Abstract
Herein, we propose a Carbopol hydrogel-based oxygen nanodelivery “nanohyperbaric” system as a wound dressing material for an enhanced wound healing process. Oxygen nanobubbles (ONBs) were used to supply oxygen, and collagenase was added in the gel as a drug model. Both oxygen and collagenase would benefit the wound healing process, and the Carbopol hydrogel serves as the matrix to load ONBs and collagenase in the wound dressing. The obtained ONB-embedded Carbopol hydrogel with collagenase (ONB-CC) could provide 12.08 ± 0.75 μg of oxygen from 1 mL of ONB-CC and exhibited a notable capacity to prolong the oxygen holding for up to 3 weeks and maintained the enzymatic activity of collagenase at more than 0.05 U per 0.1 mL of ONB-CC for up to 17 days. With HDFa cells, the ONB-CC did not show a notable effect on the cell viability. In a scratch assay, the oxygen from ONBs or collagenase aided cell migration; further, the ONB-CC induced the most obvious scratch closure, indicating an improvement in wound healing as a cocktail in the ONB-CC. The mRNA expression further demonstrated the effectiveness of the ONB-CC. Studies in rats with punched wounds treated with the ONB-CC dressing showed improved wound closure. Histopathological images showed that the ONB-CC dressing enhanced re-epithelization and formation of new blood vessels and hair follicles. The proposed ONB-CC has excellent potential as an ideal wound dressing material to accelerate wound healing by integration of multiple functions.
Introduction
Injuries to the skin that could result in both acute and chronic wounds are common. These can result from surgical incisions, lacerations, abrasions, punctures, burns, scalds, and diseases. Compared with acute wounds, chronic wounds require complex multidisciplinary and multimodal treatments that reduce patients’ productivity and increase the health care cost. Dressing materials are critical in the care of acute wounds requiring anti-infection properties, maintenance of humidity, and air permeability. Chronic wounds usually demand repeated hospital visits for debridement and refreshment of the dressing. Sometimes, expensive medicines or materials such as growth factors or cellular matrix/stem cell tissues are involved.1 Meanwhile, the requirements on wound care have caused an exacerbating burden on the public health system. A report in 2022 indicated that around 28.1 to 96.8 billion U.S. dollars were spent on wound care in the United States.2 Advances in medical materials and methods for wound care would greatly benefit patients and the public health system.
The improvement from oxygen to wound healing has been demonstrated in prior work3,4 with hyperbaric oxygen treatment (HBOT) being one of the most common treatments for chronic wounds, and some research indicates that HBOT appears to be effective for complicated acute wounds.5,6 However, HBOT requires periodic hospital visits, a protracted treatment process, and insurance approvals and is often poorly tolerated by patients due to side effects of being in a hyperbaric oxygen chamber under high atmospheric pressure. Patients have poor tolerance and difficulty in accessing this treatment due to the large size as well as the requirement of trained operators. Portable devices, such as NATROX oxygen wound therapy and EPIFLO transdermal continuous oxygen therapy, which could increase the oxygen level in the location around the wounds are alternated to hyperbaric chambers for home use. However, these devices are usually composed of an oxygen generator, inducing a potential risk of explosion due to the generated oxygen. Therefore, a facile and reliable method to store and deliver oxygen to wounds is highly desirable.
Hydrogels are good substrates for wound dressing and can deliver drugs for anti-infection, anti-inflammation, and hemostasis.7−16 Hydrogel-based oxygen delivery has also drawn increased attention. Until now, the hydrogel-based dressing materials with oxygen-accelerated wound healing are mainly based on two strategies: (i) to directly hold and deliver oxygen with the hydrogel-based products to wounds and (ii) to generate oxygen when the hydrogel-based products are applied on wounds. For the first strategy, hydrogels were made with dissolved oxygen or oxygen-embedded nanostructures.17,18 Dissolved oxygen could provide only a limited amount of oxygen, while the stored oxygen would be lost in a short period. However, with oxygen-embedded nanostructures, such as dextran-based oxygen nanobubbles (ONBs), the hydrogel could carry more oxygen, while the oxygen stored in the ONBs would be more stable in the hydrogel for a significantly increased storage time.18,19 For the second strategy, catalysts or enzymes that could accelerate oxygen-generating reactions were loaded in the hydrogels, and substrates such as H2O2 were added to generate oxygen when the hydrogels were applied to wounds.15,20−22 Meanwhile, bioactive species could also be loaded into hydrogels to generate oxygen to improve wound healing. With this strategy, the hydrogel could provide significant oxygen; meanwhile, there are concerns on the catalyzed reaction or bioactivity of wounds, especially open wounds.
Collagenase as an enzyme because of its favorable properties have been used in wound healing.23 Collagenase could benefit wound healing because it could improve cell migration, increase cell proliferation, and form new blood vessels and collagen remodeling for better re-epithelization,24 and it is also used in the treatment of burns and keloidal scars.25,26 It is notable that collagenase could be used for enzymatic debridement of wounds, which is considered to be safe and effective and has been used in medical care for over five decades.27,28 Debridement includes the removal of devitalized and necrotic or infected tissues and fibrin to result in the formation of granulation and epithelization. For chronic wounds, debridement needs to be more frequent. Collagenase-based enzymatic debridement provides a simple, safe, and cost-effective choice in the treatment of wounds and used as a model drug in this work.
In our previous work, we utilized a Carbopol hydrogel-based dressing material, where ONBs were embedded for oxygen storage and delivery to accelerate wound healing. Carbopol as a high-molecular weight polymer has been used in skin care.29−31 In a different application, we have previously demonstrated that ONBs prepared with dextran yielded excellent oxygen holding capacity with extended release characteristics for the treatment of retinal ischemia.32,33 By embedding ONBs in a Carbopol-based hydrogel, we showed that our dressing material had unique properties of oxygen storage and sustained release to the oxygen-deprived wound bed. In a porcine model, the embedded ONB hydrogel showed accelerated healing of acute wounds.
Herein, to explore dressing materials with multifunction, we integrated the ONBs and collagenase in the Carbopol-based hydrogel as the matrix and prepared an ONB-embedded Carbopol hydrogel with collagenase (ONB-CC). In the conceived ONB-CC, ONBs could hold oxygen and deliver oxygen to wounds to create a prolonged oxygen-enriched environment around the wounds. Collagenase was chosen as a drug in this demonstration to exhibit the drug delivery capability of the ONB-CC. The obtained ONB-CC could release more oxygen compared with the oxygen-saturated hydrogel for up to at least 24 h. Meanwhile, the oxygen release capacity did not show a significant change for well over 3 weeks of storage, highlighting the stability of oxygen in the ONB-CC. The enzymatic activity of the collagenase loaded in the ONB-CC was retained for ∼17 days. Further, the ONB-CC presented good absorbance to PB, suggesting the capability of the ONB-CC to absorb biological fluids for hemostasis. With HDFa cells, in vitro experiments indicated that the ONB-CC was safe for human cells and could enable cell migration under hypoxia conditions. Scratch assay demonstrated that oxygen and collagenase could improve cell migration. The ONB-CC with both oxygen and collagenase exhibited a greater improvement than that with oxygen or collagenase only, suggesting that the ONB-CC integrated the beneficial characteristics from oxygen and collagenase to result in a better wound-healing product. mRNA expression indicated that the ONB-CC could reduce the expression of PAI-1, VEGF-a, and TIMP-1 which are upregulated in hypoxic conditions.34−37 Based on the rat model, in vivo experiments demonstrated that the ONB-CC could significantly accelerate wound closure and remodeling. Histopathological results further confirmed that the ONB-CC would improve the re-epithelization and formation of new blood vessels and hair follicles. The proposed ONB-CC facilitates the use at home and renders a steady supply of oxygen around the wound environment, and the dressing can be exchanged as needed.
