Abstract
Background:
Given emerging evidence of rapid non-genomic cytoprotective effects of triiodothyronine (T3), we evaluated the resuscitative efficacy of two nanoparticle formulations of T3 (T3np) designed to prolong cell membrane receptor-mediated signaling.
Methods:
Swine (n=40) were randomized to intravenous vehicle (empty np), EPI (0.015 mg/kg), T3np (0.125 mg/kg), or T3np loaded with phosphocreatine (T3np+PCr; 0.125 mg/kg) during CPR following 7-min cardiac arrest (n=10/group). Hemodynamics and biomarkers of heart (cardiac troponin I; cTnI) and brain (neuron-specific enolase; NSE) injury were assessed for up to 4-hours post-ROSC, at which time the heart and brain were collected for post-mortem analysis.
Results:
Compared with vehicle (4/10), the rate of ROSC was higher in swine receiving T3np (10/10; p<0.01), T3np+PCr (8/10; p=0.08) or EPI (10/10; p<0.01) during CPR. Although time to ROSC and survival duration were comparable between groups, EPI was associated with a ~2-fold higher post-ROSC concentration of cTnI vs. T3np and T3np+PCr and the early post-ROSC rise in NSE and neuronal injury were attenuated in T3np-treated vs. EPI-treated animals. Analysis of hippocampal ultrastructure revealed deterioration of mitochondrial integrity, reduced active zone length, and increased axonal vacuolization in EPI-treated animals vs. controls. However, the frequency of these abnormalities was diminished in animals resuscitated with T3np.
Conclusions:
T3np achieved a ROSC rate and post-ROSC survival that was superior to vehicle and comparable to EPI. The attenuation of selected biomarkers of cardiac and neurologic injury at individual early post-ROSC timepoints in T3np-treated vs. EPI-treated animals suggests that T3np administration during CPR may lead to more favorable outcomes in cardiac arrest.
Keywords: Cardiac Arrest, Thyroid Hormone, Triiodothyronine, Nanoparticles, Phosphocreatine, Ischemic Injury
Introduction
Nearly 600,000 adults suffer from cardiac arrest in the United States each year, with the majority of events occurring suddenly in the out-of-hospital setting in seemingly healthy individuals1. Unfortunately, despite improved basic and advanced life support interventions that have increased the likelihood of achieving return of spontaneous circulation (ROSC), fewer than ~10% of these patients will survive with favorable neurologic function2. Although efforts to address this problem have been directed towards several links in the chain of survival3, the use of epinephrine (EPI) to enhance the likelihood of achieving ROSC has been increasingly scrutinized based on a growing body of evidence that questions its safety and efficacy in this setting4, 5. This was recently highlighted by the findings of the blinded and randomized PARAMEDIC2 clinical trial, in which EPI was shown to increase the rate of ROSC and 30-day survival without improving survival with favorable neurologic outcome6. As a result, there is growing interest in identifying alternative therapies that can be administered during cardiopulmonary resuscitation (CPR) to enhance the likelihood of achieving ROSC and improve clinical outcomes in patients who suffer from cardiac arrest7, 8.
Triiodothyronine (T3), the most active form of thyroid hormone, is an interesting candidate for such a therapy based on emerging evidence that T3 exerts rapid, non-genomic cytoprotective effects9, 10. These actions, which are independent of nuclear thyroid hormone receptors, are mediated by a receptor on the plasma membrane integrin αvβ3 and may promote successful cardiac resuscitation while protecting cells from ischemic injury through a number of mechanisms including regulation of membrane ion pumps, alterations in mitochondrial function, and/or activation of PI3K/AKT and ERK1/2 signaling pathways9, 11, 12. However, the short half-life of T3 in the blood, loss of non-genomic T3 activity upon translocation to the nucleus, and potentially deleterious effects of T3-mediated genomic effects have dampened enthusiasm for the therapeutic potential of free T3 or synthetic analogues in the setting of acute ischemic injury13–15. To address these limitations, we recently developed a novel nanoparticle formulation of T3 (T3np) designed to limit T3 activity to the cell surface αvβ3, prevent its translocation into the cell nucleus, and prolong cell membrane receptor-mediated effects. Our prior work describing the formulation and characterization of T3np demonstrated preferential binding to plasma membrane αvβ3 receptors, as well as the feasibility of encapsulating phosphocreatine (T3np+PCr) to provide an additional source of high-energy phosphates to support mitochondrial respiration in cells subjected to hypoxia16. In addition, T3nps exhibited cardioprotective potential in isolated cardiomyocytes subjected to hypoxia and rapid localization to the heart and brain following intravenous administration in rodent biodistribution studies16. However, the potential therapeutic effects of T3np and T3np+PCr during acute ischemia and reperfusion have yet to be investigated in a clinically relevant large animal model of cardiac arrest and resuscitation.
Accordingly, the objective of the present study was to evaluate the efficacy of T3np and T3np+PCr as novel therapeutic agents to promote successful cardiac resuscitation while minimizing multi-organ injury associated with systemic ischemia and reperfusion in the setting of cardiac arrest. To achieve this goal, a blinded, randomized, and vehicle-controlled preclinical trial was performed to directly compare the resuscitative efficacy of T3np, T3np+PCr, and EPI in a porcine model of cardiac arrest. In animals that were successfully resuscitated, assessment of early post-ROSC neuronal injury was performed to gain preliminary insight regarding the potential neuroprotective effects of T3nps. In addition, the effects of T3np and T3np+PCr were investigated in isolated neurons subjected to hypoxia in vitro to further elucidate mechanisms underlying T3np-mediated neuroprotection. The results provide strong initial support for pursuing development of T3np therapy as a novel approach to improve clinical outcomes after cardiac arrest.
Methods
All procedures and protocols utilized for in vivo animal experiments conformed to institutional guidelines for the care and use of animals in research and were approved by the Institutional Animal Care and Use Committee at the University at Buffalo in Buffalo, NY. T3np and T3np+PCr were synthesized at the Pharmaceutical Research Institute at the Albany College of Pharmacy and Health Sciences as previously described15, 17 and summarized in Supplemental Figure 1. A total of 47 male Yorkshire-cross farm-bred pigs were studied in the closed-chest state using a protocol summarized in Figure 1 and described in detail in Supplemental Materials. Briefly, animals were subjected to 7-min of cardiac arrest, followed by up to 20-min of CPR with manual chest compressions, mechanical ventilation, and defibrillation. Animals were randomized to empty NP (vehicle), EPI (0.015 mg/kg), T3np (0.125 mg/kg), or T3np+PCr (0.125 mg/kg) in a blinded fashion. Each drug was administered intravenously 1-min after initial defibrillation (2-min CPR) and every 3-min thereafter for up to 20-min or until return of spontaneous circulation (ROSC; unassisted arterial systolic blood pressure (SBP) of ≥ 80 mmHg for at least 1 min). If ROSC was achieved, hemodynamics, echocardiography, and blood sampling were performed for up to 4 hours post-ROSC, at which time the heart and brain were collected for post-mortem analysis. Please see Supplemental Materials for complete methodological details.
Figure 1: Experimental Protocol for Blinded, Randomized, and Vehicle-Controlled Preclinical Trial of T3np, T3np+PCr, and EPI in Swine Subjected to Cardiac Arrest.
