Abstract
Background and Objectives:
Current methods of intraoperative breast cancer margin assessment are labor intensive, not fully reliable, and time consuming; therefore novel strategies are necessary. We hypothesized that near infrared (NIR) intraoperative molecular imaging using systemic indocyanine green (ICG) would be helpful in discerning tumor margins.
Methods:
A mammary cancer cell line, 4T1, was used to establish tumors in mouse flanks (n=60). Tumors were excised 24 hours after intravenous ICG. Assessment of residual tumor in the wound bed was performed using a combination of NIR imaging and traditional method (by visual inspection and palpation) vs. traditional method alone. Next we performed a clinical trial to evaluate the role of NIR imaging after systemic ICG for the margin assessment of 12 patients undergoing breast-conserving surgery.
Results:
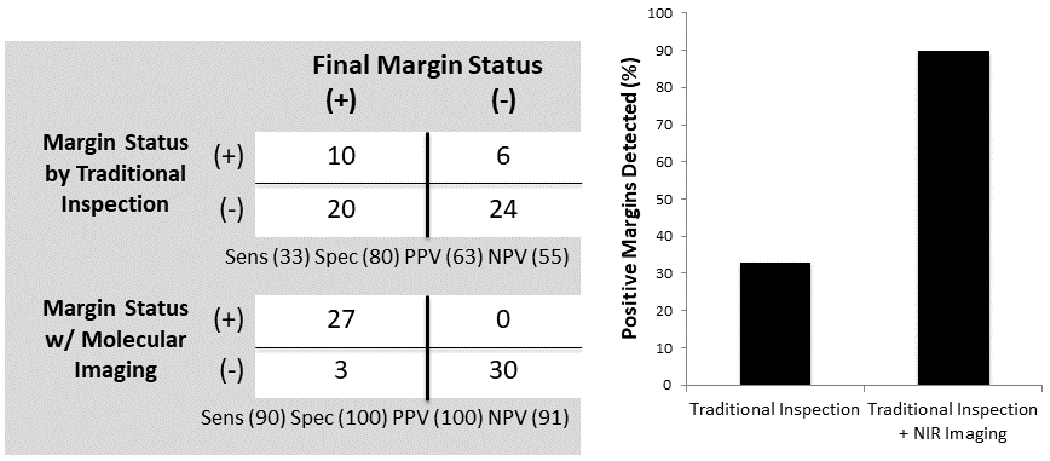
Traditional margin assessment identified 30% of positive margins while NIR imaging identified 90% of positive margins. In our clinical trial, all tumors were detected by NIR imaging and there was fluorescent evidence of residual tumor in the tumor bed in 6 of the 12 patients. None of these patients had positive margins on pathology.
Keywords: Indocyanine green, molecular imaging, solid tumor
INTRODUCTION:
Partial mastectomy followed by radiation is effective for the surgical treatment of early stage breast cancer however; positive histologic margins increase the rate of cancer recurrence, which negatively impacts patient survival 1,2. Therefore, in addition to radiation therapy, re-operation is performed for positive histological margins. Rates of re-excision for positive margins are high, and vary widely from 20-70% depending on institutional experience 3,4.
Current methods of intraoperative margin assessment include wire localization, radioactive seed localization, routine cavity shave excision, intraoperative ultrasound and intraoperative frozen section. These methods are imperfect, labor intensive, time consuming and may lead to poor cosmesis. For example, for an intraoperative frozen section to assess a typical lumpectomy specimen in its entirety, it would require 3000 slides to be developed which is logistically unrealistic 5. Thus, there is an unmet need for new approaches to identify cancer margins intraoperatively during the initial operation 6,7.
Molecular imaging utilizes fluorescent contrast agents to localize cancer cells and has been described to identify positive margins 8. Intraoperative molecular imaging has been used to detect human ovarian and lung cancers and is able to detect disease burden that would have otherwise have been missed 8–12. Recently, Tummers et al utilized a non-receptor targeted approach using intravenous methylene blue and technetium-sestamibi to localize human breast tumors using the enhanced permeability and retention (EPR) effect 13. Although promising for tumor localization, this approach demonstrated concerns about the value of NIR intraoperative molecular imaging using the EPR effect for evaluating breast cancer margins because fluorescence in the wound bed was not consistently congruent with positive margins on pathology.
We have significant experience with using NIR imaging to identify tumor margins in several cancers, but these techniques have not been investigated for breast cancer. We hypothesized that intravenous injection of a NIR small molecule contrast agent, indocyanine green (ICG), would localize to breast cancers, and the fluorescence could be utilized to assess for residual disease in the surgical wound. We tested our hypothesis in a murine model confirming the role of intraoperative NIR imaging for detecting residual tumor in the tumor bed. We then performed a pilot clinical trial to evaluate the role of ICG in intraoperative margin assessment in breast cancer surgery.
