Abstract
Objectives
This study aims to assess the seroprevalence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) IgG antibodies against the spike (S) and nucleocapsid (NP) proteins, as well as neutralizing antibodies against the receptor-binding domain (RBD). Additionally, it aims to detect viral RNA of SARS-CoV-2 in pre-pandemic archival pediatric specimens collected before the announcement of the COVID-19 pandemic spread on March 20th, 2020, in Morocco. The objective is to investigate the existence of pre-pandemic immunity to SARS-CoV-2.
Methods
We conducted a cross-sectional study, to analyze IgG antibody levels in a cohort of 106 pre-pandemic pediatric participants. Using an indirect enzyme-linked immunosorbent assay (ELISA), we measured the IgG levels against the S and NP proteins of SARS-CoV-2. Additionally, we staged a competitive ELISA assay to evaluate the neutralizing capability of these antibodies. We used reverse transcription polymerase chain reaction (rRT-PCR) to detect viral NP and ORF1ab genes of SARS-CoV-2 in oropharyngeal swabs. Moreover, we conducted on the same specimens a multiplexed RT-PCR to detect RNA of the most common 27 pathogens involved in lower respiratory tract infections.
Results
Among the 106 serum samples, 13% (nn = =14) tested positive for SARS-CoV-2 IgG antibodies using ELISA. Temporal analysis indicated varying IgG positivity levels across 2019. Neutralizing antibodies were found in 21% of the 28 samples analyzed, including two with high inhibition rates (93%). The SARS-CoV-2 RNA was detected using rRT-PCR in 14 samples. None of the samples tested positive for the other 27 pathogens associated with lower respiratory tract infections, using multiplexed RT-PCR.
Conclusion
Our study addresses the possibility, that COVID-19 infections occurred in Morocco before the recognized outbreak. On the other hand, some of the cases might reflect cross-reactivity with other coronaviruses or be influenced by previous viral exposures or vaccinations. Understanding these factors is crucial to comprehending pediatric immune responses to newly emerging infectious diseases.
Keywords: humoral immunity, SARS-CoV-2, high-affinity antibodies, neutralizing antibodies, cross-reactive immunity, human-coronaviruses, IgG
Introduction
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was noted first to have occurred in Wuhan, China in November 2019. 1 The World Health Organization (WHO) reported the first case on December 31st, 2019, 1 and announced the pandemic on March 11th. 1 By the middle of March 2020, China, the United States, and several European nations had become epicenters of the pandemic. Following the same suit, the Moroccan government declared a state of emergency on March 20th, 2020. 2 Since December 19th, 2023, the WHO reported over 1,278,055 confirmed cases of COVID-19 in Morocco, along with a total of 16,298 deaths. 3 Worldwide the virus outbreak rapidly saturated healthcare systems. Without prophylactic or therapeutic solutions, governments implemented non-pharmaceutical measures like school closures, mobility restrictions, curfews, and global shutdowns to curb viral transmission. 4 Numerous countries experienced subsequent waves of the epidemic after the lockdown ended, highlighting the importance of achieving herd immunity either through vaccination or natural infection. This strategy aimed to limit SARS-CoV-2 circulation. Hence, the immune response triggered by SARS-CoV-2 was closely monitored to guide vaccine development, establish baseline protection, and ascertain the longevity and nature of protective immunity. It is known that our immune system responds to SARS-CoV-2 through a variety of humoral and cellular effectors. By generating potent antibodies targeting various components of viral pathogens. 5 These antibodies are crucial for neutralizing the virus. 6 SARS-CoV-2 belongs to the Betacoronavirus genus of human coronaviruses, it shares similar RNA sequences with other human coronaviruses, with approximately 69% of OC43, 68% of HKU1, and around 65% of NL63 and 229E.6,7 This identity suggests a potential cross-reactivity between immune responses to these viruses, not to mention that it depends on genetic diversity, individual immune system variations, and the contexts of immune reactions. 8 These viruses are characterized by the presence of a S protein on their surfaces. It plays a crucial role in the virus’s ability to enter and infect host cells and is a primary target for the immune response. 6 Therefore, not only the anti-spike antibodies are important but also antibodies targeting other viral proteins such as the NP, E, and M. These proteins may also trigger a cross-reactive immune reaction, which is essential for understanding the broader immunity landscape about SARS-CoV-2 and other coronaviruses.6–8 However, previous research has shown minimal cross-reactivity between RBD domains from differing coronaviruses. These studies largely ignore the rest of the spike protein and the other proteins, which would be an important consideration for identifying antibodies and can be used in vitro to help identify polyclonal responses that are not detected with RBD alone. 8 The potential cross-reactivity of these antibodies is an interesting matter, particularly to understand the immunity against SARS-CoV-2. Individuals previously exposed to other betacoronaviruses may have some level of immunity to SARS-CoV-2 because of this cross-reactivity.6,9
On the other hand, it is worth mentioning that several studies draw some preliminary insights into the possible relationship between the MMR (Measles, Mumps, and Rubella) vaccine and COVID-19 immune responses, especially among children. The investigations in the cross-reactivity extended to childhood vaccines. 8 The MMR vaccine is delivered to Moroccan children in two doses according to the national vaccination schedule. The initial dose is given between 9 and 15 months old, followed by a second one from the age of 15 months to 6 years old. 10 A systematic review that included 169 studies, focusing on 11 that specifically address the MMR vaccine’s impact on COVID-19, suggests a potential link between the vaccine and a modulated immune response to COVID-19, by decreasing disease severity. 11 While most of these studies are hypothesis-driven, some evidence, including changes in immunoglobulin IgG levels and a quasi-experimental study showing mild COVID-19 symptoms in vaccinated individuals, supports this notion. 11 Understanding cross-reactive immune responses to SARS-CoV-2 may contribute to our comprehension of the heterogeneity of clinical outcomes in COVID-19 disease. This can be achieved by identifying immunological epitopes common to coronaviruses with different genetic compositions, variations, and most likely specific functions. Such understanding is relevant not only to SARS-CoV-2 but also to similar diseases and vaccinations.
