Abstract
Human cytomegalovirus (CMV) causes severe disease in immunosuppressed patients and notably infects the gastrointestinal tract. To understand the interaction of CMV with intestinal epithelial cells, which are highly susceptible to CMV infection in vivo, we used the intestinal epithelial cell line Caco-2 and demonstrated that CMV enters predominantly through the basolateral surface of polarized Caco-2 cells. As shown by expression of all three classes of CMV proteins and by visualization of nucleocapsids by transmission electron microscopy, both poorly and fully differentiated Caco-2 cells were permissive to CMV replication. However, infection failed to produce infectious particles in Caco-2 cells, irrespective of the state of differentiation.
Human cytomegalovirus (CMV) is a ubiquitous virus that is a major cause of morbidity and mortality in immunocompromised individuals. During severe CMV diseases in AIDS patients, the gastrointestinal tract is frequently involved and is the second main site of infection, after the retina (3, 8). Although any location within the gut may be affected, gastrointestinal CMV disease typically produces mucosal erosion or ulceration with inflammation, tissue necrosis, and vascular injury (8). CMV infects a broad spectrum of gastrointestinal cells, notably endothelial cells, smooth muscle cells, macrophages, fibroblasts, and epithelial cells of the mucosa (23, 25). Infection of the endothelial cells can explain ischemic mucosal injury with concomitant necrosis. However, epithelial cells are frequently infected, suggesting that they may also be involved in the pathological process.
To further understand the role of intestinal epithelial cells in gastrointestinal CMV disease, we investigated the ability of strain AD169 of human CMV to initiate infection in the human intestinal epithelial cell line Caco-2. Caco-2 cells differentiate spontaneously in culture (6, 18), as they do in the normal intestine, and exhibit many of the morphologic and functional properties of enterocytes (28). The efficiency of CMV replication in other cell systems appears to depend on the state of differentiation of the cell (4, 7, 11, 20). Thus, to examine the relationship of differentiation and infectibility, Caco-2 cultures grown on coverslips were infected 3, 6, and 14 days postseeding. Before confluency, cells are organized in islets. Peripheral cells of these islets are undifferentiated and proliferate, whereas central cells become polarized and then differentiate. After confluency, the whole monolayer is polarized, but the process of differentiation requires many more days to reach completion. At 14 days postseeding, cells were fully differentiated, since we detected expression of sucrase-isomaltase, a marker of differentiation, on the whole monolayer (data not shown) (13). Cells were infected with the AD169 strain of CMV at a multiplicity of infection (MOI) of 1, centrifuged for 45 min at 1,400 × g during the adsorption period to enhance infectivity of CMV (10), fixed in acetone-water 48 h postinfection (p.i.), and examined by immunofluorescence for the intranuclear presence of the CMV immediate-early (IE) antigens with the monoclonal antibody E13 (Biosoft).
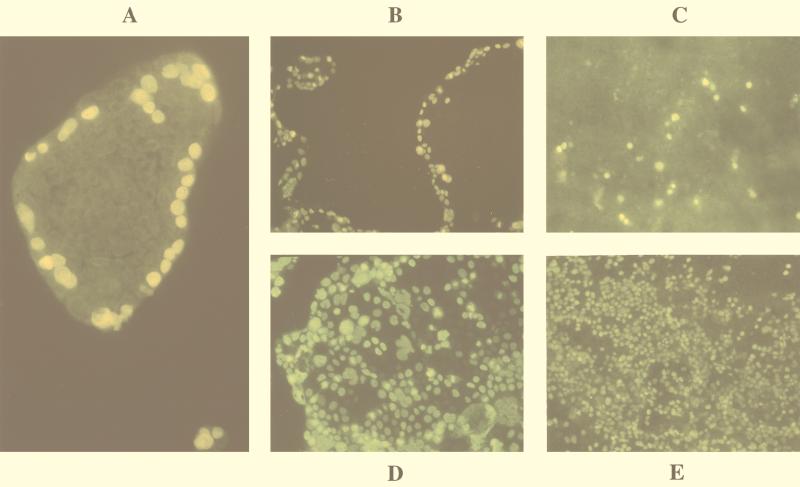
Different patterns of infection were observed when Caco-2 cells were infected with CMV at different ages. Cells grown for 3 days consisted mainly of small heterogeneous-sized islets. In small islets, nearly all cells showed IE proteins. In contrast, IE protein expression in larger islets was restricted to their outer edges (Fig. 1A). A similar pattern was obtained with monolayers grown for 6 days that consisted of large islets. After infection, IE antigens were seen almost exclusively in cells at the outer edges of the islets (Fig. 1B). Infection with CMV of 14-day-old Caco-2 monolayers showed that only a few cells expressed IE antigens (Fig. 1C).
