Abstract
The prognosis for patients with non-small cell lung cancer (NSCLC), a cancer type which represents 85% of all lung cancers, is poor with a 5-year survival rate of 19%, mainly because NSCLC is diagnosed at an advanced and metastatic stage. Despite recent therapeutic advancements, ~50% of patients with NSCLC will develop brain metastases (BMs). Either surgical BM treatment alone for symptomatic patients and patients with single cerebral metastases, or in combination with stereotactic radiotherapy (RT) for patients who are not suitable for surgery or presenting with fewer than four cerebral lesions with a diameter range of 5-30 mm, or whole-brain RT for numerous or large BMs can be administered. However, radioresistance (RR) invariably prevents the action of RT. Several mechanisms of RR have been described including hypoxia, cellular stress, presence of cancer stem cells, dysregulation of apoptosis and/or autophagy, dysregulation of the cell cycle, changes in cellular metabolism, epithelial-to-mesenchymal transition, overexpression of programmed cell death-ligand 1 and activation several signaling pathways; however, the role of the Hippo signaling pathway in RR is unclear. Dysregulation of the Hippo pathway in NSCLC confers metastatic properties, and inhibitors targeting this pathway are currently in development. It is therefore essential to evaluate the effect of inhibiting the Hippo pathway, particularly the effector yes-associated protein-1, on cerebral metastases originating from lung cancer.
Key words: brain metastasis, non-small lung cancer, radiation, Hippo pathway, radioresistance
1. Introduction
Advanced-stage non-small cell lung cancer (NSCLC)-related mortality occurs <18 months of diagnosis mainly due to metastatic spread (1). A total of ~50% of patients with NSCLC develop brain metastasis (BM) (2-4), mostly in the cerebral hemisphere (80%), the cerebellum (15%) and the brainstem (5%) (5). The management of BM includes: i) multimodal surgery, the gold standard when there are neurological symptoms or <3 BMs (6); ii) radiotherapy (RT); iii) immunotherapy; and iv) tyrosine kinase inhibitors (TKIs) (4). RT is inevitably associated with radioresistance (RR). A search for prognostic factors in NSCLC has shown that high expression of Hippo pathway effectors is associated with decreased overall survival (7). Hippo pathway dysregulation (Fig. 1) mediates cancer cell motility and subsequent metastasis formation as a drug or RT resistance in several human cancers (Fig. 2), including NSCLC (8-13). In NSCLC, the following events occur: deregulation of the Hippo pathway occurring in all NSCLC due to epigenetic dysregulation (14) and/or hypoxia (13), leads to the transformation of healthy bronchial epithelial cells into tumour cells (8,9), and subsequently promotes cell motility and subsequent metastasis of bronchial origin by hyperactivating the nuclear dbf2-related (NDR) 2 kinase and the transcription cofactor yes-associated protein-1 (YAP1), and inhibiting the antimigratory small GTPase RhoB (9). BMs of bronchial origin are treated with surgery and/or RT, but invariably, RR is established, and it is hypothesized that the deregulation of the Hippo pathway, which is the origin of bronchial cancer and its dissemination, is also involved in the RR of these BMs of bronchial origin (4,5). This finding suggested that the Hippo pathway is involved in the unsatisfactory treatment of NSCLC. Due to the large scope of Hippo-dependent biological implications, elucidating the mechanisms of Hippo-dependent treatment resistance, notably RR, in BM from NSCLC is essential. The aim of the present review is to emphasize the importance of Hippo in RR, providing a comprehensive summary of the literature and identifying the underlying mechanism involved in the treatment of BM from NSCLC.
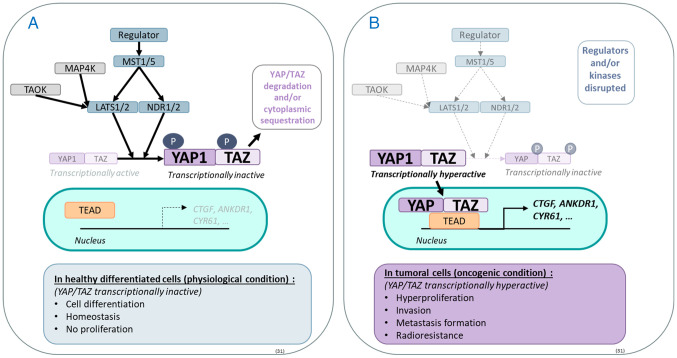
Figure 1.
Hippo pathway and its physiological and oncogenic roles. The Hippo pathway is comprised of serine/threonine kinases activated by a phosphorylation cascade, the core of which starts with MST1/5, which activates LATS1/2 and NDR1/2. The latter two regulate by phosphorylation the activation of the effector proteins YAP1 and TAZ. When phosphorylated, YAP1 and TAZ are degraded by the proteasome and/or sequestered in the cytoplasm. (A) In a physiological context, YAP1 and TAZ are found in an equilibrium of active and inactive forms that regulate the transcription of various target genes such as CTFG, ANKDR1 and CYR61. (B) In cancer, YAP1 and/or TAZ are often found in hyperactive states due to reduced regulation of Hippo kinases, thereby playing an oncogenic role. MST1/5, mammalian sterile 20-like kinase; LATS1/2, large tumour suppressor 1/2; NDR1/2, nuclear dbf2-related kinase; YAP1, yes-associated protein 1; TAZ, transcriptional coactivator with a PDZ-binding domain; TEAD, TEA DNA-binding protein; TAOK, Thousand and one amino-acid kinase.
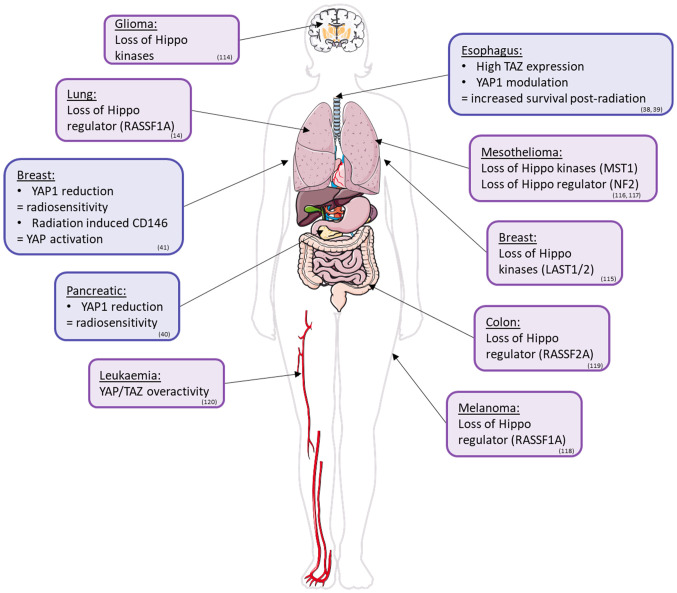
Figure 2.
A glance at the alteration of the Hippo pathway in human cancers and radioresistance. The Hippo pathway is implicated in cancer formation/maintenance (purple) and radioresistance (blue). Loss of Hippo kinases is common in gliomas (114), breast cancer (LAST1/2) (115) and mesothelioma (MST1) (116). The loss of Hippo regulators is also common for RASSF1A in lung cancer and melanoma (117), RASSF2A in colon cancer (118) and NF2 in mesothelioma (119). YAP1 and TAZ are upregulated in leukemia (120). High levels of TAZ expression and YAP1 modulation are associated with increased survival post-radiation in esophageal cancer cells. In breast cancer, radiation-induced CD146 activation leads to increased YAP activity. Reducing YAP activity enhances the radiosensitivity of breast and pancreatic cancer cells. YAP1, yes-associated protein 1; TAZ, transcriptional coactivator with a PDZ-binding domain; LATS1/2, large tumour suppressor 1/2; MST1/5, mammalian sterile 20-like kinase; NF2, Neurofibromin 2; RASSF1A, Ras association domain family 1 isoform A.
2. RR of BM from NSCLC
Biological effects of X-rays (XRs)
Ionizing radiation (IR), notably XR, induces single-strand breaks (SSBs) and double-strand breaks (DSBs) in DNA, ultimately leading to cellular death by necrosis, apoptosis or mitotic death (15). One gray (Gy) of XR results in >2,000 base damages, 30 DNA-DNA crosslinks, 1,000 SSBs and 40 DSBs per cell (16), although only the latter are considered lethal (15) and carcinogenic (16). Moreover, there are indirect effects due to the radiolysis of water, which creates reactive oxygen species (ROS), called the bystander effect, that potentiate the effect of IR by increasing the number of DSBs and propagating its effects on neighboring cells (17). After irradiation, the majority of lesions are repaired by processes such as homologous recombination repair in the synthesis (S)/growth (G)2 phase of the cell cycle (16) and non-homologous end joining (NHEJ) (15) in the G1 phase (16). The first is limited to the S and G phases of the cell cycle, and the second is used to repair DSBs independently of the cell cycle (15). IR activates the kinase activity of ataxia-telangiectasia mutated (ATM), which interacts with a large panel of proteins such P53, Brac2 and checkpoint kinase 2 (CHK2) to pause the cell cycle, allowing DNA repair (15). Despite these reparations, a number of these defects are not repaired or repaired with defects, leading to cellular apoptosis or necrosis (15).
Mechanisms of RR
RR is defined by a reduced efficiency of RT emerging through complex and interconnected mechanisms. An intrinsic RR results from genetic or phenotypic alterations in response to XR (15), whereas an extrinsic RR can be due to the tumor environment (18). Hypoxic cancers, cancer stem cells (CSCs) and altered metabolism favor RR (18-20), and modified cell cycle control and DNA repair directly impact the efficiency of XR (21-24).
Intrinsic RR
Radiation induces the activation of a number of genes, including early genes such as C-JUN, and epidermal growth factor-1 (EGF-1) serves as regulator of other protective genes (15), while later genes are mostly associated with trophic factors such as platelet-derived growth factor (PDGF), transforming growth factor-β (TGF-β) and basic fibroblast growth factor expressed to modulate radio-sensitivity (15).
Hypoxic microenvironment
Hypoxia reduces the production of ROS, which are essential for radiation-induced DNA damage (19,20), activates cellular autophagy and accelerates ROS clearance to further protect cancer cells (20). Hypoxia-inducible factor (HIF) regulates vascular endothelial growth factor (VEGF) and PDGF gene expression, protecting endothelial cells from radiation damage while stimulating tumour blood vessel growth (19,20). HIF inhibits apoptosis by: i) Stimulating NDRG2 and inhibiting BAX, a proapoptotic gene; and ii) inhibiting p53-mediated apoptosis via direct interaction with p53 (20). Furthermore, HIFs are implicated in the activation of RR signaling pathways such as CXCL8, AKT/mTOR/STAT3, MERK/ERK and DNA-protein kinase (DNA-PK) (19,20).
