Abstract
Background
Nirmatrelvir in combination with ritonavir is an antiviral treatment for mild-to-moderate coronavirus disease 2019 (Covid-19). The efficacy of this treatment in patients who are at standard risk for severe Covid-19 or who are fully vaccinated and have at least one risk factor for severe Covid-19 has not been established.
Methods
In this phase 2–3 trial, we randomly assigned adults who had confirmed Covid-19 with symptom onset within the past 5 days in a 1:1 ratio to receive nirmatrelvir–ritonavir or placebo every 12 hours for 5 days. Patients who were fully vaccinated against Covid-19 and who had at least one risk factor for severe disease, as well as patients without such risk factors who had never been vaccinated against Covid-19 or had not been vaccinated within the previous year, were eligible for participation. Participants logged the presence and severity of prespecified Covid-19 signs and symptoms daily from day 1 through day 28. The primary end point was the time to sustained alleviation of all targeted Covid-19 signs and symptoms. Covid-19–related hospitalization and death from any cause were also assessed through day 28.
Results
Among the 1296 participants who underwent randomization and were included in the full analysis population, 1288 received at least one dose of nirmatrelvir–ritonavir (654 participants) or placebo (634 participants) and had at least one postbaseline visit. The median time to sustained alleviation of all targeted signs and symptoms of Covid-19 was 12 days in the nirmatrelvir–ritonavir group and 13 days in the placebo group (P=0.60). Five participants (0.8%) in the nirmatrelvir–ritonavir group and 10 (1.6%) in the placebo group were hospitalized for Covid-19 or died from any cause (difference, −0.8 percentage points; 95% confidence interval, −2.0 to 0.4). The percentages of participants with adverse events were similar in the two groups (25.8% with nirmatrelvir–ritonavir and 24.1% with placebo). In the nirmatrelvir–ritonavir group, the most commonly reported treatment-related adverse events were dysgeusia (in 5.8% of the participants) and diarrhea (in 2.1%).
Conclusions
The time to sustained alleviation of all signs and symptoms of Covid-19 did not differ significantly between participants who received nirmatrelvir–ritonavir and those who received placebo. (Supported by Pfizer; EPIC-SR ClinicalTrials.gov number, NCT05011513.)
Although coronavirus disease 2019 (Covid-19) can be mild or asymptomatic,1,2 severe cases can lead to hospitalization and are associated with substantial morbidity and mortality.3,4 Older persons and those who have certain underlying conditions, including cardiovascular disease, obesity, and diabetes, are at increased risk for severe outcomes from Covid-19.2-5
The ongoing clinical burden of Covid-19 necessitates the availability of effective, easily accessible oral treatments for Covid-19 that can shorten the time to resolution of symptoms and reduce the risk of severe Covid-19 leading to hospitalization or resulting in death. Nirmatrelvir is an orally administered antiviral agent that inhibits the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) main protease (Mpro), which is critical for viral replication.6,7 Nirmatrelvir is administered with the pharmacokinetic enhancer ritonavir (nirmatrelvir–ritonavir) to inhibit metabolism by CYP3A4.8 In a pivotal phase 2–3 safety and efficacy trial involving unvaccinated adults with at least one risk factor for severe Covid-19, nirmatrelvir–ritonavir reduced the risk of progression to Covid-19–related hospitalization or death from any cause by 89% and 86% as compared with placebo when administered within 3 days and 5 days, respectively, after symptom onset.9,10
The current phase 2–3 trial evaluated the efficacy and safety of nirmatrelvir–ritonavir in nonhospitalized adults with symptomatic Covid-19 who either were at standard risk for severe Covid-19 (i.e., without risk factors for severe Covid-19, either unvaccinated or without vaccination within the previous 12 months) or were fully vaccinated and at high risk for progression to severe Covid-19.
Methods
Trial Design and Participants
We conducted the Evaluation of Protease Inhibition for Covid-19 in Standard-Risk Patients (EPIC-SR) trial, a phase 2–3, double-blind, randomized, placebo-controlled trial to assess the efficacy, safety, and side-effect profile of nirmatrelvir–ritonavir. Eligible participants were at least 18 years of age and had reverse-transcriptase–polymerase-chain-reaction (RT-PCR)–confirmed or rapid antigen–confirmed SARS-CoV-2 infection and associated signs or symptoms of Covid-19 (Table S1 in the Supplementary Appendix, available with the full text of this article at NEJM.org), with the onset of signs of symptoms occurring 5 or fewer days before randomization. Participants were required to have at least one prespecified Covid-19 sign or symptom on the day of randomization and to be capable of providing signed informed consent.
