Abstract
The Epstein-Barr virus (EBV) EBNA-LP and EBNA2 proteins are the first to be synthesized during establishment of latent infection in B lymphocytes. EBNA2 is a key transcriptional regulator of both viral and cellular gene expression and is essential for EBV-induced immortalization of B lymphocytes. EBNA-LP is also important for EBV-induced immortalization of B lymphocytes, but far less is known about the functional domains and cellular cofactors that mediate EBNA-LP function. While recent studies suggest that serine phosphorylation of EBNA-LP and coactivation of EBNA2-mediated transactivation are important, more detailed mutational and genetic studies are complicated by the repeat regions that comprise the majority of the EBNA-LP sequence. Therefore, we have used a comparative approach by studying the EBNA-LP homologues from baboon and rhesus macaque lymphocryptoviruses (LCVs) (baboon LCV and rhesus LCV). The predicted baboon and rhesus LCV EBNA-LP amino acid sequences are 61 and 64% identical to the EBV EBNA-LP W1 and W2 exons and 51% identical to the EBV EBNA-LP Y1 and Y2 exons. Five evolutionarily conserved regions can be defined, and four of eight potential serine residues are conserved among all three EBNA-LPs. The major internal repeat sequence also revealed a highly conserved Wp EBNA promoter with strong conservation of upstream activating sequences important for Wp transcriptional regulation. To test whether transcriptional coactivating properties were common to the rhesus LCV EBNA-LP, a rhesus LCV EBNA2 homologue was cloned and expressed. The rhesus LCV EBNA2 transcriptionally transactivates EBNA2-responsive promoters through a CBF1-dependent mechanism. The rhesus LCV EBNA-LP was able to further enhance rhesus LCV or EBV EBNA2 transactivation 5- to 12-fold. Thus, there is strong structural and functional conservation among the simian EBNA-LP homologues. Identification of evolutionarily conserved serine residues and regions in EBNA-LP homologues provides important clues for identifying the cellular cofactors and molecular mechanisms mediating these conserved viral functions.
Epstein-Barr virus (EBV) is a gammaherpesvirus and a preeminent tumor virus in humans. EBV is associated with a variety of cancers, including endemic Burkitt's lymphoma, nasopharyngeal carcinoma, Hodgkin's lymphoma, and lymphoma in the immunosuppressed (40). Consistent with its association with human malignancy, EBV also immortalizes human B lymphocytes with high efficiency in vitro (35). Efficient immortalization of B lymphocytes requires expression of only a subset of viral genes (22). These genes include several EBV nuclear antigens (EBNAs), EBNA1, EBNA2, EBNA3A and -C, and EBNA-LP, and an integral latent membrane protein, LMP-1. EBNA-LP is the first protein along with EBNA2 made during infection of lymphocytes by EBV (1). Despite a growing body of knowledge on the molecular mechanisms of latent protein functions, the role of EBNA-LP for EBV-induced immortalization remains enigmatic.
The EBNA-LP protein (also referred to as EBNA-5 or EBNA-4) contains multiple copies of a 66-amino-acid repeat domain encoded by two exons in the internal repeat 1 (IR1) repeats W1 (22 amino acids) and W2 (44 amino acids) followed by a unique 45-amino-acid domain encoded by the Y1 and Y2 exons located within the Bam Y fragment just downstream of the IR1 repeats (6, 44, 46). Genetic studies using recombinant viruses lacking the last two EBNA-LP exons (Y1 and Y2) or a stop codon placed after the first amino acid in Y1 were unable to immortalize lymphocytes unless cocultivated with fibroblast feeder cells (16, 33). While this assay was unable to determine the biochemical mechanism of EBNA-LP function, it gave rise to the hypothesis that EBNA-LP was important but not essential for EBV-induced immortalization. EBNA-LP localizes to the nucleus in distinct foci now recognized as nuclear domain 10 (ND10) bodies or promyelocytic leukemia-associated protein (PML) oncogenic domains (PODs) (21, 39). Several cellular proteins, including PML, hsp70, and an antigenically distinct form of RB, have been reported to be present in PODs or ND10 bodies (7, 21, 26, 49, 50, 54). Although little is known about the functions of proteins present in the PODs, they appear to be involved in cellular proliferation processes. Immunofluorescence and in vitro binding studies have suggested that EBNA-LP interacts with p53 and RB (51). However, coexpression of EBNA-LP and RB or p53 did not result in any functional effect on RB- or p53-dependent transcription from reporter plasmids (19). EBNA-LP also interacts with hsp72/hsc73, although the functional consequence of such an interaction is unclear (24, 34). EBNA-LP has also been shown to be phosphorylated on serine residues, and it is phosphorylated to greater amounts during the late G2 stage of the cell cycle (23, 39). Both casein kinase II (CKII) and the cyclin-dependent p34cdc2 kinase could also phosphorylate EBNA-LP in vitro (23).
Recent studies have found that while EBNA-LP has little effect on transcription alone, it stimulated EBNA2 activation of the LMP-1 promoter and a regulatory region from the latency BamHI C promoter (Cp) (17, 38). Interestingly, a minimum of two W1/W2 repeats was required for these assays, and the Y1 and Y2 exons were dispensable (17, 38). Consistent with these studies, it has also been shown that introduction of both EBNA2- and EBNA-LP-expressing plasmids into resting B lymphocytes results in activation of cyclin D2 and progression of these cells from G0 to G1 (45). These data provided direct evidence for an effect of EBNA-LP on cell phenotype.
Genetic analysis of EBNA-LP is difficult because EBNA-LP is derived from several repeated exons in the major internal repeat of the virus (IR1). An alternative approach for elucidating functional domains and their associated cellular cofactors in viral proteins is to focus on regions of the protein that are evolutionarily conserved. Several lymphocryptoviruses (LCVs) have been isolated from nonhuman primates, including rhesus macaques (rhesus LCV or cercopithicine herpesvirus 15) and baboons (herpesvirus papio or cercopithicine herpesvirus 12). For consistency, we will refer to these viruses as rhesus LCV and baboon LCV. We previously sequenced the EBNA2 homologue from a baboon LCV and identified several clustered regions of homology between the different EBV EBNA2 proteins (32). The EBNA2 protein functions as a transcriptional regulator of viral and cellular genes and is essential for EBV-induced immortalization (8, 25, 36, 48, 52, 55, 60). EBNA2 function is mediated through interactions with cellular DNA binding proteins that include CBF1 and Spi-1/Pu.1 (15, 18, 27, 31, 53, 58). EBNA2 also contains a strong acidic activation domain whose function is also mediated by cellular factors (3, 4, 32). Identification of conserved regions served as an important tool for identification of the CBF1 interaction domain, nuclear localization signals, and an important element of the transactivation domain (29–32). It seems likely that a similar comparative approach will be equally effective for dissecting important functional domains in the EBNA-LP protein. The comparative approach is also supported by data suggesting that the pathogenesis and establishment of a persistent carrier infection by rhesus LCV in rhesus macaques is similar to that observed for EBV in humans (37). Like human immunodeficiency virus-infected individuals who can develop EBV-associated lymphomas, SIV-infected macaques also can develop rhesus LCV-associated lymphomas (9, 10).
To further our understanding of functional domains in EBNA-LP, we have cloned and sequenced the genomic regions encoding EBNA-LP from rhesus and baboon LCVs. We have also isolated a cDNA for the rhesus LCV EBNA-LP and tested its ability to stimulate EBNA2-mediated transactivation of reporter plasmids. To evaluate whether EBNA-LP cooperation might be dependent on the EBNA2 derived from a syngeneic strain, we have also cloned, sequenced, and expressed the rhesus EBNA2 homologue and tested it for function in transient transfection assays. The sequence information and functional analysis of the EBNA-LP homologues will provide a framework for elucidating novel functional domains that are likely to be important for EBV immortalization of B lymphocytes. In addition, functional analysis of these proteins will further confirm the importance of cooperation between EBNA-LP and EBNA2.
MATERIALS AND METHODS
Cell culture.
DG75, BJAB, and CA46 are EBV-negative Burkitt's lymphoma cell lines. They were maintained in RPMI 1640 supplemented with 10% fetal bovine serum and incubated in 5% CO2 at 37°C. B95-8, P3HR1, 26CB-1 (baboon LCV-infected cell line; obtained from the American Type Culture Collection [ATCC] as CRL-1495), BA65 (baboon LCV-infected cell line), H254 (rhesus LCV-infected cell line), and LCL8664 (rhesus LCV-infected cell line; ATCC CRL-1805) were similarly maintained.
