Abstract
What distinguishes vulnerability and resilience to posttraumatic stress disorder (PTSD) remains unclear. Levering traumatic experiences reporting, genetic data, and electronic health records (EHR), we investigated and predicted the clinical comorbidities (co-phenome) of PTSD vulnerability and resilience in the UK Biobank (UKB) and All of Us Research Program (AoU), respectively. In 60,354 trauma-exposed UKB participants, we defined PTSD vulnerability and resilience considering PTSD symptoms, trauma burden, and polygenic risk scores. EHR-based phenome-wide association studies (PheWAS) were conducted to dissect the co-phenomes of PTSD vulnerability and resilience. Significant diagnostic endpoints were applied as weights, yielding a phenotypic risk score (PheRS) to conduct PheWAS of PTSD vulnerability and resilience PheRS in up to 95,761 AoU participants. EHR-based PheWAS revealed three significant phenotypes positively associated with PTSD vulnerability (top association “Sleep disorders”) and five outcomes inversely associated with PTSD resilience (top association “Irritable Bowel Syndrome”). In the AoU cohort, PheRS analysis showed a partial inverse relationship between vulnerability and resilience with distinct comorbid associations. While PheRSvulnerability associations were linked to multiple phenotypes, PheRSresilience showed inverse relationships with eye conditions. Our study unveils phenotypic differences in PTSD vulnerability and resilience, highlighting that these concepts are not simply the absence and presence of PTSD.
Keywords: Posttraumatic stress disorder, resilience, vulnerability, phenome, polygenic risk score, UK Biobank, All of Us Research Program
1. Introduction
During their lifetime, 90% of individuals are exposed to at least one traumatic experience that could lead to a diagnosis of posttraumatic stress disorder (PTSD) (Kessler et al., 2005). However, the global PTSD prevalence is around 3.9% and about 5.6% in a known trauma-exposed population (Koenen et al., 2017). In other words, most individuals who perceive an event as a severe trauma will not develop PTSD. While most research focuses on PTSD diagnosis and symptoms, there are limited efforts in distinguishing PTSD vulnerability (in our study: PTSD cases with relatively low trauma exposure and low genetic PTSD risk) and resilience (in our study: PTSD controls with relatively high trauma exposure and high genetic PTSD risk) as distinct phenotypic outcomes (Choi et al., 2019). Distinguishing vulnerability and resilience is crucial for comprehending PTSD pathology and implementing clinically effective interventions. To fill this gap, we used genetic data, information regarding traumatic experiences and electronic health records (EHRs) to explore the comorbid phenomes of PTSD vulnerability and resilience and define phenotype risk scores (PheRS) for prediction in independent cohorts.
Genome-wide association studies (GWAS) of PTSD identified multiple genetic risk loci associated with PTSD risk (Nievergelt et al., 2024), permitting the exploration of the pleiotropic mechanisms linking PTSD to comorbid conditions (Pathak et al., 2023). Additionally, by combining the additive effect of associated loci, polygenic risk scores (PRS) can quantify genetic susceptibility to PTSD. Correspondingly, PRS is demonstrated to be a valid tool for discriminating resilience trajectories in longitudinal studies (Schultebraucks et al., 2021). A previous study demonstrated the ability of EHR-based PheRS to predict the genetic component of PTSD and other internalizing disorders (Wendt et al., 2022). Indeed, PTSD exhibits comorbidity to various neurobehavioral and somatic disorders, including mood disorders, anxiety disorders, cardiovascular disorders, and metabolic syndrome (Hicks et al., 2023). Still, the phenome characteristics of PTSD vulnerability and resilience remain unknown.
In the present study, we advanced the understanding of PTSD vulnerability and resilience by dissecting their comorbid phenome (co-phenome) using EHR data available from UK Biobank (UKB) (Bycroft et al., 2018) and All of Us Research Program (AoU) (Denny et al., 2019). By integrating information regarding PTSD symptomatology, trauma burden, and PRS distribution, we distinguished PTSD vulnerable vs. resilient individuals among UKB participants. Further, we applied EHR data to uncover their medical phenomes through a phenome-wide association study (PheWAS). Based on their co-phenome, we applied machine learning models to calculate a phenotypic risk score (PheRS) to predict PTSD vulnerability and resilience among AoU participants.