Patients with any predefined underlying condition associated with an increased risk of severe Covid-19 were eligible for participation if they had been fully vaccinated against Covid-19 (i.e., if they had received a complete primary series of an authorized Covid-19 vaccine).2-5 In addition, patients without underlying conditions associated with an increased risk of severe Covid-19 were eligible if they had not received a Covid-19 vaccine. After authorization of nirmatrelvir–ritonavir by the Food and Drug Administration for the treatment of Covid-19 in patients with risk factors for severe disease in December 2021, the trial protocol was amended to exclude all patients with risk factors for severe disease (to ensure clinical balance) and to allow enrollment of patients without underlying conditions associated with an increased risk of severe Covid-19 who had not received a Covid-19 vaccine within the previous 12 months. Additional details are provided in the Supplementary Appendix and in the protocol (available at NEJM.org).
All participants provided written informed consent. The trial was conducted in accordance with ethical principles derived from international guidelines (see the Supplementary Appendix) and local laws and regulations. The protocol and other relevant documents were each approved by an institutional review board or ethics committee before trial initiation. Nirmatrelvir and matching placebo were manufactured by Pfizer, and ritonavir tablets were manufactured and tested by Hetero Labs. Blinding was performed by Pfizer by means of overencapsulation.
Pfizer was responsible for the trial design and conduct and for data collection, analysis, and interpretation. The first draft of the manuscript was written by medical writers (funded by Pfizer) under direction from the authors. All the data were available to the authors, who vouch for the accuracy and completeness of the data and for the adherence of the trial to the protocol.
Trial Procedures
After a screening period that lasted no longer than 48 hours, participants were randomly assigned in a 1:1 ratio with the use of an interactive response technology system to receive either nirmatrelvir–ritonavir (300 mg of nirmatrelvir and 100 mg of ritonavir) or placebo (consisting of inactive filler ingredients) every 12 hours for 5 days (10 doses in total). Randomization was stratified according to geographic region, vaccination status, and time of Covid-19 symptom onset (≤3 days vs. >3 to 5 days before randomization). Participants used an electronic diary daily to record when they took nirmatrelvir–ritonavir or placebo, as well as the presence and severity of Covid-19 signs or symptoms.11 Efficacy and safety assessments were performed through day 28 and day 34, respectively, with planned longer-term follow-up to evaluate vital status and symptoms at weeks 12 and 24.
Efficacy
The primary objective of the trial was to compare the efficacy of nirmatrelvir–ritonavir with that of placebo for the treatment of Covid-19, as measured by the difference in time to sustained alleviation of all targeted Covid-19 signs and symptoms through day 28. All efficacy end points were evaluated in the population of participants who took at least one dose of nirmatrelvir–ritonavir or placebo and had at least one postbaseline visit. The targeted symptoms were cough, shortness of breath or difficulty breathing, feeling feverish, chills or shivering, muscle or body aches, diarrhea, nausea, vomiting, headache, sore throat, and stuffy or runny nose. Participants recorded daily symptom severity on a 4-point scale (0, absent; 1, mild; 2, moderate; 3, severe). Sustained alleviation was considered to have occurred on the first of 4 consecutive days during which all symptoms that had been scored as moderate or severe and as mild or absent at baseline were scored as mild or absent and as absent, respectively.
The prespecified key secondary end point was Covid-19–related hospitalization or death from any cause through day 28. Related secondary end points were the number of medical visits and the number of days in the hospital or intensive care unit (ICU) related to Covid-19 through day 28. Viral load was evaluated as described previously.9 The day 5 viral load was assessed as at or above the lower limit of quantification or below the lower limit of quantification (<2 log10 copies per milliliter). Viral load rebound was then evaluated on day 10 or day 14 and was defined either as a viral load of 3.0 log10 copies per milliliter or higher on day 10 or day 14 in a patient with a viral load that had been below the lower limit of quantitation on day 5 or as a viral load increase by at least 0.5 log10 copies per milliliter from day 5 and a day 10 or day 14 viral load of 3.0 log10 copies per milliliter or higher in a patient with a viral load that had been at or above the lower limit of quantitation at day 5, day 10, or day 14. Symptom rebound was also evaluated and was defined as a worsening of symptoms (total symptom score increased by ≥4 points; scores range from 0 [no symptoms] to 36 [all severe symptoms]) after any abatement of symptoms.