MATERIALS AND METHODS:
Cell Line
A mouse mammary tumor cell line, 4T1, was obtained from American Type Culture Collection® (Manassas, VA, USA). Cultures were maintained in RPMI (RPMI 1640 Medium, Mediatech, Washington DC), 10% FBS, 1% penicillin/streptomycin, and 1% glutamine. The cell line was regularly tested and maintained negative for Mycoplasma spp.
Reagents
Indocyanine green (ICG) is a water-soluble anionic, amphiphilic near-infrared fluorophore with a molecular weight of 774.9 kDA. ICG has an excitation wavelength of 790nm and an emission wavelength of 830nm. Mice received 5 mg/kg of intravenous ICG dissolved in 20 uL of phosphate buffered saline via tail vein injection 24 hours prior to imaging. For our clinical trial, the same dose of ICG (5 mg/kg) was dissolved in 250 cc of sterile water (Akorn®, Lake Forest, IL, USA and Pulsion®, Feldkirchen, Germany) and administered via antecubital vein one day prior to operation.
Near-Infrared Imaging
Imaging was performed using the FloCam (BioVision®, Exeter, PA, USA and BioMedicon®, Moorestown, NJ, USA), the Artemis Fluorescence Imaging system (Quest® Medical Imaging, Middenmeer, the Netherlands), or the Iridium (Visionsense®, New York, NY, USA). All devices are capable of emitting and absorbing light in the NIR spectrum10. The FloCam is a research imaging system and is capable of dual imaging with white light and NIR. The Artemis likewise allows for dual imaging with white light in color and NIR. The Iridium is a high definition (HD) 3D color camera (λex 790 nm and λem 830 nm) and was used for all animal imaging. Positive and negative controls were used for all images.
In order to quantitate tissue fluorescence, we used region-of-interest software and HeatMap plugin within ImageJ (http://rsb.info.nih.gov/ij/; public domain free software developed by National Institutes of Health). Background readings were taken from adjacent subcutaneous tissue in order to generate a signal-to-background ratio (SBR) or tumor-to-background ratio (TBR). All readings were done in quadruplicate.
Spectroscopy
Spectroscopy was performed using a hand-held device (Spectropen, InPhotonics®, Norwood, MA, USA) to measure NIR fluorescence of tumors. The Spectropen is a stainless steel device comprised of illumination and detector fibers coupled via an FC connector to a spectrometer. It utilizes a NIR diode laser (λex 785 nm) coupled to a head unit for light excitation and collection. The device was held as close as possible to the tissue to be measured without making contact 14.
Mouse tumor model
Animal studies were approved by the University of Pennsylvania Animal Use Committee. Mean TBR was determined in flank tumors established in mice (BALB/cfC3H) (n=12) after subcutaneous injection of 1.0x106 4T1 cells suspended in 100μL PBS. When tumors reached ~500 mm3, 5 mg/kg of ICG in deionized water was given via tail vein injection. Mice were then anesthetized with intramuscular ketamine (80 mg/kg) and xylazine (10 mg/kg). The tumor was inspected, imaged in situ and biopsied. We took four spectroscopic readings of the tumor as well as the adjacent normal cutaneous tissue as background. Mice were euthanized by CO2 exposure and cervical dislocation in order to assess fluorescence in vital organs.
NIR imaging and traditional method vs. traditional method alone for residual tumor assessment
To compare NIR imaging and traditional method vs. traditional method alone, we established tumors in 60 mice as described above. Mice received ICG 24 hours prior to tumor resection when tumor size reached ~ 500 mm3. Through a 2 cm incision, thirty mice then underwent complete resection, and the remaining mice underwent partial resection in which approximately 5% rim of residual tumor was left behind15. A first investigator, blinded to the status of resection, was asked to determine the margin status of all mice by traditional inspection and palpation alone. A second investigator, blinded to the surgeon and first investigator, used the combination method, including NIR imaging, to evaluate for residual tumor. In this case suspicion for residual tumor was defined as an area of retained tissue bed fluorescence with SBR ≥ 1.5. Each true positive wound bed (determined by the initial surgeon) as well as any suspected positive margins chosen by either investigator were biopsied for histology.
Patient study design
The clinical trial protocol was approved by the University of Pennsylvania Institutional Review Board, and all patients gave informed consent. Patients with biopsy proven invasive breast cancer undergoing breast-conserving therapy were eligible for this study. Following initial dissection, the NIR imaging system was sterilely draped and positioned above the field. The lumpectomy specimen was imaged in vivo and ex vivo prior to pathology submission. The surgical bed was then re-imaged in NIR for any residual fluorescence. Wound bed fluorescence with SBR ≥ 1.5 was used as the definition of suspicious positive margin. All specimens were evaluated by standard histological examination by a breast pathologist.
