Abstract
Background:
Perioperative use of immune checkpoint blockade (ICB) improves survival in patients with early-stage cancer. Treatment-related adverse events (AEs), frequently involve the endocrine system which may increase perioperative complications and affect quality of life.
Objective:
We conducted a meta-analysis to elucidate the impact of adding ICB to conventional neoadjuvant/adjuvant therapy on the incidence of endocrine AEs.
Design:
A systematic review and meta-analysis of randomize-controlled trials (RCTs).
Data sources and methods:
A systematic search of PubMed, Embase, Web of Science, and Cochrane library was performed for RCTs comparing groups with and without the addition of ICB to conventional perioperative therapy in patients with cancer. Outcomes included all-grade and grade 3–5 thyroiditis, hyperthyroidism, hypothyroidism, adrenal insufficiency, hypophysitis, type 1 diabetes mellitus, and hyperglycemia. The odds ratios (ORs) of all-grade and grade 3–5 endocrine were pooled using the random-effect model meta-analysis.
Results:
Twenty-four RCTs comprising 12,199 patients were identified for meta-analysis. The addition of ICB was associated with higher incidence of thyroiditis [all grade: OR = 3.53 (95% confidence interval (CI): 1.88–6.64)], hyperthyroidism [all-grade: 7.18 (4.30–12.01); grade 3–5: 3.93 (1.21–12.82)], hypothyroidism [all-grade: 5.39 (3.68–7.90); grade 3–5: 3.63 (1.18–11.11)], adrenal insufficiency [all-grade: 3.82 (1.88–7.79); grade 3–5: 5.91 (2.36–14.82)], hypophysitis [all-grade: 10.29 (4.97–21.3); grade 3–5: 5.80 (1.99–16.92)], and type 1 diabetes mellitus [all-grade: 2.24 (1.06–4.74); grade 3–5: 3.49 (1.21–10.08)]. The cumulative incidence of each grade 3–5 endocrine AE was low (<1.3%). No grade 5 AEs leading to death were observed.
Conclusion:
The addition of neoadjuvant/adjuvant ICB to conventional therapy was associated with an increased incidence of several endocrine AEs. Clinicians should be aware of the risk of endocrinopathy from the perioperative ICB use to facilitate risk–benefit discussion with patients with early-stage cancer.
Trial registration:
The protocol of this research was registered in PROSPERO (CRD42022332624).
Keywords: adjuvant therapy, cancer immunotherapy, endocrine-related adverse events, immune checkpoint blockade, immune-related adverse events, neoadjuvant therapy
Introduction
In recent decades, immunotherapy including immune checkpoint blockade (ICB) and cellular therapy has emerged as the ‘fifth pillar’ of cancer therapy, expanding the ranks of surgery, chemotherapy, radiation, and targeted therapy.1,2 ICB has become one of the most important breakthroughs in cancer treatment, especially in patients with advanced, recurrent, and metastatic cancer.3–5 Four different groups of ICB, including programmed cell death protein-1 (PD-1), programmed cell death ligand-1 (PD-L1), cytotoxic T lymphocyte-associated protein-4 (CTLA-4), and lymphocyte-activation gene 3 (LAG-3) blockade have been approved by the U.S. Food and Drug Administration for the treatment of various types of cancer. ICB was approved for advanced cancer after ipilimumab showed efficacy in patients with advanced/metastatic melanoma. 6 The incorporation of ICB into neoadjuvant or adjuvant therapy with surgery and/or radiotherapy also showed survival benefits, leading to approval in the perioperative setting in 2015. 7 Multiple clinical trials have shown perioperative ICB, either monotherapy or combined with chemotherapy, resulted in improved survival in non-small cell lung cancer, breast cancer, urothelial carcinoma, and renal cell carcinoma.8–10 Therefore, ICB is currently used as adjuvant and neoadjuvant treatment for many resectable cancers.
ICB disrupts immunologic homeostasis by reactivating cellular immunity, increasing the incidence of treatment-related adverse events (trAEs), mostly immune toxicities known as immune-related adverse events (irAEs). 11 Endocrine adverse events (AEs), including thyroiditis, hyperthyroidism, hypothyroidism, hypophysitis, adrenal insufficiency, and type 1 diabetes mellitus, occur in approximately 10% of patients treated with ICB.12,13 The incidence, risk, and management of irAEs has been evaluated in previous studies in patients with unresectable/metastatic cancers. 14 Severe trAEs may lead to delay or cancellation of surgery, increased postoperative complications, and even fatal events.15,16 Endocrine AEs may necessitate life-long hormone replacement therapy and negatively affect patients’ quality of life. These risks must be balanced with the potential for prolonged survival and cure among patients with early stage disease. 17 Therefore, data are needed to assess the incidence of endocrine AEs among patients receiving neoadjuvant and adjuvant ICB for curative intent.
We performed a systematic review and meta-analysis of endocrine AEs in patients receiving neoadjuvant/adjuvant therapy with ICB to evaluate the effect of the addition of ICB on the incidence of endocrine AEs, which guides clinicians providing perioperative ICB therapy for patients with early-stage cancer.
Methods
Data source and search strategy
We conducted a systematic review and meta-analysis under Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) criteria. 18 We performed a systematic search of PubMed, EMBASE, Web of Science, and Cochrane library to identify articles up to 18 December 2022, reporting results of randomized controlled trials (RCTs) evaluating neoadjuvant and adjuvant therapy with ICB in patients with solid tumors. The search strategy is described in Supplemental Table 1. The protocol of this research was registered in PROSPERO with a registry number CRD42022332624.
Study selection
To evaluate the effect of ICB on the incidence of endocrine AEs, studies meeting the following inclusion criteria were chosen for meta-analysis19,20: (1) RCTs reporting the efficacy and safety of neoadjuvant and/or adjuvant ICB in patients with solid tumors; (2) RCTs with an experimental arm of ICB combined with conventional neoadjuvant/adjuvant therapy and a control arm of the same conventional neoadjuvant/adjuvant therapy (such as ICB versus placebo/observation, ICB plus chemotherapy versus chemotherapy, ICB-‘1’ plus ICB-‘2’versus ICB-‘2’); and (3) RCT reporting the results of endocrine AEs. If multiple articles reported results of the same RCT, we chose an article that contained the most-updated information on endocrine toxicity.
Data extraction
Two investigators (SZ and YF) independently extracted data from all eligible studies. Any discrepancies between review authors were resolved by consensus. We recorded the following information of each eligible RCT: first author’s name, publication year, study name, cancer type, cancer status, treatment setting (adjuvant and/or neoadjuvant), ICB subtype, treatment in each arm, RCT design (double-blind, open-label), reported endocrine AEs, the number of patients, the number of all-grade, and grade 3–5 endocrine AEs (thyroiditis, hyperthyroidism, hypothyroidism, hypophysitis, adrenal insufficiency, type 1 diabetes mellitus, and hyperglycemia). The Cochrane Risk of Bias Tool was used to evaluate the risk of bias for each RCT. 21 TrAEs were prioritized for data extraction and meta-analysis, but irAEs were chosen if no trAEs were reported in eligible studies.
