Abstract
Vaccine vectors derived from Venezuelan equine encephalitis virus (VEE) that expressed simian immunodeficiency virus (SIV) immunogens were tested in rhesus macaques as part of the effort to design a safe and effective vaccine for human immunodeficiency virus. Immunization with VEE replicon particles induced both humoral and cellular immune responses. Four of four vaccinated animals were protected against disease for at least 16 months following intravenous challenge with a pathogenic SIV swarm, while two of four controls required euthanasia at 10 and 11 weeks. Vaccination reduced the mean peak viral load 100-fold. The plasma viral load was reduced to below the limit of detection (1,500 genome copies/ml) in one vaccinated animal between 6 and 16 weeks postchallenge and in another from week 6 through the last sampling time (40 weeks postchallenge). The extent of reduction in challenge virus replication was directly correlated with the strength of the immune response induced by the vectors, which suggests that vaccination was effective.
The solution to the worldwide human immunodeficiency virus (HIV) epidemic will include an affordable, safe, and effective vaccine. However, safety concerns surround the development of live, attenuated, or whole killed vaccines (3, 13, 44), and recombinant protein vaccination has been only moderately immunogenic in humans (14). One strategy for balancing safety and immunogenicity is the use of virus vectors, viruses whose genomes have been engineered to express heterologous proteins. When used for vaccination, a virus vector targets specific cells in the host for in vivo production of immunogens. In the best case, this results in induction of protective immunity against the pathogen whose genes were inserted into the vector. The most widely studied virus vectors are recombinant poxviruses, and the HIV-specific immune response generated in human subjects by highly attenuated poxvirus vectors is currently under investigation (12, 17, 41). The addition of purified recombinant protein boosts strengthened the immune response, both in monkey trials and in human trials of candidate AIDS vaccines (12, 17, 27, 28, 41). Extensive experimentation in animal models with these vector systems, and several others, is proceeding with the goal of developing a compelling strategy for an effective HIV vaccine (1, 2).
Expression vectors also have been derived from three alphaviruses: Sindbis virus, Semliki Forest virus (SFV), and Venezuelan equine encephalitis virus (VEE) (16, 33, 47). Two general types of alphavirus vectors have been shown to induce immune responses to heterologous proteins in rodent models. These are (i) propagation-competent double-promoter vectors that express a foreign gene from a second viral subgenomic RNA promoter (16, 21) and (ii) single-hit nonpropagating replicon vectors that contain a foreign gene in place of the viral structural protein genes (22, 42, 43, 48, 49). The advantages of replicon vectors include increased capacity for foreign sequence, lack of reactogenicity, and reduction of anti-vector immune responses.
Replicon vectors derived from VEE (43) have properties that may be advantageous for an HIV vaccine. First, VEE replicon particles (VRP) target expression to lymphoid tissues, a preferred site for induction of immunity. The specific cell types infected in the lymph node draining the site of subcutaneous (s.c.) inoculation of mice vary with mutations in the glycoprotein genes. In the context of the parental glycoproteins, or of glycoprotein mutants at higher doses, a major target appears to be dendritic cells (35a). Targeting of VRP to a professional antigen-presenting cell (4) may be of vital importance for HIV proteins with low intrinsic immunogenicity. Second, s.c. inoculation of mice with VRP expressing the hemagglutinin (HA) protein of influenza virus (HA-VRP) gives complete protection against a lethal intranasal challenge with influenza virus (43). The immunity induced is sufficient to block challenge virus replication at the level of the mucosal target, the respiratory epithelium (N. L. Davis, K. W. Brown, and R. E. Johnston, unpublished results). The ability to protect against a mucosal challenge following s.c. immunization is characteristic of both the live, attenuated VEE vaccine (TC-83) that has been administered to thousands of people for protection against VEE infection and more recent VEE vaccine candidates (9, 15, 29, 31). Because protection of mucosal surfaces from sexually transmitted HIV will be required of an effective vaccine, the potential to protect mucosal surfaces is a key feature of VEE vaccine vectors. Third, VEE vectors produce high levels of authentic foreign proteins, including nonglycosylated and glycosylated proteins, without a requirement for a translational enhancer downstream of the AUG start codon as in other alphavirus vectors (18). The amount of protein produced by VEE vectors is comparable to that of baculovirus (35) and vaccinia virus vectors (36).
Redundant safety features have been engineered into the VEE replicon vectors. Replicon RNA is packaged into VRP by structural proteins provided in trans from two distinct helper RNAs in baby hamster kidney (BHK) cells cotransfected with all three RNAs (43). The use of two helper RNAs instead of one reduces the generation of viable virus by at least a factor of 105. The insertion of two independently attenuating mutations in the glycoprotein genes ensures that even a rare recombinant would be a nonpathogenic vaccine strain. More than 1,000 rodents, a very sensitive host for VEE, and 64 macaques have been given primary doses as high as 108 IU of VRP with no detectable clinical signs. Since the macaque model reflects human experience with VEE and VEE vaccines, this record suggests that trials of VRP vaccines for HIV in humans would demonstrate them to be safe (38) (J. F. Smith, N. L. Davis, and R. E. Johnston, unpublished results).
In primates, the efficacy of VRP vaccination has been demonstrated dramatically with VRP expressing the glycoprotein (GP) of Marburg virus, a filovirus closely related to Ebola virus. GP-VRP-vaccinated cynomolgus macaques were completely protected against a lethal challenge with Marburg virus and had no detectable viremia, while controls died within 10 days, with serum titers of 107 to 108 PFU of the challenge virus (22). Primate trials also have been performed with replicon vectors derived from SFV with a lentivirus challenge. In the first test, pigtailed macaques were vaccinated with SFV replicon particles expressing gp160 from the acutely fatal simian immunodeficiency virus (SIV) sooty mangabey (sm) strain PBj14 and challenged with a 75% fatal dose of SIVsm PBj14 virus (40). Vaccinated animals were protected against fatal acute disease. In a second study, cynomolgus macaques were vaccinated with SFV replicon particles expressing gp160 from the IIIB strain of HIV type 1 (HIV-1IIIB) and challenged with a 100% infectious dose of simian-human immunodeficiency virus 4 (6). Vaccinated animals were not protected against infection but showed some reduction in viral load.
In contrast to previous studies with SFV-derived replicon vaccines, the first test of VEE-based antilentivirus vaccines in primates, reported here, included challenge with a highly pathogenic strain of SIV that consistently induces lethal immunodeficiency in rhesus macaques. Macaques received VRP expressing proteins encoded in the SIVsm H-4i molecular clone, which is closely related to the pathogenic uncloned SIVsm E660 (19, 23, 26). Humoral and cellular immune responses to vaccination were measured, and animals were challenged intravenously (i.v.) with SIVsm E660 (grown in macaque peripheral blood mononuclear cells [PBMCs]). This pathogenic challenge virus contains a more genetically diverse population than virus derived from the SIVsm H-4i molecular clone and was therefore a more rigorous challenge than the homologous SIVsm H-4i.
