Abstract
Sperm head morphology is crucial for male factor infertility diagnosis and assessment of male reproductive potential. Several criteria are available to analyze sperm head morphology, but they are limited by poor methodology comparability and population applicability. This study aimed to explore comprehensive and new normal morphometric reference values for spermatozoa heads in fertile Asian males. An automated sperm morphology analysis system captured 23 152 stained spermatozoa from confirmed fertile males. Of these samples, 1856 sperm head images were annotated by three experienced laboratory technicians as “normal”. We employed 14 novel morphometric features to describe sperm head size (head length, head width, length/width ratio, and girth), shape (ellipse intersection over union, girth intersection over union, short-axis symmetry, and long-axis symmetry), area (head, acrosome, postacrosomal areas, and acrosome area ratio), and degrees of acrosome and nuclear uniformity. This straight-forward method for the morphometric analysis of sperm by accurate visual measurements is clinically applicable. The measured parameters present valuable information to establish morphometric reference intervals for normal sperm heads in fertile Asian males. The presented detailed measurement data will be valuable for interlaboratory comparisons and technician training. In vitro fertilization and andrology laboratory technicians can use these parameters to perform objective morphology evaluation when assessing male fertilization potential.
Keywords: fertile Asian males, head morphometric reference value, normal spermatozoa
INTRODUCTION
Sperm morphology assessment is deemed to be an indicator of male factor fertility and reproductive potential.1 The impact of sperm morphology on the outcomes of natural pregnancies, intrauterine insemination, in vitro fertilization (IVF), and intracytoplasmic sperm injection has been progressively studied over the past 40 years.1 Kruger et al.2 found a strong positive correlation between the percentage of morphologically “normal” features of sperm and fertilization and pregnancy rates. This correlation was the theoretical foundation for the Kruger or Tygerberg strict criteria of 1986.2 Sperm assessment is significant when evaluating male fertility and was regarded as the first of the top ten research priorities for male infertility in a consensus statement of 11 countries.3 However, the clinical reliability, sperm morphology assessment precision, and differences between sperm morphology classification systems remain debated.1 Although historical reports indicated that sperm morphology had a predictive value for reproductive outcomes, recent published data do not support this assertion. Investigators have reported that males with abnormal sperm morphology conceived naturally.4 No clinical differences in the success and live birth rates following intrauterine insemination were noted in patients with abnormal sperm morphology.5,6 Another study found no statistical differences in fertilization, fertilization failure, pregnancy, and live birth rates in IVF cycles between couples with isolated teratozoospermia (<5% normal morphology) and those with normal sperm morphology.7 A meta-analysis detected no statistically significant association between isolated teratozoospermia and the likelihood of assisted conception and pregnancy.8 These published data showed that, using the Kruger or Tygerberg strict criteria for sperm morphology, assessment may decrease the efficacy and utility of sperm morphology in assisted reproductive technologies and male fertility.1,5
The human spermatozoon is one of the most diverse cell types known. It has three main parts: head, neck and midpiece, and tail. Diverse morphological presentations in these three parts result in numerous combinations of features. This diversity was assumed to reflect sperm function.9 Conventionally, sperm morphology measurements were conducted visually by technicians and considered a subjective parameter in semen analysis, resulting in individual variabilities.10 Laboratory technicians need appropriate training, testing, and control procedures before evaluating sperm morphology for patients.11 Several authors stressed the lack of standardization in sperm morphology assessment, which results in intra- and interlaboratory variabilities.12,13,14 In addition, vague definitions and descriptions of normal and abnormal spermatozoa were difficult for technicians to understand during training, even when presented with schematic drawings and micrographs.15 However, an alternative novel and efficient approach emerged using a computer-controlled device to perform objective sperm morphology analysis. This approach is less time consuming and easier to control than traditional manual laboratory work. An automated sperm morphometry instrument was released in 1992 to improve the reliability of morphology assays.16 The measurements of the sperm head included length (L), width (W), area (A), perimeter (P), and the L/W ratio. The availability of this instrument suggested that a device could replace traditional sperm morphology analysis.
The current sperm head morphometric reference values are based on the World Health Organization (WHO) criteria published in 2021.17 The 5th and 6th WHO manual guidelines describe only three sperm head morphometric parameters (L, W, and L/W ratio),15,17 limiting the description of spermatozoa in various clinical conditions. The available normal sperm morphology studies were based on populations from North America, Europe, Oceania, and Africa, possibly making them inapplicable to Asian populations.2,18,19
Our research enrolled participants from China with proven fertility, making our results more practical for the Asian population. In addition, this research assessed 14 sperm characteristics, enhancing the description of the contour and content of sperm. With the improvement in laboratory instruments and techniques, which enable precise male gamete assessments, we intended to explore suitable sperm head morphometric reference values for fertile males in Asia. These measured parameters could provide a basis for subsequent sperm testing and the comparison of sperm head parameters among different male groups.
PARTICIPANTS AND METHODS
Study population
The measured sperm morphometry data were acquired from candidate donors of the Sichuan Province Human Sperm Bank (SPHSB, Chengdu, China) from May 2020 to December 2021. All participants had fathered a child naturally during the past year. We invited 59 fertile males to participate in the plan, and 54 (91.5%) agreed. All participants provided written informed consent. A trained interviewer (YBW) collected information about the demographic and anthropometric characteristics of the participants through in-person interviews. The participants were requested to supply at least two semen samples, which underwent routine semen assessment following the 5th WHO manual guidelines.15 Subsequently, images of 23 152 stained spermatozoa were acquired by an Automated Sperm Morphology Analysis (ASMA) system (Puhua Corporation, Chengdu, China). The ASMA instrument comprised dedicated hardware systems and Sperm Morphometry Analysis Software, which evaluated 14 sperm head morphometric parameters, including L (μm), W (μm), L/W ratio (R), girth (HG), ellipse intersection over union (EIoU), girth intersection over union (GIoU), short-axis symmetry (SAS), long-axis symmetry (LAS), head area (HA), acrosome area (AA), acrosome area ratio (AAR: AA/HA), postacrosomal area (PAA), acrosome uniformity (AU), and nuclear uniformity (NU), by measuring sizes in pixels. All sperm images were assessed by three experienced technicians (YLJ, YBW, and LY) who labeled 1856 spermatozoa as “normal”. The number of included normal spermatozoa per participant ranged from 16 to 86 (mean: 34). The Ethics Committee of West China Second University Hospital Affiliated with Sichuan University (WCSUH-SCU; Chengdu, China), approved the study (Approval No. IRB 2020-001).