Histopathology and Fluorescence Microscopy
Histopathology was used to evaluate resected tissues. Samples were fixed in 10% formalin, embedded in paraffin, sectioned, and evaluated by a pathologist. Fluorescence microscopy was utilized to verify the accumulation of ICG within resected tissues in several samples. 5μm-thick sections from tissue biopsies obtained during the surgery were mounted with a glycerin-based mounting media and frozen tumor sections were prepared as previously described16. The samples were examined using an Olympus® IX51 fluorescent microscope equipped with an indocyanine green specific filter set (Chroma® 49030). Fluorescence images were acquired using a PixeLink® NIR CCD camera (PL-B741EU). Each sample was then subsequently stained with hematoxylin and eosin and re-imaged using white light. Fluorescence images were processed using ImageJ® (http://rsb.info.nih.gov/ij/; public domain software developed by National Institutes of Health). The images were overlaid on white-light images to create color-NIR images.
Statistics
After measuring mean fluorescence from tumors, vital organs, margins and background in quadruplicate as previously described, the mean TBR or SBR of each subject was calculated. A Chi-square test for homogeneity was used to analyze the rate of positive margin detection between each investigator. In our human trial, a tumor bed fluorescence SBR ≥1.5 was considered suspicious for positive margin. Next, in vivo mean TBRs of human breast cancer subjects were compared to the diagnosis of cancer type (lobular vs. ductal) to evaluate for any significance using Wilcoxon-Mann-Whitney Test. Tests were considered significant if P-value ≤ 0.05. All calculations were performed using SAS ® software.
RESULTS:
Murine mammary flank tumors can be identified using NIR molecular imaging
Based on prior studies, we hypothesized that optical imaging using a near-infrared contrast agent ICG should be able to detect 4T1 murine flank tumors. Twenty-four hours after ICG tail vein injection, mice were anesthetized and fluorescence was quantified using NIR imaging and spectroscopy (Fig. 1a).
Figure 1.
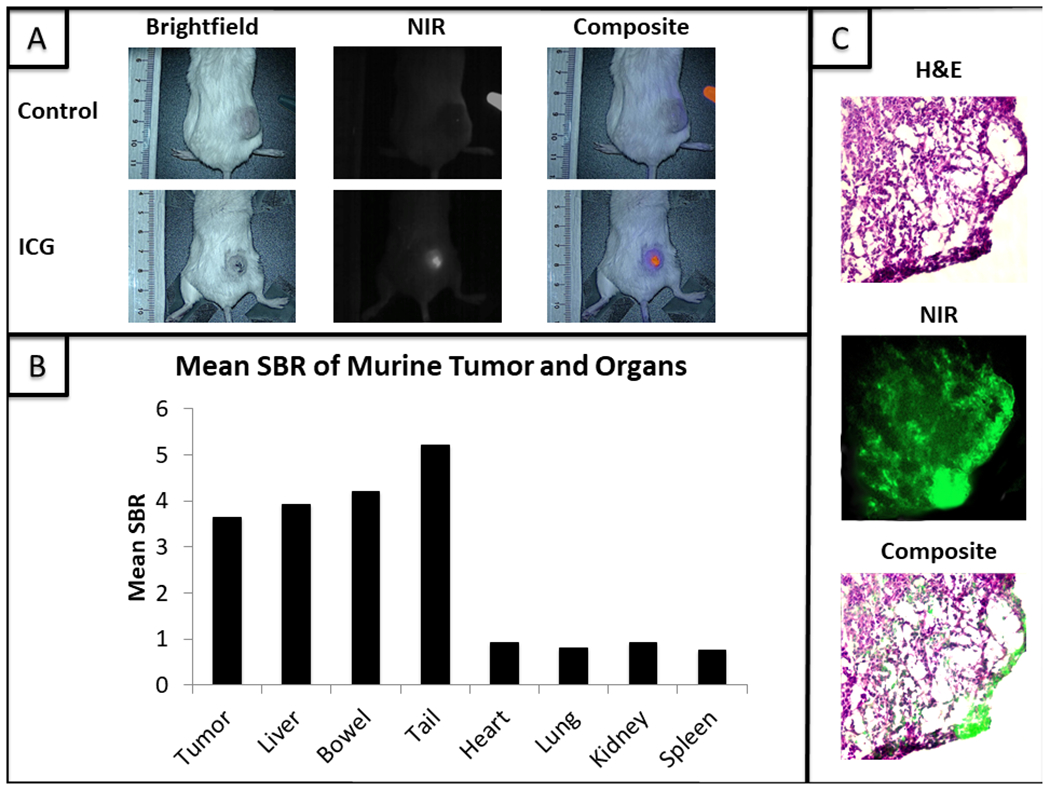
(A) 4T1 flank tumors imaged with the Iridium NIR imaging device. The ICG labeled mouse received an intravenous injection of ICG 24 hours earlier while the control mouse did not receive an injection prior to imaging. (B) Mean signal-to-background ratio (SBR) of vital organs when compared to tumors. (C) Tumor stained with H&E followed by a NIR fluorescence microscopy (100x) image. These were then layered to demonstrate fluorescence in the area of cancer cells.
