Abstract
The centriole is a defining feature of many eukaryotic cells. It nucleates a cilium, organizes microtubules as part of the centrosome, and is duplicated in coordination with the cell cycle. Centrioles have a remarkable structure, consisting of microtubules arranged in a barrel with ninefold radial symmetry. At their base, or proximal end, centrioles have unique triplet microtubules, formed from three microtubules linked to each other. This microtubule organization is not found anywhere else in the cell, is conserved in all major branches of the eukaryotic tree, and likely was present in the last eukaryotic common ancestor. At their tip, or distal end, centrioles have doublet microtubules, which template the cilium. Here, we consider the structures of the compound microtubules in centrioles and discuss potential mechanisms for their formation and their function. We propose that triplet microtubules are required for the structural integrity of centrioles, allowing the centriole to serve as the essential nucleator of the cilium.
In most animal cells, centrioles come in pairs, and in cells that are traversing the cell cycle, this pair of centrioles is precisely duplicated and segregated to ensure that each daughter cell receives exactly one pair of centrioles. Many of the molecular players in this cycle of centriole duplication have been identified and are conserved through evolution (Carvalho-Santos et al. 2011). Birth of a new centriole occurs in S phase, when a procentriole forms off the side of each existing mother centriole, near the proximal end. The triplet microtubules are added to a structural hub, known as the cartwheel, early in the process, resulting in a structure ~0.2 μm long and 0.2 μm wide (Kuriyama and Borisy 1981; Vorobjev and Chentsov 1982; Chrétien et al. 1997). In G2, the triplet microtubules elongate, reaching a maximum length of ~0.35 μm. At the G2/M transition, the inner two microtubules of the triplet elongate to their final length of 0.5 μm, forming a distal end with only doublet microtubules. In the following cell cycle, this new centriole recruits the pericentriolar material components of the centrosome and acquires appendages, allowing it to serve as a nucleator of the cilium in the subsequent G0/G1. The doublet microtubules of the centriole extend to form the axoneme of the cilium. Here, we refer to the doublet and triplet microtubules of the centriole and axoneme as compound microtubules. The centriole structure at the base of the cilium is often termed a basal body; we will use the term centriole to refer to all such structures. Although conserved in structure, centrioles, and the associated cilium and centrosome, have been lost from several key eukaryotic groups, including higher fungi and higher plants (Carvalho-Santos et al. 2011).
Centrioles can also be made without an existing centriole. The mechanisms underlying this de novo centriole formation are less understood, but have been observed in multiple species: the flatworm Planaria, the amoeba Naegleria, and the plants Ginkgo biloba and Zamia (Norstog 1967; Fulton and Dingle 1971; Gifford and Lin 1975; Azimzadeh et al. 2012). Centrioles formed de novo in these organisms have the typical ninefold structure with triplet microtubules, indicating that it is not necessary to have an existing centriole to create a new one. Human cells usually do not form centrioles de novo but can be induced to do so by experimental manipulation (Khodjakov et al. 2002; La Terra et al. 2005; Uetake et al. 2007). Centrioles formed under these conditions often have structural defects (Khodjakov et al. 2002; Wang et al. 2015). Last, specialized cells in animals can generate many centrioles by centriole amplification. In differentiating multiciliated cells, centriole amplification occurs from unique structures known as the deuterosome (Sorokin 1968; Steinman 1968; Kalnins and Porter 1969), which is formed from an existing centriole (Al Jord et al. 2014).
Regardless of the mechanism of formation, centrioles share two defining structural features: ninefold symmetry and the presence of compound microtubules (Fig. 1). Ninefold symmetry is derived from the intrinsic assembly properties of proteins making up a ninefold symmetric hub and spoke structure known as the cartwheel. The cartwheel is the first structure to form in the nascent centriole, and its major component, SASS6, is capable of self-assembling in vitro into ninefold symmetric, cartwheel-like structures (Kitagawa et al. 2011; van Breugel et al. 2011). During the normal centriole duplication cycle, the mother centriole templates cartwheel formation (Fong et al. 2014). Although the cartwheel is the basis of centriolar ninefold symmetry, there are likely other interactions that reinforce this symmetry, such as those between the cartwheel and centriolar microtubules (Hilbert et al. 2016). Interestingly, ~15% of Chlamydomonas mutants lacking SASS6 have ninefold symmetric centrioles (Nakazawa et al. 2007), and human cells expressing mutant forms of SASS6 deficient in oligomerization can form centrioles with a normal structure (Wang et al. 2015). Other mechanisms that impart ninefold symmetry may include structural constraints imposed by the A–C linker that bridges adjacent centriolar microtubule triplets (Hirono 2014; Meehl et al. 2016).
Figure 1.
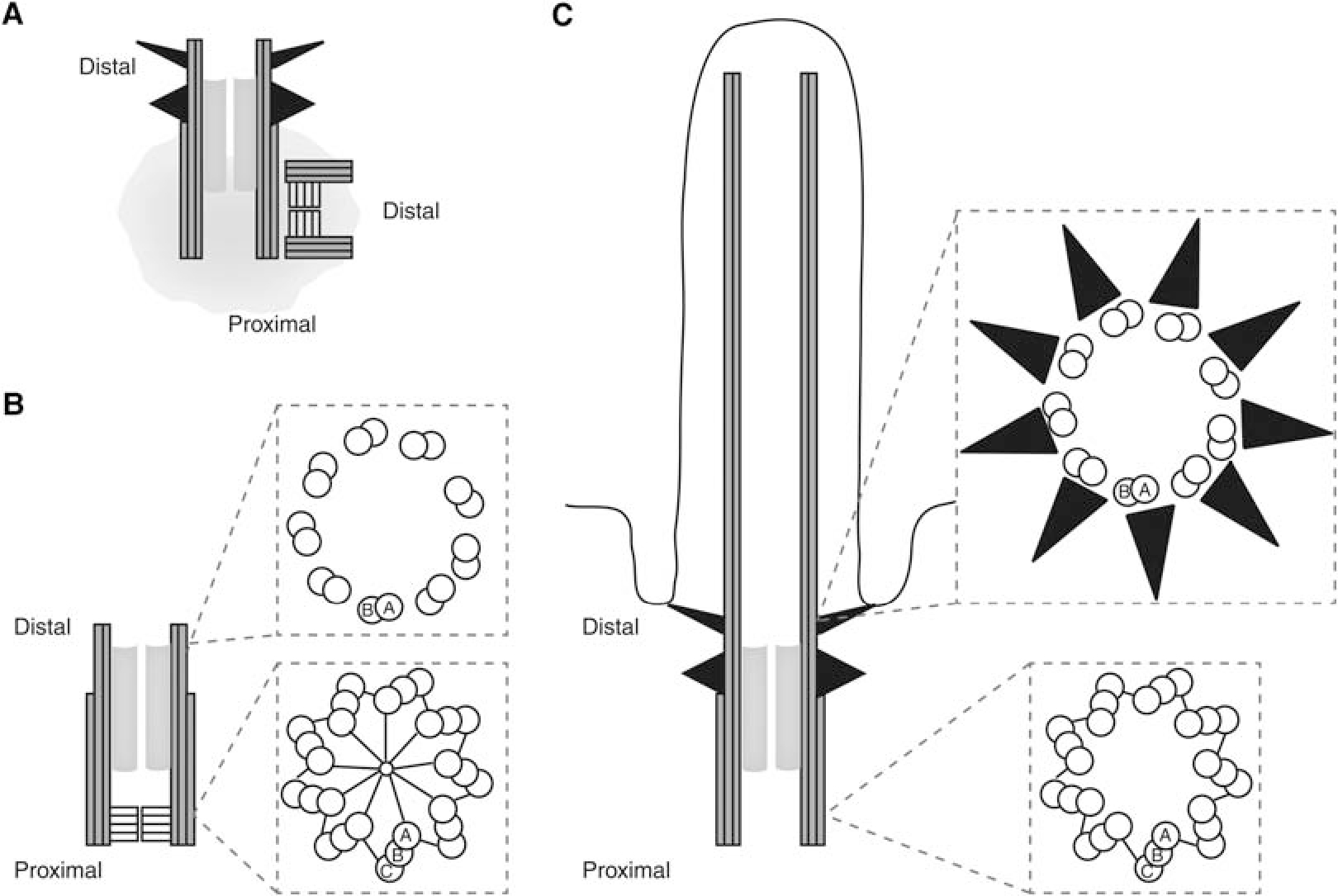
Centriole structures at key stages of the cell cycle. (A) Centrosome structure in S-phase cells. Each centrosome is composed of two centrioles, surrounded by pericentriolar material (gray cloud). The mother centriole is vertical and has appendages (black triangles) on its distal end. The procentriole is at a right angle and has a cartwheel structure within its proximal end. (B) Centriole structure in procentriole at G2–M. The centriole has elongated to its full length. Cross sections through the centriole are shown at right. A-, B-, and C-tubules are labeled. (C) Mother centriole at the base of a cilium. The centriole has appendages and lacks a cartwheel. Cross sections through the centriole are shown at right. A-, B-, and C-tubules are labeled.