Experimental Section
Chemicals and Agents
Sodium sulfite, poly(vinylpyrrolidone) (PVP) K17, potassium chloride, cobalt(II) chloride hexahydrate, dextran sulfate sodium, palmitic acid, d-α-tocopherol poly(ethylene glycol) 1000 succinate (TPGS), and collagenase were obtained from Sigma-Aldrich (MO). The synthesis of ONBs was conducted with molecular biology-grade water from Mediatech. Inc. (VA). Lecithin (DS-Soya PC80-C) is provided by Solus Advanced Materials Co., Ltd. Ethanol was purchased from Decon Laboratories (PA). Dibasic potassium phosphate was obtained from Mallinckrodt Chemicals (Dublin, Ireland). Casein peptone was obtained from Remel Products (Waltham, MA). Peptone S (Soy Peptone) was ordered from BioWorld (Dublin, OH). Propidium iodide was ordered from ThermoFisher Scientific (Waltham, MA). Calcein AM (ab141420) was purchased from Abcam (Boston, MA). The chemicals were used as received without further purification. Glassware for experiments was cleaned with detergent, rinsed with deionized (DI) water, and autoclaved.
Preparation and Characterization of the Hydrogel
The ONB synthesis was performed based on our previous work with slight modification.33 The sonication was conducted under a 30 s cycle on and 40 s off step. To 10 mL of oxygen-saturated water, oxygen was purged at a pressure of 30 psi, and then, the water was sonicated at 50 W under the cycle. Ingredients comprising 0.2 mL of 0.045% KCl, 0.6 mL of 0.23% lecithin, 0.4 mL of 0.028% TPGS, 1 mL of 0.9% dextran sulfate, 0.3 mL of 0.19% palmitic acid (ethanol solution), and 0.331 mL of 0.5% PVP were added and subjected to two circles of sonication. The obtained solution was sonicated for four more cycles. The resulting ONB solution was then filtered with a 0.22 μm filter and sealed in 20 mL vials with a rubber stopper and aluminum cap for the next step.
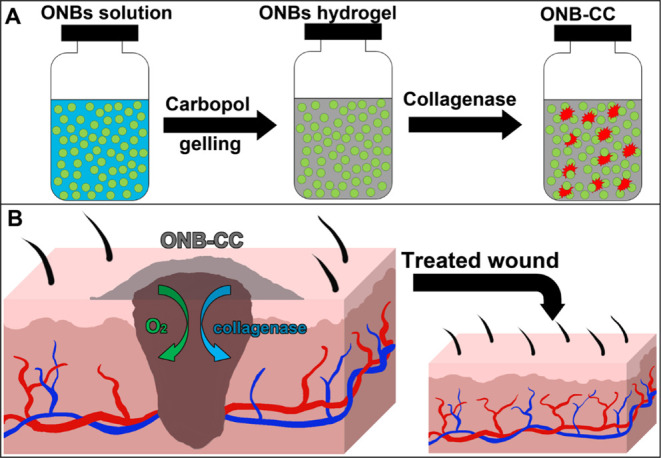
To prepare the ONB-CC, Carbopol powder was added to the ONB solution to result in 1% Carbopol solution and the obtained solution was strongly vortexed until a clear gel was generated, which took around 20 min, and the pH of the gel was adjusted to 7.4. Calculated 10 mg/mL collagenase was added to the gel to produce a gel with 1% Carbopol 940 embedded with ONBs along with 10 μg/mL collagenase. The obtained gel was vigorously stirred and centrifuged at 1000 rpm for 15 s to remove the gas generated during stirring. The ONB-CC was stored in a sealed vial to prevent oxygen loss at 4 °C. The synthesis procedure of the ONB-CC is illustrated in Scheme 1A.
Scheme 1. Illustration of the Synthesis Process of the ONB-CC (A) and the Manner of Applying the ONB-CC to a Wound (B) for Accelerated Wound Healing.
Scanning electron microscopy (SEM) images were recorded with an environmental SEM (ESEM) microscope (FEI Quanta FEG 450 ESEM) operated at 10 kV under 1.0 Torr.
Oxygen Release and Storage Test
Oxygen release experiments were conducted according to our previous work.33 Briefly, to a centrifuge tube was added 0.3 mL of 0.267 g/mL sodium sulfite containing 99 μg/mL cobalt(II) chloride hexahydrate. Then, the tube was placed in a vial with 1 mL of ONB-CC. A Thermo Scientific Orion Versa Star Pro DO Benchtop Meter with an optical dissolved oxygen probe (Ottawa, Ontario, Canada) was sealed in the vial containing the ONB-CC and the centrifuge tube. The oxygen level in the sealed space was recorded at 1 min intervals.
Collagenase Activity Test
The activity of the collagenase embedded in the hydrogel was tested with a collagenase activity colorimetric assay kit (catalog number: MAK293) from Sigma-Aldrich (MO) following the instructions. 100 μL of ONB-CC was applied to the bottom of a well on a 96-well plate, and then, the test substrates were added onto the top of the ONB-CC. The readout was obtained with a plate reader with the wells containing the ONB-CC and the colorimetric product depicting the collagenase in the ONB-CC. The results from the wells with the ONB-CC were used as the baseline.
Swelling Test
2 ml of ONB-CC was immersed in 10 mL of 10 mm PB and incubated at 37 °C. After a particular incubation time, the water was completely removed, and the weight of the ONB-CC was measured. Then, the ONB-CC was immersed in water and incubated for the next measurement.
Cell Culture
Primary human dermal fibroblast (HDFa, PCS-201–012, ATCC) cells were cultured in Dulbecco’s modified Eagle medium (DMEM) buffer with 4.5 g/L glucose and l-glutamine and sodium pyruvate (10–13-CM, Corning) supplemented with Fibroblast Growth Kit-Low Serum (PCS-201–041, ATCC) at 10% in the final medium, and 1× Antimycotic-Antibiotic (15240096, Thermo Fisher) at 1% in the final medium at 37 °C and 5% CO2.
Viability Assay
Normoxic conditions: Cells were planted in 96-well plates at a density of ×103 cells/well and incubated overnight. Medium was exchanged the next day, and the cells were treated with 2, 5, or 10 mg of ONB-CC hydrogels with eight replications. The cells without the addition of the hydrogel were used as a negative control. All the cells were incubated for 24 h, and then, MTT assay was used to assess cell viability.
Hypoxic conditions: Cells were treated as described in a manner similar to that under normoxic conditions. After treatment with the ONB-CC, the cells were cultured at 37 °C for 24 h in a humidified hypoxic chamber, which was filled with 3% O2, 5% CO2, and 92% N2. Then, MTT assay was carried out.