Statistical analysis
We recorded the number of patients and endocrine AEs in each treatment arm and calculated the odds ratio (OR) and corresponding 95% confidence interval (CI) of each all-grade and grade 3–5 endocrine AEs. We then performed a meta-analysis of each endocrine AE by pooling ORs using random-effects models. A p-value less than 0.05 was considered statistically significant. Funnel plots were applied to evaluate publication bias of each outcome with more than 10 studies. Subgroup analyses were conducted based on ICB class (PD-1, PD-L1, and CTLA-4 blockade) and clinical trial setting (neoadjuvant and/or adjuvant). Cochran’s Q-test and I2 statistics were used to evaluate the heterogeneity in each analysis. I2 values of greater than 50% were considered as substantial heterogeneity in our study. We used RevMan 5.4 (The Cochrane Collaboration, Copenhagen, Denmark) for these analyses. 22 The incidence of each AEs was calculated as the number of total events divided by the number of patients receiving ICB treatment in both experimental and control arms.
Results
Eligible studies and baseline characteristics
The systemic search identified 3602 records. After removing 1520 duplicates and 1997 records by title and abstract screening, full texts of 85 articles were reviewed in detail. Finally, 24 studies involving 12,199 patients were included for meta-analysis.7,23–45 The PRISMA flow diagram for a systematic review is shown in Figure 1.
Figure 1.
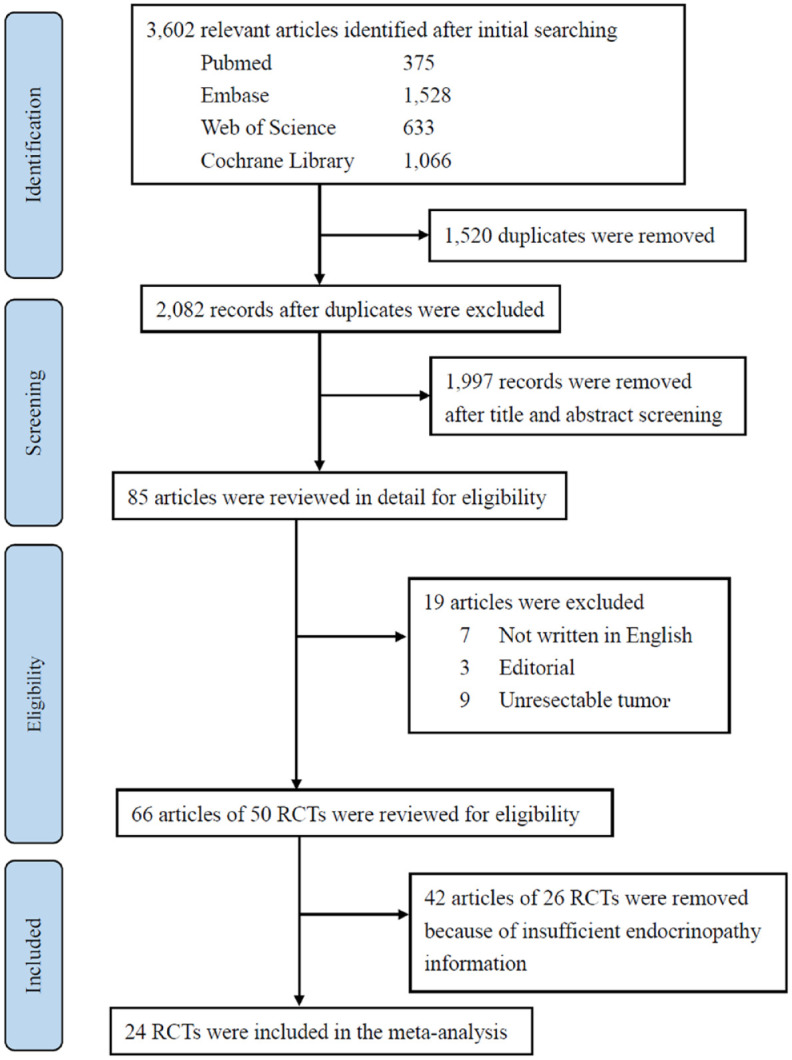
Flow diagram of study selection.
RCTs, randomized controlled trials.
The characteristics of 24 included studies are summarized in Table 1. Overall, 10, 12, and 2 studies evaluated ICB in neoadjuvant, adjuvant, and neoadjuvant/adjuvant settings, respectively. Regarding ICB subtype, 6, 10, and 8 studies assessed CTLA-4, PD-1, and PD-L1 blockade, respectively. About treatment design, 5 studies compared dual ICB therapy to ICB monotherapy, 11 studies compared ICB to placebo/observation, and 8 studies compared ICB plus chemotherapy to the same chemotherapy. Most commonly evaluated cancers were malignant melanoma (n = 5), breast cancer (n = 5), and non-small cell lung cancer (n = 3) (Table 1).
Table 1.
Main characteristics of the included randomized control trials.
| First author | Year | Study | Cancer | Cancer status | Study setting | ICB added in the experimental group | Control/baseline treatment | Analyzed endocrine irAE | Patients | Reference | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| ICB | Control | ||||||||||
| Amaria | 2018 | NCT02519322 | Melanoma | Neoadjuvant | Ipilimumab | Nivolumab | Hyperthyroidism, hypothyroidism, hypophysitis, hyperglycemia |
11 | 12 | 23 | |
| Bajorin | 2021 | CheckMate 274 | Urothelial | Muscle-invasive | Adjuvant | Nivolumab | Placebo | Thyroiditis, hyperthyroidism, hypothyroidism, adrenal insufficiency, type 1 diabetes mellitus |
351 | 348 | 24 |
| Bellmunt | 2021 | IMvigor010 | Urothelial | Muscle-invasive | Adjuvant | Atezolizumab | Observation | Hyperthyroidism, hypothyroidism, hypophysitis, type 1 diabetes mellitus, hyperglycemia |
390 | 397 | 25 |
| Cascone | 2021 | NEOSTAR | NSCLC | Neoadjuvant | Ipilimumab | Nivolumab | Hypothyroidism | 21 | 23 | 26 | |
| Choueiri | 2021 | KEYNOTE-564 | Renal | Adjuvant | Pembrolizumab | Placebo | Thyroiditis, hyperthyroidism, hypothyroidism, adrenal insufficiency, hypophysitis, type 1 diabetes mellitus | 488 | 496 | 27 | |
| Cloughesy | 2019 | Ivy Consortium | Glioblastoma | Neoadjuvant | Pembrolizumab | Adjuvant pembrolizumab | Hyperthyroidism, hypothyroidism | 16 | 16 | 28 | |
| Eggermont | 2015 | EORTC 18071 | Melanoma | Stage III | Adjuvant | Ipilimumab | Placebo | Hypothyroidism, hypophysitis | 471 | 474 | 7 |
| Eggermont | 2020 | EORTC 1325-MG/KEYNOTE-054 | Melanoma | Resected Stage III | Adjuvant | Pembrolizumab | Placebo | Thyroiditis, hyperthyroidism, hypothyroidism, adrenal insufficiency, hypophysitis, type 1 diabetes mellitus | 509 | 502 | 29 |
| Felip | 2021 | IMpower010 | NSCLC | Stage IB–IIIA | Adjuvant | Atezolizumab | BSC following adjuvant chemotherapy | Hyperthyroidism, hypothyroidism, adrenal insufficiency, hypophysitis, type 1 diabetes mellitus | 495 | 495 | 30 |