CSCs
The presence of CSCs renders RT inefficient (20). These cells express CD133 and CD44, with the latter being strongly upregulated post radiation, promoting the stem phenotype and therefore RR (20). CSCs continuously modify their environment to potentiate their survival (18); they are also implicated in tumours, increasing the complexity of tumour treatment (18). The ability of stem cell-like cells to replicate and differentiate provides more protection from RT; however, a fraction of stem cells remain quiescent and are thus unaltered (18). This process facilitates CSC survival post-irradiation, followed by proliferation and tumor invasion (18).
Altered cellular metabolism
Cellular metabolism adaptations are often observed in RR cancer cells, notably through the Warburg effect, which favors anaerobic glycolysis (20). This effect translates to an increase in the glucose consumption rate, active glycolysis and a high concentration of lactic acid (20). Lactic acid is found in large quantities in the BM of patients with NSCLC and can stimulate the release of hyaluronic acid by tumor-associated fibroblasts, which promotes VEGF secretion and cell migration (20). A reduction in oxidative phosphorylation in mitochondria reduces the rate of ROS generation and renders the cell dependent on anaerobic glycolysis (20). Glucose transporter 1 (Glut1) is stimulated under hypoxic conditions, during which its overexpression is associated with RR (20). Moreover, Glut1 expression is modulated by PI3K/AKT signaling, which is known for its oncogenic functions (20). Additionally, manganese superoxide dismutase (MnSOD) is an antioxidant enzyme that is upregulated in certain cells after radiation and reduces mortality by reducing ROS levels and preventing subsequent apoptosis (20).
Cell cycle
The cell cycle has checkpoints that regulate the passage of different phases to perform mitosis (21). The sensitivity of cells to radiation varies during these phases; RT stops the cell cycle at a checkpoint before the S phase (21). This checkpoint is used to repair DNA before any errors are copied; if irreparable, the cell dies (21). Dysregulation of the cell cycle is observed in cancer cells (21). Proteins such as ATM, P53 and CHK2 regulate this process and are thus altered in cancer cells, increasing RR (21). Activated ATM can either activate CHK2, which, in turn, phosphorylates P53 stabilizing the protein, or sequester P53 in the nucleus (21). These proteins lead to powerful cell cycle arrest in the S and G1 phases, allowing additional time for DNA repair and increasing the survival rate of cancer cells (21).
DNA repair enzymes
DNA repair enzymes are upregulated in RR cells to reverse radiation-induced DNA damage and thus allow cells to survive despite exposure to radiation (20). For instance, DNA-PKs and ATM are implicated in NHEJ DNA repair, the major repair mechanism activated post-irradiation (22). DNA-PK recognizes DSBs in DNA and relinks the broken strands in a non-homologous way, but this could favor the acquisition of new oncogenic mutations (21). Irradiation of the adenocarcinoma cell line A549 with inhibition of DNA-PK results in a higher rate of DNA damage and apoptosis, suggesting the involvement of DNA-PK in these mechanisms (22). Similarly, the inhibition of ATM in irradiated cells reduces cellular survival and increases apoptosis (22). Furthermore, in EGFR-mutated NSCLC cells, the condensation of chromatin seems to have radioprotective effects (23). Chromatin modification is a survival mechanism used by cancer cells to protect their DNA from radiation damage. The type of radiation (XR or carbon ion) does not seem to modify cellular responses (24).
Signaling pathways involved in RR
Janus kinase (JAK)/signal transducer and activator of transcription (STAT)
The JAK/STAT pathway involves fast-acting signaling from the membrane to the nucleus activated in response to cytokines and growth factors, leading to the activation of JAKs and subsequent phosphorylation and activation of STAT proteins. Activated STAT translocates to the nucleus to regulate the transcription of genes involved in various cellular functions such as differentiation, lipid metabolism, cell-cycle inhibition, cell-cycle progression, apoptosis inhibition, etc (25). RT induces the production of IFN, which binds to its receptor to activate the JAK/STAT pathway; this also activates other pathways, such as the mTOR, NF-κB and MAPK pathways (25). IFN activation of the JAK/STAT pathway upregulates genes that control cell survival and metabolism, DNA repair systems and immune protection (25). This pathway has been implicated in RR lung cancer via an increase in STAT3 activation (25).
ERK/MAPK
The ERK/MAPK pathway is altered in a large portion of NSCLCs, and confers invasive and metastatic properties to BM (26). RT leads to the phosphorylation of MEK1 and 2, which are activated following HER activation (26). ERK modulates NHEJ-mediated DNA repair by controlling ATM and ATM Rad3-related but also homologous repair via DNA-PK, promoting cell survival (26). Moreover, ERK promotes G2/M cell cycle arrest, rendering cells more RR (26). Another possible mechanism is that the proliferation signals induced by the ERK pathway stimulate cells less exposed to irradiation or are already somewhat RR such as CSCs, and these signals promote their proliferation to replace dead cells (26).
PI3K/AKT/mTOR pathway
The PI3K/AKT/mTOR pathway is another pathway upregulated and implicated in RR (27) in NSCLC and their BM (28). When this pathway is inhibited, a reduction in colony formation post-irradiation has been observed in prostate cancer cells, and these cells present more DSBs due to a reduction in NHEJ DNA repair and autophagy (27). In NSCLC, the combination of PI3K and extracellular matric (ECM) inhibitors prevents or delays RR (28).
Wnt/β-catenin
Dysregulation of the Wnt/β-catenin pathway contributes to RR by affecting the cell cycle, proliferation, DNA repair, apoptosis and invasion (29). Indeed, Wnt is expressed at high levels in CSCs known for their RR (29).
Hedgehog
The Hedgehog pathway regulates proliferation and differentiation (30); one of its members, Sonic hedgehog (Shh), exerts an inhibitory signal when associated with its transmembrane receptor patched-1 (PTC-1) (30). In addition, PTC-1 leads to the expression of a transcription factor named glioma-associated oncogene 1 (Gli1) (30). Gli1 regulates proliferation, differentiation, ECM interactions and stem cell activation (30). Inhibition of Gli1 in NSCLC slows the proliferation of RR CSCs and increases radiosensitivity (30).
Hippo pathway
The Hippo pathway is a signaling pathway that controls various biological functions, including cell growth, survival, differentiation, determination of cellular fate, organ size and tissue homeostasis (31); it is found at the crosstalk site with all the others signaling pathways involved in RR, where it confers metastatic properties in NSCLC (8,9). The role of the Hippo pathway in RR is still unclear even if a recent study of resistance to treatment induced by YAP1/transcriptional coactivator with a PDZ-binding domain (TAZ) revealed that 'whether YAP1/TAZ confers resistance to RT is an important open question' (32). The aim of the present review was to explore the potential implications of Hippo in RR.
3. Hippo pathway in cancer and RR
Hippo pathway
The well-preserved center of the Hippo pathway is primarily composed of a cascade of serine/threonine kinases, including mammalian sterile 20-like kinase (MST1/5), NDR1/2 and large tumour suppressor 1/2 (LATS1/2; Fig. 1) (31). Activated by phosphorylation, MST kinases phosphorylate and activate NDR kinases (31). Active NDR kinases phosphorylate and inhibit the downstream effectors YAP1 and TAZ by sequestering them in the cytoplasm and directing them towards the proteasome (31). In the absence of phosphorylation, YAP1 and TAZ translocate to the nucleus and bind to different transcription factors, such as TEA DNA-binding proteins (TEAD 1-4) (31), thereby regulating a variety of genes controlling proliferation, stem cell renewal and survival through genes such as CTGF, ANKDR1, CYR61, AXXL and BIRC5 (31). The panel of genes induced by YAP-1/TAZ varies during organogenesis (Fig. 1), depends on the cell fate and evolves between the non-oncogenic situation and the oncogenic situation; during malignant transformation, the transcription of the panel of genes promoted by YAP-1/TAZ is often exacerbated, reflecting the hyperactivity of YAP-1/TAZ in cancer (31,32). Notably, the Hippo pathway is also regulated by multiple pathways, resulting in the control of the transcriptional activity of YAP/TAZ (31). For example, the mitogen-activated protein kinase 4 and the thousand and one amino-acid kinase directly phosphorylate LATS1/2, thereby working in tandem with MST1/5 (31).
Hippo pathway in human cancers including metastatic lung cancer
In most human cancers, including metastatic lung cancer, the Hippo pathway is highly disrupted (Fig. 2) (31,32). In NSCLC, the upregulation of YAP1 sustains cancer cell invasion, drug resistance and metastasis (8-11). The hyperactivation of YAP1 in NSCLC results from various factors: Epigenetic dysregulation that silences Ras association domain-containing protein 1 (RASSF1) expression (8,14), hypoxia induced by tumour growth (33) or the presence of oncogenic drivers such as EGFR-activating mutations (10). Hippo pathway deregulation leads to BM formation in lung cancer cells (8-11). Hypermethylation of the RASSF1 promoter blocks the negative control of RASSF1A, a regulator of the Hippo pathway, on YAP1, leading to its nuclear accumulation (8). RASSF1A prevents the epithelial-to-mesenchymal transition (EMT) of human NSCLC cells via the inhibition of YAP1 by NDR2/Rho guanine nucleotide exchange factor H1/RhoB signaling (8). Similarly, the inhibition of YAP1 by the RASSF1A gene blocks metastasis formation in a murine model (8). In cellular models of BM from NSCLC, H2030-BrM3 and PC9-BrM3 cells, YAP1 is overexpressed compared with that in the parental cell lines H2030 and PC9 (34), and direct inhibition of YAP1 by short-hairpin RNA (sh-RNA) blocks BM formation from H2030-BrM3 in a murine model (10), which supports that YAP1 is involved in metastasis formation.
Finally, YAP1 is implicated in MAPK/ERK signaling, a pathway involved in RR, following EGFR mutation (10): Forced expression of YAP1 confers EGFR-TKI resistance in NSCLC cells, while YAP1 inhibition potentiates this effect (35). Activated YAP1 increases the expression of certain EGFR ligands, such as AREG and ERBB3/4, and thus promotes MAPK signaling to induce tumour progression and drug resistance (10). Correspondingly, EGFR/MAPK signaling regulates YAP1 via the inhibition of Hippo kinases (35). YAP1 controls the transcriptional regulation of programmed cell death-ligand 1 via its interaction with TEAD, leading to the suppression of the immune-related antitumoral response (36). These discoveries highlight the importance of different drug development approaches targeting YAP1 (10). Indeed, inhibitors of the interactions between YAP1/TAZ and TEADs have been tested using in vitro and in vivo methods (31,32), with select candidates such as VT3989 proceeding to clinical trials (37).
Role of the Hippo pathway in RR
There is previous evidence of a link between the Hippo pathway and RR in certain cancers (Table I). For instance, esophageal cancer cells strongly expressing TAZ survive longer than cells with reduced TAZ expression post-radiation (38). CD155 stimulates RR via modulation of YAP1 phosphorylation (39). Indeed, the overexpression of CD155 increases the quantity of nuclear YAP1, whereas its inhibition favors cytoplasmic localization (39). A reduction in the expression of YAP1 and its target genes following catechol treatment sensitizes pancreatic cancer cells to irradiation (40). The inhibition of YAP1 by shRNA in breast cancer cells triple negative for hormone receptors increased the sensitivity to irradiation compared to that in shRNA control cells (41). Finally, YAP1 is translocated to the nucleus, where its activity is essential for maintaining survival and proliferation signaling after irradiation (41). In the same breast cancer model, irradiation appeared to stimulate CD146, which inhibits LATS1, in turn favoring YAP1 activation (42). This is associated with RR due to DNA repair, cell cycle arrest and stem characteristics (42).