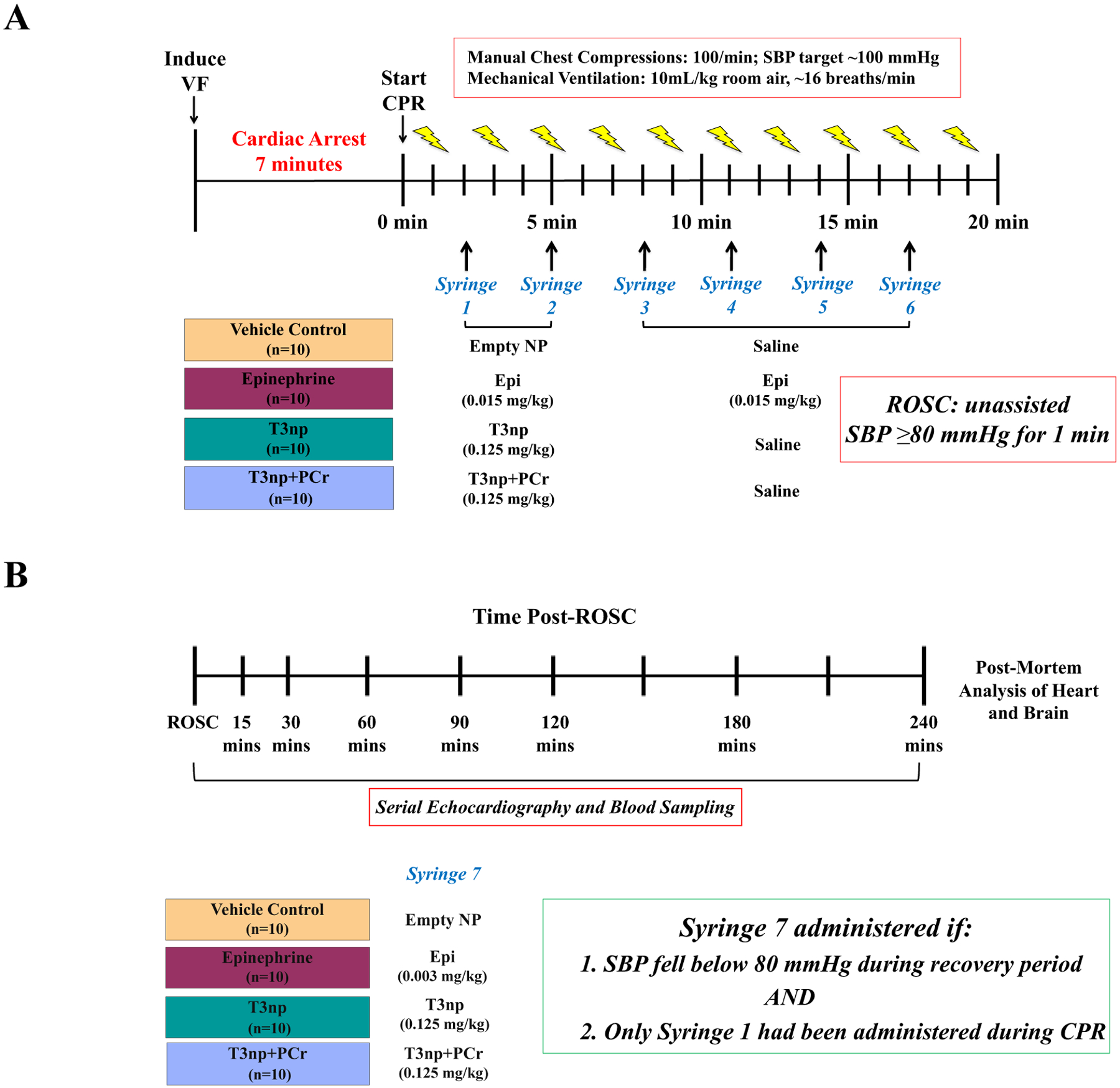
(A) Swine were subjected to electrical induction of ventricular fibrillation and 7-minutes of untreated cardiac arrest, followed by initiation of CPR with manual chest compressions and mechanical ventilation. Defibrillation was attempted 1-minute after the onset of CPR and every 2-minutes thereafter as needed. Animals that were not successfully resuscitated by CPR and defibrillation alone were randomized to receive empty NP (vehicle), EPI, T3np, or T3np+PCr at the 2-minute CPR timepoint via intravenous infusion in a blinded fashion. Resuscitation efforts continued for up to 20 minutes or until the ROSC was achieved (defined as unassisted SBP ≥ 80 mmHg for at least 1 minute). (B) Following ROSC, animals were followed for up to 4-hours, during which time an additional dose of the test drug was given if hypotension developed and only 1 dose of the drug had been given during the resuscitation period. Serial echocardiography and blood sampling were performed throughout the 4-hour post-resuscitation period, after which animals were humanely euthanized and tissue was collected for post-mortem analysis. Please see text for additional details. CPR = cardiopulmonary resuscitation; EPI = epinephrine; ROSC = return of spontaneous circulation; NP = nanoparticles; SBP = systolic blood pressure; T3np = triiodothyronine nanoparticles; T3np+PCr = triiodothyronine nanoparticles with encapsulated phosphocreatine.
Results
Comparative Resuscitative Efficacy of EPI, T3np, T3np+PCr:
Treatment groups were well-matched with respect to body mass, CPR quality (i.e., coronary perfusion pressure during CPR), number of defibrillator shocks needed to terminate VF, and ECG rhythm at the time of drug administration (Table). Animals randomized to the vehicle-treated control group exhibited a ROSC rate of 40% (4/10), with an average time to ROSC of 200±10 seconds. The rate of ROSC was higher in animals that received EPI (100%, 10/10; p<0.01), T3np (100%, 10/10; p<0.01), or T3np+PCr (80%, 8/10, p=0.08), with a similar time to ROSC in all groups (Figure 2A). All animals that achieved ROSC did so after receiving a single dose of the assigned drug treatment. As a result, a second dose of the test drug was available for all animals if hypotension developed in the post-ROSC period; this occurred in 9/10 animals in the EPI group (12.1±0.9 min post-ROSC), 10/10 animals in the T3np group (14.1±1.2 min post-ROSC), and 8/8 animals in the T3np+PCr group (20.7±5.4 min post-ROSC; p=0.35 between groups). Due to the absence of additional hemodynamic support during the post-ROSC period, a majority of animals in all treatment groups died from hemodynamic collapse prior to the 4-hour post-ROSC timepoint (Figure 2B). Gradual deterioration of arterial blood pressure progressing to asystole was the cause of death in all animals that initially achieved ROSC but failed to survive to the 4-hour post-ROSC timepoint. Average survival duration was comparable between EPI-treated (116±26 min post-ROSC), T3np-treated (122±30 min post-ROSC), and T3np+PCr-treated (119±33 min post-ROSC) animals. Early after ROSC, T3np-treated animals exhibited a lower heart rate, left ventricular (LV) dP/dtmax, and LV ejection fraction vs. EPI-treated animals, but between-group differences in hemodynamic parameters and LV function were no longer apparent ~30–60 min post-ROSC (Figure 3 and Supplemental Table 1).