| Forde | 2022 | CheckMate 816 | NSCLC | Stage IB–IIIA | Neoadjuvant | Nivolumab | platinum-doublet chemotherapy | Hyperthyroidism, hypothyroidism, adrenal insufficiency, hypophysitis, type 1 diabetes mellitus | 176 | 176 | 31 |
| Gianni | 2022 | NeoTRIP | Breast | Neoadjuvant | Atezolizumab | CBDCA + nabPTX | Thyroiditis, hyperthyroidism, hypothyroidism | 138 | 140 | 32 | |
| Kaseb | 2022 | NCT03222076 | Hepatocellular | Neoadjuvant and adjuvant | Ipilimumab | Nivolumab | Hyperthyroidism, hypothyroidism, adrenal insufficiency, hyperglycemia | 14 | 13 | 33 | |
| Kelly | 2021 | CheckMate 577 | Esophageal or GEJ | Resected stage II or III | Adjuvant | Nivolumab | Neoadjuvant CRT, surgery, adjuvant placebo | Hyperthyroidism, hypothyroidism | 532 | 260 | 34 |
| Loibl | 2019 | GeparNuevo | Breast | Neoadjuvant | Durvalumab | Placebo + nabPTX, EC | Hyperthyroidism, hypothyroidism, hypophysitis, hyperglycemia | 92 | 82 | 35 | |
| Luke | 2022 | KEYNOTE-716 | Melanoma | Stage IIB or IIC | Adjuvant | Pembrolizumab | Placebo | Thyroiditis, hyperthyroidism, hypothyroidism, adrenal insufficiency, hypophysitis, type 1 diabetes mellitus | 483 | 486 | 36 |
| Mittendorf | 2020 | IMpassion031 | TNBC | Neoadjuvant | Atezolizumab | Placebo + nabPTX followed by CPA + ADR | Hyperthyroidism, hypothyroidism, adrenal insufficiency, hypophysitis, type 1 diabetes mellitus | 164 | 167 | 37 | |
| Monk | 2021 | JAVELIN Ovarian 100 | Ovarian | Stage III or IV | After debulking surgery or neoadjuvant | Avelumab | Chemotherapy followed by observation | Thyroiditis, hyperthyroidism, hypothyroidism, adrenal insufficiency, type 1 diabetes mellitus, hyperglycemia | 328 | 334 | 38 |
| Moore Nanda |
2021 2020 |
IMagyn050 I-SPY2 |
Ovarian Breast |
Stage III or IV Stage II or III |
After debulking surgery or neoadjuvant Neoadjuvant |
Atezolizumab Pembrolizumab |
Placebo + paclitaxel + carboplatin + bevacizumab PTX, AC |
Hyperthyroidism, hypothyroidism, adrenal insufficiency, type 1 diabetes mellitus, hyperglycemia Hyperthyroidism, hypothyroidism, adrenal insufficiency, hypophysitis, hyperglycemia |
642 69 |
644 181 |
39, 40 |
| Park | 2022 | NCT02520453 | Esophageal | Adjuvant | Durvalumab | Placebo after CCRT | Hyperthyroidism, hypothyroidism, adrenal insufficiency, hyperglycemia |
45 | 41 | 41 | |
| Rahma | 2021 | NRG-GI002 | Rectal | Stage II or III | Neoadjuvant | Pembrolizumab | Neoadjuvant FOLFOX, CRT (with capecitabine) | Hyperthyroidism, hypothyroidism | 81 | 83 | 42 |
| Schmid | 2022 | KEYNOTE-522 | Breast | Neoadjuvant and adjuvant | Pembrolizumab | Placebo + CBDCA + PTX, AC/EC, placebo | Thyroiditis, hyperthyroidism, hypothyroidism, adrenal insufficiency, hypophysitis, type 1 diabetes mellitus |
783 | 389 | 43 | |
| Schoenfeld | 2020 | NCT02919683 | Oral cavity | Neoadjuvant | Ipilimumab | Nivolumab | Hyperthyroidism, hypothyroidism, type 1 diabetes mellitus, hyperglycemia |
15 | 15 | 44 | |
| Zimmer | 2020 | IMMUNED | Melanoma | Resected stage IV | Adjuvant | Ipilimumab | Nivolumab | Thyroiditis, hyperthyroidism, hypothyroidism, adrenal insufficiency, hypophysitis, type 1 diabetes mellitus, hyperglycemia | 55 | 56 | 45 |
AC, adriamycin + cyclophosphamide; ADR, adriamycin; BSC, best supportive care; CBDCA, carboplatin; CCRT, concomitant chemoradiotherapy; CPA, cyclophosphamide; CRT, chemo-radiotherapy; EC, epirubicin + cyclophosphamide; FOLFOX, 5-fluorouracil + oxaliplatin; GEJ, gastroesophageal junction; ICB, immune checkpoint blockade; irAE, immune-related adverse event; nabPTX, nab-paclitaxel; NSCLC, non-small cell lung cancer; PTX, paclitaxel; TNBC, triple negative breast cancer.
Meta-analysis of endocrine AEs
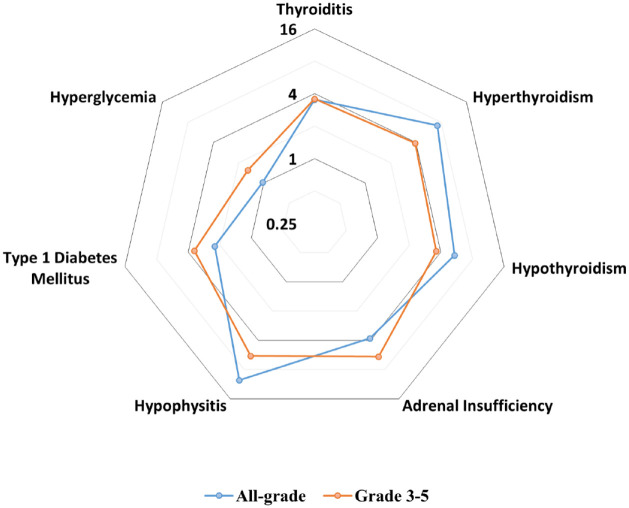
We performed meta-analyses of all-grade and grade 3–5 endocrine AEs: thyroiditis, hyperthyroidism, hypothyroidism, hypophysitis, adrenal insufficiency, type 1 diabetes mellitus, and hyperglycemia. The results of these meta-analyses are summarized in Figure 2 and Table 2. No grade 5 endocrine AEs were observed.
Figure 2.
Radar chart illustrating pooled odds ratios of endocrine adverse events associated with immune checkpoint blockade. Seven axes represent the log-transformed odds ratio of each endocrinopathies. The incidence of all-grade adverse events is represented in blue, whereas grade 3–5 adverse events are plotted in orange.
Table 2.