Scratch Assay
HDFa cells were seeded at 5 × 104 cells/well in 12-well plates with two replications and incubated overnight. Then, the cells were treated with 5 mg of ONB-CC, a hydrogel with ONBs, a hydrogel with collagenase, and the hydrogel only, while cells treated without the hydrogel were used as the negative control. Then, images of each well were taken at 4× magnification. After scratches were made in the wells, the cells were then cultured in a humidified hypoxic chamber filled with 3% O2, 5% CO2, and 92% N2 at 37 °C for 24 h. Four images of each well were recorded at 4× magnification after medium exchange. All of the images were analyzed with ImageJ for quantitative determination of the scratch closure.
Gene Expression
To evaluate the expression of relevant genes in the cells upon treatment with the proposed ONB-CC, HDF-a was cultured in a 6-well plate at 0.6 × 105 cells/well with two replications. After the exchange with fresh media, the cells were treated with 5 mg of ONB-CC. Cells treated with 5 mg of 1% Carbopol hydrogel, a hydrogel with 10 μg/mL collagenase, a hydrogel with ONBs, and without the hydrogel were also prepared as the control groups. One set of treated cells was incubated in a humidified hypoxic chamber as described before for 12 h, while another set of cells was incubated under normoxic conditions for 12 h. The total RNA was extracted with an RNA purification kit (Thermoscientific, K0731, Waltham, MA) following the instructions. Then, the extracted RNA was purified with a DNA-free kit (Thermoscientific, AM1906, Waltham, MA). cDNA as obtained with a high-capacity cDNA reverse transcription kit (Applied Biosystems, 4368814, Waltham, MA). To obtain enough target sequences, a preamplification step was conducted with the cDNA based on the methods reported before.38 Briefly, in 10 μL reactions, 5 μL of PowerUP SYBR Green Master Mix (Applied Biosystems, A25742, Waltham, MA), 0.2 μL of 10 μM forward primer, 0.2 μL of 10 μM reverse primer, and 1 μL of cDNA were mixed well in 0.2 mL tubes and then applied with the thermal profile below: 95 °C for 2 min, 45 cycles of 95 °C 15 s, 55 °C 15 s, 72 °C 1 min, and then 72 °C 10 min. After the elongation step, the samples were immediately frozen in liquid nitrogen, followed by slow thawing on ice and diluted with TE buffer (10 mM Tris and 1 mM EDTA, pH 8.0) to be at around 250 ng/μL. The preamplified samples were further evaluated with electrophoresis under 100 mV for 20 min. Reverse transcription–polymerase chain reaction (RT-PCR) tests were conducted with PowerUP SYBR Green Master Mix following the product introduction with 100 ng of the preamplified samples (Table 1).
Table 1. Primers for Target Amplification.
| primer | forward | reverse |
|---|---|---|
| PAI-1 | GCA AGG CAC CTC TGA GAA CT | GGG TGA GAA AAC CAC GTT GC |
| TIMP-1 | TCC AAG GCT CTG AAA AGG GC | ATT CAG GCT ATC TGG GAC CG |
| VEGF-A | CGA AAG CGC AAG AAA TCC CG | GCT CCA GGG CAT TAG ACA GC |
Animal Experiment
Animal experiments were conducted according to the guidelines of the Institutional Animal Care and Use Committee and the Division of Animal Resources at the University of Illinois (IACUC Protocol#: 23012). Male Sprague–Dawley rats of 250–300 g and 8-week ages were obtained from Envigo Laboratory (Indianapolis, IN). The rats were randomly assigned into two groups, no treatment (NT), and ONB-CC. Each group comprised five rats, and two wounds were created on each rat. The rats were anesthetized with an injection of a mixture of ketamine (40 mg/kg), xylazine (8 mg/kg), and acepromazine (1 mg/kg). Full-thickness wounds at 2 mm depth were made on both sides of the dorsal areas with a sterile disposable dermal biopsy punch (8 mm diameter, Medline Industries, IL). The wounds were then cleaned with povidone-iodine solution and 70% ethanol, and then, a layer of 2–4 mm hydrogel samples was applied to the wounds, which was then held in place with a piece of Tegaderm and self-adhering elastic bandage. The hydrogel and dressing were exchanged with a fresh hydrogel every 2 days. The wounds of the rats in NT groups were cleaned in the same manner as that in the ONB-CC group, and then, a piece of Tegaderm was used to cover the wounds. The healing process was monitored by imaging, and the wound area was asessed with ImageJ according to
After 14 days, the animals were euthanized humanely. The tissues of the wounds were collected and fixed with 10% formalin followed by H&E staining. The histologic images were recorded with a microscope.
Results and Discussion
The ONB-CC is a composite with the Carbopol hydrogel as the substrate to store oxygen and collagenase to benefit wound healing through the delivery of these components to the wounds in a user-friendly manner. The medicated ONB-CC gel was stored in a sealed container and applied to the wound. Scheme 1B illustrates the manner of applying the ONB-CC to the wound. We demonstrated that the oxygen release from the ONB-CC is a sustained process that could last for up to 24 h; hence, it is also possible to apply the ONB-CC to the wound without cover and exchange the hydrogel multiple times. Another possibility is to preload the ONB-CC on a sheet of Tegaderm covered with an oxygen-tight polymeric cover. The ONB-CC could be simply applied to the wound along with the Tegaderm cover after peeling off the polymeric cover. In addition to benefiting from oxygen, collagenase embedded in the hydrogel could also improve the healing of the wound. It was reported that collagenase is critical for collagen remodeling and cell migration, which are important for wound closure.24 Meanwhile, collagenase would aid in the debridement of chronic dermal and decubitus ulcers, thus accelerating the healing of chronic wounds.39 Examples of current wound dressing products in the market containing collagenase are SANTYL Ointment and Crystalia Kollagenase. We hypothesize that the combination of oxygen and collagenase in the substrate of the hydrogel would result in a dressing with improved wound healing properties.
