Abstract
Background:
Stair-climbing (SC) is an essential daily life skill, and stair-climbing exercise (SCE) serves as a valuable method for promoting physical activity in older adults. This study aimed to compare the impact of SCEs with heel contact (HC) and heel off (HO) during SC on functional mobility and trunk muscle (TM) activation amplitudes in community-dwelling older adults.
Methods:
In the pilot randomized controlled trial, participants were randomly allocated to either the HC group (n = 17; mean age 75.9 ± 6.3 years) or the HO group (n = 17; mean age 76.5 ± 4.6 years). The HC participants performed SCE with the heel of the ankle in contact with the ground, while the HO participants performed SCE with the heel of the ankle off the ground during SC. Both groups participated in progressive SCE for one hour per day, three days per week, over four consecutive weeks (totaling 12 sessions) at the community center. We measured timed stair-climbing (TSC), timed up and go (TUG), and electromyography (EMG) amplitudes of the TMs including rectus abdominis (RA), external oblique (EO), transverse abdominus and internal oblique abdominals (TrA-IO), and erector spinae (ES) during SC before and after the intervention.
Results:
Both groups showed a significant improvement in TSC and TUG after the intervention (P < .01, respectively), with no significant difference between the groups. There was no significant difference in the EMG activity of the TMs between the groups after the intervention. The amplitude of TMs significantly decreased after the intervention in both groups (P < .01, respectively).
Conclusion:
Both SCE methods could improve balance and SC ability in older adults while reducing the recruitment of TMs during SC. Both SCE strategies are effective in improving functional mobility and promoting appropriate posture control during SC in older adults.
Keywords: aging, falling, postural balance, stair-climbing, surface electromyography
1. Introduction
Stair-climbing (SC) is an essential daily life skill, and this activity may serve as a feasible opportunity to remain physically active because it is a low-cost, inconspicuous, and readily accessible form of exercise.[1] Therefore, SC could be an essential training for the older adults with aging in their local communities.[2] Stair-climbing exercise (SCE) has many general health benefits, such as improvements in cardiorespiratory fitness, bone mineral density and balance, and lower extremity strength, which may reduce fall risk in older adult.[1–4]
A previous study has reported SCE improved balance, heart rate, and endurance in individuals aged ≥ 75 years.[2] Furthermore, study with a stair negotiation training demonstrated improvements in the strength of knee extensors and functional physical fitness in older adults.[5] In a study involving SCE, improvements in peak leg power were observed in older adults after the intervention.[6] However, there are various methods of SC, and they may have different effects on leg and trunk strength and balance ability.[7,8] SC with heel contact (HC) could markedly increase the knee flexion movement with increased activity of the quadriceps while minimizing the activity of the gastrocnemius muscles (GCM), whereas plantar flexors can be more activated when ascending stairs with heel off (HO), reducing the activity of the quadriceps.[9] These different SC training approaches may lead to distinct integration of proprioceptive and biomechanical demands through variations in trunk and lower extremity muscle function and coordination, with different foot placements.[9–12]
In essence, delineating these various intervention effects will facilitate the development of a tailored SCE approach specifically catered to older adults with compromised balance and functional mobility.
Trunk muscle (TM) is necessary to maintain spinal stability during daily activities,[13] serving as the kinetic link facilitating the transfer of torque and momentum between the lower and upper extremities.[14] In environments that demand greater balance, such as irregular surfaces, individuals tend to rely excessively on co-contractions or heightened contractions of TM and leg muscles for postural control.[15–18] Greater activation of TM has been linked to impaired postural control during unstable balancing tasks.[18,19] Moreover, as SC presents a greater challenge compared to simple walking, older adults need to exert increased attention and balance during SC.[20,21] Therefore, evaluating TM activation during SC is crucial for assessing the SC performance and balance capacity needed by older adults during SC.[19]
To the best of our knowledge, no study has yet investigated the effects of SCE with 2 different foot placement methods on functional mobility and TM activity in older adults.
Therefore, this study aimed to examine the effects of SCEs with HC compared with HO on the activation amplitudes of the TM and mobility in community-dwelling older adults. It was hypothesized that SCE with both HC and HO would result in increased mobility and decreased activation amplitudes of TM during SC; and there would be difference between the 2 different training approaches in TM activity and mobility. The findings of the research would offer potential for developing tailored interventions in SCE aimed at enhancing postural control during SC and improving functional mobility among older adults facing age-related decline.