Table I.
Effects of Hippo modulation post-radiation.
| First author(s), year | Cancer type | In vitro/In vivo | Model | IR dose, Gy | Hippo modulation | Result | (Refs.) |
|---|---|---|---|---|---|---|---|
| Moon et al, 2021 | Pancreatic | In vitro | Panc-1 cells | 2, 4 | Catechol treatmenta | Decreased survival fraction | (40) |
| Zhou et al, 2020 | Esophageal | In vitro | Eca109, Kyse150 and TE1 cells | 2, 4, 6, 10 | SiTAZa | Decreased survival fraction Increased DNA damage |
(38) |
| TAZ overexpression via plasmidsb | Increased surviving fraction Reduced DNA damage |
||||||
| Xin et al, 2022 | In vitro | Eca109 and Kyse510 cells | 3, 6, 9 | ShCD155a | Decreased nuclear YAP Decreased proliferation and migration |
(39) | |
| Andrade et al, 2017 | Breast | In vitro | MDA-MB231 and MDA- MB468 cells | 2, 4, 6 | ShYAP1a | Decreased survival fraction | (41) |
| Liang et al, 2022 | In vivo | Xenomorphic 4 (x3 radiation injection of MDA-MB-231 cells in mice | cycles) | YAP overexpressionb | Increased tumour growth | (42) | |
| Zhang et al, 2021 | Glioma | In vitro | U87 and U251 cells | 4, 6, 8, 10 | YAP overexpression via vectorsb | Increased surviving (108) fraction Increased DNA repair |
|
| In vivo | Xenomorphic injection of U251 and GBM1 cells in mice | 10 | YAP overexpression via vectorsb | Decreased tumour size Decreased survival |
|||
| Zeng et al, 2020 | Non-small cell lung cancer | In vitro | A549 and H1299 cells | 2, 4, 6, 8 | SiCDK5 (Hippo modulator)a | Decreased DNA damage | (12) |
| In vivo | Xenomorphic injection of H1299 cells in mice | 10 | ShCDK5 (Hippo modulator)a | Decreased size of tumour Decreased DNA damage |
|||
| Li et al, 2022 | In vitro | A549, H1299 and H460 cells | 2, 4, 6, 8 | p130cas (YAP modulator) overexpression via vectorsb ShP130cas (YAP modulator)a | Increased survival fraction Decreased DNA damage Increased DNA damage Increased tumour size |
(44) | |
| In vivo | Xenomorphic injection of H1299 cells in mice | 8 (x3) | p130cas (YAP modulator) overexpression via vectorsb |
The Hippo pathway modulates the aoverexpression or binhibition of effector proteins and has radioresistance properties post-radiation. IR, ionizing radiation; YAP, yes-associated protein 1; TAZ, transcriptional coactivator with a PDZ-binding domain; p130cas, breast cancer anti-estrogen resistance protein 1; si; short-interfering RNA; sh, short hairpin RNA.
In SCLC, YAP1 overexpression is associated with an unfavorable prognosis in patients following an irradiation protocol because RR is modulated by CD133 expression associated with YAP1 expression (43). The Hippo pathway is also implicated in NSCLC RR via an increase in TAZ transcription (12). CDK5 is an upstream regulator of the Hippo pathway; when CDK5 is silenced by shRNA, the expression of TAZ decreases (12). This inhibition leads to an increase in DSBs (γH2AX) and a decrease in DNA repair (RAD51) after radiation in A549 cells (12). Breast cancer anti-estrogen resistance protein 1 (p130cas) interacts with and promotes via focal adhesion kinase the stabilization of YAP1 when overexpressed, resulting in RR in NSCLC cells (44). Inhibition of YAP1 with verteporfin restores the number of DSBs back to a normal level after p130cas is overexpressed, thereby restoring radiation efficiency (44). This evidence shows an implication of the Hippo pathway in RR in different types of cancers, yet its role in BM formation from NSCLC has yet to be studied.
4. Key factors in RR and the Hippo pathway
Mechanisms of RR and Hippo signaling
Numerous aspects of the cellular environment alter the effectiveness of irradiation (Fig. 3). Cells can switch to different states; for example, cells can dedifferentiate into a stem phenotype or increase control of the cell cycle (45-51). These changes favor resistance to RT (45-51). Finally, the mechanisms of DNA repair (12,38,52), apoptosis regulation (53,54) and metabolic dysregulation (55-57) also greatly alter the success of RT. All of these mechanisms interact with and alter members of the Hippo pathway in a large variety of cell types (Fig. 3).
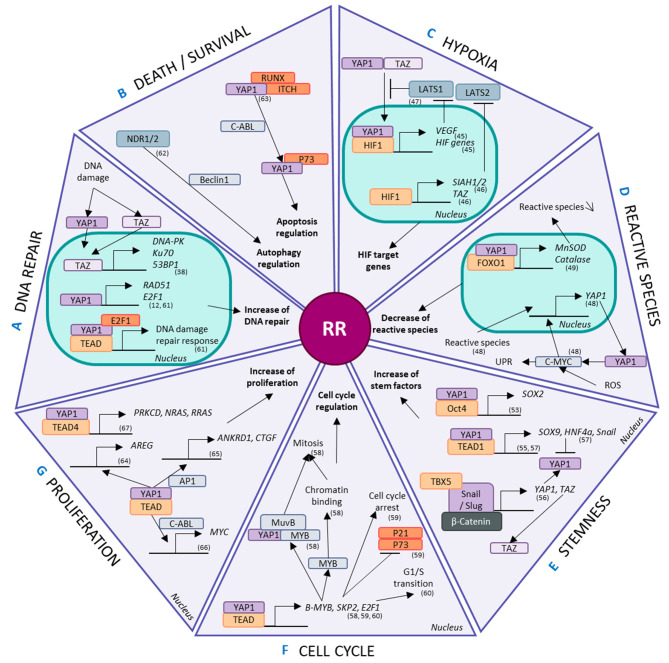
Figure 3.
Involvement of the Hippo pathway in radioresistance. Hippo effectors are modulated and implicated in radioresistant phenomena, such as (A) DNA repair, (B) cell death and survival regulation, (C) hypoxia, (D) reactive species modulation, (E) stem properties, (F) cell cycle regulation and (G) proliferation. YAP1 and/or TAZ regulate the transcription, activation and regulation of mechanisms related to these phenomena. MST1/5, mammalian sterile 20-like kinase; LATS1/2, large tumour suppressor 1/2; NDR1/2, nuclear dbf2-related kinase; YAP1, yes-associated protein 1; TAZ, transcriptional coactivator with a PDZ-binding domain; TEAD, TEA DNA-binding protein; ROS, reactive oxygen species; DNA-PK, DNA-protein kinase; MnSOD, manganese superoxide dismutase; UPR, unfolded protein response; HIF, hypoxia-inducible factor; VEGF, vascular endothelial growth factor; RUNX, Runt-related transcription factor; ITCH, Itchy E3 ubiquitin protein ligase; E2F1, E2F transcription factor 1; RAD51, RAD51 recombinase; PRKCD, protein kinase C δ; AREG, amphiregulin; ANKRD1, ankyrin repeat domain-containing protein 1; CTGF, connective tissue growth factor; AP1, activator protein 1; MYB, myeloblastosis viral oncogene homolog; SKP2, S-phase kinase-associated protein-2; SIAH, Siah E3 ubiquitin protein ligase.
The Hippo pathway interferes with hypoxia through the interaction of HIF1 with YAP1, inducing the expression of HIF target genes (58). Furthermore, in hypoxic conditions involving breast cancer stem cells, HIF1 stimulates the expression of the E2 ubiquitin ligases Siah E3 ubiquitin protein ligase (SIAH) 1 and 2, promoting the proteasomal degradation of LATS2 (59). This in turn favors the nuclear localization of YAP1 and TAZ. HIF1 increases TAZ expression by binding to the WWTR1 gene (59). Moreover, using a biosensor that monitors LATS kinase activity, VEGFR activation by VEGF has been shown to inhibit LATS and activate the Hippo effectors YAP1 and TAZ, highlighting the implication of the Hippo pathway in neoangiogenesis (60).
There is evidence showing that reactive species increase the mRNA and protein levels of YAP1, controlling the proliferation of hepatocellular carcinoma cells (55). ROS-exposed cells act via the c-myc pathway to stimulate YAP1 expression and lead to an increase in the unfolded protein response (55). Then, YAP1 coactivates the transcription of FOXO1, an essential protein for antioxidant gene expression, in myoblast cells (56). The binding of YAP1 and FOXO1 to the promotor regions of antioxidant genes such as MnSOD and catalase activates their transcription (56). These genes are also implicated in cellular metabolism and are modified in a number of cancers such as glioma, lung, breast, pancreatic, esophagus, mesothelioma, colon, melanoma and leukemia because they adapt to their hostile new microenvironment in part via the aid of YAP1 and TAZ (61). YAP1 upregulates Glut3 via binding with TEAD in its promoter region in a kidney cell line (62), prioritizing glucose uptake in these cancer cells and allowing greater energy usage. Consequently, YAP1 favors the clearance of reactive species, decreasing the efficiency of secondary IR effects and altering cellular metabolism.
CSCs are maintained by a number of factors that control stemness and dedifferentiation, such as Sox2, Sox9, Snail/Slug and HNF4a (61). In osteosarcomas, the Hippo pathway is a downstream effector of Sox2-mediated stem maintenance, and this relationship is antagonized by Nf2/WWC1 (63). This regulatory effect has also been found in other cell types, including an immortalized murine fibroblast line and primary cultures of human glioblastoma cells (63). The loss of YAP1 decreases the self-renewal potential of NSCLC stem cells (45). The interaction between the YAP1 and Oct4 transcription factors regulates Sox2 expression in these NSCLC stem cells (45). However, TEAD4 and YAP1 repress the expression of Sox2 in the early stages in murine blastocysts through LATS1 control (64), suggesting that the functions of YAP1 are altered depending on the stage of life. YAP1 and its co-factor TEAD1 have been shown to regulate the transcription of other stem factors, such as Sox9, promoting CSC-like properties in esophageal cancer cells (46). However, Hippo signaling pathways are regulated by several stem factors. For example, in mesenchymal stem/stromal cells, Snail/Slug mediate YAP1 and TAZ expression in association with β-catenin and TBX5 (47). However, YAP1 also directly controls the transcription of Snail and HNF4a via TEAD in epithelial and hepatocyte cells (48). Despite this, HNF4a negatively regulates the expression of YAP1 in hepatocytes (48). YAP1 represses the differentiation of epithelial cells and hepatocytes by regulating MET genes (48).
