Fukawa et al. (2016) 55 | Mice | Fatty acid metabolism | Fatty acid oxidation | Etomoxir |
| Viana et al. (2016) 48 | Rats | Amino acid metabolism | BCAAs | Leucine |
| Molinari et al. (2017) 97 | Mice | Mitochondrial energy metabolism | Mitochondrial succinate dehydrogenase | Trimetazidine |
| Cui et al. (2019) 30 | Mice | Glucose metabolism | Hexokinase; phosphofructokinase; phosphoglycerate mutase | |
| Koutnik et al. (2020) 66 | Mice | Fatty acid metabolism | Ketone bodies | Ketone diester |
| Cruz et al. (2020) 50 | Rats | Amino acid metabolism | BCAAs | Leucine |
| Kunz et al. (2020) 53 | Mice/C2C12 cell | Amino acid metabolism | Methylarginine | Asymmetric dimethylarginine |
| Ballaro et al. (2021) 90 | Mice | Mitochondrial energy metabolism | Mitochondrial succinate dehydrogenase | SS‐31 |
| Arneson‐Wissink and Doles (2021) 54 | C2C12 cell | Amino acid metabolism | Nitric oxide synthase 2 | 1400W |
| Potgens et al. (2021) 57 | Mice | Glucose metabolism | Glycolysis | |
| Feng et al. (2021) 59 | Mice | Fatty acid metabolism | Bile acid metabolism | Tauroursodeoxycholic acid |
| Von Renesse et al. (2023) 64 | Patients | Fatty acid metabolism | Ketone bodies; ketogenic amino acid lysine | |
| Gumpper‐Fedus et al. (2022) 56 | Patients | Fatty acid metabolism | Oleic acid; linoleic acid | |
| Zhang et al. (2022) 100 | Mice | Mitochondrial energy metabolism | Mitochondrial morphology; ATP | Curcumin |
| Wei et al. (2022) 33 | Mice | Glucose metabolism | Hexokinase | 2‐Deoxy‐d‐glucose |
| Ferrer et al. (2023) 72 | Mice | Fatty acid metabolism | Fatty acid metabolites | Dexamethasone + ketogenic diets |
| Beltra et al. (2023) 103 |
Patients Mice |
Mitochondrial energy metabolism | NAD+ | Niacin |
Abbreviations: ATP, adenosine triphosphate; BCAAs, branched‐chain amino acids; NAD+, nicotinamide adenine dinucleotide.
Significant advances have been made in preclinical research on potential therapies for CC, yet translating these therapies into clinical practice remains challenging. The primary obstacle is the inability of animal models to fully represent the complex metabolic syndrome observed in CC patients. Different animal models offer varied benefits and limitations, and the current therapeutic approaches do not address all aspects of the CC syndrome effectively.
Human CC is a multifaceted, heterogeneous condition with varying levels of inflammation, anorexia, metabolic disturbances, muscle loss and metastasis. However, current animal models fall short of fully representing these diverse clinical aspects. 104 , 105 Particularly in advanced lung cancers, anorexia emerges as a critical symptom of CC, leading to reduced food intake and exacerbating systemic metabolic issues. Unlike metabolic changes caused by starvation or malabsorption, cancer‐induced anorexia presents unique challenges. Most standard animal models for CC do not adequately mimic this aspect, 106 which hampers research into treatments aimed at improving food intake in cachexia. This gap in representation also complicates our understanding of whether the metabolic alterations observed in CC are consequences of, or inherently connected to, the anorexia driven by the cancer itself.
The complex interplay between cancer‐induced anorexia and metabolic alterations in CC can be understood as a bidirectional relationship with three key aspects:
Anorexia has effects on metabolism: Anorexia can obviously reduce food intake and significantly exacerbate the metabolic imbalances inherent in cachexia. This leads to a metabolic shift towards catabolism, where the body relies increasingly on its muscle and fat stores for energy. This catabolic state further accelerates the progression of cachexia by increasing muscle wasting and weight loss.
Metabolic changes affect appetite: The metabolic abnormalities associated with cachexia can profoundly affect the central nervous system and gastrointestinal system. These changes disrupt the normal balance of appetite‐regulating hormones and neural pathways, potentially reducing appetite and exacerbating anorexia. This demonstrates how metabolic changes in cachexia can, in turn, exacerbate anorexia.