2. Methods
2.1. Study populations
From >500,000 included participants in UKB (Bycroft et al., 2018), we extracted genetic and EHR data from 60,314 unrelated trauma-exposed individuals of European or African descent (born between 1936 – 1970, agemedian=57 [50 – 62]; 51% females) to define PTSD vulnerability and resilience. Selected trauma subtypes aligned with the Lifetime Event Checklist for DSM-5 and included being exposed to combat or war zones, witnessing sudden violent death, sexual abuse, physical violence, being diagnosed with a life-threatening illness, and being involved in a serious accident. The UKB participants investigated in the present study reported a history of one or more trauma types (Supplementary Table 1). PTSD symptoms were positively associated with all trauma items and negatively associated with male sex and age (Supplementary Table 2).
In the AoU cohort (Denny et al., 2019), 95,761 unrelated individuals of European ancestry with EHR and genetic data (born between 1905 – 2004, agemedian=63 [48 – 73]; 60% females) covering the same EHR diagnostic codes as in UKB were extracted. Detailed descriptions of UKB and AoU cohorts are described elsewhere (Bycroft et al., 2018; Denny et al., 2019). While UKB was stratified into a training and test dataset, the AoU cohort was fully used as a independent test dataset for further phenome exploration. Data processing and genetic quality control of UKB and AoU are available in Supplementary Material p 3.
The study was conducted between October 15, 2022, and December 2, 2023. The study has been conducted under UKB application number 58146 and is approved by the Norwegian Regional Committees for Medical and Health Research Ethics (REK 467496). UKB and AoU participants provided written informed consent as reported in the articles describing these cohorts (Bycroft et al., 2018; Denny et al., 2019).
2.2. Polygenic risk scoring
To quantify PTSD genetic predisposition, we calculated PRS leveraging GWAS data available from the Psychiatric Genomics Consortium PTSD (PGC-PTSD) workgroup. In the European-ancestry (EUR) PRS analysis, we used data generated from a GWAS of 37,044 PTSD cases and 903,658 controls that did not include UKB participants (Nievergelt et al., 2024). EUR PRS was calculated using the PRS-CS method (Ge et al., 2019). We also compared PRS-CS results with those obtained from a different PRS method, PRSice-2 (Choi & O’Reilly, 2019). The results obtained from the two PRS approaches were consistent with each other (Supplementary Figure 6). In African-ancestry (AFR) PRS analysis, we used data generated from a GWAS of 3,163 PTSD cases and 9,459 controls that did not include UKB participants (Nievergelt et al., 2019). AFR PRS was calculated using the PRS-CSx method to combine EUR and AFR GWAS from the PGC-PTSD workgroup. PRS estimates were corrected for age, sex, and the top-10 within-ancestry principal components (PCs) and the residuals were normalized (Supplementary Figure 3–5). Because of the lack of corresponding ancestry-specific PTSD GWAS and their limited sample size in the UKB cohort, PTSD PRS was not calculated in other ancestry groups.
2.3. Defining PTSD vulnerable and resilient individuals
In the UKB cohort, we defined PTSD vulnerability and resilience based on three criteria: PTSD symptoms, trauma burden, and PTSD genetic predisposition, as by PRS (Fig. 1). In line with previous research from the PGC-PTSD workgroup (Nievergelt et al., 2019), PTSD cases in UKB were defined as having a symptom score ≥13 in an adapted version of the PTSD checklist. Trauma burden was estimated as the number of trauma types reported considering six severe trauma items assessed in the UKB mental health questionnaire (Supplementary Table 1, Supplementary Figure 1). Reporting ≥2 trauma types was set as the cutoff to define a high trauma burden. Regression analysis revealed a positive association between PTSD PRS and the total trauma score (Supplementary Table 3). A high PTSD genetic predisposition was defined as a PTSD-PRS above the median PRS observed in PTSD cases. Conversely, a low PTSD genetic predisposition was defined as a PTSD PRS below the median PRS observed in PTSD controls.
Figure 1. Study design overview.
