Abstract
Purpose of review
Bladder cancer incidence is on the rise, and until recently, there has been little to no change in treatment regimens over the last 40 years. Hence, it is imperative to work on strategies and approaches to untangle the complexity of intra- and inter-tumour heterogeneity of bladder cancer with the aim of improving patient-specific care and treatment outcomes. The focus of this review is therefore to highlight novel targets, advances, and therapy approaches for bladder cancer patients.
Recent findings
The success of combining an antibody-drug conjugate (ADC) with immunotherapy has been recently hailed as a game changer in treating bladder cancer patients. Hence, interest in other ADCs as a treatment option is also rife. Furthermore, strategies to overcome chemoresistance to standard therapy have been described recently. In addition, other studies showed that targeting genomic alterations (e.g. mutations in FGFR3, DNA damage repair genes and loss of the Y chromosome) could also be helpful as prognostic and treatment stratification biomarkers. The use of single-cell RNA sequencing approaches has allowed better characterisation of the tumour microenvironment and subsequent identification of novel targets. Functional precision medicine could be another avenue to improve and guide personalized treatment options.
Summary
Several novel preclinical targets and treatment options have been described recently. The validation of these advances will lead to the development and implementation of robust personalized treatment regimens for bladder cancer patients.
Keywords: antibody-drug conjugate, bladder cancer, chemoresistance, precision medicine, tumour microenvironment
INTRODUCTION
The standard of care for locally progressed or metastatic muscle invasive bladder cancer (MIBC) patients has predominantly been cisplatin-based neoadjuvant chemotherapy (NAC) followed by radical cystectomy [1]. In recent years, immune checkpoint inhibitors (ICIs) directed at programmed cell-death protein 1 (PD-1) or its ligand (PD-L1) have become available in the clinics as second line or maintenance therapy [2,3]. Recently, enfortumab vedotin, a Nectin-4-targeted antibody-drug conjugate (ADC) coupled to the microtubule disrupting compound auristatin E, has been approved by the FDA (2019) and EMA (2023) up to the third-line treatment after platinum and ICI therapy [1]. More recently, a current clinical trial has shown therapeutic superiority over cisplatin-based neoadjuvant chemotherapy for the first-line administration of the combination of an ICI (pembrolizumab) with enfortumab vedotin, which has been granted breakthrough designation by the FDA for the treatment of metastatic bladder cancer and may significantly change therapy sequence of bladder cancer in the future [4▪▪].
Box 1.
no caption available
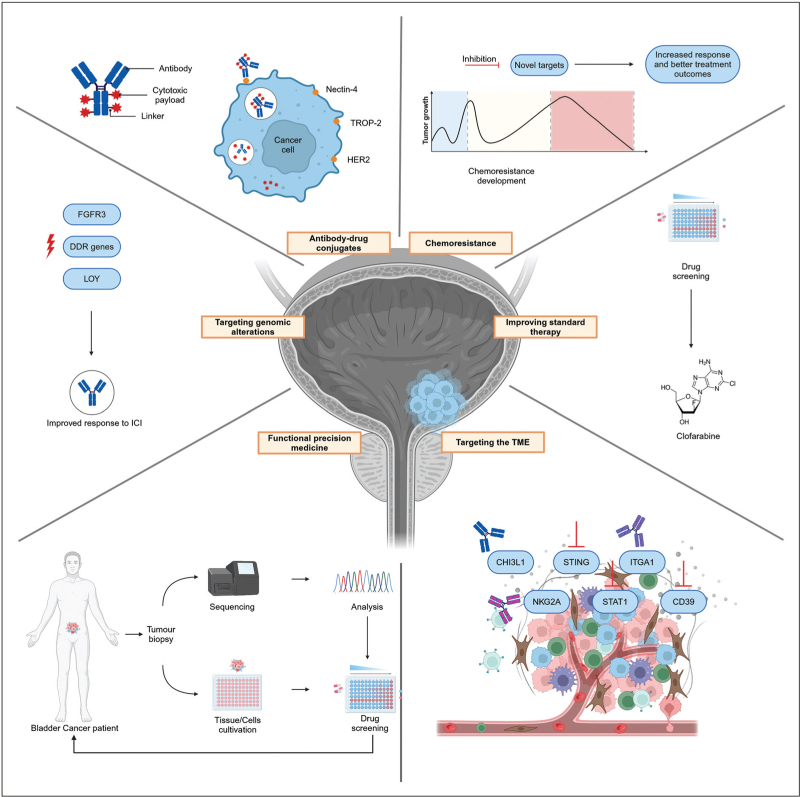
However, in spite of all innovations, treatment of patients across diverse stages of bladder cancer is often still palliative and not all patients experience (long-term) benefit from therapy. Furthermore, apart from the fact that some molecular patient stratification correlates with therapeutic value, including FGFR3 mutation status [2,3], there is still insufficient evidence for robust prognostic or predictive molecular markers in bladder cancer [1]. Histopathological substaging of bladder cancer tissue, after transurethral resection of bladder tumour (TURBT), still mainly dictates treatment regimens [1]. The aim of this review is therefore to evaluate and present recent key advances in preclinical and clinical studies of therapeutic targets and treatment options for bladder cancer (Fig. 1).