The infection of rhesus macaques with SIV is currently one of the best animal models for HIV infection of humans. Although utilization of virus vectors to protect against infection with highly pathogenic strains of SIV has proven difficult, there are encouraging data to suggest that protection against disease may be attainable (1, 24). Our goal was to correlate the relative strength of the immune response in vaccinated animals with the level of protection against a pathogenic high-dose SIV challenge. The effectiveness of SIV-VRP vaccination was evaluated in terms of mortality, peak plasma viremia, and ability to control virus replication (i.e., plasma viremia set point).
MATERIALS AND METHODS
Cells and plasmids.
BHK cells were obtained from the American Type Culture Collection in passage 52 or 53 and were used between passages 54 and 64. Cells were maintained in alpha minimal essential medium containing 10% donor calf serum, 10% tryptose phosphate broth, and 0.29 mg of l-glutamine per ml. For electroporation, cells were cultured overnight in medium containing 10% fetal calf serum, harvested when subconfluent, and prepared for electroporation as previously described (33).
SIV genes were inserted into the VEE replicon plasmid pVR2 (43) as follows. PCRs with the SIVsm H-4i plasmid as the template and appropriate primers were used to amplify (i) the region of gag encoding matrix-capsid (MA/CA; nucleotides 1049 to 2143, numbering from the 5′ end of the SIVsm H-4i genome) with a change in codon 2 from Gly to Ala to ablate the myristylation signal, (ii) the entire env open reading frame (gp160; nucleotides 6587 to 9244), and (iii) env lacking the 3′ region encoding the membrane-spanning domain and the cytoplasmic tail (gp140; nucleotides 6587 to 8626). Amplified regions were initially cloned into PCR cloning plasmids, and products were confirmed by sequencing. Flanking ClaI restriction sites contained in the primers were used to subclone gag sequences directly into pVR2. Flanking SalI restriction sites contained in the primers were used for insertion of env sequences into a shuttle plasmid containing a copy of the VEE subgenomic 26S mRNA promoter followed by a multiple cloning site and the VEE 3′ untranslated region. The resulting 26S transcription unit was moved into pVR2 by using unique restriction sites and standard techniques.
These derivatives of pVR2 containing specific SIVsm H-4i sequences were used for in vitro transcription of VEE replicon RNA. Plasmids pV3014Δ520-7505Δ8495-11229 and pV3014Δ520-7505Δ7565-8386 were transcribed in vitro to give capsid helper RNA and glycoprotein helper RNA, respectively (43). Two independently attenuating mutations are contained in the genes expressed from the glycoprotein helper: a change from Glu to Lys at E2 position 209 and a change from Ala to Thr at E1 position 272 (20).
Production and titration of VRP.
BHK cells electroporated with a mixture of replicon RNA, capsid helper RNA, and glycoprotein helper RNA, transcribed and capped in vitro, were diluted into growth medium in a 75-cm tissue culture flask and incubated at 37°C under 5% CO2 for 24 to 27 h. VRP-containing culture supernatants were clarified by centrifugation at 12,000 × g for 30 min. VRP were partially purified and concentrated by sedimentation at 72,000 × g for 4 h through a 5-ml cushion of 20% (wt/vol) sucrose dissolved in 0.0017 M KH2PO4–0.005 M Na2PO4–0.15 M NaCl (pH 7.4) (phosphate-buffered saline [PBS]), followed by overnight resuspension in PBS at 4°C. For titration of VRP, BHK cells were infected with serial dilutions of concentrated VRP for 16 h at 37°C, fixed in methanol for 10 min at 4°C, and incubated sequentially with SIV-infected macaque serum, biotinylated anti-human immunoglobulin G (IgG), and avidin conjugated to fluorescein isothiocyanate (FITC). Single replicon-infected cells were scored microscopically by fluorescence under UV illumination.
Radioimmunoprecipitation and polyacrylamide gel electrophoresis.
BHK cells that had been either infected with VRP or mock infected were incubated in methionine-free medium for 4 h between 6 and 10 h postinfection and then in medium containing 20 μCi of [35S]methionine/ml for 2 h between 10 and 12 h postinfection. At 12 h postinfection cytoplasmic extracts were prepared in the presence of protease inhibitors and then were immunoprecipitated with either SIV-infected monkey serum or normal monkey serum by using protein A-Sepharose CL-4B (Sigma) (30). Proteins were resolved by electrophoresis in 10% polyacrylamide gels containing 0.1% (wt/vol) sodium dodecyl sulfate (SDS) after denaturation in 1% SDS and 50 mM 2-mercaptoethanol were compared to molecular weight standards (14.3 to 220 kDa; Amersham Pharmacia Biotech).
Vaccination protocols.
Four macaques were inoculated s.c. in the inguinal area at week 0 with 105 IU each of SIV gp160-VRP and SIV MA/CA-VRP, boosted by the same route at week 7 with 107 IU of each VRP, and boosted i.v. at weeks 12 and 20 with 5 × 108 IU of each VRP. Two control animals were inoculated with equivalent doses of HA-VRP, and two were inoculated with diluent. The four SIV-VRP-immunized monkeys received an additional dose of 2 × 107 IU of gp140-VRP s.c. in the arm at week 41, followed by a final boost of 2 × 107 IU each of gp140-VRP and MA/CA-VRP s.c. in the arm at week 49. All animal care was in accordance with institutional guidelines.
Quantitation of anti-SIV antibody by ELISA.
The antigen used for enzyme-linked immunosorbent assay (ELISA) was a six-His-tagged version of SIVsm H-4i gp140 secreted from BHK cells infected with six-His-tagged gp140-VRP and purified over a Ni-nitrilotriacetic acid (NTA) matrix (Qiagen). Antigen dissolved in carbonate buffer (15 mM Na2CO3–34.8 mM NaHCO3 [pH 9.6]) was bound to microtiter plates (Immulon-4; Dynatech Laboratories, Inc.) by incubation at 37°C for 1 h. Serum dilutions in duplicate were incubated with the antigen, and bound antibody was quantitated with horseradish peroxidase (HRP)-linked anti-monkey IgG (Cappel) as the secondary antibody. Titers represent the highest serum dilution that gave an optical density at 450 nm of ≥0.2.
Cytotoxic T-lymphocyte (CTL) assay.
PBMCs collected from vaccinated and control macaques were cultured in vitro with autologous herpesvirus papio-transformed B lymphocytes infected with vaccinia virus vectors expressing SIV Gag-Pro-Pol and SIV Env in the presence of interleukin 2 (IL-2) and IL-7 for 7 to 8 days. PBMCs from HA-VRP or diluent controls did not grow when cultured under these conditions of in vitro stimulation. Following positive selection of CD8+ cells by using magnetic beads (Miltenyi Biotec), chromium release assays were performed against autologous B lymphocytes infected with vaccinia virus vectors expressing either SIV Gag-Pro-Pol or SIV Env by published methods (37). Control targets were infected with a wild-type vaccinia virus vector or left uninfected. The percent specific lysis was calculated according to the following formula: (experimental lysis − spontaneous lysis)/(maximal lysis − spontaneous lysis) × 100.
