Abstract
The use of patient‐reported outcomes (PROMs) of quality of life (QOL) is common in cachexia trials. Patients' self‐report on health, functioning, wellbeing, and perceptions of care, represent important measures of efficacy. This review describes the frequency, variety, and reporting of QOL endpoints used in cancer cachexia clinical trials. Electronic literature searches were performed in Medline, Embase, and Cochrane (1990–2023). Seven thousand four hundred thirty‐five papers were retained for evaluation. Eligibility criteria included QOL as a study endpoint using validated measures, controlled design, adults (>18 years), ≥40 participants randomized, and intervention exceeding 2 weeks. The Covidence software was used for review procedures and data extractions. Four independent authors screened all records for consensus. Papers were screened by titles and abstracts, prior to full‐text reading. PRISMA guidance for systematic reviews was followed. The protocol was prospectively registered via PROSPERO (CRD42022276710). Fifty papers focused on QOL. Twenty‐four (48%) were double‐blind randomized controlled trials. Sample sizes varied considerably (n = 42 to 469). Thirty‐nine trials (78%) included multiple cancer types. Twenty‐seven trials (54%) featured multimodal interventions with various drugs and dietary supplements, 11 (22%) used nutritional interventions alone and 12 (24%) used a single pharmacological intervention only. The median duration of the interventions was 12 weeks (4–96). The most frequent QOL measure was the EORTC QLQ‐C30 (60%), followed by different FACIT questionnaires (34%). QOL was a primary, secondary, or exploratory endpoint in 15, 31 and 4 trials respectively, being the single primary in six. Statistically significant results on one or more QOL items favouring the intervention group were found in 18 trials. Eleven of these used a complete multidimensional measure. Adjustments for multiple testing when using multicomponent QOL measures were not reported. Nine trials (18%) defined a statistically or clinically significant difference for QOL, five with QOL as a primary outcome, and four with QOL as a secondary outcome. Correlation statistics with other study outcomes were rarely performed. PROMs including QOL are important endpoints in cachexia trials. We recommend using well‐validated QOL measures, including cachexia‐specific items such as weight history, appetite loss, and nutritional intake. Appropriate statistical methods with definitions of clinical significance, adjustment for multiple testing and few co‐primary endpoints are encouraged, as is an understanding of how interventions may relate to changes in QOL endpoints. A strategic and scientific‐based approach to PROM research in cachexia trials is warranted, to improve the research base in this field and avoid the use of QOL as supplementary measures.
Keywords: Cachexia, Cancer, Patient‐reported outcomes, Quality of life
Introduction
Cancer cachexia is a multifactorial syndrome defined by an ongoing loss of skeletal muscle mass (with or without loss of fat mass), that cannot be fully reversed by conventional nutritional support and leads to progressive functional impairment. 1 Cachexia in patients with cancer is very common, 2 with a complex pathophysiology and multifaceted impact on patients. To date, there are no universally accepted endpoints for interventional cancer cachexia trials, and endpoints used remain highly variable. Yet if cancer cachexia is optimally treated, then this may have a direct or indirect effect on patients' quality of life (QOL) as studies have shown that improved nutritional status and/or an attenuation of inflammation correspond to improved QOL and well‐being, and better mental status. 3 , 4
The terms QOL and health related quality of life (HRQOL) are often used interchangeably 5 as both denote the overall well‐being and health aspects in life. These cover broad topics, such as health status, physical functioning, symptoms, psychosocial adjustment, wellbeing, life satisfaction, and happiness, 6 although some claim that HRQOL measures may more appropriately capture changes pertaining to health problems Broadly, both are multidimensional concepts representing an individual's perception of physical, psychological, and social aspects, and overall health (henceforth referred to as ‘QOL’). These QOL measures fall under the umbrella of patient‐reported outcome measures (PROMs) and regulatory agencies (US Food and Drug Administration (FDA), 7 European Medicines Agency (EMA) 8 ) recognizing PROMs as approvable endpoints in evaluating treatment efficacy 9 in other conditions. To date, guidance on specific QOL measures as approvable endpoints in cachexia from regulatory agencies is not clear.
PROMs supplement clinician observations and objective findings with information based on patients' own lived experience. As such, the routine integration of PROMs within clinical research aligns with patient‐centred care, defined as ‘care that is respectful of, and responsive to, individual patient preferences, needs and values, and ensuring that patient values guide all clinical decisions’. 10 PROMs have been utilized throughout cancer clinical trials, as endpoints, interventions, and prognostic markers. For example, PROMs defined the impact of integrating palliative care early in patients with advanced cancer demonstrating improved QOL, psychological distress, symptom burden, 11 and a survival benefit. 12 Additionally, empirical evidence indicates that PROMs provide independent prognostic information on survival in several cancer populations. 13 Thus, using PROMs within clinical trials in patients with cancer is highly clinically relevant, is well accepted 9 and particularly relevant to patients experiencing the multifaceted impacts of cancer cachexia.
Several types of PROMs exist, for example, the Edmonton Symptom Assessment System (ESAS), 14 the M.D. Anderson Symptom Inventory (MDASI), 15 the Spitzer Uniscale 16 and the early Priestman and Baum LASA scales 17 that all include assessments of wellbeing. Most QOL measures are multidimensional questionnaires, comprising several items that form specific scales, for example, physical, and emotional functioning, supplemented with single items. The questionnaires may measure generic QOL such as the Short Form‐36, 18 and the EuroQol‐5D (EQ 5D) 19 or may be disease‐ or condition‐specific, with the most frequent cancer‐specific PROMs being the Functional Assessment of Cancer Therapy scale (FACT‐G), 20 the European Organization for Research and Treatment Quality of Life Questionnaire (EORTC QLQ‐C30), 21 and the palliative care EORTC QLQ C15‐PAL, 22 the early Rotterdam Symptom Checklist 23 and the Japanese QOL‐ACD. 24
In terms of what has been used to measure QOL in cachexia trials there are various assessments. The EORTC QLQ‐C30 is often supplemented with condition specific measures such as the one for Head‐and‐Neck Cancer 25 corresponding to the FACIT condition specific measures 26 used together with FACT‐G 20 such as the FACT Fatigue and Anemia scales 27 and the FACT Head and Neck Symptom inventory (FHNSI‐2). 28 The content covered in these validated measures is relevant to patients with cachexia and they are commonly used together with more cachexia specific measures such as the first and subsequently revised Functional Assessment of Anorexia/Cachexia Therapy (FAACT) questionnaire 29 and the EORTC QOL cancer cachexia questionnaire (EORTC QLQ‐CAX24). 30 Despite these cachexia specific QOL assessments being available, there is no consensus about the most appropriate QOL endpoint in cachexia trials with inconsistency of assessments being used, analysis measures differing and subsequently varying reporting approaches. There is also no robust evidence to support which might be easiest to use in a trial and/or preferred by trial participants. These limitations are further compounded by the lack of a widely accepted ‘minimally clinically important difference (MCID)’, and this then impedes trial design and ultimately drug development.
This systematic review is part of a series of reviews assessing endpoints in cachexia clinical trials and aims specifically to examine QOL. The main objective was to describe the frequency and variety of QOL endpoints. This review includes descriptions of trial characteristics, interventions, QOL measures, reporting of QOL, and the relationship with significant primary and/or secondary outcomes.
Methods
Protocol and registration
This systematic review follows the Preferred Reporting Items for Systematic Reviews and Meta‐Analyzes Statement PRISMA (Supporting file S1). 31
Search strategy
The search for trials published from January 1990 until 2 June 2021, was conducted by a research librarian (University of Oslo, NO) for this review series in the following databases MEDLINE (Ovid), EMBASE (Ovid) and the Cochrane Central Register of Controlled Trials. The search was registered on the International Prospective Register of Systematic Reviews (PROSPERO ‐ CRD42022276710) where further details are available. 32 The full electronic search strategy including limits used for the OVID Medline database can be found as Supporting file S2.
The systematic review is part of a comprehensive collaboration including six reviews examining different endpoints in cachexia (body composition, oncology, physical function, PROMs, systemic inflammation, and nutritional). One search was performed for all reviews followed by central appraisal, data extraction and quality assessment. Thereon, eligible trials were reviewed and those specifically examining quality of life were included in the present review. For the present review, the search was updated from 2 June 2021 to 17 October 2023.
Eligibility criteria
Articles were considered eligible if they were controlled trials investigating interventions which aimed to treat or attenuate cachexia (defined as detailed in PROSPERO) in adults with cancer. There were no restrictions on the type of intervention (pharmacological, nutritional, exercise, multimodal, etc.) or type of comparator. To reduce bias and focus on outcomes with the most clinical impact, trials were excluded if they had randomized fewer than 40 patients, and the intervention lasted <14 days.
For the present review on QOL, some additional inclusion criteria were applied:
Patient reported QOL (used interchangeably with HRQOL) should be a stated outcome
Use of validated QOL measures, not ad‐hoc measures
Studies where QOL partial domains of PROMS (e.g., EORTC emotional functioning) were used were eligible
The following exclusion criteria were applied:
Insufficient reporting of QOL (i.e., data not shown, not compared between intervention and control groups, or lack of appropriate statistical measures)
Trials using observer‐rated measures of physical functioning, for example the Karnofsky Performance Status scale (KPS), 33 or Eastern Cooperative Oncology Group Performance Status scale (ECOG) 34 as a substitute for self‐reported QOL
The use of a single symptom scale denoting (e.g., assessing appetite and fatigue) conceptualized as a measure of QOL
Data selection and extraction
All articles identified were transferred to Covidence software. 35 Article selection based on titles and abstracts was completed by three researchers in the core team (B. L., T. S .S., and O. F. D.). Any uncertainties in assessing the eligibility of the trials were discussed among the authors until a consensus was reached.
A data extraction table was developed, pilot‐tested and refined within the review group before data were extracted from each article by two independent authors from the review group. Articles relevant to each systematic review were then identified from the data. For this paper, relevant articles assessed the specified QOL endpoints noted in this review.
Assessing the risk of bias
The methodological quality of each study was systematically assessed by four independent reviewers (J. M. D., J. S., B. L., and O. F. D.) with the Modified Downs & Black Scale. 36 The measure assesses among other criteria, study design, blinding, sample size, estimate of variance reporting, and whether the outcome is defined and robust.
Outcomes
This systematic review examines the assessment of QOL in RCTs using validated PROMs on QOL as study endpoints.
More specifically, it describes the following:
the number of identified cancer cachexia RCTs stating QOL as a primary or secondary, or identified as an exploratory outcome;
study characteristics and interventions;
the QOL measures used, including content and properties related to validation, international applicability, mode of administration, dimensionality, scoring methods and interpretation;
the reporting of QOL results, including statistical methods; and
correlations with significant primary or secondary study outcomes, as appropriate.
Data analyses
As expected, the number of retrieved trials was large and heterogeneous. Given this volume and with the main objective being to describe the frequency and diversity of QOL endpoints used, a meta‐analysis of the effect of the interventions was not relevant. Hence, the data were summarized narratively. In trials reporting significant findings on any QOL parameter, raw scores on these subjective measures and the corresponding variability were extracted (if available) to enhance the interpretation of results.
Results
Identified cancer cachexia clinical trials with quality of life as an outcome
The systematic literature search for the series of reviews on cachexia outcomes identified 8166 trials (Figure 1). After deleting duplicates, 7435 papers were retained to screen abstracts, producing 387 articles for full‐text reading.
Figure 1.
PRISMA flow chart.
Study characteristics
The characteristics of the 50 included trials with QOL outcomes are reported in Table 1. These were published between 1996 and 2023, and conducted in 20 different countries, most often the United States and China (both = 6) followed by Italy, Australia, and Iran (n = 4 for all). Three trials were multinational. 38 , 50 , 78 The total sample size based on the number of randomized patients was 6893, but varied considerably across trials, ranging from 42 42 to 469. 56
Table 1.