Candidates and sample preparation
In this prospective observational study, we collected candidate characteristics, semen parameters, and blood test screening results, including (1) age; (2) health status, including basic physical examination and psychological evaluation; (3) semen analysis results (sperm concentration, motility, viability, percentage of normal morphology, and semen volume); and (4) blood test results for sexually transmitted diseases (human immunodeficiency virus types 1 and 2, hepatitis B and C, syphilis, gonorrhea, mycoplasma, chlamydia, cytomegalovirus, Toxoplasma gondii, rubella virus, and herpes simplex virus types 1 and 2), and karyotype analysis.
The ejaculates were obtained by masturbation after 2–7 days of abstinence. We stressed the importance of accurately reporting the abstinence period, advising the participants that their samples would be discarded if they deviated from the recommended protocol. The samples were analyzed within 60 min after collection following standard procedures (5th WHO manual).15 Semen volume was measured by weighing the specimen in a standard container. A Makler chamber (Sefi Medical instruments Co., Haifa, Israel) was used to assess sperm concentration and count round cells (106 ml−1). Sperm motility analysis was performed by a computer-aided sperm analysis (CASA) system, including phase contrast microscopy (CX41, Olympus Corporation, Tokyo, Japan) and the Suiplus computer-aided sperm analysis system (Suiplus, Beijing, China). Sperm vitality (%) was assessed using eosin staining. After air-drying, fixation, and Papanicolaou staining of the sperm smears, images were captured by an ASMA system (oil immersion magnification of 1000×) to assess sperm morphology. Images of spermatozoa with normal morphology were those deemed normal by at least two of the three experienced technicians.
ASMA system
The ASMA instrument was equipped with a computer and an Olympus microscope with a C-Mount type 1.0× digital camera adapter, a 5.3× photo ocular, and a 100× oil bright field objective (CX43, Olympus Corporation, Tokyo, Japan). The video signal was acquired by a Daheng MER-231 digital camera (CMOS ½ In., Daheng Imaging, Beijing, China) mounted on the microscope and connected to an Intel® Core™ i7-10700 2.90-gigabyte processor (Intel Corporation, Santa Clara, CA, USA). The computer system configurations included the interface Sperm Morphometry Analysis Software (Puhua Corporation, Chengdu, China). Digitized images comprised 2 304 000 pixels (picture elements) and 24 color bits. The ASMA software theory was based on switching the known objective microscale to a corresponding pixel value and then converting it into the corresponding length (μm) by detecting the number of pixels delineating the sperm head.
Data collection and statistical analyses
Descriptive statistics and statistical analysis were performed using SPSS for Windows, version 15.0 (SPSS Inc., Chicago, IL, USA).
Demographic and anthropometric information (age, height, weight, body mass index, and education level) was collected and assessed after face-to-face interviews. Descriptive statistics (median, interquartile range [IQR], and percentage) of the participants’ characteristics are collected. Intraclass correlation coefficient (ICC) values for intra- and interobserver were determined for reliability analysis of the three experienced technicians. At least 200 spermatozoa per semen sample were randomly captured. Of these, 1856 “normal” spermatozoa were selected for measurements by the ASMA system. The obtained normal sperm head morphometric values described four main aspects (size, shape, area, and uniformity) and 14 other features (L, W, R, HG, EIoU, GIoU, SAS, LAS, HA, AA, AAR, PAA, AU, and NU; Supplementary Table 1).
Supplementary Table 1.
Detailed descriptions of all the measured parameters
| Sperm morphometry parameters | Full name (unit of measurement) | Description |
|---|---|---|
| L | Head length (mm) | The measurement of sperm head from acrosomal end to the nucleus end (long axis) |
| W | Head width (mm) | The measurement of sperm head short axis |
| R | Head ratio | Head length to width ratio. R=[Head length/head width] |
| HG | Head girth (mm) | The circumference length of sperm head |
| EIoU | Ellipse intersection over union (%) | EIoU=[Intersection/Union] ×100%. The ratio of a real sperm head area to a standard digital mimetic ellipse. “EIoU=1” means the real sperm figure is a standard ellipse (details in Figure 1b) |
| GIoU | Girth intersection over union (%) | GIoU=[Real sperm perimeter/a standard digital mimetic ellipse perimeter] ×100%. The ratio of a real sperm circumference length to a standard digital mimetic ellipse circumference length |
| SAS | Short-axis symmetry (%) | The sperm head was virtually divided according to the short axis. The symmetry degree of sperm head in short axis is SAS. The ratio of acrosomal end area to the nucleus end area. SAS=[area (CBD)/area (CAD)] ×100% (details in Figure 1a) |
| LAS | Long-axis symmetry (%) | The sperm head was virtually divided according to the long axis. LAS is the symmetry degree of sperm head in long axis. LAS=[area (ACB)/area (ADB)] ×100% (details in Figure 1a) |
| HA | Head area (mm2) | The area of sperm head |
| AA | Acrosome area (mm2) | The area of sperm acrosome |
| AAR | Acrosome area ratio (%) | The ratio of acrosome region area to head area. AAR=[Acrosome area/head area] ×100% |
| PAA | Postacrosomal area (mm2) | The postacrosomal region area in sperm head |
| AU | Acrosome uniformity (%) | “AU=100%” means the texture of sperm acrosome is uniformly distributed. Ideally, the acrosomal region should be optically even which contains no large vacuoles, more than two small vacuoles, or vacuoles occupy more than 20% of the sperm head. The AU represents the uniformity degree of the sperm acrosome |
| NU | Nuclear uniformity (%) | “NU=100%” means the texture of sperm nuclear is uniformly distributed. Ideally, the nuclear region should be optically even in the sperm head which contains no vacuole. The NU represents the uniformity degree of the sperm nuclear |
NU: nuclear uniformity; AU: acrosome uniformity; LAS: long-axis symmetry; SAS: short-axis symmetry; GIoU: girth intersection over union; EIoU: ellipse intersection over union; AAR: acrosome area ratio
We also reviewed 15 influential criteria reported in the literature between 1951 and 2021, nine sperm morphology classification systems from independent researchers, and the WHO manual series (1st–6th).15,17,20,21,22,23 Details of the research population, sperm staining methods, and morphometric measurement methods were assessed (Supplementary Table 2).