Transient transfection analysis.
DNA transfections were carried out by using a DEAE-dextran method for DG75 cells and electroporation for BJAB cells (13, 14, 17). Cells were transfected with the indicated amounts of target and effector plasmids. Total amounts of plasmid DNA for transfections were equalized by using SG5 (Stratagene) plasmid DNA. Transfections were harvested after 2 days of incubation, cells were lysed with reporter lysis buffer (Promega), and chloramphenicol acetyltransferase (CAT) or luciferase assays were carried out as previously described (13, 14). A constitutively expressing luciferase reporter vector (pGL2-control; Promega) was used as an internal control for transfections, and the values from CAT assays were normalized to luciferase activity. A constitutively expressing CAT reporter vector (pGL2-control; Promega) was used as an internal control for transfections, and the values from CAT assays were normalized to luciferase activity. A constitutively expressing CAT reporter vector (pCAT control; Promega) was used as an internal control for experiments using luciferase reporters, and the values from luciferase assays were normalized to CAT activity. In some experiments, transfection assay results were measured using the Promega dual-luciferase reporter assay system. Reporter plasmids expressing the firefly luciferase protein were cotransfected with a plasmid expressing the Renilla luciferase, which was used as an internal control as described by the manufacturer.
Western blot analysis.
Cells transfected with plasmids expressing EBV or rhesus LCV EBNA2 or EBNA-LP proteins were lysed in sample buffer, sonicated, and boiled. The proteins were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on an 8.0% gel and transferred to nitrocellulose. The membranes were blocked with phosphate-buffered saline containing 5% nonfat dried milk, and the blots were incubated with monoclonal antibody PE2 (DAKO) for detection of EBNA2 or monoclonal antibody JF186 (11) or M2 (anti-Flag; Sigma) to detect EBNA-LP proteins, followed by a secondary horseradish peroxidase-conjugated anti-mouse antibody. The proteins were then detected by enhanced chemoluminescence (ECL) using an Amersham ECL detection kit.
Plasmids and cDNA cloning.
RcosCC1 is a cosmid containing a fragment from the rhesus LCV genome derived from infected cell line LCL8664 and is approximately colinear between positions 1600 to 57000 of the EBV genome (unpublished observations). The cosmid was digested with restriction enzyme, XhoI, and the resulting six fragments were subcloned into the SalI site of pGH56 (pUC19 derivative containing a BglII site in the polylinker). pAG6 contains a 1.4-kb XhoI fragment encoding W1 and W2 exon regions for the EBNA-LP homologue and also 500 bp of sequence homologous to the Bam W promoter (Wp) from EBV. Plasmid pAG2 contains a 5.6-kb XhoI fragment which contains the rhesus LCV EBNA2 homologue. The Y1 and Y2 exons are also found in pAG2. The baboon LCV Xba P and K fragments containing EBNA-LP, Wp-like, and EBNA2 coding regions were generated and characterized as described previously (43). The rhesus LCV EBNA2 open reading frame (ORF) was amplified by PCR and subcloned into the SG5 expression vector (Stratagene) as an EcoRI/BglII fragment. A large internal part of this PCR-derived DNA fragment (AgeI-SapI) was subsequently replaced with the corresponding genomic DNA (derived from pAG2). Sequences flanking the AgeI and SapI restriction sites were sequenced to ensure that no PCR-generated errors were present. The final rhesus LCV EBNA2 expression plasmid is pAG115. The target reporter plasmid used for EBNA-LP cooperation experiments (BamCp8LUC) was made by excising the multimerized EBNA2 enhancer unit from pBamCp8CAT (30) and introducing it into the pGL3 promoter vector (Promega).
mRNA was prepared from the LCL8664 cell line as described previously (14). A 3′ primer (OPL321; 5′-CATTTAACCGGCAAAAATCATCTAAACC-3′) complementary to the end of the Y2 exon was annealed to the mRNA and extended by using reverse transcriptase. The cDNA was then amplified by PCR using primers OPL321 and 320 (OPL320 is complementary to the end of the C1 exon; 5′-TTAGATCTCTTCCTCCTCTTCTATGTAGACCCTTCG-3′) (12). The resulting 1.0-kb DNA products were then separated on a 0.8% agarose gel, excised and purified by using a Qiaex II kit (Qiagen), and cloned into the pGEM-T Easy vector (Promega). The resulting clones were then analyzed by sequencing. Clone pPDL398, which contained a translational initiation codon, was subsequently cloned into the eukaryotic expression plasmid SG5, yielding pTLD100. pTLD100 was then cleaved with BglII, which cuts proximal to the rhesus LCV EBNA-LP termination codon, and an oligonucleotide which encodes sequences for the Flag epitope was inserted. The SG5 rhesus LCV EBNA-LP clone containing the Flag epitope is plasmid pJT117.
DNA sequencing.
DNA sequencing of rhesus LCV plasmid clones was performed with an automated ABI sequencing system. Plasmid clones pAG2 and pAG6 were sequenced by using universal forward and reverse primers. Based on sequence information derived from these primers, new oligonucleotides were designed and used for obtaining further sequences until the end of the cloned insert was reached. Additional primers were designed and synthesized to sequence the complementary strand of DNA. Baboon LCV plasmid clones XbaI P (pPDL73) and K (pPDL74) were sequenced as described previously (43). Clones pPDL397 (cDNA.2) and 398 (cDNA.1) containing rhesus LCV EBNA-LP cDNA were sequenced by using T7 and SP6 primers.
DNA and amino acid sequences were compared and aligned by using the ClustalW program in MacVector version 6.0.
Nucleotide sequence accession numbers.
Rhesus and baboon LCV Wp, EBNA-LP, and EBNA2 sequences have been deposited in GenBank and assigned accession no. AF200821, AF200822, AF200823, AF200364, and AF200187.
RESULTS
Predicted amino acid sequence of rhesus and baboon LCV EBNA-LP homologues and identification of Wp-like sequences.
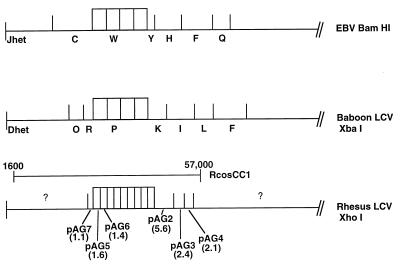
A large cosmid, RcosCC1, which is colinear to EBV sequences from positions 1600 to 57000 was cleaved by using XhoI, and the subsequent DNA fragments were cloned. Initial sequencing analysis of the ends from five of these clones was performed. Based on homology with the known EBV genome sequence, the arrangement of the XhoI DNA fragments is shown in Fig. 1. Sequence analysis of plasmid pAG6, containing a 1.4-kb XhoI fragment (see Materials and Methods), revealed that this sequence is largely colinear to part of the EBV BamHI W repeat sequence including putative EBNA-LP W1 and W2 exons. Similarly, the XbaI P fragment (pPDL73) derived from the baboon LCV cosmid JR4 also encoded the EBNA-LP W1 and W2 exons (43). Sequencing of pAG2 and XbaI K (pPDL74) revealed the presence of EBNA-LP coding exons for Y1 and Y2 from rhesus and baboon LCVs. For simplicity, the EBV, rhesus, and baboon LCV EBNA-LP amino acid sequences containing one copy of the W1 and W2 repeats are shown aligned with each other (Fig. 2). The predicted EBNA-LP ORF from type 2 EBV was derived from previously reported P3HR1 and AG876 sequences and thus represents a hypothetical type 2 protein sequence (5, 20). The type 1 sequence is represented by EBV strain B95-8. The baboon LCV EBNA-LP is 61% identical to EBV EBNA-LP W1 and W2 exons and 51% identical to the Y1 and Y2 exons. The rhesus LCV EBNA-LP is 64% identical to the W1 and W2 exons of EBV and 51% identical to the Y1 and Y2 exons of EBV EBNA-LP. Interestingly, the baboon and rhesus LCV EBNA-LP sequences are more similar to each other and are 67% identical between W1 and W2 and 69% identical between Y1 and Y2. The type 1 and 2 EBV EBNA-LP proteins appear to be 89% homologous to each other, with most of the divergence occurring in regions that also are not conserved among the other LCVs.
FIG. 1.