All animals survived the study with no adverse effects. The tumors were highly fluorescent when compared to surrounding tissues. The mean tumor fluorescence ranged from 3.19 to 4.20 a.u. (IQR 3.31-4.05 a.u.); the mean background fluorescence in these cases was 1.01 a.u. (IQR 0.95-1.09 a.u.). The mean TBR was 3.64 +/− 0.18 (IQR 3.53-3.76). Mean spectroscopic TBR was 34.9 +/− 11.1 (IQR 25.9-43.5). Fluorescent signal was highly localized to flank tumors with fluorescence ≥ 1.5 times background only in the liver (mean SBR 3.92 +/− 0.41), bowel (mean SBR 4.20 +/− 0.51) and tail (SBR 5.21 +/− 0.62) (Fig. 1b).
Fluorescence microscopy demonstrated fluorescence in the region of tumor parenchyma on H&E (Fig. 1c).
Residual fluorescence in the surgical bed identifies positive margins
After determining that 4T1 flank xenografts fluoresced using NIR imaging, we sought to determine if fluorescent imaging could detect residual tumor deposits after resection. 4T1 flank tumors were again established in BALB/cfC3H mice. Once established, animals were given ICG one day prior to surgery. Animals underwent tumor resection with intentional positive margins (n=30) or complete removal (n=30) by the first surgeon.
The second surgeon was blinded to which animals had positive margins and examined the wound bed for residual disease. Conventional inspection using visual and tactile cues correctly identified 10 residual tumors and falsely assigned 6 mice as having residual tumors. Inspection aided by intraoperative NIR imaging was able to detect the 10 positive wound beds discovered by the first investigator and an additional 17 positive wound beds for a total of 27 positive margins (Fig. 2a). The mean TBR of these residual tumor deposits was 3.63 +/− 0.21 (IQR 3.53-3.75). NIR imaging was not associated with any false positives. In summary, traditional inspection missed 20 of the 30 true positive margins while inspection aided by intraoperative NIR missed only 3 of the 30 true positive margins.
Figure 2.
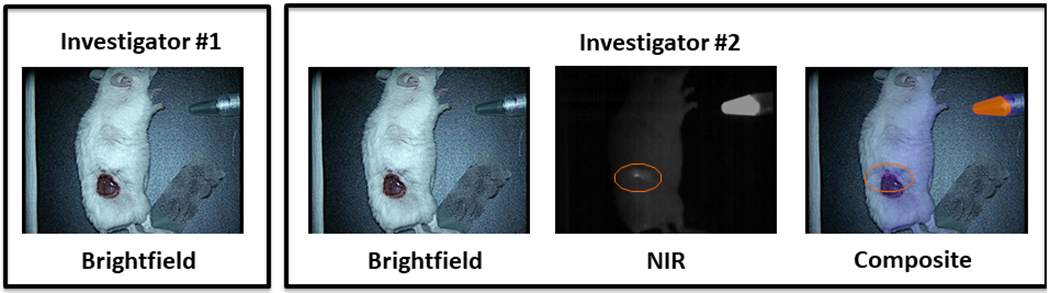
(A) The orange circle denotes a positive margin detected as an area of fluorescence with NIR imaging that was missed by traditional inspection. (B) Comparison of margin inspection by investigator 1 (using traditional inspection alone) vs. investigator 2 (aided by NIR imaging).
Human breast cancers fluoresce using systemic injection of ICG
Once we validated that NIR imaging could identify breast tumors and improve locating residual disease, we postulated our NIR contrast agent would also localize to human breast cancers. Twelve patients with biopsy-proven invasive carcinoma were enrolled in an IRB approved clinical study. The clinical characteristics of our patient cohort are summarized in Table 1. All 12 patients were given a 5 mg/kg intravenous dosage of ICG 24 hours (range 20.8 – 27.0 hours) prior to surgery. One patient had mild nausea after ICG infusion that was successfully treated with intravenous diphenhydramine. There were no other adverse events encountered.
Table 1.
Patient and Tumor Characteristics of Patients Involved in the Human Pilot Trial for Investigating NIR Intraoperative Molecular Imaging for Breast Tumor and Margin Localization
| No. of patients (n=12) | |
|---|---|
| Age, median (range) | 60 (44-70) |
| Race | |
| Caucasian | 7 |
| African-American | 4 |
| Asian | 1 |
| Tumor Size (cm), median (IQR) | 1.7 (0.7-2.6) |
| Diagnosis | |
| Invasive ductal carcinoma | 9 |
| Invasive lobular carcinoma | 3 |
| Receptor Status | |
| ER(+) PR(+) Her2(+) | 3 |
| ER(+) PR(+) Her2(−) | 5 |
| ER(+) PR(−) Her2(−) | 2 |
| ER(−) PR(−) Her2(−) | 2 |
| Nodule Palpability | |
| Palpable | 6 |
| Non-Palpable | 6 |
| Mode of Detection | |
| Screening mammogram | 10 |
| Chest X-ray | 1 |
| Ultrasound | 1 |
Of the twelve patients, six had non-palpable cancer and underwent preoperative wire localization of the cancer on the day of surgery. The women underwent standard surgical incisions ranging from 4 cm to 7 cm. All twelve patients’ breast tumors were positively identified by intraoperative molecular imaging in situ. Overall, the mean in vivo tumor fluorescence ranged from 3.07 to 4.00 a.u. (IQR 3.21-3.43 a.u.) and the mean background fluorescence was 1.01 a.u. (IQR 0.99-1.01 a.u). The in vivo mean TBR was 3.14 +/− 0.34 (IQR 2.84-3.41).
