Abstract
Salmonellosis, one of the most common foodborne infections in Europe, is monitored by food safety surveillance programmes, resulting in the generation of extensive databases. By leveraging tree-based machine learning (ML) algorithms, we exploited data from food safety audits to predict spatiotemporal patterns of salmonellosis in northwestern Italy. Data on human cases confirmed in 2015–2018 (n = 1969) and food surveillance data collected in 2014–2018 were used to develop ML algorithms. We integrated the monthly municipal human incidence with 27 potential predictors, including the observed prevalence of Salmonella in food. We applied the tree regression, random forest and gradient boosting algorithms considering different scenarios and evaluated their predictivity in terms of the mean absolute percentage error (MAPE) and R2. Using a similar dataset from the year 2019, spatiotemporal predictions and their relative sensitivities and specificities were obtained. Random forest and gradient boosting (R2 = 0.55, MAPE = 7.5%) outperformed the tree regression algorithm (R2 = 0.42, MAPE = 8.8%). Salmonella prevalence in food; spatial features; and monitoring efforts in ready-to-eat milk, fruits and vegetables, and pig meat products contributed the most to the models’ predictivity, reducing the variance by 90.5%. Conversely, the number of positive samples obtained for specific food matrices minimally influenced the predictions (2.9%). Spatiotemporal predictions for 2019 showed sensitivity and specificity levels of 46.5% (due to the lack of some infection hotspots) and 78.5%, respectively. This study demonstrates the added value of integrating data from human and veterinary health services to develop predictive models of human salmonellosis occurrence, providing early warnings useful for mitigating foodborne disease impacts on public health.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13567-024-01323-9.
Keywords: Supervised learning, decision tree algorithms, disease surveillance, food products, salmonellosis, transdisciplinarity
Introduction
The One Health concept has increasingly gained strength in recent years, stressing the need for a transdisciplinary approach to addressing public health concerns. Foodborne pathogens are globally acknowledged as among the most important health priorities due to their direct impact on public health, the economy and society [1]. In 2021, European Union (EU) Member States reported 4005 foodborne outbreaks, resulting in 32 543 cases of illness, 2495 hospitalisations and 31 deaths. Domestic and public settings, including catering, restaurants and canteens, were the main point sources of exposure to contaminated food for most of the cases. Nontyphoidal Salmonella was the second most important enteric pathogen involved in foodborne infections, accounting for 19.3% of all outbreaks. Eggs and egg products, mixed foods, bakery products, vegetables and juices and other products thereof were among the main food sources of Salmonella infection, although composite or multiingredient foods were generally responsible for the greatest number of illnesses [2].
In Europe, Salmonella surveillance is governed by Directive 2003/99/EC [3], which obliges EU Member States to collect relevant information on pathogens, antimicrobial resistance and foodborne outbreaks. In parallel, its surveillance in humans is performed by the network for the epidemiological surveillance and control of communicable diseases [4], to which EU Member States adhere. This feeds the metadata-driven platform (TESSy) of the European Centre for Disease Prevention and Control [5]. In Italy, disease surveillance benefits from standardised and functional communication channels that have been in place for a long time. The implemented animal health surveillance programmes are coordinated at the national level, ensuring an even distribution of activities throughout the territory. In northwestern Italy, a central laboratory (i.e., the Istituto Zooprofilattico Sperimentale del Piemonte, Liguria e Valle D’Aosta, IZSPLVA) manages the data generated by veterinary activities in the field and transmits them to competent regional and national authorities. As a result, the IZSPLVA maintains large databases, and the validity of these databases has been verified over the years. In parallel, human disease surveillance data are collected and collated by the Piedmont Regional Service for the Epidemiology of Infectious Diseases (SeREMI). Surveillance activities for certain zoonoses, including salmonellosis, are coordinated at the regional level for both animals and humans. However, current data on zoonoses generated by laboratories or medical and veterinary health services often result in very large and heterogeneous databases that rarely communicate with each other or have minimal opportunities for interconnection [6]. The opportunity to use such datasets (“big data”) with a multidisciplinary approach is often overlooked. However, available data analysis methods enable the processing and/or transformation of data with high epidemiological value and great significance in terms of health prevention.
Artificial intelligence techniques, such as machine learning (ML), have been widely exploited in medical and public health research [7–9] due to the potential advantages this discipline offers in terms of health protection and promotion while increasing the efficiency of health services [10]. These tools facilitate the extraction of the underlying information contained in big data, enabling the discovery of otherwise invisible patterns that are valuable for public health and epidemiological research [11–14]. When the emphasis is on prediction rather than inference (which falls under the classical domain of statistics), ML algorithms have displayed pronounced success. In the field of foodborne diseases, ML techniques have been employed to forecast the number of incident cases caused by selected foodborne pathogens [15–18], to identify food attributions or the causative agent responsible for human outbreaks [19–21] and to evaluate the spatial risk of human outbreaks [22]. The identification of common spatial and temporal features in food and human data using ML may pave the way for the early detection of warning signals and the adoption of effective prevention strategies. Despite its potential, the current data collection methods for both veterinary and human epidemiological surveillance are usually separate and often neglect data integration.
Therefore, we aimed to demonstrate the added value of integrating data on the occurrence of salmonellosis in humans and food products in the Piedmont region of northwestern Italy. In particular, we assessed the potential of food data generated by regional food safety surveillance activities to predict spatiotemporal patterns of emerging human infections by applying different tree-based ML algorithms. The data generated by both surveillance systems from 2014 to 2018 were used to develop optimal prediction models, whereas the food surveillance data from 2019 were used to predict the incidence of human salmonellosis in the same year.
Materials and methods
Data sources and processing
Data on Salmonella infections were obtained from different information databases and retrieved separately from each of the consulted information systems. We collected all the cases of human infection reported in the Piedmont region between January 1st, 2015, and December 31st, 2019. The computerised SeREMI system, called “Sistema Informatizzato Malattie Infettive” (SIMI, [23]), provided the data on human infections. The SIMI collects all probable and/or confirmed cases of infectious aetiology reported by physicians at the regional level. These data were extracted using the GeMInI web-based database [24], with the inclusion criteria based on the Code 003 of the International Classification of Diseases (ICD-9). This code identifies Salmonella infections and excludes those caused by S. typhi and S. paratyphi. To ensure comprehensive case detection, we additionally collected human salmonellosis data from the EnterNet Italia platform [25]. This portal records information concerning enteric pathogens involved in confirmed clinical cases at the national level. From EnterNet, we extracted all the records related to Piedmont’s Salmonella infections during the specified time period. The obtained human datasets were integrated by matching records based on birth date, sex, location of symptom onset, or, in the absence of this information, place of residence. This data integration provides added value by improving the characterisation of the health issue and potentially identifying human cases that may have been missed by the SeREMI system.
Data on food products were obtained from the IZSPLVA laboratory information system called SIGLA, which records all institutional activities related to animal research and routine laboratory testing. We retrieved the data using general PL/SQL queries, which is a common method used at the IZSPLVA for data analysis and routine reporting activities. The resulting dataset contained nonaggregated records, including details of sample collections, such as geographical origin, animal species, type of laboratory analyses performed, and results. Among the features retrieved from the SIGLA system, no variable was dedicated to uniquely identifying specific diseases. This required several steps of accurate data processing before the data were ready for use (Additional file 1). Our inclusion criteria focused on food products collected in the Piedmont region between July 1st, 2014, and December 31st, 2019. We selected specific laboratory tests for Salmonella detection or untargeted laboratory tests, such as bacterial isolation, in which Salmonella spp. were identified. The resulting dataset was then checked for duplicates and cleaned, giving priority to confirmed positive results when multiple laboratory tests on the same sample yielded contrasting results.
Differences in the types of data collected between the food and human datasets led to the use of different measures of disease frequency prior to data integration. The human databases contained only positive/confirmed disease cases, allowing the calculation of disease incidence based on the resident population. By contrast, the animal/food database included both positive and negative results for pathogen detection, allowing the prevalence of infection in food products to be estimated.