2. Methods
2.1. Participants
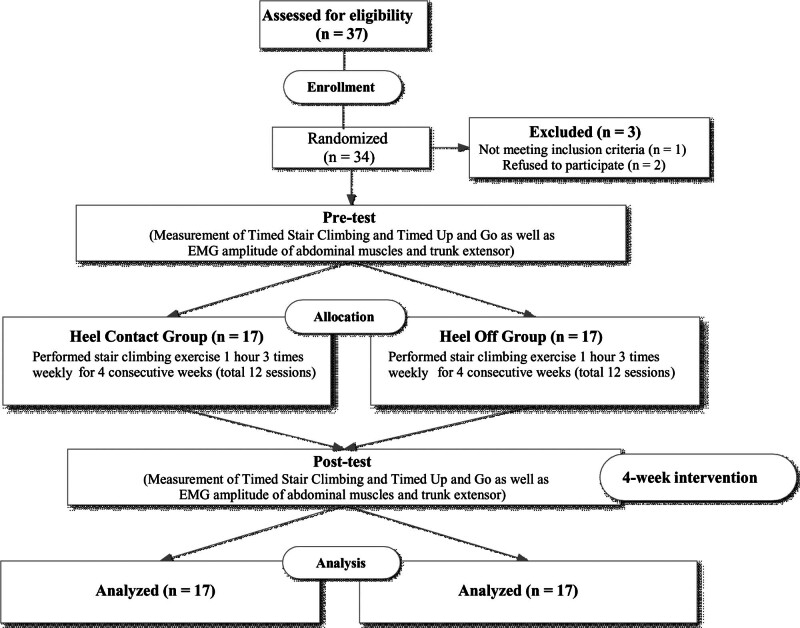
A flowchart and demographic characteristics of the participants are presented in Figure 1 and Table 1. In total, 34 subjects (22 women, 12 men) participated in this study, and they were randomly assigned either to the HC (n = 17; mean age 75.9 ± 6.3 years, range: 66–81 years) or HO group (n = 17; mean age 76.5 ± 4.6 years, range: 65–83 years) using computerized block randomization with block size and allocation ratio of 1:1 in 2 groups. Following this allocation, the participants received explanations regarding their group assignments before participating in each intervention.
Figure 1.
Flow diagram of the study.
Table 1.
Demographic characteristics of the participants.
| Characteristic | HC group (n = 17) | HO group (n = 17) |
|---|---|---|
| Sex (male/female) | 5/12 | 7/10 |
| Age, yr | 75.9 ± 6.3 | 76.5 ± 4.6 |
| Height, cm | 156.5 ± 7.7 | 157.7 ± 6.5 |
| Weight, kg | 59.0 ± 10.8 | 58.8 ± 9.0 |
| BMI, kg/m2 | 24.0 ± 3.2 | 23.6 ± 2.8 |
| Foot length, mm | 246.9 ± 15.1 | 245.8 ± 8.2 |
| MMSE | 27.6 ± 2.4 | 27.8 ± 2.0 |
Values are mean ± SD.
BMI = body mass index, HC = heel contact, HO = heel off, MMSE = mini-mental state examination.
The inclusion criteria were as follows: subjects aged 65 years or older, individuals who scored 24 or higher on the mini-mental status examination (MMSE),[22] those who were not participating in any regular self-exercise (e.g., SC and strengthening exercises) or intervention program as part of another study. The exclusion criteria were defined as follows: individuals with neurological disorders such as stroke and Parkinson disease, or unstable medical conditions, including cardiovascular disease, ischemic heart disease, orthostatic hypotension, dementia, and visual impairments affecting functional mobility; those with a history of falls within at least 3 months prior to study participation; individuals unable to walk independently without aids; and those experiencing leg pain during SC. All subjects provided written informed consent, which was approved by the Ethics Committee and Institutional Review Board of the University Medical Center (IRB No. KU-IRB-16-92-A-2).
2.2. Procedures
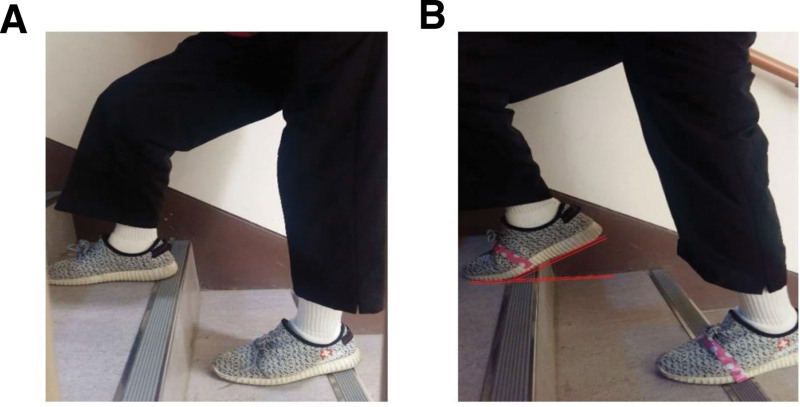
Measurements of timed stair-climbing (TSC), timed up and go (TUG), and TM activation were obtained at baseline and 4 weeks after the intervention by 2 physical therapists who were not associated with recruitment and intervention. Participants from both groups received progressive exercise training for 1 h/d, 3 d/wk, over 4 consecutive weeks (total 12 sessions) at the community center. Each training session included 10 minutes of warm-up, 40 minutes of SCE, and 10 minutes of cool-down. Subjects were asked to use either the HC method, in which the heel was in full contact with the ground (Fig. 2A), or the HO method, in which the heel was lifted from the bottom of the stairs and grounded only to the forefoot and midfoot (Fig. 2B) while maintaining trunk and head positions as neutral as possible. These motions involving ankle adjustments in SC result in a narrower base of support during the initial contact compared to ascending regular stairs.
Figure 2.
different stair-climbing exercises. (A) Heel contact method: the heel had full contact with the ground, (B) heel off method: the heel was lifted from the bottom of the stairs and was grounded only to the forefoot and midfoot.
