Abstract
Following two requests from the European Commission, the EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA) was asked to deliver a scientific opinion on the revision of the tolerable upper intake level (UL) for preformed vitamin A and β‐carotene. Systematic reviews of the literature were conducted for priority adverse health effects of excess vitamin A intake, namely teratogenicity, hepatotoxicity and endpoints related to bone health. Available data did not allow to address whether β‐carotene could potentiate preformed vitamin A toxicity. Teratogenicity was selected as the critical effect on which to base the UL for preformed vitamin A. The Panel proposes to retain the UL for preformed vitamin A of 3000 μg RE/day for adults. This UL applies to men and women, including women of child‐bearing age, pregnant and lactating women and post‐menopausal women. This value was scaled down to other population groups using allometric scaling (body weight0.75), leading to ULs between 600 μg RE/day (infants 4–11 months) and 2600 μg RE/day (adolescents 15–17 years). Based on available intake data, European populations are unlikely to exceed the UL for preformed vitamin A if consumption of liver, offal and products thereof is limited to once per month or less. Women who are planning to become pregnant or who are pregnant are advised not to consume liver products. Lung cancer risk was selected as the critical effect of excess supplemental β‐carotene. The available data were not sufficient and suitable to characterise a dose–response relationship and identify a reference point; therefore, no UL could be established. There is no indication that β‐carotene intake from the background diet is associated with adverse health effects. Smokers should avoid consuming food supplements containing β‐carotene. The use of supplemental β‐carotene by the general population should be limited to the purpose of meeting vitamin A requirements.
Keywords: β‐Carotene, adverse health effects, lung cancer, retinol, teratogenicity, tolerable upper intake level, vitamin A
1. INTRODUCTION
The term vitamin A comprises all‐trans‐retinol (also called retinol), naturally occurring molecules associated with the biological activity of retinol (such as retinal, retinoic acid and retinyl esters), and provitamin A carotenoids (such as β‐carotene, α‐carotene and β‐cryptoxanthin) that are dietary precursors of retinol. Provitamin A carotenoids are subject to specific routes regarding their absorption and metabolism, and their vitamin A activity is lower than that of preformed vitamin A, i.e. retinol and retinyl esters (EFSA NDA Panel, 2015). Considering these differences, the Scientific Committee on Food (SCF) assessed the tolerable upper intake level (UL) for β‐carotene and preformed vitamin A separately (SCF, 2000b, 2002).
The present opinion aims at updating the UL for vitamin A, including both preformed vitamin A and β‐carotene, to address the mandate received from the European Commission.
1.1. Background as provided by the European Commission
Article 6 of Regulation (EC) No 1925/2006 on the addition of vitamins and minerals and of certain other substances to foods and Article 5 of Directive 2002/46/EC on the approximation of the laws of the Member States relating to food supplements provide that maximum amount of vitamins and minerals added to foods and to food supplements, respectively, shall be set.
The above‐mentioned provisions lay down the criteria to be taken into account when establishing these maximum amounts that include the upper safe levels (ULs) of vitamins and minerals established by scientific risk assessment based on ‘generally accepted scientific data, taking into account, as appropriate, the varying degrees of sensitivity of different groups of consumers’.
To set maximum amounts of vitamins and minerals in fortified foods and food supplements, the Commission would like to ask the European Food Safety Authority (EFSA) to review the previous opinions of the Scientific Committee on Food (SCF) or the NDA Panel on the ULs for vitamin A, 1 folic acid/folate,1 vitamin D,1 vitamin E,1 vitamin B6,1 iron,1 manganese1 and β‐carotene1 to take into account recent scientific developments and evidence.
In this context, EFSA should first review the guidelines of the SCF1 for the development of tolerable upper intake levels for vitamins and minerals (adopted on 19 October 2000).
Tolerable Upper Intake Levels should be presented separately for the age group from 4/6 months onwards until 3 years of age and the general population group from 3 years onwards, taking into account, as appropriate, the varying degrees of sensitivity of different consumer groups. As foods intended for the general population are also consumed by young children, young children should be considered as a potentially sensitive consumer group.
1.2. Terms of reference as provided by the European Commission
In accordance with Article 29(1)(a) of Regulation (EC) No 178/2002, the European Commission requests the European Food Safety Authority to:
Update the guidelines of the SCF for the development of Tolerable Upper Intake Levels for vitamins and minerals in the light of available recent scientific and methodological developments.
- Review existing scientific evidence and provide advice on Tolerable Upper Intake Levels for the following vitamins and minerals including their currently authorised forms for the addition to fortified foods and food supplements for the general population and, as appropriate, for vulnerable subgroups of the population:
- vitamin A
- folic acid/folate
- vitamin D
- vitamin E
- iron
- manganese
- β‐carotene
- vitamin B6
For nutrients for which there are no, or insufficient, data on which to base the establishment of an UL, an indication should be given on the highest level of intake where there is reasonable confidence in data on the absence of adverse effects.
1.3. Overview of previous assessments of the UL for preformed vitamin A and β‐carotene
1.3.1. Preformed vitamin A
In 2002, the SCF set a UL for preformed vitamin A of 3000 μg retinol equivalent (RE)/day for women of child‐bearing age based on the teratogenic potential of vitamin A. The SCF considered that this UL was also appropriate for men, for pregnant and lactating women and for infants and children after correction for differences in metabolic rate, because it is 2.5‐fold lower than the lowest daily intake that has been associated with hepatotoxicity during chronic intake. The SCF advised that intakes of preformed vitamin A should be restricted to 1500 μg RE/day in postmenopausal women based on its effects on bone mineral density and bone fracture risk (SCF, 2002).
An overview of upper levels for preformed vitamin A established by risk assessment bodies is tabulated in Table 1. For a more detailed summary, see Appendix A of the protocol (Annex A).
TABLE 1.
Overview of existing UL values for preformed vitamin A.
| Population group | SCF (2002) UL (μg RE/day) | IOM (2001) UL (μg/day) | EVM (2003) GL (μg/day) | NNR (2014) UL (μg/day) |
|---|---|---|---|---|
| Infants | ||||
| 0–6 months | 600 | |||
| 7–12 months | 600 | |||
| Children and adolescents | ||||
| 1–3 years | 800 | 600 | ||
| 4–6 years | 1100 | |||
| 4–8 years | 900 | |||
| 7–10 years | 1500 | |||
| 9–13 years | 1700 | |||
| 11–14 years | 2000 | |||
| 14–18 years | 2800 a | |||
| 15–17 years | 2600 a | |||
| Adults | ||||
| ≥ 18 years | 3000 a | 1500b,c | 3000 a | |
| ≥ 19 years | 3000 b | |||
| Post‐menopausal women | 1500 c | 1500 c | ||
Abbreviations: GL, guidance level; RE, retinol equivalents; UL, tolerable upper intake level.
Includes pregnant and lactating women.
Includes pregnant, lactating and post‐menopausal women.
Guidance level; a safe upper level or a tolerable upper intake level could not be established with available data.
Previous ULs for preformed vitamin A have been established for different population groups using different critical endpoints. ULs for women of childbearing age (IOM, 2001; SCF, 2002) are based on a no observed adverse effect level (NOAEL) for teratogenic effects of 3000 μg/day (Mastroiacovo et al., 1999; Rothman et al., 1995). The SCF (2002) considered this dose protective for hepatotoxicity, with a lowest observed adverse effect level (LOAEL) of 7500 μg/day from case reports (Geubel et al., 1991; Kowalski et al., 1994) and an uncertainty factor (UF) of 2.5. This approach was also followed by the NNR (2014) to derive the UL for all adults, and by the IOM (2001) to derive the UL for adults. In the latter case, a LOAEL of 14,000 μg/day and an UF of 5 were applied.
Based on data from prospective studies (Feskanich et al., 2002; Melhus et al., 1998) and using the risk of osteoporotic bone (hip) fractures as a critical endpoint, the SCF (2002) advised post‐menopausal women and the EVM (2003) the whole adult population, not to exceed intakes of 1500 μg RE/day. Both bodies raised questions about the causality of the relationship at these levels of intake and found the available data for this endpoint insufficient to set a UL. The same conclusions were reached by the NNR (2014) some years later, based on a wider body of evidence (e.g. Caire‐Juvera et al., 2009; Michaëlsson et al., 2003).
Owing to the paucity of data available for this population group, ULs for children and adolescents were derived by either isometric scaling (linear with body weight (IOM, 2001)) or allometric scaling (using body surface area = body weight0.75 (SCF, 2002)) from those set for adults. Although the SCF considered that this approach was also appropriate to derive a UL for infants, no value was provided. Conversely, a UL of 600 μg/day for infants was set by the IOM based on a LOAEL of 6000 μg/day (Persson et al., 1965) and an UF of 10.
1.3.2. β‐Carotene
In 2000, the SCF concluded from trials in humans that supplemental β‐carotene (20 mg/day or more) was contraindicated for use in heavy smokers. However, data were insufficient to set a UL (no dose–response relationship between the intake of β‐carotene and lung cancer incidence or mortality in smokers could be established). It was also not possible to distinguish between the different isomeric forms of β‐carotene or specific formulations administered (SCF, 2000b).
The Expert Group on Vitamins and Minerals set a safe upper level for lifetime daily consumption of supplemental β‐carotene for the general population (excluding smokers) at 7 mg/day, deriving a LOAEL of 20 mg/day from the ATBC trial and applying an UF of 3 (EVM, 2003). On the same basis, the Panel on Nutrition, Dietetic Products, Novel Food and Allergy of the Norwegian Scientific Committee for Food Safety set a tentative upper level (TUL) at 4 mg/day for supplemental β‐carotene, using an UF of 5, with smokers being discouraged from taking β‐carotene supplements all together (VKM, 2015).
Drawing from the same body of evidence, the IOM did not set a UL for β‐carotene but, in turn, advised against the use of β‐carotene supplements for the general population (IOM, 2000).
No specific recommendations or UL has been set for β‐carotene by the Nordic Council of Ministers in its most recent Nordic Nutrition Recommendations (Blomhoff et al., 2023).
1.4. Other assessments of preformed vitamin A and β‐carotene by EFSA
Dietary reference values
In response to a request from the European Commission, EFSA derived dietary reference values (DRVs) for vitamin A for the European population (EFSA NDA Panel, 2015). For provitamin A carotenoids, the NDA Panel maintained the conversion factors proposed by the SCF, namely 1 μg retinol equivalents (RE) equals 1 μg of retinol, 6 μg of β‐carotene and 12 μg of other provitamin A carotenoids. Vitamin A requirement can be met with any mixture of preformed vitamin A and provitamin A carotenoids that provides an amount of vitamin A equivalent to the reference value in terms of μg RE/day.
The Panel set a concentration of 20 μg retinol/g liver (0.07 μmol/g) as a target value to be used for establishing the average requirement (AR) for vitamin A for all age groups. This was based on the consideration that in adults, such a value represents a level assumed to maintain adequate plasma retinol concentration, which prevents clinical signs of deficiency and provides adequate stores.
The available data on the relationship between dietary intake of vitamin A and retinol liver stores were considered insufficient to derive an AR; therefore, a factorial approach was applied. This approach considered a total body/liver retinol store ratio of 1.25, a liver/body weight ratio of 2.4%, a fractional catabolic rate of retinol of 0.7% per day of total body stores, an efficiency of storage in the whole body of ingested retinol of 50% and the reference body weights for women and men in the EU of 58.5 and 68.1 kg, respectively. Based on this, ARs of 570 μg RE/day for men and 490 μg RE/day for women were derived. A coefficient of variation (CV) of 15% was used to account for the variability in requirement and the large uncertainties in the data set to set population reference intakes (PRIs) of 750 μg RE/day for men and 650 μg RE/day for women.
For infants, the same factorial approach was applied, using specific values for reference body weight and for liver/body weight ratio. As the available data on retinol catabolic rate in children were limited, the Panel applied the value for catabolic rate in adults and corrected it on the basis of a growth factor. Estimated ARs range from 190 μg RE/day in infants aged 7–11 months to 580 μg RE/day in boys aged 15–17 years. PRIs for infants and children were estimated based on a CV of 15% and range from 250 to 750 μg RE/day.
For pregnant women, over the course of pregnancy, a total of 3600 μg retinol is accumulated in the fetus, with accretion mostly occurring in the last months of pregnancy. Assuming an efficiency of storage of 50% for the fetus, an additional daily requirement of 51 μg RE was calculated for the second half of pregnancy. The panel applied this additional requirement to the whole period of pregnancy to allow for the extra need related to the growth of maternal tissues; thus, an AR of 540 μg RE/day was estimated. Considering a CV of 15%, a PRI of 700 μg RE/day was proposed for pregnant women.
For lactating women, to compensate for the loss of retinol in breast milk, an increased AR of 1020 μg RE/day was estimated. This was based on the average amount of retinol secreted in breast milk of 424 μg/day and an absorption efficiency of retinol of 80%; hence, an additional vitamin A intake of 530 μg RE/day was considered sufficient to replace these losses. Considering a CV of 15%, a PRI of 1300 μg RE/day was derived for lactating women.
Other assessments (OpenFoodTox, link)
In 2012, EFSA's ANS Panel reassessed the safety of β‐carotene for use as food additive and in food supplements. Based on human data, the Panel concluded that exposure to β‐carotene from these sources at levels < 15 mg/day is not associated with adverse health effects in the general population, including heavy smokers (EFSA ANS Panel, 2012b).
The ANS Panel also assessed the safety of β‐carotene in the context of its use as a food additive for food colouring purposes. The available data did not allow the setting of an acceptable daily intake level (ADI); however, the panel concluded that the use of (synthetic) β‐carotene and mixed β‐carotenes as food colour is not of safety concern, provided the intake from this use as a food additive and as food supplement is not more than the amount likely to be ingested from regular consumption of foods in which they occur naturally (5–10 mg/day) (EFSA ANS Panel, 2012a). In its assessment on the safety of the extension of use of the food colour synthetic β‐carotene when added to foods for special medical purposes (FSMP) intended for young children aged 1–3 years, the ANS Panel concluded that the proposed extension of use (at the proposed level of 5 mg/L in the diluted FSMP) would not be of safety concern (EFSA ANS Panel, 2016).
In 2019, Joint FAO/WHO Expert Committee on Food Additives (JECFA) withdrew the two group ADIs of 0–5 mg/kg body weight (bw) for (1) the sum of the synthetic carotenoids β‐carotene, β‐apo‐8′‐carotenal and β‐apo‐8′‐carotenoic acid methyl and ethyl esters and (2) synthetic β‐carotene and β‐carotene derived from Blakeslea trisporae, which were based on NOAEL from a rat study because rats are not an appropriate model for deriving an ADI for β‐carotene due to the relatively low bioavailability of β‐carotene in rats compared with humans. JECFA was unable to establish a group ADI for these compounds because a group ADI is applicable to the general population, which includes heavy smokers. JECFA noted that it is very unlikely that it will ever be possible to establish a group ADI because further data from the population of heavy smokers cannot be gathered ethically (JECFA, 2019).
The FEEDAP Panel assessed the safety of vitamin A (retinyl acetate, retinyl palmitate and retinyl propionate) in the context of its use as an additive to animal feed and water for drinking for all animal specifies. According to consumer exposure calculations, liver is the only food of animal origin that poses a risk to adult consumers. The panel thus proposed a reduction of the maximum vitamin A content of feeding stuff that would in turn significantly reduce the risk for consumers. The Panel noted that adding an additional route of administration for vitamin A, such as through drinking water, would increase the risk for consumers (EFSA FEEDAP Panel, 2009, 2013). In the same context, in its assessment of β‐carotene, the Panel determined that the use of supplemental β‐carotene in feeds for food‐producing animals, with the exception of veal calves, would not result in a significant increase in consumer exposure to β‐carotene. The Panel concluded that unlimited use of β‐carotene as an additive to milk replacers used as feed for calves may be of concern as regards consumer safety in those consuming liver from pre‐ruminant calves treated with β‐carotene (EFSA FEEDAP Panel, 2012).
1.5. Interpretation of the Terms of Reference and context of the assessment
According to the mandate, EFSA has first reviewed the guidelines of the SCF for the development of tolerable upper intake levels for vitamins and minerals (SCF, 2000a). A draft guidance was endorsed by the NDA Panel and published for a 1‐year pilot phase (EFSA NDA Panel, 2022), after which it will be revised and complemented as necessary, following a public consultation.
The panel interprets that the UL for preformed vitamin A and β‐carotene should be revised according to the principles laid down in the above‐mentioned guidance.
When developing the protocol to update the ULs for vitamin A and β‐carotene (Annex A), the NDA Panel noticed that no new supplementation trials have been conducted/published with high doses of β‐carotene (≥ 15–20 mg/day) after the evaluations of the SCF (2000b) and the EFSA ANS Panel (2012b, 2012a), and that the available evidence for adverse health effects of supplemental β‐carotene (other than lung cancer risk in smokers) was limited and conflicting. Thus, in the context of deriving a UL, the Panel considered that supplemental β‐carotene should be primarily assessed as a source of vitamin A, and therefore for its potential to increase preformed vitamin A toxicity.
Throughout this opinion, vitamin A refers to the forms of preformed vitamin A authorised for addition to foods and food supplements (i.e. retinol and retinyl esters) and the provitamin A β‐carotene. As stated in the protocol (Annex A), β‐carotene is the most important carotenoid in terms of its relative provitamin A activity, being the most potent retinol precursor and the most abundant in the diet (Harrison, 2012; Rodriguez‐Amaya, 2015). Therefore, although it is acknowledged that other provitamin A carotenoids (e.g. α‐carotene and β‐cryptoxanthin) may contribute to total vitamin A intake, their dietary contribution to the overall toxicity of preformed vitamin A is expected to be marginal.
2. DATA AND METHODOLOGIES
A protocol has been developed for this assessment (Annex A).
In accordance with the draft NDA Panel guidance on establishing and applying tolerable upper intake levels for vitamins and essential minerals (EFSA NDA Panel, 2022), the assessment questions underlying the UL evaluation are as follows:
What is the maximum level of total chronic daily intake of vitamin A (including preformed vitamin A and β‐carotene) from all sources which is not expected to pose a risk of adverse health effects to humans? (Hazard identification and characterisation)
What is the daily intake of vitamin A (including preformed vitamin A and β‐carotene) from all dietary sources in EU populations? (Intake assessment)
What is the risk of adverse effects related to the intake of vitamin A in EU populations, including attendant uncertainties? (Risk characterisation)
Priority adverse health effects, i.e. those that are expected to play a critical role for establishing a UL, were identified in consultation with a panel of qualified experts on vitamin A 2 and after discussion by the ULs Working Group (WG) as follows: a) teratogenicity, b) hepatotoxicity and c) bone health, including bone fractures, bone mineral density (BMD), bone mineral content (BMC) and indices of bone strength at all ages. These will be addressed through systematic reviews of the literature. The rationale for the prioritisation of these adverse health effects is detailed in the protocol (Annex A).
The assessment of subquestions identified as the result of the problem formulation, together with the methods selected to address them, are provided in Table 2.
TABLE 2.
Assessment of subquestions and methods to address them.
| No. | Subquestion | Methods |
|---|---|---|
| sQ1 | ADME of the different forms of vitamin A | |
| 1a. What is the ADME of the different forms of vitamin A a in humans? | Narrative review | |
| 1b. What is the extent to which β‐carotene in fortified foods or supplements can contribute to ‘excess’ vitamin A? (i.e. bioavailability/bioconversion of β‐carotene in the ‘high’ range of intake in individuals with adequate vitamin A status) | Narrative review | |
| 1c. Are there differences related to age or other individual factors, e.g. genetic polymorphisms of vitamin A a metabolism? | Narrative review | |
| sQ2 | Biomarkers of intake for vitamin A | |
| 2a. How does hepatic retinol content reflect ‘high’ vitamin A intake? What is the relevance of this marker as biomarker of vitamin A toxicity? | Narrative review | |
| 2b. How does circulating fasting retinyl esters reflect ‘high’ vitamin A intake? What is the relevance of this marker as biomarker of vitamin A toxicity? | Narrative review | |
| 2c. What are other markers of ‘high’ vitamin A intake and toxicity? | Narrative review | |
| sQ3 | Teratogenicity | |
| 3a. What is the dose–response relationship between ‘high’ vitamin A a intake and teratogenicity? | Systematic review | |
| 3b. What are the potential mechanisms/mode(s) of action underlying the relationship between vitamin A intake and this endpoint? | Narrative review | |
| sQ4 | Hepatotoxicity | |
| 4a. What is the dose–response relationship between ‘high’ vitamin A a intake and hepatotoxicity? | Systematic review | |
| 4b. What are the potential mechanisms/mode(s) of action underlying the relationship between vitamin A intake and this endpoint? | Narrative review | |
| sQ5 | Bone health | |
| 5a. Does ‘high’ vitamin A a intake increase the risk of bone fractures in humans? If so, could a dose–response be characterised? | Systematic review | |
| 5b. Does ‘high’ vitamin A a intake affect BMD/BMC and/or indices of bone strength in humans? If so, could a dose–response be characterised? | Systematic review | |
| 5c. What are the potential mechanisms/mode(s) of action underlying the relationships between vitamin A intake and these endpoints? | Narrative review | |
| sQ6 | What other adverse health effects have been reported to be associated with ‘high’ intake of vitamin A a ? | Narrative review |
| sQ7 | Vitamin A intake | |
| 7a. What are the levels of vitamin A a in foods, beverages and food supplements in the EU? | Food composition and food consumption data in the EU b | |
| 7b. What is the distribution of intakes of vitamin A a from all dietary sources (including fortified foods and food supplements) by population group in the EU? |
Preformed vitamin A and provitamin A β‐carotene.
EFSA Food Composition Database, Mintel's Global New Products Database, EFSA Comprehensive Food Consumption Database.
The preparatory work for this assessment was contracted out by EFSA through a call for tender (OC/EFSA/NUTRI/2021/01) (see Section 1 of the Protocol for more details). The preparatory work to address subquestion (sQ) 1 to sQ6 has been carried out by the University of Oslo, and the technical report has been published (Olsen et al., 2024). The Panel made an independent evaluation of the evidence and adapted the outcome of the contractor's work where needed.
A draft opinion was endorsed by the NDA Panel on 30 January 2024 and was open for public consultation from 9 February to 22 March 2024. The draft opinion has been amended in view of the comments received, which have all been addressed and are published in a technical report (Annex I).
2.1. Hazard identification and characterisation
2.1.1. Data
For subquestions addressed through narrative reviews, textbooks, authoritative reviews and research papers have been retrieved through non‐systematic searches in bibliographic databases.
For subquestions addressed through systematic reviews, a brief description of the processes used for evidence retrieval, study selection and data extraction is provided below. More information on these steps is available in the contractor's technical report (Olsen et al., 2024).
2.1.1.1. Literature searches
To address sQ3a, sQ4a and sQ5a/b, relevant human studies on the selected endpoints were identified through systematic searches of the literature in MEDLINE (Ovid), Embase (Ovid) and Cochrane Central Register of Controlled Trials. Searches were targeting articles published in English only. The search strategy was developed by information specialists from the University of Oslo, and peer reviewed by information specialists at Karolinska Institutet and EFSA. Specific search strings were used in the aforementioned databases to limit by type of study and publication type, and limited to studies published in 2001 and later, as described in the protocol (Annex A of the opinion). The search strategy is further detailed in Appendix B of the technical report (Olsen et al., 2024). The searches covered literature published up to 18 March 2022.
The literature searches for sQ5 (bone health) were designed to address each type of endpoint (i.e. fractures, BMD/BMC and indices of bone strength). The results by endpoint and database were combined.
2.1.1.2. Study selection
Articles retrieved were screened in duplicate in Distiller SR® (Web‐Based Systematic Review Software; Evidence Partners, Ottawa, Canada) according to the eligibility criteria defined in the protocol (Annex A). Conflicts were resolved by discussion or by a third reviewer. To maximise the identification of relevant publications, the reference list of systematic or narrative reviews identified via the search were scrutinised for additional eligible studies. To cover the period before 2001, the literature cited in the SCF assessment (SCF, 2002) was screened using the same inclusion criteria as described in the protocol (Annex A). In addition, forward citation searches of included studies were performed using Google Scholar, to identify any studies not included in the de novo literature search.
Reviews, expert opinions, editorials, letters to the editors, abstracts, posters, theses and grey literature (i.e. literature not indexed in literature databases) were excluded.
For sQ3a on teratogenicity, eligible studies were those measuring exposure in pregnant women and teratogenic outcomes in the offspring. The inclusion criteria for exposure were quantitative estimates of dietary preformed vitamin A intake (retinol and retinyl esters), or preformed vitamin A and β‐carotene intake, with or without supplements. The comparator was lower intakes or placebo. There were no inclusion criteria restrictions on study design (Annex A). A total of 384 records were identified after removing duplicates and screened at title and abstract level, of which 35 full‐text papers were assessed for eligibility and 16 were included. In addition, four articles were identified from the forward citation search of included studies and another five were identified from the SCF (2002) UL assessment, using the same inclusion criteria as described in the protocol (Annex A). During data extraction, six references were excluded (Annex H), leaving a total of 19 publications reporting original data (Appendix A, Figure A.1).
For sQ4a on hepatotoxicity, the inclusion criteria were restricted to human intervention studies with a duration of at least 3 months and with no population restriction. Only studies comparing daily or weekly oral supplementation with vitamin A, as preformed vitamin A (retinol and retinyl esters) with or without β‐carotene (with or without a co‐intervention that was the same for both arms), were included. The endpoints of interest were valid markers of liver damage or function, including liver enzymes, liver steatosis assessed by ultrasound or MRI, transient elastography (fibroscan), histopathological signs of hepatotoxicity assessed by liver biopsy, clinically diagnosed liver cirrhosis and clinically diagnosed portal hypertension, with or without cirrhosis. After removing duplicates, a total of 918 records were identified and screened at title and abstract level. Of these, 18 full‐text papers were assessed for eligibility, and in addition, one paper was identified via citation searching, resulting in five papers that met the eligibility criteria. One paper was excluded during data extraction because of duplication (Annex H), leaving a total of four RCTs that were included in the assessment (Appendix A, Figure A.2).
For sQ5a (fractures) and sQ5b (BMD/BMC and bone strength), only prospective studies, RCTs and non‐randomised comparative studies of interventions, with a study duration of at least 12 months were eligible. All population age groups were of interest except individuals at risk of/with vitamin A deficiency receiving therapeutical doses of (preformed) vitamin A, individuals under medical therapy with topic synthetic retinoids and individuals with primary hyperparathyroidism or other disorders affecting bone health. The same eligibility criteria as for sQ3a and sQ4a were applied in relation to exposure, and in addition, prospective studies investigating serum retinyl ester concentrations as biomarker of intake were also eligible. Endpoints of interest for sQ5a were bone fractures (all sites), either self‐reported or diagnosed by a physician. For sQ5b only studies that measured BMD/BMC by dual‐energy x‐ray absorptiometry (DXA) or peripheral quantitative computed tomography (pQCT), and measures of bone strength derived from pQCT, were eligible.
For sQ5 (bone health), a total of 795 unique records were identified after removing duplicates and screened at title and abstract level. At full‐text screening, 48 papers were assessed for eligibility, and in addition, two relevant papers from the SCF (2002) assessment were identified, resulting in 20 papers that met the inclusion/exclusion criteria. At data extraction level, four papers were excluded (Annex H). No RCTs meeting the inclusion criteria were identified. Among the 17 prospective observational (3 NCC, 14 PC; 18 publications) studies that met the inclusion criteria, nine report on bone fractures only, five on BMD only and three report on both endpoints (Appendix A, Figure A.3).
Reasons for references excluded at full‐text screening, or during data extraction, are outlined in Annex H.
2.1.1.3. Data extraction
Data were extracted into Microsoft Excel® by two extractors and were jointly discussed, compared and harmonised at several time points. Evidence tables were prepared in Microsoft Word® and are provided in Appendix B.
Intakes of preformed vitamin A were extracted and converted into μg RE/day using the conversion factors depicted in Table 5 (Section 3.1). Intakes of total vitamin A, the provitamin A carotenoids included in the exposure and the conversion factors used, when reported by the authors, were extracted. For studies reporting on vitamin A intake from diet and supplements separately and combined, only data from the most aggregated exposure were extracted, unless otherwise noted. For further details on data collection and preparation methods, see the technical report (Olsen et al., 2024).
TABLE 5.
Conversion factors for preformed vitamin A.
| Vitamin A form | Vitamin A activity in IU | Vitamin A activity in RE |
|---|---|---|
| Retinol (1 mg) | 3330 | 1000 |
| Retinyl acetate (1 mg) | 2900 | 870 |
| Retinyl palmitate (1 mg) | 1830 | 550 |
Note: Adapted from SCF (2002).
Abbreviations: IU, International units; RE, retinol equivalents.
2.1.1.4. Requests for additional information
Additional data were requested from study authors when this information was pertinent to the interpretation of the study results. See Annex G for details on data requested.
2.1.2. Methodologies
The methodology for this assessment follows the draft guidance for establishing ULs developed by the NDA Panel (EFSA NDA Panel, 2022). EFSA's transversal guidance for use in scientific assessments in relation to the application of the systematic review methodology in food and feed safety (EFSA, 2010), the principles and processes for dealing with data and evidence (EFSA, 2015b), the assessment of the biological relevance of data (EFSA Scientific Committee, 2017a), the use of weight of evidence (EFSA Scientific Committee, 2017b), the appraisal and integration of evidence from epidemiological studies (EFSA Scientific Committee, 2020) and the analysis of uncertainty in scientific assessments (EFSA Scientific Committee, 2018) have also been considered.
The methodology used for the appraisal of the internal validity of included studies from the systematic reviews, for evidence synthesis and integration and for the analysis of uncertainty in the context of this assessment, is described below.
2.1.2.1. Evidence appraisal (sQ3, sQ4, sQ5)
The internal validity of eligible studies for which data were extracted in relation to subquestions 3a, 4a and 5a/b (i.e. addressed through systematic reviews) was assessed in duplicate by two independent reviewers using a customised version of the Office of Health Assessment and Translation (OHAT) risk of bias (RoB) tool developed by the US National Toxicology Program (NTP) (OHAT/NTP, 2015). Any discrepancies in the RoB assessment for each bias domain were discussed among the assessors. If there was disagreement, a third reviewer was consulted for resolution.
For observational studies, the appraisal addressed six RoB questions, covering five domains. The questions considered the most critical for the allocation of studies to RoB tiers (key questions) were those related to confounding and those related to detection bias in the exposure and outcome. For intervention studies, the appraisal addressed eight RoB questions, covering seven domains, with the key questions being related to randomisation, exposure and outcome. In accordance with the OHAT/NTP guidelines, the RoB tool was customised to fit the specific nature of the review questions. The default OHAT/NTP tiering approach, which combines the evaluations of all the RoB questions into an overall RoB judgement (i.e. low (tier 1), moderate (tier 2) or high (tier 3) RoB), was also modified (Table 3). The OHAT RoB tool proposes five response options for each RoB question: definitely low RoB (++), probably low RoB (+), not reported (NR), probably high RoB (−), definitely high RoB (−).
TABLE 3.
Modified version of the OHAT predefined algorithm.
| Tier 1 | Study must be rated as ‘definitely low’ ++ or ‘probably low’ + risk of bias for all key criteria AND have most other applicable criteria rated as ‘definitely low’ ++ or ‘probably low’ + risk of bias | Low RoB |
| Tier 2 | Study does not meet criteria for Tier 1 or Tier 3 | Moderate RoB |
| Tier 3 | Study must be rated as ‘definitely high’ −− or ‘probably high’ –/NR risk of bias for most (at least two) key criteria AND have most other applicable criteria rated as ‘definitely high’ −− or ‘probably high’ –/NR risk of bias | High RoB |
The forms used for the RoB assessment for sQ3 and sQ4, including the explanations for expert judgements, can be found in Appendix F of the technical report (Olsen et al., 2024) and for sQ5 on bone health in Annex F of the opinion.
2.1.2.2. Evidence synthesis (sQ3, sQ4, sQ5, sQ6)
For sQ3, sQ4 and sQ5, a narrative qualitative synthesis of the evidence was performed through descriptive forest plots when three or more studies were available for a given endpoint. A quantitative synthesis of the evidence through meta‐analyses or dose–response analyses was not performed for these sQs, owing to the low number of studies available for each exposure and endpoint.
A narrative synthesis of the available evidence was performed for sQ6.
2.1.2.3. Evidence integration and uncertainty analysis (sQ3, sQ4, sQ5, sQ6)
Hazard identification
For the priority endpoints of teratogenicity (sQ3), hepatotoxicity (sQ4) and bone health (sQ5), the adverse effects of preformed vitamin A at very high doses are all well established. Thus, the purpose of the hazard identification step is to assess the available evidence for a positive and causal relationship between preformed vitamin A intake and the risk of adverse health effects at doses at or below the current UL of 3000 μg RE/day. A second objective was to investigate in the context of the available data, whether the intake of β‐carotene could potentiate the toxicity of preformed vitamin A on these endpoints. Uncertainties in the body of evidence are narratively described and no comprehensive uncertainty analysis is performed.
Hazard characterisation
At this step, evidence is integrated to select the critical effect(s) and identify a reference point (RP) for establishing the UL. If the available data are not suitable for dose–response modelling, a no‐observed‐adverse‐effect level (NOAEL) or a lowest‐observed‐adverse‐effect level (LOAEL) could be identified and used as the RP. ULs are derived for different life‐stage groups using relevant data for each group, where available. The UL is derived as follows: UL = RP/UF, where UF is an uncertainty factor which accounts for the uncertainties associated with extrapolating from the observed data to the general population, as ULs should be protective for all members of the general population, including sensitive individuals, throughout their lifetime (EFSA NDA Panel, 2022). The rationale for the selection of the RP and UF is documented in the scientific opinion.
If there are no, or insufficient, data on which to base an UL, the Panel will give an indication on the highest level of intake where there is reasonable confidence in data on the absence of adverse effects, i.e. a safe level of intake.
2.2. Dietary intake assessment
The assessment follows the approach outlined in the protocol for the intake assessments performed in the context of the revision of ULs for selected nutrients (EFSA, 2022).
Briefly, the EFSA's food composition and food consumption databases were used to obtain harmonised intake estimates in EU populations of preformed vitamin A and β‐carotene from the background diet. Such intake estimates include the use of β‐carotene as food additive, as analytical data cannot differentiate between this and the natural content in foods. Other data sources were used to gather non‐harmonised intake estimates of preformed vitamin A and β‐carotene from the background diet, fortified foods and food supplements, either alone or in combination, in European countries (i.e. intake estimates from nationally representative food consumption surveys), and data on the amounts of preformed vitamin A and β‐carotene used for food fortification (excluding the use of β‐carotene as food additive) and in food supplements (i.e. Mintel Global New Product Database [GNPD]).
2.2.1. Data
2.2.1.1. EFSA's databases
Food intake data from the EFSA Comprehensive European Food Consumption Database (hereinafter referred as Comprehensive Database) and data from the EFSA food composition database (FCDB) were used to estimate the intake of preformed vitamin A 3 and β‐carotene from the background diet in EU populations (Sections 3.5.1.2 and 3.5.2.2). In food composition tables, including the EFSA FCDB, the term retinol is commonly used, although it includes also retinyl acetate and retinyl palmitate, alone or in combination. The EFSA FCDB does not provide content of retinyl esters in food.
Food consumption data
The Comprehensive Database provides a compilation of existing national information on food consumption at individual level collected through repeated non‐consecutive 24‐h dietary recalls or dietary records (EFSA, 2011b, 2011a). The latest version of the Comprehensive Database, updated in 2022, contains results from a total of 83 different dietary surveys carried out in 29 different European countries (including EU member states, pre‐accession countries and the United Kingdom) covering 154,388 individuals. In this assessment, food consumption surveys from 22 EU member states covering at least 2 days per subject were used. Dietary assessment methods used in national surveys included in the EFSA Comprehensive Database are repeated 24 or 48‐h dietary recalls (2–4 days), food records alone or in combination with 24‐h dietary recalls (2–7 days, 9 days only in the survey in lactating women in Greece) or web‐based dietary recalls (2–4 days), with the majority of the surveys covering 2 or 3 days. Food frequency questionnaires (FFQ) have not been available in EFSA for any of the surveys.
Among the 83 surveys included in the EFSA Comprehensive database, the vast majority lasted 12 months or more. Exceptions were a few national surveys, mostly conducted among specific population groups such as pregnant or lactating women and vegetarians. Overall, the duration of most surveys ensures that seasonality is considered. In particular, the methodology for National Surveys conducted under the EU Menu project (EFSA, 2014) provides for an equal distribution through the four seasons.
Food composition data
Composition data for preformed vitamin A and for β‐carotene in foods and beverages (including β‐carotene used as food additive) were derived from the EFSA FCDB, which was compiled as a deliverable of the procurement project ‘Updated food composition database for nutrient intake’ (Roe et al., 2013). The EFSA FCDB contains data for energy, macro‐ and micronutrients from national food composition databases provided by 14 national food database compiler organisations covering approximately 1750 food entries and harmonised information on the most common composite recipes of European countries up to 2012. When needed, publicly available national food composition databases and the Mintel GNPD 4 were used to complement EFSA's FCDB. More details on these data sources are described in Annex B of this opinion.
2.2.1.2. Other data sources
Food consumption data
EFSA collected intake estimates from nationally representative food consumption surveys on preformed vitamin A and β‐carotene from natural sources, from addition to foods and from food supplements. No date limits were applied. Between September and November 2021, 64 competent authorities in 37 European countries have been contacted through the EFSA Focal Points 5 and the EFSA Food Consumption Network. 6 An additional search in sources of bibliographic information (Google Scholar, PubMed) was performed to collect reports of national surveys included in the Comprehensive Database that had not been obtained through the competent authorities. Between August and October 2022, EFSA contacted all EU Member States and Norway through the European Commission Working Group on Food supplements and Fortified foods 7 and collected data on the intake of preformed vitamin A and β‐carotene, specifically from food supplements (Sections 3.5.1.3, 3.5.1.4, 3.5.2.3 and 3.5.2.4).
The majority of the national food consumption surveys covered by this data collection relied on 24‐h recalls (in combination or not with FFQs) or food records to assess dietary intake (Annex E).
Food composition data
The Mintel GNPD was used as a data source to identify the content of preformed vitamin A or β‐carotene in fortified foods and food supplements available on the EU market (Sections 3.5.1.1 and 3.5.2.1).
For the retrieval of food supplements, the search on the Mintel GNPD included products that reported vitamin A on their nutrition label under the ‘vitamins and dietary supplements’ Mintel category.
For the retrieval of fortified foods, a search for food and drink products with vitamin A in the ingredient list and in the nutrition label was performed, assuming that these were vitamin A‐fortified products. To exclude food products in which vitamin A (as β‐carotene) is used as an additive (food colour), the data set was refined as follows:
a) Disaggregation of the ingredients list to identify β‐carotene, either explicitly reported as such in text or represented by the code E160a. 8
b) Products identified as containing β‐carotene that did not report a vitamin A value (or a β‐carotene) in the nutrition label were excluded from the search.
To differentiate the products by form of vitamin A (preformed vitamin A or β‐carotene), the ingredient lists of the products have been disaggregated to identify the form of vitamin A added to the foods or food supplements. The keywords used for preformed vitamin A were ‘retinol’ and ‘retinyl esters’ (acetate and palmitate), and for β‐carotene, it was ‘β‐carotene’.
The search was conducted in November 2022 and was limited to 5 years before, i.e. between November 2017 and November 2022 (Sections 3.5.1.1 and 3.5.2.1). The Panel notes that this search allows to capture the products that were newly introduced on the market and the products for which the packaging was changed during that period. Therefore, the information collected is indicative and does not represent a comprehensive overview of the products available on the market.
2.2.2. Methodologies
2.2.2.1. Intake data
Intake assessment from natural sources
The FoodEx2 classification and description system was used to facilitate the linkage between the food consumption and food composition databases (EFSA, 2015a). Food consumption and composition data used in the assessment were checked for consistency of FoodEx2 codes and the original food name in English (freely entered text).
Composition data on preformed vitamin A and β‐carotene were extracted from the EFSA FCDB and were subject to a cleaning procedure. As the scope of the intake assessment was to consider natural sources of preformed vitamin A and β‐carotene as well as β‐carotene used as additive, a data cleaning strategy was applied to exclude fortified foods from the composition database, including foods for weight reduction, 9 and single meal replacements 10 (Annex B). This is with the exception of infant and follow‐on formulae for which data from the Mintel's GNPD were used for the calculations for preformed vitamin A. Indeed, the minimum content of vitamin A in these food categories is subject to regulatory requirements to guarantee an adequate supply of the nutrient to the consumers (Regulation (EU) 2016/127 11 and Regulation (EU) 2017/152211). β‐Carotene is not authorised for use in infant and follow‐on formulae.
Dietary intakes of preformed vitamin A in μg RE/day and β‐carotene in mg/day from natural food sources were calculated linking food consumption data at individual level to food composition data. The resulting intakes per food item were summed up to obtain total daily intakes for each individual. The mean, P5, median and P95 of intakes were subsequently calculated for each survey by population group and sex, as well as total populations.
The methodology followed for the assessment of intake from natural sources is further detailed in Annex B.
Intake assessment from fortified foods and food supplements
Data on the intake of preformed vitamin A and β‐carotene from recent national food consumption surveys, including specific estimates of intake from food supplements and/or fortified foods, were extracted and are provided in Annex E. These data have been used to evaluate the accuracy of the results obtained, comparing EFSA's estimates with published national intake estimates from the same surveys with the same (or similar) window of data collection and population groups, when available (EFSA, 2022).
2.2.2.2. Food composition data from Mintel GNPD
Information on food products fortified with preformed vitamin A and β‐carotene and on supplements containing preformed vitamin A and β‐carotene available on the EU market as reported on the labels, were extracted from the Mintel GNPD. These data were used to describe the types of fortified foods and food supplements available and to gain insight into their potential contribution to the intake of preformed vitamin A and β‐carotene.
Under EU regulation, 12 labelling of vitamin A (the combination of preformed vitamin A and provitamin A carotenoids) should be in micrograms (μg) and expressed as percentage of the nutrient reference value (NRV). The NRV to which the regulation refers is 800 μg RE (SCF, 2003). The search of products containing vitamin A revealed a range of units used, such as international units (IU), μg or μg RE. Units are sometimes reported incorrectly. For products where more than one form of vitamin A was added, it was not possible to identify the amount of each form when this was not reported on the nutrition label, as the conversion factors used for provitamin A carotenoids were not reported. There were 116 food supplements and 24 fortified foods for which the forms of vitamin A added were a mix of preformed vitamin A and provitamin A carotenoids and for which each form's contribution to the content of vitamin A is unclear. For all these reasons, some products identified as containing vitamin A have been excluded from this report.
Regarding the products retrieved and included in Sections 3.5.1.1 and 3.5.2.1, some assumptions were made:
Fortified foods declaring vitamin A in the label without further specification were assumed to contain preformed vitamin A only, except for ‘juice drinks’ and ‘flavoured water’, which were assumed to contain vitamin A as β‐carotene, on the consideration that only these foods appear to be fortified with β‐carotene in some countries, and that no mandatory β‐carotene fortification policy has been reported in the EU (Appendix D);
Food supplements declaring only vitamin A in the label were assumed to contain preformed vitamin A, since β‐carotene supplements are usually labelled as such and pertain to the categories of tanning and skin care supplements;
Only fortified foods and food supplements declaring β‐carotene explicitly in the ingredients list have been considered to contain this carotenoid, with the exception made in point 1 for ‘juice drinks’ and ‘flavoured water’.
For fortified foods and food supplements containing preformed vitamin A (retinol and retinyl esters), the units reported as μg were assumed to be μg RE.9 Where available, the units of the products identified as containing only β‐carotene were kept as milligrams (mg) or were converted from μg RE to mg using a conversion factor of 1:6.
3. ASSESSMENT
3.1. Chemistry of vitamin A and β‐carotene and definition of terms
The term vitamin A comprises all‐trans‐retinol (also called retinol), the family of naturally occurring molecules associated with the biological activity of retinol (such as retinal, retinoic acid and retinyl esters), and the group of provitamin A carotenoids (such as β‐carotene, α‐carotene and β‐cryptoxanthin) that are dietary precursors of retinal/retinol, whereas the term retinoids refer to retinol and structurally related compounds, including its metabolites (retinyl ester, retinal and retinoic acid), and synthetic analogues (EFSA NDA Panel, 2015).
Retinol is composed of a β‐ionone ring, a polyunsaturated side chain and a polar end group, which makes it poorly soluble in water but easily transferable through membrane lipid bilayers. Preformed vitamin A consists predominantly of retinol and retinyl esters, which are supplied in the diet by animal‐derived products. Carotenoids are isoprenoids that contain up to 15 conjugated double bonds, synthesised in plants, fungi and microorganisms and occurring naturally in fruits and vegetables. To exhibit provitamin A activity, the carotenoid molecule must have at least one unsubstituted β‐ionone ring and the correct number and position of methyl groups in the polyene chain (Figure 1).
FIGURE 1.
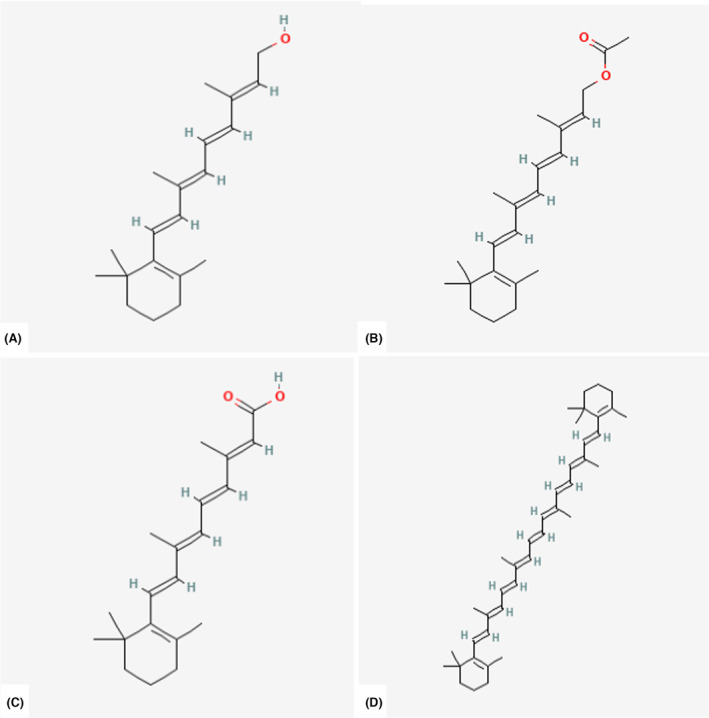
Chemical structure of different forms of vitamin A: (A) All‐trans‐retinol; (B) All‐trans‐retinyl ester (retinyl acetate); (C) All‐trans‐retinoic acid; (D) All‐trans‐β‐carotene. Source: PubChem.
In the EU, retinol, retinyl acetate, retinyl palmitate and β‐carotene are the forms of vitamin A authorised for addition to foods 13 and for use in food supplements 14 (Table 4). This includes foods for specific groups 15 with exception of infant and follow‐on formulae to which β‐carotene cannot be added.
TABLE 4.
Forms of vitamin A authorised as nutrient sources for human use in the EU.
| Terminology | Vitamin A form | Addition to foods Regulation (EC) 1925/2006 13 | Food supplements Directive 2002/46/EC 14 | |
|---|---|---|---|---|
| Vitamin A | Preformed vitamin A | Retinol | x | x |
| Retinyl acetate | x | x | ||
| Retinyl palmitate | x | x | ||
| β‐carotene | β‐carotene | x | x | |
The vitamin A activity of the above‐mentioned compounds is expressed as retinol equivalents (RE) or International Units (IU). The conversion factors used in this opinion for authorised forms of preformed vitamin A are in Table 5. Specific β‐carotene/retinol equivalency ratios have been defined to account for the less efficient absorption of β‐carotene and its bioconversion to retinol (see Section 3.3.5).
In this opinion, the following terminology is used:
Vitamin A refers to the forms of preformed vitamin A authorised for addition to foods and food supplements (retinol and retinyl esters) plus the provitamin A carotenoid β‐carotene (Table 4), and excludes retinal, retinoic acid and provitamin A carotenoids other than β‐carotene for which the dietary contribution to the overall toxicity of preformed vitamin A is expected to be marginal, unless otherwise noted. Whenever retinoids and carotenoids are mentioned, reference is made to their all‐trans‐isomers, unless indicated otherwise.
Total vitamin A has been used for data extraction from observational studies reporting on the combined intake of preformed vitamin A plus all provitamin A carotenoids considered by the authors. Whether total vitamin A intake estimates are from foods, supplements, or both, is further specified in data extraction tables.
Bioconversion denotes the fraction of absorbed β‐carotene that is converted into retinol in the body.
Bioefficacy is used to express the fraction of ingested β‐carotene that is absorbed and converted into retinol in the body (i.e. the product of absorption and bioconversion).
3.2. Absorption, distribution, metabolism and excretion
3.2.1. Intestinal absorption
3.2.1.1. Preformed vitamin A
It is generally assumed that the efficiency of absorption for preformed vitamin A over a wide range of intake is approximately 70%–90%, and that this is independent from vitamin A status (EFSA NDA Panel, 2015). However, direct measurements of preformed vitamin A absorption from the diet are scarce and current data often rely on single dose supplements (EFSA NDA Panel, 2015; Reddy & Sivakumar, 1972).
The majority of dietary preformed vitamin A is composed of long‐chain fatty acid esters of retinol, which are hydrolysed prior to intestinal absorption by the intestinal enterocytes (Harrison, 2012). Following hydrolysis, free retinol is absorbed into the enterocyte by both protein‐mediated facilitated uptake and passive diffusion mechanisms via the action of membrane‐bound lipid transporters (Reboul & Borel, 2011). The absorbed free retinol then undergoes esterification with mainly saturated long‐chain fatty acids by the enzymes lecithin:retinol acyltranferase (LRAT) and/or acyl‐CoA:retinol acyltransferase (ARAT), particularly when physiological doses of preformed vitamin A are ingested (Blomhoff et al., 1991; Harrison, 2012; O'Byrne & Blaner, 2013). The resulting retinyl esters are incorporated with dietary fat, cholesterol and absorbed carotenoids into chylomicrons, which are secreted into the lymphatic system for delivery to the bloodstream (Harrison, 2012; Ramkumar et al., 2021).
Fortified foods and food supplements may contain free retinol, long‐chain fatty acid esters of retinol and retinyl acetate, with the latter being absorbed by a similar route as long‐chain fatty acid esters of retinol. Importantly, some supplement formulations may contain either oil‐soluble or water‐miscible retinyl esters, with the latter being faster and more efficiently absorbed, particularly in patients with gastrointestinal diseases (Barnes et al., 1950; Silva et al., 2001). Water‐miscible formulations of preformed vitamin A have also been found to induce hypervitaminosis A faster and are thought to be 10 times more toxic than similar doses of oil‐based retinyl‐ester supplements (Myhre et al., 2003).
3.2.1.2. β‐Carotene
Similar to preformed vitamin A, the absorption of β‐carotene depends on the release of this provitamin A carotenoid from the food matrix followed by emulsification with dietary fatty acids and bile salts to form mixed micelles in the intestinal lumen (Iddir et al., 2022; Maurya et al., 2022; Parker, 1996). Dietary β‐carotene is then absorbed via passive diffusion or taken up by the enterocyte through facilitated transport via intestinal transport proteins such as scavenger receptor class B type I (SR‐BI), cluster determinant 36 (CD36), Niemann–Pick C1‐like 1 (NPC1L1) or ATP‐binding cassette A1 (ABCA1) (Reboul & Borel, 2011). The absorption of dietary β‐carotene appears to be highly variable (between 5% and 65%), depending on food‐ and diet‐related factors, genetic characteristics and the health and vitamin A status of the subject (Haskell, 2012; Maurya et al., 2022). Key factors that contribute to this large inter‐individual variation are the dietary release of β‐carotene from the food matrix and its micellisation, combined with its cellular uptake and intracellular metabolism in the enterocytes (Bohn et al., 2017). Inside the enterocyte, the majority (> 50%) of the absorbed β‐carotene molecules are cleaved at their central bond into all‐trans‐retinal by the enzyme β, β‐carotene‐15,15′‐dioxygenase (BCO1). Although variations in bioconversion efficiency range from 50% to 93%, the large inter‐individual variation in bioefficacy of dietary β‐carotene appears to be mostly driven by the high variability in absorption (Ford et al., 2018). Differences in absorption efficacy appear to exist also between β‐carotene in food vs. fortified foods and food supplements. In the latter two cases, β‐carotene may be present in highly soluble forms, i.e. emulsified to enhance its solubility in an aqueous environment, which translates into unusually high circulating plasma levels of β‐carotene (see Section 3.3.4.2).
All‐trans‐retinal bound to cellular retinol binding protein type II (CRBPII) is further oxidised irreversibly to retinoic acid or reduced reversibly to retinol, which is subsequently esterified by the enzymes LRAT and/or ARAT to form retinyl‐esters (Harrison, 2012). Both the remaining absorbed β‐carotene that is not cleaved in the intestine and the resulting retinyl esters are incorporated into nascent chylomicrons and secreted into the lymphatic system for delivery to the bloodstream (Harrison, 2012; Ramkumar et al., 2021).
Although extra‐intestinal tissues have the ability to cleave β‐carotene by both BCO1 or the enzyme β, β‐carotene‐9′,10′‐dioxygenase 2 (BCO2), which catalyses an asymmetrical cleavage of carotenoids, the lack of asymmetric carotenoid cleavage products in postprandial plasma suggests that BCO2 is not involved in intestinal cleavage of β‐carotene (Kopec et al., 2018). Both the liver and the intestine have BCO1 activity, around 81% of the total vitamin A formed from a β‐carotene dose is from intestinal cleavage and 19% is from extra‐intestinal cleavage (post‐absorptive conversion) (Ford et al., 2017; Tang et al., 2003). Importantly, high intakes of preformed vitamin A downregulate the bioconversion of β‐carotene via a negative feedback loop that involves the intestine‐specific transcription factor intestine‐specific homeobox (ISX), allowing ISX to act as a retinoic acid‐sensitive gatekeeper that controls vitamin A production at the intestinal level (Lobo et al., 2010).
3.2.2. Transport in blood and distribution to tissues
The majority of the absorbed retinol is delivered to the blood via lymph as retinyl esters in chylomicrons, although a small amount is also secreted directly from the enterocyte into the portal circulation (Blomhoff et al., 1991; Goodman et al., 1966; Yeung & Veen‐Baigent, 1972). Following secretion of chylomicrons into the blood stream, these lipoproteins are exposed to several processes, such as triacylglycerol hydrolysis and apolipoprotein exchanges, resulting in the formation of chylomicron remnants (Blomhoff et al., 1991; O'Byrne & Blaner, 2013). Chylomicron remnants are primarily taken up by the liver parenchymal cells. Retinyl esters obtained from these chylomicron remnants are immediately hydrolysed to retinol in hepatocytes and either directly secreted into the blood stream bound to retinol‐binding protein (RBP4) or re‐esterified to retinyl esters for storage in lipid droplets in hepatic stellate cells (Blomhoff et al., 1991; EFSA NDA Panel, 2015; O'Byrne & Blaner, 2013). It is worth to note that a small proportion (5%–10%) of retinyl esters in chylomicrons may be transferred to other lipoproteins during chylomicron catabolism (Blomhoff et al., 1991; O'Byrne & Blaner, 2013). Similarly, unaltered β‐carotene is also transported from the intestinal cell via the lymph to the blood stream where it is repartitioned between lipoproteins, so that plasma β‐carotene can be found in chylomicrons, chylomicron remnants, very low‐density lipoprotein (VLDL), low‐density lipoprotein (LDL) and high‐density lipoprotein (HDL) (Blomhoff et al., 1991; EFSA NDA Panel, 2015; O'Byrne & Blaner, 2013).
Several different forms of vitamin A are found in the circulation, which differ depending on the fasting and postprandial states. Retinyl esters can be found in chylomicrons, chylomicron remnants, VLDL, LDL and HDL; retinol is bound to RBP4; retinoic acid is bound to albumin; and water‐soluble β‐glucuronides of retinol and metabolites of retinoic acid are found in the plasma (Blomhoff et al., 1991; O'Byrne & Blaner, 2013). The delivery of retinoids to tissues is complex. The most important pathway involves retinol bound to RBP4 and retinyl esters bound to chylomicrons and chylomicron remnants during the postprandial phase. Approximately 66%–75% of chylomicron‐bound retinyl esters are cleared by the liver, with the reminder taken up by peripheral tissues (Blomhoff et al., 1991; O'Byrne & Blaner, 2013). Retinol bound to RBP4 is recognised by the cell‐surface receptor STRA6, which mediates uptake of retinol into cells (Noy, 2016). Uptake of retinol from RBP4 via the STRA6 receptor is particularly important for the retinal pigmented epithelium (RPE) cells of the eye, even though alternative pathways can also deliver retinol into the RPE to a lesser extent (O'Byrne & Blaner, 2013).
3.2.3. Storage
The main storage form of retinol is retinyl esters, with the majority of vitamin A stored in the liver. However, other tissues such as the eye, lung, adipose tissue, testes, skin and spleen also accumulate retinyl esters. Within the liver, the majority of retinyl esters is concentrated in the lipid droplets of stellate cells, with hepatocytes only storing 10%–20% of hepatic vitamin A (Blomhoff et al., 1991; EFSA NDA Panel, 2015; O'Byrne & Blaner, 2013).
Similarly for carotenoids, the liver is a major organ for carotenoid accumulation and metabolism since it is a central hub for lipoprotein assembling and release (Bohn et al., 2017). The adipose tissue is another important site of carotenoid accumulation and contains the highest total amounts due to its tissue mass (Bohn et al., 2017).
3.2.4. Metabolism
Within cells retinol can be oxidised to the transcriptionally active metabolite retinoic acid (RA), which accounts for the regulatory properties of vitamin A in more than 500 different target genes involved in cell differentiation and development, reproductive and immune functions and regulation of lipid and energy metabolism (EFSA NDA Panel, 2015). RA can be isomerised through a non‐enzymatic process to 9‐cis‐ or 13‐cis‐retinoic acid isomers. Retinyl esters are the substrate for RPE65, an enzyme that produces 11‐cis retinol, which is converted to 11‐cis retinaldehyde for transport to the photoreceptors to form rhodopsin to enable vision.
3.2.5. Excretion
The majority of retinol metabolites are excreted in the urine and faeces, with minimal amounts excreted in breath. Retinol is metabolised in the liver to numerous products, some of which are conjugated with glucuronic acid or taurine for excretion in bile. Animal data indicate that the amount of retinol metabolites excreted in bile increases as the liver retinol exceeds a critical concentration, which may serve as a protective mechanism for reducing the risk of excess vitamin A storage (Blomhoff et al., 1991; EFSA NDA Panel, 2015; O'Byrne & Blaner, 2013). Catabolism and irreversible loss of vitamin A from the body, mostly in urine and faeces, has been measured using isotopic tracer techniques (Aklamati et al., 2010). The absolute loss of vitamin A increases in relation to total body vitamin A stores, resulting in a fairly constant fractional catabolic rate of ~0.5%–0.7% per day.
Not much is known regarding carotenoid excretion pathways. Approximately 5% of an isotopically labelled β‐carotene dose was excreted in urine and 70% in faeces (Bohn et al., 2017).
3.3. Biomarkers of intake for vitamin A, including β‐carotene
3.3.1. Liver retinol concentrations
3.3.1.1. Relationship between vitamin A intake and liver retinol concentrations
In healthy individuals, about 70%–90% of the retinol in the body is stored in the liver, whereas it can decrease to ≤ 50% in severely vitamin A‐deficient individuals (EFSA NDA Panel, 2015). The predominant storage form of vitamin A are retinyl esters (> 95%), within lipid droplets of stellate cells, which store 70%–90% of liver vitamin A (Blomhoff et al., 1991; O'Byrne & Blaner, 2013). The total liver concentration of vitamin A (i.e. free and esterified retinol), expressed per weight of liver tissue, is regarded as the best available measure of vitamin A status (Tanumihardjo et al., 2016).
Liver retinol content can be measured directly by the analysis of liver biopsies (the gold standard), which are invasive and not always feasible, or indirectly by stable retinol isotope dilution (RID) methods or the relative dose–response (RDR). As the sensitivity of the RDR is notoriously limited to liver retinol concentrations in the vitamin A deficiency range, only RID methods will be described below.
The RID method is currently considered the best available indirect method to assess total body vitamin A status in humans (Gannon & Tanumihardjo, 2015; Green et al., 2020; Lietz et al., 2016). A small dose of retinol tracer labelled with stable deuterium (2H) or carbon (13C) is given orally, and the dilution of the tracer in plasma is measured when the labelled dose has mixed with endogenous stores and equilibrium is reached (14–21 days after administration). The total body exchangeable retinol pool can then be derived from an RID equation based on the measurement of the ratio of labelled retinol to total retinol in plasma at a specified time after dosing, i.e. usually after 14–21 days after dose administration (Gannon & Tanumihardjo, 2015; Green et al., 2020; Lietz et al., 2016). Liver stores can be further determined by considering that the amount of retinol stored in the liver is positively correlated with the size of the total body pool. Between 40% and 90% of the total body retinol pool is assumed to be stored in the liver, depending on the vitamin A status of the subject.
RID methods have been validated against liver biopsies in a wide range of physiological conditions, from vitamin A deficiency to excess, and are considered a sensitive method to assess vitamin A status in vivo, at least at the group level (Furr et al., 1989; Haskell et al., 1997). However, they may lack precision to assess vitamin A status at individual level, owing to the large inter‐individual variability of the factors used in the equation and limited knowledge about how individual factors such as infection, iron deficiency and/or liver disease may impact the results. Liver and total body retinol stores assessed by RID have been shown to correlate well with measures of habitual vitamin A intake in cross‐sectional studies over a wide range of intakes and to respond to vitamin A supplementation in intervention studies (EFSA NDA Panel, 2015; Lietz et al., 2016).
3.3.1.2. Relationship between liver retinol concentrations and adverse health effects
Liver concentrations of retinol of 0.07 μmol/g (as free retinol and retinyl esters) have been proposed as a criterium to define vitamin A adequacy and were used as a reference point to derive vitamin A requirements by different bodies (EFSA NDA Panel, 2015). More recently, the cut‐off for vitamin A deficiency has been revised to 0.1 μmol/g liver based on the fact that long‐term storage and biliary excretion do not occur below this liver retinol concentration (Tanumihardjo, 2021; Tanumihardjo et al., 2016).
Defining cut‐offs for excess liver retinol concentrations, however, is more controversial. In 2016, the BOND expert panel proposed provisional cut‐offs for high liver vitamin A stores as determined by stable isotope methods and excess vitamin A liver stores (i.e. vitamin A toxicity) as liver retinol concentrations > 1 μmol/g and > 10 μmol/g, respectively, until more animal studies become available on the relationship between liver retinol stores and adverse health effects (Tanumihardjo et al., 2016).
