Abstract
Background
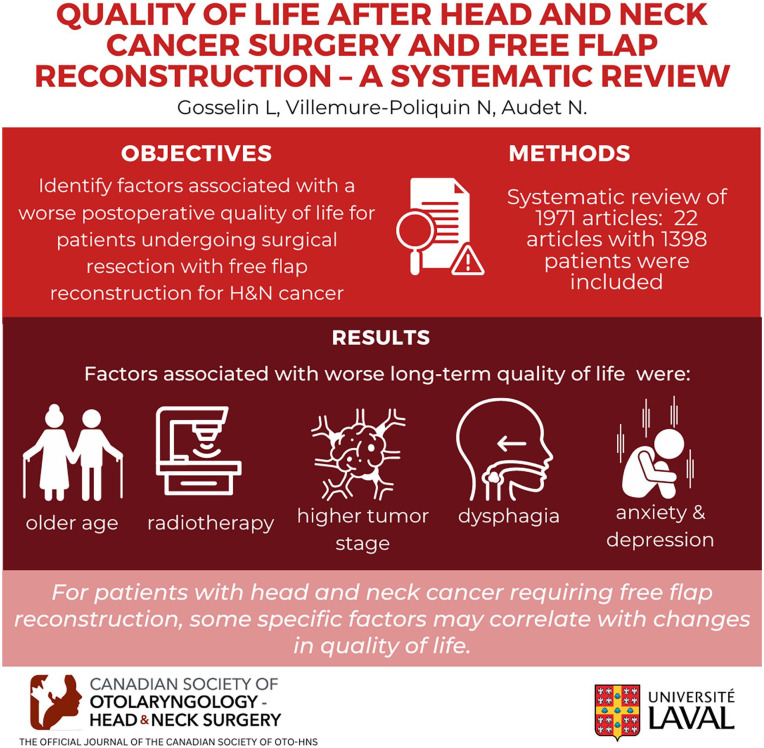
Different factors can affect the quality of life of patients treated for head and neck cancer undergoing major surgical intervention. However, it remains unclear which specific factors and what possible interventions could have the greatest influence on quality of life postoperatively for patients undergoing surgical resection with free flap reconstruction. The objective of our systematic review was to identify which factors, at the time of surgical treatment, are associated with a worse postoperative quality of life for patients undergoing surgical resection with free flap reconstruction for head and neck cancer.
Methods
We performed a systematic review of MEDLINE, Embase, CINAHL, Web of Science, and the Cochrane Central Register of Controlled Trials (CENTRAL), from their inception through November 2021. We included peer reviewed studies that evaluated the impact of specific factors on quality of life for adult patients who underwent surgery with free flap reconstruction for head and neck cancer. Two reviewers independently screened citations for eligibility and extracted data. Risk of bias of each study was evaluated using the New-Castle Ottawa Scale. Vote counting and qualitative review were used to synthesize results. All relevant findings were reported.
Results
We initially identified 1971 articles. We included 22 articles in our systematic review, totaling 1398 patients. There was a high level of variability for factors evaluated throughout studies and many studies presented small sample sizes. However, some factors were associated with worse long-term quality of life, including older age, radiotherapy, higher tumor stage, dysphagia, anxiety as well as depressive symptoms. Very few articles analyzed their data for specific tumor subsites and the impact of psychosocial factors was rarely evaluated throughout studies.
Conclusions
For patients with head and neck cancer requiring free flap reconstruction, some specific factors may correlate with changes in quality of life. However, these findings are based on very few and mostly underpowered studies. A better understanding of factors affecting quality of life could allow a more personalized and overall better quality of care for patients.
Keywords: cervicofacial cancer, free flap, head and neck cancer, quality of life, systematic review
Graphical abstract.
Background
The World Health Organization defines quality of life (QOL) as “an individual’s perception of their position in life [. . .] in relation to their goals, expectations, standards and concerns.” 1 QOL used as an outcome is a deeply complex concept that is interwoven with a patient’s values and beliefs. We know that head and neck cancer (HNC) can deeply affect QOL by depriving a patient of their most basic senses, such as the capacity to speak, to eat, and to breathe.2,3 HNC can also cause pain, as well as disfiguration. All these cancer-related effects can ultimately lead to anxious and depressive symptoms.2,3
In addition, many cases of HNC are being treated by surgical resection with free flap reconstruction. These major surgeries will have a significant impact on a patient’s QOL. 4
Due to improvements in treatments and changes in the epidemiology of HNC, notably a rise in HPV-related HNC, 3 more patients will have to live with the long-term consequences of their cancer and its treatment. HNC survivorship is now increasingly being recognized 5 and many world-renown institutions are creating survivorship guidelines for physicians.6,7 However, even though survivorship is defined to begin at the time of diagnosis by the American Head and Neck Society Committee of Survivorship, 2 the previously referred guidelines address what should be done after completion of treatment. Very few studies aim at a better understanding of which specific factors, at the time of surgical treatment, will affect long-term QOL. Furthermore, although there are known factors which can affect QOL for HNC patients, 8 there is no systematic review, to our knowledge, that presents factors affecting HNC patients who will be treated specifically with surgical resection and free flap reconstruction. This type of major surgical procedure may cure a patient’s disease but may also cause significant morbidity. Each individual patient may have a different opinion on what the most important outcome is for them: quality versus quantity of life. There is a paucity of studies evaluating patients’ preferences and decision aids in HNC. 9 Is there a way for clinicians to understand which patients will truly benefit, as per their own personal values, from a major surgical resection with free flap reconstruction? If a patient seeks QOL over quantity of life, are there any factors on which the clinician could rely to guide patients in their treatment decision? And if they choose surgical resection with a free flap, are there any factors that can be controlled by clinicians to improve QOL postoperatively?
To answer that question, we performed a systematic review focusing on factors affecting long-term QOL after major surgical resection with free flap reconstruction. We included all HNC patients, without consideration of their specific subsites, to have a better appreciation of the general outline which can affect QOL, while also portraying a realistic picture of a standard HNC practice.
Methods
Aim, Design, and Setting of the Study
Our goal was to understand which specific factors have the most significant impact on postoperative QOL for patients undergoing major surgical resection with free flap reconstruction. We conducted a systematic review of the literature. We used a predefined protocol which was registered in the international prospective register of systematic review platform, PROSPERO (CRD42023404936).
Eligibility Criteria
We selected peer-reviewed studies assessing postoperative QOL in adult patients with HNC undergoing free flap reconstruction surgery. Studies were included if they satisfied our inclusion criteria: (1) adult patients (equal to or older than 18 years old) affected by HNC; (2) patients having undergone surgical resection with free flap reconstruction; and (3) studies that evaluated the impact of specific factors on postoperative QOL at least 1 month after surgery. Studies that included a mixed population of nononcologic as well as oncologic patients were considered, but were only included if a statistical distinction could be extracted for the oncologic population, to include only HNC patients in our systematic review. Likewise, studies that evaluated other types of flap reconstructions (such as regional and local flaps) were included only if a statistical distinction was made for free flap patients, to include only free flap reconstructions in our analyses. Investigators of such studies were contacted for further information regarding specific subgroup data if it was not available in the article. We contacted authors of conference abstracts, to seek unpublished data or articles. Synthesis was made including all studies. In addition, subgroup analysis was made with HNC subsites, as defined by the American Joint Committee on Cancer.