Based on these data, our study aimed to evaluate anti-SARS-CoV-2 immunity and viral RNA detection in pre-pandemic archival blood serum samples and oropharyngeal swabs, that were collected from children clinically suspected of having eruptive fevers caused by measles and rubella. Focusing on pediatric populations, particularly those with skin rashes, we assessed to understand the humoral immune responses to determine the SARS CoV-2 prevalence before the pandemic. Given the virus’s association with skin issues in pediatric COVID-19 cases, 12 our research aimed to enhance our understanding of immune response dynamics and establish pre-pandemic immunological benchmarks. To achieve this, we conducted an ELISA test to detect IgG antibodies against S and NP proteins, as well as IgG/IgM antibodies against SARS-CoV-2 using a Lateral Flow Assay. Furthermore, we assessed neutralizing IgG levels targeting the RBD domain through a competitive ELISA assay. Additionally, we identified viral RNA using the NP and ORF1ab genes of SARS-CoV-2.
Materials and methods
Study design
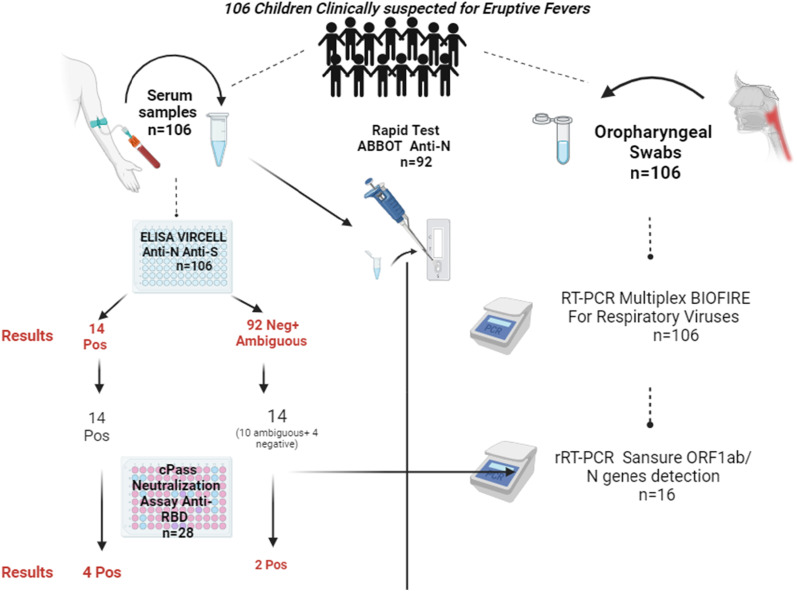
This study aims to measure the seroprevalence of different antibody types targeting SARS-CoV-2 among children attending the National Institute of Hygiene (NIH) in Morocco (Figure 1). Serum and oropharyngeal swab samples were collected between February 2019 to February 2020. The specimens were analysed with three different immunoassays for the serums and two virological tests for the swabs. The samples (collected from 106 patients) had been collected before the announcement of the spread of the COVID-19 pandemic in (March 20th, 2020). We selected our cohort study as a part of a national surveillance program investigating cases of rash fever associated with measles or rubella infections among children. This program is part of the Moroccan Ministry of Health (MoH) in Morocco public health strategies, that evaluate the impact of the measles and rubella vaccination campaign on rates incidence, by supporting the WHO global strategy against their eradication.
Figure 1.
Study Design, Population, and Results. In this investigation, 106 serum and oropharyngeal swab samples were collected from the National Institute of Hygiene virology laboratory, forming the basis of the dataset analyzed in this study. Various tests were conducted, and comprehensive details on each test and its corresponding results are elaborated in the paper.
Subject exclusion criteria
Study context
In the context of national surveillance for measles and rubella in Morocco, we receive numerous children’s samples annually, including blood, oropharyngeal swabs, and urine. The 106 children chosen tested negative for IgM antibodies against measles and rubella. Suspecting SARS-CoV-2 due to associated rash conditions, we screened the whole serums (n = 106) for IgG antibodies using the Vircell assay. 14 individuals tested positive. Due to limited resources, the 14 positive and 14 borderline sera underwent the neutralizing test. Multiplex RT-PCR on oropharyngeal swabs was conducted on the whole cohort. Subsequently, we opted for a specific test (Sansure rRT-PCR) designed to detect the NP and ORF1ab genes of SARS-CoV-2. Unfortunately, we only had 16 samples with sufficient remaining volume for the rRT-PCR test, which explains the limited number of samples tested.
Reactivity to measles and rubella
We excluded positive samples of measles and rubella IgM-type immunoglobulin, to determine the underlying causes of the rash and fever.
Clinical case definition
Only children having fever and maculopapular rash, along with at least one of the following symptoms: cough, coryza (runny nose), or conjunctivitis (red eyes), in addition to negative IgM antibodies against measles and rubella were included in the study.
Sample availability
Our study was limited to pre-pandemic samples available, ensuring relevance to our COVID-19 immunity investigation.
Testing suitability
We excluded samples that were not viable for ELISA and RT-PCR and rRT-PCR testing due to insufficient volume or quality.
Study population and samples
The study included individuals with ages ranging from 1 to 14 years old with no travel history. The analysed samples were residual serum samples collected for a primary clinical diagnosis of an eruptive fever condition; informed consent was legally signed by the authorized representatives. Oropharyngeal swab samples were also collected and used in this study. Meanwhile, information about age, gender, and sampling date was collected from the medical records and compiled with the serological results (Table 1).
Table 1.