Next, the tumors were removed by standard surgical techniques and the specimens were re-imaged prior to submitting to pathology. The ex vivo mean TBR measured from the anterior surface of these masses was 3.46 +/− 0.35 (IQR 3.17-3.71).
A representative patient is shown in Figure 3. Preoperative imaging (Fig. 3a), in vivo (Fig. 3b) and ex vivo imaging (Fig. 3c) are shown for this example patient.
Figure 3.
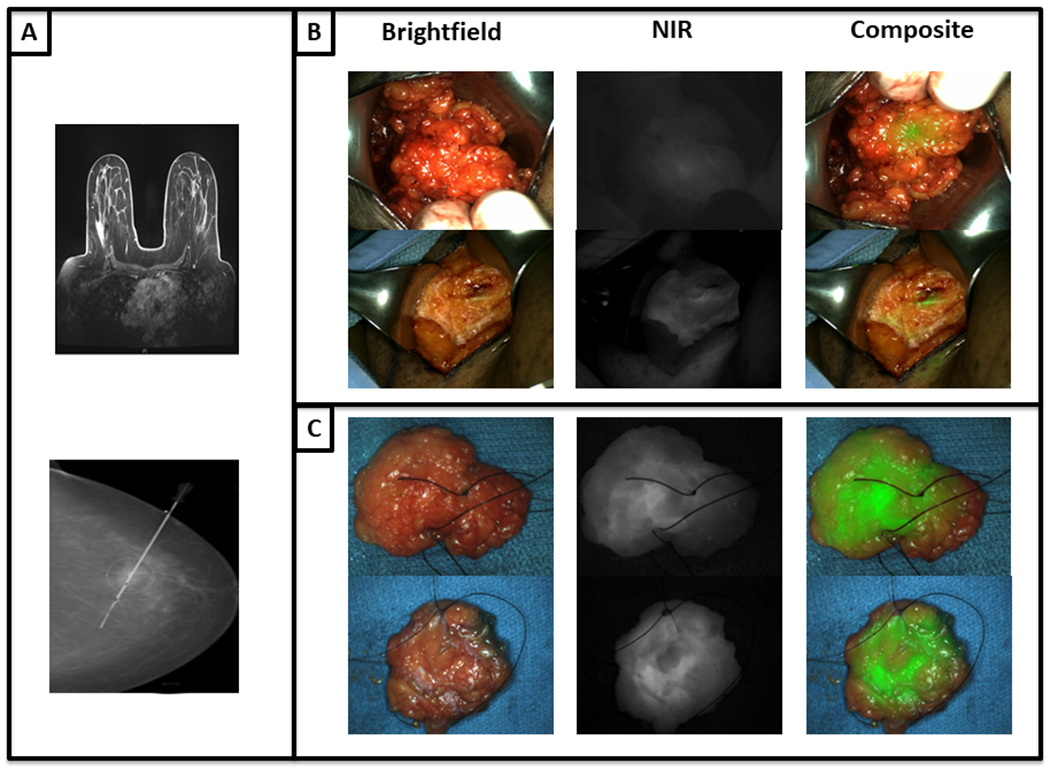
(A) Preoperative imaging of an example patient with preoperatively diagnosed invasive breast cancer. (B) In vivo and (C) ex vivo images obtained with the Artemis NIR imaging device demonstrate a markedly fluorescent tumor intraoperatively.
Systemic ICG for margin assessment in human breast cancer
In all cases, the surgeons subjectively felt that they had completely removed the tumor and had negative margins. We then sought to determine whether we could use NIR imaging to predict the margin status following lumpectomy.
First, we examined the wound bed using a NIR imaging device following specimen resection. Wound beds with retained fluorescence of SBR ≥ 1.5 were deemed suspicious for positive margins. Of the 12 patients, 6 patients were found to have retained fluorescence of the surgical bed following tumor resection, with a mean SBR of 3.11 +/− 0.32 (IQR 2.86-3.29). The remaining 6 patients had wound bed mean SBRs of < 1.5. Because of the experimental nature of this trial, the surgeons made the decision not to resect further tissue based only on the finding of NIR wound bed fluorescence.