Some recent studies seem to question these cut‐offs. Liver retinol concentrations > 1 μmol/g have been reported in 59% (Mondloch et al., 2015) and 64% (van Stuijvenberg et al., 2019) of pre‐school children with vitamin A intake below the UL of 900 μg/day for the respective age group, with no observed adverse health effects. When children were consuming vitamin A at intake levels above the UL for their respective age group, no adverse health effects were recorded even though liver retinol concentrations were > 1 μmol/g in over 78% of children (Engle‐Stone et al., 2022). In a post‐mortem study, hepatotoxicity (e.g. hypertrophy of stellate cells in one person; perisinusoidal space enlargement and lipid droplets in 3 persons) was found at liver retinol concentrations ≥ 2.87 μmol/g, suggesting a cut‐off of 3 μmol/g as indicator of vitamin A toxicity. Total liver vitamin A reserves and serum retinol were not significantly correlated in this study while they were significantly correlated with circulating retinyl ester concentrations. Lack of knowledge about other concomitant conditions inducing hypertrophy of stellate cells (e.g. hepatitis, chronic biliary disease, metabolic liver fibrosis) limit these conclusions (Hoffmann et al., 2020).
The Panel notes the paucity of available data on the relationship between vitamin A intake and hepatic retinol concentration on the one hand, and between hepatic retinol concentration and adverse health effects on the other hand, which limits the use of liver retinol concentrations both as a marker of vitamin A intake and as an endpoint for establishing a UL.
3.3.2. Plasma/serum retinol concentrations
In the bloodstream, the main form of vitamin A (> 95%) is retinol bound to retinol‐binding protein (RBP). Plasma or serum retinol concentration is under tight homeostatic control, is not related to habitual vitamin A intake (from either preformed vitamin A or provitamin A carotenoids), does not respond to vitamin A supplementation and is therefore not a sensitive indicator of vitamin A status in an individual (EFSA NDA Panel, 2015). However, the distribution of plasma retinol concentrations in a population has been used to provide information about the vitamin A status and their response to vitamin A supplementations (Sommer & Davidson, 2002).
Normal serum retinol concentration ranges between 1.7 and 4 μmol/L and is under tight homeostatic control. In addition, serum retinol concentration is affected by dietary and other factors, including inflammation and infections, which can lower mean plasma/serum retinol concentration by as much as 25%, independently of vitamin A intake (EFSA NDA Panel, 2015; IOM, 2001).
A severe vitamin A deficiency related to liver store depletion is characterised by retinol serum concentrations of < 0.7 μmol/L (Tanumihardjo et al., 2016), whereas plasma concentrations as the sum of retinol and retinyl esters > 3.5 μmol/L have been regarded as a sign of hypervitaminosis A (Olson, 1990). However, several case studies have reported hypervitaminosis A with serum retinol concentrations in the normal range (Penniston & Tanumihardjo, 2006).
The panel notes that plasma/serum retinol concentrations are maintained nearly constant over a wide range of vitamin A intakes, can be strongly affected by factors unrelated to vitamin A status and may not correlate with adverse health effects of vitamin A toxicity, which limit their use both as a marker of vitamin A intake and as an endpoint for establishing a UL.
3.3.3. Plasma/serum retinyl esters concentrations
Circulating retinyl esters have been discussed for their potential use as a marker of vitamin A toxicity, as the capacity of the liver to remove them from the circulation and store retinyl esters may be reduced at high vitamin A liver stores. However, retinyl esters can only be used as indicators for vitamin A toxicity if blood was drawn in the fasted state, as retinyl esters increase in the circulation 3–5 h after a meal. More importantly, the BOND expert panel noted that protein malnutrition, liver disease and hypertriglyceridaemia can all result in elevated retinyl ester concentrations when in fact total vitamin A status is normal (Tanumihardjo et al., 2016).
No established cut‐off with regard to normal circulating levels of retinyl esters exists. This is due to the fact that cut‐off values of 244 nmol/L or 130 nmol/L have been described for normal percentages of total serum vitamin A as retinyl esters of 10%–11% for healthy young adults (Olsen et al., 2018; Tanumihardjo et al., 2016). In the NHANES study III, normal fasting retinyl ester levels < 244 nmol/L in serum were reported (Ballew et al., 2001). Krasinski et al. (1989) reported lower normal values in 194 young adults (< 130 nmol/L) and the elderly (n = 562, < 170 nmol/L). These values were in line with the fraction of retinyl esters of total circulating vitamin A being below 10% and 11% in the two studies, respectively. Typical values of 100–200 nmol/L retinyl esters were also reported by O'Byrne and Blaner (2013).
Comparably high circulating concentrations of retinyl esters have been measured in clinically confirmed cases of vitamin A toxicity, with retinyl ester plasma concentrations ranging between 1600 and 16,000 nmol/L, with up to > 60% of circulating vitamin A present as retinyl esters (Ellis et al., 1986; Smith & Goodman, 1976).
Preformed vitamin A supplementation has been shown to increase fasting plasma concentrations of retinyl esters. Supplemental vitamin A intakes of 1500–3000 μg/day for 3 months induced a 1.5‐ and 2.5‐fold increase in fasting plasma concentrations of retinyl esters vs. non‐supplement users in young and older adults, respectively (Krasinski et al., 1989). In the same study, higher prevalence of liver damage (high AST and ALT concentrations) was observed with fasting plasma retinyl ester concentrations ≥ 380 nmol/L in adults aged ≥ 60 years. Long‐term supplemental vitamin A intake (up to 14,100 μg RE/day) was also significantly correlated with serum retinyl ester concentrations (R = 0.74) in 116 older adults, which reached values of 254 (range 180–419) nmol/L in individuals consuming 6690 μg vitamin A per day. Fasting plasma retinyl esters varied between 3.4% and 10.2% of plasma retinol plus retinyl esters (Stauber et al., 1991).
A fraction of fasting plasma retinyl esters ≥ 30% was suggested as sign of vitamin A toxicity based on three cases (Smith & Goodman, 1976). Lower cut‐off points of either 10% (Tanumihardjo et al., 2016) or 7.5% for adults based on post‐mortem histological analysis in three adults with total liver retinol concentrations > 3 μmol/g (Olsen et al., 2018) were proposed. The recent lower cut‐off values are, however, questionable. In the NHANES III study, out of 6545 participants, 37% of participants showed serum retinyl ester fractions > 10%, and 10% showed concentrations > 15%; however, serum retinyl ester fractions > 10% were not associated with abnormal liver function (Ballew et al., 2001). In addition, liver diseases such as cirrhosis have also been reported to result in elevated serum retinyl ester concentrations of up to 42 μg/L (147 nmol/L) (Ballew et al., 2001; Ukleja et al., 2002) (Ballew et al., 2001). Finally, old age alone can result in impaired clearance of retinyl esters from chylomicron circulation after a meal, and thus result in higher blood retinyl ester concentrations for longer periods of time after eating than in young adults (Krasinski et al., 1989).
For children, a 5% cut‐off level for hypervitaminosis A has been proposed based on total liver vitamin A stores by RID (Mondloch et al., 2015), and as a more prudent cut‐off level if RID is unavailable (Williams et al., 2021).
The Panel notes that, although fasting plasma retinyl ester concentration may be a useful marker of ‘high’ vitamin A intake, the proposed cut‐offs for excess vitamin A and vitamin A toxicity are based on limited data and there is currently no consensus regarding levels indicative of excess vitamin A intake or hepatic toxicity. Therefore, the Panel considers that fasting retinyl ester concentrations lack sufficient validation to be used in isolation for setting ULs for vitamin A, but data on this variable will be extracted from the observational studies included in this opinion, where available, as a marker of vitamin A intake.
3.3.4. Other markers
3.3.4.1. Preformed vitamin A
Since all‐trans‐retinoic acid appears to be the bioactive species responsible for most of the toxic effects of vitamin A (Al Tanoury et al., 2013) through the activation of a number of genes following interactions with transcription factors/nuclear receptors, specifically RAR/RXR, downstream gene expression products related to such pathways, such as FGF21 related to fatty acid oxidation or CYP27A1 involved in cholesterol breakdown, may be indicators of vitamin A toxicity (Li et al., 2021). The GloVitAS group (Engle‐Stone et al., 2022; Lietz et al., 2019) has recently engaged in the validation of such markers using multi‐omics tools, also in relation to vitamin A toxicity (Schönberger et al., 2022). Lipidomics within metabolomics has a high potential for identifying downstream individual or composite markers related to vitamin A status and toxicity (Johnson et al., 2022; Marqueño et al., 2021). Although promising and analytically well developed, there is still a lack of studies using (multi‐)omics tools to determine vitamin A status and toxicity aspects due to the complexity (analytically and statistically) and cost of such methods.
Conjugates of retinoic acid, i.e. glucuronides following phase‐2 metabolism by UDP‐glucuronyl transferase, may be measured in blood or urine. Retinyl‐, retinoic acid and oxi‐retinoic acid glucuronides have also been measured in serum, stool or urine (Barua & Sidell, 2004; Samokyszyn et al., 2000; Sass et al., 1995). However, there is no consensus on normal or elevated concentrations of such metabolites.
3.3.4.2. β‐Carotene
Plasma concentration of β‐carotene has been used as an approximate marker of dietary β‐carotene intake. Its plasma half‐life has been reported to be about 37 days (Burri et al., 2001). However, the source of β‐carotene and the pattern of consumption have shown to have a major impact on plasma β‐carotene concentrations. Data from an RCT indicate that consumption of beverages containing β‐carotene as synthetic water‐dispersible powder induced higher mean plasma β‐carotene concentrations when consumed daily for 6 weeks (3.84 μmol/L for a dose of β‐carotene of 7.2 mg/day and 5.04 μmol/L for a dose of 21.6 mg/day) compared to non‐fortified carrot juice‐based beverages (0.42 μmol/L for a dose of β‐carotene of 6 mg/day and 1.71 μmol/L for a dose of 18 mg/day) (Thürmann et al., 2002). Apparent steady‐state concentrations were reached after 40 days of supplementation and the apparent half‐life for plasma clearance was 6–11 days. Data from RCTs also indicate that daily consumption of supplements containing 20 mg of synthetic water soluble all‐trans‐β‐carotene lead to higher mean plasma β‐carotene concentrations than consumption of a different β‐carotene formulation at doses of 50 mg every other day (5.59 μmol/L vs. 2.19 μmol/L, respectively) (ATBC Study Group, 1994; Cook et al., 2000, see Section 3.6.4.2), emphasising the importance of the β‐carotene formulation and frequency of intake on bioavailability.
It is known that vitamin A status influences both the absorption and bioconversion of dietary β‐carotene to preformed vitamin A, as high vitamin A status activates the intestinal homeobox transcription factor ISX, which reduces transport‐related cellular uptake and also BCO1 cleavage activity (Widjaja‐Adhi et al., 2015). Correlation coefficients of 0.09–0.52 (average 0.29 +/−0.14 CV) between the dietary intake and plasma levels of β‐carotene have been reported (Böhm et al., 2021). It appears that, for a given intake, the larger part of the interindividual variability in plasma concentration of β‐carotene may be attributable to differences in absorption rather than bioconversion. In a study using isotopically labelled β‐carotene in 45 young individuals, a CV of 14% for bioconversion and a CV of 44% for bioefficacy (i.e. the product of absorption and conversion), were reported (Ford et al., 2018). Concentrations in adipose tissue have been proposed as a marker of long‐term β‐carotene intake, but limited data on this marker are available (Bohn, 2018).
Owing to the large CV of β‐carotene regarding its absorption and bioconversion, plasma β‐carotene has not been validated as a biomarker of β‐carotene intake from all sources or as a biomarker of status. Plasma β‐carotene concentrations, however, can provide an indication of dietary intake in populations, which could particularly be useful to interpret the potential adverse health effects of different sources of β‐carotene at high intakes.
3.3.5. Retinol equivalents
β‐Carotene is converted into vitamin A via BCO1 in various tissues, especially the intestine and the liver (dela Seña et al., 2013). As discussed in Section 3.2, the conversion efficacy of β‐carotene into retinol depends on several extrinsic factors (dose intake of β‐carotene, food composition, the food matrix), but also on host‐related factors (genetics, age, sex, nutritional status, digestive dysfunction and illness), which directly or indirectly affect BCO1 activity.
Different retinol/provitamin A carotenoids equivalency ratios have been used to derive dietary recommendations and reference values for vitamin A (Böhm et al., 2021). Conversion factors where 1 μg RE equals 1 μg of retinol, 6 μg of β‐carotene and 12 μg of other provitamin A carotenoids (i.e. equivalency ratios of 1:6 for β‐carotene 1:12 for other provitamin A carotenoids) were initially proposed since 1967 by the US FDA and the WHO and were confirmed in 1988 by the FAO/WHO (1988). The same conversion factors were proposed by the SCF some years later (SCF, 1993). In 2001, the IOM proposed retinol activity equivalency (RAE) ratios of 1:12 for β‐carotene and 1:24 for other provitamin A carotenoids, taking into account that absorption of β‐carotene from a mixed vegetable diet is 14% compared to the absorption of β‐carotene in oil, and that absorption from green leafy vegetables appears to be lower than absorption from fruits, which were a less significant source of β‐carotene in the US (IOM, 2001). These RAE were later adopted by the Nordic Nutrition Recommendations (NNR, 2014). Three years later the WHO/FAO proposed 1:14 for β‐carotene and 1:28 for other provitamin A carotenoids from usual vegetable diets based on similar considerations (WHO/FAO, 2004). In 2015, the EFSA NDA Panel (EFSA NDA Panel, 2015) noted the large variability in the bioavailability of β‐carotene from plant sources and oil in humans (and in the reported RE ratios thereof) and the high uncertainties in establishing equivalency ratios for the whole diet of large populations, concluding that new evidence was insufficient to change the conversion factors proposed by the SCF (1993).
Retinol/β‐carotene equivalency ratios have been reported to vary considerably, depending on the food source and population group, from 1:2 for corn oil in vitamin A‐deficient adults (Sauberlich et al., 1974) to 1:12 for orange fruits and 1:28 for green leafy vegetables in breastfeeding women (Khan et al., 2007). Retinol/β‐carotene equivalency ratios of 1:2 for oil, 1:4 for biofortified cassava, golden rice and yellow maize, and of 1:9–1:16 for mixed diets have been proposed based on literature reviews (Van Loo‐Bouwman et al., 2014). The main factor influencing equivalency ratios appeared to be the food matrix, with spinach, carrots and green/yellow vegetables showing equivalency ratios < 1:15 (Tang, 2010). Indeed, in a study using stable isotopes in which β‐carotene in oil was administered to elderly subjects, low absorption, rather than low bioconversion, was shown to be responsible for the low bioefficacy of β‐carotene, which was on average 7.3%, also suggesting overall equivalency ratios < 1:10 in this population group (Green et al., 2021). No significant difference in β‐carotene bioconversion between males and females was observed in humans using stable isotope techniques (Lietz et al., 2015). With regard to supplements, even the form in which β‐carotene is present has been related to various bioefficacies. Excipient emulsions showed higher bioefficacies than crystals within cell wall materials and those had higher bioefficacies when melted in oil than dispersed in oil, with bioefficacies ranging from 16% to 69%. As findings were based on a mouse model, with animals converting most of the β‐carotene into retinol, it is expected that, in humans, such differences are even magnified (Chen et al., 2020). A study in Mongolian gerbils, an animal model relevant to humans, suggests that high vitamin A liver stores (≥ 1.0 μmol/g) downregulate carotenoid absorption and cleavage‐related genes Scarb1 and BCO1, decreasing (but not preventing) further β‐carotene‐derived vitamin A accumulation in the liver. As a consequence, the retinol/β‐carotene equivalency ratio decreases, thus increasing the amount (μg) of β‐carotene needed to obtain 1 μg RE or retinol (Sowa et al., 2020). BCO1 has been proposed to be involved in the regulation of conversion of β‐carotene into retinoids, the conversion efficacy of which depends on the food matrix (amount of fat, presence of other carotenoids) and the amount of β‐carotene consumed. Lack of competing carotenoids could increase bioconversion of β‐carotene from food supplements (Bohn et al., 2019).
The Panel notes that retinol/carotenoid equivalency ratios vary widely across studies and are affected by both exogenous and endogenous factors, which makes it difficult to derive conversion factors for mixed diets of large populations. In 2015, the NDA Panel kept the conversion factors proposed for European populations by the SCF (EFSA NDA Panel, 2015; SCF, 1993), namely that 1 μg RE equals 1 μg of retinol, 6 μg of β‐carotene and 12 μg of other carotenoids with provitamin A activity. The Panel notes that β‐carotene equivalency ratios for mixed diets may be lower (and conversion factors higher) than 1:6, particularly under conditions of high vitamin A intake/status. However, the Panel considers that, for the purpose of this opinion, an equivalency ratio of 1:6 for β‐carotene would be more protective for potential toxicity than an equivalency ratio of 1:12.
3.3.6. Potential contribution of β‐carotene to preformed vitamin A toxicity
Except for the increased risk of lung cancer in heavy smokers at supplemental doses ≥ 20 mg/day, dietary β‐carotene intake has been advocated as safe, with a very low potential to induce adverse health effects in humans (Diplock, 1995; Grune et al., 2010). The only known sign of high carotenoid intake (from food and supplements) is carotenodermia, an orange discolouring of the skin, which is benign and reversible (Micozzi et al., 1988; Tanumihardjo et al., 2015). No single case of vitamin A toxicity based solely on the intake of pro‐vitamin A carotenoids has been reported in the literature.
Intake of β‐carotene could, however, contribute to high total vitamin A stores. Male Mongolian gerbils were administered orange carrots naturally rich in β‐carotene and preformed vitamin A at 100% and 200% the estimated needs in a 2 × 3 factorial design (Sowa et al., 2020). Liver vitamin A concentrations (retinol and retinyl esters) were approximately double in the groups receiving additional carrot intake compared to those receiving preformed vitamin A alone (0.4 vs. 0.8 and 0.5 vs. 1.0 μmol/g in the 100% and 200% preformed vitamin A arms, respectively). In the 200% preformed vitamin A arm plus carrots, liver concentrations were between > 1.0 μmol/g and 1.5 μmol/g in four out of 10 gerbils, with no histological liver abnormalities except mild fibrosis at 1.5 μmol/g. The intake of β‐carotene alone did not result in liver vitamin A stores > 1 μmol/g. This suggests that β‐carotene intake can contribute to high vitamin A liver stores, although the toxicological relevance of this finding in humans is unclear. In an RCT testing, the efficacy of biofortified maize, 13% of children in the intervention group had hypercarotenaemia (> 3.7 μmol/L total circulating carotenoids) and 16% had retinyl esters > 5% of circulating total vitamin A, consistent with high liver vitamin A stores (> 1 μmol/g) as determined by RID. Intakes of preformed vitamin A were, however, not reported. Only supplements were avoided for 6 months preceding the study (Mondloch et al., 2015). These studies indicate that β‐carotene intake in combination with high intakes of preformed vitamin A could contribute to high liver vitamin A stores in humans.
The Panel notes that β‐carotene could contribute to vitamin A liver stores, even with repleted liver stores. The Panel also notes that high intakes of β‐carotene in combination with (regular or irregular) high intakes of preformed vitamin A could contribute to high liver vitamin A stores. For this reason, eligible studies in the systematic reviews conducted for this opinion include those with exposures to preformed vitamin A alone or in combination with β‐carotene.
3.3.7. Conclusions on biomarkers of intake
The total liver concentration of vitamin A (i.e. free and esterified retinol), expressed per weight of liver tissue, is regarded as the best available measure of vitamin A status. Stable isotope dilution methods are less invasive than liver biopsies and are considered a sensitive method to assess vitamin A status at the group level, although they may lack precision to assess vitamin A status at individual level. Defining cut‐offs for excess liver retinol concentrations remains controversial, with recent studies questioning previously proposed values. The Panel notes the scarcity of data linking preformed vitamin A intake, hepatic retinol levels and adverse health effects, limiting the use of liver retinol concentrations both as a biomarker of vitamin A status and as an endpoint for establishing a UL.
Plasma/serum retinol concentrations lack sensitivity to habitual intake and may not correlate with adverse health effects of vitamin A toxicity, whereas fasting plasma retinyl ester concentrations, while potentially informative, lack consensus on cut‐off values for excess intake or toxicity. Other markers, such as retinoic acid glucuronides, lack consensus on normal or elevated concentrations.
Although plasma β‐carotene concentrations can provide an insight into population dietary intake, it lacks validation as a biomarker of β‐carotene intake and may not reflect β‐carotene status accurately. The Panel notes the variability of retinol/β‐carotene equivalency ratios derived for different sources (food, food supplements) and retains the 1:6 ratio, which is considered more protective for potential toxicity in the context of this opinion.
3.4. Dietary assessment methods used in observational studies and associated uncertainties
Food frequency questionnaires (FFQs) are the most used dietary assessment method to estimate the intake of vitamin A in human observational studies. Multiple 24‐h recalls are also frequently used and, less commonly, multiple‐day dietary records or dietary histories/interviews.
Consumption of the few food sources that are particularly rich in preformed vitamin A, such as liver, other offal and liver oil, is typically very irregular and infrequent. However, if consumed, these food items contribute the most to vitamin A intake. In 24‐h recalls and dietary records, consumption occasions could be missed or, if picked up, vitamin A intake may be grossly overestimated at individual level. These items could also be completely missed in FFQs if not listed. Some FFQs, however, have been developed and validated for vitamin A intake assessments (Henríquez‐Sánchez et al., 2009) and, when available, are preferred over 24‐h recalls and dietary records to assess habitual vitamin A intake in observational studies, unless the latter cover a sufficient number of days or are repeated overtime at least in a subset of participants (Willett, 2013).
Another source of uncertainty associated with vitamin A intake estimates in observational studies relates to the contribution of provitamin A carotenoids to total vitamin A intake. Lack of information about provitamin A carotenoids considered and the conversions factors may preclude direct comparison across studies. Some studies calculate intake of β‐carotene only, primarily because food composition tables, particularly in Europe, do not provide sufficient information about the content of α‐carotene and β‐cryptoxanthin in foods (EFSA NDA Panel, 2015), whereas other studies, mainly in the US, account for all provitamin A carotenoids, but this is not always specified in the publications. In addition, the factors used to convert provitamin A carotenoids into retinol (see Section 3.3.5) are often not specified but can vary widely across studies and have a significant impact on vitamin A intake estimates. For example, mean vitamin A intake from food ranged from 684 and 2000 μg RE/day [RE = μg retinol+(μg β‐carotene/6) + (μg α‐carotene/12) + (μg β‐cryptoxanthin/12)] and from 404 to 1417 μg RAE/day [RAE = μg retinol+(μg β‐carotene/12) + (μg α‐carotene/24) + (μg β‐cryptoxanthin/24)] across quintiles of intake in the same population of Dutch elderly subjects (de Jonge et al., 2015). The Panel notes that, whereas the different terminologies used in Europe and the US (RE vs. RAE) could imply the conversions factors applied, it is unclear whether these have been used consistently across studies not reporting on the conversion factors used.
All these aspects have been considered in the appraisal of the RoB of the human studies included in this opinion.
3.5. Intake assessment
This section provides information on the main sources of preformed vitamin A and β‐carotene. It also provides harmonised estimates of the intake of preformed vitamin A naturally present in foods (i.e. from the background diet) and on the combined intake of β‐carotene naturally present in foods and used as food additive (E160a) across EU countries. These intake values have been calculated using the EFSA Comprehensive food consumption database and the EFSA FCDB, following data cleaning to exclude fortified foods (Section 2.2.2). Data available to EFSA in such databases were insufficient to provide harmonised intake estimates of preformed vitamin A and β‐carotene from fortified food and/or food supplements; thus, data from national food consumption surveys (Section 2.2.1) are presented instead.
3.5.1. Intake assessment for preformed vitamin A
3.5.1.1. Sources of dietary preformed vitamin A
Natural sources
Dietary sources of preformed vitamin A are of animal origin, primarily animal liver and offal. Other foods naturally rich in preformed vitamin A are dairy products such as butter and some cheese, and eggs.
In the EFSA FCDB, highest concentrations were observed in liver, other edible offal and offal‐based processed products (Table 6). Further information on the composition levels together with the number of values from different national databases are available in Annex C, Table 2.
TABLE 6.
Average content of retinol in EFSA's FCDB in selected food categories.
| Food category | Average composition levels (μg/100 g) |
|---|---|
| Animal liver | 17,268 |
| Pig liver | 19,925 |
| Bovine liver | 18,837 |
| Poultry liver | 13,992 |
| Chicken liver | 15,513 |
| Poultry edible offal, non‐muscle, other than liver and kidney | 20,258 |
| Mammals' edible offal, non‐muscle, other than liver and kidney | 6391 |
| Bovine kidney | 149 |
| Spreadable cooked sausages | 5545 |
| Liver based spreadable‐textured specialities | 5357 |
| Meat specialties | 4592 |
| Milk | 43 |
| Cheese | 223 |
| Butter | 701 |
| Whole eggs | 277 |
Fortified foods
In the Mintel GNPD, a total of 3137 packaged food products were identified as containing vitamin A in the ingredients list. Of these, 760 foods reported containing preformed vitamin A (retinol, retinyl acetate or retinyl palmitate, and not provitamin A carotenoids) in their ingredients list, while 1991 products only reported ‘vitamin A' and were assumed to contain vitamin A as retinol (see search and cleaning strategy Sections 2.2.1.1 and 2.2.2.1).
Most of the products containing preformed vitamin A belong to the Mintel categories ‘baby foods’ 16 (47%, n = 1168), ‘nutritional drinks and other beverages’ (21%, n = 585) and ‘dairy’ (including desserts and ice cream, 20%, n = 560). Data on content per serving (as recommended by the manufacturer) were available for 20% (n = 555) of all products retrieved containing preformed vitamin A. The median for ‘nutritional drinks and other beverages’ in liquid form ranged between 200 and 269 μg RE/serving for those reported in the liquid form (n = 46) and in the powder form (n = 160), respectively. The median for ‘dairy’ was 80 μg RE/serving. The highest preformed vitamin A content declared on the label was found in six meal replacement drinks in powder form (640 to 1204 μg RE/serving) under the category ‘nutritional drinks and other beverages’, two protein bars under the category ‘snacks’ (600 μg RE/serving) and a ‘sports drink’ (800 μg RE/serving).
Food supplements
The Mintel GNPD search yielded a total of 1017 products that contained vitamin A, of which 767 products had only preformed vitamin A in the forms of retinol, retinyl acetate or retinyl palmitate and not provitamin A carotenoids. The vitamin A dose per serving declared on labels was available for 765 products, with a median dose per serving 17 of 630 μg RE. About 55% of supplements contained less than 750 μg RE per serving, and about 40% had doses between 750 and 1000 μg RE per serving. Only 6% (n = 45) of the products contained more than 1000 μg RE per serving, with a maximum of 2400 μg RE per serving (Figure 2).
FIGURE 2.
Distribution of preformed vitamin A content in food supplements as displayed on labels of products in EU Member States and Norway (μg RE per serving).
Source: Mintel GNPD. Search for vitamin A‐containing supplements available in the EU market from November 2017 to November 2022. Database refined to include only supplements with preformed vitamin A and not provitamin A carotenoids.
Among the analysed food supplements, only 12 products were single nutrient supplements, containing preformed vitamin A only, while the remaining 753 were multivitamins. For single nutrient supplements, the content of preformed vitamin A per serving ranged between 120 and 1800 μg RE, while for multivitamins, the content per serving ranged between 20 and 2400 μg RE.
3.5.1.2. EFSA's assessment of background intake for preformed vitamin A
Dietary intakes of preformed vitamin A in μg RE/day from natural food sources (background intake) were calculated linking food consumption data at individual level in the EFSA Comprehensive Database to food composition data in the EFSA FCDB and using the observed individual means method.
The standard intake assessment (i.e. using data as reported in food consumption surveys) led to very high 95th percentile (P95) intake values across surveys for all population groups, except vegetarians (Tables 7 and 8). The top contributing food to preformed vitamin A intake was offal (including liver and other edible offal and offal‐based processed products), which accounted for up to 72% of the intake in adults and up to 76% in older adults. Food consumption surveys recording only 2 or 3 days (i.e. 46 out of the 53 food consumption surveys present in the EFSA Comprehensive database; Section 2.2.1.1) cannot accurately capture the habitual intake of foods consumed with a lower frequency, as is typically the case of offal and products thereof. When offal consumption is reported in one of the two to three survey days, intakes can be overestimated if offal is consumed less frequently than on a weekly basis. Conversely, if offal is consumed but not captured in the two to three survey days, actual intakes will be underestimated. Whereas these two errors are expected to compensate for each other in large samples regarding mean intakes, substantial errors at both the low and high percentiles of intake are expected.
TABLE 7.
Daily intake of preformed vitamin A (μg RE/day) from food sources (supplements and fortified foods excluded) for males across EU dietary surveys by population group.
| Population group (age range) | N of surveys | Standard intake assessment | Offal consumption scenarios – P95 | No offal – P95 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | P95 a | 1×/week | 1×/month | ||||||||
| Min. b | Max. b | Min. b | Max. b | Min. | Max. | Min. | Max. | Min. | Max. | ||
| Infants (≥ 4 to < 12 months) | 12 | 123 | 569 | 304 | 1257 | 300 | 1257 | 300 | 769 | 300 | 769 |
| Toddlers (≥ 1 to < 3 years) | 15 | 213 | 795 | 402 | 3214 | 366 | 1744 | 310 | 666 | 293 | 613 |
| Young children (≥ 3 to < 7 years) | 20 | 241 | 847 | 436 | 3987 | 436 | 1583 | 436 | 777 | 379 | 678 |
| Older children (≥ 7 to < 10 years) | 15 | 237 | 903 | 420 | 3384 | 420 | 2009 | 420 | 870 | 419 | 826 |
| Young adolescents (≥ 10 to < 14 years) | 20 | 238 | 917 | 454 | 4744 | 454 | 1806 | 454 | 850 | 444 | 835 |
| Older adolescents (≥ 14 to < 18 years) | 19 | 289 | 1374 | 526 | 4885 | 526 | 1661 | 521 | 1040 | 519 | 1036 |
| Adults (≥ 18 to < 65 years) | 22 | 322 | 1241 | 521 | 6850 | 521 | 2896 | 521 | 1203 | 466 | 1087 |
| Older adults (≥ 65 years) | 23 | 326 | 1808 | 524 | 8210 | 524 | 3503 | 494 | 1252 | 387 | 950 |
| Vegetarians c | 1 | 215 | 215 | 604 | 604 | 604 | 604 | 604 | 604 | 604 | 604 |
Abbreviations: n, number, P, percentile.
The 95th percentile estimates obtained from dietary surveys and population groups with fewer than 60 subjects may not be statistically robust (EFSA, 2011) and consequently are not considered in this table.
Minimum and maximum mean and 95th percentile estimates across EU surveys, for each population group.
Age range (12–70 years).
TABLE 8.
Daily intake of preformed vitamin A (μg RE/day) from food sources (supplements and fortified foods excluded) for females across EU dietary surveys by population group.
| Population group (age range) | N of surveys | Standard intake assessment | Offal consumption scenarios – P95 | No offal – P95 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | P95 a | 1×/week | 1×/month | ||||||||
| Min. b | Max. b | Min. b | Max. b | Min. | Max. | Min. | Max. | Min. | Max. | ||
| Infants (≥ 4 to < 12 months) | 12 | 111 | 517 | 283 | 1249 | 283 | 1249 | 283 | 753 | 283 | 748 |
| Toddlers (≥ 1 to < 3 years) | 15 | 191 | 708 | 359 | 4881 | 359 | 1606 | 306 | 653 | 284 | 632 |
| Young children (≥ 3 to < 7 years) | 20 | 176 | 737 | 305 | 2900 | 301 | 1625 | 301 | 804 | 301 | 631 |
| Older children (≥ 7 to < 10 years) | 15 | 213 | 858 | 378 | 3804 | 378 | 1760 | 378 | 828 | 364 | 669 |
| Young adolescents (≥ 10 to < 14 years) | 20 | 229 | 735 | 433 | 3118 | 433 | 1669 | 409 | 806 | 399 | 771 |
| Older adolescents (≥ 14 to < 18 years) | 19 | 223 | 650 | 417 | 2431 | 417 | 1228 | 417 | 1130 | 392 | 1130 |
| Adults (≥ 18 to < 65 years) | 22 | 274 | 936 | 428 | 4048 | 424 | 1542 | 414 | 823 | 405 | 777 |
| Older adults (≥ 65 years) | 23 | 209 | 1705 | 386 | 3551 | 386 | 2044 | 382 | 908 | 343 | 714 |
| Pregnant women | 6 | 265 | 732 | 493 | 3529 | 493 | 1470 | 488 | 961 | 486 | 955 |
| Lactating women | 2 | 731 | 824 | 2464 | 4601 | 1182 | 2296 | 923 | 1020 | 738 | 840 |
| Vegetarians c | 1 | 154 | 154 | 433 | 433 | 433 | 433 | 433 | 433 | 433 | 433 |
Abbreviations: n, number, P, percentile.
The 95th percentile estimates obtained from dietary surveys and population groups with fewer than 60 subjects may not be statistically robust (EFSA, 2011a, 2011b) and consequently are not considered in this table.
Minimum and maximum mean and 95th percentile estimates across EU surveys, for each population group.
Age range (12–70 years).
The number and percentage of offal consumers (i.e. those reporting offal consumption in at least one survey day) by survey, and intake results for offal consumers only by population group, country and survey are presented in Annex C Table 6. The percentage of offal consumers varies widely across surveys and population groups, and for adults' ranges from 2% in Cyprus to 78% in Denmark.
To obtain a more realistic picture on the P95 intake of preformed vitamin A for the whole population, the frequency of offal consumption in offal consumers was adjusted to once per month, twice per month and once per week to build three different intake scenarios using the portion sizes for each individual as reported in the EFSA Comprehensive Database to be consumed (Annex B). For liver, offal and products thereof, consumption occasions (n) and portion sizes (mean, minimum and maximum) per food item, survey, age group and country are shown in Annex C, Table 16. Intake estimates without offal consumption were also calculated.
Preformed vitamin A intake estimates for the whole population for the standard intake assessment (means and P95) and P95 for the four different intake scenarios are presented by age group, sex and country in Appendix E and Annex C (Tables 5 and 6).
A summary overview providing the range of means and P95 across EU surveys for the standard intake assessment, and the range of P95 for three out of the four offal consumption scenarios, is given in Tables 7 and 8 for the whole population.
The main food groups contributing to background preformed vitamin A intake are meat and meat products (represented mainly by liver, other offal and products thereof), followed by milk and dairy products (mainly milk, butter and some cheese). Among infants, the highest contributors were infant and follow‐on formulae and processed cereal‐based foods.
According to the intake assessment protocol (EFSA, 2022), the accuracy of the results obtained should be evaluated by comparing EFSA's estimates with published national background intake estimates. These comparisons are made with the same surveys, similar data collection windows and population groups, when available (Section 2.2.1). Presently, only Belgium has published intake estimates for preformed vitamin A that correspond to the same surveys and population groups as in EFSA's Comprehensive database. However, the use of two 24‐h recalls in combination with dietary assessment methods (FFQ or dietary interview) to adjust for the frequency of food consumption, which is relevant to rarely consumed foods such as liver, offal and products thereof, invalidates comparisons with EFSA's standard intake estimates for that survey (two 24‐h recalls only).
Sources of uncertainty
Sources of uncertainty and their potential impact on the intake estimates, where possible, are identified and further discussed in Annex B.
Specific to this intake assessment, the most important uncertainties in relation to the P95 intake estimates, which are the most relevant for risk characterisation in the context of the UL for preformed vitamin A, are the true frequency of consumption of offal and products thereof in individuals identified as offal consumers in food consumption surveys, and the true percentage of offal consumers in those surveys, which is likely to be underestimated.
Animal feeding practices can impact the preformed vitamin A content in liver and other offal and products thereof. However, this uncertainty is more relevant for products where consumers typically show brand‐loyalty. In other cases, using average values, such as for preformed vitamin A, is likely to provide a realistic estimate since the offal consumed is sourced from different suppliers.
3.5.1.3. Data on the intake of preformed vitamin A excluding food supplements
Mandatory or voluntary preformed vitamin A fortification policies are in place in different EU countries for margarine, cooking oils, other fat products for baking and frying and/or fat blends and mixtures (see Appendix D). These data suggest that only fat‐based food products (i.e. excluding water‐based low‐fat food products) are fortified with retinol. Details on the amount of preformed vitamin A mandatorily or voluntarily added to foods in EU country can be found in Appendix D.
The following paragraphs summarise key information on preformed vitamin A intake from foods, including fortified foods and excluding food supplements, available in national survey reports or other scientific publications collected from national competent authorities, as described in Section 2.2.1. Additional information on the survey characteristics, as well as mean and P95 intake estimates, are presented in Annex E.
Intake estimates from national food consumption surveys
Reports from national consumption surveys providing estimates of preformed vitamin A intake from foods, including fortified foods, are available for 14 surveys conducted in 13 countries: Belgium, Finland, France, Hungary, Iceland, Ireland, Latvia, Lithuania, the Netherlands, Norway, Slovenia, Spain and Sweden (Annex E). Only the French, Dutch, Norwegian, Belgian and Irish surveys report P95 intake estimates in the publications identified.
For surveys that did not clearly indicate whether fortified foods were included/excluded in the estimates, it was assumed that they were not excluded. Separate intake estimates for background diet and for fortified foods were provided in the Belgian survey only. These food consumption surveys were conducted using different dietary assessment methods (24‐h recalls, dietary records, FFQs, dietary interviews and different combinations of these) and number of survey days (from 1 to 7 days). Five surveys (Finland, France, Hungary, Lithuania, the Netherlands) had 1, 2 or 3 survey days (24‐h recalls and/or dietary records) not coupled with other methods (FFQs, semi‐quantitative FFQ, dietary interviews) to adjust for the frequency of consumption of foods such as offal and products thereof.
Retinol intake from foods including fortified foods in infants was only assessed in a national survey in France (highest P95 intake in males, 608 μg RE/day). The highest P95 intake was reported in the Netherlands for male toddlers (994 μg RE/day), and in Sweden for male children aged 9 years (1545 μg RE/day) and male adolescents (1206 μg RE/day). For adults, the highest P95 was reported for males in France (18–79 years, 1870 μg RE/day). The French and Dutch surveys used 3‐ and 2‐day 24‐h recalls as dietary assessment method, respectively. As expected, these P95 intake estimates are much lower than the highest P95 intake estimates from the background diet obtained using the EFSA Comprehensive database in the standard intake scenario.
Contribution of fortified foods to preformed vitamin A intake
National reports providing additional information on preformed vitamin A intake from fortified foods are available for Belgium and the Netherlands.
In Belgium, it is mandatory to fortify margarines, low‐fat margarines and fats for baking with preformed vitamin A (Appendix D). Estimated P95 intakes of preformed vitamin A from food, including foods fortified with preformed vitamin A, ranged between 869 μg RE/day in children 3–6 years and 1152 μg RE/day in adults, of which 8%–14% was attributable to fortified foods (range across all population groups) (Belgian Food Consumption Survey (BFCS) 2014–2015) (Moyersoen et al., 2017) (Annex E).
In the Netherlands, voluntary fortification of margarines and other plant‐based fats with ‘retinoid form’ is encouraged through national covenants (Appendix D). Based on data from the Dutch National Food Consumption Survey (DNFCS) (2012–2016), it is estimated that fortified fats contribute to 29% of total retinol intake among Dutch consumers of fortified foods (de Jong et al., 2022a). Data provided in the report from the same national survey showed that intakes from all sources ranged between 1085 μg RE/day in male toddlers and 2047 μg RE/day in male adults (Annex E).
3.5.1.4. Data on the intake of preformed vitamin A including food supplements
The following paragraphs summarise key information available in reports or other scientific publications collected from national competent authorities (see Section 2.2.1). Additional information on the surveys, as well as mean and P95 intake estimates, are presented in Annex E.
A total of eight dietary surveys conducted in five countries (Belgium, Denmark, Ireland, Netherlands and Norway) provided information on the intake of either preformed vitamin A from all sources including food supplements, or from food supplements only. All reports referred to retinol and did not provide further information on the chemical form.
Intake data from cohorts of supplement users only were provided by Denmark, Ireland, Netherlands and Norway.
Intake data of preformed vitamin A from supplements among infants and toddlers retinol supplement users are not available. Among young age groups, the highest P95 of retinol intake from food supplements only in retinol supplement users only was reported in Ireland (males and females combined), 800 μg RE/day for children (1–3 years old) and 833 μg RE/day for adolescents (13–18 years old). For retinol supplement users adults and older adults, the highest P95 was reported in the Netherlands, with 1233 μg RE/day in male participants aged 65–80 years (Table 9).
TABLE 9.
Percent retinol supplement users in EU surveys and retinol intake from food supplements among users.
|
Country Survey name (N subjects) Reference |
Dietary method, (N of days) | Sex |
Age Range (years) |
% Retinol supplement users in total survey sample/among supplements users | Retinol intake from supplements only, P95 (μg RE/day) | Contribution of supplements to retinol intake, mean (%) |
|---|---|---|---|---|---|---|
|
Denmark DANSDA 2011–2013 (n = 3936) (Hindborg, 2015, unpublished) |
Face‐to‐face interview |
m + f m f m f m f |
4–10 11–17 11–17 18–50 18–50 51–75 51–75 |
61 a /NR 47 a /NR 43 a /NR 42 a /NR 51 a /NR 44 a /NR 58 a /NR |
NR |
26 37 60 35 50 37 54 |
|
Ireland NPNS 2011–2012 (n = 500) NCFS II 2017–2018 (n = 600) NTFS II 2019–2020 (n = 428) NANS 2008–2010 (n = 1500) (Kehoe & Walton, 2022) |
Weighted food diary (4d) | m + f |
1–4 5–12 13–18 18–64 65–90 |
12.4/57.9 14/64.6 4.1/29.3 12.4/41.7 16.8/44.7 |
800 480 833 1001 1092 |
34 36 17 25 25 |
|
Netherlands b DNFCS 2012–2016 (n = 4313) (van Rossum et al., 2022) |
Questionnaire (online/paper) | m |
1–3 3–10 10–14 14–18 18–65 65–80 |
15/17 23/40 15/36 9/27 13/35 13/37 |
368 778 772 746 1159 1233 |
NR NR NR NR NR NR |
| f c |
1–3 3–10 10–14 14–18 18–65 65–80 |
15/17 20/33 18/38 9/21 18/30 18/30 |
581 596 789 744 1114 1114 |
NR NR NR NR NR NR |
Abbreviations: DANSDA, Danish National Survey of Diet and Physical Activity; DNFCS, Dutch National Food Consumption Survey; NANS, National Adult Nutrition Survey; NCFS, National Children's Food Survey; NPNS, National Pre‐School Nutrition Survey; NR, not reported; NTFS, National Teens Food Survey.
% users of multivitamin/mineral supplements. By default, multivitamin/mineral supplements were considered to contain retinol based on Danish households purchases data.
Assumed that all vitamin A is retinol. The % of retinol supplement users was calculated among all multivitamin supplement users.
Excluding pregnant and lactating women.
Among retinol supplement users, the intake from all sources was reported in Denmark (only from food and food supplements, excluding fortified foods) and Norway. The highest P95 intake across all age groups was observed in men 18–50 years old in Denmark (3669 μg RE/day) (Annex E). In this age group, 50% of the overall intake was from retinol supplements (Table 9).
3.5.1.5. Conclusions on intake data for preformed vitamin A
EFSA's standard intake estimates (i.e. using data as reported in food consumption surveys) of preformed vitamin A from the background diet, excluding fortified foods and food supplements, led to very high P95 intakes across surveys for all population groups, except vegetarians (Section 3.5.1.2). Mean and P95 intakes were higher for males than for females in virtually all population groups and will be taken as the worst‐case scenario for the discussion of the results. The highest contribution to preformed vitamin A intake was from liver, other offal and products thereof. Excluding offal, the highest P95 for all population groups were from two to eight times lower depending on the population group, except vegetarians.
The highest P95 of EFSA's standard intake estimates of preformed vitamin A from the background diet are much higher than the highest P95 intake estimates across national surveys (published data) which considered also fortified foods or supplements in the intake assessment, even if data available for the latter are scarce (Sections 3.5.1.3 and 3.5.1.4). The reason for the discrepancy may be the fact that most food consumption surveys in the EFSA database include 2–3 consumption days only (24‐h recalls and/or dietary records), whereas most national surveys from which intake estimates have been published used, in addition, other dietary assessment methods (FFQs, dietary interviews) to adjust for the frequency of consumption of foods such as offal and products thereof. To obtain more realistic P95 intake estimates of preformed vitamin A for the whole population, the frequency of offal consumption was adjustsed to obtain three consumption scenarios (1 serving/week, 2 servings/month, 1 serving/month) (Section 3.5.1.2).
Assuming a frequency of consumption in offal consumers of 1 serving/week, the highest P95 estimated across surveys for males (taken as the highest observed intakes) is two to three times lower than those obtained using the standard intake assessment for virtually all population groups, whereas a frequency of consumption of 1 serving/month leads to P95 intake estimates close to intake estimates excluding offal (Section 3.5.1.2, Appendix E.1). The two servings/month scenario leads to a mixed picture (results can be found in Annex C).
Mandatory or voluntary preformed vitamin A fortification policies are in place in different EU countries for margarine, cooking oils, other fat products for baking and frying and/or fat blends and mixtures (Appendix D). A search in the Mintel GNPD database, however, indicates that also meal replacements and sports drinks and snacks are fortified with preformed vitamin A, with highest values ranging from 600 to 1200 μg RE/serving. Preformed vitamin A can be found in food supplements as a single ingredient or in multivitamin products. A search in the Mintel GNPD database showed that single‐nutrient supplements contain from 120 to 1800 μg RE per serving, while in multivitamins, the content per serving ranged between 20 and 2400 μg RE. However, the unclear labelling of vitamin A added to foods and food supplements (specific form often not reported, unclear units) precludes an accurate mapping of fortified foods and food supplements in the market containing preformed vitamin A (Section 2.2.2).
The Panel notes that estimates of the contribution of fortified foods and food supplements to vitamin A intake in EU populations are scarce. In the BFCS (Belgium) and DNFCS 2012–2016 (the Netherlands) surveys, the contribution of fortified foods to preformed vitamin A intake ranged from 8% to 14% (Belgium – mandatory fortification for margarines, low‐fat margarines and fats for baking) to 29% (the Netherlands – voluntary fortification of margarines and other plant‐based fats). Data on the contribution of food supplements to total retinol intake are only available from Denmark (obtained using 7‐day food records), where 50% of retinol intake in adult supplement users was from supplements (P95 for total intake from food and supplements, excluding fortified foods, was 3669 μg RE/day).
3.5.2. Intake assessment for β‐carotene
3.5.2.1. Sources of β‐carotene
Natural sources
Dietary sources of β‐carotene are fruits and vegetables. Foods naturally rich in β‐carotene are carrots, pumpkins, sweet potato, dark leafy vegetables, apricots, mangoes, melons and some spices such as paprika powder and dried peppers (Annex D, Table 1).
Food additives
β‐carotene is included in the Union list of food additives approved for use in foods as colour quantum satis (E160a). 18
Fortified foods
In the Mintel GNPD (Sections 2.2.1.1 and 2.2.2.1), a total of 3137 packaged food products were identified as containing vitamin A in the ingredients list. Of these, a total of 326 packaged food products were identified the source of vitamin A was only β‐carotene 19 (and not other preformed vitamin A forms or other provitamin A carotenoids). These products explicitly report the β‐carotene nutritional value in their nutrition label, and do not contain β‐carotene as food colour (E160a). Some products were assumed to be fortified with β‐carotene as they are not usually fortified with preformed vitamin A, as described in Section 2.2.1.1.
Most products containing only β‐carotene as source of vitamin A belong to the Mintel categories ‘juice drinks’ (77%), ‘snacks’ (8%), ‘dairy’ (5%) and ‘nutritional drinks and other beverages’ (4%).
Data on the content per serving (as recommended by the manufacturer) were available for 23% (n = 74) of the products retrieved. The products in the ‘juice drinks’ category (n = 55) contained from 0.72 to 4.8 mg per serving (converted from 120 to 800 μg RE) with a median of 1.8 mg per serving (converted from 300 μg RE). The content in ‘snacks’ was 1.08–1.9 mg per serving (n = 2), in ‘dairy’ was 0.072–0.48 mg per serving (n = 3), and in ‘nutritional drinks and other beverages’ was 1.4–5 mg per serving (n = 4).
Food supplements
The Mintel GNPD search yielded a total of 1017 products containing vitamin A, of which 46 had only β‐carotene added. The β‐carotene dose declared on labels ranged from 0.7 to 18 mg per serving, with a median of 3.5 mg per serving. 20 The majority (74%) of supplements declared ≤ 5 mg per serving. About 2% contained more than 15 mg per serving with a maximum of 18 mg per serving. One supplement containing only natural ingredients (Hawaiian spirulina) contained 3143 μg RE per serving from different carotenoids, with β‐carotene as or among the most prominent carotenoid(s).
3.5.2.2. EFSA's assessment of background intake of β‐carotene
Dietary intakes of β‐carotene in mg/day from the combined intake of β‐carotene naturally present in foods and used as food additive across EU countries were calculated linking food consumption data at individual level in the EFSA Comprehensive Database to food composition data and by using the observed individual means method.
The intake estimates are presented by age group, sex and country in Appendix E.2, see also Annex D). A summary overview providing the range of means and P95 across EU surveys is given in Table 10.
TABLE 10.
Daily intake of β‐carotene (mg/day) from food sources (supplements and fortified foods excluded) across EU dietary surveys by population group.
| Population group, age range | N of surveys | Males | Females | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | P95 a | Mean | P95 a | ||||||
| Min. b | Max. b | Min. b | Max. b | Min. b | Max. b | Min. b | Max. b | ||
| Infants, ≥ 4 to < 12 months | 12 | 1.4 | 3.3 | 3.9 | 6.7 | 1.3 | 3.3 | 3.2 | 6.6 |
| Toddlers, ≥ 1 to < 3 years | 15 | 1.3 | 4.1 | 3.1 | 7.9 | 1.2 | 3.8 | 2.6 | 7.6 |
| Young children, ≥ 3 to < 7 years | 20 | 1.4 | 3.5 | 2.9 | 8.2 | 1.2 | 3.5 | 2.7 | 7.8 |
| Older children, ≥ 7 to < 10 years | 15 | 1.5 | 3.8 | 4.5 | 9.3 | 1.2 | 3.4 | 3.4 | 8.8 |
| Young adolescents, ≥ 10 to < 14 years | 20 | 1.3 | 3.7 | 4.0 | 10.7 | 1.2 | 3.3 | 3.2 | 8.8 |
| Older adolescents, ≥ 14 to <18 years | 19 | 1.6 | 3.6 | 3.6 | 9.4 | 1.3 | 3.9 | 3.3 | 11.0 |
| Adults, ≥ 18 to < 65 years | 22 | 1.9 | 3.9 | 4.9 | 10.2 | 1.7 | 4.6 | 4.2 | 12.5 |
| Older adults, ≥ 65 years | 23 | 2.0 | 5.2 | 4.7 | 10.8 | 1.8 | 4.8 | 4.1 | 10.7 |
| Pregnant women | 6 | 1.8 | 3.7 | 3.9 | 10.5 | ||||
| Lactating women | 2 | 2.8 | 3.1 | 8.3 | 8.5 | ||||
| Vegetarians c | 1 | 5.4 | 5.4 | 13.2 | 13.2 | 4.3 | 4.3 | 10.1 | 10.1 |
Abbreviations: n, number, P, percentile.
The 95th percentile estimates obtained from dietary surveys and population groups with fewer than 60 subjects may not be statistically robust (EFSA, 2011a, 2011b) and consequently are not considered in this table.
Minimum and maximum mean and 95th percentile estimates across EU surveys, for each population group.
Age range (12–70 years).
The main food groups contributing to background β‐carotene intake are vegetables and vegetable products (mainly carrots, tomatoes, spinach), and fruit/vegetable juices and nectars, in all age groups.
According to the intake assessment protocol (EFSA, 2022), the accuracy of the results obtained should be evaluated by comparing EFSA's estimates with published national background intake estimates. These comparisons are made with the same surveys, similar data collection windows and population groups, when available (Section 2.2.1). For the purpose of this comparison, only estimates from national surveys in Austria, and Estonia could be used. In these surveys, mean and P95 intakes were in line with the estimates calculated by EFSA for most population groups.
Sources of uncertainty
Sources of uncertainty and their potential impact on the intake estimates, where possible, are identified and further discussed in Annex B.
Specific to this intake assessment the following uncertainties were found concerning the composition data for β‐carotene:
The β‐carotene content in fruits and vegetables, in addition to the type of vegetable, also depends on the maturity and colour variety (e.g. peaches with white or yellow flesh, green or white asparagus), parameters that are not recorded in food composition tables or the food consumption database. This uncertainty could lead to both under‐ and overestimation of individual intakes. However, the effect of this uncertainty at a population level is expected to be low.
A specific approach was required for checking fortified foods for β‐carotene, given its use as a food additive (food colour under the number E 160a) in various composite food products such as pastries, biscuits, confectionary or fruit soft drinks. β‐carotene content in foods where it is used as food colour cannot be distinguished analytically from its content as fortifying agent. Thus, β‐carotene content in certain composite foods reported in composition databases may include both. This assessment aimed to include the intake of β‐carotene used as food additive but exclude its use for fortification purposes, but this was not always possible. Consequently, a more inclusive approach was adopted, potentially leading to the inclusion of some unidentified fortified foods.
3.5.2.3. Data on β‐carotene intake excluding food supplements
There is no mandatory β‐carotene fortification policy among EU countries. In Sweden and Greece, the addition of β‐carotene to margarines and fat blends is permissible (Appendix D).
The following paragraphs summarise key information on β‐carotene intake from foods, including fortified foods and excluding food supplements, available in national survey reports or other scientific publications collected from national competent authorities, as described in Section 2.2.1. Additional information on the survey characteristics, as well as mean and P95 intake estimates, are presented in Annex E.
Data from national consumption surveys
Reports from national consumption surveys providing estimates of β‐carotene intake from foods, including fortified foods, are available from six surveys conducted in five countries: France, Germany, Hungary, Iceland and Sweden (Annex E). For surveys that did not clearly indicate whether fortified foods were included/excluded in the estimates, it was assumed that they were not excluded. Separate intake estimates for background diet and for fortified foods were not provided in any survey. Only the French, German and Swedish surveys report P95 intake estimates.
The highest P95 intakes for infants and toddlers were reported in males in France (8.2 mg/day and 3.4 mg/day, respectively). For children and adolescents, the highest P95 intakes were reported in Germany (7.3 mg/day for male children and 15.2 mg/day for adolescent females). For adults, the highest P95 was reported in females (65–80 years) in Sweden (7.1 mg/day).
In the Netherlands, results of an analysis based on consumption data from the national survey DNFCS 2012–2016 showed that, the median contribution of voluntary fortified foods to total β‐carotene intake among users of voluntary fortified foods (all age groups, 1–79 years) was 78% (de Jong et al., 2022b). The group ‘drink mixed fruit (not 100% juice)’ was the most consumed of all foods voluntarily fortified with β‐carotene in the DNFCS 2012–2016.
In Germany, an analysis performed with consumption data from the national adult survey (NVS II) showed that ‘foods fortified with β‐carotene often are important contributors (up to 30%) to the daily supply of vitamin A’ (Grune et al., 2010). In Germany, voluntary fortification of multivitamin juices on a wide scale is in place since 1980s (Sichert‐Hellert et al., 2001).
3.5.2.4. Data on β‐carotene intake including food supplements
The following paragraphs summarise key information available in reports or other scientific publications collected from National Competent Authorities, as described in Section 2.2.1. Additional information on the surveys, as well as mean and P95 intake estimates, are presented in Annex E.
There are no policies advising supplementation with β‐carotene.
A total of nine dietary surveys conducted in five countries (Denmark, Estonia, Ireland, Poland and Sweden) provided information on the intake of β‐carotene from all sources, including food supplements, or from food supplements only. Information on supplemental intake in cohorts of supplements users only were provided by Denmark, Ireland and Poland.
Data on β‐carotene intakes from supplements in infants and toddlers supplement users are not available. β‐carotene intake estimates from food supplements only were available from the surveys in Ireland (all age groups) and Poland (adults only). Intakes in high consumers (P95, males and females combined) were only calculated for the national survey in Ireland. P95 intake estimates ranged between about 0.5 and 2.9 mg/day in children and adolescents and were 1.1 and 2.4 in adults and older adults, respectively, and the percent contribution of β‐carotene containing supplements to total β‐carotene intake was below 4% in all age groups (Table 11). The estimated mean intake in the national survey in Poland for adults aged > 18 years was 1.1 mg/day.
TABLE 11.
Percent β‐carotene supplement users in European surveys and β‐carotene intake from food supplements among users.
|
Country Survey name (N subjects) Reference |
Dietary method, (N of days) | Sex |
Age Range (years) |
% β‐carotene supplement users in total survey sample/among supplements users | β‐Carotene intake from supplements only, P95 (μg RE/day) | Contribution of supplements to β‐carotene intake, mean (%) |
|---|---|---|---|---|---|---|
|
Denmark DANSDA 2011–2013 (n = 3936) (Hindborg, 2015, unpublished) |
Face‐to‐face interview |
m + f m f m f m f |
4–10 11–17 11–17 18–50 18–50 51–75 51–75 |
60 a /NR 47 a /NR 42 a /NR 41 a /NR 49 a /NR 42 a /NR 53 a /NR |
NR |
2.1 0.5 1.5 1.2 0.8 1.0 1.0 |
|
Ireland NCFS II 2017–2018 (n = 600) NTFS II 2019–2020 (n = 428) NANS 2008–2010 (n = 1500) (Kehoe & Walton, 2022) |
Weighted food diary (4d) | m + f |
1–4 5–12 13–18 18–64 65–90 |
3.7/0.8 11/2.4 14.7/2.1 8.4/2.5 10.6/4.0 |
NR 0.52 2.87 1.13 2.40 |
1.6 3.7 3.9 3.5 3.1 |
|
Poland National Dietary Survey 2019–2020 (n = 1831) (Stos et al., 2021) |
FPQ |
m f |
18–65+ | NR |
Mean ± SD 1.52 ± 1.84 0.11 ± 0.13 |
NA |
Abbreviations: DANSDA, Danish National Survey of Diet and Physical Activity; FPQ, food propensity questionnaire; NANS, National Adult Nutrition Survey; NCFS, National Children's Food Survey; NR, not reported; NTFS, National Teens Food Survey.
% users of multivitamin/mineral supplements. By default, multivitamin/mineral supplements were considered to contain retinol based on Danish households purchases data.
P95 intakes from foods (not fortified) and food supplements in consumers of β‐carotene supplements were estimated only in the national survey in Denmark. The estimated P95 intakes were 8.6 and 15 mg/day for children and adolescents, respectively, and 11 and 14 mg/day for adults and older adults, respectively (Annex E). The contribution of food supplements to total β‐carotene intake in this survey was between 0.5 and 2.1% across age groups (Table 11).
3.5.2.5. Conclusions on intake data for β‐carotene
The Panel notes that P95 estimated background β‐carotene intake from natural food sources, and from its use as food additive (but without fortified foods and food supplements), in males is up to 6.7 mg/day, in infants (4 to < 12 months), up to 7.9 mg/day in toddlers (1 to < 3 years), up to 8.2 mg/day in young children (3 to < 7 years), up to 9.3 mg/day in older children (7 to < 10 years), up to 10.7 mg/day in young adolescents (10 to < 14 years), up to 9.4 mg/day in older adolescents (14 to < 18 years), up to 10.2 mg/day in adults (≥ 18 years), up to 10.5 mg/day in pregnant women and up to 8.5 mg/day in lactating women across surveys included in EFSA's intake assessment (Table 10) (see also Annex D). Except for the adult and older adolescent population groups, estimated intakes were higher in males than in females, presumably due to higher energy intakes.
Fortification with β‐carotene is not mandatory in EU countries, and only in Sweden, it is permitted to add it to margarines and fat blends with a maximum limit of 400 μg per 100 g. Voluntary food fortification practices are in place in different EU countries, where products such as fruit and vegetable drinks are the most frequently fortified. The median β‐carotene content in fortified products belonging to the Mintel category ‘juice drinks’ is 1.8 mg per serving (range 0.72–4.8 mg per serving) (Section 3.5.2.3).
There are no policies advising supplementation with β‐carotene in the EU. In the EU market, the majority of the supplements declared a β‐carotene content of ≤ 5 mg per serving, and only 2% of the totality of the products exceeded a per serving content of 15 mg (with a maximum of 18 mg) (Section 3.5.2.4).
The Panel notes that estimates of the contribution of fortified foods and food supplements to β‐carotene intake in EU populations are scarce. In the national survey in the Netherlands (DNFCS 2012–2016), the median contribution of voluntary fortified foods to total β‐carotene intake was 78% in users of fortified foods, with ‘drink mixed fruit (not 100% juice)’ being the most consumed among all food groups fortified with β‐carotene. In the national adult survey (NVS II) in Germany, where voluntary fortification of multivitamin juices is in place since 1980s, it was calculated that foods fortified with β‐carotene may contribute up to 30% to the total daily vitamin A intake (Section 3.5.2.3).
Information on the use of β‐carotene containing supplements was also scarce. Among users of β‐carotene containing supplements analysed in two national surveys in Denmark and Ireland, the contribution of these supplements to total β‐carotene intake was below 4% in all age groups. The absolute β‐carotene P95 intake from supplements only ranged between 0.5 mg/day in toddlers and 2.9 mg/day in adolescents (1 survey) (Section 3.5.2.4).
3.6. Hazard identification
3.6.1. Teratogenicity (sQ3)
3.6.1.1. Introduction and mechanisms of toxicity
The teratogenic potential of retinoic acids (RA), the active oxidised metabolites of vitamin A, is well established in both animals and humans (IOM, 2001; SCF, 2002). Whereas both vitamin A deficiency and toxicity during pregnancy have been shown to affect the normal development of the fetus, only mechanisms of toxicity are discussed below.
The teratogenic effects of excess RA and other (synthetic) retinoids have been documented in various experimental animal models, including mice, rats, rabbits and non‐human primates, showing large differences in susceptibility among species (Quemelo et al., 2007; SCF, 2002). In humans, in utero exposure to high doses of preformed vitamin A leads to congenital malformations, craniofacial (small or absent external ears and auditory canals, cleft palate, micrognathia, low set ears), of the cardiovascular system (transposition of the heart vessels, aortic arch hypoplasia, ventricular septal defects), the thymus and the central nervous system (micro‐ or anophthalmia, cerebellar or cortical defects, microcephaly). Most of these anatomical defects in the embryo appear to be associated with alterations in the migration of cells from the neural crest. The most critical period of susceptibility to high vitamin A intake appears to be the first trimester of pregnancy, especially during the first 60 days (Bastos Maia et al., 2019). Concentrations of 13‐cis RA and 4‐oxo‐13‐cis RA decrease over the pregnancy and rebound after delivery, while all‐trans RA concentrations exhibited a unique temporal pattern with levels peaking at mid‐pregnancy (Jeong et al., 2023).
Over‐expression of wild type or mutant nuclear receptors, loss‐of‐function studies in knockout mice and further genetic manipulations have shown that RA is involved in many critical processes regarding early organ development, emphasising its teratogenic potential (Knudsen et al., 2021; Kumar & Duester, 2011; Rhinn & Dolle, 2012; Shannon et al., 2017). Through its interaction with the nuclear receptor RAR/RXR, RA signalling is paramount for the normal development of many organs and tissues in the embryo (Berenguer & Duester, 2022), regulating the expression of several hundred downstream targets (Balmer & Blomhoff, 2002). RA‐related signalling is prevalent in all chordates during embryogenesis over other cellular signalling pathways (Duester, 2008; Marlétaz et al., 2006), whereas other animals lack retinaldehyde dehydrogenase (RALDH) to synthesise RA.