Supplementary Table 2.
Population, staining method, and morphology classification in sperm morphology classification systems during 1951–2021
| Year | Sperm morphology classification system | Population (City, Country, Continent) | Staining method | Sperm head morphology classification | Morphometric data (measurement method) |
|---|---|---|---|---|---|
| 1951 | MacLeod and Gold18 | 1000 fertile men (female partner currently pregnant) and 1000 men in infertile marriage (New York, the United States of America, North America) | Kaufman staining (formalin, hematoxylin) | Oval (normal), large (megalo), small, tapering, amorphous, duplicate | None |
| 1962 | MacLeod42 | normal males after orally took bis (dichloroacetyl) diamines (New York, the United States of America, North America) | Papanicolaou staining | Normal, small, megalo-form, acute tapering, moderate taper, tendency to taper, duplicate | None |
| 1966 | Freund31 | Unclear, 47 laboratories (the United States of America, North America) | Unknown | Too large (macrocephales), too small (microcephales), amorphous, duplicate, tapering, pear-shaped head, pyknotic (darkened nucleus, dense nucleus) | None |
| 1971 | Eliasson24 | Unknown (Stockholm, Sweden, Europe) | Papanicolaou staining | Normal, too large, too small, tapering, amorphous, duplicate, pear-shaped heads | Available (measured by one eyepiece with a scale in the optic pathway) |
| 1972 | Schirren39 | Unknown (Berlin, Germany, Europe) | Papanicolaou staining | Normal, tapered head, giant/large head, double head, deformed head, phantom | None |
| 1975 | David19 | 394 infertile men (Paris, France, Europe) | Shorr staining | Normal, tapered, thin, microcephalous, macrocephalous, amorphous, double, lysis, abnormal postacrosomal region, abnormal or absent acrosome | None |
| 1983 | Rogers40 | 30 fertile men (fathered children and their sperm showed penetration of >20% of the zona-free hamster eggs with the IVF test) and 65 infertile men (from infertile couples whose wives showed no evidence of infertile, and the IVF ability was ≤10% (Honolulu, the United States of America, Oceania) | Casarett staining and Modified Papanicolaou staining | Normal type, bicephalic, large amorphous head, slight head abnormalities, gross head, “bullet-like” head, immature forms | None |
| 1986 | Katz25 | 30 proven fertile donors and 30 men in marriages of long-standing infertility (these patients were defined as infertile since their wives had conceived after artificial insemination with donor semen) (California, the United States of America, North America) | Papanicolaou staining | Not described | Available [videomicrographic system, the cursor of a Numonics Model 1224 digitizer (Lansdale, PA) to the screen] |
| 1986 | Kruger (Tygerberg strict criteria)2 | 96 (in 1987) and 45 (in 1988) IVF couples (women had bilateral tubal damage, male partners) (Tygerberg, South Africa, Africa) | Papanicolaou staining | Same as Macleod and Gold18 and Eliasson24 classification: normal, large, small, tapering, duplicated, amorphous heads | Available (use a micrometer in the eyepiece of the microscope) |
| 1980–1987 | WHO (1st and 2ed)20,21 | Unknown | Modified Papanicolaou staining | Normal, large, small, tapering, pyriform, round, amorphous, amorphous, duplicate, or double head, pin head | Available |
| 1992 | WHO 3rd22 | Unknown | Modified Papanicolaou staining | Normal, large, small, tapering, pyriform, round, amorphous, vacuolated, double heads, pin head | Available (videomicrographic system) |
| 1999 | WHO 4th23 | Unknown | Modified Papanicolaou staining | Similar as WHO 3rd | Available (videomicrographic system) |
| 2010 | WHO 5th15 | Unknown (77 spermatozoa classified as normal) | Modified Papanicolaou staining | Normal, large, small, tapered, pyriform, round, amorphous, vacuolated, acrosomal defects | Available (computerized system) |
| 2021 | WHO 6th17 | Unknown (77 spermatozoa classified as normal) | Modified Papanicolaou staining | Normal, large, small, tapered, pyriform, round, amorphous, asymmetrical or nonoval shape in the apical part, vacuolated, acrosomal defects, double heads | Available (computerized system) |
IVF: in vitro fertilization
RESULTS
Study participants and data collection
Fifty-four fertile participants were included in this study and completed the survey. All blood tests for sexually transmitted disease and karyotype analysis were negative. The general demographic characteristics and detailed routine semen parameters are summarized in Table 1. The median age of the participants at semen collection was 29 years, with most in their middle 20s to early 30s (IQR: 26–33 years). The median height, weight, and body mass index were 1.72 m, 67.0 kg, and 22.5 kg m−2, respectively. Most participants (42, 77.8%) were highly educated (college graduates, master’s degrees, and doctorate/professional degrees). All candidates identified as Asian. The median normal sperm morphology rate was 8.2% (IQR: 6.1%–9.5%).
Table 1.
The semen parameters of fertile male participants (n=54)
| Parameter | Value |
|---|---|
| Demographic/anthropometric | |
| Age (year), median (IQR) | 29 (26–33) |
| Height (m), median (IQR) | 1.72 (1.70–1.75) |
| Weight (kg), median (IQR) | 67.0 (62.3–70.0) |
| BMI (kg m−2), median (IQR) | 22.5 (20.5–24.5) |
| Education level (% high) | 77.8 |
| Semen parameters, median (IQR) | |
| Sexual abstinence (day) | 4 (4–5) |
| Semen volume (ml) | 3.3 (2.6–4.4) |
| Sperm concentration (×106 ml−1) | 105.0 (74.5–162.0) |
| Progressive motility (%) | 68.0 (57.3–74.0) |
| Viability (%) | 67.0 (55.0–74.0) |
| Normal sperm morphology (%) | 8.2 (6.1–9.5) |
| Round cell (×106 ml−1) | 0.2 (0.1–0.2) |
IQR: interquartile range; BMI: body mass index
Capture of sperm images
We assessed 23 410 objects in the captured images. After removing noise (heavy background staining, faintly stained images, debris, and non-sperm cells) from the images, 23 152 objects were confirmed as spermatozoa. The ASMA sperm detection accuracy (detected sperm/detected objects) was 98.9%. The 23 152 sperm images were analyzed to morphometrically characterize the sperm heads.