Search Strategy
We searched MEDLINE, EMBASE, CINAHL, Web of Science, and the Cochrane Central Register of Controlled Trials (CENTRAL) databases. Medical Subject Headings (MeSH), Emtree words, and free text words were used to identify articles. An example of our search strategy (using MEDLINE) is available in Supplemental File 1. There was no language or time of publication restrictions. We performed forward searching through the bibliographies of all included studies and related systematic reviews to retrieve relevant publications.
Selection Process
Two reviewers (L.-E.G. and N.V.-P.) independently screened citations based on titles and abstracts for potentially relevant studies. Full-text articles were then independently assessed for eligibility by the same reviewers for final inclusion. A third-party reviewer (N.A.) was consulted as needed for any disagreements. The final decisions were based on common consensus among all reviewers. The date of final study search and the beginning of study selection was November 7, 2021.
Data Collection Process
Data was collected through a standardized and pretested collection form. All data was collected by 2 reviewers (L.-E.G. and N.V.-P.) independently. Relevant study characteristics were collected. Extracted data included details about the postoperative hospitalization, surgery, type of free flap, and the tumor pathology. Any outcome deemed relevant to this review topic was also extracted. Details about the evaluation of QOL were collected. All factors that could influence QOL were collected. This included, but was not limited to, means of age, sex, alcohol or tobacco consumption, ASA (American Society of Anaesthesiologists) score, TNM (Tumor, Node, Metastasis) staging, cancer subsites, adjuvant treatment, tracheostomy, blood transfusions, postoperative complications, pain, dysphagia, dysphonia, anxious, and depressive symptoms.
Study Risk of Bias Assessment
The New-Castle Ottawa Scale (NOS) 10 was used to evaluate the quality of study design for each included trial. Two reviewers (LEG and NVP) independently evaluated the risk of bias of every trial. The NOS comprises the following domains: selection, comparability, and outcome.
Statistical Analysis and Synthesis Methods
Each eligible study was analyzed by 2 reviewers (LEG and NVP) independently. QOL being a patient-reported outcome (PRO), we retrieved objective data from patient-reported outcome measures (PROMs) with constructs related to QOL. The validity of each PROM was carefully reviewed before the start of synthesis. Correlation coefficients measuring the effect of different factors on QOL scores, used as a continuous variable, were retrieved, and were used as effect measures to synthesize data. A P-value of less than .05 was deemed statistically significant. Data were prepared for analysis using our standardized collection form, which also allowed us to decide on eligibility for HNC subsite group analysis. We contacted authors of every study that included missing data or statistics. All study’s characteristics and results were tabulated and visually displayed in the collection form. Vote counting based on the direction of effect was used as the favored method for synthesizing results, but proper calculation of a P-value for our vote counting results was impossible, since studies did not evaluate all the same factors and because each factor was defined differently by different authors.
All relevant findings for factors associated with QOL were reported using text, tabulation, and visual display. We considered the possibility of doing further analysis to explore possible causes of heterogeneity, but none were deemed possible. Sensitivity analysis was not deemed possible either.
The risk of bias due to missing results in our synthesis was taken into consideration, but due to the qualitative nature of our review, no standardized method could be used to assess that risk. 11 Certainty in the body of evidence for factors affecting QOL will be assessed using the GRADE (Grading of Recommendations Assessment, Development and Evaluation) approach. 12
Results
Study Selection
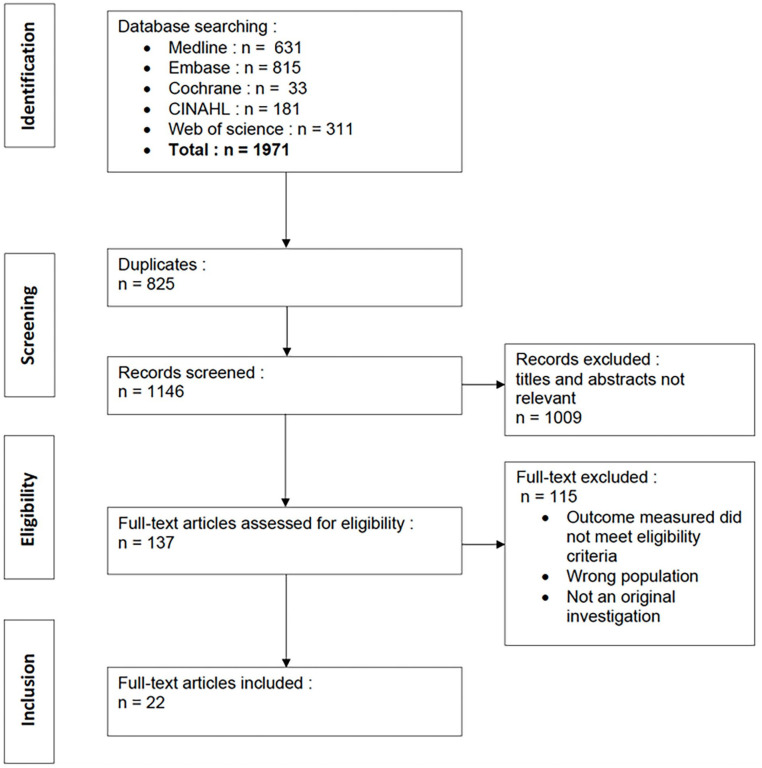
Our search strategy yielded a total of 1971 articles, from which 825 duplicates were removed. We screened 1146 articles, and 137 full-text articles were reviewed. After exclusions, 22 articles were included in the study, enrolling a total of 1398 patients. We did not find any unpublished data or articles relevant to our study topic. A study flow diagram is presented in Figure 1. Fifty-two studies were excluded because they did not specify the type of reconstruction for each patient. Sixty-eight articles were excluded because they only reported QOL scores, without evaluating any factors that could have an impact on QOL. Eight studies were excluded because their study population was not limited to an oncologic population.
Figure 1.
Flow chart of literature search methodology.
Study Characteristics and Methodologic Quality
Relevant study characteristics of every article included in this systematic review are shown in Table 1. Most of the included studies evaluated multiple HNC subsites but the vast majority included oral cavity or oropharyngeal cancer. Eight studies focused on oral cavity tumors and 2 studies only evaluated oropharyngeal tumors. Seven articles included both oral cavity and oropharyngeal tumors, but none made statistical distinction for each subsite. Six articles included multiple cancer subsites (≥3) but none of them made statistical distinction for each subsite.