RESULTS
Expression of SIV genes from SIV-VRP vectors.
The region of the gag gene encoding the matrix and capsid polypeptides (MA/CA), the env gene, and a derivative of env (lacking the region encoding its membrane-spanning and cytoplasmic portions) of the molecular clone SIVsm H-4i were individually inserted into VEE replicon plasmids. Individual VRP preparations expressing each of these SIV sequences (MA/CA-VRP, gp160-VRP, and gp140-VRP, respectively) were prepared as previously described (43), and expression in VRP-infected cell cultures was examined (Fig. 1). Proteins of the expected size and antibody reactivity were expressed in all three cases. The size and amount of MA/CA protein was estimated by comparison to molecular weight markers and to a known quantity of purified six-His-tagged SIV MA/CA protein synthesized in Escherichia coli. The apparent molecular size of MA/CA was 42 kDa, and the approximate intracellular level of MA/CA was 60 μg per 107 cells (9 × 107 copies per cell) at 26 h after infection (Fig. 1A). The amount of gp140 was estimated by comparing the Western blot reactivity with SIV-infected monkey serum to that of a known quantity of SIVmac239 rgp130 standard protein produced in vaccinia virus vector-infected Chinese hamster ovary cells (Quality Biological, Inc.). About 0.5 μg of gp140 was secreted per 107 cells during a 12-h infection (Fig. 1C). These amounts are consistent with levels of heterologous protein production in VRP-infected cells reported earlier (43). gp140 was secreted in an oligomeric form, as shown by gradient centrifugation and chemical cross-linking studies (I. J. Caley, K. W. Brown, and R. E. Johnston, unpublished results). gp160 accumulated in much lower amounts, possibly due to toxic effects or turnover of this protein in gp160-VRP-infected cells.
FIG. 1.
Expression of SIV proteins in VRP-infected cultured cells. (A) BHK cells were mock infected (lane 1) or infected with SIV MA/CA-VRP at a multiplicity of infection (MOI, infectious units [IU] per cell) of 5 (lane 2), and 26 h later cytoplasmic extracts were prepared. Proteins resolved by SDS-polyacrylamide gel electrophoresis were visualized by staining with Coomassie brilliant blue (8). Samples each contained 3 × 105 cell equivalents. The MA/CA band (arrowhead) migrated in the region of a coelectrophoresed six-His-tagged SIV MA/CA standard synthesized in E. coli. The intensities of the bands given by 0.825 μg of the standard and by the MA/CA-VRP-infected cell lysate were compared by using densitometry to estimate the amount of MA/CA produced. (B) BHK cells were infected with SIV gp140-VRP at an MOI of 4 (lanes 1 and 2) or with SIV gp160-VRP at an MOI of 4 (lanes 3 and 4) or were mock infected (lanes 5 and 6). [35S]methionine-labeled samples (8 × 106 cell equivalents) were immunoprecipitated with either SIV-infected monkey serum (lanes 1, 3, and 5) or normal monkey serum (lanes 2, 4, and 6). Proteins were visualized by autoradiography, and molecular weight standards were used to identify gp160 and gp140 bands (arrowheads). (C) Culture supernatants (from cells described in the legend to panel B) from the gp140-VRP-infected cells (lanes 1 and 2) or gp160-VRP-infected cells (lanes 3 and 4) were collected, concentrated, immunoprecipitated with either SIV-infected monkey serum (lanes 1 and 3) or uninfected monkey serum (lanes 2 and 4), and subjected to SDS-polyacrylamide gel electrophoresis. Samples each contained supernatant protein secreted from 8 × 106 cells, less any protein lost during the concentration process. The fully glycosylated gp140 band is marked with an arrowhead.
Vaccination of macaques with an SIV-VRP vector cocktail: humoral immune response.
A cocktail of SIV MA/CA-VRP and gp160-VRP was used to vaccinate four juvenile rhesus macaques (Macaca mulatta) in an initial series of four inoculations by two different routes (Fig. 2). Two control animals received equivalent doses of HA-VRP, and two received diluent. The first two inoculations, administered s.c. in the inguinal area, were designed for efficient delivery of the VRP vaccine to the inguinal lymph nodes. The third and fourth inoculations, administered i.v. at a high dose, were designed to direct the VRP to as many lymphoid tissues as possible. That a sufficient amount of VRP had reached these tissues by the third inoculation was indicated by the response to VRP expressing HA, a viral protein that is immunogenic in primates (11). Following the third inoculation, the two HA-VRP control monkeys had anti-influenza serum IgG titers of 16,000 and 8,000 by ELISA and hemagglutination inhibition titers (80 and 160) equivalent to those that are protective in humans (10). It was more difficult to elicit a detectable humoral response to the SIV proteins (Fig. 2). After four inoculations, two of four vaccinated animals showed reactivity to SIV envelope protein by ELISA, and one had detectable levels of neutralizing antibody against virus generated from the SIVsm H-4i molecular clone.
FIG. 2.
Titers of anti-SIV antibody in sera of vaccinated and control macaques. Four macaques were inoculated four times with a cocktail of SIV gp160-VRP and SIV MA/CA-VRP (▵ on the x axis). Control animals were inoculated with equal doses of HA-VRP or diluent. The four SIV-VRP-immunized monkeys received a booster containing gp140-VRP (◊ on the x axis), followed by a final boost of gp140-VRP and MA/CA-VRP (▿ on the x axis). The time of challenge is indicated (▴ on the x axis). (A) Sera collected at the indicated times were tested by ELISA. Vaccinated macaques were VW6 (●), PE9 (■), WON (▾), and K2F (▴). Control macaques were N2P (receiving HA-VRP) (○), N8X (receiving HA-VRP) (▿), N9K (receiving diluent) (□), and W1A (receiving diluent) (▵). (B) Sera were tested for neutralization against SIVsm H-4i in a CEM×174 cell killing (cytopathic effect) assay (39). (C) Postchallenge titers regraphed to show the anamnestic neutralizing antibody responses in vaccinated animals compared to control animals. In each panel, an asterisk on the y axis indicates the limit of detection.
We reasoned that the production of large amounts of the secreted, oligomeric form of the envelope protein within the lymph node might be a more efficient inducer of anti-Env antibody. Therefore, additional immunizations used gp140-VRP. The fifth inoculation was gp140-VRP alone and the sixth was a cocktail of gp140-VRP and MA/CA-VRP, both given by a third route, s.c. in the arm. The choice of a subcutaneous route was based on the recent identification of Langerhans' cells as a primary target of VEE infection (35a). The fifth immunization resulted in a boost in anti-Env IgG (Fig. 2A) and anti-SIVsm H-4i neutralizing titer (Fig. 2B) in three of four vaccinated animals. The final immunization did not have a dramatic effect but did increase the anti-Env ELISA titer of the lowest responder.