FIGURE 1.
Graphical overview of recent advances in bladder cancer precision medicine.
OVERCOMING CHEMORESISTANCE AND IMPROVEMENT OF STANDARD THERAPY
Despite the recent success of ICI and ADC strategies that will likely shift therapy lines in the near future, chemotherapy is expected to remain a pillar in bladder cancer management. However, many patients fail to respond to standard chemotherapy (Gemcitabine and cisplatin), due to the development of chemoresistance. Several drug resistance mechanisms have been described before [5], including a genome-wide CRISPR screen demonstrating the involvement of the heterogeneous nuclear ribonucleoprotein U (HNRNPU) in cisplatin resistance, in a panel of established bladder cancer cell lines. Mechanistically, HNRNPU expression correlates with cisplatin resistance by regulating the chromatin structure and the transcription of DNA-damage repair genes (DDR). Inhibition of HNRNPU could be a promising target to restore cisplatin sensitivity in advanced MIBC patients [6▪]. A similar finding was reported about the transcription factor ZBTB11. Targeting ZBTB11 increased the sensitivity of bladder cell lines to cisplatin by inhibiting the transcription of the RNA helicase DDX1, leading to the accumulation of R-loops and subsequent cell death [7].
Another study focused more on the plasticity of cancer cells during the course of resistance acquisition [8▪▪]. Chemoresistance to gemcitabine and cisplatin treatment leads to a partial squamous differentiation in murine and human MIBC. A multiomics approach revealed that the lysosomal cysteine proteinase cathepsin H (CTSH) is responsible for this phenotype. Pharmacological blockage of CTSH with the proteinase inhibitor E64 induces full squamous differentiation and cell death via pyroptosis. Mechanistically, this proposed therapy depends on the activation of the tumour necrosis factor pathway [8▪▪]. This concept of inducing differentiation in cancer cells could be an encouraging new way of treating chemoresistant MIBC.
A different approach was used by Ertl et al.[9▪]. A high-throughput drug screen containing over 1700 compounds identified the antimetabolite drug clofarabine as a potent antineoplastic compound in a panel of commercially available bladder cancer cell lines. In addition, in patient-derived xenograft (PDX) models, clofarabine also showed high antitumour activity [9▪]. Clofarabine and gemcitabine are both antimetabolite drugs with similar, but not identical way of action. The former is a purine and the latter a pyrimidine-analogue. Although direct comparison of the two drugs revealed a slightly enhanced activity of gemcitabine in vitro, PDX models showed the superiority of clofarabine over gemcitabine in vivo, by displaying higher anticancer efficacy and less toxic side effects (M. Gutmann, I. Ertl, et al., unpublished data). Therefore, this finding has the potential to transform standard therapy for MIBC patients.
TARGETING GENOMIC ALTERATIONS
Bladder cancer finds itself among the most mutated human cancer types and major genomic alterations have been identified [2]. Further, MIBC has also been categorized into six distinctive molecular subtypes based on molecular profiling analysis and identified signatures [10]. Despite these excessive research efforts, the molecular subtypes of bladder cancer and known genomic alterations are still barely used to guide treatment regimens [3].