OR of endocrine AEs with subgroup analysis based on ICB subtype.
| ICB subgroup | Overall | CTLA-4 blockade | PD-1 blockade | PD-L1 blockade | Subgroup difference | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Endocrine AEs | No. studies | OR (95% CI) | p | No. studies | OR (95% CI) | p | No. studies | OR (95% CI) | p | No. studies | OR (95% CI) | p | I2 (%) | p |
| All-grade | ||||||||||||||
| Thyroiditis | 8 | 3.53 (1.88–6.64) | <0.001 | 1 | 9.87 (0.52–187.9) | 0.13 | 5 | 3.23 (1.64–6.35) | <0.001 | 2 | 5.13 (0.60–44.15) | 0.14 | 0 | 0.74 |
| Hyperthyroidism | 22 | 7.18 (4.30–12.01) | <0.001 | 4 | 1.99 (0.54–7.33) | 0.30 | 10 | 11.21 (5.89–21.32) | <0.001 | 8 | 6.25 (2.69–14.50) | <0.001 | 64.6 | 0.06 |
| Hypothyroidism | 24 | 5.39 (3.68–7.90) | <0.001 | 6 | 1.70 (0.51–5.68) | 0.39 | 10 | 5.72 (4.21–7.77) | <0.001 | 8 | 8.02 (3.11–20.71) | <0.001 | 53.6 | 0.12 |
| Adrenal insufficiency | 14 | 3.82 (1.88–7.79) | <0.001 | 2 | 1.58 (0.18–13.56) | 0.67 | 7 | 6.91 (2.10–22.73) | 0.001 | 5 | 2.64 (0.85–8.18) | 0.09 | 0.2 | 0.37 |
| Hypophysitis | 12 | 10.29 (4.97–21.3) | <0.001 | 3 | 9.15 (0.78–106.86) | 0.08 | 7 | 8.96 (3.35–23.96) | <0.001 | 2 | 2.85 (0.29–27.58) | 0.37 | 0 | 0.66 |
| Type 1 diabetes mellitus | 13 | 2.24 (1.06–4.74) | 0.03 | 2 | 1.01 (0.10–10.01) | 1.00 | 6 | 6.17 (1.81–21.03) | 0.004 | 5 | 1.28 (0.45–3.61) | 0.64 | 52.5 | 0.12 |
| Hyperglycemia | 10 | 1.03 (0.76–1.39) | 0.87 | 4 | 2.12 (0.42–10.63) | 0.36 | 1 | 2.01 (0.44–9.23) | 0.37 | 5 | 1.0 (0.66–1.52) | 0.99 | 0 | 0.49 |
| Grade 3–5 | ||||||||||||||
| Thyroiditis | 2 | 3.57 (0.42–30.58) | 0.25 | – | – | – | 2 | 3.57 (0.42–30.58) | 0.25 | – | – | – | – | – |
| Hyperthyroidism | 7 | 3.93 (1.21–12.82) | 0.02 | 2 | 6.29 (0.70–56.73) | 0.10 | 3 | 2.83 (0.46–17.41) | 0.26 | 2 | 3.97 (0.44–36.04) | 0.22 | 0 | 0.86 |
| Hypothyroidism | 7 | 3.63 (1.18–11.11) | 0.02 | 1 | 3.03 (0.12–74.46) | 0.50 | 4 | 4.30 (0.90–20.58) | 0.07 | 2 | 3.03 (0.48–19.29) | 0.24 | 0 | 0.95 |
| Adrenal insufficiency | 10 | 5.91 (2.36–14.82) | <0.001 | 1 | 3.11 (0.12–78.01) | 0.49 | 6 | 7.79 (2.52–24.07) | <0.001 | 3 | 3.53 (0.57–21.83) | 0.17 | 0 | 0.71 |
| Hypophysitis | 8 | 5.80 (1.99–16.92) | 0.001 | 3 | 4.27 (0.18–99.76) | 0.37 | 5 | 6.24 (1.63–23.82) | 0.007 | – | – | – | 0 | 0.83 |
| Type 1 diabetes mellitus | 8 | 3.49 (1.21–10.08) | 0.02 | 1 | 0.31 (0.01–8.28) | 0.49 | 4 | 7.54 (1.72–32.98) | 0.007 | 3 | 2.37 (0.42–13.33) | 0.33 | 39.8 | 0.19 |
| Hyperglycemia | 4 | 1.55 (0.77–3.10) | 0.22 | 2 | 1.05 (0.10–11.41) | 0.97 | – | – | – | 2 | 1.61 (0.78–3.33) | 0.20 | 0 | 0.74 |
CI, confidence interval; CTLA-4, cytotoxic T-lymphocyte antigen 4; ICB, immune checkpoint blockade; OR, odds ratio; PD-1, programmed cell death protein-1; PD-L1, programmed cell death ligand 1.
P values less than 0.05 and I2 values 50 or more are bolded in the table.
Thyroid dysfunction
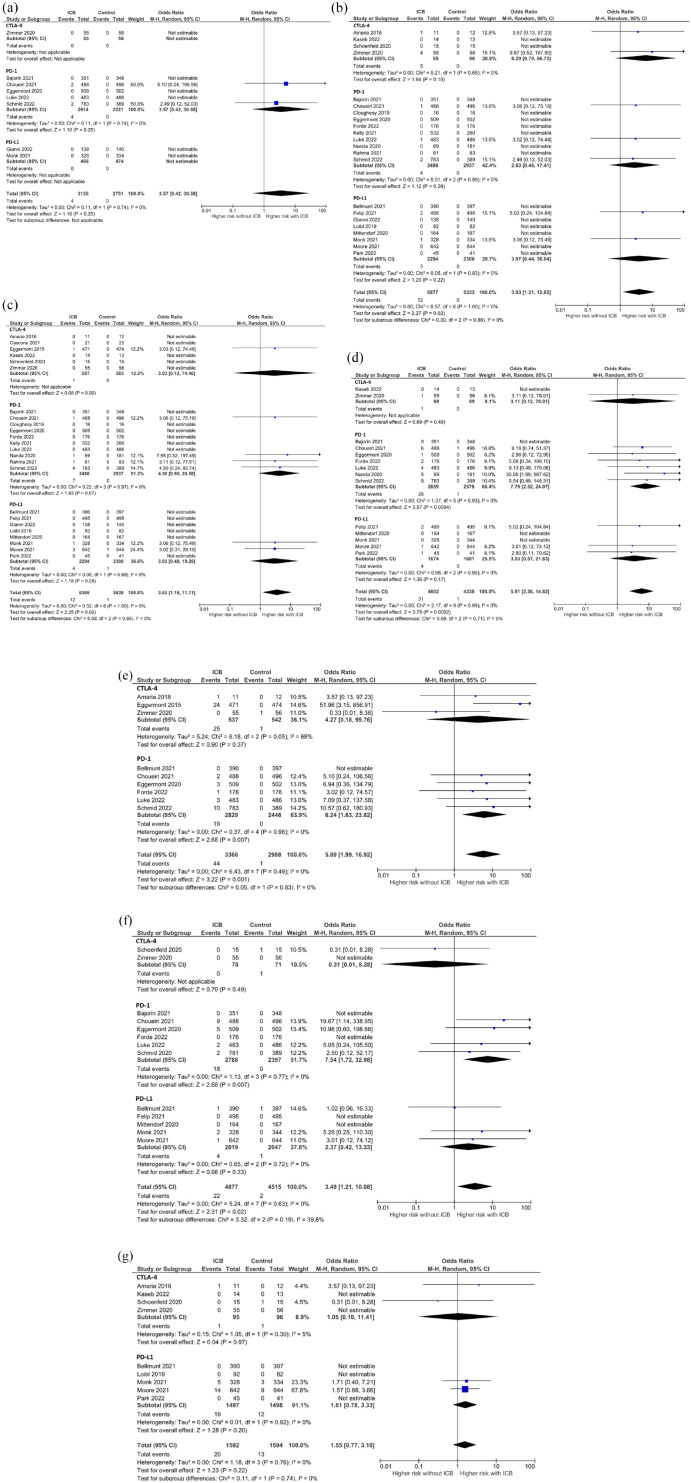
The addition of ICB to conventional neoadjuvant or adjuvant therapy was associated with an increase in the incidence of all-grade thyroiditis (OR: 3.53, 95% CI: 1.88–6.64, p < 0.001), hyperthyroidism (OR: 7.18, 95% CI: 4.30–12.01, p < 0.001), and hypothyroidism (OR: 5.39, 95% CI: 3.68–7.90, p < 0.001) [Table 2 and Supplemental Figure 1(A)–(C)]. For grade 3–5 thyroid dysfunction, the addition of ICB to conventional perioperative treatment significantly increased the incidence of hyperthyroidism (OR: 3.93, 95% CI: 1.21–12.82, p = 0.02) and hypothyroidism (OR: 3.63, 95% CI: 1.18–11.11, p = 0.02), but did not increase the incidence of thyroiditis (OR: 3.57, 95% CI: 0.42–30.58, p = 0.25) [Table 2 and Figure 3(a)–(c)]. The incidence of grade 3–5 thyroid-related AEs in patients treated with ICB was low: 0.13% (N = 4/3191) for thyroiditis, 0.20% (N = 12/5973) for hyperthyroidism, and 0.19% (N = 12/6448) for hypothyroidism.