It should be noted that no clinical side effects of VRP vaccination were seen in any individual at any time during any of the vaccination regimens.
The antibody responses to VEE, measured following each immunization, were below the level of detection after the first two s.c. inoculations. This was expected, since the mass of particles inoculated was small and the vector does not produce VEE structural proteins. However, VEE-specific antibody began to appear after the first i.v. inoculation. Serum samples taken 3 weeks after the second i.v. inoculation and tested by ELISA against VEE showed a geometric mean titer of 1:17,947. Compared to the first two immunizations, the third and fourth inoculations were given at a higher dose (5 × 108 IU) and by a different route (i.v.), either or both of which may have contributed to the appearance of anti-VEE antibody. However, the presence of this antivector response did not prevent the fifth immunization, consisting of SIV gp140-VRP given s.c. in the arm, from boosting the SIV-specific antibody response (Fig. 2).
In standard assays, none of the sera neutralized the challenge virus, SIVsm E660, an SIV isolate that is less sensitive than SIVsm H-4i to neutralization in vitro (unpublished data). However, anti-SIV E660 neutralizing antibodies were detected in two of the controls and all vaccinated animals following challenge (see below).
Cellular immune response.
Previous experiments with mice demonstrated that VEE-based vectors expressing HIV MA/CA, including the HIV MA/CA-VRP, induced a strong HIV-specific CTL response (K. W. Brown, I. J. Caley, M. R. Betts, J. A. Frelinger, and R. E. Johnston, unpublished results) (8). However, the ability of VEE-based vaccine vectors to induce CTLs in primates was unknown. PBMCs harvested 3 to 4 weeks following the fourth immunization were tested for the presence of SIV Gag- and Env-specific CD8+ CTLs. Two of the four vaccinated monkeys showed strong cellular immune responses to both SIV proteins (Fig. 3A and B), and a third had a low level of SIV-specific CTLs against either Gag or Env (Fig. 3C). CD8+ T lymphocytes isolated from the fourth monkey gave no detectable SIV-specific cellular immune response (Fig. 3D). This was the same animal (K2F) that showed the lowest titer of anti-Env antibody by ELISA and no neutralizing antibody against SIVsm H-4i. Calculation of lytic units (7), which integrated the dose-response curves shown in Fig. 3, also showed that two of the vaccinated animals (VW6 and PE9) had substantial responses to both Gag and Env, while two (WON and K2F) had much lower responses (Table 1). These CTL assays were done following one in vitro stimulation and therefore do not directly measure precursor frequency. However, the relative levels of specific lysis given by CD-8+ T lymphocytes from the four vaccinated macaques likely reflect the relative number of SIV-specific precursor cells that they each carried. These findings, in conjunction with the serum antibody titrations described above, demonstrated that immunization with the SIV-VRP cocktail activated both humoral and cellular arms of the immune response, although the responses were not uniform in all animals. The induction of both antibodies and CTLs is critical for a lentivirus vaccine, because the correlates of protection from SIV- or HIV-induced disease have not been clearly determined (45).
FIG. 3.
SIV-specific CD8+ CTLs in SIV-VRP-vaccinated macaques. PBMCs were taken from the four SIV-VRP-immunized monkeys at 3 to 4 weeks after the fourth inoculation, stimulated in vitro, and tested for lytic activity against autologous target cells infected with vaccinia virus expressing SIV Gag (●) or SIV Env (■). Control target cells were infected with vaccinia virus alone (○) or left uninfected (□). Vaccinated monkeys were VW6 (A), PE9 (B), WON (C), and K2F (D). Similar results were obtained in two independent assays of PBMCs from VW6 and PE9.
TABLE 1.
Summary of immunological and challenge results
| Treatment | Macaque | Anti-SIVsm H-4i neutralizing antibody titera | CTLs (lytic units)
|
SIVsm E660 viremiad
|
||
|---|---|---|---|---|---|---|
| SIV Gag specificb | SIV Env specificc | Peak | Post-acute phase | |||
| SIV-VRP | VW6 | 121 | 85 | 43 | 79,210 | ≤1,500 |
| PE9 | 78 | 59 | 53 | 73,130 | ≤1,500 | |
| WON | 259 | 18 | 14 | 1,899,000 | 40,726 | |
| K2F | ≤20 | 16 | 25 | 943,500 | 25,581 | |
| HA-VRP | N2P | ≤20 | NAe | NA | 42,460,500 | 44,339,026f |
| N8X | ≤20 | NA | NA | 6,539,000 | 126,093 | |
| PBS | N9K | ≤20 | NA | NA | 499,917,700 | 1,281,454 |
| W1A | ≤20 | NA | NA | 60,226,300 | 102,877,164f | |
On the day of challenge (Fig. 2B). The limit of detection was 20.
Targets were infected with vaccinia virus expressing Gag-Pro-Pol (Fig. 3).
Targets were infected with vaccinia virus expressing Env (Fig. 3).
Genome equivalents per milliliter of plasma at peak viremia (2 or 3 weeks postchallenge) or geometric mean of two measurements taken during post-acute phase (6 to 8 weeks postchallenge [Fig. 4]). The limit of detection was 1,500.
NA, not applicable. PBMCs from control animals could not be stimulated in vitro.
Early mortality from SIV-induced disease.
Challenge with pathogenic SIVsm E660.
Four weeks after the final immunization all eight macaques were challenged i.v. with 50 50% monkey infectious doses (MID50) of the pathogenic SIVsm E660. This large dose and i.v. route ensured that disease would be evident in the majority of unvaccinated macaques (24). All of the animals were infected by the challenge virus, as judged by clinical findings and recovery of SIV from cultures of PBMCs within the first 2 weeks after challenge (data not shown). By 4 weeks after challenge, two of the control macaques (one from the diluent group and one from the HA-VRP group) were showing clear signs of SIV-induced illness. At weeks 10 and 11, respectively, these animals were sacrificed because of generalized wasting, diarrhea, dehydration, and signs of central nervous system disease. Necropsy results were consistent with a rapidly progressive disease course as previously described (25). As expected, SIV-specific antibodies were not detected either by ELISA or by neutralization assay in these two animals at any time after challenge (Fig. 2) (26).