Safety
The secondary objective was to describe the safety and side-effect profile of nirmatrelvir–ritonavir as compared with placebo, with safety measured as the incidence of adverse events that emerged during the treatment period, serious adverse events, and adverse events that led to discontinuation of nirmatrelvir–ritonavir or placebo. Safety data were evaluated in the safety analysis population, which included all participants who received at least one dose of nirmatrelvir–ritonavir or placebo. The safety follow-up period lasted through day 34.
Statistical Analysis
Initially, we planned to enroll 1140 participants to ensure that 800 participants would be enrolled within 3 days after symptom onset, which would provide 90% power to detect a 25% difference in the time to sustained alleviation of all targeted Covid-19 signs and symptoms (8 days vs. 6 days), assuming that 18% of the participants would discontinue participation and that approximately 30% of the participants would undergo randomization more than 3 days after symptom onset. The protocol was later amended to include more participants to allow for better evaluation of the secondary efficacy end point (a composite of Covid-19–related hospitalization or death from any cause during the 28 days after enrollment).
The primary end point, the time to sustained alleviation of all Covid-19–related signs or symptoms, was compared between the groups with the use of a log-rank test. The median time to sustained alleviation was estimated by the Kaplan–Meier method within each group. For the key secondary end point, the cumulative percentage of participants who were hospitalized for Covid-19 or died during the first 28 days of the trial was estimated for each group with the use of the Kaplan–Meier method and was compared between the groups with the use of the Wald test. For 95% confidence intervals, the corresponding estimate of the standard error was computed with Greenwood’s formula. The number of Covid-19–related medical visits through day 28 was compared between the groups with a negative binomial regression model. The mean number of days spent in the hospital in each group was summarized and compared with the use of the bootstrap method with 100,000 replicates, with each replicate randomly selected (with replacement) from the analysis set.
The primary end point was tested at a significance level of 0.05. If the results of this test were not significant, only 95% confidence intervals would be reported for secondary end points. These 95% confidence intervals were not adjusted for multiplicity and should not be used to infer treatment effects.
Results
Participants
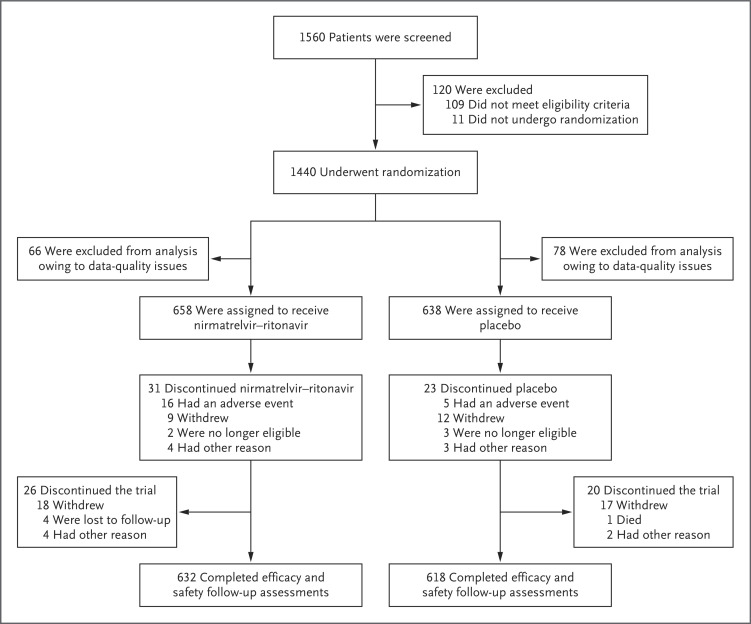
From August 25, 2021, to July 25, 2022, a total of 1296 participants underwent randomization and were included in the full analysis population (Figure 1), and 1288 received at least one dose of nirmatrelvir–ritonavir (654 participants) or placebo (634 participants) and had at least one postbaseline visit. A total of 1250 participants completed efficacy and safety follow-up assessments. Subgroup analyses were performed in the subgroup of 636 participants with at least one risk factor for severe Covid-19 who had been vaccinated and in the subgroup of 649 participants who were at standard risk (i.e., without risk factors).
Figure 1. Enrollment and Randomization.
Demographic and Covid-19–related characteristics were similar in the two groups (Table 1 and Table S2) and were largely representative of the expected patient population (Table S3). More than half the participants were women (54.0%), and the median age at enrollment was 42 years. Most participants were White (78.5%), and 41.4% identified as Hispanic or Latino; 48.6% had a body-mass index (BMI, the weight in kilograms divided by the square of the height in meters) of 25 or higher at screening (mean, 26.3). All the participants had confirmed SARS-CoV-2 infection, and 72.5% underwent randomization within 3 days after symptom onset. Slightly more than half the participants (56.9%) had received previous Covid-19 vaccination (most commonly with mRNA vaccines), and 49.9% had at least one risk factor for severe Covid-19; the most common risk factor was cigarette smoking (in 13.3% of the participants), and the most common coexisting condition was hypertension (in 12.3%). Eleven participants who had at least one risk factor and were unvaccinated were not included in the subgroup analyses. Adherence (defined as having taken 80 to 115% of the expected number of tablets) was 94.8% in the nirmatrelvir–ritonavir group and 96.5% in the placebo group.