Psychological factors play roles in cachexia: The psychological burden of a cancer diagnosis and associated stress can significantly affect both metabolism and appetite. Stress and emotional distress can suppress appetite while affecting metabolic processes, often exacerbating cachexia symptoms. This creates a cyclical feedback loop in which psychological factors exacerbate both anorexia and metabolic dysregulation in cachexia.
This bidirectional relationship underscores the complexity of CC and the need for comprehensive treatment strategies that address both the physical and psychological aspects of the disease. Effective treatment of CC requires multimodal approaches that address both the direct metabolic effects of cachexia and the contributing role of anorexia. These include nutritional support, pharmacological interventions and psychological therapies.
Given the complexity and heterogeneity of CC, current animal models fall short of mirroring the intricate metabolic syndrome seen in CC humans. Furthermore, potential therapies, including those not driven by metabolomics, often fail to alleviate most CC symptoms. For better translation into clinical applications, it is essential to choose or develop animal models that closely match the specific characteristics of CC. Utilizing multiple CC models for research and adopting multimodal approaches to therapy are recommended strategies.
CC presents as a complex syndrome affecting multiple organs, posing considerable challenges in its treatment. Despite extensive research, no drugs have been approved in Europe or the United States specifically to improve functional parameters and body composition in CC patients. Historically, treatments have included human growth hormone for AIDS‐related cachexia in the United States and megestrol acetate for cachexia and anorexia syndrome, although its use is restricted to Korea. In 2020, the American Society of Clinical Oncology (ASCO) guidelines suggested corticosteroids and progesterone analogues to enhance appetite and body weight in CC patients. 74 However, the use of corticosteroids may result in adverse side effects and is recommended only in low doses and for short periods. Progestins have not been shown to significantly increase lean body mass. 107
In a significant development, Japan approved anamorelin in 2020 to treat CC, marking the first such approval for this condition. Anamorelin, a ghrelin mimetic agonist, has demonstrated efficacy in improving anorexia and increasing muscle mass and body weight in CC patients. However, it does not appear to improve muscle strength or life expectancy and is associated with side effects like nausea and constipation. 108
Metabolomics has emerged as a powerful tool in CC treatment, allowing for the precise identification of metabolic alterations and the development of personalized treatments. By targeting specific metabolic pathways, it is possible to restore metabolic homeostasis and alleviate CC symptoms. This approach includes the use of metabolites like BCAAs, ketone bodies and ketone diesters as nutritional supplements. Additionally, agents such as traditional herbal medicines (e.g., RKT and curcumin), clinically approved drugs (e.g., TMZ, tauroursodeoxycholic acid and ursodeoxycholic acid), peptides (e.g., SS‐31) and common chemical compounds (e.g., niacin) are being explored (Figure 2 ). These diverse interventions offer promising avenues for developing effective therapeutic strategies against CC.
Figure 2.
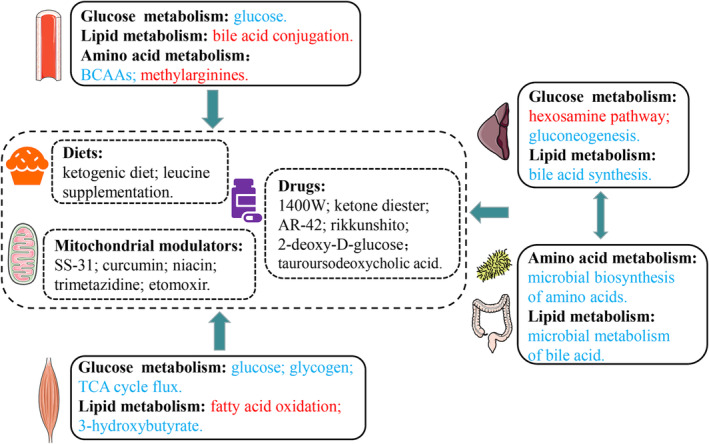
Overview of potential therapeutic targets discovered through metabolomics studies and their corresponding evaluation in cancer cachexia (CC). Up‐regulated metabolites/pathways in the CC group compared with the normal control group are represented in red, while down‐regulated metabolites/pathways are shown in blue. These findings offer insights into the molecular alterations associated with CC and highlight potential targets for therapeutic interventions. BCAAs, branched‐chain amino acids; TCA, tricarboxylic acid.
For future clinical treatments, a multidisciplinary approach that combines pharmacological therapy, exercise and nutritional intervention is strongly recommended. This holistic approach aligns with international guidelines for managing CC patients. Metabolomics‐driven potential targets, such as BCAAs, ketogenic diets and clinically approved drugs like TMZ, have shown beneficial effects on skeletal muscle metabolism. These may act as adjuncts to nutritional interventions and exercise, complementing existing therapies like anamorelin in human trials.