In contrast with the radial symmetry of centrioles, the mechanisms by which centrioles form compound microtubule are not understood. The triplet microtubules, in particular, define centrioles in most organisms, differentiating them from the ciliary axoneme, which has doublet microtubules that extend directly from the doublets at the distal end of the centriole. Here, we discuss the structure of compound microtubules, potential mechanisms for their formation and elongation, and their function. Because centriole structure is largely conserved in eukaryotes, we have incorporated information from many organisms, unless otherwise noted.
COMPOUND MICROTUBULE STRUCTURE: TRIPLETS AND DOUBLETS
The proximal end of the centriole in most organisms has a unique triplet microtubule structure not found anywhere else in the cell. These triplets consist of three linked microtubules, named the A-, B-, and C-tubules (Fig. 1). The A-tubule is connected to the cartwheel through pinhead structures (Anderson and Brenner 1971; Cavalier-Smith 1974; Paintrand et al. 1992; Guichard et al. 2013). In human centrioles, the triplets are ~350 nm long (Paintrand et al. 1992), and only the A-tubule and B-tubule extend through the remaining one-third of the centriole. When the centriole forms a cilium, the A- and B-tubules extend to form the axonemal doublet microtubules (Fig. 1C).
The compound microtubules of the centriole share some similarities with standard microtubules. Each is composed of α-tubulin and β-tubulin subunits (Kuriyama 1976; Nogales et al. 1998), which form heterodimers that associate head-to-tail in a protofilament of a microtubule. Standard microtubules are a tube formed from 13 protofilaments (Tilney et al. 1973), each associating with its neighboring protofilaments through lateral contacts between the M-loop and H1–S2/H2–S3 lateral surfaces of tubulin (Nogales et al. 1999; Sui and Downing 2010). Most of these lateral contacts are formed from connections between like tubulins: between α-tubulin and α-tubulin or between β-tubulin and β-tubulin, with the exception of the seam that closes the tube, where α-tubulin of one protofilament associates laterally with β-tubulin of its neighbor (Song and Mandelkow 1993; Kikkawa et al. 1994; McIntosh et al. 2009).
In the centriolar compound microtubules, the A-tubule has a very similar structure to standard microtubules: 13-protofilaments form a ring (Fig. 2A; Linck and Stephens 2007; Li et al. 2012; Maheshwari et al. 2015). Unlike standard microtubules, which are circular in cross section, the A-tubule is a deformed oval (Li et al. 2012; Ichikawa et al. 2017). The lateral contacts between protofilaments have been proposed to be similar to those of standard microtubules, consisting of connections between like tubulin subunits (Song and Mandelkow 1995; Maheshwari et al. 2015). The seam has been proposed to lie between protofilaments A1 and A2, between A9 and A10, or between A10 and A11 (Song and Mandelkow 1995; Maheshwari et al. 2015; Ichikawa et al. 2017). Unlike the A-tubule, the B-, and C-tubules are incomplete microtubules, each sharing walls with the adjoining tubules: the B-tubule is made of 10 protofilaments (B1–B10) and shares four additional protofilaments (A10–A13) with the A-tubule, and the C-tubule is also made of 10 protofilaments (C1–C10) and shares four additional protofilaments with the B-tubule (B5–B8). Within each tubule, the protofilaments interact through their M-loop and H1–S2/H2–S3 lateral surfaces, as in standard microtubules, and lateral interactions between protofilaments consist of connections between like tubulin subunits (Song and Mandelkow 1995; Maheshwari et al. 2015).
Figure 2.
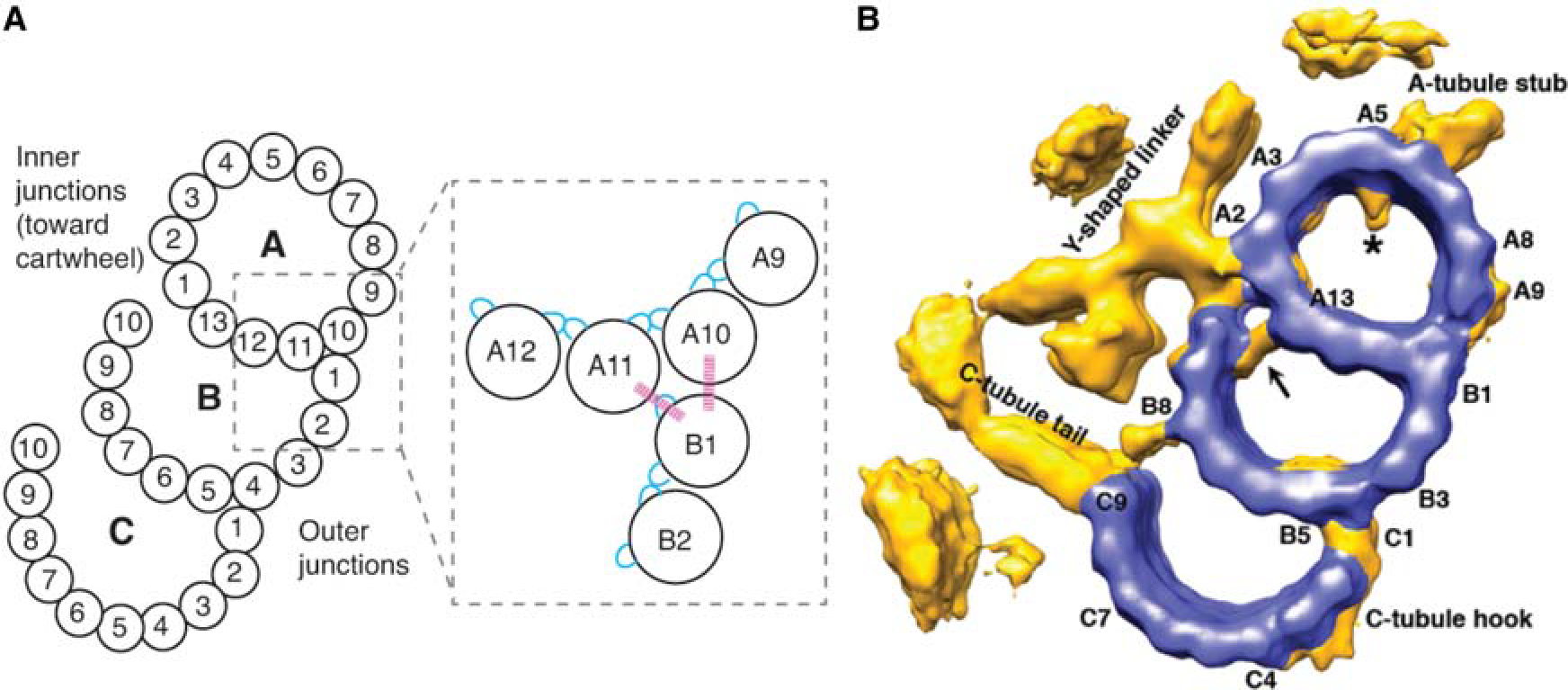
Triplet microtubule structure. (A) (Left) Protofilaments of the triplet microtubules, numbered according to convention by Linck and Stephens (2007). Inner junctions and outer junctions are labeled. (Right) Enlarged region of the outer junction between the A-tubule and B-tubule. Blue, M-loop and H1–S2/H2–S3 loop connections in canonical lateral interactions; pink lines, noncanonical external side wall interactions between protofilament B1 and protofilaments A10 and A11. (B) Centriole triplet microtubule cryo-EM structure. Blue, microtubule protofilaments; yellow, nonmicrotubule densities. (B, Reprinted from Li et al. 2012, with permission from John Wiley and Sons, © 2012 European Molecular Biology Organization.)
The defining features of the triplet microtubules are the junctions between the three tubules. The inner junction, nearest the cartwheel, differs from the outer junction, located toward the cytoplasm. The outer junction between the A- and B-tubules consists of interactions between protofilament B1 of the B-tubule, and protofilaments A10 and A11 of the A-tubule (Fig. 2A). The interactions between these protofilaments differ from those between standard microtubule protofilaments (Li et al. 2012; Ichikawa et al. 2017) in that here protofilament B1 interacts with the cytoplasm-facing sidewalls of protofilaments A10 and A11. Though the amino acid residues involved in these side wall interactions have not been identified in centrioles, recent high-resolution cryo-EM of ciliary axonemes has identified several conserved residues that may be involved (Ichikawa et al. 2017). At the inner junction between the A-tubule and B-tubule, nontubulin proteins form additional structure, known as the Y-shaped linker (Li et al. 2012). The identities of these proteins are unknown, though a recent report suggests that Poc16/WDR90 may form part of this structure (Hamel et al. 2017).
