Editor—After publication of the Cochrane Injuries Group Albumin Reviewers’ systematic review of albumin administration in critically ill patients1 the Committee on Safety of Medicines convened an expert working party to consider the implications for the use of albumin in the United Kingdom. To date the committee has not made any announcement. The results of the review were widely reported in the medical and lay press,2 and this may have influenced the use of albumin.
We requested data on the monthly issues of albumin solutions to regional blood centres and hospitals between 1993 and 1999 from the Bio Products Laboratory (which serves England and Wales) and the Protein Fractionation Centre of the Scottish National Blood Transfusion Service (which serves Scotland and Northern Ireland). Issues were expressed in kg of albumin, reflecting the total albumin content of the various dose units. A three month moving average of albumin issues was calculated.
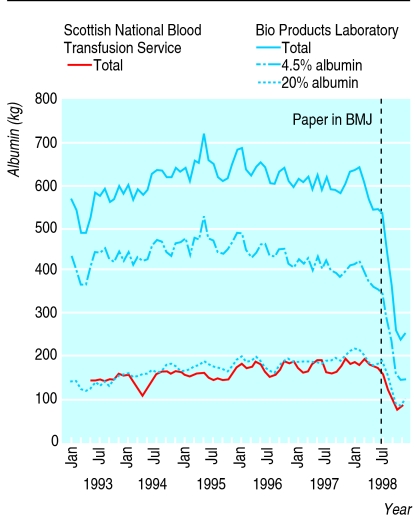
From May 1993 to June 1998 albumin issues from the Scottish National Blood Transfusion Service increased by about 17%. In July 1998, after publication in the BMJ and Cochrane Library of the systematic review, issues fell steeply, from 180 kg in June to 62 kg in December (figure). Issues from Bio Products Laboratory had been stable from January 1994 to July 1998, after which they fell dramatically. By the end of December 1998 issues of albumin from the Bio Products Laboratory had levelled out, those of 4.5% albumin having dropped by 40-45% and those of 20% albumin by around 40% (figure).
The Protein Fractionation Centre’s data reflect virtually all albumin used in Scotland and Northern Ireland. Data on Bio Products Laboratory’s share of the market in England and Wales are not available.
Demand for albumin fell steeply after publication of the systematic review. This raises important questions about the alternatives being used—non-albumin colloids (gelatins, starches, dextrans) and crystalloid solutions. Although the review included trials comparing albumin with crystalloids, there should be some concern that albumin may have been replaced by non-albumin colloids as there is no compelling evidence that these are superior and these products may also have important adverse effects.3,4 In view of the rapid and substantial changes in albumin issues there is a case for monitoring changing patterns of use of colloid and providing guidance for clinicians on fluid management.
Figure.
Three month moving average of albumin issues from Scottish National Blood Transfusion Service between May 1993 and December 1998 and from Bio Products Laboratory between January 1993 and December 1998
References
- 1.Cochrane Injuries Group Albumin Reviewers. Human albumin administration in critically ill patients: a systematic review of randomised controlled trials. BMJ. 1998;317:235–240. doi: 10.1136/bmj.317.7153.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nadel S, Marriage S, de Munter C, Britto J, Levin M, Habbibi P. Human albumin administration in critically ill patients. BMJ. 1998;317:882–886. . (26 September.) [PubMed] [Google Scholar]
- 3.McLelland B. Albumin: don’t confuse us with the facts. BMJ. 1998;317:829–830. doi: 10.1136/bmj.317.7162.829. . (26 September.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schierhout G, Roberts I. Crystalloid verses colloids in the fluid resuscitation of critically ill patients: a systematic review of randomised controlled trials. BMJ. 1998;316:961–964. doi: 10.1136/bmj.316.7136.961. [DOI] [PMC free article] [PubMed] [Google Scholar]