Figure 3.
Forest plot of grade 3–5 endocrine adverse events with subgroup analyses based on ICB subtype. (a) Thyroiditis. (b) Hyperthyroidism. (c) Hypothyroidism. (d) Adrenal insufficiency. (e) Hypophysitis. (f) Type 1 diabetes mellitus. (g) Hyperglycemia.
CI, confidence interval; CTLA-4, T lymphocyte-associated protein 4; ICB, immune checkpoint blockade; PD-1, programmed cell death protein-1; PD-L1, programmed cell death ligand-1.
In subgroup analysis according to ICB subtype, the addition of PD-1 or PD-L1 blockade was associated with a higher incidence of all-grade thyroid dysfunction. In contrast, CTLA-4 blockade was not associated with increased incidence of any all-grade thyroid AEs. None of the ICB subtypes were associated with higher incidence of grade 3–5 thyroid dysfunction (Table 2). Moderate heterogeneity among subgroups of ICB subtype was observed for all-grade hyperthyroidism (I2 = 64.6%) and hypothyroidism (I2 = 53.6%), but when analysis was limited to 18 studies of PD-1/PD-L1 blockade, heterogeneity between subgroups became low (I2 = 14.5% for hyperthyroidism and 0% for hypothyroidism), suggesting high heterogeneity derived from discrepancy between CTLA-4 and PD-1/PD-L1 subgroups.
Adrenal insufficiency and hypophysitis
The addition of ICB resulted in a significantly higher incidence of adrenal insufficiency (all-grade: OR: 3.82, 95% CI: 1.88–7.79, p < 0.001; grade 3–5: OR: 5.91, 95% CI: 2.36–14.82, p < 0.001) and hypophysitis (all-grade: OR: 10.29, 95% CI: 4.97–21.3, p < 0.001; grade 3–5: OR: 5.80, 95% CI: 1.99–16.92, p = 0.001). The incidence of grade 3–5 adrenal insufficiency and hypophysitis in patients treated with ICB was 0.66% (N = 31/46711) and 1.28% (N = 44/3434), respectively. Subgroup analysis by ICB subtype showed that the incidence of all-grade and grade 3–5 adrenal insufficiency and hypophysitis were significantly increased by the addition of PD-1 blockade but not by the addition of CTLA-4 or PD-L1 blockade [Table 2, Figure 3(d) and (e), and Supplemental Figure 1(D) and (E)]. Heterogeneity was not high among ICB subtypes for these AEs (Table 2).
Type 1 diabetes mellitus and hyperglycemia
The addition of ICB to conventional perioperative therapy resulted in an increase in the incidence of type 1 diabetes mellitus (all-grade: OR: 2.24, 95% CI: 1.06–4.74, p = 0.03; grade 3–5: OR: 3.49, 95% CI: 1.21–10.08, p = 0.02). Moderately high heterogeneity among ICB subtypes was found for all-grade type 1 diabetes mellitus (I2 = 52.5%). On the other hand, the incidence of both all-grade and grade 3–5 hyperglycemia was not significantly increased by the addition of ICB (all-grade: OR: 1.03, 95% CI: 0.76–1.39, p = 0.87; grade 3–5: OR: 1.55, 95% CI: 0.77–3.10, p = 0.22). The incidence of grade 3–5 type 1 diabetes mellitus and hyperglycemia in patients treated with ICB was 0.44% (N = 22/4948) and 1.18% (N = 20/1688), respectively. The summary of subgroup analysis based on ICB subtype is shown in Table 2, Figure 3(f) and (g), and Supplemental Figure 1(F) and (G).
Subgroup analysis based on clinical trial setting
We next conducted subgroup analyses based on clinical trial setting (neoadjuvant and adjuvant therapy). Only a single small study included patients who received ICB in the neoadjuvant setting, therefore this result should be interpreted with caution. The addition of ICB in the adjuvant setting was associated with a significant increase in the incidence of grade 3–5 type 1 diabetes mellitus (OR: 5.10, 95% CI: 1.52–17.05, p = 0.008), but this increase was not seen in the neoadjuvant setting (OR: 0.31, 95% CI: 0.01–8.28, p = 0.49) with moderate subgroup differences (I2 = 56.3%, p = 0.12). Otherwise, no significant subgroup heterogeneity between neoadjuvant and adjuvant groups was observed for all-grade and grade 3–5 endocrinopathies (Supplemental Table 2).
Comparison of dual ICB with ICB monotherapy
We also compared the incidence of endocrine AEs from dual ICB (PD-1 and CTLA-4 blockade) to that from PD-1 blockade alone. As shown in Supplemental Table 3, the incidence of all-grade and grade 3–5 endocrine AEs was not significantly different between patients on dual ICB and those on ICB monotherapy although the number of RCTs included in an analysis of each endocrine AE was low (all grade: n = 1–5, grade 3–5: n = 1–2).
Risk of bias and publication bias
According to the Cochrane risk-of-bias tool, 12, 8, and 4 RCTs were judged at a low, moderate, and high risk of bias, respectively. Twelve RCTs with open-label design were at high risk of bias in outcome measurement. A summary of the risk of bias assessment is presented in Supplemental Figure 2. Funnel plots evaluating publication bias showed a symmetrical distribution, suggesting there was no obvious publication bias among the studies (Supplemental Figure 3).
Discussion
With this meta-analysis, we investigated the effect of the addition of ICB to conventional neoadjuvant/adjuvant therapy on the incidence of endocrine toxicities in patients with solid tumors. Incorporating ICB as a part of perioperative therapy significantly increased the incidence of all-grade and grade 3–5 thyroid dysfunction, hypophysitis, adrenal insufficiency, and type 1 diabetes mellitus. Because these AEs often require life-long hormone replacement therapy, our work supports risk and benefit discussion with patients who receive neoadjuvant/adjuvant ICB therapy.