The outer junction between the B- and C-tubules varies between organisms. In the proximal region of Chlamydomonas centrioles, protofilament C1 is a microtubule protofilament like B1 (Li et al. 2012), and the interface between protofilaments C1 and B4/B5 is likely similar to that between B1 and A10/A11. Toward the distal end, however, the C1 microtubule protofilament is absent and in its place is an elongated linker, which has ~8-nm periodicity along the centriole, suggesting that it may bind to only one tubulin molecule in the heterodimer. In Trichonympha centrioles, an extended linker with 8.5-nm periodicity spans the B4 and C1 protofilaments in the proximal region of the centriole (Guichard et al. 2013). The identities of the proteins that form these structures are unknown. It is also unknown whether similar structures are present in human centrioles. The inner junction between the B- and C-tubules consists of structures, unique to the C-tubule, bridging protofilaments B8 and C10 (Li et al. 2012; Guichard et al. 2013).
In addition to the junctional structures in the centriolar triplets, multiple nonmicrotubule densities are associated with the triplet microtubule structure (Li et al. 2012). These include densities both inside and outside the microtubule protofilaments, some spanning the protofilaments longitudinally, and others bridging protofilaments. Some of these structures are also present in ciliary axonemes (Sui and Downing 2006; Nicastro et al. 2011; Pigino et al. 2012; Maheshwari et al. 2015; Ichikawa et al. 2017). Although the proteins that form these densities have not been definitively assigned, they may include ribbon proteins and tektins, intermediate-filament-like proteins that localize to both the centriole and cilium (Amos 2008; Linck et al. 2014). Altogether, we suggest three nonexclusive possibilities for the function of these proteins: formation of the B- and C-tubules, stabilization of the triplet structure, or recruitment of other proteins for centriole function. Further work will be needed to distinguish between these possibilities.
FORMING THE TRIPLETS
The triplet microtubules of the centriole form in the context of the centriole duplication cycle (Firat-Karalar and Stearns 2014). Centriole assembly occurs at the G1–S transition and begins with formation of the cartwheel structure, followed by attachment of microtubules (Kalnins and Porter 1969; Vorobjev and Chentsov 1982). The pinhead structure, which attaches the A-tubule to the cartwheel (Anderson and Brenner 1971; Cavalier-Smith 1974; Guichard et al. 2013), is likely composed of the carboxyl terminus of SASS6 and CEP135 (Matsuura et al. 2004; Hiraki et al. 2007; Guichard et al. 2017). CEP135 is able to bind and bundle microtubules (Kraatz et al. 2016) and interacts with CPAP/CenpJ/Sas-4, another microtubule-binding protein that affects centriolar microtubule dynamics (Lin et al. 2013a; Sharma et al. 2016; Zheng et al. 2016). Thus, it is very likely that these proteins are involved in recruitment and stabilization of microtubules at the proximal end of the centriole.
In human cells, a conical cap, proposed to be the γ-tubulin ring complex, is present at the base of the A-tubule (Guichard et al. 2010). The distal end of the A-tubule during elongation appears similar to the plus-end of a growing standard microtubule. Together, these results suggest that the A-tubule grows uni-directionally from a γ-tubulin ring complex at the proximal end of the centriole. Once the A-tubule is formed, the B-tubule and C-tubules are then added, although this does not occur synchronously at each of the nine A-tubules (Vorobjev and Chentsov 1982). γ-Tubulin ring complex structures have not been observed at the base of the B- or C-tubules (Guichard et al. 2010), suggesting that they form by different methods. The outside wall of the B-tubule forms first, growing from the sidewall of the A-tubule (Fig. 2; Dippell 1968; Kalnins and Porter 1969; Anderson and Brenner 1971), indicating that attachment of protofilament B1 is the initiating event in B-tubule formation. C-tubule assembly begins while the B-tubule is still elongating, likely from the outer junction (Kalnins and Porter 1969; Anderson and Brenner 1971; Guichard et al. 2010). The B-and C-tubules of human centrioles are initiated about 50 to 100 nm from the proximal base of the A-tubule, then grow bidirectionally, elongating toward both the proximal and distal ends (Guichard et al. 2010).
Any mechanism for triplet microtubule assembly must incorporate the observations that these structures are only found at centrioles, and that only triplets form, not higher-order microtubule structures. We speculate that the following factors may be involved: (1) centriole-specific tubulin proteins, and (2) the cartwheel.
Centriolar Tubulins
A possible explanation for the unique structure of the compound microtubules is that they are assembled from centriole-specific tubulin superfamily members, or from their isoforms. α-Tubulin and β-tubulin form heterodimers that are the basis of all microtubules, including the compound microtubules of axonemes and centrioles (Kuriyama 1976; Nogales et al. 1998). Most organisms have multiple α-tubulin and β-tubulin isoforms, which mainly differ in the amino acid composition of their disordered, highly charged carboxy-terminal tails that project from the cytoplasmic surface of the microtubule (Redeker 2010). Different α-tubulin and β-tubulin isoforms impart different dynamics to standard microtubules (Vemu et al. 2017), and it is possible that specific isoforms are required for the formation of compound microtubules. Indeed, early work on axonemes from multiple organisms suggests that the axonemal microtubules may contain specific isoforms relative to standard microtubules (Stephens 1970; Bibring et al. 1976; Lai et al. 1979). In Drosophila, a sperm-specific β-tubulin isoform forms the axonemal compound microtubules, as well as other microtubules in sperm (Kemphues et al. 1982; Raff et al. 2000; Nielsen et al. 2001). A more divergent β-tubulin isoform, found in a minority of tissues, cannot compensate for loss of the sperm-specific β-tubulin (Hoyle and Raff 1990). It is not clear whether any specific isoforms are strictly necessary for the formation of compound microtubules in other organisms.
Regulation of the carboxy-terminal tails of tubulin is likely critical to compound microtubule formation. This region is highly charged, and is also the site of several post-translational modifications (Wloga and Gaertig 2010; Yu et al. 2015; Gadadhar et al. 2017). Remarkably, sperm of a Drosophila mutant that expresses β-tubulin lacking its carboxy-terminal tail were shown to have compound microtubules with many additional tubules (Fackenthal et al. 1993), suggesting that the carboxyl terminus is normally inhibitory to formation. In vitro experiments showing that removal of the tails with subtilisin facilitated the formation of “hooks” growing from the sidewalls of microtubules (Serrano et al. 1984), a structure similar to that occurring during the early stages of B-tubule assembly. The formation of hooks on microtubules has been used as a means of determining microtubule polarity in cells, as the handedness of the hook reveals the polarity of the microtubule on which it has formed (Euteneuer and McIntosh 1980; Heidemann and McIntosh 1980; McIntosh and Euteneuer 1984; Baas and Lin 2011). Interestingly, the standard protocol for hook formation in these experiments involved high ionic-strength buffer conditions, which we propose might facilitate hook formation by neutralizing the inhibitory effect of the carboxy-terminal tails on the unique sidewall interaction that defines compound microtubules. Indeed, the first observation of higher-order microtubule assembly in vitro was by Burton and Himes (1978), who showed that pH regulates their formation. These results suggest that regulation of electrostatic interactions that involve the carboxy-terminal tails of tubulin is important for compound microtubule formation.
In addition to α-tubulin and β-tubulin, the tubulin superfamily contains four additional members: γ-tubulin, delta (δ)-tubulin, epsilon (ε)-tubulin, and zeta (ζ)-tubulin. γ-Tubulin is absolutely conserved in all eukaryotes, including those that have lost the ability to make centrioles and cilia in evolution. However, the other three, termed the ZED-tubulins, are found only in a subset of eukaryotes, all of which form centrioles and cilia. The ZED-tubulins form an evolutionarily coconserved module, suggesting that they may function together (Turk et al. 2015). δ-Tubulin was identified in Chlamydomonas as the protein encoded by UNI3, (Dutcher and Trabuco 1998), ε-tubulin was first found in the human genome by sequence-alignment (Chang and Stearns 2000), and ζ-tubulin was found by evolutionary comparison of sequenced genomes (Findeisen et al. 2014; Turk et al. 2015).
Experiments probing the function of δ-tubulin and ε-tubulin reveal that they are required for triplet microtubule formation and/or stability in a range of organisms from protists to humans (Goodenough and St Clair 1975; Dutcher and Trabuco 1998; Garreau de Loubresse et al. 2001; Chang et al. 2002; Dupuis-Williams et al. 2002; Dutcher et al. 2002; Gadelha et al. 2006; Ross et al. 2013; Wang et al. 2017). The limited information on localization of these tubulins indicates that they are limited to centrioles, suggesting that they might be involved in interactions that constrain the occurrence of triplet microtubules to the centriole. The functions of δ-tubulin and ε-tubulin have been tested by a variety of methods, from depletion of the protein to mutations likely to be strong loss-of-function alleles. Although some aspects of the phenotypes observed differ, there are commonalities in most of the experiments that suggest a conserved function. In most cases, cells in which either δ-tubulin or ε-tubulin has been lost make centrioles that lack triplet microtubules and are unstable over time.