It is well known that genes participating in DDR pathways and somatic alterations present in these genes correlate with better pathologic response to platinum compounds interfering with DNA replication and integrity. Deletion in the ERCC2, RB1, ATM and FANCC genes has been reported to correlate with patient response to cisplatin-based therapy, including NAC [11]. Other studies focused predominately on the role of the Ataxia-telangiectasia mutated (ATM) gene since it has an essential role in persevering genomic integrity and in the cellular response to DNA damage [12]. ATM loss increases the sensitivity of preclinical bladder cancer models to DNA repair-targeting agents, especially to ATR and PARP inhibitors. Moreover, the loss of ATM expression reshapes the tumour microenvironment (TME), conferring the sensitivity of the preclinical models to ICI therapy, while surprisingly having no effect in clinical cohorts [13▪]. In addition, Gil-Jimenez et al.[14▪] showed that only patients harbouring mutations in ERCC2 had a better response to NAC, even though a positive association with recurrence-free or overall survival (OS) was not found. Thus, genetic alterations in DDR pathways can still not be used as a prognostic marker of the response rate of bladder cancer patients to chemotherapy.
Fibroblast growth factor receptor 3 (FGFR3) is often hyperactivated by point mutations and amplified in MIBC [2]. The phase III clinical trial THOR revealed that erdafitinib, a pan-FGFR inhibitor, significantly improved progression-free survival (PFS), and OS in metastatic urothelial carcinoma patients with FGFR perturbations previously treated with ICI therapy [15]. Erdafitinib has been FDA-approved since 2019 for treating metastatic urothelial carcinoma and since January 2024 for treating locally advanced and/or metastatic urothelial carcinoma with FGFR3 alterations even without previous ICI therapy [16]. Furthermore, in their recent study, Jin et al.[17▪▪] revealed a potential mechanism underlying the synergistic effect of ICI and erdafitinib that suggests a rationale for an immuno-targeted combination treatment for FGFR3-mutated bladder cancer: erdafitinib downregulates the expression of E3-ubiquitin ligase NEDD4, preventing it from targeting PD-L1 for ubiquitination. Furthermore, another study in a murine model of bladder cancer investigated cooperativity between ICI and FGFR inhibition. The results indicate a synergism where the FGFR inhibition with erdafitinib possibly reverses anti-PD-1 induced immunosuppression by abolishing Treg expansion [18].
Another major genomic alteration found in bladder cancer is the loss of the Y chromosome (LOY) [19]. LOY is connected to ageing and has been reported in various health conditions, including bladder cancer, where the loss rate varies between 10 and 40% and correlates with poor prognosis [20]. It was shown that Y- tumours are more aggressive than their Y+ counterparts in vivo in immune-competent hosts due to their ability to evade adaptive immunity by inducing CD8+ T cell exhaustion in the TME. This phenotype is at least partly driven by the loss of the Y chromosome-encoded genes UTY and KDM5D. Patients with LOY have a better response to anti-PD1-targeted ICI, since it drives T cell differentiation from exhaustion to effector function [21▪▪]. This study proposed the use of LOY as a potential biomarker for the use of ICI in bladder cancer.
TARGETING THE TUMOUR MICROENVIRONMENT
The TME consists of a heterogeneous population of cancer, stromal and immune cells. The composition and the interplay of the TME can influence disease progression and therapy response, especially in the context of ICI therapy [22]. Furthermore, the broader availability of single-cell RNA sequencing (scRNA-seq) technologies has contributed to a better characterization of the TME in bladder cancer [23].
The importance of the TME composition was illustrated in a study describing overexpression of CD39 in bladder cancer. This ectonucleoside triphosphate diphosphohydrolase-1 converts extracellular ATP and ADP to immunosuppressive extracellular adenosine. Pharmacological inhibition of CD39 was able to reduce the growth of tumour cells in a murine orthotopic bladder cancer model. Furthermore, CD39 inhibition significantly increased the abundance of immune cells in the TME. Depletion of specific immune subpopulations showed that this effect depends on natural killer (NK) cells and conventional type 1 dendritic cells. Moreover, CD39 inhibition had a synergistic effect with cisplatin in vivo, suggesting the potential use of CD39 as a target in bladder cancer [24▪].
In addition, there is also increased research interest beyond the classical ICI targets (PD-1, PD-1L and CTLA-4) [25]. NKG2A (together with its co-receptor CD94) acts as an inhibitory receptor on NK and CD8+ T cells when it binds to its ligand HLA-E (a nonclassical MHC class I molecule) [26]. Surprisingly, high NKG2A expression levels correlated with better survival and response to PD-L1 blockage in MIBC patients. Mechanistically, NKG2A+ PD1+ CD8+ T cells use T cell receptor-independent innate-like function to exert antitumour activity. Nevertheless, in the context of high HLA-E expression in bladder cancer, pharmacological blockage of NKG2A enhances the cytotoxicity of T cells. This discovery could potentially also transform ICI therapy, by targeting the NKG2A/HLA-E interaction as an alternative immune checkpoint axis [27▪].