In addition, we chose to use the open-source dataset provided by the Italian National Institute of Statistics (ISTAT, [26]) to compile the demographic and spatial data of the Italian territory. These data were needed to calculate the denominators of the resident population and to integrate the human and food datasets.
The dataset
We initiated the construction of the working dataset by focusing on human data collected from 2015 to 2018. These data were aggregated at the municipality level, calculating the monthly incidence rates of Salmonella infections (shown as the dependent variable, H_INC), and standardised by sex and age. Consequently, the epidemiological unit of the dataset consisted of a specific combination of a municipality where salmonellosis cases arose and a one-month interval (H_MONTH). Next, we assigned a value of Salmonella prevalence detected in food products (the predictive variable, F_PREV) for each epidemiological unit. This was determined by considering a hypothetical exposure area (the potential area of food supply) and a time lag that took into account the municipalities where consumers were most likely to purchase food products, the incubation period of the disease (from pathogen exposure to illness onset), and the time elapsed between the onset of symptoms, disease case detection and notification of health authorities.
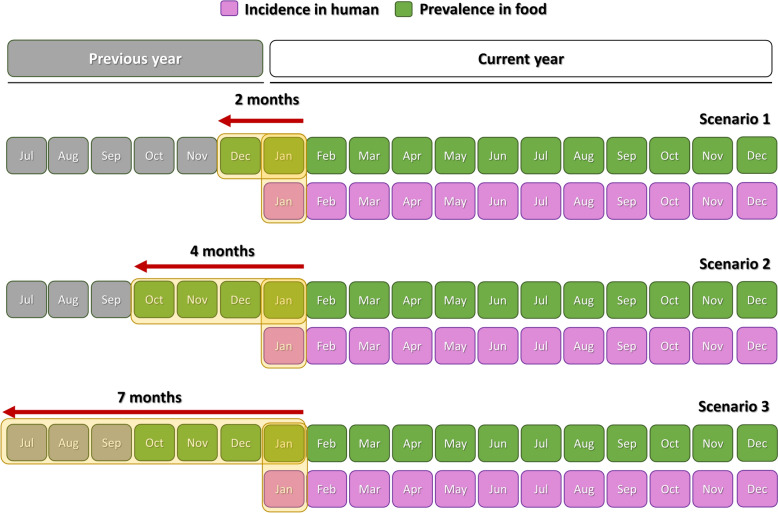
Foodborne disease outbreaks generally involve contamination from a single point source in localised areas, and the infection only occasionally spreads through the supply chain to geographically distant locations [27]. Hence, we determined the potential exposure area based on the average size of the municipalities as well as the distance between them. The calculation of F_PREV, reflecting the proportion of positive food samples out of the total tested, was performed in the area encompassing the municipality where human cases emerged and among their nearest neighbours. Both the incubation period and the notification process were considered when accounting for the time lag between infection and detection as a case in the information systems. All infectious diseases exhibit an incubation period. In nontyphoidal Salmonella infections, the typical reported duration of infection is between 6 and 72 h [28]. Nonetheless, longer incubation periods of 9 to 16 days have been recorded [29, 30]. Additionally, due to the delay associated with case identification, confirmation and subsequent reporting to health authorities, the time lag can be quite long [31]. To address these complexities, we devised three different temporal scenarios by linking the H_INC recorded in a specific municipality and month with the F_PREV determined in the exposure area during three predefined time lags (i.e., lags of two months, four months and seven months) from the emergence of human cases (Figure 1). Therefore, taking into account the 2-month time lag, the F_PREV expressed for H_INC occurring in March 2015 was calculated by considering all the food products tested and exhibiting in positive results from an exposure area during February and March 2015.
Figure 1.
Time intervals established for estimating the prevalence of Salmonella contamination in food products from the hypothetical food supply areas (areas of exposure) within each spatial–temporal scenario.
We excluded municipalities where no human salmonellosis cases were recorded by health services or where no food product monitoring was conducted during the specific time interval. Each record was enriched with additional explanatory features intended to offer a more comprehensive understanding of the observed human incidence (Table 1). These features included spatial characteristics such as the centroid coordinates (DDcoordY and DDcoordX) of the municipalities where human cases emerged; the total surface area of the exposure area (SUPKM2), expressed in km2; the quantity of food samples with positive results; and the total number of laboratory assays conducted for each exposure area across 11 different food categories.
Table 1.
Description of the explanatory features used as inputs for the ML models in the study
| Predictors | Name of the features | Description |
|---|---|---|
| Time | ||
| Month | HMONTH | Actual month in which incident cases emerged or may have emerged |
| Spatial | ||
| Longitude | DDcoordX | Centroid coordinates of municipalities in which human cases have emerged |
| Latitude | DDcoordY | |
| Area of exposure | SUPKM2 | Area of pathogen exposure, expressed in km2 |
| Food | ||
| Food prevalence | F_PREV | Prevalence of Salmonella contamination in food products |
| Food categories | Type of foods tested within the food safety surveillance | |
| Name of the food category | N. of tests | N. of positive samples | Note | |
|---|---|---|---|---|
| 1 | Eggs | EGGt | EGGp | Including their products and derivatives |
| 2 | Milk | MILKt | MILKp | |
| 3 | Cereal-based products and legumes | CERELEGUMt | CERELEGUMp | Cereals, flour, pasta, dough, legumes |
| 4 | Fruits and vegetables | FRUITt | FRUITp | Fresh and frozen products and vegetable sauces |
| 5 | Bakery products | BAKERYt | BAKERYp | Creams, fruit jams, syrup and candied fruits, cookies |
| 6 | Seafood | FISHt | FISHp | Fresh, frozen and canned products and fish-based sauces |
| 7 | Poultry meat | POULTRYt | POULTRYp | Fresh, frozen, cured meats and processed meat products, including animal byproducts |
| 8 | Beef | BEEFt | BEEFp | |
| 9 | Pig meat | PIGt | PIGp | |
| 10 | Various meats | V_MEATt | V_MEATp | Fresh, frozen, cured and minced meats and processed products, including animal byproducts, from different animal species (e.g., horse, lamb, goat) |
| 11 | Ready-to-eat foods | READYt | READYp | Products for direct consumption without the need for cooking or other processing |
The entire dataset consisted of 220 observations, all of which contained complete data on both the dependent variable (H_INC) and the 27 predictors.
A significant challenge faced during this study was to find sufficient data on food products to develop parameter estimates. After integrating the data, only 220 out of the initial 1377 records provided complete information. To enhance the performance of the ML algorithms, we therefore simulated a scenario where we had Salmonella prevalence data for food products available for all the epidemiological units and time lags under investigation. This methodology allowed us to develop three ML algorithms that were later used to pursue our objectives and evaluate their suitability for our dataset. To achieve this, we applied Laplace smoothing to the initial tests conducted and positive outcome tallies [32]. This procedure assumed the requirement of further tests or samplings () for detecting pathogens in foodstuffs per sampling area and lag time, irrespective of the food category. By including a minimum ‘corrected’ prevalence (), we could integrate the previously excluded records that lacked prevalence information. Here, was calculated as follows:
where.
represents the observed number of Salmonella-positive food products tested;
represents the hypothetical number of additional tests needed;
denotes the mean prevalence observed in food products within a given sampling area and time lag; and.
represents the actual number of tests conducted in a given sampling area and period of time.
A set of -values was defined based on the municipality’s population size and the specified time lag for each scenario (Additional file 2, Sect. 4, Table S2). For this purpose, we classified the municipalities in the Piedmont region into five distinct groups: (1) those with a population ≤ 5000 inhabitants, (2) those with a population between 5001 and 9999, (3) those with a population between 10 000 and 19 999, (4) those with a population between 20 000 and 29 999, and (5) those with a population ≥ 30 000 inhabitants. The parameters estimated for the model using this approach, along with the temporal scenario, which exhibited the best fit and the lowest mean absolute percentage error (MAPE), were considered the best results in the simulated modelling performance assessment.