Table 1.
Study Characteristics of Included Studies.
| Author (Ref.) | Year of publication | Country | Type of study | Instrument used to quantify QOL | No. of patients included in the study (n) | Mean age of patients included in the study | Tumor stage of patients included in the study | Tumor subsite (% of included patients, if available) | Mean follow-up duration | Adjuvant radiotx (% of included patients) | Adjuvant chemotx (% of included patients) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Airoldi et al 13 | 2011 | Italy | C | EORTC QLQ-C30 and EORTC QLQ-H&N35 | 36 | Info not available | I to IVB | OC | 63 ± 18 mo | 100% | Info not available |
| Borggreven et al 14 | 2007 | Netherlands | L | EORTC QLQ-C30 and EORTC QLQ-H&N35 | 80 | 58 | Info not available | OC (47%), OPX (53%) | Info not available | 93% | Info not available |
| Bozec et al 15 | 2018 | France | L | EORTC QLQ-C30 and EORTC QLQ-H&N35 | 58 | 61.5 | II to IV | OPX | >1 y | 76% | 35% |
| Bozec et al 16 | 2020 | France | C | EORTC QLQ-C30 and EORTC QLQ-H&N35 | 64 | 75.4 | Info not available | OC (75%), OPX (25%) | >1 y | 78% | 17% |
| Bozec et al 17 | 2009 | France | L | EORTC QLQ-C30 and EORTC QLQ-H&N35 | 41 | 68% had less than 70 y old | II to IV | OC (51%), OPX (49%) | >1 y | 75% | 48% |
| Bozec et al 18 | 2008 | France | L | EORTC QLQ-C30 and EORTC QLQ-H&N35 | 65 | 61.2 | Info not available | OC/OPX (87.3%), HPX (11.1%), sinus (1.6%) | >1 y | 81.50% | Info not available |
| Chang et al 19 | 2012 | Taiwan | L | UW-QOL | 32 | 53.53 | Info not available | OC | >2 y | Info not available | Info not available |
| Dimovska et al 20 | 2016 | England | L | UW-QOL | 96 (34 evaluation of QOL) | 84 | I to IV | Skin, OC, LX | Info not available | Info not available | Info not available |
| Elfring et al 21 | 2014 | Canada | L | EORTC QLQ-H&N35 | 30 | Info not available | I to III | OPX | Info not available | 36.70% | 56.60% |
| Hartl et al 22 | 2009 | France | L | EORTC QLQ-H&N35 | 9 | 51 | Info not available | OC, OPX | 43 mo | 77.80% | 22.20% |
| Jimenez et al 23 | 2021 | USA | C | UW-QOL | 80 | 60 | I to IVB | OC | 6 mo to 17 y | 38% | 41% |
| Klug et al 24 | 2002 | Austria | L | EORTC QLQ-C30 and EORTC QLQ-H&N35 | 67 | 56 | Info not available | OC, OPX | Info not available | 100% | 100% |
| Lahtinen et al 25 | 2018 | England | C | EORTC QLQ-C30 and EORTC QLQ-H&N35 and UW-QOL | 53 | 62.7 | Info not available | OC (43.4%), maxilla (9.4%), mandible (20.8%), LX/pharynx (3.8%), skin (3.8%), palate (7.5%), buccal mucosa (9.4%), other (2.6%) | >1 y | 67.90% | Info not available |
| Markkanen-Leppänen et al 26 | 2006 | Finland | L | UW-QOL | 44 | 56.2 | II to IV | OC (64%), pharynx (29%), HPX (7%) | >1 y | 88% | 9% |
| Momeni et al 27 | 2013 | USA | L | EORTC QLQ-C30 and EORTC QLQ-H&N35 and UW-QOL | 21 | 57.9 | Info not available | OC, OPX, esophagus, sinus, skin | >1 y | 61.90% | Info not available |
| Oskam et al 28 | 2010 | Nether-lands | L | EORTC QLQ-C30 and EORTC QLQ-H&N35 | 80 (55 evaluation of QOL) | 58 | II to IV | OC (47%), OPX (53%) | > 6 mo | 93% | Info not available |
| Pierre et al 29 | 2014 | France | L | EORTC QLQ-C30 and EORTC QLQ-H&N35 | 80 | Info not available | Info not available | OC (46%), OPX (54%) | >1 y | 69% | Info not available |
| Rhemrev et al 30 | 2007 | Nether-lands | L | EORTC QLQ-H&N35 | 85 | 57 | Info not available | OC | 43 mo | 79% | Info not available |
| Segna et al 31 | 2018 | Italy | L | SF-36 and SF-12 | 30 | 65.5 | I/II: 33.3% III/IV: 66.7% | OC, skin | >1 y | 60% | 16.70% |
| Smith et al 32 | 2006 | Australia | L | FACT-G, FACT-H&N, UW-QOL, and PSS-HN | 63 | 65.2 | Info not available | OC | >1 y | 47.60% | Info not available |
| Tamer et al 33 | 2020 | China | L | MDADI, EAT-10, and FACT-H&N | 256 | 46.7 | I: 29.1% II: 48.3% III: 22.3% IV: 0.4% | OC | >1 mo | 71.70% | 10.20% |
| Yang et al 34 | 2016 | China | C | UW-QOL | 61 (28 free flap) | Info not available | Info not available | OC | >1 y | 68% | Info not available |
Abbreviations: C, cross-sectional; EAT-10, Eating Assessment Tool; EORTC, European Organization for Research and Treatment of Cancer Questionnaires; FACT-G, Functional Assessment of Cancer Therapy – General; FACT-H&N, Functional Assessment of Cancer Therapy—Head and Neck; HPX, hypopharynx; L, longitudinal; LX, larynx; MDADI, M.D. Anderson Dysphagia Inventory; OC, oral cavity; OPX, oropharynx; QOL, quality of life; UW-QOL, University of Washington Quality of Life Questionnaire..
The methodological quality of all included studies was rated according to the NOS and is detailed in Table 2. Two important items emerged as a common pitfall in many studies: sample size was often inadequate, and investigators often failed to control their analyses for significant confounders, such as age, sex, tobacco, and alcohol consumption. Certainty in the body of evidence of every factor presented in this systematic review was globally graded as low, according to the GRADE approach, due to the risk of bias inherent to nonrandomized cohort studies, as well as the inconsistency of some results.
Table 2.