Each participant climbed 4 flights of stairs, totaling 52 stairs (riser height, 17 cm; a single flight of stairs, 13 stairs), at a self-determined pace while wearing walking shoes. During each exercise session, each participant initially ascended 40 flights (a total of 520 stairs) divided into 10 sets of 4 flights, with a 1-minute rest period between each set. The training volume was gradually increased over 4 weeks by increasing the number of repetitions every week (1st week, 10 repetitions per session; 4th week, 13 repetitions per session; 1 repetition, 52 stairs). Each participant was guided by an instructor who carefully observed them to ensure their safety. Furthermore, participants were informed and encouraged to grasp the stair handrail when only necessary. An elevator was used to take participants from the top floor to the first floor to avoid walking downstairs for the exercise intervention.
2.3. Outcome measures
Mobility measures included TSC and TUG. In TSC, the time taken to climb up and down an isolated set of 6 steps (step height, 18 cm; step depth, 30 cm) was measured using the handrail in a usual manner and at a safe and comfortable pace to assess SC performance and the functional strength of lower extremity.[23] The completed time was evaluated using a stopwatch by a physical therapist with over 10 years of experience working with older adults. These measures were carried out by the same evaluator in the same stair environment.
During the TUG test, participants were also instructed to get up from the armchair, walk 3 m forward, turn around, and walk back to the chair with their backs against the chair. The completed time was recorded in seconds.[24] The TSC and TUG have been shown to have good test-retest reliability (r = .98; r = .93 to r = .98, respectively).[25,26] The electromyography (EMG) parameters in TM included activations of the rectus abdominis (RA), external oblique (EO), transverse abdominis and internal oblique abdominals (TrA-IO), and erector spinae (ES) during SC. Each test was performed 3 times, and the average value was recorded.
2.4. Electromyography processing
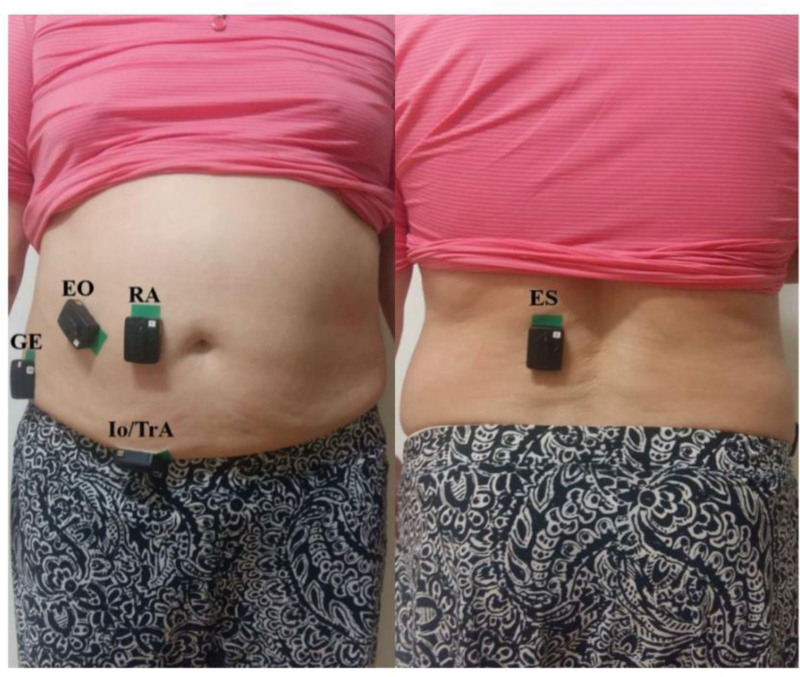
The surface EMG signals of the TM were measured during SC using a Trigno wireless EMG system (Delsys, Inc., Boston, MA, USA). Each EMG sensor (27 mm × 37 mm × 15 mm) had a 4-bar formation electrode (5 mm × 10 mm) with an interelectrode distance of 20 mm and 2 patent-pending stabilizing references. The EMG signals were amplified and sampled at 1024 Hz using a 12 bit A/D converter. The signals were band-pass filtered at 20 to 450 Hz twice using a 4th-order Butterworth filter. Raw EMG signals were numerically rectified, and then the root mean square (RMS) of the EMG amplitude was calculated for each trial with a 10 seconds interval using EMG Works 4.0 software (Delsys). A common-mode rejection ratio of 80 dB, with an input impedance of 100 MΩ, was employed. The skin at the electrode placement sites was shaved and cleaned using alcohol pads before electrode placement. All EMG electrodes were attached to the dominant side of the measured muscle. Electrode placement was verified through a series of functional tests and was placed on the RA, EO, TrA-IO, and ES (Fig. 3).
Figure 3.
EMG electrodes on abdominal muscles and the erector spine. EO = external oblique; ES = erector spinae muscle; GE, ground electrode, Io/TrA = internal oblique and transversus abdominis, RA = rectus abdominis.
