Abstract
A 19-month-old Korean native cow died the following day after consuming new silage. Grossly, the liver showed enlargement, redness, and haemorrhages in all the lobes. Additionally, many of the bur-shaped fruits of the cocklebur (Xanthium strumarium) were found in the gastric contents. The histological evaluation confirmed centrilobular hepatic necrosis. Additionally, carboxyatractyloside (CATR), a material fatal to animals found in the cocklebur, was detected in the gastric contents using high-performance liquid chromatography-quadrupole-time of flight mass spectrometry (HPLC-Q-TOF-MS). Based on the pathological findings and analytical confirmation, CATR intoxication was diagnosed. Therefore, careful feeding and elimination of the cocklebur is essential for minimising economic loss.
Keywords: centrilobular hepatic necrosis, cow, silage
The cocklebur consists of two valid species (X. strumarium and X. spinosum). It is an exotic weed in the Korean peninsula and widely distributed throughout the world (Turgut et al. 2005). It grows in temperate regions, between the latitudes 53 °N and 33 °S. The fruits are hard, woody bur covered with hooked spines, ovoid-globose, and 1–2 cm long (Weaver and Lechowicz 1983). Cocklebur seeds and seedlings are rich in carboxyatractyloside (CATR), which is used as a plant growth inhibitor and is the toxic principle in the common cocklebur. Cocklebur fruits are rarely consumed by animals due to the burs; however, contamination of animal feed with mechanically harvested crops can cause accidental poisoning. Seed consumption of 0.30–0.45% of the bodyweight is usually fatal (Turgut et al. 2005).
Carboxyatractyloside, a sulfated diterpenoid glycoside, is an analogue of atractyloside (ATR) and a potent inhibitor of oxidative phosphorylation across the mitochondrial membranes in various organs (Kedrov et al. 2010). Due to the geometry and charge distribution that is similar to adenosine diphosphate (ADP), CATR inhibits the translocation of ADP and adenosine triphosphate (ATP) by phosphoryl transferase, blocking the oxidative phosphorylation and oxidative reactions in the Krebs cycle. The inhibition of the oxidative phosphorylation leads to consequences, such as severe hypoglycaemia, depression of the respiration, hypoxaemia, and tissue hypoxia throughout the whole body. Additionally, these consequences cause acute abdominal pain, nausea, vomiting, ataxia, convulsions, coma, and even death (Obatomi and Bach 1998; Turgut et al. 2005).
The liver is the primary target organ. Distinguished gross lesions include a pale liver with a spotted haemorrhagic pattern on the cut surface, and the accumulation of ascites in the abdominal cavity, related to the liver injury. Prominent histopathological findings are acute (or subacute), severe centrilobular to massive hepatic necrosis with haemorrhage, gastroenteritis, cardiac haemorrhage, and renal necrosis (Sebastian 2007). The clinical signs usually appear within a few hours or could take up to three days after ingestion. Although some animals recover from the poisoning, it could take weeks, and additional chronic hepatitis is often observed (Turgut et al. 2005).
Case description
In this case, we describe the pathological findings and present the analytical confirmation of CATR poisoning from cocklebur ingestion in a Korean native cow which is, to the best of our knowledge, the first case of such a poisoning in South Korea (Republic of Korea).
A new silage was fed to a farm breeding Korean native cattle. Most of them avoided the new silage. However, two cows ate well, and died the next day. The carcass of one of the cows, a 19-month-old, was requested by the Animal and Plant Quarantine Agency (APQA) for disease diagnosis in December 2019. The cowshed was a farm that bred 27 cattle in Ulsan, South Korea.