Risk of Bias of Included Studies.
| Authors | Selection | Comparability | Outcome | Overall quality |
|---|---|---|---|---|
| Airoldi et al 13 | ** | * | * | **/*/* |
| Borggreven et al 14 | *** | * | ** | ***/*/** |
| Bozec et al 15 | **** | * | ** | ****/*/** |
| Bozec et al 16 | **** | ** | ****/ /** | |
| Bozec et al 17 | ** | * | ** | **/*/** |
| Bozec et al 18 | ** | ** | **/ /** | |
| Chang et al 19 | *** | ** | ***/ /** | |
| Dimovska et al 20 | ** | * | ** | **/*/** |
| Elfring et al 21 | *** | * | * | ***/*/* |
| Hartl et al 22 | ** | ** | **/ /** | |
| Jimenez et al 23 | **** | * | ** | ****/*/** |
| Klug et al 24 | *** | * | * | ***/*/* |
| Lahtinen et al 25 | **** | * | ** | ****/*/** |
| Markkanen-Leppänen et al 26 | ** | * | **/ /* | |
| Momeni et al 27 | ** | ** | **/ /** | |
| Oskam et al 28 | *** | * | * | ***/*/* |
| Pierre et al 29 | *** | * | ** | ***/*/** |
| Rhemrev et al 30 | **** | * | ** | ****/*/** |
| Segna et al 31 | ** | * | * | **/*/* |
| Smith et al 32 | *** | * | * | ***/*/* |
| Tamer et al 33 | *** | * | ** | ***/*/** |
| Yang et al 34 | ** | ** | **/ /** |
Each asterix represents the number of points accorded in each category. A maximum of 4 points could be given in the Selection category, a maximum of 2 points could be given in the Comparability category and a maximum of 3 points could be given in the Outcome category. Overall quality represents the number of points given in each category : Selection / Comparability / Outcome.
QOL Questionnaires
All included articles used a validated QOL questionnaire. Fourteen articles used the European Organisation for Research and Treatment of Cancer Questionnaires (EORTC—QLQ-C30 and QLQ-H&N35). 35 Eight studies used the University of Washington Quality of Life Questionnaire (UW-QOL), 36 2 studies used the Functional Assessment of Cancer Therapy—Head and Neck Questionnaire (FACT-H&N), 37 1 study used the RAND 36-items Short Form Survey (SF-36) 38 as well as the RAND 12-items Short Form Survey (SF-12) 39 and 1 study used the M.D. Anderson Dysphagia Inventory (MDADI). 40 Three studies used multiple QOL questionnaires. Table 1 details which questionnaires were used in every included study. Studies that evaluated anxiety and depression symptoms frequently used the Hospital Anxiety and Depression Scale (HADS) questionnaire. 41
Longitudinal Evaluation of QOL Across Studies
Six of our included studies chose to repeat QOL evaluation over time14,17,18,26,31,33 to compare the evolution of QOL before and after surgery. Only 2 of them presented statistically significant results.
Markkanen-Leppänen et al 26 showed that the UW-QOL scores declined at 6 weeks (76.2), 3 months (75.6), 6 months (76.6), and 12 months (79.8) after surgery and were significantly lower than preoperative level (83.5, P < .0001).
Tamer et al demonstrated an increase in dysphagia 1 month after surgery (47.77 ± 19.08), compared to the preoperative evaluation (27.36 ± 14.67, P = .00) using the MDADI questionnaire. Symptoms of dysphagia were significantly improved 3 months postoperatively and were better than before surgery (7.05 ± 2.11, P = .00). 33
Factors Affecting QOL, All Cancer Subsites Included
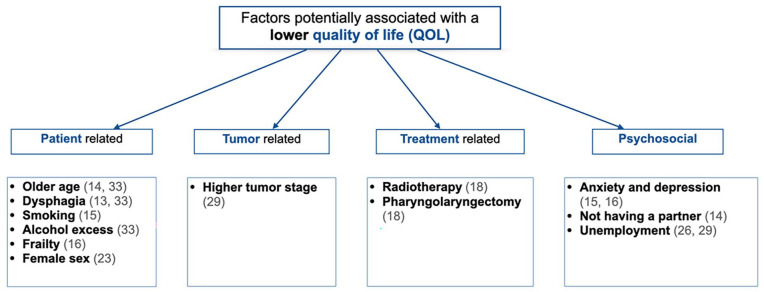
Some factors were found to have a statistically significant association with general QOL. Factors that were correlated with QOL varied across included studies, as some studies yielded significant results and others failed to show the same associations. Figure 2 provides a summary of all significant factors statistically associated with a lower global QOL.
Figure 2.
Factors potentially associated with a lower quality of life, all cancer subsites included.
Factors Affecting QOL for Patients With Oral Cavity Cancer
Seven articles analyzed QOL data only for patients presenting with an oral cavity cancer.13,19,23,30,32-34 Four studies found statistically significant factors associated with a worse postoperative QOL (Table 3).
Table 3.
Factors Negatively Affecting QOL for Patients With Oral Cavity Cancer.
| Factor negatively affecting QOL in oral cavity cancer | Study | Instrument used to quantify QOL | P |
|---|---|---|---|
| Severe dysphagia | Airoldi et al 13 | EORTC QLQ-C30/H&N35 | <.001 |
| Female sex | Jiminez et al 23 | UW-QOL (physical domain) | .0076 |
| UW-QOL (social domain) | .003 | ||
| Worrying about cancer recurrence | Smith et al 32 | UW-QOL | .016 |
| Denture use | Tamer et al 33 | FACT-H&N | .000 |
| Age | Tamer et al 33 | MDADI | .017 |
| Drinking habit | Tamer et al 33 | MDADI | .023 |
| Diet | Tamer et al 33 | FACT-H&N | .007 |
Abbreviations: FACT-H&N, Functional Assessment of Cancer Therapy—Head and Neck; MDADI, M.D. Anderson Dysphagia Inventory; QOL, quality of life; UW-QOL, University of Washington Quality of Life Questionnaire.
Factors Affecting QOL for Patients With Oropharyngeal Cancer
Two articles exclusively analyzed QOL data for patients presenting with an oropharyngeal cancer.15,21 Only the study by Bozec et al 15 identified factors significantly associated with a decreased QOL: tobacco consumption (P = .04), anxiety (HADS, P < .001), depression (HADS, P < .001), lateral pharyngeal wall involvement (P = .01), and soft palate involvement (P = .009).
Effect of Patient-Related Factors on QOL, All Cancer Subsites Included
Older age was a factor commonly evaluated across studies. Yet, the definition of “older age” was variable. One study used “more than 50 years old,” 34 another “more than 55.5 years old,” 26 one used “more than 60 years old,” 27 and another study used “more than 70 years old.” 31 Borggreven et al 14 and Tamer et al 33 did not specify their definition of older age. Yet, they found a statistically significant association between older age and QOL (P = .041; P = .017, respectively). On the counterpart, Yang et al 34 demonstrated that younger patients were significantly more anxious than older patients.
Frailty, defined by a G8 score of more than 15, 42 was significantly associated with worse postoperative QOL in a study by Bozec et al 16 (P < .001).
Smoking (P = .04), 15 drinking (P = .023), 33 and female sex (UW-QOL physical score P = .0076, UW-QOL social-emotional score P = .003) 23 were among the factors associated with a worse long-term QOL. Yang et al 34 also found that women were more anxious (UW-QOL).