Key characteristics of eligible trials
| 1st author | Publ year | Country | Quality a | Design | N b | Cancer | Intervention | Comparator | Primary outcome (s) | Secondary outcome (s) | QOL Endpoints |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Agteresch 37 | 2000 | Netherlands | 7 | Open RCT | 58 | Lung (NSCLC) | Pharmacological:Adenosine 58‐triphosphate | None | QOLWeight loss/gainAlbuminMuscle strength | Rotterdam Symptom Checklist (RSCL) | |
| Bauer 38 | 2005 | Multinational | 8 | Double‐blind RCT | 200 | Pancreatic | Nutritional: Dietary nutritional intervention (protein and energy dense, n‐3 fatty acid, EPA oral) | Isocaloric, isonitrogenous control supplement without n‐3 fatty acids | QOLDietary intakeBody composition | EORTC QLQ‐C30 | |
| Beller 39 | 1997 | Australia | 4 | Double‐blind RCT | 240 | GI, mixedHead and NeckHaematological Lung/Pleura | Pharmacological:Arm 1: MA 480 mg per day Arm 2: MA 160 mg per day | Placebo | QOL composite score (LASA + Spitzer QLI‐Index + combined nutritional status score | Separate QOL scoresNutritional status Survival time | LASA |
| Bouleuc 40 | 2020 | France | 7 | Open RCT | 148 | BreastGI mixedLungMelanomaPelvisProstateSarcomaOther | Nutritional: Parenteral | Oral feeding | QOL (overall QOL, physical functioning, fatigue) |
Other QOL scores Nutritional parametersSurvival |
EORTC QLQ‐C15‐PAL |
| Britton 41 | 2019 | Australia | 8 | Stepped‐wedge, cluster, RCT | 307 | Head and Neck, mixed |
Multimodal: Motivational interview cognitive behavioural therapy (Eating As Treatment) |
Usual standard care | Nutritional status |
QOLDietitian SGA ComplianceRe‐admissionsLength of stayDepression |
EORTC QLQ‐C30 |
| Bumrungpert 42 | 2018 | Thailand | 6 | Double‐blind RCT | 42 | BreastGI, mixedLungLymphoma |
Nutritional: Whey protein isolates |
Placebo (maltodextrin) as a daytime snack | Nutritional statusGlutathione levelsImmunityInflammatory status | QOL (explorative)Symptoms | EORTC QLQ‐ C30 |
| Capozzi 43 | 2015 | Canada | 8 | Single‐blinded RCT | 60 | Head and neck Unknown primary |
Multimodal Exercise/Lifestyle: Immediate lifestyle intervention |
Delayed lifestyle intervention | Body composition BMI, lean body mass, % body fat | QOLFitnessDepressionNutritional status | FACT‐AnFHNSI‐22 |
| Cereda 44 | 2019 | Italy | 8 | Double‐blind RCT | 166 |
BloodBreastGI mixed Head and NeckLung |
Nutritional: Nutritional counselling + whey protein isolate supplement | Nutritional counselling | Phase‐angle (3 months) |
QOLPhase angle (1 month) Standardized phase angleFat‐free mass indexWeightHGSChemotherapy toxicity |
EORTC QLQ‐C30 |
| Chen 45 | 2023 | China | 7 | Open RCT | 108 | GI, mixed | Nutritional:Five‐step intervention, education, diet, enteral nutrition, partial enteral/parenteral nutrition, parenteral nutrition | Standard nutritional care | Nutritional status |
QOL |
EORTC QLQ‐C30 |
| Currow 46 | 2021 | Australia | 6 | Double‐blind RCT (phase III) | 190 |
GI, mixed Lung Prostate |
Pharmacological:Arm 1: MA |
Arm 2: Dexamethasone Arm 3: Placebo |
Appetite score | QOLWeightECOG‐PS/KPS | FACT‐G |
| Dehghani 47 | 2020 | Iran | 7 | Single‐blinded RCT | 43 | GI, mixed | Pharmacological: Angiotensin‐converting enzyme inhibitor | Placebo starch pills | QOL | Weight | EORTC QLQ‐C30 |
| Del Fabbro 48 | 2013 | USA | 10 | Double‐blind RCT (phase III) | 73 | Advanced cancers mixed, | Pharmacological: Melatonin | Placebo | Appetite score | QOLSymptomsFatigueBody compositionWeight | FACIT‐F13FAACT 12 itemESAS |
| Famil‐Dardashti 49 | 2020 | Iran | 8 | Double‐blind RCT | 67 |
Breast GI, mixed Lung Other |
Pharmacological: Herbal combination (Fenugreek, Fennel, Chicory) + MA |
Placebo +MA | Weight gain | QOLAnthropometric indexes HGSSymptom burdenAnorexia/cachexia symptoms |
EORTC QLQ‐C30 FAACT 12 item ESAS |
| Fearon 50 | 2003 | Multinational | 8 | Double‐blind RCT | 200 | Pancreatic | Nutritional: Protein and energy dense n‐3 PUFA enriched oral supplement | Oral supplement (without n‐3 PUFAs | WeightBody compositionDietary intake | QOL |
EORTC QLQ‐C30 EQ‐5D |
| Gavazzi 51 | 2016 | Italy | 7 | Open RCT | 79 | GI, mixed | Nutritional: Home enteral nutrition | Nutritional counselling | Nutritional status (weight, biomarkers, muscle strength) | QOL (explorative)Treatment compliance | FAACT 12 item |
| Hong 52 | 2020 | China | 9 | Open RCT | 204 |
Breast GI, mixed |
Multimodal Exercise/Lifestyle: Resistance exercise | Relaxation | Physical function | QOL | EORTC QLQ‐C30 |
| Hunter 53 | 2021 | Egypt | 7 | Double‐blind RCT(phase III) | 120 |
Breast GI, mixed Lung Pleura Other |
Pharmacological: Mirtazapine | Placebo | Appetite score | QOLFatigueDepressive symptomsWeightLean body massHGSOverall survivalCRP, IL‐6, YKL‐40 | FAACT 12 itemFACT‐GESAS |
| Isenring 54 | 2004 | Australia | 8 | Open RCT | 60 | GIHead and Neck | Nutritional: Nutrition intervention | Usual standard care | QOLWeightFoot‐to‐foot bioelectrical impedance Nutritional status | EORTC QLQ‐C30 | |
| Izumi 55 | 2021 | Japan | 6 | Open RCT | 81 | GI, mixedBladderCarcinoma, unknown LeukaemiaLungMal. mesotheliomaSoft tissue sarcomaThyroidUrological | Pharmacological: Testosterone enanthate administration | None | QOL | Cancer cachexia‐related biomarkersSurvival |
FAACT 12 item ESAS |
| Jatoi 56 | 2002 | USA | 10 | Double‐blind RCT | 469 | GI, mixedLungOther | Pharmacological: Arm 1: MA liquid suspension 800 mg orally daily + capsule placebosArm 2: Dronabinol capsules 2.5 mg orally twice a day + liquid placeboArm 3: Combination of Arm 1&2 medications and dosage | Across arms | AppetiteWeight gain | QOL Toxicity data | FAACTSpitzer QOL index |
| Jatoi 57 | 2010 | USA | 7 | Double‐blind RCT | 61 | Lung (NSCLC) | Pharmacological: Infliximab + docetaxel | Placebo + docetaxel | Weight gain | QOL (explorative)Appetite changesTumour response rates | FACT‐G |
| Jatoi 58 | 2017 | USA | 8 | Double‐blind RCT | 263 | GI, mixed LungOther | Nutritional:Creatine | Placebo | Weight gain | QOLWeight stabilityAppetite changesHGSBioelectrical impedance | FAACT 12 item LASA scales |
| Kanat 59 | 2013 | Turkey | 8 | Open RCT | 62 | Breast GI mixedLung Urogenital Other | Pharmacological:Arm 1: MA + meloxicamArm 2: MA + meloxicam + oral eicosapentaenoic acid‐enriched nutritional supplementArm 3: Meloxicam + oral eicosapentaenoic acid‐enriched nutritional supplement | Comparisons across arms | Weight Lean body mass | QOLBMIIL‐6, TNF‐α | FAACT 12 itemVAS (0–100 for appetite) |
| Katakami 60 | 2018 | Japan | 8 | Double‐blind RCT | 174 | Lung (NSCLC) | Pharmacological: Anamorelin | Placebo | Lean body mass |
QOLWeightBody compositionAppetiteFatigue scoreECOG‐PS/KPS HGS 6‐minute walk testBiomarkers |
QOL‐ACD |
| Kouchaki 61 | 2018 | Iran | 8 | Double‐blind RCT (phase III) | 90 | GI, mixed | Pharmacological: MA + celecoxib | MA + placebo | Weight | QOLHGSAppetite scoreECOG ‐PS Plasma albuminCRP, IL‐6Glasgow Prognostic Score | EORTC QLQ‐C30 |
| Maccio 62 | 2012 | Italy | 8 | Open RCT (phase III) | 144 | Gynaecological, mixed | Pharmacological: Antioxidant agents + L‐carnitine + celecoxib + MA | MA | QOLLean body massResting energy expenditureFatigue | AppetiteGrip strengthGlasgow Prognostic ScorePerformance statusCRP, IL‐6, TNF‐a | EORTC QLQ‐C30 |
| Mantovani 63 | 2010 | Italy | 7 | Open RCT (phase III) | 332 |
BreastGI, mixedGynaecological Head and neckLungUrogenital |
PharmacologicalArm 1: MPA (500 mg/day) or MA (320 mg/day)Arm 2: EPA‐enriched (2.2 g/day) ProSure and Resource Support or 3 Forticare cartons/day Arm 3: L‐carnitine 4 g/dayArm 4: Thalidomide 200 mg/day Arm 5: MPA or MA plus EPA‐enriched nutritional supplement + L‐carnitine plus thalidomide |
Comparisons across arms | Lean body massResting energy expenditureFatigue | QOLAppetiteGrip strengthGlasgow prognostic scoreProinflammatory cytokines | EORTC QLQ‐C30EQ‐5D index/VAS |
| McMillan 64 | 1999 | UK | 7 | Double‐blind RCT | 73 | GI, mixed | Pharmacological: MA + ibuprofen | MA + placebo | QOLWeight gain | AlbuminCRP |
EORTC QLQ‐C30 EQ‐5D |
| Mehrzad 65 | 2016 | Iran | 8 | Double‐blind RCT | 70 | Advanced cancer, mixed, | Pharmacological: Pentoxifylline | Placebo | Weight loss/gainArm circumstance | QOL | SF‐36 |
| Meng 66 | 2021 | China | 8 | Open RCT | 353 | GI, mixed |
Nutritional: Post‐discharge oral nutritional supplements (ONS) with dietary advice |
Dietary advice |
Nutritional outcomes (BMI,SMI) Sarcopenia prevalence |
QOL Chemotherapy tolerance 90‐day readmission rate |
EORTC QLQ‐C30 |
| Navari 67 | 2010 | USA | 7 | Open RCT | 80 | ColonLung | Pharmacological:MA + Olanzapine | MA | Weight gainAppetite stimulation | QOLNausea | FACT‐GMDASI |
| Obling 68 | 2019 | Denmark | 7 | Open RCT | 47 | GI, mixed | Nutritional: Dietic counselling, supplemental home parenteral nutrition | Best practice nutritional care and dietetic counselling | Fat‐free mass | QOLHGSSix minute walk testSkinfold thicknessOverall survival | EORTC QLQ‐C15‐PAL |
| Persson 69 | 2002 | Sweden | 6 | Open RCT | 142 | GI, mixed | Multimodal: Arm 1: Individual nutritional support Arm 2: Group rehabilitationArm 3: Individual support + group rehabilitation | Arm 4: Usual standard care | QOLWeight changes Food intakeSurvival | EORTC QLQ‐C30 | |
| Poulsen 70 | 2014 | Denmark | 5 | Open RCT | 61 | GI, mixed Gynaecological |
Nutritional: Nutritional counselling High‐protein nutrition supplement with 3‐fatty acids |
Nutritional advice nurses or dieticians |
Weight‐loss % weight gain |
QOLTreatment related side effects | EORTC QLQ‐C30 |
| Qiu 71 | 2020 | China | 6 | Open RCT | 96 | Oesophageal |
Nutritional: Whole‐course nutritional management by nutrition support team |
Nutritional supplements (protein, fat, carbohydrate, dietary fibre, minerals, vitamins) | PrognosisChemoradiotherapy complications | QOLNutritional statusIncidence of complications | EORTC QLQ‐C30 |