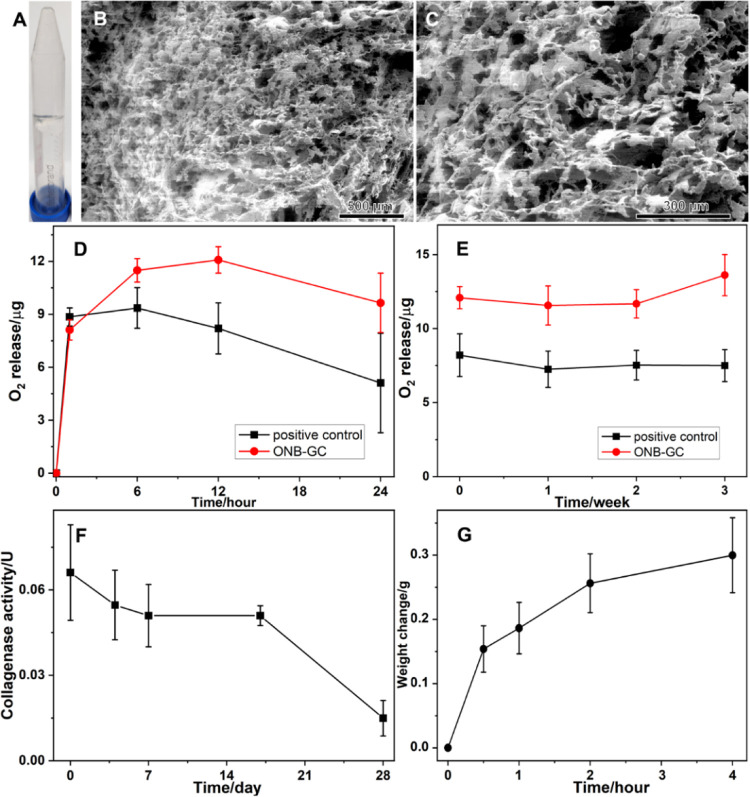
It can be seen in Figure 1A that the ONB-CC is a colorless and transparent gel system. SEM images depict the microstructure of the ONB-CC. As shown in Figure 1B,C of the ONB-CC, a porous structure can be noted. The oxygen release capacity of the ONB-CC determines the ability of the dressing to create an oxygen-rich environment around the wound when applied on the wounds. To characterize the oxygen release behavior, 1 mL of freshly prepared ONB-CC was placed in a sealed glass container, where a solution of sodium sulfite and cobalt chloride continuously consumed the oxygen in the container. An oxygen probe was introduced and sealed in the container to track the oxygen level in the headspace of the container. Oxygen-saturated water-prepared hydrogels without ONBs and collagenase were used as the positive control, while the container with the solution of sodium sulfite and cobalt chloride but without the hydrogel was used as the negative control. The oxygen concentration difference in the container headspace between the sample and negative control was attributed to the oxygen released from hydrogels. Based on the volume of air in the container, the oxygen released from hydrogels could be calculated (Figure 1D). It can be seen that the released oxygen from the ONB-CC referred to as the negative control increased during the first 12 h. Beyond the consumed oxygen in the solution of sodium sulfite and cobalt chloride, 1 mL of ONB-CC provided 12.08 ± 0.75 μg of extra oxygen over a 12 h release. After 12 h, the oxygen released decreased, which could be attributed to the fact that oxygen released from the ONB-CC was slower than the consumption of oxygen due to the solution of sodium sulfite and cobalt chloride. However, the released oxygen from the ONB-CC is still greater than that from the positive control over a 24 h release, indicating the oxygen release capacity of the ONB-CC over a period of 24 h. The oxygen release behavior from the positive control samples with hydrogels prepared with oxygen-saturated water was similar to the one from the ONB-CC. An increase in the amount of oxygen released from the positive control was noted in the first 6 h, but at 12 h, the oxygen released was lesser than that at 6 h, suggesting that the oxygen stored in the hydrogels prepared with oxygen-saturated water was lower than that in the ONB-CC. Meanwhile, in Figure 1D, the amount of oxygen released at 1 h from the ONB-CC was slightly lesser than that from hydrogels prepared with oxygen-saturated water. In the ONB-CC preparation, the ONBs needed to be filtrated and then added to Carbopol 940 powder for hydrogel development. In contrast, the oxygen-saturated water was directly used after oxygenation for the hydrogel preparation. It is possible that the filtration step would induce oxygen loss in the solution as noted in previous studies.40 Therefore, the oxygen concentration in the ONB-CC would be slightly lower than that in the hydrogels prepared with oxygen-saturated water. However, the total amount of oxygen in the ONB-CC would be greater, resulting in the higher amount of released oxygen in Figure 1D.
Figure 1.
Photo (A) and SEM images (B, C) of the ONB-CC. Oxygen released from the ONB-CC (D) and oxygen amount in the ONB-CC changing upon storage time (E), collagenase activity of the ONB-CC upon storage (F), and ONB-CC swelling behavior in 10 mM PB (G).
The total amount of oxygen in the ONB-CC is critical for the acceleration of wound healing. More oxygen wrapped in the ONB-CC creates a prolonged oxygen-enriched environment around the wound when the dressing is applied to the wound; meanwhile, a larger amount of oxygen in the ONB-CC extends the shelf life of the dressing. To characterize the amount of oxygen in the ONBs, the oxygen released for 12 h referred to as the negative control was tracked, and the results are shown in Figure 1E. The weight of the oxygen from the ONB-CC is determined based on
where woxygen is the amount of oxygen released from the ONB-CC hydrogel, the oxygen concentration at the beginning and after 12 h in the test with the negative control is C0hNC and C12hNC, respectively, oxygen concentrations at the beginning and 12 h in the test with the ONB-CC are C0hONBs and C12hONBs, respectively, and the volume of the space in the test setup is V. Because the volume of the hydrogel in the release test is 1 mL, the volume of air in the test of the ONB-CC is (V – 1). The equation above estimates the total oxygen released from the ONB-CC. It can be seen that there is no significant change in oxygen released over a 3-week storage period at 4 °C. A slight increase in the oxygen release over the storage period was noted, which is similar to that observed in the ONB-embedded Carbopol hydrogel that was proposed in our prior work.18 This increase could be attributed to the aging process of the Carbopol-based hydrogel.41 The results shown in Figure 1E demonstrated that the proposed ONB-CC could hold oxygen in the hydrogel substrates for 3 weeks without a significant decrease.
Collagenase plays a key role in cell migration and tissue repair. Its activity would be important to wound healing when the ONB-CC dressing was applied to the wound. A colorimetric test kit was used to demonstrate the activity of the collagenase wrapped in the ONB-CC, and the results are plotted in Figure 1F. It can be seen that the activity of the collagenase in the ONB-CC was similar in the first 17 days when stored at 4 °C. Meanwhile, a decrease in activity was noted when the storage time was extended to 27 days. Comparing the freshly prepared ONB-CC and the ones stored for 7 days, a relatively large deviation in the results was observed, while when the storage time increased from 7 to 17 days, the deviation was much smaller. Considering the synthesis of the ONB-CC, when the pH of the ONB-embedded hydrogel was adjusted to 7.4, the hydrogel became much thicker, making the added collagenase not very uniform in the ONB-CC. Upon storage, the aging of the hydrogel and the diffusion of collagenase in the hydrogel make the distribution of collagenase in the ONB-CC fairly uniform, resulting in a small deviation in the activity.