Conflict of interest statement
The authors declare no conflict of interest.
Funding
This work was supported by the Scientific Research Foundation of Xuchang University (No. 962005), the Education Department of Henan Province (No. 24A550016) and the National Natural Science Foundation of China (Nos 31971357 and 32171174).
Supporting information
Data S1. Supporting Information.
Acknowledgements
The authors comply with the ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle. 109
Cui P, Li X, Huang C, Lin D. Metabolomics‐driven discovery of therapeutic targets for cancer cachexia. Journal of Cachexia, Sarcopenia and Muscle 2024; 10.1002/jcsm.13465.
References
- 1. Schmidt SF, Rohm M, Herzig S, Berriel DM. Cancer cachexia: more than skeletal muscle wasting. Trends Cancer 2018;4:849–860. [DOI] [PubMed] [Google Scholar]
- 2. Vudatha V, Devarakonda T, Liu C, Freudenberger DC, Riner AN, Herremans KM, et al. Review of mechanisms and treatment of cancer‐induced cardiac cachexia. Cells 2022;11:1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Siff T, Parajuli P, Razzaque MS, Atfi A. Cancer‐mediated muscle cachexia: etiology and clinical management. Trends Endocrinol Metab 2021;32:382–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 2011;12:489–495. [DOI] [PubMed] [Google Scholar]
- 5. Bozzetti F. Forcing the vicious circle: sarcopenia increases toxicity, decreases response to chemotherapy and worsens with chemotherapy. Ann Oncol 2017;28:2107–2118. [DOI] [PubMed] [Google Scholar]
- 6. Baracos VE, Martin L, Korc M, Guttridge DC, Fearon KCH. Cancer‐associated cachexia. Nat Rev Dis Primers 2018;4:17105. [DOI] [PubMed] [Google Scholar]
- 7. Fearon KC, Glass DJ, Guttridge DC. Cancer cachexia: mediators, signaling, and metabolic pathways. Cell Metab 2012;16:153–166. [DOI] [PubMed] [Google Scholar]
- 8. Argiles JM, Busquets S, Stemmler B, Lopez‐Soriano FJ. Cancer cachexia: understanding the molecular basis. Nat Rev Cancer 2014;14:754–762. [DOI] [PubMed] [Google Scholar]
- 9. Porporato PE. Understanding cachexia as a cancer metabolism syndrome. Oncogene 2016;5:e200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rohm M, Zeigerer A, Machado J, Herzig S. Energy metabolism in cachexia. EMBO Rep 2019;20:47258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhou X, Wang JL, Lu J, Song Y, Kwak KS, Jiao Q, et al. Reversal of cancer cachexia and muscle wasting by ActRIIB antagonism leads to prolonged survival. Cell 2010;142:531–543. [DOI] [PubMed] [Google Scholar]
- 12. Johnston AJ, Murphy KT, Jenkinson L, Laine D, Emmrich K, Faou P, et al. Targeting of Fn14 prevents cancer‐induced cachexia and prolongs survival. Cell 2015;162:1365–1378. [DOI] [PubMed] [Google Scholar]
- 13. Chiappalupi S, Sorci G, Vukasinovic A, Salvadori L, Sagheddu R, Coletti D, et al. Targeting RAGE prevents muscle wasting and prolongs survival in cancer cachexia. J Cachexia Sarcopenia Muscle 2020;11:929–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Geremia A, Sartori R, Baraldo M, Nogara L, Balmaceda V, Dumitras GA, et al. Activation of Akt‐mTORC1 signalling reverts cancer‐dependent muscle wasting. J Cachexia Sarcopenia Muscle 2022;13:648–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lambert CP. Anti‐cachexia therapy should target metabolism, inflammatory cytokines, and androgens in hormone‐independent cancers. J Cachexia Sarcopenia Muscle 2021;12:1352–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wishart DS. Emerging applications of metabolomics in drug discovery and precision medicine. Nat Rev Drug Discov 2016;15:473–484. [DOI] [PubMed] [Google Scholar]