On necropsy, redness all over the lungs and multiple petechial haemorrhages in the heart were observed (Figure 1A). One-centimetre-long haemorrhages were also observed in the vascular region near the heart and omasal mucosa. The liver was enlarged, red, and showed haemorrhages in all the lobes (Figure 1B), and bur-shaped fruits were found in the ruminal contents (Figure 1C). The gastric contents were collected for toxicological analysis during the post-mortem examination and stored at –20 °C until analysis. For the histopathological examination, various visceral organs were fixed in 10% neutral buffered formalin, and paraffin-embedded samples were stained with haematoxylin and eosin (H&E) (Roh et al. 2020). The histopathological examination showed severe haemorrhages and congestion in the alveolar lumen, and haemorrhages, and infiltration of neutrophils in the myocardium. Centrilobular hepatic necrosis and multiple round-shaped brownish granules were also observed in the liver (Figure 1D). In addition, the hepatic blood vessels were damaged, resulting in leakage of red blood cells and fibrin. Eosinophilic materials were partially filled between the renal tubules of the kidney.
Figure 1. Gross and histopathological findings on necropsy.
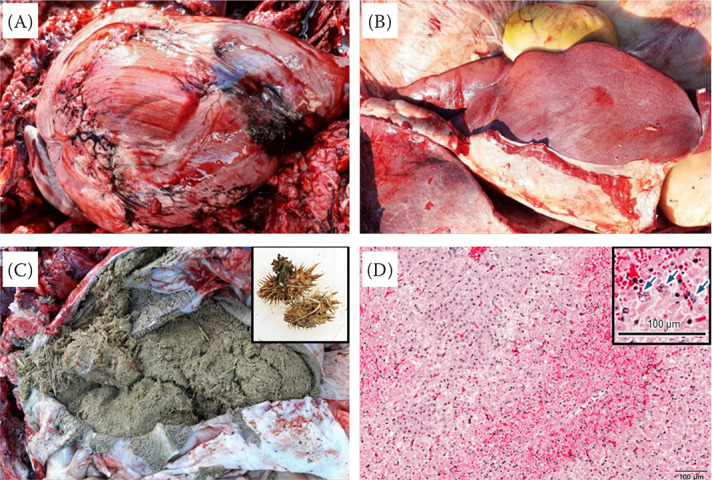
(A) Haemorrhage in the heart. Multiple petechial haemorrhages were observed in the myocardium and cardiac adipose tissue. (B) Damaged liver of the cow. Enlargement, redness, and haemorrhage were seen in all the hepatic lobes. (C) Gastric contents in the rumen. Numerous bur-shaped fruits of cocklebur were found in rumen. Inset shows the cocklebur fruits in the rumen. (D) Centrilobular hepatic necrosis. Round-shaped brownish granules and damaged hepatic blood vessels were observed on the H&E-stained sections. The inset shows round-shaped brownish granules (arrows)
The extraction and detection of poisonous substances from the gastric contents, cocklebur-supplemented silage, and dried cocklebur fruits were performed for confirmation of CATR. Twenty grams of the gastric contents and silage were extracted with 100 ml of ethanol and one gram of dried cocklebur fruits underwent 5 ml of methanol extraction under ultra-sonication for 1 hour. Then they were filtered and dried under an N2 stream. The residue was reconstituted with 200 μl methanol and a 5 μl aliquot was injected into the high-performance liquid chromatography-quadrupole-time of flight mass spectrometry (HPLC-Q-TOF-MS) system. The CATR was analysed with an AB Sciex X500R QTOF high-resolution mass spectrometer (AB Sciex, Framingham, MA, USA), coupled with an Agilent 1290 infinity ultra-high performance liquid chromatography (UHPLC) system (Agilent, Santa Clara, CA, USA). SCIEX OS (v1.5; AB Sciex, Framingham, MA, USA) was used for the data acquisition and identification. A Cadenza CD-18TM column (3 μm, 150 mm × 2.0 mm) was used for the analysis. The mobile phases were tested with 0.1% formic acid in distilled water (A) and 