When the ONB-CC is applied to wounds that are bleeding, the biological fluids could dilute the ONB-CC, thus influencing the properties of the dressing. Blood hemolysis is a factor in evaluating blood compatibility.42 In the reported work, the Carbopol-based hydrogel has been demonstrated with a low hemolysis rate, less than 5%,43−45 indicating excellent blood compatibility of the Carbopol-based hydrogel. Thus, the swelling behavior of the ONB-CC would affect the stability of the ONB-CC in biological fluids. 2 mL of ONB-CC was immersed in 10 mM PB to evaluate the swelling behavior. The initial weight of the ONB-CC and the container was set as zero, and the increase in the weight of the system was recorded with respect to the incubation time. The change in weight with respect to time is plotted in Figure 1G. It can be noted that the weight of the ONB-CC increased to around 0.30 ± 0.05 g over a 4 h incubation period. The absorption of the solvent implies that the ONB-CC could absorb tissue exudates and benefit the hemostasis of bleeding wounds.46
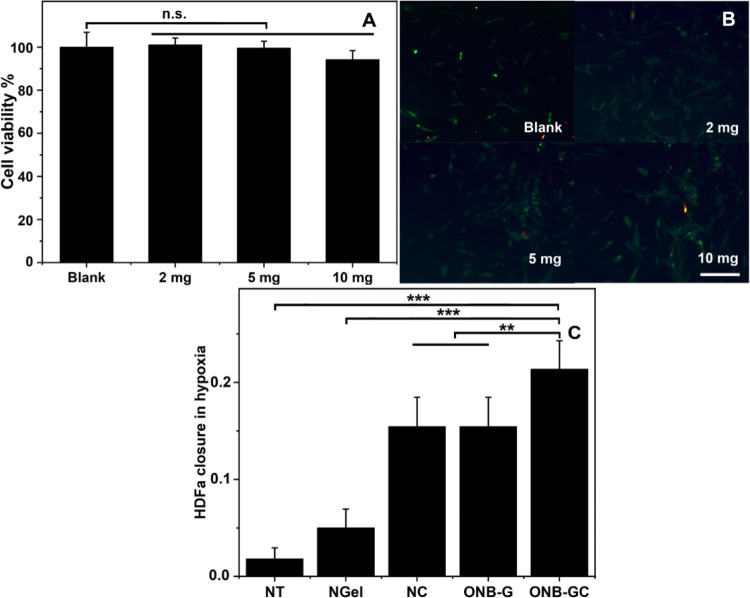
To demonstrate the potential effect of the ONB-CC on the dermis, the viability of HDFa, a type of skin cells, was investigated with serial doses of ONB-CC from 2 to 10 mg. The cells treated without the ONB-CC were noted as blank with 100% viability. The results are illustrated in Figure 2A. It can be seen that there was no significant change in the viability when the HDFa cells were treated with 2 and 5 mg of ONB-CC. When the dose of the ONB-CC increased to 10 mg, a slight decrease in the ability of HDFa was observed, from 100.0 ± 6.88% (blank) to 94.2 ± 4.19% (with 10 mg ONB-CC). Fluorescent images as shown in Figure 2B from the live/dead staining assay exhibit no significant difference between the blank and HDFa treated with serial amounts of ONB-CC. Results indicate that the ONB-CC would not induce significant cell death even with a high-dose treatment, indicating that the ONB-CC is safe.
Figure 2.
HDFa viability upon treatment with the blank and 2, 5, and 10 mg of ONB-CC (A) and fluorescent images of live/dead staining of HDFa cells (B), scale bar = 500 μm. Scratch assay with 5 mg of ONB-CC under hypoxic conditions (C). **: p < 0.01, ***: p < 0.001.
Under hypoxic conditions, oxygen supply would aid in cell migration for an accelerated wound closure, which was presented with a fast closure in a cell scratch assay.18,47 Meanwhile, in the proposed ONB-CC, the loaded collagenase would also improve cell migration for better wound closure.24 Thus, it could be expected that the ONB-CC would exhibit an enhanced capacity to improve wound healing. To assess cell migration, a scratch assay was conducted under hypoxic conditions with HDFa treated with 5 mg of ONB-CC. As a reference, cells treated without the hydrogel were used as “no treatment” (NT). The Carbopol gel prepared with deoxygenated water without ONBs and collagenase was used as a “negative hydrogel” (NGel), and the hydrogel with collagenases was used as the “negative collagenase hydrogel” (NC). Meanwhile, the hydrogel with ONBs was used as an “ONB-embedded hydrogel” (ONB-G). The scratch closure of HDFa cells was estimated based on the equation cell closure = (Dinitialdistance – Dfinaldistance)/Dinitialdistance. The HDFa cell closure results are listed in Figure 2C. A slightly higher closure could be noted from the NGel group, indicating that the hydrogel itself could aid in the HDFa closure. However, this benefit is much lower than that of the hydrogel loaded with oxygen or collagenase, which was presented by the much higher HDFa closure from NC and ONB-G groups than from the NGel group. Furthermore, by combining the ONBs and collagenase in the hydrogel, the proposed ONB-CC yielded an even higher closure compared with the groups of NC and ONB-G, indicating that the Carbopol-based hydrogel can be a good platform to integrate the effective elements for improved wound healing.
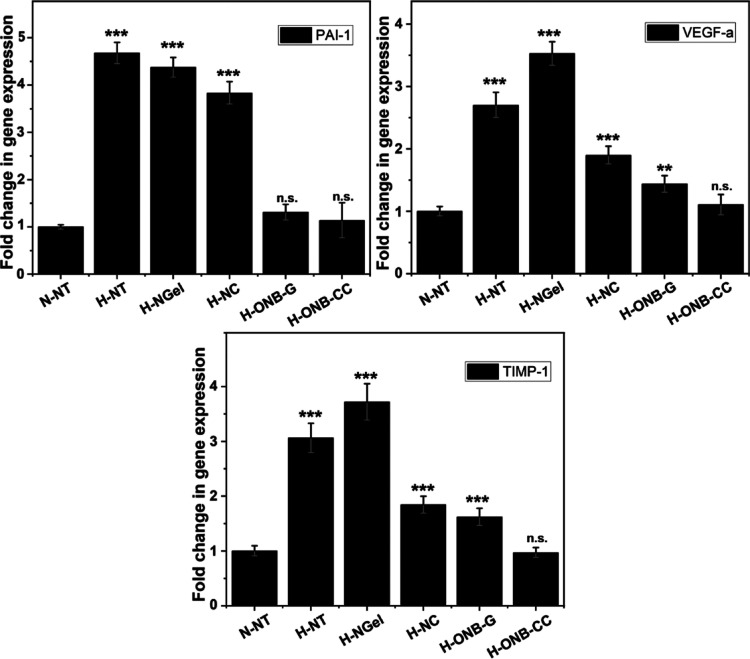
Cell activity would be affected under hypoxic conditions, along with the change in gene expression. To understand the influence of the ONB-CC on HDFa cells under hypoxic conditions, PAI-1, VEGF-a, and TIMP-1 genes were evaluated in HDFa cells treated with 5 mg of ONB-CC. PAI-1, VEGF-a, and TIPM-1 have been reported to be upregulated in response to hypoxic conditions because these genes promote pathways, such as vasculogenesis and erythropoiesis, among other to improve oxygen delivery under hypoxia.34−37 As shown in Figure 3, compared with the NT group under normoxic conditions, the NT group under hypoxic conditions exhibited a significant increase in the expression of PAI-1, VEGF-a, and TIMP-1. The cells treated with NGel showed a comparable or higher expression level because there is no oxygen supply from NGel. It can be noted that the treatment of NC exhibited increased expression levels of PAI-1, VEGF-a, and TIMP-1 compared to that with NT under normoxic conditions, but they were lower than in the NT group under hypoxic conditions. The gene expression results also showed that the ONB-G groups and ONB-CC groups induced similar changes in the gene expression levels, indicating that the integration of collagenase did not influence the amount of loaded oxygen in the ONB-CC.
Figure 3.
RT-PCR analysis of hypoxic genes of PAI-1, VEGF-a, and TIMP-1 with HDFa treated with 5 mg of ONB-CC. Significant difference from the N-NT group, **: p < 0.01 and ***: p < 0.001.