- 17. Newgard CB. Metabolomics and metabolic diseases: where do we stand? Cell Metab 2017;25:43–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Larkin JR, Anthony S, Johanssen VA, Yeo T, Sealey M, Yates AG, et al. Metabolomic biomarkers in blood samples identify cancers in a mixed population of patients with nonspecific symptoms. Clin Cancer Res 2022;28:1651–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schmidt DR, Patel R, Kirsch DG, Lewis CA, Vander Heiden MG, Locasale JW. Metabolomics in cancer research and emerging applications in clinical oncology. CA Cancer J Clin 2021;71:333–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Danlos FX, Grajeda‐Iglesias C, Durand S, Sauvat A, Roumier M, Cantin D, et al. Metabolomic analyses of COVID‐19 patients unravel stage‐dependent and prognostic biomarkers. Cell Death Dis 2021;12:258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shen B, Yi X, Sun Y, Bi X, Du J, Zhang C, et al. Proteomic and metabolomic characterization of COVID‐19 patient sera. Cell 2020;182:59–72.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Opazo R, Angel B, Marquez C, Lera L, Cardoso Dos Santos GR, Monnerat G, et al. Sarcopenic metabolomic profile reflected a sarcopenic phenotype associated with amino acid and essential fatty acid changes. Metabolomics 2021;17:83. [DOI] [PubMed] [Google Scholar]
- 23. Tsai JS, Wang SY, Chang CH, Chen CY, Wen CJ, Chen GY, et al. Identification of traumatic acid as a potential plasma biomarker for sarcopenia using a metabolomics‐based approach. J Cachexia Sarcopenia Muscle 2022;13:276–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Talmor‐Barkan Y, Bar N, Shaul AA, Shahaf N, Godneva A, Bussi Y, et al. Metabolomic and microbiome profiling reveals personalized risk factors for coronary artery disease. Nat Med 2022;28:295–302. [DOI] [PubMed] [Google Scholar]
- 25. Yang H, Yang F, Luo M, Chen Q, Liu X, Zhang Y, et al. Metabolomic profile reveals that ceramide metabolic disturbance plays an important role in thoracic aortic dissection. Front Cardiovasc Med 2022;9:826861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jin Q, Ma RCW. Metabolomics in diabetes and diabetic complications: insights from epidemiological studies. Cells 2021;10:2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sun Y, Zou H, Li X, Xu S, Liu C. Plasma metabolomics reveals metabolic profiling for diabetic retinopathy and disease progression. Front Endocrinol (Lausanne) 2021;12:757088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cui P, Li X, Huang C, Li Q, Lin D. Metabolomics and its applications in cancer cachexia. Front Mol Biosci 2022;9:789889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cui P, Shao W, Huang C, Wu CJ, Jiang B, Lin D. Metabolic derangements of skeletal muscle from a murine model of glioma cachexia. Skelet Muscle 2019;9:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cui P, Huang C, Guo J, Wang Q, Liu Z, Zhuo H, et al. Metabolic profiling of tumors, sera, and skeletal muscles from an orthotopic murine model of gastric cancer associated‐cachexia. J Proteome Res 2019;18:1880–1892. [DOI] [PubMed] [Google Scholar]
- 31. QuanJun Y, GenJin Y, LiLi W, Yan H, YongLong H, Jin L, et al. Integrated analysis of serum and intact muscle metabonomics identify metabolic profiles of cancer cachexia in a dynamic mouse model. RSC Adv 2015;5:92438–92448. [Google Scholar]
- 32. Ohbuchi K, Nishiumi S, Fujitsuka N, Hattori T, Yamamoto M, Inui A, et al. Rikkunshito ameliorates cancer cachexia partly through elevation of glucarate in plasma. Evid Based Complement Alternat Med 2015;2015:871832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wei L, Wang R, Wazir J, Lin K, Song S, Li L, et al. 2‐Deoxy‐d‐glucose alleviates cancer cachexia‐induced muscle wasting by enhancing ketone metabolism and inhibiting the Cori cycle. Cells 2022;11:2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ralser M, Wamelink MM, Struys EA, Joppich C, Krobitsch S, Jakobs C, et al. A catabolic block does not sufficiently explain how 2‐deoxy‐d‐glucose inhibits cell growth. Proc Natl Acad Sci U S A 2008;105:17807–17811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhu Z, Jiang W, McGinley JN, Thompson HJ. 2‐Deoxyglucose as an energy restriction mimetic agent: effects on mammary carcinogenesis and on mammary tumor cell growth in vitro. Cancer Res 2005;65:7023–7030. [DOI] [PubMed] [Google Scholar]
- 36. Laussel C, Leon S. Cellular toxicity of the metabolic inhibitor 2‐deoxyglucose and associated resistance mechanisms. Biochem Pharmacol 2020;182:114213. [DOI] [PubMed] [Google Scholar]