We recently tested the roles of δ-tubulin and ε-tubulin in human cells by using CRISPR/Cas9 to make null mutations (Wang et al. 2017). Null mutations in δ-tubulin or ε-tubulin could only be recovered in a p53-null background, indicating that cells had defects in creating functional centrioles, which is known to trigger a p53-dependent cell cycle arrest in mammalian cells (Lambrus et al. 2015; Wong et al. 2015). Cells with mutations in either δ-tubulin or ε-tubulin have in a similar phenotype, with centrioles that have only have singlet microtubules, and that disintegrate each time the cell transitions from mitosis to interphase (Wang et al. 2017). Less is known about the function of the more recently described ζ-tubulin, but experiments in Xenopus multiciliated cells suggest that, in that cell type at least, it is involved in elaboration of centriolar appendages rather than centriole formation (Turk et al. 2015).
Mutations in δ-tubulin and ε-tubulin are among the only mutations known to specifically affect the formation of the triplet microtubules. How might they function in forming compound microtubules? A defining feature of all characterized tubulins is that they interact with other tubulins in the context of structures of the microtubule cytoskeleton. δ-Tubulin and ε-tubulin have been shown to interact with each other in directed experiments (Breslow et al. 2017; Wang et al. 2017) and high-throughput screens (Hein et al. 2015) and have been proposed to share particular interaction interfaces with α-tubulin and β-tubulin (Inclán and Nogales 2001). A simple hypothesis for the function of δ-tubulin and ε-tubulin is that they replace α-tubulin and/or β-tubulin in specific sites critical to the interactions that uniquely occur in compound microtubules. Another model would be that they form a nonprotofilament structure, such as the extended linker found between the B- and C-tubules (Li et al. 2012). Breslow et al. (2017) identified two previously uncharacterized proteins as stoichiometric interactors with δ-tubulin and ε-tubulin, which might also take part in such structures.
As attractive as these models are for the function of δ-tubulin and ε-tubulin, there are indications from evolutionary analysis that they cannot fully account for all instances of compound microtubules. First, nematodes and dipteran insects lack any of the ZED tubulins but make doublet microtubules as part of ciliary axonemes. Second, Drosophila and the primitive plant Ginkgo biloba lack the ZED tubulins, but form centrioles with triplet microtubules in their sperm (Gifford and Lin 1975; Gottardo et al. 2015; Wang et al. 2017). Since the mechanism of action of δ-tubulin and ε-tubulin at centrioles is not known, we cannot yet say whether these are examples in which the requirement for δ-tubulin and ε-tubulin has been bypassed evolutionarily, perhaps by gain-of-function in another tubulin, or in which the compound microtubules differ from those in other organisms that have δ-tubulin and ε-tubulin. We note that a mutation in α-tubulin can partially suppress the centriole defects of the uni3 δ-tubulin mutation in Chlamydomonas, consistent with the possibility of evolutionary adaptation (Fromherz et al. 2004).
The Role of the Cartwheel
One possible mechanism to limit triplet microtubule assembly to centrioles would be to restrict formation to preexisting structures already formed at the nascent procentriole, such as the cartwheel. In theory, the cartwheel may help to promote triplet microtubule assembly in several ways, including: modification of the nascent A-tubule, alteration of microtubule growth kinetics, creation of a template for the triplet microtubules, or stabilization of the triplets against depolymerization.
The A-tubule differs subtly in structure from a standard microtubule—it is a deformed oval in cross section, rather than round (Li et al. 2012). If this deformation were the result of attachment of the cartwheel or its associated proteins, it might limit compound microtubule formation to that site by altering the lattice of the A-tubule, and making it a suitable substrate for addition of the B1 protofilament. However, it is not known whether A-tubule deformation is due to cartwheel interaction, or whether such deformation aids in B-tubule formation.
The cartwheel, or associated proteins, may alter the growth kinetics of centriolar microtubules, aiding in compound microtubule formation. Compared to standard dynamic microtubules, the centriolar microtubules appear to grow very slowly (Kuriyama and Borisy 1981; Chrétien et al. 1997; Kinoshita et al. 2001), and it is possible that rapid dynamic growth would be incompatible with protofilament–sidewall interactions. Recently, it has been proposed that the centriole assembly protein CPAP enforces slow, processive growth on microtubules (Sharma et al. 2016; Zheng et al. 2016). CPAP interacts with the pinhead protein CEP135 (Lin et al. 2013a), and thus the cartwheel may spatially constrain CPAP activity by localization. It is not clear whether CPAP acts on all three microtubules of the triplet, or whether other mechanisms are involved in restricting the growth of the B- and C-tubules.
Other, yet more speculative possibilities might also involve the cartwheel. For example, the cartwheel, or associated proteins, could provide a direct template for the triplet microtubules, controlling both their structure and their spatial distribution. Or it might stabilize triplet microtubules that form constantly in the cytoplasm, but disassemble before being observed without that stabilization. Finally, the localization of the cartwheel adjacent to the mother centriole during centriole duplication places it within the pericentriolar material of the centrosome, which might be a permissive environment for triplet microtubule assembly. The pericentriolar material has been proposed to act as a tubulin concentrator (Woodruff et al. 2017), and high concentrations of tubulin are used to form microtubule hook structures in vitro (Euteneuer and McIntosh 1980; Heidemann and McIntosh 1980; McIntosh and Euteneuer 1984; Baas and Lin 2011). We note that the pericentriolar material is unlikely to be instructive for triplet microtubule assembly: centrioles with triplet microtubules can form de novo without a mother centriole in many contexts. However, in cycling cells, de novo formation of centrioles often leads to centriole structural aberrations, including loss of whole triplets (Khodjakov et al. 2002; Wang et al. 2015).
REGULATING THE MICROTUBULES: ELONGATION
The compound microtubules of the centriole are initiated in G1/S, soon after the initiation of the procentrioles, but they continue to elongate slowly until the completion of cell division. Given the slow rate of elongation, compared to the growth rate of standard microtubules, this suggests that centriolar triplet growth is regulated, perhaps coordinately with formation of other centriole structures. In human cells, the C-tubule is only a partial tubule near the end of the triplets (Paintrand et al. 1992), and in many contexts, including human cells, the C-tubule terminates before the doublets end, forming a distal end extension consisting only of doublets. The doublets extend during ciliogenesis, forming the doublet axoneme of the cilium. It seems likely that there is a structural distinction between the centriole and axoneme, because the centriolar microtubules are stable over time spans as long as the life of the organism (Kochanski and Borisy 1990; Balestra et al. 2015), whereas the axoneme is capable of disassembly and assembly within a single cell cycle (Sánchez and Dynlacht 2016).
The picture of growth of the centriolar compound microtubules is a complex one, with several points of regulation likely required to limit growth of one or more tubules but also to relieve that limitation for axoneme formation. Several proteins have been implicated in control of centriole length, perhaps directly though controlling centriole microtubule growth. Overexpression or depletion of several proteins (CPAP, CEP120, or CEP295, CP110, OFD1, RTTN, POC5) results in centrioles with a variety of length defects (Azimzadeh et al. 2009; Kohlmaier et al. 2009; Schmidt et al. 2009; Tang et al. 2009; Singla et al. 2010; Comartin et al. 2013; Lin et al. 2013b; Chang et al. 2016; Chen et al. 2017).These proteins have been placed in a recruitment pathway (Chen et al. 2017). Among these, CPAP, OFD1, CEP295, and CEP120 have been reported to directly interact with standard microtubules, and therefore might be candidates for direct action on centriolar compound microtubules (Hsu et al. 2008; Cormier et al. 2009; Singla et al. 2010; Lin et al. 2013b; Chang et al. 2016). CPAP may be a particularly critical protein for the regulation of centriolar microtubules, with recent work showing that it acts both to promote and to limit microtubule growth during growth of the centriole (Sharma et al. 2016; Zheng et al. 2016). Members of the kinesin-13 family—KIF24 in humans and Klp10A in flies—have also been shown to be involved in centriolar microtubule structure (Kobayashi et al. 2011; Delgehyr et al. 2012). Kinesins of this family depolymerize microtubules, and such an activity might be important for limiting the extent of centriole microtubules relative to other centriole structures, or in enforcing equal length across all nine of the centriolar compound microtubules.
Finally, axoneme growth is restricted to mother centrioles that are primed to form a cilium (Fig. 1C). Initiation of axonemal microtubule growth from the centriolar microtubules is a multistep process, initiated by vesicle docking and the recruitment of a kinase, TTBK2, to distal appendages (Sorokin 1962; Goetz et al. 2012; Schmidt et al. 2012; Joo et al. 2013; Sillibourne et al. 2013; Tanos et al. 2013; Čajánek and Nigg 2014; Kobayashi et al. 2014; Lu et al. 2015). These processes result in the removal of CP110, which is thought to form a cap at the distal end of the centriole, limiting elongation of the microtubules (Kleylein-Sohn et al. 2007; Spektor et al. 2007; Kobayashi and Dynlacht 2011). CP110 interacts with other proteins that are important for its function, including KIF24 (Tsang et al. 2006, 2008, 2009; Spektor et al. 2007; Kobayashi et al. 2011, 2014; Franz et al. 2013; Al-Jassar et al. 2017).