Table:
Baseline Characteristics of Swine Randomized to Receive Vehicle, EPI, T3np, or T3np+PCr During CPR
| Vehicle (n=10) |
EPI (n=10) |
T3np (n=10) |
T3np+PCr (n=10) |
|
|---|---|---|---|---|
|
Body Mass
(kg) |
31.8 ± 2.6 | 30.8 ± 2.2 | 29.6 ± 2.5 | 29.4 ± 1.9 |
|
CPP at 1-min CPR
(mmHg) |
10.9 ± 1.0 | 9.1 ± 1.1 | 11.8 ± 1.5 | 11.4 ± 1.3 |
| # of Shocks Needed to Terminate VF | 1.1 ± 0.1 | 1.1 ± 0.1 | 1.2 ± 0.1 | 1.4 ± 0.4 |
|
ECG at 2-min CPR
(VF/PEA/Pseudo-PEA) |
0/5/5 | 0/7/3 | 0/5/5 | 1/5/4 |
Values are Mean ± SEM; EPI: epinephrine, T3np: triiodothyronine nanoparticles, PCr: phosphocreatine, CPP: coronary perfusion pressure, CPR: cardiopulmonary resuscitation, VF: ventricular fibrillation, PEA: pulseless electrical activity.
Figure 2: Rate of ROSC and Post-ROSC Survival in Swine Resuscitated with T3np, T3np+PCR, and EPI After Cardiac Arrest.
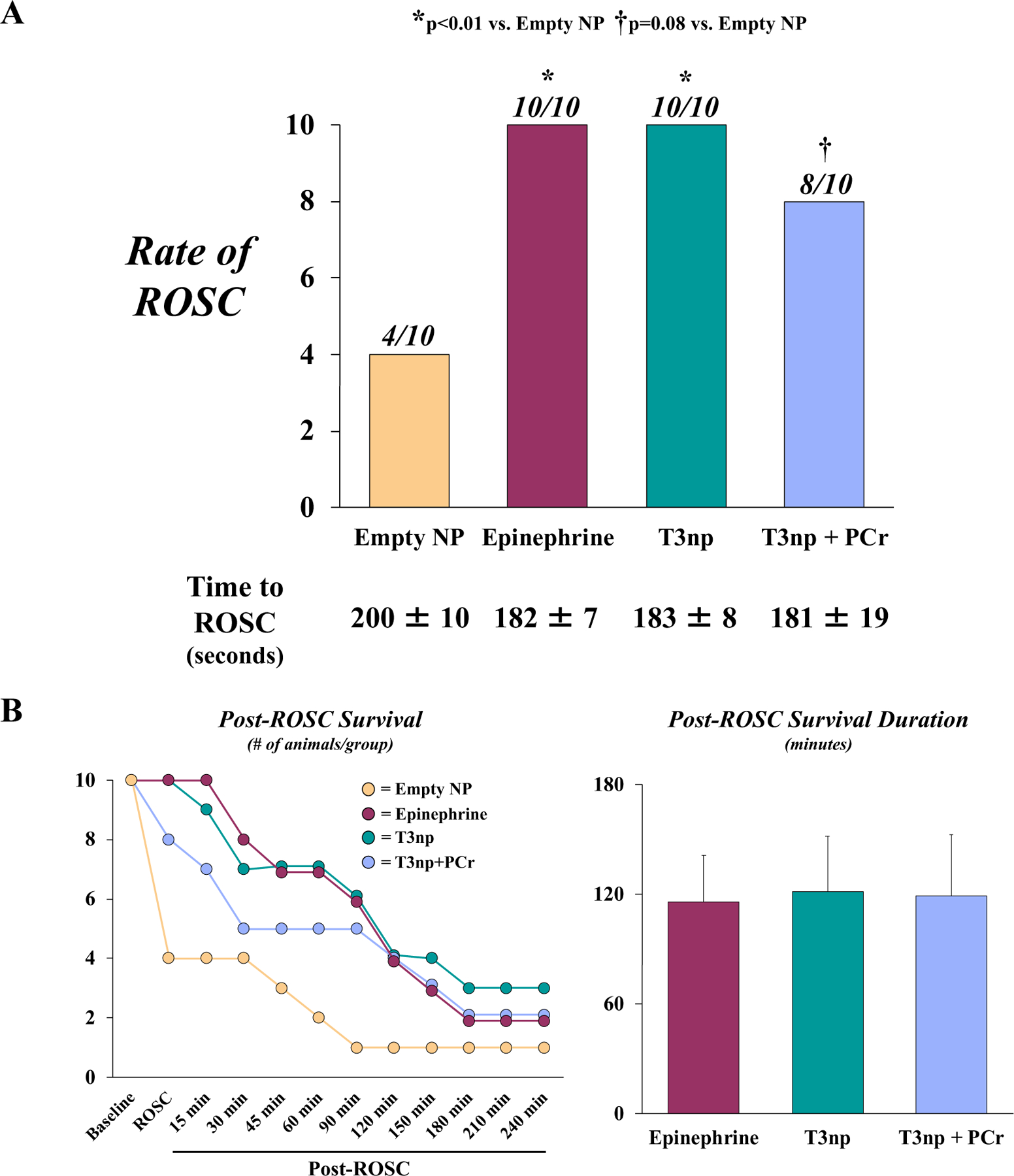
(A) Compared with vehicle treatment, EPI, T3np, and T3np+PCr administration during CPR resulted in a significant improvement in the rate of ROSC that was not different between treatment groups. The time needed to achieve ROSC was also similar among treatment groups. (B) The timecourse of post-ROSC survival among each group (left panel) reveals a similar pattern among EPI-, T3np-, and T3np+PCR-treated animals such that a majority of animals died prior to the 4-hour post-ROSC timepoint. Overall, average post-ROSC survival duration did not differ among treatment groups (right panel). Values are mean±SEM. *p<0.05 vs. empty NP; †p=0.08 vs. empty NP. ROSC = return of spontaneous circulation; NP = nanoparticles; T3np = triiodothyronine nanoparticles; T3np+PCr = triiodothyronine nanoparticles with encapsulated phosphocreatine.
Figure 3: Post-ROSC Hemodynamics and Left Ventricular Function in Swine Resuscitated with T3np, T3np+PCR, and EPI After Cardiac Arrest.