The molecular mechanisms of teratogenicity induced by excess vitamin A are, however, not fully elucidated, and may depend on the action of RA on different tissues. The potential mechanisms proposed involve activation of target genes for RA and/or related metabolites (e.g. 13‐cis RA) involved in early organogenesis (Duester, 2008), the covalent binding of RA to proteins (retinoylation) (Takahashi, 2022) and the effect of retinoid metabolites (e.g. retro‐retinoids, anhydro‐retinoids) on the immune system (O'Byrne & Blaner, 2013).
β‐Carotene alone is not teratogenic (Woutersen et al., 1999). In an animal model with chicks, preformed vitamin A induced malformations at 0.03–0.3 μg/embryo exposure, whereas β‐carotene at 100 μg/embryo did not cause any adverse effects (Peterka et al., 1997). It is likely that, under such circumstances, only a small proportion of β‐carotene is cleaved into vitamin A. Conversely, it has been hypothesised that, as maternal β‐carotene crosses the placenta and is converted to 13‐trans retinoic acid by the fetus owing to its limited capacity to store β‐carotene, maternal β‐carotene supplementation could contribute to the teratogenic effects of retinoids (Goldberg, 2011). However, no experimental data to support this hypothesis are currently available.
The SCF (2002) set a UL for preformed vitamin A based on a NOAEL of 3000 μg RE/day for teratogenicity. No uncertainty factor was applied. The SCF also kept a previous recommendation (SCF, 1992) for women who are planning to become pregnant or who are pregnant not to consume animal livers. The purpose of this systematic review was to evaluate human data published since then, to characterise the dose–response relationship between the intake of preformed vitamin A and teratogenicity in humans if data allow, and to address whether β‐carotene intake from diet or supplements could potentiate the teratogenic effect of excess preformed vitamin A.
3.6.1.2. Evidence from human studies
A total of 19 publications were identified to be eligible for this systematic review, which had no limit for study design. Three publications report on prospective cohort studies, one on a case‐cohort study, and 15 on case–control studies. Evidence tables can be found in Appendix B.
Case–control studies
Out of the 15 publications reporting results of case–control studies, 10 found no increased risk of congenital birth defects in newborns from mothers consuming higher vs. lower doses of vitamin A (preformed or total; from diet, supplements or both) before and up to the first trimester of pregnancy. Study populations, however, reported relatively low vitamin A intakes (cut‐off for the highest vitamin A intake category generally < 1000 μg RE/day and up to 2000 μg RE/day). These include four publications from the National Birth Defects Prevention Study in the UK (Chandler et al., 2012; Feldkamp et al., 2011; Weber et al., 2018; Yang et al., 2008), three publications from a birth cohort in California (USA; Carmichael et al. (2010); Shaw et al. (2010); Wallenstein et al. (2013)) and three publications from a birth cohort in Bangladesh (Obrycki et al., 2019), Denmark (Mitchell et al., 2003) and the Netherlands (Beurskens et al., 2013). These studies will not be discussed further owing to the limited information they provide for the update of the UL for vitamin A.
The remaining five publications report on three case–control studies that had already been considered by the SCF (2002) (Martínez‐Frías & Salvador, 1990; Mills et al., 1997; Shaw et al., 1997) and two additional case–control studies identified in this systematic review (Botto et al., 2001; Johansen et al., 2008). Whereas all studies had preformed vitamin A as the exposure, only one assessed total vitamin A in relation to the outcome (Johansen et al., 2008). The main characteristics of the studies and the results for preformed vitamin A are shown in Figure 3. Whenever intakes from different sources were reported in the same publication (from food, supplements, or food and supplements combined), only the highest combined intake has been plotted. Evidence tables can be found in Appendix B.2 and the heat map for the appraisal of the RoB can be found in Appendix C.1.
FIGURE 3.
Preformed vitamin A and congenital birth defects. BD, birth defect; D + S, diet and supplements; FC, fortified cereals; NCCD, neural crest cell defects; NRA, normally related arteries; NTD, neural tube defects; OFC, oral facial clefts; OTD, outflow tract defects; S, supplements; TGA, transposition of great arteries.
These case–control studies are very heterogeneous regarding both the exposure and the endpoints assessed. In the study conducted in Spain (Martínez‐Frías & Salvador, 1990), only maternal exposure to preformed vitamin A from supplements > 12,000 μg RE/day was associated with an increased risk of birth defects in newborns with no chromosomal anomalies, while intakes between 3000 and 12,000 μg RE/day did not increase the risk as compared to no supplement consumption. In the context of the National Institute of Child Health and Human Development Neural Tube Defects (NTD) Study in California (USA; Mills et al. (1997)), NTD, major (non‐cosmetic, non‐NTD) congenital malformations and the subgroup of neural crest defects (NCD) including cranial and cardiac defects were evaluated in relation to the periconceptional intake of preformed vitamin A from supplements alone or supplements plus fortified cereals. Subjects were excluded from the abnormal pregnancy group if the malformations could not have been caused by vitamin A (i.e. chromosomal defects were not allowed to exceed 15% of subjects). No increased risk was observed for intakes > 2400 or > 3000 μg RE/day as compared to intakes < 1500 μg RE/day for any of the endpoints. In another study conducted in the same geographical region (Shaw et al., 1997), periconceptional exposure to preformed vitamin A from food and supplements or from supplements alone was not associated with an increased risk of NTD. The cut‐off for the highest intake category from diet and supplements was 4500 μg RE/day. The Panel notes the low number of subjects in the high‐intake categories in all these studies where participants retrospectively report their intake, which had a moderate RoB (tier 2). Critical domains were exposure assessment and confounding.
Of the remaining two case–control studies identified in this systematic review, the one conducted in the USA (East Coast, Botto et al. (2001)) focused on outflow tract defects (OTD, n = 126) with either transposition of great arteries (TGA, n = 53), mostly dextro‐transposition (D‐TGA, n = 47 out of 53) or with normally related arteries (NRA, n = 73), mostly tetralogy of Fallot (n = 58 out of 73), in relation to retinol intake from diet and supplements during the year prior to conception. Control babies were a representative sample of the underlying birth cohort and were selected randomly from obstetric logbooks after stratification by hospital and year of birth. Intakes ≥ 3000 μg RE/day were associated with a higher risk of OTD‐TGA (OR, 95% CI = 2.4, 1.1–4.8) but not OTD‐NRA compared to intakes < 3000 μg RE/day. As the increased risk was confined to high intakes of retinol from supplements and not diet when these data were analysed separately, a sensitivity analysis was conducted for four categories of supplemental retinol intake: 0–1499 (reference), 1500–2399, 2400–2999 and ≥ 3000 μg RE/day. As compared to the reference category, only retinol intakes from supplements ≥ 3000 μg RE/day were associated with an increased risk and only of OTD‐TGA (7 cases and 11 controls vs. 40 cases and 553 controls in the reference group; OR, 95% CI = 8.8, 3.8–20.5).
The second study (Norway, Johansen et al. (2008)) examined oral facial clefts (OFC, i.e. cleft lip with or without cleft palate and cleft palate alone) with or with other birth defects in relation to total vitamin A (retinol +1/12 β‐carotene) from diet and supplements. Control mothers of livebirths were randomly selected via the national Medical Birth Registry within 6 weeks of delivery. As for the publications discussed at the beginning of this section, no increased risk was observed for total vitamin A across quartiles of intake (defined on the distribution of intakes among controls), but the cut‐off for the highest quartile was relatively low (< 2000 μg RE/day). Data were also analysed comparing the 95th percentile of total vitamin A and retinol intake from diet and supplements with the 40–60th percentiles for isolated OFC (without other birth defects), including cleft lip with or without cleft palate and cleft palate only, as these are two distinct genetic malformations. No increased risk of OFC or categories thereof was observed for high intakes of either retinol (> 3398 μg RE/day) or total vitamin A (> 3763 μg RE/day) as compared to lower intakes (Figure 3). These two studies were at low RoB (tier 1).
The Panel considers that these case–control studies support a NOAEL for preformed vitamin A of 3000 μg RE/day for teratogenicity and notes that they do not allow addressing the question of whether β‐carotene from diet or supplements could potentiate the teratogenic effect of preformed vitamin A at high doses.
Prospective cohort and case‐cohort studies
Three prospective cohort (Mastroiacovo et al., 1999; Michikawa et al., 2019; Rothman et al., 1995) and one case‐cohort (Bille et al., 2007) studies were identified. Evidence tables are in Appendix B.1 and heatmap for RoB in Appendix C.1.
Of the four studies identified, two (Mastroiacovo et al., 1999; Rothman et al., 1995) had been already assessed by the SCF in 2002 and used as the basis to derive a NOAEL of 3000 μg RE/day for pregnant women based on teratogenicity outcomes (SCF, 2002).
In the prospective cohort (PC) study conducted in the USA (Rothman et al., 1995), 22,755 women undergoing prenatal screening between weeks 15 and 20 of pregnancy were interviewed to gather data on retinol intakes from food and supplements in the 12 weeks after the last menses. Data on pregnancy outcomes was retrieved from physicians (76.5%) or the mothers through a mailed questionnaire at the time of delivery. Birth defects were classified by two coders unaware of the exposure. Chromosomal defects were not coded. Cranial NCD (i.e. craniofacial, central nervous system, thymic and heart defects), NTD, musculoskeletal and urogenital defects and other defects were considered separately and combined for data analysis. Data were analysed by considering retinol from food (categories of intake: 0–1500, 1501–3000 and ≥ 3001 μg RE/day) and supplements (categories of intake: 0–1500, 1501–2400, 2401–3000 and ≥ 3001 μg RE/day) separately and combined (total retinol; categories of intake: 0–1500, 1501–3000, 3001–4500 and ≥ 4501 μg RE/day). Intakes of retinol from food and supplements were almost uncorrelated (r = 0.005).
Women who consumed ≥ 4501 μg RE/day from diet and supplements had a higher proportion of babies with birth defects (prevalence ratio (PR), 95% CI = 2.2, 1.3–3.8), and particularly cranial NCD (PR, 95% CI = 3.5, 1.7–7.3), than women with intakes ≤ 1500 μg RE/day, whereas the proportion on newborns with birth defects was relatively stable in the first categories of intake. PR for categories of retinol intake from food only were unstable owing to the low number of women exceeding 3000 μg RE/day. For intakes from supplements only, the PR (95% CI) was 2.4 (1.3–4.4) for all birth defects combined and 4.8 (2.2–10.5) for cranial NCD. Multiple logistic regression models adjusting for confounders and other sensitivity analyses did not change the results. In dose–response analysis using the midpoint of the intake categories and the mean of the highest category (6502 μg RE/day for retinol from supplements), an apparent threshold of 3000 μg RE/day for supplements and cranial NCD was identified. All mothers of newborns (n = 7) with cranial NTD in the highest intake category of retinol intake from supplements consumed these between the 2 weeks prior to conception and the first months of pregnancy. The dose–response for total retinol raised less steeply. This study was at low RoB (tier 1).
The other PC was a multicentre study conducted in Europe (Mastroiacovo et al., 1999). The study population was selected among women contacting the European Network of the Teratology Information Services (ENTIS). Women were selected if reporting intake of preformed vitamin A supplements at doses ≥ 3000 μg RE/day during (n = 423 exposed; n = 311 with complete data, a live birth and no chromosomal abnormalities) or after (n = 116; exposed controls) the first 9 weeks of pregnancy, or if they were exposed to non‐teratogenic agents (n = 679; non‐exposed controls). No information was provided about the selection of the exposed women and control groups (time frame, inclusion/exclusion criteria). The outcome of the study was major malformations (i.e. excluding chromosomal or genetic diseases and minor anomalies), ascertained by telephone interview with the women or their doctors, with the interviewer blinded to the exposure. In the exposed group, median intakes (IQR) of retinol from supplements were 15,000 (7500–18,000) μg RE/day, with 120 women exposed to > 15,000 μg RE/day and 32 women to > 30,000 μg RE/day. Only three cases of major malformations (pulmonary stenosis, anterior and stenotic anus with perineal fistula and bilateral inguinal hernia) were identified in the exposed group, as compared to four cases in exposed controls (at intakes > 7500 μg RE/day) and 13 in non‐exposed controls (rate ratio and 95% CI for early exposed vs. exposed and non‐exposed controls = 0.28, 0.06–1.23 and 0.50, 0.14–1.76, respectively). This study was at high RoB (tier 3), critical domains being outcome assessment, confounding, selection bias and attrition.
The remaining two studies investigated OFC in relation to preformed vitamin A supplements (Bille et al., 2007) and congenital diaphragmatic hernia (CDH) in relation to total vitamin A (as μg RAE/day; conversion factors for provitamin A carotenoids not reported) and retinol from the diet (Michikawa et al., 2019). Neither of these two studies reported an increased risk of congenital birth defects for higher vs. lower intakes of preformed or total vitamin A. However, preformed vitamin A intakes in these studies were relatively low, with cut‐offs for the highest intake category largely < 1000 μg RE/day. Both studies were at moderate RoB (tier 2), critical domains being exposure assessment and confounding.
The Panel notes that no new data from prospective observational studies have become available since 2002 (SCF, 2002) on the relationship between high intakes (i.e. > 3000 μg RE/day) of preformed or total vitamin A by women in child‐bearing age and risk of congenital birth defects in their offspring.
3.6.1.3. Conclusions on teratogenicity
Although molecular mechanisms of teratogenicity induced by excess vitamin A are not fully elucidated, the teratogenic effects of preformed vitamin A at high intakes are well documented in animals and humans. The only PC that addressed the dose–response relationship between the intake of preformed vitamin A and birth defects (Rothman et al., 1995) identified a threshold of 3000 μg RE/day supplemental retinol in the first trimester of pregnancy for cranial NCD, which can be considered as a NOAEL for teratogenicity for preformed vitamin A from all sources (RoB tier 1). This is supported by the findings of another PC (Mastroiacovo et al. (1999); RoB tier 3), which reports a negligible risk of teratogenicity at higher levels of intake (no cases up to 7500 μg RE/day, with twice as many women exposed to ≥ 6000 μg RE/day), and by data from five case–control studies at moderate RoB (tier 2; Martínez‐Frías and Salvador (1990); Mills et al. (1997); Shaw et al. (1997)) and low RoB (tier 1; Botto et al. (2001); Johansen et al. (2008)) that have assessed intakes of preformed vitamin A from all sources or from supplements only above and below this threshold.
The Panel notes that β‐carotene per se is not considered to be teratogenic. The Panel also notes that, owing to the downregulation of β‐carotene absorption and conversion to retinol in vitamin A‐repleted states, it is also unlikely that maternal β‐carotene intake from food or supplements would potentiate the teratogenic effects of preformed vitamin A, although the available data in humans does not allow to address this question.
3.6.2. Hepatotoxicity (sQ4)
3.6.2.1. Introduction and mechanisms of toxicity
It is well established that hepatotoxicity is a late symptom of hypervitaminosis A but one of the most severe outcomes. Liver abnormalities associated with chronic intakes of excess preformed vitamin A range from reversibly elevated liver enzymes to widespread fibrosis, cirrhosis and sometimes death (EVM, 2003; IOM, 2001; SCF, 2002). Histological features of vitamin A‐induced hepatotoxicity include hepatic stellate cell hyperplasia and hyperproliferation, collagen diffusion within the space of Disse, which can evolve in a portal hypertension, and perisinusoidal dilation and sinusoidal barrier abnormalities that can lead to peliosis hepatitis (SCF, 2002; Zafrani et al., 1984). However, the relationship between preformed vitamin A storage in stellate cells and fibrosis is complex, since retinyl ester storage may protect stellate cells from trans‐differentiation, while trans‐differentiation to myofibroblasts may lead to depletion of retinyl ester storage capacity (Nollevaux et al., 2006; Tsuchida & Friedman, 2017).
Based on case reports of individuals consuming high doses of preformed vitamin A for several years, the SCF (2002) concluded that an intake of 7500 μg RE/day taken over 6 years was the lowest dose reported to cause hepatotoxicity in humans (Geubel et al., 1991; Kowalski et al., 1994), although it was unclear whether lower doses taken for longer periods of time could also induce hepatotoxicity.
The purpose of this systematic review was therefore to characterise the dose–response relationship between the intake of vitamin A and early signs of hepatotoxicity using evidence from intervention studies in humans. Eligible studies were those providing daily or weekly vitamin A supplementation (as preformed vitamin A with or without β‐carotene) vs. placebo or lower vitamin A doses and lasting at least 3 months, the minimum time estimated to detect morphological changes in the liver using imaging techniques. Among the endpoints of interest, liver enzymes, liver steatosis using imaging techniques (ultrasound, MRI) and liver fibrosis (transient elastography) were included, in addition to clinical diagnosis of liver cirrhosis and portal hypertension. The Panel notes, however, that liver enzymes are only moderately elevated in hypervitaminosis A‐induced hepatotoxicity, and that localised liver fibrosis may precede widespread liver damage and the increase of circulating liver enzymes, suggesting that liver enzymes may not be an appropriate marker of early liver damage associated to excess intake of preformed vitamin A (Newsome et al., 2018). Prospective observational studies in humans were not searched for because it was anticipated that the levels of preformed vitamin A intake reported in the general population would not be relevant for the endpoints described above.
3.6.2.2. Evidence from human studies
Only four RCTs meeting the inclusion criteria were identified. The RCTs were conducted in Iran (Bitarafan et al., 2015; Farhangi et al., 2013) and the USA (Alberts et al., 2004; Dougherty et al., 2012), used retinyl palmitate as intervention and measured liver enzymes as safety markers for liver damage. The evidence tables are available in Appendix B.3.
One 12‐month RCT (Dougherty et al., 2012) was designed to assess the efficacy of retinyl palmitate at doses around the recommended levels for vitamin A adequacy (i.e. 300, 400 or 600 μg/day depending on age and sex, corresponding to 164, 218 and 328 μg RE/day, respectively) to optimise vitamin A status in children with type SS sickle cell disease and suboptimal vitamin A status as compared to placebo (n ~ 20 per group). Gamma‐glutamyl transferase (GGT) was the only marker of liver function measured. This study will not be discussed further owing to the low doses of preformed vitamin A used.
In two of the remaining RCTs, the duration of the intervention was 12 months. One RCT (Alberts et al., 2004) provided either placebo or retinyl palmitate at doses of 25,000, 50,000 or 75,000 IU/day (corresponding to about 7500, 15,000 or 22,500 μg RE/day) for the prevention of skin cancer to subjects at risk (n ~ 32 per group) and measured aspartate aminotransferase (AST), alanine aminotransferase (ALT) and alkaline phosphatase (ALP) as markers of liver function. Liver damage was defined as an increase in any liver enzyme × 3 the normal range, which occurred in one subject consuming 22,500 μg RE/day (for AST and ALT) and one subject consuming 15,000 μg RE/day (AST only). The authors report that differences were not significant across intervention groups. Follow‐up liver nuclear scans, foreseen in case of clinical or laboratory‐related toxicity, were not performed in any patient. In the other RCT (Bitarafan et al., 2015), patients with relapsing remitting multiple sclerosis received either placebo or retinyl palmitate at doses of 25,000 IU/day (7500 μg RE/day) for the first 6 months and 10,000 IU/day (3000 μg RE/day) for the second 6 months (n ~ 47 per group) and measured AST and ALT, which were stable and within the normal range in both groups during the trial.
In the last RCT (Farhangi et al., 2013), the intervention lasted 4 months. Obese women were randomised to consume either placebo (n = 23) or retinyl palmitate (n = 27) at doses of 25,000 IU/day (7500 μg RE/day). One other group of normal‐weight women (n = 25) received the same dose of retinyl palmitate for the duration of the study. Although the study reports a statistically significant increase in AST within the retinyl palmitate supplemented groups and a statistically significant increase in the normal weight group compared to obese controls when expressed as percent change from baseline, absolute values did not differ across groups either at baseline or at the end of the study, and were always within the normal range (mean (SD) = 26.26 (2.60) U/L in the normal‐weight group at the end of the trial).
The RoB was low (Tier 1) for one (Bitarafan et al., 2015) and moderate (Tier 2) for three RCTs (Alberts et al., 2004; Dougherty et al., 2012; Farhangi et al., 2013), critical domains being exposure, allocation concealment and selective reporting. The heatmap is in Appendix C.2.
The Panel notes that the available RCTs do not report an adverse effect of preformed vitamin A supplementation on liver enzymes in adults at doses between 7500 and 22,500 μg RE/day. The Panel also notes, however, the short duration of the studies (up to 12 months), the small sample size (from ~20 to 47 per group per study group), and that no RCTs tested high doses of preformed vitamin A below the LOAEL for liver damage (i.e. 7500 μg RE/day consumed for 6 years). The Panel also notes that none of the RCTs used histological endpoints to assess hepatoxicity, particularly in the light that liver enzymes may not be adequate indicators of early liver damage associated with excess intake of preformed vitamin A.
3.6.2.3. Conclusions on hepatotoxicity
The Panel notes that the available evidence does not allow characterising the dose–response relationship between the intake of preformed vitamin A and liver damage or explore whether high intakes of β‐carotene could potentiate preformed vitamin A‐induced hepatotoxicity. The currently available RCTs, conducted with doses of retinyl palmitate up to 22,500 μg RE/day for up to 12 months, do not allow to address the question raised by the SCF (2002) of whether intake levels of preformed vitamin A < 7500 μg RE/day consumed for six years are hepatotoxic.
3.6.3. Bone health (sQ5)
3.6.3.1. Introduction and mechanisms of toxicity
It is well established that hypervitaminosis A in humans leads to hypercalcemia, impaired bone remodelling, bone alterations, decreased bone mineral density (BMD) and increased risk for bone fractures (Frame et al., 1974; Hathcock et al., 1990). Case reports come from Arctic populations consuming very high doses of dietary retinol (Moore & Wang, 1945) and patients on high doses of synthetic retinoids for the treatment of skin conditions (DiGiovanna et al., 1995; Okada et al., 1994). In laboratory animals, preformed vitamin A toxicity has a teratogenic effect on bone, whereas anomalies in the skeleton and bone fractures have been described during growth (Abu‐Hijleh & Padmanabhan, 1997; Binkley & Krueger, 2000; Lionikaite et al., 2019).
Several mechanisms have been proposed by which excess of preformed vitamin A could adversely affect bone remodelling, BMD and bone strength.
First, retinol could have a direct effect on bone remodelling. Bone remodelling is driven by a complex interplay among cellular phenotypes of different origin and function (bone‐marrow stromal cells, osteoblasts, osteocytes, osteoclasts), and it is tightly regulated by a network of hormones, cytokines and growth factors acting both at systemic and paracrine/autocrine level. Receptors for retinoic acid have been demonstrated in cells of osteoblastic and osteoclastic lineage (Harada et al., 1995). It has been shown that excess retinol can have a direct effect on osteoclast formation, on the stimulation of mature osteoclast activity, on the inhibition of collagen formation and on the synthesis and growth of osteoblast‐like cells (Oreffo et al., 1988; Scheven & Hamilton, 1990). In a murine model treated with high doses of preformed vitamin A, an increased number and size of osteoclasts was shown, together with accelerated bone resorption and reduced bone formation, resulting in bone loss and associated spontaneous bone fractures (Binkley & Krueger, 2000). While at low concentration retinol promotes osteoblast differentiation in vitro, a net detrimental effect on osteoblast differentiation and mineralisation via its concerted effects on osteogenic gene inhibition, osteoclastogenic gene activation and modulation of osteocyte/osteoblast‐related bone peptides can be observed at high concentration, possibly via RAR/RXR signalling (Green et al., 2017; Lind et al., 2013; Yee et al., 2021).
Second, it has been suggested that preformed vitamin A may impair vitamin D‐mediated calcium absorption. In animals, high doses of preformed vitamin A reduce the toxicity of hypervitaminosis D (Clark & Bassett, 1962; Metz et al., 1985) and increase vitamin D dietary requirements (Aburto & Britton, 1998). In humans, a single dose of retinyl palmitate (corresponding to 8190 μg RE) decreased the serum calcium response to an oral dose of 1,25(OH)D3 (Johansson & Melhus, 2001). However, in vivo studies in rats have demonstrated that low bone mass and bone fractures in preformed vitamin A toxicity occur independently of vitamin D status (Moore & Wang, 1945; Rohde & DeLuca, 2003), whereas synergistic, additive and antagonistic interactions between the two vitamins on bone have been described in vitro (Haussler et al., 1998).
Third, retinol could have a direct effect on bone vascularisation. In a rat model, high doses of preformed vitamin A induced impaired endosteal/marrow blood flow that resulted in hypoxia and pathological endosteal mineralisation. Expression of hypoxia‐associated genes was impaired (Lind et al., 2011). Finally, it has also been suggested that retinoic acid could induce PTH release from cultured parathyroid cells (Chertow et al., 1977).
Conversely, no adverse associations between β‐carotene intake and bone health have been reported in human observational studies (Gao & Zhao, 2023). β‐Carotene has been shown to enhance osteoblast differentiation and inhibit osteoclastic activity in vitro. In murine osteoblastic cells, β‐carotene increased cell growth, ALP, Runx‐2 and OPN expression. Early osteoblastic differentiation induced by β‐carotene was mediated through RAR signalling (Nishide et al., 2015). β‐Carotene given at concentrations of 400–600 nM in vitro has also been shown to decrease the viability of bone marrow‐derived monocytes/macrophages when stimulated using RANKL, to reduce the density of TRAP‐positive areas, osteoclast numbers and resorption pit formation, and to increase lactate dehydrogenase release as an indicator of cell apoptosis (Wang et al., 2017).
Although the mechanisms of bone toxicity induced by high doses of preformed vitamin A have not been completely elucidated, decreased bone mass and increased risk of bone fractures are well‐established hazards of excess preformed vitamin A intake. However, it is unclear whether the adverse effects of preformed vitamin A on bone occur at levels that are below the current UL of 3000 μg RE/day, and whether β‐carotene intake could contribute to such an effect.
The SCF (2002) advised post‐menopausal women not to exceed 1500 μg RE/day of preformed vitamin A owing to an association with an increased risk of hip fractures observed in two prospective observational studies above this level of intake (Feskanich et al., 2002; Melhus et al., 1998). The SCF, however, expressed uncertainties about the causality of the positive relationship between the intake of preformed vitamin A and the risk of hip fracture at these levels of intake and found the data available insufficient to set a UL.
Therefore, the purpose of this systematic review was to investigate whether the intake of preformed vitamin A, either alone or in combination with β‐carotene, could adversely affect BMD and the risk of bone fractures in humans at daily doses ≤ 3000 μg RE/day. To that end, a common literature search was performed for sub‐question 5 on bone fractures (sQ5a) and BMD (sQ5b) because the inclusion/exclusion criteria were identical for the two endpoints and some studies could report on both (see Annex A). No eligible RCTs were identified. Among the 17 prospective observational (three NCC, 14 PC; 18 publications) studies that met the inclusion criteria, 9 report on bone fractures only, 5 on BMD only and 3 report on both endpoints.
3.6.3.2. Bone fractures (sQ5a)
Bone fractures are a complex endpoint influenced by several host, environmental and age‐related conditions. The Fracture Risk Assessment Tool (FRAX) (Kanis, 2008), used to predict 10‐year fragility (or osteoporotic, low‐trauma) risk of hip fracture and other major osteoporotic fractures (i.e. vertebral, wrist, humerus), relies on 12 personal, lifestyle and medical factors, namely: (a) age, sex, weight and height; (b) prior fracture, parental hip fracture, smoking, corticosteroid use, rheumatoid arthritis, secondary osteoporosis, alcohol intake ≥ 3 units per day (yes/no answer to each factor); and (c) areal BMD at the femoral neck. Adjustments to the FRAX probability (FRAX‐plus) can be made by adding information on other risk factors for fractures if available (i.e. trabecular bone score, bone fracture in the last 2 years, the number of self‐reported falls in the previous year, glucocorticoid dose and duration, duration of type 2 diabetes mellitus). In the context of the ongoing update of the FRAX (Vandenput et al., 2022), a prior bone fracture appears to double the risk for a future fracture, and such risk is largely independent of areal BMD assessed by DXA (Kanis et al., 2023).
The ratio between the amount of trabecular to cortical bone is much higher in the lumbar spine and the wrist than in the femur, and higher in the diaphysis than in the metaphysis of long bones. Since bone turnover depends on the bone surface available for remodelling, bone loss with aging occurs primarily on trabecular bone and then in cortical bone as the latter becomes more porous. The transition from early trabecular to later cortical bone loss is consistent with the epidemiological data on osteoporotic fractures, with higher incidence of fractures at trabecular sites (e.g. wrist, lumbar spine) in individuals < 65 years of age and higher incidence of fractures at cortical sites (e.g. hip) in individuals ≥ 65 years of age (Osterhoff et al., 2016).
A total of 12 (3 NCC, 9 PC) studies assessed the relationship between vitamin A intake and bone fractures. The studies differ regarding the exposure of interest (preformed and/or total vitamin A from diet, supplements or both), the exposure assessment methods used single or multiple semi‐quantitative FFQs (sFFQ); single or multiple 4 to 7‐day food records; blood retinyl esters), the categorisation of the exposure for data analysis (e.g. continuous, quintiles with/without previous adjustment for energy intake, fixed categories of intake, supplement vs. non‐supplement users), the type of fractures (low trauma, osteoporotic, stress or any type of fracture) and fracture site (any site, hip, lumbar spine, wrist, apical). See evidence table in Appendix B.4. The heat map for the appraisal of the RoB is in Appendix C.3.
Hip fractures
A total of 7 (2 NCC, 5 PC) studies had hip fractures as an endpoint. Four studies were conducted in the USA, two in the UK and one in Sweden. Five studies were in females only and two (Hayhoe et al., 2017; White et al., 2006) included both sexes analysed separately.
The exposure variable was preformed vitamin A in all the studies (of which three also report on total vitamin A) from either diet only (Melhus et al., 1998), supplements only (White et al., 2006) or both diet and supplements (Caire‐Juvera et al., 2009; Feskanich et al., 2002; Hayhoe et al., 2017; Lim et al., 2004). Blood retinyl ester concentration was used as the marker of intake in the remaining study (Barker et al., 2005). Five studies analysed the exposure as a categorical variable (either as fixed categories, quartiles or quintiles of intake) and two as a continuous variable only (Barker et al., 2005; White et al., 2006).
Preformed vitamin A
In a NCC (Barker et al. (2005); UK) in women > 75 years of age (92 cases and 273 controls; mean follow‐up 3.7 years), baseline serum concentrations of retinyl palmitate or supplementation with preformed vitamin A (multivitamin or cod liver oil, intakes not reported) were not positively associated with the risk of incident osteoporotic hip fractures in multivariate analysis. The Panel notes that this study was at moderate RoB (Tier 2). Critical domains were confounding, selection bias and other potential bias in relation to the statistical analysis.
The Leisure World Cohort Study (White et al., 2006) is a PC conducted in the USA for 20 years in male (n = 4769; 278 hip fractures) and female (n = 6850; 949 hip fractures) older adults (mean age (SD) 74.9 (7.2) years for men and 73.7 (7.4) years for women). In a multivariate analysis, preformed vitamin A from supplements was found to be a significant predictor of hip fracture in females (HR, 95% CI = 1.07, 1.00–1.15 for each 3000 μg RE/day increase), but not in males. The Panel notes that the HR refers to a level of intake beyond the current UL for preformed vitamin A and that the risk at lower intakes is not reported. This study was at high RoB (tier 3). Critical domains were exposure, confounding, attrition and other potential sources of bias in relation to the statistical analysis.
Figure 4 depicts the characteristics and results of the five studies that report on preformed vitamin A (μg RE/day) and analysed the exposure as a categorical variable.
FIGURE 4.
Preformed vitamin A (μg RE/day) and risk of hip fractures. C, fixed categories; D, diet; F, females; HR, hazard ratio; IWHS, Iowa Women's Health Study; M, males; NHS, Nurses' Health Study; OR, odds ratio; PC, prospective cohort; Q, quantiles; RoB, risk of bias; RR, relative risk; S, supplements; SMC, Swedish Mammography Cohort; WHIOS, Women's Health Initiative Observational Study. Study duration represents either mean, median or maximum follow‐up. 1The intake was adjusted for energy using the residual method before categorisation; 2Numbers per category were estimated from the total sample assuming equal distribution of participants per category; 3The total number of study participants is reported instead of N per category.
Based on data from one NCC (Melhus et al., 1998) and one PC (Feskanich et al., 2002), the SCF (2002) advised post‐menopausal women not to exceed 1500 μg RE/day of preformed vitamin A.
In the NCC (Melhus et al., 1998), 247 women with a first hip fracture within 2 to 64 months after enrolment and 873 age‐matched controls were selected from the Swedish Mammography Cohort (n = 66,651 women 40–76 years of age). Hip factures due to cancer or high trauma were excluded. Dietary intake of retinol in the previous 6 months was assessed at baseline using a sFFQ. Data on potential risk factors for bone fractures were retrospectively obtained for cases and controls through a mailed questionnaire. In multivariate analysis 21 (conditional logistic regression) with the intake as continuous variable, the OR for the risk for hip fracture (95% CI) was 1.68 (1.18, 2.40) for each 1000 μg RE/day of preformed vitamin A. Risk estimates did not change when use of vitamin supplements was introduced as dichotomous variable (yes/no). A dose–response relationship (P for trend = 0.006) was also observed across predefined categories of non‐energy‐adjusted dietary intake (Figure 4). The OR for the risk for hip fracture (95% CI) for the highest (> 1500 μg RE/day) vs. the lowest‐reference (< 500 μg RE/day) category was 2.05 (1.05, 3.98). Additional adjustment for iron, magnesium, vitamin C and calcium intake attenuated the relationship (OR 1.54, 95% CI: 1.06, 2.24). No women in the lowest category had a retinol intake from the diet < 270 μg RE/day. The number of cases and controls in each intake category, and the mean/upper bound of retinol intake in the highest category, were not reported. This study was at moderate RoB (Tier 2). The critical domain was confounding, owing that data on potential risk factors for fractures were obtained retrospectively, after cases and controls had been identified.
Within the Nurses' Health Study (NHS, USA; Feskanich et al. (2002)), the PC study included 72,337 postmenopausal women (natural or surgical menopause) 34 to 77 years of age, 98% of which were Caucasian, with a maximum follow‐up of 18 years. Intakes of retinol from diet plus supplements were assessed at baseline and every 4 years (5 times in total, one per follow‐up cycle) through a validated sFFQ and were adjusted for energy intake using the residual method prior to categorisation into quintiles for data analysis. At baseline, about 34% of women consumed vitamin A‐containing multivitamins, 3% consumed supplements containing retinol only and 3% consumed supplements with β‐carotene only. These figures increased to 53%, 5% and 10%, respectively, at the end of the follow‐up. These percentages increased intake data were cumulatively updated during analysis for each follow‐up cycle. The endpoint of interest was incident low‐trauma hip fractures, which were self‐reported and confirmed by medical records in a validation study of 30 reported fractures. In multivariate analysis (proportional hazards models), 22 a dose–response relationship between the intake of energy‐adjusted retinol intake from food and supplements and the risk of hip fracture was observed (P for trend < 0.001). The RR (95% CI) for the highest categories of intake (1300–1999 μg RE/day and ≥ 2000 μg RE/day) vs. the lowest (< 500 μg RE/day) were 1.43 (1.04, 1.96) and 1.89 (1.33, 2.68), respectively. Users of supplements containing only retinol had a non‐significant higher risk of hip fractures than non‐users (RR, 1.40; 95% CI: 0.99, 1.99). Retinol from food, mostly from liver, was significantly associated with hip fracture risk (RR, 1.69; 95% CI: 1.05, 2.75 for ≥ 1000 μg/day vs. < 400 μg/day; P for trend = 0.05).
In sensitivity analyses:
considering the intake categories of dietary retinol assessed in Melhus et al. (1998), a RR of 1.64 (95% CI: 1.14, 2.35) was found for the highest (> 1500 μg RE/day) vs. the lowest reference (< 500 μg RE/day) category;
the increased risk for hip fractures was stronger in postmenopausal women not using hormone replacement therapy (HRT) (RR, 95% CI for the highest vs. the lowest category of energy‐adjusted retinol intake was 2.52, 1.48–4.31 and 1.26, 0.68–2.33 for non‐users and users, respectively).
analyses using only baseline energy‐adjusted retinol intakes (rather than cumulative intakes updated overtime) attenuated the relationship between retinol intake and hip fracture risk, leading to a non‐statistically significant RR of 1.17 (95% CI, 0.87–1.58) when comparing the highest vs. the lowest category.
no significant differences in the relationship between retinol intake and risk of hip fracture was seen within strata in stratified analyses for energy‐adjusted calcium and vitamin D intake from diet and supplements.
The Panel notes that multivariate analyses did not control for some important risk factors for bone fractures, including use of corticosteroids, diabetes medication or previous osteoporotic fractures. This study was at low RoB (Tier 1).
Among the new data that have become available since 2002, only one PC with data from the Women's Health Initiative Observational Study (WHIOS; USA, Caire‐Juvera et al. (2009) also used repeated measurements of total retinol intake. A total of 75,747 postmenopausal women 50–79 years of age from various ethnic groups (18% Caucasian) were followed up for a mean of 6.6 years. Dietary intake was assessed at baseline and at year 3 of follow‐up using a validated sFFQ, as well as the intake of vitamin and mineral supplements through an ad‐hoc interview. The proportion of participants consuming preformed vitamin A‐containing supplements is not reported. The mean intake from supplements was 1075 ug RE/day at baseline and 1149 ug RE/day at year 3 of follow up. Retinol intake from food plus supplements was calculated as the mean of baseline and 3 years. Hip fractures (spontaneous and traumatic) were self‐reported and then confirmed through medical records. The risk of hip fractures (HR) was determined using energy‐adjusted multivariate Cox proportional hazards models 23 across quintiles of absolute retinol intake. Retinol intake was not significantly associated with the risk of hip fracture in any of the age‐ or multivariate‐adjusted models, and no dose–response relationship was observed (Figure 4). The cut‐offs for the lowest and upper quintiles of retinol intake from food and supplements were 474 and 1426 μg RE/day, respectively. The results did not change by excluding women with a history of bone fractures or diagnosis of osteoporosis. The Panel notes that the multivariate analyses did not control for the use of medications (other than HRT) that may affect bone fracture risk (e.g. corticosteroids, diabetes medication). The Panel also notes that, although the duration of follow‐up was about one third the follow‐up in the NHS (6.6 vs. 18 years), the number of subjects and the number of events per quintile of intake were comparable, suggesting a similar power. Stratified analyses by vitamin D and calcium intake were not conducted for hip fracture owing to the small number of events. This study was at low RoB (Tier 1).
Similar results were reported using data from the IWHS (USA, Lim et al. (2004)). A cohort of 34,703 postmenopausal women (99% Caucasian) 55–69 years of age was followed‐up for a mean of 9.5 years. Intake of retinol from food plus supplements was assessed at baseline through the same validated sFFQ used in the NHS. About 35% of the women consumed vitamin A‐containing supplements. Hip fractures (traumatic and spontaneous fractures) were self‐reported. The risk of hip fractures was determined using an energy‐adjusted multivariate Cox proportional hazards model 24 across quintiles of absolute retinol intake. There was no indication for a dose–response relationship across quintiles of retinol intake and hip fracture risk (Figure 4). Calcium or vitamin D intakes were not found to be independent risk factors for hip fracture in the models and were not included as covariates. The cut‐offs for the lowest and upper quintiles of retinol intake from food and supplements were 422 and 2101 μg RE/day, respectively. In a subsequent analysis that used the reference category non‐supplement users, HR for hip fractures in all intake categories of supplement users (< 1500, 1500–2999 and ≥ 3000 μg RE/day) were higher (not statistically significant) than 1 (HR, 95% CI = 1.18, 0.94–1.48; 1.24, 0.96–1.59 and 1.10, 0.78–1.55, respectively), and no dose–response relationship was observed. Furthermore, no relationship between total retinol intake and risk of hip fractures was observed either in sensitivity analyses comparing the highest vs. the lowest deciles of intake or using cut‐offs proposed by the NHS. The Panel notes that multivariate analyses did not control for previous fractures. This study was at moderate RoB (Tier 2). Critical domains were outcome assessment and attrition.
The intake of retinol from food and supplements assessed at baseline through a 7‐day weighed food record was also investigated in relation to the risk of hip fractures in males and females (39 to 79 years of age) using data form the EPIC‐Norfolk cohort (Hayhoe et al., 2017). The proportion of participants consuming preformed vitamin A‐containing supplements is not reported. Mean follow‐up was 12.5 years. In women, sample size was considerably lower than in the American cohorts but the number of events per category of intake was higher. No association between retinol intake and risk of hip fractures was observed. The cut‐offs for the lowest and upper quintiles of retinol intake from food and supplements were 216 and 1109 μg RE/day, respectively. In men, there was a non‐statistically significant higher risk of hip fractures in all quintiles of intake (Q2–Q5) vs. the reference, with no evidence for a dose–response. Cut‐offs for the lowest and upper quintiles of retinol intake from food and supplements were 265 and 1158 μg RE/day, respectively (Figure 4). This study was at low RoB (Tier 1).
Owing to the low number of studies available that precludes the characterisation of the sources of heterogeneity and to the type of potential sources, which does not meet the assumptions for considering a mean risk in a distribution of risks across studies (random‐effects model), the Panel decided not to conduct meta‐analyses or dose–response analyses for this endpoint.
Total vitamin A
Three of the prospective studies described above (Caire‐Juvera et al., 2009; Feskanich et al., 2002; Lim et al., 2004) also assessed the relationship between total vitamin A (including retinol and pro‐vitamin A carotenoids) from food and supplements and the risk of hip fracture. The results obtained within each study were similar to those for retinol (Figure 5). In the only study showing a positive dose–response relationship between total vitamin A intake and risk of hip fracture, no relationship was observed between β‐carotene intake and hip fracture risk and the risk was mostly attributed to retinol (Feskanich et al., 2002). Mean intakes in the highest quintile of β‐carotene intake in this study were close to 7 mg/day.
FIGURE 5.
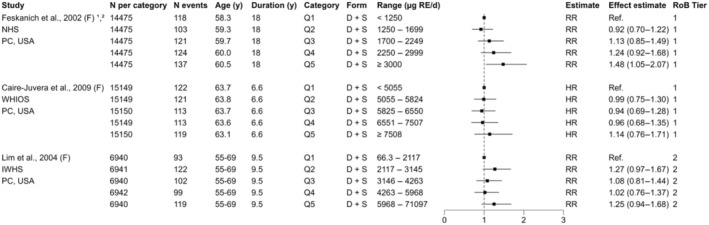
Total vitamin A (μg RE/day) and risk of hip fractures. D, diet; HR, hazard ratio; F, females; IWHS, Iowa Women's Health Study; NHS, Nurse's Health Study; PC, prospective cohort; Q, quantiles; RoB, Risk of Bias; RR, relative risk; S, supplements; WHIOS, Women's Health Initiative Observational Study. Study duration represents either mean, median or maximum follow‐up. 1The intake was adjusted for energy using the residual method before categorisation; 2 Numbers per category were estimated from the total sample assuming equal distribution of participants per category.
Bone fractures at other specific sites
Two of the PC described above also addressed the relationship between the intake of preformed vitamin A and the risk of bone fractures at the wrist and the lumbar spine (Hayhoe et al., 2017; White et al., 2006). Whereas no increased risk of fractures was found at either site in the EPIC‐Norfolk cohort in men or women (Hayhoe et al. (2017); RoB tier 1), preformed vitamin A from supplements was identified in multivariate analysis as a risk factor for wrist fractures in women in the Leisure World Cohort Study (White et al., 2006). The HR (95% CI) was 1.15 (1.07–1.23) for each 3000 μg RE/day increase in preformed vitamin A from supplements, whereas the risk at lower intakes was not reported. Preformed vitamin A from supplements was not identified as a risk factor for wrist fractures in men, or for lumbar spine fractures in men or women. This study was at high RoB (tier 3).
The Panel notes the low number of studies available on the relationship between preformed vitamin A intake and risk of fracture at specific bone sites mainly composed of trabecular bone, namely the wrist and the lumbar spine. The Panel also notes the conflicting results, and that the HR for wrist fracture in women in the Leisure World Cohort Study (White et al., 2006) corresponds to a level of intake beyond the current UL for preformed vitamin A (i.e. for each 3000 μg RE/day increase in intake from supplements only).
Bone fractures at any site
A total of 9 (2 NCC, 7 PC) studies considered first bone fracture at any site as a study endpoint. Two studies were conducted in the USA, one in Japan, and six in Europe. Three studies were in females only, two in males only, two included both sexes analysed separately, and one both sexes analysed together.
The exposure variable was preformed vitamin A in seven studies (of which three also report on total vitamin A) from either diet only or both diet and supplements (Figure 6), blood retinyl esters as marker of preformed vitamin A in one (Barker et al., 2005), and total vitamin A in the remaining study (Toraishi et al., 2021). Except in one study (Toraishi et al., 2021), where the endpoint of interest was stress fractures, the variable of interest was osteoporotic fractures. While using record linkage to identify fracture cases precludes the ability to distinguish between osteoporotic and high‐trauma fractures, some studies excluded bone sites where typically high‐trauma fractures occur (e.g. skull, ribs, hands/feet (Barker et al., 2005; de Jonge et al., 2015; Hayhoe et al., 2017; Key et al., 2007)). When this was not possible (Caire‐Juvera et al., 2009; Lim et al., 2004; Rejnmark et al., 2004), it was assumed that high‐trauma fractures in post‐menopausal women and older men were a minority of all fractures (< 10% in the studies reporting on them separately, e.g. (de Jonge et al., 2015; Michaëlsson et al., 2003).
FIGURE 6.
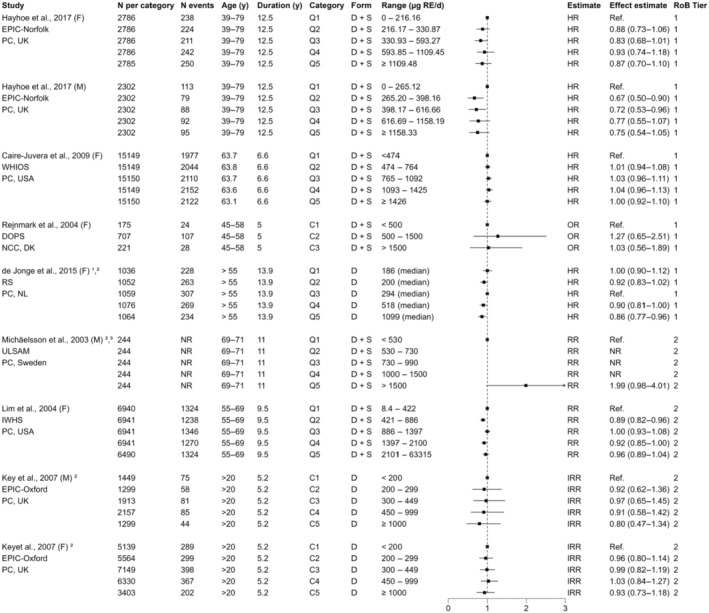
Preformed vitamin A (μg RE/day) and risk of any fracture. C, fixed categories; D, diet; HR, hazard ratio; IRR, incidence risk ratio; F, females; IWHS, Iowa Women's Health Study; NCC, nested case‐cohort; NR, Not reported; M, males; OR, odds ratio; PC, prospective cohort; Q, quantiles; RoB, Risk of Bias; RR, relative risk; RS, Rotterdam Study; S, supplements; SMC, Swedish Mammography Cohort; ULSAM, Uppsala Longitudinal Study of Adult Men; WHIOS, Women's Health Initiative Observational Study. Study duration represents either mean, median or maximum follow‐up. 1The intake was adjusted for energy using the residual method before categorisation; 2Information was obtained from the study authors; 3The age of study participants at the time of intake assessment was estimated from the age at recruitment.
Preformed vitamin A
As for hip fractures (Barker et al., 2005), baseline serum concentrations of retinyl palmitate or supplementation with preformed vitamin A (multivitamin or cod liver oil; intakes not reported) were not significantly associated with the risk of incident osteoporotic fractures at any site in multivariate analysis in women > 75 years of age (312 cases and 934 controls; mean follow‐up 3.7 years). The Panel notes that this study was at moderate RoB (Tier 2). Critical domains were confounding, selection bias and other potential bias in relation to the statistical analysis.
The characteristics and results of the 7 studies (1 NCC, 6 PC) that assessed the relationship between the intake of preformed vitamin A and risk of any fracture using a categorical analysis are illustrated in Figure 6. Four studies were in females only, one in males only, and two were in males and females analysed separately. Three studies used a single validated sFFQ at baseline (de Jonge et al., 2015; Key et al., 2007; Lim et al., 2004), one a repeated validated sFFQ (twice, at baseline and at 3 years (Caire‐Juvera et al., 2009)), two a single 7‐day food record at baseline (Hayhoe et al., 2017; Michaëlsson et al., 2003) and one two 4–7 days food records (Rejnmark et al., 2004). All the studies analysed the exposure as categorical variable (by quartiles or quintiles, or fixed categories) and all selected the lowest category of intake as the reference for comparisons except de Jonge et al. (2015), which selected the middle quintile.
Only one study (Michaëlsson et al., 2003) reports an increased risk of any fracture with higher vs. lower intakes of preformed vitamin A. The study was conducted among participants (n = 2322) of the Uppsala Longitudinal Study of Adult Men (ULSAM). The publication focuses on serum retinol concentrations as a marker of intake, which was not eligible for the present systematic review (see Section 3.3.2). Data on preformed vitamin A intake was available only for half of the study sample (n = 1138), of which 49 (4%) consumed vitamin A‐containing supplements. Only the HR (95% CI) for the highest (> 1500 μg RE/day) vs. the lowest (< 530 μg RE/day) category of preformed vitamin A intake in relation to any fracture is reported in the publication. The HR (95% CI) for energy‐adjusted preformed vitamin A from diet only was 2.00 (1.00–3.99), and 1.99 (0.98–4.01) when supplements were included in the nutrient calculation. The authors informed EFSA that the original data were no longer available to them (see Annex G). This study was at moderate RoB (tier 2) for the exposure (dietary assessment of preformed vitamin A intake) and endpoint (any bone fracture) assessed in this opinion. Critical domains were exposure assessment, confounding and selection bias.
The remaining six studies do not report an increased risk of any fracture with higher vs. lower intakes of preformed vitamin A from diet only or from diet plus supplements (Figure 6). In the two studies reporting on males (Hayhoe et al., 2017; Key et al., 2007), the cut‐offs for the lowest (reference) intake category (265 and 200 μg RE/day, respectively) and the highest intake category (1158 and 1000 μg RE/day, respectively) were lower than in the ULSAM cohort (Michaëlsson et al., 2003), and the lack of risk estimates for intermediate intake categories in the latter precludes direct comparisons. These studies were at low ([Hayhoe et al., 2017]; tier 1) and moderate ([Key et al., 2007]; tier 2) RoB. Critical domains in the latter were outcome assessment and attrition.
Among the six studies reporting on women, the cut‐offs for the lowest and highest categories of intake in the WHIOS ((Caire‐Juvera et al., 2009); RoB tier 1) and the DOPS ((Rejnmark et al., 2004); RoB tier 1) are comparable to those in the ULSAM cohort (Michaëlsson et al., 2003), and even higher (2101 μg RE/day) for the highest category in the IWHS cohort ((Lim et al., 2004); RoB tier 2). It is worth noting that, in the PC selecting the middle quintile of intake (median 294 μg RE/day) as the reference category ((de Jonge et al., 2015); RoB tier 1), no increased risk of fracture was observed in the lower (median 186 and 200 μg RE/day) and higher (median 518 and 1099 μg RE/day) quintiles vs. the reference. The Panel notes that particularly the WHIOS (Caire‐Juvera et al., 2009) and the IWHS (Lim et al., 2004) cohorts, which include thousands of cases, reported null associations between retinol intake form diet and supplements and fracture risk at any site.
In the WHIOS (Caire‐Juvera et al., 2009), stratified analyses by levels of intake above and below the median of calcium, vitamin D and the combination of these were conducted. Among women in the lower vitamin D strata (intake ≤ 11 μg/day), there was a slightly higher risk of fractures in the highest quintile of retinol intake (HR: 1.15; 95% CI: 1.03, 1.29; P for trend: 0.056; median intake 2488 μg RE/day) compared with the lowest quintile. There were no significant risks associated with retinol intake levels in the groups with higher (> 11 μg/day) or lower (< 11 μg/day) vitamin D intake and higher (> 1236 mg/day) calcium intakes. The combination of lower vitamin D and lower calcium intakes resulted in an HR of 1.17 (95% CI: 1.01, 1.36; P for trend 0.258) for total fractures among women in the highest compared with the lowest quintile of retinol. The authors report no increased risk in any ethnic group in stratified analysis by calcium and vitamin D intake, but sample size was probably insufficient for this analysis (data not shown). The Panel notes that the relative risk of any facture at median intakes of retinol ~2500 μg RE/day in these subgroup analyses was several times lower than the relative risk reported for intakes > 1500 μg RE/day in the ULSAM cohort, and that vitamin D intake does not necessarily reflect vitamin D status.
The only other study that assessed the interaction between retinol intake and vitamin D intake (and status, measured as serum 25(OH)D) did not show a significant interaction on fracture risk in women ((de Jonge et al., 2015); RoB tier 1).
Total vitamin A
Three of the above‐mentioned PCs, all conducted in women, also report on total vitamin A in relation to the risk of any facture (Figure 7). As for preformed vitamin A, none report an increased risk of fracture with higher vitamin A intakes in the main analysis. In stratified analyses for calcium and vitamin D intakes above and below the median in the WHIOS (Caire‐Juvera et al., 2009), there was a slightly higher risk of fractures in the highest quintile of total vitamin A (HR: 1.19, 95% CI: 1.04, 1.37; P for trend 0.022; median intake 8902 μg RAE/day – conversion factor for provitamin A carotenoids not reported) compared with the lowest quintile in the low vitamin D intake group (≤ 11 μg/day), a relationship likely driven by retinol intake. There were no significant risks associated with total vitamin A intake in the other vitamin D, calcium or calcium/vitamin D stratified analyses.
FIGURE 7.
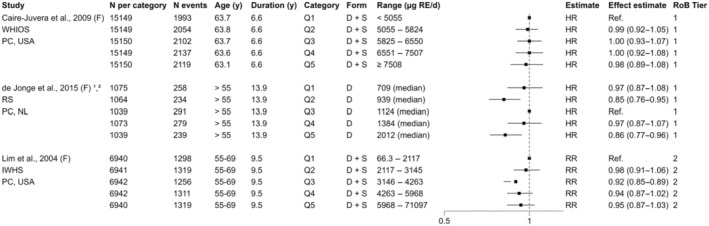
Total vitamin A (μg RE/day) and risk of any fracture. D, diet; F, females; HR, hazard ratio; IWHS, Iowa Women's Health Study; M, males; PC, prospective cohort; Q, quantiles; RR, relative risk; RS, Rotterdam Study; S, supplements; WHIOS, Women's Health Initiative Observational Study. Study duration represents either mean, median or maximum follow‐up. 1Intake adjusted for energy before categorisation; 2 Information was obtained from the study authors.
A fourth PC investigated the relationship between total vitamin A from food and supplements as μg RAE/day (conversion factor for provitamin A carotenoids not reported) and the risk of stress fractures in 41 male collegiate long‐distance runners 18–25 years of age during a 1‐year follow‐up (Toraishi et al., 2021). Intake of total vitamin A was assessed using a semiquantitative FFQ at baseline and end of the study, but only the baseline intake was used in prospective data analysis. Four subjects had a stress fracture during follow‐up. In logistic regression analysis, an OR (95% CI) of 1.22 (0.99–1.50) is reported for each 100 μg RAE/day increase in total vitamin A. The Panel notes that this study was at high RoB (tier 3), and that the results are difficult to interpret. Critical domains were outcome assessment, confounding, selection bias, and other sources of bias related to the statistical analysis.
3.6.3.3. Bone mineral density (sQ5b)
Among the pertinent studies identified, only eight (1 NCC, 7 PC) assessed the relationship between vitamin A intake and BMD at specific bone sites prospectively (Table 12) or the risk of osteoporosis (Sugiura et al., 2016) (see also evidence table in Appendix B.4). Seven studies assessed preformed vitamin A (four from diet only, three from diet and supplements), of which three also report on total vitamin A, and one assessed only total vitamin A from the diet. The studies differ regarding the study population (younger and older male and female adults analysed separately or together, peri‐menopausal women, post‐menopausal women), the dietary method used to estimate vitamin A intake (single or repeated sFFQ, 4–7 day dietary records administered once or multiple times), the bone site at which BMD was measured (total hip, femoral neck, lumbar spine, radial), the endpoint BMD variable (percent change from baseline over the study period or per year, absolute change over the study period, BMD values at different time points) and the statistical analyses conducted. In all the studies, BMD was assessed using DXA.
TABLE 12.
Characteristics of the prospective observational studies reporting on the relationship between vitamin A intake and BMD.
| Study | Houtkooper et al. (1995) | Promislow et al. (2002) | Kaptoge et al. (2003) | Macdonald et al. (2004) | Rejnmark et al. (2004) | Chan et al. (2011) | de Jonge et al. (2015) |
|---|---|---|---|---|---|---|---|
| Design | PC | PC | PC | PC | NCC | PC | PC |
| Country | USA | USA | UK | UK | Denmark | China | The Netherlands |
| Age (years) a | 28–39 | 55–92 | 67–79 | 45–55 | 45–58 | > 65 | > 55 |
| Sex/N |
– F = 66 |
M = 388 F = 570 |
M = 470 F = 474 |
– F = 891 |
– F = 1694 |
M = 1225 F = 992 |
M/F = 5288 |
| Follow‐up | 12–18 months | 4 years | 2–5 years | 5–7 years | 5 years | 4 years | 14 years |
| Vitamin A (exposure) |
Preformed – |
Preformed – |
Preformed – |
Preformed Total |
Preformed Total |
– Total |
Preformed Total |
| Source | Diet | Diet + supplements | Diet | Diet + supplements | Diet + supplements | Diet | Diet |
| Dietary assessment | 1–3 × 4‐day dietary records | 1 × sFFQ | 1 × 7‐day food diary | 2 × sFFQ | 2 × 4 to 7‐day FR | 1 × sFFQ | 1 × sFFQ |
| Preformed vitamin A (μg RE/day) |
Mean (SD) D: 1220 (472) |
Mean (SD) D/F: 497 (460) D/M: 624 (585) D + S/F: 1247 (1573) D + S/M: 1242 (1442) |
Mean (5th ‐ 95th) M: 358 (109–3836) F: 289 (98–3517) |
Mean (SD) D: 820 (602) D + S: 924 (666) |
Median (IQR) D: 530 (309–750) D + S: 1210 (680–1450) |
– |
Median (IQR) Q1: 194 (135–298) Q3 (ref): 356 (212–523) Q5: 1021 (598–1518) |
|
Total vitamin A (μg RE/day) |
– |
– |
– |
NR |
Median (IQR) 1740 (1290‐2360) |
Median (IQR) M: 940 (667–1315) F: 939 (676–1277) |
Median (IQR) Q1: 684 (568–793) Q3(ref):1141 (1050–1257) Q5: 2000 (1712–2485) |
| BMD site |
Total body 3 hip sites(2) Lumbar spine |
Total hip Femoral neck Lumbar spine |
Total hip – – |
– Femoral neck Lumbar spine |
– Femoral neck Lumbar spine |
Total hip Femoral neck – |
– Femoral neck – |
| BMD endpoint | Change (mg/cm2)/year |
% change/year 4‐year change (g/cm2) |
% change/year | % change/year | % change/5 years | % change/4 years | BMD (mg/cm2) at four visits |
| RoB tier | 1 | 1 | 1 | 1 | 1 | 2 | 2 |
Abbreviations: BMD, bone mineral density; FR, food record; IQR, interquartile range; NCC, nested case–control; NR, not reported; PC, prospective cohort; RE, retinol equivalents; SD, standard deviation; sFFQ, semiquantitative food frequency questionnaire; UK, United Kingdom; USA, United States of America.
Age as recruitment target at baseline; (2) femoral neck, trochanter, Ward's triangle.
Preformed vitamin A
Among the six studies that addressed the relationship between preformed vitamin A and prospective changes in BMD (Table 12), two report an adverse effect of higher (vs lower) retinol intake.
The population‐based cohort Rancho Bernardo Heart and Chronic Disease Study (RBHCDS) includes mostly middle and middle‐upper class older men and postmenopausal women (mean age 70–71 years) of Caucasian ancestry (Promislow et al., 2002). Retinol intake from diet (energy‐adjusted) and supplements was assessed at baseline using a validated sFFQ, and BMD at the total hip, femoral neck and lumbar spine was measured at baseline and at the end of the 4‐year follow‐up. One‐half of the women and 39% of men reported taking supplements containing retinol. Of these, the majority (87% of women and 86% of men) had supplemental retinol from multivitamins alone, 5% of women and 6% of men took vitamin A pills only, and 8% of women and men took both.
There was no evidence for linear association between retinol intake and either changes in BMD (g/cm2) or annual % change in BMD in either males or females in the main analysis. There was, however, a significant effect modification by supplemental retinol intake in linear regression models 25 (p for the interaction ≤ 0.01 at all sites for BMD changes; p = 0.02 for total hip and p = 0.06 for femoral BMD % change) in females, but not in males, whereas age was not an effect modifier. Stratified analysis by retinol supplement use showed positive associations between retinol intake and BMD (g/cm2) in female supplement non‐users at all sites, which were statistically significant at the total hip and femoral neck. Statistically significant negative associations between retinol intake and BMD % change among female supplement users were reported at the total hip only. There was little overlap in retinol intake between supplement users and non‐users, and the dietary and supplement components of retinol intake in users had very similar (negative) associations with BMD. Adjusted non‐linear (spline) regression models showed an inverted U‐shape relationship between retinol intake from food and supplements and BMD variables in women, which was more pronounced and statistically significant at the total hip (p = 0.01) and femoral neck (p = 0.02) using BMD % change as outcome variable. BMD variables reached a peak at retinol intakes between 600 and 840 μg RE/day depending on the BMD endpoint and sex. This study was at low RoB (Tier 1) (Appendix C.4). The Panel notes that, in this elderly population, retinol intakes from food and supplements close to the PRI for vitamin A and below those that had been associated with an increased risk of hip fractures in women (1000–1500 μg RE/day) showed a negative relationship with BMD % change at the total hip and femoral neck in women.
Conversely, in the cohort of the Aberdeen Prospective Osteoporosis Screening Study (Macdonald et al., 2004) of mostly (90%) premenopausal women at baseline (mean age 47.5 years), energy‐adjusted retinol intake (mean of two sFFQ at baseline and 5 years later) from diet was significantly and negatively correlated with changes in femoral neck BMD (%/year) in multiple regression linear models 26 (Pearson's correlation coefficient = −0.084, p < 0.05), but not with changes in lumbar spine or femoral neck BMD when supplements (mostly as cod liver oil) were added to dietary intakes. The Panel notes, however, that the difference in mean (SD) intake estimates from diet only (820 (602) μg RE/day) and diet plus supplements (924 (666) μg RE/day) was small. Energy‐adjusted retinol intake from the diet accounted for 0.4% of the variability in BMD changes at the femoral neck. In a subgroup of 146 women still menstruating at the end of follow‐up that had never used HRT, retinol was not associated with BMD changes at either bone site. This study was at low RoB (Tier 1) (Appendix C.4).