Normal sperm morphology selection
The intraobserver (ICC: 0.938, 95% confidence interval [CI]: 0.875–0.973) and interobserver (ICC: 0.978, 95% CI: 0.954–0.991) correlation coefficients of the three experienced technicians were considered good and excellent, respectively (ICC>0.9, P<0.001). The captured sperm images were annotated three times by three experienced laboratory technicians (YLJ, YBW, and LY) with at least 5 years of experience evaluating sperm morphology. The heads of 23 152 spermatozoa were classified as normal, abnormal, or borderline. A complete or partial agreement (three or two experts) was achieved for 1856 spermatozoa considered to exhibit normal sperm heads.
Characteristics of sperm head size and shape
We present eight sperm head size and shape parameters for the 1856 visually normal spermatozoa (Table 2). The median head size parameters included L of 4.02 (95% CI: 4.01–4.05) μm, W of 2.52 (95% CI: 2.52–2.54) μm, R of 1.59 (95% CI: 1.59–1.62), and HG of 11.18 (95% CI: 11.17–11.25) μm. The median head symmetry parameters included SAS of 92.1% (95% CI: 88.3%–89.2%) and the LAS of 94.3% (95% CI: 91.6%–92.3%; Table 2 and Figure 1a). The median head shape parameters included EIoU of 88.9% (95% CI: 85.4%–86.3%) and GIoU of 102.4% (95% CI: 102.4%–102.5%; Table 2 and Figure 1b).
Table 2.
Distributions of the morphometry in normal sperm heads (size and shape)
| Morphometry parameter | Mean (s.d.) | Percentile | 95% CI | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| 5th | 25th | 50th | 75th | 95th | |||
| L (µm) | 4.03 (0.39) | 3.41 | 3.78 | 4.02 | 4.27 | 4.69 | 4.01–4.05 |
| W (µm) | 2.53 (0.27) | 2.13 | 2.34 | 2.52 | 2.70 | 3.00 | 2.52–2.54 |
| L/W ratio (R) | 1.61 (0.20) | 1.30 | 1.46 | 1.59 | 1.72 | 1.97 | 1.59–1.62 |
| HG (µm) | 11.21 (0.93) | 9.80 | 10.61 | 11.18 | 11.78 | 12.73 | 11.17–11.25 |
| EIoU (%) | 85.8 (10.4) | 66.1 | 82.1 | 88.9 | 92.6 | 96.0 | 85.4–86.3 |
| GIoU (%) | 102.4 (1.5) | 100.2 | 101.5 | 102.4 | 103.3 | 104.8 | 102.4–102.5 |
| SAS (%) | 88.8 (9.8) | 69.6 | 85.5 | 92.1 | 95.2 | 97.4 | 88.3–89.2 |
| LAS (%) | 92.0 (7.7) | 78.4 | 90.7 | 94.3 | 96.2 | 97.9 | 91.6–92.3 |
s.d.: standard deviation; CI: confidence interval; L: head length; W: head width; L/W ratio (R): length/width ratio; HG: head girth; EIoU: Ellipse Intersection over Union; GIoU: Girth Intersection over Union; SAS: short-axis symmetry; LAS: long-axis symmetry
Figure 1.
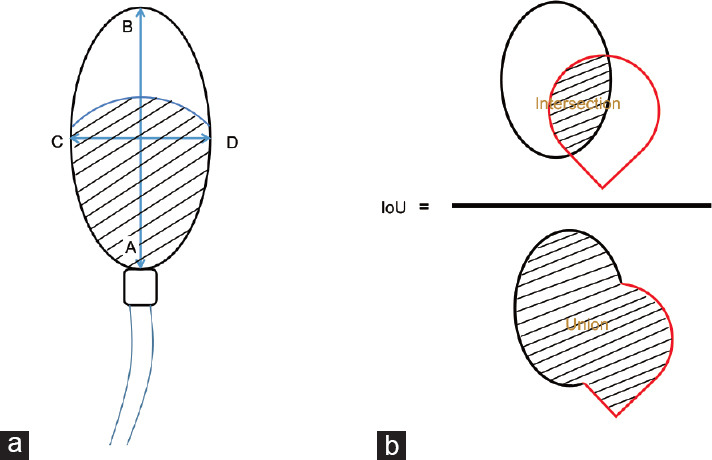
Schematic drawings of human sperm definition of size, shape, and area. (a) Schematic descriptions for sperm head parameters. Head length (L): AB. Head width (W): CD. Ratio (R): AB/CD. Head girth: circumference of a sperm head (length of ACBD). Short-axis symmetry (SAS): area (CBD)/area (CAD). Long-axis symmetry (LAS): area (ACB)/area (ADB). Head area (HA): area (ACBD). Postacrosomal area (PAA): the black lines region. Acrosome area (AA): area above the PPA in the sperm head. Acrosome area ratio (AAR): AA/HA. Acrosome uniformity (AU): the uniformity degree of the acrosome region (as a supplementary parameter for vacuole measurement in acrosomal region). Nuclear uniformity (NU): the uniformity degree of the postacrosomal region (as a supplementary parameter for vacuole measurement in postacrosomal region). All area and perimeter data were measured based on digital pixel measurement. (b) Schematic descriptions for sperm head shape parameters. Ellipse IoU=(Intersection/Union). The ellipse drawn by the black line represents a standard digital mimetic ellipse. The drop-like figure drawn by the red line represents a real sperm figure. “Ellipse IoU=1” means the real sperm figure is a standard ellipse. Girth IoU=(real sperm perimeter/a standard digital mimetic ellipse perimeter). The sperm head perimeter measurement is based on digital pixel measurement.