FIG. 1.
Cells were infected with CMV, fixed 48 h p.i., and examined by indirect immunofluorescence with antibody clone E13, directed against CMV IE proteins, and fluorescein isothiocyanate-conjugated secondary antibody. (A) Infection of 3-day-old Caco-2 cell islets appeared to be restricted to the outer edge. Six-day-old Caco-2 cells, which are subconfluent and consist of large islets, were infected with CMV without any treatment (B) or treated with EGTA (Sigma) (D). Fourteen-day-old Caco-2 cells grown on glass coverslips infected with CMV that were untreated (C) or treated with EGTA (E).
Restriction of infection to the outer edges of islets raised the possibility that CMV infects polarized Caco-2 cells preferentially through the basolateral surface. Indeed, in peripheral islet cells, the virus has access to the entire cellular surface. In contrast, central cells of the islet, which are already polarized and have tight junctions, are accessible only by the apical domain.
Virus enters polarized Caco-2 cells preferentially through the basolateral membrane domain.
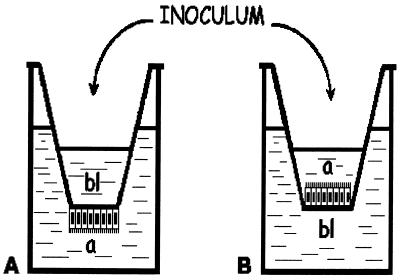
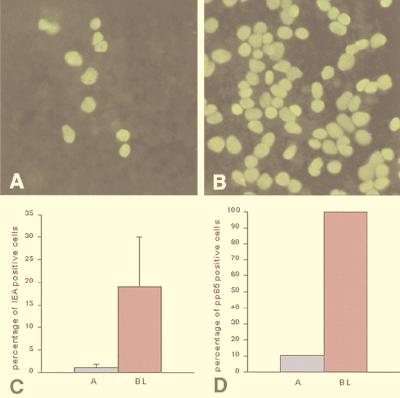
We tested the polarity in the infection process by applying virus either to the apical surface of cells grown on porous filters (Transwell-clear; pore size, 0.4 μm; Costar, Brumath, France) or to their basolateral surface. Fourteen-day-old cells were infected with CMV at an MOI of 1 and examined for expression of IE antigens by indirect immunofluorescence. To centrifuge filters during the adsorption period, virus inocula must be added in the upper reservoir of the filter. Cells were therefore seeded on the lower side of the filter as previously described by Perdomo et al. (Fig. 2) (17). Of the differentiated Caco-2 cells infected with CMV through the apical membrane and fixed 48 h p.i., only a small number expressed IE antigens (Fig. 3A). In contrast, infection of Caco-2 cells through the basolateral membrane led to a large number of cells expressing CMV IE antigens (Fig. 3B). Figure 3C presents the percentage of cells expressing IE antigens 24 h p.i. and shows that the preference CMV displayed for the basolateral membrane was 17-fold.
FIG. 2.
(A) Caco-2 cells were seeded onto inverted inserts to allow centrifugation during basolateral infection. Cells were allowed to attach overnight, after which the filters were placed upright in 12-well culture plates, thus orienting the basolateral side of the monolayer upward. The apical (a) surfaces of monolayers were suspended in the lower chamber. The CMV inoculum was placed in the upper chamber for infection through the basolateral (bl) surface. (B) Apical infection was performed on cells grown normally on filters. Adsorption was carried out by centrifugation at 350 × g for 45 min. Cellular integrity was verified after centrifugation by measuring lactate dehydrogenase activity and transepithelial resistance.