Cancer-associated fibroblasts (CAFs) are another important cell population in the TME of solid tumours. These highly heterogeneous stromal cells can play a tumour promoting or suppressing role in cancer [28]. A scRNA-seq analysis of bladder cancer from treatment-naive patients identified a novel subtype of CAFs in bladder cancer [29▪]. This subpopulation was characterized by expression of the urea transporter SLC14A1 and upregulation of interferon responsive genes. Therefore, this subpopulation was named interferon-regulated CAF (irCAF). The abundance of irCAFs correlated with poor prognosis and chemoresistance of bladder cancer patients. Furthermore, they also contributed to enhanced cancer stemness by releasing WNT5A to influence β-Catenin signalling in tumour cells in a paracrine way. On the other hand, chemotherapy can activate the cGAS-STING pathway that leads to the production of interferon and subsequently influences irCAF differentiation through STAT1. Thus, this finding supports the rationale to use STING or STAT1 inhibitors to improve response rates to chemotherapy in bladder cancer [29▪].
Another previously unknown CAF subset has been identified recently [30▪▪]. PDGFRa+ ITGA11+ CAFs play a major role in early-stage bladder cancer, by aiding lymphovascular invasion and subsequent lymph node metastasis. Spatial transcriptomics revealed that PDGFRa+ ITGA11+ CAFs enhance extravasation of bladder cancer cells by binding to E-selectin (SELE) expressed on lymphatic endothelial cells. The release of the cytokine CHI3L1, which causes extracellular matrix remodelling, further enhances this phenotype. mAbs targeting ITGA11 and/or CHI3L1 significantly reduced lymphovascular invasion in a carcinogen-induced early-stage bladder cancer mouse model and a PDX model. Therefore, targeting PDGFRa+ ITGA11+ CAFs in early-stage bladder cancer patient could be an effective treatment option to reduce lymph node metastasis [30▪▪].
ANTIBODY-DRUG CONJUGATES
Many cytotoxic drugs cannot be used as chemotherapeutic agents in the clinics, because of their systemic side effects. The discovery of tumour-specific antigens led to the development of ADCs. This novel treatment approach uses mAbs coupled with a cytotoxic payload to specifically target and kill cancer cells [31]. As already mentioned above, approval of enfortumab vedotin with the anti-PD1 ICI pembrolizumab has the potential to change the standard of care for bladder cancer patients [4▪▪]. The success of this novel combination therapy might also partially be explained by the notion that Nectin-4 is a ligand of the inhibitory TIGIT receptor (T cell immunoreceptor with Ig and ITIM domains), expressed on most immune cells. Preclinical studies have shown that inhibitory Nectin-4 antibodies enhance tumour cell killing by blocking Nectin-4 TIGIT interactions NK cells [32]. The role of this novel immune checkpoint axis in bladder cancer has not been investigated yet.
Another ADC that was approved for bladder cancer patients is sacituzumab govitecan [33]. This ADC consists of an antibody targeting the transmembrane calcium signal transducer protein TROP-2, coupled with several molecules of SN-38, a topoisomerase-1 inhibitor. Several clinical trials are currently investigating the efficacy of using sacituzumab govitecan as a single agent or in combination with ICI [34]. Furthermore, a phase III trial to test the efficacy of sacituzumab govitecan as a single agent is ongoing [35]. Datopotamab deruxtecan (Dato-DXd) is an additional ADC, consisting of an antibody targeting TROP-2 and the topoisomerase-1 inhibitor deruxtecan, which showed potent antitumour activity in preclinical models of solid tumours [36]. Preliminary results from a phase 1 study demonstrated the tolerability, safety and encouraging efficacy of Dato-DXd in advanced or metastatic urothelial cancer patients [37]. Human epidermal growth factor 2 (HER2) is also overexpressed in a significant amount of MIBC patients [38]. Different ADCs targeting HER2 (disitamab vedotin, trastuzumab deruxtecan and trastuzumab emtasine) coupled with distinct cytotoxic compounds are also currently being investigated in clinical trials for their use in bladder cancer [34].