No negative relationship between retinol intake from diet (de Jonge et al., 2015; Houtkooper et al., 1995; Kaptoge et al., 2003) or from diet and supplements (Rejnmark et al., 2004) and changes in BMD at any bone site were observed in the remaining four studies.
In the EPIC‐Norfolk (Kaptoge et al., 2003) and Rotterdam (de Jonge et al., 2015) cohorts of older men and postmenopausal women (mean age 72 and 67 years, respectively) dietary retinol was not associated with BMD, but mean intakes were considerably lower (Table 12) than in the above‐mentioned studies (Macdonald et al., 2004; Promislow et al., 2002). In the DOPS (Rejnmark et al., 2004), a prospective open‐label multicentre study in perimenopausal women (mean age 50 years) on the effect of HRT on lumbar spine and femoral neck BMD and fracture risk, multiple regression analyses adjusted for relevant confounders showed no association between baseline retinol intake from food, with or without supplements, and changes in BMD during the 5‐year follow‐up period. HRT was not a modifying factor of the relationship. No differences in BMD were found either between those at the 5th and the 95th percentile of retinol intake from either food or food and supplements. Median (IQR) retinol intakes from diet (530 (309–750) μg RE/day) and diet and supplements (1210 (680–1450) μg RE/day) were comparable to those in the RBHCDS study (Promislow et al., 2002).
Finally, no relationship between vitamin A (assumed preformed) intake from the diet and changes in BMD (mg/cm2) at any specific bone site were observed in 66 premenopausal and normally menstruating women (mean age 34.4 years) participating in a RCT on the effect of calcium supplementation (500 mg/day; all participants) and resistance training (1 h 3 times per week; n = 27) on BMD ((Houtkooper et al., 1995); RoB tier 1). Preformed vitamin A was positively associated with total body BMD changes. Mean retinol intakes from diet were comparable to those from diet and supplements in the RBHCDS (Promislow et al., 2002) and the DOPS (Rejnmark et al., 2004) studies.
One additional PC (Sugiura et al., 2016) conducted in Japan investigated retinol intake from the diet in relation to BMD changes at the radius and the risk of developing osteoporosis at this site (results only reported for the latter endpoint) in 187 post‐menopausal women with no osteoporosis at baseline. No relationship between dietary retinol intake and the risk of developing osteoporosis was observed across tertiles of intake, although mean intakes in the highest tertile were relatively low (538 μg RE/day). This study was at moderate RoB (tier 2). Critical domains were exposure assessment, selection bias and other sources of bias (i.e. selective reporting) (Appendix C.4).
Total vitamin A
Three of the above‐mentioned studies also assessed the relationship between total vitamin A and changes in BMD (de Jonge et al., 2015; Macdonald et al., 2004; Rejnmark et al., 2004). The results and conclusions were similar to those described for retinol.
An additional study meeting the inclusion criteria (Chan et al., 2011) reports only on total vitamin A from the diet in relation to changes in BMD at the total hip and femoral neck over 4 years in a cohort of men (n = 1225) and women (n = 992) > 65 years of age living in Hong Kong (Appendix B.4). Total vitamin A was assessed using a single sFFQ at baseline. Total vitamin A was not significantly associated with 4‐year % changes in BMD at any site in multivariate, adjusted linear regression models. This study was at moderate RoB (tier 2). Critical domains were exposure assessment, selection bias, attrition, and other sources of bias related to the statistical analysis.
3.6.3.4. Evidence integration and uncertainty analysis
No eligible RCTs that had investigated the effect of either total or preformed vitamin A on BMD or the risk of bone fractures were identified. Evidence from prospective observational studies in humans on the relationship between the intake of preformed vitamin A at levels below the current UL of 3000 μg RE/day and these endpoints are conflicting.
Two studies in post‐menopausal women report an increased risk of hip fractures of about 1.5 times for retinol intakes > 1000–1300 μg RE/day (Feskanich et al., 2002) and > 1500 μg RE/day (Melhus et al., 1998) vs. < 500 μg RE/day either from diet only or from diet and supplements. Another study in older men (Michaëlsson et al., 2003) observed an increased risk of any fracture of about 2 times at intakes > 1500 μg RE/day vs. < 500 μg RE/day from either diet or diet and supplements. These studies were at low (tier 1 (Feskanich et al., 2002)) and moderate (tier 2 (Melhus et al., 1998; Michaëlsson et al., 2003)) RoB (Appendix C.3). Conversely, most of the available studies did not provide consistent evidence for an increased risk of bone fractures in relation to retinol intake from diet or diet and supplements, three of which had cut‐offs for the highest category of retinol intake ranging between 1426 and 2101 μg RE/day ((Caire‐Juvera et al., 2009; Rejnmark et al., 2004); RoB tier 1 (Lim et al., 2004); RoB tier 2).
Available data on BMD are likewise fragmented. Whereas one study (Promislow et al., 2002) reports lower BMD at the hip and femoral neck in older females with retinol intakes of 600–840 μg RE/day from diet and supplements, most studies do not show such negative association in older males or females, although mean intakes of retinol were relatively lower in these populations and supplemental intake was not assessed ((Kaptoge et al., 2003), mean dietary intakes were 358 and 289 μg RE/day in males and females, respectively (de Jonge et al., 2015), median intake for the highest quintile was 1021 μg RE/day). In the same study (Promislow et al., 2002), intakes of retinol in supplement users showed a negative relationship with BMD at the hip and femoral neck, whereas in another study (Macdonald et al., 2004) an inverse relationship between retinol intake with hip and femoral neck BMD was only observed when retinol from the diet was considered, and not when supplemental retinol was added to the intake estimates. The difference between intakes of retinol from diet (mean intake = 820 μg RE/day) and diet plus supplements (mean intake = 924 μg RE/day), however, was small in this study. The negative association between retinol intake and BMD was not observed in perimenopausal (Rejnmark et al., 2004) or premenopausal (Houtkooper et al., 1995) women at mean levels of intake (1210 and 1220 μg RE/day, respectively) similar to those in Promislow et al. (2002). These studies were mostly at low RoB (tier 1) (Appendix C.4).
Different reasons have been offered by the authors of the above‐mentioned studies for the conflicting results. Differences in age, sex, race, menopausal status, characterisation of the exposure (e.g. type and frequency of the dietary assessment method used, dietary sources of preformed vitamin A including fortification of water‐based vs. fat‐based foods, supplements included/not included in the intake estimates, supplemental sources, e.g. pills vs. cod liver oil), background (and supplemental) intake (or status) of other nutrients (e.g. calcium, vitamin D) or medicines (e.g. HRT) likely to affect BMD, and choices made by the authors for data analysis, among others, have been identified as sources of heterogeneity across studies which, however, cannot be quantified or systematically addressed with the data available.
The available studies do not provide evidence that dietary β‐carotene could contribute to preformed vitamin A toxicity on bone, as the direction and magnitude of the association between retinol and total vitamin A in relation to BMD and bone fractures in the subset of studies that assessed both exposures is similar.
3.6.3.5. Conclusions on bone health
Taking into account the variety of genetic, environmental and age‐related conditions (including diet and medication use) that have been shown to affect bone metabolism and bone fracture risk, the fact that these factors are only partially and heterogeneously adjusted for in observational studies, the uncertainties in the characterisation of the exposure, and the divergent evidence available from human prospective observational studies, the Panel considers that the causality of the relationship between the intake of preformed vitamin A at levels that are below the current UL (i.e. in the range 1000–3000 μg RE/day) and an increased risk of bone fractures cannot be established. The Panel also considers that the evidence that has become available since the assessment of the SCF (2002) does not support the association between preformed vitamin A at intakes ≤ 3000 μg RE/day and impaired bone health.
3.6.4. Other endpoints (sQ6)
At protocol level (Annex A), a series of adverse health effects that had been associated with excess vitamin A (all chemical forms) were listed and identified as non‐priority endpoints. Of these, only endpoints for which publications were found in relation to chemical forms of vitamin A authorised for addition to foods and food supplements that report adverse health effects in humans upon oral consumption are presented in the following subsections.
3.6.4.1. Preformed vitamin A
Bulging fontanelle in infants
Bulging fontanelle is a well‐established and reversible adverse effect of single or repeated, large doses of preformed vitamin A administered at birth and up to 6–9 months of age to infants at risk of vitamin A deficiency in developing countries. The effect occurs in a small proportion of babies and the dose–response relationship is apparent for cumulative doses, reported at 15,000 μg RE given orally at 6, 10 and 14 weeks and of 7500 μg RE given at 6, 12 and 17 weeks of age, but not at 30,000 μg RE when given at both 6 and 9 months (SCF, 2002).
Two recent systematic reviews and meta‐analysis of RCTs have addressed the acute and long‐term effects (beneficial and adverse) of high‐dose supplementation with preformed vitamin A at birth for the prevention and treatment of vitamin A deficiency in developing countries (Haider et al., 2017; Imdad et al., 2021). The effect of single doses of preformed vitamin A (15,000 μg RE as retinyl palmitate) given in the first 48 to 72 h after birth on the risk of bulging fontanelle within that time period was also assessed. Both systematic reviews included virtually the same studies for that endpoint (i.e. the five RCTs included in Haider et al. (2017) were among the six RCTs included in Imdad et al. (2021); total number of participants = 100,562) and report a 53% higher risk for bulging fontanelle for neonates supplemented with preformed vitamin A compared to controls (RR = 1.53; 95% CI = 1.12–2.09; I 2 = 65% and RR = 1.53; 95% CI = 1.11–2.11; I 2 = 71%, respectively).
Impaired growth
A recent study of vitamin A‐replete preschool children (n = 94; age range 36 to 60 months) in South Africa reported a significant inverse relationship between estimated total liver vitamin A reserves, both at baseline and after a single mandatory supplemental dose (60,000 μg RE) of preformed vitamin A, and growth (weight‐for‐age z‐score, weight‐for‐height z‐score) in the 4 weeks post administration (Sheftel et al., 2022). The Panel notes the high dose of vitamin A administered and that the short follow‐up limits conclusions on growth, which is non‐linear at that age (Wake et al., 2021).
Lipid metabolism
Several reports suggest that retinol increases plasma triacylglycerol concentrations in humans (SCF, 2002). In an RCT, 2297 subjects with a moderate risk of skin cancer (actinic keratoses), received 7500 μg RE/day of retinol or placebo for approximately 4 years. Retinol intake significantly increased fasting serum triacylglycerol concentrations (by 11%), total cholesterol (by 3%) and decreased HDL‐cholesterol (by 1%) as compared to placebo (Cartmel et al., 1999). A similar increase in fasting triacylglycerol concentrations was observed in 146 patients with retinitis pigmentosa consuming supplemental preformed vitamin A (4500 μg RE/day) for 12 years as compared to a control group receiving trace doses (Sibulesky et al., 1999). Total or HDL‐cholesterol concentrations were not measured. No other adverse effects were reported in this long‐term supplementation study, where mean total consumption of preformed vitamin A in the supplemented group was 5583 μg RE/day, an intake well above the current UL.
3.6.4.2. β‐Carotene
Several RCTs have been conducted with supplemental β‐carotene, either alone or in combination with other nutrients (antioxidant vitamins and/or minerals) or aspirin for the primary prevention of cardiovascular diseases (CVD) and cancer.
Lung cancer incidence and mortality
The SCF (2000b) selected three RCTs in humans with β‐carotene supplementation, either administered alone or in co‐supplementation with other nutrients or aspirin, for the prevention of cancer and/or CVD, as the critical data set for the safety assessment of β‐carotene. In the Physicians' Health Study (PHS), β‐carotene (50 mg every other day; Lurotin manufactured by BASF Corporation) was added in a 2 × 2 factorial design to aspirin and given for 12.9 years to 22,071 US male physicians with 39% being past and 11% current smokers. β‐Carotene supplementation did not increase lung cancer risk (RR 0.9, 95% CI: 0.7, 1.2) and no effect modification was found with smoking or alcohol use, but the number of incident lung cancer cases (n = 178; 85 in β‐carotene group and 93 in non‐β‐carotene group) was small (Cook et al., 2000). In the β‐Carotene and Retinol Efficacy Trial (CARET), 30 mg of β‐carotene (manufactured by Hoffmann‐La Roche) plus 7500 μg RE of retinyl palmitate or placebo were administered daily to 18,314 men and women at high risk of developing lung cancer (heavy current or former smokers, and asbestos‐exposed male workers). After a mean intervention period of four years, the active treatment group showed an increased risk of incident lung cancer (RR 1.28, 95% CI: 1.04, 1.57) and lung cancer mortality (RR 1.46, 95% CI: 1.07, 2.00) as compared to placebo. There was an estimated weighted RR of 1.36 (95% CI: 1.07, 1.73) for lung cancer incidence and of 1.59 (95% CI: 1.13, 2.23) for lung cancer mortality for the active treatment group compared with the placebo group, with the weighting accounting for the varying impact of the intervention over time, i.e. with greater emphasis placed on cases occurring in the period two years after randomisation (Omenn et al., 1996). However, it was not possible to distinguish the contribution of β‐carotene and retinyl palmitate to the adverse effects, as they were given in co‐supplementation (Omenn et al., 1996). Finally, in the Alpha‐Tocopherol, Β‐Carotene Cancer Prevention (ATBC) trial (ATBC Study Group, 1994), 29,133 Finnish male smokers (age 50–69 years) with a smoking history averaging one pack/day for 36 years were randomised to consume 20 mg β‐carotene (manufactured by Hoffman‐La Roche) and/or 50 IU α‐tocopherol (vitamin E) daily for a median of 6.1 years in a 2 × 2 factorial design. Participants receiving β‐carotene had higher lung cancer incidence compared to non‐recipients (474 vs. 402 cases; RR 1.18, 95% CI: 1.03, 1.36). Based on these data and the numerous animal studies carried out to elucidate the potential mechanisms for the effect of β‐carotene, the SCF concluded that supplemental β‐carotene at doses ≥ 20 mg/day was contraindicated for use in current, heavy smokers.
Two reports in hamsters (Beems, 1987; Wolterbeek et al., 1995) and one in ferrets (Wang et al., 1999) describe the potential enhancement of chemically induced respiratory tract tumorigenesis, although a statistically significant increase in the incidence of malignant tumours has not been reported. The study in ferrets, which was specifically designed to mimic the human trials regarding the dose of β‐carotene administered and the exposure to smoking and is extensively described in the SCF opinion (SCF, 2000b), clearly showed a strong proliferative response in lung tissue in all β‐carotene‐supplemented animals (exposed and not exposed to tobacco smoke), a response that was enhanced by exposure to tobacco smoke (Wang et al., 1999). The SCF noted, however, that there was insufficient scientific basis to set a UL for isolated β‐carotene, as no dose–response relationship for the adverse effects of β‐carotene was available either from the intervention trials in humans or from appropriate animal models (e.g. ferrets).
A systematic review by Druesne‐Pecollo et al. (2010) identified 8 RCTs on β‐carotene supplementation, either alone or in combination with other antioxidant nutrients, with primary lung cancer incidence as the outcome of interest. The meta‐analysis included 180,702 subjects and 1852 incident lung cancer cases with supplemental β‐carotene doses ranging from 6 to 30 mg/day and an average follow‐up duration between 2.1 and 12.9 years.
Overall, a RR of 1.13 (95% CI: 1.04, 1.24) was reported in subjects (smokers and non‐smokers combined) supplemented with β‐carotene compared to placebo. In sensitivity analyses, the risk of incident lung cancer increased when β‐carotene was given at doses ≥ 20 mg/day in combination with antioxidants (20–30 mg/day; RR 1.16, 95% CI: 1.06, 1.27; 6 RCTs with 138,101 subjects and 1682 cases). Such increase in risk was not observed when β‐carotene was given alone at similar doses (20–25 mg/day; RR 1.09, 95% CI: 0.94, 1.26; 3 RCTs with 91,080 subjects and 680 cases) or when β‐carotene was given at lower doses in combination with antioxidants (6 and 15 mg/day; RR 0.93, 95% CI: 0.69, 1.25; 2 RCTs with 42,601 subjects and 680 cases). The risk was higher when the analysis was restricted to populations of exclusively smokers or asbestos workers (RR 1.20, 95% CI: 1.07, 1.34; 2 RCT with 47,447 subjects and 1078 cases) and to trials of mostly males (ATBC, CARET). The effect of β‐carotene supplementation on lung cancer risk was substantially attenuated when the ATBC and CARET trials were not included in the analysis (RR 1.05, 95% CI: 0.91, 1.20; 6 RCTs with 133,255 subjects and 774 cases). The p‐value for heterogeneity was greater than 0.05 in all the analyses.
The Panel notes that, in the two RCTs conducted with lower supplemental doses (6 and 15 mg/day; personal communication on the SU.VI.MAX Study (Hercberg et al., 2004) and Kamangar et al. (2006), respectively), β‐carotene was given in co‐supplementation with other vitamins and/or minerals (ascorbic acid, zinc, α‐tocopherol and selenium in SU.VI.MAX (Hercberg et al., 2004); α‐tocopherol and selenium, with or without other fixed combinations of vitamins and/or minerals, in Kamangar et al. (2006)). Co‐supplementation with other vitamins and minerals in the above‐mentioned studies precludes conclusions on the effect of β‐carotene alone. The Panel also notes that the study by Kamangar et al. (2006) reports on lung cancer mortality rates rather than on the incidence of lung cancer. Specifically, the publication provides hazard ratios for lung cancer mortality during the post‐trial follow‐up period, which spans 15 years (5 years of the initial trial and an additional 10 years of follow‐up). In a publication reporting on the results for the 5‐year intervention period only (Blot et al., 1993), overall cancer incidence is also reported, though cases of lung cancer in particular are not reported and are categorised under ‘other cancers’.
The Panel also notes that only four of the RCTs identified allow conclusions on supplemental β‐carotene compared to no supplemental β‐carotene (i.e. β‐carotene was provided in the context of factorial designs). Their main characteristics are summarised in Table 13. Two RCTs (the ATBC and the PHS) had been already identified and discussed by the SCF. The third was the Women's Health Study (WHS (Lee et al., 1999)), a RCT giving vitamin C, vitamin E and β‐carotene in a 2 × 2 × 2 factorial design (≥ 45 years of age, 13% smokers; 19,939 β‐carotene recipients vs. 19,937 non‐recipients). In this RCT, β‐carotene supplementation (50 mg every other day) was terminated early after a median intervention duration of 2.1 years (follow‐up 4.1 years) owing to the results of the ATBC, CARET and PHS trials, where an increased risk or no benefit of β‐carotene supplementation was observed. No increased risk of cancer associated with β‐carotene supplementation was observed in this population up to trial termination and follow‐up, even adjusting for multiple comparisons (Lee et al., 1999). Regarding lung cancer, 30 and 21 cases were diagnosed in the β‐carotene and the no β‐carotene groups, respectively, with an RR of 1.42 (95% CI 0.82–2.49) as calculated in Druesne‐Pecollo et al. (2010) (Lee et al., 1999). The fourth RCT was the Women's Antioxidant Cardiovascular Study (WACS), which used the same study design as the WHS, a 2 × 2 × 2 factorial design (≥ 40 years of age, 15% current and 41% past smokers; 3807 β‐carotene recipients vs. 3820 non‐recipients) (Lin et al., 2009). After an average follow‐up duration of 9.4 years, β‐carotene supplementation (50 mg every other day) lead to an increased, though not statistically significant, risk of lung cancer (41 vs. 33 cases in the β‐carotene vs. the no β‐carotene group; RR 1.26, 95% CI: 0.80, 1.99). The Panel however notes that Druesne‐Pecollo et al. (2010) incorporated WACS into their review categorising the exposure as β‐carotene in combination with other antioxidants.
TABLE 13.
Main characteristics of randomised controlled trials which have investigated the effect of supplemental β‐carotene on the risk of lung cancer.
| ATBC (ATBC Study Group, 1994) | PHS (Cook et al., 2000) | WHS (Lee et al., 1999) | WACS (Lin et al., 2009) | |
|---|---|---|---|---|
| Country | Finland | USA | USA | USA |
| Sex/population |
Males Heavy smokers |
Males Physicians |
Females Health professionals |
Females Post‐menopausal |
| Age at recruitment | 50–69 years | 40–84 years | ≥ 45 years | ≥ 40 years |
| Health status | No prior cancer or serious illness | Apparently healthy | Apparently healthy | History of CVD or at least three risk factors for CVD |
| Smoking status | 100% heavy smokers (~ 1 pack/day for 36 years) | 11% current and 39% past smokers | 13% current smokers | 15% current and 41% past smokers |
| Intervention |
2 × 2 factorial Vitamin E, β‐carotene (20 mg/day) |
2 × 2 factorial Aspirin, β‐carotene (50 mg e.o.d) |
2 × 2 × 2 factorial Aspirin, vitamin E, β‐carotene (50 mg e.o.d) |
2 × 2 × 2 factorial Vitamin C, Vitamin E, β‐carotene (50 mg e.o.d) |
| β‐Carotene supplement (manufacturer) | Synthetic water soluble β‐catotene (Hoffman‐La Roche) | Lurotin, microencapsulated, water‐dispersible synthetic β‐carotene (BASF corporation) | Lurotin, microencapsulated, water‐dispersible synthetic β‐carotene (BASF corporation) | Lurotin, microencapsulated, water‐dispersible synthetic β‐carotene (BASF corporation) |
| Duration of the intervention | 6.1 years (median) | 12.9 years (mean) | 2.1 years (median) | 9.4 years (mean) |
| n on β‐carotene/n not on β‐carotene | 14,560/14,573 | 11,034/11,037 | 19,937/19,936 | 3807/3820 |
| Lung cancer cases on β‐carotene/not on β‐carotene (n) | 474/402 | 85/93 | 30/21a | 41/33 |
| Serum β‐carotene levels reached | 5.59 μmol/L | 2.19 μmol/L | NR | NR |
| Results | RR 1.18 (95% CI: 1.03, 1.36) | RR 0.9 (95% CI: 0.7, 1.2) | RR 1.43 (95% CI: 0.82, 2.49)b | RR 1.26 (95% CI: 0.80, 1.99) |
Abbreviations: CI, confidence interval; CVD, cardiovascular disease; e.o.d, every other day; NR, not reported; RR, relative risk.
Lung cancer cases reported for the trial period plus the 2 year follow‐up, i.e. for a total median duration of 4.1 years.
The RR and 95% CIs were calculated by Druesne‐Pecollo et al. (2010) using the number of cases and number of participants provided in the publication.
As shown in Table 13, higher RRs for lung cancer incidence are reported for supplemental β‐carotene vs. non‐β‐carotene in three out of the four RCTs available. The increased risk of lung cancer was only statistically significant in the ATBC trial of heavy male smokers, possibly the only trial powered for this endpoint owing to the notably higher number of lung cancer cases reported. The ATBC trial also differed from the other three RCTs on the type of supplement administered, the amount of supplemental β‐carotene, the pattern of administration, and the plasma concentrations of β‐carotene reached.
In the ATBC and CARET trials, synthetic water soluble β‐carotene supplements manufactured by Hoffman‐La Roche were administered daily (20 and 30 mg/day, respectively). In the PHS, WHS and WACS, 50 mg of microencapsulated, water‐dispersible synthetic all‐trans β‐carotene (Lurotin) supplements manufactured by BASF corporation were given every other day. In the ATBC, mean baseline plasma β‐carotene concentrations increased from 17 μg/dL to 300 μg/dL (from 0.32 to 5.59 27 μmol/L) at the 3‐year mark, while in the CARET study the median post‐intervention plasma concentration of β‐carotene was 210 μg/dL (3.91 μmol/L). The PHS trial showed a comparatively lower increase from baseline, reaching a median level of 118 μg/dL (2.19 μmol/L). The increase in plasma β‐carotene concentrations in the ATBC and CARET trials significantly exceeded those observed in the PHS, with a respective 17‐fold and 12‐fold rise from baseline compared to a four‐fold increase in the PHS study. The increase in plasma β‐carotene concentrations in the ATBC and CARET trials also exceeded the 10‐fold increase observed in the Skin Cancer Prevention Study (SCPS), which used 50 mg of Lurotin daily (see section on Cardiovascular disease incidence and mortality).
The disparity in the achieved plasma β‐carotene concentrations may be attributed to a lower bioavailability of supplemental β‐carotene in the PHS in comparison to the ATBC, owing to the different formulation used and to the pattern of supplementation. Plasma β‐carotene concentrations were not reported in WHS and WACS, which used the same dose, type of supplement and supplementation pattern as PHS. The Panel notes that the mean plasma concentration of β‐carotene reached in the ATBC trial is comparable to that reported with daily consumption of a similar amount (21.6 mg/day) of β‐carotene as synthetic water dispersible powder in beverages (5.04 μmol/L) and well above the level reached by consuming similar daily amounts (18 mg/day) of β‐carotene from non‐fortified carrot juice (1.71 μmol/L) ((Thürmann et al., 2002); see Section 3.3.4.2).
The most recent systematic review and meta‐analysis reporting on β‐carotene supplementation and risk of lung cancer (O'Connor et al., 2022) did not identify additional RCTs on primary cancer incidence. An increased risk for lung cancer in smokers and non‐smokers combined at daily doses of 20–30 mg of supplemental β‐carotene, with or without vitamin A co‐supplementation (OR 1.20, 95% CI: 1.01, 1.42; 4 RCTs [n = 94,830]; I 2 = 38.8%), is reported in this analysis. Another recent systematic review and meta‐analysis (Kordiak et al., 2022), based on the same studies identified by Druesne‐Pecollo et al. (2010), reported similar risk estimates for lung cancer with supplemental β‐carotene alone or in combination with other antioxidants (RR 1.16, 95% CI: 1.06, 1.26; 8 RCTs [n = 167,141]; I 2 = 0%). The authors also conducted a meta‐regression analysis that did not identify a dose–response relationship between β‐carotene supplementation and lung cancer risk.
Similarly, increased lung cancer mortality associated with β‐carotene supplementation, alone or in combination with other antioxidants, was reported in a meta‐analysis of 5 RCTs (RR 1.14, 95% CI: 1.02, 1.27, I 2 = 3%) (Corbi et al., 2022). However, the publication does not allow identifying the individual RCTs considered for the analysis.
Other cancer incidence and mortality
The systematic review of RCTs by Druesne‐Pecollo et al. (2010) also addressed the effect of β‐carotene, either alone or in combination with other antioxidant nutrients, on the incidence of all cancers and cancer at other sites, including stomach, pancreatic, colorectal, prostate, breast, and skin cancers, including melanoma. With a total of 180,702 participants and 10,600 cancer cases (8 RCTs), the effect of β‐carotene supplementation versus no β‐carotene was not significant (RR 1.01, 95% CI: 0.98, 1.04). In sensitivity analyses, β‐carotene supplementation increased the relative risk of cancer at any site only in the ATBC and CARET trials, which included exclusively smokers and/or asbestos workers (RR 1.08, 95% CI: 1.01, 1.15). No effect of β‐carotene supplementation was observed on the incidence of pancreatic, colorectal, prostate, breast or skin cancer in any analysis.
Likewise, no effect of β‐carotene supplementation given alone or in combination with other antioxidant nutrients was observed for stomach cancer (6–30 mg/day; RR 0.99, 95% CI: 0.86, 1.13; 7 RCTs with 172,531 subjects and 808 cases). In sensitivity analysis, the risk of incident stomach cancer increased when β‐carotene was given at doses ≥ 20 mg/day in combination with antioxidants (20–30 mg/day; RR 1.34, 95% CI: 1.06, 1.70; 5 RCTs with 129,390 subjects and 265 cases). Such increase in risk was not observed when β‐carotene was given alone at similar doses (20–25 mg/day; RR 1.16, 95% CI: 0.78, 1.73; 3 RCTs with 91,080 subjects and 680 cases). The risk was higher when the analysis was restricted to populations of exclusively smokers or asbestos workers in the ATBC and CARET trials (RR 1.54, 95% CI: 1.08, 2.19; 2 RCTs with 47,447 subjects and 106 cases). The p‐value for heterogeneity was greater than 0.05 in all the analyses.
Another systematic review of RCTs (Jeon et al., 2011) focused on the effect of β‐carotene in mono‐supplementation for the primary or secondary prevention of cancer. Among the 6 RCTs identified, three were on primary prevention (already included in Druesne‐Pecollo et al. (2010)) and three were on secondary prevention (skin or head and neck cancer). Doses of β‐carotene ranged from 20 mg/day to approximately 45 mg/day (75 mg/day supplementation in 3‐month cycles with one‐month inter‐cycle intervals), with mean treatment and follow‐up periods of 6 and 6.3 years, respectively. The increase in cancer risk in relation to β‐carotene supplementation was attenuated as compared to that reported in previous meta‐analyses (RR 1.08, 95% CI: 0.99, 1.18; I 2 = 54.0%, 6 RCTs) and there was no association with mortality (RR 1.00, 95% CI: 0.87, 1.15; I 2 = 0.0%, 4 RCTs), as compared to the control group. Among the cancer sites assessed (lung, colorectal, head and neck cancer, skin, and prostate cancer), β‐carotene supplementation increased the incidence of urothelial cancer (RR 1.35, 95% CI: 1.01, 1.81, I 2 = 0.0%, 3 RCTs), and specifically bladder cancer (RR 1.52, 95% CI: 1.03, 2.24, I 2 = 0.0%, 2 RCTs). Doses of β‐carotene ranged from 20 mg/day to approximately 45 mg/day and follow‐up duration from 5.1 to 12.9 years.
Cardiovascular disease incidence and mortality
One systematic review assessed the risk of adverse events, including CVD incidence and mortality, of β‐carotene alone or in combination with preformed vitamin A (O'Connor et al., 2022). Right after lung cancer incidence, an increased risk for CVD mortality was also observed for β‐carotene alone (at doses between 25 to 50 mg/day) or in combination with preformed vitamin A (CARET study included), although of smaller magnitude (OR 1.09; 95% CI, 1.01 to 1.18; 5 RCTs; 94,506 subjects; I 2 = 0%), whereas no increased risk of CVD incidence was seen for β‐carotene alone at doses of 50 mg every other day (2 RCTs available: PHS and WHS; myocardial infarction, stroke, and CVD events‐composite endpoint).
The highest administered dose of β‐carotene, at 50 mg/day, was in the SCPS. This study compared β‐carotene (Lurotin) to a placebo over a median intervention period of 4.3 years, involving 1720 individuals at high risk for nonmelanoma skin cancer. Over the entire period of observation (median 8.2 years), the RR for CVD mortality and all‐cause mortality were 1.16 (95% CI, 0.82–1.64) and 1.03 (95% CI, 0.82–1.30), respectively. The Panel notes that, despite the larger administered dose, the increase in plasma β‐carotene concentrations from baseline in the SCPS was approximately 10‐fold, reaching median plasma β‐carotene concentrations of 172 μg/dL (3.2 μmol/L), in contrast to the ATBC and CARET which administered lower doses (20 and 30 mg/day, respectively) and observed 17‐fold and 12‐fold increase, respectively. This level of increase in plasma β‐carotene is consistent to that observed in the PHS, which used the same supplement formulation but administered it every other day, resulting in a fourfold increase in plasma concentrations.
In another recent systematic review by Yang et al. (2022), β‐carotene supplementation at doses ranging from 6 to 25 mg/day, alone or in combination with other antioxidant vitamins or minerals, showed no effect on major CVD incidence (myocardial infarction, stroke, peripheral arterial disease, ischemic CVD, and revascularisation procedures) when compared to placebo (7 RCTs; RR 1.03, 95% CI: 0.99, 1.08; I 2 = 0%). However, doses of 15–50 mg/day were associated with an increase in cardiovascular mortality risk 9 RCTs (RR 1.12, 95% CI: 1.04, 1.19; I 2 = 24%). Subgroup analyses indicated that the elevated risk of cardiovascular mortality was primarily observed in at‐risk populations (T2DM, angina pectoris, previous myocardial infarction, history of CVD or ≥ 3 CVD risk factors, peripheral arterial disease, coronary disease) with no discernible impact on cardiovascular mortality in apparently healthy populations (RR 1.05, 95% CI: 0.92, 1.19; I 2 = 0%). Conversely, in at‐risk populations, an increased risk was evident (RR 1.15, 95% CI: 1.05, 1.26; I 2 = 45%).
In subgroup analyses, β‐carotene given alone was associated with a modest increased risk of cardiovascular disease incidence (20–25 mg/day; 4 RCTs; RR 1.06, 95% CI: 1.01, 1.12; I 2 = 5%) and cardiovascular mortality (20–50 mg/day; 7 RCTs; RR 1.10, 95% CI: 1.02, 1.19; I 2 = 11%). The Panel notes however that in the meta‐analysis for β‐carotene given alone and cardiovascular mortality, the study authors had erroneously included two studies (Catalano et al., 2007; Heart Protection Study Collaborative Group, 2002) that had in fact given β‐carotene in co‐supplementation with other antioxidant vitamins or minerals (i.e. the Heart Protection Study and the Critical Leg Ischaemia Prevention Study). The Panel also notes that these analyses included some studies with at‐risk populations for CVD and that the meta‐analyses included multiple publications from the ATBC study, and two publications from the CARET study which reported results on various types of CVD endpoints, making it challenging to determine the extent of participant overlap.
All‐cause mortality
In a meta‐analysis of 7 RCTs including 43,019 participants at doses of β‐carotene ranging from 20 to 50 mg (Bjelakovic et al., 2013), an increased risk for all‐cause mortality with β‐carotene supplementation alone (RR 1.06, 95% CI: 1.02, 1.10, I 2 = 0%) compared to placebo was reported. A more recent meta‐analysis of five RCTs including data from 112,820 individuals found a similar risk for all‐cause mortality associated with β‐carotene given alone (20–50 mg/day; OR 1.06, 95% CI: 1.00, 1.12; I 2 = 6.4%) (O'Connor et al., 2022).
β‐Carotene alone or in combination with other antioxidants had no effect on all‐cause mortality (RR 0.90, 95% CI: 0.69, 1.17; I 2 = 23%; 6 RCT, 14,285 participants) at doses ≤ 9.6 mg/day (corresponding to the US RDA for vitamin A of 800 μg RE/day using a conversion factor of 1/12) versus placebo, whereas the risk was increased at doses > 9.6 mg/day (RR 1.06, 95% CI: 1.02, 1.09; I 2 = 13%; 20 RCTs, 158,721 participants) (Bjelakovic et al., 2013). The Panel notes that studies on supplemental β‐carotene in combination with other vitamins/minerals limit conclusions on supplemental β‐carotene alone.
3.6.4.3. Conclusions
Preformed vitamin A
Bulging fontanelle in infants and adverse effects on the blood lipid profile in adults have been reported in relation to preformed vitamin A supplementation, either as single or repeated high doses well above the current UL for preformed vitamin A for adults of 3000 μg RE (≥ 7500 μg RE). The Panel considers that these endpoints cannot be used to derive a UL for preformed vitamin A.
β‐Carotene
An increased risk of cancer (primarily lung cancer) and all‐cause mortality, presumably driven by CVD mortality, has been reported in RCTs with supplemental β‐carotene alone at doses ranging from 20 to 50 mg/day. This increased risk is primarily evident among male heavy smokers (ATBC), with little evidence from studies involving mostly non‐smokers or females (PHS, WHS, WACS, SCPS).
The Panel notes the heterogeneity of these studies regarding the duration of the intervention (e.g. the WHS with only a median duration of 2.1 years), the β‐carotene formulations used and the pattern of administration (i.e. 20 mg daily in ATBC vs. 50 mg every other day in PHS, WHS, WACS, SCPS), and the study population regarding sex and smoking habits, which may have impacted the power of the studies in relation to lung cancer incidence and all‐cause, including CVD, mortality. The Panel also notes that the available data do not allow identifying a dose–response relationship between the intake of supplemental β‐carotene and cancer risk and/or all‐cause mortality (i.e. the RCTs available used a single, fixed dose, and no RCTs are available with doses < 20 mg/day), and that the potential sources of heterogeneity for the effect cannot be characterised (i.e. unclear whether differences in risk across studies depend on sex, baseline risk, supplemental dose, type of supplement, pattern of administration, duration of the intervention, and/or other factors).
3.7. Hazard characterisation
3.7.1. Selection of the critical effect
3.7.1.1. Preformed vitamin A
The critical effect on which the UL for preformed vitamin A from all sources was established in 2002 for all population groups (except post‐menopausal women) is teratogenicity (SCF, 2002). The UL was set at 3000 μg RE/day for adults (men and women of child‐bearing age, including pregnant and lactating women), based on a NOAEL of 3000 μg/day derived from the dose–response relationship between the intake of preformed vitamin A and birth defects reported in one PC study (Rothman et al., 1995). An uncertainty factor was not considered necessary, because the data from other studies indicated that the true threshold for an effect could be higher than this value. These were one PC (Mastroiacovo et al. (1999); RoB tier 3) and three cases‐control studies (Martínez‐Frías and Salvador (1990); Mills et al. (1997); Shaw et al. (1997); all RoB tier 2). Two additional case–control studies at low RoB (tier 1), published since the SCF assessment support that conclusion (Botto et al. (2001); Johansen et al. (2008). The UL for children and adolescents was set based on the UL of 3000 μg RE/day for adults with correction for differences in basal metabolic rate using allometric scaling according to body surface area (body weight0.75).
The Panel notes that β‐carotene per se is not considered to be teratogenic. The Panel also notes that, owing to the downregulation of β‐carotene absorption and conversion to retinol in vitamin A‐repleted states, it is also unlikely that maternal β‐carotene intake from food or supplements would potentiate the teratogenic effects of preformed vitamin A, although the available data in humans do not allow to address this question. Therefore, the Panel considers that a UL based on teratogenicity should apply to preformed vitamin A only (Section 3.6.1).
The lowest dose reported to cause hepatotoxicity in humans (Geubel et al., 1991; Kowalski et al., 1994), an adverse effect of excess preformed vitamin A that is relevant for all population groups, is 2.5 times higher than the NOAEL for teratogenicity. The available evidence from RCTs does not allow addressing the question of whether lower intakes for longer periods of time are hepatotoxic, or to characterise the dose–response relationship for hepatotoxicity (Section 3.6.2).
With respect to bone health, the SCF (2002) recommended post‐menopausal women not to exceed 1500 ug RE/day of preformed vitamin A and further called for data to clarify the possible contribution of confounding to the reported increased risk of bone fractures that would provide greater confidence in a true cause‐effect relationship at such low levels of intake. The Panel considers that the causality of the relationship between the intake of preformed vitamin A in the range 1000–3000 μg RE/day and an increased risk of bone fractures cannot be established with the available data. The Panel also considers that the evidence that has become available since the assessment of the SCF (2002) does not support the association between preformed vitamin A at intakes ≤ 3000 μg RE/day and impaired bone health (Section 3.6.3).
Bulging fontanelle in infants and adverse effects on the blood lipid profile in adults have been reported in relation to preformed vitamin A supplementation, either as single or repeated high doses well above the current UL for preformed vitamin A for adults of 3000 μg RE (≥ 7500 μg RE).
Based on the available evidence, the Panel selects teratogenicity as the critical effect on which to base the UL for preformed vitamin A. The Panel considers that this endpoint is only relevant for women of child‐bearing age. The Panel however notes that this is an irreversible form of toxicity that occurs at relatively low intakes, and that a UL based on this effect would also cover for other adverse effects of excess preformed vitamin A.
3.7.1.2. β‐Carotene
The Panel selects lung cancer risk as the critical effect of excess supplemental β‐carotene.
Higher RRs for lung cancer incidence are reported for β‐carotene vs. no β‐carotene supplements in three out of the four RCTs available from which conclusions on β‐carotene alone can be drawn for this endpoint. The biological plausibility for an effect of supplemental β‐carotene on lung cancer risk is provided by one study in ferrets specifically designed to mimic the human trials regarding the dose of β‐carotene administered and the exposure to smoking. That study showed a strong proliferative response in lung tissue in all β‐carotene‐supplemented animals (exposed and not exposed to tobacco smoke), the response being enhanced by exposure to tobacco smoke (Wang et al. (1999), extensively described in SCF (2000b)).
The increased risk of lung cancer was only statistically significant in the ATBC trial of heavy male smokers using supplemental β‐carotene at doses of 20 mg/day for 6 years (ATBC Study Group, 1994), possibly the only trial powered for this endpoint owing to the notably higher number of lung cancer cases ascertained in this population compared to those reported in RCTs of men (PHS; Cook et al. (2000)) and women (WHS; Lee et al. (1999); WACS; Lin et al. (2009)) with a lower proportion of current and past smokers. The ATBC trial also differed from the other three RCTs on the type of supplement administered (more bioavailable), the amount of supplemental β‐carotene (20 mg vs. 50 mg), the pattern of administration (daily vs. every other day), and the plasma concentrations of β‐carotene reached (17‐fold and four‐fold increase in the ATBC and PHS trials, respectively). The Panel notes that, with the available data, the potential sources of heterogeneity for the effect of supplemental β‐carotene on lung cancer risk cannot be characterised (i.e. unclear whether differences in risk across studies depend on sex, baseline risk, supplemental dose, type of supplement and its bioavailability, pattern of administration, duration of the intervention, and/or other factors, or any combination of these).
No data for supplemental β‐carotene given alone are available at doses < 20 mg/day in any population group. Therefore, the Panel considers that the available data are not sufficient and suitable to characterise a dose–response relationship and identify a reference point for supplemental β‐carotene in relation to lung cancer risk (Sections 3.6.4.2 and 3.6.4.3).
3.7.2. Derivation of the UL
3.7.2.1. Preformed vitamin A
The Panel proposes to retain the UL of 3000 μg RE/day for adults, based on a NOAEL for teratogenicity (Section 3.7.1.1). This UL applies to men and women, including women of child‐bearing age, pregnant and lactating women, and post‐menopausal women.
The Panel also proposes to retain the ULs for children and adolescents that were extrapolated from the UL of 3000 μg RE/day for adults by the SCF (2002) using allometric scaling (body weight0.75) to account for differences in basal metabolic rate. These are 2600 μg RE/day for adolescents 15 to 17 years; 2000 μg RE/day for adolescents 11 to 14 years; 1500 μg RE/day for children 7 to 10 years; 1100 μg RE/day; and 800 μg RE/day for children 1 to 3 years.
Applying allometric scaling for the derivation of a UL for infants aged 4 to 6 months 28 and 7 to 11 months 29 based on the UL for adults would result in a value of ca. 545 and 623 μg RE/day, respectively, which rounding down to the lowest 100 would lead to a UL of 500 and 600 μg RE/day, respectively. Considering that a secretion of 424 μg RE/day of retinol in breast milk has been estimated during the first 6 months of lactation using the midpoint concentration (530 μg/L) of the range of means (229–831 μg/L) reported in breast milk of mothers from Western countries (EFSA NDA Panel, 2015), the Panel considers that a UL of 500 μg RE/day for infants 4–6 months would be overconservative and proposes a UL of 600 μg RE/day for all infants (aged 4–11 months).
3.7.2.2. β‐Carotene
In the absence of adequate data to characterise a dose–response relationship and identify a reference point for supplemental β‐carotene in relation to lung cancer risk, no UL for supplemental β‐carotene intake can be established for any population group. For nutrients for which there are no, or insufficient, data on which to base an UL, the Panel is requested to ‘give an indication on the highest level of intake where there is reasonable confidence in data on the absence of adverse effects’ (see Section 1.2), i.e. a safe level of intake.
The Panel notes that there is no indication that β‐carotene intake from the background diet, including its use as a food additive, is associated with adverse health effects (Section 3.3.6). The estimated background dietary intake of β‐carotene observed among high consumers (95th percentile) in representative groups of the population can be found in Section 3.5.2.2. The main contributors to β‐carotene intake from the background diet are fruits, vegetables and products thereof (Section 3.5.2.1). Specific subgroups of the population, such as high consumers of fruits and vegetables, vegetarians and vegans, may have habitual intakes of β‐carotene in the higher range of the intake distribution in the general population (Section 3.3.4.2).
An increased risk of lung cancer has been observed among male smokers consuming food supplements at doses of 20 mg/day. No data for supplemental β‐carotene given alone are available at doses < 20 mg/day in any population group. In addition, supplemental forms of β‐carotene have markedly greater bioavailability than β‐carotene from foods and its bioavailability can also vary depending on the formulation, administration pattern, and other individual (dietary and non‐dietary) factors. Therefore, the available data do not allow characterising the hazard or deriving a safe level of intake for supplemental β‐carotene (i.e. identifying the highest level of intake where there is reasonable confidence in data on the absence of adverse effects).
No data are available about the potential risk of lung cancer associated with the consumption of β‐carotene added to fortified foods. The Panel notes, however, that the mean plasma levels of β‐carotene achieved with the consumption of 20 mg/day of synthetic water soluble β‐carotene from either food supplements in the ATBC study or fortified beverages are similar, and well above the levels achieved with the same amount of β‐carotene from non‐fortified carrot juice in beverages (Sections 3.3.4.2 and 3.6.4.2).
3.8. Risk characterisation
3.8.1. Preformed vitamin A
The ULs for preformed vitamin A apply to the general European population, cover dietary intake of preformed vitamin A from all sources, including fortified foods and food supplements, and apply to all forms of preformed vitamin A authorised for addition to foods and food supplements (i.e. retinol, retinyl acetate and retinyl palmitate).
The Panel considers that the UL for preformed vitamin A is not expected to be exceeded in European populations if consumption of ‘liver, offal and products thereof’ is limited to once per month or less. However, a higher frequency of offal consumption, and/or the consumption of high‐dose supplements, may lead to intakes above the UL (Section 3.5.1 and Sections thereof). For ‘liver, offal and products thereof’, consumption occasions (n) and portion sizes (mean, minimum and maximum) per food item and food consumption survey, age group and country as found in the EFSA Comprehensive Database are shown in Annex C, Table 16.
Since both acute and chronic intakes of preformed vitamin A and the timing of consumption by pregnant women (i.e. during embryogenesis) may be critical for teratogenicity (Section 3.6.1), the Panel maintains the general recommendation for women who are planning to become pregnant or who are pregnant not to consume liver, offal or products thereof (SCF, 1992, 2002).
3.8.2. β‐Carotene
There is no indication that β‐carotene intake from the background diet, including its use as food additive for technological purposes (food colour), is associated with adverse health effects.
In the absence of a UL, the risk associated with the consumption of supplemental β‐carotene cannot be characterised. The Panel considers that smokers should avoid consuming food supplements containing β‐carotene. The Panel also considers that the use of supplemental β‐carotene (i.e. in fortified foods and/or food supplements) by the general population should be limited to the purpose of meeting vitamin A requirements. PRIs for vitamin A for all population groups have been set by EFSA (EFSA NDA Panel, 2015). Using a conversion factor of 6:1, PRIs for vitamin A for adult men (750 μg RE/day) and women (650 μg RE/day) correspond to 4.5 and 3.9 mg/day of β‐carotene, respectively. The Panel notes that data collected from the Mintel GNPD indicates that the β‐carotene content of some fortified foods and food supplements available on the EU market exceeds the PRIs for vitamin A for several population groups (Section 3.5.2.1).
4. CONCLUSIONS
4.1. Preformed vitamin A
The following ULs are established for the intake of preformed vitamin A from all sources, including fortified foods and food supplements (Table 14).
TABLE 14.
ULs for preformed vitamin A from all sources, including fortified foods and food supplements, for all population groups.
| Age group | UL males and females (μg RE/day) |
|---|---|
| 4–6 months | 600 |
| 7–11 months a | 600 |
| 1–3 years | 800 |
| 4–6 years | 1100 |
| 7–10 years | 1500 |
| 11–14 years | 2000 |
| 15–17 years | 2600 |
| Adults (≥ 18 years) | 3000 |
| Pregnant women | 3000 |
| Lactating women | 3000 |
Abbreviations: RE, retinol equivalent; UL, Tolerable Upper Intake Level.
Age range covers the second half of the first year of life, i.e. from the beginning of the 7th month to the 1st birthday.
4.2. β‐Carotene
There is no indication that β‐carotene intake from the background diet, including its use as food additive for technological purposes, is associated with adverse health effects. Based on the available data, no UL or safe level of intake can be established for supplemental β‐carotene.
The Panel considers that smokers should avoid consuming food supplements containing β‐carotene. The Panel also considers that the use of supplemental β‐carotene (i.e. in fortified foods and/or food supplements) by the general population should be limited to the purpose of meeting vitamin A requirements. Using a conversion factor of 6:1, PRIs for vitamin A for adult men (750 μg RE/day) and women (650 μg RE/day) correspond to 4.5 and 3.9 mg/day of β‐carotene, respectively.
This conclusion does not apply to the possible use of supplemental β‐carotene for therapeutic purposes under medical supervision (e.g. as source of provitamin A in vitamin A deficiency, for the treatment of erythropoietic protoporphyria).
5. RECOMMENDATIONS FOR RESEARCH
The Panel considers that the priorities for research to inform a future revision of the ULs for preformed vitamin A and β‐carotene are as follows:
To validate existing and new non‐invasive biomarkers for adverse health effects resulting from excess intake of preformed vitamin A.
To assess liver retinol and serum retinyl‐ester concentrations in consumers with regular liver intake.
To assess whether β‐carotene intake can increase preformed vitamin A liver stores in vitamin A‐repleted states.
To generate data on bioequivalence for β‐carotene in foods, food additives, fortified foods and food supplements versus retinol, accounting for differences in β‐carotene formulations and the effect of the food matrix, among others.
To investigate the relationship between the intake of preformed vitamin A in the range of 1000–3000 μg RE/day and relevant bone‐health outcomes (e.g. areal and volumetric BMD at specific bone sites; osteoporotic bone fractures at specific sites) in humans, and the potential interactions between preformed vitamin A and vitamin D on these endpoints.
To gather data in humans on adverse effects of different β‐carotene isoforms and supplemental β‐carotene formulations when consumed at high doses and/or frequency, and the mechanisms through which adverse effects could occur.
To obtain most updated and representative across Member States food composition data on the levels of preformed vitamin A in the food products within the category ‘liver, other offal and products thereof’.
To foster ongoing efforts on the collection of accurate food composition and food consumption data on fortified foods and food supplements (i.e. vitamin A forms and concentrations/amounts).
To investigate the application of statistical modelling to estimate the usual intake of irregularly/less frequently consumed food items that contain high levels of preformed vitamin A (e.g. liver) based on short‐term measurements (few reporting days in 24‐h recalls or food records).
ABBREVIATIONS
- 25(OH)D
25‐hydroxyvitamin D
- ADI
Acceptable daily intake
- ADME
Absorption, distribution, metabolism and excretion
- ALP
Alkaline Phosphatase
- ALT
alanine aminotransferase
- ANS Panel
Panel on Food Additives and Flavourings
- AR
Average requirement
- ARAT
Acyl‐CoA: retinol acyltransferase
- AST
Aspartate Aminotransferase
- ATBC study
The Alpha‐Tocopherol, Beta Carotene Cancer Prevention study
- BCO1
β‐carotene‐15,15′‐dioxygenase
- BCO2
β, β‐carotene‐9′,10′‐dioxygenase 2
- BFCS
Belgian Food Consumption Survey
- BMC
Bone mineral content
- BMD
Bone mineral density
- BMI
Body Mass Index
- BOND panel
Biomarkers of Nutrition for Development panel
- CARET
the β‐Carotene and Retinol Efficacy Trial
- CDH
Congenital Diaphragmatic Hernia
- CI
Confidence interval
- CV
Coefficient of Variation
- CVD
Cardiovascular disease
- CYP27A1
Cytochrome P450 27A1
- DNFCS
Dutch National Food Consumption Survey
- DOPS
Danish Osteoporosis Prevention Study
- DXA
Dual‐energy X‐ray absorptiometry
- EC
European Commission
- EFSA
European Food Safety Authority
- EPIC
European Prospective Investigation into Cancer and Nutrition
- EU
European Union
- EVM
Expert Group on Vitamins and Minerals
- FAO
Food and Agriculture Organisation of the United Nations
- FCDB
EFSA Food composition database
- FDA
Food and Drug Administration
- FEEDAP Panel
Panel on Additives and Products or Substances used in Animal Feed
- FFQ
Food Frequency Questionnaire
- FGF 21
Fibroblast‐growth factor 21
- FRAX
Fracture Risk Assessment Tool
- FSMP
Foods for special medical purposes
- GloVitAS
Global Vitamin A Safety Assessment
- GNPD
Global New Products Database
- HDL
High Density Lipoprotein
- HR
Hazard ratio
- HRT
hormone replacement therapy
- IOM
Institute of Medicine
- ISX
Intestinal Homeobox Transcription Factor
- IU
International units
- IWHS
Iowa Women's Health Study
- JECFA
Joint FAO/WHO Expert Committee on Food Additives
- LDL
Low Density Lipoprotein
- LOAEL
Lowest observed adverse effect level
- LRAT
Lecithin: retinol acyltranferase
- MRI
Magnetic Resonance Imaging
- NCC
Nested Case Control studies
- NCD
Neural Crest Defects
- NDA Panel
EFSA Panel on Nutrition, Novel Foods and Food Allergens
- NHANES
National Health and Nutrition Examination Survey
- NHS
Nurses' Health Study
- NNR
Nordic Nutrition Recommendations
- NOAEL
No Observed Adverse Effect Level
- NR
Not Reported
- NRA
Normally related arteries
- NRV
Nutrient Reference Value
- NTD
Neural Tube Defects
- NTP
National Toxicology Program
- OFC
Oral Facial Clefts
- OHAT
Office of Health Assessment and Translation
- OPN
Osteopontin
- OR
Odds ratio
- OTD
Outflow Tract Defects
- PC
Prospective Cohort
- PCs
Prospective cohort studies
- PHS
Physicians' Health Study
- PR
Prevalence Ratio
- PRI
Population reference intake
- pQCT
Peripheral quantitative computed tomography
- PTH
Parathyroid hormone
- RA
Retinoic Acid
- RAE
Retinol Activity Equivalents
- RAR
Retinoic Acid Receptor
- RBHCDS
Rancho Bernardo Heart and Chronic Disease Study
- RBP
Retinol Binding Protein
- RCT
Randomised controlled trial
- RDA
Recommended Daily Allowance
- RDR
Relative Dose Response
- RE
Retinol Equivalent
- RID
Retinol Isotope Dilution
- RoB
Risk of bias
- RP
Reference point
- RPE
Retinal Pigmented Epithelium
- RR
Relative risk
- RXR
Retinoid X Receptor
- SCF
Scientific Committee on Food
- SCPS
Skin Cancer Prevention Study
- SD
Standard deviation
- sFFQ
Semi‐quantitative Food Frequency Questionnaire
- sQ
Sub‐question
- SU.VI.MAX study
Supplementation en Vitamines et Minéraux Antioxydants study
- T2DM
Type 2 Diabetes Mellitus
- TGA
Transposition of great arteries
- TRAP‐positive
Tartrate‐Resistant Acid Phosphatase positive
- UF
Uncertainty factor
- UL
Tolerable Upper Intake Level
- ULSAM
Uppsala Longitudinal Study of Adult Men
- UK
United Kingdom
- US
United States
- USA
United States of America
- VLDL
Very low density lipoprotein
- WACS
Women's Antioxidant Cardiovascular Study
- WG
Working Group
- WHIOS
Women's Health Initiative Observational Study
- WHO
World Health Organisation
- WHS
Women's Health Study
GLOSSARY
- Adequate intake (AI)
The value estimated when a population reference intake cannot be established because an average requirement cannot be determined. An adequate intake is the average observed daily level of intake by a population group (or groups) of apparently healthy people that is assumed to be adequate.
- Adverse (health) effects
Change in the morphology, physiology, growth, development, reproduction or lifespan of an organism, system or (sub)population that results in an impairment of functional capacity to compensate for additional stress or an increase in susceptibility to other influences (EFSA Scientific Committee, 2017a; FAO/WHO, 2009).
- Bioavailability
Nutrient fraction which is absorbed and becomes available to normal metabolic and physiological processes.
- Bioconversion
Fraction of absorbed β‐carotene that is converted into retinol in the body.
- Bioefficacy
Fraction of ingested β‐carotene that is absorbed and converted into retinol in the body (i.e. the product of absorption and bioconversion).
- Biomarker of intake
An exogenous substance or its metabolite or the product of an interaction between a xenobiotic agent and some target molecule or cell that is measured in a compartment within an organism (EFSA Scientific Committee, 2017a; WHO/IPCS, 1993). Urine, blood, faeces or nails are common media for the measurements of biomarkers of exposure (EFSA Scientific Committee, 2017a).
- Critical effect
Effect selected for the derivation of a health‐based guidance value.
- Dietary reference values (DRVs)
A set of nutrient reference values that includes the average requirement, the population reference intake, the adequate intake and the reference intake range for macronutrients.
- Endpoint
Qualitative or quantitative expression of a specific factor with which a risk may be associated as determined through an appropriate risk assessment.
- Hazard
Inherent property of an agent or situation having the potential to cause adverse effects when an organism, system, or (sub)population is exposed to that agent (FAO/WHO, 2009; WHO/IPCS, 2004).
- Lowest‐observed‐adverse‐effect level (LOAEL)
The lowest concentration or amount of a substance, found by experiment or observation, that causes an adverse alteration of morphology, functional capacity, growth, development or lifespan of the target organism distinguishable from normal (control) organisms of the same species and strain under the same defined conditions of exposure (FAO/WHO, 2009).
- No‐observed‐adverse‐effect level (NOAEL)
The greatest concentration or amount of a substance, found by experiment or observation, that causes no adverse alteration of morphology, functional capacity, growth, development or lifespan of the target organism distinguishable from those observed in normal (control) organisms of the same species and strain under the same defined conditions of exposure (FAO/WHO, 2009).
- Tolerable Upper Intake Level (UL)
The maximum level of total chronic daily intake of a nutrient (from all sources) which is not expected to pose a risk of adverse health effects to humans.
- Population reference intakes (PRI)
The level of (nutrient) intake that is enough for virtually all healthy people in a group.
CONFLICT OF INTEREST
If you wish to access the declaration of interests of any expert contributing to an EFSA scientific assessment, please contact interestmanagement@efsa.europa.eu.
REQUESTOR
European Commission
QUESTION NUMBERS
EFSA‐Q‐2021‐00365; EFSA‐Q‐2021‐00372.
PANEL MEMBERS
Dominique Turck, Torsten Bohn, Jacqueline Castenmiller, Stefaan De Henauw, Karen Ildico Hirsch‐Ernst, Helle Katrine Knutsen, Alexandre Maciuk, Inge Mangelsdorf, Harry J McArdle, Androniki Naska, Kristina Pentieva, Alfonso Siani, Frank Thies, Sophia Tsabouri and Marco Vinceti.
COPYRIGHT FOR NON‐EFSA CONTENT
EFSA may include images or other content for which it does not hold copyright. In such cases, EFSA indicates the copyright holder and users should seek permission to reproduce the content from the original source.
DECLARATIONS OF INTEREST
The declarations of interest of all scientific experts active in EFSA's work are available at https://ess.efsa.europa.eu/doi/doiweb/doisearch.
Supporting information
Protocol for the Scientific Opinion on tolerable upper intake level for preformed vitamin A and β‐carotene
Methodological considerations in the calculation of intake estimates for preformed vitamin A and β‐carotene from the background diet in EU countries
EFSA’s intake assessment for preformed vitamin A
EFSA’s intake assessment for β‐carotene
Vitamin A intake data from competent authorities in European countries
ROB appraisal of studies on bone health
Additional information requested from study authors
Studies excluded at full‐text screening and during data extraction
Outcome of the public consultation
ACKNOWLEDGEMENTS
The Panel wishes to thank for their contribution to this output the following members of the WG on Upper Levels: Peter Aggett, Marta Crous Bou, Francesco Cubadda, Aymeric Dopter, Susan Fairweather‐Tait, Rex FitzGerald and Misha Vrolijk; and EFSA staff members: Charlotte Marie Bercovici, Laura Ciccolallo, Pedro Daniel das Neves Ferreira, Agnès de Sesmaisons Lecarré, Rita Ferreira De Sousa, Thibault Fiolet, Nena Karavasiloglou, Laura Martino, Roanne Marie Saad and Angeliki Sofroniou. The Panel also wishes to thank Carmen Peláez for her contribution as member of the NDA Panel until June 2023. The Panel wishes to acknowledge Erik Kristoffer Arnesen, Jacob J. Christiansen, Vegard Lysne, Thomas Olsen and Rune Blomhoff for the preparatory work as part of a procurement procedure. The Panel also wishes to acknowledge the contribution of all national institutions in European countries that provided consumption data for this scientific output and the authors of published papers on vitamin A who provided additional information upon request.
APPENDIX A. Flow charts for the selection of studies
A.1.
FIGURE A.1.
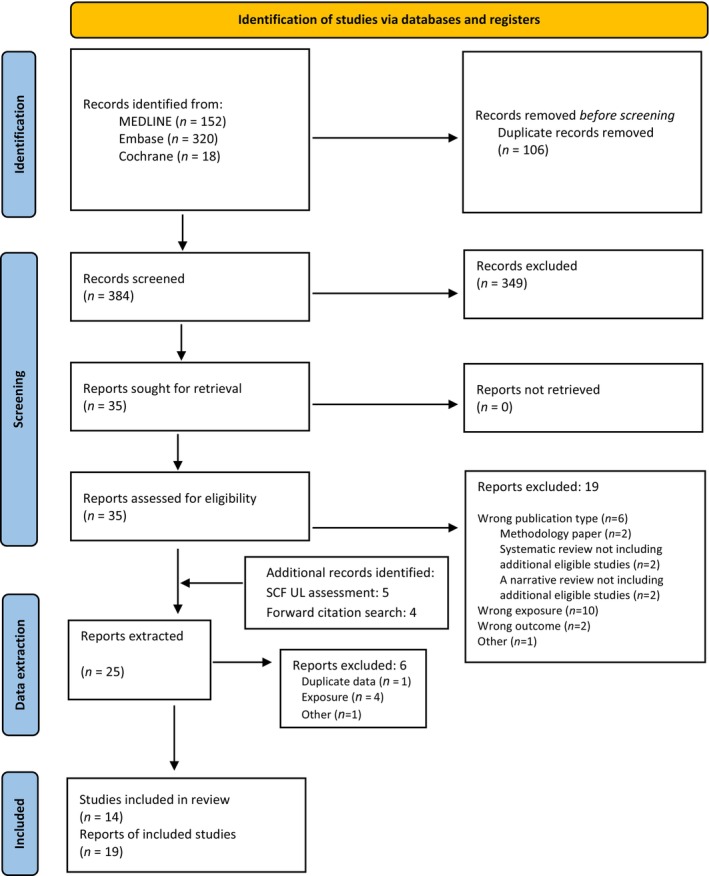
Flow chart for the selection of studies on teratogenicity.
FIGURE A.2.