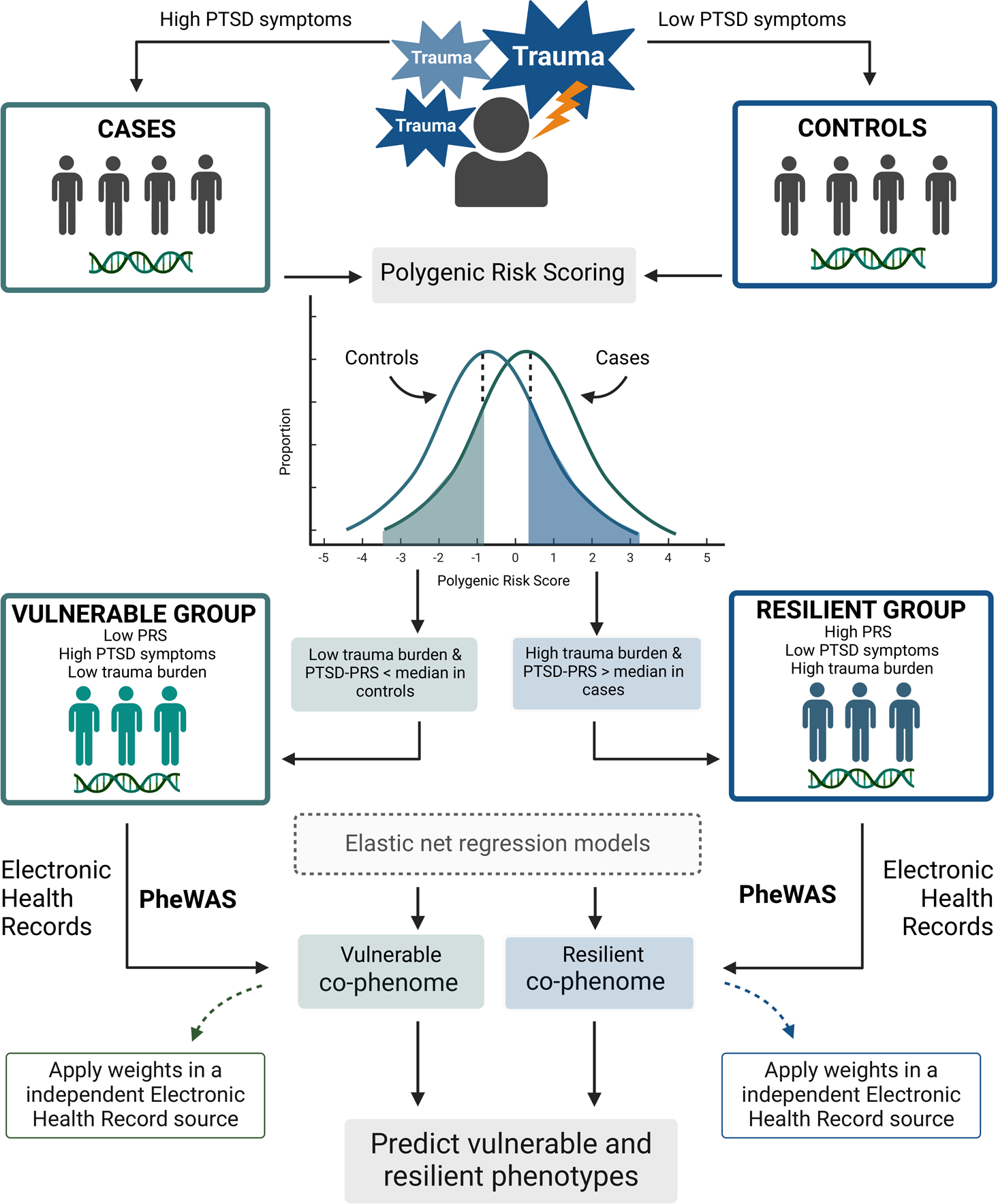
First, only trauma-exposed individuals were investigated to define PTSD+ cases and PTSD- controls based on PTSD symptom burden. Contrasting these groups, weighted by GWAS summary statistics of PTSD, we calculated a polygenic risk score for each study participant. Assessing three criteria – trauma burden, PTSD symptom levels and polygenic risk score – we defined PTSD vulnerability and resilience. An electronic health record-based phenome-wide association study (PheWAS) was applied to each group, characterizing their medical co-phenome. Further, elastic net regression models were performed to generate phenotype weights to derive a phenotypic risk score (PheRS) applied in an independent EHR source to perform PheWAS of PTSD vulnerability and resilience PheRS. Created with BioRender.
The PTSD resilient group included UKB participants with high PTSD genetic risk (i.e., PRS>median PRS in PTSD cases), high trauma burden (≥two trauma types reported), and low PTSD symptom severity (PTSD symptom score <13). The PTSD resilient group was compared to equal-risk non-resilient individuals defined as UKB participants with high PTSD genetic risk (i.e., PRS>median PRS in PTSD cases), high trauma burden (two or more trauma types reported) but high PTSD symptom severity (PTSD symptom score ≥13).