Demographic characteristics of 106 subjects sampled in NIH Rabat, Morocco.
| N | % | |
|---|---|---|
| Gender | ||
| Female | 45 | 42 |
| Male | 61 | 58 |
| Sampling date | ||
| February-May 2019 | 44 | 41,5 |
| June-September 2019 | 49 | 46,2 |
| October-November 2019 | 4 | 3,7 |
| December 2019-February-2020 | 9 | 8,5 |
| Age | ||
| 1 year-2 years | 22 | 20,7 |
| 3 years-5 years | 46 | 43,4 |
| 6 years- 10 years | 28 | 26,4 |
| 11 years- 12 years | 7 | 6,6 |
| 13 years- 14 years | 3 | 2,8 |
| Vaccine type | ||
| Measles and rubella vaccine (live) (attenuated, freeze dried) by biological E. Limited, India | 106 | 100 |
| Number of doses | ||
| 1 dose | 30 | 28,3 |
| 2 doses | 69 | 65,1 |
| Unknown | 7 | 6,6 |
Enzyme-linked immunosorbent assay ELISA
All serum samples were tested for IgG recognizing SARS-CoV-2 using the Vircell COVID-19 ELISA IgG (Vircell Spain S.L.U., Granada, Spain) against two SARS-CoV-2 antigens (S and NP). According to manufacturer instructions, a 1/20 dilution of serum was prepared by adding 5 μL of the sample to 95 μL of the dilution solution. Then 80 μL of sample dilution solution was added into all wells except those assigned to controls. Finally, 20 μL of the 1/20 dilutions of serum samples, 100 μL of positive control, 100 μL of cut-off control, and 100 μL of negative control were added into the corresponding wells. After 45 min of incubation at 37°C, wells were washed according to the manufacturer’s recommendation, and 100 ul of anti-IgG human conjugate to Horseradish Peroxidase (HRP) was added. After 30 min of incubation and washing, we added 100 μL of substrate 5,5′-Tétraméthylbenzidine (TMB) to each well and then incubated it in the dark. The reaction was stopped with 50ul of stop solution. The absorbance (OD) was measured in 450/620 nm and results were expressed as a ratio using the following equation (sample OD/cutoff serum mean OD) × 10 to determine the index for positive, negative, and ambiguous value thresholds.
Lateral flow assay (LFA)
Panbio IgG/IgM rapid test COVID-19 (Abbott) is a rapid lateral flow assay (LFA) for qualitative detection of IgG and IgM against SARS-CoV-2 antigens in whole blood plasma. Two drops of the assay buffer for migration were added to 20 μL from every sample. Revelation occurred after 20 min of incubation for 92 samples.
Neutralization antibody detection assay
The cPass SARS-CoV-2 Neutralization Antibody Detection Kit by GenScript is a competitive ELISA-based detection tool. The host cell ACE2 receptor is immobilized into the ELISA plate. The assay was conducted according to the manufacturer’s instructions on a cohort comprising 28 samples. The HRP-RBD complex was prepared, with the dilution of 10 µl of RBD conjugated to HRP in 10 mL of buffer. Subsequently, 5 µl of samples, along with positive and negative controls, were combined with a dilution buffer (1/9). The HRP-RBD solution was then added, followed by incubation at 37°C for 30 min. After washing steps, 100 µl of TMB solution was introduced to each well and incubated in darkness at 20/25°C for 15 min. The reaction was stopped by adding 50 µl of stop solution to each well. The microtiter plate reader was employed to measure absorbance at 450 nm. The neutralizing antibodies detection of SARS-CoV-2 was determined using a 30% threshold signal inhibition according to the manufacturer’s instructions. The inhibition signal percentage was calculated as (1-OD of sample/mean OD of negative controls) * 100. The neutralizing antibodies' presence was affirmed when the signal of inhibition exceeded 30%.
Virological tests: RT-PCR assays
Two distinct types of RT-PCR assays were conducted on oropharyngeal swabs. A real-time PCR test using the CORONAVIRUS (2019-nCoV) Sansure Biotech, was conducted on a subset of 16 samples to identify SARS-CoV-2 genes of NP and ORF1ab according to the manufacturer’s instructions. Ct values were collected to detect the viral RNA. The Coronavirus (2019-nCoV) Nucleic Acid Diagnostic Kit (PCR-Fluorescence Probing) uses real-time reverse transcription polymerase chain reaction (rRT-PCR) for the qualitative detection of ORF1ab and NP genes of SARS-CoV-2 RNA in respiratory specimens. For RNA extraction, we used the Sample Release Reagent RNA fast-releasing technology provided in the kit. As recommended by manufacturers, patient results were interpreted using a Ct cutoff of 40, the gene’s absence is indicated by a Ct value exceeding 40. While a Ct value of 40 or lower underlined the presence of the gene. A Multiplex RT-PCR assay, the BIOFIRE® FILMARRAY® Pneumonia plus Panel Test, was conducted on the entire cohort (n = 106), according to the manufacturer’s instructions, to screen for 27 of the most common pathogens involved in lower respiratory tract infections (see supplementary Data in Table S1).
Statistical methods
Statistical analyses and graphical visualizations were performed using GraphPad Prism8 (GraphPad Software, LLC, San Diego, CA, USA, EE. UU.) and Microsoft Excel. Some Diagrams were edited on Biorender Graph Editor.