FIG. 3.
Polarized entry of CMV into Caco-2 cells. Expression of CMV IE antigens in polarized Caco-2 cells grown on porous filters inoculated through either the apical (A) or basolateral (B) surface with the AD169 strain at an MOI of 1 is shown. Only cultures with transepithelial electrical resistance greater than 800 Ω · cm2 were used. Cells were fixed 48 h p.i. and examined by indirect immunofluorescence for expression of IE proteins. (C) Infection of Caco-2 cells occurred essentially through the basolateral membrane. (D) Expression of pp65 in 14-day-old Caco-2 cells cultured on permeable filters infected with CMV at an MOI of 1 and fixed 6 h p.i. We used monoclonal antibody 95/30, which reacts with the E phosphoprotein pp65 and was a gift from Susan Michelson (Institut Pasteur, Paris, France). This antibody can detect entry of virions and dense bodies. A, apical infection; BL, basolateral infection.
It is well known that tight junctions of polarized cells can be disrupted by treating monolayers with EGTA, a Ca2+ chelator, thus exposing the lateral pole of the cells (19). Such treatment has been used with Caco-2 cells to demonstrate the lateral entry of Shigella flexneri, an enterovirulent bacterium (16). We therefore investigated the effects of disrupting tight junctions by treatment with 100 μM EGTA on CMV entry into Caco-2 cells grown on glass coverslips. Treatment with EGTA for 1 h prior to infection and throughout the adsorption period caused a significant increase in the number of infected cells among 6-day-old Caco-2 cells compared to those in untreated cultures. It abolished the restricted expression of IE antigens, resulting in homogeneous distribution (Fig. 1B and D). Identical treatment of 14-day-old differentiated cells dramatically increased the number of cells expressing IE antigens (Fig. 1C and E).
To verify that the differential expression of IE antigens between apical and basolateral infection reflects differences in viral entry, we used an antibody directed against pp65. Indeed, pp65, a constituent of virions and dense bodies, accumulates in the nuclei of infected cells immediately after virus-cell contact, before the onset of viral genome expression (5). Caco-2 cells cultured on permeable filters were infected apically or basolaterally with CMV at an MOI of 1. Cells were fixed 6 h p.i. and stained with anti-pp65. Figure 3D shows that basolateral infection resulted in 100% pp65-positive cells versus only 10.45% for apical infection.
Taken together, these results strongly indicate that initiation of CMV infection in epithelial polarized Caco-2 cells is preferentially restricted to the basolateral membrane. This observation supports a clinical characteristic of intestinal CMV disease. A number of observations have clearly demonstrated that CMV is primarily disseminated hematogenously (24). Moreover, most symptomatic CMV disease manifestations are due to reactivation of latent virus, which is associated with viremia. In peripheral blood, infectious virus is predominantly cell associated; endothelial cells (9) and the monocyte/macrophage system (26), which are infected by CMV, may contribute to hematogenous dissemination of infection. These infected blood cells have access to the basolateral surface of epithelial cells and thus could transmit CMV to intestinal epithelial cells.
Comparison of the sequential expression of CMV antigens in 6- and 14-day-old Caco-2 cells and in fibroblasts.
Depending on the cell type, CMV undergoes permissive or abortive infection. In permissive infection, CMV proteins are sequentially expressed in three phases: IE, early (E), and late (L). In abortive infection, viral expression is limited to those proteins appearing before viral DNA synthesis. Caco-2 cells were used at various stages of differentiation. Six and 14-day-old Caco-2 cultures grown on glass coverslips and on permeable filters, respectively, were infected with CMV at an MOI of 1. Expression of IE, E, and L antigens was studied by immunofluorescence with monoclonal antibody clone E13 (Biosoft), CCH2 (Dako), and SL-20 (Biosoft) (1), directed against IE, E, and L antigens, respectively. The results were compared with those for CMV-infected MRC-5 fibroblasts.