Table 1. Demographic and Clinical Characteristics of Participants Included in the Full Analysis Population.*.
| Characteristic | Nirmatrelvir–Ritonavir (N=658) |
Placebo (N=638) |
Total (N=1296) |
|---|---|---|---|
| Sex — no. (%) | |||
| Male | 312 (47.4) | 284 (44.5) | 596 (46.0) |
| Female | 346 (52.6) | 354 (55.5) | 700 (54.0) |
| Median age (range) — yr | 41 (18–87) | 42 (18–82) | 42 (18–87) |
| Race or ethnic group — no. (%)† | |||
| White | 515 (78.3) | 502 (78.7) | 1017 (78.5) |
| Black | 28 (4.3) | 23 (3.6) | 51 (3.9) |
| Asian | 70 (10.6) | 72 (11.3) | 142 (11.0) |
| American Indian or Alaska Native | 39 (5.9) | 32 (5.0) | 71 (5.5) |
| Not reported or unknown | 6 (0.9) | 9 (1.4) | 15 (1.2) |
| Hispanic or Latino | 274 (41.6) | 262 (41.1) | 536 (41.4) |
| Not reported | 4 (0.6) | 6 (0.9) | 10 (0.8) |
| Geographic region — no. (%) | |||
| United States | 216 (32.8) | 206 (32.3) | 422 (32.6) |
| Europe | 222 (33.7) | 215 (33.7) | 437 (33.7) |
| Rest of world | 220 (33.4) | 217 (34.0) | 437 (33.7) |
| Median BMI (range)‡ | 24.9 (17.4–58.8) | 24.9 (14.2–53.1) | 24.9 (14.2–58.8) |
| Vaccinated against Covid-19 — no. (%) | 374 (56.8) | 364 (57.1) | 738 (56.9) |
| Serologic testing for SARS-CoV-2 — no. (%)§ | |||
| Positive | 482 (73.3) | 475 (74.5) | 957 (73.8) |
| Negative | 161 (24.5) | 149 (23.4) | 310 (23.9) |
| Unknown | 15 (2.3) | 14 (2.2) | 29 (2.2) |
| Baseline Covid-19 severity — no. (%)¶ | |||
| None | 8 (1.2) | 11 (1.7) | 19 (1.5) |
| Mild | 205 (31.2) | 178 (27.9) | 383 (29.6) |
| Moderate | 308 (46.8) | 307 (48.1) | 615 (47.5) |
| Severe | 114 (17.3) | 129 (20.2) | 243 (18.8) |
| Missing data | 23 (3.5) | 13 (2.0) | 36 (2.8) |
| Median time from first symptom to start of treatment (range) — days | 3 (0–5) | 3 (0–6) | 3 (0–6) |
| Risk status — no. (%)‖ | |||
| High risk | 319 (48.5) | 317 (49.7) | 636 (49.1) |
| Standard risk | 335 (50.9) | 314 (49.2) | 649 (50.1) |
| Other | 4 (0.6) | 7 (1.1) | 11 (0.8) |
| Most common risk factors — no. (%)** | |||
| BMI ≥30 | 109 (16.6) | 122 (19.1) | 231 (17.8) |
| Smoking | 86 (13.1) | 86 (13.5) | 172 (13.3) |
| Hypertension | 83 (12.6) | 77 (12.1) | 160 (12.3) |
| Diabetes mellitus | 34 (5.2) | 32 (5.0) | 66 (5.1) |
| ≥65 yr of age | 36 (5.5) | 29 (4.5) | 65 (5.0) |
The full analysis population included all participants who underwent randomization, regardless of whether they received the assigned intervention, not including those who were excluded from the analysis because of data-quality issues. Percentages may not total 100 because of rounding. BMI denotes body-mass index, Covid-19 coronavirus disease 2019, and SARS-CoV-2 severe acute respiratory syndrome coronavirus 2.
Race and ethnic group were reported by the participants.
Data were missing for 2 participants in the placebo group.
Participants were considered to be seropositive for SARS-CoV-2 if they had evidence of antibodies to either the nucleocapsid antigen or the spike antigen.