0.1% formic acid in acetonitrile (B). To initiate, solvent B was used to equilibrate the system at 10% for 0.5 min, and it was then increased to 90% within 10 min, after which it was maintained for 5 minutes. The flow rate was 0.4 ml/min, and the column temperature was fixed at 40 °C. The electrospray ionisation (ESI) spray mode was operated in a negative mode. The other liquid chromatography–tandem mass spectrometry (LC-MS/MS) analytical conditions were as follows: source temperature, 550 °C; capillary voltage, 5 500 V; curtain gas pressure, 20 psi; nebuliser gas pressure, 40 psi; and auxiliary gas pressure, 70 psi. In the HPLC-Q-TOF-MS analysis, each CATR was eluted at approximately 6.26, 6.44, and 6.42 min from the dried cocklebur fruits, cocklebur-supplemented silage, and gastric contents, respectively. Furthermore, mono-desulfated CATR (CATR-SO3) was co-eluted at approximately 7.53, 7.51, and 7.52 min in each sample (Figure 2). In addition, the CATR and CATR-SO3 were detected in direct infusion after chromatographic separation at the above-mentioned retention times. Although a quantitative analysis was not carried out in this study, the relative peak intensities of the CATR and CATR-SO3 in the chromatograms showed that the contents of the target analytes were increased in order of the gastric contents, silage, and dried cocklebur fruits. In the mass spectra of the CATR (Figure 3A,C,E), the unfragmented CATR showed a mass/charge (m/z) of approximately 769.2, with major fragmented forms, which were 689.2 m/z of CATR-SO3 and 645.2 m/z of mono-decarboxylated CATR-SO3 (CATR-COOH-SO3). The mass spectra of CATR-SO3 (Figure 3B,D,F) showed an m/z of approximately 689.2 and 645.2 of CATR-COOH-SO3 with various fragmented derivatives detected. The CATR derivatives increase during degradation by ageing and desiccation of the cocklebur (Chen et al. 2013). In this study, a desulfated CATR derivative (CATR-SO3) was also identified in the dried cocklebur fruits, silage, and gastric contents (Figure 2). Considering these findings, it was assumed that internal degradation occurred in the cocklebur.
Figure 2. Chromatograms of the CATR and CATR-SO3 in the dried cocklebur fruits, silage, and gastric contents based on liquid chromatography.
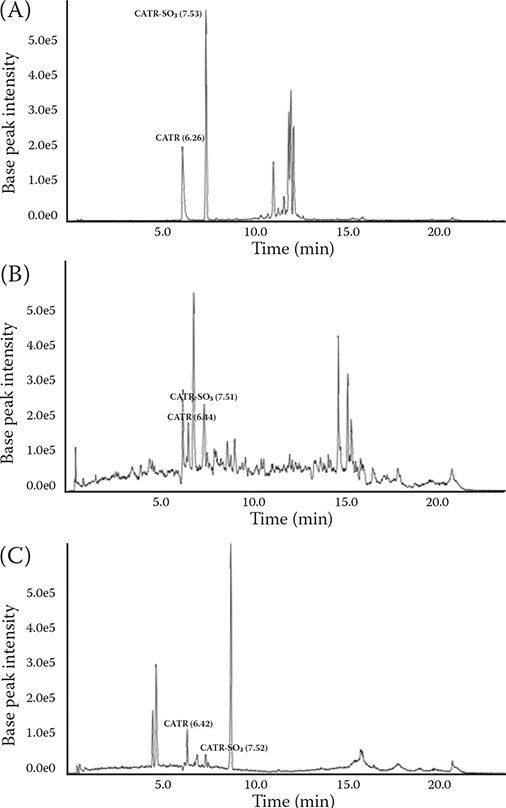
(A) Chromatogram in the dried cocklebur fruits. CATR and its mono-desulfated derivatives were observed at 6.26 and 7.53 min, respectively. (B) Chromatogram of the silage mixed by cocklebur. CATR and its mono-desulfated derivatives were observed at 6.44 and 7.51 min, respectively. (C) Chromatogram of the gastric contents. CATR and its mono-desulfated derivatives were observed at 6.42 and 7.52 min, respectively. CATR, carboxyatractyloside; CATR-SO3, mono-desulfated carboxyatractyloside
Figure 3. Mass spectra of the CATR and its derivatives separated from the cocklebur fruits, silage, and gastric contents based on the QTOF (quadrupole time-of-flight) high-resolution mass spectrometry.