Data obtained by immunofluorescence microscopy and flow cytometry (21) are summarized in Table 1. Although the percentages of Caco-2 cells expressing IE, E, and L proteins were lower than those of MRC-5 cells, the kinetics of expression of CMV antigens were similar to those observed in MRC-5 cells. From these experiments, it was evident that both 6- and 14-day-old Caco-2 cells were permissive for all phases of CMV gene expression. The presence of the L protein, which is expressed only after viral DNA synthesis (1), showed that viral DNA synthesis occurred in Caco-2 cells, irrespective of the state of differentiation.
TABLE 1.
Comparison of the expression of CMV IE, E, and L proteins in MRC-5 cells and 6- and 14-day-old Caco-2 cells
| Day(s) of incubation | Reactivity (% of positive cells)a
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| IE protein
|
E protein
|
L protein
|
|||||||
| MRC-5 | 6-day-old Caco-2 | 14-day-old Caco-2 | MRC-5 | 6-day-old Caco-2 | 14-day-old Caco-2 | MRC-5 | 6-day-old Caco-2 | 14-day-old Caco-2 | |
| 1 | ++ (>90) | + (25) | + (19) | − (0) | − (0) | − (0) | − (0) | − (0) | − (0) |
| 2 | ++ (>90) | ++ (34) | ++ (30) | + (50) | + (<1) | + (<1) | + (50) | +/− (<1) | − (0) |
| 3 | ++ (100) | ++ (33) | ++ (34) | ++ (>90) | + (5) | + (5) | ++ (70) | + (1) | + (1) |
| 4 | ++ (100) | ++ (35) | ++ (42) | ++ (100) | ++ (5) | ++ (5) | ++ (100) | ++ (5) | ++ (2) |
+, positive; ++, positive with increased intensity; −, negative; +/−, weakly positive.
CMV-infected Caco-2 cells produce nucleocapsids but no infectious particles.
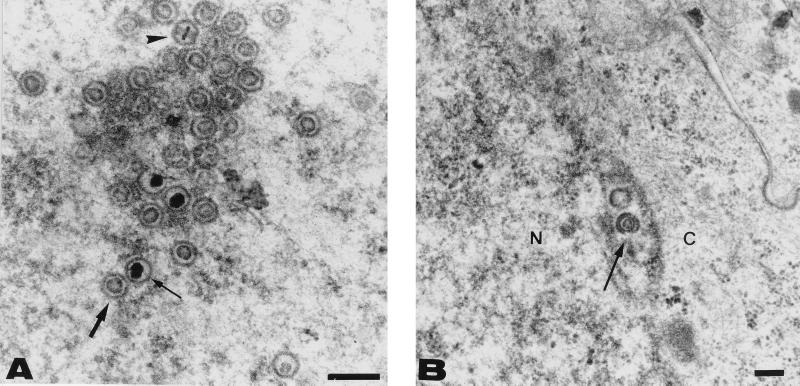
To gain further insight into the assembly and maturation processes of CMV in Caco-2 cells, differentiated infected cells grown on permeable filters were fixed 14 days after basolateral infection (MOI of 1) and prepared for transmission electron microscopy, as previously described (14). Many nucleocapsids with electron-translucent and electron-dense cores were observed in the nuclei (Fig. 4A). Nucleocapsids were seen acquiring an envelope during budding through the inner leaflet of the nuclear membrane (Fig. 4B). However, no cytoplasmic particles or dense bodies, previously described as composed of pp65 aggregates surrounded by membranes (22), were observed in infected Caco-2 cells.
FIG. 4.
Electron micrographs of basolaterally infected, filter-grown Caco-2 cells 14 days p.i. Filters were fixed with glutaraldehyde, postfixed with osmium, dehydrated in ethanol, and embedded in epoxy resin. Thin sections of Caco-2 monolayers are shown at different magnifications. (A) A nucleus containing numerous nucleocapsids with a ring-shaped core (thick arrow) and a dense core (thin arrow). A toroidal core was also visible (arrowhead). Important morphological changes in nuclear structures that are characteristic of late-stage CMV infection were observed. These changes consisted notably of nuclear enlargement, condensation and margination of heterochromatin, and irregularities of the nuclear outline, which was frequently tortuous. (B) A nucleocapsid (thin arrow) acquiring an envelope by budding through the inner leaflet of the nuclear membrane was seen. N, nucleus; C, cytoplasm. Bars, 200 nm.