The PTSD vulnerable group included UKB participants with low PTSD genetic risk (i.e., PRS<median PRS in PTSD controls), low trauma burden (only one trauma type reported), and high PTSD symptom severity (PTSD symptom score ≥13). PTSD vulnerable group was compared to equal-risk non-vulnerable individuals defined as UKB participants with low PTSD genetic risk (i.e., PRS<median PRS in PTSD controls), and low trauma burden (only one trauma type reported) but with low PTSD symptom severity (PTSD symptom score <13).
2.4. Electronic Health Records
UKB EHR data were extracted from hospital inpatient records, cancer registers, death registers, self-reported medical conditions, and primary healthcare records. The registries included ICD10 and ICD9 codes, which were converted to 1,987 unique phecodes (EHR-derived phenotypes intended to capture clinically meaningful concepts for research [R]) using maps provided by UKB (UK Biobank, 2019) and further to 589 phecodes through publicly available phecode maps (Zheng et al., 2020). Similar to past analyses (Hyppönen et al., 2019; Xu et al., 2022; Zheutlin et al., 2019), these PheMap algorithms permitted us to convert redundant and heterogenous ICD codes into phecodes (Zheng et al., 2020). In AoU, ICD10 and ICD9 codes mapped to 1600 and 1661 phecodes, respectively, using the PheWAS catalog resources (Wei et al., 2017; Wu et al., 2019). In total, 1707 unique phecodes were extracted. In line with previous studies (Zheutlin et al., 2019), we considered only phecodes with more than 100 cases in both UKB and AoU to maximise the statistical power of the PheRS analysis and avoid biases due to extreme case-control imbalance.
2.5. EHR-based phenome-wide association studies of PTSD vulnerability and resilience
We conducted two parallel EHR-based PheWAS in UKB. First, one for PTSD vulnerability (vulnerable vs. equal-risk non-vulnerable individuals) and second, for PTSD resilience (resilient vs. equal-risk non-resilient individuals), applying logistic regression models where the phecode was the independent and vulnerability or resilience status was the dependent variable. Vulnerability or resilience status was coded 1, while non-vulnerable or non-resilient was coded 0. We also included sex, age, birth year, recruitment centre, Townsend Deprivation Index (as a proxy for socioeconomic status, SES), and within-ancestry genetic PCs 1–10 as covariates. False discovery rate (FDR) correction was applied for the number of phecodes tested in each PheWAS (FDR q<0.05).
2.6. Elastic net regression to investigate PTSD vulnerability and resilience
To investigate PTSD vulnerability and resilience based on their co-phenomes, we used glmnet R package (Friedman et al., 2010) to apply elastic net regression (ENR) models due to the ability to select relevant features while handling multicollinearity (Lebovitch et al., 2021). The ENR models combined L1 (lasso) and L2 (ridge) penalties, setting alpha=0.5. UKB cohort was divided into training and testing samples (75% and 25%, respectively). Phecodes showing nominally significant associations (p<0.05) in the PheWAS were entered in the ENR models. As mentioned, vulnerability and resilience analyses were tested separately (i.e., vulnerability-associated phecodes were entered in the ENR model for vulnerability prediction and resilience-associated phecodes were entered in the ENR model for resilience prediction).
2.7. Phenotypic risk scoring
A phenotype risk score (PheRS) represents an individual-level estimate of the likelihood of a trait derived from EHR data (Lebovitch et al., 2021). In the present study, PheRS were calculated as the weighted sum of the co-phenome, where weights were the elastic net beta-values issued from the vulnerability or resilience PheWAS. Specifically, the PheRS formula was: , where Np is the number of phecodes associated with the trait of interest, xi,p is 0 if the phecode was not related to the phenotype of interest, and wp is the elastic net beta-value (Wendt et al., 2022).
2.8. Testing UKB-derived PheRS scores in All of Us Research Program
After defining PheRSvulnerability and PheRSresilience in the UKB cohort, we tested them in AoU participants. Considering both UKB and AoU cohorts, 176 and 144 shared phecodes were available for the EHR-based PheWAS of PTSD vulnerability and resilience analysis. To perform PheRS PheWAS, phecodes with more than 100 cases in AoU were investigated, excluding the phecodes applied to calculate PheRS. This included 950 and 936 phecodes for the PheWAS of PheRSvulnerability and PheRSresilience, respectively. In each PheWAS, we tested each phecode as the dependent variable and the PheRS as the independent variable. Sex, age, income (as a proxy for SES) and within-ancestry genetic PCs 1–10 were included in the logistic regression models as covariates. Differently from the discovery analysis in UKB cohort, we did not include birth year as a covariate to maximize the statistical power of the analysis performed in AoU cohort. FDR correction was applied to adjust for multiple testing across phecodes tested in each PheWAS (FDR q<0.05). The correlation between PheRSvulnerablility and PheRSresilience was calculated by Spearman’s rank correlation coefficient. Because no information regarding trauma burden and PTSD symptom severity is available in the AoU cohort, we could not calculate PTSD vulnerability and resilience phenotypes to further test the PheRS. However, to assess their PheRS transferability in an independent cohort, we tested PTSD vulnerability and resilience PheRS with respect to phecodes not entered as inputs in the ENR models.