We then examined the specimens using our NIR imaging techniques prior to sending them to pathology. We imaged each specimen from 6 perspectives, including anterior, posterior, superior, inferior, medial and lateral. Of the 12 specimens, we discovered significant NIR fluorescence in all 6 perspectives of each tumor.
On final pathology, no patients were found to have tumor at the specimen margin. However, two patients were discovered to have close positive margins (tumor within 1 mm of the edge of the specimen) on pathology. Both of these patients had retained fluorescence in the surgical bed (SBR 2.91 and 3.29, respectively). The remaining 10 patients had negative surgical margins (tumor present ≥ 1cm from specimen edge) by pathology. However, 4 of these 10 patients had retained fluorescence in the surgical bed following lumpectomy, with a mean SBR of 3.02 +/− 0.27.
One representative patient is discussed here. BA is a 55-year-old female who presented with a 1.7 cm biopsy-proven infiltrative ductal carcinoma of her right breast that was imaged with preoperative MRI (Fig. 4a). In the operating room, the mass was markedly fluorescent with a mean in vivo TBR of 3.30. As the tumor was resected and when imaged ex vivo, strong fluorescence was seen from the posterior margin of the tumor (Fig. 4b–c). After excision, the mass was reimaged on the back table (ex vivo mean TBR was 3.67) (Fig. 4d–e). During inspection of the posterior margin, the surgeon deemed this margin of the specimen to be clinically clear. However the lumpectomy bed demonstrated significant retained fluorescence (mean SBR 3.27) (Fig. 4f–g). On pathological review, the margin was negative for tumor cells, however invasive ductal carcinoma was less than 1 mm from the posterior margin in the original surgical specimen (Fig. 4h).
Figure 4.
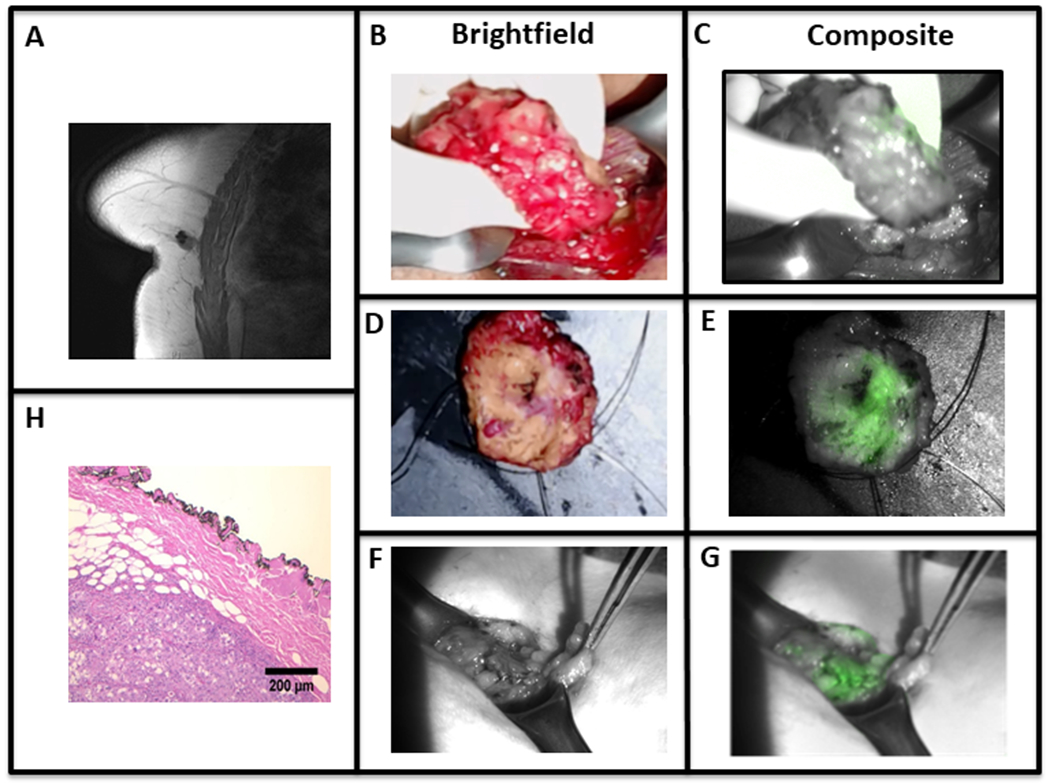
A patient with an invasive ductal carcinoma as seen with (A) preoperative MRI. (B-E) ICG accumulation is noted with in vivo and ex vivo brightfield and overlay fluorescence images. Additionally (F-G) imaging of the wound bed demonstrates retained fluorescence. (H) The H&E of the tumor specimen shows a close but negative posterior margin on final pathology.