We compiled a final dataset containing exclusively data from the 2019 food safety surveillance. This was achieved by following the aforementioned procedure and adhering to the optimal temporal scenario. The dataset that resulted contains a total of 1035 observations, each equipped with complete information on the 27 explanatory features that are outlined in Table 1. Considering our aim to predict the emergence of human salmonellosis, this dataset, which was not used for model testing purposes, was treated as unlabelled data. This means that information on human salmonellosis, which was treated separately and later used for comparisons with the models’ predictions, was lacking.
Statistical analyses
All data management, preprocessing and analyses were performed using Stata 17 [33], whereas graphical representation of the results was obtained by using R (version 4.2.2) and QGIS3 (version 3.4 Madeira) software. We calculated the proportion of human salmonellosis detected by the human health system as well as the prevalence of Salmonella in food products and 95% exact binomial confidence intervals (CIs). Initially, we evaluated the potential relationship between H_INC and F_PREV (Additional file 2, Sect. 1, Figure S4). For this purpose, we utilised data from the entire study period (2014–2019), considering the different designed time scenarios. A log–log linear regression model was used to fit the natural logarithm transformation of both variables, with H_INC representing the dependent variable and F_PREV serving as the explanatory variable.
A range of epidemiological studies on foodborne diseases have employed tree-based ML algorithms [15, 17, 19–21]. We ran and fitted tree regression (TR), random forest (RM) and gradient boosting (GB) algorithms using the recently developed r_ml_stata_cv command [34]. This command makes use of the Python Scikit-learn API for both cross-validation and outcome prediction. To determine the model with the best performance, we conducted five-fold cross-validation. This method randomly splits the training dataset into five equal-size portions, called folds. Here, four folds were used for model training (in-sample), and the remaining fold was used to estimate model performance (out-of-sample). This procedure is repeated until all five folds have been used for testing five distinct models trained on the remaining folds, each using unique and separate training and testing folds. The prediction error estimates are obtained by averaging all out–of–sample mean square errors obtained fold–by–fold. K-fold cross-validation also provides an estimation of the true test error (i.e., mean absolute percentage error, MAPE), which enables us to evaluate the uncertainty of the best-optimised model. The tuning of the hyperparameters of each ML algorithm was modified from the default values based on the grid search strategy [35] using the values reported in Table 2 to optimise algorithm performance.
Table 2.
The parameters used in tree regression (TR), random forest (RF) and gradient boosting (GB) ML algorithms
| ML algorithm | Parameter | Real prevalence | Simulated prevalence |
|---|---|---|---|
| TR | Maximum tree depth | 25 | 20 |
| RF | Maximum tree depth | 25 | 20 |
| Max. no. of splitting features | 27 | 5 | |
| Max. no. of bootstrapped trees | 50–250 | 50–250 | |
| GB | Maximum tree depth | 25 | 20 |
| Learning rate | 0.1–0.3 | 0.1–0.3 | |
| Number of sequential trees | 50–250 | 50–250 |
To develop our ML algorithms, we used two collections of data: human data from 2015 to 2018 and food data from 2014 to 2018. These data were treated as both training and test datasets by randomly selecting data from the original dataset at a 7:3 ratio. The scenario that yielded the best model fit and precision was selected for the prediction of human salmonellosis in 2019. The unlabelled dataset for this task was the 2019 food safety surveillance data, which was employed to evaluate the generalisation performance of the predictive model that had been trained on data from 2015 to 2018 (Additional file 2, Sects. 3 and 5). The predictions obtained were then compared with the incidence of human salmonellosis recorded in 2019 by human health surveillance systems. In addition, we evaluated the sensitivity and specificity of the models when used to predict the observed disease occurrence status (in terms of the presence or absence of at least one case) of each municipality.
Results
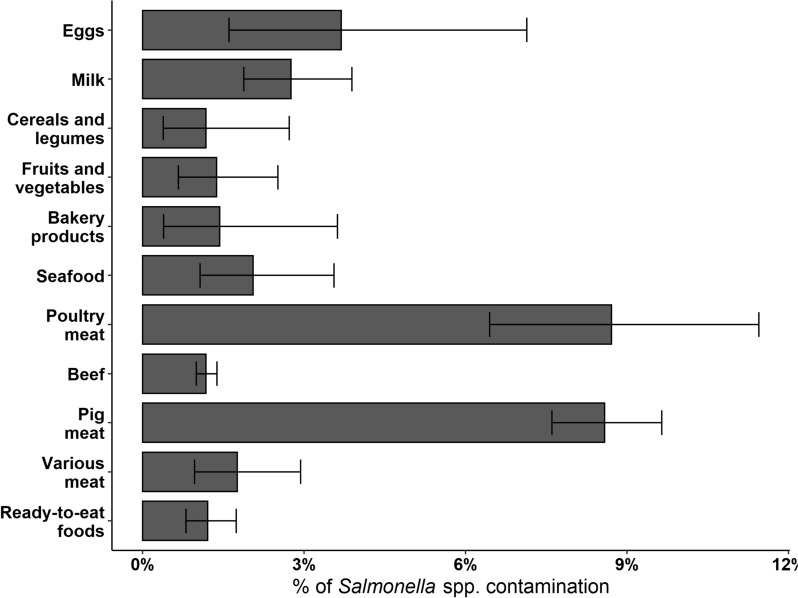
The regional health services recorded 2560 Salmonella infections in the human population from 2015 to 2019, resulting in an average incidence rate of 5.8 per 10 000 person-years. The infections were distributed among all age and sex strata, with the youngest population displaying the highest infection rates (Table 3). We noted differences in the retrieval of disease occurrence records depending on which human data sources were used. Specifically, only 36.5% (n = 935) of the cases were commonly shared between both human databases, whereas the remaining 22.3% (n = 572) and 41.1% (n = 1053) of the cases specifically originated from the SeREMI and Enternet databases, respectively. Regarding food safety surveillance, the system revealed a Salmonella spp. prevalence of 2.5% (95% CI 2.3–2.7) in food products monitored between 2014 and 2019. The highest levels of Salmonella spp. contamination were found in poultry and swine meat products, with other food categories not exceeding a prevalence of 3.7% (Figure 2).
Table 3.
Salmonellosis incidence rates (IRs) in the Piedmont region from 2015 to 2019
| Males | Females | |||||
|---|---|---|---|---|---|---|
| Age strata | n | Average population | IRm | n | Average population | IRf |
| 0–9 | 594 | 187 047.4 | 31.8 | 526 | 176 086.6 | 29.9 |
| 10–24 | 160 | 299 289.4 | 5.3 | 120 | 279 169 | 4.3 |
| 25–49 | 95 | 701 997.4 | 1.4 | 122 | 698 011.4 | 1.7 |
| 50–74 | 243 | 707 284.2 | 3.4 | 197 | 755 683.4 | 2.6 |
| ≥ 75 | 186 | 227 447.6 | 8.2 | 188 | 348 495.8 | 5.4 |
Incidence rates are presented as cases per 10 000 person-years and stratified by sex and age. A total of 129 cases of salmonellosis were omitted from this table because both sex and age data were unavailable. IRm represents the incidence rate for males, and IRf represents the incidence rate for females.
Figure 2.
Prevalence and 95% CIs of Salmonella spp. contamination in food products, the Piedmont region 2014–2019.
We observed a positive association between the prevalence of Salmonella in food products and the incidence of human salmonellosis recorded during the study period (β = 0.59; R2 = 0.28; p < 0.001): the expected percentage change in human illness increased by 0.5% for each one-unit increase in the prevalence of Salmonella in food products (Additional file 2, Figure S4).