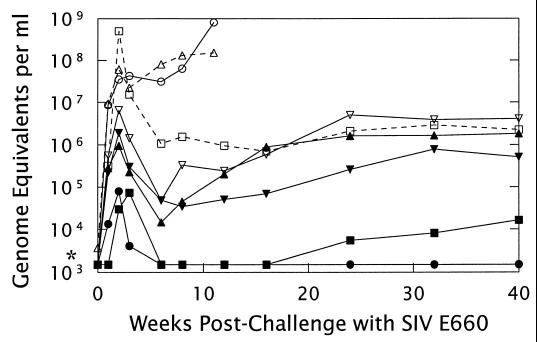
The protection from disease conferred by SIV-VRP vaccination was clear from the lack of early mortality and from the short duration of clinical signs in all members of the vaccinated group. However, a more precise picture of the patterns of virus replication in unvaccinated and vaccinated animals was obtained by using the branched-chain DNA assay (Chiron Corp.) to determine the virus load in the blood (Fig. 4) (46). By this measure, challenge virus replication was significantly lower in the monkeys that received the SIV-VRP vaccine. The geometric mean peak viral load in the vaccinated animals was reduced by more than 2 orders of magnitude from that in the controls (geometric mean peak titers of 3.2 × 105 versus 5.4 × 107 genome equivalents per ml of plasma; P < 0.05), and the highest of the peak viral loads in the vaccinated group was lower than the lowest of those in the controls.
FIG. 4.
Challenge of SIV-VRP-vaccinated and control macaques with SIVsm E660. Monkeys inoculated with either diluent (N9K [□] and W1A [▵]), HA-VRP (N2P [○] and N8X [▿]) or a cocktail of SIV-VRP (MA/CA plus gp160 and MA/CA plus gp140) (VW6 [●], PE9 [■], WON [▾], and K2F [▴]) were challenged i.v. with 50 MID50 of SIVsm E660 at 4 weeks following the final booster. Plasma taken at the indicated times was analyzed by the branched-chain DNA assay (46) for quantitation of SIV genome equivalents. One monkey from the diluent group (W1A) and one from the HA-VRP group (N2P) were euthanized with severe symptoms of SIV infection at 10 and 11 weeks, respectively. *, limit of detection (1,500 genomes per ml).
The vaccinated animals showed a range of abilities to suppress challenge virus replication, and the level of suppression correlated with the relative strength of the immune response induced by VRP vaccination (Table 1). For example, one of the vaccinated animals (VW6, the one with a high ELISA antibody titer, detectable neutralizing antibody, and a strong CTL response), showed the lowest peak viral load and had undetectable plasma viral loads from 6 to 40 weeks postchallenge. Except for a sample taken 1 week postchallenge, no virus replication was detected in cultures of PBMCs taken from this macaque through week 40 postchallenge. At the other extreme, the animal without detectable neutralizing antibody or a significant CTL response at the time of challenge (K2F) had a plasma viral load similar to those of the surviving unvaccinated controls by 16 weeks postchallenge (Fig. 4). The immune responses and abilities to suppress challenge virus replication of the remaining two vaccinated animals fell between these two extremes.
In the unvaccinated controls, two distinct patterns were apparent after only 8 weeks of infection. One HA-VRP control and one diluent control showed high viremia by 2 weeks postchallenge, and their titers increased steadily until they were euthanized as described above. This pattern of serum viremia has been observed previously with SIVsm E660 infection of rhesus macaques (24, 32). The other two unvaccinated animals were able to reduce challenge virus replication, although their virus loads remained nearly 3 orders of magnitude above the limit of detection. At 63 weeks postchallenge, the time at which the experiment was terminated, all four vaccinated animals and the two remaining controls were clinically healthy.
A clear anamnestic neutralizing antibody response was seen in all of the vaccinated animals (Fig. 2C), suggesting that the VRP vaccine consistently primed a humoral immune response against SIVsm H-4i. This was true even for the animal (K2F) that showed an ELISA titer but no neutralizing antibody on the day of challenge. As this anamnestic neutralizing antibody response was directed against the vaccine virus and not the challenge virus, its contribution to the observed protection against SIVsm E660-induced disease is unclear. Much later, at 16 weeks postchallenge, sera from all surviving animals also neutralized the challenge virus, SIVsm E660. However, SIVsm E660-neutralizing activity appeared no sooner in vaccinated animals than in the two surviving controls.
DISCUSSION
We have described in this report the first trial of a multicomponent VEE-based SIV vaccine in rhesus macaques, including a high-dose i.v. challenge with a pathogenic uncloned stock of SIV. The results of this trial demonstrated the safety and immunogenicity of SIV-VRP vaccines and also gave a first measure of their efficacy. All of the vaccinated animals remained healthy at 16 months postchallenge. Two of four controls were euthanized with symptoms of severe SIV infection at 10 and 11 weeks. The mean peak viral load was 2 orders of magnitude lower in the vaccinated animals than in the controls, and the mean viral load in the vaccinated animals during the post-acute phase of infection (6 to 8 weeks postchallenge) (32) was 750-fold lower. The ability to control SIV replication and reduce viral load to undetectable levels was closely correlated with the strongest measurable antibody responses and the strongest relative anti-SIV CD8+ CTL responses. This correlation between protection and the immune response to VRP-expressed proteins suggests that VRP vaccination was effective in these individuals. Humoral and cellular immune responses that are strong enough to give long-term control of virus replication may be a sufficient goal for an HIV vaccine, in that this could delay or possibly prevent immune suppression and disease as well as reduce the rate of transmission.
Optimum route and dose for VRP vaccination.
Inoculation routes and vaccine doses were varied during this trial. Changes were based on interim immune responses and on data obtained from parallel studies in the mouse model for VRP vaccination. In addition, a separate concurrent experiment was performed with naive macaques to compare different doses of VRP given s.c. in the arm. In this separate experiment, two rhesus macaques per dose were inoculated with 106, 107, or 108 IU of HA-VRP and boosted at 5 weeks with an equal amount. Mean serum antibody titers induced by two inoculations of the 106 dose were 1:2,000 as measured by ELISA or 1:120 by hemagglutination inhibition (HAI), similar to those induced by the higher doses. These titers were comparable to those seen in the HA-VRP controls described above, in which four vaccinations by a combination of s.c. (inguinal) and i.v. routes at doses ranging up to 5 × 108 IU produced mean titers of 1:3,000 (by ELISA) and 1:120 (by HAI). These preliminary results point to a likely effective dose (106 IU) and route of vaccination (s.c. in the arm).
Comparison of alphavirus replicon vector challenge experiments.
Replicon vaccine vectors derived from SFV, another alphavirus, have been tested in two macaque studies. In the first, pigtailed macaques were vaccinated four times with SFV replicons expressing PBj14 Env gp160 (40). All of the vaccinated animals developed anti-gp160 antibodies detectable by ELISA but showed no neutralizing antibodies or T-cell proliferative responses. SIV-specific CTLs were not assayed. Upon challenge with a 100% infectious, 75% fatal dose of SIV-PBj14-bcl3, three of four controls died, while all four vaccinated animals became ill but were protected from acute lethal disease. Plasma viral loads, reported as 50% tissue culture infectious doses per milliliter, were 10-fold lower in the vaccinated animals than in two controls on their day of death. (The equivalent differential in the SIV-VRP experiment reported here, between mean viral loads of the vaccinated animals and the two controls that died, was greater than 10,000-fold.) Although the SFV replicon vaccine afforded protection from the acute death syndrome caused by this strain of SIV and a reduction in plasma virus load, the relevance of this unique SIV-macaque model to models of SIV-induced simian AIDS and HIV-induced human AIDS is uncertain.