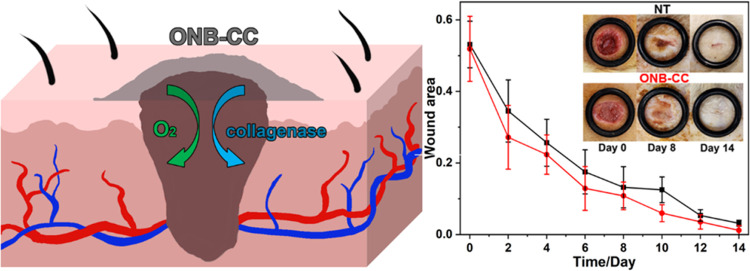
The Sprague–Dawley rat model was employed to demonstrate the effectiveness of the ONB-CC dressing on full-thickness skin wounds. The ONB-CC was applied on day 0 after the creation of the wounds and then reapplied every 2 days, while the wounds treated with Tegaderm only at the same frequency were used as the NT group. The images of the wounds were recorded when refreshing the dressing and are shown in Figure 4. It can be noted that for each group, the wound area decreased upon healing due to the epidermal migration. Similar to the scratch assay results, the ONB-CC dressing significantly reduced the wound area more than that with a Tegaderm cover only. On day 14, an observable residual wound could be noted; in contrast, the wound treated with the ONB-CC showed excellent healing. The calculated wound area results are plotted in Figure 4. The area of the wound treated with the ONB-CC was always smaller than that of the NT groups at each recorded time point, indicating accelerated healing due to the ONB-CC.
Figure 4.
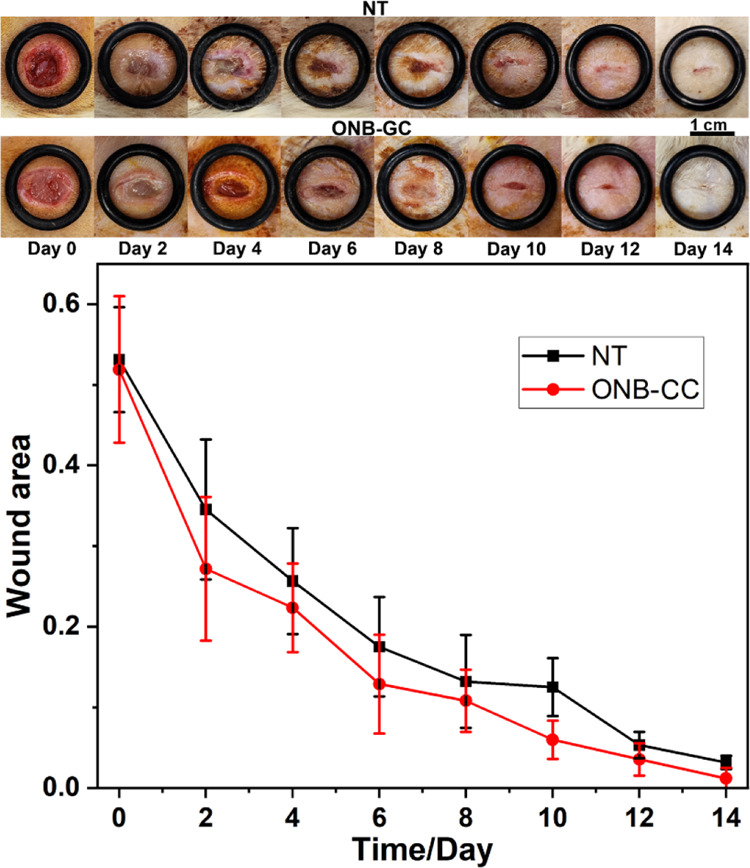
Representative images of wounds at serial healing time from in vivo wound healing experiments with the ONB-CC and evaluation of wound closure.
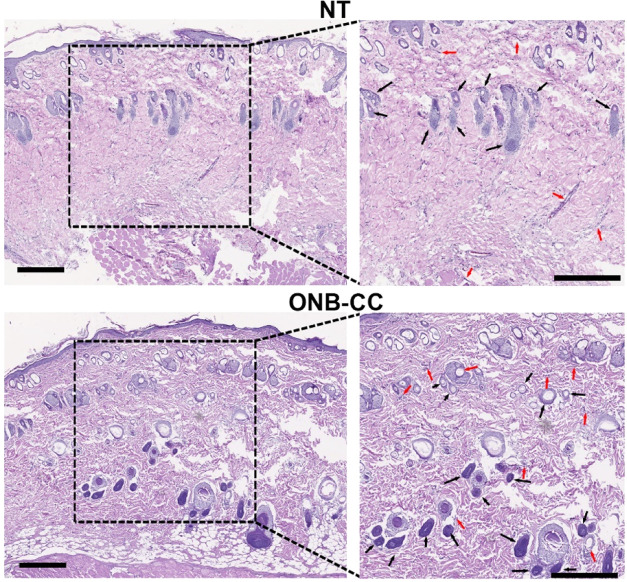
After 14 days of healing with ONB-CC dressing, the tissues of the wounds were collected to evaluate the therapeutic efficacy of the ONB-CC. After hematoxylin and eosin (H&E) staining, the obtained histopathological results were recorded with a microscope. As shown in Figure 5, both NT groups and ONB-CC groups showed wound closure with newly formed epidermis after a 14-day healing period. However, the ONB-CC groups exhibited a notably flatter and more uniform epidermis layer. Meanwhile, more structures such as blood vessels and hair follicles were observed in the epidermis, especially in the deeper section in the histopathological image from the ONB-CC group, indicating a significantly improved healing in the presence of ONB-CC dressing. Overall, our results demonstrated that the combination of oxygen from ONBs and collagenase in a Carbopol hydrogel-based platform could result in an ideal dressing material for accelerated wound healing.
Figure 5.
Histopathological evaluation of wound tissues from the NT group and ONB-CC group after 14-day healing. Arrows highlight typical hair follicles (black) and blood vessels (red); scale bar = 500 μm.
Conclusions
Herein, we propose an integrated dressing material with the Carbopol hydrogel, ONBs, and collagenase dressing for improved wound healing. The Carbopol hydrogel was used as the matrix to embed ONBs and load collagenase. ONBs were introduced to store oxygen for a prolonged period of sustained release when the ONB-CC was applied to the wound. Collagenase, which is currently used in wound healing, was chosen as a drug model in the proposed design. In the system developed, 1 mL of ONB-CC could release 12.08 ± 0.75 μg of oxygen over a 12 h period. We showed that the ONB-CC could provide an oxygen-enriched environment for up to 24 h and can hold oxygen in the dressing material for at least 3 weeks. Collagenase activity could be maintained higher than at more than 0.05 U per 0.1 mL of ONB-CC over 17 days. A HDFa cell-based in vitro test showed a minor influence of the delivery system on cell viability, and the closure results from scratch assay exhibited that the integration of oxygen from ONBs and collagenase would induce the most rapid closure compared with that with ONBs or collagenase only. mRNA expression further showed the effectiveness of the ONB-CC. With the Sprague–Dawley rat model, ONB-CC dressing exhibited a significant improvement in wound closure, while an obvious increase in re-epithelization as well as the formation of new blood vessels and hair follicles was observed in the histopathological images from the wounds treated with ONB-CC dressing. The proposed ONB-CC integrates the benefits of oxygen and collagenase as a cost-effective treatment for acute wounds and chronic wounds.
Acknowledgments
We acknowledge the partial seed funding from the Health Maker Lab (HML) of the Carle-Illinois College of Medicine and support from the NSF-SBIR Award# 2031313 and NSF STTR Phase II 2236857.
The authors declare the following competing financial interest(s): We have filed a provisional patent application.