The application of the different ML algorithms to the initial dataset (n = 220 records) yielded notable differences in model performance. Table 4 summarises the optimal tuning parameters, the fit and the MAPE results obtained by each algorithm in different scenarios on the test set. GB and RF generally outperformed the TR algorithm; however, all models achieved only low-to-moderate fit levels. No improvements in the performances of the RF and GB algorithms were observed when they were applied to scenarios with extended time lags; all of these algorithms achieved the highest level of fit in scenario 1. Furthermore, the MAPE for all the models did not decrease but instead increased, especially in scenario 2. We recorded the lowest MAPEs with the highest model fit in scenario 1 (Table 4), indicating that this was the most suitable time lag for prediction with minimal error. Based on this latter result, we fitted the three models with the 2019 integrated dataset containing the time slots and municipalities for which the H_INC and F_PREV data were complete. This highlighted the differences observed earlier between the three algorithms (Figure 3). We obtained a comparatively low average H_INC compared to the total average H_INC observed in the study municipalities (n = 39; 3.09 per 10 000 person-years). The TR algorithm yielded an average incidence rate of 2.07 per 10 000 person-years, and the incidence rates calculated using RF and GB were 1.85/10 000 and 2.16/10 000, respectively. Among the 27 features included in the models, the relevance of spatial data and the testing effort performed in particular food matrices were prominent (Figure 4), resulting in a 90.5% reduction in the variance of Salmonella incidence estimates. The sampling/testing effort employed in ready-to-eat foods (READYt), milk (MILKt), fruit and vegetables (FRUITt) and pig meat (PIGt) provided the greatest contribution to the models’ prediction ability. By contrast, the contribution of positive outcomes ascertained for each food category was generally low (2.9%), with the number of positive pig meat samples (PIGp) obtaining the highest level of importance (1.5%; Figure 4).
Table 4.
Optimal tuning parameters obtained after conducting five-fold cross-validation for tree regression (TR), random forest (RF) and gradient boosting (GB) algorithms
| ML algorithm | Scenario | Optimal tuning parameters | Log-scale | ||||
|---|---|---|---|---|---|---|---|
| Tree depth | No. splitting features | N. of trees | Learning rate | Fit | MAPE (%) | ||
| TR | 1 | 2 | 0.42 | 8.8 | |||
| 2 | 2 | 0.18 | 8.6 | ||||
| 3 | 1 | 0.12 | 9.1 | ||||
| RF | 1 | 20 | 20 | 150 | 0.55 | 7.5 | |
| 2 | 5 | 4 | 50 | 0.32 | 8.3 | ||
| 3 | 5 | 8 | 50 | 0.31 | 5.8 | ||
| GB | 1 | 3 | 50 | 0.1 | 0.55 | 7.5 | |
| 2 | 1 | 50 | 0.1 | 0.35 | 8.3 | ||
| 3 | 1 | 50 | 0.1 | 0.27 | 6.3 | ||
The dataset for the years 2015–2018 (n = 220 observations) was used, including municipalities with complete information on H_INC and F_PREV
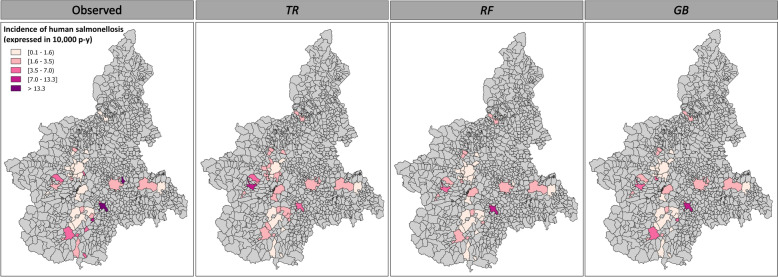
Figure 3.
Observed and predicted incidence rates of human salmonellosis recorded across 39 municipalities in the Piedmont region during 2019. These data provide a partial representation of the actual distribution of human cases, as they only include the epidemiological units (a combination of time intervals and municipalities) for which data on both human salmonellosis (H_INC) and Salmonella contamination prevalence in food products (F_PREV) were available.
Figure 4.
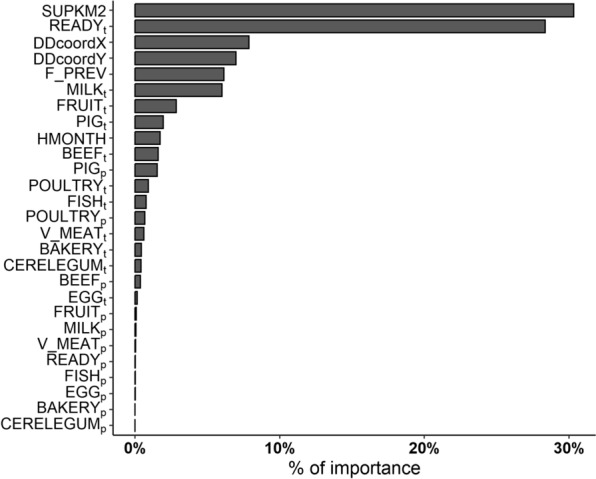
Feature importance displayed by the random forest (RF) algorithm in scenario 1, expressed as the total percentage of variance reduction that results from predictor splits.
By simulating greater data availability regarding Salmonella prevalence in food products, we observed that the prediction models displayed better performance and reduced the percentage error. Even minor increases in the simulated sampling/testing effort ( sufficed to maximise the models’ performance with increased data availability. Nevertheless, no further improvement in model fit occurred with gradual increases in levels (Figure 5). RF and GB were verified as the best performing algorithms, achieving comparable levels of fit and attaining optimal performance at an level = 4 (R2 ≈ 0.74; Additional file 2, Sect. 4, Table S3), with MAPE values of 5.50% and 5.39%, respectively. By contrast, TR seemed to require more food sampling ( level = 10) to achieve optimal performance (R2 ≈ 0.60; Additional file 2, Sect. 4, Table S3) with the lowest error (MAPE = 7.29%).
Figure 5.
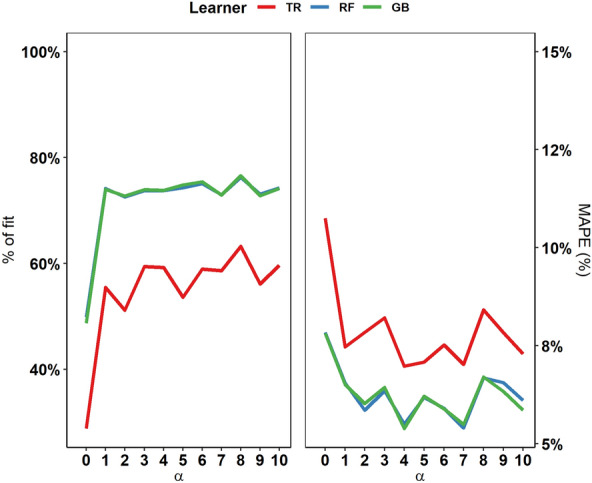
Calibration of the tree regression (TR), random forest (RF) and gradient boosting (GB) algorithms in scenario 1 at different levels of simulated sampling/testing effort (α).
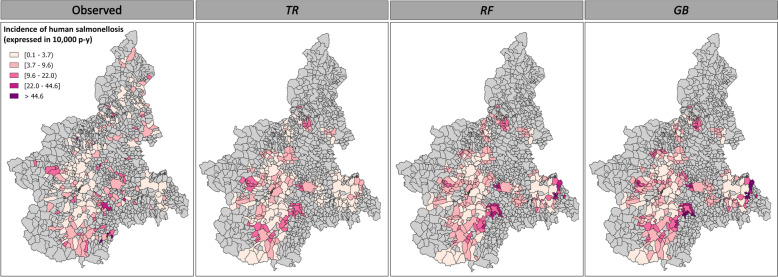
In 2019, health services recorded an average H_INC of 7.03 per 10 000 person-years, encompassing 213 out of 1181 municipalities in the Piedmont region. Food safety surveillance covered 337 municipalities and revealed a total food contamination prevalence of 4.1% (95% CI 3.6–4.7) involving 48 municipalities (Additional file 3). Based on the food-recorded data, our models predicted infection rates similar to those observed by the health services, especially when using RF (8.08/10 000) and GB (10. 4/10 000). However, we obtained lower incidence rates with TR, with an estimated average incidence rate of 4.99/10 000. The fit and MAPE of the algorithms for the predicted incidence are illustrated in Table 5. In our dataset, human cases of salmonellosis were officially reported in 213 of a total of 1181 municipalities. As described above, our models were used to predict the occurrence status (in terms of the presence or absence of at least one case) of each municipality. When the disease was reported by the health services, our models showed a sensitivity of 46.5% (99/213). Of the 968 municipalities where no human cases were recorded, the absence of the disease was correctly predicted in 760, yielding a specificity of 78.5% (Figure 6).