The electrode was placed parallel to the RA muscle fibers and situated approximately 3 cm lateral to the umbilicus.[27] The electrode for the EO was attached beneath the rib cage, at the most inferior point of the costal margin, and on the line opposite to the pubic tubercle. The TrA-IO muscles are measured concurrently at the same location because they have similar functions and shrinkage times for body stability, and cannot be accurately separated using surface EMG.[28] For TrA-IO, the electrode was positioned 1 to 2 cm medial and below the anterior superior iliac spine (ASIS).[29] The electrode was placed parallel to and approximately 3 cm lateral to the L3 spinous process to measure ES.[30] The ground electrode was then placed on the ASIS.
RMS values were normalized with respect to maximal voluntary isometric contraction (MVIC) as a percentage of MVIC (% MVIC).[31] Before SC, all participants performed manual muscle tests (MMTs) for corresponding muscles against manual resistance 3 times for 5 seconds, as recommended by Kendall et al.[32] Visual inspection was performed before data collection to reduce crosstalk, and Delsys sensors have an interface and interelectrode distance to optimize crosstalk suppression.[33] The order of the normalization procedures for each muscle was randomized, with each repeated at least 3 times, and a 2-minute rest between trials (each muscle test) to minimize muscle fatigue.[34] The remaining middle 3 seconds contraction, obtained after removing 1 second at the first and last MVIC, was used, and the mean value of the middle 3 seconds contraction of the 3 trials was considered as the MVIC.[31]
After conducting several trials to determine the stair ascent speed, a target testing pace of 2 seconds/step was established for the SC task. An audible metronome was employed to synchronize with the target pace. TM activation was measured during the ascent of 5 stairs for 10 seconds, without restrictions on foot placement. Each subject performed 3 trials with a 1-minute rest period between trials. The EMG data were collected during the middle 6 seconds, which corresponded to the middle 3 steps, excluding every 2 seconds at the beginning (1st step) and end (5th step).[31,35] The mean RMS amplitude for each trial of SC was normalized as %MVIC, and the mean of 3 trials of %MVIC for each muscle was used for statistical data analyses.[31]
2.5. Data analysis
Data distribution was analyzed using the Shapiro–Wilk test. The differences in participant characteristics between groups were compared using independent t tests, Mann–Whitney U tests, and chi-square tests. In comparison of dependent variables, activation of the EO was compared using independent t tests and paired t test, whereas those of RA, TrA-IO, ES, and TSC as well as TUG measurements were compared using the Mann–Whitney U test and Wilcoxon signed-rank test. Statistical significance was set at a P value of < .05. Statistical analyses were performed using SPSS version 20.0 (SPSS Inc., Chicago, IL, USA). The sample size of this study was determined based on the available literature,[36] and calculated using G-power analysis with a significance level of 0.05, group number of 2, effect size of 0.5, and statistical power of 0.8. The minimum sample size satisfying all the requirements was 17 per group.
3. Results
All subjects tolerated the training protocol well and completed all sessions over 4 consecutive weeks without dropouts; no adverse events were reported. Initially, a total of 37 individuals were considered as potential participants. However, only 34 met the inclusion criteria and were consequently enrolled in the study (1 did not meet the inclusion criteria; 2 declined participation). They were then randomly assigned to either the HC group (n = 17) or the HO group (n = 17). There were no significant differences in the characteristics or baseline outcome variables between the groups (Table 1).
For the TSC and TUG measurements, significant improvements were observed after the intervention in both groups (P < .01). In the HC group, enhancements of 10.17% and 18.18% were noted in the corresponding parameters. Meanwhile, the HO group exhibited improvements of 15.79% and 24.69% in the relevant measures. However, no significant difference was found between the groups after the intervention (Table 2). Within each group, significant improvements were observed in the activations of RA, EO, TrA-IO, and ES (P < .01), but no significant differences were found between the groups (Table 3).
Table 2.
Changes in the TSC and TUG by intervention conditions for each group.
| Parameter | HC group (n = 17) | HO group (n = 17) | P | ||
|---|---|---|---|---|---|
| Pre-intervention | Post-intervention | Pre-intervention | Post-intervention | ||
| TSC, s | 12.4 ± 3.8 | 11.2 ± 3.5* | 10.8 ± 3.5 | 9.0 ± 2.8* | >.05 |
| TUG, s | 10.0 ± 2.3 | 8.7 ± 2.2* | 10.1 ± 3.5 | 8.1 ± 2.6* | >.05 |
Values are mean ± SD.
HC = heel contact, HO = heel off, TSC = timed stair-climbing, TUG = timed up and go.
Significant change (P < .01) within the group by Wilcoxon signed-rank test.
Table 3.
Changes in EMG amplitudes in percentage of MVIC for each muscle by intervention conditions for each group.
| Parameter | HC group (n = 17) |
HO group (n = 17) |
P | ||
|---|---|---|---|---|---|
| Pre-intervention | Post- intervention |
Pre-intervention | Post-intervention | ||
| RA, % | 16.8 ± 15.1 | 5.8 ± 3.4* | 19.9 ± 17.7 | 8.65 ± 7.53* | >.05 |
| EO, % | 18.1 ± 15.4 | 8.2 ± 4.9† | 18.5 ± 9.6 | 9.3 ± 5.5† | >.05 |
| TrA-IO, % | 22.8 ± 15.8 | 15.3 ± 10.5* | 16.8 ± 13.2 | 13.8 ± 12.5* | >.05 |
| ES, % | 38.8 ± 28.8 | 25.0 ± 17.5* | 39.0 ± 27.4 | 24.5 ± 9.1* | >.05 |
Values are mean ± SD.