Characteristics of sperm head area and acrosome uniformity
Descriptive analysis of the sperm head area characteristics (Table 3) included a median HA of 7.72 (95% CI: 7.74–7.85) μm2, AA of 3.67 (95% CI: 3.77–3.88) μm2, AAR of 48.2% (95% CI: 47.8%–48.6%), PAA of 3.95 (95% CI: 3.95–4.00) μm2, AU of 88.6% (95% CI: 88.6%–89.1%), and NU of 100.0% (95% CI: 99.8%–99.9%; Supplementary Figure 1 (74.1KB, tif) ).
Table 3.
Distributions of the morphometry in normal sperm heads (area and uniformity)
| Morphometry parameter | Mean (s.d.) | Percentile | 95% CI | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| 5th | 25th | 50th | 75th | 95th | |||
| HA (µm2) | 7.80 (1.20) | 5.98 | 6.99 | 7.72 | 8.56 | 9.88 | 7.74–7.85 |
| AA (µm2) | 3.83 (1.18) | 2.13 | 2.96 | 3.67 | 4.57 | 6.07 | 3.77–3.88 |
| AAR (%) | 48.2 (8.7) | 34.0 | 41.4 | 48.2 | 54.5 | 62.5 | 47.8–48.6 |
| PAA (µm2) | 3.97 (0.53) | 3.21 | 3.60 | 3.95 | 4.28 | 4.87 | 3.95–4.00 |
| AU (%) | 88.8 (6.4) | 78.4 | 84.1 | 88.6 | 93.8 | 100.0 | 88.6–89.1 |
| NU (%) | 99.9 (0.6) | 99.1 | 100.0 | 100.0 | 100.0 | 100.0 | 99.8–99.9 |
s.d.: standard deviation; CI: confidence interval; HA: head area; AA: acrosome area; AAR: acrosome area ratio; PAA: postacrosomal area; AU: acrosome uniformity; NU: nuclear uniformity
Summary of the sperm morphology classification systems
We identified 15 influential criteria reported in the literature between 1951 and 2021 (Supplementary Table 2). The population source was recorded in six out of the 15 criteria. Five sperm staining methods were mentioned, with hematoxylin the most used staining ingredient for sperm morphology over the past 70 years. Sperm head morphology types ranged between 6 and 11 categories, depending on the taxonomy method. The ideal/normal sperm type was the only category that appeared in all classifications. Large, small, tapering, and amorphous sperm heads appeared with consistent frequencies since 1951. The sperm morphology classification system in the WHO manual series has changed little during 1980–2021.
Historical normal sperm head morphometric data
The available normal sperm head morphometric data are listed in Table 4. The earliest data referred only to the L and W.24 Katz et al.25 added the R and A. Kruger et al.2 and WHO manuals (1st–6th) illustrated sperm heads using three parameters (L, W, and R). New data from our study provide ten more sperm head characteristics (Table 2 and 3).
Table 4.
Morphometric items of normal sperm heads
| Sperm morphology classification | Eliasson24 | Katz et al.25 | Kruger et al.2 | WHO (1st and 2nd)20,21 | WHO 3rd22 | WHO 4th23 | WHO (5th and 6th)15,17 | This study |
|---|---|---|---|---|---|---|---|---|
| Morphometric items (95% CI) | ||||||||
| Head length (µm) | 3–5 | 4.26–4.49 | 5–6 | 3–5 | 4.0–5.5 | 4.0–5.0 | 3.7–4.7 | 4.01–4.05 |
| Head width (µm) | 2–3 | 2.77–2.89 | 2.5–3.5 | 2–3 | 2.5–3.5 | 2.5–3.5 | 2.5–3.2 | 2.52–2.54 |
| Length/width ratio | - | 1.52–1.61 | 1.49–1.67 | 1.5–2.0 | 1.50–1.75 | 1.50–1.75 | 1.3–1.8 | 1.59–1.62 |
| Head area (µm2) | - | 11.9–12.86 | - | - | - | - | - | 7.74–7.85 |
| Data sources | ||||||||
| Population | Unknown | Fertile donors | IVF couples | Unknown | Unknown | Unknown | Fertile male | Fertile males |
| Geographic area | Europe | USA | Africa | Unknown | Unknown | Unknown | Unknown | Asia |
| Number of sperm for morphometry | Unknown | 50 | Unknown | Unknown | Unknown | Unknown | 77 | 1856 |
-: no data were available for the item; WHO: World Health Organization; CI: confidence interval
DISCUSSION
Male fertility is a complex process that involves the participation of a wide range of sperm characteristics.26 Sperm morphology has been identified as a predictive characteristic of its ability to fertilize an egg under competitive and noncompetitive conditions.27,28 The use of sperm morphology to assess male fertility potential began in the early 1900s.29 Unlike other cells, human spermatozoa present extreme heterogeneity and pleomorphism throughout spermatogenesis and spermiogenesis. Many researchers have devoted considerable effort to studying sperm morphology, and assessment methods were enriched by Williams,30 MacLeod and Gold,18 Freund,31 Eliasson,24 and the WHO manuals during 1980–2021.15,17,20,21,22,23 Various sperm morphology classification systems and assessment methods were proposed over the years. However, various opinions on how to assess sperm morphology are still under debate. Some laboratories only assess normal forms, whereas others claim that the type, location, and extent of the abnormality are more important.15 Based on an early and liberal assessment consensus, investigators described obvious sperm abnormalities, and the remaining spermatozoa were considered normal. The most distorted forms of spermatozoa were considered abnormal,18 whereas morphologically normal spermatozoa were identified by default.32 This approach increases the uncertainty and inhomogeneity in the definition and assessment of “normal sperm morphology”. Some investigators regarded the “borderline” or “slightly abnormal” sperm heads as normal, whereas the Tygerberg strict criteria considered the “borderline” spermatozoa as abnormal.2,20,21,24 The attribution of “borderline” spermatozoa leads to a huge variability in the results of sperm morphology analysis. Owing to the subjective nature of sperm morphology analysis, manual assessment methods were described in a comparative study of 47 laboratories31 as “personally oriented” and “difficult to teach to students and technicians”. In addition, the increasingly stringent sperm morphology assessment standards led to a corresponding decrease in the average number of morphologically normal sperm and decreased the productivity of sperm morphology analysis. The low reference value limit (5th percentile in the strict and WHO 5th/6th manual criteria) seems to lose clinical application for assessing subfertile and fertile males. Notably, the variability in sperm morphology assessment appears to diminish its value in the evaluation of male fertility and assisted reproductive technologies outcomes. Recent evidence suggested that males with poor sperm morphology, even those without a single spermatozoon with normal morphology, perform as well as those with normal sperm morphology.5,6,7,8 Therefore, objective sperm morphology assessment based on accurate and precise measurements will require new approaches for future practical application.