In a second SFV replicon vaccine trial, an SFV replicon expressing HIV-1IIIB gp160 was used in four inoculations of four cynomolgus macaques (6). Vaccination induced anti-gp160 antibodies, measured by ELISA, in one of four vaccinated animals, no neutralizing antibody, and transient T-cell proliferative responses in two of the four. CTLs were not assayed. All of the macaques became infected upon challenge with the chimeric SHIV-4. Three of the vaccinated animals at 1 month postchallenge showed a 30-fold reduction in virus load (measured by a limiting dilution cocultivation assay) compared to three controls. This vaccine was less immunogenic than that used in the first study, possibly due to differences between the two expressed gp160 molecules. Vaccination did not prevent infection, but the anamnestic antibody and T-cell proliferative responses may have modulated the growth of the challenge virus. However, no correlation could be made between prechallenge immune responses and the outcome of the challenge. This nonpathogenic virus produces only transient viremia, making assessment of efficacy difficult in the absence of sterilizing immunity.
The SIVsm E660 challenge used in this experiment was a highly stringent test of efficacy, since an HIV vaccine would not be required to protect a human against such a large i.v. dose of HIV. Previously reported primate trials using vaccine vectors and similar high-dose i.v. challenges with pathogenic SIV did not prevent infection (5, 24, 34). Results obtained in one of these studies suggested that an immune response that did not protect against i.v. challenge was partially protective against an intrarectal challenge (5). Therefore, the protection afforded by the SIV-VRP vaccination against a pathogenic i.v. challenge might be more effective against a less stringent mucosal challenge. More importantly, a mucosal challenge mimics the most common route of HIV infection and for this reason will be included in future trials.
A more consistent immune response and increased protection may be achieved by one or more improvements in the VRP vaccine. These could include the incorporation of the remainder of the gag gene into the MA/CA VRP and the addition of a Pol-VRP to the cocktail. This would expand the number of available epitopes and thereby increase the opportunity for an immune response in members of an outbred population. Moreover, a measurable Pol-specific CTL response has been correlated with lowered viral loads in long-term survivors of HIV infection (7). The use of a protein booster also may improve the response to VRP vaccination. Prime-boost protocols, in which a vaccine vector is followed by a recombinant protein booster, have been shown to induce a better immune response than the vector alone against both SIV and HIV (1, 12). Additional SIV-macaque trials combining optimum dose and route, added immunogens, and a mucosal challenge will further define the potential of the VRP vector system as a candidate HIV vaccine.
ACKNOWLEDGMENTS
This work was supported by grant DAMD17-94-J-4430 from the U.S. Army Research and Development Command, PHS-NIH grant R21-AI42644, and a supplement to PHS-NIH grant RO1-NS 26681. I.J.C. was supported on an ASSERT training grant, DAAH04-95-1-0224, from the Army Research Office. M.R.B. was supported by a Pre-Doctoral Traineeship, PHS-NIH grant T32-AI07273. K.M.M. was supported by NIH training grant T32-GM07092.
We thank Vanessa M. Hirsch for providing the SIVsm E660 challenge stock and Gene H. MacDonald, Jonathan F. Smith, Peter Pushko, and Mike Parker for sharing unpublished results and for helpful discussions. We also gratefully acknowledge Anne D. Lewis for help with pathology and Cherice Connor, Michael Hawley, Todd Cross, and Joseph Holsinger for excellent technical assistance.
REFERENCES
- 1.Almond N M, Heeney J L. AIDS vaccine development in primate models. AIDS. 1998;12(Suppl. A):S133–S140. [PubMed] [Google Scholar]
- 2.Anderson M J, Porter D C, Moldoveanue Z, Fletcher III T M, McPherson S, Morrow C D. Characterization of the expression and immunogenicity of poliovirus replicons that encode simian immunodeficiency virus SIVmac239 Gag or envelope SU proteins. AIDS Res Hum Retrovir. 1997;13:53–62. doi: 10.1089/aid.1997.13.53. [DOI] [PubMed] [Google Scholar]
- 3.Baba T W, Jeong Y S, Penninck D, Bronson R, Greene M F, Ruprecht R M. Pathogenicity of live, attenuated SIV after mucosal infection of neonatal macaques. Science. 1995;267:1820–1824. doi: 10.1126/science.7892606. [DOI] [PubMed] [Google Scholar]
- 4.Banchereau J, Steinman R M. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 5.Benson J, Chougnet C, Robert-Guroff M, Montefiori D, Markham P, Shearer G, Gallo R C, Cranage M, Paoletti E, Limbach K, Venzon D, Tartaglia J, Franchini G. Recombinant vaccine-induced protection against the highly pathogenic simian immunodeficiency virus SIVmac251: dependence on route of challenge exposure. J Virol. 1998;72:4170–4182. doi: 10.1128/jvi.72.5.4170-4182.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berglund P, Quesada-Rolander M, Putkonen P, Biberfeld G, Thorstensson R, Liljestrom P. Outcome of immunization of cynomolgus monkeys with recombinant Semliki Forest virus encoding human immunodeficiency virus type 1 envelope protein and challenge with a high dose of SHIV-4 virus. AIDS Res Hum Retrovir. 1997;13:1487–1495. doi: 10.1089/aid.1997.13.1487. [DOI] [PubMed] [Google Scholar]
- 7.Betts M R, Krowka J F, Kepler T B, Davidian M, Christopherson C, Kwok S, Louie L, Eron J, Sheppard H, Frelinger J A. Human immunodeficiency virus type 1 cytotoxic T lymphocyte activity is inversely correlated with HIV type 1 viral load in HIV type 1-infected long-term survivors. AIDS Res Hum Retrovir. 1999;15:1219–1228. doi: 10.1089/088922299310313. [DOI] [PubMed] [Google Scholar]
- 8.Caley I J, Betts M R, Irlbeck D M, Davis N L, Swanstrom R, Frelinger J A, Johnston R E. Humoral, mucosal, and cellular immunity in response to a human immunodeficiency virus type 1 immunogen expressed by a Venezuelan equine encephalitis virus vaccine vector. J Virol. 1997;71:3031–3038. doi: 10.1128/jvi.71.4.3031-3038.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Charles P C, Brown K W, Davis N L, Hart M K, Johnston R E. Mucosal immunity induced by parenteral immunization with a live attenuated Venezuelan equine encephalitis virus vaccine candidate. Virology. 1997;228:153–160. doi: 10.1006/viro.1996.8381. [DOI] [PubMed] [Google Scholar]