References
- Frykberg R. G.; Banks J. Challenges in the treatment of chronic wounds. Adv. Wound Care 2015, 4, 560–582. 10.1089/wound.2015.0635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monika P.; Chandraprabha M. N.; Rangarajan A.; Waiker P. V.; Chidambara Murthy K. N. Challenges in healing wound: Role of complementary and alternative medicine. Front. Nutr. 2022, 8, 791899 10.3389/fnut.2021.791899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castilla D. M.; Liu Z.-J.; Velazquez O. C. Oxygen: implications for wound healing. Adv. Wound Care 2012, 1 (6), 225–230. 10.1089/wound.2011.0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Smet G. H.; Kroese L. F.; Menon A. G.; Jeekel J.; van Pelt A. W.; Kleinrensink G. J.; Lange J. F. Oxygen therapies and their effects on wound healing. Wound Repair Regener. 2017, 25 (4), 591–608. 10.1111/wrr.12561. [DOI] [PubMed] [Google Scholar]
- Eskes A. M.; Ubbink D. T.; Lubbers M. J.; Lucas C.; Vermeulen H. Hyperbaric oxygen therapy: solution for difficult to heal acute wounds? Systematic review. World J. Surg. 2011, 35 (3), 535–542. 10.1007/s00268-010-0923-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauwe P. B.; Pulikkottil B. J.; Lavery L.; Stuzin J. M.; Rohrich R. J. Does hyperbaric oxygen therapy work in facilitating acute wound healing: a systematic review. Plast. Reconstr. Surg. 2014, 133 (2), 208e–215e. 10.1097/01.prs.0000436849.79161.a4. [DOI] [PubMed] [Google Scholar]
- Fan F.; Saha S.; Hanjaya-Putra D. Biomimetic hydrogels to promote wound healing. Front. Bioeng. Biotechnol. 2021, 9, 718377 10.3389/fbioe.2021.718377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y.; He J.; Guo B. Functional hydrogels as wound dressing to enhance wound healing. ACS Nano 2021, 15 (8), 12687–12722. 10.1021/acsnano.1c04206. [DOI] [PubMed] [Google Scholar]
- Xiang J.; Shen L.; Hong Y. Status and future scope of hydrogels in wound healing: Synthesis, materials and evaluation. Eur. Polym. J. 2020, 130, 109609 10.1016/j.eurpolymj.2020.109609. [DOI] [Google Scholar]
- Gao G.; Jiang Y. W.; Jia H. R.; Wu F. G. Near-infrared light-controllable on-demand antibiotics release using thermo-sensitive hydrogel-based drug reservoir for combating bacterial infection. Biomaterials 2019, 188, 83–95. 10.1016/j.biomaterials.2018.09.045. [DOI] [PubMed] [Google Scholar]
- Sang Y. J.; Li W.; Liu H.; Zhang L.; Wang H.; Liu Z. W.; Ren J. S.; Qu X. G. Construction of Nanozyme-Hydrogel for Enhanced Capture and Elimination of Bacteria. Adv. Funct. Mater. 2019, 29 (22), 1900518 10.1002/adfm.201900518. [DOI] [Google Scholar]
- Pourshahrestani S.; Zeimaran E.; Kadri N. A.; Mutlu N.; Boccaccini A. R. Polymeric hydrogel systems as emerging biomaterial platforms to enable hemostasis and wound healing. Adv. Healthcare Mater. 2020, 9 (20), 2000905 10.1002/adhm.202000905. [DOI] [PubMed] [Google Scholar]
- Huang H.; Dong Z.; Ren X.; Jia B.; Li G.; Zhou S.; Zhao X.; Wang W. High-strength hydrogels: Fabrication, reinforcement mechanisms, and applications. Nano Res. 2023, 16 (2), 3475–3515. 10.1007/s12274-022-5129-1. [DOI] [Google Scholar]
- Jia B.; Li G.; Cao E.; Luo J.; Zhao X.; Huang H. Recent progress of antibacterial hydrogels in wound dressings. Mater. Today Bio 2023, 19, 100582 10.1016/j.mtbio.2023.100582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X.; Saengow C.; Ju L.; Ren W.; Ewoldt R. H.; Irudayaraj J. Exosome-coated oxygen nanobubble-laden hydrogel augments intracellular delivery of exosomes for enhanced wound healing. Nat. Commun. 2024, 15 (1), 3435 10.1038/s41467-024-47696-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu J.; Zhao X.; Liang Y.; Xu Y.; Ma P. X.; Guo B. Degradable conductive injectable hydrogels as novel antibacterial, anti-oxidant wound dressings for wound healing. Chem. Eng. J. 2019, 362, 548–560. 10.1016/j.cej.2019.01.028. [DOI] [Google Scholar]
- Patil P. S.; Fountas-Davis N.; Huang H.; Evancho-Chapman M. M.; Fulton J. A.; Shriver L. P.; Leipzig N. D. Fluorinated methacrylamide chitosan hydrogels enhance collagen synthesis in wound healing through increased oxygen availability. Acta Biomater. 2016, 36, 164–174. 10.1016/j.actbio.2016.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren W.; Messerschmidt V.; Tsipursky M.; Irudayaraj J. Oxygen Nanobubbles-Embedded Hydrogel as Wound Dressing to Accelerate Healing. ACS Appl. Nano Mater. 2023, 6 (14), 13116–13126. 10.1021/acsanm.3c01812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhandari P. N.; Cui Y.; Elzey B. D.; Goergen C. J.; Long C. M.; Irudayaraj J. Oxygen nanobubbles revert hypoxia by methylation programming. Sci. Rep. 2017, 7 (1), 9268 10.1038/s41598-017-08988-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X.; Ju L.; Saengow C.; Ren W.; Ewoldt R.; Fan T.; Irudayaraj J. Nano oxygen chamber by cascade reaction for hypoxia mitigation and reactive oxygen species scavenging in wound healing. Bioact. Mater. 2024, 35, 67–81. 10.1016/j.bioactmat.2024.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochoa M.; Rahimi R.; Zhou J.; Jiang H.; Yoon C. K.; Maddipatla D.; Narakathu B. B.; Jain V.; Oscai M. M.; Morken T. J.; et al. Integrated sensing and delivery of oxygen for next-generation smart wound dressings. Microsyst. Nanoeng. 2020, 6 (1), 46 10.1038/s41378-020-0141-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y.; Fu R.; Duan Z.; Zhu C.; Fan D. Adaptive hydrogels based on nanozyme with dual-enhanced triple enzyme-like activities for wound disinfection and mimicking antioxidant defense system. Adv. Healthcare Mater. 2022, 11 (2), 2101849 10.1002/adhm.202101849. [DOI] [PubMed] [Google Scholar]
- Alipour H.; Raz A.; Zakeri S.; Djadid N. D. Therapeutic applications of collagenase (metalloproteases): A review. Asian Pac. J. Trop. Biomed. 2016, 6 (11), 975–981. 10.1016/j.apjtb.2016.07.017. [DOI] [Google Scholar]
- Clark R. A.The Molecular and Cellular Biology of Wound Repair; Springer Science & Business Media, 2013. [Google Scholar]