| Ravasco 72 | 2005 | Portugal | 7 | Open RCT | 75 | Head and Neck | Multimodal: Arm 1: Dietary counselling with regular foodsArm 2: Usual diet plus supplements | Maintained intake ad lib. | Weight | QOLNutritional intake | EORTC QLQ‐C30 |
| Rowland 73 | 1996 | USA | 10 | Double‐blind RCT | 243 | Lung (SCLC) | Pharmacological:MA | Placebo | Survival | QOL (explorative)Response rateWeightToxicity | Spitzer QOL index |
| Silander 74 | 2011 | Sweden | 6 | Open RCT | 134 | Head and NeckUnknown primary | Nutritional:PEG before start of treatment and individual nutritional support | Usual standard care | Malnutrition | QOLHospital stay | EORTC QLQ‐C30QLQ‐H&N35 |
| Sim 75 | 2022 | Korea | 8 | Open RCT | 58 | GI, mixed |
Nutritional: ONS enriched with omega‐3 fatty acids |
Standard nutritional care | Nutritional status |
QOL Cytokine levels |
EORTC QLQ‐C30 |
| Simons 76 | 1996 | Netherlands | 7 | Double‐blind RCT | 206 | GI, mixedLung (NSCLC)Other | Pharmacological: Medroxyprogesterone acetate | Placebo | AppetiteWeight | QOLSide effects | EORTC‐QQL‐C30 |
| Storck 77 | 2020 | Switzerland | 10 | Open RCT | 52 |
BreastGI, mixed OvarianLung Urothelial |
Multimodal: Leucine‐rich supplement combined with nutritional counselling and physical exercise program | Standard care | Physical function | QOLNutritional statusDietary intakeFatigueCRP | EORTC QLQ‐C30 |
| Strasser 78 | 2006 | Multinational | 8 | Double‐blind RCT (phase III) | 243 | GI, mixedHead and NeckHematologic‐lymphogenicLungUrogenitalOther | Pharmacological:Arm 1: Cannabis extract Arm 2: Delta‐9‐tetrahydrocannabinol | Arm 3: Placebo | QOLAppetite score | EORTC‐QLQ C30 | |
| Takayama 79 | 2016 | Japan | 8 | Double‐blind RCT (phase II) | 181 | Lung (NSCLC) | Pharmacological:Arm 1: Anamorelin 50 mgArm 2: Anamorelin 100 mg | Arm 3: Placebo | Lean body massHGS | QOLBody composition WeightSymptomsECOG‐PSKPS Serum biomarkers | QOL‐ACD |
| Uster 80 | 2018 | Switzerland | 9 | Open RCT | 58 |
GI, mixedLung Other |
Multimodal: Standardized individual nutritional counselling + exercise program | Usual standard care | QOL (overall QOL) | Dietary intakeNutritional statusPhysical function tests (HGS, lower limb strength, walking capacity, maximal muscle strength)Performance status | EORTC QLQ‐C30 |
| Van der Werf 81 | 2020 | Netherlands | 9 | Single blinded RCT | 107 | GI metastatic, mixed |
Nutritional: Nutritional counselling Encouragement of physical activity |
Standard care |
Muscle mass |
QOL Weight Muscle density Hand grip strength Treatment toxicity, intensity, response Progression free overall survival |
EORTC QLQ‐C30 |
| Wen 82 | 2012 | China | 5 | Open RCT | 108 | BreastGI, mixedLung | Pharmacological:MA + Thalidomide | MA | QOLWeightFatigue | AppetiteGrip strengthIL‐6 or TNF‐αGlasgow prognostic scorePerformance status | EORTC QLQ‐C30 |
| Westman 83 | 1999 | Sweden | 7 | Double‐blind RCT | 255 |
BreastGI mixedGynaecological Head and neckHepatocellularLeiomyosarcomaLung LymphomaMesothelioma MelanomaUrogenital |
Pharmacological: MA | Placebo | QOL | SurvivalWeightMA side‐effects | EORTC QLQ‐C30 |
| Wiedenmann 84 | 2008 | Germany | 7 | Double‐blind RCT (phase II) | 89 | Pancreatic | Pharmacological: Infliximab | Placebo | Lean body mass | QOLOverall + progression free survivalKPS6‐minute walk test FatigueNutritional healthPainPhysical + mental functioningTNF‐alpha, CRP, IL‐6, IL‐2 | FACIT–F13FAACT SF‐36 |
| Woo 85 | 2016 | Korea | 9 | Double‐blind RCT (phase III) | 67 | Pancreatic | Pharmacological: Pancreatic Exocrine Replacement Therapy Pancreatine‐digestive enzymes (proteins) | Placebo | Weight | QOLPG‐SGA scoreDietary intake Abdominal painFlatulenceOverall survival | EORTC QLQ‐C30 |
| Xie 86 | 2018 | China | 8 | Double‐blind RCT | 54 | Lung (NSCLC) | Pharmacological: Thalidomide and cinobufagin | Cinobufagin | QOLNutritional statusSide effects | EORTC QLQ‐C30 |
By the Downs & Black checklist.
No. of patients randomized.
ECOG‐PS, European Cooperative Oncology Group Performance Status; EORTC QLQ‐C30, European Organization for Research and Treatment Quality of Life Questionnaire; EORTC QLQ‐C15‐PAL, European Organization for Research and Treatment Quality of Life Palliative Care; EORTC QLQ‐C30 QLQ‐H&N35, EORTC QLQ‐C30 QLQ head and neck module; EPA, eicosapentaenoic acid; EQ‐5D, EuroQoL 5D‐Health‐Related Quality Of Life; ESAS, Edmonton Symptom Assessment System; FACT‐An, Functional Assessment of Cancer Therapy – Anaemia scale; FACT‐G, Functional Assessment of Cancer Therapy ‐ General; FAACT 12 item, Functional Assessment of Anorexia/Cachexia Treatment 12 item version; FHNSI‐22, FACT Head/Neck Symptom Index‐22; FACT‐G, Functional Assessment of Cancer Therapy ‐ General; FACIT‐F13, Functional Assessment of Cancer Therapy; GI, Gastro intestinal; HGS, Hand‐grip strength; KPS, Karnofsky Performance Status; LASA, Linear analogue Self‐Assessment scales; MA, Megestrol acetate; MDASI, M.D. Anderson Symptom Inventory MPA, Medroxyprogesterone acetate; PEG, Percutaneous endoscopic gastrostomy; PG‐SGA, patient‐generated subjective global assessment; QOL‐ACD, Quality of Life Questionnaire for Cancer Patients Treated with Anti‐Cancer Drugs; QOL, Quality of Life; SF‐36, MOS short‐form 36‐survey; WL, weight loss.
Most trials (39/50, 78%) included multiple diagnostic groups. Thirty‐one trials mentioned all cancer diagnoses involved, while eight were less specific using broad terms such as gastrointestinal or advanced cancer (Table 1). Twenty‐five of the 50 trials (50%) included patients with lung cancer while pancreatic cancer (42%) was the second most common diagnostic group; Lung (n = 6) and pancreatic cancers (n = 4) were the two diagnoses most used in the trials limited to one cancer type (Table 1).
The interventions
Pharmacological interventions dominated (27/50, 54%) with diverse pharmacological agents, that is, anticancer drugs, appetite stimulants, anti‐inflammatory drugs, and dietary supplements (Table 1). Seventeen trials (34%) were categorized as nutritional interventions, and composed with different nutritional agents, and dietary counselling. Nutritional interventions were, for example, whole‐course nutritional management programme provided by a specialized or multiprofessional teams, 45 , 71 protein and energy‐dense oral nutritional supplement with n‐3 fatty acids, 38 , 75 whey protein isolate supplements, 44 or thorough follow up of nutritional status with tube feeding or parenteral nutrition as necessary. 74 , 81 Nutritional advice was also included in some of the six multimodal programmes, for example, the cognitive behavioural intervention by Britton et al. 41 and combined with physical exercise. 77 , 80 The median duration of the interventions was 12 weeks (range 4–96).
Study outcomes
Trials with quality of life as the primary outcome
Fifteen trials (30%) had QOL as the primary study outcome. 37 , 38 , 39 , 40 , 47 , 54 , 55 , 62 , 64 , 69 , 78 , 80 , 82 , 83 , 86 QOL was the single primary outcome in six of these trials 39 , 40 , 47 , 55 , 80 , 83 and one of three or four co‐primary outcomes in the remaining nine (Table 1). Five of the 15 trials (27%) defined a clinically meaningful change for QOL, either as a 5 or 10% change in the scales or item scores 38 , 40 , 80 or specified as a difference of .45 or .5 SD. 39 , 83 All except four 37 , 39 , 40 , 55 of these 15 trials used the EORTC QLQ‐C30, either alone (n = 7) or in combination (n = 4) with other PROMs (Table 1).
The reporting of QOL results varied. Mean (standard [SD]) scores with corresponding p‐values for patient groups were used in eight trials. 47 , 54 , 62 , 64 , 78 , 80 , 83 , 86 Two trials reported mean (standard error of the mean [SEM]) or mean (95% confidence interval [CI]) values 38 , 54 and one presented the median and range of scores. 69 Four trials 39 , 40 , 55 , 82 reported the absolute or per cent change in mean scores at the different assessment points, while one study presented both mean (SD) and per cent change. 39
Trials with quality of life as secondary or exploratory outcomes
Thirty‐one trials (62%) used QOL as a secondary outcome. 41 , 43 , 44 , 46 , 48 , 49 , 50 , 52 , 53 , 58 , 59 , 60 , 61 , 65 , 67 , 68 , 70 , 71 , 72 , 76 , 77 , 79 , 84 , 85 Four of these specified a clinically significant difference for the QOL measures, being a 10%, 20% or 25% difference on the 0–100 scales between groups. This difference was assessed either at a specific assessment point or as a within‐group change over time. 49 , 61 , 72 , 74
Sometimes QOL measures were not specified as a study objective even if the QOL results were presented in the results section. However, if the latter applied these data were assessed and we defined QOL as an exploratory outcome in four trials. 42 , 51 , 57 , 73 None of these trials defined a clinically significant difference for the QOL measures.
The quality of life measures
Seventeen different QOL measures were used in these 50 trials. Two of these, the SF‐36 18 and the EQ‐5D 19 are generic QOL measures while the remaining 15 are cancer‐specific. The most commonly used measure was the EORTC QLQ‐C30 21 in 60% of the trials, while 17 trials (34%) used different versions of the FACIT. Thirteen trials (26%) used multiple measures of QOL, often including diagnosis or condition specific measures, such as the EORTC H&N35 25 and the anaemia and fatigue FACIT measures. 27 FAACT was the only cachexia‐specific measure used, either in the 18‐ or 12‐Item versions. 29 Supporting file S3 presents the measures used, their content, assessment period, scoring, number of scales and items and whether a summary measure could be calculated. Figure 2 indicates how often different measures were reported together. With the exception of the measure developed in Japan by Kurihara et al., 24 all measures were validated in an international context and demonstrated cross‐cultural applicability.