Airoldi et al 13 demonstrated that dysphagia negatively affects QOL (P > .001), as did Tamer et al 33 (P = .035). Patients with severe dysphagia showed significantly higher levels of anxiety and depression (HADS score, P < .001). 13
The use of dentures failed to show a significant association with general QOL. However, Tamer et al 33 demonstrated that the presence of denture predicted dysphagia-specific QOL (MDADI scores, P = .000), as well as functional status (FACT-H&N, P = .000) 1 month after surgery.
Effect of Tumor-Related Factors on QOL, All Cancer Subsites Included
Tumor stage (T3-T4) had a significantly negative impact on global QOL in the study by Pierre et al (P = .04). 29 Bozec et al 15 and Smith et al 32 failed to show the same association. Higher tumor stage (T3-T4) was also associated with higher score for pain at 12 months in a study by Borggreven et al 14 (P = .031).
Early cancer stage (stage 1-2) was associated with a better physical function postoperatively according to Dimovksa et al 20 (UW-QOL, P = .046).
The association of specific HNC subsites with QOL was evaluated, but no study found an association with general QOL. Pierre et al 29 showed that tumor involvement of the tongue base had a negative impact on head and neck-specific symptoms (P = .04).
Effect of Treatment-Related Factors on QOL, All Cancer Subsites Included
Bozec et al 18 found that radiotherapy (pre- or postoperative) was associated with a worse global QOL (EORTC QLQ-C30) 6 months postoperatively (P = .04). Smith et al 32 showed that postoperative radiotherapy could potentially be associated with a worse UW-QOL score (P = .08). Dimovska et al 20 and Yang et al 34 failed to demonstrate an association.
The extent of surgical resection was not associated with a decreased general QOL in the studies by Hartl et al 22 and Klug et al. 24 However, Bozec et al 18 demonstrated that patients who had a total circular pharyngolaryngectomy presented worse global QOL (P = .02).
In the study by Hartl et al, 22 the extent of tongue base resection was associated with worse swallowing (P = .037) and worse aspiration scores (EORTC QLQ-H&N35, P = .042), as well as a higher incidence of depressive symptoms (HADS depression scale, P = .028). Jimenez et al 23 also found that larger tongue resections were associated with a worse UW-QOL physical score (P < .0001).
Elfring et al 21 showed that transection of the lingual and hypoglossal nerve was associated with worse swallowing scores, trouble with social eating and social contact, as well as xerostomia (EORTC QLQ-H&N35).
Furthermore, selective neck dissection, compared to radical neck dissection, produced fewer problems with appearance (P = .01) and better shoulder function (UW-QOL questionnaire, P = .00) according to Yang et al. 34
Few studies evaluated the impact of postoperative complications on QOL. Lahtinen et al 25 did find that patients with postoperative medical complications presented with increased pain, insomnia, and increased financial difficulties (EORTC QLQ-C30). Markkanen-Leppänen et al 26 demonstrated that patients with surgical complications presented less pain 6 weeks after surgery (P < .01) and a greater decrease in the recreation domain (P < .05), as well as the chewing domain (P < .01) of the UW-QOL questionnaire, 3 months after surgery. Patients who presented postoperative complications at large also seemed to present worse cognitive functioning (P = .04), worse insomnia (P = .04), greater problems with social contact (P = .03), and felt more ill (P = .03) in the study by Momeni et al. 27
Effect of Psychosocial-Related Factors on QOL, All Cancer Subsites Included
Psychosocial factors were significantly less studied than other types of factors. Two studies by Bozec et al15,16 demonstrated that anxiety and depression (evaluated with the HADS questionnaire) were associated with a worse postoperative QOL in the EORTC QLQ-C30 (P < .001, both studies) and in the EORTC QLQ-H&N35 (P < .001, both studies). Resumption of professional activity correlated with a better global QOL (EORTC QLQ-C30, r = .40) according to Pierre et al. 29 Markkanen-Leppänen et al 26 also found that unemployed patients reported lower QOL scores (P < .05).
Not having a partner was among the potential factors associated with a worse global QOL 6 months after surgery (EORTC QLQ-C30, P = .017) according to Borggreven et al. 14
Facial appearance and disfigurement were not addressed in our included studies, as they included mostly aerodigestive HNC. Hence, no study analyzed the possible impact of facial reanimation procedures.
Discussion
Our systematic review identified and analyzed all relevant literature on postoperative QOL for HNC patients who underwent major surgical resection with free flap reconstruction. Even though systematic reviews on this topic have been published,8,43-46 we present the first systematic review focusing specifically on the QOL of HNC patients who underwent surgery with free flap reconstruction.
We selected this specific population for the potentially severe impact of free flap reconstruction on QOL to aim toward a better understanding of how clinicians can offer specific patient-centered care. By doing so, we selected patients who are most likely to have highly staged tumors and to require adjuvant therapy (shown in Table 1).
The inclusion was limited to an oncologic population, because cancer patients experience a different set of challenges and psychological burden. 47 The decision to include all HNC subsites allowed us to include more studies and more patients in our systematic review, as most of the included studies presented multiple cancer subsites in their analyses. It also allowed us to compare factors affecting different types of cancer. We gained a comprehensive picture of all the possible factors affecting QOL, while portraying a standard HNC practice.
Our systematic review first demonstrates that some factors are associated with a worse postoperative QOL. Although no single factor was constantly associated with QOL across all studies, some factors did appear to correlate with a deterioration of QOL, notably older age, radiotherapy, dysphagia, anxiety, depression, and higher tumor stage. The variability of associations found between studies may be explained by the extensive variation of chosen factors evaluated in each study. Factor and outcome definitions were also different from one study to another, which limited possible comparisons. Many studies also made their statistical analyses with symptom-specific questionnaires related to QOL, instead of a general or a health-related QOL questionnaire.
The second important finding of this systematic review is that few studies evaluated factors affecting QOL for specific cancer subsites. Indeed, most studies included multiple subsites in their analysis. Major differences can be observed in the postoperative outcomes and the possible associated morbidity for different tumor sites. Ultimately, we believe that studies included in this systematic review can help clinicians but may not guide them toward truly adapting their care for each individual patient. Therefore, further research is needed to find specific factors affecting QOL for each HNC subsites.
The third important aspect displayed by this systematic review is the paucity of research on psychosocial factors. However, studies who did evaluate these factors (anxiety, depression, lack of a life partner, unemployment) found a significant deterioration of QOL. Assessment of these factors is crucial since psychological distress, as well as anxious and depressive symptoms have been overly associated with the HNC population.48-53 In fact, the incidence of preoperative depressive symptoms is particularly high among HNC patients. 50
Compared to survivors of other cancer sites, HNC patients are up to 2 times more likely to die from suicide. 54 Surveillance and management of psychosocial effects are now an integral part of survivorship, as defined by the “Quality of Cancer Survivorship Care Framework.” 55 Also, anxiety and depression are factors on which clinicians can provide help for their patients. Therefore, psychosocial factors should be addressed and evaluated in future research on QOL.