FUNCTIONS OF COMPOUND MICROTUBULES
What properties do compound microtubules impart on the structures that have them? We propose several possibilities: centriole structure stabilization, recruitment of distinct proteins to these structures, elaboration of tracks for protein movement in the axoneme, and differentiation of the basal body from the axoneme.
First, it is possible that compound microtubules are intrinsically more stable than standard microtubules, and that the triplet centriolar microtubules are required for long-term stability of the centriole structure. Axonemal microtubules are relatively stable to depolymerization in vitro, but these have many associated proteins that might affect their stability, and remarkably little is known about the dynamic properties of unadorned doublet or triplet microtubules. In cells, mutations in δ-tubulin or ε-tubulin, which lack triplet microtubules, result in centriole instability in Chlamydomonas, Tetrahymena, and human cells (Goodenough and St Clair 1975; Ross et al. 2013; Wang et al. 2017). In human cells lacking δ-tubulin or ε-tubulin, centrioles with singlet microtubules formed in S phase, and elongated in G2–M, forming centrioles of approximately normal length (Wang et al. 2017). However, these centrioles disintegrated during the transition from mitosis to interphase of the next cell cycle. Treating these cells with the microtubule-stabilizing drug paclitaxel suppressed centriole disintegration, suggesting that centriolar microtubules are essential to the integrity of the centriole. This is consistent with the previous work of Bobinnec et al. (1998), indicating that the triplet microtubules are required for normal centriole stability. We note that Caenorhabditis elegans has singlet microtubule centrioles in most of its cells (Pelletier et al. 2006), yet has long-lived centrioles (Balestra et al. 2015), challenging the idea that the triplet microtubules are universally required for centriole stability.
Second, it is possible that the compound microtubules are required to recruit proteins that help stabilize the centriole, or contribute to centriolar function. These might include many of the proteins and structures known to localize to centrioles, including the A–C linker that connects triplet microtubules to each other, the centriolar appendages, and the pericentriolar material. Each of these structures fail to form in centrioles with only singlet microtubules from δ-tubulin or ε-tubulin mutant cells (Wang et al. 2017). These mutants also failed to recruit POC5, a component of the distal extension, and Chlamydomonas ε-tubulin mutants failed to localize katanin (Esparza et al. 2013). It is possible that these missing structures help stabilize the centriole, and such a role has been identified for the pericentriolar material (for review, see Werner et al. 2017). Presumably, each of these structures involves a protein directly interacting with the compound microtubules. In the pericentriolar material, a protein that interacts with both polyglutamylated tubulin and pericentrin, ATF5, has been proposed to link it to the centriole (Madarampalli et al. 2015). The mechanisms by which specificity for doublet or triplet microtubules is achieved are unknown.
Third, the centriole is the essential nucleator of the cilium and determinant of axoneme structure. Every organism that makes cilia has compound microtubules as part of the cilium, even C. elegans (Nechipurenko et al. 2017; Serwas et al. 2017). Thus, compound microtubules are likely intrinsic to the function of the cilium. This is especially apparent in motile cilia, in which many proteins complexes associated with motility are attached to the doublet microtubules (Nicastro et al. 2011; Pigino et al. 2012). In addition, the doublets of the axoneme in motile cilia have recently been shown to be double-track “railways” for movement of cargoes (Stepanek and Pigino 2016), and kinesin-2 motors move differently upon standard microtubules and doublet microtubules from motile cilia (Stepp et al. 2017). Axonemes have doublets, never triplets, and it is possible that the triplet microtubules of the centriole are required to distinguish the centriole from the cilium. The cilium assembles and disassembles each cell cycle, but the centriole is stable and is segregated in cell division. Thus, the triplet microtubules may serve as a point of recognition to limit depolymerization, allowing the axoneme to be dynamic and its nucleator stable.
CONCLUSION
Much work has been done on the architecture of the centriole, the basis for the ninefold symmetry of the structure, and the means by which it is duplicated each cell cycle. Less is understood about the compound microtubules found at the centriole, which are also defining properties of the organelle. Here we have considered possible mechanisms for the formation of compound microtubules, and for limiting such microtubule structures to centrioles and axonemes, as well as possible functions imparted by compound microtubules on the structures that have them. Much of this review is necessarily speculative, because, although there is much morphological information, there is little information bearing on mechanism. Future work will focus on the molecular basis for compound microtubule structure, function and regulation. This information will be critical to understanding the centrosome and cilium, key organelles with strong ties to human disease.
ACKNOWLEDGMENTS
We thank members of the Stearns laboratory for com ments on the manuscript and helpful discussions. This work was supported by National Research Service Award grant 5 F32 GM117678 to J.T.W. and National Institutes of Health (NIH) grant R01GM052022 to T.S.
REFERENCES
- Al-Jassar C, Andreeva A, Barnabas DD, McLaughlin SH, Johnson CM, Yu M, van Breugel M. 2017. The ciliopathy-associated Cep104 protein interacts with tubulin and Nek1 kinase. Structure 25: 146–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Jord A, Lemaître A-I, Delgehyr N, Faucourt M, Spassky N, Meunier A. 2014. Centriole amplification by mother and daughter centrioles differs in multiciliated cells. Nature 516: 104–107. [DOI] [PubMed] [Google Scholar]
- Amos LA. 2008. The tektin family of microtubule-stabilizing proteins. Genome Biol 9: 229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RG, Brenner RM. 1971. The formation of basal bodies (centrioles) in the Rhesus monkey oviduct. J Cell Biol 50: 10–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azimzadeh J, Hergert P, Delouvée A, Euteneuer U, Formstecher E, Khodjakov A, Bornens M. 2009. hPOC5 is a centrin-bind ing protein required for assembly of full-length centrioles. J Cell Biol 185: 101–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azimzadeh J, Wong ML, Downhour DM, Sánchez Alvarado A, Marshall WF. 2012. Centrosome loss in the evolution of planarians. Science 335: 461–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas PW, Lin S. 2011. Hooks and comets: The story of microtubule polarity orientation in the neuron. Dev Neurobiol 71: 403–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balestra FR, von Tobel L, Gönczy P. 2015. Paternally contributed centrioles exhibit exceptional persistence in C. elegans embryos. Cell Res 25: 642–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibring T, Baxandall J, Denslow S, Walker B. 1976. Heterogeneity of the α subunit of tubulin and the variability of tubulin within a single organism. J Cell Biol 69: 301–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobinnec Y, Khodjakov A, Mir LM, Rieder CL, Eddé B, Bornens M. 1998. Centriole disassembly in vivo and its effect on centrosome structure and function in vertebrate cells. J Cell Biol 143: 1575–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslow DK, Hoogendoorn S, Kopp AR, Morgens DW, Vu BK, Han K, Li A, Hess GT, Bassik MC, Chen JK, et al. 2017. A comprehensive portrait of cilia and ciliopathies from a CRISPR-based screen for Hedgehog signaling. bioRxiv doi: 10.1101/156059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton PR, Himes RH. 1978. Electron microscope studies of pH effects on assembly of tubulin free of associated proteins. Delineation of substructure by tannic acid staining. J Cell Biol 77: 120–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Čajánek L, Nigg EA. 2014. Cep164 triggers ciliogenesis by recruiting Tau tubulin kinase 2 to the mother centriole. Proc Natl Acad Sci 111: E2841–E2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho-Santos Z, Azimzadeh J, Pereira-Leal JB, Bettencourt-Dias M. 2011. Evolution: Tracing the origins of centrioles, cilia, and flagella. J Cell Biol 194: 165–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalier-Smith T 1974. Basal body and flagellar development during the vegetative cell cycle and the sexual cycle of Chlamydomonas reinhardii. J Cell Sci 16: 529–556. [DOI] [PubMed] [Google Scholar]