Figure 2.
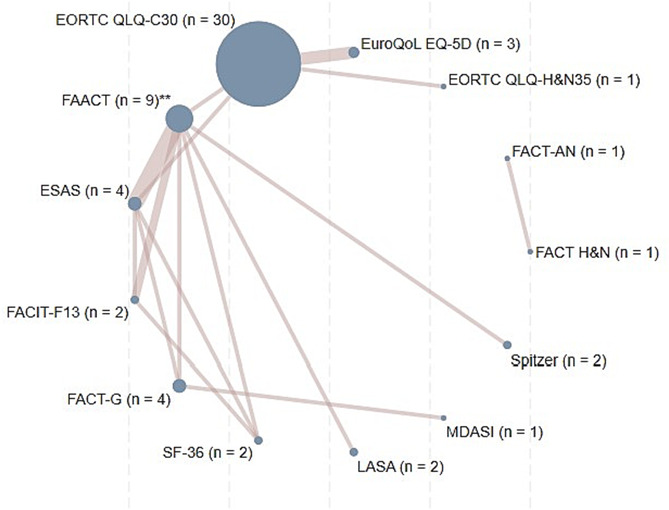
Network diagram reporting of QOL measures. The size of the circles represents the frequency of each measure, and the width of the connecting lines reflects the number of studies reporting each pair of measures. The measures QOL‐ACD, RSCL, and EORTC QLQ‐C15‐PAL are not shown as these have not been presented in combination with other measures of QOL. **FAACT includes both the 12‐ and 18‐item version of this measure. Numerical details are reported in Table 1.
Trials reporting statistically significant quality of life results
Eighteen trials reported statistically significant QOL benefits in favour of the intervention arm. 37 , 39 , 41 , 45 , 49 , 52 , 54 , 56 , 60 , 62 , 64 , 66 , 67 , 68 , 74 , 75 , 79 , 82 Nine of these 18 studies (50%) used pharmacological interventions, and had a total sample size of 2895 (ranging from 47 to 469). The EORTC QLQ‐C30 was the most common measure; used in 61% (11/18) of the trials. The length of the intervention in these trials varied from eight to 28 weeks. Two trials had a pre‐set definition of a clinically significant difference, that is, a difference of 10 points or more on the EORTC‐QLQ‐C30 52 , 74 and on the QLQ‐H&N35. 74
QOL was the primary outcome in six (32%) of these trials 37 , 39 , 54 , 62 , 64 , 82 and a secondary outcome in 12. 41 , 45 , 49 , 52 , 56 , 60 , 66 , 67 , 68 , 74 , 75 , 79 Authors' interpretation of the QOL results are summarized in Table 2, with the statistical presentation of significant results in Table 3. None of these 18 trials reported statistical correlations between the QOL outcomes and other outcome measures. If a potential relationship was mentioned, this appeared in the discussion section and was vaguely described as ‘being associated with’ symptom items or other from the intervention endpoints, for example weight gain in the questionnaires.
Table 2.
Studies reporting significant QOL results
| Studies with QOL as their primary outcome (N = 6) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1st author | Publ year | Sample size a | Type of intervention | Duration of intervention | Assessment points | QOL measure(s) | Use of QOL measure(s) | Use of other PROM | Significant results b | Effect measures c | Authors' interpretation |
| Agteresch 37 | 2000 | 58 | Pharma |
Max 10 infusions (2–4 week intervals) |
Week 0, 4, 8, 12, 16, 20, 24, 28 | RSCL | Complete measure | ‐ | Less decline in PF, functional/psychologic state, overall QOL, sustainable over 4‐week periods | Mean (SD), log‐rank test, GEE4 | Marked beneficial effect of ATP on QOL |
| Beller 39 | 1997 | 240 | Pharma | 12 weeks | Week 0, 4, 8, 12 |
LASA Spitzer (physician rated) |
Complete measure | ‐ | Better appetite, mood, Overall QOL | Mean (SD), log‐rank test, Cox regression, GEE4 | Patient‐reports disclose important QOL dimensions, not captured by physician rating |
| Isenring 54 | 2004 | 60 | Nutritional | 12 weeks | Week 0, 4, 8, 12 | EORTC QLQ‐C30 | Global QOL scale, PF | ‐ | Better QOL and PF in control group | Mean (SEM) χ2, GEE | Less global QOL/PF decline in control group. Weight maintenance may impact PF |
| Maccio 62 | 2012 | 144 | Pharma | 4 months | Month 0–4 | EORTC QLQ‐C30 | Global QOL scale | ‐ | Greater change in QOL over time | Mean (SD), χ2, Student's t‐/Wilcoxon/rank sum | Multimodal interventions favourable |
| McMillan 64 | 1999 | 73 | Pharma | 12 weeks | Week 0, 4–6, 12 |
EORTC QLQ‐C30 EQ 5D |
Complete measures | ‐ |
Weeks 4–6: sign. Better appetite in both groups Week 12: Better QOL (EQ 5D) |
Median (range), Mann–Whitney U, Fisher's exact test, Wilcoxon signed rank Freidman test | Weight gain may be associated with better QOL |
| Wen 82 | 2012 | 108 | Pharma | 8 weeks | Week 0–8 | EORTC QLQ‐C30 | Global QOL scale | Appetite (VAS) | Greater change in QOL over time | Mean scores (SD), Mean change (SD) Student's t, χ2 | Adequate fat‐free mass may contribute to better QOL |
| Studies with QOL as a secondary outcome (n = 12) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1st author | Publ year | Sample size a | Type of intervention | Duration of intervention | Assessment points | QOL measure(s) | Use of QOL measure(s) | Use of other PROM | Significant results b | Effect measures c | Authors' interpretation |
| Britton 41 | 2019 | 307 | Nutritional | 6 weeks | Week 0, 1 12 post‐RT | EORTC QLQ‐C30 | Complete measure |
PG‐SGA |
Better overall QOL post radiation therapy | Linear mixed model of QOL mean scores over time | Effective interventions in H&N patients during RT |
| Chen 45 | 2023 | 108 | Nutritional | 10 weeks | Week 0–10 | EORTC QLQ‐C30 | Complete measures |
PG‐SGA NRS‐2002 |
Improved PF and SF, reduced fatigue, pain, appetite loss, constipation | Mean (SD), Shapiro–Wilk test, Chi‐square, Fisher exact test, Wilcoxon Sign‐Rank Test, Mann–Whitney U Test | Nutritional intervention improved the nutrition status and showed positive impact on quality of life |
| Famil‐Dardashti 49 | 2020 | 67 | Nutritional | 8 weeks | Week 0, 8 | EORTC QLQ‐C30, FAACT |
FAACT total score Global QOL score |
ESAS | Better QOL after 8 weeks | Mean (SD) paired‐sample/and independent t‐test, Mann Whitney u | Reaching QOL improvement needs a longer duration of follow up in comparison with other indices |
| Hong 52 | 2020 | 204 | Multimodal Exercise/Lifestyle: Resistance exercise | 12 weeks | Week 0, 12 | EORTC QLQ‐C30 | Complete measure | ‐ | Better PF and RF after 12 weeks | Mean (SD) Student t‐test | Interventions is effective on symptoms, PF and QOL during chemotherapy |
| Jatoi 56 | 2002 | 469 | Pharma (three arms) | Median 80 days | Weekly for 4 weeks monthly thereafter |
UNISCALE FAACT‐AN |
FAACT‐AN total score | ‐ | Greater improvement in QOL over time | Independent sample t‐test, Wilcoxon rank sum tests | Better QOL may reflect the emphasis on anorexia of the FAACT‐AN |
| Katakami 60 | 2018 | 174 | Pharma | 12 weeks | Week 1, 3, 6, 9, 12 | QOL‐ACD | QOL‐ACD scores | ‐ | Better QOL‐ACD scores on PF, meals, appetite, weight loss | Mean (SD) Least square means (+/‐SD) difference from baseline | Interventions are effective on multiple outcomes in NSCLC patients with CC |
| Meng 66 | 2021 | 353 | Nutritional | 3 months | Month 0–3 | EORTC QLQ‐C30 | Complete measure | ‐ | Lower weight loss, less sarcopenia, and chemo‐therapy modifications, less fatigue, and appetite loss | Mean (SD), independent‐samples t test, Mann–Whitney U test, χ2, Fisher exact test | Post‐discharge ONS and dietary advice improved nutritional outcomes, skeletal muscle maintenance, chemotherapy tolerance and some QOL variables |
| Navari 46 | 2010 | 80 | Pharma | 8 weeks | Week 0, 4, 8 | FACT‐G | Complete measure | MDASI | Better QOL at 4 and 8 weeks | Percentage of patients with improvement | Interventions are effective on multiple outcomes in cachectic patients |
| Obling 69 | 2019 | 47 | Nutritional | 24 weeks | Week 0, 6, 12, 18, 24 | EORTC QLQ‐C15‐PAL | Global QOL scale | ‐ | Better QOL at week 12, then stabilized | Mean (SD) difference | Home parenteral nutrition may be feasible in select patients |
| Silander 74 | 2012 | 134 | Nutritional Percutaneous Endoscopic Gastrostomy (PEG) | NA | Month 0, 3, 6, 12, 24 |
EORTC QLQ‐C30 QLQ‐H&N35 |
Complete measures | ‐ | Better overall QOL, PF, SF and CF at 6 months | Mean, Mann–Whitney U test | Prophylactic PEG reduced malnutrition improved QOL |
| Sim 75 | 2022 | 58 | Nutritional | 3 months | Month 0–3 | EORTC QLQ‐C30 | Complete measure |
PG‐SGA |
Worsening of symptoms in control group, Better better Global QOL, RF, sleep, fatigue constipation at week 8 | Mean (SD), independent‐samples t test, Mann–Whitney U test, χ2, Fisher exact test | Intervention improves PG‐SGA scores and QOL during chemotherapy |
| Takayama 79 | 2016 | 181 | Pharma | 12 weeks | Week 0, 4, 8, 12 | QOL‐ACD | Complete measure | MDASI | Greater improvement in QOL over time | Mean (SD) Least squares (LS) mean change from baseline | Promising results on multiple outcomes, especially QOL |
Number of randomized patients.
Defined as a statistically significant difference across groups or within groups over time. Significance is in favour of the intervention group, unless otherwise stated.
Statistics used for QOL scores.
CF, cognitive functioning (function scale EORTC QLQ‐C30); GEE, generalized estimating equation; PG‐SGA, patient‐generated subjective global assessment; PF, physical functioning (function scale EORTC QLQ‐C30); RF, role functioning (function scale EORTC QLQ‐C30); RT, radiation therapy; SD, standard deviation; SF, social functioning (function scale EORTC QLQ‐C30).
Table 3.