Restriction enzyme maps of the left end of the EBV, baboon LCV and rhesus LCV genomes. Four IR1 repeats are shown for EBV for purposes of comparison to the other LCVs, and it should be noted that the prototype B95-8 published sequence contains 11 repeats. The restriction enzymes used are shown at the right. Since a complete analysis of the rhesus LCV genome has not been described, the plasmid name containing each XhoI-derived DNA fragment is indicated below the rhesus LCV genome, and the size of each fragment is indicated in kilobases below the plasmid name. Preliminary restriction digests for RcosCC1 indicate approximately four copies of the internal repeats for rhesus LCV (unpublished observations), while similar analysis for the baboon LCV clone JR4 has also indicated four repeats (43). The XhoI restriction enzyme cleaves the homologous IR1 repeats (approximately 3.0 kb) in the rhesus LCV clone twice so that each repeat consists of both pAG5 and pAG6 fragments (1.6 and 1.4 kb, respectively). The approximate boundaries of the rhesus LCV cosmid used for these studies are shown above the rhesus LCV map.
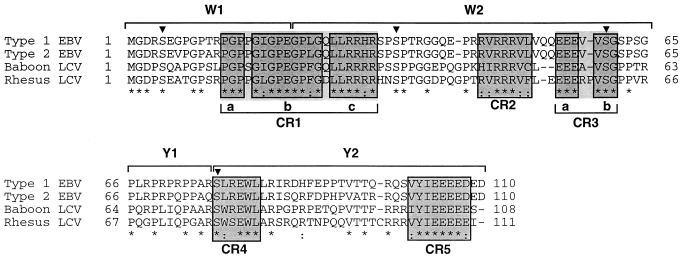
FIG. 2.
Alignment of the predicted amino acid sequences from EBV type 2, baboon LCV, and rhesus LCV EBNA-LP proteins with the EBV type 1 EBNA-LP amino acid sequence. Amino acid residues identical between sequences are indicated by asterisks, and similar nonidentical residues are indicated by a dotted line. Conserved regions are boxed and numbered in consecutive order as CR1 to CR5. Amino acid numbers are indicated at the beginning and end of each line. The boundaries of the W1, W2, Y1, and Y2 exons are shown above the sequences. The triangles indicate conserved serine residues that are potential phosphorylation sites.
For purely heuristic value, we chose to focus on conserved regions in the EBNA-LP protein that consisted of three or more consecutively conserved amino acid residues. With this criterion, some regions were separated by only one or two nonconserved amino acids, and we have chosen to combine some of these groups into a single conserved region which is then subdivided into smaller regions. By this criterion, we have identified five conserved regions in the EBNA-LP protein (Fig. 2). Conserved region 1 (CR1) and CR3 consist of three and two smaller subregions, respectively. After taking into consideration that a methionine is required for translation initiation, the three conserved amino acid residues at the N-terminal end of the protein were not identified as a conserved region since that region would then include only two conserved residues. It should be noted, however, that a minimal functional EBNA-LP protein consists of two repeat sequences and the N terminus of the second W1 exon contains an extra amino acid, PRGD versus MGD, which is also conserved in all LCVs (Fig. 4B and unpublished data). Earlier identification of conserved regions for EBNA2 was postulated from sequence inspection (32). This was possible since the sequence of EBNA2 is considerably larger, and regions of conserved amino acids that fell into clusters were easily apparent. Using the criteria that we chose for EBNA-LP, we would have identified all conserved regions outlined in Fig. 6 for EBNA2 but would have also subdivided some of the conserved regions into two or more subregions.
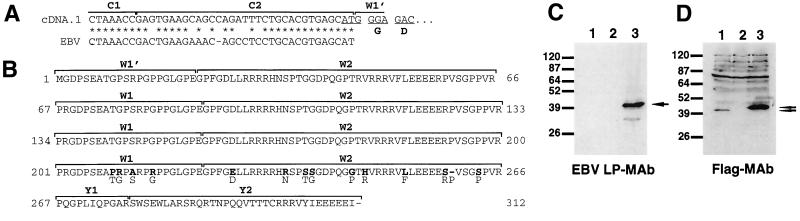
FIG. 4.
Sequence and expression of a rhesus LCV EBNA-LP cDNA clone. (A) Sequence of rLPcDNA-1 5′ untranslated termini. rLPcDNA-1 contains an in-frame splice to generate an initiation codon. The EBV C2 exon sequence is shown below the rLPcDNA-1 sequence for comparison. (B) The entire predicted amino acid sequence of rLPcDNA-1. The W1, W2, Y1, and Y2 boundaries are shown above each line. Amino acid numbers are shown at the beginning and end of each line. Sequence changes in the last W1/W2 exons leading to amino acid changes are indicated in bold. The predicted amino acids at those positions based on genomic sequencing are indicated below in plain type. (C) Western blot analysis of DG75 cells transfected with a rhesus LCV EBNA-LP expression plasmid (lane 1), vector expression plasmid only (lane 2), and an EBV EBNA-LP expression plasmid (lane 3). The blot was probed with a monoclonal antibody reactive with the EBV EBNA-LP protein (JF186). The arrow designates the detected EBNA-LP band. (D) Same as panel C except that the blot was probed with monoclonal antibody M2, which reacts with the Flag epitope that was engineered to be expressed on both EBV and rhesus LCV EBNA-LP proteins. Both EBNA-LP cDNAs used in these assays contain four BamHI W repeats. The two arrows indicate the detected EBV EBNA-LP and rhesus LCV EBNA-LP bands.
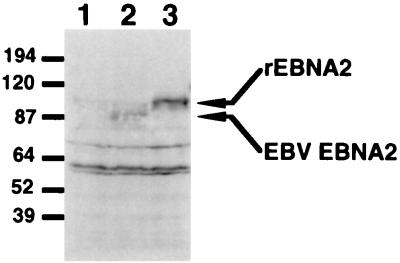
FIG. 6.
Detection of the rhesus LCV EBNA2 protein in transiently transfected lymphoid cells. Western blot analysis of transfected cell lysates was performed with monoclonal antibody PE2. The cell lysates were prepared from cells transfected with SG5 vector (lane 1), pPDL176A (EBNA2 expression plasmid) (lane 2), and pAG115 (rhesus LCV EBNA2 expression plasmid) (lane 3). The arrows indicate the detected EBNA2 and rhesus LCV EBNA2 (rEBNA2) proteins, which are approximately 87 and 100 kDa, respectively.
Phosphorylation of EBNA-LP has been suggested to be important for function. EBNA-LP appears to be only serine phosphorylated. Four of eight serine residues are well conserved. The serine at EBV EBNA-LP amino acid 76 is contained within a predicted CKII consensus site ([S/T]X2[D/E]). There are two conserved serines at amino acids 35 and 60 in the EBV W2 exon that may also be phosphorylated by cdc2 kinase (24). The conserved serine at position 5 and the other nonconserved serine residues are not contained in any apparent phosphorylation consensus sites. Two positively charged regions which may encode karyophilic signals (CR1c and CR2) and two negatively charged regions of unknown function (CR3a and CR5) also are well conserved.
The 5′ end of pAG6 contains approximately 500 bp of sequence similar to the EBV Wp. The baboon LCV XbaI P fragment also contained similar sequences. Bell et al. (2) recently reported that Wp activity was primarily modulated by three upstream regions termed UAS1 to UAS3. The region most upstream to the transcription start site, UAS3, is likely to be located in plasmid pAG5 from the rhesus LCV, and thus our Wp comparisons are limited to regions including UAS1 and UAS2. An alignment of the predicted rhesus and baboon LCV Wp sequences is shown in Fig. 3A. The baboon LCV Wp is 80% identical to the EBV Wp and 82% identical to the rhesus LCV Wp. The rhesus LCV Wp is 83% identical to the EBV Wp. This high level of homology is similar to that found between the lytic origin of replication and the Qp promoters from baboon and rhesus LCVs (42, 43). The regulation of Wp has only recently been explored in detail (2). UAS1 appears to confer tissue-specific augmentation of Wp activity in B cells, while UAS2 and UAS3 function in a cell lineage-independent manner. The studies by Bell et al. have identified a YY1 site in UAS2 (2). In addition, two subdomains within UAS1 also bound unidentified cellular factors that are required for Wp activity. Cellular proteins binding to one end of UAS1 that could be recognized by CREB/ATF antibodies in gel mobility shift assays were also identified (2). The different cellular factor binding sites within UAS1 and UAS2 are indicated in Fig. 3A. Notably, analysis of the Wp sequences from the different LCVs by using a transcription factor database (NCBI TFD database available on MacVector 6.0) also identified a consensus TATA box, CTF/CAAT, and ETS/PU-box regulatory motifs (Fig. 3A).