3. Results
Considering information regarding PTSD symptom severity, trauma burden, and PTSD PRS distribution in 60,314 trauma-exposed UKB participants, we defined 1,232 vulnerable and 16,543 equal-risk non-vulnerable subjects of European ancestry. In resilience analysis, we classified 7,990 resilient and 1,833 equal-risk non-resilient subjects of European descent that were investigated in the PTSD resilience analysis. In UKB participants of African descent, we classified 77 resilient individuals, 19 equal-risk non-resilient subjects, 15 vulnerable individuals, and 108 equal-risk non-vulnerable subjects. Because of the limited sample size in the African ancestry sample, we could not investigate phecode associations and derive African-specific PheRS.
After applying FDR multiple-testing correction (FDR q<0.05), we identified three phecodes associated with PTSD vulnerability and five phecodes associated with PTSD resilience (Fig. 2, Supplementary Table 4 and 5). Two phecodes were significantly associated with both traits, showing opposite effect directions. “Type 2 diabetes” (phecode 250.2) and “Sleep disorders” (phecode 327) were both positively associated with vulnerability (beta=0.56, p=6.43×10−4 and beta=0.72, p=2.65×10−5, respectively) and inversely associated with resilience (beta=−0.40, p=0.001 and beta=−0.49, p=0.005, respectively). “Cerebral artery occlusion, with cerebral infarction” was specifically associated with PTSD vulnerability (phecode 433.21 beta=0.95, p=8.2×10−4), while resilience-associated phecodes comprised “Irritable Bowel Syndrome” (phecode 564.1 beta=−0.46, p=3.44×10−5), “Spondylosis and allied disorders” (phecode 721 beta=−0.43, p=4.82×10−4) and “Migraine” (phecode 340, beta=−0.40, p=7.96×10−4).
Figure 2. Electronic Health Record (EHR)-based phenome-wide association studies of PTSD vulnerability (panel A) and resilience (panel B) in trauma-exposed UK Biobank participants.
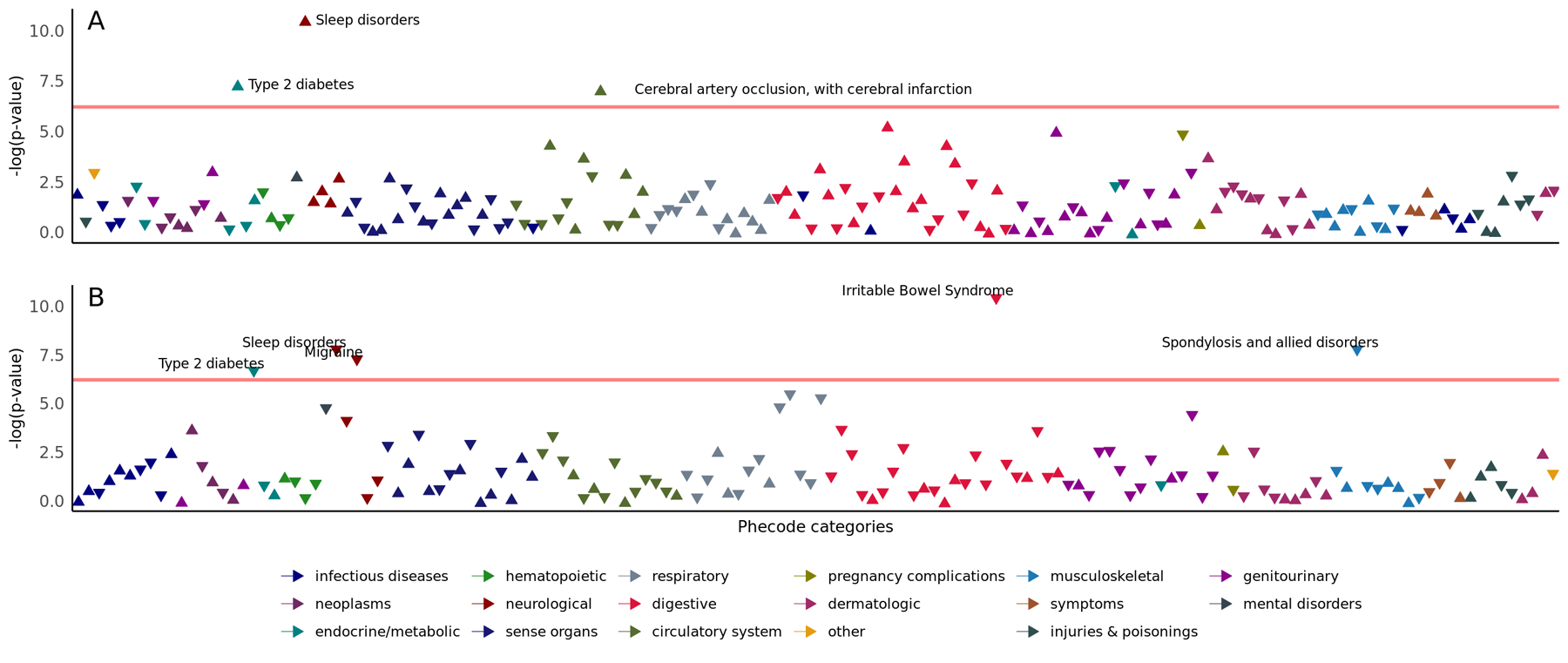