Since many nucleocapsids with dense cores were observed, we asked whether Caco-2 cells produce infectious particles. To analyze whether extracellular and cell-associated infectious virus was produced, we infected 6- and 14-day-old Caco-2 cells grown on permeable filters. Six-day-old Caco-2 cells were infected at an MOI of 0.2 and 2, and 14-day-old cells were infected at an MOI of 1. Titers of virus in apical and basolateral media and cell lysates collected at 2 to 24 days p.i. were determined on MRC-5 cells. Neither extracellular nor intracellular infectious virus was found in Caco-2 cells, even 24 days p.i., irrespective of the state of differentiation at the time of infection.
Although IE, E, and L antigens were expressed in Caco-2 cells, this did not correlate with virus production in either differentiated or undifferentiated cells. This is in striking contrast to the results described by Jarvis et al. (12), published after our paper was originally submitted. They showed that basolateral infection of Caco-2 cells is productive, with progeny virus localized intracellularly and released from the apical membrane domain. This difference may be related to the CMV strains used. Indeed, we used the AD169 strain, whereas they used another laboratory strain, the Towne strain. It has been reported that infection of differentiated THP-1 monocytes with the Towne strain, as opposed to the AD169 strain, results in productive infection (27). Another explanation may be the very high MOI (a total of 25) used by Jarvis et al. to infect Caco-2 cells, which still allowed only a low level of production. After infection with the Towne strain, progeny virus was released from the apical membrane domain, which was consistent with an apical localization of an essential glycoprotein, gB. Upon infection by the AD169 strain, expression of gB was observed only within permeabilized cells in our laboratory studies, suggesting intracellular sequestration of this protein (data not shown). This correlates with the observed lack of virus production. To gain further insight into the assembly and maturation processes of CMV in Caco-2 cells, electron microscopic analyses were performed. Many nucleocapsids were observed in the nucleus and in the perinuclear space, while neither virus particles nor dense bodies were seen in the cytoplasm. The absence of dense bodies may be related to the low level of pp65 expression we observed after 24 days of infection (data not shown). This again correlates with the nonproductive state.
Other differences between the AD169 and Towne strains can be noted. Infection of Caco-2 cells with the AD169 strain at an MOI of 1 leads to percentages of cells expressing CMV IE, E, and L proteins similar to the percentages obtained with the Towne strain at an MOI of 25 (12). After 4 days of infection, we observed about 40% IE-positive cells and 5% E- and L-positive cells. Similar percentages of expression were observed by Jarvis et al. at 8 days p.i. for IE proteins and at 12 days p.i. for E and L proteins. Surprisingly, in their study, Jarvis et al. detected L protein after the appearance of intracellular and extracellular infectious virus.
Although CMV does not seem to replicate in Caco-2 cells, it is well known that CMV can modify and damage cells in the absence of a complete replicative cycle (2). It would thus be of interest to study whether Caco-2 cells are altered functionally and/or morphologically by CMV infection.
Finally, it was reported that, in vitro, the efficiency of CMV replication seems to depend on the state of cellular differentiation (4, 20). However, we showed here that infection with CMV of Caco-2 cells is independent of their state of differentiation. Jarvis et al. (12) failed to infect fully differentiated Caco-2 cells, but this is perhaps related to the laboratory strain used. Laboratory strains of CMV have deletions in their viral genomes compared to those in clinical isolates (15). Subsequently, it will be important to compare infectivity of the laboratory strain AD169 with that of fresh clinical isolates.
Acknowledgments
We are very grateful to Susan Michelson for helpful discussions and assistance in the preparation of the manuscript.