FIG. 3.
Alignment of the DNA sequence for the predicted EBV, baboon, and rhesus LCV Wp sequences. (A) UAS1 and UAS2 are indicated by brackets above the sequence. The dark and light shaded sequences in UAS1 indicate important distinct cis-acting elements that bind unknown cellular factors (2). Conserved cis-acting elements that bind cellular factors are boxed. The putative elements are also labeled. The W0 exon is shown by the arrow. The putative W0 splice donor site is indicated by a dashed box. The putative splice acceptor sites are indicated by the underlined bases and black (W1-generated splice acceptor) and white (W1′-generated splice acceptor) boxes. Nucleic acid numbers are indicated at the beginning and end of each line. The first number is arbitrary and begins at the XhoI restriction site for rhesus LCV clone pAG6. The EBV and baboon LCV sequences were then given consistent numbers based on the alignment. For EBV, base 1 corresponds to position 44547 and base 742 corresponds to position 45289 of the last W repeat in the EBV genome. (B) Alignment of EBV splice donor and acceptor sites and the predicted homologous sites for baboon LCV (bLCV) and rhesus LCV (rLCV). Consensus donor and acceptor sites are shown at the top, and the exon junctions are shown at the left.
Initial transcription from the EBV Wp results in an initial short exon termed W0 that is spliced to the W1 exon through the use of alternative splice acceptor sites termed W1 and W1′ (Fig. 3A) (41). A W0/W1 splice results in a transcript without an initiation codon for EBNA-LP, whereas a W0/W1′ splicing event gives rise to a message containing an initiation codon and thus codes for EBNA-LP. The sequences comprising the putative transcription initiation sites are well conserved, as are the first splice donor and acceptor sites that give rise to W0/W1 and W0/W1′ spliced transcripts (Fig. 3A). Genomic sequencing downstream of the Wp for both rhesus and baboon LCVs revealed several well-conserved splice donor and acceptor sites that flank putative coding regions for the W1, W2, Y1, and Y2 exons (Fig. 3B).
Cloning and expression of a rhesus LCV EBNA-LP cDNA.
To verify that structural features of rhesus or baboon LCV cDNA were analogous to EBV, we attempted to clone and sequence their cDNAs. Using primer pairs complementary to the Y2 coding exon and the C1 exons, we were able to clone two cDNAs (rLPcDNA-1 and -2) from the rhesus LCV-infected cell line LCL8664 but were unable to obtain an EBNA-LP cDNA by using a W0-Y2 primer pair. rLPcDNA-1 contains an in-frame splice that utilizes the W1′ alternative splice site and generates an initiation codon for EBNA-LP (Fig. 4). rLPcDNA-2 contains a C1/C2 exon splice to the W1 exon and is unable to code for EBNA-LP because it lacks an ATG initiation codon (data not shown). Sequencing of both cDNAs revealed that they have four copies of the W1/W2 repeats which correspond to genomic sequences except for the final W1/W2 exon, where several amino acid changes were identified in both clones (Fig. 4B). These changes are unlikely to be from PCR-generated errors, as these clones were obtained from independent PCRs. Since only one genomic fragment containing the W1 and W2 exons was sequenced, it remains a strong possibility that the last IR1 repeat in the LCL8664 strain is different from the first three copies. The functional relevance of this has yet to be determined.
To test whether we could express rLPcDNA-1, it was engineered to encode a carboxy-terminal Flag epitope tag, cloned into a eukaryotic expression vector (SG5), and transfected into EBV-negative DG75 cells. A similar EBV EBNA-LP was also cloned and expressed. As shown in Fig. 4C, the EBV EBNA-LP but not the rhesus LCV EBNA-LP protein was detected by Western blot analysis with monoclonal antibody JF186, which recognizes the EBV EBNA-LP protein. This finding is consistent with earlier reports that JF186 reacts only with EBV type 1 EBNA-LP (11). Both EBV and rhesus LCV EBNA-LP proteins were detected by monoclonal antibody M2, which recognizes the Flag epitope tag, although the abundance of the rhesus LCV EBNA-LP protein is significantly less (Fig. 4D). Some of this difference may be due to differential reactivity of the anti-FLAG antibody to amino-terminal (EBV EBNA-LP) versus carboxy-terminal (rhesus LCV EBNA-LP) Flag locations or possibly protein stability or mRNA stability. We conclude from analysis of these clones that the predicted rhesus LCV EBNA-LP sequence is transcribed in infected cells and the corresponding cDNA can be expressed to produce a protein of expected molecular weight.
Predicted amino acid sequence of a rhesus LCV EBNA2.
Before testing the rhesus LCV EBNA-LP homologue for cooperativity with EBNA2, we considered it important to identify and express a functional EBNA2 protein from the same LCV species. Plasmid pAG2 from the rhesus LCV contained sequences homologous to the EBNA2 ORF. Further sequence analysis of this clone revealed an ORF with homology to EBV EBNA2. The amino acid sequence is shown in Fig. 5 in alignment with other known EBNA2 protein sequences. Previous analysis has shown that the EBNA2 homologue in rhesus LCV-infected cells is significantly larger than its EBV counterpart (37). Consistent with this, the rhesus LCV EBNA2 is the largest EBNA2 isolate to date and contains 605 amino acid residues. The rhesus LCV EBNA2 is 38% identical to type 1 (B95-8), 34% identical to type 2 (AG876), and 40% identical to the baboon LCV EBNA2. The rhesus LCV EBNA2 has a 33-residue polyproline region that is intermediate to type 1 (42-residue) and type 2 (16-residue) polyproline stretches but slightly larger than the baboon LCV (21-residue) EBNA2. Similar to EBV and baboon LCV EBNA2s (32), the rhesus LCV EBNA2 amino acid homology is not evenly dispersed throughout the protein but rather consists of small clustered blocks of homology interspersed with larger regions of little homology. The central region of these proteins contains the greatest amount of divergence. The predominant reason for the large size difference between the rhesus LCV EBNA2 ORF and other EBNA2 proteins is a large insertion in the divergent region. Several well-conserved regions known to contain functional domains, such as the CBF1 interaction domain (CR6) and an important component of the transactivation domain (CR8), are well conserved in the rhesus LCV EBNA2 isolate. We would suggest, however, that CR4 may be slightly larger than previously predicted (Fig. 5) (32). Interestingly, EBV recombinants carrying deletions in EBNA2 CR4 have significantly reduced ability to immortalize B lymphocytes (4).
FIG. 5.
Alignment of the rhesus LCV EBNA2 amino acid sequence with the EBV type 1 (B95-8), type 2 (AG876), and baboon LCV EBNA2 amino acid sequences. Amino acid residues identical between sequences are indicated by asterisks, and amino acid residues with overall similarity are indicated by a dotted line. Conserved regions have been boxed and shaded and numbered in consecutive order as CR1 to CR9. Amino acid numbers are indicated at the beginning and end of each line.
Functional analysis of the rhesus LCV EBNA2 protein.
We cloned and expressed the rhesus LCV EBNA2 ORF in DG75 cells (Fig. 6). The protein product was made in slightly higher amounts than was type 1 EBNA2 expressed from a similar clone. It also matched the predicted size, as judged by its migration on SDS-PAGE (37). It is unclear why the rhesus LCV protein is made in larger amounts, but it may in part be because this protein contains a smaller polyproline region. Derivatives of EBV EBNA2 that contain deletions in the polyproline region, or lack this region altogether, also appear to accumulate to larger amounts than the wild-type protein in transfected cells (unpublished observations).
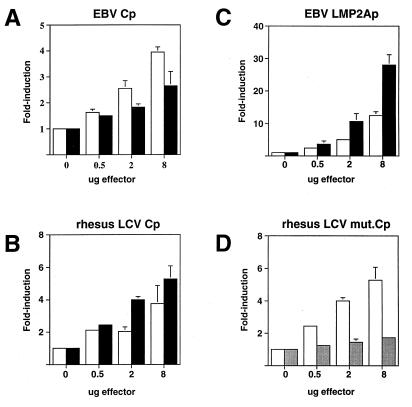
The rhesus LCV EBNA2 protein was tested for its ability to transactivate several reporter plasmids that have previously been shown to be activated by EBV EBNA2 in transient cotransfection analysis (29–32, 59). Rhesus LCV EBNA2 stimulated expression from both the EBV Cp (−1024 to +3) and rhesus LCV Cp (−1024 to +3) three- and sixfold, respectively, similar to the levels obtained with EBV EBNA2 (Fig. 7A and B). In general, EBV EBNA2 transactivated EBV Cp better than rhesus LCV EBNA2, while the reverse was true for rhesus LCV Cp. The ability of each EBNA2 to stimulate Cp better when it was derived from the same virus may be due to subtle evolutionary changes that are optimal for their interactions. In addition, EBV EBNA2 stimulated the LMP2A promoter up to 15-fold, whereas rhesus LCV EBNA2 gave a close to 30-fold effect (Fig. 7C). While these results are statistically significant, it is unclear at this time why rhesus LCV EBNA2 displayed higher transactivating activity than the EBV EBNA2 protein, but this may be related to its overall greater accumulation in transfected cells.