Results
Cohort characteristics
Identification of SARS-CoV-2 IgG antibody in human serum collected before the COVID-19 pandemic
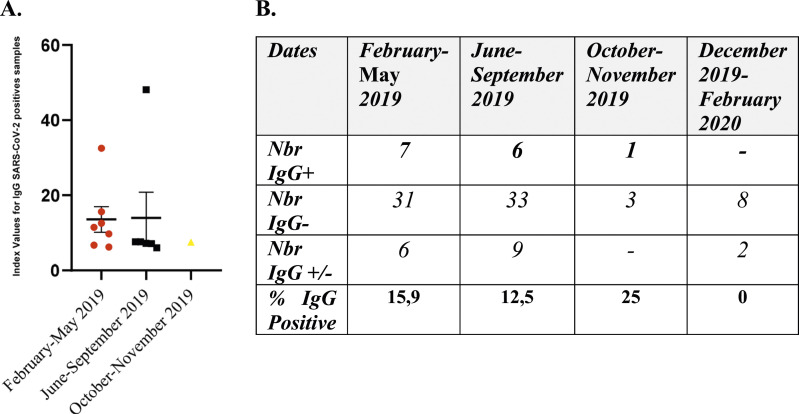
The humoral activity was determined using ELISAs for IgG in all samples (n = 106). Overall, 14 samples representing 13%, showed positive results, revealing the presence of anti-SARS-CoV-2 antibodies anti-S and anti-NP. Additionally, it was observed that 15.74% (n = 17) of the cases showed ambiguous results, with an index value ranging between four and 6. (Figure 2(a) and (b)). Meanwhile, in the samples collected between February and May 2019, 15,9% (7/44) tested positive for IgG antibodies. While those collected from June to September 2019, 12,5% (6/48), were IgG-positive. Only, one sample collected between October and November 2019 tested positive for IgG antibodies. The median index value for the positive samples collected from February to May 2019 was 11.45, while for the period between June and September 2019, was 7.42. (Figure 2(b)). The rapid test was conducted on 92 specimens, resulting in two positive samples: one for IgM (indicating recent exposure) and another for IgG (suggesting past infection). These samples showed divergent results in the ELISA test, with the IgM-positive sample testing negative and the IgG-positive sample for the rapid test testing positive. Remarkably, the sample that tested positive in both assessments was the earliest one, collected on February 2, 2019, and it was from an individual who had received two doses of the MMR vaccine. (Table 1)
Figure 2.
(a) Serologic positivity in different periods: Immunoglobulin G presence for SARS-CoV-2 in pre-2019 children’s serums (n = 14). The different categories of index values are determined using the test recommendations. Index= (sample OD/average serum OD Threshold) * 10. (b) Number of individuals of each IgG status; positive, negative, and ambiguous for different times of sampling. Percentages of IgG-positive samples were calculated from the total samples collected within each period.
Children with pre-pandemic SARS-CoV-2 detectable antibodies had elevated levels of neutralizing antibodies
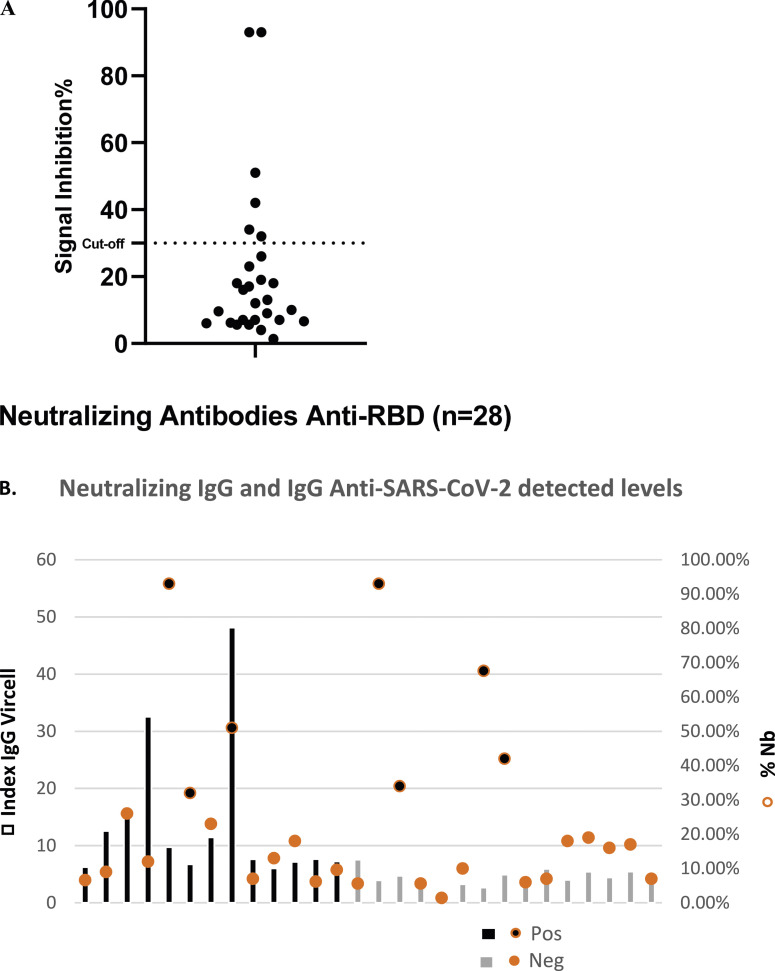
Since several samples had a detectable rate of anti-S and anti-NP IgG levels, we opted to analyse their nature. To fulfill that, we assessed the cPass SARS-CoV-2 antibody neutralization test among 28 chosen samples to identify their function. Among them, 14 samples tested positive in the ELISA Vircell test, 10 were ambiguous, and four were strictly negative. We noted, that six samples (21%) showed neutralizing antibody levels against the RBD protein (Figure 3(a)). Among those, three tested positive in the ELISA Vircell showing neutralizing antibody inhibition rates of 51%, 32%, and 93%, while the other three samples generated ambiguous results in the ELISA Vircell Test (93%, 42%, 34%) (Figure 3(b)).
Figure 3.
The detected levels of anti-RBD neutralizing antibodies against SARS-CoV-2 and IgG levels against the S and NP in children’s sera. (a) Neutralizing Antibodies (NAbs) levels were assessed via a competitive ELISA neutralization test. The presence of neutralizing antibodies was determined as follows: If the inhibition signal exceeded 30%, it indicated the presence of NAbs. Conversely, if the inhibition signal was less than 30%, neutralizing antibodies are undetectable. (b) Comparison of the IgG levels between two tests: the neutralization test and the Vircell ELISA.
Pre-existing SARS-CoV-2 RNA in oropharyngeal swabs
All samples underwent analysis using the BIOFIRE test, a multiplex RT-PCR assay designed to detect 27 common pathogens associated with lower respiratory tract infections. The results showed that all 106 samples tested negative for this test (see supplementary Table S1).