Despite the success rate of ADCs in the clinics, there is still some ambiguity about the use of ADC targets as prognostic biomarkers. One clinical trial detected uniformly high expression levels of Nectin-4 in a cohort of metastatic bladder cancer patients [39]. On the other hand, the recent analysis of Nectin-4 and TROP-2 protein expression in an advanced urothelial bladder cancer cohort showed that both ADC targets are widely detected via IHC, but their expression decreases in more aggressive subtypes (e.g. neuroendocrine-like MIBC) [40▪]. Furthermore, Nectin-4 protein expression was significantly lower in metastatic tumour samples compared to their patient-matched primary tumours. Downregulation of Nectin-4 in the metastatic tissue also correlated with a shortened PFS of metastatic patients in a multicentre enfortumab vedotin treated cohort [41▪]. It would therefore be beneficial to stratify patients according to their expression levels of target proteins before initiating ADC therapy. However, the identification and validation of robust additional biomarkers is required to predict responsiveness of ADCs beyond expression levels of target surface markers, especially in the context of metastatic disease where tissue material is usually scarce. Moreover, there will also be the clinical need to understand unresponsiveness to ADC treatments and to investigate potential resistance mechanisms.
FUNCTIONAL PRECISION MEDICINE
The lack of prognostic or predictive molecular markers is the prime cause of treatment failure in bladder cancer patients. Inter-patient heterogeneity further exacerbates this problem. Highly personalized drug screens, also known as functional precision medicine, could be an avenue to improve response rates to cancer treatments [42]. In hematologic cancers, functional precision medicine has already been shown to provide clinical benefit to patients [43]. Hence, efforts have been made to implement this approach in the field of bladder cancer, especially in the light of frequent poor fitness rendering patients ineligible for platinum-based chemotherapy. Patient-derived organoids that preserve molecular and phenotypic features of the primary tumour have been screened to determine their sensitivity to a panel of drugs. The various response rates reinforced the notion of bladder cancer as a very heterogeneous disease [44▪]. A similar study used organoids from sarcomatoid urothelial bladder cancer patients to identify personalized vulnerabilities in this aggressive form of bladder cancer. Again, this organoid model resembled molecular and phenotypic characteristics of the primary tumour. A high-throughput screen with 1567 compounds revealed high sensitivity of these organoids against glucocorticoids, compared to organoids from other subtypes of bladder cancer [45]. Unfortunately, both studies reported potential vulnerabilities just retrospectively and were not designed to use these results in the treatment of the corresponding bladder cancer patients. Further, despite technological advances, screening of patient-derived organoids is still expensive and requires trained personnel and equipment. In addition, difficulties in cultivating cancer cells with their TME ex vivo is also an obstacle to implement this approach as standard of care in the clinics for solid tumours. Nevertheless, functional precision therapy could be an avenue to guide treatment options in advanced and metastatic bladder cancer in the future.
CONCLUSION
The recent presentation of promising preliminary data from a phase III clinical trial combining enfortumab vedotin with pembrolizumab [4▪▪] caused a buzz in the field of uro-oncology. This finding will undoubtedly transform the management of advanced and metastatic bladder cancer. Nevertheless, further research will be needed to assess limitations (e.g. development of resistance) and to identify patients that will benefit most from this novel treatment approach. Further, the search for prognostic or predictive biomarkers in bladder cancer patients will continue. Technological advances in scRNA-seq [23] and also spatial transcriptomics [46] will aid in the identification and validation of molecular markers. In addition, advancements in detecting circulating and urinary tumour DNA in liquid biopsies (plasma and urine) will further improve the care, diagnosis and monitoring of bladder cancer patients [47]. These recent advances, together with the future development of fast and cost-effective drug screening methods [42], will hopefully lead to the generation of robust guidelines for personalized treatment options.
Acknowledgements
None.
Financial support and sponsorship
None.