The geographic area (city, country, and continent) is one of the reasons for sperm morphometric variations. Nine of the 15 assessed criteria recorded the geographic location, covering only four continents (North America, Europe, Oceania, and Africa; Supplementary Table 2). Sperm morphology criteria data were from studies performed on populations from various sources (fertile males, normal males orally taking bis compounds as a part of a clinical trial, infertile males, fertile and infertile males from an IVF experiment, fertile donors and infertile males from an IVF clinic, and male partners of IVF couples; Supplementary Table 2). The selection of geographical area and source population determines the scope of data applicability. Given the population characteristics of Asian males, the previous 15 criteria are likely to be inapplicable or unsuitable for this population. The sperm morphometric data in the current study were from fertile Asian males selected as sperm donor candidates (Table 4), facilitating the determination of representative reference values suitable for evaluating the fertility of Asian males. The new sperm morphometric dimensions, derived from the 1856 spermatozoa in our study, are predicted to be applicable as a reference for fertile Asian males.
Metric standards for normal human sperm head characteristics have been cited in several sperm morphology standards.15,17,24,25,33 In recent decades, methodological changes strongly affected the percentage of morphologically normal spermatozoa.34 It was reported that the staining technique affects the morphometric dimensions of the human sperm head, presumably because the fixatives or dyes are not iso-osmotic relative to sperm.33 It is important to also note differences between ejaculated and swim-up-selected spermatozoa.35 Researchers observed wider sperm heads and larger AA, R, and perimeter/area ratio values in native spermatozoa.36 The morphometric values varied among morphology classifications probably because of differences in the staining materials used.33,37 Papanicolaou staining was reported to cause sperm shrinkage.33 Some smears stained by rapid procedures such as the Diff-Quik stain, which highlights the background, resulted in sperm heads appearing larger than those stained by Papanicolaou.17,37 We noticed that the sperm metric data varied among studies and measurement methodologies (e.g., eyepiece with a scale in the optic pathway and videomicrographic and computerized systems), possibly resulting in subtle differences at the micron level (Table 4).
Most normal sperm head morphometric descriptions include three parameters (L, W, and R; Table 4). Eliasson24 pioneered sperm size measurements and advocated their importance. Katz et al.25 (L: 4.26–4.49 μm; W: 2.77–2.89 μm; R: 1.52–1.61; and HA: 11.90–12.86 μm2), Menkveld38 (L: 3.88–4.26 μm and W: 2.84–3.12μm), WHO 5th/6th manuals (L: 3.7–4.7 μm and W: 2.5–3.2 μm),15,17 and Maree et al.33 (L: 4.01–4.55 μm and W: 2.46–2.84 μm) reported morphometric data using computer-assisted sperm morphology analysis and Papanicolaou staining. In the present study, we described eight sperm head size and shape parameters and six sperm head area and uniformity parameters, increasing the range of sperm description parameters. The current L, W, and R reference values were “narrow” compared with data in the WHO 5th/6th manual. This finding indicated that “normal” spermatozoa in fertile Asians were more homogeneous than those described in other standards (Table 4). Sperm acrosome uniformity, a novel indicator stated in this study, is considered a supplementary parameter for acrosome vacuole assessment that could digitally reveal overall acrosomal transparency (Supplementary Figure 1a (74.1KB, tif) and 1b (74.1KB, tif) ). The new morphometric parameters (LAS, SAS, EIoU, and GIoU) reflect the degree of fitness between the tested sperm head contour and a standard ellipse. The area measurement data (Table 3) were improved upon past vague expressions such as “larger sperm head” and “acrosome under 40% or over 70% of a normal head area” by replacing them with a precise and viable approach.
In past research, the status of subjects used for several sperm morphology classifications were not stated clearly. Seven of 13 classifications, including all the WHO criteria, did not cite their population sources. In the WHO 5th/6th manual criteria,15,17 only 77 spermatozoa classified as normal were measured by a computerized system (Table 4). This limited number of spermatozoa for the WHO sperm morphometric data is controversial, debated, and may cause bias. Although many studies included a large number of participants (e.g., Freund,31 Eliasson et al.,24 Schirren,39 David et al.,19 Rogers et al.,40 Katz et al.,25 Kruger et al.,2 and the WHO manual series 1–615,17,20,21,22,23), these studies usually did not consider the time required for donor subjects to achieve a pregnancy. MacLeod and Gold18 mentioned “currently pregnant”, but no accurate time before pregnancy was recorded. In our study, all recruited participants produced a child naturally within the past year, which improves the reliability of a normal sperm source from “fertile” donors.
The sperm head is difficult to evaluate, with a reported broad coefficient of variation range (4.80%–132.97%),41 making it imperative to find an objective measurement approach. Our study measured a wide range of sperm head parameters in fertile Asian males. The measurement technique was accurate, objective, and highly informative. The current data provide extremely detailed information for reference studies and comparison of sperm head parameters among different male groups. We hope these new parameters will be applied in routine semen analysis when assessing sperm morphology and male fertility potential.
The current study had several limitations that should be noted. The use of samples from a single center, covering relatively young participants from only seven provinces in China, could limit the usage of our findings. The use of only three technicians to assess the samples may have introduced a subjective bias. Future studies should apply our reported criteria to assess their predictive value for fertility or reproductive treatment success.