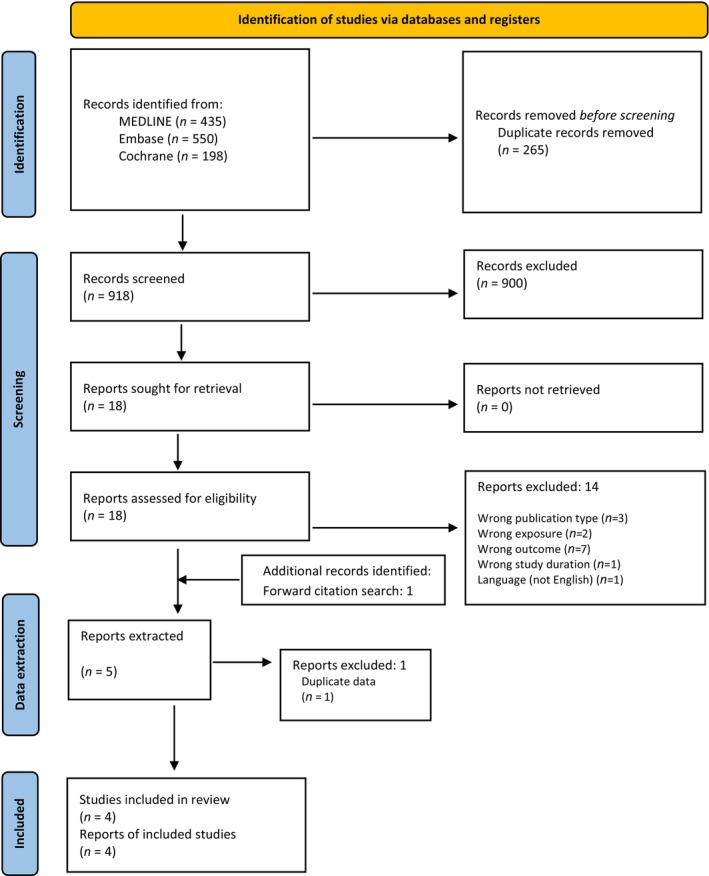
Flow chart for the selection of studies on hepatotoxicity.
FIGURE A.3.
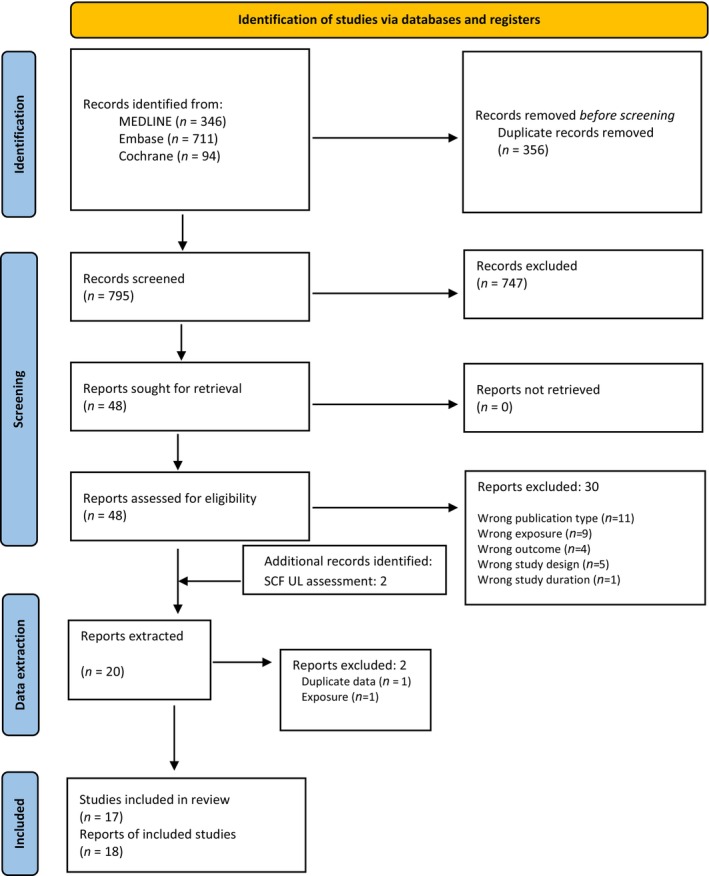
Flow chart for the selection of studies on bone health.
APPENDIX B. Evidence tables
B.1. Prospective studies reporting on tera togenic outcomes
|
Reference Study name Country Study design Follow‐up Funding |
Original cohort (N total) Exclusion criteria Study population |
Ascertainment of outcome |
Exposure groups n/person‐years Exposure assessment method |
Incident cases | Model covariates | Results |
|---|---|---|---|---|---|---|
|
Michikawa et al. (2019) The Japan Environment and Children's Study Japan PC Follow‐up from first trimester to birth Funding: public |
N = 103,099 pregnancies Population sampled: General population of pregnant women Exclusion criteria: residing outside of study areas, restricting to first pregnancy per woman, and excluding twins and triplets, miscarriages and stillbirths, did not respond to FFQ in first trimester, reported extreme energy intake (lower and upper percentile), and missing information of maternal age at delivery % lost to follow up: NR n = 89,658 Age at delivery, mean (SD): 31.2 (5.0) Infant sex (% females): 48.7 |
Outcome Identification of congenital diaphragmatic hernia (CDH) cases was made from medical records (ICD‐10 code Q79.0) CDH reported either at delivery or at the end of the first month |
Total vitamin A (retinol + provitamin A carotenoids – from diet only) Median (IQR), μg RAE/day: Q1 (ref): 230 (185, 264) Q2: 346 (320, 373) Q3: 468 (433, 509) Q4: 738 (631, 940) N per quartile of vitamin A: Q1 (ref): 22,414 Q2: 22,415 Q3: 22,414 Q4: 22,415 Total vitamin A intake, Median, μg RAE/day (N): Q1 (ref): 230 (22,414) Q2‐Q4: 468 (67,244) Preformed vitamin A intake – from the diet only Median, μg/day: Q1 (ref): 81 Q2–Q4: 185 Exposure assessment method: 2 × FFQ: first trimester FFQ used as main exposure (median fill‐in week of gestation = 15), and second/third trimester FFQ used in sensitivity analyses (median fill‐in week 27 of gestation). Total vitamin A reported as μg RAE (retinol + provitamin A carotenoids), conversion factors NR |
Cases of CDH per quartiles of total vitamin A intake (N cases per 10,000 live births): Q1 (ref): 14 (6.2) Q2: 8 (3.6) Q3: 9 (4) Q4: 9 (4) Q1 (ref): 14 Q2–Q4: 26 By preformed vitamin A intake: Q1 (ref): 10 Q2–Q4: 30 In women with BMI 18.5–25 kg/m 2 Total vitamin A Q1 (ref): 12 Q2–Q4: 18 Preformed vitamin A: Q1 (ref): 7 Q2–Q4: 23 |
Model 1: adjusted for maternal age at delivery Model 2: Adjusted for maternal age at delivery, smoking habits, alcohol consumption, pre‐pregnancy BMI, current history of diabetes or gestational diabetes and infertility treatment Many sensitivity analyses with similar results (adjusting for socio‐economic factors, excluding supplement users, excluding mothers with morning sickness, only isolated cases of CDH, adjusted for dietary folate and vitamin C, adjusting for vitamin A intake in mid/late pregnancy) |
Total vitamin A intake and CDH, OR (95% CI) Model 1 Q1: ref Q2‐Q4: 0.6 (0.3–1.2) Model 2 (N = 89,481) Q1: ref Q2‐Q4: 0.6 (0.3–1.2) Retinol intake and CDH, OR (95% CI) Model 1 Q1: ref Q2‐Q4: 1.0 (0.5–2.1) Model 2 (N = 89,481) Q1: ref Q2‐Q4: 1.0 (0.5–2.0) Total vitamin A intake and CDH in women with BMI 18.5–25 kg/m 2 , OR (95% CI) Model 1 Q1: ref Q2‐Q4: 0.5 (0.2–1.0) Model 2 (N = 65,568) Q1: ref Q2‐Q4: 0.5 (0.2–1.0) Retinol intake and CDH in women with BMI 18.5–25 kg/m 2 , OR (95% CI) Model 1 Q1: ref Q2‐Q4: 1.1 (0.5–2.5) Model 2 (N = 65,568) Q1: ref Q2‐Q4: 1.0 (0.4–2.4) |
|
Mastroiacovo et al. (1999) 13 European Teratology Information Services (TIS) Follow‐up: from first trimester to 3–6 weeks after expected delivery (by mail or phone) Funding: NR |
Original cohort N = 423 pregnancies exposed to ‘high’ dose of vitamin A (10 000 IU per day [3000 μg/day] or 50,000 IU per week [15,000 μg/week]) for at least one week during first 9 weeks of gestation Women contacted TIS either directly or through their doctor Control groups were mothers who consulted a TIS in the same period as the study subjects Control group 1: all infants exposed to ‘high’ vitamin A doses after 9 weeks of gestation Control group 2: all infants exposed to a documented nonteratogenic agent Exclusion: chromosomal or genetic diseases, as well as deformations (e.g. clubfoot), minor anomalies (e.g. preauricular tag), birth marks (e.g. nevus or angiomas), functional problems (e.g. pyloric stenosis, gastroesophageal reflux), and mild findings (e.g. hydronephrosis identified by prenatal sonography and not demanding treatment during the neonatal period), were excluded from the analysis N with complete follow‐up (study group) = 394 N for analysis Study group = 311 Control group 1 = 116 Control group 2 = 679 |
Outcome Prevalence rate of major malformations, defined as a structural abnormality that has an adverse effect on either the functional or the social acceptability of the individual, and which called for medical or surgical treatment Ascertainment of the outcome was done by mail or telephone interviews from doctors or mothers |
Preformed vitamin A, μg RE/day (from supplements) Cases Median = 15,000 IQR = 7500–18,000 Min‐max = 3000–90,000 N above 15,000 μg/day = 120 N above 30,000 μg/day = 32 Main reasons for high exposures were various dermatologic conditions and breast fibrocystic disease Dietary assessment method: NR. Preformed vitamin A expressed as IU/day, here converted to μg RE/day by a factor of 0.3 |
Cases of major malformations Study group: 3 Control group 1: 4 Control group 2: 13 |
Model 1: No covariates Model 2: Stratified by potential confounding factors (age, education, race, family history of birth defects) (NR) Model 3: Regression models adjusted for age, education, race, family history of birth defects, use of folate supplements during early pregnancy, diabetes, alcohol consumption, genital herpes infection, fever, use of antiseizure medication, retinoids or exogenous hormones (NR) Model 2 and 3 were similar to model 1, and therefore NR |
Birth prevalence, % (95% CI) Study group: 0.96 (0.25–2.60) Control group 1: 3.45 (1.11–8.11) Control group 2: 1.91 (1.07–3.17) Rate ratio (95% CI) Study group vs. control group 1: 0.28 (0.06–1.23) Study group vs. control group 2: 0.50 (0.14–1.76) |
|
Rothman et al. (1995) USA PC Funding: Mixed |
N = 22,755 Pregnant women undergoing screening were recruited around gestational week 15–20 (1984–1987) Exclusion: missing information on more than half of retinol‐containing foods N for analysis = 22,748 N Any birth defect = 339 Of which cranial neural crest defects = 121 |
The outcome of pregnancy was obtained from a questionnaire mailed to the obstetrician around expected time of delivery (76.5% responded); if the physician did not respond, the questionnaire was mailed to the mother (all remaining responded: 23.5%) Outcome Outcome was any birth defect, cranial neural‐crest defects, neural tube defects (NTDs), musculoskeletal or urogenital defects, or other defects |
Preformed vitamin A, μg RE/day Total intake (from diet + supplements) C1: 0–1500 C2: 1501–3000 C3: 3001‐4500 C4: > 4500 Total pregnancies (n per category) C1: 6410 C2: 12688 C3: 3150 C4: 500 From supplements only C1: 0–1500 C2: 1501‐2400 C3: 2401–3000 C4: > 3000 Total pregnancies C1: 11083 C2: 10585 C3: 763 C4: 317 Dietary assessment method: Assessment of diet (incl. 50 food items containing retinol) and supplement use (incl. vitamin A) conduced by telephone, focusing on the last 3 months before conception and first 8 weeks of pregnancy. Preformed vitamin A expressed as IU/day, here converted to μg/day by a factor of 0.3 |
Total intake (from diet + supplements) Total defects C1: 86 (1.3) C2: 196 (1.5) C3: 42 (1.3) C4: 15 (3.0) Cranial neural‐crest defects C1: 33 (0.51) C2: 59 (0.47) C3: 20 (0.63) C4: 9 (1.80) NTDs C1: 13 (0.20) C2: 29 (0.23) C3: 5 (0.16) C4: 1 (0.20) Musculoskeletal or urogenital defects C1: 24 (0.37) C2: 62 (0.49) C3: 10 (0.32) C4: 4 (0.80) Other defects C1: 16 (0.25) C2: 46 (0.36) C3: 7 (0.22) C4: 1 (0.20) From supplements Total defects C1: 148 (1.3) C2: 168 (1.6) C3: 13 (1.7) C4: 10 (3.2) Cranial neural‐crest defects C1: 51 (0.46) C2: 54 (0.51) C3: 9 (1.18) C4: 7 (2.21) |
Model 1: No covariates Model 2: Stratified by potential confounding factors (age, education, race, family history of birth defects) (NR) Model 3: Regression models adjusted for age, education, race, family history of birth defects, use of folate supplements during early pregnancy, diabetes, alcohol consumption, genital herpes infection, fever, use of antiseizure medication, retinoids or exogenous hormones (NR) |
Preformed vitamin A (from diet + supplements) Prevalence ratio (95% CI) Model 1: Cranial neural crest defects C4 vs. C1: 3.5 (1.7, 7.3) All birth defects C4 vs. C1: 2.2 (1.3, 3.8) Preformed vitamin A (from supplements only) Prevalence ratio (95% CI) All birth defects C4 vs. C1: 2.4 (1.3–4.4) Cranial neural crest defects C4 vs. C1: 4.8 (2.2–10.5) Models 2 and 3 were similar to model 1, and therefore NR Smoothed curves (unrestricted quadratic splines) indicated a threshold at ~ 3000 μg RE/day where the prevalence ratio increased (both total preformed vitamin A and preformed vitamin A from supplements only) |
|
NTDs C1: 21 (0.19) C2: 26 (0.25) C3: 1 (0.13) C4: 0 Musculoskeletal or urogenital defects C1: 44 (0.40) C2: 52 (0.49) C3: 2 (0.26) C4: 2 (0.63) Other defects C1: 32 (0.29) C2: 36 (0.34) C3: 1 (0.13) C4: 1 (0.32) |
||||||
|
Bille et al. (2007) Denmark Danish National Birth Cohort Prospective case‐cohort study Funding: Public |
N Total birth cohort ~100,000 Cases = 220 Controls = 880 Exclusion criteria: Twins were excluded and pregnancies not leading to births. Controls: Randomly selected from the birth cohort. % lost to follow up Cases: 8.2 Controls: 6 Periconceptional vitamin A supplement use (first weeks of pregnancy), %: Cases: 53 Controls: 58 |
Cases identified through maternally reported or a discharge diagnosis of oral clefts, including cleft lip with or without (±) cleft palate and isolated cleft palate cases, or an ICD code for reconstructive surgery on lips or palate. |
Preformed vitamin A intake from supplements only (μg/day) Binary (yes/no) Cases: 102 /90 Control: 480/348 Categorical (μg/day, from suppl. only), C1: 0 C2: 0–400 C3: 400–800 C4: ≥ 800 n cases/controls per category of intake: C1: 90/348 C2: 40 /173 C3: 25/154 C4: 37/153 Exposure assessment method: Data on daily supplement use was obtained through a questionnaire at enrolment |
Total cases = 192 Cleft lip ± cleft palate cases = 134 Cleft palate cases = 58 Controls = 828 |
Model 1: No covariates, NR Model 2: Adjusted for parental age and social class Results reported for binary, continuous (unit not reported, probably per 1 μg increment), and categorical (3 and 4 categories). |
OR (95% CI) All oral clefts Binary: 0.82 (0.59–1.13) Continuous: 0.99 (0.99–1.00) Categorical analysis C1: ref C2: 0.93 (0.61–1.43) C3: 0.62 (0.38–1.02) C4: 0.90 (0.57–1.40) Cleft lip ± cleft palate Binary: 0.73 (0.50–1.06) Continuous: 0.99 (0.99–1) Categorical analysis C1: ref C2: 0.69 (0.41–1.18) C3: 0.51 (0.28–0.93) C4: 1.00 (0.61–1.63) Isolated cleft palate Binary: 1.06 (0.6–1.86) Continuous: 0.99 (0.99–1.00) Categorical analysis C1: ref C2: 1.60 (0.82–3.11) C3: 0.93 (0.41–2.10) C4: 0.60 (0.24–1.50) |
Abbreviations: BMI, body mass index; CDH, congenital diaphragmatic hernia; FFQ, food frequency questionnaire; NR, not reported; NTD, Neural tube defects; OR, odds ratio; RAE, retinol activity equivalents; RE, retinol equivalents.
B.2. Case–control studies reporting on teratogenic outcomes
|
Reference Study name Country Funding |
Study population Endpoint Selection of cases and controls |
Exposure groups N cases/controls per exposure group Exposure assessment method |
Model covariates | Results |
|---|---|---|---|---|
|
Weber et al. (2018) USA The National Birth Defects Prevention Study (NBDPS) Funding: Public |
N Cases = 47,832 Controls 18,272 Cases: infants diagnosed with anophthalmia or microphthalmia . Exclusion criteria: ‘small eyes’ or ‘small palpebral fissures’, anophthalmia/microphthalmia associated with anterior encephalocele, anencephaly, holoprosencephaly or amniotic band syndrome, suspected to have a chromosomal abnormality or single‐gene condition. Controls: non‐malformed infants randomly selected from vital records, yearly by each study centre with complete dietary information from the mother Mothers for cases and controls were excluded if the FFQ was incomplete, or if reported energy intake exceeded 5000 kcal/day or was below 500 kcal/day % lost to follow up: Cases: 32% Controls: 36% N Cases = 224, Controls = 11,109 Maternal age group (Case/Control, %): < 20 years: 9.4/9.7 20–24 years: 24.1/22.4 25–29 years: 32.1/27.9 30–34 years: 21.4/25.9 > 34 years: 13.0/14.1 Periconceptional vitamin supplement use (2 months before through 2 months after conception): Cases: 78.6% Controls: 77.0% Obesity: Cases: 21% Controls: 17.5% |
Intake categories determined from intake among control mothers Quartiles of total vitamin A intake in periconceptional vitamin supplement users Range, μg RAE/day: Q1 (ref): ≤ 453.91 Q2: 453.92–660.51 Q3: 660.52–953.79 Q4: ≥ 953.79 N, cases/controls Q1 (ref): 39/2117 Q2: 50/2201 Q3: 48/2119 Q4: 39/2039 Quartiles of preformed vitamin A intake in periconceptional vitamin supplement users Range, μg RE/day: Q1 (ref): ≤ 264.12 Q2: 264.13–407.89 Q3: 407.90–615.13 Q4: ≥ 615.13 N, cases/controls Q1 (ref): 32/2165 Q2: 49/2208 Q3: 55/2194 Q4: 40/1989 Total vitamin A intake in vitamin supplement non‐users Range, μg RAE/day: Q1 (ref): ≤ 453.91 Q2‐4: > 453.91 N, cases/controls Q1: 11/631 Q2‐4: 32/1789 Preformed vitamin A intake (μg/day) in vitamin supplement non‐users: Q1 (ref): ≤ 264.12 Q2‐4: > 264.12 N, cases/controls Q1: 19/579 Q2‐4: 26/1841 Dietary assessment method: Shortened version of the Willett FFQ to capture nutrient intake during 1 year prior to pregnancy. Total vitamin A expressed as μg RAE, conversion factors NR |
Model 1: maternal total energy intake Model 2: Adjusted for maternal total energy intake, maternal age, race/ethnicity, and obesity Results for Model 2 NR |
Total vitamin A (μg RAE) intake in periconceptional vitamin supplement users; OR (95% CI) Model 1 Q1: ref Q2: 1.17 (0.76–1.80) Q3: 1.06 (0.68–1.67) Q4: 0.84 (0.49–1.43) Retinol intake in periconceptional vitamin supplement users; OR (95% CI) Model 1 Q1: ref Q2: 1.46 (0.93–2.30) Q3: 1.62 (1.02–2.55 Q4: 1.23 (0.72–2.11) By total vitamin A (μg RAE) intake in vitamin supplement non‐users; OR (95% CI) Model 1 Q1: ref Q2‐4: 1.16 (0.55–2.44) By retinol intake in vitamin supplement non‐users; OR (95% CI) Model 1 Q1: ref Q2‐4: 0.37 (0.19–0.73) ‘Further adjustment for maternal age, race/ethnicity, and obesity among vitamin users and nonvitamin users did not substantially alter the results (data not shown)’ |
|
Obrycki et al. (2019) Bangladesh Funding: public |
N Cases = 55 Controls = 55 Cases: Infants with any type of neural tube defect presenting for clinical care or identified otherwise by midwives or other health officials. Controls: live‐born infants with no neural tube defects or other congenital anomalies, randomly enrolled from pregnancy registries of Dhaka Community Hospital‐affiliated clinics, matched on infant sex and age. Lost to follow up: NR N Cases = 55, Controls = 55 Maternal age (mean): Cases: 24.5 years Controls: 22.3 years Periconceptional multivitamin supplement use (within 2 months prior to conception, %): Cases: 38 Controls: 49 |
Total vitamin A intake (from diet only), μg RAE/day Mean (SD) Cases: 471 (211) Controls: 406 (151) Preformed vitamin A intake (from diet only), μg RE/day Mean (SD) Cases: 343 (183) Controls: 293 (122) Dietary assessment method: A validated 39‐item semi‐quantitative FFQ, conducted when infants were enrolled, to capture the diet throughout pregnancy. Conversion factors for RAE NR. |
No models reported |
‘Mothers of cases and mothers of controls were estimated to have diets deficient in vitamin A, based on the FFQ results.’ The intake of retinol (μg/day) and total vitamin A (μg RAE/day) was lower in mothers of controls (293 and 406) vs. cases (343 and 471), p = 0.049 and 0.065. |
|
Beurskens et al. (2013) Netherlands The Congenital Diaphragmatic Hernia, Environment, Retinoids, Nutrition, Inheritance, and other Associations (HERNIA) study Funding: Unclear |
N Cases = 50 Controls = 46 Cases: Mothers with fetuses being diagnosed with congenital diaphragmatic hernia . Exclusion criteria: Did not complete questionnaires, language problems, did not consent, other. Controls: control mothers with non‐malformed fetuses recruited from the same tertiary centre by risk set sampling. Lost to follow up: Cases: 19 (38%) Controls: 0 N Cases = 31 Controls = 46 |
Median (IQR) total vitamin A intake (from diet only), μg RAE/day: Cases: 695 (366) Controls: 805 (388) Median (IQR) total vitamin A intake stratified by BMI category Cases < 18.5: NR 18.5–24.9: 685 (343) > 25: 734 (438) Controls < 18.5: NR 18.5–24.9: 843 (441) > 25: 613 (368) Dietary assessment method: A validated semi‐quantitative FFQ to capture the diet during 4 weeks prior to enrolment. Total vitamin A intake estimated using the following conversion formulas: 1 RAE = 1 μg retinol = 12 μg β‐carotene = 24 μg α‐carotene and β‐cryptoxanthin. |
Model 1: Unadjusted Model 2: Adjusted for maternal energy intake Model 3: Adjusted for maternal energy intake, age, and education level Results presented for total population and in BMI category subgroups |
OR (95% CI) for Vitamin A intakes < 800 compared to > 800 μg RAE/day Total population Model 1: NR Model 2: 2.3 (0.8–6.4), p = 0.122 Model 3: NR BMI < 18.5 kg/m 2 Model 1: NR Model 2: NR Model 3: NR BMI 18.5–24.9 kg/m 2 Model 1: 5.4 (1.4–20.5), p = 0.01 Model 2: 7.2 (1.5–34.4), p = 0.01 Model 3: 1.6 (1.1–20.8), p = 0.03 BMI > 25 kg/m 2 Model 1: NR Model 2: 0.35 (0.05–2.42), p = 0.29 Model 3: NR |
|
Maternal age, mean (sd): Cases: 31.6 (5.9) Controls: 34.7 (4.3) BMI category (kg/m2, case/control, %) < 18.5: 3.2/0 18.5–24.9: 54.8/56.5 > 25: 25.8/30.4 Unknown: 16.1/13 Periconceptional multivitamin supplement use (4 weeks before through 8 weeks after conception), %: Cases: 67.7 Controls: 60.9 Parity ≥1 (%): Cases: 32.3 Controls: 69.6 |
||||
|
Chandler et al. (2012) The National Birth Defects Prevention Study (NBDPS) USA Funding: Public |
N Cases = 1082 Controls = 6807 Cases: neural tube defects (NTD) diagnoses were verified by clinical geneticists based on information obtained from medical records. Exclusion criteria: Single gene disorders and chromosomal abnormalities, multiple births, maternal diabetes mellitus, use of various medications, missing FFQ, extreme energy intakes Controls: Control women were randomly selected and had a pregnancy resulting in a live‐born with no major birth defect. N included Cases = 954 (300 anencephaly, 654 spina bifida) (88%), Controls = 6268 (92%) |
Quartiles of preformed vitamin A (from diet only), μg RE/day Q1: < 218.60 Q2: 218.60–348.59 Q3: 348.60–535.10 Q4: > 535.10 Quartiles of total vitamin A (from diet only), μg RAE/day Q1: < 390.88 Q2: 390.88–592.60 Q3: 592.61–858.76 Q4: > 858.76 N per quartile: NR Anencephaly, n cases/controls Non‐suppl users: 142/2874 Suppl users: 151/3288 White, non‐Hispanic: 143/3748 Hispanic: 108/1404 Black, non‐Hispanic: 23/700 Normal weight: 151/3373 Overweight: 64/1347 Obesity: 49/966 |
Model 1: adjusted for centre and energy intake All results stratified by: 1) periconceptional supplement use (extracted), (2) ethnicity (not extracted), or (3) maternal pre‐pregnancy BMI (not extracted) |
OR (95% CI) Preformed vitamin A and anencephaly Folic acid non‐suppl users: Q1: ref Q2: 0.71 (0.44–1.15) Q3: 0.62 (0.37–1.04) Q4: 0.74 (0.44–1.23) Folic acid suppl users: Q1: ref Q2: 0.96 (0.61–1.51) Q3: 0.86 (0.53–1.38) Q4: 0.69 (0.40–1.21) Preformed vitamin A and spina bifida Folic acid non‐suppl users: Q1: ref Q2: 0.82 (0.58–1.16) Q3: 0.96 (0.68–1.35) Q4: 0.94 (0.65–1.36) Folic acid suppl users: Q1: ref Q2: 0.91 (0.66–1.25) Q3: 0.74 (0.52–1.04) Q4: 0.88 (0.61–1.29) |
|
Maternal age group (years, all cases/controls, %): < 20: 14.4/13.8 20–25: 27.8/28.6 26–30: 31.6/28.3 31–35: 17.5/21.1 > 35: 8.8/8.2 |
Spina bifida, n cases/controls Non‐suppl users: 312/2874 Suppl users: 326/3288 White, non‐Hispanic: 351/3748 Hispanic: 211/1404 Black, non‐Hispanic: 53/700 Normal weight: 291/3373 Overweight: 150/1347 Obesity: 152/966 Dietary assessment method: A 58‐item modified Willett FFQ to capture dietary intake during the last year. Total vitamin A intake expressed as μg RAE/day, conversion factors NR. |
Model 1: adjusted for centre and energy intake All results stratified by: 1) periconceptional supplement use (extracted), (2) ethnicity (not extracted), or (3) maternal pre‐pregnancy BMI (not extracted) |
Vitamin A and anencephaly Folic acid non‐suppl users: Q1: ref Q2: 0.75 (0.46–1.25) Q3: 1.07 (0.65–1.75) Q4: 0.85 (0.49–1.48) Folic acid suppl users: Q1: ref Q2: 0.70 (0.44–1.12) Q3: 0.76 (0.47–1.22) Q4: 0.71 (0.40–1.23) Vitamin A and spina bifida Folic acid non‐suppl users: Q1: ref Q2: 0.93 (0.66–1.30) Q3: 0.94 (0.66–1.35) Q4: 1.00 (0.68–1.46) Folic acid suppl users: Q1: ref Q2: 0.95 (0.69–1.30) Q3: 0.69 (0.48–0.98) Q4: 0.81 (0.55–1.20) |
|
|
Carmichael et al. (2010) USA Funding: Public |
N Cases = 441 Controls = 786 Cases: live born, stillborn (fetal deaths at ≥ 20 weeks' gestation), and prenatally diagnosed, electively terminated case fetuses with anencephaly or spina bifida. Exclusion criteria: Infants diagnosed with single gene disorders or aneusomies. Birth mothers were excluded if their primary language was not English or Spanish. Controls: Nonmalformed, live born controls were selected randomly from birth hospitals to represent the population from which cases were derived. |
Intake categories determined from intake among control mothers (in supplement non‐users) Total vitamin A intake, μg RE/day C1: < 1040 C2‐3 (ref): 1040–2134 C4: > 2134 N, anencephaly cases/spina bifida cases/controls C1: 13/21/48 C2‐3 (ref): 28/32/105 C4: 10/14/47 Dietary assessment method: A validated 107‐item semi‐quantitative FFQ to capture dietary intake during the periconceptional period. Total vitamin A intake expressed as RE/day, conversion factor NR. |
Adjusted for maternal energy intake, race/ethnicity, age, education, BMI, gravidity, smoking, and alcohol use |
OR (95% CI) for anencephaly C1: 1.3 (0.5–3.2) C2‐3 (ref): 1 Q4: 0.5 (0.2–1.3) OR (95% CI) for Spina bifida C1: 2.2 (1.0–4.9) C2‐3 (ref): 1 C4: 0.7 (0.3–1.6) |
|
% lost to follow up: Anencephaly Cases: 27 Spina bifida cases: 21 Controls: 20 N Cases = 330 (141 anencephaly, 189 spina bifida) Controls = 625 |
||||
|
Maternal age group (years, Anencephaly Case/Spina bifida case/Control, %): < 25: 33/28/30 25–29: 23/26/23 30–34:27/26/29 > 34: 18/20/18 Periconceptional folic acid containing vitamin supplement use (2 months before through 2 months after conception), %: Anencephaly cases: 52 Spina bifida cases: 57 Controls: 62 |
||||
|
Johansen et al. (2008) Norway Funding: Public |
Cases: All babies born from 1996 to 2001 who were referred for treatment for either cleft lip with or without cleft palate or cleft palate only Exclusion criteria: non‐Norwegian speaking women, infants who died after birth Controls: recruited in the same period by randomly selecting four births per thousand from the National Medical Birth Registry covering all births in the country. N Cases: 535 Controls: 693 Maternal age, mean (SD): ~29 (5) years for all groups |
Quartiles of total vitamin A (from diet + supplements), μg RAE/day: Q1: 32–833.5 Q2: 833.6–1257.0 Q3: 1257.1–1911.0 Q4: 1911.1–9640.0 N controls in quartiles Q1: 173 Q2: 174 Q3: 173 Q4: 173 Total vitamin A in RAE/day C1(ref): 40–60th percentile: 1083–1485 C2: > 95th percentile: > 3763 Preformed vitamin A intake in RAE/day C1(ref): 40–60th percentile: 799–1142 C2: > 95th percentile: > 3398 N controls in percentiles: C1: 40–60th p: 139 C2: > 95th p: 34 Cleft palate only, all cases Q1: 70 Q2: 46 Q3: 34 Q4: 38 Cleft palate only, isolated cases (model 1/model 2) Q1: 46/43 Q2: 29/26 Q3: 17/16 Q4: 23/23 Cleft lip with or without cleft palate, all cases Q1: 95 |
Model 1: No covariates Model 2: Adjusted for maternal energy intake, dietary folate, folic acid supplements, alcohol consumption, smoking, working during first trimester, educational level, father's income, and year of birth |
OR (95% CI) Quartiles of total vitamin A (from diet + supplements) Cleft palate only, all cases Model 1 Q1 (ref): 1.0 Q2: 0.65 (0.43–1.00) Q3: 0.49 (0.31–0.77) Q4: 0.54 (0.35–0.85) P for trend: 0.002 Cleft palate only, isolated cases Model 1 Q1 (ref): 1.0 Q2: 0.63 (0.38–1.04) Q3: 0.37 (0.20–0.67) Q4: 0.50 (0.29–0.86) P for trend: 0.002 Model 2 Q1 (ref): 1.0 Q2: 0.57 (0.32–1.03) Q3: 0.33 (0.16–0.68) Q4: 0.47 (0.24–0.94) P for trend: 0.02 Cleft lip with or without cleft palate, all cases Model 1 Q1 (ref): 1.0 Q2: 1.01 (0.71–1.43) Q3: 0.87 (0.61–1.26) Q4: 0.77 (0.53–1.11) P for trend: 0.122 Cleft lip with or without cleft palate, isolated cases Model 1 Q1 (ref): 1.0 |
|
Q2: 96 Q3: 83 Q4: 73 Cleft lip with or without cleft palate, isolated cases (model 1/model 2) Q1: 76/71 Q2: 77/72 Q3: 73/67 Q4: 61/53 All isolated cases (total vitamin A/preformed vitamin A) C1: 40–60th p: 86 C2: > 95th p: 8/7 Isolated cleft lip with or without cleft palate (total vitamin A/preformed vitamin A) C1: 40–60th p: 62/67 C2: > 95th p: 6/5 Isolated cleft palate only (total vitamin A/preformed vitamin A) C1: 40–60th p: 24/19 C2: > 95th p: 2/2 Dietary assessment method: An Validated 11‐page quantitative FFQ to capture dietary intake during the previous year. Total vitamin A was expressed as RAE, using the conversion factor 1 μg RAE = 1μg retinol = 12μg β‐carotene. |
Q2: 1.01 (0.69–1.47) Q3: 0.96 (0.65–1.41) Q4: 0.80 (0.54–1.19) P for trend: 0.278 Model 2 Q1 (ref): 1.0 Q2: 1.13 (0.74–1.72) Q3: 1.13 (0.71–1.80) Q4: 0.88 (0.54–1.44) P for trend: 0.59 Total vitamin A intake, 95th vs. 40–60th percentiles All isolated cases Model 1 40–60th‐p (ref): 1.0 > 95th‐p: 0.38 (0.17–0.86) Model 2 > 95th p: 0.48 (0.20–1.14) Isolated cleft lip with or without cleft palate Model 1 40–60th‐p (ref): 1.0 > 95th p: 0.40 (0.16–0.99) Model 2 > 95th p: 0.51 (0.19–1.38) Isolated cleft palate only Model 1 40–60th‐p (ref): 1.0 > 95th p: 0.34 (0.08–1.51) Model 2 > 95th p: 0.34 (0.07–1.68) Preformed vitamin A intake, 95th vs. 40–60th percentiles All isolated cases Model 1 40–60th p (ref): 1.0 > 95th p: 0.33 (0.14–0.78) Model 2 > 95th p: 0.40 (0.15–1.02) Isolated cleft lip with or without cleft palate Model 1 40–60th p (ref): 1.0 > 95th p: 0.31 (0.11–0.82) Model 2 > 95th p: 0.36 (0.12–1.07) Isolated cleft palate only Model 1 40–60th‐p (ref): 1.0 > 95th p: 0.43 (0.10–1.94) Model 2 > 95th p: 0.55 (0.09–3.26) |
|||
|
Yang et al. (2008) The National Birth Defects Prevention Study (NBDPS) USA Funding: Public |
N (assessed for eligibility) Cases = 377 Controls = 5008 Cases: Clinical information on infants and fetuses diagnosed with congenital diaphragmatic hernia (CDH) was reviewed and confirmed by clinical description, surgical or autopsy report to establish their eligibility for study. Exclusion criteria: Single‐gene conditions and chromosomal abnormalities; diaphragmatic eventration, amniotic band sequence or limb–body wall complex were also excluded; missing dietary intake data, extreme energy intake, CDH in a first degree relative, mothers with type 1 or 2 diabetes, non‐isolated cases Controls: Each study site randomly selected 100 children without birth defects per year as controls. N for analysis Cases = 290, Controls = 4863 Maternal age (years, Case/Control, %): < 20: 8.8/11.2 20–24: 21.2/22.2 25–29: 28.0/26.1 30–34: 26.9/26.8 35–39: 13.5/11.7 > = 40: 1.7/2.0 |
Total vitamin A intake (from diet only), RAE/day C1: 10th percentile (low): ≤ 253.19 C2: 10th‐90th‐percentile (mid): 253.20–1169.06 C3: 90th percentile (high): ≥ 1169.07 N cases/controls, suppl users C1: 35/337 C2: 164/2980 C3: 19/327 N cases/controls, non‐suppl users C1: 10/149 C2: 53/909 C3: 9/160 Preformed vitamin A intake (from diet only), ug/day C1: 10th percentile (low): ≤ 130.10 C2: 10th‐90th‐percentile (mid): 130.11–751.41 C3: 90th‐percentile (high): ≥ 751.42 N cases/controls, suppl users C1: 22/346 C2: 180/2970 C3: 16/328 N cases/controls, non‐suppl users C1: 16/140 C2: 46/919 C3: 10/159 Dietary assessment method: A validated modified 58‐item Willett FFQ to capture dietary intake the year before pregnancy. Total vitamin A expressed as μg RAE/day, conversion factors NR. |
Model 1: Adjusted for total energy intake. Model 2: Adjusted for maternal race/ethnicity, education, age, BMI, cigarette smoking, alcohol intake, nausea, infant sex, number of previous live births, and total energy intake. |
OR (95% CI) Total Vit A Isolated CDH, suppl users Model 1 C1: 1.7 (1.2–2.6) C2: ref C3: 1.2 (0.7–2.0) Model 2 C1: 1.8 (1.2–2.7) C2: ref C3: 1.3 (0.7–2.3) Isolated CDH, non‐suppl users Model 1 C1: 1.0 (0.5–2.1) C2: ref C3: 1.2 (0.5–2.5) Model 2 C1: 0.9 (0.4–2.0) C2: ref C3: 1.0 (0.4–2.5) Preformed vitamin A Isolated CDH, suppl users Model 1 C1: 0.9 (0.6–1.5) C2: ref C3: 1.0 (0.5–1.7) Model 2 C1: 0.9 (0.6–1.5) C2: ref C3: 1.0 (0.6–1.8) Isolated CDH, non‐suppl users Model 1 C1: 2.1 (1.1–3.9) C2: ref C3: 1.4 (0.7–3.0) Model 2 C1: 1.8 (0.9–3.7) C2: ref C3: 1.3 (0.6–3.0) |
|
Mitchell et al. (2003) Denmark Funding: public |
N Cases = 302 Controls = 567 Cases: liveborn infants with cleft lip with or without (±) cleft palate, or isolated cleft palate. Exclusion criteria: If the child had other congenital malformations or syndromes, and if the parents did not speak Danish fluently Controls: The mothers of the two preceding children (or the next) born in the hospital where case mothers delivered. Controls were eligible if the child was liveborn and had no congenital malformations, and if the parents spoke Danish fluently. N Cleft lip ± cleft palate cases = 222 Cleft palate cases = 80 Controls = 567 Maternal age: NR Multivitamin use, % (daily/less than daily) Cleft lip ± cleft palate cases: 64.5/35.5 Cleft palate cases: 76.8/23.2 Controls: 75.3/24.7 |
Preformed vitamin A intake (μg RE /day) From liver products and supplements, mean (SE) Cleft lip ± cleft palate cases: 1146 (678) Cleft palate cases: 1242 (606) Controls: 1276 (763) From liver products, mean (SE) Cleft lip ± cleft palate cases: 586 (587) Cleft palate cases: 618 (574) Controls: 646 (696) From liver products, quartiles (RE/day): Q1: < 214.3 Q2: 214.3–535.7 Q3: 535.7–857.1 Q4: > 857.1 N (cleft lip ± cleft palate cases/controls) Total population: Q1: 51/142 Q2: 24/54 Q3: 53/106 Q4: 24/95 Supplement users: Q1: 35/105 Q2: 14/44 Q3: 33/82 Q4: 16/68 Supplement non‐users: Q1: 11/28 Q2: 7/8 Q3: 14/19 Q4: 6/21 Exposure assessment method: Vitamin A intake estimated based on interviews (asking about supplement use and use of liver product intakes), from reported multivitamin use (assumed dose 800 RE), liver meals (assumed 15,000 RE/portion), and liver paste sandwich (assumed 750 RAE/portion) |
Model 1: unadjusted Model 2: Adjusted for maternal cigarette smoking and alcohol consumption Results for model 2 NR |
OR (95% CI) per increment of 500 RE/day From liver products and supplements Cleft lip ± cleft palate 0.88 (0.77–1.01) Cleft palate 0.97 (0.80–1.18) From liver products alone Cleft lip ± cleft palate 0.93 (0.80–1.08) Cleft palate 0.97 (0.79–1.20) OR (95% CI) for cleft lip ± cleft palate for Q4 vs. Q1–3: Total population: Q1–3: ref Q4: 0.60 (0.36–0.98) Daily supplement users: Q1‐3: ref Q4: 0.66 (0.36–1.21) Supplement nonusers: Q1‐3: ref Q4: 0.49 (0.18–1.34) |
|
Botto et al. (2001) The Baltimore‐ Washington Infant Study USA Funding: Public |
Cases: liveborn infants with CV malformations diagnosed before 1 year of age. Outcomes were transposition of the great arteries (TGA), double‐outlet right ventricle (with normally related or transposed great arteries), tetralogy of Fallot, truncus arteriosus and supracristal ventricular septal defect . Exclusion: infants with syndromes or selected congenital abnormalities, or corrected TGA. Controls: a sample of the underlying birth cohort selected randomly from obstetric logbooks after stratification by hospital and year of birth. N Cases: 126 Controls: 679 College education, Cases: 45% Controls: 54% Married Cases: 67% Controls: 77% |
Dietary (average daily) intake of total preformed vitamin A, preformed vitamin A from foods only, or preformed vitamin A from supplements: < 3000 μg RE/day (low), or > = 3000 μg RE/day (high) Total preformed vitamin A intake, n controls Low: 618 High: 61 Preformed vitamin A intake from foods only, n controls Low: 661 High: 18 Preformed vitamin A intake from supplements, n controls Low: 668 High: 11 Preformed vitamin A intake from supplements in four categories (μg RE/day), n controls 0–1499 μg RE/day (low): 553 1500–2399 μg RE/day (mid‐low): 81 2400–2999 μg RE/day (mid‐high): 34 > = 3000 μg RE/day (high): 11 N cases stratified by measurement, low/high or by four categories (preformed vitamin A from suppl only) All outflow tract defects: Total preformed vitamin A: 109/17 Preformed vitamin A (foods): 122/4 Preformed vitamin A (suppl): 118/8 Preformed vitamin A (suppl): 103/10/5/8 Transposition of the great arteries, all: Total preformed vitamin A: 43/10 Preformed vitamin A (foods): 51/2 Preformed vitamin A (suppl):46/7 Preformed vitamin A (suppl): 40/4/2/7 Transposition of the great arteries, D‐TGA: Total preformed vitamin A: 38/9 Preformed vitamin A (foods): 45/2 Preformed vitamin A (suppl): 41/6 Preformed vitamin A (suppl): 36/3/2/6 Normally related arteries, all: Total preformed vitamin A: 66/7 Preformed vitamin A (foods): 71/2 Preformed vitamin A (suppl): 72/1 Preformed vitamin A (suppl): 63/6/3/1 Normally related arteries, TOF: Total preformed vitamin A: 51/7 Preformed vitamin A (foods): 56/2 Preformed vitamin A (suppl): 57/1 Preformed vitamin A (suppl): 48/6/3/1 Dietary assessment method: A validated FFQ with > 100 items. |
Model 1: No covariates, NR Model 2: adjusted for maternal education, marital status, race, age, smoking, alcohol use, diabetes mellitus, fever, and folic acid supplement use. |
OR (95% CI) High vs. low All outflow tract defects: Total preformed vitamin A: 1.6 (0.9–2.8) Preformed vitamin A (foods): 1.2 (0.4–3.6) Preformed vitamin A (suppl): 4.1 (1.7–9.8) Transposition of the great arteries, all: Total preformed vitamin A: 2.4 (1.1–4.8) Preformed vitamin A (foods): 1.4 (0.3–6.3) Preformed vitamin A (suppl): 9.2 (4.0–21.2) Transposition of the great arteries, D‐TGA: Total preformed vitamin A: 2.4 (1.1–5.1) Preformed vitamin A (foods): 1.6 (0.4–7.2) Preformed vitamin A (suppl): 8.9 (3.7–21.4) Normally related arteries, all: Total preformed vitamin A: 1.1 (0.5–2.4) Retinol (foods): 1.0 (0.2–4.6) Retinol (suppl): 0.8 (0.1–6.6) Normally related arteries, TOF: Total preformed vitamin A: 1.4 (0.6–3.2) Preformed vitamin A (foods): 1.3 (0.3–5.8) Preformed vitamin A (suppl): 1.1 (1.4–8.4) Preformed vitamin A as suppl in four categories All outflow tract defects: Low: ref Mid‐low: 0.7 (0.3–1.3) Mid‐high: 0.8 (0.3–2.1) High: 3.9 (0.6–9.3) Transposition of the great arteries, all: Low: ref Mid‐low: 0.7 (0.2–1.9) Mid‐high: 0.8 (0.2–3.5) High: 8.8 (3.8–20.5) Transposition of the great arteries, D‐TGA: Low: ref Mid‐low: 0.6 (0.2–1.9) Mid‐high: 0.9 (0.3–3.9) High: 8.4 (3.4–20.5) Normally related arteries, all: Low: ref Mid‐low: 0.7 (0.3–1.5) Mid‐high: 0.8 (0.2–2.6) High: 0.8 (0.1–6.3) Normally related arteries, TOF: Low: ref Mid‐low: 0.9 (0.4–2.1) Mid‐high: 1.0 (0.3–3.4) High: 1.0 (0.1–8.3) |
|
Shaw et al. (2010) USA Funding: Public |
Cases: Case information was abstracted from multiple hospital reports and medical records, which were reviewed by a clinical geneticist. Cases of conotruncal heart defects including day‐transposition of great arteries (dTGA) and tetralogy of Fallot (TOF) , and were confirmed by reviewing echocardiography, cardiac catheterisation, surgery or autopsy reports. Exclusion criteria: Diagnoses of single gene disorders or chromosomal aneusomy; infants with dTGA or TOF associated with an endocardial cushion defect or with double outlet right ventricle were excluded. Cases and controls with mothers who had type I or II diabetes were excluded from analyses, given that those subjects may be different etiologically. Controls: Nonmalformed, liveborn controls were selected randomly from birth hospitals, to represent the population from which the cases were derived. N Controls: 698 Cases: 163 with TOF, 140 with dTGA Maternal age, dTGA/TOF/controls, %: < 25: 26/26/31 25–29: 21/23/23 30–34: 31/26/28 > 34: 21/24/18 |
Quartiles of total vitamin A intake determined by intake distribution in the control group Total vitamin A (from diet only), μg RE/day Q1: < 1044.7 Q2‐Q3 (ref): 1044.7–2152.5 Q4: > 2152.5 N controls Q1: 63 Q2‐Q3 (ref): 122 Q4: 66 N cases dTGA Q1: 22 Q2‐Q3 (ref): 19 Q4: 11 N cases TOF Q1: 20 Q2‐Q3 (ref): 29 Q4: 17 Dietary assessment method: A validated modified semi‐quantitative FFQ. Vitamin A expressed as RE, conversion factors NR. |
Model 1: Adjusted for total energy intake Model 2: Adjusted for energy intake, maternal ethnicity, age, education, body mass index, smoking, alcohol use, and gravidity. |
OR (95% CI) dTGA Model 1 Q1: 2.7 (1.2–5.9) Q2‐Q3 (ref): Q4: 0.9 (0.4–2.2) Model 2 Q1: 3.4 (1.4–8.6) Q2‐Q3 (ref): Q4: 0.6 (0.2–1.8) TOF Model 1 Q1: 1.1 (0.6–2.3) Q2‐Q3 (ref): Q4: 1.3 (0.6–2.9) Model 2 Q1: 1.5 (0.6–3.4) Q2‐Q3 (ref): Q4: 1.8 (0.7–4.6) |
|
Feldkamp et al. (2011) The National Birth Defects Prevention Study (NBDPS) USA Population‐based case–control study Funding: Public |
N Cases = 694 Controls = 6157 Cases: Cases of gastroschisis were ascertained from 10 birth defect surveillance systems covering the general population (1997–2005) Controls: Controls were randomly selected from birth certificates or hospital records Maternal age, cases, n (%) < 20 years = 326 (47.0) 20–24 = 240 (34.6) 25–29 = 88 (12.7) > 29 = 40 (5.8) Maternal age, controls, n (%) < 20 years = 863 (14.0) 20–24 = 1469 (23.9) 25–29 = 1656 (26.9) > 29 = 2169 (35.2) |
Tertiles determined by intake distribution in control mothers Total vitamin A (from diet only), μg RAE/day Q1: 15.9–448.1 Q2: 448.2–751.9 Q3: 752.2–11516.1 n controls Q1: 2032 Q2: 2094 Q3: 2031 n all cases Q1: 283 Q2: 211 Q3: 200 n isolated cases Q1: 258 Q2: 194 Q3: 181 |
Model 1: No covariates (NR) Model 2: Adjusted for maternal age, race/ethnicity, education, preconception body mass index, smoking, alcohol, energy intake (kilocalories), time to interview, and centre. |
OR (95% CI) All cases Q1: ref Q2: 0.92 (0.74–1.14) Q3: 0.89 (0.70–1.14) Isolated cases Q1: ref Q2: 0.92 (0.73–1.16) Q3: 0.89 (0.69–1.15) Multiple cases Q1: ref Q2: 0.82 (0.41–1.63) Q3: 0.92 (0.44–1.94) |
|
n multiple cases Q1: 25 Q2: 17 Q3: 19 Dietary assessment method: A valid modified Willett, 58‐item FFQ, to capture diet 1 year prior to pregnancy. Vitamin A expressed as μg RAE/day, conversion factors NR. |
||||
|
Wallenstein et al. (2013) USA Population‐based case–control study Funding: Public |
N Cleft palate = 170 Cleft lip with or without (±) cleft palate = 425 n controls = 534 Cases: cleft palate and cleft lip ± cleft palate occurring among mothers residing in Los Angeles, San Francisco, and Santa Clara counties (1999–2003) Controls: Controls were selected randomly from birth hospitals to represent the population from which the cases were derived Exclusion: diagnoses of single‐gene disorders or chromosomal aneusomy Maternal age of cleft palate cases, % < 25 years = 20.6 25–29 = 25.3 30–34 = 34.1 > 34 = 20.0 Maternal age of cleft lip ± cleft palate cases, % < 25 years = 29.9 25–29 = 26.1 30–34 = 24.9 > 34 = 19.1 Maternal age of controls, % < 25 years = 29.6 25–29 = 22.1 30–34 = 28.3 > 34 = 20.0 |
Quartiles of total vitamin A intake determined by intake in the control group Total vitamin A (from diet only), μg RE/day Q1: < 1040.1 Q2‐Q3: 1040.1–2134.2 Q4: ≥ 2134.3 n controls (Vitamin/mineral supplement users) Q1: 86 Q2‐Q3: 169 Q4: 79 n controls (Vitamin/mineral supplement non‐users) Q1: 48 Q2‐Q3: 105 Q4: 47 n cleft palate (Vitamin/mineral supplement users) Q1: 30 Q2‐Q3: 54 Q4: 21 n cleft palate (Vitamin/mineral supplement non‐users) Q1: 20 Q2‐Q3: 36 Q4: 9 n cleft lip ± cleft palate (Vitamin/mineral supplement users) Q1: 61 Q2‐Q3: 127 Q4: 49 n cleft lip ± cleft palate (Vitamin/mineral supplement non‐users) Q1: 61 Q2‐Q3: 92 Q4: 35 Dietary assessment method: Dietary intake 2‐months before and 2‐months after conception was estimated with a semi‐quantitative FFQ (a modified version of the National Cancer Institute Health Habits and History Questionnaire). Vitamin A expressed as RE, conversion factors NR |
Adjusted for maternal race/ethnicity, age, education, BMI, gravidity, smoking, alcohol use, and energy intake |
OR (95% CI) Cleft palate (Vitamin/mineral supplement users) Q1: 0.8 (0.4, 1.4) Q2‐Q3: ref Q4: 1.4 (0.7, 2.7) Cleft palate (Vitamin/mineral supplement non‐users) Q1: 1.1 (0.5, 2.3) Q2‐Q3: ref Q4: 0.6 (0.2, 1.6) Cleft lip ± cleft palate (Vitamin/mineral supplement users) Q1: 0.9 (0.6, 1.4) Q2‐Q3: ref Q4: 0.8 (0.5, 1.3) Cleft lip ± cleft palate (Vitamin/mineral supplement non‐users) Q1: 1.9 (1.1, 3.3) Q2‐Q3: ref Q4: 0.7 (0.4, 1.2) |
|
Shaw et al. (1997) USA Funding: NR |
N study population Cases = 653 Controls = 644 Cases: Cases were infants with an eligible neural tube defect (NTD) diagnosis in California (1989–1991) Controls: Controls were randomly selected from each area hospital in proportion to the hospital's estimated contribution to the total population of infants born live Exclusion: language other than English or Spanish or who had a previous NTD‐affected pregnancy N after exclusion Cases = 613 Controls = 611 N follow‐up with interview Cases = 538 Controls = 539 N with complete data for analysis Cases = 448 Controls = 451 |
Preformed vitamin A intake (from diet and supplements), μg RE/day C1 (ref): 0–3000 C2: 3000–4500 C3: ≥ 4500 N cases/controls per category of intake C1: 426/432 C2: 16/12 C3: 6/7 Dietary assessment method: Dietary intake 3 months before conception was assessed with a validated 100‐item FFQ, and by asking about supplement use. Preformed vitamin A intake expressed as IU/day, here converted to μg/day by a conversion factor of 0.3 |
Model 1: No covariates |
OR (95% CI) Cases vs. controls C1: ref C2: 1.4 (0.6, 2.8) C3: 0.9 (0.3, 2.5) |
|
Mills et al. (1997) The National Institute of Child Health and Human Development Neural Tube Defects Study USA Funding: Public |
N Cases (NTDs) = 548 Cases (non‐NTD major malformations) = 387 (of which cranial neural crest malformations = 89) Controls = 573 Cases: Cases of neural tube defects (NTDs) identified by ultrasound and amniocentesis records at prenatal diagnostic centres in California and Illinois (1985–1987) Controls: Normal and malformed controls matched by searching records of the same prenatal diagnostic centre. Normal pregnancies randomly selected in California from the neonatal screening program and in Illinois from birth certificates |
Preformed vitamin A exposure rates by malformation status, μg RE/day From supplement only > 2400, % Major malformations: 3.4 NTDs: 2.9 Cranial neural crest malformations: 3.4 Normal controls: 3.1 From supplement and fortified cereals > 2400, % Major malformations: 3.6 NTDs: 3.3 Cranial neural crest malformations: 3.4 Normal controls: 4.5 > 3000, % Major malformations: 1.6 NTDs: 2.0 Cranial neural crest malformations: 2.2 Normal controls: 2.1 Dietary assessment method: Use of supplements and cereals around time of conception was assessed by interview. Preformed vitamin A intake expressed as IU/day, here converted to μg/day by a conversion factor of 0.3 |
Model 1: No covariates |
OR (95% CI) Comparison group included women receiving < 1500 μg/day Supplement only > 2400 Major malformations: 1.05 (0.51–2.18) NTDs: 0.91 (0.46–1.81) Cranial neural crest malformations: 1.06 (0.31–3.68) Supplement and fortified cereals > 2400 Major malformations: 0.79 (0.40–1.53) NTDs: 0.70 (0.38–1.28) Cranial neural crest malformations: 0.76 (0.22–2.56) > 3000 Major malformations: 0.73 (0.27–1.96) NTDs: 0.92 (0.40–2.11) Cranial neural crest malformations: 1.09 (0.24–4.98) |
|
Martínez‐Frías and Salvador (1990) The Spanish Collaborative Study of Congenital Malformations Spain Funding: public |
N Cases: 12,625 Controls: 12,525 Cases: Malformed infants were ascertained in participating hospitals through newborn examination of all livebirths during the first 3 days of life Controls: The next non‐malformed baby of the same sex, born in the same hospital, was selected as control Exclusion criteria: Cases with a chromosomal anomaly, and their controls, were excluded. Lost to follow up: Cases: 2.5% Controls: 2.6% N Cases: 11,293 Controls: 11,193 |
Preformed vitamin A from supplements* (alone or as part of multivitamin) Cases/Controls Non‐exposed: 11277/11,179 Any vitamin A (> 3000 μg): 16/14 3000–12,000 μg: 5/10 > 12,000 μg: 11/4 Vitamin A (> 3000 μg) alone: 10/1 Vitamin A in multivitamin: 6/13 * Drugs were also considered in the any vitamin A group Exposure assessment method: Mothers of case and control infants were asked open‐ended questions on the use of drugs and dietary supplements. |
Unadjusted models reported Sensitivity analyses conducted for vitamin A supplements alone vs. vitamin A as part of a multivitamin complex. |
OR (95% CI), p (one‐tail): Any vs. no vitamin A supplement: 1.1 (0.5, 2.5), p = 0.4 Unexposed: ref. 3000–12,000 μg: 0.5 (0.1, 1.6), p = 0.15 > 12,000 μg: 2.7 (0.8, 11.7), p = 0.06 Total vitamin A alone vs. unexposed: 9.9 (1.4, 430.1), p = 0.006 Total vitamin A in multivitamin vs. unexposed: 0.5 (0.2, 1.3), p = 0.08 |
Abbreviations: BMI, body mass index; CDH, congenital diaphragmatic hernia; CV, cardiovascular; FFQ, food frequency questionnaire; NR, not reported; NTD, Neural tube defects; OR, odds ratio; RAE, retinol activity equivalents; RE, retinol equivalents; TGA, transportation of great arteries, TOF, tetralogy of fallot.
B.3. Intervention studies reporting on hepatotoxicity
|
Reference Study Country Duration Funding |
Design | Subject characteristics at baseline | Intervention | Endpoint assessed | Results |
|---|---|---|---|---|---|
|
Farhangi et al. (2013) Iran 4 months Funding: public |
RCT (parallel) Inclusion criteria: 20–52 years of age, BMI 30–39.9 kg/m2 for obese and 18.5–24.9 kg/m2 for non‐obese women. Exclusion criteria: history of diabetes, thyroid abnormalities, liver or renal disease and autoimmune disease, consumption of any dietary vitamin A supplements or treatment with drugs that may interfere with absorption or bioavailability of the supplement. N randomised = 84 N completed = 75 G1: N intervention (obese) = 27 G2: N control (obese) = 23 G3: N intervention (normal weight) = 25 |
Sex (% female): 100 Age (range): 20–52 years Background Vitamin A intake at baseline, mean (SEM): G1: 566 (150) G2: 444 (232) G3: 399 (173) |
Vitamin A as retinyl palmitate Doses (IU/day): G1: 25,000 (~7500 μg RE/day) G2: 0 G3: 25,000 (~7500 μg RE/day) Background nutrient intake (RE/day) at end‐of‐study, mean (SEM): G1: 637 (234) G2: 674 (325) G3: 535 (101) Exposure assessment: three‐day 24‐h dietary recall questionnaire at baseline and end. Compliance: NR |
Serum AST and ALT Determined by enzymatic methods. Mean inter‐ and intra‐assay CV for these tests were 4.40, 3.25 and 3.08, 6.22, respectively. |
Type of analysis extracted: PP Units: U/L Values: mean (SEM) AST at baseline G1: 20.25 (0.86) G2: 22.46 (1.48) G3: 19.07 (1.47) P = NS AST at end‐of‐study G1: 23.60 (1.11) (P = 0.008) G2: 22.84 (0.76) (P = NS) G3: 26.26 (2.60) (P = 0.001) P = NS ALT at baseline G1: 17.64 (1.49) G2: 17.82 (1.40) G3: 15.75 (1.84) P = NS ALT at end‐of‐study G1: 17.08 (1.80) (P = NS) G2: 16.00 (1.05) (P = NS) G3: 17.73 (3.18) (P = NS) P = NS % change in AST G1: 16.72 (8.75) G2: 2.25 (8.80) G3: 37.06 (11.66) P = 0.036 % change in ALT G1: −3.68 (11.85) G2: −6.19 (7.06) G3: 13.28 (10.11) P = NS |
|
Dougherty et al. (2012) USA 12 months Funding: Public |
RCT (parallel) Inclusion criteria: Subjects with SCD‐SS aged 2.0–12.9 years. Exclusion criteria: Chronic transfusion therapy or a transfusion within the past 2 months, hydroxyurea therapy, history of stroke, liver enzymes > 3 times the reference range, height > 2.0 SDs above the age and sex mean (> 98th percentile, CDC 2000 reference standards), participation in another intervention study, pregnancy, and other chronic conditions known to affect growth, dietary intake or nutritional status. In addition, subjects taking daily vitamins or commercial nutritional supplements containing vitamin A were not eligible for the study, unless willing to discontinue supplementation and have a 2‐months washout period. N participants with sub‐optimal vitamin A status randomised/completed/analysed: G1 (vitamin A): 23/18/18 G2 (vitamin A + zinc): 18/15/15 G3 (placebo): 21/19/19 |
Sex at baseline (as % female) G1: 39 G2: 39 G3: 52 Age at baseline, years, mean (SD) G1: 7.5 (2.9) G2: 7.6 (2.4) G3: 7.8 (3.2) Serum retinol at baseline, μg/dL G1: 17.6 (4.0) G2: 18.2 (3.3) G3: 19.4 (4.9) Serum retinol at end‐of‐study, μg/dL G1: 18.9 (4.5) G2: 19.4 (3.2) G3: 19.0 (4.3) |
Vitamin A as retinyl palmitate Vitamin A groups received dose by age: 2.0–3.9 years: 300 μg/day (164 μg RE/day) 4.0–8.9 years: 400 μg/day (218 μg RE/day) 9.0–12.9 years: 600 μg/day (328 μg RE/day) Background nutrient intake, total vitamin A (IU/day), median (range) by serum retinol status: All (N = 96): 1876 (159, 14,729) Retinol ≥ 30 μg/dL (n = 26): 1748 (159, 4362) Retinol 20–29 μg/dL (N = 48): 2100 (481, 14,729) Retinol <20 μg/dL (N = 22): 1689 (317, 4359) Exposure assessment: 24‐h recall at screening and three 24‐h recalls during the supplementation study at baseline and 3 and 12 months. Compliance: 64% took suppl ≥85% of the time; 36% took suppl <85% of the time |
Serum gamma‐glutamyl transferase (gGT) gGT was measured by the Clinical Chemistry Laboratory at CHOP |
Type of analysis extracted: PP Units: U/L, median (range) gGT at baseline G1: 20 (8, 50) G2: 23 (11, 43) G3: 21 (8, 68) P = NR gGT at end‐of‐study G1: 23 (11, 63) G2: 21 (11, 62) G3: 19 (12, 49) P = NR No significant group differences over time (change score) were detected. |
|
Alberts et al. (2004) USA 12 months Funding: Public |
RCT (parallel) Inclusion criteria: Moderate to severe sun damage with or without actinic keratoses on posterior forearms, and potentially experienced nonmelanoma skin cancer, minimum 50 years of age, postmenopausal (for women). Exclusion criteria: History of invasive cancers, CVD, stroke or other serious disease. Participants randomised: N = 129 Participants completed: N = 116 Participants analysed: N = 129 (ITT) N participants randomised/completed/analysed G1: 31/30/31 G2: 32/29/32 G3: 33/27/33 G4: 33/30/33 |
Sex (as % female) G1: 42% G2: 38% G3: 33% G4: 33% Age males, years (mean) G1: 63.6 G2: 62.9 G3: 64.0 G4: 64.5 Age females, years (mean) G1: 64.3 G2: 61.2 G3: 61.9 G4: 64.3 |
Vitamin A as retinyl palmitate Doses in IU/day: G1: Placebo G2: 25,000 (~7500 μg RE/day) G3: 50,000 (~15,000 μg RE/day) G4: 75,000 (~22,500 μg RE/day) Background nutrient intake: NR Compliance: NR |
AST, ALT, ALP Analysis method: NR Liver scan performed at baseline. According to protocol: second scan for subjects with ASAT or ALAT > 3x upper normal limit, but this applied to none. |
Type of analysis extracted: ITT AST, N subjects who experienced toxicity G1: 0 G2: 0 G3: 0 G4: 1 (3%) ALT, N subjects who experienced toxicity G1: 0 G2: 0 G3: 1 (3% G4: 1 (3%) ALP, N subjects who experienced toxicity G1: 0 G2: 0 G3: 0 G4: 0 |
|
Bitarafan et al. (2015) Iran 12 months Funding: Public |
RCT (Parallel) Inclusion: Multiple sclerosis patients in relapsing–remitting phase, using Interferon beta‐1a (Avonex) as a treatment, aged 20–45 years old. Exclusion criteria: any addiction, alcohol intake, dysphagia, history of myocardial infarction, stroke, other autoimmune diseases, hypersensitivity to vitamin A compounds, liver, pancreatic and biliary disorders N participants randomised/completed: 101/93 G1: 51/47 G2: 50/46 |
Sex: 74% female Age (range): 20–45 years (mean, SD): G1: 30.4 ± 6.9 G2: 32.3 ± 5.9 Other background variables: BMI (kg/m 2 ) (mean, SD) G1: 23.9 ± 3.1 G2: 24.5 ± 4.3 Range: 18.5–30 Dietary vitamin A intake (μg/day) (mean, SD): G1: 737.4 ± 483.78 G2: 744.8 ± 541.91 |
Vitamin A as retinyl palmitate Doses (IU/day) G1: 25,000 (~7500 μg RE for the first 6 months, 10,000 IU/~3000 μg RE for the second 6 months) G2: 0 (placebo) Background vitamin A intake, change (μg/day): G1: −11.2 ± 68.04 G2: +17.8 ± 90.85 (p for change between groups = 0.08) Compliance: NR. |
ALT, AST Determined with colorimetric method |
Type of analysis: NR ALT, change (mean, SD) G1: 0.57 ± 9.44 G2: −0.85 ± 7.91 p = 0.53 AST, change (mean, SD) G1: 0.15 ± 1.85 G2: 0.24 ± 1.48 p = 0.99 |
Abreviations: ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CT, controlled trial; GGT, Gamma‐glutamyl transferase; ITT: Intention‐to‐treat; NR, Not reported; PP, Per protocol; RCT: randomised controlled trial; SCD, sickle cell disease; SEM, standard error mean.