Then, 16 samples were subjected to rRT-PCR to detect the virus RNA by targeting the N and ORF1ab genes. Only samples that tested positive for IgG or showed ambiguous results were included in this test. The outcome revealed that 14 of these samples tested positive, indicating the presence of NP and/or ORF1ab genes specific to SARS-CoV-2, with Ct values ranging between 34 and 39 (see Table 2). Moreover, nine rRT-PCR-positive samples (n = 8) tested positive for the IgG anti-Spike ELISA test.
Table 2.
The Ct values of existing RNA for SARS-CoV-2 NP and ORF genes, accompanied by corresponding IgG values obtained from Vircell ELISA (Index) Test and Neutralizing ELISA assay (% of inhibition), along with the dates of sample collection.
| Samples | Ct N | Ct ORF1ab | IgG index | NAb% (%) | Sample collection date |
|---|---|---|---|---|---|
| sample1 | — | — | 4,297 | 6 | 06/02/2019 |
| sample2 | 35 | 37 | 48,128 | 51 | 27/03/2019 |
| sample3 | — | 38,9 | 12,554 | 9 | 20/05/2019 |
| sample4 | 35 | 38 | 4,722 | 34 | 20/05/2019 |
| sample5 | 35 | — | 6,722 | 32 | 12/06/2019 |
| sample6 | 35 | 37 | 11,455 | 23 | 11/07/2019 |
| sample7 | 35,8 | 39,5 | 4,92 | 42 | 17/07/2019 |
| sample8 | 37 | 38 | 5,91 | 7 | 31/07/2019 |
| sample9 | 37 | — | 6,009 | 13 | 01/04/2019 |
| sample10 | 35 | 36 | 7,148 | 18 | 23/05/2019 |
| sample11 | 34 | 38 | 4 | 18 | 03/06/2019 |
| sample12 | 36,6 | 36,5 | 7,643 | 6,20 | 12/06/2019 |
| sample13 | 36,4 | 35,4 | 5,435 | 19 | 17/07/2019 |
| sample14 | 36,1 | 38,8 | 4,435 | 16 | 28/08/2019 |
| sample15 | — | 39 | 5,445 | 17 | 12/09/2019 |
| sample16 | — | — | 6,237 | 6,60 | 23/09/2019 |
Values with bold highlighting indicate the positive for IgG ELISA and Neutralizing antibodies samples and the presence of the genes.
Discussion
In this study, we found that 13% of the children (n = 106) with confirmed eruptive fevers and clinical symptoms exhibited anti-SARS-CoV-2 IgG antibodies (anti-S and anti-NP), indicating a prior humoral immune response to SARS-CoV-2. The detectable IgG levels in the serum indicate that B lymphocytes have undergone class-switch recombination within germinal centers, indicating a mature phase of the immune response after the infection. 5 The presence of this immunity raises several hypotheses regarding its origin. It could be due to cross-reactivity with other human coronaviruses that may have previously infected these children. Otherwise, it might be linked to the possibility that the virus circulated in the community before the first confirmed case was reported in Morocco. To address the first hypothesis, studies identified the presence of this cross-immunity when comparing serum from healthy volunteers collected pre-2019 to those from a high-exposure community. These studies have shown that antibodies against SARS-CoV-2 can recognize spike proteins of HCoV-OC43 and HCoV-HKU1, suggesting possible cross-immunity from previous infections with these common coronaviruses. 13 A research study provided data about the percentage similarity of the RBD protein among various Coronaviruses (CoVs). The RBD exhibits more diversity when compared with different CoVs. It is known that SARS-CoV-2 shares a 73 to 76% identity with SARS-CoV, 24% with HKU1, and 23% with OC43. 8 Furthermore, other studies have confirmed identities between SARS-CoV-2 and H-CoVs OC43/HKU1, with 30% and 29% identity, respectively.13,14 Moreover, a substantial degree of identity is observed towards the C-terminus of the spike protein in all CoVs. This region is the primary structural segment of the protein, which includes the heptad repeat regions responsible for facilitating the insertion of the fusion peptide into the host cell membrane. 13 In previous reports, the majority of children are exposed to H-CoV-OC43 as well as HCoV-NL63 and seroconvert early in life. 15 To support this hypothesis, several studies have indicated that children are less affected by COVID-19. 16 This phenomenon might be attributed to distinct immunological responses characteristic of the younger age groups. In Piccaluga et al. children’s frequent exposure to common respiratory coronaviruses and their inherently more reactive immune systems may lead to more effective cross-immunization. 17 Supporting this, Ng et al. observed elevated neutralizing antibodies in children, possibly stemming from a varied antibody repertoire formed through consistent interactions with seasonal coronaviruses. 18 Notably, these antibodies often target the S2 subunit of the coronavirus spike protein, suggesting an age-related immunological memory. 18
All our patients had received the Measles and Rubella Vaccine. It’s crucial to highlight that while some clinical presentations resembled those of measles or rubella, they were not cases of Measles vaccine-associated reaction, as stated by the WHO through a positive IgM measles-rubella test. 19 None of the selected cases in our study tested positive for IgM for measles and rubella. The potential role of the MMR (Measles, Mumps, Rubella) vaccine may provide significant protection against COVID-19 in children. Gold et al. have reported a significant inverse correlation between MMR vaccination and the severity of COVID-19 symptoms. 20 Furthermore, Sidiq et al. proposed that MMR vaccine components might offer a protective effect against COVID-19, a theory that continues to be explored. 21 While these findings offer promising insights, definitive proof confirming the MMR vaccine’s role in safeguarding children against COVID-19 remains to be established.
The two positive results for the ABBOT test for both IgM and IgG support that these individuals' responses are related to a potential virus infection. According to the manufacturer’s instructions, a positive result could indicate a current or previous infection with coronaviruses other than SARS-CoV-2, including strains like HKU1, NL63, OC43, or 229E. 22 This underscores the variability in antibody detection depending on the test used. A related study on the clinical performance of this test highlighted a high sensitivity (95.2%) for IgG detection in samples collected more than 14 days post-symptom onset. 22 However, IgM detection was considerably lower, with a maximum sensitivity of 20.5%. Specificity rates were notably high, at 98.7% for IgG and 100% for IgM. 22 These findings highlight the complexities and variability in antibody testing and underscore the importance of test selection in accurately assessing immune response to SARS-CoV-2.