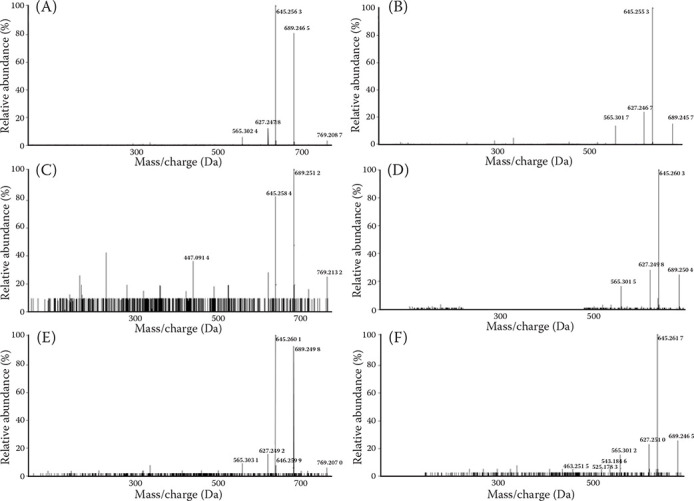
(A) Mass spectrum of the CATR separated from the cocklebur fruits. (B) Mass spectrum of the CATR-SO3 separated from the cocklebur fruits. (C) Mass spectrum of the CATR separated from the silage mixed with the cocklebur. (D) Mass spectrum of the CATR-SO3 separated from the silage mixed with the cocklebur. (E) Mass spectrum of the CATR separated from the gastric contents. (F) Mass spectrum of the CATR-SO3 separated from the gastric contents. CATR, carboxyatractyloside; CATR-SO3, mono-desulfated carboxyatractyloside
The pathological findings, presence of cocklebur in the silage and gastric contents, and analytical confirmation of CATR by HPLC-Q-TOF-MS suggested CATR poisoning as the cause of death. This case was diagnosed as a case of acute death with severe haemorrhagic hepatitis and systemic damage caused by CATR poisoning.
DISCUSSION
Plants of the genera Atractylis, Callilepsis, Iphiona, Pascalia, and Xanthium, commonly affiliated with the family Asteraceae, contain poisonous substances such as ATR and CATR (Obatomi and Bach 1998). In animals, cocklebur poisoning is reported mainly in cattle and pigs and rarely in dogs and horses (Machado et al. 2021). Previously described cases of cocklebur intoxication included clinical signs, such as nausea, ataxia, weakness, prostration, depression, dyspnoea, hypothermia, opisthotonos, blindness, hypersensitivity, limb and neck spasms, and convulsions (Witte et al. 1990). Furthermore, the bur-shaped fruits in ruminal contents caused hepatic congestion, myocardial haemorrhage, pulmonary oedema, and haemorrhage as gross lesions during necropsy. Hepatic centrilobular degeneration with necrosis has also been observed on histopathological examinations (Witte et al. 1990; Turgut et al. 2005; Botha et al. 2014).