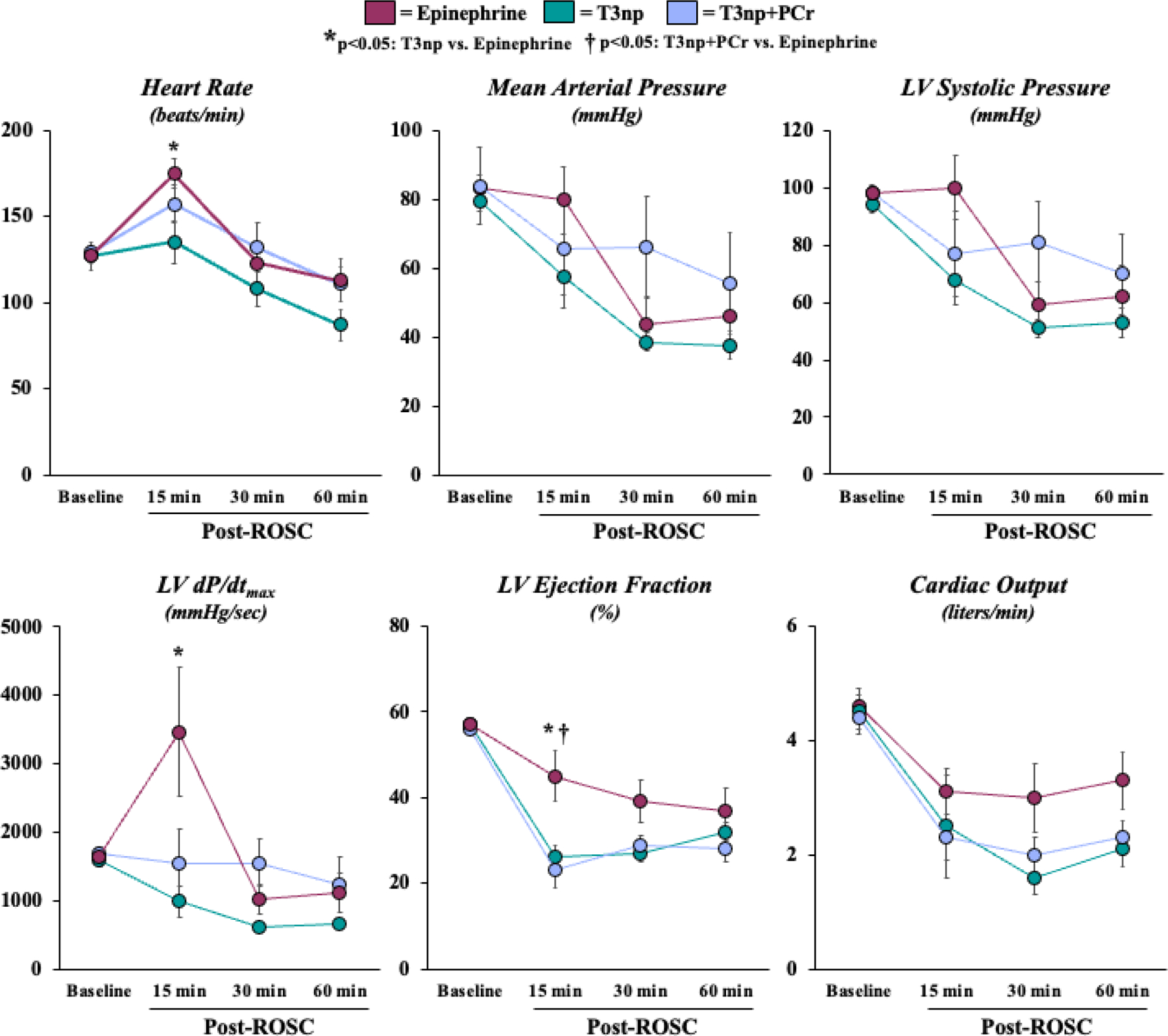
Hemodynamic and echocardiographic parameters were similar among treatment groups prior to cardiac arrest and were generally similar throughout the post-resuscitation period. Notable differences among groups were observed only at the 15-minute post-ROSC timepoint, as EPI-treated animals exhibited an elevated heart rate, LV dP/dtmax, and LV ejection fraction relative to T3np-treated animals. Values are mean±SEM. *p<0.05: T3np vs. EPI; †p<0.05: T3np+PCr vs. EPI. LV = left ventricular; ROSC = return of spontaneous circulation; T3np = triiodothyronine nanoparticles; T3np+PCr = triiodothyronine nanoparticles with encapsulated phosphocreatine.
Post-ROSC Injury to the Heart and Brain:
During the first hour post-ROSC, EPI-treated animals exhibited a significant rise in cTnI concentrations in the peripheral venous (from 57±21 ng/L at baseline to 882±153 ng/L 60 min post-ROSC, p<0.001) and coronary venous (from 104±41 ng/L at baseline to 1516±257 ng/L 60 min post-ROSC, p<0.001) blood (trans-coronary gradient from 49±21 ng/L at baseline to 634±144 ng/L 60 min post-ROSC, p<0.001). This rise in circulating cTnI at the 60 min post-ROSC period was attenuated in T3np-treated (387±148 ng/L; p=0.04 vs. EPI) and T3np+PCr-treated (385±55 ng/L; p=0.06 vs. EPI) animals (Figure 4), with parallel reductions in coronary venous cTnI (T3np: 645±226 ng/L; T3np+PCr: 752±131 ng/L; both p<0.05 vs. EPI) and the trans-coronary cTnI gradient (T3np: 258±85 ng/L, p<0.05 vs. EPI; T3np+PCr: 367±120 ng/L, p=0.20 vs. EPI) at the 60 min post-ROSC timepoint.
Figure 4: Circulating Cardiac Troponin I Concentrations in Swine Resuscitated with T3np, T3np+PCR, and EPI Before and After Cardiac Arrest.
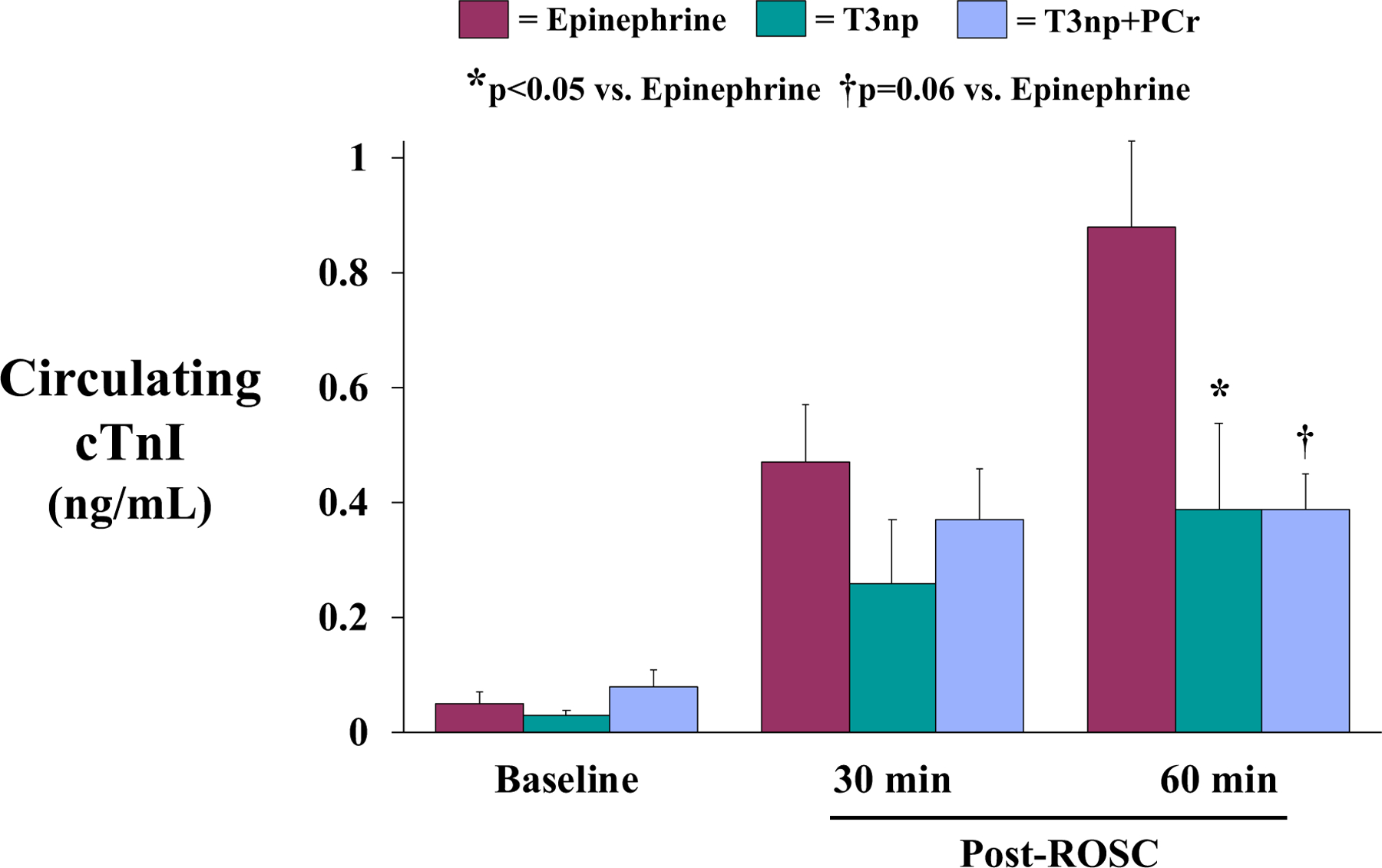
Circulating cTnI concentrations were low, but detectable, at baseline and did not exceed the 99th percentile of normal animals (0.04 ng/mL). Within the first hour after ROSC, circulating cTnI levels rose in all animals, but the magnitude of cTnI elevation was greater in EPI-treated animals compared with animals treated with either T3np formulation. Values are mean±SEM. *p<0.05 vs. EPI; †p=0.06 vs. EPI. cTnI = cardiac troponin I; ROSC = return of spontaneous circulation; T3np = triiodothyronine nanoparticles; T3np+PCr = triiodothyronine nanoparticles with encapsulated phosphocreatine
To gain insight regarding potential neuroprotective effects of T3nps, early post-ROSC brain injury was quantified via measurement of circulating neuron-specific enolase (NSE) levels, post-mortem histopathological assessment of H&E-stained tissue collected from various brain regions known to be susceptible to ischemic injury, and transmission electron microscopy (TEM)-based ultrastructural analysis of the hippocampus. However, given the relatively short duration of post-ROSC follow-up and small number of animals that survived to the 4-hour post-ROSC timepoint, data pertaining to brain injury should be viewed as preliminary and must be confirmed in future studies. In the subset of animals that survived to the 2-hour post-ROSC timepoint, circulating