Statistical presentation of significant QOL results a
| Studies with QOL as a PRIMARY outcome (n = 6) | |||||
|---|---|---|---|---|---|
| 1st author | P‐value, difference between‐groups b , c | Control baseline (mean ± SD) (median + ICR/range) | Control endpoint (mean ± SD) (median + ICR/range) | Intervention baseline (mean ± SD) (median + ICR/range) | Intervention endpoint (mean ± SD) (median + ICR/range) |
|
Agteresch 37 |
RSCL Physical QOL; 0.0002RSCL Functional QOL; 0.02RSCL Overall QOL; 0.001 | 77.9 ± 13.9 | Not reported | 78.1 ± 12.3 |
Changes in scores, intervention vs. controlsPhysical (−0.2% vs. − 2.4%; Functional + 0.4% vs. −5.5Overall QOL + 0.1% vs. −3.5% |
|
Beller 39 |
LASAP for trend: <0.001Mood: 0.001 Appetite: 0.001Overall QOL: <0.001QOL categorized: <0.001 | Average difference in scores between baseline and subsequent weeks 4, 8, 12 Mood; placebo −4.1, low: 0.4, high: 10.9 Appetite: placebo: 9.7, low: 17.0, high: 31.3 Overall QOL: placebo: −2.1, low: 2.4, high: 12.3QOL categorized: placebo: −2.37, low: 2.66, high: 3.05 | |||
|
Isenring 54 |
EORTC QLQ‐C30, global QOL: 0.009 | 75.3 ± 19.2 | 62.6 (SEM in Figure) | 67.7 ± 18.8 | 72.7 (SEM in Figure) |
| Maccio 62 | EORTC QLQ‐C30, global QOL: 0.042 | 57.0 ± 12.8 | 61.1 ± 15.5 | 53.8 ± 17.4 | 61.3 ± 20.9 |
|
McMillan 64 |
EORTC QLQ‐C30: NSEQ 5D: <0.05 | QLQ‐30: 33.3 (0–91.7)EQ‐5D: 0.630 (−0.095–1.000) | Not reported | EORTC QLQ‐30:33.3 (0–83.3)EQ‐5D: 0.689 (−0.261–1.000) | Not reported |
| Wen 82 | EORTC QLQ‐C30, within group over timeIntervention, Global score: 0.02Control, appetite: 0.02Between groups: Global QOL: <0.01 | 50.3 ± 16.6 | 51.4 ± 19.7 | 49.0 ± 23.2 | 56.9 ± 26.3 |
| Studies with QOL as a secondary outcome (n = 12) | |||||
|---|---|---|---|---|---|
| 1st author | P‐value, difference between‐groups 2 , 3 | Control baseline (mean ± SD) (median + ICR/range) | Control endpoint (mean ± SD) median + ICR/range) | Intervention baseline (mean ± SD) (median + ICR/range) | Intervention endpoint (mean ± SD) (median + ICR/range) |
| Britton 41 | EORTC QLQ‐C30:Global QOL < 0.01CF < 0.01PF 0.01Nausea/vomiting: <0.01Appetite loss: 0.02 | Mean EORTC QLQ‐C30 scores from a linear mixed model tabulated across end of treatment, 1 month post‐RT, and 3 months post RT | |||
| Chen 45 |
EORTC QLQ‐C30 Global QOL: <0.001 SF: 0.0023 Fatigue: 0.023 Pain: 0.0127 Appetite: 0.0228 Constipation: 0.0004 |
Global QOL: 62.7 ± 10.1 SF: 83.3 ± 16.4 Fatigue: 7.5 ± 9.1 Pain: 18.1 ± 10.7 Appetite: 18.3 ± 11.8 Constipation: 17.3 ± 12.2 |
Global QOL: 48.7 ± 15.9 SF: 57.3 ± 15.6 Fatigue: 55.4 ± 21.2 Pain: 61.8 ± 20.1 Appetite: 47.7 ± 14.4 Constipation: 50.7 ± 12.3 |
Global QOL: 60.5 ± 10.5 SF: 92.1 ± 17.7 Fatigue: 10.2 ± 6.3 Pain: 18.2 ± 15.1 Appetite: 19.8 ± 15.6 Constipation: 16.2 ± 10.3 |
Global QOL: 66.6 ± 18.1 SF: 88.5 ± 19.6 Fatigue: 30.5 ± 14.8 Pain: 49.1 ± 15.8 Appetite: 22.2 ± 15.4 Constipation: 19.1 ± 9.7 |
| Famil‐Dardashti 49 | EORTC QLQ‐C30 Global QOL: 0.001FAACT Global score: 0.05 | 64.4 ± 4.9 | Not reported | 66.9 ± 7.7 | Not reported |
| Hong 67 |
EORTC QLQ‐C30 PF: 0.035 RF: 0.041 Fatigue: 0.024 |
Global QOL: 58.6 ± 20.3PF: 84.7 ± 18.2RF: 64.1 ± 21.3 |
Global QOL:55.2 ± 22.6PF: 72.4 ± 15.9RF: 54.9 ± 17.9 | Global QOL:56.8 ± 19.5PF:83.6 ± 15.7RF:62.3 ± 24.7 | Global QOL 60.3 ± 21.7PF: 86.3 ± 17.4RF: 66.7 ± 19.4 |
| Jatoi 56 |
FAACTFAACT‐AN QOL: 0.002Dronabinol+MA vs. MA: 0.003FAACT Appetite: <.001FAACT QOL: .009Dronabinol vs MA: 0.003 |
Megestrol acetate55 (26–84) | Results visualized in Figure only | Dronabinol: 56 (27–92)Megestrol Acetat + Dronabinol:57 (27–92) | Difference between megestrol acetate and dronabinol groups median, 7.8 [range, 0 to 41] v 2.6 [range, 0 to 59] |
| Katakami 60 | QOL‐ACDPF: 0.017Enjoying meals: 0.032Appetite: 0.0029Total score: NS | 70.9 ± 13.0 | Least square means ± SD shown in figures every 3 months | 74.9 ± 13.0 | Least square means ± SD shown in figures every 3 months |
| Meng 66 | EORTC QLQ‐C30Global QOL: 0.256Fatigue: 0.035Appetite loss: 0.013 | Not reported | Global QOL: 73 (50–100)Fatigue: 22 (0–44)Appetite: 8 (0–33) | Not reported | Global QOL: 75 (58–100)Fatigue: 11 (0–33)Appetite: 0 (0–33) |
| Navari 67 | MDASI summary score:<0.01 | Number of patients with improvement in figures, significant value reported in text | |||
| Obling 68 | EORTC QLQ C15‐PAL‐15: Global QOL: <0.05 (after 12 weeks)NS (24 weeks) | Global QOL: 64 ± 17 | Global QOL: 56 ± 18 | Global QOL: 60 ± 23 | Global QOL: 69 ± 24 |
| Silander 74 | EORTC QLQ‐C30Global QOL 0.02 (6 months)(NS 3, 12, 24 months) | Global QOL: 63 (SD not reported) | Global QOL: 52 (SD not reported) | Global QOL: 64 (SD not reported) | Global QOL: 77 (SD not reported) |
| Sim 75 |
EORTC QLQ‐C30 Fatigue: 0.020 |
Fatigue: 18.52 ± 4.67 |
Fatigue: 35.21 ± 8.15 | Fatigue: 28.28 ± 6.15 | Fatigue: 16.66 ± 4.18 |
| Takayama 79 |
QOL ‐ACD 0.05 (100 mg vs. placebo) NS (50 mg vs. placebo) |
73.4 ± 13.9 | Least square means ± SD shown in figures every 4 weeks | 50 mg anamorelin: 71.3 ± 14.9100 mg: 70.6 ± 13.9 | Least square means ± SD shown in figures every 4 weeks |
Number of randomized patients.
Defined as a statistically significant difference across groups or within groups over time. Significance is in favour of the intervention group, unless otherwise stated.
Statistics used for QOL scores.
CF = Cognitive functioning (Function scale EORTC QLQ‐C30), GEE = Generalized estimating equation, PG‐SGA = Patient Generated Subjective Global Assessment, PF = Physical functioning (Function scale EORTC QLQ‐C30), RF = Role functioning (Function scale EORTC QLQ‐C30), RT = Radiation therapy, SD = Standard deviation, SF = Social functioning, (Function scale EORTC QLQ‐C30)
Only three of the 18 trials presenting significant results had defined a magnitude of a statistically and/or clinically significant difference, that is, a 0.45 SD corresponding to an 11% change on the 0–100 overall LASA or Uniscale scores 39 or a difference of 10 points or more on the EORTC‐QLQ‐C30 measures. 52 , 74 None of the trials reported adjustments for multiple testing in the statistical significance analyses, even if most QOL measures were composed of several items and domains.
Discussion
This review identified 50 RCTs in cancer cachexia where QOL was assessed as an outcome. Overall, 18 trials reported statistically significant differences in QOL outcomes, in favour of the intervention groups. Of these, six had QOL as the primary study outcome, and 12 had it as a secondary outcome. These findings, although encouraging, indicate many considerations are needed when incorporating QOL in cachexia clinical trials.
Firstly, defining what a clinically significant difference represents is challenging, and this was seldom reported. Only one trial (QOL was the primary outcome) defined a clinically meaningful difference in QOL (11%/0.5 SD), 39 while another used a 10% difference on the 0–100 numerical scales, but did not specify which of the multiple outcomes this applied to. 82 A ‘rule of thumb’ is that a difference of 7–15% on the 0–100 scale, or a 0.5 SD is meaningful to patients. 87 , 88 However, the difference between minimally clinically important differences (MCID) at a patient level versus at the group level is not clear. The latter relates to mean differences between groups or mean change over time reaching a level of significant difference, whereas individual patient change over time categorize, for example, non‐responders/responders to a particular treatment effect is the focus at the patient level. These approaches require different thresholds for correct interpretations as emphasized in ongoing international projects aiming to standardize the measurement and interpretation of PROMs. 89 , 90 As cachexia is a multifactorial syndrome, it is important to understand how changes in QOL relate to changes in other endpoints. For example, does improved QOL correlate with improved physical function and vice versa? An understanding of such relationships is critical both for patient benefit and also to know how pathophysiological changes (and therefore potential mechanisms of action of interventions) relate to changes in endpoint(s). None of the 18 studies where QOL improved examined how this related to other endpoints. This represents an area that should be addressed in future trials.
Secondly, sample size calculations need to be applied when QOL endpoints are assessed, although QOL improved in a proportion of studies, sample size estimations in relation to this was uncommon, as were effect sizes.
Thirdly, the optimal time point for measuring QOL needs to be clarified. Usually, these are assessed over time with multiple QOL assessments, and this was the case for some trials included where for example QOL improved after 12 weeks. This finding could mean that a significantly improved QOL at 4 weeks may be sustainable for the next 2 months as well, that it was a random finding, or maybe that it was not attributable to the intervention per se but to other factors influencing QOL. Yet other factors may impact QOL and as does the expected deterioration in patients with cancer cachexia. 3
Finally, the complex intervention(s) complicates the interpretation of results as disentangling which affects which outcome, is challenging; particularly with QOL. Yet these multimodal interventions in cachexia trials are recommended in cachexia treatment 91 and practical guidelines. 3 Additionally regulatory bodies advocate QOL endpoints, so ways to integrate appropriately and assess QOL in cachexia trials is essential and a research priority. Taken together, much work remains to integrate QOL measures optimally and meaningfully into cancer cachexia clinical trials. Some proposals can be found in Supporting file S4.
As QOL assessment is likely to remain a central tenet of the cachexia trial endpoint spectrum, the question remains as to which measure should be used. We noted that the EORTC QLQ‐C30 21 was the most frequently used measure (as evidenced in other reviews 4 , 92 followed by the FACT‐G 20 (part of the specific FACT‐modules). These are multidimensional, internationally validated and developed through rigorous and stepwise scientific processes. The EORTC QLQ‐C30 and FACT‐G have been adapted to include cachexia‐specific QOL assessment via the EORTC Cachexia‐24 module 30 and the 12‐item FACIT cachexia‐specific instrument (FAACT). 29 Whilst these adaptions are welcome further evaluation of these is needed before they can be recommended as being the preferred QOL assessment; specifically, regarding response to change in patients with cachexia. 4 It could also be proposed that single items from QOL assessments could be assessed or even single items from multiple assessments combined; yet one of the limitations of such an approach is that when tools are dissected in terms of their component parts, the validity is often questioned, and these tools have usually been developed and assessed as a whole.