EO = external oblique, ES = erector spinae, HC = heel contact, HO = heel off, MVIC = maximal voluntary isometric contraction, RA = rectus abdominis, TrA-IO = transverse abdominis and internal oblique.
Significant change (P < .01) within the group by Wilcoxon signed-rank test.
Significant change (P < .01) within the group by a paired t test.
4. Discussion
The purpose of this study is to investigate the effects of 2 different SCE approaches on functional mobility and TM activity during SC in older adults. Furthermore, this is the first study to compare SCEs with HC and HO in terms of measurements of TSC, TUG, and TM activity. The findings of the study demonstrated that diverse SCEs led to a significant improvement in mobility and a significant decrease in activation of the TM during the SC task in both groups. These different SCEs showed similar effects for the corresponding outcome measures. In this study, older adults showed a significant improvement in TSC (SC performance) after engaging in SCE with HC. This is consistent with many previous studies involving SCE with HC, demonstrating that the SCE led to significant improvements in SC performance, including step and time, in individuals aged 65 years or older.[2,6,37] Other studies have also documented improvements in functional lower limb strength for SC among older adults following SCE.[6,38] Our findings suggest that repetitively conducting SC task-oriented training could lead to improvements in functional mobility, involving increased functional leg strength for SC in older adults.[39]
Furthermore, the HO group also showed similar effects to those in the HC group on SC performance. Ankle-HO during SCE likely induced activation primarily in the calf muscles.[7,9] Strengthening the calf muscles through HO training, alongside functional training, has been shown to enhance functional movement in older adults and stroke patients.[40,41] While there was no difference between HO and HC training in enhancing SC performance itself, we posit that there are likely biomechanical differences. This underscores the importance of considering various parameters such as timing, force, and activation patterns in different lower leg muscles during SC, rather than solely focusing on SC performance. Future research should consider these factors for further validation.
Previous studies have demonstrated that SC training with HC positively affects the static and dynamic balance of the older adults and individuals with neurological disorders.[2,42,43] Additionally, the combination of functional movement training with ankle control training, eliciting heightened calf muscle activation, yielded favorable outcomes on dynamic balance in young adults with ankle instability and older adults.[40,44] It is postulated that the positive effects derived from our SCE incorporating HO may be similar to those observed.
Both SCs could require a higher level of 1-leg standing ability during the stance phase of the stair cycle compared to walking, thus necessitating significant balance abilities, such as mediolateral postural stability.[20,45] Additionally, the diverse SCE protocols, which incorporate challenging ankle control in both stance and swing phases during SC, may affect the kinetics and kinematics of the hip and knee joints, as well as the ankle. This could create a more demanding environment that necessitates increased postural control.[10–12] Hence, our findings suggest that dynamic balance could be improved through both SCEs in older adults. While, prior to the intervention, the TUG values of both groups did not reach the reference values for the specified age group (75 years and older), they reached reference values for the 65 to 70 age range (8.2–8.7 seconds) even after only 4 consecutive weeks.[24] However, as mentioned above, there was no significant difference in TUG between the 2 SCEs. To address this aspect, future research should encompass different variables that cover the measurement of mobility involving dynamic balance ability.
Previous studies have indicated that challenging environments lead to increased co-activation during postural control tasks and higher percentages of MVIC in the TM (i.e., excessive activity).[19,46] Specifically, reduced activation of TMs and decreased involvement of adjacent lower joints during challenging postural control tasks indicated an improvement in balance.[15–19] In this study, both groups showed reduced activation of all TMs, including RA, EO, TrA-IO, and ES during SC in both groups, consistent with the study by Nagai et al demonstrating that balance training effectively diminishes excessive activity of muscles for postural control among older adults.[16] Furthermore, the improved postural adjustments through training mitigated unnecessary activation patterns mobilized to sustain balance, as reported by previous studies involving individuals with stroke, anterior cruciate ligament rupture, and young adults.[47–49] Thus, we considered that both SCEs would have led to improved functional mobility, such as SC performance and enhanced balance ability, thereby alleviating unnecessary and excessive TM activation during the SC task. This implies that the SC tasks may no longer be perceived as challenging for the participants, as they were before the training intervention. Therefore, our results suggest that with the improvement in balance, along with enhanced SC performance, there has been sufficient postural adjustment in the SC task environment, as indicated by a reduction in the excessive recruitment of TMs. Furthermore, in EO, significant changes are observed, accompanied by a large effect size in both group (Cohen d: 0.94, 2.24, respectively). However, similar to the results of TSC and TUG, no distinct differences were observed between the groups. As mentioned above, future studies should consider assessing both the MVIC and co-activation levels of various lower limb muscles and TMs to confirm any distinct differences between SCEs with HC and HO. This study has several limitations. First, generalizing the results is challenging due to the limited sample size. Furthermore, the sample size was not directly calculated using findings from comparable studies, due to the absence of studies addressing similar outcomes and methodological designs (HO vs HC). Second, there is no follow-up examination available to assess the sustained impact. Third, the study lacked a control group as a reference for assessing the effects of different SCEs. Fourth, we exclusively utilized stair ascent as the intervention method. Consequently, there is a limitation in generalizing the effects to encompass all stair training, including stair descent. Future research should explore the effects of SC training integrated with various stair descent intervention methods. Fifth, kinetic and kinematic values of various lower limb joints were not evaluated during SC. To clearly identify differences between various SCEs, a variety of tools for assessing balance (both static and dynamic balances) should have been utilized. Sixth, limitations in EMG channel availability prevented the measurement of overall TM activity and lower limb muscles during SC. Additionally, muscle co-activation index was not evaluated in various EMG measurement techniques that could allow for the interpretation of various results. These limitations might restrict the elucidation of clear differences in SCEs. Since this is the first study, there is a scarcity of data from comparable studies for a thorough comparison with the findings of the present research. Furthermore, the results of this study should be carefully considered and interpreted in future research endeavors.