In combination, myeloblastosis viral oncogene homolog (Myb)-MuvB and YAP1 regulate genes implicated in the cell cycle and, notably, mitosis via direct action of YAP1 (49). B-MyB expression is under the control of YAP1 and TEAD via a distal enhancer, favoring the chromatin-binding activities of B-MyB required for mitosis (49). Moreover, the physical interaction of MyB-MuvB with YAP1 seems necessary for YAP1-induced cell cycle progression (49). YAP1 and TEAD respond to mechanical stress signals to control the transcription of S-phase kinase-associated protein-2 (SKP2) (50). SKP2 overexpression inhibits its substrates, p21 and p73, and mediates cell cycle arrest induced by YAP1 depletion (50). Furthermore, E2F1 is a downstream target of YAP1 that regulates the G1/S transition through TEAD (51). YAP1 and TEAD therefore regulate the cell cycle.
The ability of Hippo pathway effectors to increase DNA repair has already been explored in a number of different models, including NSCLC (12). YAP1 forms a complex with TEAD2 and E2F1 to regulate the cellular response to DNA damage via the expression of Fanconi anemia components (52). Similarly, TAZ overexpression increases the expression of TP53BP1, PRKCD and XRCC6 which are associated with the 53BP1, DNA-PK and Ku70 proteins, respectively, which are implicated in the NHEJ DNA repair mechanism in esophageal cancer (38).
NDR1/2, upstream regulators of YAP1 and TAZ, contribute to the autophagic response to stress through a process thought to involve Beclin1, a major player in autophagy (53). C-ABL favors the formation of a YAP1/p73 complex that dissociates both RUNX and ITCH from YAP1 (51). The p73/YAP1 complex controls apoptosis, particularly after DNA damage via C-ABLs (51). In hepatocellular carcinoma, YAP1 inhibition induces apoptosis under hypoxic conditions compared normoxic conditions, which appears to be associated with HIF1 (54). Thus, the Hippo pathway is intertwined with autophagy and apoptosis regulation, which controls cell survival.
Despite all these factors participating in RR, a simple increase in proliferation also plays a part, and controlling proliferation is a well-known aspect of the Hippo pathway. YAP1 and TEAD regulate the expression of AREG (65), ANKRD1 and CTGF with the aid of AP1 (66), SKP2 stabilized by p300 (50) and Myc via the interaction of YAP1 with C-ABL (57), all of which play a role in proliferation. Additionally, proliferation is controlled by Hippo signaling through the interaction of YAP1 with TEAD4, which specifically regulates the expression of PRLCD, NRAS and RRAS (67).
Signaling pathways implicated in RR and Hippo signaling
The driving pathways of RR involve varying levels of interaction and crosstalk with the Hippo pathway and its components, which could lead to RT failure (Fig. 4).
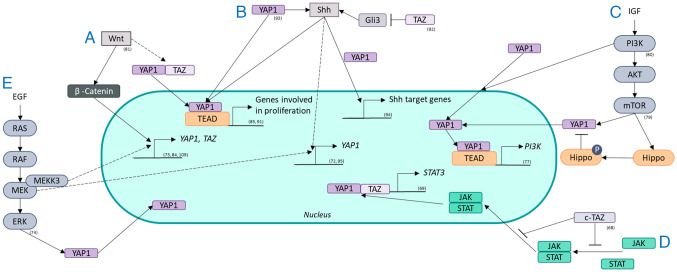
Figure 4.
Crosstalk between radioresistance signaling pathways and the Hippo pathway. The Hippo pathway interacts with pathways known for radioresistance, such as (A) the Wnt pathway, (B) the Shh pathway, (C) the PI3K/AKT/mTOR pathway, (D) the JAK/STAT axis and (E) the RAS/RAF/MEK/ERK pathway. The Hippo pathway and its effectors YAP1 and/or TAZ are regulated by these pathways through transcription, localization and activity. YAP1 and/or TAZ also regulate the activity of these pathways through transcriptional regulation. YAP1, yes-associated protein 1; TAZ, transcriptional coactivator with a PDZ-binding domain; EGF, epidermal growth factor; Shh, Sonic hedgehog; P, phosphorylated; JAK, Janus kinase; STAT, signal transducer and activation of transcription; Gli, glioma-associated oncogene; IGF, insulin-like growth factor.
cTAZ is an isoform of TAZ that is not regulated by TEAD which suppresses the JAK/STAT pathway by blocking dimerization and nuclear transport of STAT factors that control antiviral responses (68). YAP1 and TAZ increase the transcription of STAT3 components capable of reacting to oncogenic RAS and inflammation in pancreatitis (69) revealing the possible interplay of these factors in radiation-stressed cells. The JAK/STAT pathway is constitutively active in NSCLC (70). In NSCLC cells, an RR effect is potentiated by the microenvironment of the cells via JAK/STAT signaling (71).
EGF and insulin stimulate YAP in different human and Drosophila cells via MAPK signaling; however, this interaction has not been systematically investigated in another review (58). Specifically, MEK1 inhibition reduces the expression and activity of YAP1, revealing that YAP1 is regulated via MEK1, which is independent of the core Hippo pathway and promotes tumorigenesis in liver cancer cells (72). MEKK3 regulates YAP1 and TAZ at the transcriptional level in pancreatic cancer cells, promoting EMT and stemness (73). Moreover, ERK1/2 regulate YAP1 protein expression to increase the viability and invasion of NSCLC cells (74). The regulation of YAP1 and TAZ by MEK in NSCLC cells was found (74). YAP1 is implicated in MAPK/ERK signaling via EGFR mutations in NSCLC through an increase in the expression of EGFR ligands, such as AREG and ERBB3/4, subsequently increasing MAPK signaling (10). Furthermore, EGFR/MAPK signaling inhibits the phosphorylation and degradation of YAP1 by the Hippo kinase (35). These interactions between ERK/MAPK and the Hippo pathway play a part in stimulating proliferation signals after stress, limiting the effects of RT.
Along with Src and PDK1, PI3K regulates the nuclear localization of YAP1, favoring its activity (75). PI3K regulates both YAP1 and TAZ in breast cancer via PDK1 and AKT signaling which play a role in tumorigenesis (76). YAP1, in turn, activates PI3K/AKT/mTOR signaling in human bronchial epithelial cells via TEAD, leading to increased proliferation (77). In medulloblastoma cells, YAP1 increases the RR via IGF2/AKT signaling (78). An effector of this pathway, mTOR, phosphorylates the Hippo pathway and favors YAP1 activity, stimulating the proliferation and invasion of glioblastoma cells (79). In colorectal cancer, the PI3K/AKT pathway activates YAP1, leading to increased invasion and migration (80).
The Wnt pathway regulates YAP1 and TAZ, similar to β-catenin (58); ligands from this pathway activate YAP1 and TAZ through the frizzled receptor LATS1/2 and Rho-GTPases instead of the typical β-catenin pathway (81). This TEAD-mediated signaling leads to the expression of various genes: Osteogenic differentiation and cell migration (81). In liver cancer, Tribbles pseudokinase 2, a direct target of the Wnt pathway, stabilizes the coactivation factor of YAP1 transcription (82). YAP1 and TAZ are activated by oncogenic pathways such as the Wnt pathway (83). YAP1 transcription is elevated by Wnt/β-catenin signaling in colorectal carcinoma cells (84). TAZ is regulated by the Wnt pathway and increases the proliferation of colorectal cancer cells but also controls mesenchymal stem cell differentiation (85). YAP1 stimulates Wnt/β-catenin signaling in epithelial cells experiencing inflammation and regeneration through the targeting of CDK5 (86). Specifically, YAP1 regulates Wnt pathway activity differently depending on its localization; cytoplasmic YAP1 inhibits Wnt pathway activity, whereas nucleic YAP1 activates Wnt pathway activity (87). Inhibition of the Wnt pathway increases sensitivity to radiation in NSCL (88). The interplay of inhibition or activation of one another by the Hippo and Wnt pathways indicates that there is a complex relationship between these pathways possibly causing RR.
YAP1 blocks Shh-induced differentiation in smooth muscle cells (89). However, when YAP1 is inhibited in embryonic lung cells, the expression of Shh and its target genes decreases (90). YAP1 and TAZ also regulate the Shh pathway in epithelial lung cells (90). Human medulloblastoma cells develop from cerebellar granule neuron precursors activated by the Shh pathway and from high levels of YAP1 (91). In mouse Shh-induced medulloblastomas, YAP1 is also upregulated (91). In both of these cell types, YAP1 interacts with TEAD1, leading to Shh-driven proliferation (91), confirming the regulation of Shh by YAP1. On the other hand, TAZ suppresses the Shh pathway in in vitro and in in vivo models, potentially through Gli3 repression (92). The cell density regulation of Shh is regulated by the Hippo pathway effector YAP1 (93). YAP1 controls proliferation and inhibits differentiation in a mouse embryonic carcinoma cell line and increases the expression of Shh signaling and patched 1, a downstream effector of Shh (94). However, the relationship between these two pathways is not unilateral. Shh also regulates YAP1 activity via the hedgehog protein in regenerating murine liver cells (95), revealing a feedback loop. Since Shh activation increases the RR in NSCLC cells, these interactions are noteworthy possible mechanisms (30).
Influence of radiation on the regulation or dysregulation of the Hippo pathway
Although glycosylation, methylation and hypermethylation of Hippo members can influence their function and activity, to our knowledge, the roles they play in response to radiation exposure have not been reported.
Ubiquitination is an alternative modification permitting the control of various signaling pathways, including the Hippo pathway (96). β-transducin repeat containing E3 ubiquitin protein ligase is an E3 ligase that targets YAP1 and TAZ, leading to a reduction in their activity (96). Contrary to the regulation of LATS1/2 by other upstream E3 ligases, ITCH promotes growth and survival, and a hypoxia-activated E3 ligase, SIAH, promotes oncogenic YAP1 activation (97). Ubiquitin is overexpressed in a number of different NSCLC cell lines and implicated in increased growth (98). Silencing UBB and UBC, two key genes involved in the ubiquitination process, decreases cellular growth and increases radiosensitivity, as shown through pH2AX staining (98). Furthermore, ubiquitination of the Hippo pathway is regulated at both the transcriptional and post-translational levels and is implicated in the maintenance of CSC stemness (99).