FIG. 7.
The rhesus LCV EBNA2 protein stimulates the latency C and LMP2a promoters in transient cotransfections and is dependent on CBF1. Both EBV EBNA2 and rhesus LCV EBNA2 were tested for the ability to activate reporter plasmids containing EBNA2-responsive promoters. The amount of target plasmid in all experiments was 2.0 μg. The results are shown as an average of three experiments; the T-bars indicate standard errors. (A) Transactivation of the EBV Cp (−1024 to +3) (11). EBV EBNA2 is shown as white bars, and rhesus LCV EBNA2 is shown as black bars. (B) Transactivation of the rhesus LCV Cp (−1024 to +3) (12). EBV EBNA2 is shown as white bars, and rhesus LCV EBNA2 is shown as black bars. (C) Transactivation of the EBV LMP2A promoter (60). EBV EBNA2 is shown as white bars, and rhesus LCV EBNA2 is shown as black bars. (D) The rhesus LCV EBNA2 was the only effector plasmid used in these experiments. The white bars indicate rhesus LCV induction of a wild-type rhesus LCV Cp, and the shaded bars indicate the level of rhesus LCV EBNA2 induction of a rhesus LCV Cp containing a mutant CBF1 binding site (12).
The major cellular cofactor that mediates EBNA2 promoter targeting activity is the cellular DNA binding protein CBF1. The primary motif for binding to the CBF1 protein is located in CR6, which is well conserved in both the rhesus and baboon LCV EBNA2 proteins (29, 57). To test whether rhesus LCV EBNA2 also depended on CBF1 for transcriptional activation, we tested its ability to transactivate Cp reporter plasmids that contained mutant, nonfunctional CBF1 binding sites. As seen in Fig. 7D, a functional CBF1 binding site in the rhesus LCV Cp is required for rhesus LCV EBNA2 to transactivate this promoter. This finding is consistent with previous studies utilizing EBV EBNA2 and suggests that transactivation signaling through CBF1 has been maintained during the evolutionary divergence of these proteins.
Functional analysis of the rhesus LCV EBNA-LP protein.
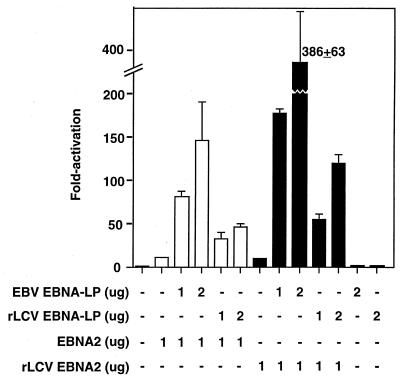
Having cloned and expressed rhesus LCV EBNA-LP and EBNA2 proteins (Fig. 4 and 6), we attempted to address two specific questions. First, does the rhesus LCV EBNA-LP coactivate rhesus LCV EBNA2-mediated transactivation, and does it do so to a similar order of magnitude as EBV EBNA-LP stimulation of EBV EBNA2? Second, are the EBNA-LP and EBNA2 homologues from different species interchangeable for coactivation? To carry out these experiments, we used a reporter plasmid containing the EBNA2 enhancer element from the latency C promoter which has been previously shown to be synergistically activated by EBNA2 and EBNA-LP (17). The rhesus LCV EBNA-LP protein was able to stimulate transcription with either rhesus or EBV EBNA2 to levels 5- to 12-fold above those obtained with EBNA2 alone (Fig. 8). Likewise, the EBV EBNA-LP protein was also able to stimulate both EBV and rhesus LCV EBNA2 proteins in transient cotransfection assays. Neither of the EBNA-LP expression plasmids activated the Cp reporter plasmid on their own (Fig. 8), and in some cases they actually repressed basal activity (unpublished observations). Consistent with the fact that the EBV EBNA-LP protein accumulates in larger amounts in transfected cells, it stimulated EBNA2 activation to higher levels than the rhesus LCV EBNA-LP. Subsequent experiments using larger amounts of the rhesus LCV EBNA-LP-expressing plasmid have indicated that we can approach levels of synergy similar to those achieved with the EBV EBNA-LP protein (data not shown). These results indicate that costimulation of EBNA2 by EBNA-LP is not dependent on both proteins being derived from the same viral species and that the magnitude of rhesus LCV stimulation of EBNA2 transactivation approaches that of EBV EBNA-LP.
FIG. 8.
EBNA-LP from either rhesus LCV or EBV enhances EBNA2-mediated transactivation of the BamCpLUC reporter gene. Plasmids expressing EBNA2 (white bars) or rhesus LCV (rLCV) EBNA2 (black bars) were transfected into DG75 cells (1.0 μg). The reporter plasmid was BamCp8LUC, which contains eight copies of the 100-bp EBNA2 enhancer from the latency C promoter (see Materials and Methods for details). In some samples, EBV EBNA-LP or rhesus LCV EBNA-LP expression plasmids were cotransfected. The presence or absence of the EBNA-LP or EBNA2 plasmids is indicated below the graph.
DISCUSSION
This study demonstrates that the EBNA-LP and EBNA2 proteins encoded by the nonhuman primate LCVs from baboon and rhesus macaques have strong sequence and functional conservation. Sequences colinear to the EBV BamHI Wp were also identified and are highly conserved between EBV and baboon and rhesus LCVs. We also show that the EBNA-LP homologue from rhesus LCV is transcribed in rhesus LCV-infected cells and the RNA is structurally similar to EBV EBNA-LP mRNAs. In addition, we show that the rhesus LCV EBNA2 is functionally similar to EBV EBNA2 and stimulates EBNA2-responsive promoters through a CBF1-dependent mechanism. Finally, the rhesus LCV EBNA-LP protein stimulates EBNA2-mediated transactivation similarly to EBV EBNA-LP, and transcriptional cooperation is not restricted between human and rhesus macaque LCV-encoded EBNA-LP and EBNA2 proteins.
Alignment of the EBNA-LP protein sequences from baboon and rhesus LCVs with EBV type 1 and 2 EBNA-LP sequences reveals several well-conserved regions and serine residues (Fig. 2). Although somewhat divergent at the sequence level compared to EBV, other latent proteins from nonhuman primate LCVs demonstrate a high degree of functional conservation. Examples include EBNA2 interaction with CBF1 and LMP-1 interaction with tumor necrosis factor receptor-associated factors (12, 31). This new comparative sequence information will allow us to circumvent a random approach toward identification of novel EBNA-LP functional domains by highlighting potentially important conserved amino acid regions and phosphorylated residues. Thus, we anticipate that some of the conserved regions identified from our comparison of EBNA-LP from EBV with other LCV EBNA-LP proteins will provide important clues for identifying the cellular cofactors and molecular mechanisms mediating EBNA-LP function.
Some attempts have been made to characterize phosphorylation of EBNA-LP (23, 39). EBNA-LP appears to be exclusively serine phosphorylated (23, 39). Only four of eight serines are conserved between the LCV EBNA-LP proteins (Fig. 2). None of the conserved serines in the repeats appears to be associated with a recognizable phosphorylation site. Since the Y1 and Y2 exons are dispensable for cooperation with EBNA2-mediated transactivation, phosphorylated serines at positions 5, 35, and 60 (Fig. 2) are likely candidates for potentially modulating cooperativity function with EBNA2. CR4 contains a consensus CKII site, (S/T)X2(D/E), and can be phosphorylated in vitro by CKII. The exact role of phosphorylation at this position is unclear and is probably not required for cooperation with EBNA2 since this region of the protein can be deleted without affecting cooperative function. By using the comparative approach, the number of potential serine mutants needed to determine the role of phosphorylation required for EBNA-LP function can be substantially reduced.