NSE levels were measured to assess early neurological injury. In EPI-treated animals, NSE levels rose from 0.65±0.18 ng/mL at baseline to 1.18 ng/mL at 2-hours post-ROSC (p=0.11). This trend in NSE was absent in T3np-treated (from 0.65±0.07 ng/mL at baseline to 0.77±0.19 ng/mL at 2-hours post-ROSC; p=0.37) and T3np+PCr-treated (from 0.98±0.10 ng/mL at baseline to 1.05±0.15 ng/mL at 2-hours post-ROSC; p=0.76) animals. Thus, the ~65% rise in NSE levels in EPI-treated animals (ΔNSE: 0.52±0.25 ng/mL) at this early post-ROSC timepoint tended to be attenuated in T3np-treated (ΔNSE: 0.13±0.14 ng/mL; p=0.20 vs. EPI) and T3np+PCr-treated (ΔNSE: 0.07±0.06 ng/mL; p=0.11 vs. EPI) animals (Supplemental Table 2). Consistent with a potential neuroprotective effect of T3np and T3np+PCr, neuronal injury tended to be lower in post-mortem H&E-stained brain tissue sections from animals treated with either T3np formulation compared with EPI-treated animals (Supplemental Figure 2). These differences were most notable in the putamen (p=0.09, T3np/T3np+PCr vs. EPI), parietal cortex (p=0.11, T3np/T3np+PCr vs. EPI), and hippocampus (p=0.18, T3np/T3np+PCr vs. EPI).
TEM-based ultrastructural assessment of the hippocampus provided additional preliminary evidence of T3np-mediated neuroprotection after ROSC (Figure 5). Compared with sham controls (mitochondrial integrity score of 4.1±0.2), animals subjected to cardiac arrest and resuscitated with EPI exhibited a significant reduction in mitochondrial integrity (3.5±0.1, p<0.01 vs. sham), characterized by disorganization and fragmentation of cristae (Figure 5A). However, this reduction in mitochondrial integrity was attenuated in animals treated with T3np (3.9±0.1) and T3np+PCr (3.8±0.1, both p=0.07 vs. EPI). Examination of neuronal synapses revealed that active zone length was significantly reduced in EPI-treated animals (223±14 nm) vs. sham controls (271±9 nm, p=0.02), suggesting that neurotransmitter release from synaptic vesicles would be impaired in these animals (Figure 5B). However, the active zone length was not significantly reduced in T3np-treated (268±27 nm) or T3np+PCr-treated (245±12 nm) animals compared with sham controls. Axonal injury was also more prevalent in EPI-treated animals, as illustrated by visible evidence of collapsed axons as well as a significant increase in axonal vacuolization (0.63±0.18 vacuoles/axon) compared with sham controls (0.08±0.03 vacuoles/axon, p=0.01, Figure 5C). This was attenuated in animals treated with T3np (0.28±0.09 vacuoles/axon) or T3np+PCr (0.29±0.14 vacuoles/axon), indicating better preservation of axonal structure after ROSC in T3np-treated animals. Additional in vitro studies in neurons subjected to hypoxia revealed that T3nps preserved ATP levels and attenuated LDH release while increasing expression of neural protection markers PAX6 and DLX2 by ~60% and 40%, respectively (described in detail in Supplemental Materials with results shown in Supplemental Figure 3).
Figure 5: Post-ROSC Hippocampal Ultrastructure in Swine Resuscitated with T3np, T3np+PCR, and EPI After Cardiac Arrest.