Clinical Outcomes
No patients were lost to follow up, and no patients underwent re-excision following surgery. All 12 patients received postoperative radiation as planned. Each patient has undergone routine postoperative exams, and there have been no recurrences to date with a mean follow up time of 1.2 years (Range 7 months – 1.6 years).
DISCUSSION:
Identification of breast tumor margins can be challenging, as surgeons are primarily limited to fallible methods including finger palpation, wire localization, seed localization, and intraoperative ultrasound for localizing tumors and making decisions regarding margin resection. We proposed intraoperative molecular imaging using systemic ICG to localize breast cancer tumors as well as evaluate tumor margins.
In this translational study, we showed that systemic ICG prior to surgery localizes to cancerous mammary tissue in mice. We have also shown that this imaging protocol was superior to traditional methods for the detection of positive tumor margins in a murine model. Next, we demonstrated that this technique is clinically translatable, as it is safely and accurately able to detect human breast cancers. However, unlike our murine model, retained fluorescence in the surgical bed did not correlate with positive breast tumor margins.
We have several potential explanations for the false positive signal in the tumor bed. First, we made an important observation during the ex vivo imaging of the breast specimen. If we inadvertently squeezed or overly manipulated the specimen, a small pool of fluid would smear the tray that contained the specimen. Interestingly, this fluid was fluorescent. On several more patients, we reproduced this ability to draw fluorescent fluid out of the tumor by manipulation. Prior evidence from our lab shows that NIR intraoperative imaging using ICG for well-encapsulated tumors, such as sarcoma, is able to localize and accurately predict margin status. Unlike mouse flank tumors, human breast tumors lack a well-defined capsule, which may affect the ability of ICG to remain contained to the extracellular space around the tumor, which affects our ability to predict margins.
Another important consideration is related to our choice of tracer. ICG is a NIR fluorophore that when given systemically binds to plasma proteins forming a nanoparticle17. ICG is thought to accumulate in tumors by the enhanced permeability and retention (EPR) effect, which takes advantage of leaky vasculature and dysfunctional lymphatics 18. Although ICG did localize to the breast tumors, it is likely that the lack of ligand specific binding allowed it to leak out of the tumor and easily infiltrate surrounding tissues over 24 hours. This may be overcome by reducing the amount of time from ICG injection to imaging making margins ill defined.
One potential solution to overcome the limitations of ICG as a localizing contrast agent is to utilize a targeted NIR dye. Several targeted dyes are currently under investigation, although few are approved for clinical research. Our group has studied folate-FITC (EC17) for the intraoperative imaging of triple negative breast cancer, which is a visible wavelength probe conjugated with a folate receptor alpha agonist19. This allows the targeted fluorescent probe to bind folate receptors on a tumor cell. However, unlike EC17, ICG is excited and absorbed in the NIR spectrum, which provides several advantages over tracers in the visible wavelength. For example, NIR tracers should allow for less autofluorescence and greater depth of penetration in human tissue when compared to imaging in the visible spectrum20. In this study, the breast surgeon first dissected out the lumpectomy specimen by traditional methods prior to using NIR imaging, so depth of penetration was not an issue. However, we speculate that the depth of penetration using ICG in breast tissue is approximately 1 cm. An interesting follow up study using ICG as a localization technique prior to traditional dissection would be helpful to understand the depth of penetration of NIR tracers in breast tissue.
Currently, the cost of conducting intraoperative molecular imaging is not insignificant. A typical high-quality commercial near-infrared imaging device can cost approximately $100,000. These camera systems can be sterilized and used indefinitely. Additionally, with continued research and interest in this field it is expected further competition may reduce this price. The equipment is simple to use and requires the use of a specialized camera and screen. The learning curve for the surgeon takes approximately one use, however, the interpretation of real-time imaging is still experimental.
The future of intraoperative molecular imaging in the localization of primary breast tumors and margins is promising. Tumor-specific tracers with greater affinity for binding breast cancer will certainly be important to move forward. In addition to intraoperative molecular imaging of tumors and margins, there is a role for intraoperative localization of ductal carcinoma in situ, which will provide an additional area of potential research.
CONCLUSIONS:
ICG accumulates in breast cancers in both a murine model and human patients. However, NIR imaging for margin detection using systemic ICG will need further refinement before clinical value can be obtained.
Synopsis:
Intraoperative molecular imaging using systemic indocyanine green identifies breast cancer. However, fluorescence does not reliably predict breast cancer tumor margins.