The rhesus and baboon LCV EBNA-LP proteins show greater than 60% homology to EBV EBNA-LP between the W1 and W2 exons. In contrast, a previous study has reported the sequence of putative W1 and W2 exons for the EBNA-LP protein from herpesvirus macaca fascularis (HVMF1), which shows only 51% identity to EBV EBNA-LP (28). It should be noted however, that HVFM1 and rhesus LCV EBNA-LP proteins are 71% identical between their W1 and W2 exons and are slightly more related to each other than to the other EBNA-LP proteins. HVMF1 infects cynomolgus macaques, a species related to rhesus macaques. The reason for such a marked difference in homology compared to other primate LCVs is unclear but is due in part to several nonconserved amino acid changes and a four-amino-acid deletion in the W1 exon (28). The HVFM1 sequence was obtained from DNA isolated from a cell line (H50) derived from a lymphoma. Additional mutations introduced during lymphomagenesis, PCR amplification, or sequencing errors (only a single strand was sequenced) may have contributed to sequence changes. Analysis of additional HVMF1 isolates should help to resolve whether the HVFM1 EBNA-LP diverges significantly from EBV EBNA-LP relative to the other LCV EBNA-LP proteins. Nonetheless, with the exception of an absence of a serine at position 5 and no homology in CR1a, the HVMF1 EBNA-LP repeat exon protein sequence retains amino acid residues conserved among the other LCV EBNA-LP proteins presented here (e.g., CR1b and -c, CR2, and CR3).
The putative Wp sequences from EBV and rhesus and baboon LCVs are more highly conserved overall (greater than 80%) than the latency C promoter sequence (14). The latency Q promoter and the origin of lytic replication are the only sequences between LCVs that are this highly conserved (42, 43). Recent analysis of the Wp has revealed three distinct regions, termed UAS1 to UAS3, that contribute substantially to Wp activity (2). A YY1 site within UAS2 which has been found to be important for Wp activation is well conserved (2). Three cis-acting elements within UAS1 have been found to bind cellular factors and are also well conserved (2). One of these is a partially conserved CREB/ATF site that binds cellular factors reactive with CREB/ATF1 antibodies (Fig. 3). Two other elements within UAS1 binding unidentified cellular factors have also been found (Fig. 3) (2). Since a paucity of conserved transcription factor binding sites was found in a search of a transcription factor database, the sequence comparison is likely to be an informative tool for elucidating additional and possibly novel cellular regulatory proteins.
In addition to identification of homologous Wp sequences, it appears that the strategy for alternative splicing and generation of EBNA-LP coding and noncoding transcripts has been evolutionarily conserved (47). All potential splice donor and acceptor sites identified from analysis of EBV genomic and EBNA-LP cDNAs also are present in the nonhuman primate LCVs (Fig. 4A and B) (47). Cloning and sequence analysis of two rhesus LCV EBNA-LP-encoding cDNAs also confirm that these viruses likely utilize similar transcription strategies. Since the number of cDNAs isolated in our study is small, additional EBNA-LP cDNAs will need to be isolated to confirm if the structure of EBNA-LP cDNA found in rhesus or baboon LCV-infected cells is generally identical to that of EBV-infected cell mRNAs.
Several functional domains have been identified in the EBNA2 protein. These include a nuclear localization domain, a transactivation domain, and a domain that interacts with the cellular DNA binding protein CBF1. All of these functional domains are well conserved among EBV and baboon LCV EBNA2 proteins (32). In this study, we report the amino acid sequence of another EBNA2 protein derived from the rhesus LCV. All of the characterized functional domains are also well conserved in the rhesus LCV EBNA2 protein sequence. In addition, several conserved regions (CR1 to CR4) in the amino-terminal half of the EBNA2 which have yet to be assigned functions are also conserved in the rhesus LCV EBNA2 protein. Previous genetic analysis has indicated that parts of the amino-terminal half of EBNA2 can be deleted without disrupting immortalizing function (56). At least one essential domain appears to be the polyproline domain, although it is not clear whether the 3′ acidic region proximal to the polyprolines or CR4 may also be essential (4, 56). Notably, the rhesus LCV EBNA2 contains a large stretch of polyprolines that is intermediate in size to the type 1 and type 2 EBV EBNA2 proteins. The usefulness of the EBNA2 sequence comparisons would be strengthened if the EBNA2s from the other related LCVs could be functionally tested. To this end, we cloned and expressed the rhesus LCV EBNA2 protein in lymphocytes and tested it for the ability to stimulate a variety of EBNA2-responsive promoters. Like EBV EBNA2, rhesus EBNA2 was able to transactivate both the EBV and rhesus LCV Cp and EBV LMP2a promoters. In addition, transactivation of the Cp required a functional CBF1 binding site (Fig. 7). Despite only 38% amino acid identity between rhesus LCV EBNA2 and EBV EBNA2, the rhesus LCV EBNA2 appears to retain a similar transactivation function at least, which is likely to be modulated through conserved domains. This is consistent with our earlier studies which have shown that CBF1 and CBF2 binding sites are important elements required for rhesus LCV Cp activation by EBV EBNA2 (13). Characterization of the rhesus LCV EBNA2 will serve as an important tool for further development of the rhesus animal model for EBV infection and disease.
To verify that other LCV EBNA-LP proteins can function like EBV EBNA-LP, we tested the rhesus LCV EBNA-LP protein for the ability to stimulate EBNA2 transactivation in transient cotransfection assays. Both rhesus LCV EBNA-LP and EBV EBNA-LP proved capable of stimulating transcription mediated by an EBNA2 derived from either EBV or rhesus LCV. Currently, there are no genetic data that link EBNA-LP synergy function to EBV immortalization. However, retention of this synergistic function between rhesus LCV EBNA-LP and EBNA2 suggests that it is a universal function important for the LCV life cycle. The conserved EBNA-LP function also validates an approach for future studies targeting conserved regions for mutagenesis that will allow elucidation of novel EBNA-LP functional domains, which in the future can be used to assess EBNA-LP function in genetically based assays.
ACKNOWLEDGMENTS
This work was supported by NIH grant R29 CA69437 and an award from the William Stamps Farish Foundation to P.D.L.
We thank Georg Bornkamm for generously providing the LMP2A reporter plasmid and Elliott Kieff for the EBV EBNA-LP expression plasmid.