The most severe targeted sign or symptom was considered for this category.
High risk was defined by the presence of one or more risk factors for severe Covid-19, and standard risk was defined by the absence of such risk factors. “Other” includes participants who were at high risk but had never been vaccinated or had not been vaccinated per protocol.
Risk factors occurring in at least 5% of the participants in either group are listed.
Efficacy
Among the 1288 participants who received at least one dose of nirmatrelvir–ritonavir or placebo and had at least one postbaseline visit, the median time to sustained alleviation of all targeted Covid-19 signs or symptoms through day 28 was 12 days in the nirmatrelvir–ritonavir group and 13 days in the placebo group, a difference that was not significant (P=0.60) (Table 2). Similar results were observed in the high-risk subgroup (i.e., participants who had been vaccinated and had at least one risk factor for severe illness) and in the standard-risk subgroup (i.e., those who had no risk factors for severe illness and had never been vaccinated or had not been vaccinated within the previous 12 months).
Table 2. Time to Sustained Alleviation of All Targeted Signs and Symptoms through Day 28.*.
| Variable | Nirmatrelvir–Ritonavir (N=654) |
Placebo (N=634) |
|---|---|---|
| Mean trial follow-up — days | 27.4 | 27.5 |
| Mean time at risk for event — days | 13.1 | 13.7 |
| No. of participants with sustained alleviation (%) | 477 (72.9) | 470 (74.1) |
| Time to sustained alleviation | ||
| Median (95% CI) — days | 12 (11–13) | 13 (12–14) |
| Mean — days | 13.8 | 14.1 |
| P value by log-rank test | 0.60 |
Sustained alleviation of all targeted signs and symptoms was considered to have occurred on the first of 4 consecutive days during which all targeted symptoms that had been scored as moderate or severe at trial entry were scored as mild or absent and all symptoms that had been scored as mild or absent at trial entry were scored as absent. The targeted symptoms were cough, shortness of breath or difficulty breathing, feeling feverish, chills or shivering, muscle or body aches, diarrhea, nausea, vomiting, headache, sore throat, and stuffy or runny nose. Efficacy end points were evaluated in the population of participants who took at least one dose of nirmatrelvir–ritonavir or placebo had at least one postbaseline visit.
A key secondary end point in the trial was Covid-19–related hospitalization or death from any cause (see Table S4 for data pertaining to this end point, including subgroup analyses). A total of 5 of the 654 participants (0.8%) who received nirmatrelvir–ritonavir and 10 of the 634 (1.6%) who received placebo were hospitalized for Covid-19 or died from any cause through day 28, which corresponded to a difference of −0.8 percentage points (95% confidence interval [CI], −2.0 to 0.4). One high-risk vaccinated participant in the placebo group died. None of the 5 participants in the nirmatrelvir–ritonavir group who were hospitalized were admitted to the ICU, whereas 3 of 10 participants in the placebo group were admitted to the ICU. In a planned subgroup analysis involving high-risk participants, hospitalization or death occurred in 3 (0.9%) in the nirmaltrelvir–ritonavir group and 7 (2.2%) in the placebo group (difference, −1.3 percentage points; 95% CI, −3.3 to 0.7).
In subgroup analyses of Covid-19–related medical visits (i.e., emergency department, practitioner’s office, home health care services, urgent care, telephone consultation, outpatient infusion center, or other medical visit), the number of participants with medical visits was lower in the nirmatrelvir–ritonavir group than in the placebo group (24 visits among 15 participants vs. 36 visits among 25 participants; least-squares mean ratio vs. placebo, 0.50; 95% CI, 0.22 to 1.13). In an assessment according to baseline risk, 6 Covid-19–related medical visits occurred among 6 high-risk participants in the nirmatrelvir–ritonavir group, as compared with 26 visits among 17 high-risk participants in the placebo group (least-squares mean ratio, 0.18; 95% CI, 0.06 to 0.56).
Among the 15 participants who were hospitalized, the mean number of days in the hospital per 100 participants was 5 in the nirmatrelvir–ritonavir group and 18 in the placebo group. The longest hospital stay was 9 days in the nirmatrelvir–ritonavir group and 32 days in the placebo group.
By day 14, viral load rebound had occurred in 4.3% of the participants in the nirmatrelvir–ritonavir group and 4.1% of those in the placebo group; symptom rebound occurred in 11.4% and 16.1%, respectively, and symptom and viral load rebound together occurred in 1.2% and 0.5%, respectively. Three participants who had viral load rebound were hospitalized (one in the nirmatrelvir–ritonavir group and two in the placebo group); there was no consistent temporal relationship between viral load rebound and hospitalization.