We further performed an Antibody neutralization assay on 28 samples to check the nature of the antibodies found earlier. We therefore observed that 21% (n = 6) of the children had neutralizing immune responses against the RBD domain of the spike protein. Not only are these antibodies specific to antigens of SARS-CoV-2, but they also demonstrate neutralizing capacity against the virus. The SARS-CoV-2 Neutralization Antibody Detection GenScript assay exhibited a sensitivity of 96.1% (95% CI, 94.9%–97.3%), and a specificity of 100% (95% CI, 98.0%–100.0%). 23 Detectable IgG antibodies in response to a virus typically indicate either a prior infection that has generated memory B cells, leading to the reactivation of those cells upon encountering the virus again, or the activation of naïve B cells by a new virus. These memory B cells can be activated by an antigenically related virus.24–26 This concept is well documented for influenza infections whereby humans are repeatedly exposed to antigenically distinct viruses. 24 Humans are repeatedly infected with endemic H-CoVs.27,28 Our findings suggest that children from the pre-pandemic period exhibited specific immune responses to SARS-CoV-2. This observation may align with the hypothesis of an early circulation of SARS-CoV-2 before the first reported case in Morocco. However, the antibodies detected in these children may be cross-reactive IgG. However, further testing is necessary to substantiate this conclusion.
On the other hand, the result of the RT-PCR Multiplex BIOFIRE test exhibited no detectable viral RNA against several pneumonia viruses. This suggests that the observed immune responses may not be attributed to the respiratory viruses identified by the assay. However, once the virus has been cleared, it might not be detectable with PCR, while the immune response could persist. 29 Therefore, RT-PCR results could explain the immune response, especially in the context of infectious diseases. 30 However, RT-PCR does not measure the immune response directly; it only indicates whether the pathogen’s genetic material is present. When a pathogen is identified in a sample via RT-PCR, and there is an ongoing immune response, it is often inferred that the detected pathogen is the cause of this response. 31 However, this relationship is not always straightforward, as other factors (co-infections, pre-existing conditions, etc.) might influence the immune response.
During our investigation, we tested 16 oropharyngeal swabs and assessed the potential of SARS-CoV-2 RNA presence by the detection of the NP and ORF genes using Sansure rRT-PCR assay. 14 of the samples were identified as positive, indicating the presence of NP and ORF1ab genes specific to SARS-CoV-2. Out of the 16 samples analyzed, 10 showed the presence of both the NP and ORF1ab genes of SARS-CoV-2 (Samples 2, 4, 6, 7, 8, 10, 11, 12, 13, 14; see Table 2). Among these, four samples also displayed IgG antibodies against the virus (Samples 2, 6, 10, 12; see Table 2), with Sample 2 testing positive for neutralizing antibodies (NAbs), while the remaining six samples had results that were ambiguous or borderline (Samples 4, 7, 8, 11, 13, 14; see Table 2). These findings may suggest that these 10 cases provide evidence supporting the hypothesis of early circulation of the virus in Morocco. Nevertheless, it’s important to acknowledge the limitations of this experiment. Factors such as the possibility of false positives, high Ct values, lack of sequencing performed, an unusually high positivity rate, and the relatively low number of samples tested are among the key limitations to be considered when interpreting these results.
However, the four cases that tested positive for only one of the SARS-CoV-2 genes (Samples 3, 5, 9, 15; see Table 2) may potentially indicate cross-reactivity. Among these cases, three also exhibited positive results for IgG antibodies (Samples 3, 5, 9; see Table 2), with sample 5 showing levels of NAbs. The fourth case yielded borderline results for IgG antibodies. The gene ORF1ab in SARS-CoV-2, as well as in other Human Coronaviruses (H-CoVs), encodes multiple non-structural proteins (nsps), along with the NP protein, that has been noted to have a high degree of identity and similarity. 32 Various comparative studies have indicated that the Sansure Biotech assay exhibits slightly superior diagnostic effectiveness compared to other RT-qPCR methods, although they are generally similar in performance. 33 However, among the limitations of the kit is that the ORF1ab and NP gene primer/probes may detect bat coronaviruses based on in silico analysis, additionally, no cross-reactivity was observed for the four H-CoVs. 34
A study conducted in Japan on the serum of 92 healthcare workers noted that during the COVID-19 pre-pandemic phase, five (5.4%) and fifteen (16.3%) participants tested positive and borderline for SARS-CoV-2 IgG antibodies, respectively. Based on their government status reports, these rates were greater than predicted. They stated that these results imply that during the beginning of the pandemic, 35 COVID-19 had already begun to spread throughout the southern section of Kyoto city. 29 In a comparative study in Angola, researchers analyzed the immune response to SARS-CoV-2 in 442 participants with both confirmed and non-confirmed clinical cases of measles, by using ELISA to test blood samples collected between September 23, 2019, and February 28th, 2020. Interestingly, within the seropositive and vaccinated measles group, 40 samples showed a positive reaction to SARS-CoV-2 viral proteins in both IgG and IgM tests, which occurred more than 2 weeks before the first confirmed COVID-19 case in Angola. These findings indicated significant differences in IgG and IgM levels, both in measles patients who were vaccinated and those who were not. 36 These differences suggest the possibility of an early and unnoticed spread of SARS-CoV-2 when compared to our study conducted during a similar timeframe. An additional study involved analyzing blood samples from 169 deceased individuals who had autopsies performed between October 1, 2019, and March 27, 2020 and reported five cases with positive anti-SARS-CoV-2 antibodies using LFIA and ELISA tests, as well as SARS-CoV-2 RNA in blood and available lung tissues through RT-PCR and ddPCR. The search of SARS-CoV-2 RNA by RT-PCR resulted in negative in all seropositive subjects but one. 37 A comprehensive analysis of multiple independent studies found retrospective evidence of the existence of antibodies and viral RNA in clinical samples. Additionally, these studies proved that SARS-CoV-2’s community transmission by detecting viral RNA in wastewater during periods that do not coincide with the virus’s initial emergence in November 2019. 38 This is consistent with our research, another study examined 235 serum samples collected from March 2017 to March 2022. These samples were obtained as part of the measles/rubella surveillance program from patients who had tested negative for measles and rubella viruses. Interestingly, they observed that 5.5% of these samples tested positive for IgG antibodies against SARS-CoV-2. 39
Overall, the results obtained in this study confirm the presence of immunity against SARS-CoV-2 immunity in children before the pandemic, with specific and neutralizing immunity. It is worth indicating that further experiments, larger cohorts, and different types of proteins (H-CoV proteins), are necessary to establish more precisely the protection duration and the antibodies nature together with their neutralizing effect against SARS-CoV-2 by investigating different epitopes. All the results obtained during this study highlight the relevance of the anti-SARS-CoV2 serological status in presuming SARS-CoV2 circulation, regardless of the cohort conducting context.