The findings of this study offer promise for creating personalized interventions to improve postural control during SC and functional mobility among older adults facing age-related decline. This could potentially reduce the risk of falls among older individuals in the future.
5. Conclusions
The study findings demonstrate the efficacy of both SCE methods in improving functional mobility among older adults while causing significantly less recruitment of TMs during SC. However, no significant differences were observed between the 2 groups in terms of functional mobility and TM activation. Both SCE methods are effective in improving functional mobility and promoting appropriate posture control during SC in older adults.
Author contributions
Conceptualization: Min Kang Kim, Chang Yong Kim.
Data curation: Min Kang Kim.
Visualization: Chang Yong Kim.
Supervision: Chang Yoon Baek.
Writing – original draft: Chang Yoon Baek, Hyeong Dong Kim.
Writing – review & editing: Chang Yoon Baek, Hyeong Dong Kim.
Methodology: Suhng Wook Kim, Hyun Dong Je.
Formal analysis: Ji Hoon Jeong.
Investigation: Ji Hoon Jeong.
Abbreviations:
- ASIS
- anterior superior iliac spine
- EMG
- electromyography
- EO
- external oblique
- GCM
- gastrocnemius muscles
- HC
- heel contact
- HO
- heel off
- MMSE
- mini-mental status examination
- MMT
- manual muscle tests
- MVIC
- maximal voluntary isometric contraction
- RMS
- root mean square
- SC
- stair-climbing
- SCE
- stair-climbing exercise
- TM
- trunk muscle
- TrA-IO
- transverse abdominis and internal oblique abdominals
- TSC
- timed stair-climbing
- TUG
- timed up and go
This study was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (NRF-2022R1F1A1072343).
The study was approved by Korea University Institutional Review Board (KU-IRB-13-102-A-2).
The authors have no conflicts of interest to disclose.
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
How to cite this article: Kim M-K, Kim C-Y, Baek C-Y, Kim S-W, Je HD, Jeong JH, Kim H-D. The effects of various stair-climbing exercises on functional mobility and trunk muscle activation in community-dwelling older adults: A pilot randomized controlled trial. Medicine 2024;103:23(e38446).
MK, CK, and CY contributed equally to this work.
Contributor Information
Min-Kang Kim, Email: hdkimx0286@korea.ac.kr.
Chang-Yong Kim, Email: hdkimx0286@korea.ac.kr.
Suhng-Wook Kim, Email: hdkimx0286@korea.ac.kr.
Hyun Dong Je, Email: 211057@nhis.or.kr.
Ji Hoon Jeong, Email: jyj305@naver.com.
Hyeong-Dong Kim, Email: hdkimx0286@korea.ac.kr.