REFERENCES
- 1.Alain S, Mazeron M C, Pepin J M, Morinet F, Raskine L, Sanson-Le Pors M J. Rapid detection of cytomegalovirus strains resistant to ganciclovir through mutations within the gene UL97. Mol Cell Probes. 1993;7:487–495. doi: 10.1006/mcpr.1993.1072. [DOI] [PubMed] [Google Scholar]
- 2.Albrecht T, Boldogh I, Fons M, Lee C H, AbuBakar S, Russell J M, Au W W. Cell-activation responses to cytomegalovirus infection relationship to the phasing of CMV replication and to the induction of cellular damage. Subcell Biochem. 1989;15:157–202. [PubMed] [Google Scholar]
- 3.Britt W J, Alford C A. Cytomegalovirus. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 2493–2523. [Google Scholar]
- 4.Cinalt J, Jr, Cinalt J, Radsak K, Rabenau H, Weber B, Novak M, Benda R, Kornhuber B, Doerr H W. Replication of human cytomegalovirus in a rhabdomyosarcoma cell line depends on the state of differentiation of the cells. Arch Virol. 1994;138:391–401. doi: 10.1007/BF01379143. [DOI] [PubMed] [Google Scholar]
- 5.Dal Monte P, Bessia C, Landini M P, Michelson S. Expression of human cytomegalovirus ppUL83 (pp65) in a stable cell line and its association with metaphase chromosomes. J Gen Virol. 1996;77:2591–2596. doi: 10.1099/0022-1317-77-10-2591. [DOI] [PubMed] [Google Scholar]
- 6.Fogh J, Fogh J M, Orfeo T. One hundred and twenty-seven cultured human tumor cell lines producing tumors in nude mice. J Natl Cancer Inst. 1977;59:221–226. doi: 10.1093/jnci/59.1.221. [DOI] [PubMed] [Google Scholar]
- 7.Gonczol E, Andrews P W, Plotkin S A. Cytomegalovirus infection of human teratocarcinoma cells in culture. J Gen Virol. 1985;66:509–515. doi: 10.1099/0022-1317-66-3-509. [DOI] [PubMed] [Google Scholar]
- 8.Goodgame R W. Gastrointestinal cytomegalovirus disease. Ann Intern Med. 1993;119:924–935. doi: 10.7326/0003-4819-119-9-199311010-00010. [DOI] [PubMed] [Google Scholar]
- 9.Grefte A, Blom N, van der Giessen M, van Son W, The T H. Ultrastructural analysis of circulating cytomegalic cells in patients with active cytomegalovirus infection: evidence for virus production and endothelial origin. J Infect Dis. 1993;168:1110–1118. doi: 10.1093/infdis/168.5.1110. [DOI] [PubMed] [Google Scholar]
- 10.Hudson J B. Further studies on the mechanism of centrifugal enhancement of cytomegalovirus infectivity. J Virol Methods. 1988;19:97–108. doi: 10.1016/0166-0934(88)90153-x. [DOI] [PubMed] [Google Scholar]
- 11.Ibanez C E, Schrier R, Ghazal P, Wiley C, Nelson J A. Human cytomegalovirus productively infects primary differentiated macrophages. J Virol. 1991;65:6581–6588. doi: 10.1128/jvi.65.12.6581-6588.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jarvis M A, Wang C E, Meyers H L, Smith P P, Corless C L, Henderson G J, Vieira J, Britt W J, Nelson J A. Human cytomegalovirus infection of Caco-2 cells occurs at the basolateral membrane and is differentiation state dependent. J Virol. 1999;73:4552–4560. doi: 10.1128/jvi.73.6.4552-4560.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jourdan N, Cotte-Laffitte J, Forestier F, Servin A L, Quero A M. Infection of cultured human intestinal cells by monkey RRV and human Wa rotavirus as a function of intestinal epithelial cell differentiation. Res Virol. 1995;146:325–331. doi: 10.1016/0923-2516(96)80595-4. [DOI] [PubMed] [Google Scholar]