- 37. Tseng YC, Kulp SK, Lai IL, Hsu EC, He WA, Frankhouser DE, et al. Preclinical investigation of the novel histone deacetylase inhibitor AR‐42 in the treatment of cancer‐induced cachexia. J Natl Cancer Inst 2015;107:djv274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Viana LR, Lopes‐Aguiar L, Rossi Rosolen R, Willians Dos Santos R, Cintra Gomes‐Marcondes MC. 1H‐NMR based serum metabolomics identifies different profile between sarcopenia and cancer cachexia in ageing Walker 256 tumour‐bearing rats. Metabolites 2020;10:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lautaoja JH, Lalowski M, Nissinen TA, Hentila J, Shi Y, Ritvos O, et al. Muscle and serum metabolomes are dysregulated in colon‐26 tumor‐bearing mice despite amelioration of cachexia with activin receptor type 2B ligand blockade. Am J Physiol Endocrinol Metab 2019;316:E852–E865. [DOI] [PubMed] [Google Scholar]
- 40. Quanjun Y, Genjin Y, Lili W, Bin L, Jin L, Qi Y, et al. Serum metabolic profiles reveal the effect of formoterol on cachexia in tumor‐bearing mice. Mol Biosyst 2013;9:3015–3025. [DOI] [PubMed] [Google Scholar]
- 41. Newgard CB, An J, Bain JR, Muehlbauer MJ, Stevens RD, Lien LF, et al. A branched‐chain amino acid‐related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab 2009;9:311–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gojda J, Cahova M. Gut microbiota as the link between elevated BCAA serum levels and insulin resistance. Biomolecules 2021;11:1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Der‐Torossian H, Asher SA, Winnike JH, Wysong A, Yin X, Willis MS, et al. Cancer cachexia's metabolic signature in a murine model confirms a distinct entity. Metabolomics 2012;9:730–739. [Google Scholar]
- 44. Valdes AM, Walter J, Segal E, Spector TD. Role of the gut microbiota in nutrition and health. BMJ 2018;361:k2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ni Y, Lohinai Z, Heshiki Y, Dome B, Moldvay J, Dulka E, et al. Distinct composition and metabolic functions of human gut microbiota are associated with cachexia in lung cancer patients. ISME J 2021;15:3207–3220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Columbus DA, Fiorotto ML, Davis TA. Leucine is a major regulator of muscle protein synthesis in neonates. Amino Acids 2015;47:259–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Moro T, Ebert SM, Adams CM, Rasmussen BB. Amino acid sensing in skeletal muscle. Trends Endocrinol Metab 2016;27:796–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Viana LR, Canevarolo R, Luiz AC, Soares RF, Lubaczeuski C, Zeri AC, et al. Leucine‐rich diet alters the 1H‐NMR based metabolomic profile without changing the Walker‐256 tumour mass in rats. BMC Cancer 2016;16:764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Miyaguti N, Oliveira SCP, Gomes‐Marcondes MCC. Maternal leucine‐rich diet minimises muscle mass loss in tumour‐bearing adult rat offspring by improving the balance of muscle protein synthesis and degradation. Biomolecules 2019;9:229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cruz B, Oliveira A, Viana LR, Lopes‐Aguiar L, Canevarolo R, Colombera MC, et al. Leucine‐rich diet modulates the metabolomic and proteomic profile of skeletal muscle during cancer cachexia. Cancers (Basel) 2020;12:1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Beaudry AG, Law ML. Leucine supplementation in cancer cachexia: mechanisms and a review of the pre‐clinical literature. Nutrients 2022;14:2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Prado CM, Orsso CE, Pereira SL, Atherton PJ, Deutz NEP. Effects of β‐hydroxy β‐methylbutyrate (HMB) supplementation on muscle mass, function, and other outcomes in patients with cancer: a systematic review. J Cachexia Sarcopenia Muscle 2022;13:1623–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kunz HE, Dorschner JM, Berent TE, Meyer T, Wang X, Jatoi A, et al. Methylarginine metabolites are associated with attenuated muscle protein synthesis in cancer‐associated muscle wasting. J Biol Chem 2020;295:17441–17459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Arneson‐Wissink PC, Doles JD. Disrupted NOS2 metabolism drives myoblast response to wasting‐associated cytokines. Exp Cell Res 2021;407:112779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Fukawa T, Yan‐Jiang BC, Min‐Wen JC, Jun‐Hao ET, Huang D, Qian CN, et al. Excessive fatty acid oxidation induces muscle atrophy in cancer cachexia. Nat Med 2016;22:666–671. [DOI] [PubMed] [Google Scholar]