References
- [1].Meyer P, Kayser B, Mach F. Stair use for cardiovascular disease prevention. Eur J Cardiovasc Prev Rehabil. 2009;16(2_Suppl):S17–8. [DOI] [PubMed] [Google Scholar]
- [2].Donath L, Faude O, Roth R, Zahner L. Effects of stair-climbing on balance, gait, strength, resting heart rate, and submaximal endurance in healthy seniors. Scand J Med Sci Sports. 2014;24:e93–101. [DOI] [PubMed] [Google Scholar]
- [3].Granacher U, Muehlbauer T, Gruber M. A qualitative review of balance and strength performance in healthy older adults: impact for testing and training. J Aging Res. 2012;2012:708905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Dostan A, Dobson CA, Vanicek N. Relationship between stair ascent gait speed, bone density and gait characteristics of postmenopausal women. PLoS One. 2023;18:e0283333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Chen TC, Hsieh C-C, Tseng K-W, Ho C-C, Nosaka K. Effects of descending stair walking on health and fitness of elderly obese women. Med Sci Sports Exerc. 2017;49:1614–22. [DOI] [PubMed] [Google Scholar]
- [6].Jonathan Bean M, Seth Herman B, Mph DKK, et al. Weighted stair climbing in mobility-limited older people: a pilot study. J Am Geriatr Soc. 2002;50:663–70. [DOI] [PubMed] [Google Scholar]
- [7].Kang J-H, Kim C-Y, Kim J-H, Kim H-D. Effects of heel contact methods of stair ascent on abdominal muscle activation in healthy subjects: a cross-sectional pilot study. J Korean Soc Phys Med. 2017;12:1–8. [Google Scholar]
- [8].Park J-W, Kim Y-J. A study on changes in lower limb joint angles during stair walking with high heel. J Korean Phys Ther. 2013;25:379–85. [Google Scholar]
- [9].Riener R, Rabuffetti M, Frigo C. Stair ascent and descent at different inclinations. Gait Posture. 2002;15:32–44. [DOI] [PubMed] [Google Scholar]
- [10].Sinitski EH, Hansen AH, Wilken JM. Biomechanics of the ankle–foot system during stair ambulation: implications for design of advanced ankle–foot prostheses. J Biomech. 2012;45:588–94. [DOI] [PubMed] [Google Scholar]
- [11].Whatling GM, Holt CA. Does the choice of stair gait cycle affect resulting knee joint kinematics and moments? Proc Inst Mech Eng H. 2010;224:1085–93. [DOI] [PubMed] [Google Scholar]
- [12].Antonio PJ, Perry SD. Quantifying stair gait stability in young and older adults, with modifications to insole hardness. Gait Posture. 2014;40:429–34. [DOI] [PubMed] [Google Scholar]
- [13].Hodges PW. Is there a role for transversus abdominis in lumbo-pelvic stability? Man Ther. 1999;4:74–86. [DOI] [PubMed] [Google Scholar]
- [14].Behm DG, Drinkwater EJ, Willardson JM, Cowley PM. The use of instability to train the core musculature. Appl Physiol Nutr Metab. 2010;35:91–108. [DOI] [PubMed] [Google Scholar]
- [15].Nagai K, Okita Y, Ogaya S, Tsuboyama T. Effect of higher muscle coactivation on standing postural response to perturbation in older adults. Aging Clin Exp Res. 2017;29:231–7. [DOI] [PubMed] [Google Scholar]
- [16].Nagai K, Yamada M, Tanaka B, et al. Effects of balance training on muscle coactivation during postural control in older adults: a randomized controlled trial. J Gerontol A Biol Sci Med Sci. 2012;67:882–9. [DOI] [PubMed] [Google Scholar]
- [17].Reeves NP, Everding VQ, Cholewicki J, Morrisette DC. The effects of trunk stiffness on postural control during unstable seated balance. Exp Brain Res. 2006;174:694–700. [DOI] [PubMed] [Google Scholar]
- [18].Donath L, Kurz E, Roth R, Zahner L, Faude O. Leg and trunk muscle coordination and postural sway during increasingly difficult standing balance tasks in young and older adults. Maturitas. 2016;91:60–8. [DOI] [PubMed] [Google Scholar]
- [19].Kaur N, Bhanot K, Ferreira G. Lower extremity and trunk electromyographic muscle activity during performance of the Y-balance test on stable and unstable surfaces. Int J Sports Phys Ther. 2022;17:483–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Casaña J, Calatayud J, Silvestre A, et al. Knee extensor muscle strength is more important than postural balance for stair-climbing ability in elderly patients with severe knee osteoarthritis. Int J Environ Res Public Health. 2021;18:3637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Cesari P, Formenti F, Olivato P. A common perceptual parameter for stair climbing for children, young and old adults. Hum Mov Sci. 2003;22:111–24. [DOI] [PubMed] [Google Scholar]
- [22].Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. [DOI] [PubMed] [Google Scholar]
- [23].Bennell K, Dobson F, Hinman R. Measures of physical performance assessments: self-paced walk test (SPWT), stair climb test (SCT), six-minute walk test (6MWT), chair stand test (CST), timed up & go (TUG), sock test, lift and carry test (LCT), and car task. Arthritis Care Res. 2011;63:S350–70. [DOI] [PubMed] [Google Scholar]
- [24].Svinøy O-E, Hilde G, Bergland A, Strand BH. Timed up and go: reference values for community-dwelling older adults with and without arthritis and non-communicable diseases: the Tromsø study. Clin Interv Aging. 2021;16:335–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Lin MR, Hwang HF, Hu MH, Wu HDI, Wang YW, Huang FC. Psychometric comparisons of the timed up and go, one-leg stand, functional reach, and Tinetti balance measures in community-dwelling older people. J Am Geriatr Soc. 2004;52:1343–8. [DOI] [PubMed] [Google Scholar]
- [26].Flansbjer U-B, Holmbäck AM, Downham D, Patten C, Lexell J. Reliability of gait performance tests in men and women with hemiparesis after stroke. J Rehabil Med. 2005;37:75–82. [DOI] [PubMed] [Google Scholar]