QOL is increasingly recognized as being paramount to treatment decisions and quality of care for cancer patients. 56 QOL is an important factor to be included in the patient-clinician decision-making, prognostication, and posttreatment care. 57 Historically, PROs were generally used as secondary outcomes. 58 However, we found an increasing number of articles published in HNC literature with QOL as a primary outcome. 59 Moreover, although there has been an overall decrease in HNC incidence in the United States, 60 there has been a significant increase in the incidence of late-stage HNC. 60 Older patients were found to be more likely to present with stage IV diseases, compared to younger patients. 60 This raises important new dilemmas for clinicians. We believe that a better understanding of factors having an impact on QOL for patients with HNC who are contemplating surgical resection could help them understand how their treatments will affect their QOL. This lack of knowledge is currently driving many studies in HNC. 61
Limitations
There are some limitations in this systematic review. First, our results are limited by the lack of statistical power in many of our included studies, which could be explained by a small number of patients included in most trials.
Most studies also included many HNC subsites in their analysis, which limited comparability and prevented the use of comparison groups.
The high level of heterogeneity across included studies and the use of different QOL questionnaires also limited possible comparisons, since specific details about responsiveness and reliability could not be found for every questionnaire.62,63 Another limitation is intricate with the rise in HPV-related oropharyngeal squamous cell carcinoma. Eight of our included studies were published before 2010, which may have affected the decision to go forward with major surgical resection. Despite these limitations, our systematic review exposes all relevant factors associated with postoperative QOL.
Conclusion
This systematic review identified factors associated with a decreased QOL for patients with HNC treated with major surgery and free flap reconstruction. These factors include older age, use of radiotherapy, higher tumor stage, and the presence of dysphagia. Few studies evaluated specific HNC subsites. Psychosocial factors were seldomly evaluated, but anxiety, depression, and unemployment were significantly associated with a lower postoperative QOL.
HNC resection and free flap reconstruction can have major long-term consequences for patients, which may greatly affect their overall QOL. We know that a patient’s QOL is an intrinsic part of their prognosis. Psychosocial factors need to be equally incorporated in future research on QOL. Specific analysis must be made for every specific HNC subsites. Overall, more research is still needed to properly identify which factors will have the highest impact on QOL for patients undergoing major surgery with free flap reconstruction.
Supplemental Material
Supplemental material, sj-docx-1-ohn-10.1177_19160216241248666 for Quality of Life After Head and Neck Cancer Surgery and Free Flap Reconstruction: A Systematic Review by Laura-Elisabeth Gosselin, Noémie Villemure-Poliquin and Nathalie Audet in Journal of Otolaryngology - Head & Neck Surgery
Acknowledgments
None.
Footnotes
Author Contributions: LEG: project design, data analysis, manuscript drafting, revisions, final approval. NVP: project design, data analysis, revisions, final approval. NA: project design, data analysis, revisions, final approval. All authors read and approved the final manuscript.
Availability of Data and Materials: An example of our search strategy is available in Supplemental file 1. The research study protocol is available on Prospero: CRD42023404936.
Consent for Publication: Not applicable.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Ethics Approval and Consent to Participate: Not applicable.
ORCID iDs: Laura-Elisabeth Gosselin  https://orcid.org/0009-0007-6111-0288
https://orcid.org/0009-0007-6111-0288
Nathalie Audet  https://orcid.org/0000-0002-9977-9493
https://orcid.org/0000-0002-9977-9493
Supplemental Material: Additional supporting information is available in the online version of the article.
References
- 1. Skevington SM, Lotfy M, O’Connell KA. The World Health Organization’s WHOQOL-BREF quality of life assessment: psychometric properties and results of the international field trial. A report from the WHOQOL group. Qual Life Res. 2004;13(2):299-310. [DOI] [PubMed] [Google Scholar]
- 2. Miller MC, Shuman AG. Survivorship in head and neck cancer: a primer. JAMA Otolaryngol Head Neck Surg. 2016;142(10):1002-1008. [DOI] [PubMed] [Google Scholar]
- 3. Margalit DN, Salz T, Venchiarutti R, et al. Interventions for head and neck cancer survivors: systematic review. Head Neck. 2022;44(11):2579-2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ringash J, Bernstein LJ, Devins G, et al. Head and neck cancer survivorship: learning the needs, meeting the needs. Semin Radiat Oncol. 2018;28(1):64-74. [DOI] [PubMed] [Google Scholar]
- 5. Shapiro CL. Cancer survivorship. N Engl J Med. 2018;379(25):2438-2450. [DOI] [PubMed] [Google Scholar]
- 6. Cohen EE, LaMonte SJ, Erb NL, et al. American cancer society head and neck cancer survivorship care guideline. CA Cancer J Clin. 2016;66(3):203-239. [DOI] [PubMed] [Google Scholar]
- 7. Verdonck-de Leeuw I, Dawson C, Licitra L, et al. European Head and Neck Society recommendations for head and neck cancer survivorship care. Oral Oncol. 2022;133:106047. [DOI] [PubMed] [Google Scholar]
- 8. Murphy BA, Ridner S, Wells N, Dietrich M. Quality of life research in head and neck cancer: a review of the current state of the science. Crit Rev Oncol Hematol. 2007;62(3):251-267. [DOI] [PubMed] [Google Scholar]
- 9. Blanchard P, Volk RJ, Ringash J, Peterson SK, Hutcheson KA, Frank SJ. Assessing head and neck cancer patient preferences and expectations: a systematic review. Oral Oncol. 2016;62:44-53. [DOI] [PubMed] [Google Scholar]
- 10. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603-605. [DOI] [PubMed] [Google Scholar]
- 11. Toews I, Booth A, Berg RC, et al. Further exploration of dissemination bias in qualitative research required to facilitate assessment within qualitative evidence syntheses. J Clin Epidemiol. 2017;88:133-139. [DOI] [PubMed] [Google Scholar]
- 12. Atkins D, Best D, Briss PA, et al. Grading quality of evidence and strength of recommendations. BMJ. 2004;328(7454):1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Airoldi M, Garzaro M, Raimondo L, et al. Functional and psychological evaluation after flap reconstruction plus radiotherapy in oral cancer. Head Neck. 2011;33(4):458-468. [DOI] [PubMed] [Google Scholar]