It is acknowledged that patients with cancer cachexia are often frail or deteriorate rapidly. Thus, the balance between the need for short measures to reduce patient burden and the need for comprehensive assessments may be challenging. However, technological development with digital measures and computer adaptive testing methods of the EORTC and FACIT measurement systems that tailor the questions to the individual patients, represents a major step forward in the monitoring and follow‐up of patients, in research and clinical practice.
Both the EORTC QLQ‐C30 and the FACT‐G/FAACT have composite scores, calculated as the mean of the combined scale and item scores for the EORTC QLQ‐C30, and by totalling subscale FACT‐G scores. For the EORTC QLQ‐C30; however, the distinction between the Global QOL score and the composite score is important, as the former consists of only two items that combine physical health and the patient perception of overall QOL. Only one reviewed paper used the composite EORTC score, while the Global QOL Score was used as the only outcome measure in two of the nine trials reporting significant QOL results with QOL as a primary outcome 62 , 82 and supplemented with the physical functioning score. 54 This is problematic because the Global QOL scale score may not correspond well to specific item or scale scores, as it does not appear to be sufficiently sensitive. A probable explanation might be a response shift over time in patients with advanced disease: ‘taken together my situation is not that bad’, despite reporting a relatively high symptom burden. On the other hand, it is acknowledged that an improvement in one specific scale within a multidimensional QOL measure denotes a generally improved QOL. The well‐validated tools contain both single items and multidimensional scales assessing different aspects of QOL including symptom burden, and possess a reasonable sensitivity and specificity. Thus, a sole focus on symptoms such as appetite or fatigue should not be used alone to indicate a multidimensional construct such as QOL. Thus, trials that used a single item to denote QOL in this review were not included. Also, it is discouraging that associations between QOL outcomes and other more objective results were not emphasized in any of the reviewed trials. Further, trials using weight loss or gain as outcomes face several challenges that were rarely elaborated on in the trials. Examples are the variability in measures (per cent, kilograms, and slopes on the weight curves) and the association with body composition variables that may affect muscle mass, strength, and functioning that may affect specific QOL scores, to which a global score is not sufficiently sensitive.
Removing confounding factors, particularly in the context of a clinical trial where QOL is measured, is worthy of note. Of the 15 trials reporting significant QOL differences across groups, nine were pharmaceutical trials, while the remaining six had a more individualized approach. It can be hypothesized that a direct patient‐centred approach with individual or group follow‐ups in terms of meetings, exercise groups, frequent phone calls, or digital consultations may improve the patients' emotional wellbeing and mental state. However, this ‘attention’ effect would be controlled for, though not blinded against, if there were some sort of active intervention in the control group, as opposed to conventional care. To our knowledge, the direct benefits of being in a study are rarely investigated, but it is likely that being ‘seen’ is a positive factor. In cachexia trials where counselling on nutrition and physical activity are commonplace, therapeutic relationships will develop and these are likely to influence QOL for trial participants. It is also reasonable to assume that other aspects may influence QOL, independent of the intervention being assessed. Examples include psychological distress caused by disease progression, side‐effects from anti‐cancer therapy, or financial worries. Disentangling these facets from a ‘pure’ QOL assessment in a clinical trial is complex and may be challenging to truly understand.
Strengths and limitations
A key limitation of the review was that the heterogeneity of trial designs, populations studied, and variation in interventions prevented direct comparisons of results or a meta‐analysis. This limitation was partly due to the decision to include trials that assessed QOL in varying hierarchies of endpoints. As such, where QOL was not a primary endpoint, the trial was not powered to conclude on QOL results. This approach was justified to ensure that no important information was missed.
The multifactorial origin of cancer cachexia calls for multimodal interventions, even if it makes it difficult to prove an exact relationship between interventions and outcomes. Related to this is that the use of aggregated data limits a detailed assessment of the relationship between different QOL measures and other study outcomes. It is therefore not possible to draw firm conclusions regarding which measure to use, given the QOL measures are multidimensional and their sensitivity to changes in other outcomes is unknown. We also have to acknowledge that in patients with cachexia, other symptoms related to cancer will be common, in addition to co morbidities, in older patients in particular. Choice and use of QOL instruments within the context of clinical trials must be cognizant of these factors.
We purposefully chose to use a quality assessment tool that was general rather than specific to certain endpoints, and we regard this as an appropriate methodology. Further, we decided to do quality assessments at the initial level by reviewers to limit bias. We believe that the selection of another approach for reporting quality assessment endpoints would have had only a minor influence on our conclusion.
It was challenging to distinguish between studies trying to prevent or treat cachexia per se (involuntary weight loss) versus those trying to treat the symptoms caused by cachexia. There was no uniform definition of cachexia used throughout to the extent that some studies examined cancer anorexia (a component of cachexia syndrome) versus targeting lean mass versus targeting multiple components. Indeed, the lack of uniform trial definition reflects the current status quo of cachexia clinical practice whereby there are several, alternative operational diagnostic criteria (e.g., Fearon definition) 1 or the Global Leadership Initiative in Malnutrition (GLIM) criteria 93 each of which has been established by expert consensus alone. From a QOL perspective however, it should be acknowledged that objective changes such as weight gain or improved performance status might contribute to improvement in one or more QOL aspects, even if it does not change the patient's cachectic state. Future work should be clear as to the primary aim of any intervention as potentially the term ‘cancer cachexia’ may be too vague in the context of a clinical trial intervention.
One study strength is the review of trials using PROMs that span more than 30 years of research, coupled with the fact that the most frequently used and well‐validated QOL measures in the reviewed trials date back to the early 1990s, that is, the EORTC QLQ‐C30 and FACT‐G. The EORTC QLQ‐C30 was used in the two oldest trials in this review, published in 1999. Also, the thorough approaches used for evaluating scientific quality, extraction, and appraisal of papers is a major strength. This also applies to the careful registration of relevant variables in a common database for a series of reviews of cachexia trials involving double or triple appraisals for paper retention.
Conclusions
QOL is an important endpoint in cancer cachexia trials, regardless of whether an improvement is due to direct effects from a specific drug or results from synergistic effects of the multiple components in complex interventions. Thus, it makes sense to include patient‐reported QOL endpoints in cancer clinical trials. As demonstrated in this review, however, comprehensive descriptions of the patient samples and characteristics varied, as did the presentation of statistical considerations related to sample size, power estimations, presentation of results and correlations with other study outcomes, and adjusting for multiple significance testing.
Thus, we call for a more rigorous approach to assessing QOL as an endpoint in cancer cachexia trials, including defining what a MCID is, how QOL relates to mechanism of action of the intervention, other key endpoints (e.g., physical function), and learning from other areas where regulatory approval has been given on the basis of a PROM of QOL. As cancer cachexia has a profound impact on patients' QOL, and as it is a multidimensional construct, we recommend the use of well‐validated comprehensive QOL measures with cachexia‐specific modifiers and advise against using single items as surrogate indices of QOL. Taken together, these will inform future trials and clinical practice.
Conflict of interest
Eric. J. Roeland has served as a member of the scientific advisory board for Napo Pharmaceuticals, Care4ward, Actimed Therapeutics, and Meter Health in the last 2 years, as a consultant for Veloxis Therapeutics, Aileron, and BYOMass, and as a member of the advisory board for Takeda. He has also served as a member on the data safety monitoring boards for Enzychem Lifesciences Pharmaceutical Company. Barry Laird has served as a member of the scientific advisory board for Actimed and Artelo. He has undertaken consultancy for Faraday, Kyona Kirin, and Grunenthal. Andrew S. J. Coats declares to have received honoraria and/or lecture fees from: Astra Zeneca, Bayer, Boehringer Ingelheim, Edwards, Eli Lilly, Menarini, Novartis, Servier, Vifor, Abbott, Actimed, Cardiac Dimensions, Corvia, CVRx, Enopace, ESN Cleer, Faraday, Impulse Dynamics, Respicardia, and Viatris. Richard Skipworth has received grant funding from Novartis, has been an advisory board member for Helsinn and Faraday Pharamaceuticals, and has provided consultancy work for Helsinn, Actimed Therapeutics, and Avidity Biosciences. Marianne J. Hjermstad, Gunnhild Jakobsen, Jann Arends, Trude R. Balstad, Leo R. Brown, Asta Bye, Olav F. Dajani, Ross D. Dolan, Marie T. Fallon, Christine Greil, Alexandra Grzyb, Stein Kaasa, Lisa Koteng, Anne M. May, James McDonald, Inger Ottestad, Iain Philips, Judith Sayers, Melanie R. Simpson, Tora S. Solheim, Mariana S. Sousa, Lisa H Koteng, and Ola M. Vagnildhaug declare that they have no conflict of interest.
Supporting information
Data S1. Supporting Information.
Data S2. Supporting Information.
Data S3. Supporting Information.
Data S4. Supporting Information.
Hjermstad M. J., Jakobsen G., Arends J., Balstad T., Brown L. R., Bye A., et al (2024) Quality of life endpoints in cancer cachexia clinical trials: Systematic review 3 of the cachexia endpoints series, Journal of Cachexia, Sarcopenia and Muscle, doi: 10.1002/jcsm.13453.
References
- 1. Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 2011;12:489–495. [DOI] [PubMed] [Google Scholar]
- 2. Baracos VE. Cancer‐associated malnutrition. Eur J Clin Nutr 2018;72:1255–1259. [DOI] [PubMed] [Google Scholar]
- 3. Arends J, Strasser F, Gonella S, Solheim TS, Madeddu C, Ravasco P, et al. Cancer cachexia in adult patients: ESMO Clinical Practice Guidelines(☆). ESMO open 2021;6:100092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wheelwright SJ, Darlington AS, Hopkinson JB, Fitzsimmons D, White A, Johnson CD. A systematic review to establish health‐related quality‐of‐life domains for intervention targets in cancer cachexia. BMJ Support Palliat Care 2016;6:307–314. [DOI] [PubMed] [Google Scholar]
- 5. Karimi M, Brazier J. Health, health‐related quality of life, and quality of life: what is the difference? Pharmacoeconomics 2016;34:645–649. [DOI] [PubMed] [Google Scholar]
- 6. Ferrans CE, Zerwic JJ, Wilbur JE, Larson JL. Conceptual model of health‐related quality of life. J Nurs Scholarsh 2005;37:336–342. [DOI] [PubMed] [Google Scholar]
- 7. FDA . Guidance for industry. Patient‐reported outcome measures: use in medical product development to support labeling claims. 2009. [DOI] [PMC free article] [PubMed]
- 8. Patrick DL, Burke LB, Gwaltney CJ, Leidy NK, Martin ML, Molsen E, et al. Content validity—establishing and reporting the evidence in newly developed patient‐reported outcomes (PRO) instruments for medical product evaluation: ISPOR PRO good research practices task force report: Part 2—assessing respondent understanding. Value Health 2011;14:978–988. [DOI] [PubMed] [Google Scholar]
- 9. Bottomley A, Reijneveld JC, Koller M, Flechtner H, Tomaszewski KA, Greimel E, et al. Current state of quality of life and patient‐reported outcomes research. Eur J Cancer 2019;121:55–63. [DOI] [PubMed] [Google Scholar]
- 10. Baker A, Institute of Medicine (IOM) . Crossing the quality chasm: a new health system for the 21st century. 2001;323.(7322):1192.