- 56. Gumpper‐Fedus K, Hart PA, Belury MA, Crowe O, Cole RM, Pita Grisanti V, et al. Altered plasma fatty acid abundance is associated with cachexia in treatment‐naive pancreatic cancer. Cells 2022;11:910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Potgens SA, Thibaut MM, Joudiou N, Sboarina M, Neyrinck AM, Cani PD, et al. Multi‐compartment metabolomics and metagenomics reveal major hepatic and intestinal disturbances in cancer cachectic mice. J Cachexia Sarcopenia Muscle 2021;12:456–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Thibaut MM, Sboarina M, Roumain M, Potgens SA, Neyrinck AM, Destree F, et al. Inflammation‐induced cholestasis in cancer cachexia. J Cachexia Sarcopenia Muscle 2021;12:70–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Feng L, Zhang W, Shen Q, Miao C, Chen L, Li Y, et al. Bile acid metabolism dysregulation associates with cancer cachexia: roles of liver and gut microbiome. J Cachexia Sarcopenia Muscle 2021;12:1553–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Thibaut MM, Gillard J, Dolly A, Roumain M, Leclercq IA, Delzenne NM, et al. Bile acid dysregulation is intrinsically related to cachexia in tumor‐bearing mice. Cancers (Basel) 2021;13:6389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Han YM, Bedarida T, Ding Y, Somba BK, Lu Q, Wang Q, et al. β‐Hydroxybutyrate prevents vascular senescence through hnRNP A1‐mediated upregulation of Oct4. Mol Cell 2018;71:1064–1078.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Youm YH, Nguyen KY, Grant RW, Goldberg EL, Bodogai M, Kim D, et al. The ketone metabolite β‐hydroxybutyrate blocks NLRP3 inflammasome‐mediated inflammatory disease. Nat Med 2015;21:263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Shukla SK, Gebregiworgis T, Purohit V, Chaika NV, Gunda V, Radhakrishnan P, et al. Metabolic reprogramming induced by ketone bodies diminishes pancreatic cancer cachexia. Cancer Metab 2014;2:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. von Renesse J, von Bechtolsheim F, Jonas S, Seifert L, Alves TC, Seifert AM, et al. Tumour catabolism independent of malnutrition and inflammation in upper GI cancer patients revealed by longitudinal metabolomics. J Cachexia Sarcopenia Muscle 2023;14:298–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Chen J, Li Z, Zhang Y, Zhang X, Zhang S, Liu Z, et al. Mechanism of reduced muscle atrophy via ketone body (D)‐3‐hydroxybutyrate. Cell Biosci 2022;12:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Koutnik AP, Poff AM, Ward NP, DeBlasi JM, Soliven MA, Romero MA, et al. Ketone bodies attenuate wasting in models of atrophy. J Cachexia Sarcopenia Muscle 2020;11:973–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Lane J, Brown NI, Williams S, Plaisance EP, Fontaine KR. Ketogenic diet for cancer: critical assessment and research recommendations. Nutrients 2021;13:3562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Cortez NE, Mackenzie GG. Ketogenic diets in pancreatic cancer and associated cachexia: cellular mechanisms and clinical perspectives. Nutrients 2021;13:3202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Chai Y, Grebe SKG, Maus A. Improving LC‐MS/MS measurements of steroids with differential mobility spectrometry. J Mass Spectrom Adv Clin Lab 2023;30:30–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Cole TJ, Short KL, Hooper SB. The science of steroids. Semin Fetal Neonatal Med 2019;24:170–175. [DOI] [PubMed] [Google Scholar]
- 71. Bowen TS, Adams V, Werner S, Fischer T, Vinke P, Brogger MN, et al. Small‐molecule inhibition of MuRF1 attenuates skeletal muscle atrophy and dysfunction in cardiac cachexia. J Cachexia Sarcopenia Muscle 2017;8:939–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Ferrer M, Mourikis N, Davidson EE, Kleeman SO, Zaccaria M, Habel J, et al. Ketogenic diet promotes tumor ferroptosis but induces relative corticosterone deficiency that accelerates cachexia. Cell Metab 2023;35:1147–1162.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Watanabe H, Oshima T. The latest treatments for cancer cachexia: an overview. Anticancer Res 2023;43:511–521. [DOI] [PubMed] [Google Scholar]