REFERENCES
- 1.Alfieri C, Birkenbach M, Kieff E. Early events in Epstein-Barr virus infection of human B lymphocytes. Virology. 1991;181:595–608. doi: 10.1016/0042-6822(91)90893-g. . (Erratum, 185:946.) [DOI] [PubMed] [Google Scholar]
- 2.Bell A, Skinner J, Kirby H, Rickinson A. Characterisation of regulatory sequences at the Epstein-Barr virus BamHI W promoter. Virology. 1998;252:149–161. doi: 10.1006/viro.1998.9440. [DOI] [PubMed] [Google Scholar]
- 3.Cohen J I, Kieff E. An Epstein-Barr virus nuclear protein 2 domain essential for transformation is a direct transcriptional activator. J Virol. 1991;65:5880–5885. doi: 10.1128/jvi.65.11.5880-5885.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen J I, Wang F, Kieff E. Epstein-Barr virus nuclear protein 2 mutations define essential domains for transformation and transactivation. J Virol. 1991;65:2545–2554. doi: 10.1128/jvi.65.5.2545-2554.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dambaugh T, Hennessy K, Chamnankit L, Kieff E. U2 region of Epstein-Barr virus DNA may encode Epstein-Barr nuclear antigen 2. Proc Natl Acad Sci USA. 1984;81:7632–7636. doi: 10.1073/pnas.81.23.7632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dillner J, Kallin B, Alexander H, Ernberg I, Uno M, Ono Y, Klein G, Lerner R A. An Epstein-Barr virus (EBV)-determined nuclear antigen (EBNA5) partly encoded by the transformation-associated Bam WYH region of EBV DNA: preferential expression in lymphoblastoid cell lines. Proc Natl Acad Sci USA. 1986;83:6641–6646. doi: 10.1073/pnas.83.17.6641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dyck J A, Maul G G, Miller W H, Jr, Chen J D, Kakizuka A, Evans R M. A novel macromolecular structure is a target of the promyelocyte-retinoic acid receptor oncoprotein. Cell. 1994;76:333–343. doi: 10.1016/0092-8674(94)90340-9. [DOI] [PubMed] [Google Scholar]
- 8.Fahraeus R, Jansson A, Ricksten A, Sjoblom A, Rymo L. Epstein-Barr virus-encoded nuclear antigen 2 activates the viral latent membrane protein promoter by modulating the activity of a negative regulatory element. Proc Natl Acad Sci USA. 1990;87:7390–7394. doi: 10.1073/pnas.87.19.7390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feichtinger H, Kaaya E, Putkonen P, Li S L, Ekman M, Gendelman R, Biberfeld G, Biberfeld P. Malignant lymphoma associated with human AIDS and with SIV-induced immunodeficiency in macaques. AIDS Res Hum Retroviruses. 1992;8:339–348. doi: 10.1089/aid.1992.8.339. [DOI] [PubMed] [Google Scholar]
- 10.Feichtinger H, Li S L, Kaaya E, Putkonen P, Grunewald K, Weyrer K, Bottiger D, Ernberg I, Linde A, Biberfeld G, et al. A monkey model for Epstein Barr virus-associated lymphomagenesis in human acquired immunodeficiency syndrome. J Exp Med. 1992;176:281–286. doi: 10.1084/jem.176.1.281. . (Erratum, 176: following 634.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Finke J, Rowe M, Kallin B, Ernberg I, Rosen A, Dillner J, Klein G. Monoclonal and polyclonal antibodies against Epstein-Barr virus nuclear antigen 5 (EBNA-5) detect multiple protein species in Burkitt's lymphoma and lymphoblastoid cell lines. J Virol. 1987;61:3870–3878. doi: 10.1128/jvi.61.12.3870-3878.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Franken M, Devergne O, Rosenzweig M, Annis B, Kieff E, Wang F. Comparative analysis identifies conserved tumor necrosis factor receptor-associated factor 3 binding sites in the human and simian Epstein-Barr virus oncogene LMP1. J Virol. 1996;70:7819–7826. doi: 10.1128/jvi.70.11.7819-7826.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fuentes-Pananá E M, Ling P D. Characterization of the CBF2 binding site within the Epstein-Barr virus latency C promoter and its role in modulating EBNA2-mediated transactivation. J Virol. 1998;72:693–700. doi: 10.1128/jvi.72.1.693-700.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fuentes-Pananá E M, Swaminathan S, Ling P D. Transcriptional activation signals found in the Epstein-Barr virus (EBV) latency C promoter are conserved in the latency C promoter sequences from baboon and rhesus monkey EBV-like lymphocryptoviruses (cercopithicine herpesviruses 12 and 15) J Virol. 1999;73:826–833. doi: 10.1128/jvi.73.1.826-833.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grossman S R, Johannsen E, Tong X, Yalamanchili R, Kieff E. The Epstein-Barr virus nuclear antigen 2 transactivator is directed to response elements by the J kappa recombination signal binding protein. Proc Natl Acad Sci USA. 1994;91:7568–7572. doi: 10.1073/pnas.91.16.7568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hammerschmidt W, Sugden B. Genetic analysis of immortalizing functions of Epstein-Barr virus in human B lymphocytes. Nature. 1989;340:393–397. doi: 10.1038/340393a0. [DOI] [PubMed] [Google Scholar]
- 17.Harada S, Kieff E. Epstein-Barr virus nuclear protein LP stimulates EBNA-2 acidic domain-mediated transcriptional activation. J Virol. 1997;71:6611–6618. doi: 10.1128/jvi.71.9.6611-6618.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henkel T, Ling P D, Hayward S D, Peterson M G. Mediation of Epstein-Barr virus EBNA2 transactivation by recombination signal-binding protein J kappa. Science. 1994;265:92–95. doi: 10.1126/science.8016657. [DOI] [PubMed] [Google Scholar]
- 19.Inman G J, Farrell P J. Epstein-Barr virus EBNA-LP and transcription regulation properties of pRB, p107 and p53 in transfection assays. J Gen Virol. 1995;76:2141–2149. doi: 10.1099/0022-1317-76-9-2141. [DOI] [PubMed] [Google Scholar]
- 20.Jenson H B, Farrell P J, Miller G. Sequences of the Epstein-Barr virus (EBV) large internal repeat form the center of a 16-kilobase-pair palindrome of EBV (P3HR-1) heterogeneous DNA. J Virol. 1987;61:1495–1506. doi: 10.1128/jvi.61.5.1495-1506.1987. . (Erratum, 61:2950.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang W Q, Szekely L, Wendel-Hansen V, Ringertz N, Klein G, Rosen A. Co-localization of the retinoblastoma protein and the Epstein-Barr virus-encoded nuclear antigen EBNA-5. Exp Cell Res. 1991;197:314–318. doi: 10.1016/0014-4827(91)90438-z. [DOI] [PubMed] [Google Scholar]
- 22.Kieff E. Epstein-Barr virus and its replication. In: Fields B N, Knipe D M, Howley P M, editors. Virology. 3rd ed. Vol. 2. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 107–172. [Google Scholar]
- 23.Kitay M K, Rowe D T. Cell cycle stage-specific phosphorylation of the Epstein-Barr virus immortalization protein EBNA-LP. J Virol. 1996;70:7885–7893. doi: 10.1128/jvi.70.11.7885-7893.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kitay M K, Rowe D T. Protein-protein interactions between Epstein-Barr virus nuclear antigen-LP and cellular gene products: binding of 70-kilodalton heat shock proteins. Virology. 1996;220:91–99. doi: 10.1006/viro.1996.0289. [DOI] [PubMed] [Google Scholar]
- 25.Knutson J C. The level of c-fgr RNA is increased by EBNA-2, an Epstein-Barr virus gene required for B-cell immortalization. J Virol. 1990;64:2530–2536. doi: 10.1128/jvi.64.6.2530-2536.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koken M H, Puvion-Dutilleul F, Guillemin M C, Viron A, Linares-Cruz G, Stuurman N, de Jong L, Szostecki C, Calvo F, Chomienne C, et al. The t(15;17) translocation alters a nuclear body in a retinoic acid-reversible fashion. EMBO J. 1994;13:1073–1083. doi: 10.1002/j.1460-2075.1994.tb06356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laux G, Adam B, Strobl L J, Moreau-Gachelin F. The Spi-1/PU.1 and Spi-B ets family transcription factors and the recombination signal binding protein RBP-J kappa interact with an Epstein-Barr virus nuclear antigen 2 responsive cis-element. EMBO J. 1994;13:5624–5632. doi: 10.1002/j.1460-2075.1994.tb06900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li S L, Biberfeld P, Ernberg I. DNA of lymphoma-associated herpesvirus (HVMF1) in SIV-infected monkeys (Macaca fascicularis) shows homologies to EBNA-1, -2 and -5 genes. Int J Cancer. 1994;59:287–295. doi: 10.1002/ijc.2910590223. [DOI] [PubMed] [Google Scholar]