- 11. Ferrell BR, Temel JS, Temin S, Alesi ER, Balboni TA, Basch EM, et al. Integration of palliative care into standard oncology care: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol 2017;35:96–112. [DOI] [PubMed] [Google Scholar]
- 12. Basch E, Deal AM, Dueck AC, Scher HI, Kris MG, Hudis C, et al. Overall survival results of a trial assessing patient‐reported outcomes for symptom monitoring during routine cancer treatment. JAMA 2017;318:197–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ediebah DE, Quinten C, Coens C, Ringash J, Dancey J, Zikos E, et al. Quality of life as a prognostic indicator of survival: a pooled analysis of individual patient data from Canadian Cancer Trials Group clinical trials. Cancer 2018;124:3409–3416. [DOI] [PubMed] [Google Scholar]
- 14. Bruera E, Kuehn N, Miller MJ, Selmser P, Macmillan K. The Edmonton Symptom Assessment System (ESAS): a simple method for the assessment of palliative care patients. J Palliat Care 1991;7:6–9. [PubMed] [Google Scholar]
- 15. Cleeland CS, Mendoza TR, Wang XS, Chou C, Harle MT, Morrissey M, et al. Assessing symptom distress in cancer patients: the M.D. Anderson Symptom Inventory. Cancer 2000;89:1634–1646. [DOI] [PubMed] [Google Scholar]
- 16. Spitzer WO, Dobson AJ, Hall J, Chesterman E, Levi J, Shepherd R, et al. Measuring the quality of life of cancer patients: a concise QL‐index for use by physicians. J Chronic Dis 1981;34:585–597. [DOI] [PubMed] [Google Scholar]
- 17. Priestman TJ, Baum M. Evaluation of quality of life in patients receiving treatment for advanced breast cancer. Lancet 1976;1:899–900. [DOI] [PubMed] [Google Scholar]
- 18. Ware JE Jr, Sherbourne CD. The MOS 36‐item short‐form health survey (SF‐36). I. Conceptual framework and item selection. Med Care 1992;30:473–483. [PubMed] [Google Scholar]
- 19. EuroQol G. EuroQol ‐ a new facility for the measurement of health‐related quality of life. Health Policy 1990;16:199–208. [DOI] [PubMed] [Google Scholar]
- 20. Cella DF, Tulsky DS, Gray G, Sarafian B, Linn E, Bonomi A, et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol 1993;11:570–579. [DOI] [PubMed] [Google Scholar]
- 21. Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ‐C30: a quality‐of‐life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993;85:365–376. [DOI] [PubMed] [Google Scholar]
- 22. Groenvold M, Petersen MA, Aaronson NK, Arraras JI, Blazeby JM, Bottomley A, et al. EORTC QLQ‐C15‐PAL: the new standard in the assessment of health‐related quality of life in advanced cancer? Palliat Med 2006;20:59–61. [DOI] [PubMed] [Google Scholar]
- 23. de Haes JC, van Knippenberg FC, Neijt JP. Measuring psychological and physical distress in cancer patients: structure and application of the Rotterdam Symptom Checklist. Br J Cancer 1990;62:1034–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kurihara M, Shimizu H, Tsuboi K, Kobayashi K, Murakami M, Eguchi K, et al. Development of quality of life questionnaire in Japan: quality of life assessment of cancer patients receiving chemotherapy. Psychooncology 1999;8:355–363. [DOI] [PubMed] [Google Scholar]
- 25. Bjordal K, Ahlner‐Elmqvist M, Tollesson E, Jensen AB, Razavi D, Maher EJ, et al. Development of a European Organization for Research and Treatment of Cancer (EORTC) questionnaire module to be used in quality of life assessments in head and neck cancer patients. EORTC Quality of Life Study Group. Acta Oncol 1994;33:879–885. [DOI] [PubMed] [Google Scholar]
- 26. FACIT . FACIT GROUP. Available from: https://www.facit.org/. Accessed Oct, 20, 2023.
- 27. Yellen SB, Cella DF, Webster K, Blendowski C, Kaplan E. Measuring fatigue and other anemia‐related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. J Pain Symptom Manage 1997;13:63–74. [DOI] [PubMed] [Google Scholar]
- 28. Cella D, Rosenbloom SK, Beaumont JL, Yount SE, Paul D, Hampton D, et al. Development and validation of 11 symptom indexes to evaluate response to chemotherapy for advanced cancer. J Natl Compr Canc Netw 2011;9:268–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ribaudo JM, Cella D, Hahn EA, Lloyd SR, Tchekmedyian NS, Von Roenn J, et al. Re‐validation and shortening of the Functional Assessment of Anorexia/Cachexia Therapy (FAACT) questionnaire. Qual Life Res 2000;9:1137–1146. [DOI] [PubMed] [Google Scholar]
- 30. Wheelwright SH, Hopkinson JB, Darlington AS, Fitzsimmons DF, Fayers P, Balstad TR, et al. Development of the EORTC QLQ‐CAX24, A Questionnaire for Cancer Patients With Cachexia. J Pain Symptom Manage 2017;53:232–242. [DOI] [PubMed] [Google Scholar]
- 31. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. PLoS Med 2021;18:e1003583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. University of York. PROSPERO – International prospective register of systematic reviews. York, UK: University of York; Available from: http://www.crd.york.ac.uk/PROSPERO/
- 33. Karnofsky D, Abelmann W, Craver L, Burchenal J. The use of nitrogen mustard in the palliative treatment of cancer. Cancer 1948;1:634–656. [Google Scholar]
- 34. Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982;5:649–655. [PubMed] [Google Scholar]
- 35. Covidence . Covidence largest systematic review community ‐ Covidence Australia. Available from: http://www.covidence.org. Accessed Oct, 20, 2023.
- 36. Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non‐randomised studies of health care interventions. J Epidemiol Community Health 1998;52:377–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Agteresch HJ, Leij‐Halfwerk S, Van Den Berg JW, Hordijk‐Luijk CH, Wilson JH, Dagnelie PC. Effects of ATP infusion on glucose turnover and gluconeogenesis in patients with advanced non‐small‐cell lung cancer. Clin Sci 2000;98:689–695. [PubMed] [Google Scholar]
- 38. Bauer J, Capra S, Battistutta D, Davidson W, Ash S, Cancer Cachexia Study Group . Compliance with nutrition prescription improves outcomes in patients with unresectable pancreatic cancer. Clin Nutr 2005;24:998–1004. [DOI] [PubMed] [Google Scholar]
- 39. Beller E, Tattersall M, Lumley T, Levi J, Dalley D, Olver I, et al. Improved quality of life with megestrol acetate in patients with endocrine‐insensitive advanced cancer: a randomised placebo‐controlled trial. Australasian Megestrol Acetate Cooperative Study Group. Ann Oncol 1997;8:277–283. [DOI] [PubMed] [Google Scholar]
- 40. Bouleuc C, Anota A, Cornet C, Grodard G, Thiery‐Vuillemin A, Dubroeucq O, et al. Impact on health‐related quality of life of parenteral nutrition for patients with advanced cancer cachexia: results from a randomized controlled trial. Oncologist 2020;25:e843–e851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Britton B, Baker AL, Wolfenden L, Wratten C, Bauer J, Beck AK, et al. Eating as treatment (EAT): a stepped‐wedge, randomized controlled trial of a health behavior change intervention provided by dietitians to improve nutrition in patients with head and neck cancer undergoing radiation therapy (TROG 12.03). Int J Radiat Oncol Biol Phys 2019;103:353–362. [DOI] [PubMed] [Google Scholar]
- 42. Bumrungpert A, Pavadhgul P, Nunthanawanich P, Sirikanchanarod A, Adulbhan A. Whey protein supplementation improves nutritional status, glutathione levels, and immune function in cancer patients: a randomized, double‐blind controlled trial. J Med Food 2018;21:612–616. [DOI] [PubMed] [Google Scholar]
- 43. Capozzi LC, McNeely ML, Lau HY, Reimer RA, Giese‐Davis J, Fung TS, et al. Patient‐reported outcomes, body composition, and nutrition status in patients with head and neck cancer: Results from an exploratory randomized controlled exercise trial. Cancer 2016;122:1185–1200. [DOI] [PubMed] [Google Scholar]
- 44. Cereda E, Turri A, Klersy C, Cappello S, Ferrari A, Filippi AR, et al. Whey protein isolate supplementation improves body composition, muscle strength, and treatment tolerance in malnourished advanced cancer patients undergoing chemotherapy. Cancer Med 2019;8:6923–6932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chen L, Zhao M, Tan L, Zhang Y. Effects of five‐step nutritional interventions conducted by a multidisciplinary care team on gastroenteric cancer patients undergoing chemotherapy: a randomized clinical trial. Nutr Cancer 2023;75:197–206. [DOI] [PubMed] [Google Scholar]
- 46. Currow DC, Glare P, Louw S, Martin P, Clark K, Fazekas B, et al. A randomised, double blind, placebo‐controlled trial of megestrol acetate or dexamethasone in treating symptomatic anorexia in people with advanced cancer. Sci Rep 2021;11:2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Dehghani M, Mirzaie M, Farhadi P, Rezvani A. The effect of ACE inhibitor on the quality of life amongst patients with cancer cachexia. Asian Pac J Cancer Prev 2020;21:325–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Del Fabbro E, Dev R, Hui D, Palmer L, Bruera E. Effects of melatonin on appetite and other symptoms in patients with advanced cancer and cachexia: a double‐blind placebo‐controlled trial. J Clin Oncol 2013;31:1271–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Famil‐Dardashti A, Hajigholami A, Badri S, Yekdaneh A, Moghaddas A. The role of trigonella, cichorium, and foeniculum herbal combination in the treatment of cancer‐induced anorexia/cachexia: a quasi‐experimental study. Int J Cancer Manag 2020;13. [Google Scholar]
- 50. Fearon KC, Von Meyenfeldt MF, Moses AG, Van Geenen R, Roy A, Gouma DJ, et al. Effect of a protein and energy dense N‐3 fatty acid enriched oral supplement on loss of weight and lean tissue in cancer cachexia: a randomised double blind trial. Gut 2003;52:1479–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gavazzi C, Colatruglio S, Valoriani F, Mazzaferro V, Sabbatini A, Biffi R, et al. Impact of home enteral nutrition in malnourished patients with upper gastrointestinal cancer: a multicentre randomised clinical trial. Eur J Cancer 2016;64:107–112. [DOI] [PubMed] [Google Scholar]
- 52. Hong Y, Wu C, Wu B. Effects of resistance exercise on symptoms, physical function, and quality of life in gastrointestinal cancer patients undergoing chemotherapy. Integr Cancer Ther 2020;19:1534735420954912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hunter CN, Abdel‐Aal HH, Elsherief WA, Farag DE, Riad NM, Alsirafy SA. Mirtazapine in cancer‐associated anorexia and cachexia: a double‐blind placebo‐controlled randomized trial. J Pain Symptom Manage 2021;62:1207–1215. [DOI] [PubMed] [Google Scholar]