- [27].Cholewicki J, Vanvliet JJ, Iv. Relative contribution of trunk muscles to the stability of the lumbar spine during isometric exertions. Clin Biomec (Bristol, Avon). 2002;17:99–105. [DOI] [PubMed] [Google Scholar]
- [28].Ng J, Kippers V, Richardson C. Muscle fibre orientation of abdominal muscles and suggested surface EMG electrode positions. Electromyogr Clin Neurophysiol. 1998;38:51–8. [PubMed] [Google Scholar]
- [29].Ng J, Kippers V, Parnianpour M, Richardson CA. EMG activity normalization for trunk muscles in subjects with and without back pain. Med Sci Sports Exerc. 2002;34:1082–6. [DOI] [PubMed] [Google Scholar]
- [30].Juker D, McGill S, Kropf P, Steffen T. Quantitative intramuscular myoelectric activity of lumbar portions of psoas and the abdominal wall during a wide variety of tasks. Med Sci Sports Exerc. 1998;30:301–10. [DOI] [PubMed] [Google Scholar]
- [31].Fatima S, Bhati P, Singla D, Choudhary S, Hussain ME. Electromyographic activity of hip musculature during functional exercises in participants with and without chronic ankle instability. J Chiropr Med. 2020;19:82–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Kendall FP, McCreary EK, Provance PG, Rodgers MM, Romani WA. Muscles: testing and function with posture and pain. MD: Lippincott Williams & Wilkins Baltimore; 2005:5. [Google Scholar]
- [33].Balasukumaran T, Gottlieb U, Springer S. Muscle activation patterns during backward walking in people with chronic ankle instability. BMC Musculoskelet Disord. 2020;21:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Zarrouk N, Rebai H, Yahia A, Souissi N, Hug F, Dogui M. Comparison of Recovery Strategies on Maximal Force-Generating Capacity and Electromyographic Activity Level of the Knee Extensor Muscles. National Athletic Trainers’ Association, Inc. Journal of Athletic Training. 2011;46:386–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Feger MA, Donovan L, Hart JM, Hertel J. Lower extremity muscle activation in patients with or without chronic ankle instability during walking. J Athl Train. 2015;50:350–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Faul F, Erdfelder E, Lang A-G, Buchner A. G* Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–91. [DOI] [PubMed] [Google Scholar]
- [37].Hongu N, Shimada M, Miyake R, Nakajima Y, Nakajima I, Yoshitake Y. Promoting stair climbing as an exercise routine among healthy older adults attending a community-based physical activity program. Sports (Basel). 2019;7:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Mair JL, De Vito G, Boreham CA. Low volume, home-based weighted step exercise training can improve lower limb muscle power and functional ability in community-dwelling older women. J Clin Med. 2019;8:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Manini T, Marko M, VanArnam T, et al. Efficacy of resistance and task-specific exercise in older adults who modify tasks of everyday life. J Gerontol A Biol Sci Med Sci. 2007;62:616–23. [DOI] [PubMed] [Google Scholar]
- [40].Maritz CA, Silbernagel KG. A prospective cohort study on the effect of a balance training program, including calf muscle strengthening, in community-dwelling older adults. J Geriatr Phys Ther. 2016;39:125–31. [DOI] [PubMed] [Google Scholar]
- [41].Lee S-M, Cynn H-S, Yoon T-L, Lee J-H. Effects of different heel-raise-lower exercise interventions on the strength of plantarflexion, balance, and gait parameters in stroke survivors. Physiother Theory Pract. 2017;33:706–15. [DOI] [PubMed] [Google Scholar]
- [42].Kang S-J, Ahn C-H. The effects of home-based stair and normal walking exercises on lower extremity functional ability, fall risk factors, and cardiovascular health risk factors in middle-aged older women. J Exerc Rehabil. 2019;15:584–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Huang W-Y, Li M-H, Lee C-H, Tuan S-H, Sun S-F, Liou I-H. Efficacy of lateral stair walking training in patients with chronic stroke: a pilot randomized controlled study. Gait Posture. 2021;88:10–5. [DOI] [PubMed] [Google Scholar]
- [44].Seo JH, Lee MY. Effects of quarter heel raising exercise on balance and ankle strength in functional ankle instability subjects. Medicine (Baltimore). 2022;101:e30672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Nadeau S, McFadyen BJ, Malouin F. Frontal and sagittal plane analyses of the stair climbing task in healthy adults aged over 40 years: what are the challenges compared to level walking? Clin Biomec (Bristol, Avon). 2003;18:950–9. [DOI] [PubMed] [Google Scholar]
- [46].Lee T-G, Park C-H, Son H-H. The effects of a bridging exercise applying changes in the base of support for the shoulders on trunk muscle activation. J Korean Soc Phys Med. 2016;11:97–104. [Google Scholar]
- [47].Kitatani R, Ohata K, Sakuma K, et al. Ankl e muscle coactivation during gait is decreased immediately after anterior weight shift practice in adults after stroke. Gait Posture. 2016;45:35–40. [DOI] [PubMed] [Google Scholar]
- [48].Behrens M, Mau-Moeller A, Wassermann F, Bader R, Bruhn S. Effect of balance training on neuromuscular function at rest and during isometric maximum voluntary contraction. Eur J Appl Physiol. 2015;115:1075–85. [DOI] [PubMed] [Google Scholar]
- [49].Chmielewski TL, Hurd WJ, Rudolph KS, Axe MJ, Snyder-Mackler L. Perturbation training improves knee kinematics and reduces muscle co-contraction after complete unilateral anterior cruciate ligament rupture. Phys Ther. 2005;85:740–9 [discussion 750]. [PubMed] [Google Scholar]