- Chang P, Stearns T. 2000. δ-tubulin and ε-tubulin: Two new human centrosomal tubulins reveal new aspects of centrosome structure and function. Nat Cell Biol 2: 30–35. [DOI] [PubMed] [Google Scholar]
- Chang P, Giddings TH Jr, Winey M, Stearns T. 2002. ɛ-Tubulin is required for centriole duplication and microtubule organization. Nat Cell Biol 5: 71–76. [DOI] [PubMed] [Google Scholar]
- Chang C-W, Hsu W-B, Tsai J-J, Tang C-JC, Tang TK. 2016. CEP295 interacts with microtubules and is required for centriole elongation. J Cell Sci 129: 2501–2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H-Y, Wu C-T, Tang C-JC, Lin Y-N, Wang W-J, Tang TK. 2017. Human microcephaly protein RTTN interacts with STIL and is required to build full-length centrioles. Nat Commun 8: 247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrétien D, Buendia B, Fuller SD, Karsenti E. 1997. Reconstruction of the centrosome cycle from cryoelectron micrographs. J Struct Biol 120: 117–133. [DOI] [PubMed] [Google Scholar]
- Comartin D, Gupta GD, Fussner E, Coyaud É, Hasegan M, Archinti M, Cheung SWT, Pinchev D, Lawo S, Raught B, et al. 2013. CEP120 and SPICE1 cooperate with CPAP in centriole elongation. Curr Biol 23: 1360–1366. [DOI] [PubMed] [Google Scholar]
- Cormier A, Clément M-J, Knossow M, Lachkar S, Savarin P, Toma F, Sobel A, Gigant B, Curmi PA. 2009. The PN2–3 domain of centrosomal P4.1-associated protein implements a novel mechanism for tubulin sequestration. J Biol Chem 284: 6909–6917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgehyr N, Rangone H, Fu J, Mao G, Tom B, Riparbelli MG, Callaini G, Glover DM. 2012. Klp10A, a microtubule-depolymerizing kinesin-13, cooperates with CP110 to control Drosophila centriole length. Curr Biol 22: 502–509. [DOI] [PubMed] [Google Scholar]
- Dippell RV. 1968. The development of basal bodies in paramecium. Proc Natl Acad Sci 61: 461–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupuis-Williams P, Fleury-Aubusson A, De Loubresse NG, Geoffroy H, Vayssié L, Galvani A, Espigat A, Rossier J. 2002. Functional role of ε-tubulin in the assembly of the centriolar microtubule scaffold. J Cell Biol 158: 1183–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutcher SK, Trabuco EC. 1998. The UNI3 gene is required for assembly of basal bodies of Chlamydomonas and encodes δ-tubulin, a new member of the tubulin superfamily. Mol Biol Cell 9: 1293–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutcher SK, Morrissette NS, Preble AM, Rackley C, Stanga J. 2002. ε-Tubulin is an essential component of the centriole. Mol Biol Cell 13: 3859–3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esparza JM, O’Toole E, Li L, Giddings TH, Kozak B, Albee AJ, Dutcher SK. 2013. Katanin localization requires triplet microtubules in Chlamydomonas reinhardtii. PLoS One 8: e53940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euteneuer U, McIntosh JR. 1980. Polarity of midbody and phragmoplast microtubules. J Cell Biol 87: 509–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fackenthal JD, Turner FR, Raff EC. 1993. Tissue-specific microtubule functions in Drosophila spermatogenesis require the β 2-tubulin isotype-specific carboxy terminus. Dev Biol 158: 213–227. [DOI] [PubMed] [Google Scholar]
- Findeisen P, Mühlhausen S, Dempewolf S, Hertzog J, Zietlow A, Carlomagno T, Kollmar M. 2014. Six subgroups and extensive recent duplications characterize the evolution of the eukaryotic tubulin protein family. Genome Biol Evol 6: 2274–2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firat-Karalar EN, Stearns T. 2014. The centriole duplication cycle. Philos Trans R Soc Lond B Biol Sci 369: 20130460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong CS, Kim M, Yang TT, Liao JC, Tsou MFB. 2014. SAS-6 assembly templated by the lumen of cartwheel-less centrioles precedes centriole duplication. Dev Cell 30: 238–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz A, Roque H, Saurya S, Dobbelaere J, Raff JW. 2013. CP110 exhibits novel regulatory activities during centriole assembly in Drosophila. J Cell Biol 203: 785–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromherz S, Giddings TH Jr, Gomez-Ospina N, Dutcher SK. 2004. Mutations in α-tubulin promote basal body maturation and flagellar assembly in the absence of δ-tubulin. J Cell Sci 117(Pt 2): 303–314. [DOI] [PubMed] [Google Scholar]
- Fulton C, Dingle AD. 1971. Basal bodies, but not centrioles, in Naegleria. J Cell Biol 51: 826–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadadhar S, Bodakuntla S, Natarajan K, Janke C. 2017. The tubulin code at a glance. J Cell Sci 130: 1347–1353. [DOI] [PubMed] [Google Scholar]
- Gadelha C, Wickstead B, McKean PG, Gull K. 2006. Basal body and flagellum mutants reveal a rotational constraint of the central pair microtubules in the axonemes of trypanosomes. J Cell Sci 119(Pt 12): 2405–2413. [DOI] [PubMed] [Google Scholar]
- Garreau de Loubresse N, Ruiz F, Beisson J, Klotz C. 2001. Role of δ-tubulin and the C-tubule in assembly of Paramecium basal bodies. BMC Cell Biol 2: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gifford EM Jr, Lin J. 1975. Light microscope and ultrastructural studies of the male gametophyte in Ginkgo biloba: The spermatogenous cell. Am J Bot 62: 974–981. [Google Scholar]
- Goetz SC, Liem KF Jr, Anderson KV. 2012. The spinocerebellar ataxia-associated gene Tau tubulin kinase 2 controls the initiation of ciliogenesis. Cell 151: 847–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodenough UW, St Clair HS. 1975. BALD-2: A mutation affecting the formation of doublet and triplet sets of microtubules in Chlamydomonas reinhardtii. J Cell Biol 66: 480–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottardo M, Callaini G, Riparbelli MG. 2015. The Drosophila centriole—Conversion of doublets into triplets within the stem cell niche. J Cell Sci 128: 2437–2442. [DOI] [PubMed] [Google Scholar]
- Guichard P, Chrétien D, Marco S, Tassin A-M. 2010. Procentriole assembly revealed by cryo-electron tomography. EMBO J 29: 1565–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guichard P, Hachet V, Majubu N, Neves A, Demurtas D, Olieric N, Fluckiger I, Yamada A, Kihara K, Nishida Y, et al. 2013. Native architecture of the centriole proximal region reveals features underlying its 9-fold radial symmetry. Curr Biol 23: 1620–1628. [DOI] [PubMed] [Google Scholar]
- Guichard P, Hamel V, Le Guennec M, Banterle N, Iacovache I, Nemčíková V, Flückiger I, Goldie KN, Stahlberg H, Lévy D, et al. 2017. Cell-free reconstitution reveals centriole cartwheel assembly mechanisms. Nat Commun 8: 14813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamel V, Steib E, Hamelin R, Armand F, Borgers S, Flückiger I, Busso C, Olieric N, Sorzano COS, Steinmetz MO, et al. 2017. Identification of Chlamydomonas central core centriolar proteins reveals a role for human WDR90 in ciliogenesis. Curr Biol 27: 2486–2498.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidemann SR, McIntosh JR. 1980. Visualization of the structural polarity of microtubules. Nature 286: 517–519. [DOI] [PubMed] [Google Scholar]
- Hein MY, Hubner NC, Poser I, Cox J, Nagaraj N, Toyoda Y, Gak IA, Weisswange I, Mansfeld J, Buchholz F, et al. 2015. A human interactome in three quantitative dimensions organized by stoichiometries and abundances. Cell 163: 712–723. [DOI] [PubMed] [Google Scholar]
- Hilbert M, Noga A, Frey D, Hamel V, Guichard P, Kraatz SHW, Pfreundschuh M, Hosner S, Flückiger I, Jaussi R, et al. 2016. SAS-6 engineering reveals interdependence between cartwheel and microtubules in determining centriole architecture. Nat Cell Biol 18: 393–403. [DOI] [PubMed] [Google Scholar]
- Hiraki M, Nakazawa Y, Kamiya R, Hirono M. 2007. Bld10p constitutes the cartwheel-spoke tip and stabilizes the 9-fold symmetry of the centriole. Curr Biol 17: 1778–1783. [DOI] [PubMed] [Google Scholar]
- Hirono M 2014. Cartwheel assembly. Philos Trans R Soc Lond B Biol Sci 369: 20130458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyle HD, Raff EC. 1990. Two Drosophila β tubulin isoforms are not functionally equivalent. J Cell Biol 111: 1009–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu W-B, Hung L-Y, Tang C-JC, Su C-L, Chang Y, Tang TK. 2008. Functional characterization of the microtubule-binding and -destabilizing domains of CPAP and d-SAS-4. Exp Cell Res 314: 2591–2602. [DOI] [PubMed] [Google Scholar]
- Ichikawa M, Liu D, Kastritis PL, Basu K, Hsu TC, Yang S, Bui KH. 2017. Subnanometre-resolution structure of the doublet microtubule reveals new classes of microtubule-associated proteins. Nat Commun 8: 15035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inclán YF, Nogales E. 2001. Structural models for the self-assembly and microtubule interactions of γ-, δ- and ε-tubulin. J Cell Sci 114(Pt 2): 413–422. [DOI] [PubMed] [Google Scholar]