- 14. Borggreven PA, Aaronson NK, Verdonck-de Leeuw IM, et al. Quality of life after surgical treatment for oral and oropharyngeal cancer: a prospective longitudinal assessment of patients reconstructed by a microvascular flap. Oral Oncol. 2007;43(10):1034-1042. [DOI] [PubMed] [Google Scholar]
- 15. Bozec A, Demez P, Gal J, et al. Long-term quality of life and psycho-social outcomes after oropharyngeal cancer surgery and radial forearm free-flap reconstruction: a GETTEC prospective multicentric study. Surg Oncol. 2018;27(1):23-30. [DOI] [PubMed] [Google Scholar]
- 16. Bozec A, Majoufre C, De Boutray M, et al. Oral and oropharyngeal cancer surgery with free-flap reconstruction in the elderly: factors associated with long-term quality of life, patient needs and concerns. A GETTEC cross-sectional study. Surg Oncol. 2020;35:81-88. [DOI] [PubMed] [Google Scholar]
- 17. Bozec A, Poissonnet G, Chamorey E, et al. Quality of life after oral and oropharyngeal reconstruction with a radial forearm free flap: prospective study. J Otolaryngol Head Neck Surg. 2009;38(3):401-408. [PubMed] [Google Scholar]
- 18. Bozec A, Poissonnet G, Chamorey E, et al. Free-flap head and neck reconstruction and quality of life: a 2-year prospective study. Laryngoscope. 2008;118(5):874-880. [DOI] [PubMed] [Google Scholar]
- 19. Chang KP, Lai CS, Hsieh TY, Wu YC, Chang CH. Two-year quality of life after free flap reconstruction in tumor-site discrepancy among Taiwanese with moderately advanced oral squamous cell carcinoma. World J Surg Oncol. 2012;10:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dimovska EO, Clibbon JJ, Moncrieff MD, Heaton MJ, Figus A. Microsurgical reconstructions for head and neck cancers in elderly aged >80 years: an analysis of surgical outcomes and quality of life. Ann Surg Oncol. 2016;23(5):1684-1692. [DOI] [PubMed] [Google Scholar]
- 21. Elfring T, Boliek CA, Winget M, Paulsen C, Seikaly H, Rieger JM. The relationship between lingual and hypoglossal nerve function and quality of life in head and neck cancer. J Oral Rehabil. 2014;41(2):133-140. [DOI] [PubMed] [Google Scholar]
- 22. Hartl DM, Dauchy S, Escande C, Bretagne E, Janot F, Kolb F. Quality of life after free-flap tongue reconstruction. J Laryngol Otol. 2009;123(5):550-554. [DOI] [PubMed] [Google Scholar]
- 23. Jimenez JE, Nilsen ML, Gooding WE, et al. Surgical factors associated with patient-reported quality of life outcomes after free flap reconstruction of the oral cavity. Oral Oncol. 2021;123:105574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Klug C, Neuburg J, Glaser C, Schwarz B, Kermer C, Millesi W. Quality of life 2-10 years after combined treatment for advanced oral and oropharyngeal cancer. Int J Oral Maxillofac Surg. 2002;31(6):664-669. [DOI] [PubMed] [Google Scholar]
- 25. Lahtinen S, Koivunen P, Ala-Kokko T, Laurila P, Kaarela O, Liisanantti JH. Quality of life after free flap surgery for cancer of the head and neck in patients with or without postoperative complications. Eur Arch Otorhinolaryngol. 2018;275(10):2575-2584. [DOI] [PubMed] [Google Scholar]
- 26. Markkanen-Leppänen M, Mäkitie AA, Haapanen ML, Suominen E, Asko-Seljavaara S. Quality of life after free-flap reconstruction in patients with oral and pharyngeal cancer. Head Neck. 2006;28(3):210-216. [DOI] [PubMed] [Google Scholar]
- 27. Momeni A, Kim RY, Kattan A, Lee GK. Microsurgical head and neck reconstruction after oncologic ablation: a study analyzing health-related quality of life. Ann Plast Surg. 2013;70(4):462-469. [DOI] [PubMed] [Google Scholar]
- 28. Oskam IM, Verdonck-De Leeuw IM, Aaronson NK, et al. Quality of life as predictor of survival: a prospective study on patients treated with combined surgery and radiotherapy for advanced oral and oropharyngeal cancer. Radiother Oncol. 2010;97(2):258-262. [DOI] [PubMed] [Google Scholar]
- 29. Pierre CS, Dassonville O, Chamorey E, et al. Long-term quality of life and its predictive factors after oncologic surgery and microvascular reconstruction in patients with oral or oropharyngeal cancer. Eur Arch Otorhinolaryngol. 2014;271(4):801-807. [DOI] [PubMed] [Google Scholar]
- 30. Rhemrev R, Rakhorst HA, Zuidam JM, Mureau MA, Hovius SE, Hofer SO. Long-term functional outcome and satisfaction after radial forearm free flap reconstructions of intraoral malignancy resections. J Plast Reconstr Aesthet Surg. 2007;60(6):588-592. [DOI] [PubMed] [Google Scholar]
- 31. Segna E, Bolzoni AR, Giannì AB, Baj A, Beltramini GA. Impact of reconstructive microsurgery on patients with cancer of the head and neck: a prospective study of quality of life, particularly in older patients. Br J Oral Maxillofac Surg. 2018;56(9):830-834. [DOI] [PubMed] [Google Scholar]
- 32. Smith GI, Yeo D, Clark J, et al. Measures of health-related quality of life and functional status in survivors of oral cavity cancer who have had defects reconstructed with radial forearm free flaps. Br J Oral Maxillofac Surg. 2006;44(3):187-192. [DOI] [PubMed] [Google Scholar]
- 33. Tamer R, Chen Y, Xu X, Xie C, Swai J. Short-term quality of life, functional status, and their predictors in tongue cancer patients after anterolateral thigh free flap reconstruction: a single-center, prospective, comparative study. Cancer Manag Res. 2020;12:11663-11673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yang Y, Li F, Li W. Factors that affect the quality of life of patients with oral cancer who have had their defects reconstructed immediately after excision of the tumour. Br J Oral Maxillofac Surg. 2016;54(4):410-414. [DOI] [PubMed] [Google Scholar]
- 35. Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365-376. [DOI] [PubMed] [Google Scholar]
- 36. Weymuller EA, Jr, Alsarraf R, Yueh B, Deleyiannis FW, Coltrera MD. Analysis of the performance characteristics of the University of Washington Quality of Life instrument and its modification (UW-QOL-R). Arch Otolaryngol Head Neck Surg. 2001;127(5):489-493. [DOI] [PubMed] [Google Scholar]
- 37. List MA, D’Antonio LL, Cella DF, et al. The performance status scale for head and neck cancer patients and the functional assessment of cancer therapy-head and neck scale. A study of utility and validity. Cancer. 1996;77(11):2294-2301. [DOI] [PubMed] [Google Scholar]