In the present case, the two dead cows had no history of disease during the previous six months before the incident; they were healthy cattle, and died suddenly. The dead cows showed clinical signs similar to previous cases of CATR poisoning, such as depression, inappetence, ataxia, and weakness, after consuming a new silage. The symptoms were acute and surfaced within a few hours. The above-mentioned signs that appeared in the present case were supposedly associated with insufficient glucose supply in various organs. Based on previous studies, ATP production by oxidative phosphorylation in the mitochondrial membrane is hindered by CATR activity, and it accelerates glycolysis as a feedback mechanism, continuously reducing the blood glucose levels. Sequentially, to replenish the low glucose level, glycogenolysis is accelerated, which causes a temporary hyperglycaemic state. During this state, lactic acidosis is further potentiated, causing reduced glucose levels by CATR via the gluconeogenesis pathway (Krejci and Koechel 1992; Turgut et al. 2005). Moreover, in ruminants, since only 10% of the glucose is absorbed through the digestive tract, gluconeogenesis is more important than in non-ruminants (Young 1977). Therefore, the CATR activity is very deleterious in cattle. However, as blood samples were not collected due to the sudden death, the blood data, including the glucose level and acidity, were not analysed in this case.
In the present case, the gross and histopathological findings, such as severe haemorrhages and congestion in the alveolar lumen, myocardial haemorrhages, and centrilobular hepatic necrosis, were similar to lesions of previously reported cases of cocklebur poisoning. However, since centrilobular hepatic necrosis commonly occurs during intoxication, a differential diagnosis with several poisonous plants is essential. Plants of the genera Senecio, Crotalaria, and Heliotropium, are known to cause centrilobular hepatic necrosis, similar to cocklebur (Benninger et al. 1999; Schuppan et al. 1999; Botha et al. 2014). However, Cestrum and Heliotropium were ruled out in this case because they cannot survive the winter in South Korea. Other plants, including those of the Asteraceae family (containing ATR and CATR), were ruled out as none were found in the silage and gastric contents of the cow. The analysis for bacteria, viruses, and parasites capable of causing hepatic necrosis also showed negative results, except for Escherichia coli and Clostridium perfringens type A in the large intestine (data not shown). The exclusion of other potentials causes for centrilobular hepatic necrosis using a differential diagnosis, along with the large number of cockleburs found in the cow and analytical confirmation of CATR led to the confirmation of CATR intoxication.
The minimal lethal doses per day of cocklebur are reported as 0.3% and 1.5% of the body weight for the seeds and the seedlings, respectively (Witte et al. 1990). In this case, CATR and its derivatives were qualitatively confirmed using HPLC-Q-TOF-MS. Based on the statistical data of National Institute of Animal Science (NIAS) for standard body weight of Korean native cattle, the minimal lethal dose per day of cocklebur for a 19-month-old cow was calculated as 1.23 kg. In this case, the rumen was full of gastric contents and the amount of cocklebur found in the gastric contents was sufficient to kill the animal.
To the best of our knowledge, this critical report provides the first case of CATR poisoning in cattle in South Korea. CATR poisoning cases of other countries, the grazing livestock ingested cocklebur. In this case, however, the cattle consumed a silage containing cocklebur, which likely got mixed during the production process. Cattle usually avoid the burr-shaped fruit; however, they can eat it even if it has hooked spines, as in this case. Therefore, it is important to produce and manage feed carefully. The cocklebur widely inhabits wild fields as the climate in South Korea supports its growth. It should, therefore, be controlled in the cowshed using chemical methods with herbicides, such as glyphosate and dicamba, and physically controlled with its elimination before flowering to prevent mixing into the bulky feed that includes silage (Corbett et al. 2004; Soltani et al. 2010). Additionally, in case of grazing, as grazers have a higher potential for exposure to poisonous plants, including cockleburs, more careful feeding, and the broad elimination of poisonous plants in the pasture is essential for minimising economic losses due to death.
Funding Statement
Supported by a grant (F-1543069-2022-23-01) from the Animal and Plant Quarantine Agency (APQA), Ministry of Agriculture, Food and Rural Affairs (MAFRA), Republic of Korea.
Conflict of interest
The authors declare no conflict of interest.