- 29.Ling P D, Hayward S D. Contribution of conserved amino acids in mediating the interaction between EBNA2 and CBF1/RBPJk. J Virol. 1995;69:1944–1950. doi: 10.1128/jvi.69.3.1944-1950.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ling P D, Hsieh J J, Ruf I K, Rawlins D R, Hayward S D. EBNA-2 upregulation of Epstein-Barr virus latency promoters and the cellular CD23 promoter utilizes a common targeting intermediate, CBF1. J Virol. 1994;68:5375–5383. doi: 10.1128/jvi.68.9.5375-5383.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ling P D, Rawlins D R, Hayward S D. The Epstein-Barr virus immortalizing protein EBNA-2 is targeted to DNA by a cellular enhancer-binding protein. Proc Natl Acad Sci USA. 1993;90:9237–9241. doi: 10.1073/pnas.90.20.9237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ling P D, Ryon J J, Hayward S D. EBNA-2 of herpesvirus papio diverges significantly from the type A and type B EBNA-2 proteins of Epstein-Barr virus but retains an efficient transactivation domain with a conserved hydrophobic motif. J Virol. 1993;67:2990–3003. doi: 10.1128/jvi.67.6.2990-3003.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mannick J B, Cohen J I, Birkenbach M, Marchini A, Kieff E. The Epstein-Barr virus nuclear protein encoded by the leader of the EBNA RNAs is important in B-lymphocyte transformation. J Virol. 1991;65:6826–6837. doi: 10.1128/jvi.65.12.6826-6837.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mannick J B, Tong X, Hemnes A, Kieff E. The Epstein-Barr virus nuclear antigen leader protein associates with hsp72/hsc73. J Virol. 1995;69:8169–8172. doi: 10.1128/jvi.69.12.8169-8172.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mark W, Sugden B. Transformation of lymphocytes by Epstein-Barr virus requires only one-fourth of the viral genome. Virology. 1982;122:431–443. doi: 10.1016/0042-6822(82)90242-2. [DOI] [PubMed] [Google Scholar]
- 36.Meitinger C, Strobl L J, Marschall G, Bornkamm G W, Zimber S U. Crucial sequences within the Epstein-Barr virus TP1 promoter for EBNA2-mediated transactivation and interaction of EBNA2 with its responsive element. J Virol. 1994;68:7497–7506. doi: 10.1128/jvi.68.11.7497-7506.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moghaddam A, Rosenzweig M, Lee-Parritz D, Annis B, Johnson R P, Wang F. An animal model for acute and persistent Epstein-Barr virus infection. Science. 1997;276:2030–2033. doi: 10.1126/science.276.5321.2030. [DOI] [PubMed] [Google Scholar]
- 38.Nitsche F, Bell A, Rickinson A. Epstein-Barr virus leader protein enhances EBNA-2-mediated transactivation of latent membrane protein 1 expression: a role for the W1W2 repeat domain. J Virol. 1997;71:6619–6628. doi: 10.1128/jvi.71.9.6619-6628.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Petti L, Sample C, Kieff E. Subnuclear localization and phosphorylation of Epstein-Barr virus latent infection nuclear proteins. Virology. 1990;176:563–574. doi: 10.1016/0042-6822(90)90027-o. [DOI] [PubMed] [Google Scholar]
- 40.Rickinson A B, Kieff E. Epstein-Barr virus. In: Fields B N, Knipe D M, Howley P M, editors. Virology. 3rd ed. Vol. 2. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 2397–2446. [Google Scholar]
- 41.Rogers R P, Woisetschlaeger M, Speck S H. Alternative splicing dictates translational start in Epstein-Barr virus transcripts. EMBO J. 1990;9:2273–2277. doi: 10.1002/j.1460-2075.1990.tb07398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ruf I K, Moghaddam A, Wang F, Sample J. Mechanisms that regulate Epstein-Barr virus EBNA-1 gene transcription during restricted latency are conserved among lymphocryptoviruses of Old World primates. J Virol. 1999;73:1980–1989. doi: 10.1128/jvi.73.3.1980-1989.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ryon J J, Fizman E D, Houchens C, Zong J, Lieberman P M, Chang Y-N, Hayward G S, Hayward S D. The lytic origin of herpesvirus papio is highly homologous to Epstein-Barr virus ori-Lyt: evolutionary conservation of transcriptional activation and replication signals. J Virol. 1993;67:4006–4016. doi: 10.1128/jvi.67.7.4006-4016.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sample J, Hummel M, Braun D, Birkenbach M, Kieff E. Nucleotide sequences of mRNAs encoding Epstein-Barr virus nuclear proteins: a probable transcriptional initiation site. Proc Natl Acad Sci USA. 1986;83:5096–5100. doi: 10.1073/pnas.83.14.5096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sinclair A J, Palmero I, Peters G, Farrell P J. EBNA-2 and EBNA-LP cooperate to cause G0 to G1 transition during immortalization of resting human B lymphocytes by Epstein-Barr virus. EMBO J. 1994;13:3321–3328. doi: 10.1002/j.1460-2075.1994.tb06634.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Speck S H, Pfitzner A, Strominger J L. An Epstein-Barr virus transcript from a latently infected, growth-transformed B-cell line encodes a highly repetitive polypeptide. Proc Natl Acad Sci USA. 1986;83:9298–9302. doi: 10.1073/pnas.83.24.9298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Speck S H, Strominger J L. Transcription of Epstein-Barr virus in latently infected growth-transformed lymphocytes. In: Klein G, editor. Advances in oncology. Vol. 8. New York, N.Y: Raven Press, Ltd.; 1989. pp. 133–150. [Google Scholar]
- 48.Sung N S, Kenney S, Gutsch D, Pagano J S. EBNA-2 transactivates a lymphoid-specific enhancer in the BamHI C promoter of Epstein-Barr virus. J Virol. 1991;65:2164–2169. doi: 10.1128/jvi.65.5.2164-2169.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Szekely L, Jiang W Q, Pokrovskaja K, Wiman K G, Klein G, Ringertz N. Reversible nucleolar translocation of Epstein-Barr virus-encoded EBNA-5 and hsp70 proteins after exposure to heat shock or cell density congestion. J Gen Virol. 1995;76:2423–2432. doi: 10.1099/0022-1317-76-10-2423. [DOI] [PubMed] [Google Scholar]
- 50.Szekely L, Pokrovskaja K, Jiang W Q, de The H, Ringertz N, Klein G. The Epstein-Barr virus-encoded nuclear antigen EBNA-5 accumulates in PML-containing bodies. J Virol. 1996;70:2562–2568. doi: 10.1128/jvi.70.4.2562-2568.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Szekely L, Selivanova G, Magnusson K P, Klein G, Wiman K G. EBNA-5, an Epstein-Barr virus-encoded nuclear antigen, binds to the retinoblastoma and p53 proteins. Proc Natl Acad Sci USA. 1993;90:5455–5459. doi: 10.1073/pnas.90.12.5455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tsang S F, Wang F, Izumi K M, Kieff E. Delineation of the cis-acting element mediating EBNA-2 transactivation of latent infection membrane protein expression. J Virol. 1991;65:6765–6771. doi: 10.1128/jvi.65.12.6765-6771.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Waltzer L, Logeat F, Brou C, Israel A, Sergeant A, Manet E. The human J kappa recombination signal sequence binding protein (RBP-J kappa) targets the Epstein-Barr virus EBNA2 protein to its DNA responsive elements. EMBO J. 1994;13:5633–5638. doi: 10.1002/j.1460-2075.1994.tb06901.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weis K, Rambaud S, Lavau C, Jansen J, Carvalho T, Carmo-Fonseca M, Lamond A, Dejean A. Retinoic acid regulates aberrant nuclear localization of PML-RAR alpha in acute promyelocytic leukemia cells. Cell. 1994;76:345–356. doi: 10.1016/0092-8674(94)90341-7. [DOI] [PubMed] [Google Scholar]
- 55.Woisetschlaeger M, Jin X W, Yandava C N, Furmanski L A, Strominger J L, Speck S H. Role for the Epstein-Barr virus nuclear antigen 2 in viral promoter switching during initial stages of infection. Proc Natl Acad Sci USA. 1991;88:3942–3946. doi: 10.1073/pnas.88.9.3942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yalamanchili R, Harada S, Kieff E. The N-terminal half of EBNA2, except for seven prolines, is not essential for primary B-lymphocyte growth transformation. J Virol. 1996;70:2468–2473. doi: 10.1128/jvi.70.4.2468-2473.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yalamanchili R, Tong X, Grossman S, Johannsen E, Mosialos G, Kieff E. Genetic and biochemical evidence that EBNA 2 interaction with a 63-kDa cellular GTG-binding protein is essential for B lymphocyte growth transformation by EBV. Virology. 1994;204:634–641. doi: 10.1006/viro.1994.1578. [DOI] [PubMed] [Google Scholar]
- 58.Zimber-Strobl U, Strobl L J, Meitinger C, Hinrichs R, Sakai T, Furukawa T, Honjo T, Bornkamm G W. Epstein-Barr virus nuclear antigen 2 exerts its transactivating function through interaction with recombination signal binding protein RBP-J kappa, the homologue of Drosophila Suppressor of Hairless. EMBO J. 1994;13:4973–4982. doi: 10.1002/j.1460-2075.1994.tb06824.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zimber-Strobl U, Suentzenich K, Falk M, Laux G, Cordier M, Calender A, Billaud M, Lenoir G M, Bornkamm G W. Epstein-Barr virus terminal protein gene transcription is dependent on EBNA2 expression and provides evidence for viral integration into the host genome. Curr Top Microbiol Immunol. 1990;166:359–366. doi: 10.1007/978-3-642-75889-8_44. [DOI] [PubMed] [Google Scholar]
- 60.Zimber-Strobl U, Suentzenich K O, Laux G, Eick D, Cordier M, Calender A, Billaud M, Lenoir G M, Bornkamm G W. Epstein-Barr virus nuclear antigen 2 activates transcription of the terminal protein gene. J Virol. 1991;65:415–423. doi: 10.1128/jvi.65.1.415-423.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]