Safety
Adverse events that emerged during the treatment period, serious adverse events, and adverse events leading to discontinuation occurred in similar percentages of participants in the two groups (Table 3). The percentages of participants with adverse events of any cause during the treatment period were 25.8% in the nirmatrelvir–ritonavir group and 24.1% in the placebo group and did not differ markedly between vaccinated and unvaccinated participants. In the nirmatrelvir–ritonavir group, 24 participants (3.7%) reported grade 3 or 4 adverse events, and no grade 5 adverse events occurred; the corresponding values in the placebo group were 25 (3.9%) and 1 (0.2%). Serious adverse events were reported by 8 participants (1.2%) receiving nirmatrelvir–ritonavir and by 12 (1.9%) receiving placebo; none of these events were considered by the investigator to be related to nirmatrelvir–ritonavir or placebo. The most common adverse events reported by nirmatrelvir–ritonavir recipients were dysgeusia (in 6.7% of the participants), diarrhea (in 4.0%), and nausea (in 3.1%); the corresponding percentages of placebo recipients with these events were 0.5%, 3.0%, and 2.7%.
Table 3. Summary of Adverse Events through Day 34.*.
| Adverse Event | Nirmatrelvir–Ritonavir (N=654) |
Placebo (N=634) |
|---|---|---|
| no. of participants (%) | ||
| Events that emerged during the treatment period† | ||
| Any event | 169 (25.8) | 153 (24.1) |
| Serious event | 8 (1.2) | 12 (1.9) |
| Maximum event grade of 3 or 4 | 24 (3.7) | 25 (3.9) |
| Maximum event grade of 5 | 0 | 1 (0.2) |
| Event leading to discontinuation of participation in trial | 0 | 1 (0.2) |
| Event leading to discontinuation of nirmatrelvir–ritonavir or placebo; trial participation continued | 16 (2.4) | 5 (0.8) |
| Event leading to dose reduction or temporary discontinuation of nirmatrelvir–ritonavir or placebo | 2 (0.3) | 2 (0.3) |
| Events related to nirmatrelvir–ritonavir or placebo‡ | ||
| Any event | 83 (12.7) | 31 (4.9) |
| Serious event | 0 | 0 |
| Maximum event grade of 3 or 4 | 3 (0.5) | 2 (0.3) |
| Maximum event grade of 5 | 0 | 0 |
| Event leading to discontinuation of participation in trial | 0 | 0 |
| Event leading to discontinuation of nirmatrelvir–ritonavir or placebo; trial participation continued | 11 (1.7) | 2 (0.3) |
| Event leading to dose reduction or temporary discontinuation of nirmatrelvir–ritonavir or placebo | 0 | 1 (0.2) |
Safety was evaluated in the population of participants who took at least one dose of nirmatrelvir–ritonavir or placebo and had at least one postbaseline visit. The severity of adverse events was graded according to the Division of AIDS Table for Grading the Severity of Adult and Pediatric Adverse Events, version 2.1 (July 2017).
A total of 323 events occurred in the nirmatrelvir–ritonavir group, and 280 occurred in the placebo group.
A total of 109 treatment-related events occurred in the nirmatrelvir–ritonavir group, and 49 occurred in the placebo group.
A total of 83 participants (12.7%) had adverse events that were considered by the investigator to be related to nirmatrelvir–ritonavir, as compared with 31 (4.9%) with adverse events that were considered to be related to placebo; the difference was primarily due to higher percentages of participants in the nirmatrelvir–ritonavir group than in the placebo group having dysgeusia (5.8% vs. 0.2%) and diarrhea (2.1% vs. 1.3%). Adverse events and treatment-related adverse events of grade 3 or higher and all serious adverse events are summarized in Tables S5, S6, and S7.
Discussion
In adult participants with Covid-19 who were at standard or high risk for severe Covid-19, there was no significant difference in the time to symptom alleviation between the nirmatrelvir–ritonavir group and the placebo group.