- 38. Brazier JE, Harper R, Jones NM, et al. Validating the SF-36 health survey questionnaire: new outcome measure for primary care. BMJ. 1992;305(6846):160-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gandek B, Ware JE, Aaronson NK, et al. Cross-validation of item selection and scoring for the SF-12 Health Survey in nine countries: results from the IQOLA Project. International Quality of Life Assessment. J Clin Epidemiol. 1998;51(11):1171-1178. [DOI] [PubMed] [Google Scholar]
- 40. Chen AY, Frankowski R, Bishop-Leone J, et al. The development and validation of a dysphagia-specific quality-of-life questionnaire for patients with head and neck cancer: the M. D. Anderson dysphagia inventory. Arch Otolaryngol Head Neck Surg. 2001;127(7):870-876. [PubMed] [Google Scholar]
- 41. Herrmann C. International experiences with the Hospital Anxiety and Depression Scale—a review of validation data and clinical results. J Psychosom Res. 1997;42(1):17-41. [DOI] [PubMed] [Google Scholar]
- 42. Bellera CA, Rainfray M, Mathoulin-Pélissier S, et al. Screening older cancer patients: first evaluation of the G-8 geriatric screening tool. Ann Oncol. 2012;23(8):2166-2172. [DOI] [PubMed] [Google Scholar]
- 43. Rathod S, Livergant J, Klein J, Witterick I, Ringash J. A systematic review of quality of life in head and neck cancer treated with surgery with or without adjuvant treatment. Oral Oncol. 2015;51(10):888-900. [DOI] [PubMed] [Google Scholar]
- 44. Wijbenga JG, Schepers RH, Werker PM, Witjes MJ, Dijkstra PU. A systematic review of functional outcome and quality of life following reconstruction of maxillofacial defects using vascularized free fibula flaps and dental rehabilitation reveals poor data quality. J Plast Reconstr Aesthet Surg. 2016;69(8):1024-1036. [DOI] [PubMed] [Google Scholar]
- 45. Mahalingam S, Srinivasan R, Spielmann P. Quality-of-life and functional outcomes following pharyngolaryngectomy: a systematic review of literature. Clin Otolaryngol. 2016;41(1):25-43. [DOI] [PubMed] [Google Scholar]
- 46. Chandu A, Smith AC, Rogers SN. Health-related quality of life in oral cancer: a review. J Oral Maxillofac Surg. 2006;64(3):495-502. [DOI] [PubMed] [Google Scholar]
- 47. Lang H, France E, Williams B, Humphris G, Wells M. The psychological experience of living with head and neck cancer: a systematic review and meta-synthesis. Psychooncology. 2013;22(12):2648-2663. [DOI] [PubMed] [Google Scholar]
- 48. Hammerlid E, Ahlner-Elmqvist M, Bjordal K, et al. A prospective multicentre study in Sweden and Norway of mental distress and psychiatric morbidity in head and neck cancer patients. Br J Cancer. 1999;80(5-6):766-774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Holloway RL, Hellewell JL, Marbella AM, Layde PM, Myers KB, Campbell BH. Psychosocial effects in long-term head and neck cancer survivors. Head Neck. 2005;27(4):281-288. [DOI] [PubMed] [Google Scholar]
- 50. Barber B, Dergousoff J, Nesbitt M, et al. Depression as a predictor of postoperative functional performance status (PFPS) and treatment adherence in head and neck cancer patients: a prospective study. J Otolaryngol Head Neck Surg. 2015;44:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Krebber AM, Jansen F, Cuijpers P, Leemans CR, Verdonck-de Leeuw IM. Screening for psychological distress in follow-up care to identify head and neck cancer patients with untreated distress. Support Care Cancer. 2016;24(6):2541-2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kugaya A, Akechi T, Okuyama T, et al. Prevalence, predictive factors, and screening for psychologic distress in patients with newly diagnosed head and neck cancer. Cancer. 2000;88(12):2817-2823. [DOI] [PubMed] [Google Scholar]
- 53. De Boer MF, McCormick LK, Pruyn JF, Ryckman RM, van den Borne BW. Physical and psychosocial correlates of head and neck cancer: a review of the literature. Otolaryngol Head Neck Surg. 1999;120(3):427-436. [DOI] [PubMed] [Google Scholar]
- 54. Osazuwa-Peters N, Simpson MC, Zhao L, et al. Suicide risk among cancer survivors: head and neck versus other cancers. Cancer. 2018;124(20):4072-4079. [DOI] [PubMed] [Google Scholar]
- 55. Nekhlyudov L, Mollica MA, Jacobsen PB, Mayer DK, Shulman LN, Geiger AM. Developing a quality of cancer survivorship care framework: implications for clinical care, research, and policy. J Natl Cancer Inst. 2019;111(11):1120-1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sayed SI, Elmiyeh B, Rhys-Evans P, et al. Quality of life and outcomes research in head and neck cancer: a review of the state of the discipline and likely future directions. Cancer Treat Rev. 2009;35(5):397-402. [DOI] [PubMed] [Google Scholar]
- 57. Rogers SN, Waylen AE, Thomas S, et al. Quality of life, cognitive, physical and emotional function at diagnosis predicts head and neck cancer survival: analysis of cases from the Head and Neck 5000 study. Eur Arch Otorhinolaryngol. 2020;277(5):1515-1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Boyes H, Barraclough J, Ratansi R, Rogers SN, Kanatas A. Structured review of the patient-reported outcome instruments used in clinical trials in head and neck surgery. Br J Oral Maxillofac Surg. 2018;56(3):161-167. [DOI] [PubMed] [Google Scholar]
- 59. Rogers SN, Ahad SA, Murphy AP. A structured review and theme analysis of papers published on ‘quality of life’ in head and neck cancer: 2000-2005. Oral Oncol. 2007;43(9):843-868. [DOI] [PubMed] [Google Scholar]
- 60. Thompson-Harvey A, Yetukuri M, Hansen AR, et al. Rising incidence of late-stage head and neck cancer in the United States. Cancer. 2020;126(5):1090-1101. [DOI] [PubMed] [Google Scholar]
- 61. Alonso I, Lopez-Perez L, Martin Guirado JC, Fernanda Cabrera-Umpierrez M, Arredondo MT, Fico G. Data analytics for predicting quality of life changes in head and neck cancer survivors: a scoping review. In: Sacristan E, (ed.) Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Mexico, 2021; 2021:2262-2265. [DOI] [PubMed] [Google Scholar]
- 62. Kuenstner S, Langelotz C, Budach V, Possinger K, Krause B, Sezer O. The comparability of quality of life scores. a multitrait multimethod analysis of the EORTC QLQ-C30, SF-36 and FLIC questionnaires. Eur J Cancer. 2002;38(3):339-348. [DOI] [PubMed] [Google Scholar]
- 63. Johnston BC, Thorlund K, Schünemann HJ, et al. Improving the interpretation of quality of life evidence in meta-analyses: the application of minimal important difference units. Health Qual Life Outcomes. 2010;8:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-ohn-10.1177_19160216241248666 for Quality of Life After Head and Neck Cancer Surgery and Free Flap Reconstruction: A Systematic Review by Laura-Elisabeth Gosselin, Noémie Villemure-Poliquin and Nathalie Audet in Journal of Otolaryngology - Head & Neck Surgery