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.Alfred Witjes J, Max Bruins H, Carrión A, et al. European Association of Urology Guidelines on muscle-invasive and metastatic bladder cancer: summary of the 2023 guidelines. Eur Urol 2024; 85:17–31. [DOI] [PubMed] [Google Scholar]
- 2.Tran L, Xiao J-F, Agarwal N, et al. Advances in bladder cancer biology and therapy. Nat Rev Cancer 2021; 21:104–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dyrskjøt L, Hansel DE, Efstathiou JA, et al. Bladder cancer. Nat Rev Dis Primers 2023; 9:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4▪▪.Powles TB, Valderrama BP, Gupta S, et al. LBA6 EV-302/KEYNOTE-A39: open-label, randomized phase III study of enfortumab vedotin in combination with pembrolizumab (EV+P) vs chemotherapy (Chemo) in previously untreated locally advanced metastatic urothelial carcinoma (la/mUC). Ann Oncol 2023; 34:S1340. [Google Scholar]; This is the first clinical trial demonstrating that EV+P combination almost doubles OS in previously untreated locally advanced metastatic urothelial carcinoma (la/mUC) patients compared to chemotherapy. EV+P could become the new standard of care in 1L treatment of la/mUC.
- 5.Vasan N, Baselga J, Hyman DM. A view on drug resistance in cancer. Nature 2019; 575:299–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6▪.Shi ZD, Hao L, Han XX, et al. Targeting HNRNPU to overcome cisplatin resistance in bladder cancer. Mol Cancer 2022; 21:37. [DOI] [PMC free article] [PubMed] [Google Scholar]; In this study, a CRISPR-screen showed that HNRNPU expression plays a role in cisplatin resistance of bladder cancer cell lines and that this finding could be a potential target.
- 7.Chen L, Liu Z, Tang H, et al. Knockdown of ZBTB11 impedes R-loop elimination and increases the sensitivity to cisplatin by inhibiting DDX1 transcription in bladder cancer. Cell Prolif 2022; 55:e13325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8▪▪.Wang M, Chen X, Tan P, et al. Acquired semi-squamatization during chemotherapy suggests differentiation as a therapeutic strategy for bladder cancer. Cancer Cell 2022; 40:1044–1059. e8. [DOI] [PubMed] [Google Scholar]; This is the first study demonstrating that chemoresistance is driven by a semi-squamatization phenotype in MIBC and that induction of differentiation via targeting CTSH is a potential treatment strategy.
- 9▪.Ertl IE, Lemberger U, Ilijazi D, et al. Molecular and pharmacological bladder cancer therapy screening: discovery of clofarabine as a highly active compound. Eur Urol 2022; 82:261–270. [DOI] [PubMed] [Google Scholar]; This study revealed that the antimetabolite drug clofarabine could be repurposed as a treatment option for bladder cancer patients.
- 10.Kamoun A, de Reyniès A, Allory Y, et al. A consensus molecular classification of muscle-invasive bladder cancer. Eur Urol 2020; 77:420–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Teo MY, Bambury RM, Zabor EC, et al. DNA damage response and repair gene alterations are associated with improved survival in patients with platinum-treated advanced urothelial carcinoma. Clin Cancer Res 2017; 23:3610–3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shiloh Y, Ziv Y. The ATM protein kinase: regulating the cellular response to genotoxic stress, and more. Nat Rev Mol Cell Biol 2013; 14:197–210. [PubMed] [Google Scholar]
- 13▪.Zhou Y, Börcsök J, Adib E, et al. ATM deficiency confers specific therapeutic vulnerabilities in bladder cancer. Sci Adv 2023; 9:eadg2263. [DOI] [PMC free article] [PubMed] [Google Scholar]; In this paper the authors demonstrated that ATM deficiency makes bladder cancer cells more susceptible to DNA-damaging agents and DNA repair-targeted agents. ATM deficiency could be used as a predictive biomarker in bladder cancer.
- 14▪.Gil-Jimenez A, van Dorp J, Contreras-Sanz A, et al. Assessment of predictive genomic biomarkers for response to cisplatin-based neoadjuvant chemotherapy in bladder cancer. Eur Urol 2023; 83:313–317. [DOI] [PubMed] [Google Scholar]; This multicentre retrospective cohort study revealed that only deleterious mutations in ERCC2 are associated with pathological response to NAC, but not with OS. The use of other genomic alterations as biomarkers, tested in this study, have been dismissed by the authors.