Table 5.
Performance of the ML algorithms for predictions in 2019
| ML algorithm | Optimal tuning parameters | Log-scale | Natural scale | |||||
|---|---|---|---|---|---|---|---|---|
| Tree depth | No. splitting features | N. of trees | Learning rate | Fit | MAPE (%) | Fit | MAPE (%) | |
| TR | 2 | 0.9998 | 8.21 | 0.9840 | 66.3 | |||
| RF | 20 | 20 | 150 | 0.9998 | 7.37 | 0.9872 | 59.9 | |
| GB | 3 | 50 | 0.1 | 0.9999 | 7.07 | 0.9887 | 64.4 | |
Figure 6.
Comparison of the observed and predicted incidence rates of salmonellosis in humans using tree regression (TR), random forest (RF) and gradient boosting (GB) algorithms with food safety surveillance data from 2019.
Discussion
By fitting tree-based ML algorithms to real-world data produced by regional food safety surveillance activity, we successfully forecasted spatiotemporal patterns of emerging Salmonella infections in the local population of the Piedmont region of northwestern Italy. This novel approach highlights the essential role of veterinarians in the animal-based food supply chain and emphasises the importance of interdisciplinary collaboration in protecting public health [36]. In addition, the method provides a consistent approach that can be implemented in food surveillance databases for hazards beyond Salmonella spp.
Despite ongoing efforts to reduce foodborne salmonellosis, its complexity derives from the various pathways that lead to foodborne illnesses and the different food products that can be involved in human outbreaks [37, 38]. ML techniques can support prevention efforts against salmonellosis because these algorithms can detect complex patterns that can be overlooked by conventional methods, especially when dealing with intricate interactions and patterns [39]. Among the tree-based algorithms used, random forest (RF) and gradient boosting (GB) algorithms exhibited comparable abilities to predict human salmonellosis. Both algorithms outperformed tree regression (TR) algorithms in terms of accuracy and reduced uncertainty. TR algorithms are known to yield highly unstable predictions, as they are prone to overfitting and show high variance errors [40]. However, the incorporation of bootstrap aggregation techniques into TR (resulting in RF and GB algorithms) enhanced its predictive power and generalisation capabilities [41, 42].
Although the sensitivity levels were quite low and there were quite a few instances where our models inaccurately failed to predict the occurrence of at least one case at the municipality level, both the RF and GB algorithms were able to identify significant occurrences of actual human salmonellosis. Although the predictions of these ML algorithms closely resembled the infection rates reported by regional clinical laboratories and public health authorities, there were notable discrepancies in the distribution of the disease compared to the observed data. Several reasons may explain these discrepancies. Disease dynamics play a significant role, as cases may arise in municipalities escaping detection by the healthcare system, or the origin of contaminated food may be traced to a different region [43]. Additionally, the high rates of false-negative results recorded may be partly due to constraints imposed by the quality and quantity of collected data available for developing the models [44]. Salmonella outbreaks primarily occur in domestic settings. However, disease cases are usually reported based on the municipality of the individual’s official residence. This practice may not accurately reflect the actual home location of the patients, potentially leading to some geographical misclassification of the disease distribution. Such imprecision could impede our efforts to improve the accuracy of our predictions and have a direct impact on the development of health system policies and their subsequent evaluation [45].
Despite these challenges, it is important to acknowledge that our analysis assumes a solid and efficient health care system; however, limitations of the surveillance strategies employed for humans and food products, administrative challenges within the health system, and intrinsic factors related to the natural progression of the illness may also account for the obtained results. Human salmonellosis surveillance operates under a passive monitoring approach, which inherently limits its ability to effectively identify disease cases. This strategy heavily relies on interactions with the health care system to detect cases and is thus subject to such interactions. However, various factors, including an individual’s attitude towards seeking medical care, the prevalence of subclinical or self-limiting infections, and challenges related to diagnosing, communicating and investigating cases within the healthcare system, can undermine the efficiency of case detection [46–49]. Although salmonellosis infections typically resolve spontaneously, certain vulnerable individuals, such as infants, elderly individuals, and immunocompromised individuals, may develop severe forms of the disease that require medical intervention [50, 51]. As a result, only a fraction of illness events within the population are detected, reported and communicated to health authorities [52]. In Greece, it was estimated that only 47.7% of human salmonellosis cases are officially reported, highlighting notable regional differences in disease reporting practices [53]. We noted a comparable situation when incorporating data from health authorities with records from regional laboratories. Our study revealed that although approximately 60% of the cases were officially reported, a significant number of cases were recognised by health services but not conveyed to health authorities. This difference exemplifies the difficulty of achieving extensive and precise data integration across the healthcare system and adequately capturing and measuring the real burden of Salmonella infections in the population [54, 55].
On the other hand, food safety surveillance is built upon standardised active monitoring aimed at the timely detection and resolution of potential foodborne hazards [56]. However, the main challenge to this approach has centred on sampling considerations. As microbial contaminants can occur at multiple stages of the food supply chain, the effectiveness of the active surveillance system depends on the accuracy of the sampling process [57, 58]. Therefore, any gaps in active monitoring activities or reduced sampling efforts for food products in certain areas could lead to oversights. This could cause our ML models to miss certain clusters of human salmonellosis (as we noticed in the northeastern and southeastern parts of the Piedmont region). These findings highlight the crucial role of sampling decisions in achieving successful results and emphasise the need to increase efforts within surveillance systems to reduce the risk of contaminated food reaching consumers and to protect public health. There are many types of food in which Salmonella spp. are actively searched for, and the accuracy of this search significantly influences the probability of detection. The total number of tests performed on ready-to-eat foods, milk and milk products, fruits and vegetables, and pig meat and its byproducts were among the most important features for predicting human salmonellosis. These findings are consistent with recent European-level zoonotic surveillance data, which highlight mixed foods and pig meat as the primary food categories frequently implicated in human outbreaks [2]. In our study, a prevalence of 1.7% was found in pig meat and its byproducts, indicating a higher level of Salmonella contamination compared to the average prevalence observed at both the Italian and European levels. These contrasting results may rely on the broad food categorisation used, as we did not differentiate between different meat products such as carcasses, fresh meat, or minced meat. Consequently, the higher prevalence observed may be due to the more frequent occurrence of the pathogen in pig meat products other than carcasses. In fact, Salmonella contamination is most common in non-ready-to-eat foods derived from poultry and pig meat [2]. Nonetheless, the impact of the presence of Salmonella in food samples on the prediction of human cases appears to be rather limited, and it is important to be cautious in our interpretation. We recognise that the inclusion of this information contributed to our ability to predict the geographical distribution of documented human outbreaks and the observed incidence rates. However, it is important to recognise that the performance of our models may be influenced by a complex interplay of factors, and the relative importance of certain variables may vary [59].