- 54. Isenring EA, Capra S, Bauer JD. Nutrition intervention is beneficial in oncology outpatients receiving radiotherapy to the gastrointestinal or head and neck area. Br J Cancer 2004;91:447–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Izumi K, Iwamoto H, Yaegashi H, Nohara T, Shigehara K, Kadono Y, et al. Androgen replacement therapy for cancer‐related symptoms in male: result of prospective randomized trial (ARTFORM study). J Cachexia Sarcopenia Muscle 2021;12:831–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Jatoi A, Windschitl HE, Loprinzi CL, Sloan JA, Dakhil SR, Mailliard JA, et al. Dronabinol versus megestrol acetate versus combination therapy for cancer‐associated anorexia: a North Central Cancer Treatment Group study. J Clin Oncol 2002;20:567–573. [DOI] [PubMed] [Google Scholar]
- 57. Jatoi A, Ritter HL, Dueck A, Nguyen PL, Nikcevich DA, Luyun RF, et al. A placebo‐controlled, double‐blind trial of infliximab for cancer‐associated weight loss in elderly and/or poor performance non‐small cell lung cancer patients (N01C9). Lung Cancer 2010;68:234–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Jatoi A, Steen PD, Atherton PJ, Moore DF, Rowland KM, Le‐Lindqwister NA, et al. A double‐blind, placebo‐controlled randomized trial of creatine for the cancer anorexia/weight loss syndrome (N02C4): an alliance trial. Ann Oncol 2017;28:1957–1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kanat O, Cubukcu E, Avci N, Budak F, Ercan I, Canhoroz M, et al. Comparison of three different treatment modalities in the management of cancer cachexia. Tumori 2013;99:229–233. [DOI] [PubMed] [Google Scholar]
- 60. Katakami N, Uchino J, Yokoyama T, Naito T, Kondo M, Yamada K, et al. Anamorelin (ONO‐7643) for the treatment of patients with non‐small cell lung cancer and cachexia: results from a randomized, double‐blind, placebo‐controlled, multicenter study of Japanese patients (ONO‐7643‐04). Cancer 2018;124:606–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kouchaki B, Janbabai G, Alipour A, Ala S, Borhani S, Salehifar E. Randomized double‐blind clinical trial of combined treatment with megestrol acetate plus celecoxib versus megestrol acetate alone in cachexia‐anorexia syndrome induced by GI cancers. Support Care Cancer 2018;26:2479–2489. [DOI] [PubMed] [Google Scholar]
- 62. Maccio A, Madeddu C, Gramignano G, Mulas C, Floris C, Sanna E, et al. A randomized phase III clinical trial of a combined treatment for cachexia in patients with gynecological cancers: evaluating the impact on metabolic and inflammatory profiles and quality of life. Gynecol Oncol 2012;124:417–425. [DOI] [PubMed] [Google Scholar]
- 63. Mantovani G, Maccio A, Madeddu C, Serpe R, Massa E, Dessi M, et al. Randomized phase III clinical trial of five different arms of treatment in 332 patients with cancer cachexia. Oncologist 2010;15:200–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. McMillan DC, Wigmore SJ, Fearon KC, O'Gorman P, Wright CE, McArdle CS. A prospective randomized study of megestrol acetate and ibuprofen in gastrointestinal cancer patients with weight loss. Br J Cancer 1999;79:495–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Mehrzad V, Afshar R, Akbari M. Pentoxifylline treatment in patients with cancer cachexia: a double‐blind, randomized, placebo‐controlled clinical trial. Adv Biomed Res 2016;5:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Meng Q, Tan S, Jiang Y, Han J, Xi Q, Zhuang Q, et al. Post‐discharge oral nutritional supplements with dietary advice in patients at nutritional risk after surgery for gastric cancer: a randomized clinical trial. Clin Nutr 2021;40:40–46. [DOI] [PubMed] [Google Scholar]
- 67. Navari RM, Brenner MC. Treatment of cancer‐related anorexia with olanzapine and megestrol acetate: a randomized trial. Support Care Cancer 2010;18:951–956. [DOI] [PubMed] [Google Scholar]
- 68. Obling SR, Wilson BV, Pfeiffer P, Kjeldsen J. Home parenteral nutrition increases fat free mass in patients with incurable gastrointestinal cancer. Results of a randomized controlled trial. Clin Nutr 2019;38:182–190. [DOI] [PubMed] [Google Scholar]
- 69. Persson CR, Johansson BB, Sjoden PO, Glimelius BL. A randomized study of nutritional support in patients with colorectal and gastric cancer. Nutr Cancer 2002;42:48–58. [DOI] [PubMed] [Google Scholar]
- 70. Poulsen GM, Pedersen LL, Osterlind K, Baeksgaard L, Andersen JR. Randomized trial of the effects of individual nutritional counseling in cancer patients. Clin Nutr 2014;33:749–753. [DOI] [PubMed] [Google Scholar]
- 71. Qiu Y, You J, Wang K, Cao Y, Hu Y, Zhang H, et al. Effect of whole‐course nutrition management on patients with esophageal cancer undergoing concurrent chemoradiotherapy: a randomized control trial. Nutrition 2020;69:110558. [DOI] [PubMed] [Google Scholar]
- 72. Ravasco P, Monteiro‐Grillo I, Marques Vidal P, Camilo ME. Impact of nutrition on outcome: a prospective randomized controlled trial in patients with head and neck cancer undergoing radiotherapy. Head Neck 2005;27:659–668. [DOI] [PubMed] [Google Scholar]
- 73. Rowland KM Jr, Loprinzi CL, Shaw EG, Maksymiuk AW, Kuross SA, Jung S‐H, et al. Randomized double‐blind placebo‐controlled trial of cisplatin and etoposide plus megestrol acetate/placebo in extensive‐stage small‐cell lung cancer: a North Central Cancer Treatment Group study. J Clin Oncol 1996;14:135–141. [DOI] [PubMed] [Google Scholar]
- 74. Silander E, Nyman J, Bove M, Johansson L, Larsson S, Hammerlid E. Impact of prophylactic percutaneous endoscopic gastrostomy on malnutrition and quality of life in patients with head and neck cancer: a randomized study. Head Neck 2012;34:1–9. [DOI] [PubMed] [Google Scholar]
- 75. Sim E, Kim JM, Lee SM, Chung MJ, Song SY, Kim ES, et al. The effect of omega‐3 enriched oral nutrition supplement on nutritional indices and quality of life in gastrointestinal cancer patients: a randomized clinical trial. Asian Pac J Cancer Prev 2022;23:485–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Simons JP, Schols AM, Hoefnagels JM, Westerterp KR, ten Velde GP, Wouters EF. Effects of medroxyprogesterone acetate on food intake, body composition, and resting energy expenditure in patients with advanced, nonhormone‐sensitive cancer: a randomized, placebo‐controlled trial. Cancer 1998;82:553–560. [PubMed] [Google Scholar]
- 77. Storck LJ, Ruehlin M, Gaeumann S, Gisi D, Schmocker M, Meffert PJ, et al. Effect of a leucine‐rich supplement in combination with nutrition and physical exercise in advanced cancer patients: a randomized controlled intervention trial. Clin Nutr 2020;39:3637–3644. [DOI] [PubMed] [Google Scholar]
- 78. Strasser F, Luftner D, Possinger K, Ernst G, Ruhstaller T, Meissner W, et al. Comparison of orally administered cannabis extract and delta‐9‐tetrahydrocannabinol in treating patients with cancer‐related anorexia‐cachexia syndrome: a multicenter, phase III, randomized, double‐blind, placebo‐controlled clinical trial from the Cannabis‐In‐Cachexia‐Study‐Group. J Clin Oncol 2006;24:3394–3400. [DOI] [PubMed] [Google Scholar]
- 79. Takayama K, Katakami N, Yokoyama T, Atagi S, Yoshimori K, Kagamu H, et al. Anamorelin (ONO‐7643) in Japanese patients with non‐small cell lung cancer and cachexia: results of a randomized phase 2 trial. Support Care Cancer 2016;24:3495–3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Uster A, Ruehlin M, Mey S, Gisi D, Knols R, Imoberdorf R, et al. Effects of nutrition and physical exercise intervention in palliative cancer patients: A randomized controlled trial. Clin Nutr 2018;37:1202–1209. [DOI] [PubMed] [Google Scholar]
- 81. van der Werf A, Langius JAE, Beeker A, Ten Tije AJ, Vulink AJ, Haringhuizen A, et al. The effect of nutritional counseling on muscle mass and treatment outcome in patients with metastatic colorectal cancer undergoing chemotherapy: a randomized controlled trial. Clin Nutr 2020;39:3005–3013. [DOI] [PubMed] [Google Scholar]
- 82. Wen HS, Li X, Cao YZ, Zhang CC, Yang F, Shi YM, et al. Clinical studies on the treatment of cancer cachexia with megestrol acetate plus thalidomide. Chemotherapy 2012;58:461–467. [DOI] [PubMed] [Google Scholar]
- 83. Westman G, Bergman B, Albertsson M, Kadar L, Gustavsson G, Thaning L, et al. Megestrol acetate in advanced, progressive, hormone‐insensitive cancer. Effects on the quality of life: a placebo‐controlled, randomised, multicentre trial. Eur J Cancer 1999;35:586–595. [DOI] [PubMed] [Google Scholar]
- 84. Wiedenmann B, Malfertheiner P, Friess H, Ritch P, Arseneau J, Mantovani G, et al. A multicenter, phase II study of infliximab plus gemcitabine in pancreatic cancer cachexia. J Support Oncol 2008;6:18–25. [PubMed] [Google Scholar]
- 85. Woo SM, Joo J, Kim SY, Park SJ, Han SS, Kim TH, et al. Efficacy of pancreatic exocrine replacement therapy for patients with unresectable pancreatic cancer in a randomized trial. Pancreatology 2016;16:1099–1105. [DOI] [PubMed] [Google Scholar]
- 86. Xie M, Chen X, Qin S, Bao Y, Bu K, Lu Y. Clinical study on thalidomide combined with cinobufagin to treat lung cancer cachexia. J Cancer Res Ther 2018;14:226–232. [DOI] [PubMed] [Google Scholar]
- 87. Osoba D, Rodrigues G, Myles J, Zee B, Pater J. Interpreting the significance of changes in health‐related quality‐of‐life scores. J Clin Oncol 1998;16:139–144. [DOI] [PubMed] [Google Scholar]
- 88. Cocks K, King MT, Velikova G, de Castro G, Martyn St‐James M, Fayers PM, et al. Evidence‐based guidelines for interpreting change scores for the European Organisation for the Research and Treatment of Cancer Quality of Life Questionnaire Core 30. Eur J Cancer 2012;48:1713–1721. [DOI] [PubMed] [Google Scholar]
- 89. Pilz MJ, Aaronson NK, Arraras JI, Caocci G, Efficace F, Groenvold M, et al. Evaluating the thresholds for clinical importance of the EORTC QLQ‐C15‐PAL in patients receiving palliative treatment. J Palliat Med 2021;24:397–404. [DOI] [PubMed] [Google Scholar]
- 90. Coens C, Pe M, Dueck AC, Sloan J, Basch E, Calvert M, et al. International standards for the analysis of quality‐of‐life and patient‐reported outcome endpoints in cancer randomised controlled trials: recommendations of the SISAQOL Consortium. Lancet Oncol 2020;21:e83–e96. [DOI] [PubMed] [Google Scholar]
- 91. Arends J, Bachmann P, Baracos V, Barthelemy N, Bertz H, Bozzetti F, et al. ESPEN guidelines on nutrition in cancer patients. Clin Nutr 2017;36:11–48. [DOI] [PubMed] [Google Scholar]
- 92. Tarricone R, Abu Koush D, Nyanzi‐Wakholi B, Medina‐Lara A. A systematic literature review of the economic implications of chemotherapy‐induced diarrhea and its impact on quality of life. Crit Rev Oncol Hematol 2016;99:37–48. [DOI] [PubMed] [Google Scholar]
- 93. Cederholm T, Jensen GL, Correia M, Gonzalez MC, Fukushima R, Higashiguchi T, et al. GLIM criteria for the diagnosis of malnutrition ‐ a consensus report from the global clinical nutrition community. J Cachexia Sarcopenia Muscle 2019;10:207–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supporting Information.
Data S2. Supporting Information.
Data S3. Supporting Information.
Data S4. Supporting Information.