Thyroid AEs are among the most common endocrine toxicities related to ICB therapy. 46 Consistent with our previous finding, this study showed an increase in thyroiditis, hyperthyroidism, and hypothyroidism associated with the addition of ICB. 47 Subgroup analysis revealed hyperthyroidism and hypothyroidism to be more likely with addition of PD-1 or PD-L1 blockade than with CTLA-4 blockade. Although our work did not focus on the incidence of endocrine AEs from dual checkpoint blockade, this is consistent with prior data that dual CTLA-4 and PD-1 blockade had the highest incidence of thyroid issues, followed by PD-1, PD-L1, and CTLA-4 blockade in advanced disease.11,48 The difference in the incidence of thyroid dysfunction based on ICB subtype may derive from expression of PD-1 ligands including PD-L1 and PD-L2 on normal thyroid tissue. 49
In our study, ICB was associated with a significantly higher incidence of both all-grade and grade 3–5 hypophysitis and adrenal insufficiency. Subgroup analysis revealed an increase in the incidence of hypophysitis and adrenal insufficiency associated with addition of PD-1 blockade, but not CTLA-4 blockade statistically. The reliability of these results is limited by the small number of studies utilizing CTLA-4 blockade included in the subgroup analysis. Hypophysitis has been described as more frequently associated with CTLA-4 blockade than with PD-1 or PD-L1 blockade in studies evaluating patients with advanced disease.50,51 CTLA-4 expression on the pituitary gland has been implicated in CTLA-4 blockade-induced hypophysitis, but the association between the PD-1–PD-L1 axis and hypophysitis has not been fully explored yet.52,53 Adrenocorticotropic hormone (ACTH) deficiency is occasionally seen in patients treated with PD-1 blockade; therefore, expression of PD-1 on ACTH-secreting cells may be involved in the pathogenesis of hypophysitis induced by PD-1 blockade. 54 These AEs require prolonged hormone replacement therapy, which causes a significant burden and impairs quality of life, particularly in patients with early-stage disease. Therefore, further study is warranted to elucidate the pathophysiology and incidence of hypophysitis and adrenal insufficiency associated with ICB.
Type 1 diabetes mellitus is an endocrine AE oftentimes associated with PD-1/PD-L1 blockade.54,55 In our study, the addition of ICB was associated with a higher incidence of type 1 diabetes, particularly with the addition of PD-1 blockade, consistent with previous research in the advanced disease setting.55,56 The finding of our study supports further research investigating risk factors, incidence, and pathophysiology of immune-related diabetes mellitus to guide discussion about the risk of neoadjuvant/adjuvant ICB therapy.
Unlike irAEs involving other organ systems, where steroids are often used as first-line treatment, managing endocrine AEs may require a unique approach. For endocrine AEs, high-dose steroids usually play a limited role, and endocrine organ failure from ICB is often irreversible, requiring lifelong treatment with hormone replacement or insulin therapy.57,58 Patients receiving neoadjuvant and adjuvant ICB have potentially curable cancer; however, they may experience a negative impact in their quality of life as a result of an endocrine AE. Hypophysitis and type 1 diabetes mellitus may be life-threatening if unrecognized. Clinicians should strive for early detection of ICB-mediated endocrinopathies through vigilant monitoring of signs and symptoms and serial laboratory surveillance.
Our study has several limitations. First, the effect of each ICB subtype on endocrinopathies was not compared head-to-head because the aim of this study was to investigate the effect of the addition ICB to conventional neoadjuvant/adjuvant therapy on the incidence of endocrine AEs. Subgroup analyses based on ICB subtype may give an insight on differences in the incidence of endocrine AEs among ICB mechanisms; however, this subgroup analysis was based on a small number of RCTs, limiting the statistical power to assess some subgroups, particularly CTLA-4 blockade. The number of studies was insufficient to compare the incidence of endocrine AEs according to cancer type or individual ICB agent (nivolumab, pembrolizumab, etc.). Additionally, risk factors associated with the development of endocrine AEs, such as genetic predisposition, were not reported in the studies included in this meta-analysis; therefore, the impact of patients’ risk factors on the analysis cannot be estimated. Further studies utilizing individual patient data could elucidate risk factors for development of endocrine AEs associated with use of ICB. Lastly, the included RCTs did not include information on the association between endocrine AEs and surgical delays and cancellations; thus, our study was unable to perform an analysis investigating the impact of endocrine AEs on the surgery itself. The occurrence of endocrine AEs in the neoadjuvant setting may affect the surgical schedule, which could lead to worse surgical outcomes. Future studies are needed to evaluate the impact of these AEs on surgery delays and cancellations.
Conclusion
Addition of ICB to conventional neoadjuvant/adjuvant therapy for treatment of solid tumors was associated with an increase in the incidence of a variety of endocrine AEs. Patients receiving ICB in the perioperative setting have an elevated risk of thyroid dysfunction, hypophysitis, adrenal insufficiency, and type 1 diabetes mellitus. Clinicians utilizing neoadjuvant and adjuvant ICB for treatment of early stage cancer must balance the risk of irreversible endocrinopathy with the potential for cure and guide risk–benefit discussion with patients given the risk of life-long complications from endocrine AEs associated with ICB.
Supplemental Material
Supplemental material, sj-docx-1-tam-10.1177_17588359241257874 for Endocrine adverse events in patients with cancer receiving perioperative immune checkpoint blockade: a meta-analysis of randomized controlled trials by Susu Zhou, Nobuyuki Horita, Theresa Shao, Matthew Harrington and Yu Fujiwara in Therapeutic Advances in Medical Oncology
Supplemental material, sj-docx-2-tam-10.1177_17588359241257874 for Endocrine adverse events in patients with cancer receiving perioperative immune checkpoint blockade: a meta-analysis of randomized controlled trials by Susu Zhou, Nobuyuki Horita, Theresa Shao, Matthew Harrington and Yu Fujiwara in Therapeutic Advances in Medical Oncology
Acknowledgments
None.
Footnotes
ORCID iDs: Nobuyuki Horita  https://orcid.org/0000-0002-8200-0340
https://orcid.org/0000-0002-8200-0340
Yu Fujiwara  https://orcid.org/0000-0003-1406-2352
https://orcid.org/0000-0003-1406-2352
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Susu Zhou, Department of Medicine, Icahn School of Medicine at Mount Sinai, Mount Sinai Beth Israel, New York, NY, USA.
Nobuyuki Horita, Chemotherapy Center, Yokohama City University Hospital, Yokohama, Japan.
Theresa Shao, Division of Hematology and Medical Oncology, The Tisch Cancer Institute, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Matthew Harrington, Department of Medicine, Icahn School of Medicine at Mount Sinai, Mount Sinai Beth Israel, New York, NY, USA.
Yu Fujiwara, Department of Medicine, Roswell Park Comprehensive Cancer Center, Elm and Carlton Street, Buffalo, NY 14263, USA; Department of Medicine, Icahn School of Medicine at Mount Sinai, Mount Sinai Beth Israel, New York, NY, USA.
Declarations
Ethics approval and consent to participate: Not applicable.
Consent for publication: Not applicable.
Author contributions: Susu Zhou: Data curation; Formal analysis; Investigation; Resources; Visualization; Writing – original draft.