Data integration is a crucial aspect of gaining insights from real-time data [60]. Surveillance platforms are a reliable source of information on confirmed cases of disease and/or infection compared to other data sources. However, merging data from various sources can be challenging [61]. In our case, the integration of human and food databases was successful due to their similar structure and common fields that facilitated data merging. The challenge at hand was to obtain sufficient data for estimating ML model parameters. It is well known that the size of the dataset used for ML techniques has a significant impact on the precision and accuracy of the predicted outcomes [62, 63]. The first training dataset used in this study contained a limited number of records to ensure data completeness, resulting in models with moderate-to-low performance and low prediction accuracy. To evaluate the models’ effectiveness in predicting disease cases, we simulated increased availability of complete data. The resulting increase in prediction accuracy confirms the models’ suitability for our stated objective. Our implementation of ML techniques underscores their potential to enhance the efficiency of health services [10]. Although our predictive models do not have optimal sensitivity and specificity, the usefulness of these techniques is significant in regard to addressing evolving diseases and changing transmission patterns [64]. ML models can learn and adapt from new data continuously, enhancing their overall usefulness by refining their predictions as new data surfaces [65]. These methods can identify anomalous shifts in data that may indicate an emerging outbreak. Although these models may overlook specific cases, the overall detection of these shifts can help authorities take proactive measures to prevent larger outbreaks [66]. Moreover, the timely identification of potential high-risk outbreak areas and particular food categories that significantly contribute to disease transmission can offer guidance for targeted interventions. This enables health services to focus their efforts on the areas in greatest need, optimising resource allocation in a more responsive and data-driven manner and mitigating the impact of disease spread.
Our findings highlight the significance of interdisciplinary collaboration, reliable data integration, and the utilisation of ML techniques to enhance preparedness to effectively manage risks from foodborne salmonellosis. We have gained valuable insights into the potential of food safety surveillance data in predicting foodborne Salmonella outbreaks. Additionally, challenging issues have been identified within healthcare services regarding data transmission and integration, emphasising the complexity of managing epidemiological data. The use of ML algorithms, particularly random forest (RF) and gradient boosting (GB), on our dataset has shown considerable success in predicting cases of human salmonellosis. Despite some inherent shortcomings, such as limited sensitivity and specificity, these ML algorithms nevertheless represent valuable operational tools. As such, they hold great promise as a warning resource for public health interventions, thereby facilitating a proactive response. The methodology outlined here offers potential for adaptation to other contexts and communicable diseases. However, any extension of this method should be undertaken carefully, taking into account the specific characteristics and challenges of particular epidemiological scenarios. This approach, if implemented with care and consideration of local epidemiological circumstances, can provide insightful guidance and support in protecting public health.
Supplementary Information
Additional file 1. Management and processing of food safety surveillance data. Flowchart illustrating the retrieval and processing of food safety surveillance data from the SIGLA database, the electronic system of the Istituto Zooprofilattico Sperimentale del Piemonte, Ligura e Valle d’Aosta.
Additional file 2. Codes used to perform the analyses presented in the current manuscript and their outputs.
Additional file 3. Geographical distribution of food surveillance activity in the Piedmont region in 2019. Figure illustrating municipalities that were subjected to food surveillance in 2019.
Additional file 4. Data supporting the results and conclusions of the present manuscript. Details on the datasets provided are explained in Additional file 2.
Acknowledgements
This work was performed as part of the Master’s Programme in Epidemiology at the University of Turin. We express our gratitude to Prof. Mario Dante Lucio Giacobini and Dr Giovenale Moirano for their valuable critique and insights that improved the present work. We also acknowledge the contributions of Vittorio Paragallo, Manuela Migotto and Davide Loccisano, students of the Department of Computer Sciences, who were involved in the initial stages of the study.
Authors' contributions
CM, LAC and GR conceived the study. AG-V, CM, and GR actively participated and discussed the study design. AG-V and LAC managed the initial steps of data cleaning and management. AG-V concluded the data cleaning phase, conducted the data integration and analyses, and wrote the first draft of the manuscript. EC and RM assisted in the application of ML modelling. DL and CP provided human surveillance data. AG-V, CM, LAC, EC, RM, WM, MP, DL, DM, CP, and GR participated in the writing, review and editing of the manuscript. All the authors have read and approved the final manuscript.
Funding
This research was supported by the Ministry of Health of Italy under the National Programme of Health Research 2020 (Grant Number: IZS PLV 07/20 RC; CUP: J16I20000080001).
Availability of data and materials
The raw data were generated by the Servizio di riferimento Regionale di Epidemiologia per la sorveglianza, la prevenzione e il controllo delle Malattie Infettive (SeREMI) and Istituto Zooprofilattico Sperimentale del Piemonte, Liguria e Valle d’Aosta (IZSPLVA). Access to these data is limited due to licensing agreements for the current study, and these data are not publicly available. The derived data and code scripts on which the conclusions of this study are based are openly available in the Additional files.
Declarations
Ethics approval and consent to participate
Informed consent for medical intervention was obtained from patients who visited medical doctors in accordance with the current Italian legislation. All human data were treated in anonymized manner and were used exclusively for the purposes of scientific research.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organization (2015) WHO estimates of the global burden of foodborne diseases: Foodborne Disease Burden Epidemiology Reference Group 2007–2015. https://apps.who.int/iris/handle/10665/199350 Accessed 20 Apr 2024
- 2.European Food Safety Authority EFSA, and European Centre for Disease Prevention and Control, ECDC The European Union One Health 2021 Zoonoses Report. EFSA J. 2022;20:7666. doi: 10.2903/j.efsa.2022.7666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Directive 2003/99/EC of the European Parliament and of the Council of 17 November 2003 on the monitoring of zoonoses and zoonotic agents, amending Council Decision 90/424/EEC and repealing Council Directive 92/117/EEC
- 4.Decision No. 1082/2013/EU of the European Parliament and of the Council of 22 October 2013 on serious cross-border threats to health and repealing Decision No 2119/98/EC Text with EEA relevance
- 5.European Centre for Disease Prevention and Control, ECDC (2022) The European Surveillance System (TESSy). https://www.ecdc.europa.eu/en/publications-data/european-surveillance-system-tessy. Accessed 18 Apr 2024