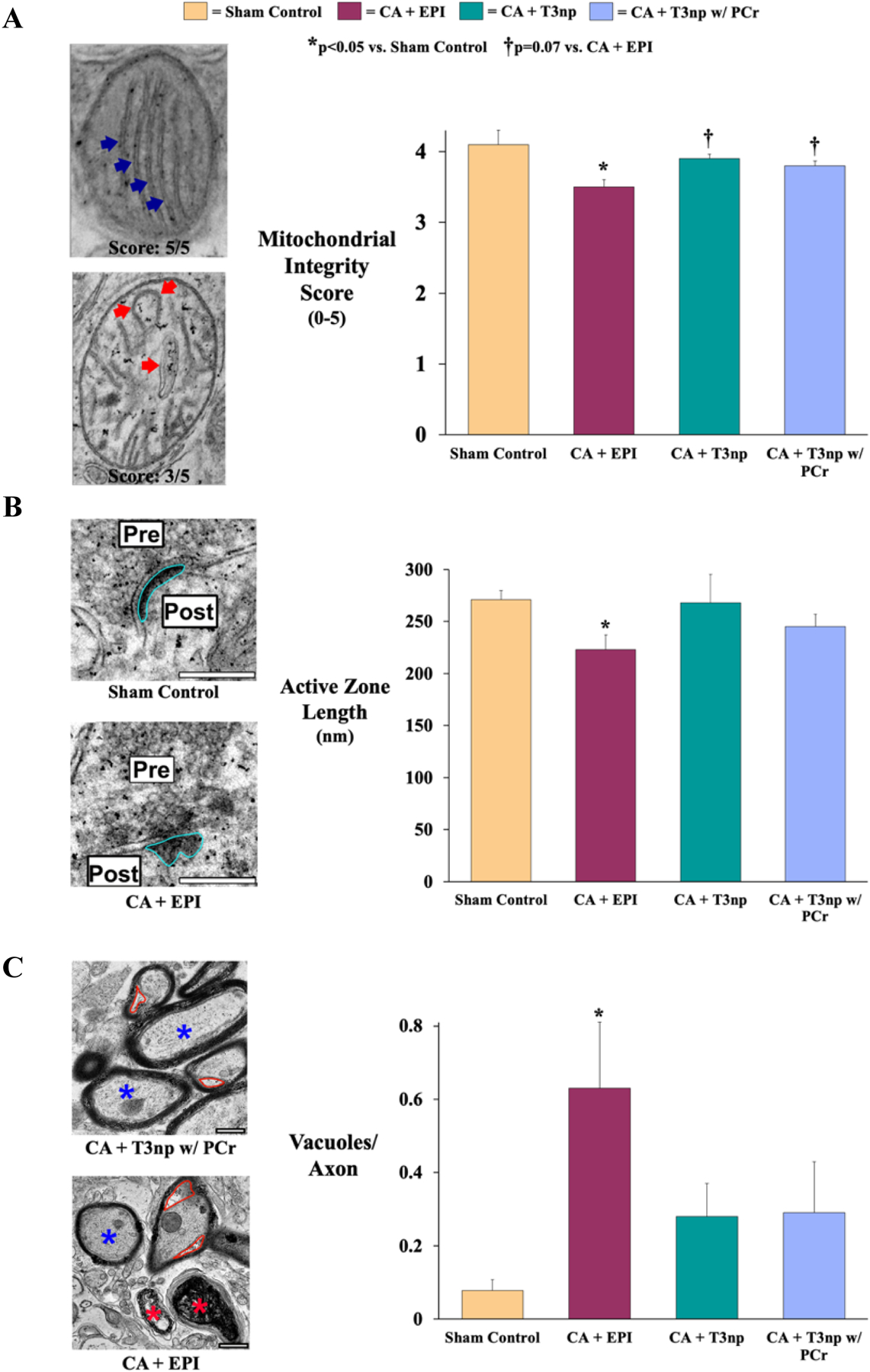
(A) Hippocampal mitochondrial integrity was assessed by examining cristae organization and fragmentation (left panel; blue arrows), with assignment of a score on a scale from 1–5 (5 = mitochondria with preserved ultrastructural integrity). Based on this scoring system, mitochondrial integrity was significantly reduced in EPI-treated animals (left panel; red arrows) compared with sham controls but was largely preserved in T3np-treated animals. (B) Active zone length (left panel; outlined in blue) at neuronal synapses was significantly reduced in EPI-treated animals vs. sham controls, suggestive of impaired neurotransmitter release from synaptic vesicles in these animals. However, T3np-treated animals did not exhibit significant changes in active zone length compared with sham controls. (C) Ultrastructural changes to hippocampal axons were assessed as shown in the left panel, with a normal axon labeled with a blue asterisk, dysfunctional axons labeled with red asterisks, and vacuoles outlined in red. Dysfunctional axons were more prevalent in EPI-treated animals and this group also exhibited a significant increase in axonal vacuolization, an early sign of axonal injury. However, this was attenuated in animals treated with either formulation of T3np, indicating better preservation of axonal structure in these groups. Values are mean±SEM. *p<0.05 vs. sham control; †p=0.07 vs. CA+EPI. CA = cardiac arrest; EPI = epinephrine; T3np = triiodothyronine nanoparticles; T3np+PCr = triiodothyronine nanoparticles with encapsulated phosphocreatine.
Discussion
Despite decades of effort to improve CPR delivery and access to defibrillation, rates of survival to hospital discharge after cardiac arrest remain low, with fewer than 20% of cardiac arrest victims returning home to their families2. A majority of cardiac arrest victims do not survive early resuscitation efforts, which encompass the acute delivery of CPR and initial pharmacologic intervention (primarily EPI), as well as the first 24–72 hours following resuscitation. Over the past several decades, limited progress has been made to develop new pharmacologic treatment strategies, while clinicians have continued to rely on EPI despite the absence of rigorous scientific data supporting its use4, 5. Growing concerns regarding the efficacy of EPI in cardiac arrest led Perkins et al. to conduct the PARAMEDIC2 trial, in which ~8,000 patients with out-of-hospital cardiac arrest were randomized to receive either EPI or placebo6. Although EPI was shown to increase overall 30-day survival rates, a larger proportion of patients in the EPI group exhibited severe neurological impairment (i.e., modified Rankin score of 4–5) and neurologically intact survival was not different between groups6. Although the mechanistic explanation for these findings is unclear, it has been suggested that EPI may exacerbate anoxic brain injury by constricting the cerebral microvasculature18 and/or decreasing post-resuscitation cerebral perfusion pressures via worsening of post-resuscitation myocardial dysfunction19. Regardless of the underlying mechanism, these findings have fueled renewed efforts at identifying novel alternatives to EPI that improve the likelihood of successful resuscitation from cardiac arrest while also minimizing post-resuscitation injury to the vital organs.
The findings of the present study provide initial in vivo support for the notion that T3nps are an attractive candidate to achieve this goal. Consistent with clinical data, administration of EPI during CPR led to a significant improvement in the rate of ROSC compared with vehicle treatment but was also associated with an elevation in selected circulating biomarkers of heart (cTnI) and brain (NSE) injury during the early post-resuscitation period. Furthermore, detailed post-mortem evaluation of regional brain tissue sections revealed structural changes indicative of increased neuronal injury, reduced mitochondrial integrity, impaired neurotransmitter release, and more prevalent axonal vacuolization. However, these pathological changes were largely attenuated in animals that were resuscitated with T3nps, despite a similar rate of ROSC, time to ROSC, and duration of post-ROSC survival among treatment groups. For example, we observed a significant reduction in circulating concentrations of cTnI in T3np- and T3np+PCr-treated animals compared with EPI-treated animals during the post-ROSC period, consistent with reduced myocardial injury after resuscitation with T3nps. This may be attributed to the fact that EPI treatment was associated with an increase in heart rate and contractility (LV dP/dtmax) relative to T3np treatment during the early post-ROSC period, which could exacerbate cardiac injury by raising myocardial oxygen demand and eliciting demand-induced ischemia. In addition, T3np resuscitation was associated with a relative reduction in several indices of brain injury: post-ROSC NSE levels rose ~65% in EPI-treated animals but only ~10% after T3np, and the frequency of abnormalities in hippocampal ultrastructure was attenuated in T3np-treated animals relative to the EPI-treated group. Initial insight regarding the mechanistic basis of these beneficial effects on the brain was provided by in vitro studies demonstrating T3np-mediated preservation of neuronal ATP levels, attenuation of LDH release, and expression of the neuroprotective markers PAX6 and DLX2 in isolated neurons subjected to hypoxia. Taken together, these results demonstrate that T3nps improve the likelihood of achieving ROSC to a similar extent as EPI, while avoiding the deleterious effects of EPI on the heart and brain during the early post-resuscitation period.