Results from the trial provide some intriguing observations about the use of nirmatrelvir–ritonavir in vaccinated patients who have risk factors for severe Covid-19. Among the patients who underwent randomization, Covid-19–related hospitalization or death from any cause occurred in 5 participants in the nirmatrelvir–ritonavir group and 10 in the placebo group (with the only death occurring in the placebo group). In the subgroup of high-risk participants, the numbers with this outcome were 3 and 7, respectively (difference in risk, −1.3 percentage points; 95% CI, −3.3 to 0.7). These data complement those from the Evaluation of Protease Inhibition for Covid-19 in High-Risk Patients (EPIC-HR) trial, which enrolled unvaccinated patients with risk factors for severe Covid-19.9
The results with respect to the numbers of Covid-19–related hospitalizations and deaths from any cause in this trial, although not significant, are consistent with and supported by recent real-world data.10 Five real-world cohort studies have investigated nirmatrelvir–ritonavir in SARS-CoV-2–positive nonhospitalized patients during the period of omicron-variant dominance.12-16 In these studies — which were conducted between December 2021 and October 2022, when omicron BA.1, BA.2, and BA.4–BA.5 were the predominant SARS-CoV-2 variants in circulation in the United States — more than half a million patients with risk factors consistent with the Centers for Disease Control and Prevention criteria were studied. In four of the studies, most of the patient populations were vaccinated (up to 85%), including administration of booster doses.12,14-16 The relative effectiveness of nirmatrelvir–ritonavir against hospitalization ranged from 53 to 76% when estimated without consideration of the time from symptom onset to the start of treatment.12-14,16 Fewer Covid-19–related medical visits were also observed among participants receiving nirmatrelvir–ritonavir in the current trial.
The safety of nirmatrelvir–ritonavir in this trial is consistent with that in the EPIC-HR trial,9 with no new safety findings. Frequencies of adverse events that emerged during the treatment period, serious adverse events, and adverse events that led to discontinuation were similar in the two groups, with dysgeusia the most frequent event reported by nirmatrelvir–ritonavir recipients, followed by diarrhea and nausea.
The limitations of the trial include the statistical analysis of the key secondary end point (Covid-19–related hospitalization or death from any cause), which was only a descriptive analysis because the results for the primary efficacy end point were not significant. Moreover, participants in the vaccinated high-risk subgroup were enrolled irrespective of the time since their last administered dose of vaccine and irrespective of the effect of time since vaccination on the efficacy of nirmatrelvir–ritonavir. Given the distinctive taste of nirmatrelvir–ritonavir, participants may have suspected that they were taking that medication, which may have limited the effectiveness of the blinding. Another limitation is that the trial was started during the period of predominance of the B.1.617.2 (delta) variant; however, more recent real-world studies have provided evidence for the efficacy of nirmatrelvir–ritonavir across SARS-CoV-2 variants.10 As vaccine-induced immunity wanes, new variants emerge, and vaccination uptake declines, the risk of severe Covid-19–associated outcomes among high-risk patients may increase. The strengths of the trial include the enrollment of participants from diverse global locations, which enables generalizability of the results. These results are important, since infections continue to occur even among persons with previous Covid-19 infection or vaccination.
In this trial, we assessed the safety and efficacy of nirmatrelvir–ritonavir as an antiviral agent against SARS-CoV-2 in symptomatic, nonhospitalized, vaccinated or unvaccinated adults. Nirmatrelvir–ritonavir was not associated with a significantly shorter time to sustained alleviation of Covid-19 symptoms than placebo, and the usefulness of nirmatrelvir–ritonavir in patients who are not at high risk for severe Covid-19 has not been established.
Acknowledgments
We thank the participants who enrolled in the trial and the staff at the trial sites; the ACTIV2b investigators for their collaboration during design of the trial; the following Pfizer colleagues: Charlotte Allerton, Annaliesa Anderson, Jeremias Antinew, Daniel Arenson, Ayman Ayoub, Karen Baker, Elizabeth Bateman, Arthur Bergman, Mary Boylan Bost, Jade Brennan, Kevin Brown, Carol Buck, Gina Buckley, Paul Butler, Victoria Butler, Regina Calderon, Rhonda Cardin, Kara Chew, Kristopher Chrisman, Carol Connell, Darren Cowan, Donna Cox, Bharat Damle, Gretchen Dean, Vanessa Dell, Mikael Dolsten, Elizabeth Dushin, Courtney Fletcher, Michael T. Gaffney, Ramakanth GSH, Eileen Mary Girten, Heidi Goldfarb, Mark Griffths, Shunjie Guan, Wen He, Marie-Pierre Hellio Le Graverand-Gastineau, Jim Huang, Michael Hughes, Craig Hyde, David Keller, Catherine Lee, Cathy Q. Li, Jia Yi Liang, Katherine Liau, Carlos Linn, Marianne Lorenzen, Rod MacKenzie, Carlos Malvestutto, Anita Moradoghli-Haftvani, Seleen Ong, Lesley O’Regan, Russ Orrico, Dafydd Owen, Kannan Natarajan, Mary Anne Nemeth, Demetris Neophytou Zambas, Susan Parish, Shyam Parvatini, Claire Penick, Amanda Radola, Kathleen Reilly Thompson, Amy Robey, Liz Rodgers, Glenda Rojas, Eve Rowe, Jean Satish, Cara Sclafani, Ravi Shankar Singh, Karen Singletary, Lisa Skeens, Patricia Smith, Holly Soares, Karan Somasundar, Anna Spolnik, Kathleen Szymczak, Manjit Toor, Sarah Tweedy, Sophie Van De Leest, Zhenyu Wang, Christine West, and all the colleagues not named here who contributed to the success of this trial; and Sheena Hunt and Kandyss Najjar of ICON, who wrote the first draft of the manuscript.