- 6.Zinsstag J, Schelling E, Waltner-Toews D, Tanner M. From "one medicine" to "one health" and systemic approaches to health and well-being. Prev Vet Med. 2011;101:148–156. doi: 10.1016/j.prevetmed.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vilne B, Meistere I, Grantiņa-Ieviņa L, Ķibilds J. Machine Learning approaches for epidemiological investigations of food-borne disease outbreaks. Front Microbiol. 2019;10:1722. doi: 10.3389/fmicb.2019.01722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cabatuan M, Manguerra M (2020) Machine learning for disease surveillance or outbreak monitoring: A review. In: 2020 IEEE Proceedings of the 12th International Conference on Humanoid, Nanotechnology, Information Technology, Communication and Control, Environment, and Management (HNICEM), December 2020, pp 1–6. 10.1109/HNICEM51456.2020.9400088
- 9.Weissler EH, Naumann T, Andersson T, Ranganath R, Elemento O, Luo Y, Freitag DF, Benoit J, Hughes MC, Khan F, Slater P, Shameer K, Roe M, Hutchison E, Kollins S, Broedl U, Meng Z, Wong JL, Curtis L, Huang E, Ghassemi M. The role of machine learning in clinical research: transforming the future of evidence generation. Trials. 2021;22:537. doi: 10.1186/s13063-021-05489-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Panch T, Pearson-Stuttard J, Greaves F, Atun R. Artificial intelligence: opportunities and risks for public health. Lancet Digit Health. 2019;1:e13–e14. doi: 10.1016/S2589-7500(19)30002-0. [DOI] [PubMed] [Google Scholar]
- 11.Ru G, Crescio MI, Ingravalle F, Maurella C, Gregori D, Lanera C, Azzolina D, Lorenzoni G, Soriani N, Zec P, Berchialla P, Mercadante S, Zobec F, Ghidina M, Baldas S, Bonifacio B, Kinkopf A, Kozina D, Nicolandi L, Rosat L. Machine Learning techniques applied in risk assessment related to food safety. EFSA J. 2017;14:1254. doi: 10.2903/sp.efsa.2017.EN-1254. [DOI] [Google Scholar]
- 12.Munck NSM (2019) Tracing sources of zoonotic Salmonella Infections and contamination using Whole Genome Sequencing data and Machine Learning. PhD Thesis, Technical University of Denmark. Available online: https://orbit.dtu.dk/en/publications/tracing-sources-of-zoonotic-salmonella-infections-and-contaminati. Accessed 20 Apr 2024
- 13.Wardeh M, Sharkey KJ, Baylis M. Integration of shared-pathogen networks and machine learning reveals the key aspects of zoonoses and predicts mammalian reservoirs. Proc Biol Sci. 2020;287:20192882. doi: 10.1098/rspb.2019.2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Agany DDM, Pietri JE, Gnimpieba EZ. Assessment of vector-host-pathogen relationships using data mining and machine learning. Comput Struct Biotechnol J. 2020;18:1704–1721. doi: 10.1016/j.csbj.2020.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sadilek A, Caty S, DiPrete L, Mansour R, Schenk T, Jr, Bergtholdt M, Jha A, Ramaswami P, Gabrilovich E. Machine-learned epidemiology: real-time detection of foodborne illness at scale. NPJ Digital Med. 2018;1:36. doi: 10.1038/s41746-018-0045-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang X, Zhou M, Jia J, Geng Z, Xiao G. A Bayesian approach to real-time monitoring and forecasting of Chinese foodborne diseases. Int J Environ Res Public Health. 2018;15:1740. doi: 10.3390/ijerph15081740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang M, Guo D, Hu J (2019) Risk prediction and assessment of foodborne disease based on big data. In Proceedings of the 5th ACM SIGSPATIAL International Workshop on the Use of GIS in Emergency Management, Chicago, November 2019. Article N. 8, pp 1–6. 10.1145/3356998.3365776
- 18.Li S, Peng Z, Zhou Y, Zhang J. Time series analysis of foodborne diseases during 2012–2018 in Shenzhen, China. J Consum Prot Food Saf. 2022;17:83–91. doi: 10.1007/s00003-021-01346-w. [DOI] [Google Scholar]
- 19.Gu W, Vieira AR, Hoekstra RM, Griffin PM, Cole D. Use of random forest to estimate population attributable fractions from a case-control study of Salmonella enterica serotype Enteritidis infections. Epidemiol Infect. 2015;143:2786–2794. doi: 10.1017/S095026881500014X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alianell AT (2017) Analysis of food exposures in foodborne disease outbreaks. PhD Thesis, University of North Carolina. https://scholarcommons.sc.edu/etd/4096?utm_source=scholarcommons.sc.edu%2Fetd%2F4096&utm_medium=PDF&utm_campaign=PDFCoverPages. Accessed 20 Apr 2024
- 21.Tanui CK, Benefo EO, Karanth S, Pradhan AK. A machine learning model for food source attribution of Listeria monocytogenes. Pathogens. 2022;11:691. doi: 10.3390/pathogens11060691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bian W, Hou H, Chen J, Zhou B, Xia J, Xie S, Liu T. Evaluating the spatial risk of bacterial foodborne diseases using vulnerability assessment and geographically weighted logistic regression. Remote Sensing. 2022;14:3613. doi: 10.3390/rs14153613. [DOI] [Google Scholar]
- 23.Decreto Ministeriale 15 dicembre 1990. Sistema informativo delle malattie infettive e diffusive. Gazzetta Ufficiale 8 gennaio 1991, n. 6.
- 24.GeMInI web-based Database. https://progetto-gemini.it/. Accessed 20 Apr 2024
- 25.Enteric Pathogen Network (EnterNet) Italia Database. https://enternet.iss.it/. Accessed 20 Apr 2024
- 26.Italian National Institute of Statistics (ISTAT) Database. https://www.istat.it/. Accessed 20 Apr 2024
- 27.Horn AL, Friedrich H. Locating the source of large-scale outbreaks of foodborne disease. J R Soc Inferface. 2019;16:20180624. doi: 10.1098/rsif.2018.0624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ethelberg S, Mølbak K, Josefsen MH. Bacteria: Salmonella Non-Typhi. In: Motarjemi Y, Moy G, Todd E, editors. Encyclopedia of Food Safety. London: Academic Press; 2014. [Google Scholar]
- 29.Eikmeier D, Medus C, Smith K. Incubation period for outbreak-associated, non-typhoidal salmonellosis cases, Minnesota, 2000–2015. Epidemiol Infect. 2018;146:423–429. doi: 10.1017/S0950268818000079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Siira L, MacDonald E, Holmbakken GM, Sundar T, Meyer-Myklestad L, Lange H, Brandal LT, Naseer U, Johannessen GS, Bergsjø B, Espenhain L, Vold L, Nygård K. Increasing incubation periods during a prolonged monophasic Salmonella Typhimurium outbreak with environmental contamination of a commercial kitchen at Oslo Airport, Norway, 2017. Euro Surveill. 2019;24:1900207. doi: 10.2807/1560-7917.ES.2019.24.34.1900207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Centers for Disease Prevention and Control, CDC (2022) Timeline for Identifying and reporting illnesses in foodborne outbreaks. https://www.cdc.gov/foodsafety/outbreaks/basics/reporting-timeline.html. Accessed 22 Nov 2022
- 32.Manning CD, Raghavan P, Schütze H. Introduction to information retrieval. Cambridge: Cambridge University Press; 2008. [Google Scholar]
- 33.StataCorp . Stata statistical software: release 17. College Station: StataCorp LLC; 2021. [Google Scholar]
- 34.Cerulli G. Machine learning using stata/python. arXiv. 2021 doi: 10.48550/arXiv.2103.03122. [DOI] [Google Scholar]
- 35.Bergstra J, Bengio Y. Random search for hyper-parameter optimization. J Machine Learn Res. 2012;13:281–305. [Google Scholar]
- 36.van Herten J, Meijboom FLB. Veterinary responsibilities within the one health framework. Food Ethics. 2019;3:109–123. doi: 10.1007/s41055-019-00034-8. [DOI] [Google Scholar]
- 37.Hodges JR, Kimball AM. The global diet: trade and novel infections. Global Health. 2005;1:4. doi: 10.1186/1744-8603-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ferrari RG, Rosario DKA, Cunha-Neto A, Mano SB, Figueiredo EES, Conte-Junior CA. Worldwide epidemiology of Salmonella serovars in animal-based foods: a meta-analysis. Appl Environ Microbiol. 2019;85:e00591–e619. doi: 10.1128/AEM.00591-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang P, Cui W, Wang H, Du Y, Zhou Y. High-efficiency machine learning method for identifying foodborne disease outbreaks and confounding factors. Foodborne Pathog Dis. 2021;18:590–598. doi: 10.1089/fpd.2020.2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bramer M. Avoiding overfitting of decision trees principles of data mining. London: Springer-Verlag, London Ltd; 2016. [Google Scholar]
- 41.Breiman L. Random forests. Mach Learn. 2001;45:5–32. doi: 10.1023/A:1010933404324. [DOI] [Google Scholar]
- 42.Chen T, Guestrin C. XGBoost: a scalable tree boosting system. arXiv. 2016 doi: 10.1145/29396722939785. [DOI] [Google Scholar]