B.4. Prospective observational studies reporting on bone fractures and/or BMD
|
Reference Study name Country Study design Follow‐up Funding |
Original cohort (N total) Exclusion criteria Study population |
Ascertainment of outcome |
Exposure groups n/person‐years Exposure assessment method |
Incident cases | Model covariates |
Results |
|---|---|---|---|---|---|---|
|
Melhus et al. (1998) Swedish Mammography Cohort Sweden NCC 2–64 months Funding: Public |
N = 1247 Population sampled: Females, 40–76 years old living in two counties in Sweden from 1987 to 1990 Exclusion criteria: Incorrect diagnosis of hip fractures, fractures due to cancer or high‐energy trauma n = 1120 Sex: Females Age: Cases: 67.6 ± 7.3 years Controls: 67.7 ± 7.3 years Other characteristics BMI (kg/m2): Cases: 24.4 ± 3.9 Controls: 25.9 ± 4.3 Current smokers: n = 137 Alcohol consumption: NR Vitamin D intake (μg/day): NR Calcium intake (mg/day): NR β‐carotene intake (mg/day): Cases: 800 ± 700 Controls: 750 ± 600 Current HRT users: 56 Previous osteoporotic fracture= Cases: 82/247 Controls: 165/874 |
Hip fractures (cervical, trochanteric or subtrochanteric femoral fracture): from hospital records |
Preformed vitamin A intake (diet only), μg RE/day: Mean ± SD Cases: 960 ± 480 Controls: 880 ± 430 Categories 1: ≤ 500 (ref.) 2: 510–1000 3: 1010‐1500 4: > 1500 No participant had intake less than 270 μg RE/day n/person‐years per category: NR Total person‐years: NR Exposure assessment: A 60‐item FFQ at baseline (validation not published) |
Hip fractures: Cases = 247 Controls = 873 Matching criteria = age, county of residence |
Model 1: Univariate Model 2: Adjusted for BMI, energy intake, age at menopause, lifetime physical activity during leisure time, cigarette smoking, hormone replacement therapy, diabetes mellitus, use of oral contraceptives or cortisone, previous osteoporotic fracture of the distal forearm or proximal humerus, menopause at time of the 2. questionnaire, former athletic activity |
OR (95% CI) Per 1000 μg RE preformed vitamin A: Univariate model: 1.56 (1.15–2.11) Adjusted model: 1.68 (1.18–2.40) Per category: Univariate model: C1. 1.0 (ref) C2. 0.93 (0.61–1.41) C3. 1.27 (0.80–2.02) C4. 1.95 (1.11–3.43) Adjusted model: C1. 1.0 (ref) C2. 0.92 (0.57–1.46) C3. 1.34 (0.77–2.31) C4. 2.05 (1.05–3.98) Additionally adjusted for iron, magnesium, vitamin C, and calcium intake: OR high vs. low: 1.54 (1.06–2.24), p = 0.02 |
|
Feskanich et al. (2002) Nurses' health study USA PC Up to 18 years Funding: Mixed |
N = 121,700 Population sampled: Postmenopausal registered female nurses Exclusion criteria: Premenopausal, previous hip fracture, diagnosis of cancer, heart disease, stroke or osteoporosis % lost to follow up: NR n = 72,377 Sex: Females Age (mean, no SD given) per quintile of total vitamin A intake Q1: 58.3 Q2: 59.3 Q3: 59.7 Q4: 60.0 Q5: 60.5 Other characteristics (mean, no SD given) BMI, kg/m2 Q1: 26.0 Q2: 26.0 Q3: 25.9 Q4: 25.8 Q5: 25.7 Physical activity, h/wk Q1: 2.4 Q2: 2.7 Q3: 2.9 Q4: 3.0 Q5: 3.2 |
Hip fractures: Self‐reported (questionnaire). Only fractures due to low or moderate trauma were considered cases |
Cumulative average intake across quintiles (μg RE/day): Preformed vitamin A intake (diet and supplements): Q1 (ref) (< 500): 487 Q2 (500–849): 763 Q3 (850–1299): 1085 Q4 (1300‐1999): 1607 Q5 (≥ 2000): 3206 n/Person‐years: 72,377/313,308 Preformed vitamin A intake (diet only): Q1 (ref) (< 400): 425 Q2 (400–549): 553 Q3 (550–699): 666 Q4 (700–999): 802 Q5 (≥ 1000):1014 n/Person‐years: 34,386/313,138 Total vitamin A intake (diet and supplements) Q1 (ref) (< 1250): 965 Q2 (1250‐1699): 1442 Q3 (1700‐2249): 1890 Q4 (2250‐2999): 2491 Q5 (≥ 3000): 4274 n/Person‐years: 72,377/313,308 Total vitamin A intake (diet only): Q1 (ref) (< 1000): 811 Q2 (1000‐1299): 1146 |
Hip fracture cases Preformed vitamin A intake (diet and supplements) Q1 (ref): 102 Q2: 122 Q3: 111 Q4: 122 Q5: 146 Preformed vitamin A intake (diet only) Q1 (ref): 31 Q2: 36 Q3: 29 Q4: 42 Q5: 52 Total vitamin A intake (diet and supplements) Q1 (ref): 118 Q2: 123 Q3: 121 Q4: 124 Q5: 137 Total vitamin A intake (diet only) Q1 (ref): 22 Q2: 30 Q3: 25 Q4: 32 Q5: 30 |
Model 1: Adjusted for age Model 2: Adjusted for age, follow‐up cycle, body mass index, use of postmenopausal hormones, smoking, hours of leisure‐time activity per week, use of thiazide diuretics, and intakes of calcium, protein, vitamin D, vitamin K, alcohol, and caffeine + total energy |
Preformed vitamin A intake (diet and supplements) Model 1 Q1 (ref): 1.00 Q2: 1.12 (0.86–1.46) Q3: 0.99 (0.76–1.30) Q4: 1.08 (0.83–1.40) Q5: 1.25 (0.97–1.60) p for trend = 0.03 Model 2 Q1 (ref): 1.00 Q2: 1.25 (0.95–1.65) Q3: 1.18 (0.88–1.59) Q4: 1.43 (1.04–1.96) Q5: 1.89 (1.33–2.68) p for trend <0.001 Preformed vitamin A intake (diet only) Model 1 Q1 (ref): 1.00 Q2: 1.20 (0.74–1.94) Q3: 0.92 (0.55–1.53) Q4: 1.34 (0.84–2.15) Q5: 1.67 (1.07–2.61) p for trend = 0.05 Model 2 Q1 (ref): 1.00 Q2: 1.27 (0.77–2.07) Q3: 0.96 (0.57–1.63) Q4: 1.41 (0.86–2.32) Q5: 1.69 (1.05–2.74) p for trend = 0.05t Total vitamin A intake (diet and supplements) HR (95% CI) |
|
Total calcium intake, mg/day Q1: 719 Q2: 827 Q3: 887 Q4: 947 Q5: 1058 Total vitamin D intake, μg/day Q1: 4.2 Q2: 5.6 Q3: 7.1 Q4: 9.5 Q5: 13.8 Current use of postmenopausal hormones, % Q1: 29 Q2: 30 Q3: 33 Q4: 34 Q5: 34 |
Q3 (1300‐1599): 1427 Q4 (1600‐1999): 1763 Q5 (≥ 2000): 2507 n/Person‐years: 28,676/217,635 Exposure assessment Up to 5 times repeated (61 to > 130‐items, depending on the iteration) semi‐quantitative validated FFQs Conversion factor for pro‐vitamin A carotenoids: NR |
Model 1 Q1 (ref): 1.00 Q2: 0.79 (0.60–1.02) Q3: 0.89 (0.69–1.14) Q4: 0.88 (0.69–1.14) Q5: 0.94 (0.74–1.21) p for trend = 0.55 Model 2 Q1 (ref): 1.00 Q2: 0.92 (0.70–1.22) Q3: 1.13 (0.85–1.49) Q4: 1.24 (0.92–1.68) Q5: 1.48 (1.05–2.07) p for trend = 0.003 Total vitamin A intake (diet only) Model 1 Q1 (ref): 1.00 Q2: 1.33 (0.77–2.31) Q3: 1.16 (0.66–2.05) Q4: 1.34 (0.77–2.34) Q5: 1.40 (0.81–2.42) p for trend = 0.53 Model 2 Q1 (ref): 1.00 Q2: 1.51 (0.86–2.66) Q3: 1.37 (0.74–2.51) Q4: 1.74 (0.96–3.14) Q5: 1.82 (0.97–3.40) p for trend = 0.24 |
||||
|
Michaëlsson et al. (2003) Uppsala Longitudinal Study of Adult Men Sweden PC up to 11 years Funding: Mixed |
N = 2322 Population sampled: General population born between 1920 and 1924, living in Uppsala Exclusion criteria: Missing serum retinol samples % lost to follow up: NR n = 2032/1221 a Sex: Males Baseline variables reported according to quintiles of serum retinol Age, years Q1: 49.7 ± 0.6 Q2: 49.6 ± 0.6 Q3: 49.7 ± 0.6 Q4: 49.7 ± 0.6 Q5: 49.7 ± 0.6 Other characteristics BMI, kg/m2 Q1: 24.1 ± 3.0 Q2: 25.1 ± 3.3 Q3: 24.9 ± 3.2 Q4: 25.5 ± 3.4 Q5: 25.6 ± 3.1 Leisure physical activity ≥3 h per week, % Q1: 42 Q2: 48 Q3: 49 Q4: 47 Q5: 41 Vitamin D intake: NR Calcium intake: NR |
Any fracture site Ascertained through medical records and linkage to the Hospital Discharge Register. Cases of fracture caused by cancer were excluded |
Preformed vitamin A intake (diet and supplements), RE μg/day Mean ± SD (Range) Q1 (ref): 410 ± 90 (< 530) Q2: 640 ± 60 (540–740) Q3: 880 ± 80 (750–1040) Q4: 1290 ± 150 (1050–1560) Q5: 2370 ± 770 (> 1560) Preformed vitamin A intake (diet only) Q1 (ref): 410 ± 90 (< 530) Q2: 640 ± 60 (530–730) Q3: 860 ± 70 (730–990) Q4: 1230 ± 150 (1000‐1500) Q5: 2250 ± 720 (> 1500) n/Person‐years: NR Exposure assessment method: 7‐day dietary records in subsample. |
111, among those with dietary information (n = 1138) Cases per quintile of intake NR |
Model 1: Adjusted for total energy intake. No other model covariates reported for models with dietary vitamin A. |
RR (95% CI) of any fracture for Preformed vitamin A (diet + supplements) Q1 (ref): 1 Q5: 1.99 (0.98, 4.01) p for trend: NR Preformed vitamin A (diet only) Q1 (ref): 1 Q5: 2.00 (1.00, 3.99) p for trend: NR Intermediate quartile RRs not reported |
|
Lim et al. (2004) Iowa Women's Health Study USA PC 9.5 years (mean) Funding: Public |
N = 41,836 Population sampled: Females, aged 55–69 from the general population Exclusion criteria: Premenopausal at baseline, implausible energy intakes, incomplete FFQ, history of cancer (except skin cancer) % lost to follow up: 21 n = 34,703 Sex: Females Median age 61 years Age‐adjusted characteristics stratified by vitamin A supplementation and total vitamin A intake (measure of central tendency or dispersion not given for all variables): Supplement users: BMI, kg/m2: 26.6 Physically active (%): 56.4 Prior fracture (%): 14.1 Mean calcium intake, mg/day: 1295 Mean vitamin D intake, IU/day: 671 Non‐supplement users: BMI, kg/m2: 27.2 Physically active (%): 49.2 Prior fracture (%): 13.4 Mean calcium intake, mg/day: 984 Mean vitamin D intake, IU/day: 269 |
Hip and total fractures: Self‐reported (via questionnaire) |
Quintiles of Preformed vitamin A (food and supplements), mean (range), μg RE/day Q1 (ref): 274 (8–422) Q2: 609 (422–886) Q3: 1157 (886–1397) Q4: 1730 (1397‐2100) Q5: 3783 (2101‐63,315) n/Person‐years hip fractures: Q1: 6940/65,807 Q2: 6941/67,194 Q3: 6941/65,468 Q4: 6941/66,052 Q5: 6940/65,290 n/Person‐years all fractures: Q1: 6940/58,648 Q2: 6941/60,455 Q3: 6941/58,304 Q4: 6941/59,129 Q5: 6940/58,527 Quintiles of total vitamin A (food and supplements) (Mean (range), IU/day [μg RE/day]) Q1 (ref): 1534 (66.3–2117) Q2: 2631 (2117–3145) Q3: 3679 (3146–4263) Q4: 5029 (4263–5968) Q5: 8771 (5968–71,097) n/Person‐years hip fractures: Q1: 6940/64,989 Q2: 6941/65,688 Q3: 6941/58672 Q4: 6942/66,724 Q5: 6940/66,068 |
Hip fractures Preformed vitamin A (food and supplements) Q1: 109 Q2: 84 Q3: 116 Q4: 101 Q5: 125 Total vitamin A (food and supplements) Q1: 93 Q2: 122 Q3: 102 Q4: 99 Q5: 119 All fractures Total Vitamin A (food and supplement) Q1: 1298 Q2: 1319 Q3: 1256 Q4: 1311 Q5: 1319 Preformed vitamin A (food and supplement) Q1: 1324 Q2: 1238 Q3: 1346 Q4: 1270 Q5: 1324 |
Hip fractures Total and preformed retinol dietary intake Model 1: Age Model 2: Age, BMI, waist‐to‐hip‐ratio, diabetes mellitus, past irregular menstrual duration, physical activity, steroid medication, oestrogen replacement and energy intake Total and preformed vitamin A supplements Model 1: Age Model 2: Age, BMI, waist‐to‐hip ratio, diabetes mellitus, physical activity, steroid medication and oestrogen replacement therapy All fractures Total and preformed vitamin A dietary intake Model 1: Age Model 2: Age, BMI, diabetes mellitus, cirrhosis, past irregular menstrual duration, thyrotropic medication, sedative medication, steroid medication, antiepileptic medication, diuretic medication, education, alcohol use, energy intake Total and preformed vitamin A supplements Model 1: Age Model 2: Age, BMI, diabetes mellitus, cirrhosis, thyrotropic medication, antiepileptic medication, sedative medication, steroid medication, education |
Hip fractures RR (95% CI) Preformed vitamin A intake quintiles (food and supplements) Model 1 Q1: 1.00 (ref) Q2: 0.72 (0.54–0.96) Q3: 1.03 (0.79–1.33) Q4: 0.88 (0.67–1.15) Q5: 1.10 (0.85–1.42) p for trend = 0.21 Model 2 Q1: 1.00 (ref) Q2: 0.69 (0.52–0.93) Q3: 1.03 (0.79–1.34) Q4: 0.86 (0.65–1.14) Q5: 1.10 (0.84–1.43) p for trend = 0.19 Total vitamin A intake quintiles (food and supplements) Model 1 Q1: 1.00 (ref) Q2: 1.26 (0.96–1.65) Q3: 1.03 (0.78–1.37) Q4: 0.97 (0.73–1.29) Q5: 1.17 (0.89–1.54) p for trend = 0.85 Model 2 Q1: 1.00 (ref) Q2: 1.27 (0.97–1.67) Q3: 1.08 (0.81–1.44) Q4: 1.02 (0.76–1.37) Q5: 1.25 (0.94–1.68) p for trend = 0.49 All fractures Preformed vitamin A intake quintiles (food and supplements) |
|
1st and 5th quintile of vitamin A intake BMI, kg/m2: Q1: 27.1 Q5: 26.9 Physically active, %: Q1: 40.0 Q5: 62.0 Prior fracture, % Q1: 13.4 Q5: 14.0 Mean calcium intake, mg/day: Q1: 874 Q5: 1318 Mean vitamin D intake, IU/day: Q1: 219 Q5: 632 |
n/Person‐years all fractures: Q1: 6940/58,073 Q2: 6940/66,434 Q3: 6940/59,622 Q4: 6942/59,717 Q5: 6940/58,979 Exposure assessment 127‐item validated semi‐quantitative FFQ Conversion factors for carotenoids not reported. |
Non‐users only Preformed vitamin A intake Model 1: Age Model 2: Age, body mass index, diabetes mellitus, cirrhosis, past irregular menstrual duration, thyrotropic medication, sedative medication, steroid medication, antiepileptic medication, diuretic medication, education, alcohol use, and energy intake Total vitamin A intake Model 1: Age Model 2: Age, body mass index, waist‐to‐hip ratio, diabetes mellitus, past irregular menstrual duration, physical activity, steroid medication, oestrogen replacement, and energy intake |
Model 1 Q1: 1.00 (ref) Q2: 0.90 (0.83–0.97) Q3: 1.01 (0.94–1.09) Q4: 0.94 (0.87–1.01) Q5: 0.99 (0.91–1.06) p for trend = 0.86 Model 2 Q1: 1.00 (ref) Q2: 0.89 (0.82–0.96) Q3: 1.00 (0.93–1.08) Q4: 0.92 (0.85–1.00) Q5: 0.96 (0.89–1.04) p for trend = 0.61 Total Vitamin A intake quintiles (food and supplements) Model 1 Q1: 1.00 (ref) Q2: 1.00 (0.92–1.06) Q3: 0.93 (0.86–1.01) Q4: 0.97 (0.89–1.04) Q5: 0.98 (0.91–1.06) p for trend = 0.43 Model 2 Q1: 1.00 (ref) Q2: 0.98 (0.91–1.06) Q3: 0.92 (0.85–0.99) Q4: 0.94 (0.87–1.02) Q5: 0.95 (0.87–1.03) p for trend = 0.098 |
|||
|
White et al. (2006) Leisure World Cohort Study USA PC Median follow‐up times for males: Hip fractures: 9.8 years Wrist fractures: 4.7 years Spine fractures: 5.3 years Median follow‐up times for females: Hip fractures: 11.8 years Wrist fractures: 7.4 years Spine fractures: 9.1 years Funding: Mixed |
N = 13,978 Population sampled: Residents of a retirement community, mostly white, upper‐middle socioeconomic class Exclusion criteria: NR % lost to follow up: NR n = 13,978 Sex: 63.5% females Age, years: Males: 74.9 ± 7.2 Females: 73.7 ± 7.4 Other characteristics Males : BMI 24.1 ± 2.9 kg/m2 Smoking pack‐years 25.9 ± 29.7 Alcohol consumption drinks/day: 1.64 ± 1.6 Active activities, h/day: 1.1 ± 1.3 Previous fracture after age 40, but before study entry: 14% Females: BMI: 23.1 ± 3.5 kg/m2 Smoking pack‐years: 12.7 ± 22.2 Alcohol consumption drinks/day: 1.17 ± 1.22 Postmenopausal oestrogen: 54% Active activities h/day: 0.9 ± 1.1 Previous fracture after age 40, but before study entry: 30% Vitamin D intake: NR Calcium intake: NR |
Hip, wrist and spine fractures: Self‐reported (follow‐up surveys), hospital discharge records and death certificates |
Preformed vitamin A supplement, b μg RE/day Mean ± SD Males 14,610 ± 26,010 Females 15,930 ± 24,120 N/person‐years Males Hip fractures: 4769/49,586 Wrist fractures: 4177/31,654 Spine fractures: 3890/32,570 Females Hip fractures: 6850/98,290 Wrist fractures: 6393/62,734 Spine fractures: 7153/67,239 Exposure assessment method: Non‐validated survey/questionnaire. |
Males Hip: 278 Wrist: 56 Spine: 167 Females Hip: 949 Wrist: 389 Spine: 562 |
Model 1, all fracture sites (hip, wrist, spine): Age Model 2 (females only) Hip: age, previous fracture, BMI, current smoker, pack‐years of smoking, diabetes, glaucoma, attitude, ever pregnant Wrist: age, previous fracture, BMI, hysterectomy, heart attack, alcohol,, cola consumption |
HR (95% CI) of fracture per 3000 μg RE/day increase in supplemental preformed vitamin A: Males Model 1 Hip: 1.00 (1.00–1.00) Wrist: 1.00 (0.99–1.00) Spine: 1.00 (1.00–1.00) Model 2 NR for any fracture site Females Model 1 Hip: 1.00 (1.00–1.00) Wrist: 1.00 (1.00–1.00) Spine: 1.00 (1.00–1.00) Model 2 Hip: 1.07 (1.00–1.15) Wrist: 1.15 (1.07–1.23) Spine: NR Note: The multiple regression models used a backwards elimination procedure and retained variables significant at 0.05 in the models. Vitamin A supplement use was not a significant variable in model 2 for males (any fracture site) or females (spine fractures) |
|
Hayhoe et al. (2017) EPIC‐Norfolk UK PC 12.5 years (mean) Funding: Public |
N = 25,639 Population sampled: Aged 39–79, between 1993 and 1997 Exclusion criteria: NR % lost to follow up: NR n = 25,439 Sex: 54.8% females Age, years: Females: 58.9 ± 9.3 Males: 59.7 ± 9.3 BMI, kg/m2 Females: 26.2 ± 4.3 Males: 26.5 ± 3.3 Dietary calcium intake (mg/day): Females: 766 ± 249 Males: 919 ± 298 Current smoker (%) Females: 12.1 Males: 12.8 Physically active (%) Females: 15.3 Males: 21.5 Corticosteroid use, > 3 months (%) Females: 3.4 Males: 3.0 |
Osteoporotic hip, spine or wrist fractures: Self‐reported, corroborated via linkage to hospital attendance database |
Quintiles of preformed vitamin A (diet and supplements), μg RE/day Mean ± SD, Range Females: Q1: 146 ± 49 (0–216) Q2: 271 ± 33 (217–331) Q3: 431 ± 71 (331–593) Q4: 889 ± 140 (594–1109) Q5: 2505 ± 2179 (1109–43,483) Males: Q1: 184 ± 59 (0–265) Q2: 330 ± 38 (265–398) Q3: 493 ± 60 (398–617) Q4: 880 ± 157 (617–1158) Q5: 2911 ± 2832 (1158–57,714) N per quintile: Females: Q1: 2786 Q2: 2786 Q3: 2786 Q4: 2786 Q5: 2785 Males: Q1: 2302 Q2: 2302 Q3: 2302 Q4: 2302 Q5: 2302 Exposure assessment method: 7‐day food diaries |
Diet + supplements analyses Hip Fractures Females: Q1: 132 Q2: 128 Q3: 114 Q4: 136 Q5: 155 Males: Q1: 41 Q2: 45 Q3: 46 Q4: 55 Q5: 44 Total fractures (hip, wrist and spine) Females: Q1: 238 Q2: 224 Q3: 211 Q4: 242 Q5: 250 Males: Q1: 113 Q2: 79 Q3: 88 Q4: 92 Q5: 95 Wrist Fractures Females: Q1: 91 Q2: 75 Q3: 73 Q4: 83 Q5: 76 Males: Q1: 31 Q2: 27 Q3: 17 Q4: 24 Q5: 16 |
Age, BMI, family history of osteoporosis, menopausal and hormone replacement therapy status in women, corticosteroid use, smoking status, physical activity, calcium intake, total energy intake, calcium and vitamin D‐containing supplement use, days of food diary completed and the ratio of energy intake: estimated energy requirement |
Preformed vitamin A (diet + supplements) HR (95% CI) Hip Fractures Females: Q1: 1 Q2: 0.89 (0.69, 1.14) Q3: 0.78 (0.60, 1.02) Q4: 0.98 (0.72, 1.33) Q5: 0.97 (0.72, 1.30) Males: Q1: 1 Q2: 1.16 (0.75, 1.79) Q3: 1.20 (0.77, 1.88) Q4: 1.17 (0.71, 1.92) Q5: 1.32 (0.81, 2.16) Total Fractures Females: Q1: 1 Q2: 0.88 (0.73, 1.06) Q3: 0.83 (0.68, 1.01) Q4: 0.93 (0.74, 1.18) Q5: 0.87 (0.70, 1.10) Males: Q1: 1 Q2: 0.67 (0.50, 0.90) Q3: 0.72 (0.53, 0.96) Q4: 0.77 (0.55, 1.07) Q5: 0.75 (0.54, 1.05) Wrist Fractures Females: Q1: 1 Q2: 0.80 (0.58, 1.09) Q3: 0.78 (0.56, 1.10) Q4: 0.73 (0.49, 1.10) Q5: 0.64 (0.43, 0.96) Males: Q1: 1 Q2: 0.72 (0.42, 1.21) Q3: 0.37 (0.20, 0.69) Q4: 0.52 (0.27, 0.99) Q5: 0.35 (0.17, 0.75) |
|
Spine Fractures Females: Q1: 57 Q2: 48 Q3: 41 Q4: 56 Q5: 47 Males: Q1: 46 Q2: 15 Q3: 30 Q4: 28 Q5: 30 |
Spine Fractures Females: Q1: 1 Q2: 0.82 (0.55, 1.23) Q3: 0.72 (0.47, 1.12) Q4: 1.07 (0.65, 1.75) Q5: 0.82 (0.50, 1.34) Males: Q1: 1 Q2: 0.31 (0.17, 0.56) Q3: 0.59 (0.36, 0.96) Q4: 0.54 (0.31, 0.97) Q5: 0.54 (0.30, 0.97) |
|||||
|
Key et al. (2007) EPIC‐Oxford UK PC 5.2 years (mean) Funding: Public |
N: 57,450 Population sampled: General population and vegetarians living in the UK, aged 20 and above between 1993 and 2000 Exclusion criteria: did not answer follow‐up question about fractures; reported fractures of the digits or ribs, had any type of fracture before recruitment; unreliable nutrient intake (≥ 20% FFQ missing, or daily energy intakes <500 kcal or > 3500 kcal for females or < 800 kcal or > 4000 kcal for males) |
All fractures: Self‐reported fractures in bones other than the digits or ribs, accruing after study recruitment |
Categories of preformed vitamin A (food only) (μg RE/day) C1: < 200 C2: 200–299 C3: 300–449 C4: 450–999 C5: ≥ 1000 Mean (SD) intakes per category of preformed vitamin A (food only) (μg RE/day)* Females: C1: 127 (52) C2: 251 (29) C3: 369 (43) C4: 627 (160) C5: 1463 (879) |
Incident fractures: Female: n = 1555 Male: n = 343 Females: C1: 289 C2: 299 C3: 398 C4: 367 C5: 202 Males: C1: 75 C2: 58 C3: 81 C4: 85 C5: 44 |
Age at recruitment, smoking, intakes of energy and calcium, protein, vitamins D and C, carotene, potassium and magnesium, alcohol consumption, BMI, walking, cycling, vigorous exercise, other exercise, physical activity at work, marital status and, for females, parity and use of hormone replacement therapy |
Incidence rate ratio (95% CI) Females: C1: (ref) 1.00 C2: 0.96 (0.80–1.14) C3: 0.99 (0.82–1.19) C4: 1.03 (0.84–1.27) C5: 0.93 (0.73–1.18) p for trend = 0.97 Males: C1: (ref) 1.00 C2: 0.92 (0.62–1.36) C3: 0.97 (0.65–1.45) C4: 0.91 (0.58–1.42) C5: 0.80 (0.47–1.34) p for trend = 0.54 |
|
Lost to follow up: NR n = 34,696 Sex: 77% female Age: Female: 45.8 ± 13.1 Male: 49.5 ± 13.5 Other characteristics BMI (kg/m2): Female: 23.6 ± 3.9 Male: 24.2 ± 3.3% Current smokers: Female: 8.9 Male: 11.5 Alcohol consumption (g/day): Female: 7.7 ± 9.6 Male: 15.1 ± 17.8 Vitamin D intake (μg/day): Female: 2.7 ± 1.9 Male: 2.73 ± 1.99 Calcium intake (mg/day): Female: 996 ± 329 Male. 1046 ± 363% current HRT users: 13.5% ≥ 3h vigorous exercise/week: Female: 27.6 Male: 34.3 |
Males C1: 111 (58) C2: 251 (28) C3: 370 (43) C4: 636 (153) C5: 1531 (1189) N/person‐years per category*: Females: C1: 5139 C2: 5564 C3: 7149 C4: 6330 C5: 3403 Males: C1: 1449 C2: 1299 C3: 1913 C4: 2157 C5: 1299 *Data received from study authors for total N of 35,702, which is ~ 1000 more participants than what is reported in the publication Exposure assessment: 130‐item, validated FFQ, covering the previous 12 months, at baseline |
|||||
|
Caire‐Juvera et al. (2009) Women's' Health Initiative Observational Study USA PC 6.6 years (mean) Funding: Public |
N: 93,676 Population sampled: Postmenopausal females aged 50–79 years, unlikely to move or die ≤ 3, not participating in other clinical trials Exclusion criteria: Missing FFQ data; previous fractures or diagnosis of osteoporosis Lost to follow up: 1,9% n = 75,747 Sex: female Age (years) per quintile of vitamin A intake: Q1: 63.7 ± 7.3 Q2: 63.8 ± 7.3 Q3: 63.7 ± 7.3 Q4: 63.6 ± 7.2 Q5: 63.1 ± 7.2 Other characteristics BMI (kg/m2) per quintile of vitamin A intake: Q1: 27.1 ± 5.8 Q2: 26.8 ± 5.5 Q3: 26.7 ± 5.5 Q4: 27.0 ± 5.6 Q5: 27.6 ± 6.1 Vitamin D intake (ug/day) per quintile of vitamin A intake: Q1: 5.6 ± 3.3 Q2: 8.9 ± 4.2 Q3: 11.5 ± 4.7 Q4: 13.4 ± 4.6 Q5: 15.4 ± 5.6 |
Hip fractures: self‐reported (by participants or proxy) and adjudicated by central review of radiology and other medical reports Other fractures: self‐report (by participants or proxy |
Quintiles of preformed vitamin A (food and supplements), μg RE/day: Q1 (ref): < 474 Q2: 474–764 Q3: 765–1092 Q4: 1093–1425 Q5: ≥ 1426 μg N Q1 (ref): 15,149 Q2: 15,149 Q3: 15,150 Q4: 15,149 Q5: 15,150 Quintiles of total vitamin A (food and supplements) (μg RE/day): Q1 (ref): < 5055 Q2: 5055–5824 Q3: 5825–6550 Q4: 6551–7507 Q5: ≥ 7508 N Q1 (ref): 15,149 Q2: 15,149 Q3: 15,150 Q4: 15,149 Q5: 15,150 Nutrient intakes were calculated as the mean of the intakes at baseline and year 3 of follow‐up |
Total fractures: 10,405 Hip fractures: 588 Per quintile: Hip fractures Preformed vitamin A Q1: 112 Q2: 129 Q3: 94 Q4: 124 Q5: 129 Total vitamin A Q1: 122 Q2: 121 Q3: 113 Q4: 113 Q5: 119 Total fractures Preformed vitamin A Q1: 1977 Q2: 2044 Q3: 2110 Q4: 2152 Q5: 2122 Total vitamin A Q1: 1993 Q2: 2054 Q3: 2102 Q4: 2137 Q5: 2119 Incidence of total fractures: 221/10,000 person‐years |
Model 1: Adjusted for age Model 2: Adjusted for age, energy, vitamin K, protein, alcohol, and caffeine intake; smoking; BMI; hormone therapy use; total METs per week; ethnic group; region Model 3: Model 2 plus vitamin D and calcium |
HR (95% CI) Hip fractures: Preformed vitamin A Model 1: Q1: 1 Q2: 1.22 (0.95, 1.57) Q3: 0.87 (0.66, 1.15) Q4: 1.13 (0.87, 1.46) Q5: 1.21 (0.94, 1.55) p for trend: 0.373 Model 2: Q1: 1 Q2: 1.23 (0.94, 1.60) Q3: 0.89 (0.67, 1.19) Q4: 1.10 (0.84, 1.45) Q5: 1.25 (0.95, 1.64) p for trend: 0.415 Model 3: Q1: 1 Q2: 1.19 (0.91, 1.57) Q3: 0.84 (0.61, 1.14) Q4: 1.00 (0.73, 1.39) Q5: 1.13 (0.81, 1.59) p for trend: 0.925 Total vitamin A Model 1: Q1: 1 Q2: 0.99 (0.77, 1.27) Q3: 0.94 (0.73, 1.22) Q4: 0.96 (0.74, 1.23) Q5: 1.06 (0.83, 1.37) p for trend: 0.612 Model 2: Q1: 1 Q2: 1.01 (0.78, 1.32) Q3: 0.99 (0.75, 1.31) Q4: 1.03 (0.76, 1.38) Q5: 1.24 (0.88, 1.73) p for trend: 0.2 |
|
Calcium intake (mg/day) per quintile of vitamin A intake: Q1: 847 ± 429 Q2: 1081 ± 607 Q3: 1240 ± 498 Q4: 1390 ± 462 Q5: 1622 ± 547 Alcohol intake (g/day) per quintile of vitamin A intake: Q1: 4.1 ± 7.9 Q2: 5.2 ± 9.2 Q3: 5.9 ± 10.2 Q4: 6.4 ± 10.9 Q5: 6.8 ± 12.5% current smokers per quintile of vitamin A intake: Q1: 6.8 Q2: 6.1 Q3: 5.0 Q4: 4.8 Q5: 4.9 Physical activity (METs/week) per quintile of vitamin A intake: Q1: 12.9 ± 4.1 Q2: 13.8 ± 14.3 Q3: 14.5 ± 14.3 Q4: 14.7 ± 14.4 Q5: 14.8 ± 14.8% current HRT users, per quintile of vitamin A intake: Q1: 41 Q2: 45 Q3: 47 Q4: 49 Q5: 48 |
Exposure assessment: 122‐item FFQ at baseline and year 3. Intakes were averaged across the two FFQs. Supplement intake was assessed by a computerised inventory and by participants bringing supplements to the clinic for an in‐person interview. Conversion factors for carotenoids not reported |
Model 3: Q1: 1 Q2: 0.99 (0.75, 1.30) Q3: 0.94 (0.69, 1.28) Q4: 0.96 (0.68, 1.35) Q5: 1.14 (0.76, 1.71) p for trend: 0.445 Total fractures: Preformed vitamin A Model 1: Q1: 1 Q2: 1.05 (0.99, 1.12) Q3: 1.09 (1.03, 1.16) Q4: 1.13 (1.06, 1.20) Q5: 1.10 (1.03, 1.17) p for trend: < 0.001 Model 2: Q1: 1 Q2: 1.03 (0.96, 1.11) Q3: 1.08 (1.00, 1.15) Q4: 1.11 (1.04, 1.18) Q5: 1.08 (1.01, 1.16) p for trend: 0.002 Model 3: Q1: 1 Q2: 1.01 (0.94, 1.08) Q3: 1.03 (0.96, 1.11) Q4: 1.04 (0.96, 1.13) Q5: 1.00 (0.92, 1.10) p for trend: 0.8 Total vitamin A Model 1: Q1: 1 (ref) Q2: 1.03 (0.97, 1.10) Q3: 1.07 (1.00, 1.14) Q4: 1.09 (1.02, 1.16) Q5: 1.09 (1.02, 1.16) p for trend: < 0.001 |
||||
|
Model 2: Q1: 1 Q2: 1.02 (0.96, 1.09) Q3: 1.06 (0.99, 1.13) Q4: 1.08 (1.01, 1.16) Q5: 1.09 (1.01, 1.19) p for trend: < 0.001 Model 3: Q1: 1 Q2: 0.99 (0.92, 1.05) Q3: 1.00 (0.93, 1.07) Q4: 1.00 (0.92, 1.08) Q5: 0.98 (0.89, 1.08) p for trend: 0.330 ‘Among the females in the lower vitamin D strata, there was a modest risk of fractures in the highest quintile of both total vitamin A (HR: 1.19, 95% CI: 1.04, 1.37; P for trend <0.05) and retinol (HR: 1.15; 95% CI: 1.03, 1.29; P for trend: 0.056), compared with the lowest quintile. There were no significant risks in the groups with higher vitamin D intakes or lower and higher calcium intakes. The combination of lower vitamin D and calcium intakes resulted in an HR of 1.17 (95% CI: 1.01, 1.36; P for trend <0.05) for total fractures among females in the highest compared with the lowest quintile of retinol.’ |
||||||
|
Rejnmark et al. (2004) Danish Osteoporosis Prevention Study Denmark PC/NCC 5 years Funding: Mixed |
N = 2016 Population sampled: Perimenopausal females, participating in an open‐label trial, aged 45–58 years, Caucasian, 3–24 months after the last menstrual bleeding or having experienced perimenopausal symptoms and having elevated serum FSH Exclusion criteria: Known metabolic bone disease, osteoporosis (defined as nontraumatic vertebral fractures), current oestrogen use, ever treatment with glucocorticoids for ≥ 6 months, current or past malignancy, newly diagnosed or uncontrolled chronic disease, hospitalisation due to alcohol or drug addiction |
NCC Fractures: Self‐reported validated against hospital discharge records Some cases were added upon spinal x‐ray review A fracture was defined as more than 20% reduction in the height of a vertebra compared with the highest vertical distance of that vertebrae PC Femoral neck and lumbar spine BMD measurements were performed by using DXA |
NCC – Fractures analyses Preformed vitamin A intake (food and supplements) Median (IQR), μg RE/day For cases (n = 163) 1190 (700–1420) For controls (n = 978) 1210 (740–1430) Categories of Preformed vitamin A intake (food and supplements), μg RE/day C1: < 500 (ref) C2: 500–1500 C3: > 1500 n per category C1: 175 C2: 707 C3: 219 Quintile of preformed vitamin A intakes or n per quintile = NR PC – BMD analyses Median (IQR), μg RE/day Total vitamin A intake (food and supplements): 1740 (1290‐2360) |
163 fractures Cases per category of intake: C1 (ref): 24 C2: 107 C3: 28 Cases per quintile of intake: NR |
NCC – Fractures Model 1: Crude Model 2: Adjusted for age, years postmenopausal, previous fracture, body weight, physical activity, total energy intake, dietary calcium intake, dietary vitamin D intake, use of vitamin D supplements, alcohol intake, smoking, thiazide diuretics, loop diruetics, thyroid hormones, antipsychotic/anxiolytic/antidepressant therapy, diagnosis of thyretoxicosis, chronic obstructive lung disease, lumbar spine and femoral neck BMD PC ‐ BMD Adjusted for age, years postmenopausal, hormone replacement therapy status, previous fracture, body weight, baseline lumbar spine and femoral neck BMD, physical activity, total energy intake, dietary calcium intake, dietary vitamin D intake, use of vitamin D supplements, alcohol intake, smoking, use of thiazide diuretics, loop diruetics, thyroid hormones or antipsychotic/anxiolytic/antidepressant therapy, diagnosis of thyretoxicosis, non‐insulin dependent diabetes mellitus, insulin‐dependent diabetes mellitus, chronic obstructive lung disease, the other dietary vitamin A intake variables |
NCC – Fractures Logistic regression for preformed vitamin A and fractures, OR (95% CI) Categories of preformed vitamin A intake (food and supplements) Model 1 C1: 1 (ref) C2: 1.12 (0.70, 1.81) C3: 0.96 (0.71, 1.28) Model 2 C1: 1 (ref) C2: 1.27 (0.65, 2.51) C3: 1.03 (0.56, 1.89) Quintiles of preformed vitamin A intake (food and supplements) Model 1 Q1: 1 (ref) Q2: 1.37 (0.81, 2.32) Q3: 1.04 (0.79, 1.36) Q4: 1.05 (0.88, 1.25) Q5: 0.99 (0.86, 1.14) Model 2 Q1: 1 (ref) Q2: 1.79 (0.86, 3.75) Q3: 1.05 (0.78, 1.42) Q4: 0.94 (0.43, 2.07) Q5: 1.00 (0.75, 1.35) PC ‐ BMD Regression models for total and preformed vitamin A intake with lumbar spine and femoral neck BMD, (β (95% CI)) |
|
% lost to follow up: NR n = 1690 (longitudinal analysis on lumbar spine BMD) n = 1694 (longitudinal analyses on femoral neck BMD). n = 1141 NCC Sex: Females Age, years (median (IQR)) PC: 50 (48–52) NCC: Cases 50 (48–52) Controls 50 (48–52) Other characteristics at baseline, Median (IQR) PC: Years postmenopausal: 0.5 (0.2–1.5) Body weight, kg: 65.7 (59.8–74) Physical activity, h/week: 19 (9–30) Dietary calcium intake, mg/day: 807 (626–1040) Dietary vitamin D intake, μg/day: 2.2 (1.6–3.2) Dietary β‐carotene intake, mg/day: 2.94 (1.44–5.50) |
Preformed vitamin A intake (food and supplements): 1210 (680–1450) n/Person‐years: NR Exposure assessment: 4‐ or 7‐day food records. A dietician used food models and photographs during a 15‐min validation interview to evaluate serving sizes and cooking habits. Conversion factor for β‐carotene: 6:1 |
Change in BMD at the lumbar spine (g/cm 2 ) per total vitamin A intake (food and supplements) (mg RE/day) increase: 0.043 (−0.193, 0.284) Change in BMD at the lumbar spine (g/cm 2 ) per total preformed vitamin A intake (food and supplements), (mg RE/day) increase: 0.101 (−0.180, 0.390) Change in BMD at the femoral neck (g/cm 2 ) per total vitamin A intake (food and supplements) (mg RE/day) increase: −0.122 (−0.349, 0.105) Change in BMD at the femoral neck (g/cm 2 ) per total preformed vitamin A intake (food and supplements) (mg RE/day) increase: −0.065 (−0.340, 0.209), p = NR |
||||
|
Barker et al. (2005) UK NCC 3.7 years (mean) Funding: Public |
N = 2602 Population sampled: Cases and controls sampled from the placebo arm of a study, general population of females > 75 years Exclusion criteria: bilateral hip arthroplasty, hypocalcaemia, leucopoenia, impaired hepatic function, known malignancy, inflammatory bowel disease, impaired renal function, and use of bisphosphonate or calcium supplement of > 500 mg/day % lost to follow up: NR n = 1611 Any osteoporotic fracture Cases: 312 Controls: 934 Hip fracture Cases: 92, Controls: 273 Sex: Females Age, years, mean (95% CI) Any osteoporotic fracture Cases: 80.1 (79.6–80.6) Controls 79.3 (79.1–79.5) Hip fracture Cases: 80.8 (80.0–81.7) Controls 79.2 (78.7–80.0) |
Hip and any fractures: self‐reports (house visits), medical records with radiology or surgical report confirmation. Verification in a subsample by comparing ICD codes |
Serum retinyl palmitate (upper quartile vs. rest of population). Concentrations not reported. Measured by HPLC‐MS/MS Retinol supplement use. Concentrations or contrast in analysis not reported. Intake estimated from multivitamin/cod liver oil use Exposure assessment method: Serum retinyl palmitate and surveys for multivitamin/cod liver oil use which was in turn used to estimate retinol supplement use |
Any osteoporotic fracture: 312 Hip fracture: 92 |
Models were unadjusted Retinyl palmitate and retinol supplementation was only evaluated in univariate Cox PH models with fracture as outcome. Because they did not satisfy the authors' criterion of p < 0.1 on fracture risk they were not included in multivariable models. The final model only included age, total hip BMD and weight and no dietary variables |
Serum retinyl palmitate HR (95% CI) Any fracture: Q1‐3: ref. Q4: 0.97 (0.74–1.26) p = 0.800 Hip fracture: Q1‐3: ref. Q4: 0.91 (0.56–1.46) p = 0.687 Preformed vitamin A supplement use HR (95% CI) Any fracture: Non‐users: ref. Users: 0.76 (0.60–0.95) p = 0.021 Hip fracture: Non‐users: ref. Users: 0.86 (0.56–1.33) p = 0.507 In the multivariate step‐wise analysis, serum retinyl palmitate, and retinol supplement use, was not associated with fracture risk |
|
Other characteristics, mean (95% CI) Any osteoporotic fracture cases Height, cm: 156 (155–157) Weight, kg: 61.7 (60.6–62.9) Total hip BMD, g/cm2: 0.68 (0.66–0.69) Serum 25(OH)D, nM [geometric mean (95% CI)]: 40.1 (38.3–42) Any osteoporotic fracture controls Height, cm: 156 (156–157) Weight, kg: 65.4 (64.6–66.1) Total hip BMD, g/cm2: 0.76 (0.76–0.77) Serum 25(OH)D, nM [geometric mean (95% CI)]: 41.9 (40.8–43.0) Hip fracture cases Height, cm: 155 (154–156) Weight, kg: 58.8 (56.8–60.7) Total hip BMD, g/cm2: 0.65 (0.62–0.68) Serum 25(OH)D, nM [geometric mean (95% CI)]: 37.5 (34.5–40.7) Controls Height, cm: 157 (156–157) Weight, kg: 65.7 (64.1–67.1) Total hip BMD, g/cm2: 0.76 (0.74–0.78) Serum 25(OH)D, nM [geometric mean (95% CI)]: 39.6 (37.7–41.7) |
||||||
|
de Jonge et al. (2015) The Rotterdam study The Netherlands PC 13.9 years (mean) Funding: Mixed |
N: 7983 Population sampled: Aged 55 years and older from the general population Exclusion criteria: No dietary intake data/unreliable data, missing BMD data Lost to follow up: NR n = 5288 Sex: 59% female Age: ≥ 55 years Other characteristics (median (IQR) per quintile of vitamin A intake) Vitamin D intake, μg/day: Q1: 3.62 (2.68–4.61) Q2: 3.29 (2.36–4.45) Q3: 3.01 (2.18–4.12) Q4: 3.02 (2.20–4.15) Q5: 3.16 (2.25–4.32) Calcium intake, mg/day: Q1: 1009 (804–1279) Q2: 1046 (857–1282) Q3: 1097 (878–1341) Q4: 1097 (894–1340) Q5: 1130 (872–1374) Height, cm: Q1: 168 (161–174) Q2: 167 (161–175) Q3: 167 (161–174) Q4: 166 (161–173) Q5: 166 (160–174) |
Radius/ulna, tibia/fibula, hand/foot, femur and other fractures: Reported by general practitioners; verified by two research physicians Femoral neck BMD: By DXA, assessed at four visits |
Quintiles of energy‐adjusted total vitamin A intake (food only), μg RE/day Median Q1: 709 Q2: 939 Q3: 1124 Q4: 1384 Q5: 2012 Energy‐adjusted preformed vitamin A intake, median μg RE/day: Q1: 186 Q2: 200 Q3: 294 Q4: 518 Q5: 1099 N/person‐years: NR (author requested) Exposure assessment: 170‐item, validated, semi‐quantitative FFQ through interviews at baseline ‘Energy‐adjusted’ nutrient intakes = unstandardised residuals from linear regression Conversion factor for β‐carotene 6:1 |
Fractures: 1301 Cases per quintile: Total vitamin A, energy‐adjusted: Q1: 258 Q2: 234 Q3: 291 Q4: 279 Q5: 239 Retinol, energy‐adjusted Q1: 228 Q2: 263 Q3: 307 Q4: 269 Q5: 234 |
Model 1: Age, sex Model 2: Model 1 + smoking, dietary calcium, alcohol intake, education, net income, disability index, physical activity Model 3: Model 2 + BMI Model 4: Model 3 + Baseline BMD |
Total fractures: HR (95% CI) Total vitamin A: Model 3: Q5 vs. Q3: 0.82 (0.69, 0.97) p for trend: NR Retinol: Model 3: Q5 vs. Q3: 0.81 (0.68, 0.96) p for trend: NR Model 4: Not significant (data not shown) Significant lower fracture risk in subjects in the highest quintile of retinol intake only in those with a BMI ≥ 25 kg/m2 (HR (95% CI) = 0.78 (0.68–0.89) versus 1.04 (0.87–1.24) with BMI ⩽25 kg/m2) No significant interaction between total vitamin A, retinol or β‐carotene and vitamin D intake (p for all interactions > 0.45) on fracture risk. No significant interactions between dietary intake of vitamin A and vitamin D plasma concentrations BMD (g/cm 2 ) Baseline (median (IQR)): 0.86 (0.77–0.96) At follow‐up: Per 100 μg/day RE total vitamin A (β (95% CI): Model 1: 0.53 (0.06–0.99) |
|
BMI, kg/m2: Q1: 25.6 (23.7–27.8) Q2: 25.9 (23.8–28.1) Q3: 25.9 (23.8–28.4) Q4: 26.0 (24.2–28.5) Q5: 26.4 (24.2–29.1) Current smokers, %: Q1: 25 Q2: 23 Q3: 22 Q4: 20 Q5: 25 Alcohol intake, g/day: Q1: 3.5 (0.1–16.9) Q2: 3.2 (0.2–14.4) Q3: 3.1 (0.2–14.4) Q4: 3.5 (0.2–14.8) Q5: 3.9 (0.2–15.0) Physical activity, h/day: Q1: 5.8 (4.2–7.5) Q2: 5.8 (4.1–7.8) Q3: 5.9 (4.2–7.8) Q4: 5.8 (4.3–7.9) Q5: 5.9 (4.2–7.9) Prevalent osteoporosis, % Q1: 11 Q2: 10 Q3: 10 Q4: 10 Q5: 11 Current or past HRT use, %: Q1: 13 Q2: 13 Q3: 13 Q4: 17 Q5: 12 |
Model 2: 0.46 (0.00–0.91) Model 3: 0.14 (−0.28–0.56) (model 3 n.s.) Per 100 μg/day retinol: Model 1: 0.31 (−0.23, 0.87) Model 2: 0.45 (−0.09, 1.01) Model 3: 0.13 (−0.40, 0.75) (model 3 n.s.) Significant interaction between dietary intake of vitamin D and total vitamin A (p for interaction = 0.016) in relation to BMD. However, stratified analysis for dietary vitamin D intake above or below the median did not show significant associations between total vitamin A and BMD. No significant interactions between dietary intake of vitamin A and vitamin D plasma concentrations |
|||||
|
Toraishi et al. (2021) Japan PC 1 year Funding: Public |
N = 41 Population sampled: Collegiate distance runners Exclusion criteria: NR % lost to follow up: 0 n = 41 Sex: Males Age, years: 19.4 ± 1.0 Other characteristics BMI, kg/m2: 19.3 ± 1.2 Baseline vitamin D intake, μg/day: Overall: 9.6 ± 2.2 Baseline calcium intake, mg/day: Overall: 515.1 ± 175.8 Stress‐fracture group: 684 ± 320 Non‐stress‐fracture group: 497 ± 150 |
Stress fractures at any site, i.e. due to repeated mechanical load: Self‐reported |
Mean intake of total vitamin A (food only), μg RAE/day All subjects: 1441.3 ± 802.4 Stress‐fracture group: 2792 ± 1136 No stress‐fracture group: 1295 ± 619 Mean intake at follow‐up: Stress‐fracture group: 3747 ± 309 No stress‐fracture group: 2943 ± 1204 Exposure assessment: Semi‐quantitative FFQ Conversion factors for carotenoids not reported |
N = 4 (3 at tibia and one at metatarsus) |
NR In logistic regression: Calcium and iron intake |
OR (95% CI) for stress fracture (at any site) Per 100 μg RAE increase: 1.22 (0.99–1.50) Threshold intake for stress fracture (from ROC): 3206 μg |
|
Kaptoge et al. (2003) EPIC‐Norfolk UK PC 3 years (mean; range 2–5 years) Funding: Public |
N = 30,411 Population sampled: Elderly males and females Exclusion criteria: < 2 BMD scans, treatment with bone active medication, different side of the hip scanned at follow‐up, incomplete food diaries % lost to follow up: NR n = 944 (470 males, 474 females) Sex: 50.2% female Mean (5th, 95th percentile) age, years Males: 72.0 (68.0, 77.4) Females: 71.9 (67.9, 77.0) |
Total hip BMD: DXA |
Preformed vitamin A intake (food only), μg RE/day: Mean (5th, 95th percentile) Males: 358 (109, 3836) Females: 289 (98, 3517) Analyses are also reported by tertiles but intakes are not reported T1: NR T2: NR T3: NR |
NA |
Continuous analyses Model 1: Weight change, FEV, Stair climbing, Activities in daily living (ADL) score change, Past activity score Categorical analyses Estimated mean BMD loss (% per annum) Males Model 1: Rate of weight change and FEV Females Model 1: Rate of weight change, stair climbing, change in ADL score and past activity |
Continuous analyses on rate of total hip BMD percentage loss per annum (β coefficient (SE)) Model 1 Vitamin A (/1000 μg): Males: 0.029 (0.043) p = 0.508 Females: −0.024 (0.039) p = 0.539 Categorical analyses on total hip BMD percentage loss per annum (mean rates) Males Model 1 T1: −0.11 T2: −0.14 T3: −0.21 p for trend = 0.773 |
|
Other characteristics Mean (5th, 95th percentile) Males: BMI, kg/m2: 27.0 (21.9, 32.3) Past physical activity, z‐score: 0.31 (−1.18, 2.37) Grip strength, kg: 39 (27, 52) Weight loss, kg/year: −0.06 (−2.30, 2.10) Ever fractured (% yes): 39 Vitamin D intake, μg/day: 3.4 (1.1,8.0) Calcium intake, mg/day: 886 (505, 1364) Carotene intake μg/day: 1871 (556, 4664) Females: BMI, kg/m2: 26.8 (21.0, 35.0) Past physical activity, z‐score: −0.21(−1.77, 1.19) Grip strength, kg: 24 (15, 32) Weight loss, kg/year: −1.7 (−7.5, 3.5) Ever fractured (% yes): 34 Vitamin D intake, μg/day: 2.5 (0.9,6.0) Calcium intake, mg/day: 755 (399, 1188) Carotene intake μg/day: 1658 (458, 3885) |
The nutrient intakes for categorical analyses were adjusted for total energy intake by taking the residuals from regressing each nutrient on total energy Exposure assessment 7‐day food diary with a 24‐h recall the first day. The food diary has been validated against biomarkers and 16‐day weighted food records. Conversion factors for carotenoids not reported |
Females Model 1 T1: −0.42 T2: −0.49 T3: −0.33 p for trend = 0.517 |
||||
|
Promislow et al. (2002) The Rancho Bernardo Study USA PC Mean ± SD follow‐up time: 4.1 (0.5) years Funding: Public |
N = 1526 Population sampled: Ambulatory community‐dwelling elderly Exclusion criteria: NR % lost to follow up: 37.2% n = 958 females = 570 males = 388 Sex: 59.5% female Age, years Females: 71.3 ± 8.7 Males: 70 ± 8.5 Other characteristics Mean ± SD Females BMI, kg/m2: 24.6 ± 3.7 Total calcium intake, mg/day: 984 ± 573.8 Oestrogen users, %: 39.7 Vitamin D: NR Males BMI, kg/m2: 26.4 ± 3.4 Total calcium intake, mg/day: 797.2 ± 458.2 Vitamin D: NR |
Total hip, femoral neck and lumbar spine BMD: DXA Total hip BMD was obtained by summing the bone mineral content at the femoral neck, intertrochanter, and greater trochanter and dividing by the composite area of the three sites. Spine BMD was defined as the average BMD of lumbar vertebrae L1–L4. Instruments were calibrated daily and had measurement precisions of ≤ 1% for the spine and ≤ 1.5% for the Hip |
Preformed vitamin A (food and supplements), μg RE/day Mean (SD) Females: 1247 (1573) Males: 1242 (1442) Preformed vitamin A (food only), μg RE/day Mean (SD) Females: 497 (460) Males: 624 (585) Supplement users, n Females: 281 Males: 150 Supplement use defined as taking either a multivitamin or a retinol supplement Exposure assessment 61‐item FFQ validated against four one‐week food records. Conversion factors for carotenoids not reported |
NA |
Model 1: Adjusted for age Model 2: Adjusted for age, BMI, calcium intake (including supplements), diabetes status; years postmenopausal (females only), current exercise, and current use of cigarettes, alcohol, thiazides, thyroid hormones, steroids, supplemental retinol, and oestrogen, percent change in body weight |
Change (%) in BMD per unit increase in energy‐adjusted log preformed vitamin A intake (retinol) (β (95% CI)) Total hip BMD Females Model 1 All: −0.05 (−0.016, 0.07), p = 0.43 Supplement users: −0.28 (−0.5, −0.06), p = 0.01 Non‐users: 0.10 (−0.12, 0.33), p = 0.36 Model 2 All: NR Supplement users: −0.27 (−0.48, −0.04), p = 0.02 Non‐users: 0.13 (−0.09, 0.35), p = 0.25 Males Model 1 All: −0.01 (−0.13 0.10), p = 0.84 Supplement users: −0.15 (−0.41, −0.10), p = 0.24 Non‐users: −0.06 (−0.24, 0.13), p = 0.55 Model 2 All: −0.08 (−0.23, 0.08), p = 0.32 Supplement users: −0.19 (−0.46, 0.08), p = 0.17 Non‐users: −0.04 (−0.23, 0.15), p = 0.68 Femoral neck BMD Females Model 1 All: −0.04 (−0.019, 0.10), p = 0.56 Supplement users: −0.21 (−0.44, −0.02), p = 0.07 Non‐users: 0.13 (−0.20, 0.46), p = 0.44 Model 2 All: NR Supplement users: −0.23 (−0.46, 0.00), p = 0.05 Non‐users: 0.22 (−0.11, 0.56), p = 0.19 |
|
Males Model 1 All: 0.01 (−0.13, 0.15), p = 0.87 Supplement users: −0.10 (−0.37, −0.17), p = 0.45 Non‐users: 0.01 (−0.24, 0.26), p = 0.95 Model 2 All: 0.06 (−0.14,0.25), p = 0.57 Supplement users: −0.15 (−0.42, 0.12), p = 0.28 Non‐users: 0.16 (−0.12, 0.44), p = 0.25 Total spine Females Model 1 All: −0.03 (−0.13, 0.07), p = 0.50 Supplement users: −0.10 (−0.29, 0.08), p = 0.27 Non‐users: 0.04 (−0.15, 0.23), p = 0.67 Model 2 All: NR Supplement users: −0.10 (−0.28, 0.09), p = 0.30 Non‐users: 0.01 (−0.17, 0.20), p = 0.89 Males Model 1 All: 0.00 (−0.12, 0.12), p = 0.97 Supplement users: −0.05 (−0.28, 0.17), p = 0.64 Non‐users: −0.06 (−0.27, 0.14), p = 0.95 Model 2 All: 0.01 (−0.15,0.16), p = 0.92 Supplement users: 0.00 (−0.24, 0.24), p = 0.98 Non‐users: 0.02 (−0.19, 0.23), p = 0.87 ‘Regression models showed an analogous inverse U‐shaped association of retinol intake with percent bone change, suggesting that those with low or high retinol intakes suffered greater bone loss, although statistical evidence for this pattern was weak in men’ |
||||||
|
Macdonald et al. (2004) Aberdeen Prospective Osteoporosis Screening Study UK Up to 7 years PC Funding: Public |
N = 5119 Population sampled: Premenopausal women Exclusion criteria: Chronic medication use, conditions likely to affect bone metabolism, bisphosphonate therapy, wheelchair use, outlying dietary calcium intake % lost to follow up: 17.3 n = 891 Sex: Females Age, years 57.5 ± 1.5 Other characteristics BMI, kg/m2: 24.6 ± 4.0 BMD, g/cm2 Lumbar spine: 1.064 ± 0.16 Femoral neck: 0.886 ± 0.13 Total calcium intake, mg/day 1070 ± 334 Total protein intake, g/day 81.4 ± 22.5 Total vitamin D intake, μg/day 4.5 ± 3.1 Liver enzymes: NR |
Femoral neck and lumbar spine BMD: DXA |
Preformed vitamin A intake (food only), μg RE/day: Mean ± SD: 820 ± 602 Median (range): 588 (39–4354) Preformed vitamin A intake (food and supplements), μg RE/day: Mean ± SD: 924 ± 666 Median (range): 702 (85–4354) Total vitamin A intake NR Exposure assessment 98‐item semi‐quantitative FFQ validated against weighted food records. Results reported using 6:1 conversion |
NA |
Model 1: Unadjusted Model 2: Adjusted for age, weight, annual percentage change in weight, height, smoking status, socioeconomic status, physical activity level, baseline BMD at appropriate site, menopausal status and hormonal replacement therapy use Multivariable regression model for dietary retinol and vitamin A and femoral neck BMD: Baseline femoral neck BMD, age, annual percentage weight change, height, hormone replacement therapy use, menopausal status Multivariable regression model for lumbar spine BMD: Vitamin A was not part of this model. It included weight, age, hormone replacement therapy use, menopausal status and alcohol intake. |
Pearson's correlation analyses Energy‐adjusted vitamin A and change in femoral neck BMD Model 1: Preformed vitamin A (food only): r = −0.072, p < 0.05 Preformed vitamin A (food and supplements): r = −0.071, p < 0.05 Total vitamin A (food only): r = −0.090, p < 0.01 Total vitamin A (food and supplements): r = −0.004, p = NS (value NR) Model 2 Preformed vitamin A (food only): r = −0.067, p < 0.05 Preformed vitamin A (food and supplements): r = −0.032, p = NS (value NR) Total vitamin A (food only): r = −0.090, p < 0.01 Total vitamin A (food and supplements): r = −0.012, p = NS (value NR) Energy‐adjusted vitamin A and change in lumbar spine BMD Model 1: Preformed vitamin A (food only): r = −0.021, p = NS (value NR) Preformed vitamin A (food and supplements): r = −0.019, p = NS (value NR) Total vitamin A (food only): r = −0.041, p = NS (value NR) Total vitamin A (food and supplements): r = −0.029, p = NS (value NR) |
|
Model 2 Preformed vitamin A (food only): r = −0.036, p = NS (value NR) Preformed vitamin A (food and supplements): r = −0.004, p = NS (value NR) Total vitamin A (food only): r = −0.061, p < 0.08 Total vitamin A (food and supplements): r = −0.032, p = NS (value NR) Multivariable regression model Femoral neck BMD, β coefficient (95% CI) ‐ Standardised to 8 MJ energy intake Preformed vitamin A (food only) (mg × 10−4): −1.73 (−3.20, −0.30), p = 0.018 Total vitamin A (food only) (mg × 10−4): −1.24 (−2.47, 0.17), p = 0.047 Including the nutrient intake from dietary supplements, the relation between retinol and vitamin A and FN BMD change was no longer significant Lumbar spine BMD Of dietary factors, only alcohol intake was significantly associated with lumbar spine BMD: 0.0893 (0.034, 0.145) p = 0.002 |
||||||
|
Chan et al. (2011) China PC 4 years Funding: Public |
N = 4000 Population sampled: Elderly (≥ 65 years) living in the community Exclusion criteria: Detectable disease or medication known to affect bone mass; incomplete dietary data; extreme energy intake |
Total hip and femoral neck BMD: measured by DEXA |
Total Vitamin A intake (food only) at baseline, μg RE/day Median (IQR) Males: 940 (667–1315) Females: 939 (676–1277) |
NA |
Nutrient intakes were adjusted for dietary energy intake by the residual method Model 1: Adjusted for age, baseline weight, baseline height, % change in body weight, education, current drinker, current smoker, use of calcium supplements, physical activity, total energy intake |
Change (%) in BMD over the 4‐year follow‐up per IU/day vitamin A increase (β) Males Total Hip: Univariate: −0.653, p = 0.116 Model 1: −0.433, p = 0.259 Model 2: 0.035, p = 0.932 Femoral neck: Univariate: −0.316, p = 0.618 Model 1: 0.068%, p = 0.914 |
|
Lost to follow up: 25% n = 2217 Sex: 45% female Age, years: Male: 71.6 ± 4.6 Female: 72.0 ± 5.1 Other characteristics BMI, kg/m2: Male: 23.5 ± 3.1 Female: 24.0 ± 3.5 Height, cm: Male: 163.2 ± 5.6 Female: 151.2 ± 5.3 Physical activity score, PASE: Male: 101.7 ± 51.3 Female: 87.6 ± 33.9 Baseline BMD, g/cm2: Hip: Males: 0.875 ± 0.122 Females: 0.725 ± 0.114 Femoral neck: Males: 0.696 ± 0.106 Females: 0.594 ± 0.098 Current smoker, % Males: 11.9 Females: 1.8 Current drinker, % Males: 25.2 Females: 2.9 Vitamin D intake (IU/day), median (IQR): Male: 8.2 (2.9–16.6) [0.2 (0.07–0.42 μg] Female: 7.2 (3.1–15.0) [0.2 (0.08–0.38) μg] Calcium intake (mg/day) Male: 638.8 ± 294.4 Female: 571.8 ± 260.0 |
Exposure assessment: Semi‐quantitative FFQ including 13 food groups at baseline, validated with basal metabolic rate calculation and 24‐h sodium/creatinine and potassium/creatinine Conversion factors for carotenoids not reported |
Model 2: As model 1 + energy‐adjusted calcium and vitamin D intake |
Model 2: 0.742%, p = 0.274 Females No associations (data not shown) |
|||
|
Houtkooper et al. (1995) USA PC 12–18 months Funding: Mixed |
N = 66 Population sampled: Pre‐menopausal women who participated in a RCT on resistance exercise training (and were taking 500 mg of calcium supplements per day). 27 participated in resistance training, rest were sedentary Exclusion criteria: Pregnancy, lactation, < 10 normal menses in previous year, contraceptive use, medication affecting bone metabolism for two years prior to study, smoking > 10 cigarettes per day, regular exercise > 6 months during the 5 years preceding enrolment, weight fluctuations > 3 kg the previous year excluding pregnancy, had intentions of changing weight status by this magnitude within the next year, BMI < 5th or >95th percentile (NHANES 1976–80), history of anorexia, bulimia, cancer, diabetes, thyroid disease or myocardial infarction % lost to follow up: 15.2% for 18 month visit n = 66 at 12 months, 56 at 18 months |
Total body, lumbar spine, femoral neck, trochanter and Ward's triangle BMD: DXA at four timepoints: Baseline and at 5, 12 and 18 months |
Preformed vitamin A intake (food only), μg RE/day Mean ± SD 1220 ± 472 Exposure assessment: Repeated 4‐day records before 5–12‐ and 18‐months testing periods |
NA |
Total body BMD: Adjusted for exercise group in original study, baseline fat mass, fat mass annual rate of change and baseline total body BMD |
Total body BMD annual rate of change (β, no CI reported) 0.007, p = 0.002 Vitamin A was only included in the model assessing total BMD. Vitamin A was not a significant variables in regression models predicting bone mineral density slopes (rates of change) at any femur site or at the lumbar spine |
|
Sex: Females Age, years: 34.4 ± 2.7 Other characteristics: Height, cm: 165.4 ± 6.4 Weight, kg: 59.9 ± 9.8 BMD, g/cm 3 Total body: 1.15 ± 0.08 Lumbar spine: 1.22 ± 0.13 Femoral neck: 0.96 ± 0.13 Trochanter: 0.76 ± 0.10 Ward's triangle: 0.89 ± 0.15 Total calcium intake (diet + supplement), mg/day: 1326 ± 232 Vitamin D: NR |
||||||
|
Sugiura et al. (2016) Mikkabi Cohort Study Japan PC 4 years Funding: Public |
N = 457 Population sampled: post‐menopausal women with T‐scores > 70% at baseline Exclusion criteria: those with T‐scores < 70% % lost to follow up: NR n = 187 Sex: females Age, years: 59–61 (mean range across tertiles) Other characteristics: BMD (g/cm2) Mean (SD) T1: 0.568 (0.068) T2: 0.586 (0.077) T3: 0.562 (0.075) T‐score (%) Mean (SD) T1: 88.0 (10.5) T2: 90.8 (11.9) T3: 87.0 11.6) |
Osteroporosis was defined as T‐score less than 70%. Radial BMD at baseline and follow‐up survey using DXA |
Tertiles of preformed vitamin A intake (from food only), μg RE/day Means (Range) T1(ref): 138 (29–199) T2: 265 (200–349) T3: 538 (351–2320) n per tertile T1: 62 T2: 62 T3: 63 Exposure assessment: Semi‐quantitative FFQ at baseline |
Cases of osteoporosis n, (%) T1: 6 (9.7) T2: 3 (4.8) T3: 8 (12.7) |
Model 1: Age, weight, height, years since menopause, current tobacco use, regular alcohol intake, exercise habits, supplement use, and total energy intake Model 2: Model 1 + intakes of calcium, magnesium, potassium, and vitamin D |
OR (95% CI) for osteoporosis per tertile of preformed vitamin A Model 1: T1(ref): 1 T2: 0.57 (0.12, 2.78) T3: 1.49 (0.36, 6.22) Model 2: T1(ref): 1 T2: 0.34 (0.06, 1.84) T3: 0.91 (0.19, 4.29) |
Abbreviations: ADL, activities in daily living; BMD, bone mineral density; BMI, Body mass index; CC, case‐cohort; DXA, dual X‐ray absorptiometry; FEV, forced expiratory volume; FFQ, food frequency questionnaire; HRT, hormone replacement therapy; IU, international units; NA, not available; NR, Not reported; NCC, nested case–control; PC, prospective cohort; RAE, retinol activity equivalents; RE, retinol equivalents.