Nobuyuki Horita: Investigation; Methodology; Writing – review & editing.
Theresa Shao: Investigation; Writing – review & editing.
Matthew Harrington: Investigation; Writing – review & editing.
Yu Fujiwara: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Validation; Visualization; Writing – review & editing.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
The authors declare that there is no conflict of interest.
Availability of data and materials: At the request to the corresponding author.
References
- 1. Topalian SL, Weiner GJ, Pardoll DM. Cancer immunotherapy comes of age. J Clin Oncol 2011; 29: 4828–4836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kaufman HL, Atkins MB, Subedi P, et al. The promise of immuno-oncology: implications for defining the value of cancer treatment. J Immunother Cancer 2019; 7: 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ishida Y, Agata Y, Shibahara K, et al. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J 1992; 11: 3887–3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science 1996; 271: 1734–1736. [DOI] [PubMed] [Google Scholar]
- 5. Kwon ED, Hurwitz AA, Foster BA, et al. Manipulation of T cell costimulatory and inhibitory signals for immunotherapy of prostate cancer. Proc Natl Acad Sci U S A 1997; 94: 8099–8103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010; 363: 711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Eggermont AM, Chiarion-Sileni V, Grob JJ, et al. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): a randomised, double-blind, phase 3 trial. Lancet Oncol 2015; 16: 522–530. [DOI] [PubMed] [Google Scholar]
- 8. Gao S, Li N, Gao S, et al. Neoadjuvant PD-1 inhibitor (Sintilimab) in NSCLC. J Thorac Oncol 2020; 15: 816–826. [DOI] [PubMed] [Google Scholar]
- 9. Li C, Zhao S, Zheng Y, et al. Preoperative pembrolizumab combined with chemoradiotherapy for oesophageal squamous cell carcinoma (PALACE-1). Eur J Cancer 2021; 144: 232–241. [DOI] [PubMed] [Google Scholar]
- 10. Rizzo A, Cusmai A, Massafra R, et al. Pathological complete response to neoadjuvant chemoimmunotherapy for early triple-negative breast cancer: an updated meta-analysis. Cells 2022; 11: 1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ramos-Casals M, Brahmer JR, Callahan MK, et al. Immune-related adverse events of checkpoint inhibitors. Nat Rev Dis Primers 2020; 6: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Byun DJ, Wolchok JD, Rosenberg LM, et al. Cancer immunotherapy – immune checkpoint blockade and associated endocrinopathies. Nat Rev Endocrinol 2017; 13: 195–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Barroso-Sousa R, Barry WT, Garrido-Castro AC, et al. Incidence of endocrine dysfunction following the use of different immune checkpoint inhibitor regimens: a systematic review and meta-analysis. JAMA Oncol 2018; 4: 173–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Darnell EP, Mooradian MJ, Baruch EN, et al. Immune-related adverse events (irAEs): diagnosis, management, and clinical pearls. Curr Oncol Rep 2020; 22: 39. [DOI] [PubMed] [Google Scholar]
- 15. Ni J, Huang M, Zhang L, et al. Clinical recommendations for perioperative immunotherapy-induced adverse events in patients with non-small cell lung cancer. Thorac Cancer 2021; 12: 1469–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Patrinely JR, Jr, Johnson R, Lawless AR, et al. Chronic immune-related adverse events following adjuvant anti-PD-1 therapy for high-risk resected melanoma. JAMA Oncol 2021; 7: 744–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fujiwara Y, Horita N, Adib E, et al. Treatment-related adverse events, including fatal toxicities, in patients with solid tumours receiving neoadjuvant and adjuvant immune checkpoint blockade: a systematic review and meta-analysis of randomised controlled trials. Lancet Oncol 2024; 25: P62–P75. [DOI] [PubMed] [Google Scholar]
- 18. Page MJ, Moher D, Bossuyt PM, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ 2021; 372: n160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fujiwara Y, Horita N, Namkoong H, et al. The effect of adding immune checkpoint inhibitors on the risk of pneumonitis for solid tumours: a meta-analysis of phase III randomised controlled trials. Eur J Cancer 2021; 150: 168–178. [DOI] [PubMed] [Google Scholar]
- 20. Fujiwara Y, Horita N, Harrington M, et al. Incidence of hepatotoxicity associated with addition of immune checkpoint blockade to systemic solid tumor therapy: a meta-analysis of phase 3 randomized controlled trials. Cancer Immunol Immunother 2022; 71: 2837–2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Higgins JP, Altman DG, Gøtzsche PC, et al. Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011; 343: d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Review Manager (RevMan). Version 5.4. The Cochrane Collaboration, revman.cochrane.org (2020).
- 23. Amaria RN, Reddy SM, Tawbi HA, et al. Neoadjuvant immune checkpoint blockade in high-risk resectable melanoma. Nat Med 2018; 24: 1649–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bajorin DF, Witjes JA, Gschwend JE, et al. Adjuvant nivolumab versus placebo in muscle-invasive urothelial carcinoma. N Engl J Med 2021; 384: 2102–2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bellmunt J, Hussain M, Gschwend JE, et al. Adjuvant atezolizumab versus observation in muscle-invasive urothelial carcinoma (IMvigor010): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol 2021; 22: 525–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cascone T, William WN, Jr, Weissferdt A, et al. Neoadjuvant nivolumab or nivolumab plus ipilimumab in operable non-small cell lung cancer: the phase 2 randomized NEOSTAR trial. Nat Med 2021; 27: 504–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Choueiri TK, Tomczak P, Park SH, et al. Adjuvant pembrolizumab after nephrectomy in renal-cell carcinoma. N Engl J Med 2021; 385: 683–694. [DOI] [PubMed] [Google Scholar]
- 28. Cloughesy TF, Mochizuki AY, Orpilla JR, et al. Neoadjuvant anti-PD-1 immunotherapy promotes a survival benefit with intratumoral and systemic immune responses in recurrent glioblastoma. Nat Med 2019; 25: 477–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Eggermont AMM, Blank CU, Mandala M, et al. Longer follow-up confirms recurrence-free survival benefit of adjuvant pembrolizumab in high-risk stage III melanoma: updated results from the EORTC 1325-MG/KEYNOTE-054 trial. J Clin Oncol 2020; 38: 3925–3936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Felip E, Altorki N, Zhou C, et al. Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB–IIIA non-small-cell lung cancer (IMpower010): a randomised, multicentre, open-label, phase 3 trial. Lancet 2021; 398: 1344–1357. [DOI] [PubMed] [Google Scholar]
- 31. Forde PM, Spicer J, Lu S, et al. Neoadjuvant nivolumab plus chemotherapy in resectable lung cancer. N Engl J Med 2022; 386: 1973–1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gianni L, Huang CS, Egle D, et al. Pathologic complete response (pCR) to neoadjuvant treatment with or without atezolizumab in triple-negative, early high-risk and locally advanced breast cancer: neoTRIP Michelangelo randomized study. Ann Oncol 2022; 33: 534–543. [DOI] [PubMed] [Google Scholar]