Arrows pointing upwards represent positive associations, while arrows pointing downwards represent negative associations. Each logistic regression model included vulnerability or resilience status as the dependent variable, phecodes as the independent variable, and included sex, age, Townsend deprivation index, birth year, recruitment centre and genetically derived principal components 1–10 as covariates. Phecodes are displayed by their estimate on a negative logarithmic scale of p-value on the y-axis, and phecode categories are represented on the x-axis. The red horizontal line indicates a significant threshold at FDR q<0.05. Significant associations are annotated only.
Based on the PheWAS results and the optimal feature inclusion settings, 14 phecodes were selected in the vulnerability-specific ENR model (Supplementary Table 6) and 15 phecodes in the resilience-specific ENR model (Supplementary Table 8). Three phecodes were included in both models but displayed orthogonal effects (Supplementary Table 9). In the UKB testing sample, PheRSvulnerability was significantly higher in vulnerable individuals than in equal-risk non-vulnerable sample (meanvulnerable=0.14, meannon-vulnerable=0.09, t-test=3.5, p=3.7×10−4). Similarly, PheRSresilience was significantly higher in resilient sample than equal-risk non-resilient subjects (meanresilient=−0.15, meannon-resilient=−0.27, t-test=5.8, p=9.2×10−9). Consistent results were observed when normalizing PheRS distributions using different procedures and comparing samples using a non-parametric test (Supplementary Table 9). To avoid possible biases due to the PheRS distributions, the subsequent analyses considered variables normalized by applying quantile normalization (Supplementary Figures 7 and 8). ENR prediction performance for PTSD vulnerability showed mean absolute error=0.13, mean squared error=0.066, and root mean squared error=0.26. ENR prediction performance for PTSD resilience revealed mean absolute error=0.29, mean squared error =0.15 and root mean squared error =0.38.