- 43.Jacobs R, Teunis P, van de Kassteele J. Tracing the origin of food-borne disease outbreaks: a network model approach. Epidemiology. 2020;31:327–333. doi: 10.1097/EDE.0000000000001169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Obermeyer Z, Emanuel EJ. Predicting the future - big data, machine learning, and clinical medicine. N Engl J Med. 2016;375:1216–1219. doi: 10.1056/NEJMp1606181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Groseclose SL, Buckeridge DL. Public health surveillance systems: recent advances in their use and evaluation. Annu Rev Public Health. 2017;38:57–79. doi: 10.1146/annurev-publhealth-031816-044348. [DOI] [PubMed] [Google Scholar]
- 46.Quilliam RS, Cross P, Williams AP, Edwards-Jones G, Salmon RL, Rigby D, Chalmers RM, Thomas DR, Jones DL. Subclinical infection and asymptomatic carriage of gastrointestinal zoonoses: occupational exposure, environmental pathways, and the anonymous spread of disease. Epidemiol Infect. 2013;141:2011–2021. doi: 10.1017/S0950268813001131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gibbons CL, Mangen MJ, Plass D, Havelaar AH, Brooke RJ, Kramarz P, Peterson KL, Stuurman AL, Cassini A, Fèvre EM, Kretzschmar ME, Burden of Communicable diseases in Europe (BCoDE) consortium Measuring underreporting and under-ascertainment in infectious disease datasets: a comparison of methods. BMC Public Health. 2014;14:147. doi: 10.1186/1471-2458-14-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Steele L, Orefuwa E, Dickmann P. Drivers of earlier infectious disease outbreak detection: a systematic literature review. Int J Infect Dis. 2016;53:15–20. doi: 10.1016/j.ijid.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 49.Joseph B (2018) Lack of laboratory confirmation to identify food borne diseases. In Proceedings: 10th TEPHINET Americas (2018, Colombia). https://www.tephinet.org/learning/fead/lack-of-laboratory-con%EF%AC%81rmation-to-identify-food-borne-diseases. Accessed 15 Feb 2023
- 50.Scallan E, Hoekstra RM, Angulo FJ, Tauxe RV, Widdowson MA, Roy SL, Jones JL, Griffin PM. Foodborne illness acquired in the United States—major pathogens. Emerg Infect Dis. 2011;17:7–15. doi: 10.3201/eid1701.P11101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grivas G, Lagousi T, Mandilara G. Epidemiological data, serovar distribution and antimicrobial resistance patterns of Salmonella species in children, Greece 2011–2017: a retrospective study. Acta Med Acad. 2020;49:255–264. doi: 10.5644/ama2006-124.315. [DOI] [PubMed] [Google Scholar]
- 52.Gibbons CL, Mangen MJ, Plass D, Havelaar AH, Brooke RJ, Kramarz P, Peterson KL, Stuurman AL, Cassini A, Fèvre EM, Kretzschmar ME. Burden of Communicable diseases in Europe (BCoDE) consortium measuring underreporting and under-ascertainment in infectious disease datasets: a comparison of methods. BMC Public Health. 2014 doi: 10.1186/1471-2458-14-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mellou K, Sideroglou T, Kallimani A, Potamiti-Komi M, Pervanidou D, Lillakou E, Georgakopoulou T, Mandilara G, Lambiri M, Vatopoulos A, Hadjichristodoulou C. Evaluation of underreporting of salmonellosis and shigellosis hospitalised cases in Greece, 2011: results of a capture-recapture study and a hospital registry review. BMC Public Health. 2013;13:875. doi: 10.1186/1471-2458-13-875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Field E, Strathearn M, Boyd-Skinner C, Dyda A. Usefulness of linked data for infectious disease events: a systematic review. Epidemiol Infect. 2023;151:e46. doi: 10.1017/S0950268823000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lorenzen MC, Weiser AA, Pieper R, Lahrssen-Wiederholt M, Numata J. Introducing the rapid alert supply network extractor (RASNEX) tool to mine supply chain information from food and feed contamination notifications in Europe. PLoS ONE. 2021;16:e0254301. doi: 10.1371/journal.pone.0254301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Commission Regulation (EC) No. 2073/2005 of 15 November 2005 on microbiological criteria for foodstuffs. http://data.europa.eu/eli/reg/2005/2073/2020-03-08. Accessed on 7 Sep 2023
- 57.Institute of Medicine (US) (2006) Forum on Microbial Threats. In: Chapter 5: Surveillance of the food supply. Addressing foodborne threats to health: policies, practices, and global coordination: Workshop summary. National Academies Press (US), Washington (DC), United States of America. Available online: https://www.ncbi.nlm.nih.gov/books/NBK57083. Accessed on 4 Sep 2023 [PubMed]
- 58.Sorbo A, Pucci E, Nobili C, Taglieri A, Passeri D, Zoani C. Food safety assessment: overview of metrological issues and regulatory aspects in the European Union. Separations. 2022;9:53. doi: 10.3390/separations9020053. [DOI] [Google Scholar]
- 59.Markus AF, Kors JA, Rijnbeek PR. The role of explainability in creating trustworthy artificial intelligence for health care: a comprehensive survey of the terminology, design choices, and evaluation strategies. J Biomed Inform. 2021;113:103655. doi: 10.1016/j.jbi.2020.103655. [DOI] [PubMed] [Google Scholar]
- 60.Dórea FC, Revie CW. Data-driven surveillance: effective collection, integration, and interpretation of data to support decision making. Front Vet Sci. 2021;8:789696. doi: 10.3389/fvets.2021.633977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bansal S, Chowell G, Simonsen L, Vespignani A, Viboud C. Big data for infectious disease surveillance and modeling. J Infect Dis. 2016;214:S375–S379. doi: 10.1093/infdis/jiw400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ajiboye AR, Abdullah-Arshah R, Qin H, Isah-Kebbe H (2015) Evaluating the effect of dataset size on predictive model using supervised learning technique. IJSECS 1:75–84. 10.15282/ijsecs.1.2015.6.0006
- 63.Althnian A, AlSaeed D, Al-Baity H, Samha A, Dris AB, Alzakari N, Abou Elwafa A, Kurdi H. Impact of dataset size on classification performance: an empirical evaluation in the medical domain. Appl Sci. 2021;11:796. doi: 10.3390/app11020796. [DOI] [Google Scholar]
- 64.Morris DH, Gostic KM, Pompei S, Bedford T, Łuksza M, Neher RA, Grenfell BT, Lässig M, McCauley JW. Predictive modeling of influenza shows the promise of applied evolutionary biology. Trends Microbiol. 2018;26:102–118. doi: 10.1016/j.tim.2017.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rajkomar A, Oren E, Chen K, Dai AM, Hajaj N, Hardt M, Liu PJ, Liu X, Marcus J, Sun M, Sundberg P, Yee H, Zhang K, Zhang Y, Flores G, Duggan GE, Irvine J, Le Q, Litsch K, Mossin A, Tansuwan J, Wang D, Wexler J, Wilson J, Ludwig D, Volchenboum SL, Chou K, Pearson M, Madabushi S, Shah NH, Butte AJ, Howell MD, Cui C, Corrado GS, Dean J. Scalable and accurate deep learning with electronic health records. NPJ Digit Med. 2018;1:18. doi: 10.1038/s41746-018-0029-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Razzak MI, Imran M, Xu G. Big data analytics for preventive medicine. Neural Comput Appl. 2020;32:4417–4451. doi: 10.1007/s00521-019-04095-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Management and processing of food safety surveillance data. Flowchart illustrating the retrieval and processing of food safety surveillance data from the SIGLA database, the electronic system of the Istituto Zooprofilattico Sperimentale del Piemonte, Ligura e Valle d’Aosta.
Additional file 2. Codes used to perform the analyses presented in the current manuscript and their outputs.
Additional file 3. Geographical distribution of food surveillance activity in the Piedmont region in 2019. Figure illustrating municipalities that were subjected to food surveillance in 2019.
Additional file 4. Data supporting the results and conclusions of the present manuscript. Details on the datasets provided are explained in Additional file 2.
Data Availability Statement
The raw data were generated by the Servizio di riferimento Regionale di Epidemiologia per la sorveglianza, la prevenzione e il controllo delle Malattie Infettive (SeREMI) and Istituto Zooprofilattico Sperimentale del Piemonte, Liguria e Valle d’Aosta (IZSPLVA). Access to these data is limited due to licensing agreements for the current study, and these data are not publicly available. The derived data and code scripts on which the conclusions of this study are based are openly available in the Additional files.