Note: Unless otherwise reported, values are mean ± SD. Preformed vitamin A refers to retinol intake, total vitamin A refers to retinol and β‐carotene intakes.
n = 2032 in the full cohort for which baseline covariates are presented, n = 1221 for dietary records.
As per study author likely to be preformed vitamin A.
APPENDIX C. Outcome of the appraisal for the risk of bias of included studies
C.1. RoB appraisal for SR3a on teratogenicity
| References | Outcomes | Risk of bias domains a | Tier b | |||||
|---|---|---|---|---|---|---|---|---|
| KEY – detection bias ‐ exposure | KEY – detection bias ‐ outcome | KEY – confounding bias | Selection bias | Attrition/exclusion bias | Other sources of bias | |||
| Case–control studies | ||||||||
| Johansen et al. ( 2008 ) | Cleft lip with or without cleft palate or cleft palate only | + | ++ | + | ++ | ++ | + | 1 |
| Botto et al. ( 2001 ) | Transposition of the great arteries, double‐outlet right ventricle, tetralogy of Fallot, truncus arteriosus, and supracristal ventricular septal defect | + | ++ | + | ++ | ++ | + | 1 |
| Shaw et al. ( 1997 ) | Neural tube defects | + | + | –– | + | + | + | 2 |
| Mills et al. ( 1997 ) | Neural tube defects | – | ++ | –– | – | ++ | + | 2 |
| Martínez‐Frías and Salvador ( 1990 ) | Any birth defect | – | + | –– | + | + | + | 2 |
| Prospective studies | ||||||||
| Michikawa et al. ( 2019 ) | Congenital diaphragmatic hernia | – | ++ | + | ++ | + | + | 2 |
| Mastroiacovo et al. ( 1999 ) | Major malformations | NR | – | –– | – | – | – | 3 |
| Rothman et al. ( 1995 ) | Any birth defect, cranial neural‐crest defects, neural tube defects, musculoskeletal or urogenital defects or other defects | ++ | + | ++ | + | ++ | + | 1 |
| Bille et al. ( 2007 ) | Cleft lip with or without cleft palate or cleft palate only | – | + | + | + | + | + | 2 |
Expert judgement was translated into a rating scale for each question to be answered as follows: (++): definitely low RoB; (+): probably low RoB; (NR): not reported; (−): probably high RoB; (− −): definitively high RoB.
The individual rating for each question was combined by an algorithm and translated to an overall tier of reliability for each individual study (RoB tier 1: low RoB; RoB tier 2: moderate RoB; RoB tier 3: high RoB).
C.2. RoB appraisal of RCTs for sQ4a on hepatoxicity
| References | Outcomes | Risk of bias domains a | Tier b | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Exposure KEY | Outcome KEY | Randomisation KEY | Allocation concealment | Blinding | Attrition | Reporting | Other threats to internal validity | |||
| Farhangi et al. ( 2013 ) | ASAT, ALAT | NR | + | + | NR | ++ | + | NR | + | 2 |
| Dougherty et al. ( 2012 ) | gGT | + | + | + | NR | ++ | – | – | + | 2 |
| Alberts et al. ( 2004 ) | Toxicity (based on ASAT, ALAT, and ALP) | NR | + | + | NR | NR | ++ | + | + | 2 |
| Bitarafan et al. ( 2015 ) | ASAT, ALAT | + | + | + | NR | ++ | + | NR | ++ | 1 |
Expert judgement was translated into a rating scale for each question to be answered as follows: (++): definitely low RoB; (+): probably low RoB; (NR): not reported; (−): probably high RoB; (− −): definitively high RoB.
The individual rating for each question was combined by an algorithm and translated to an overall tier of reliability for each individual study (RoB tier 1: low RoB; RoB tier 2: moderate RoB; RoB tier 3: high RoB).
C.3. RoB appraisal of prospective studies for sQ5a on bone fractures
| References | Risk of bias domains a | Tier b | |||||
|---|---|---|---|---|---|---|---|
| Exposure KEY | Outcome KEY | Confounding KEY | Inappropriate selection | Attrition | Other sources of bias/statistics | ||
| Melhus et al. ( 1998 ) | + | ++ | – – | ++ | ++ | + | 2 |
| Feskanich et al. ( 2002 ) | ++ | ++ | + | ++ | ++ | + | 1 |
| Michaëlsson et al. ( 2003 ) | NR | + | NR | –– | ++ | + | 2 |
| Lim et al. ( 2004 ) | + | – | + | + | – | + | 2 |
| White et al. ( 2006 ) | – | + | – | + | – | –– | 3 |
| Key et al. ( 2007 ) | + | – | + | + | –– | + | 2 |
| Caire‐Juvera et al. ( 2009 ) | ++ | + | ++ | ++ | ++ | + | 1 |
| Rejnmark et al. ( 2004 ) | + | ++ | ++ | ++ | NR | + | 1 |
| Barker et al. ( 2005 ) | + | ++ | – | NR | + | – | 2 |
| de Jonge et al. ( 2015 ) | + | ++ | + | ++ | ++ | + | 1 |
| Toraishi et al. ( 2021 ) | + | – | – – | – | ++ | – | 3 |
| Hayhoe et al. ( 2017 ) | + | + | + | ++ | ++ | ++ | 1 |
Expert judgement was translated into a rating scale for each question to be answered as follows: (++): definitely low RoB; (+): probably low RoB; (NR): not reported; (−): probably high RoB; (− −): definitively high RoB.
The individual rating for each question was combined by an algorithm and translated to an overall tier of reliability for each individual study (RoB tier 1: low RoB; RoB tier 2: moderate RoB; RoB tier 3: high RoB).
C.4. RoB appraisal of prospective studies for sQ5b on BMD
| References | Risk of bias domains a | Tier b | |||||
|---|---|---|---|---|---|---|---|
| Exposure KEY | Outcome KEY | Confounding KEY | Inappropriate selection | Attrition | Other sources of bias/statistics | ||
| Houtkooper et al. ( 1995 ) | ++ | ++ | + | + | NR | + | 1 |
| Chan et al. ( 2011 ) | NR | + | + | NR | – – | – | 2 |
| de Jonge et al. ( 2015 ) | + | NR | ++ | ++ | – | + | 2 |
| Kaptoge et al. ( 2003 ) | + | + | + | ++ | + | + | 1 |
| Macdonald et al. ( 2004 ) | ++ | ++ | + | ++ | + | – | 1 |
| Promislow et al. ( 2002 ) | + | + | + | + | – – | + | 1 |
| Rejnmark et al. ( 2004 ) | + | ++ | ++ | ++ | + | + | 1 |
| Sugiura et al. ( 2016 ) | – | ++ | + | NR | NR | – | 2 |
Expert judgement was translated into a rating scale for each question to be answered as follows: (++): definitely low RoB; (+): probably low RoB; (NR): not reported; (−): probably high RoB; (− −): definitively high RoB.
The individual rating for each question was combined by an algorithm and translated to an overall tier of reliability for each individual study (RoB tier 1: low RoB; RoB tier 2: moderate RoB; RoB tier 3: high RoB).
APPENDIX D. National provisions on the mandatory and voluntary addition of vitamin A to foods and national nutritional guidelines/recommendations for supplementing the diet with vitamin A
D.1. Data as provided by the European Commission, unpublished
TABLE D.1 National provisions on the mandatory addition of vitamin A to food.
| Country | Food | Amount |
|---|---|---|
| Sweden | Margarine and fat blends. Also applies to liquid products and products with other fat content which otherwise correspond to margarine or fat blends. Certain exceptions apply | 900–1500 μg retinol equivalent (RE a ) (of which maximum 400 μg of beta carotene) per 100 g |
| Belgium | Margarine, low‐fat margarine, and fats for baking | 25–30 International Units (IU)a per gram |
| Poland | Margarine with normal and reduced contents of fat, mixture of butter and oil | ≤ 900 μg/100 g |
1 mg retinol equivalent (RE) = 1 mg retinol = 1.15 mg all‐trans‐retinyl acetate = 1.83 mg all‐trans‐retinyl palmitate = 3333.33 International Units (IU).
TABLE D.2 National covenants.
| Country | Food | Amount |
|---|---|---|
| Netherlands | Margarines and other spreadable fats, and baking fat products (excl. oils and 100% fat) | 6–8 μg/g (retinoid form) |
TABLE D.3 National voluntary vitamin A fortification policies.
| Country | Food | Amount |
|---|---|---|
| Germany | Margarine and margarine‐like spreads | ≤ 10 mg/kg |
| Norway | Margarine, cooking may be lowers and other fats for eating | 900 μg/100 g |
| Netherlands | Fats, liquid products with the same purpose, baking and frying products | Max 8 μg retinol equivalents (RE)a/g |
1 mg retinol equivalent (RE) = 1 mg retinol = 1.15 mg all‐trans‐retinyl acetate = 1.83 mg all‐trans‐retinyl palmitate = 3333.33 International Units (IU)
D.2. Additional data identified during the public consultation
TABLE D.4 National voluntary vitamin A fortification policies.
| Country | Food | Amount |
|---|---|---|
| Greece | Margarines and spreadable fats | 7500 μg/kg (can be a mixture of vitamin A and carotene) |
APPENDIX E. Background Diet Intake Plots for EU Populations
E.1. Preformed vitamin A
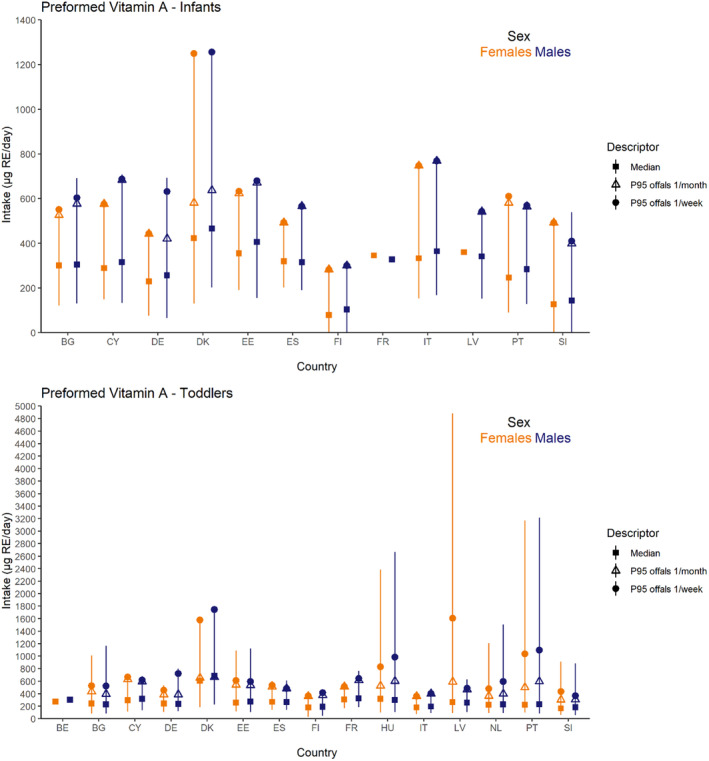
FIGURE E.1 Background diet intake estimates for preformed vitamin A, including offal consumption scenarios, for infants and toddlers, by sex and country. Estimates for females in orange and for males in blue. Squares correspond to medians, and triangles and circles to P95 offal consumption scenarios of once per month and once per week, respectively. Lines represent the range between the 5th and 95th percentiles. Estimated intakes from 5th and 95th percentiles are not presented when sample size is below 60 participants. Countries for which more than one survey was available, estimates presented in the plot are those of the most recent survey; when surveys covered the same period those with the highest number of participants are displayed. BE, Belgium; BG, Bulgaria; CY, Cyprus; DE, Germany; DK, Denmark; EE, Estonia; ES, Spain; FI, Finland; FR, France; HU, Hungary; IT, Italy; LV, Latvia; NL, the Netherlands; PT, Portugal; SI, Slovenia.
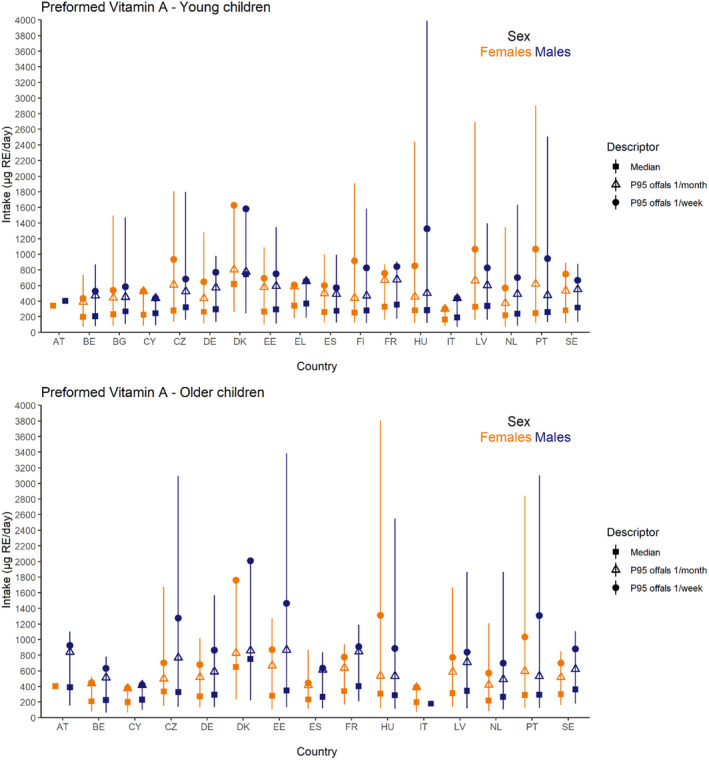
FIGURE E.2 Background diet intake estimates for preformed vitamin A, including offal consumption scenarios, for young and older children, by sex and country. Estimates for females in orange and for males in blue. Squares correspond to medians, and triangles and circles to P95 offal consumption scenarios of once per month and once per week, respectively. Lines represent the range between the 5th and 95th percentiles. Estimated intakes from 5th and 95th percentiles are not presented when sample size is below 60 participants. Countries for which more than one survey was available, estimates presented in the plot are those of the most recent survey; when surveys covered the same period those with the highest number of participants are displayed. AT, Austria; BE, Belgium; BG, Bulgaria; CY, Cyprus; CZ, Czech Republic; DE, Germany; DK, Denmark; EE, Estonia; EL, Greece; ES, Spain; FI, Finland; FR, France; HU, Hungary; IT, Italy; LV, Latvia; NL, the Netherlands; PT, Portugal; SE, Sweden.
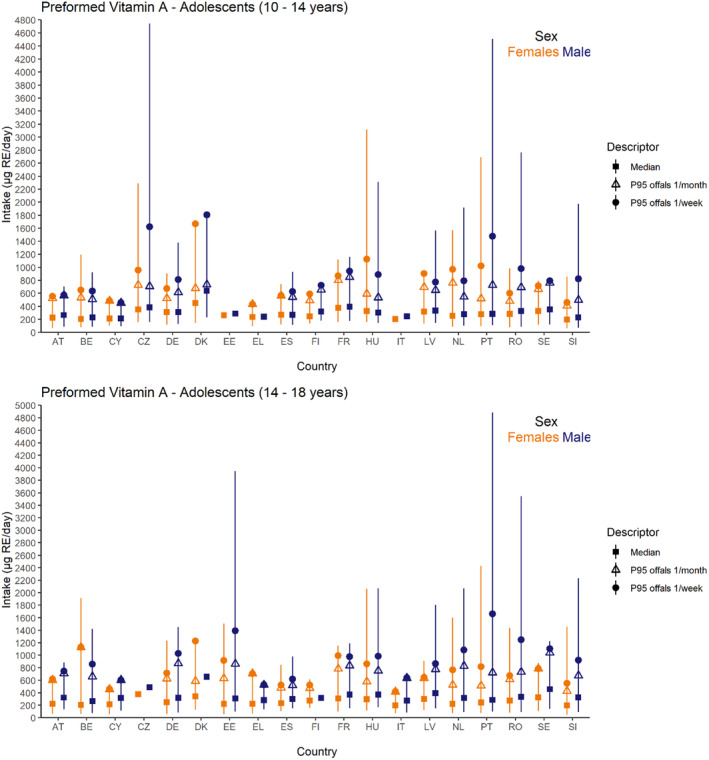
FIGURE E.3 Background diet intake estimates for preformed vitamin A, including offal consumption scenarios, for young and older adolescents, by sex and country. Estimates for females in orange and for males in blue. Squares correspond to medians, and triangles and circles to P95 offal consumption scenarios of once per month and once per week, respectively. Lines represent the range between the 5th and 95th percentiles. Estimated intakes from 5th and 95th percentiles are not presented when sample size is below 60 participants. Countries for which more than one survey was available, estimates presented in the plot are those of the most recent survey; when surveys covered the same period those with the highest number of participants are displayed. AT, Austria; BE, Belgium; CY, Cyprus; CZ, Czech Republic; DE, Germany; DK, Denmark; EE, Estonia; EL, Greece; ES, Spain; FI, Finland; FR, France; HU, Hungary; IT, Italy; LV, Latvia; NL, the Netherlands; PT, Portugal; RO, Romania; SE, Sweden; SI, Slovenia.
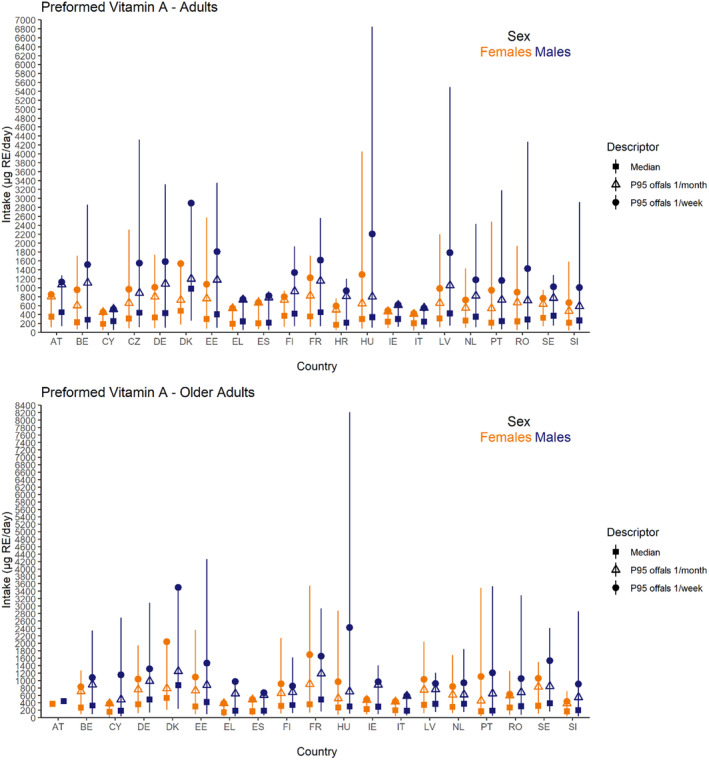
FIGURE E.4 Background diet intake estimates for preformed vitamin A, including offal consumption scenarios, for adults and older adults, by sex and country. Estimates for females in orange and for males in blue. Squares correspond to medians, and triangles and circles to P95 offal consumption scenarios of once per month and once per week, respectively. Lines represent the range between the 5th and 95th percentiles. Estimated intakes from 5th and 95th percentiles are not presented when sample size is below 60 participants. Countries for which more than one survey was available, estimates presented in the plot are those of the most recent survey; when surveys covered the same period those with the highest number of participants are displayed. AT, Austria; BE, Belgium; CY, Cyprus; CZ, Czech Republic; DE, Germany; DK, Denmark; EE, Estonia; EL, Greece; ES, Spain; FI, Finland; FR, France; HU, Hungary; IE, Ireland; IT, Italy; LV, Latvia; NL, the Netherlands; PT, Portugal; RO, Romania; SE, Sweden; SI, Slovenia.
E.2. β‐Carotene
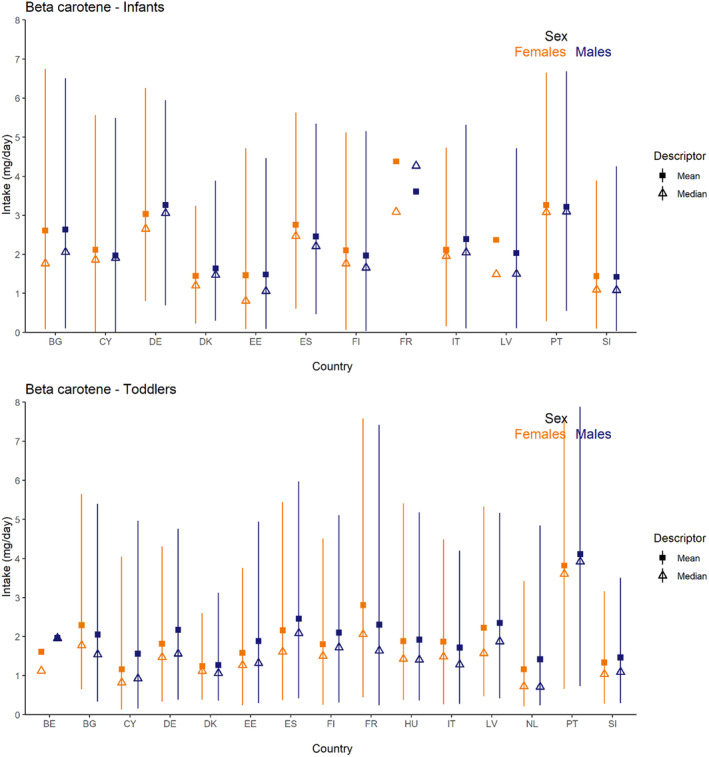
FIGURE E.5 Background diet intake estimates for β‐carotene (mg/day) for infants and toddlers, by sex and country. Estimates for females in orange and for males in blue. Squares correspond to means and triangles to medians. Lines represent the range between the 5th and 95th percentiles. Estimated intakes from 5th and 95th percentiles are not presented when sample size is below 60 participants. Countries for which more than one survey was available, estimates presented in the plot are those of the most recent survey; when surveys covered the same period those with the highest number of participants are displayed. BE, Belgium; BG, Bulgaria; CY, Cyprus; DE, Germany; DK, Denmark; EE, Estonia; ES, Spain; FI, Finland; FR, France; HU, Hungary; IT, Italy; LV, Latvia; NL, the Netherlands; PT, Portugal; SI, Slovenia.
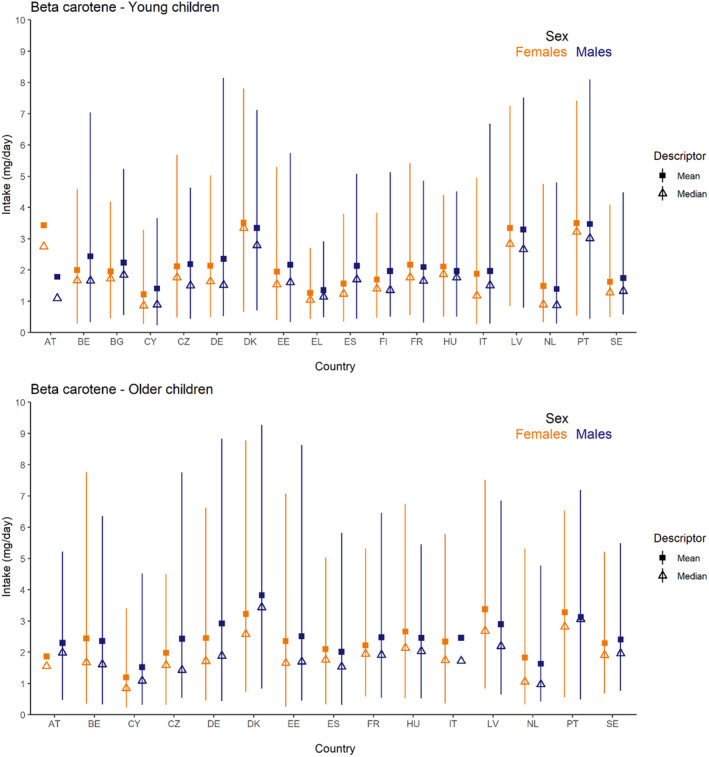
FIGURE E.6 Background diet intake estimates for β‐carotene (mg/day) for younger and older children, by sex and country. Estimates for females in orange and for males in blue. Squares correspond to means and triangles to medians. Lines represent the range between the 5th and 95th percentiles. Estimated intakes from 5th and 95th percentiles are not presented when sample size is below 60 participants. Countries for which more than one survey was available, estimates presented in the plot are those of the most recent survey; when surveys covered the same period those with the highest number of participants are displayed. AT, Austria; BE, Belgium; BG, Bulgaria; CY, Cyprus; CZ, Czech Republic; DE, Germany; DK, Denmark; EE, Estonia; EL, Greece; ES, Spain; FI, Finland; FR, France; HU, Hungary; IE, Ireland; IT, Italy; LV, Latvia; NL, the Netherlands; PT, Portugal; RO, Romania; SE, Sweden; SI, Slovenia.
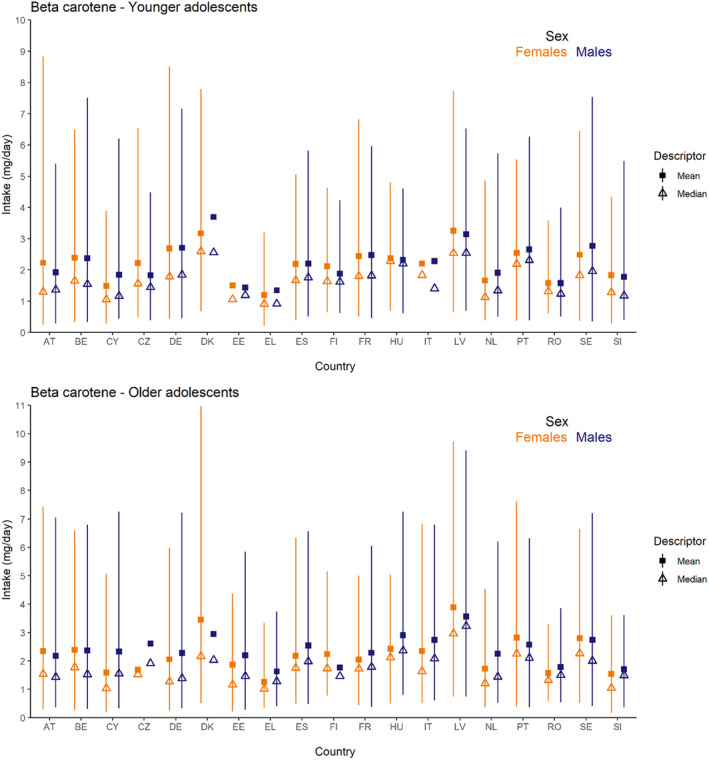
FIGURE E.7 Background diet intake estimates for β‐carotene (mg/day) for young and older adolescents, by sex and country. Estimates for females in orange and for males in blue. Squares correspond to means and triangles to medians. Lines represent the range between the 5th and 95th percentiles. Estimated intakes from 5th and 95th percentiles are not presented when sample size is below 60 participants. Countries for which more than one survey was available, estimates presented in the plot are those of the most recent survey; when surveys covered the same period those with the highest number of participants are displayed. AT, Austria; BE, Belgium; CY, Cyprus; CZ, Czech Republic; DE, Germany; DK, Denmark; EE, Estonia; EL, Greece; ES, Spain; FI, Finland; FR, France; HU, Hungary; IE, Ireland; IT, Italy; LV, Latvia; NL, the Netherlands; PT, Portugal; RO, Romania; SE, Sweden; SI, Slovenia.
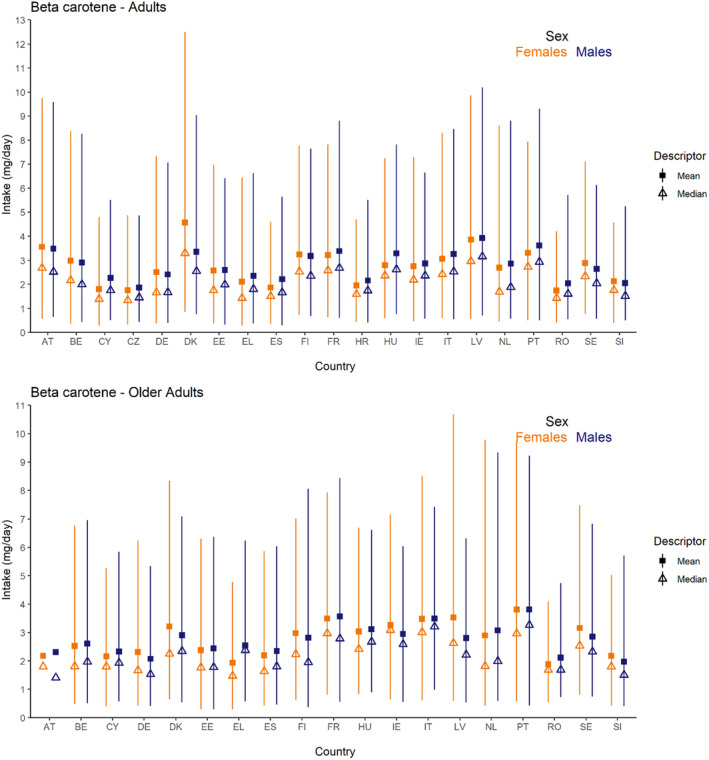
FIGURE E.8 Background diet intake estimates for β‐carotene (mg/day) for adults and older adults, by sex and country. Estimates for females in orange and for males in blue. Squares correspond to means and triangles to medians. Lines represent the range between the 5th and 95th percentiles. Estimated intakes from 5th and 95th percentiles are not presented when sample size is below 60 participants. Countries for which more than one survey was available, estimates presented in the plot are those of the most recent survey; when surveys covered the same period those with the highest number of participants are displayed. AT, Austria; BE, Belgium; CY, Cyprus; CZ, Czech Republic; DE, Germany; DK, Denmark; EE, Estonia; EL, Greece; ES, Spain; FI, Finland; FR, France; HU, Hungary; IE, Ireland; IT, Italy; LV, Latvia; NL, the Netherlands; PT, Portugal; RO, Romania; SE, Sweden; SI, Slovenia.
List of Annexes
Annex A – Protocol for the Scientific Opinion on Tolerable Upper Intake Level for preformed vitamin A and β‐carotene
Annex B – Methodological considerations in the calculation of intake estimates for preformed vitamin A and β‐carotene from the background diet in EU countries
Annex C – EFSA's intake assessment for preformed vitamin A
Annex D – EFSA's intake assessment for β‐carotene
Annex E – Vitamin A intake data from Competent Authorities in European countries
Annex F – ROB appraisal of studies on bone health
Annex G – Additional information requested from study authors
Annex H – Studies excluded at full‐text screening and during data extraction
Annex I – Outcome of the public consultation
Annexes A–I can be found in the online version of this output (in the ‘Supporting information’ section)
EFSA NDA Panel (EFSA Panel on Nutrition, Novel Foods and Food Allergens) , Turck, D. , Bohn, T. , Castenmiller, J. , de Henauw, S. , Hirsch‐Ernst, K.‐I. , Knutsen, H. K. , Maciuk, A. , Mangelsdorf, I. , McArdle, H. J. , Pentieva, K. , Siani, A. , Thies, F. , Tsabouri, S. , Vinceti, M. , Lietz, G. , Passeri, G. , Craciun, I. , Fabiani, L. , … Naska, A. (2024). Scientific opinion on the tolerable upper intake level for preformed vitamin A and β‐carotene. EFSA Journal, 22(6), e8814. 10.2903/j.efsa.2024.8814
Adopted: 30 April 2024
Notes
SCF (2000a, 2000b). Scientific Committee on Food. Guidelines of the Scientific Committee on Food for the Development of Tolerable Upper Intake Levels for Vitamins and Minerals. in: Scientific Committee on Food, Scientific Panel on Dietetic Products, Nutrition and Allergies (2006).
The expert panel was composed of William Blaner (Columbia University, USA), Georg Lietz (Newcastle University, UK), Sherry Tanumihardjo (University of Wisconsin‐Madison, USA) and Johannes von Lintig (Case Western Reserve University, USA).
In the nutrient composition database.
The Mintel GNPD contains information on over three million food and beverage products, of which more than one million are or have been available on the European food market. Twenty‐five out of the 27 EU Member States and Norway are present in the database. The database provides the compulsory ingredient information reported on product labels and the nutrition declaration when available. http://www.mintel.com/globalnew‐products‐database
Working Group consisting of representatives of 27 EU Member States and Norway.
Regulation (EC) No 1333/2008 of the European Parliament and of the Council of 16 December 2008 on food additives. OJ L 354 31.12.2008, p. 16 and Commission Regulation (EU) No 231/2012 of 9 March 2012 laying down specifications for food additives listed in Annexes II and III to Regulation (EC) No 1333/2008 of the European Parliament and of the Council. OJ L 083 22.3.2012, p. 1.
Commission Directive 96/8/EC of 26 February 1996 on foods intended for use in energy‐restricted diets for weight reduction. OJ L 055 6.3.1996, p. 22.
Commission Delegated Regulation (EU) 2017/1522 of 2 June 2017 supplementing Regulation (EU) No 609/2013 of the European Parliament and of the Council as regards the specific compositional and information requirements for total diet replacement for weight control. C/2017/3664. OJ L 230, 6.9.2017, p. 1–9.
Commission Delegated Regulation (EU) 2016/127 of 25 September 2015 supplementing Regulation (EU) No 609/2013 of the European Parliament and of the Council as regards the specific compositional and information requirements for infant formula and follow‐on formula and as regards requirements on information relating to infant and young child feeding. OJ L 25, 2.2.2016, p. 1–29.
According to Annex XIII of Regulation (EU) No 1169/2011, the generic term vitamin A is used to designate the vitamin on nutrition declarations, irrespective of the actual form added to foods or food supplements. The value declared can be expressed as % of the NRV of 800 μg RE. Regulation (EU) No 1169/2011 of the European Parliament and of the Council of 25 October 2011 on the provision of food information to consumers, amending Regulations (EC) No 1924/2006 and (EC) No 1925/2006 of the European Parliament and of the Council, and repealing Commission Directive 87/250/EEC, Council Directive 90/496/EEC, Commission Directive 1999/10/EC, Directive 2000/13/EC of the European Parliament and of the Council, Commission Directives 2002/67/EC and 2008/5/EC and Commission Regulation (EC) No 608/2004 Text with EEA relevance. OJ L 304, 22.11.2011, p. 18–63.
Regulation (EC) No 1925/2006 of the European Parliament and of the Council of 20 December 2006 on the addition of vitamins and minerals and of certain other substances to foods. OJ L 404, 30.12.2006, p. 26–38.
Directive 2002/46/EC of the European Parliament and of the Council of 10 June 2002 on the approximation of the laws of the Member States relating to food supplements. OJ L 183, 12.7.2002, p. 51–57.
Regulation (EU) No 609/2013 of the European Parliament and of the Council of 12 June 2013 on food intended for infants and young children, food for special medical purposes, and total diet replacement for weight control and repealing Council Directive 92/52/EEC, Commission Directives 96/8/EC, 1999/21/EC, 2006/125/EC and 2006/141/EC, Directive 2009/39/EC of the European Parliament and of the Council and Commission Regulations (EC) No 41/2009 and (EC) No 953/2009. OJ L 181, 29.6.2013, p. 35–56.
Baby formulae and growing up milks (n = 876, median = 60 μg/100g), baby cereals, biscuits and rusks (n = 258, median = 378 μg/100 g), and baby fruit products, yogurts or desserts and juices (n = 19, median = 127 μg/100 g).
The Mintel GNPD provides data on the content of supplements per serving which may not always reflect the daily dose recommended by the manufacturer.
Regulation (EC) No 1333/2008 of the European Parliament and of the Council of 16 December 2008 on food additives. OJ L 354, 31.12.2008, p. 16 and Commission Regulation (EU) No 231/2012 of 9 March 2012 laying down specifications for food additives listed in Annexes II and III to Regulation (EC) No 1333/2008 of the European Parliament and of the Council. OJ L 083, 22.3.2012, p. 1.
According to Annex XIII of Regulation (EU) No 1169/2011, the generic term vitamin A is used to designate the vitamin on nutrition declarations, irrespective of the actual form added to foods or food supplements. The value declared can be expressed as % of the NRV of 800 μg RE. Regulation (EU) No 1169/2011 of the European Parliament and of the Council of 25 October 2011 on the provision of food information to consumers, amending Regulations (EC) No 1924/2006 and (EC) No 1925/2006 of the European Parliament and of the Council, and repealing Commission Directive 87/250/EEC, Council Directive 90/496/EEC, Commission Directive 1999/10/EC, Directive 2000/13/EC of the European Parliament and of the Council, Commission Directives 2002/67/EC and 2008/5/EC and Commission Regulation (EC) No 608/2004 Text with EEA relevance. OJ L 304, 22.11.2011, p. 18–63.
The Mintel GNPD provides data on the content of supplements per serving which may not always reflect the daily dose recommended by the manufacturer.
Models were adjusted for physical activity in leisure time, body mass index, cigarette smoking, use of hormone replacement therapy or oral contraceptives, diabetes, former athletic activity, any use of cortisone, menopausal status and menopausal age, previous osteoporotic fractures, and energy intake.
Models were adjusted for age, follow‐up cycle, BMI, use of postmenopausal hormones, smoking, hours of leisure‐time activity/week, use of thiazide diuretics, and energy‐adjusted intakes of calcium, protein, vitamin D, vitamin K, alcohol and caffeine.
Adjusted for age, energy, calcium, vitamin D, vitamin K, protein, alcohol and caffeine intakes, smoking, BMI, HT use, physical activity, ethnic group and region.
Adjusted for age, BMI, diabetes mellitus, cirrhosis, past irregular menstrual duration, thyrotropic medication, sedative medication, steroid medication, antiepileptic medication, diuretic medication, education, alcohol use and energy intake.
Adjusted for BMI, calcium intake; years menopausal (women only), diabetes status, current exercise, current use of oestrogen (women only), steroids, cigarettes, alcohol, thiazides, thyroid hormones and supplemental retinol. Change in BMD (%) models additionally include weight change.
Adjusted for age, weight, annual percentage change in weight, height, smoking status, socioeconomic status, physical activity level, baseline BMD measurement at appropriate site, menopausal status and hormone replacement therapy use.
Using a factor of 0.01863.
ULadult × (weightinfant 4‐6mo/weightadult) = 3000 × (7.2/70) = 545.
ULadult × (weightinfant 7‐11mo/weightadult) = 3000 × (8.6/70) = 623.
REFERENCES
- Abu‐Hijleh, G. , & Padmanabhan, R. (1997). Retinoic acid‐induced abnormal development of hindlimb joints in the mouse. European Journal of Morphology, 35, 327–336. 10.1076/ejom.35.5.327.13088 [DOI] [PubMed] [Google Scholar]
- Aburto, A. , & Britton, W. M. (1998). Effects of different levels of vitamins a and E on the utilization of cholecalciferol by broiler chickens. Poultry Science, 77, 570–577. 10.1093/ps/77.4.570 [DOI] [PubMed] [Google Scholar]
- Aklamati, E. K. , Mulenga, M. , Dueker, S. R. , Buchholz, B. A. , Peerson, J. M. , Kafwembe, E. , Brown, K. H. , & Haskell, M. J. (2010). Accelerator mass spectrometry can be used to assess vitamin A metabolism quantitatively in boys in a community setting. The Journal of Nutrition, 140, 1588–1594. 10.3945/jn.110.125500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Tanoury, Z. , Piskunov, A. , & Rochette‐Egly, C. (2013). Vitamin a and retinoid signaling: Genomic and nongenomic effects. Journal of Lipid Research, 54, 1761–1775. 10.1194/jlr.R030833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberts, D. , Ranger‐Moore, J. , Einspahr, J. , Saboda, K. , Bozzo, P. , Liu, Y. , Xu, X. C. , Lotan, R. , Warneke, J. , Salasche, S. , Stratton, S. , Levine, N. , Goldman, R. , Islas, M. , Duckett, L. , Thompson, D. , Bartels, P. , & Foote, J. (2004). Safety and efficacy of dose‐intensive oral vitamin A in subjects with sun‐damaged skin. Clinical Cancer Research, 10, 1875–1880. 10.1158/1078-0432.ccr-03-0188 [DOI] [PubMed] [Google Scholar]
- ATBC Study Group . (1994). The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. The New England Journal of Medicine, 330, 1029–1035. 10.1056/NEJM199404143301501 [DOI] [PubMed] [Google Scholar]
- Ballew, C. , Bowman, B. A. , Russell, R. M. , Sowell, A. L. , & Gillespie, C. (2001). Serum retinyl esters are not associated with biochemical markers of liver dysfunction in adult participants in the third National Health and nutrition examination survey (NHANES III), 1988–1994. American Journal of Clinical Nutrition, 73, 934–940. [DOI] [PubMed] [Google Scholar]
- Balmer, J. E. , & Blomhoff, R. (2002). Gene expression regulation by retinoic acid. Journal of Lipid Research, 43, 1773–1808. 10.1194/jlr.r100015-jlr200 [DOI] [PubMed] [Google Scholar]
- Barker, M. E. , McCloskey, E. , Saha, S. , Gossiel, F. , Charlesworth, D. , Powers, H. J. , & Blumsohn, A. (2005). Serum retinoids and beta‐carotene as predictors of hip and other fractures in elderly women. Journal of Bone and Mineral Research, 20, 913–920. 10.1359/jbmr.050112 [DOI] [PubMed] [Google Scholar]
- Barnes, B. C. , Wollaeger, E. E. , & Mason, H. L. (1950). The comparative absorption of vitamin A from a water‐miscible and an oily preparation by normal human adults and patients with steatorrhea. The Journal of Clinical Investigation, 29, 982–987. 10.1172/jci102345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barua, A. B. , & Sidell, N. (2004). Retinoyl beta‐glucuronide: A biologically active interesting retinoid. The Journal of Nutrition, 134, 286s–289s. 10.1093/jn/134.1.286S [DOI] [PubMed] [Google Scholar]
- Bastos Maia, S. , Rolland Souza, A. S. , Costa Caminha, M. F. , Lins da Silva, S. , Callou Cruz, R. , Carvalho Dos Santos, C. , & Batista Filho, M. (2019). Vitamin a and pregnancy: A narrative review. Nutrients, 11, 681. 10.3390/nu11030681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beems, R. B. (1987). The effect of beta‐carotene on BP‐induced respiratory tract tumors in hamsters. Nutrition and Cancer, 10, 197–204. 10.1080/01635588709513957 [DOI] [PubMed] [Google Scholar]
- Berenguer, M. , & Duester, G. (2022). Retinoic acid, RARs and early development. Journal of Molecular Endocrinology, 69, T59–t67. 10.1530/jme-22-0041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurskens, L. W. , Schrijver, L. H. , Tibboel, D. , Wildhagen, M. F. , Knapen, M. F. , Lindemans, J. , de Vries, J. , & Steegers‐Theunissen, R. P. (2013). Dietary vitamin A intake below the recommended daily intake during pregnancy and the risk of congenital diaphragmatic hernia in the offspring. Birth defects research. Part A, Clinical and Molecular Teratology, 97, 60–66. 10.1002/bdra.23093 [DOI] [PubMed] [Google Scholar]
- Bille, C. , Olsen, J. , Vach, W. , Knudsen, V. K. , Olsen, S. F. , Rasmussen, K. , Murray, J. C. , Andersen, A. M. , & Christensen, K. (2007). Oral clefts and life style factors–a case‐cohort study based on prospective Danish data. European Journal of Epidemiology, 22, 173–181. 10.1007/s10654-006-9099-5 [DOI] [PubMed] [Google Scholar]
- Binkley, N. , & Krueger, D. (2000). Hypervitaminosis a and bone. Nutrition Reviews, 58, 138–144. 10.1111/j.1753-4887.2000.tb01848.x [DOI] [PubMed] [Google Scholar]
- Bitarafan, S. , Saboor‐Yaraghi, A. , Sahraian, M. A. , Nafissi, S. , Togha, M. , Beladi Moghadam, N. , Roostaei, T. , Siassi, F. , Eshraghian, M. R. , Ghanaati, H. , Jafarirad, S. , Rafiei, B. , & Harirchian, M. H. (2015). Impact of vitamin a supplementation on disease progression in patients with multiple sclerosis. Archives of Iranian Medicine, 18, 435–440. [PubMed] [Google Scholar]
- Bjelakovic, G. , Nikolova, D. , & Gluud, C. (2013). Meta‐regression analyses, meta‐analyses, and trial sequential analyses of the effects of supplementation with Beta‐carotene, vitamin a, and vitamin E singly or in different combinations on all‐cause mortality: Do we have evidence for lack of harm? PLoS One, 8(9), e74558. 10.1371/journal.pone.0074558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomhoff R, Andersen R, Arnesen EK, Christensen JJ, Enorth H, Erkkola M, Gudanaviciene I, Halldorsson TI, Hoyer‐Lund A, Lemming EW, Meltzer HM, Pitsi T, Schwab U, Siksna I, Thorsdottir I and Trolle E (Nordic Council of Ministers ), 2023. Nordic nutrition recommendations 2023: Integrating environmental aspects. [Google Scholar]
- Blomhoff, R. , Green, M. H. , Green, J. B. , Berg, T. , & Norum, K. R. (1991). Vitamin a metabolism: New perspectives on absorption, transport, and storage. Physiological Reviews, 71, 951–990. 10.1152/physrev.1991.71.4.951 [DOI] [PubMed] [Google Scholar]
- Blot, W. J. , Li, J. Y. , Taylor, P. R. , Guo, W. , Dawsey, S. , Wang, G. Q. , Yang, C. S. , Zheng, S. F. , Gail, M. , Li, G. Y. , et al. (1993). Nutrition intervention trials in Linxian, China: Supplementation with specific vitamin/mineral combinations, cancer incidence, and disease‐specific mortality in the general population. Journal of the National Cancer Institute, 85, 1483–1492. 10.1093/jnci/85.18.1483 [DOI] [PubMed] [Google Scholar]
- Böhm, V. , Lietz, G. , Olmedilla‐Alonso, B. , Phelan, D. , Reboul, E. , Bánati, D. , Borel, P. , Corte‐Real, J. , de Lera, A. R. , Desmarchelier, C. , Dulinska‐Litewka, J. , Landrier, J. F. , Milisav, I. , Nolan, J. , Porrini, M. , Riso, P. , Roob, J. M. , Valanou, E. , Wawrzyniak, A. , … Bohn, T. (2021). From carotenoid intake to carotenoid blood and tissue concentrations ‐ implications for dietary intake recommendations. Nutrition Reviews, 79, 544–573. 10.1093/nutrit/nuaa008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohn, T. (2018). Metabolic fate of bioaccessible and non‐bioaccessible carotenoids. In Saura‐Calixto F. & Pérez‐Jiménez J. (Eds.), Non‐extractable Polyphenols and Carotenoids: Importance in Human Nutrition and Health (p. 82). The Royal Society of Chemistry. [Google Scholar]
- Bohn, T. , Desmarchelier, C. , Dragsted, L. O. , Nielsen, C. S. , Stahl, W. , Rühl, R. , Keijer, J. , & Borel, P. (2017). Host‐related factors explaining interindividual variability of carotenoid bioavailability and tissue concentrations in humans. Molecular Nutrition & Food Research, 61(6), 1600685. 10.1002/mnfr.201600685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohn, T. , Desmarchelier, C. , El, S. N. , Keijer, J. , van Schothorst, E. , Rühl, R. , & Borel, P. (2019). β‐Carotene in the human body: Metabolic bioactivation pathways ‐ from digestion to tissue distribution and excretion. The Proceedings of the Nutrition Society, 78, 68–87. 10.1017/s0029665118002641 [DOI] [PubMed] [Google Scholar]
- Botto, L. D. , Loffredo, C. , Scanlon, K. S. , Ferencz, C. , Khoury, M. J. , David Wilson, P. , & Correa, A. (2001). Vitamin a and cardiac outflow tract defects. Epidemiology, 12, 491–496. 10.1097/00001648-200109000-00005 [DOI] [PubMed] [Google Scholar]
- Burri, B. J. , Neidlinger, T. R. , & Clifford, A. J. (2001). Serum carotenoid depletion follows first‐order kinetics in healthy adult women fed naturally low carotenoid diets. The Journal of Nutrition, 131, 2096–2100. 10.1093/jn/131.8.2096 [DOI] [PubMed] [Google Scholar]
- Caire‐Juvera, G. , Ritenbaugh, C. , Wactawski‐Wende, J. , Snetselaar, L. G. , & Chen, Z. (2009). Vitamin a and retinol intakes and the risk of fractures among participants of the Women's Health Initiative observational study. American Journal of Clinical Nutrition, 89, 323–330. 10.3945/ajcn.2008.26451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael, S. L. , Yang, W. , & Shaw, G. M. (2010). Periconceptional nutrient intakes and risks of neural tube defects in California. Birth defects research. Part A, Clinical and Molecular Teratology, 88, 670–678. 10.1002/bdra.20675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartmel, B. , Moon, T. E. , & Levine, N. (1999). Effects of long‐term intake of retinol on selected clinical and laboratory indexes. American Journal of Clinical Nutrition, 69, 937–943. 10.1093/ajcn/69.5.937 [DOI] [PubMed] [Google Scholar]
- Catalano, M. , Born, G. , & Peto, R. (2007). Prevention of serious vascular events by aspirin amongst patients with peripheral arterial disease: Randomized, double‐blind trial. Journal of Internal Medicine, 261, 276–284. 10.1111/j.1365-2796.2006.01763.x [DOI] [PubMed] [Google Scholar]
- Chan, R. , Woo, J. , & Leung, J. (2011). Effects of food groups and dietary nutrients on bone loss in elderly Chinese population. The Journal of Nutrition, Health & Aging, 15, 287–294. 10.1007/s12603-010-0279-3 [DOI] [PubMed] [Google Scholar]
- Chandler, A. L. , Hobbs, C. A. , Mosley, B. S. , Berry, R. J. , Canfield, M. A. , Qi, Y. P. , Siega‐Riz, A. M. , & Shaw, G. M. (2012). Neural tube defects and maternal intake of micronutrients related to one‐carbon metabolism or antioxidant activity. Birth Defects Research Part A, Clinical and Molecular Teratology, 94, 864–874. 10.1002/bdra.23068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, X. , Liang, R. , Zhong, F. , & Yokoyama, W. (2020). Effect of Beta‐carotene status in microcapsules on its in vivo bioefficacy and in vitro bioaccessibility. Food Hydrocolloids, 106, 105848. 10.1016/j.foodhyd.2020.105848 [DOI] [Google Scholar]
- Chertow, B. S. , Williams, G. A. , Norris, R. M. , Baker, G. R. , & Hargis, G. K. (1977). Vitamin a stimulation of parathyroid hormone: Interactions with calcium, hydrocortisone, and vitamin E in bovine parathyroid tissues and effects of vitamin A in man. European Journal of Clinical Investigation, 7, 307–314. 10.1111/j.1365-2362.1977.tb01610.x [DOI] [PubMed] [Google Scholar]
- Clark, I. , & Bassett, C. A. (1962). The amelioration of hypervitaminosis D in rats with vitamin A. The Journal of Experimental Medicine, 115, 147–156. 10.1084/jem.115.1.147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook, N. R. , Le, I. M. , Manson, J. E. , Buring, J. E. , & Hennekens, C. H. (2000). Effects of beta‐carotene supplementation on cancer incidence by baseline characteristics in the Physicians' health study (United States). Cancer Causes & Control, 11, 617–626. 10.1023/a:1008995430664 [DOI] [PubMed] [Google Scholar]
- Corbi, G. , Ali, S. , Intrieri, M. , Modaferri, S. , Calabrese, V. , Davinelli, S. , & Scapagnini, G. (2022). Association between Beta‐carotene supplementation and mortality: A systematic review and meta‐analysis of randomized controlled trials. Frontiers Medicine (Lausanne), 9, 872310. 10.3389/fmed.2022.872310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong, M. H. , Nawijn, E. L. , & Verkaik‐Kloosterman, J. (2022a). Contribution of fortified margarines and other plant‐based fats to micronutrient intake in The Netherlands. European Journal of Nutrition, 61, 1893–1904. 10.1007/s00394-021-02757-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong, M. H. , Nawijn, E. L. , & Verkaik‐Kloosterman, J. (2022b). Contribution of voluntary fortified foods to micronutrient intake in The Netherlands. European Journal of Nutrition, 61, 1649–1663. 10.1007/s00394-021-02728-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jonge, E. A. , Kiefte‐de Jong, J. C. , Campos‐Obando, N. , Booij, L. , Franco, O. H. , Hofman, A. , Uitterlinden, A. G. , Rivadeneira, F. , & Zillikens, M. C. (2015). Dietary vitamin A intake and bone health in the elderly: The Rotterdam study. European Journal of Clinical Nutrition, 69, 1360–1368. 10.1038/ejcn.2015.154 [DOI] [PubMed] [Google Scholar]
- dela Seña, C. , Narayanasamy, S. , Riedl, K. M. , Curley, R. W., Jr. , Schwartz, S. J. , & Harrison, E. H. (2013). Substrate specificity of purified recombinant human β‐carotene 15,15′‐oxygenase (BCO1). The Journal of Biological Chemistry, 288, 37094–37103. 10.1074/jbc.M113.507160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiGiovanna, J. J. , Sollitto, R. B. , Abangan, D. L. , Steinberg, S. M. , & Reynolds, J. C. (1995). Osteoporosis is a toxic effect of long‐term etretinate therapy. Archives of Dermatology, 131, 1263–1267. [PubMed] [Google Scholar]
- Diplock, A. T. (1995). Safety of antioxidant vitamins and beta‐carotene. The American Journal of Clinical Nutrition, 62, 1510s–1516s. 10.1093/ajcn/62.6.1510S [DOI] [PubMed] [Google Scholar]
- Dougherty, K. A. , Schall, J. I. , Kawchak, D. A. , Green, M. H. , Ohene‐Frempong, K. , Zemel, B. S. , & Stallings, V. A. (2012). No improvement in suboptimal vitamin A status with a randomized, double‐blind, placebo‐controlled trial of vitamin A supplementation in children with sickle cell disease. The American Journal of Clinical Nutrition, 96, 932–940. 10.3945/ajcn.112.035725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druesne‐Pecollo, N. , Latino‐Martel, P. , Norat, T. , Barrandon, E. , Bertrais, S. , Galan, P. , & Hercberg, S. (2010). Beta‐carotene supplementation and cancer risk: A systematic review and metaanalysis of randomized controlled trials. International Journal of Cancer, 127, 172–184. 10.1002/ijc.25008 [DOI] [PubMed] [Google Scholar]
- Duester, G. (2008). Retinoic acid synthesis and signaling during early organogenesis. Cell, 134, 921–931. 10.1016/j.cell.2008.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) . (2010). Application of systematic review methodology to food and feed safety assessments to support decision making. EFSA Journal, 8(6), 1637. 10.2903/j.efsa.2010.1637 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) . (2011a). Methodological characteristics of the national dietary surveys carried out in the European Union as included in the European Food Safety Authority (EFSA) Comprehensive European Food Consumption Database. Food Additives & Contaminants. Part A, Chemistry, Analysis, Control, Exposure & Risk Assessment, 8(6), 1637. 10.1080/19440049.2011.576440 [DOI] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) . (2011b). Use of the EFSA comprehensive European food consumption database in exposure assessment. EFSA Journal, 9(3), 2097. 10.2903/j.efsa.2011.2097 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) . (2014). Guidance on the EU menu methodology. EFSA Journal, 12(12), 3944. 10.2903/j.efsa.2014.3944 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) . (2015a). The food classification and description system FoodEx 2 (revision 2). EFSA Supporting Publications, 12(5), EN‐804. 10.2903/sp.efsa.2015.EN-804 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) . (2015b). Principles and process for dealing with data and evidence in scientific assessments. EFSA Journal, 13(6), 4121. 10.2903/sp.efsa.2015.4121 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) . (2022). Protocol for the intake assessments performed in the context of the revision of tolerable upper intake levels for selected nutrients. EFSA Supporting Publication, 19(8), e200801. 10.2903/sp.efsa.2022.e200801 [DOI] [Google Scholar]
- EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources added to Food) . (2012a). Scientific opinion on the re‐evaluation of mixed carotenes (E 160a (i)) and beta‐carotene (E 160a (ii)) as a food additive. EFSA Journal, 10(3), 2593. 10.2903/j.efsa.2012.2593 [DOI] [Google Scholar]
- EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources added to Food) . (2012b). Statement on the safety of β‐carotene use in heavy smokers. EFSA Journal, 10(12), 2953. 10.2903/j.efsa.2012.2953 [DOI] [Google Scholar]
- EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources added to Food) . (2016). Safety of the proposed extension of use of synthetic β‐carotene [E 160a(ii)] in foods for special medical purposes in young children. EFSA Journal, 14(3), 4434. 10.2903/j.efsa.2016.4434 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) . (2009). Consequences for the consumer of the use of vitamin A in animal nutrition. EFSA Journal, 7(2), 873. 10.2903/j.efsa.2009.873 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) . (2012). Scientific opinion on the safety and efficacy of beta‐carotene as a feed additive for all animal species and categories. EFSA Journal, 10(6), 2737. 10.2903/j.efsa.2012.2737 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) . (2013). Scientific opinion on the safety and efficacy of vitamin A (retinyl acetate, retinyl palmitate and retinyl propionate) as a feed additive for all animal species and categories. EFSA Journal, 11(1), 3037. 10.2903/j.efsa.2013.3037 [DOI] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Nutrition, Novel Foods and Food Allergens) . (2015). Scientific Opinion on Dietary Reference Values for Vitamin A. EFSA Journal, 3(3), 4028. 10.2903/j.efsa.2015.4028 [DOI] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Nutrition, Novel Foods and Food Allergens) . (2022). Guidance for establishing and applying tolerable upper intake levels for vitamins and essential minerals. Draft for Internal Testing. EFSA Journal, 20(1), 200102. 10.2903/j.efsa.2022.e200102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee . (2017a). Guidance on the assessment of the biological relevance of data in scientific assessments. EFSA Journal, 15(8), e04970. 10.2903/j.efsa.2017.4970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee . (2017b). Guidance on the use of the weight of evidence approach in scientific assessments. EFSA Journal, 15(8), e04971. 10.2903/j.efsa.2017.4971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee . (2018). Guidance on uncertainty analysis in scientific assessments. EFSA Journal, 16(1), e05123. 10.2903/j.efsa.2018.5123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee . (2020). Draft for internal testing scientific committee guidance on appraising and integrating evidence from epidemiological studies for use in EFSA's scientific assessments. EFSA Journal, 18(8), e06221. 10.2903/j.efsa.2020.6221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis, J. K. , Russell, R. M. , Makrauer, F. L. , & Schaefer, E. J. (1986). Increased risk for vitamin A toxicity in severe hypertriglyceridemia. Annals of Internal Medicine, 105, 877–879. 10.7326/0003-4819-105-6-877 [DOI] [PubMed] [Google Scholar]
- Engle‐Stone, R. , Miller, J. C. , Reario, M. F. D. , Arnold, C. D. , Stormer, A. , Lafuente, E. , Oxley, A. , Capanzana, M. V. , Cabanilla, C. V. D. , Ford, J. L. , Clark, A. , Velavan, T. P. , Brown, K. H. , Lietz, G. , & Haskell, M. J. (2022). Filipino children with high usual vitamin a intakes and exposure to multiple sources of vitamin a have elevated Total body Stores of Vitamin a but do not Show Clear Evidence of vitamin a toxicity. Current Developments in Nutrition, 6, nzac115. 10.1093/cdn/nzac115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EVM (The Expert Group on Vitamins and Minerals) . (2003). Safe upper levels for vitamins and minerals. https://cot.food.gov.uk/sites/default/files/vitmin2003.pdf
- FAO/WHO (Food and Agriculture Organization of the United Nations & World Health Organization) . (1988). Requirements of vitamin a, iron, folate, and vitamin B12: Report of a joint FAO/WHO expert consultation (p. 107). Italy. [Google Scholar]
- FAO/WHO (Food and Agriculture Organization of the United Nations/World Health Organization) . (2009). Principles and methods for the risk assessment of Chemicals in Food. Environmental Health Criteria, 240. [Google Scholar]
- Farhangi, M. A. , Keshavarz, S. A. , Eshraghian, M. , Ostadrahimi, A. , & Saboor‐Yaraghi, A. A. (2013). Vitamin a supplementation, serum lipids, liver enzymes and C‐reactive protein concentrations in obese women of reproductive age. Annals of Clinical Biochemistry, 50, 25–30. 10.1258/acb.2012.012096 [DOI] [PubMed] [Google Scholar]
- Feldkamp, M. L. , Carmichael, S. L. , Shaw, G. M. , Panichello, J. D. , Moore, C. A. , & Botto, L. D. (2011). Maternal nutrition and gastroschisis: Findings from the National Birth Defects Prevention Study. American Journal of Obstetrics and Gynecology, 204, 404.e401–404.e410. 10.1016/j.ajog.2010.12.053 [DOI] [PubMed] [Google Scholar]
- Feskanich, D. , Singh, V. , Willett, W. C. , & Colditz, G. A. (2002). Vitamin a intake and hip fractures among postmenopausal women. Jama‐Journal of the American Medical Association, 287, 47–54. 10.1001/jama.287.1.47 [DOI] [PubMed] [Google Scholar]
- Ford, J. L. , Green, J. B. , Lietz, G. , Oxley, A. , & Green, M. H. (2017). A simple plasma retinol isotope ratio method for estimating β‐carotene relative bioefficacy in humans: Validation with the use of model‐based compartmental analysis. The Journal of Nutrition, 147, 1806–1814. 10.3945/jn.117.252361 [DOI] [PubMed] [Google Scholar]
- Ford, J. L. , Green, M. H. , Green, J. B. , Oxley, A. , & Lietz, G. (2018). Intestinal β‐carotene bioconversion in humans is determined by a new single‐sample, plasma isotope ratio method and compared with traditional and modified area‐under‐the‐curve methods. Archives of Biochemistry and Biophysics, 653, 121–126. 10.1016/j.abb.2018.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frame, B. , Jackson, C. E. , Reynolds, W. A. , & Umphrey, J. E. (1974). Hypercalcemia and skeletal effects in chronic hypervitaminosis a. Annals of Internal Medicine, 80, 44–48. 10.7326/0003-4819-80-1-44 [DOI] [PubMed] [Google Scholar]
- Furr, H. C. , Amedee‐Manesme, O. , Clifford, A. J. , Bergen, H. R., 3rd , Jones, A. D. , Anderson, D. P. , & Olson, J. A. (1989). Vitamin A concentrations in liver determined by isotope dilution assay with tetradeuterated vitamin A and by biopsy in generally healthy adult humans. The American Journal of Clinical Nutrition, 49, 713–716. 10.1093/ajcn/49.4.713 [DOI] [PubMed] [Google Scholar]
- Gannon, B. M. , & Tanumihardjo, S. A. (2015). Comparisons among equations used for retinol isotope dilution in the assessment of Total body stores and Total liver reserves. The Journal of Nutrition, 145, 847–854. 10.3945/jn.114.208132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao, S. S. , & Zhao, Y. (2023). The effects of β‐carotene on osteoporosis: A systematic review and meta‐analysis of observational studies. Osteoporosis International, 34, 627–639. 10.1007/s00198-022-06593-7 [DOI] [PubMed] [Google Scholar]
- Geubel, A. P. , De Galocsy, C. , Alves, N. , Rahier, J. , & Dive, C. (1991). Liver damage caused by therapeutic vitamin A administration: Estimate of dose‐related toxicity in 41 cases. Gastroenterology, 100, 1701–1709. 10.1016/0016-5085(91)90672-8 [DOI] [PubMed] [Google Scholar]
- Goldberg, J. S. (2011). Monitoring maternal Beta carotene and retinol consumption may decrease the incidence of neurodevelopmental disorders in offspring. Clinical Medicine Insights: Reproductive Health, 6, CMRH.S8372. 10.4137/CMRH.S8372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman, D. S. , Blomstrand, R. , Werner, B. , Huang, H. S. , & Shiratori, T. (1966). The intestinal absorption and metabolism of vitamin A and beta‐carotene in man. The Journal of Clinical Investigation, 45, 1615–1623. 10.1172/jci105468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green, A. C. , Kocovski, P. , Jovic, T. , Walia, M. K. , Chandraratna, R. A. S. , Martin, T. J. , Baker, E. K. , & Purton, L. E. (2017). Retinoic acid receptor signalling directly regulates osteoblast and adipocyte differentiation from mesenchymal progenitor cells. Experimental Cell Research, 350, 284–297. 10.1016/j.yexcr.2016.12.007 [DOI] [PubMed] [Google Scholar]
- Green, M. H. , Ford, J. L. , & Green, J. B. (2021). A compartmental model describing the kinetics of β‐carotene and β‐carotene‐derived retinol in healthy older adults. The Journal of Nutrition, 151, 434–444. 10.1093/jn/nxaa306 [DOI] [PubMed] [Google Scholar]
- Green, M. H. , Green, J. B. , & Ford, J. L. (2020). Better predictions of vitamin a Total body stores by the retinol isotope dilution method are possible with deeper understanding of the mathematics and by applying compartmental modeling. The Journal of Nutrition, 150, 989–993. 10.1093/jn/nxz321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grune, T. , Lietz, G. , Palou, A. , Ross, A. C. , Stahl, W. , Tang, G. , Thurnham, D. , Yin, S. A. , & Biesalski, H. K. (2010). Beta‐carotene is an important vitamin A source for humans. The Journal of Nutrition, 140, 2268s–2285s. 10.3945/jn.109.119024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haider, B. A. , Sharma, R. , & Bhutta, Z. A. (2017). Neonatal vitamin A supplementation for the prevention of mortality and morbidity in term neonates in low and middle income countries. Cochrane Database of Systematic Reviews, 2, Cd006980. 10.1002/14651858.CD006980.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada, H. , Miki, R. , Masushige, S. , & Kato, S. (1995). Gene expression of retinoic acid receptors, retinoid‐X receptors, and cellular retinol‐binding protein I in bone and its regulation by vitamin A. Endocrinology, 136, 5329–5335. 10.1210/endo.136.12.7588278 [DOI] [PubMed] [Google Scholar]
- Harrison, E. H. (2012). Mechanisms involved in the intestinal absorption of dietary vitamin A and provitamin A carotenoids. Biochimica et Biophysica Acta, 1821, 70–77. 10.1016/j.bbalip.2011.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haskell, M. J. (2012). The challenge to reach nutritional adequacy for vitamin A: β‐carotene bioavailability and conversion–evidence in humans. The American Journal of Clinical Nutrition, 96, 1193s–1203s. 10.3945/ajcn.112.034850 [DOI] [PubMed] [Google Scholar]
- Haskell, M. J. , Handelman, G. J. , Peerson, J. M. , Jones, A. D. , Rabbi, M. A. , Awal, M. A. , Wahed, M. A. , Mahalanabis, D. , & Brown, K. H. (1997). Assessment of vitamin A status by the deuterated‐retinol‐dilution technique and comparison with hepatic vitamin A concentration in Bangladeshi surgical patients. The American Journal of Clinical Nutrition, 66, 67–74. 10.1093/ajcn/66.1.67 [DOI] [PubMed] [Google Scholar]
- Hathcock, J. N. , Hattan, D. G. , Jenkins, M. Y. , McDonald, J. T. , Sundaresan, P. R. , & Wilkening, V. L. (1990). Evaluation of vitamin a toxicity. The American Journal of Clinical Nutrition, 52, 183–202. 10.1093/ajcn/52.2.183 [DOI] [PubMed] [Google Scholar]
- Haussler, M. R. , Whitfield, G. K. , Haussler, C. A. , Hsieh, J. C. , Thompson, P. D. , Selznick, S. H. , Dominguez, C. E. , & Jurutka, P. W. (1998). The nuclear vitamin D receptor: Biological and molecular regulatory properties revealed. Journal of Bone and Mineral Research, 13, 325–349. 10.1359/jbmr.1998.13.3.325 [DOI] [PubMed] [Google Scholar]
- Hayhoe, R. P. G. , Lentjes, M. A. H. , Mulligan, A. A. , Luben, R. N. , Khaw, K. T. , & Welch, A. A. (2017). Carotenoid dietary intakes and plasma concentrations are associated with heel bone ultrasound attenuation and osteoporotic fracture risk in the European prospective investigation into cancer and nutrition (EPIC)‐Norfolk cohort. he British Journal of Nutrition, 117, 1439–1453. 10.1017/s0007114517001180 [DOI] [PubMed] [Google Scholar]
- Heart Protection Study Collaborative Group . (2002). MRC/BHF heart protection study of antioxidant vitamin supplementation in 20,536 high‐risk individuals: A randomised placebo‐controlled trial. Lancet, 360, 23–33. 10.1016/s0140-6736(02)09328-5 [DOI] [PubMed] [Google Scholar]
- Henríquez‐Sánchez, P. , Sánchez‐Villegas, A. , Doreste‐Alonso, J. , Ortiz‐Andrellucchi, A. , Pfrimer, K. , & Serra‐Majem, L. (2009). Dietary assessment methods for micronutrient intake: A systematic review on vitamins. The British Journal of Nutrition, 102(Suppl 1), S10–S37. 10.1017/s0007114509993126 [DOI] [PubMed] [Google Scholar]
- Hercberg, S. , Galan, P. , Preziosi, P. , Bertrais, S. , Mennen, L. , Malvy, D. , Roussel, A. M. , Favier, A. , & Briançon, S. (2004). The SU.VI.MAX study: A randomized, placebo‐controlled trial of the health effects of antioxidant vitamins and minerals. Archives of Internal Medicine, 164, 2335–2342. 10.1001/archinte.164.21.2335 [DOI] [PubMed] [Google Scholar]
- Hindborg H (DTU Food National Institute) . (2015, Unpublished). Dietary supplements may contribute to an adequate micronutrient intake in Danish adults but increase the risk of overdose in children.