5. Radiopotentiation and the Hippo pathway
Known methods of radiopotentiation
For advanced solid cancers, chemoRT refers to the use of irradiation combined with molecular targeting to render the tumour more radiosensitive (100). These molecular targets fall into four major categories: i) Growth factor receptor signaling inhibition; ii) targets of the DNA damage response and cell cycle checkpoints; iii) cell adhesion molecules; and iv) heat shock proteins (100). The most commonly used drugs for the treatment of NSCLC or BM from NSCLC fall into the first and second categories. For clinical treatment of BM from NSCLC in patients with EGFR mutations, third-generation TKIs, such as osimertinib, are used; these drugs fall into the first category of targeted drugs because of their greater ability to penetrate the central nervous system compared with previous generations (6). In the case of ALK rearrangements, lorlatinib, another third-generation TKI, is used to target the BM of patients with NSCLC after the failure of second generation TKIs such as alectinib and clertinib (6). The TGF-β1 inhibitor, SB431542, also induces radiopotentiation in NSCLC cell lines depending on the p53 status of the cells (101). Inhibition of P1K1 in p53 wild-type NSCLC cells induced radiosensitivity, but this effect was not found in mutated p53 cells (102). In the second category, the effects of various combinations of DNA damage response inhibitors on NSCLC cell lines have been investigated through the profiling of biomarkers and different genetic alterations (103). Eurycomalactone induces G2/M cell cycle arrest, a known radiosensitive phase of the cell cycle, and delays the repair of DSBs in NSCLC cells (104). A poly (ADP-ribose) polymerase inhibitor increases the radiosensitivity of the NSCLC cell line A549 (105). DNA damage response inhibitors are also being studied for their potential radiopotentiating effects on glioblastomas (106), demonstrating their ability to potentially cross the blood brain barrier which is the critical step in treating brain cancers such as BM from NSCLC.
Radiopotentiation using the Hippo pathway
Targeting the Hippo pathway to sensitize cancer cells to irradiation is a promising idea. As it was aforementioned, high levels of YAP1 and/or TAZ are associated with a poor RT response in most cancers, including NSCLC (43). Post-radiation activation of these factors has also been found in breast cancer (42) and metastatic breast cancer (107), and their expression is stable in NSCLC cells (44). YAP1 inhibition has radiopotentiating effects on pancreatic cancer (40), gliomas (108) and NSCLC (12).
The Hippo pathway is implicated in a number of the processes and pathways sustaining RR that are already targeted by specific drugs. YAP1 and/or TAZ have been shown to respond to hypoxia (58-60) and be induced by and decrease reactive species (55,56,61,62). These factors also play important roles in a feedback loop to maintain stem cell properties (45-48,63,64) and increase DNA repair (12,38,42,52,78,108). YAP1 and TAZ also regulate the cell cycle (22,49,51), autophagy (53) and apoptosis (54,96). The Hippo pathway effectors regulate certain factors in the JAK/STAT pathway (68,69) and the PI3K/AKT/mTOR pathway, stimulating and activating YAP1 (75-80). YAP1 is increased and activated by the ERK/MAPK pathway (72-74), the Wnt pathway (58,81-87) and the Hedgehog pathway, which also uses YAP1 as a transcription factor (89-95).
Inhibition of YAP1/TAZ
The use of drugs to inhibit YAP1 and TAZ is a common technique for studying their implications. For example, catechol treatment reduces the protein levels of YAP1 and its target genes through AMPK phosphorylation, sensitizing pancreatic cancer cells to irradiation (40). Verteporfin, a small inhibitor of the interaction of YAP1 with TEADs, decreases the number of DSBs back to a normal level after p130cas is overexpressed, restoring radiation efficiency in NSCLC (44). This drug is safe when administered via intraperitoneal injection at a dose of 100 mg/kg in mice (44). Furthermore, verteporfin is approved by the Food and Drug Administration (FDA), and is known to decrease the proliferation and migration of glioma cell lines (109). As a lipophile, verteporfin can penetrate the brain at nontoxic doses and is capable of inhibiting nuclear YAP1 in mouse models in vivo (109).
Anti-YAP1/TAZ treatments are not yet available, but IK-930, an oral TEAD inhibitor, is currently in phase 1 (NCT05228015) clinical trial for treating solid tumours. IK-930 blocks autopalmitoylation of TEAD by inhibiting TEAD-dependent transcription of YAP1 and TAZ (110). TEAD palmitoylation inhibitors stop mesothelioma cell line proliferation and block xenograft growth (111). However, TEAD palmitoylation inhibition increases VGLL3-mediated transcription of PIK3C2B and Sox4, which activate AKT signaling, contributing to cancer survival (112).
6. Summary and perspectives
NSCLC is a harsh disease in which 50% of patients develop BM (2-4), resulting in a mere 19% 5-year survival rate (113) despite the use of a treatment plan that includes both surgery and RT (4). RR remains a major hurdle in the treatment of BM from NSCLC. Notably, targeting the Hippo pathway to provoke radiopotentiation of BM from NSCLC due to its a number of potential implications for RR phenomena and the existence of inhibitors, such as IK-930, in a phase I clinical trial (110) or with FDA approval, such as verteporfin (109) is promising. However, a better understanding of the role of Hippo in RR and thus the potentially unforeseen side effects of targeting this pathway in cancer treatment of healthy cells would also be beneficial.
Acknowledgements
The authors thank the University Hospital of Caen for its financial and institutional support. Figures were partly created using Servier Medical Art, provided by Servier, licenced under a Creative Commons Attribution 3.0 Unported Licence (https://creativecommons.org/licenses/by/3.0/).
Abbreviations
- ATM
ataxia-telangiectasia mutated
- BM
brain metastasis
- CHK2
checkpoint kinase 2
- CSC
cancer stem cell
- DNA-PK
DNA-protein kinase
- DSB
double-strand break
- EGF
epidermal growth factor
- EMT
epithelial-to-mesenchymal transition
- GF
growth factor
- Gy
gray
- GLI
glioma-associated oncogene
- HIF
hypoxia-inducible factor
- IR
ionizing radiation
- JAK
Janus kinase
- LATS
large tumour suppressor
- MnSOD
manganese superoxide dismutase
- MST
mammalian sterile 20-like kinase
- NDR
nuclear dbf2-related
- NHEJ
non-homologous end joining
- NSCLC
non-small cell lung cancer
- p130cas
breast cancer anti-estrogen resistance protein 1
- PDGF
platelet-derived growth factor
- PTC1
patched 1
- ROS
reactive oxygen species
- RR
radioresistance/radioresistant
- RT
radiotherapy
- S
synthesis
- Shh
Sonic hedgehog
- SSB
single-strand break
- STAT
signal transducer and activator of transcription
- TAZ
transcriptional coactivator with a PDZ-binding domain
- TEAD
TEA DNA-binding protein
- TGF-β
transforming growth factor-β
- TKI
tyrosine kinase inhibitor
- VEGF
vascular endothelial growth factor
- XR
x-ray
- YAP1
yes-associated protein-1
Funding Statement
The present study was funded by the French Foundation for Medical Research (grant no. FRM2022).
Availability of data and materials
Not applicable.
Authors' contributions
JT and GL conceptualized the study; EB and GL acquired funding, provided project administration and supervised the study. Data were validated by JT, GL, FD and EB. JT and GL wrote the original draft which was reviewed and edited by JT, GL, FD and EB. All authors have read and approved the final version of the manuscript. Data authentication is not applicable.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Wood SL, Pernemalm M, Crosbie PA, Whetton AD. The role of the tumor-microenvironment in lung cancer-metastasis and its relationship to potential therapeutic targets. Cancer Treat Rev. 2014;40:558–566. doi: 10.1016/j.ctrv.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Dawe DE, Greenspoon JN, Ellis PM. Brain metastases in non-small-cell lung cancer. Clin Lung Cancer. 2014;15:249–257. doi: 10.1016/j.cllc.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 3.Fenske DC, Price GL, Hess LM, John WJ, Kim ES. Systematic review of brain metastases in patients with non-small-cell lung cancer in the United States, European Union, and Japan. Clin Lung Cancer. 2017;18:607–614. doi: 10.1016/j.cllc.2017.04.011. [DOI] [PubMed] [Google Scholar]
- 4.Myall NJ, Yu H, Soltys SG, Wakelee HA, Pollom E. Management of brain metastases in lung cancer: Evolving roles for radiation and systemic treatment in the era of targeted and immune therapies. Neurooncol Adv. 2021;3(Suppl 5):v52–v62. doi: 10.1093/noajnl/vdab106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dempke WCM, Edvardsen K, Lu S, Reinmuth N, Reck M, Inoue A. Brain metastases in NSCLC-are TKIs changing the treatment strategy? Anticancer Res. 2015;35:5797. [PubMed] [Google Scholar]
- 6.Ernani V, Stinchcombe TE. Management of brain metastases in non-small-cell lung cancer. J Oncol Pract. 2019;15:563–570. doi: 10.1200/JOP.19.00357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiang Y, Xie WJ, Chen RW, You WW, Ye WL, Chen H, Chen WX, Xu JP. The Hippo signaling core components YAP and TAZ as new prognostic factors in lung cancer. Front Surg. 2022;9:813123. doi: 10.3389/fsurg.2022.813123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dubois F, Keller M, Calvayrac O, Soncin F, Hoa L, Hergovich A, Parrini MC, Mazières J, Vaisse-Lesteven M, Camonis J, et al. RASSF1A suppresses the invasion and metastatic potential of human non-small cell lung cancer cells by inhibiting YAP activation through the GEF-H1/RhoB pathway. Cancer Res. 2016;76:1627–1640. doi: 10.1158/0008-5472.CAN-15-1008. [DOI] [PubMed] [Google Scholar]