- Sharp N. E.; Aguayo P.; Marx D. J.; Polak E. E.; Rash D. E.; Peter S. D.; Ostlie D. J.; Juang D. Nursing preference of topical silver sulfadiazine versus collagenase ointment for treatment of partial thickness burns in children: survey follow-up of a prospective randomized trial. J. Trauma Nurs. 2014, 21 (5), 253–257. 10.1097/jtn.0000000000000073. [DOI] [PubMed] [Google Scholar]
- Bae-Harboe Y.-S. C.; Harboe-Schmidt J. E.; Graber E.; Gilchrest B. A. Collagenase followed by compression for the treatment of earlobe keloids. Dermatol. Surg. 2014, 40 (5), 519–524. 10.1111/dsu.12465. [DOI] [PubMed] [Google Scholar]
- Duarte A. S.; Correia A.; Esteves A. C. Bacterial collagenases–a review. Crit. Rev. Microbiol. 2016, 42 (1), 106–126. 10.3109/1040841X.2014.904270. [DOI] [PubMed] [Google Scholar]
- Hoppe I. C.; Granick M. S. Debridement of chronic wounds: a qualitative systematic review of randomized controlled trials. Clin. Plast. Surg. 2012, 39 (3), 221–228. 10.1016/j.cps.2012.04.001. [DOI] [PubMed] [Google Scholar]
- Mahmoud R. A.; Hussein A. K.; Nasef G. A.; Mansour H. F. Oxiconazole nitrate solid lipid nanoparticles: formulation, in-vitro characterization and clinical assessment of an analogous loaded Carbopol gel. Drug Dev. Ind. Pharm. 2020, 46 (5), 706–716. 10.1080/03639045.2020.1752707. [DOI] [PubMed] [Google Scholar]
- Rahman S. A.; Abdelmalak N. S.; Badawi A.; Elbayoumy T.; Sabry N.; El Ramly A. Tretinoin-loaded liposomal formulations: from lab to comparative clinical study in acne patients. Drug Delivery 2016, 23 (4), 1184–1193. 10.3109/10717544.2015.1041578. [DOI] [PubMed] [Google Scholar]
- Saeedi M.; Morteza-Semnani K.; Ghoreishi M. R. The treatment of atopic dermatitis with licorice gel. J. Dermatol. Treat. 2003, 14 (3), 153–157. 10.1080/09546630310014369. [DOI] [PubMed] [Google Scholar]
- Fayyaz M.; Jabeen M.; Tsipursky M. S.; Irudayaraj J. Dextran-based oxygen nanobubbles for treating inner retinal hypoxia. ACS Appl. Nano Mater. 2021, 4 (7), 6583–6593. 10.1021/acsanm.1c00084. [DOI] [Google Scholar]
- Messerschmidt V.; Ren W.; Tsipursky M.; Irudayaraj J. Characterization of Oxygen Nanobubbles and In Vitro Evaluation of Retinal Cells in Hypoxia. Transl. Vision Sci. Technol. 2023, 12 (2), 16 10.1167/tvst.12.2.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbrech D. S.; Longaker M. T.; Mehrara B. J.; Saadeh P. B.; Chin G. S.; Gerrets R. P.; Chau D. C.; Rowe N. M.; Gittes G. K. Fibroblast response to hypoxia: the relationship between angiogenesis and matrix regulation. J. Surg. Res. 1999, 84 (2), 127–133. 10.1006/jsre.1999.5627. [DOI] [PubMed] [Google Scholar]
- Norman J. T.; Clark I. M.; Garcia P. L. Hypoxia promotes fibrogenesis in human renal fibroblasts. Kidney Int. 2000, 58 (6), 2351–2366. 10.1046/j.1523-1755.2000.00419.x. [DOI] [PubMed] [Google Scholar]
- Misra S.; Shergill U.; Yang B.; Janardhanan R.; Misra K. D. Increased expression of HIF-1α, VEGF-A and its receptors, MMP-2, TIMP-1, and ADAMTS-1 at the venous stenosis of arteriovenous fistula in a mouse model with renal insufficiency. J. Vasc. Interventional Radiol. 2010, 21 (8), 1255–1261. 10.1016/j.jvir.2010.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muth M.; Theophile K.; Hussein K.; Jacobi C.; Kreipe H.; Bock O. Hypoxia-induced down-regulation of microRNA-449a/b impairs control over targeted SERPINE1 (PAI-1) mRNA-a mechanism involved in SERPINE1 (PAI-1) overexpression. J. Transl. Med. 2010, 8, 33 10.1186/1479-5876-8-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson D.; Akrap N.; Svec D.; Godfrey T. E.; Kubista M.; Landberg G.; Ståhlberg A. Properties of targeted preamplification in DNA and cDNA quantification. Expert Rev. Mol. Diagn. 2015, 15 (8), 1085–1100. 10.1586/14737159.2015.1057124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patry J.; Blanchette V. Enzymatic debridement with collagenase in wounds and ulcers: a systematic review and meta-analysis. Int. Wound J. 2017, 14 (6), 1055–1065. 10.1111/iwj.12760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue S.; Zhang Y.; Marhaba T.; Zhang W. Aeration and dissolution behavior of oxygen nanobubbles in water. J. Colloid Interface Sci. 2022, 609, 584–591. 10.1016/j.jcis.2021.11.061. [DOI] [PubMed] [Google Scholar]
- Kolman M.; Smith C.; Chakrabarty D.; Amin S. Rheological stability of carbomer in hydroalcoholic gels: Influence of alcohol type. Int. J. Cosmet. Sci. 2021, 43 (6), 748–763. 10.1111/ics.12750. [DOI] [PubMed] [Google Scholar]
- Liang J.; Zhang K.; Li J.; Su J.; Guan F.; Li J. Injectable protocatechuic acid based composite hydrogel with hemostatic and antioxidant properties for skin regeneration. Mater. Des. 2022, 222, 111109 10.1016/j.matdes.2022.111109. [DOI] [Google Scholar]
- Jangde R.; Srivastava S.; Singh M. R.; Singh D. In vitro and In vivo characterization of quercetin loaded multiphase hydrogel for wound healing application. Int. J. Biol. Macromol. 2018, 115, 1211–1217. 10.1016/j.ijbiomac.2018.05.010. [DOI] [PubMed] [Google Scholar]
- Huang Y.; Shi F.; Wang L.; Yang Y.; Khan B. M.; Cheong K.-L.; Liu Y. Preparation and evaluation of Bletilla striata polysaccharide/carboxymethyl chitosan/Carbomer 940 hydrogel for wound healing. Int. J. Biol. Macromol. 2019, 132, 729–737. 10.1016/j.ijbiomac.2019.03.157. [DOI] [PubMed] [Google Scholar]
- Huang X.; Yang J.; Zhang R.; Ye L.; Li M.; Chen W. Phloroglucinol Derivative Carbomer Hydrogel Accelerates MRSA-Infected Wounds’ Healing. Int. J. Mol. Sci. 2022, 23 (15), 8682 10.3390/ijms23158682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens A. M.; Sikorski M. J.; Li T.; Wu Z. J.; Griffith B. P.; Kofinas P. Blood-aggregating hydrogel particles for use as a hemostatic agent. Acta Biomater. 2014, 10 (2), 701–708. 10.1016/j.actbio.2013.10.029. [DOI] [PubMed] [Google Scholar]
- Xia Y. P.; Zhao Y.; Tyrone J. W.; Chen A.; Mustoe T. A. Differential activation of migration by hypoxia in keratinocytes isolated from donors of increasing age: implication for chronic wounds in the elderly. J. Invest. Dermatol. 2001, 116 (1), 50–56. 10.1046/j.1523-1747.2001.00209.x. [DOI] [PubMed] [Google Scholar]