In AoU, PheRSvulnerability and PheRSresilience were calculated in 16,193 and 17,385 individuals of Europen descent, respectively, representing the specific phecodes needed in the weight-generating ENR model. As mentioned above, we could not derive African-specific PheRS due to the limited sample size of this population group in our discovery cohort. Because performing cross-ancestry PheRS analysis can introduce biases when applied to minority groups [R], we decided to limit the analysis to AoU participants of European descent. In this population group, a partial inverse correlation was shown between PheRSvulnerability and PheRSresilience (Spearman’s ρ=−0.33), demonstrating that the phenotype scores capture two distinct aspects of PTSD psychopathology (Supplementary Table 13). After evaluating distinct normalization procedures for PheRS, we applied quantile normalization for the PheRS scores (Supplementary Figure 9 and 10). After multiple testing corrections (FDR q<0.05), the PheRSvulnerability and PheRSresilience PheWAS revealed associations to 35 and 17 distinct phecodes, respectively (Fig. 3, Supplementary Tables 10 and 11). There was no overlap among the FDR-significant associations identified in the two analyses. In the AoU cohort, PheRSvulnerability was positively associated with phecodes related to multiple health domains, including the circulatory system (e.g., phecode 426.21 “First degree AV block” beta=0.41, p=1.09×10−5), genitourinary (e.g., phecode 586.4 “Stricture/obstruction of ureter” beta=0.41, p=1.09×10−5), musculoskeletal (e.g., phecode 711.1 “Pyogenic arthritis” beta=0.48, p=4.56×10−4), endocrine/metabolic (phecode 279.8 “Other specified disorders involving the immune mechanism” beta=0.4, p=5.15×10−4), respiratory (phecode 506 “Empyema and pneumothorax” beta=0.41, p=5.57×10−4), sense organs (phecode 376 “Disorders of the orbit” beta=1.73, p=0.001), and neoplasms (phecode 189.2 “Cancer of bladder” beta=0.72, p=0.001). Beyond positive associations, PheRSvulnerability showed an inverse relationship with 13 phecodes. Eleven of them were related to the upper gastrointestinal tract with the top associations being phecode 353.2 “Atrophic gastritis” (beta=−0.50, p=2.8×10−16), phecode 530.3 “Stricture and stenosis of esophagus” (beta=−0.68, p=2.4×10−13) and phecode 530.2 “Esophageal bleeding (varices/hemorrhage) (beta=−0.55, p=1.1×10−12). There was a statistically significant overrepresentation of phecodes related to the digestive system among FDR-significant PheRSvulnerability associations (enrichment=2.93, p=5.9×10−4, Supplementary Table 12). The results were consistent (Supplementary Table 9) by applying Dunn’s test, a non-parametric test that is not affected by PheRS distribution.
Figure 3. Phenome-wide association studies of PTSD vulnerability and resilience phenotypic risk scores (PheRS; Panels A and B, respectively) in the All of Us Research Program cohort.
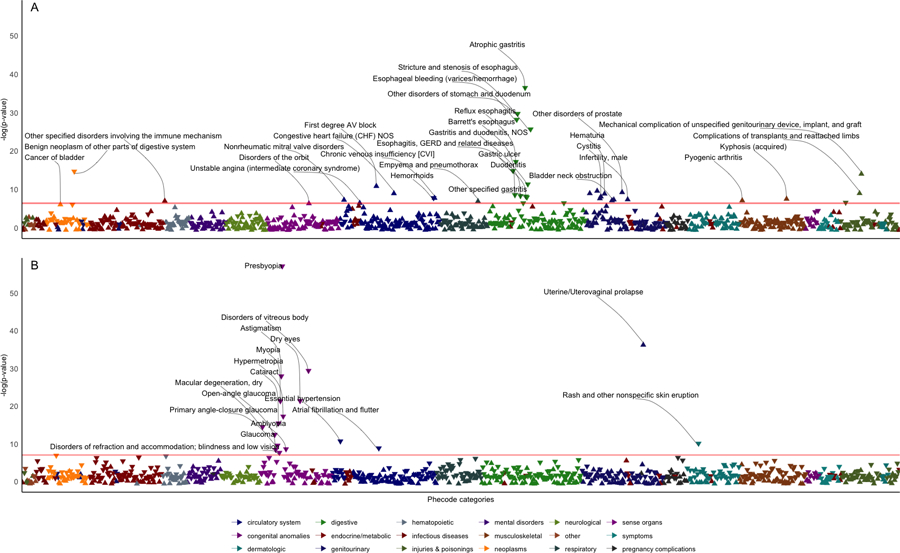
Arrows pointing upwards represent positive associations, while arrows pointing downwards represent negative associations. Each logistic regression model included phecode as the dependent variable, PheRSvulnerability (A) or PheRSresilience (B) as the independent variable and sex, age, income, and within-ancestry genetically derived principal components 1–10 as covariates. Phecodes are displayed by their estimate on a negative logarithmic scale of p-value on the y-axis, and phecode categories are represented on the x-axis. The red horizontal line indicates a significant threshold at FDR q<0.05. Labels are reported only for FDR-significant associations.