- 9.Keller M, Dubois F, Teulier S, Martin APJ, Levallet J, Maille E, Brosseau S, Elie N, Hergovich A, Bergot E, et al. NDR2 kinase contributes to cell invasion and cytokinesis defects induced by the inactivation of RASSF1A tumor-suppressor gene in lung cancer cells. J Exp Clin Cancer Res. 2019;38:158. doi: 10.1186/s13046-019-1145-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hsu PC, Jablons DM, Yang CT, You L. Epidermal growth factor receptor (EGFR) pathway, yes-associated protein (YAP) and the regulation of programmed death-ligand 1 (PD-L1) in non-small cell lung cancer (NSCLC) Int J Mol Sci. 2019;20:3821. doi: 10.3390/ijms20153821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dubois F, Bergot E, Levallet G. Cancer and RASSF1A/RASSF1C, the two faces of Janus. Trends Cancer. 2019;5:662–665. doi: 10.1016/j.trecan.2019.10.001. [DOI] [PubMed] [Google Scholar]
- 12.Zeng Y, Liu Q, Wang Y, Tian C, Yang Q, Zhao Y, Liu L, Wu G, Xu S. CDK5 activates hippo signaling to confer resistance to radiation therapy via upregulating TAZ in lung cancer. Int J Radiat Oncol Biol Phys. 2020;108:758–769. doi: 10.1016/j.ijrobp.2020.05.005. [DOI] [PubMed] [Google Scholar]
- 13.Levallet J, Biojout T, Bazille C, Douyère M, Dubois F, Ferreira DL, Taylor J, Teulier S, Toutain J, Elie N, et al. Hypoxia-induced activation of NDR2 underlies brain metastases from non-small cell lung cancer. Cell Death Dis. 2023;14:823. doi: 10.1038/s41419-023-06345-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Fraipont F, Levallet G, Creveuil C, Bergot E, Beau-Faller M, Mounawar M, Richard N, Antoine M, Rouquette I, Favrot MC, et al. An apoptosis methylation prognostic signature for early lung cancer in the IFCT-0002 trial. Clin Cancer Res. 2012;18:2976–2986. doi: 10.1158/1078-0432.CCR-11-2797. [DOI] [PubMed] [Google Scholar]
- 15.Minniti G, Goldsmith C, Brada M. Chapter 16-radiotherapy. Handb Clin Neurol. 2012;104:215–228. doi: 10.1016/B978-0-444-52138-5.00016-5. [DOI] [PubMed] [Google Scholar]
- 16.Hall EJ, Giaccia AJ. Radiobiology for the radiologist. 8th. Philadelphia Baltimore New York London Buenos Aires: LWW; 2018. p. 624. [Google Scholar]
- 17.Loh ZH, Doumy G, Arnold C, Kjellsson L, Southworth SH, Al Haddad A, Kumagai Y, Tu MF, Ho PJ, March AM, et al. Observation of the fastest chemical processes in the radiolysis of water. Science. 2020;367:179–182. doi: 10.1126/science.aaz4740. [DOI] [PubMed] [Google Scholar]
- 18.Huang T, Song X, Xu D, Tiek D, Goenka A, Wu B, Sastry N, Hu B, Cheng SY. Stem cell programs in cancer initiation, progression, and therapy resistance. Theranostics. 2020;10:8721–8743. doi: 10.7150/thno.41648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen Z, Han F, Du Y, Shi H, Zhou W. Hypoxic microenvironment in cancer: Molecular mechanisms and therapeutic interventions. Signal Transduct Target Ther. 2023;8:70. doi: 10.1038/s41392-023-01332-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tang L, Wei F, Wu Y, He Y, Shi L, Xiong F, Gong Z, Guo C, Li X, Deng H, et al. Role of metabolism in cancer cell radioresistance and radiosensitization methods. J Exp Clin Cancer Res. 2018;37:87. doi: 10.1186/s13046-018-0758-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kelley K, Knisely J, Symons M, Ruggieri R. Radioresistance of brain tumors. Cancers (Basel) 2016;8:42. doi: 10.3390/cancers8040042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang Y, Gao Y, Mutter-Rottmayer L, Zlatanou A, Durando M, Ding W, Wyatt D, Ramsden D, Tanoue Y, Tateishi S, Vaziri C. DNA repair factor RAD18 and DNA polymerase Polκ confer tolerance of oncogenic DNA replication stress. J Cell Biol. 2017;216:3097–3115. doi: 10.1083/jcb.201702006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang M, Kern AM, Hülskötter M, Greninger P, Singh A, Pan Y, Chowdhury D, Krause M, Baumann M, Benes CH, et al. EGFR-mediated chromatin condensation protects KRAS-mutant cancer cells against ionizing radiation. Cancer Res. 2014;74:2825–2834. doi: 10.1158/0008-5472.CAN-13-3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carlos-Reyes A, Muñiz-Lino MA, Romero-Garcia S, López-Camarillo C, Hernández-de la Cruz ON. Biological adaptations of tumor cells to radiation therapy. Front Oncol. 2021;11:718636. doi: 10.3389/fonc.2021.718636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shi LZ, Bonner JA. Bridging radiotherapy to immunotherapy: The IFN-JAK-STAT axis. Int J Mol Sci. 2021;22:12295. doi: 10.3390/ijms222212295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marampon F, Ciccarelli C, Zani BM. Biological rationale for targeting MEK/ERK pathways in anti-cancer therapy and to potentiate tumour responses to radiation. Int J Mol Sci. 2019;20:2530. doi: 10.3390/ijms20102530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chang L, Graham PH, Hao J, Ni J, Bucci J, Cozzi PJ, Kearsley JH, Li Y. PI3K/Akt/mTOR pathway inhibitors enhance radiosensitivity in radioresistant prostate cancer cells through inducing apoptosis, reducing autophagy, suppressing NHEJ and HR repair pathways. Cell Death Dis. 2014;5:e1437. doi: 10.1038/cddis.2014.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen K, Shang Z, Dai AL, Dai PL. Novel PI3K/Akt/mTOR pathway inhibitors plus radiotherapy: Strategy for non-small cell lung cancer with mutant RAS gene. Life Sci. 2020;255:117816. doi: 10.1016/j.lfs.2020.117816. [DOI] [PubMed] [Google Scholar]
- 29.Yang Y, Zhou H, Zhang G, Xue X. Targeting the canonical Wnt/β-catenin pathway in cancer radioresistance: Updates on the molecular mechanisms. J Cancer Res Ther. 2019;15:272–277. doi: 10.4103/jcrt.JCRT_421_18. [DOI] [PubMed] [Google Scholar]
- 30.Xie SY, Li G, Han C, Yu YY, Li N. RKIP reduction enhances radioresistance by activating the Shh signaling pathway in non-small-cell lung cancer. OncoTargets Ther. 2017;10:5605–5619. doi: 10.2147/OTT.S149200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Calses PC, Crawford JJ, Lill JR, Dey A. Hippo pathway in cancer: Aberrant regulation and therapeutic opportunities. Trends Cancer. 2019;5:297–307. doi: 10.1016/j.trecan.2019.04.001. [DOI] [PubMed] [Google Scholar]
- 32.Thompson BJ. YAP/TAZ: Drivers of tumor growth, metastasis, and resistance to therapy. Bioessays. 2020;42:e1900162. doi: 10.1002/bies.201900162. [DOI] [PubMed] [Google Scholar]
- 33.Salem A, Asselin MC, Reymen B, Jackson A, Lambin P, West CML, O'Connor JPB, Faivre-Finn C. Targeting hypoxia to improve non-small cell lung cancer outcome. J Natl Cancer Inst. 2018;110:14–30. doi: 10.1093/jnci/djx160. [DOI] [PubMed] [Google Scholar]
- 34.Nguyen DX, Chiang AC, Zhang XHF, Kim JY, Kris MG, Ladanyi M, Gerald WL, Massagué J. WNT/TCF signaling through LEF1 and HOXB9 mediates lung adenocarcinoma metastasis. Cell. 2009;138:51–62. doi: 10.1016/j.cell.2009.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hsu PC, You B, Yang YL, Zhang WQ, Wang YC, Xu Z, Dai Y, Liu S, Yang CT, Li H, et al. YAP promotes erlotinib resistance in human non-small cell lung cancer cells. Oncotarget. 2016;7:51922–51933. doi: 10.18632/oncotarget.10458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miao J, Hsu PC, Yang YL, Xu Z, Dai Y, Wang Y, Chan G, Huang Z, Hu B, Li H, et al. YAP regulates PD-L1 expression in human NSCLC cells. Oncotarget. 2017;8:114576–114587. doi: 10.18632/oncotarget.23051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xiao Y, Dong J. The Hippo signaling pathway in cancer: A cell cycle perspective. Cancers (Basel) 2021;13:6214. doi: 10.3390/cancers13246214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou W, Zhang L, Chen P, Li S, Cheng Y. Thymine DNA glycosylase-regulated TAZ promotes radioresistance by targeting nonhomologous end joining and tumor progression in esophageal cancer. Cancer Sci. 2020;111:3613–3625. doi: 10.1111/cas.14622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xin H, Liu Y, Chen P, Yin T, Wang M, Liu T, Wen Z, Cheng Y. CD155 promotes radioresistance and malignancy of esophageal cancer by regulating Hippo-YAP pathway. Discov Oncol. 2022;13:53. doi: 10.1007/s12672-022-00515-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moon JY, Ediriweera MK, Ryu JY, Kim HY, Cho SK. Catechol enhances chemo- and radio-sensitivity by targeting AMPK/Hippo signaling in pancreatic cancer cells. Oncol Rep. 2021;45:1133–1141. doi: 10.3892/or.2021.7924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Andrade D, Mehta M, Griffith J, Panneerselvam J, Srivastava A, Kim TD, Janknecht R, Herman T, Ramesh R, Munshi A. YAP1 inhibition radiosensitizes triple negative breast cancer cells by targeting the DNA damage response and cell survival pathways. Oncotarget. 2017;8:98495–98508. doi: 10.18632/oncotarget.21913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liang Y, Zhou X, Xie Q, Sun H, Huang K, Chen H, Wang W, Zhou B, Wei X, Zeng D, Lin H. CD146 interaction with integrin β1 activates LATS1-YAP signaling and induces radiation-resistance in breast cancer cells. Cancer Lett. 2022;546:215856. doi: 10.1016/j.canlet.2022.215856. [DOI] [PubMed] [Google Scholar]
- 43.Yang K, Zhao Y, Du Y, Tang R. Evaluation of Hippo pathway and CD133 in radiation resistance in small-cell lung cancer. J Oncol. 2021;2021:8842554. doi: 10.1155/2021/8842554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li J, Zhang X, Hou Z, Cai S, Guo Y, Sun L, Li A, Li Q, Wang E, Miao Y. P130cas-FAK interaction is essential for YAP-mediated radioresistance of non-small cell lung cancer. Cell Death Dis. 2022;13:783. doi: 10.1038/s41419-022-05224-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bora-Singhal N, Nguyen J, Schaal C, Perumal D, Singh S, Coppola D, Chellappan S. YAP1 regulates Oct4 activity and Sox2 expression to facilitate self-renewal and vascular mimicry of stem-like cells. Stem Cells. 2015;33:1705–1718. doi: 10.1002/stem.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang L, Zhang Z, Yu X, Huang X, Liu Z, Chai Y, Yang L, Wang Q, Li M, Zhao J, et al. Unbalanced YAP-SOX9 circuit drives stemness and malignant progression in esophageal squamous cell carcinoma. Oncogene. 2019;38:2042–2055. doi: 10.1038/s41388-018-0476-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tang Y, Weiss SJ. Snail/Slug-YAP/TAZ complexes cooperatively regulate mesenchymal stem cell function and bone formation. Cell Cycle. 2017;16:399–405. doi: 10.1080/15384101.2017.1280643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Noce V, Battistelli C, Cozzolino AM, Consalvi V, Cicchini C, Strippoli R, Tripodi M, Marchetti A, Amicone L. YAP integrates the regulatory Snail/HNF4α circuitry controlling epithelial/hepatocyte differentiation. Cell Death Dis. 2019;10:768. doi: 10.1038/s41419-019-2000-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pattschull G, Walz S, Gründl M, Schwab M, Rühl E, Baluapuri A, Cindric-Vranesic A, Kneitz S, Wolf E, Ade CP, et al. The Myb-MuvB complex is required for YAP-dependent transcription of mitotic genes. Cell Rep. 2019;27:3533–3546.e7. doi: 10.1016/j.celrep.2019.05.071. [DOI] [PubMed] [Google Scholar]