Funding:
NIH R01 CA163256
List of Acronyms:
- NIR
Near-infrared
- ICG
Indocyanine green
- TBR
Tumor-to-background ratio
- SBR
Signal-to-background ratio
References
- 1.Moran MS, Schnitt SJ, Giuliano AE, Harris JR, Khan SA, Horton J, et al. Society of Surgical Oncology–American Society for Radiation Oncology Consensus Guideline on Margins for Breast-Conserving Surgery With Whole-Breast Irradiation in Stages I and II Invasive Breast Cancer. Ann Surg Oncol. 2014;21:704–716. [DOI] [PubMed] [Google Scholar]
- 2.Singletary SE. Surgical margins in patients with early-stage breast cancer treated with breast conservation therapy. Am J Surg. 2002;184(5):383–93. [DOI] [PubMed] [Google Scholar]
- 3.Mansell J, Monypenny IJ, Skene AI, Abram P, Carpenter R, Gattuso JM, et al. Patterns and predictors of early recurrence in postmenopausal women with estrogen receptor-positive early breast cancer. Breast Cancer Res Treat. 2009;117(1):91–8. [DOI] [PubMed] [Google Scholar]
- 4.McCahill LE, Single RM, Aiello Bowles EJ, Feigelson HS, James TA, Barney T, et al. Variability in reexcision following breast conservation surgery. JAMA. 2012;307(5):467–75. [DOI] [PubMed] [Google Scholar]
- 5.Klimberg VS, Harms S, Korourian S. Assessing margin status. Surg Oncol. 1999;8(2):77–84. [DOI] [PubMed] [Google Scholar]
- 6.Meric F, Mirza NQ, Vlastos G, Buchholz TA, Kuerer HM, Babiera GV, et al. Positive surgical margins and ipsilateral breast tumor recurrence predict disease-specific survival after breast-conserving therapy. Cancer. 2003;97(4):926–33. [DOI] [PubMed] [Google Scholar]
- 7.Boughey JC, Hieken TJ, Jakub JW, Degnim AC, Grant CS, Farley DR, et al. Impact of analysis of frozen-section margin on reoperation rates in women undergoing lumpectomy for breast cancer: evaluation of the National Surgical Quality Improvement Program data. Surgery. 2014;156(1):190–7. [DOI] [PubMed] [Google Scholar]
- 8.Madajewski B, Judy BF, Mouchli A, Kapoor V, Holt D, Wang MD, et al. Intraoperative near-infrared imaging of surgical wounds after tumor resections can detect residual disease. Clin Cancer Res. 2012;18(20):5741–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Dam GM, Themelis G, Crane LM, Harlaar NJ, Pleijhuis RG, Kelder W, et al. Intraoperative tumor-specific fluorescence imaging in ovarian cancer by folate receptor-alpha targeting: first in-human results. Nat Med. 2011;17:1315–1319. [DOI] [PubMed] [Google Scholar]
- 10.Okusanya OT, Holt D, Heitjan D, Deshpande C, Venegas O, Jiang J, et al. Intraoperative Near-Infrared Imaging Can Identify Pulmonary Nodules. Ann Thorac Surg. 2014;98:1223–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singhal S, Nie S, Wang MD. Nanotechnology applications in surgical oncology. Annu Rev Med. 2010;61:359–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holt D, Parthasarathy AB, Okusanya O, Keating J, Venegas O, Deshpande C, et al. Intraoperative near-infrared fluorescence imaging and spectroscopy identifies residual tumor cells in wounds. J Biomed Opt. 2015;20(7):76002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tummers QR, Verbeek FP, Schaafsma BE, Boonstra MC, van der Vorst JR, Liefers GJ, et al. Real-time intraoperative detection of breast cancer using near-infrared fluorescence imaging and Methylene Blue. Eur J Surg Oncol. 2014;40(7):850–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mohs AM, Mancini MC, Singhal S, Provenzale JM, Leyland-Jones B, Wang MD, et al. Hand-held Spectroscopic Device for In Vivo and Intraoperative Tumor Detection: Contrast Enhancement, Detection Sensitivity, and Tissue Penetration. Anal Chem. 2010;82:9058–9065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Predina JD, Judy B, Fridlender ZG, Aliperti LA, Madajewski B, Kapoor V, et al. A positive-margin resection model recreates the postsurgical tumor microenvironment and is a reliable model for adjuvant therapy evaluation. Cancer Biol Ther. 2012;13(9):745–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Predina J, Eruslanov E, Judy B, Kapoor V, Cheng GJ, Wang LC, et al. Changes in the local tumor microenvironment in recurrent cancers may explain the failure of vaccines after surgery. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(5):E415–E424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoneya S, Saito T, Komatsu Y, Koyama I, Takahashi K, Duvoll-Young J. Binding properties of indocyanine green in human blood. Invest Ophthalmol Vis Sci. 1998;39(7):1286–90. [PubMed] [Google Scholar]
- 18.Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J Control Release. 2000;65(1-2):271–84. [DOI] [PubMed] [Google Scholar]
- 19.Low PS, Kularatne SA. Folate-targeted therapeutic and imaging agents for cancer. Curr Opin Chem Biol. 2009;13(3):256–62. [DOI] [PubMed] [Google Scholar]
- 20.Luo S, Zhang E, Su Y, Cheng T, Shi C. A review of NIR dyes in cancer targeting and imaging. Biomaterials. 2011;32(29):7127–38. [DOI] [PubMed] [Google Scholar]