- Joo K, Kim CG, Lee M-S, Moon H-Y, Lee S-H, Kim MJ, Kweon H-S, Park W-Y, Kim C-H, Gleeson JG, et al. 2013. CCDC41 is required for ciliary vesicle docking to the mother centriole. Proc Natl Acad Sci 110: 5987–5992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalnins VI, Porter KR. 1969. Centriole replication during ciliogenesis in the chick tracheal epithelium. Z Zellforsch Mikrosk Anat 100: 1–30. [DOI] [PubMed] [Google Scholar]
- Kemphues KJ, Kaufman TC, Raff RA, Raff EC. 1982. The testis-specific β-tubulin subunit in Drosophila melanogaster has multiple functions in spermatogenesis. Cell 31(3 Pt 2): 655–670. [DOI] [PubMed] [Google Scholar]
- Khodjakov A, Rieder CL, Sluder G, Cassels G, Sibon O, Wang CL. 2002. De novo formation of centrosomes in vertebrate cells arrested during S phase. J Cell Biol 158: 1171–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikkawa M, Ishikawa T, Nakata T, Wakabayashi T, Hirokawa N. 1994. Direct visualization of the microtubule lattice seam both in vitro and in vivo. J Cell Biol 127(6 Pt 2): 1965–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita K, Arnal I, Desai A, Drechsel DN, Hyman AA. 2001. Reconstitution of physiological microtubule dynamics using purified components. Science 294: 1340–1343. [DOI] [PubMed] [Google Scholar]
- Kitagawa D, Vakonakis I, Olieric N, Hilbert M, Keller D, Olieric V, Bortfeld M, Erat MC, Flückiger I, et al. 2011. Structural basis of the 9-fold symmetry of centrioles. Cell 144: 364–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleylein-Sohn J, Westendorf J, Le Clech M, Habedanck R, Stierhof YD, Nigg EA. 2007. Plk4-induced centriole biogenesis in human cells. Dev Cell 13: 190–202. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Dynlacht BD. 2011. Regulating the transition from centriole to basal body. J Cell Biol 193: 435–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T, Tsang WY, Li J, Lane W, Dynlacht BD. 2011. Centriolar kinesin Kif24 interacts with CP110 to remodel microtubules and regulate ciliogenesis. Cell 145: 914–925. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Kim S, Lin Y-C, Inoue T, Dynlacht BD. 2014. The CP110-interacting proteins Talpid3 and Cep290 play overlap ping and distinct roles in cilia assembly. J Cell Biol 204: 215–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochanski RS, Borisy GG. 1990. Mode of centriole duplication and distribution. J Cell Biol 110: 1599–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlmaier G, Lončarek J, Meng X, McEwen BF, Mogensen MM, Spektor A, Dynlacht BD, Khodjakov A, Gönczy P. 2009. Overly long centrioles and defective cell division upon excess of the SAS-4-related protein CPAP. Curr Biol 19: 1012–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraatz S, Guichard P, Obbineni JM, Olieric N, Hatzopoulos GN, Hilbert M, Sen I, Missimer J, Gönczy P, Steinmetz MO. 2016. The human centriolar protein CEP135 contains a two-stranded coiled-coil domain critical for microtubule binding. Structure 24: 1358–1371. [DOI] [PubMed] [Google Scholar]
- Kuriyama R 1976. In vitro polymerization of flagellar and ciliary outer fiber tubulin into microtubules. J Biochem 80: 153–165. [DOI] [PubMed] [Google Scholar]
- Kuriyama R, Borisy GG. 1981. Centriole cycle in Chinese hamster ovary cells as determined by whole-mount electron microscopy. J Cell Biol 91(3 Pt 1): 814–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai EY, Walsh C, Wardell D, Fulton C. 1979. Programmed appearance of translatable flagellar tubulin mRNA during cell differentiation in Naegleria. Cell 17: 867–878. [DOI] [PubMed] [Google Scholar]
- Lambrus BG, Uetake Y, Clutario KM, Daggubati V, Snyder M, Sluder G, Holland AJ. 2015. P53 protects against genome instability following centriole duplication failure. J Cell Biol 210: 63–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Terra S, English CN, Hergert P, McEwen BF, Sluder G, Khodjakov A. 2005. The de novo centriole assembly pathway in HeLa cells: Cell cycle progression and centriole assembly/maturation. J Cell Biol 168: 713–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Fernandez J-J, Marshall WF, Agard DA. 2012. Three-dimensional structure of basal body triplet revealed by electron cryo-tomography. EMBO J 31: 552–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y-C, Chang C-W, Hsu W-B, Tang C-JC, Lin Y-N, Chou E-J, Wu C-T, Tang TK. 2013a. Human microcephaly protein CEP135 binds to hSAS-6 and CPAP, and is required for centriole assembly. EMBO J 32: 1141–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YN, Wu CT, Lin YC, Hsu WB, Tang CJC, Chang CW, Tang TK. 2013b. CEP120 interacts with CPAP and positively regulates centriole elongation. J Cell Biol 202: 211–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linck RW, Stephens RE. 2007. Functional protofilament numbering of ciliary, flagellar, and centriolar microtubules. Cell Motil Cytoskeleton 64: 489–495. [DOI] [PubMed] [Google Scholar]
- Linck R, Fu X, Lin J, Ouch C, Schefter A, Steffen W, Warren P, Nicastro D. 2014. Insights into the structure and function of ciliary and flagellar doublet microtubules: Tektins, Ca2+-binding proteins, and stable protofilaments. J Biol Chem 289: 17427–17444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Q, Insinna C, Ott C, Stauffer J, Pintado PA, Rahajeng J, Baxa U, Walia V, Cuenca A, Hwang Y-S, et al. 2015. Early steps in primary cilium assembly require EHD1/EHD3-dependent ciliary vesicle formation. Nat Cell Biol 17: 531. [DOI] [PubMed] [Google Scholar]
- Madarampalli B, Yuan Y, Liu D, Lengel K, Xu Y, Li G, Yang J, Liu X, Lu Z, Liu DX. 2015. ATF5 connects the pericentriolar materials to the proximal end of the mother centriole. Cell 162: 580–592. [DOI] [PubMed] [Google Scholar]
- Maheshwari A, Obbineni JM, Bui KH, Shibata K, Toyoshima YY, Ishikawa T. 2015. α- and β-tubulin lattice of the axonemal microtubule doublet and binding proteins revealed by single particle cryo-electron microscopy and tomography. Structure 23: 1584–1595. [DOI] [PubMed] [Google Scholar]
- Matsuura K, Lefebvre PA, Kamiya R, Hirono M. 2004. Bld10p, a novel protein essential for basal body assembly in Chlamydomonas: Localization to the cartwheel, the first ninefold symmetrical structure appearing during assembly. J Cell Biol 165: 663–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh JR, Euteneuer U. 1984. Tubulin hooks as probes for microtubule polarity: An analysis of the method and an evaluation of data on microtubule polarity in the mitotic spindle. J Cell Biol 98: 525–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh JR, Morphew MK, Grissom PM, Gilbert SP, Hoenger A. 2009. Lattice structure of cytoplasmic microtubules in a cultured Mammalian cell. J Mol Biol 394: 177–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meehl JB, Bayless BA, Giddings TH Jr, Pearson CG, Winey M. 2016. Tetrahymena Poc1 ensures proper intertriplet microtubule linkages to maintain basal body integrity. Mol Biol Cell 27: 2394–2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa Y, Hiraki M, Kamiya R, Hirono M. 2007. SAS-6 is a cartwheel protein that establishes the 9-fold symmetry of the centriole. Curr Biol 17: 2169–2174. [DOI] [PubMed] [Google Scholar]
- Nechipurenko IV, Berciu C, Sengupta P, Nicastro D. 2017. Centriolar remodeling underlies basal body maturation during ciliogenesis in Caenorhabditis elegans. Elife 6: e25686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicastro D, Fu X, Heuser T, Tso A, Porter ME, Linck RW. 2011. Cryo-electron tomography reveals conserved features of doublet microtubules in flagella. Proc Natl Acad Sci 108: E845–E853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen MG, Turner FR, Hutchens JA, Raff EC. 2001. Axoneme-specific β-tubulin specialization: A conserved C-terminal mo tif specifies the central pair. Curr Biol 11: 529–533. [DOI] [PubMed] [Google Scholar]
- Nogales E, Wolf SG, Downing KH. 1998. Structure of the α β tubulin dimer by electron crystallography. Nature 391: 199–203. [DOI] [PubMed] [Google Scholar]
- Nogales E, Whittaker M, Milligan RA, Downing KH. 1999. High-resolution model of the microtubule. Cell 96: 79–88. [DOI] [PubMed] [Google Scholar]
- Norstog K 1967. Fine structure of the spermatozoid of Zamia with special reference to the flagellar apparatus. Am J Bot 54: 831–840. [Google Scholar]
- Paintrand M, Moudjou M, Delacroix H, Bornens M. 1992. Centrosome organization and centriole architecture: Their sensitivity to divalent cations. J Struct Biol 108: 107–128. [DOI] [PubMed] [Google Scholar]
- Pelletier L, O’Toole E, Schwager A, Hyman AA, Müller-Reichert T. 2006. Centriole assembly in Caenorhabditis elegans. Nature 444: 619–623. [DOI] [PubMed] [Google Scholar]