- 50.Jang W, Kim T, Koo JS, Kim S, Lim D. Mechanical cue-induced YAP instructs Skp2-dependent cell cycle exit and oncogenic signaling. EMBO J. 2017;36:2510–2528. doi: 10.15252/embj.201696089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim W, Cho YS, Wang X, Park O, Ma X, Kim H, Gan W, Jho EH, Cha B, Jeung YJ, et al. Hippo signaling is intrinsically regulated during cell cycle progression by APC/CCdh1. Proc Natl Acad Sci USA. 2019;116:9423–9432. doi: 10.1073/pnas.1821370116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Oku Y, Nishiya N, Tazawa T, Kobayashi T, Umezawa N, Sugawara Y, Uehara Y. Augmentation of the therapeutic efficacy of WEE1 kinase inhibitor AZD1775 by inhibiting the YAP-E2F1-DNA damage response pathway axis. FEBS Open Bio. 2018;8:1001–1012. doi: 10.1002/2211-5463.12440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hergovich A. The roles of NDR protein kinases in Hippo signalling. Genes (Basel) 2016;7:21. doi: 10.3390/genes7050021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhu H, Wang DD, Yuan T, Yan FJ, Zeng CM, Dai XY, Chen ZB, Chen Y, Zhou T, Fan GH, et al. Multikinase inhibitor CT-707 targets liver cancer by interrupting the hypoxia-activated IGF-1R-YAP axis. Cancer Res. 2018;78:3995–4006. doi: 10.1158/0008-5472.CAN-17-1548. [DOI] [PubMed] [Google Scholar]
- 55.Cho Y, Park MJ, Kim K, Kim SW, Kim W, Oh S, Lee JH. Reactive oxygen species-induced activation of yes-associated protein-1 through the c-Myc pathway is a therapeutic target in hepatocellular carcinoma. World J Gastroenterol. 2020;26:6599–6613. doi: 10.3748/wjg.v26.i42.6599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shao D, Zhai P, Del Re DP, Sciarretta S, Yabuta N, Nojima H, Lim DS, Pan D, Sadoshima J. A functional interaction between Hippo-YAP signalling and FoxO1 mediates the oxidative stress response. Nat Commun. 2014;5:3315. doi: 10.1038/ncomms4315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xiao W, Wang J, Ou C, Zhang Y, Ma L, Weng W, Pan Q, Sun F. Mutual interaction between YAP and c-Myc is critical for carcinogenesis in liver cancer. Biochem Biophys Res Commun. 2013;439:167–172. doi: 10.1016/j.bbrc.2013.08.071. [DOI] [PubMed] [Google Scholar]
- 58.Yu FX, Zhao B, Guan KL. Hippo pathway in organ size control, tissue homeostasis, and cancer. Cell. 2015;163:811–828. doi: 10.1016/j.cell.2015.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xiang L, Gilkes DM, Hu H, Takano N, Luo W, Lu H, Bullen JW, Samanta D, Liang H, Semenza GL. Hypoxia-inducible factor 1 mediates TAZ expression and nuclear localization to induce the breast cancer stem cell phenotype. Oncotarget. 2014;5:12509–12527. doi: 10.18632/oncotarget.2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Azad T, Janse van Rensburg HJ, Lightbody ED, Neveu B, Champagne A, Ghaffari A, Kay VR, Hao Y, Shen H, Yeung B, et al. A LATS biosensor screen identifies VEGFR as a regulator of the Hippo pathway in angiogenesis. Nat Commun. 2018;9:1061. doi: 10.1038/s41467-018-03278-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lopez-Hernandez A, Sberna S, Campaner S. Emerging principles in the transcriptional control by YAP and TAZ. Cancers (Basel) 2021;13:4242. doi: 10.3390/cancers13164242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang W, Xiao ZD, Li X, Aziz KE, Gan B, Johnson RL, Chen J. AMPK modulates Hippo pathway activity to regulate energy homeostasis. Nat Cell Biol. 2015;17:490–499. doi: 10.1038/ncb3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Basu-Roy U, Bayin NS, Rattanakorn K, Han E, Placantonakis DG, Mansukhani A, Basilico C. Sox2 antagonizes the Hippo pathway to maintain stemness in cancer cells. Nat Commun. 2015;6:6411. doi: 10.1038/ncomms7411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Frum T, Watts JL, Ralston A. TEAD4, YAP1 and WWTR1 prevent the premature onset of pluripotency prior to the 16-cell stage. Development. 2019;146:dev179861. doi: 10.1242/dev.179861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang J, Ji JY, Yu M, Overholtzer M, Smolen GA, Wang R, Brugge JS, Dyson NJ, Haber DA. YAP-dependent induction of amphiregulin identifies a non-cell-autonomous component of the Hippo pathway. Nat Cell Biol. 2009;11:1444–1450. doi: 10.1038/ncb1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Koo JH, Plouffe SW, Meng Z, Lee DH, Yang D, Lim DS, Wang CY, Guan KL. Induction of AP-1 by YAP/TAZ contributes to cell proliferation and organ growth. Genes Dev. 2020;34:72–86. doi: 10.1101/gad.331546.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li H, Li Q, Dang K, Ma S, Cotton JL, Yang S, Zhu LJ, Deng AC, Ip YT, Johnson RL, et al. YAP/TAZ activation drives uveal melanoma initiation and progression. Cell Rep. 2019;29:3200–3211.e4. doi: 10.1016/j.celrep.2019.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fang C, Li J, Qi S, Lei Y, Zeng Y, Yu P, Hu Z, Zhou Y, Wang Y, Dai R, et al. An alternatively transcribed TAZ variant negatively regulates JAK-STAT signaling. EMBO Rep. 2019;20:e47227. doi: 10.15252/embr.201847227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gruber R, Panayiotou R, Nye E, Spencer-Dene B, Stamp G, Behrens A. YAP1 and TAZ control pancreatic cancer initiation in mice by direct up-regulation of JAK-STAT3 signaling. Gastroenterology. 2016;151:526–539. doi: 10.1053/j.gastro.2016.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Prabhu KS, Bhat AA, Siveen KS, Kuttikrishnan S, Raza SS, Raheed T, Jochebeth A, Khan AQ, Chawdhery MZ, Haris M, et al. Sanguinarine mediated apoptosis in non-small cell lung cancer via generation of reactive oxygen species and suppression of JAK/STAT pathway. Biomed Pharmacother. 2021;144:112358. doi: 10.1016/j.biopha.2021.112358. [DOI] [PubMed] [Google Scholar]
- 71.Meng J, Li Y, Wan C, Sun Y, Dai X, Huang J, Hu Y, Gao Y, Wu B, Zhang Z, et al. Targeting senescence-like fibroblasts radiosensitizes non-small cell lung cancer and reduces radiation-induced pulmonary fibrosis. JCI Insight. 2021;6:e146334. doi: 10.1172/jci.insight.146334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li L, Wang J, Zhang Y, Zhang Y, Ma L, Weng W, Qiao Y, Xiao W, Wang H, Yu W, et al. MEK1 promotes YAP and their interaction is critical for tumorigenesis in liver cancer. FEBS Lett. 2013;587:3921–3927. doi: 10.1016/j.febslet.2013.10.042. [DOI] [PubMed] [Google Scholar]
- 73.Santoro R, Zanotto M, Carbone C, Piro G, Tortora G, Melisi D. MEKK3 sustains EMT and stemness in pancreatic cancer by regulating YAP and TAZ transcriptional activity. Anticancer Res. 2018;38:1937–1946. doi: 10.21873/anticanres.12431. [DOI] [PubMed] [Google Scholar]
- 74.You B, Yang YL, Xu Z, Dai Y, Liu S, Mao JH, Tetsu O, Li H, Jablons DM, You L. Inhibition of ERK1/2 down-regulates the Hippo/YAP signaling pathway in human NSCLC cells. Oncotarget. 2015;6:4357–4368. doi: 10.18632/oncotarget.2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kim NG, Gumbiner BM. Adhesion to fibronectin regulates Hippo signaling via the FAK-Src-PI3K pathway. J Cell Biol. 2015;210:503–515. doi: 10.1083/jcb.201501025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhao Y, Montminy T, Azad T, Lightbody E, Hao Y, SenGupta S, Asselin E, Nicol C, Yang X. PI3K positively regulates YAP and TAZ in mammary tumorigenesis through multiple signaling pathways. Mol Cancer Res. 2018;16:1046–1058. doi: 10.1158/1541-7786.MCR-17-0593. [DOI] [PubMed] [Google Scholar]
- 77.Gokey JJ, Sridharan A, Xu Y, Green J, Carraro G, Stripp BR, Perl AT, Whitsett JA. Active epithelial Hippo signaling in idiopathic pulmonary fibrosis. JCI Insight. 2018;3:e98738. doi: 10.1172/jci.insight.98738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fernandez-L A, Squatrito M, Northcott P, Awan A, Holland EC, Taylor MD, Nahlé Z, Kenney AM. Oncogenic YAP promotes radioresistance and genomic instability in medulloblastoma through IGF2-mediated Akt activation. Oncogene. 2012;31:1923–1937. doi: 10.1038/onc.2011.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Artinian N, Cloninger C, Holmes B, Benavides-Serrato A, Bashir T, Gera J. Phosphorylation of the Hippo pathway component AMOTL2 by the mTORC2 kinase promotes YAP signaling, resulting in enhanced glioblastoma growth and invasiveness. J Biol Chem. 2015;290:19387–19401. doi: 10.1074/jbc.M115.656587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Takeda T, Yamamoto Y, Tsubaki M, Matsuda T, Kimura A, Shimo N, Nishida S. PI3K/Akt/YAP signaling promotes migration and invasion of DLD-1 colorectal cancer cells. Oncol Lett. 2022;23:106. doi: 10.3892/ol.2022.13226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Park HW, Kim YC, Yu B, Moroishi T, Mo JS, Plouffe SW, Meng Z, Lin KC, Yu FX, Alexander CM, et al. Alternative Wnt signaling activates YAP/TAZ. Cell. 2015;162:780–794. doi: 10.1016/j.cell.2015.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang J, Park JS, Wei Y, Rajurkar M, Cotton JL, Fan Q, Lewis BC, Ji H, Mao J. TRIB2 acts downstream of Wnt/TCF in liver cancer cells to regulate YAP and C/EBPα function. Mol Cell. 2013;51:211–225. doi: 10.1016/j.molcel.2013.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Simula L, Alifano M, Icard P. How phosphofructokinase-1 promotes PI3K and YAP/TAZ in cancer: Therapeutic perspectives. Cancers (Basel) 2022;14:2478. doi: 10.3390/cancers14102478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Konsavage WM, Jr, Kyler SL, Rennoll SA, Jin G, Yochum GS. Wnt/β-catenin signaling regulates Yes-associated protein (YAP) gene expression in colorectal carcinoma cells. J Biol Chem. 2012;287:11730–11739. doi: 10.1074/jbc.M111.327767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Azzolin L, Zanconato F, Bresolin S, Forcato M, Basso G, Bicciato S, Cordenonsi M, Piccolo S. Role of TAZ as Mediator of Wnt signaling. Cell. 2012;151:1443–1456. doi: 10.1016/j.cell.2012.11.027. [DOI] [PubMed] [Google Scholar]