- 74. Roeland EJ, Bohlke K, Baracos VE, Bruera E, Del Fabbro E, Dixon S, et al. Management of cancer cachexia: ASCO guideline. J Clin Oncol 2020;38:2438–2453. [DOI] [PubMed] [Google Scholar]
- 75. Cao Z, Scott AM, Hoogenraad NJ, Osellame LD. Mediators and clinical treatment for cancer cachexia: a systematic review. JCSM Rapid Commun 2021;4:166–186. [Google Scholar]
- 76. Sato AY, Richardson D, Cregor M, Davis HM, Au ED, McAndrews K, et al. Glucocorticoids induce bone and muscle atrophy by tissue‐specific mechanisms upstream of E3 ubiquitin ligases. Endocrinology 2017;158:664–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Rivadeneira DE, Naama HA, McCarter MD, Fujita J, Evoy D, Mackrell P, et al. Glucocorticoid blockade does not abrogate tumor‐induced cachexia. Nutr Cancer 1999;35:202–206. [DOI] [PubMed] [Google Scholar]
- 78. Martin A, Castells J, Allibert V, Emerit A, Zolotoff C, Cardot‐Ruffino V, et al. Hypothalamic‐pituitary‐adrenal axis activation and glucocorticoid‐responsive gene expression in skeletal muscle and liver of Apc mice. J Cachexia Sarcopenia Muscle 2022;13:1686–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Burney BO, Hayes TG, Smiechowska J, Cardwell G, Papusha V, Bhargava P, et al. Low testosterone levels and increased inflammatory markers in patients with cancer and relationship with cachexia. J Clin Endocrinol Metab 2012;97:E700–E709. [DOI] [PubMed] [Google Scholar]
- 80. Bhasin S, Woodhouse L, Casaburi R, Singh AB, Bhasin D, Berman N, et al. Testosterone dose‐response relationships in healthy young men. Am J Physiol Endocrinol Metab 2001;281:E1172–E1181. [DOI] [PubMed] [Google Scholar]
- 81. Celichowska M, Miedziaszczyk M, Lacka K. Pharmacotherapy in cachexia: a review of endocrine abnormalities and steroid pharmacotherapy. J Pain Palliat Care Pharmacother 2022;36:117–131. [DOI] [PubMed] [Google Scholar]
- 82. Osmolak AM, Klatt‐Cromwell CN, Price AM, Sanclement JA, Krempl GA. Does perioperative oxandrolone improve nutritional status in patients with cachexia related to head and neck carcinoma? Laryngoscope Investig Otolaryngol 2019;4:314–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Sun L, Zhang H, Gao P. Metabolic reprogramming and epigenetic modifications on the path to cancer. Protein Cell 2022;13:877–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Mi Y, Qi G, Vitali F, Shang Y, Raikes AC, Wang T, et al. Loss of fatty acid degradation by astrocytic mitochondria triggers neuroinflammation and neurodegeneration. Nat Metab 2023;5:445–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Barreto R, Mandili G, Witzmann FA, Novelli F, Zimmers TA, Bonetto A. Cancer and chemotherapy contribute to muscle loss by activating common signaling pathways. Front Physiol 2016;7:472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Argiles JM, Lopez‐Soriano FJ, Busquets S. Muscle wasting in cancer: the role of mitochondria. Curr Opin Clin Nutr Metab Care 2015;18:221–225. [DOI] [PubMed] [Google Scholar]
- 87. de Vos‐Geelen J, Fearon KC, Schols AM. The energy balance in cancer cachexia revisited. Curr Opin Clin Nutr Metab Care 2014;17:509–514. [DOI] [PubMed] [Google Scholar]
- 88. Chiocchetti GME, Lopes‐Aguiar L, Miyaguti N, Viana LR, Salgado CM, Orvoen OO, et al. A time‐course comparison of skeletal muscle metabolomic alterations in Walker‐256 tumour‐bearing rats at different stages of life. Metabolites 2021;11:404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Pin F, Barreto R, Couch ME, Bonetto A, O'Connell TM. Cachexia induced by cancer and chemotherapy yield distinct perturbations to energy metabolism. J Cachexia Sarcopenia Muscle 2019;10:140–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Ballaro R, Lopalco P, Audrito V, Beltra M, Pin F, Angelini R, et al. Targeting mitochondria by SS‐31 ameliorates the whole body energy status in cancer‐ and chemotherapy‐induced cachexia. Cancers (Basel) 2021;13:850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Chavez JD, Tang X, Campbell MD, Reyes G, Kramer PA, Stuppard R, et al. Mitochondrial protein interaction landscape of SS‐31. Proc Natl Acad Sci U S A 2020;117:15363–15373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Chiao YA, Zhang H, Sweetwyne M, Whitson J, Ting YS, Basisty N, et al. Late‐life restoration of