- 15.Loriot Y, Matsubara N, Park SH, et al. Phase 3 THOR study: results of erdafitinib (erda) versus chemotherapy (chemo) in patients (pts) with advanced or metastatic urothelial cancer (mUC) with select fibroblast growth factor receptor alterations (FGFRalt). JCO 2023; 41:LBA4619. [Google Scholar]
- 16. Research C for DE and: FDA approves erdafitinib for locally advanced or metastatic urothelial carcinoma. FDA 2024. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-erdafitinib-locally-advanced-or-metastatic-urothelial-carcinoma. [Google Scholar]
- 17▪▪.Jing W, Wang G, Cui Z, et al. FGFR3 destabilizes PD-L1 via NEDD4 to control T-cell–mediated bladder cancer immune surveillance. Cancer Res 2022; 82:114–129. [DOI] [PubMed] [Google Scholar]; This study describes the molecular background and the mechanistic rationale for combining ICI with targeted therapy in FGFR3-activated bladder cancer.
- 18.Okato A, Utsumi T, Ranieri M, et al. FGFR inhibition augments anti–PD-1 efficacy in murine FGFR3-mutant bladder cancer by abrogating immunosuppression. J Clin Invest 2024; 134:e169241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sauter G, Moch H, Wagner U, et al. Y chromosome loss detected by FISH in bladder cancer. Cancer Genet Cytogenet 1995; 82:163–169. [DOI] [PubMed] [Google Scholar]
- 20.Cáceres A, Jene A, Esko T, et al. Extreme downregulation of chromosome Y and cancer risk in men. J Natl Cancer Inst 2020; 112:913–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21▪▪.Abdel-Hafiz HA, Schafer JM, Chen X, et al. Y chromosome loss in cancer drives growth by evasion of adaptive immunity. Nature 2023; 619:624–631. [DOI] [PMC free article] [PubMed] [Google Scholar]; This is the first study describing the molecular implications of the loss of Y chromosome (LOY) in bladder cancer. The data suggest that LOY alters the TME and is a potential predictive biomarker for effective ICI therapy.
- 22.van Dorp J, van der Heijden MS. The bladder cancer immune micro-environment in the context of response to immune checkpoint inhibition. Front Immunol 2023; 14:1235884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lyu T, Lin Y, Wu K, et al. Single-cell sequencing technologies in bladder cancer research: applications and challenges. Front Genet 2022; 13:1027909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24▪.Liu L, Hou Y, Deng C, et al. Single cell sequencing reveals that CD39 inhibition mediates changes to the tumor microenvironment. Nat Commun 2022; 13:6740. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study describes the molecular rationale to use CD39 inhibitors in bladder cancer patients.
- 25.Borgeaud M, Sandoval J, Obeid M, et al. Novel targets for immune-checkpoint inhibition in cancer. Cancer Treat Rev 2023; 120:102614. [DOI] [PubMed] [Google Scholar]
- 26.Borst L, van der Burg SH, van Hall T. The NKG2A–HLA-E axis as a novel checkpoint in the tumor microenvironment. Clin Cancer Res 2020; 26:5549–5556. [DOI] [PubMed] [Google Scholar]
- 27▪.Salomé B, Sfakianos JP, Ranti D, et al. NKG2A and HLA-E define an alternative immune checkpoint axis in bladder cancer. Cancer Cell 2022; 40:1027–1043. e9. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study provides a framework for future clinical trials to target the NKG2A and HLA-E immune checkpoint axis in bladder cancer, especially in the context of high HLA-E expressing tumours.
- 28.Chhabra Y, Weeraratna AT. Fibroblasts in cancer: unity in heterogeneity. Cell 2023; 186:1580–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29▪.Ma Z, Li X, Mao Y, et al. Interferon-dependent SLC14A1+ cancer-associated fibroblasts promote cancer stemness via WNT5A in bladder cancer. Cancer Cell 2022; 40:1550–1565. e7. [DOI] [PubMed] [Google Scholar]; The mechanistic investigation of a novel subpopulation of CAFs described in this study provides a rationale to use STING or STAT1 inhibitors to overcome chemoresistance in bladder cancer.
- 30▪▪.Zheng H, An M, Luo Y, et al. PDGFRα+ITGA11+ fibroblasts foster early-stage cancer lymphovascular invasion and lymphatic metastasis via ITGA11-SELE interplay. Cancer Cell 2024; 10.1016/j.ccell.2024.02.002 [DOI] [PubMed] [Google Scholar]; This study suggests to inhibit a specific subpopulation of CAFs in early bladder cancer to block lymphovascular invasion and lymphatic metastasis.