- 86.Deng F, Peng L, Li Z, Tan G, Liang E, Chen S, Zhao X, Zhi F. YAP triggers the Wnt/β-catenin signalling pathway and promotes enterocyte self-renewal, regeneration and tumorigenesis after DSS-induced injury. Cell Death Dis. 2018;9:153. doi: 10.1038/s41419-017-0244-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jiang L, Li J, Zhang C, Shang Y, Lin J. YAP-mediated crosstalk between the Wnt and Hippo signaling pathways (review) Mol Med Rep. 2020;22:4101–4106. doi: 10.3892/mmr.2020.11529. [DOI] [PubMed] [Google Scholar]
- 88.Chen Y, Jin Y, Ying H, Zhang P, Chen M, Hu X. Synergistic effect of PAF inhibition and X-ray irradiation in non-small cell lung cancer cells. Strahlenther Onkol. 2021;197:343–352. doi: 10.1007/s00066-020-01708-7. [DOI] [PubMed] [Google Scholar]
- 89.Cotton JL, Li Q, Ma L, Park JS, Wang J, Ou J, Zhu LJ, Ip YT, Johnson RL, Mao J. YAP/TAZ and hedgehog coordinate growth and patterning in gastrointestinal mesenchyme. Dev Cell. 2017;43:35–47.e4. doi: 10.1016/j.devcel.2017.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Isago H, Mitani A, Mikami Y, Horie M, Urushiyama H, Hamamoto R, Terasaki Y, Nagase T. Epithelial expression of YAP and TAZ is sequentially required in lung development. Am J Respir Cell Mol Biol. 2020;62:256–266. doi: 10.1165/rcmb.2019-0218OC. [DOI] [PubMed] [Google Scholar]
- 91.Fernandez-L A, Northcott PA, Dalton J, Fraga C, Ellison D, Angers S, Taylor MD, Kenney AM. YAP1 is amplified and up-regulated in hedgehog-associated medulloblastomas and mediates Sonic hedgehog-driven neural precursor proliferation. Genes Dev. 2009;23:2729–2741. doi: 10.1101/gad.1824509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tang C, Wang J, Yao M, Ji X, Shi W, Xu C, Zeng LH, Wu X. Hippo signaling activates hedgehog signaling by Taz-driven Gli3 processing. Cell Regen. 2023;12:3. doi: 10.1186/s13619-022-00151-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tariki M, Dhanyamraju PK, Fendrich V, Borggrefe T, Feldmann G, Lauth M. The yes-associated protein controls the cell density regulation of Hedgehog signaling. Oncogenesis. 2014;3:e112. doi: 10.1038/oncsis.2014.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lin YT, Ding JY, Li MY, Yeh TS, Wang TW, Yu JY. YAP regulates neuronal differentiation through Sonic hedgehog signaling pathway. Exp Cell Res. 2012;318:1877–1888. doi: 10.1016/j.yexcr.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 95.Swiderska-Syn M, Xie G, Michelotti GA, Jewell ML, Premont RT, Syn WK, Diehl AM. Hedgehog regulates yes-associated protein 1 in regenerating mouse liver. Hepatology. 2016;64:232–244. doi: 10.1002/hep.28542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kim Y, Jho EH. Regulation of the Hippo signaling pathway by ubiquitin modification. BMB Rep. 2018;51:143–150. doi: 10.5483/BMBRep.2018.51.3.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Meng Z, Moroishi T, Guan KL. Mechanisms of Hippo pathway regulation. Genes Dev. 2016;30:1–17. doi: 10.1101/gad.274027.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tang Y, Geng Y, Luo J, Shen W, Zhu W, Meng C, Li M, Zhou X, Zhang S, Cao J. Downregulation of ubiquitin inhibits the proliferation and radioresistance of non-small cell lung cancer cells in vitro and in vivo. Sci Rep. 2015;5:9476. doi: 10.1038/srep09476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Deng L, Meng T, Chen L, Wei W, Wang P. The role of ubiquitination in tumorigenesis and targeted drug discovery. Signal Transduct Target Ther. 2020;5:11. doi: 10.1038/s41392-020-0107-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hintelmann K, Kriegs M, Rothkamm K, Rieckmann T. Improving the efficacy of tumor radiosensitization through combined molecular targeting. Front Oncol. 2020;10:1260. doi: 10.3389/fonc.2020.01260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhao Y, Wang L, Huang Q, Jiang Y, Wang J, Zhang L, Tian Y, Yang H. Radiosensitization of non-small cell lung cancer cells by inhibition of TGF-β1 signaling with SB431542 is dependent on p53 status. Oncol Res. 2016;24:1–7. doi: 10.3727/096504016X14570992647087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Van den Bossche J, Domen A, Peeters M, Deben C, De Pauw I, Jacobs J, De Bruycker S, Specenier P, Pauwels P, Vermorken JB, et al. Radiosensitization of non-small cell lung cancer cells by the Plk1 inhibitor volasertib is dependent on the p53 status. Cancers (Basel) 2019;11:1893. doi: 10.3390/cancers11121893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gill SJ, Wijnhoven PWG, Fok JHL, Lloyd RL, Cairns J, Armenia J, Nikkilä J, Lau A, Bakkenist CJ, Galbraith SM, et al. Radiopotentiation profiling of multiple inhibitors of the DNA damage response for early clinical development. Mol Cancer Ther. 2021;20:1614–1626. doi: 10.1158/1535-7163.MCT-20-0502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dukaew N, Konishi T, Chairatvit K, Autsavapromporn N, Soonthornchareonnon N, Wongnoppavich A. Enhancement of radiosensitivity by eurycomalactone in human NSCLC cells through G2/M Cell cycle arrest and delayed DNA double-strand break repair. Oncol Res. 2020;28:161–175. doi: 10.3727/096504019X15736439848765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ryu H, Kim HJ, Song JY, Hwang SG, Kim JS, Kim J, Bui THN, Choi HK, Ahn J. A small compound KJ-28d enhances the sensitivity of non-small cell lung cancer to radio- and chemotherapy. Int J Mol Sci. 2019;20:6026. doi: 10.3390/ijms20236026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Majd NK, Yap TA, Koul D, Balasubramaniyan V, Li X, Khan S, Gandy KS, Yung WKA, de Groot JF. The promise of DNA damage response inhibitors for the treatment of glioblastoma. Neurooncol Adv. 2021;3:vdab015. doi: 10.1093/noajnl/vdab015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.La Verde G, Artiola V, Pugliese M, La Commara M, Arrichiello C, Muto P, Netti PA, Fusco S, Panzetta V. Radiation therapy affects YAP expression and intracellular localization by modulating lamin A/C levels in breast cancer. Front Bioeng Biotechnol. 2022;10:969004. doi: 10.3389/fbioe.2022.969004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhang Y, Wang Y, Zhou D, Wang K, Wang X, Wang X, Jiang Y, Zhao M, Yu R, Zhou X. Radiation-induced YAP activation confers glioma radioresistance via promoting FGF2 transcription and DNA damage repair. Oncogene. 2021;40:4580–4591. doi: 10.1038/s41388-021-01878-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Barrette AM, Ronk H, Joshi T, Mussa Z, Mehrotra M, Bouras A, Nudelman G, Jesu Raj JG, Bozec D, Lam W, et al. Anti-invasive efficacy and survival benefit of the YAP-TEAD inhibitor verteporfin in preclinical glioblastoma models. Neuro Oncol. 2022;24:694–707. doi: 10.1093/neuonc/noab244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Amidon BS, Sanchez-Martin M, Bartolini W, Syed S, McGovern K, Xu L, Ecsedy J, Zhang XM, Constan A, Castro AC. Abstract 2156: IK-930 is a novel TEAD inhibitor for the treatment of cancers harboring mutations in the Hippo signal transduction pathway. Cancer Res. 2022;82(12 Suppl):S2156. doi: 10.1158/1538-7445.AM2022-2156. [DOI] [Google Scholar]
- 111.Tang TT, Konradi AW, Feng Y, Peng X, Ma M, Li J, Yu FX, Guan KL, Post L. Small molecule inhibitors of TEAD auto-palmitoylation selectively inhibit proliferation and tumor growth of NF2-deficient mesothelioma. Mol Cancer Ther. 2021;20:986–998. doi: 10.1158/1535-7163.MCT-20-0717. [DOI] [PubMed] [Google Scholar]
- 112.Sun Y, Hu L, Tao Z, Jarugumilli GK, Erb H, Singh A, Li Q, Cotton JL, Greninger P, Egan RK, et al. Pharmacological blockade of TEAD-YAP reveals its therapeutic limitation in cancer cells. Nat Commun. 2022;13:6744. doi: 10.1038/s41467-022-34559-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 114.Levallet G, Creveuil C, Bekaert L, Péres E, Planchard G, Lecot-Cotigny S, Guillamo JS, Emery E, Zalcman G, Lechapt-Zalcman E. Promoter hypermethylation of genes encoding for RASSF/Hippo pathway members reveals specific alteration pattern in diffuse gliomas. J Mol Diagn. 2019;21:695–704. doi: 10.1016/j.jmoldx.2019.03.007. [DOI] [PubMed] [Google Scholar]
- 115.Wei C, Wang Y, Li X. The role of Hippo signal pathway in breast cancer metastasis. Onco Targets Ther. 2018;11:2185–2193. doi: 10.2147/OTT.S157058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Maille E, Brosseau S, Hanoux V, Creveuil C, Danel C, Bergot E, Scherpereel A, Mazières J, Margery J, Greillier L, et al. MST1/Hippo promoter gene methylation predicts poor survival in patients with malignant pleural mesothelioma in the IFCT-GFPC-0701 MAPS phase 3 trial. Br J Cancer. 2019;120:387–397. doi: 10.1038/s41416-019-0379-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Spugnardi M, Tommasi S, Dammann R, Pfeifer GP, Hoon DSB. Epigenetic inactivation of RAS association domain family protein 1 (RASSF1A) in malignant cutaneous melanoma. Cancer Res. 2003;63:1639–1643. [PubMed] [Google Scholar]
- 118.Riffet M, Eid Y, Faisant M, Fohlen A, Menahem B, Alves A, Dubois F, Levallet G, Bazille C. Deciphering promoter hypermethylation of genes encoding for RASSF/Hippo pathway reveals the poor prognostic factor of RASSF2 gene silencing in colon cancers. Cancers (Basel) 2021;13:5957. doi: 10.3390/cancers13235957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Thurneysen C, Opitz I, Kurtz S, Weder W, Stahel RA, Felley-Bosco E. Functional inactivation of NF2/merlin in human mesothelioma. Lung Cancer. 2009;64:140–147. doi: 10.1016/j.lungcan.2008.08.014. [DOI] [PubMed] [Google Scholar]
- 120.Noorbakhsh N, Hayatmoghadam B, Jamali M, Golmohammadi M, Kavianpour M. The Hippo signaling pathway in leukemia: function, interaction, and carcinogenesis. Cancer cell international. 2021;21:705. doi: 10.1186/s12935-021-02408-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.