REFERENCES
- Benninger J, Schneider HT, Schuppan D, Kirchner T, Hahn EG. Acute hepatitis induced by greater celandine (Chelidonium majus). Gastroenterology. 1999 Nov;117(5):1234-7. [DOI] [PubMed] [Google Scholar]
- Botha CJ, Lessing D, Rosemann M, van Wilpe E, Williams JH. Analytical confirmation of Xanthium strumarium poisoning in cattle. J Vet Diagn Invest. 2014 Sep;26(5):640-5. [DOI] [PubMed] [Google Scholar]
- Chen LY, Hu A, Chang CJ. The degradation mechanism of toxic atractyloside in herbal medicines by decoction. Molecules. 2013 Feb 5;18(2):2018-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett JL, Askew SD, Thomas WE, Wilcut JW. Weed efficiency evaluations for bromoxynil, glufosinate, glyphosate, pyrithiobac, and sulfosate. Weed Technol. 2004;18(2):443-53. [Google Scholar]
- Kedrov A, Hellawell AM, Klosin A, Broadhurst RB, Kunji ER, Muller DJ. Probing the interactions of carboxy-atractyloside and atractyloside with the yeast mitochondrial ADP/ATP carrier. Structure. 2010 Jan 13;18(1):39-46. [DOI] [PubMed] [Google Scholar]
- Krejci ME, Koechel DA. Acute effects of carboxyatractyloside and stevioside, inhibitors of mitochondrial ADP/ATP translocation, on renal function and ultrastructure in pentobarbital-anesthetized dogs. Toxicology. 1992;72(3):299-313. [DOI] [PubMed] [Google Scholar]
- Machado M, Queiroz CRR, Wilson TM, Sousa DER, Castro MB, Saravia A, Lee ST, Armien AG, Barros SS, Riet-Correa F. Endemic Xanthium strumarium poisoning in cattle in flooded areas of the Araguari River, Minas Gerais, Brazil. Toxicon. 2021 Sep;200:23-9. [DOI] [PubMed] [Google Scholar]
- Obatomi DK, Bach PH. Biochemistry and toxicology of the diterpenoid glycoside atractyloside. Food Chem Toxicol. 1998 Apr;36(4):335-46. [DOI] [PubMed] [Google Scholar]
- Roh SG, Jang YH, Kim J, Lee K, So B, Choi EJ. A rare case of bovine tuberculosis caused by Mycobacterium bovis in a domestic rabbit. Korean J Vet Res. 2020 Jun 2;60(2):85-8. [Google Scholar]
- Sebastian MM. Chapter 90. Role of pathology in diagnosis. In: Gupta RC, editor. Veterinary toxicology: Basic and clinical principles. Amsterdam: Elsevier; 2007. p. 1100-36. [Google Scholar]
- Schuppan D, Jia JD, Brinkhaus B, Hahn EG. Herbal products for liver diseases: A therapeutic challenge for the new millennium. Hepatology. 1999 Oct;30(4):1099-104. [DOI] [PubMed] [Google Scholar]
- Soltani N, Shropshire C, Sikkema PH. Control of common cocklebur (Xanthium strumarium L.) in corn. Can J Plant Sci. 2010;90(6):933-8. [Google Scholar]
- Turgut M, Alhan CC, Gurgoze M, Kurt A, Dogan Y, Tekatli M, Akpolat N, Aygun AD. Carboxyatractyloside poisoning in humans. Ann Trop Paediatr. 2005 Jun;25(2):125-34. [DOI] [PubMed] [Google Scholar]
- Weaver SE, Lechowicz MJ. The biology of canandian weeds: 56. Xanthium strumarium L. Can J Plant Sci. 1983;63(1):211-25. [Google Scholar]
- Witte ST, Osweiler GD, Stahr HM, Mobley G. Cocklebur toxicosis in cattle associated with the consumption of mature Xanthium strumarium. J Vet Diagn Invest. 1990 Oct;2(4):263-7. [DOI] [PubMed] [Google Scholar]
- Young JW. Gluconeogenesis in cattle: Significance and methodology. J Dairy Sci. 1977 Jan;60(1):1-15. [DOI] [PubMed] [Google Scholar]