Interest in the use of T3 for the acute treatment of cardiac arrest first developed in the early 1990s following the publication of several studies reporting abnormally low serum thyroid hormone concentrations following major systemic perturbations such as cardiac arrest20, 21. However, initial preclinical studies evaluating the efficacy of acute free T3 administration during CPR or early in the post-resuscitation period were largely unsuccessful21–23. Though not recognized at the time, the failure of intravenous free T3 therapy in cardiac arrest may be attributed to its poor solubility at physiological pH and/or wide cellular and nuclear distribution24. The latter mechanism may be particularly important in the setting of cardiac resuscitation, as studies comparing labeled free T3 vs. a polymer-conjugated T3 mimetic have shown that free T3 rapidly translocates to the nucleus, thereby losing the ability to exert non-genomic effects via receptors on the plasma membrane integrin αvβ315. These rapid, non-genomic actions are hypothesized to play a key role in protecting cells from ischemic injury through several potential mechanisms, including stabilization of Na+/K+-ATPase, Ca++-ATPase, and/or Na+/H+ exchanger plasma membrane ion pumps, as well as activation of cytoprotective PI3K/AKT and ERK1/2 signaling pathways9, 11, 12. By conjugating T3 with PLGA, the T3nps used in the present study exhibit prolonged activation of thyrointegrin αvβ3 receptor-mediated signaling at the plasma membrane and translocation to the cytoplasm, but not the nucleus16. Furthermore, T3nps can bind to plasma pre-albumin or transthyretin, which act as transporters across the blood-brain barrier to facilitate T3np-mediated neuroprotection16, 25. Although not directly tested in the present study, these unique properties of T3nps relative to currently available free T3 formulations may explain the discrepancy between our results and the negative studies reported ~30 years ago in preclinical models of cardiac arrest21–23.
While the results of the present study provide encouraging support for the therapeutic potential of T3nps, there are several experimental limitations that should be considered when interpreting our findings. First, like most preclinical studies investigating novel therapeutics, we studied young, otherwise healthy swine without pre-existing co-morbidities that are often present in humans that suffer cardiac arrest. Thus, it is unclear whether advanced age and/or the presence of underlying cardiometabolic disease may modify the therapeutic efficacy of T3nps. Second, we induced ventricular fibrillation via direct electrical stimulation of the right ventricular endocardium to enhance the reproducibility and consistency of the time of cardiac arrest onset. Although this approach recapitulates electrocution-induced cardiac arrest, future studies evaluating the efficacy of T3nps in animals with cardiac arrest arising from acute myocardial ischemia may be necessary given the significantly higher prevalence of ischemia-induced cardiac arrest in the clinical setting. Similarly, all of the animals in the present study initially exhibited a shockable rhythm at the onset of CPR efforts, thus the efficacy of T3nps when subjects present with a non-shockable rhythm will require further study. Third, although an identical CPR protocol was employed in all animals and research personnel were blinded to treatment status, the results of the study may not be generalizable to situations in which alternative resuscitation parameters are utilized (e.g., asynchronous ventilation, mechanical chest compressions). Fourth, we employed a protocol with a relatively short post-resuscitation follow-up period (4-hours) to allow insight into the early effects of T3nps vs. EPI in a large animal model of cardiac arrest. Because we did not employ clinically relevant post-ROSC care (e.g., low-dose vasopressors), a majority of animals that were successfully resuscitated did not survive for the 4-hour post-resuscitation period, regardless of which treatment was administered. Although these aspects of the protocol were specifically designed to allow maximal insight regarding the potential therapeutic effects of T3nps in the absence of concomitant post-ROSC interventions, our results indicate that additional hemodynamic support would likely be required within the first several hours after ROSC to avoid hypotension. Fortunately, this type of treatment is readily available in most clinical settings and can be administered within the first several hours after resuscitation in most patients. Nevertheless, additional experiments employing post-ROSC hemodynamic support and a longer follow-up period will be necessary to establish that early neuroprotective effects of T3nps translate to improved neurologic function when implemented alongside current clinical protocols. The inclusion of histopathological analysis of neuronal injury in these studies will be particularly important to confirm that our initial findings at a very early post-ROSC timepoint extend to later timepoints given the protracted evolution of post-ischemic brain injury. As mentioned above, the ultra-early timepoint used in the present study prohibits definitive determination of T3np-mediated neuroprotection, particularly because several common histopathologic and biomarker-based techniques cannot reliably detect brain injury until later post-resuscitation timepoints. Thus, the present data pertaining to brain injury should be viewed as preliminary evidence suggestive of a potential neuroprotective effect that must be confirmed in future studies. Finally, the beneficial effects exhibited by animals treated with T3np+PCr were generally also evident in animals treated with T3np. Thus, further studies are necessary to determine whether encapsulation of PCr offers additional therapeutic benefit beyond that offered by T3np alone.
Conclusions
In conclusion, the results of the present study provide promising initial evidence that intravenous administration of T3nps during CPR represents a viable therapeutic strategy to improve outcomes after cardiac arrest. Direct comparison of T3nps with EPI, the current clinical standard-of-care, revealed that T3nps increase the likelihood of achieving ROSC to a similar extent as EPI, while attenuating selected biomarkers of cardiac and neurologic injury at individual early post-ROSC timepoints. These findings offer support for future translational studies aimed at optimizing the use of T3nps in clinical settings, a novel approach that may result in significant improvements in neurologically intact survival among the hundreds of thousands of individuals who suffer from cardiac arrest each year.
Supplementary Material
Acknowledgements -
These studies could not have been completed without the assistance of Elaine Granica, Rebeccah Young, MA, Beth Palka, Noureldien Darwish, MD, PhD, and Thangirala Sudha, PhD.
Funding Sources -
The National Heart Lung and Blood Institute (R01HL-160538 and F32HL-114335), the American Heart Association (17SDG33660200), the National Center for Advancing Translational Sciences (UL1TR001412), the ZOLL Foundation, the Albert and Elizabeth Rekate Fund in Cardiovascular Medicine, Empire State Development’s Division of Science, Technology and Innovation (NYSTAR), and the Pharmaceutical Research Institute at Albany College of Pharmacy and Health Sciences.
Abbreviations:
- ANOVA
analysis of variance
- CPP
coronary perfusion pressure
- CPR
cardiopulmonary resuscitation
- cTnI
cardiac troponin I
- EPI
epinephrine
- LDH
lactate dehydrogenase
- LV
left ventricular
- NSE
neuron-specific enolase
- PCr
phosphocreatine
- PEA
pulseless electrical activity
- PLGA
polylactic acid-co-glycolic acid
- ROSC
return of spontaneous circulation
- T3
triiodothyronine
- T3np
triiodothyronine nanoparticles
- TEM
transmission electron microscopy
- VF
ventricular fibrillation
Footnotes
Disclosures – The present study was supported, in part, by the University at Buffalo Center for Advanced Technology in Big Data and Health Sciences (UB CAT), a project cost-sharing grant program through which Pro-Al Medico Technologies, Inc. provided research funds. Dr. Dickinson is a Director at Pro-Al Medico Technologies, Inc.