While the presence of anti-SARS-CoV-2 antibodies suggests possible early exposure, it’s challenging to definitively rule out cross-reactivity with other human coronaviruses without specific tests. The use of different tests, a larger sample size, and a single-time snapshot may affect results and not provide conclusive evidence on vaccination effects.
Conclusions
In this study, we discovered that 13% of children with eruptive fevers possessed anti-SARS-CoV-2 IgG antibodies, hinting at either early virus exposure or cross-reactivity with other coronaviruses. Intriguingly, 21% showed a specific neutralizing immune response against SARS-CoV-2, emphasizing the pediatric population’s unique immune capabilities. The potential impact of childhood vaccinations, like MMR, in shaping responses to COVID-19 was thoroughly highlighted. This research, along with future studies on antibodies nature and their neutralizing effects remains crucial for refining SARS-CoV-2 vaccine strategy and understanding the immune response to coronaviruses.
Supplemental Material
Supplemental Material for Pre-pandemic antibodies screening against SARS-CoV-2 and virus detection among children diagnosed with eruptive fevers by Nouhaila Najimi, Latifa Tajount, Zakia Regragui, Chaimae Remz, Rokaya Ait-Lhaj-Mhand, Chaimae Kadi, Lamiae Belayachi, Fouad Seghrouchni, Nadia dakka, Rabii Ameziane El Hassani, Elmir Elharti, Hicham Oumzil and Youssef Bakri in International Journal of Immunopathology and Pharmacology.
Acknowledgements
We would like to thank the Hassan II academy of science and technology for the doctoral scholarship for NN and CK.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Center for Scientific and Technical Research through the Program for Scientific and Technological Research Support related to COVID‐19. Grant number: Project N° 7/2020 CNRST Morocco.
Supplemental Material: Supplemental material for this article is available online.
Ethical statement
Ethical approval
Ethical approval for this study was obtained from Biomedical Research (CERB) of the Faculty of Medicine and Pharmacy in Rabat BOARD (APPROVAL NUMBER/ID): 28/20.
Informed consent
Written informed consent was obtained from legally authorized representatives before the study.
ORCID iD
Nouhaila Najimi https://orcid.org/0009-0008-5536-0237
References
- 1.Adil MT, Rahman R, Whitelaw D, et al. (2021) SARS-CoV-2 and the pandemic of COVID-19. Postgrad Med 97(1144): 110–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morocco's official coronavirus portal. https://www.covidmaroc.ma/Pages/CommuniquesAR.aspx [Google Scholar]
- 3.Morocco country overview. World Health Organization. https://www.who.int/countries/mar [Google Scholar]
- 4.Flaxman S, Mishra S, Gandy A, et al. (2020) Estimating the effects of non-pharmaceutical interventions on COVID-19 in Europe. Nature 584(7820): 257–261. [DOI] [PubMed] [Google Scholar]
- 5.Spellberg B, Nielsen TB, Casadevall A. (2021) Antibodies, immunity, and COVID-19. JAMA Intern Med 181(4): 460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bajpai P, Singh V, Chandele A, et al. (2022) Broadly neutralizing antibodies to SARS-CoV-2 provide novel insights into the neutralization of variants and other human coronaviruses. Front Cell Infect Microbiol 12: 928279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Segondy M. (2020) Les coronavirus humains. Revue francophone des laboratoires: Rundsch für Fleischhygiene Leb (RFL) 2020(526): 32–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murray SM, Ansari AM, Frater J, et al. (2023) The impact of pre-existing cross-reactive immunity on SARS-CoV-2 infection and vaccine responses. Nat Rev Immunol 23(5): 304–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Le Bert N, Tan AT, Kunasegaran K, et al. (2020) SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature 584(7821): 457–462. [DOI] [PubMed] [Google Scholar]
- 10.table_ 2_feb_2023_English.pdf. https://cdn.who.int/media/docs/default-source/immunization/immunization_schedules/table_2_feb_2023_english.pdf?sfvrsn=3e27ab48_11 [Google Scholar]
- 11.Soodejani MT, Basti M, Tabatabaei SM, et al. (2021) Measles, mumps, and rubella (MMR) vaccine and COVID-19: a systematic review. Int J Mol Epidemiol Genet 12(3): 35–39 [PMC free article] [PubMed] [Google Scholar]
- 12.Andina D, Belloni-Fortina A, Bodemer C, et al. (2021) Skin manifestations of COVID‐19 in children: Part 2. Clin Exp Dermatol 46(3): 451–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hicks J, Klumpp-Thomas C, Kalish H, et al. (2021) Serologic cross-reactivity of SARS-CoV-2 with endemic and seasonal betacoronaviruses. J Clin Immunol 41(5): 906–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harrison CM, Doster JM, Landwehr EH, et al. (2023) Evaluating the virology and evolution of seasonal human coronaviruses associated with the common cold in the COVID-19 era. Microorganisms 11(2): 445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Woudenberg T, Pelleau S, Anna F, et al. (2021) Humoral immunity to SARS-CoV-2 and seasonal coronaviruses in children and adults in north-eastern France. EBioMedicine 70: 103495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haileamlak A. (2020) Why COVID-19 is less severe in pediatric patients? Ethiopian journal of health sciences 30(4): 467–468. https://www.ajol.info/index.php/ejhs/article/view/199833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Piccaluga PP, Malerba G, Navari M, et al. (2020) Cross-immunization against respiratory coronaviruses may protect children from SARS-CoV2: more than a simple hypothesis? Frontiers in pediatrics 8: 595539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ng KW, Faulkner N, Cornish GH, et al. (2020) Preexisting and de novo humoral immunity to SARS-CoV-2 in humans. Science 370(6522): 1339–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.who-surveillancevaccinepreventable-11-measles-r2.pdf. https://cdn.who.int/media/docs/default-source/immunization/vpd_surveillance/vpd-surveillance-standards-publication/who-surveillancevaccinepreventable-11-measles-r2.pdf?sfvrsn=6d8879f9_10&download=true [Google Scholar]