AUTHOR CONTRIBUTIONS
FPL led the study, and all authors contributed to the study design. YBW, YZ, and TTY collected the data. LZ, LY and YYW analyzed and interpreted the data. YLJ drafted the manuscript, and LZ provided administrative support. BZ and FPL critically revised the manuscript. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declare no competing interests.
Sperm acrosome uniformity. (a) The sperm in the blue frame is considered having poor acrosome uniformity. A light transparency area was observed in the middle of the acrosome. (b) The sperm in the blue frame is considered normal acrosome uniformity. The sperm in the red frame is a typical sperm with small vacuoles in the acrosomal region.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (No. 81974226, No.32171264, and No. 81974365) and the Sichuan Science and Technology Program (No. 2021YFS0026 and No. 2022YFS0045).
Supplementary Information is linked to the online version of the paper on the Asian Journal of Andrology website.
REFERENCES
- 1.Danis RB, Samplaski MK. Sperm morphology: history, challenges, and impact on natural and assisted fertility. Curr Urol Rep. 2019;20:43. doi: 10.1007/s11934-019-0911-7. [DOI] [PubMed] [Google Scholar]
- 2.Kruger TF, Menkveld R, Stander FS, Lombard CJ, Van der Merwe JP, et al. Sperm morphologic features as a prognostic factor in in vitro fertilization. Fertil Steril. 1986;46:1118–23. doi: 10.1016/s0015-0282(16)49891-2. [DOI] [PubMed] [Google Scholar]
- 3.Duffy JM, Adamson GD, Benson E, Bhattacharya S, Bhattacharya S, et al. Top 10 priorities for future infertility research: an international consensus development study. Fertil Steril. 2021;115:180–90. doi: 10.1016/j.fertnstert.2020.11.014. [DOI] [PubMed] [Google Scholar]
- 4.Kovac JR, Smith RP, Cajipe M, Lamb DJ, Lipshultz LI. Men with a complete absence of normal sperm morphology exhibit high rates of success without assisted reproduction. Asian J Androl. 2017;19:39–42. doi: 10.4103/1008-682X.189211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kohn TP, Kohn JR, Ramasamy R. Effect of sperm morphology on pregnancy success via intrauterine insemination: a systematic review and meta-analysis. J Urol. 2018;199:812–22. doi: 10.1016/j.juro.2017.11.045. [DOI] [PubMed] [Google Scholar]
- 6.Patel P, Carrasquillo R, Madhusoodanan V, Dadoun S, Patel A, et al. Impact of abnormal sperm morphology on live birth rates following intrauterine insemination. J Urol. 2019;202:801–5. doi: 10.1097/JU.0000000000000288. [DOI] [PubMed] [Google Scholar]
- 7.Keegan BR, Barton S, Sanchez X, Berkeley AS, Krey LC, et al. Isolated teratozoospermia does not affect in vitro fertilization outcome and is not an indication for intracytoplasmic sperm injection. Fertil Steril. 2007;88:1583–8. doi: 10.1016/j.fertnstert.2007.01.057. [DOI] [PubMed] [Google Scholar]
- 8.Hotaling JM, Smith JF, Rosen M, Muller CH, Walsh TJ. The relationship between isolated teratozoospermia and clinical pregnancy after in vitro fertilization with or without intracytoplasmic sperm injection: a systematic review and meta-analysis. Fertil Steril. 2011;95:1141–5. doi: 10.1016/j.fertnstert.2010.09.029. [DOI] [PubMed] [Google Scholar]
- 9.Maroto-Morales A, García-Álvarez O, Ramón M, Martínez-Pastor F, Fernández-Santos MR, et al. Current status and potential of morphometric sperm analysis. Asian J Androl. 2016;18:863–70. doi: 10.4103/1008-682X.187581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eustache F, Auger J. Inter-individual variability in the morphological assessment of human sperm: effect of the level of experience and the use of standard methods. Hum Reprod. 2003;18:1018–22. doi: 10.1093/humrep/deg197. [DOI] [PubMed] [Google Scholar]
- 11.Kruger T. Computer-assisted sperm analysis systems: morphometric aspects. Hum Reprod. 1995;10:46–52. doi: 10.1093/humrep/10.suppl_1.46. [DOI] [PubMed] [Google Scholar]
- 12.Walczak-Jedrzejowska R, Marchlewska K, Oszukowska E, Filipiak E, Bergier L, et al. Semen analysis standardization: is there any problem in Polish laboratories? Asian J Androl. 2013;15:616–21. doi: 10.1038/aja.2013.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Auger J, Eustache F, Ducot B, Blandin T, Daudin M, et al. Intra-and inter-individual variability in human sperm concentration, motility and vitality assessment during a workshop involving ten laboratories. Hum Reprod. 2000;15:2360–8. doi: 10.1093/humrep/15.11.2360. [DOI] [PubMed] [Google Scholar]
- 14.Filimberti E, Degl’Innocenti S, Borsotti M, Quercioli M, Piomboni P, et al. High variability in results of semen analysis in andrology laboratories in Tuscany (Italy): the experience of an external quality control (EQC) programme. Andrology. 2013;1:401–7. doi: 10.1111/j.2047-2927.2012.00042.x. [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen. 5th ed. Geneva: World Health Organization; 2010. [Google Scholar]
- 16.Davis RO, Bain DE, Siemers RJ, Thal DM, Andrew JB, et al. Accuracy and precision of the cellform-human automated sperm morphometry instrument. Fertil Steril. 1992;58:763–9. [PubMed] [Google Scholar]
- 17.World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen. 6th ed. Geneva: World Health Organization; 2021. [Google Scholar]
- 18.MacLeod J, Gold RZ. The male factor in fertility and infertility. IV. Sperm morphology in fertile and infertile marriage. Fertil Steril. 1951;2:394–414. doi: 10.1016/s0015-0282(16)30661-6. [DOI] [PubMed] [Google Scholar]