- Hoffmann, C. , Djerir, N. E. , Danckaert, A. , Fernandes, J. , Roux, P. , Charrueau, C. , Lachages, A. M. , Charlotte, F. , Brocheriou, I. , Clement, K. , Aron‐Wisnewsky, J. , Foufelle, F. , Ratziu, V. , Hainque, B. , Bonnefont‐Rousselot, D. , Bigey, P. , & Escriou, V. (2020). Hepatic stellate cell hypertrophy is associated with metabolic liver fibrosis. Scientific Reports, 10(1), 3850. 10.1038/s41598-020-60615-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houtkooper, L. B. , Ritenbaugh, C. , Aickin, M. , Lohman, T. G. , Going, S. B. , Weber, J. L. , Greaves, K. A. , Boyden, T. W. , Pamenter, R. W. , & Hall, M. C. (1995). Nutrients, body composition and exercise are related to change in bone mineral density in premenopausal women. The Journal of Nutrition, 125, 1229–1237. 10.1093/jn/125.5.1229 [DOI] [PubMed] [Google Scholar]
- Iddir, M. , Vahid, F. , Merten, D. , Larondelle, Y. , & Bohn, T. (2022). Influence of proteins on the absorption of lipophilic vitamins, carotenoids and curcumin ‐ a review. Molecular Nutrition & Food Research, 66, e2200076. 10.1002/mnfr.202200076 [DOI] [PubMed] [Google Scholar]
- Imdad, A. , Rehman, F. , Davis, E. , Ranjit, D. , Surin, G. S. S. , Attia, S. L. , Lawler, S. , Smith, A. A. , & Bhutta, Z. A. (2021). Effects of neonatal nutrition interventions on neonatal mortality and child health and development outcomes: A systematic review. Campbell Systematic Reviews, 17, e1141. 10.1002/cl2.1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- IOM (Institute of Medicine Panel on Dietary, Antioxidants Related, Compounds) . (2000). Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. (US) NAP and reserved. CbtNAoSAr.
- IOM (Institute of Medicine) . (2001). Dietary reference intakes for vitamin a, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. PRESS NA. [PubMed] [Google Scholar]
- JECFA . (2019). Evaluation of Certain Food Additives: Eighty‐Seventh Report of the Joint FAO/WHO Expert Committee on Food Additives. Geneva: World Health Organization and Food and Agriculture Organization of the United Nations; 2019 (WHO technical report series; no. 1020).
- Jeon, Y. J. , Myung, S. K. , Lee, E. H. , Kim, Y. , Chang, Y. J. , Ju, W. , Cho, H. J. , Seo, H. G. , & Huh, B. Y. (2011). Effects of beta‐carotene supplements on cancer prevention: Meta‐analysis of randomized controlled trials. Nutrition and Cancer, 63, 1196–1207. 10.1080/01635581.2011.607541 [DOI] [PubMed] [Google Scholar]
- Jeong, H. , Armstrong, A. T. , Isoherranen, N. , Czuba, L. , Yang, A. , Zumpf, K. , Ciolino, J. , Torres, E. , Stika, C. S. , & Wisner, K. L. (2023). Temporal changes in the systemic concentrations of retinoids in pregnant and postpartum women. PLoS One, 18, e0280424. 10.1371/journal.pone.0280424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen, A. M. , Lie, R. T. , Wilcox, A. J. , Andersen, L. F. , & Drevon, C. A. (2008). Maternal dietary intake of vitamin A and risk of orofacial clefts: A population‐based case‐control study in Norway. American Journal of Epidemiology, 167, 1164–1170. 10.1093/aje/kwn035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson, S. , & Melhus, H. (2001). Vitamin A antagonizes calcium response to vitamin D in man. Journal of Bone and Mineral Research, 16, 1899–1905. 10.1359/jbmr.2001.16.10.1899 [DOI] [PubMed] [Google Scholar]
- Johnson, C. M. , Rosario, R. , Brito, A. , Agrawal, K. , Fanter, R. , Lietz, G. , Haskell, M. , Engle‐Stone, R. , Newman, J. W. , & La Frano, M. R. (2022). Multiassay nutritional metabolomics profiling of low vitamin A status versus adequacy is characterized by reduced plasma lipid mediators among lactating women in The Philippines: A pilot study. Nutrition Research, 104, 118–127. 10.1016/j.nutres.2022.05.007 [DOI] [PubMed] [Google Scholar]
- Kamangar, F. , Qiao, Y. L. , Yu, B. , Sun, X. D. , Abnet, C. C. , Fan, J. H. , Mark, S. D. , Zhao, P. , Dawsey, S. M. , & Taylor, P. R. (2006). Lung cancer chemoprevention: A randomized, double‐blind trial in Linxian, China. Cancer Epidemiology, Biomarkers & Prevention, 15, 1562–1564. 10.1158/1055-9965.Epi-06-0316 [DOI] [PubMed] [Google Scholar]
- Kanis, J. A. , (2008). Assessment of osteoporosis at the primary health‐care level. Technical Report. WHO Collaborating Centre for Metabolic Bone Diseases, University of Sheffield. [Google Scholar]
- Kanis, J. A. , Johansson, H. , McCloskey, E. V. , Liu, E. , Åkesson, K. E. , Anderson, F. A. , Azagra, R. , Bager, C. L. , Beaudart, C. , Bischoff‐Ferrari, H. A. , Biver, E. , Bruyère, O. , Cauley, J. A. , Center, J. R. , Chapurlat, R. , Christiansen, C. , Cooper, C. , Crandall, C. J. , Cummings, S. R. , … Leslie, W. D. (2023). Previous fracture and subsequent fracture risk: A meta‐analysis to update FRAX. Osteoporosis International. 34(12), 2027–2045 10.1007/s00198-023-06870-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaptoge, S. , Welch, A. , McTaggart, A. , Mulligan, A. , Dalzell, N. , Day, N. E. , Bingham, S. , Khaw, K. T. , & Reeve, J. (2003). Effects of dietary nutrients and food groups on bone loss from the proximal femur in men and women in the 7th and 8th decades of age. Osteoporosis International, 14, 418–428. 10.1007/s00198-003-1391-6 [DOI] [PubMed] [Google Scholar]
- Kehoe, L. , & Walton, J. (Munster Technological University). (2022). Report on the intake of micronutrients (Vitamin A, β‐carotene, retinol, vitamin D, vitamin E, vitamin B6, folic acid, iron and manganese) from food supplements in the Irish population.
- Key, T. J. , Appleby, P. N. , Spencer, E. A. , Roddam, A. W. , Neale, R. E. , & Allen, N. E. (2007). Calcium, diet and fracture risk: A prospective study of 1898 incident fractures among 34 696 British women and men. Public Health Nutrition, 10, 1314–1320. 10.1017/s1368980007696402 [DOI] [PubMed] [Google Scholar]
- Khan, N. C. , West, C. E. , de Pee, S. , Bosch, D. , Phuong, H. D. , Hulshof, P. J. , Khoi, H. H. , Verhoef, H. , & Hautvast, J. G. (2007). The contribution of plant foods to the vitamin A supply of lactating women in Vietnam: A randomized controlled trial. American Journal of Clinical Nutrition, 85, 1112–1120. 10.1093/ajcn/85.4.1112 [DOI] [PubMed] [Google Scholar]
- Knudsen, T. B. , Pierro, J. D. , & Baker, N. C. (2021). Retinoid signaling in skeletal development: Scoping the system for predictive toxicology. Reproductive Toxicology, 99, 109–130. 10.1016/j.reprotox.2020.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopec, R. E. , Caris‐Veyrat, C. , Nowicki, M. , Gleize, B. , Carail, M. , & Borel, P. (2018). Production of asymmetric oxidative metabolites of [13C]‐β‐carotene during digestion in the gastrointestinal lumen of healthy men. The American Journal of Clinical Nutrition, 108, 803–813. 10.1093/ajcn/nqy183 [DOI] [PubMed] [Google Scholar]
- Kordiak, J. , Bielec, F. , Jabłoński, S. , & Pastuszak‐Lewandoska, D. (2022). Role of Beta‐carotene in lung cancer primary chemoprevention: A systematic review with meta‐analysis and meta‐regression. Nutrients, 14(7), 1361. 10.3390/nu14071361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalski, T. E. , Falestiny, M. , Furth, E. , & Malet, P. F. (1994). Vitamin a hepatotoxicity: A cautionary note regarding 25,000 IU supplements. The American Journal of Medicine, 97, 523–528. 10.1016/0002-9343(94)90347-6 [DOI] [PubMed] [Google Scholar]
- Krasinski, S. D. , Russell, R. M. , Otradovec, C. L. , Sadowski, J. A. , Hartz, S. C. , Jacob, R. A. , & McGandy, R. B. (1989). Relationship of vitamin A and vitamin E intake to fasting plasma retinol, retinol‐binding protein, retinyl esters, carotene, alpha‐tocopherol, and cholesterol among elderly people and young adults: Increased plasma retinyl esters among vitamin A‐supplement users. American Journal of Clinical Nutrition, 49, 112–120. 10.1093/ajcn/49.1.112 [DOI] [PubMed] [Google Scholar]
- Kumar, S. , & Duester, G. (2011). SnapShot: retinoic acid signaling. Cell, 147, 1422‐U1233. 10.1016/j.cell.2011.11.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, I. M. , Cook, N. R. , Manson, J. E. , Buring, J. E. , & Hennekens, C. H. (1999). Beta‐carotene supplementation and incidence of cancer and cardiovascular disease: The Women's health study. Journal of the National Cancer Institute, 91, 2102–2106. 10.1093/jnci/91.24.2102 [DOI] [PubMed] [Google Scholar]
- Li, B. , Cai, S. Y. , & Boyer, J. L. (2021). The role of the retinoid receptor, RAR/RXR heterodimer, in liver physiology. Biochimica et biophysica acta. Molecular Basis of Disease, 1867, 166085. 10.1016/j.bbadis.2021.166085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lietz, G. , Furr, H. C. , Gannon, B. M. , Green, M. H. , Haskell, M. , Lopez‐Teros, V. , Novotny, J. A. , Palmer, A. C. , Russell, R. M. , Tanumihardjo, S. A. , & Van Loo‐Bouwman, C. A. (2016). Current capabilities and limitations of stable isotope techniques and applied mathematical equations in determining whole‐body vitamin a status. Food and Nutrition Bulletin, 37, S87–S103. 10.1177/0379572116630642 [DOI] [PubMed] [Google Scholar]
- Lietz, G. , Oxley, A. , Berry, P. , Hesketh, J. , Tourniaire, F. , Park, H. , Green, M. H. , & Boddy, A. (2015). β‐Carotene (BC) bioconversion is similar in men and women. The FASEB Journal, 29(604), 604. 10.1096/fasebj.29.1_supplement.604.4 [DOI] [Google Scholar]
- Lietz, G. , Oxley, A. , Finney, K. , Clark, A. , Giles, T. , Foster, N. , Southam, A. , Jankevics, A. , Lloyd, G. , Winder, C. , & Dunn, W. (2019). Effects of chronic Hypervitaminosis a on global plasma metabolome changes and liver gene expression (OR05‐06‐19). Current Developments in Nutrition, 3(1), nzz029. 10.1093/cdn/nzz029.OR05-06-19 [DOI] [Google Scholar]
- Lim, L. S. , Harnack, L. J. , Lazovich, D. , & Folsom, A. R. (2004). Vitamin a intake and the risk of hip fracture in postmenopausal women: The Iowa Women's health study. Osteoporosis International, 15, 552–559. 10.1007/s00198-003-1577-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, J. , Cook, N. R. , Albert, C. , Zaharris, E. , Gaziano, J. M. , Van Denburgh, M. , Buring, J. E. , & Manson, J. E. (2009). Vitamins C and E and beta carotene supplementation and cancer risk: A randomized controlled trial. Journal of the National Cancer Institute, 101, 14–23. 10.1093/jnci/djn438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind, T. , Lind, P. M. , Jacobson, A. , Hu, L. , Sundqvist, A. , Risteli, J. , Yebra‐Rodriguez, A. , Rodriguez‐Navarro, A. , Andersson, G. , & Melhus, H. (2011). High dietary intake of retinol leads to bone marrow hypoxia and diaphyseal endosteal mineralization in rats. Bone, 48, 496–506. 10.1016/j.bone.2010.10.169 [DOI] [PubMed] [Google Scholar]
- Lind, T. , Sundqvist, A. , Hu, L. , Pejler, G. , Andersson, G. , Jacobson, A. , & Melhus, H. (2013). Vitamin a is a negative regulator of osteoblast mineralization. PLoS One, 8, e82388. 10.1371/journal.pone.0082388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lionikaite, V. , Henning, P. , Drevinge, C. , Shah, F. A. , Palmquist, A. , Wikstrom, P. , Windahl, S. H. , & Lerner, U. H. (2019). Vitamin a decreases the anabolic bone response to mechanical loading by suppressing bone formation. FASEB Journal, 33, 5237–5247. 10.1096/fj.201802040R [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo, G. P. , Hessel, S. , Eichinger, A. , Noy, N. , Moise, A. R. , Wyss, A. , Palczewski, K. , & von Lintig, J. (2010). ISX is a retinoic acid‐sensitive gatekeeper that controls intestinal beta,beta‐carotene absorption and vitamin A production. The FASEB Journal, 24, 1656–1666. 10.1096/fj.09-150995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald, H. M. , New, S. A. , Golden, M. H. , Campbell, M. K. , & Reid, D. M. (2004). Nutritional associations with bone loss during the menopausal transition: Evidence of a beneficial effect of calcium, alcohol, and fruit and vegetable nutrients and of a detrimental effect of fatty acids. The American Journal of Clinical Nutrition, 79, 155–165. 10.1093/ajcn/79.1.155 [DOI] [PubMed] [Google Scholar]
- Marlétaz, F. , Holland, L. Z. , Laudet, V. , & Schubert, M. (2006). Retinoic acid signaling and the evolution of chordates. International Journal of Biological Sciences, 2, 38–47. 10.7150/ijbs.2.38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marqueño, A. , Flores, C. , Casado, M. , & Porte, C. (2021). Dysregulation of lipid metabolism in PLHC‐1 and ZFL cells exposed to tributyltin an all‐trans retinoic acid. Aquatic Toxicology, 231, 105733. 10.1016/j.aquatox.2020.105733 [DOI] [PubMed] [Google Scholar]
- Martínez‐Frías, M. L. , & Salvador, J. (1990). Epidemiological aspects of prenatal exposure to high doses of vitamin A in Spain. European Journal of Epidemiology, 6, 118–123. 10.1007/bf00145783 [DOI] [PubMed] [Google Scholar]
- Mastroiacovo, P. , Mazzone, T. , Addis, A. , Elephant, E. , Carlier, P. , Vial, T. , Garbis, H. , Robert, E. , Bonati, M. , Ornoy, A. , Finardi, A. , Schaffer, C. , Caramelli, L. , Rodriguez‐Pinilla, E. , & Clementi, M. (1999). High vitamin A intake in early pregnancy and major malformations: A multicenter prospective controlled study. Teratology, 59, 7–11. [DOI] [PubMed] [Google Scholar]
- Maurya, V. K. , Singh, J. , Ranjan, V. , Gothandam, K. M. , Bohn, T. , & Pareek, S. (2022). Factors affecting the fate of β‐carotene in the human gastrointestinal tract: A narrative review. International Journal for Vitamin and Nutrition Research, 92, 385–405. 10.1024/0300-9831/a000674 [DOI] [PubMed] [Google Scholar]
- Melhus, H. , Michaelsson, K. , Kindmark, A. , Bergstrom, R. , Holmberg, L. , Mallmin, H. , Wolk, A. , & Ljunghall, S. (1998). Excessive dietary intake of vitamin A is associated with reduced bone mineral density and increased risk for hip fracture. Annals of Internal Medicine, 129, 770−+. 10.7326/0003-4819-129-10-199811150-00003 [DOI] [PubMed] [Google Scholar]
- Metz, A. L. , Walser, M. M. , & Olson, W. G. (1985). The interaction of dietary vitamin A and vitamin D related to skeletal development in the Turkey poult. The Journal of Nutrition, 115, 929–935. 10.1093/jn/115.7.929 [DOI] [PubMed] [Google Scholar]
- Michaëlsson, K. , Lithell, H. , Vessby, B. , & Melhus, H. (2003). Serum retinol levels and the risk of fracture. New England Journal of Medicine, 348, 287–294. 10.1056/NEJMoa021171 [DOI] [PubMed] [Google Scholar]
- Michikawa, T. , Yamazaki, S. , Sekiyama, M. , Kuroda, T. , Nakayama, S. F. , Isobe, T. , Kobayashi, Y. , Iwai‐Shimada, M. , Suda, E. , Kawamoto, T. , & Nitta, H. (2019). Maternal dietary intake of vitamin a during pregnancy was inversely associated with congenital diaphragmatic hernia: The Japan environment and Children's study. The British Journal of Nutrition, 122, 1295–1302. 10.1017/s0007114519002204 [DOI] [PubMed] [Google Scholar]
- Micozzi, M. S. , Brown, E. D. , Taylor, P. R. , & Wolfe, E. (1988). Carotenodermia in men with elevated carotenoid intake from foods and beta‐carotene supplements. The American Journal of Clinical Nutrition, 48, 1061–1064. 10.1093/ajcn/48.4.1061 [DOI] [PubMed] [Google Scholar]
- Mills, J. L. , Simpson, J. L. , Cunningham, G. C. , Conley, M. R. , & Rhoads, G. G. (1997). Vitamin a and birth defects. American Journal of Obstetrics and Gynecology, 177, 31–36. 10.1016/s0002-9378(97)70434-4 [DOI] [PubMed] [Google Scholar]
- Mitchell, L. E. , Murray, J. C. , O'Brien, S. , & Christensen, K. (2003). Retinoic acid receptor alpha gene variants, multivitamin use, and liver intake as risk factors for oral clefts: A population‐based case‐control study in Denmark, 1991–1994. American Journal of Epidemiology, 158, 69–76. 10.1093/aje/kwg102 [DOI] [PubMed] [Google Scholar]
- Mondloch, S. , Gannon, B. M. , Davis, C. R. , Chileshe, J. , Kaliwile, C. , Masi, C. , Rios‐Avila, L. , Gregory, J. F. , & Tanumihardjo, S. A. (2015). High provitamin a carotenoid serum concentrations, elevated retinyl esters, and saturated retinol‐binding protein in Zambian preschool children are consistent with the presence of high liver vitamin a stores. American Journal of Clinical Nutrition, 102, 497–504. 10.3945/ajcn.115.112383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore, T. , & Wang, Y. L. (1945). Hypervitaminosis A. The Biochemical Journal, 39, 222–228. 10.1042/bj0390222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyersoen, I. , Devleesschauwer, B. , Dekkers, A. , de Ridder, K. , Tafforeau, J. , van Camp, J. , van Oyen, H. , & Lachat, C. (2017). Intake of fat‐soluble vitamins in the Belgian population: Adequacy and contribution of foods. Fortified Foods and Supplements. Nutrients, 9(8), 860. 10.3390/nu9080860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myhre, A. M. , Carlsen, M. H. , Bohn, S. K. , Wold, H. L. , Laake, P. , & Blomhoff, R. (2003). Water‐miscible, emulsified, and solid forms of retinol supplements are more toxic than oil‐based preparations. American Journal of Clinical Nutrition, 78, 1152–1159. 10.1093/ajcn/78.6.1152 [DOI] [PubMed] [Google Scholar]
- Newsome, P. N. , Cramb, R. , Davison, S. M. , Dillon, J. F. , Foulerton, M. , Godfrey, E. M. , Hall, R. , Harrower, U. , Hudson, M. , Langford, A. , Mackie, A. , Mitchell‐Thain, R. , Sennett, K. , Sheron, N. C. , Verne, J. , Walmsley, M. , & Yeoman, A. (2018). Guidelines on the management of abnormal liver blood tests. Gut, 67, 6. 10.1136/gutjnl-2017-314924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishide, Y. , Tousen, Y. , Tadaishi, M. , Inada, M. , Miyaura, C. , Kruger, M. C. , & Ishimi, Y. (2015). Combined effects of soy Isoflavones and β‐carotene on osteoblast differentiation. International Journal of Environmental Research and Public Health, 12, 13750–13761. 10.3390/ijerph121113750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- NNR . (2014). Nordic nutrition recommendations 2012: Integrating nutrition and physical activity (5th ed.). Copenhagen K. [Google Scholar]
- Nollevaux, M. C. , Guiot, Y. , Horsmans, Y. , Leclercq, I. , Rahier, J. , Geubel, A. P. , & Sempoux, C. (2006). Hypervitaminosis A‐induced liver fibrosis: Stellate cell activation and daily dose consumption. Liver International, 26, 182–186. 10.1111/j.1478-3231.2005.01207.x [DOI] [PubMed] [Google Scholar]
- Noy, N. (2016). Vitamin a transport and cell signaling by the retinol‐binding protein receptor STRA6. Sub‐Cellular Biochemistry, 81, 77–93. 10.1007/978-94-024-0945-1_3 [DOI] [PubMed] [Google Scholar]
- Obrycki, J. F. , Lee, J. J. , Kapur, K. , Paul, L. , Hasan, M. , Mia, S. , Quamruzzaman, Q. , Christiani, D. C. , & Mazumdar, M. (2019). A case‐control analysis of maternal diet and risk of neural tube defects in Bangladesh. Birth Defects Research, 111, 967–981. 10.1002/bdr2.1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Byrne, S. M. , & Blaner, W. S. (2013). Retinol and retinyl esters: Biochemistry and physiology. Journal of Lipid Research, 54, 1731–1743. 10.1194/jlr.R037648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor, E. A. , Evans, C. V. , Ivlev, I. , Rushkin, M. C. , Thomas, R. G. , Martin, A. , & Lin, J. S. (2022). Vitamin and mineral supplements for the primary prevention of cardiovascular disease and cancer: Updated evidence report and systematic review for the US preventive services task force. JAMA, 327, 2334–2347. 10.1001/jama.2021.15650 [DOI] [PubMed] [Google Scholar]
- OHAT/NTP (Office of Health Assessment and Translation, Division of the National Toxicology Program) . (2015). OHAT Risk of Bias Rating Tool for Human and Animal Studies. 37 pp.
- Okada, N. , Nomura, M. , Morimoto, S. , Ogihara, T. , & Yoshikawa, K. (1994). Bone mineral density of the lumbar spine in psoriatic patients with long term etretinate therapy. The Journal of Dermatology, 21, 308–311. 10.1111/j.1346-8138.1994.tb01744.x [DOI] [PubMed] [Google Scholar]
- Olsen, K. , Suri, D. J. , Davis, C. , Sheftel, J. , Nishimoto, K. , Yamaoka, Y. , Toya, Y. , Welham, N. V. , & Tanumihardjo, S. A. (2018). Serum retinyl esters are positively correlated with analyzed total liver vitamin a reserves collected from US adults at time of death. American Journal of Clinical Nutrition, 108, 997–1005. 10.1093/ajcn/nqy190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen, T. , Christensen, J. J. , Arnesen, E. K. , Lysne, V. , & Blomhoff, R. (2024). Preparatory work for the update of the tolerable upper intake levels for vitamin a. EFSA Supporting Publication, EN‐8651. 10.2903/sp.efsa.2024.EN-8651 [DOI] [Google Scholar]
- Olson, J. A. (1990). Vitamin A. In Brown M. (Ed.), Present knowledge in nutrition (6th ed.). [Google Scholar]
- Omenn, G. S. , Goodman, G. E. , Thornquist, M. D. , Balmes, J. , Cullen, M. R. , Glass, A. , Keogh, J. P. , Meyskens, F. L., Jr. , Valanis, B. , Williams, J. H., Jr. , Barnhart, S. , Cherniack, M. G. , Brodkin, C. A. , & Hammar, S. (1996). Risk factors for lung cancer and for intervention effects in CARET, the Beta‐carotene and retinol efficacy trial. Journal of the National Cancer Institute, 88, 1550–1559. 10.1093/jnci/88.21.1550 [DOI] [PubMed] [Google Scholar]
- Oreffo, R. O. , Teti, A. , Triffitt, J. T. , Francis, M. J. , Carano, A. , & Zallone, A. Z. (1988). Effect of vitamin a on bone resorption: Evidence for direct stimulation of isolated chicken osteoclasts by retinol and retinoic acid. Journal of Bone and Mineral Research, 3, 203–210. 10.1002/jbmr.5650030213 [DOI] [PubMed] [Google Scholar]
- Osterhoff, G. , Morgan, E. F. , Shefelbine, S. J. , Karim, L. , McNamara, L. M. , & Augat, P. (2016). Bone mechanical properties and changes with osteoporosis. Injury, 47(Suppl 2), S11–S20. 10.1016/s0020-1383(16)47003-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker, R. S. (1996). Absorption, metabolism, and transport of carotenoids. The FASEB Journal, 10, 542–551. [PubMed] [Google Scholar]
- Penniston, K. L. , & Tanumihardjo, S. A. (2006). The acute and chronic toxic effects of vitamin a. American Journal of Clinical Nutrition, 83, 191–201. [DOI] [PubMed] [Google Scholar]
- Persson, B. , Tunell, R. , & Ekengren, K. (1965). Chronic vitamin a intoxication during the first half year of life; description of 5 cases. Acta Paediatrica Scandinavica, 54, 49–60. 10.1111/j.1651-2227.1965.tb06345.x [DOI] [PubMed] [Google Scholar]
- Peterka, M. , Peterková, R. , & Likovský, Z. (1997). Different embryotoxic effect of vitamin a and B‐carotene detected in the chick embryo. Acta Chirurgiae Plasticae, 39, 91–96. [PubMed] [Google Scholar]
- Promislow, J. H. , Goodman‐Gruen, D. , Slymen, D. J. , & Barrett‐Connor, E. (2002). Retinol intake and bone mineral density in the elderly: The rancho Bernardo study. Journal of Bone and Mineral Research, 17, 1349–1358. 10.1359/jbmr.2002.17.8.1349 [DOI] [PubMed] [Google Scholar]
- Quemelo, P. R. , Lourenço, C. M. , & Peres, L. C. (2007). Teratogenic effect of retinoic acid in swiss mice. Acta Cirurgica Brasileira, 22, 451–456. 10.1590/s0102-86502007000600007 [DOI] [PubMed] [Google Scholar]
- Ramkumar, S. , Moon, J. , Golczak, M. , & von Lintig, J. (2021). LRAT coordinates the negative‐feedback regulation of intestinal retinoid biosynthesis from β‐carotene. Journal of Lipid Research, 62, 100055. 10.1016/j.jlr.2021.100055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reboul, E. , & Borel, P. (2011). Proteins involved in uptake, intracellular transport and basolateral secretion of fat‐soluble vitamins and carotenoids by mammalian enterocytes. Progress in Lipid Research, 50, 388–402. 10.1016/j.plipres.2011.07.001 [DOI] [PubMed] [Google Scholar]
- Reddy, V. , & Sivakumar, B. (1972). Studies on vitamin a absorption in children. Indian Pediatrics, 9, 307–310. [PubMed] [Google Scholar]
- Rejnmark, L. , Vestergaard, P. , Charles, P. , Hermann, A. P. , Brot, C. , Eiken, P. , & Mosekilde, L. (2004). No effect of vitamin a intake on bone mineral density and fracture risk in perimenopausal women. Osteoporosis International, 15, 872–880. 10.1007/s00198-004-1618-1 [DOI] [PubMed] [Google Scholar]
- Rhinn, M. , & Dolle, P. (2012). Retinoic Acid Signalling during Development. Development, 139, 843–858. 10.1242/dev.065938 [DOI] [PubMed] [Google Scholar]
- Rodriguez‐Amaya D, 2015. Provitamin A activity. 255‐281.
- Roe, M. A. , Bell, S. , Oseredczuk, M. , Christensen, T. , Westenbrink, S. , Pakkala, H. , Presser, K. , & Finglas, P. M. (2013). Updated food composition database for nutrient intake. Project developed on the procurement project CFT/EFSA/DCM/2011/03. EFSA Supporting Publications, 10(6), EN‐355. 10.2903/sp.efsa.2013.EN-355 [DOI] [Google Scholar]
- Rohde, C. M. , & DeLuca, H. (2003). Bone resorption activity of all‐trans retinoic acid is independent of vitamin D in rats. The Journal of Nutrition, 133, 777–783. 10.1093/jn/133.3.777 [DOI] [PubMed] [Google Scholar]
- Rothman, K. J. , Moore, L. L. , Singer, M. R. , Nguyen, U. , Mannino, S. , & Milunsky, A. (1995). Teratogenicity of high vitamin‐a intake. New England Journal of Medicine, 333, 1369–1373. 10.1056/nejm199511233332101 [DOI] [PubMed] [Google Scholar]
- Samokyszyn, V. M. , Gall, W. E. , Zawada, G. , Freyaldenhoven, M. A. , Chen, G. , Mackenzie, P. I. , Tephly, T. R. , & Radominska‐Pandya, A. (2000). 4‐hydroxyretinoic acid, a novel substrate for human liver microsomal UDP‐glucuronosyltransferase(s) and recombinant UGT2B7. The Journal of Biological Chemistry, 275, 6908–6914. 10.1074/jbc.275.10.6908 [DOI] [PubMed] [Google Scholar]
- Sass, J. O. , Masgrau, E. , Saurat, J. H. , & Nau, H. (1995). Metabolism of oral 9‐cis‐retinoic acid in the human. Identification of 9‐cis‐retinoyl‐beta‐glucuronide and 9‐cis‐4‐oxo‐retinoyl‐beta‐glucuronide as urinary metabolites. Drug Metabolism and Disposition: The Biological Fate of Chemicals, 23, 887–891. [PubMed] [Google Scholar]
- Sauberlich, H. E. , Hodges, R. E. , Wallace, D. L. , Kolder, H. , Canham, J. E. , Hood, J. , Raica, N., Jr. , & Lowry, L. K. (1974). Vitamin a metabolism and requirements in the human studied with the use of labeled retinol. Vitamins and Hormones, 32, 251–275. 10.1016/s0083-6729(08)60015-1 [DOI] [PubMed] [Google Scholar]
- SCF (Scientific Committee on Food) . (1992). Report on the risks of hypervitaminosis A. Reports of the Scientific Committee for Food (Twenty‐seventh series). European Commission, Luxembourg.
- SCF (Scientific Committee on Food) . (1993). Nutrient and energy intakes for the European Community. Reports of the scientific Committee for Food, 31st series. Food ‐ science and technique. European Commission. [Google Scholar]
- SCF (Scientific Committee on Food) . (2000a). Guidelines of the Scientific Committee on Food for the development of Tolerable Upper Intake Levels for vitamins and minerals.
- SCF (Scientific Committee on Food) . (2000b). Opinion of the Scientific Committee on Food on the Tolerable Upper Intake Level of Beta Carotene.
- SCF (Scientific Committee on Food) . (2002). Opinion of the Scientific Committee on Food on the Tolerable Upper Intake Level of preformed Vitamin A (retinol and retinyl esters).
- SCF (Scientific Committee on Food) . (2003). Opinion of the scientific committee on food on the revision of reference values for nutrition labelling. https://food.ec.europa.eu/system/files/2020‐12/sci‐com_scf_out171_en.pdf
- Scheven, B. A. , & Hamilton, N. J. (1990). Retinoic acid and 1,25‐dihydroxyvitamin D3 stimulate osteoclast formation by different mechanisms. Bone, 11, 53–59. 10.1016/8756-3282(90)90072-7 [DOI] [PubMed] [Google Scholar]
- Schönberger, K. , Obier, N. , Romero‐Mulero, M. C. , Cauchy, P. , Mess, J. , Pavlovich, P. V. , Zhang, Y. W. , Mitterer, M. , Rettkowski, J. , Lalioti, M. E. , Jäcklein, K. , Curtis, J. D. , Féret, B. , Sommerkamp, P. , Morganti, C. , Ito, K. , Ghyselinck, N. B. , Trompouki, E. , Buescher, J. M. , … Cabezas‐Wallscheid, N. (2022). Multilayer omics analysis reveals a non‐classical retinoic acid signaling axis that regulates hematopoietic stem cell identity. Cell Stem Cell, 29, 131–148.e110. 10.1016/j.stem.2021.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon, S. R. , Moise, A. R. , & Trainor, P. A. (2017). New insights and changing paradigms in the regulation of vitamin a metabolism in development. Wiley Interdisciplinary Reviews: Developmental Biology, 6(3), e264. 10.1002/wdev.264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw, G. M. , Carmichael, S. L. , Yang, W. , & Lammer, E. J. (2010). Periconceptional nutrient intakes and risks of conotruncal heart defects. Birth defects research. Part A, Clinical and Molecular Teratology, 88, 144–151. 10.1002/bdra.20648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw, G. M. , Velie, E. M. , Schaffer, D. , & Lammer, E. J. (1997). Periconceptional intake of vitamin A among women and risk of neural tube defect‐affected pregnancies. Teratology, 55, 132–133. [DOI] [PubMed] [Google Scholar]
- Sheftel, J. , van Stuijvenberg, M. E. , Dhansay, M. A. , Suri, D. , Grahn, M. , Keuler, N. S. , Binkley, N. C. , & Tanumihardjo, S. A. (2022). Chronic and acute hypervitaminosis a are associated with suboptimal anthropometric measurements in a cohort of south African preschool children. The American Journal of Clinical Nutrition, 115, 1059–1068. 10.1093/ajcn/nqab422 [DOI] [PubMed] [Google Scholar]
- Sibulesky, L. , Hayes, K. C. , Pronczuk, A. , Weigel‐DiFranco, C. , Rosner, B. , & Berson, E. L. (1999). Safety of < 7500RE (< 25000IU) vitamin a daily in adults with retinitis pigmentosa. American Journal of Clinical Nutrition, 69, 656–663. 10.1093/ajcn/69.4.656 [DOI] [PubMed] [Google Scholar]
- Sichert‐Hellert, W. , Kersting, M. , & Manz, F. (2001). Changes in time‐trends of nutrient intake from fortified and non‐fortified food in German children and adolescents–15 year results of the DONALD study. Dortmund Nutritional and Anthropometric Longitudinally Designed Study. European Journal of Nutrition, 40, 49–55. 10.1007/pl00007385 [DOI] [PubMed] [Google Scholar]
- Silva, K. D. , Williams, C. M. , & Lovegrove, J. A. (2001). Use of water‐miscible retinyl palmitate as markers of chylomicrons gives earlier peak response of plasma retinyl esters compared with oil‐soluble retinyl palmitate. The British Journal of Nutrition, 86, 427–432. 10.1079/bjn2001433 [DOI] [PubMed] [Google Scholar]
- Smith, F. R. , & Goodman, D. W. S. (1976). Vitamin A transport in human vitamin A toxicity. New England Journal of Medicine, 294, 805–808. 10.1056/nejm197604082941503 [DOI] [PubMed] [Google Scholar]
- Sommer, A. , & Davidson, F. R. (2002). Assessment and control of vitamin A deficiency: The Annecy accords. Journal of Nutrition, 132, 2845s–2850s. 10.1093/jn/132.9.2845S [DOI] [PubMed] [Google Scholar]
- Sowa, M. , Mourao, L. , Sheftel, J. , Kaeppler, M. , Simons, G. , Grahn, M. , Davis, C. R. , von Lintig, J. , Simon, P. W. , Pixley, K. V. , & Tanumihardjo, S. A. (2020). Overlapping vitamin A interventions with Provitamin A carotenoids and preformed vitamin A cause excessive liver retinol Stores in Male Mongolian Gerbils. The Journal of Nutrition, 150, 2912–2923. 10.1093/jn/nxaa142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauber, P. M. , Sherry, B. , VanderJagt, D. J. , Bhagavan, H. N. , & Garry, P. J. (1991). A longitudinal study of the relationship between vitamin A supplementation and plasma retinol, retinyl esters, and liver enzyme activities in a healthy elderly population. American Journal of Clinical Nutrition, 54, 878–883. 10.1093/ajcn/54.5.878 [DOI] [PubMed] [Google Scholar]
- Stos, K. , Wozniak, A. , Rychlik, E. , Ziolkowska, I. , Glowala, A. , & Oltarzewski, M. (2021). Assessment of food supplement consumption in polish population of adults. Frontiers in Nutrition, 8, 733951. 10.3389/fnut.2021.733951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura, M. , Nakamura, M. , Ogawa, K. , Ikoma, Y. , & Yano, M. (2016). High vitamin C intake with high serum β‐Cryptoxanthin associated with lower risk for osteoporosis in post‐menopausal Japanese female subjects: Mikkabi cohort study. Journal of Nutritional Science and Vitaminology (Tokyo), 62, 185–191. 10.3177/jnsv.62.185 [DOI] [PubMed] [Google Scholar]
- Takahashi, N. (2022). Inhibitory effects of vitamin a and its derivatives on cancer cell growth not mediated by retinoic acid receptors. Biological & Pharmaceutical Bulletin, 45, 1213–1224. 10.1248/bpb.b22-00315 [DOI] [PubMed] [Google Scholar]
- Tang, G. (2010). Bioconversion of dietary provitamin a carotenoids to vitamin a in humans. The American Journal of Clinical Nutrition, 91, 1468s–1473s. 10.3945/ajcn.2010.28674G [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, G. , Qin, J. , Dolnikowski, G. G. , & Russell, R. M. (2003). Short‐term (intestinal) and long‐term (postintestinal) conversion of beta‐carotene to retinol in adults as assessed by a stable‐isotope reference method. The American Journal of Clinical Nutrition, 78, 259–266. 10.1093/ajcn/78.2.259 [DOI] [PubMed] [Google Scholar]
- Tanumihardjo, S. A. (2021). Biological evidence to define a vitamin A deficiency cutoff using total liver vitamin A reserves. Experimental Biology and Medicine (Maywood, N.J.), 246, 1045–1053. 10.1177/1535370221992731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanumihardjo, S. A. , Gannon, B. M. , Kaliwile, C. , & Chileshe, J. (2015). Hypercarotenodermia in Zambia: Which children turned orange during mango season? European Journal of Clinical Nutrition, 69, 1346–1349. 10.1038/ejcn.2015.143 [DOI] [PubMed] [Google Scholar]
- Tanumihardjo, S. A. , Russe, R. M. , Stephensen, C. B. , Gannon, B. M. , Craft, N. E. , Haskell, M. J. , Lietz, G. , Schulze, K. , & Raiten, D. J. (2016). Biomarkers of nutrition for development (BOND)‐vitamin a review. Journal of Nutrition, 146, 1816–1848. 10.3945/jn.115.229708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thürmann, P. A. , Steffen, J. , Zwernemann, C. , Aebischer, C.‐P. , Cohn, W. , Wendt, G. , & Schalch, W. (2002). Plasma concentration response to drinks containing β‐carotene as carrot juice or formulated as a water dispersible powder. European Journal of Nutrition, 41, 228–235. 10.1007/s00394-002-0381-3 [DOI] [PubMed] [Google Scholar]
- Toraishi, M. , Uenishi, K. , Iwamoto, J. , & Otani, T. (2021). Vitamin a intake is related to stress fracture occurrence in male collegiate long‐distance runners. The Journal of Sports Medicine and Physical Fitness, 61, 1509–1514. 10.23736/s0022-4707.20.11792-4 [DOI] [PubMed] [Google Scholar]
- Tsuchida, T. , & Friedman, S. L. (2017). Mechanisms of hepatic stellate cell activation. Nature Reviews Gastroenterology & Hepatology, 14, 397–411. 10.1038/nrgastro.2017.38 [DOI] [PubMed] [Google Scholar]
- Ukleja, A. , Scolapio, J. S. , McConnell, J. P. , Spivey, J. R. , Dickson, R. C. , Nguyen, J. H. , & O'Brien, P. C. (2002). Nutritional assessment of serum and hepatic vitamin a levels in patients with cirrhosis. JPEN: Journal of Parenteral and Enteral Nutrition, 26, 184–188. 10.1177/0148607102026003184 [DOI] [PubMed] [Google Scholar]
- Van Loo‐Bouwman, C. A. , Naber, T. H. , & Schaafsma, G. (2014). A review of vitamin a equivalency of β‐carotene in various food matrices for human consumption. The British Journal of Nutrition, 111, 2153–2166. 10.1017/s0007114514000166 [DOI] [PubMed] [Google Scholar]
- van Rossum, B.‐R. , Dinnissen, B. , Brants, D. , & Ocké . (2022). The diet of the Dutch. In Results of the Dutch National Food Consumption Survey 2012–2016. National Institute for Public Health and the Environment R. [Google Scholar]
- van Stuijvenberg, M. E. , Dhansay, M. A. , Nel, J. , Suri, D. , Grahn, M. , Davis, C. R. , & Tanumihardjo, S. A. (2019). South African preschool children habitually consuming sheep liver and exposed to vitamin a supplementation and fortification have hypervitaminotic a liver stores: A cohort study. American Journal of Clinical Nutrition, 110, 91–101. 10.1093/ajcn/nqy382 [DOI] [PubMed] [Google Scholar]
- Vandenput, L. , Johansson, H. , McCloskey, E. V. , Liu, E. , Åkesson, K. E. , Anderson, F. A. , Azagra, R. , Bager, C. L. , Beaudart, C. , Bischoff‐Ferrari, H. A. , Biver, E. , Bruyère, O. , Cauley, J. A. , Center, J. R. , Chapurlat, R. , Christiansen, C. , Cooper, C. , Crandall, C. J. , Cummings, S. R. , … Kanis, J. A. (2022). Update of the fracture risk prediction tool FRAX: A systematic review of potential cohorts and analysis plan. Osteoporosis International, 33, 2103–2136. 10.1007/s00198-022-06435-6 [DOI] [PubMed] [Google Scholar]
- VKM (Norwegian Scientific Committee for Food Safety) , 2015. Risk assessment of beta‐carotene in food supplements, opinion of the panel on nutrition, dietetic products, novel food and allergy of the Norwegian scientific Committee for Food Safety. https://vkm.no/download/18.2994e95b15cc54507161546f/1498143188656/e967639e8a.pdf [Google Scholar]
- Wake, S. K. , Zewotir, T. , & Muluneh, E. K. (2021). Nonlinear physical growth of children from infancy to middle adolescence in low‐ and middle‐income countries. Journal of Research in Health Sciences, 21, e00533. 10.34172/jrhs.2021.69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallenstein, M. B. , Shaw, G. M. , Yang, W. , & Carmichael, S. L. (2013). Periconceptional nutrient intakes and risks of orofacial clefts in California. Pediatric Research, 74, 457–465. 10.1038/pr.2013.115 [DOI] [PubMed] [Google Scholar]
- Wang, F. , Wang, N. , Gao, Y. , Zhou, Z. , Liu, W. , Pan, C. , Yin, P. , Yu, X. , & Tang, M. (2017). β‐Carotene suppresses osteoclastogenesis and bone resorption by suppressing NF‐κB signaling pathway. Life Sciences, 174, 15–20. 10.1016/j.lfs.2017.03.002 [DOI] [PubMed] [Google Scholar]
- Wang, X. D. , Liu, C. , Bronson, R. T. , Smith, D. E. , Krinsky, N. I. , & Russell, M. (1999). Retinoid signaling and activator protein‐1 expression in ferrets given beta‐carotene supplements and exposed to tobacco smoke. Journal of the National Cancer Institute, 91, 60–66. 10.1093/jnci/91.1.60 [DOI] [PubMed] [Google Scholar]
- Weber, K. A. , Yang, W. , Carmichael, S. L. , & Shaw, G. M. (2018). Nutrient intake in women before conception and risks of anophthalmia and microphthalmia in their offspring. Birth Defects Research, 110, 863–870. 10.1002/bdr2.1201 [DOI] [PubMed] [Google Scholar]
- White, S. C. , Atchison, K. A. , Gornbein, J. A. , Nattiv, A. , Paganini‐Hill, A. , & Service SK . (2006). Risk factors for fractures in older men and women: The leisure world cohort study. Gender Medicine, 3, 110–123. 10.1016/s1550-8579(06)80200-7 [DOI] [PubMed] [Google Scholar]
- WHO/FAO (World Health Organization/Food and Agriculture Organization of the United Nations) , 2004. Vitamin and mineral requirements in human nutrition: Report of a joint FAO/WHO expert consultation. [PubMed] [Google Scholar]
- WHO/IPCS (World Health Organization and International Programme on Chemical Safety) . (2004). IPCS risk assessment terminology. World Health Organization. https://apps.who.int/iris/handle/10665/42908 [Google Scholar]
- WHO/IPCS (World Health Organization/International Programme on Chemical Safety) . (1993). Biomarkers and risk assessment: Concepts and principles. Environmental Health Criteria, 155, 86. https://apps.who.int/iris/handle/10665/39037 [Google Scholar]
- Widjaja‐Adhi, M. A. K. , Lobo, G. P. , Golczak, M. , & Von Lintig, J. (2015). A genetic dissection of intestinal fat‐soluble vitamin and carotenoid absorption. Human Molecular Genetics, 24, 3206–3219. 10.1093/hmg/ddv072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willett, W. (2013). Nutritional epidemiology. Oxford University Press. [Google Scholar]
- Williams, A. M. , Tanumihardjo, S. A. , Rhodes, E. C. , Mapango, C. , Kazembe, B. , Phiri, F. , Kang'ombe, D. D. , Sheftel, J. , Orchardson, V. , Tripp, K. , & Suchdev, P. S. (2021). Vitamin A deficiency has declined in Malawi, but with evidence of elevated vitamin A in children. American Journal of Clinical Nutrition, 113, 854–864. 10.1093/ajcn/nqab004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolterbeek, A. P. , Schoevers, E. J. , Bruyntjes, J. P. , Rutten, A. A. , & Feron, V. J. (1995). Benzo[a]pyrene‐induced respiratory tract cancer in hamsters fed a diet rich in beta‐carotene. A histomorphological study. Journal of Environmental Pathology, Toxicology and Oncology, 14, 35–43. [PubMed] [Google Scholar]
- Woutersen, R. A. , Wolterbeek, A. P. , Appel, M. J. , van den Berg, H. , Goldbohm, R. A. , & Feron, V. J. (1999). Safety evaluation of synthetic beta‐carotene. Critical Reviews in Toxicology, 29, 515–542. 10.1080/10408449991349267 [DOI] [PubMed] [Google Scholar]
- Yang, J. , Zhang, Y. , Na, X. , & Zhao, A. (2022). β‐Carotene supplementation and risk of cardiovascular disease: A systematic review and meta‐analysis of randomized controlled trials. Nutrients, 14(6), 1284. 10.3390/nu14061284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, W. , Shaw, G. M. , Carmichael, S. L. , Rasmussen, S. A. , Waller, D. K. , Pober, B. R. , & Anderka, M. (2008). Nutrient intakes in women and congenital diaphragmatic hernia in their offspring. Birth defects research. Part A, Clinical and Molecular Teratology, 82, 131–138. 10.1002/bdra.20436 [DOI] [PubMed] [Google Scholar]
- Yee, M. M. F. , Chin, K. Y. , Ima‐Nirwana, S. , & Wong, S. K. (2021). Vitamin a and bone health: A review on current evidence. Molecules, 26(6), 1757. 10.3390/molecules26061757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung, D. L. , & Veen‐Baigent, M. J. (1972). Absorption of retinol and retinyl esters via lymph and the portal vein in the rat. Canadian Journal of Physiology and Pharmacology, 50, 753–760. 10.1139/y72-110 [DOI] [PubMed] [Google Scholar]
- Zafrani, E. S. , Bernuau, D. , & Feldmann, G. (1984). Peliosis‐like ultrastructural changes of the hepatic sinusoids in human chronic hypervitaminosis a: Report of three cases. Human Pathology, 15, 1166–1170. 10.1016/s0046-8177(84)80311-1 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Protocol for the Scientific Opinion on tolerable upper intake level for preformed vitamin A and β‐carotene
Methodological considerations in the calculation of intake estimates for preformed vitamin A and β‐carotene from the background diet in EU countries
EFSA’s intake assessment for preformed vitamin A
EFSA’s intake assessment for β‐carotene
Vitamin A intake data from competent authorities in European countries
ROB appraisal of studies on bone health
Additional information requested from study authors
Studies excluded at full‐text screening and during data extraction
Outcome of the public consultation