Protocol
Supplementary Appendix
Disclosure Forms
Data Sharing Statement
A data sharing statement provided by the authors is available with the full text of this article at NEJM.org.
Footnotes
Supported by Pfizer.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.He J, Guo Y, Mao R, Zhang J. Proportion of asymptomatic coronavirus disease 2019: a systematic review and meta-analysis. J Med Virol 2021;93:820-830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020;323:1239-1242. [DOI] [PubMed] [Google Scholar]
- 3.Kim L, Garg S, O’Halloran A, et al. Risk factors for intensive care unit admission and in-hospital mortality among hospitalized adults identified through the US Coronavirus Disease 2019 (COVID-19)-Associated Hospitalization Surveillance Network (COVID-NET). Clin Infect Dis 2021;72(9):e206-e214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Docherty AB, Harrison EM, Green CA, et al. Features of 20133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ 2020;369:m1985-m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thakur B, Dubey P, Benitez J, et al. A systematic review and meta-analysis of geographic differences in comorbidities and associated severity and mortality among individuals with COVID-19. Sci Rep 2021;11:8562-8562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hilgenfeld R. From SARS to MERS: crystallographic studies on coronaviral proteases enable antiviral drug design. FEBS J 2014;281:4085-4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Owen DR, Allerton CMN, Anderson AS, et al. An oral SARS-CoV-2 Mpro inhibitor clinical candidate for the treatment of COVID-19. Science 2021;374:1586-1593. [DOI] [PubMed] [Google Scholar]
- 8.Food and Drug Administration. Fact sheet for healthcare providers: emergency use authorization for Paxlovid. 2024. (https://labeling.pfizer.com/ShowLabeling.aspx?id=16474).
- 9.Hammond J, Leister-Tebbe H, Gardner A, et al. Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19. N Engl J Med 2022;386:1397-1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Food and Drug Administration. FDA briefing document. Antimicrobial Drugs Advisory Committee meeting, March 16, 2023 (https://www.fda.gov/media/166197/download).
- 11.Food and Drug Administration. Assessing COVID-19-related symptoms in outpatient adult and adolescent subjects in clinical trials of drugs and biological products for COVID-19 prevention or treatment. Guidance for industry. February 2024. (https://www.fda.gov/regulatory-information/search-fda-guidance-documents/assessing-covid-19-related-symptoms-outpatient-adult-and-adolescent-subjects-clinical-trials-drugs).
- 12.Lewnard JA, McLaughlin JM, Malden D, et al. Effectiveness of nirmatrelvir-ritonavir against hospital admission or death: a cohort study in a large US healthcare system. January 10, 2023. (https://www.medrxiv.org/content/10.1101/2022.10.02.22280623v2). preprint. [DOI] [PMC free article] [PubMed]
- 13.Wong CKH, Au ICH, Lau KTK, Lau EHY, Cowling BJ, Leung GM. Real-world effectiveness of molnupiravir and nirmatrelvir plus ritonavir against mortality, hospitalisation, and in-hospital outcomes among community-dwelling, ambulatory patients with confirmed SARS-CoV-2 infection during the omicron wave in Hong Kong: an observational study. Lancet 2022;400:1213-1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bajema KL, Berry K, Streja E, et al. Effectiveness of COVID-19 treatment with nirmatrelvir-ritonavir or molnupiravir among U.S. veterans: target trial emulation studies with one-month and six-month outcomes. Ann Intern Med 2023;176:807-816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schwartz KL, Wang J, Tadrous M, et al. Population-based evaluation of the effectiveness of nirmatrelvir-ritonavir for reducing hospital admissions and mortality from COVID-19. CMAJ 2023;195(6):E220-E226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arbel R, Wolff Sagy Y, Hoshen M, et al. Nirmatrelvir use and severe Covid-19 outcomes during the omicron surge. N Engl J Med 2022;387:790-798. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.