- 14.Jourdan N, Maurice M, Delautier D, Quero A M, Servin A L, Trugnan G. Rotavirus is released from the apical surface of cultured human intestinal cells through nonconventional vesicular transport that bypasses the Golgi apparatus. J Virol. 1997;71:8268–8278. doi: 10.1128/jvi.71.11.8268-8278.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.MacCormac L P, Grundy J E. Two clinical isolates and the Toledo strain of cytomegalovirus contain endothelial cell tropic variants that are not present in the AD169, Towne, or Davis strains. J Med Virol. 1999;57:298–307. doi: 10.1002/(sici)1096-9071(199903)57:3<298::aid-jmv14>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 16.Mounier J, Vasselon T, Hellio R, Lesourd M, Sansonetti P J. Shigella flexneri enters human colonic Caco-2 epithelial cells through the basolateral pole. Infect Immun. 1992;60:237–248. doi: 10.1128/iai.60.1.237-248.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perdomo J J, Gounon P, Sansonetti P J. Polymorphonuclear leukocyte transmigration promotes invasion of colonic epithelial monolayer by Shigella flexneri. J Clin Investig. 1994;93:633–643. doi: 10.1172/JCI117015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pinto M, Robine-Leon S, Appay M D, Kedinger M, Triadou N, Dussaulx E, Lacroix B, Simon-Assman P, Haffen K, Fogh J, Zweibaum A. Enterocyte-like differentiation and polarization of the human colon carcinoma cell line Caco-2 in culture. Biol Cell. 1983;47:323–330. [Google Scholar]
- 19.Pitelka D R, Taggart B N, Hamamoto S T. Effects of extracellular calcium depletion on membrane topography and occluding junctions of mammary epithelial cells in culture. J Cell Biol. 1983;96:613–624. doi: 10.1083/jcb.96.3.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poland S D, Bambrick L, Dekaban G A, Rice G P A. The extent of human cytomegalovirus replication in primary neurons is dependent on host cell differentiation. J Infect Dis. 1994;170:1267–1271. doi: 10.1093/infdis/170.5.1267. [DOI] [PubMed] [Google Scholar]
- 21.Schols D, Snoeck R, Neyts J, de Clercq E. Detection of immediate early, early and late antigens of human cytomegalovirus by flow cytometry. J Virol Methods. 1989;26:247–254. doi: 10.1016/0166-0934(89)90107-9. [DOI] [PubMed] [Google Scholar]
- 22.Severi B, Landini M P, Cenacchi G, Zini N, Maraldi N M. Human cytomegalovirus nuclear and cytoplasmic dense bodies. Arch Virol. 1992;123:193–207. doi: 10.1007/BF01317149. [DOI] [PubMed] [Google Scholar]
- 23.Sinzger C, Grefte A, Platcher B, Gouw A S H, The T H, Jahn G. Fibroblasts, epithelial cells, endothelial cells and smooth muscle cells are major targets of human cytomegalovirus infection in lung and gastrointestinal tissues. J Gen Virol. 1995;76:741–750. doi: 10.1099/0022-1317-76-4-741. [DOI] [PubMed] [Google Scholar]
- 24.Sinzger C, Jahn G. Human cytomegalovirus cell tropism and pathogenesis. Intervirology. 1996;39:302–319. doi: 10.1159/000150502. [DOI] [PubMed] [Google Scholar]
- 25.Sinzger C, Platcher B, Stenglein S, Jahn G. Immunohistochemical detection of viral antigens in smooth muscle, stromal, and epithelial cells from acute human cytomegalovirus gastritis. J Infect Dis. 1993;167:1427–1432. doi: 10.1093/infdis/167.6.1427. [DOI] [PubMed] [Google Scholar]
- 26.Taylor-Wiedeman J, Sissons J G, Borysiewicz L K, Sinclair J H. Monocytes are a major site of persistence of human cytomegalovirus in peripheral blood mononuclear cells. J Gen Virol. 1991;72:2059–2064. doi: 10.1099/0022-1317-72-9-2059. [DOI] [PubMed] [Google Scholar]
- 27.Turtinen L W, Seufzer B J. Selective permissiveness of TPA differentiated THP-1 myelomonocytic cells for human cytomegalovirus strains AD169 and Towne. Microb Pathog. 1994;16:373–378. doi: 10.1006/mpat.1994.1037. [DOI] [PubMed] [Google Scholar]
- 28.Zweibaum A, Laburthe M, Grasset E, Louvard D. Use of cultured cell lines in studies of intestinal cell differentiation and function. In: Schultz S G, Field M, Frizzell R A, editors. Handbook of physiology. The gastrointestinal system, vol. IV. Intestinal absorption and secretion. Bethesda, Md: American Physiological Society; 1991. pp. 223–225. [Google Scholar]