- 33. Kaseb AO, Hasanov E, Cao HST, et al. Perioperative nivolumab monotherapy versus nivolumab plus ipilimumab in resectable hepatocellular carcinoma: a randomised, open-label, phase 2 trial. Lancet Gastroenterol Hepatol 2022; 7: 208–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kelly RJ, Ajani JA, Kuzdzal J, et al. Adjuvant nivolumab in resected esophageal or gastroesophageal junction cancer. N Engl J Med 2021; 384: 1191–1203. [DOI] [PubMed] [Google Scholar]
- 35. Loibl S, Untch M, Burchardi N, et al. A randomised phase II study investigating durvalumab in addition to an anthracycline taxane-based neoadjuvant therapy in early triple-negative breast cancer: clinical results and biomarker analysis of GeparNuevo study. Ann Oncol 2019; 30: 1279–1288. [DOI] [PubMed] [Google Scholar]
- 36. Luke JJ, Rutkowski P, Queirolo P, et al. Pembrolizumab versus placebo as adjuvant therapy in completely resected stage IIB or IIC melanoma (KEYNOTE-716): a randomised, double-blind, phase 3 trial. Lancet 2022; 399: 1718–1729. [DOI] [PubMed] [Google Scholar]
- 37. Mittendorf EA, Zhang H, Barrios CH, et al. Neoadjuvant atezolizumab in combination with sequential nab-paclitaxel and anthracycline-based chemotherapy versus placebo and chemotherapy in patients with early-stage triple-negative breast cancer (IMpassion031): a randomised, double-blind, phase 3 trial. Lancet 2020; 396: 1090–1100. [DOI] [PubMed] [Google Scholar]
- 38. Monk BJ, Colombo N, Oza AM, et al. Chemotherapy with or without avelumab followed by avelumab maintenance versus chemotherapy alone in patients with previously untreated epithelial ovarian cancer (JAVELIN Ovarian 100): an open-label, randomised, phase 3 trial. Lancet Oncol 2021; 22: 1275–1289. [DOI] [PubMed] [Google Scholar]
- 39. Moore KN, Bookman M, Sehouli J, et al. Atezolizumab, bevacizumab, and chemotherapy for newly diagnosed stage III or IV ovarian cancer: placebo-controlled randomized phase III trial (IMagyn050/GOG 3015/ENGOT-OV39). J Clin Oncol 2021; 39: 1842–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nanda R, Liu MC, Yau C, et al. Effect of pembrolizumab plus neoadjuvant chemotherapy on pathologic complete response in women with early-stage breast cancer: an analysis of the ongoing phase 2 adaptively randomized I-SPY2 trial. JAMA Oncol 2020; 6: 676–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Park S, Sun JM, Choi YL, et al. Adjuvant durvalumab for esophageal squamous cell carcinoma after neoadjuvant chemoradiotherapy: a placebo-controlled, randomized, double-blind, phase II study. ESMO Open 2022; 7: 100385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rahma OE, Yothers G, Hong TS, et al. Use of total neoadjuvant therapy for locally advanced rectal cancer: initial results from the pembrolizumab arm of a phase 2 randomized clinical trial. JAMA Oncol 2021; 7: 1225–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Schmid P, Cortes J, Dent R, et al. Event-free survival with pembrolizumab in early triple-negative breast cancer. N Engl J Med 2022; 386: 556–567. [DOI] [PubMed] [Google Scholar]
- 44. Schoenfeld JD, Hanna GJ, Jo VY, et al. Neoadjuvant nivolumab or nivolumab plus ipilimumab in untreated oral cavity squamous cell carcinoma: a phase 2 open-label randomized clinical trial. JAMA Oncol 2020; 6: 1563–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zimmer L, Livingstone E, Hassel JC, et al. Adjuvant nivolumab plus ipilimumab or nivolumab monotherapy versus placebo in patients with resected stage IV melanoma with no evidence of disease (IMMUNED): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 2020; 395: 1558–1568. [DOI] [PubMed] [Google Scholar]
- 46. Chang LS, Barroso-Sousa R, Tolaney SM, et al. Endocrine toxicity of cancer immunotherapy targeting immune checkpoints. Endocr Rev 2019; 40: 17–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhou S, Horita N, Shao T, et al. The incidence of thyroid-related adverse events in solid tumors receiving immune checkpoint blockade with curative intent: a meta-analysis. J Clin Oncol 2023; 41: 2641. [Google Scholar]
- 48. Lu D, Yao J, Yuan G, et al. Immune checkpoint inhibitor-related new-onset thyroid dysfunction: a retrospective analysis using the US FDA adverse event reporting system. Oncologist 2022; 27: e126–e132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yamauchi I, Sakane Y, Fukuda Y, et al. Clinical features of nivolumab-induced thyroiditis: a case series study. Thyroid 2017; 27: 894–901. [DOI] [PubMed] [Google Scholar]
- 50. Weber JS, Kähler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol 2012; 30: 2691–2697. [DOI] [PubMed] [Google Scholar]
- 51. Ji HH, Tang XW, Dong Z, et al. Adverse event profiles of anti-CTLA-4 and anti-PD-1 monoclonal antibodies alone or in combination: analysis of spontaneous reports submitted to FAERS. Clin Drug Investig 2019; 39: 319–330. [DOI] [PubMed] [Google Scholar]
- 52. Iwama S, De Remigis A, Callahan MK, et al. Pituitary expression of CTLA-4 mediates hypophysitis secondary to administration of CTLA-4 blocking antibody. Sci Transl Med 2014; 6: 230ra45. [DOI] [PubMed] [Google Scholar]
- 53. Caturegli P, Di Dalmazi G, Lombardi M, et al. Hypophysitis secondary to cytotoxic T-lymphocyte-associated protein 4 blockade: insights into pathogenesis from an autopsy series. Am J Pathol 2016; 186: 3225–3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Di Dalmazi G, Ippolito S, Lupi I, et al. Hypophysitis induced by immune checkpoint inhibitors: a 10-year assessment. Expert Rev Endocrinol Metab 2019; 14: 381–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Akturk HK, Kahramangil D, Sarwal A, et al. Immune checkpoint inhibitor-induced Type 1 diabetes: a systematic review and meta-analysis. Diabet Med 2019; 36: 1075–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. de Filette JMK, Pen JJ, Decoster L, et al. Immune checkpoint inhibitors and type 1 diabetes mellitus: a case report and systematic review. Eur J Endocrinol 2019; 181: 363–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Reid PD, Cifu AS, Bass AR. Management of immunotherapy-related toxicities in patients treated with immune checkpoint inhibitor therapy. JAMA 2021; 325: 482–483. [DOI] [PubMed] [Google Scholar]
- 58. Thompson JA. New NCCN guidelines: recognition and management of immunotherapy-related toxicity. J Natl Compr Canc Netw 2018; 16: 594–596. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tam-10.1177_17588359241257874 for Endocrine adverse events in patients with cancer receiving perioperative immune checkpoint blockade: a meta-analysis of randomized controlled trials by Susu Zhou, Nobuyuki Horita, Theresa Shao, Matthew Harrington and Yu Fujiwara in Therapeutic Advances in Medical Oncology
Supplemental material, sj-docx-2-tam-10.1177_17588359241257874 for Endocrine adverse events in patients with cancer receiving perioperative immune checkpoint blockade: a meta-analysis of randomized controlled trials by Susu Zhou, Nobuyuki Horita, Theresa Shao, Matthew Harrington and Yu Fujiwara in Therapeutic Advances in Medical Oncology