References
- 1.Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Jordan LC, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, O’Flaherty M, Pandey A, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Spartano NL, Stokes A, Tirschwell DL, Tsao CW, Turakhia MP, VanWagner LB, Wilkins JT, Wong SS, Virani SS, American Heart Association Council on E, Prevention Statistics C and Stroke Statistics S. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation. 2019;139:e56–e528. [DOI] [PubMed] [Google Scholar]
- 2.Becker LB, Aufderheide TP and Graham R. Strategies to Improve Survival From Cardiac Arrest: A Report From the Institute of Medicine. JAMA. 2015;314:223–4. [DOI] [PubMed] [Google Scholar]
- 3.Rea T, Kudenchuk PJ, Sayre MR, Doll A and Eisenberg M. Out of hospital cardiac arrest: Past, present, and future. Resuscitation. 2021;165:101–109. [DOI] [PubMed] [Google Scholar]
- 4.Jung J, Rice J and Bord S. Rethinking the role of epinephrine in cardiac arrest: the PARAMEDIC2 trial. Ann Transl Med. 2018;6:S129–S129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gough CJR and Nolan JP. The role of adrenaline in cardiopulmonary resuscitation. Critical Care. 2018;22:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perkins GD, Ji C, Deakin CD, Quinn T, Nolan JP, Scomparin C, Regan S, Long J, Slowther A, Pocock H, Black JJM, Moore F, Fothergill RT, Rees N, O’Shea L, Docherty M, Gunson I, Han K, Charlton K, Finn J, Petrou S, Stallard N, Gates S, Lall R and Collaborators P. A Randomized Trial of Epinephrine in Out-of-Hospital Cardiac Arrest. N Engl J Med. 2018;379:711–721. [DOI] [PubMed] [Google Scholar]
- 7.Navab E, Esmaeili M, Poorkhorshidi N, Salimi R, Khazaei A and Moghimbeigi A. Predictors of Out of Hospital Cardiac Arrest Outcomes in Pre-Hospital Settings; a Retrospective Cross-sectional Study. Arch Acad Emerg Med. 2019;7:36–36. [PMC free article] [PubMed] [Google Scholar]
- 8.McCarthy JJ, Carr B, Sasson C, Bobrow BJ, Callaway CW, Neumar RW, Ferrer JME, Garvey JL, Ornato JP, Gonzales L, Granger CB, Kleinman ME, Bjerke C, Nichol G, American Heart Association Emergency Cardiovascular Care C, Council on Cardiopulmonary CCP, Resuscitation and the Mission: Lifeline Resuscitation S. Out-of-Hospital Cardiac Arrest Resuscitation Systems of Care: A Scientific Statement From the American Heart Association. Circulation. 2018;137:e645–e660. [DOI] [PubMed] [Google Scholar]
- 9.Davis PJ, Lin HY, Hercbergs AA, Keating KA and Mousa SA. How thyroid hormone works depends upon cell type, receptor type, and hormone analogue: implications in cancer growth. Discov Med. 2019;27:111–117. [PubMed] [Google Scholar]
- 10.Davis PJ, Ashur-Fabian O, Incerpi S and Mousa SA. Editorial: Non Genomic Actions of Thyroid Hormones in Cancer. Frontiers in endocrinology. 2019;10:847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng SY, Leonard JL and Davis PJ. Molecular aspects of thyroid hormone actions. Endocr Rev. 2010;31:139–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davis PJ, Leonard JL, Lin HY, Leinung M and Mousa SA. Molecular Basis of Nongenomic Actions of Thyroid Hormone. Vitam Horm. 2018;106:67–96. [DOI] [PubMed] [Google Scholar]
- 13.Davis PJ and Davis FB. Nongenomic actions of thyroid hormone on the heart. Thyroid : official journal of the American Thyroid Association. 2002;12:459–66. [DOI] [PubMed] [Google Scholar]
- 14.Liu Y-C, Yeh C-T and Lin K-H. Molecular functions of thyroid hormone signaling in regulation of cancer progression and anti-apoptosis. International journal of molecular sciences. 2019;20:4986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bharali DJ, Yalcin M, Davis PJ and Mousa SA. Tetraiodothyroacetic acid-conjugated PLGA nanoparticles: a nanomedicine approach to treat drug-resistant breast cancer. Nanomedicine (Lond). 2013;8:1943–54. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 16.Karakus OO, Darwish NHE, Sudha T, Salaheldin TA, Fujioka K, Dickinson PCT, Weil B and Mousa SA. Development of Triiodothyronine Polymeric Nanoparticles for Targeted Delivery in the Cardioprotection against Ischemic Insult. Biomedicines. 2021;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mousa SA, Sallam A and Darwish N. Abstract 133: Cardiovascular Protective Effect of Thyroxine and Nano- Thyroxine Against Ischemic Insults. Circulation. 2019;140:A133–A133. [Google Scholar]
- 18.Ristagno G, Sun S, Tang W, Castillo C and Weil MH. Effects of epinephrine and vasopressin on cerebral microcirculatory flows during and after cardiopulmonary resuscitation. Crit Care Med. 2007;35:2145–9. [DOI] [PubMed] [Google Scholar]
- 19.Angelos MG, Butke RL, Panchal AR, Torres CA, Blumberg A, Schneider JE and Aune SE. Cardiovascular response to epinephrine varies with increasing duration of cardiac arrest. Resuscitation. 2008;77:101–10. [DOI] [PubMed] [Google Scholar]
- 20.Wortsman J, Premachandra BN, Chopra IJ and Murphy JE. Hypothyroxinemia in cardiac arrest. Arch Intern Med. 1987;147:245–8. [PubMed] [Google Scholar]
- 21.Facktor MA, Mayor GH, Nachreiner RF and D’Alecy LG. Thyroid hormone loss and replacement during resuscitation from cardiac arrest in dogs. Resuscitation. 1993;26:141–62. [DOI] [PubMed] [Google Scholar]
- 22.Whitesall SE, Mayor GH, Nachreiner RF, Zwemer CF and D’Alecy LG. Acute administration of T3 or rT3 failed to improve outcome following resuscitation from cardiac arrest in dogs. Resuscitation. 1996;33:53–62. [DOI] [PubMed] [Google Scholar]
- 23.Zwemer CF, Whitesall SE, Nachreiner RF, Mayor GH and D’Alecy LG. Acute thyroid hormone administration increases systemic oxygen delivery and consumption immediately following resuscitation from cardiac arrest without changes in thyroid-stimulating hormone. Resuscitation. 1997;33:271–80. [DOI] [PubMed] [Google Scholar]
- 24.Davis PJ, Mousa SA and Lin HY. Nongenomic Actions of Thyroid Hormone: The Integrin Component. Physiol Rev. 2021;101:319–352. [DOI] [PubMed] [Google Scholar]
- 25.Lin HY, Davis FB, Luidens MK, Mousa SA, Cao JH, Zhou M and Davis PJ. Molecular basis for certain neuroprotective effects of thyroid hormone. Front Mol Neurosci. 2011;4:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