mitochondrial function reverses cardiac dysfunction in old mice. Elife 2020;9:e55513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Zhao W, Xu Z, Cao J, Fu Q, Wu Y, Zhang X, et al. Elamipretide (SS‐31) improves mitochondrial dysfunction, synaptic and memory impairment induced by lipopolysaccharide in mice. J Neuroinflammation 2019;16:230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Zhang W, You B, Qi D, Qiu L, Ripley‐Gonzalez JW, Zheng F, et al. Trimetazidine and exercise provide comparable improvements to high fat diet‐induced muscle dysfunction through enhancement of mitochondrial quality control. Sci Rep 2021;11:19116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Wang L, Jiao XF, Wu C, Li XQ, Sun HX, Shen XY, et al. Trimetazidine attenuates dexamethasone‐induced muscle atrophy via inhibiting NLRP3/GSDMD pathway‐mediated pyroptosis. Cell Death Dis 2021;7:251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Song M, Chen FF, Li YH, Zhang L, Wang F, Qin RR, et al. Trimetazidine restores the positive adaptation to exercise training by mitigating statin‐induced skeletal muscle injury. J Cachexia Sarcopenia Muscle 2018;9:106–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Molinari F, Pin F, Gorini S, Chiandotto S, Pontecorvo L, Penna F, et al. The mitochondrial metabolic reprogramming agent trimetazidine as an ‘exercise mimetic’ in cachectic C26‐bearing mice. J Cachexia Sarcopenia Muscle 2017;8:954–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Epstein J, Sanderson IR, Macdonald TT. Curcumin as a therapeutic agent: the evidence from in vitro, animal and human studies. Br J Nutr 2010;103:1545–1557. [DOI] [PubMed] [Google Scholar]
- 99. Corson TW, Crews CM. Molecular understanding and modern application of traditional medicines: triumphs and trials. Cell 2007;130:769–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Zhang J, Zheng J, Chen H, Li X, Ye C, Zhang F, et al. Curcumin targeting NF‐κB/ubiquitin‐proteasome‐system axis ameliorates muscle atrophy in triple‐negative breast cancer cachexia mice. Mediators Inflamm 2022;2022:2567150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Penedo‐Vázquez A, Duran X, Mateu J, López‐Postigo A, Barreiro E. Curcumin and resveratrol improve muscle function and structure through attenuation of proteolytic markers in experimental cancer‐induced cachexia. Molecules 2021;26:4904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Chaiworramukkul A, Seetalarom K, Saichamchan S, Prasongsook N. A double‐blind, placebo‐controlled randomized phase IIa study: evaluating the effect of curcumin for treatment of cancer anorexia‐cachexia syndrome in solid cancer patients. Asian Pac J Cancer Prev 2022;23:2333–2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Beltra M, Pollanen N, Fornelli C, Tonttila K, Hsu MY, Zampieri S, et al. NAD+ repletion with niacin counteracts cancer cachexia. Nat Commun 2023;14:1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Li L, Wazir J, Huang Z, Wang Y, Wang H. A comprehensive review of animal models for cancer cachexia: implications for translational research. Genes Dis 2023;101080. [Google Scholar]
- 105. Suzuki T, von Haehling S, Springer J. Promising models for cancer‐induced cachexia drug discovery. Expert Opin Drug Discovery 2020;15:627–637. [DOI] [PubMed] [Google Scholar]
- 106. Queiroz AL, Dantas E, Ramsamooj S, Murthy A, Ahmed M, Zunica ERM, et al. Blocking ActRIIB and restoring appetite reverses cachexia and improves survival in mice with lung cancer. Nat Commun 2022;13:4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Fonseca G, von Haehling S. An overview of anamorelin as a treatment option for cancer‐associated anorexia and cachexia. Expert Opin Pharmacother 2021;22:889–895. [DOI] [PubMed] [Google Scholar]
- 108. Wakabayashi H, Arai H, Inui A. The regulatory approval of anamorelin for treatment of cachexia in patients with non‐small cell lung cancer, gastric cancer, pancreatic cancer, and colorectal cancer in Japan: facts and numbers. J Cachexia Sarcopenia Muscle 2021;12:14–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2021. J Cachexia Sarcopenia Muscle 2021;12:2259–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supporting Information.