- 31.Dumontet C, Reichert JM, Senter PD, et al. Antibody–drug conjugates come of age in oncology. Nat Rev Drug Discov 2023; 22:641–661. [DOI] [PubMed] [Google Scholar]
- 32.Reches A, Ophir Y, Stein N, et al. Nectin4 is a novel TIGIT ligand which combines checkpoint inhibition and tumor specificity. J Immunother Cancer 2020; 8:e000266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Research C for DE and: FDA grants accelerated approval to sacituzumab govitecan for advanced urothelial cancer. FDA 2021. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-sacituzumab-govitecan-advanced-urothelial-cancer. [Google Scholar]
- 34.Thomas J, Sun M, Getz T, et al. Antibody-drug conjugates for urothelial carcinoma. Urol Oncol 2023; 41:420–428. [DOI] [PubMed] [Google Scholar]
- 35. Gilead Sciences. A randomized open-label Phase III study of sacituzumab Govitecan versus treatment of physician's choice in subjects with metastatic or locally advanced unresectable urothelial cancer. clinicaltrials.gov; 2024. https://clinicaltrials.gov/study/NCT04527991?id=NCT04527991&rank=1. [Google Scholar]
- 36.Okajima D, Yasuda S, Maejima T, et al. Datopotamab deruxtecan, a novel TROP2-directed antibody–drug conjugate, demonstrates potent antitumor activity by efficient drug delivery to tumor cells. Mol Cancer Ther 2021; 20:2329–2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lisberg A, Drakaki A, Meric-Bernstam F, et al. Datopotamab deruxtecan in locally advanced/metastatic urothelial cancer: preliminary results from the phase 1 TROPION-PanTumor01 study. JCO 2024; 42:603–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krüger S, Weitsch G, Büttner H, et al. HER2 overexpression in muscle-invasive urothelial carcinoma of the bladder: prognostic implications. Int J Cancer 2002; 102:514–518. [DOI] [PubMed] [Google Scholar]
- 39.Rosenberg JE, O’Donnell PH, Balar AV, et al. Pivotal trial of enfortumab vedotin in urothelial carcinoma after platinum and anti-programmed death 1/programmed death ligand 1 therapy. J Clin Oncol 2019; 37:2592–2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40▪.Bahlinger V, Branz A, Strissel PL, et al. Associations of TACSTD2/TROP2 and NECTIN-4/NECTIN-4 with molecular subtypes, PD-L1 expression, and FGFR3 mutational status in two advanced urothelial bladder cancer cohorts. Histopathology 2024; 84:863–876. [DOI] [PubMed] [Google Scholar]; This study shows that NECTIN-4 and TROP-2 expression decreases in aggressive bladder cancer subtypes. The use of clinically approved ADCs, targeting these proteins, might be limited in these subtypes.
- 41▪.Klümper N, Ralser DJ, Ellinger J, et al. Membranous NECTIN-4 expression frequently decreases during metastatic spread of urothelial carcinoma and is associated with enfortumab vedotin resistance. Clin Cancer Res 2023; 29:1496–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study revealed the decreased expression of Nectin-4 in metastatic lesions of bladder cancer patients. The authors suggest to stratify patient according to Nectin-4 status before enfortumab vedotin treatment.
- 42.Dolgin E. The future of precision cancer therapy might be to try everything. Nature 2024; 626:470–473. [DOI] [PubMed] [Google Scholar]
- 43.Kornauth C, Pemovska T, Vladimer GI, et al. Functional precision medicine provides clinical benefit in advanced aggressive hematologic cancers and identifies exceptional responders. Cancer Discov 2022; 12:372–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44▪.Minoli M, Cantore T, Hanhart D, et al. Bladder cancer organoids as a functional system to model different disease stages and therapy response. Nat Commun 2023; 14:2214. [DOI] [PMC free article] [PubMed] [Google Scholar]; In this proof-of-principle study, the authors confirmed the potential to use patient derived primary bladder cancer organoids for functional precision therapy.
- 45.Garioni M, Tschan VJ, Blukacz L, et al. Patient-derived organoids identify tailored therapeutic options and determinants of plasticity in sarcomatoid urothelial bladder cancer. NPJ Precis Oncol 2023; 7:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tian L, Chen F, Macosko EZ. The expanding vistas of spatial transcriptomics. Nat Biotechnol 2023; 41:773–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rose KM, Huelster HL, Meeks JJ, et al. Circulating and urinary tumour DNA in urothelial carcinoma — upper tract, lower tract and metastatic disease. Nat Rev Urol 2023; 20:406–419. [DOI] [PubMed] [Google Scholar]