- 19.David G, Bisson JP, Czyglik F, Jounannet P, Gernigon C. [Anomalies morphologiques du spermatozoïde humain. 1. Prognitionen pour un système de classification] J Gynéc Obstet Biol Reprod. 1975;4:17–36. [Article in French] [Google Scholar]
- 20.World Health Organization. WHO Laboratory Manual for the Examination of Human Semen and Semen-Cervical Mucus Interaction. 1st ed. Singapore: World Health Organization; 1980. [Google Scholar]
- 21.World Health Organization. WHO Laboratory Manual for the Examination of Human Semen and Semen-Cervical Mucus Interaction. 2nd ed. Cambridge: World Health Organization; 1987. [Google Scholar]
- 22.World Health Organization. WHO Laboratory Manual for the Examination of Human Semen and Sperm-Cervical Mucus Interaction. 3rd ed. Cambridge: World Health Organization; 1992. [Google Scholar]
- 23.World Health Organization. WHO Laboratory Manual for the Examination of Human Semen and Sperm-Cervical Mucus Interaction. 4th ed. Cambridge: World Health Organization; 1999. [Google Scholar]
- 24.Eliasson R. Standards for investigation of human semen. Andrologie. 1971;3:49–64. [Google Scholar]
- 25.Katz DF, Overstreet JW, Samuels SJ, Niswander PW, Bloom TD, et al. Morphometric analysis of spermatozoa in the assessment of human male fertility. J Androl. 1986;7:203–10. doi: 10.1002/j.1939-4640.1986.tb00913.x. [DOI] [PubMed] [Google Scholar]
- 26.Kölle S, Reese S, Kummer W. New aspects of gamete transport, fertilization, and embryonic development in the oviduct gained by means of live cell imaging. Theriogenology. 2010;73:786–95. doi: 10.1016/j.theriogenology.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 27.Kleven O, Laskemoen T, Fossøy F, Robertson RJ, Lifjeld JT. Intraspecific variation in sperm length is negatively related to sperm competition in passerine birds. Evolution. 2008;62:494–9. doi: 10.1111/j.1558-5646.2007.00287.x. [DOI] [PubMed] [Google Scholar]
- 28.Tsakmakidis IA, Lymberopoulos AG, Khalifa TA. Relationship between sperm quality traits and field-fertility of porcine semen. J Vet Sci. 2010;11:151–4. doi: 10.4142/jvs.2010.11.2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Menkveld R, Kruger TF. Evaluation of sperm morphology by light microscopy. In: Acosta AA, Kruger TF, editors. Human Spermatozoa in Assisted Reproduction. Carnforth: Parthenon Publishers; 1996. pp. 89–107. [Google Scholar]
- 30.Williams WW. Spermatic abnormalities. N Engl J Med. 1937;217:946–51. [Google Scholar]
- 31.Freund M. Standards for the rating of human sperm morphology. A cooperative study. Int J Fertil. 1966;11:97–180. [PubMed] [Google Scholar]
- 32.Mortimer D, Menkveld R. Sperm morphology assessment–historical perspectives and current opinions. J Androl. 2001;22:192–205. [PubMed] [Google Scholar]
- 33.Maree L, du Plessis SS, Menkveld R, van der Horst G. Morphometric dimensions of the human sperm head depend on the staining method used. Hum Reprod. 2010;25:1369–82. doi: 10.1093/humrep/deq075. [DOI] [PubMed] [Google Scholar]
- 34.van den Hoven L, Hendriks JC, Verbeet JG, Westphal JR, Wetzels AM. Status of sperm morphology assessment: an evaluation of methodology and clinical value. Fertil Steril. 2015;103:53–8. doi: 10.1016/j.fertnstert.2014.09.036. [DOI] [PubMed] [Google Scholar]
- 35.Yeung CH, Pérez-Sánchez F, Soler C, Poser D, Kliesch S, et al. Maturation of human spermatozoa (from selected epididymides of prostatic carcinoma patients) with respect to their morphology and ability to undergo the acrosome reaction. Hum Reprod Update. 1997;3:205–13. doi: 10.1093/humupd/3.3.205. [DOI] [PubMed] [Google Scholar]
- 36.Soler C, Pérez-Sánchez F, Schulze H, Bergmann M, Oberpenning F, et al. Objective evaluation of the morphology of human epididymal sperm heads. Int J Androl. 2000;23:77–84. doi: 10.1046/j.1365-2605.2000.00211.x. [DOI] [PubMed] [Google Scholar]
- 37.Kruger TF, Ackerman SB, Simmons KF, Swanson RJ, Brugo SS, et al. A quick, reliable staining technique for human sperm morphology. Arch Androl. 1987;18:275–7. doi: 10.3109/01485018708988493. [DOI] [PubMed] [Google Scholar]
- 38.Menkveld R. The basic semen analysis. In: Oehninger S, Kruger TF, editors. Male Infertility: Diagnosis and Treatment. Oxon: Informa Healthcare; 2007. pp. 141–70. [Google Scholar]
- 39.Schirren C. Practical Andrology: Diagnosis Clinical Examination Morphology of Spermatozoa Biochemistry of Sperm Plasma Testicular Histology Treatment. Berlin: Schering AG Publishers; 1972. [Google Scholar]
- 40.Rogers BJ, Bentwood BJ, Van Campen H, Helmbrecht G, Soderdahl D, et al. Sperm morphology assessment as an indicator of human fertilizing capacity. J Androl. 1983;4:119–25. doi: 10.1002/j.1939-4640.1983.tb00735.x. [DOI] [PubMed] [Google Scholar]
- 41.Wang Y, Yang J, Jia Y, Xiong C, Meng T, et al. Variability in the morphologic assessment of human sperm: use of the strict criteria recommended by the World Health Organization in 2010. Fertil Steril. 2014;101:945–9. doi: 10.1016/j.fertnstert.2013.12.047. [DOI] [PubMed] [Google Scholar]
- 42.MacLeod J. A possible factor in the etiology of human male infertility: preliminary report. Fertil Steril. 1962;13:29–33. doi: 10.1016/s0015-0282(16)34383-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sperm acrosome uniformity. (a) The sperm in the blue frame is considered having poor acrosome uniformity. A light transparency area was observed in the middle of the acrosome. (b) The sperm in the blue frame is considered normal acrosome uniformity. The sperm in the red frame is a typical sperm with small vacuoles in the acrosomal region.