- Pigino G, Maheshwari A, Bui KH, Shingyoji C, Kamimura S, Ishikawa T. 2012. Comparative structural analysis of eukaryotic flagella and cilia from Chlamydomonas, Tetrahymena, and sea urchins. J Struct Biol 178: 199–206. [DOI] [PubMed] [Google Scholar]
- Raff EC, Hutchens JA, Hoyle HD, Nielsen MG, Turner FR. 2000. Conserved axoneme symmetry altered by a component β-tubulin. Curr Biol 10: 1391–1394. [DOI] [PubMed] [Google Scholar]
- Redeker V 2010. Mass spectrometry analysis of C-terminal post-translational modifications of tubulins. Methods Cell Biol 95: 77–103. [DOI] [PubMed] [Google Scholar]
- Ross I, Clarissa C, Giddings TH Jr, Winey M. 2013. ε-tubulin is essential in Tetrahymena thermophila for the assembly and stability of basal bodies. J Cell Sci 126: 3441–3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez I, Dynlacht BD. 2016. Cilium assembly and disassembly. Nat Cell Biol 18: 711–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt TI, Kleylein-Sohn J, Westendorf J, Le Clech M, Lavoie SB, Stierhof YD, Nigg EA. 2009. Control of centriole length by CPAP and CP110. Curr Biol 19: 1005–1011. [DOI] [PubMed] [Google Scholar]
- Schmidt KN, Kuhns S, Neuner A, Hub B, Zentgraf H, Pereira G. 2012. Cep164 mediates vesicular docking to the mother centriole during early steps of ciliogenesis. J Cell Biol 199: 1083–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano L, de la Torre J, Maccioni RB, Avila J. 1984. Involvement of the carboxyl-terminal domain of tubulin in the regulation of its assembly. Proc Natl Acad Sci 81: 5989–5993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serwas D, Su TY, Roessler M, Wang S, Dammermann A. 2017. Centrioles initiate cilia assembly but are dispensable for maturation and maintenance in C. elegans. J Cell Biol 216: 1659–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A, Aher A, Dynes NJ, Frey D, Katrukha EA, Jaussi R, Grigoriev I, Croisier M, Kammerer RA, Akhmanova A. 2016. Centriolar CPAP/SAS-4 imparts slow processive microtubule growth. Dev Cell 37: 362–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillibourne JE, Hurbain I, Grand-Perret T, Goud B, Tran P, Bornens M. 2013. Primary ciliogenesis requires the distal appendage component Cep123. Biol Open 2: 535–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singla V, Romaguera-Ros M, Garcia-Verdugo JM, Reiter JF. 2010. Ofd1, a human disease gene, regulates the length and distal structure of centrioles. Dev Cell 18: 410–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song YH, Mandelkow E. 1993. Recombinant kinesin motor domain binds to β-tubulin and decorates microtubules with a B surface lattice. Proc Natl Acad Sci 90: 1671–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song YH, Mandelkow E. 1995. The anatomy of flagellar microtubules: Polarity, seam, junctions, and lattice. J Cell Biol 128: 81–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorokin S 1962. Centrioles and the formation of rudimentary cilia by fibroblasts and smooth muscle cells. J Cell Biol 15: 363–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorokin SP. 1968. Reconstructions of centriole formation and ciliogenesis in mammalian lungs. J Cell Sci 3: 207–230. [DOI] [PubMed] [Google Scholar]
- Spektor A, Tsang WY, Khoo D, Dynlacht BD. 2007. Cep97 and CP110 suppress a cilia assembly program. Cell 130: 678–690. [DOI] [PubMed] [Google Scholar]
- Steinman RM. 1968. An electron microscopic study of ciliogenesis in developing epidermis and trachea in the embryo of Xenopus laevis. Am J Anat 122: 19–55. [DOI] [PubMed] [Google Scholar]
- Stepanek L, Pigino G. 2016. Microtubule doublets are double-track railways for intraflagellar transport trains. Science 352: 721–724. [DOI] [PubMed] [Google Scholar]
- Stephens RE. 1970. Thermal fractionation of outer fiber doublet microtubules into A- and B-subfiber components. A- and B-tubulin. J Mol Biol 47: 353–363. [DOI] [PubMed] [Google Scholar]
- Stepp WL, Merck G, Mueller-Planitz F, Ökten Z. 2017. Kinesin-2 motors adapt their stepping behavior for processive transport on axonemes and microtubules. EMBO Rep 18: 1947–1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui H, Downing KH. 2006. Molecular architecture of axonemal microtubule doublets revealed by cryo-electron tomography. Nature 442: 475–478. [DOI] [PubMed] [Google Scholar]
- Sui H, Downing KH. 2010. Structural basis of interprotofilament interaction and lateral deformation of microtubules. Structure 18: 1022–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang C-JC, Fu R-H, Wu K-S, Hsu W-B, Tang TK. 2009. CPAP is a cell-cycle regulated protein that controls centriole length. Nat Cell Biol 11: 825–831. [DOI] [PubMed] [Google Scholar]
- Tanos BE, Yang H-J, Soni R, Wang W-J, Macaluso FP, Asara JM, Tsou M-FB. 2013. Centriole distal appendages promote membrane docking, leading to cilia initiation. Genes Dev 27: 163–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilney LG, Bryan J, Bush DJ, Fujiwara K, Mooseker MS, Murphy DB, Snyder DH. 1973. Microtubules: Evidence for 13 protofilaments. J Cell Biol 59(2 Pt 1): 267–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang WY, Spektor A, Luciano DJ, Indjeian VB, Chen Z, Salisbury JL, Sánchez I, Dynlacht BD. 2006. CP110 cooperates with two calcium-binding proteins to regulate cytokinesis and genome stability. Mol Biol Cell 17: 3423–3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang WY, Bossard C, Khanna H, Peränen J, Swaroop A, Malhotra V, Dynlacht BD. 2008. CP110 suppresses primary cilia formation through its interaction with CEP290, a protein deficient in human ciliary disease. Dev Cell 15: 187–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang WY, Spektor A, Vijayakumar S, Bista BR, Li J, Sanchez I, Duensing S, Dynlacht BD. 2009. Cep76, a centrosomal protein that specifically restrains centriole reduplication. Dev Cell 16: 649–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turk E, Wills AA, Kwon T, Sedzinski J, Wallingford JB, Stearns T. 2015. ζ-tubulin is a member of a conserved tubulin module and is a component of the centriolar basal foot in multiciliated cells. Curr Biol 25: 2177–2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uetake Y, Lončarek J, Nordberg JJ, English CN, La Terra S, Khodjakov A, Sluder G. 2007. Cell cycle progression and de novo centriole assembly after centrosomal removal in untransformed human cells. J Cell Biol 176: 173–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Breugel M, Hirono M, Andreeva A, Yanagisawa H-A, Yamaguchi S, Nakazawa Y, Morgner N, Petrovich M, Ebong I-O, Robinson CV, et al. 2011. Structures of SAS-6 suggest its organization in centrioles. Science 331: 1196–1199. [DOI] [PubMed] [Google Scholar]
- Vemu A, Atherton J, Spector JO, Moores CA, Roll-Mecak A. 2017. Tubulin isoform composition tunes microtubule dynamics. Mol Biol Cell 28: 3564–3572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorobjev IA, Chentsov Y. 1982. Centrioles in the cell cycle. I. Epithelial cells. J Cell Biol 93: 938–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W-J, Acehan D, Kao C-H, Jane W-N, Uryu K, Tsou M-FB. 2015. De novo centriole formation in human cells is errorprone and does not require SAS-6 self-assembly. Elife 4: e10586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JT, Kong D, Hoerner CR, Loncarek J, Stearns T. 2017. Centriole triplet microtubules are required for stable centriole formation and inheritance in human cells. Elife 6: e29061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner S, Pimenta-Marques A, Bettencourt-Dias M. 2017. Main taining centrosomes and cilia. J Cell Sci 130: 3789–3800. [DOI] [PubMed] [Google Scholar]
- Wloga D, Gaertig J. 2010. Post-translational modifications of microtubules. J Cell Sci 123: 3447–3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong YL, Anzola JV, Davis RL, Yoon M, Motamedi A, Kroll A, Seo CP, Hsia JE, Kim SK, Mitchell JW, et al. 2015. Cell biology. Reversible centriole depletion with an inhibitor of Polo-like kinase 4. Science 348: 1155–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff JB, Ferreira Gomes B, Widlund PO, Mahamid J, Honigmann A, Hyman AA. 2017. The centrosome is a selective condensate that nucleates microtubules by concentrating tubu lin. Cell 169: 1066–1077.e10. [DOI] [PubMed] [Google Scholar]
- Yu I, Garnham CP, Roll-Mecak A. 2015. Writing and reading the tubulin code. J Biol Chem 290: 17163–17172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X, Ramani A, Soni K, Gottardo M, Zheng S, Gooi LM, Li W, Feng S, Mariappan A, Wason A, et al. 2016. Molecular basis for CPAP-tubulin interaction in controlling centriolar and ciliary length. Nat Commun 7: 11874. [DOI] [PMC free article] [PubMed] [Google Scholar]