- 10.Clark A, Potter C W, Jennings R, Nicholl J P, Langrick A F, Schild G C, Wood J M, Tyrrell D A. A comparison of live and inactivated influenza A (H1N1) virus vaccines. 1. Short-term immunity. J Hyg. 1983;90:351–359. doi: 10.1017/s0022172400028989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clements M L, Snyder M H, Buckler-White A J, Tierney E L, London W T, Murphy B R. Evaluation of avian-human reassortant influenza A/Washington/897/80 × A/Pintail/119/79 virus in monkeys and adult volunteers. J Clin Microbiol. 1986;24:47–51. doi: 10.1128/jcm.24.1.47-51.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clements-Mann M L, Weinhold K, Matthews T J, Graham B S, Gorse G J, Keefer M C, McElrath M J, Hsieh R H, Mestecky J, Zolla-Pazner S, Mascola J, Schwartz D, Siliciano R, Corey L, Wright P F, Belshe R, Dolin R, Jackson S, Xu S, Fast P, Walker M C, Stablein D, Excler J-L, Tartaglia J, Paoletti E, et al. Immune responses to human immunodeficiency virus (HIV) type 1 induced by canarypox expressing HIV-1MN gp120, HIV-1SF2 recombinant gp120, or both vaccines in seronegative adults. NIAID AIDS Vaccine Evaluation Group. J Infect Dis. 1998;177:1230–1246. doi: 10.1086/515288. [DOI] [PubMed] [Google Scholar]
- 13.Cohen J. Weakened SIV vaccine still kills. Science. 1997;278:24–25. doi: 10.1126/science.278.5335.24. [DOI] [PubMed] [Google Scholar]
- 14.Conner R I, Korber B T M, Graham B S, Hahn B H, Ho D D, Walker B D, Neumann A U, Vermund S H, Mestecky J, Jackson S, Fenamore E, Cao Y, Gao F, Kalams S, Kunstman K J, McDonald D, McWilliams N, Trkola A, Moore J P, Wolinsky S M. Immunological and virological analyses of persons infected by human immunodeficiency virus type 1 while participating in trials of recombinant gp120 subunit vaccines. J Virol. 1998;72:1552–1576. doi: 10.1128/jvi.72.2.1552-1576.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davis N L, Brown K W, Greenwald G F, Zajac A J, Zacny V L, Smith J F, Johnston R E. Attenuated mutants of Venezuelan equine encephalitis virus containing lethal mutations in the PE2 cleavage signal combined with a second-site suppressor mutation in E1. Virology. 1995;212:102–110. doi: 10.1006/viro.1995.1458. [DOI] [PubMed] [Google Scholar]
- 16.Davis N L, Brown K W, Johnston R E. A viral vaccine vector that expresses foreign genes in lymph nodes and protects against mucosal challenge. J Virol. 1996;70:3781–3787. doi: 10.1128/jvi.70.6.3781-3787.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferrari G, Humphrey W, McElrath M J, Excler J-L, Duliege A-M, Clements M L, Corey L C, Bolognesi D P, Weinhold K J. Clade B-based HIV-1 vaccines elicit cross-clade cytotoxic T lymphocyte reactivities in uninfected volunteers. Proc Natl Acad Sci USA. 1997;94:1396–1401. doi: 10.1073/pnas.94.4.1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frolov I, Schlesinger S. Translation of Sindbis virus mRNA: analysis of sequences downstream of the initiating AUG codon that enhance translation. J Virol. 1996;70:1182–1190. doi: 10.1128/jvi.70.2.1182-1190.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldstein S, Elkins W R, London W T, Hahn A, Goeken R, Martin J E, Hirsch V M. Immunization with whole inactivated vaccine protects from infection by SIV grown in human but not macaque cells. J Med Primatol. 1994;23:75–82. doi: 10.1111/j.1600-0684.1994.tb00105.x. [DOI] [PubMed] [Google Scholar]
- 20.Grieder F B, Davis N L, Aronson J F, Charles P C, Sellon D C, Suzuki K, Johnston R E. Specific restrictions in the progression of Venezuelan equine encephalitis virus-induced disease resulting from single amino acid changes in the glycoproteins. Virology. 1995;206:994–1006. doi: 10.1006/viro.1995.1022. [DOI] [PubMed] [Google Scholar]
- 21.Hahn C S, Hahn Y S, Braciale T J, Rice C M. Infectious Sindbis virus transient expression vectors for studying antigen processing and presentation. Proc Natl Acad Sci USA. 1992;89:2679–2683. doi: 10.1073/pnas.89.7.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hevey M, Negley D, Pushko P, Smith J, Schmaljohn A. Marburg virus vaccines based upon alphavirus replicons protect guinea pigs and nonhuman primates. Virology. 1998;251:28–37. doi: 10.1006/viro.1998.9367. [DOI] [PubMed] [Google Scholar]
- 23.Hirsch V M, Dapolito G, McGann C, Olmsted R A, Purcell R H, Johnson P R. Molecular cloning of SIV from sooty mangabey monkeys. J Med Primatol. 1989;18:279–285. [PubMed] [Google Scholar]
- 24.Hirsch V M, Fuerst T R, Sutter G, Carroll M W, Yang L C, Goldstein S, Piatak M, Jr, Elkins W R, Alvord W G, Montefiori D C, Moss B, Lifson J D. Patterns of viral replication correlate with outcome in simian immunodeficiency virus (SIV)-infected macaques: effect of prior immunization with a trivalent SIV vaccine in modified vaccinia virus Ankara. J Virol. 1996;70:3741–3751. doi: 10.1128/jvi.70.6.3741-3752.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hirsch V M, Johnson P R. Pathogenesis of experimental SIV infection of macaques. Semin Virol. 1992;3:175–183. [Google Scholar]
- 26.Hirsch V M, Johnson P R. Pathogenic diversity of simian immunodeficiency viruses. Virus Res. 1994;32:183–203. doi: 10.1016/0168-1702(94)90041-8. [DOI] [PubMed] [Google Scholar]
- 27.Hu S-L, Abrams K, Barber G N, Moran P, Zarling J M, Langlois A J, Kuller L, Morton W R, Benveniste R E. Protection of macaques against SIV infection by subunit vaccines of SIV envelope glycoprotein gp160. Science. 1992;255:456–459. doi: 10.1126/science.1531159. [DOI] [PubMed] [Google Scholar]
- 28.Hu S-L, Polacino P, Stallard V, Klaniecki J, Pennathur S, Travis B M, Misher L, Kornas H, Langlois A J, Morton W R, Benveniste R E. Recombinant subunit vaccines as an approach to study correlates of protection against primate lentivirus infection. Immunol Let. 1996;51:115–119. doi: 10.1016/0165-2478(96)02564-3. [DOI] [PubMed] [Google Scholar]