- 20.Gold JE, Baumgartl WH, Okyay RA, et al. (2020) Analysis of measles-mumps-rubella (MMR) titers of recovered COVID-19 patients. Pirofski L anne. mBio 11(6): e02628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sidiq KR, Sabir DK, Ali SM, et al. (2020) Does early childhood vaccination protect against COVID-19? Front Mol Biosci 7: 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haguet H, Douxfils J, Eucher C, et al. (2021) Clinical performance of the panbio assay for the detection of SARS‐CoV‐2 IgM and IgG in COVID‐19 patients. J Med Virol 93(5): 3277–3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nandakumar V, Profaizer T, Lozier BK, et al. (2021) Evaluation of a surrogate enzyme-linked immunosorbent assay–based severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) cPass neutralization antibody detection assay and correlation with immunoglobulin G commercial serology assays. Arch Pathol Lab Med 145(10): 1212–1220. [DOI] [PubMed] [Google Scholar]
- 24.Guthmiller JJ, Wilson PC. (2018) Harnessing immune history to combat influenza viruses. Curr Opin Immunol 53: 187–195. [DOI] [PubMed] [Google Scholar]
- 25.Henry C, Palm AKE, Krammer F, et al. (2018) From original antigenic sin to the universal influenza virus vaccine. Trends Immunol 39(1): 70–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Monto AS, Ohmit SE, Petrie JG, et al. (2009) Comparative efficacy of inactivated and live attenuated influenza vaccines. N Engl J Med 361(13): 1260–1267. [DOI] [PubMed] [Google Scholar]
- 27.Kiyuka PK, Agoti CN, Munywoki PK, et al. (2018) Human coronavirus NL63 molecular epidemiology and evolutionary patterns in rural coastal Kenya. J Infect Dis 217(11): 1728–1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Edridge AWD, Kaczorowska J, Hoste ACR, et al. (2020) Seasonal coronavirus protective immunity is short-lasting. Nat Med 26(11): 1691–1693. [DOI] [PubMed] [Google Scholar]
- 29.Fujita K, Kada S, Kanai O, et al. (2020) Quantitative SARS-CoV-2 antibody screening of healthcare workers in the southern part of Kyoto city during the COVID-19 pre-pandemic period. Front Public Health 8: 595348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Mello Malta F, Amgarten D, Marra AR, et al. (2023) Nucleocapsid single point-mutation associated with drop-out on RT-PCR assay for SARS-CoV-2 detection. BMC Infect Dis 23(1): 714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Y, Zhang L, Sang L, et al. (2020) Kinetics of viral load and antibody response in relation to COVID-19 severity. J Clin Invest 130(10): 5235–5244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Forni D, Cagliani R, Mozzi A, et al. (2016) Extensive positive selection drives the evolution of nonstructural proteins in lineage C betacoronaviruses. J Virol 90(7): 3627–3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Banko A, Petrovic G, Miljanovic D, et al. (2021) Comparison and sensitivity evaluation of three different commercial real-time quantitative PCR kits for SARS-CoV-2 detection. Viruses 13(7): 1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.download.pdf. https://www.fda.gov/media/137651/download [Google Scholar]
- 35.Teymouri M, Mollazadeh S, Mortazavi H, et al. (2021) Recent advances and challenges of RT-PCR tests for the diagnosis of COVID-19. Pathol Res Pract 221: 153443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paixao J, Galangue M, Gaston C, et al. (2022) Early evidence of circulating SARS-CoV-2 in unvaccinated and vaccinated measles patients, september 2019–february 2020. Infect Drug Resist 15: 533–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lai A, Tambuzzi S, Bergna A, et al. (2022) Evidence of SARS-CoV-2 antibodies and RNA on autopsy cases in the pre-pandemic period in milan (Italy). Front Microbiol 13: 886317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Canuti M, Bianchi S, Kolbl O, et al. (2022) Waiting for the truth: is reluctance in accepting an early origin hypothesis for SARS-CoV-2 delaying our understanding of viral emergence? BMJ Glob Health 7(3): e008386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bianchi S, Fappani C, Gori M, et al. (2023) Serological investigation of SARS-CoV-2 infection in patients with suspect measles, 2017–2022. Virol J 20(1): 160. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for Pre-pandemic antibodies screening against SARS-CoV-2 and virus detection among children diagnosed with eruptive fevers by Nouhaila Najimi, Latifa Tajount, Zakia Regragui, Chaimae Remz, Rokaya Ait-Lhaj-Mhand, Chaimae Kadi, Lamiae Belayachi, Fouad Seghrouchni, Nadia dakka, Rabii Ameziane El Hassani, Elmir Elharti, Hicham Oumzil and Youssef Bakri in International Journal of Immunopathology and Pharmacology.