- 29.Jahrling P B, Stephenson E H. Protective efficacies of live attenuated and formaldehyde-inactivated Venezuelan equine encephalitis virus vaccines against aerosol challenge in hamsters. J Clin Microbiol. 1984;19:429–431. doi: 10.1128/jcm.19.3.429-431.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaplan A H, Swanstrom R. Human immunodeficiency virus type 1 Gag proteins are processed in two cellular compartments. Proc Natl Acad Sci USA. 1991;88:4528–4532. doi: 10.1073/pnas.88.10.4528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kinney R M, Esposito J J, Mathews J H, Johnson B J B, Roehrig J T, Barrett A D T, Trent D W. Recombinant vaccinia virus/Venezuelan equine encephalitis (VEE) virus protects mice from peripheral VEE virus challenge. J Virol. 1988;62:4697–4702. doi: 10.1128/jvi.62.12.4697-4702.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lifson J, Nowak M A, Goldstein S, Rossio J L, Kinter A, Vasquez G, Wiltrout T A, Brown C, Schneider D, Wahl L, Lloyd A L, Williams J, Elkins W R, Fauci A S, Hirsch V M. The extent of early viral replication is a critical determinant of the natural history of simian immunodeficiency virus infection. J Virol. 1997;71:9508–9514. doi: 10.1128/jvi.71.12.9508-9514.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liljestrom P, Garoff H. A new generation of animal cell expression vectors based on the Semliki Forest virus replicon. Bio/Technology. 1991;9:1356–1361. doi: 10.1038/nbt1291-1356. [DOI] [PubMed] [Google Scholar]
- 34.Lu S, Arthos J, Montefiori D C, Yasutomi Y, Manson K, Mustafa F, Johnson E, Santoro J C, Wissink J, Mullins J I, Haynes J R, Letvin N L, Wyand M, Robinson H L. Simian immunodeficiency virus DNA vaccine trial in macaques. J Virol. 1996;70:3978–3991. doi: 10.1128/jvi.70.6.3978-3991.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luckow V A, Summers M D. Trends in the development of baculovirus expression vectors. Bio/Technology. 1988;6:47–55. [Google Scholar]
- 35a.MacDonald, G. H., and R. E. Johnston. The role of dendritic cell targeting in Venezuelan equine encephalitis virus pathogenesis. J. Virol., in press. [DOI] [PMC free article] [PubMed]
- 36.Mackett M, Smith G L, Moss B. General method for production and selection of infectious vaccinia virus recombinants expressing foreign genes. J Virol. 1984;49:857–864. doi: 10.1128/jvi.49.3.857-864.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matsui M, Hioe C E, Frelinger J A. Roles of the six peptide-binding pockets of the HLA-A2 molecule in allorecognition by human cytotoxic T-cell clones. Proc Natl Acad Sci USA. 1993;90:674–678. doi: 10.1073/pnas.90.2.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Monath T, Calisher C H, Davis M, Bowen G S, White J. Experimental studies of rhesus monkeys infected with epizootic and enzootic subtypes of Venezuelan equine encephalitis virus. J Infect Dis. 1974;129:194–200. doi: 10.1093/infdis/129.2.194. [DOI] [PubMed] [Google Scholar]
- 39.Montefiori D, Baba T W, Li A, Bilska M, Ruprecht R M. Neutralizing and infection-enhancing antibody responses do not correlate with the differential pathogenicity of SIVmac239delta3 in adult and infant rhesus monkeys. J Immunol. 1996;157:5528–5535. [PubMed] [Google Scholar]
- 40.Mossman S P, Bex F, Berglund P, Arthos J, O'Neil S P, Riley D, Maul D H, Bruck C, Momin P, Burny A, Fultz P N, Mullins J I, Liljestrom P, Hoover E A. Protection against lethal simian immunodeficiency virus SIVsmmPBj14 disease by a recombinant Semliki Forest virus gp160 vaccine and by a gp120 subunit vaccine. J Virol. 1996;70:1953–1960. doi: 10.1128/jvi.70.3.1953-1960.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pialoux G, Excler J-L, Riviere Y, Gonzalez-Canali G, Feuillie V, Coulaud P, Gluckman J-C, Matthews T J, Meignier B, Kieny M-P, Gonnet P, Diaz I, Meric C, Paoletti E, Tartaglia J, Salomon H, Plotkin S The AGIS Group, and L'Agence Nationale de Recherche sur le SIDA. A prime-boost approach to HIV preventive vaccine using a recombinant canarypox virus expressing glycoprotein 160(MN) followed by a recombinant glycoprotein 160(MN/LAI) AIDS Res Hum Retrovir. 1995;11:373–381. doi: 10.1089/aid.1995.11.373. [DOI] [PubMed] [Google Scholar]
- 42.Polo J M, Belli B A, Driver D A, Frolov I, Sherrill S, Hariharan M J, Townsend K, Perri S, Mento S J, Jolly D J, Chang S M W, Schlesinger S, Dubensky T W., Jr Stable alphavirus packaging cell lines for Sindbis virus- and Semliki Forest virus-derived vectors. Proc Natl Acad Sci USA. 1999;96:4598–4603. doi: 10.1073/pnas.96.8.4598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pushko P, Parker M, Ludwig G V, Davis N L, Johnston R E, Smith J F. Replicon-helper systems from attenuated Venezuelan equine encephalitis virus: expression of heterologous genes in vitro and immunization against heterologous pathogens in vivo. Virology. 1997;239:389–401. doi: 10.1006/viro.1997.8878. [DOI] [PubMed] [Google Scholar]
- 44.Stott E J, Schild G C. Strategies for AIDS vaccines. J Antimicrob Chemother. 1996;37:185–198. doi: 10.1093/jac/37.suppl_b.185. [DOI] [PubMed] [Google Scholar]
- 45.Stott J, Hu S-L. Vaccines and immunology. AIDS. 1998;12(Suppl. A):S95–S96. [PubMed] [Google Scholar]
- 46.Urdea M S, Wilber J C, Yeghiazarian T, Todd J A, Kern D G, Fong S-J, Besemer D, Hoo B, Sheridan P J, Kokka R, Neuwald P, Pachl C A. Direct and quantitative detection of HIV-1 RNA in human plasma with a branched DNA signal amplification assay. AIDS. 1993;7(Suppl. 2):S11–S14. doi: 10.1097/00002030-199311002-00004. [DOI] [PubMed] [Google Scholar]
- 47.Xiong C, Levis R, Shen P, Schlesinger S, Rice C M, Huang H V. Sindbis virus: an efficient, broad host range vector for gene expression in animal cells. Science. 1989;243:1188–1191. doi: 10.1126/science.2922607. [DOI] [PubMed] [Google Scholar]
- 48.Zhou S, Berglund P, Zhao H, Liljestrom P, Jondal M. Generation of cytotoxic and humoral immune responses by nonreplicative recombinant Semliki Forest virus. Proc Natl Acad Sci USA. 1995;92:3009–3013. doi: 10.1073/pnas.92.7.3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhou X, Berglund P, Rhodes G, Parker S E, Jondal M, Liljestrom P. Self-replicating Semliki Forest virus RNA as recombinant vaccine. Vaccine. 1994;12:1510–1514. doi: 10.1016/0264-410x(94)90074-4. [DOI] [PubMed] [Google Scholar]