In the AoU cohort, PheRSresilience was associated with 17 phecodes after FDR multiple testing corrections (FDR q<0.05, Supplementary Table 11). Among inverse associations (i.e., i.e., higher PheRSresilience was associated with less adverse health outcomes - that is, resilience to PTSD was associated with resilience to these other traits as well), thirteen of them were related to eye conditions and disorders with the most significant results being phecode 367.4 “Presbyopia” (beta=−0.56, p=3.2×10−25), phecode 379.2 “Disorders of vitreous body” (beta=−0.47, p=1.0×10−16) and phecode 367.2 “Astigmatism” (beta=−0.39, p=3.3×10−16). Other phecodes negatively associated with PheRSresilience included phecodes related to the skin (phecode 687.1 “Rash and other nonspecific skin eruption”, beta=−0.17, p=7.9×10−5) and the circulatory system (phecode 401.1 “Essential hypertension” beta=−0.17, p=4.3×10−5; phecode 427.2 “Atrial fibrillation and flutter” beta=−0.85, p=2.7×10−4). While these phecodes showed an inverse relationship with PheRSresilience, we also observed a positive FDR-significant association between PheRSresilience and phecode 618.2 “Uterine/Uterovaginal prolapse” (beta=1.23, p=1.0×10−16). Because of the associations with eye conditions and disorders, PheRSresilience FDR-significant results were enriched for phecodes related to sense organs (enrichment=9.67, presilience=3.09×10−12). Non-parametric analysis via Dunn’s test confirmed the robustness of the PheRSresilience associations (Supplementary Table 9).
4. Discussion
In this hypothesis-free data-driven investigation, we investigated a total of 156,075 participants from two cohorts, developing a novel strategy to i) define PTSD resilience and vulnerability, ii) assess their co-phenomes, and iii) test the association of weighted co-phenome scores with an independent set of phecodes. In line with known PTSD comorbidities (Hicks et al., 2023), the analysis of PTSD resilience and vulnerability in UKB and AoU cohorts revealed significant associations with multiple health outcomes. While most of the associations showed directions in line with PTSD expected comorbidities (PTSD resilience associated with less adverse outcomes, PTSD vulnerability associated with more adverse outcomes), we observed different association patterns highlighting that PTSD resilience should not be viewed as the inverse of PTSD vulnerability, but rather, these are two distinct aspects reflecting the complexity of PTSD psychopathology.
In UKB, our EHR PheWAS analysis revealed multiple associations. Type 2 diabetes and sleep disorders were associated with both PTSD vulnerability and resilience with effect directions in line with previous research (i.e., positive association with PTSD vulnerability and an inverse relationship with PTSD resilience) (Cowdin et al., 2014; Germain et al., 2017; Roberts et al., 2015; Scherrer et al., 2019; Vaccarino et al., 2014). There is consistent comorbidity between PTSD and type 2 diabetes, supported by multiple study designs (Roberts et al., 2015; Scherrer et al., 2019; Vaccarino et al., 2014). Similarly, prior research has shown a bidirectional relationship between PTSD and sleep disturbances (Cowdin et al., 2014; Germain et al., 2017).
Beyond shared associations, we also observed distinct relationships. In particular, PTSD vulnerability was explicitly positively associated with “Cerebral artery occlusion, with cerebral infarction”. There is a known association between PTSD and stroke-related outcomes pointing to different dynamics, including the role of PTSD in biological processes related to cerebral ischaemia injuries (Polopalli et al., 2022), the convergence between PTSD and stroke risk factors (Perkins et al., 2021), the development of PTSD symptoms after stroke events (Ben Assayag et al., 2022), and the impact of PTSD in increasing transient ischemic attack and ischemic stroke later in life (Rosman et al., 2019). In this context, our findings contribute to pointing to a unique aspect of PTSD vulnerability, not shared with PTSD resilience, that could play an important role in explaining the complex interplay between PTSD psychopathology and cerebral infarction risk. Conversely, PTSD resilience showed specific inverse associations with irritable bowel syndrome, spondylosis-related disorders, and migraine. PTSD relationship with irritable bowel syndrome has been confirmed by a meta-analysis including 648,375 subjects, highlighting the role of shared pathophysiology and disease management (Ng et al., 2019). The relationship between PTSD and spondylosis-related disorders has been previously linked to autoimmune processes (Lee et al., 2016). Epidemiological studies highlighted a high prevalence of PTSD among patients with migraine, suggesting its role as a potential risk factor (Minen et al., 2016; Zarei et al., 2016). Our study expands further on these previous findings, suggesting a direct relationship with PTSD resilience.
In the AoU cohort, the PheRS PheWAS revealed associations with multiple traits. Interesting associations with PheRSresilience included its inverse relationship with essential hypertension and atrial flutter, which is in line with the known comorbidity between PTSD and cardiovascular diseases (Edmondson & von Känel, 2017). A possible explanation of the association to resilience and not vulnerability, given previous association to PTSD, could be due to the heterogeneous nature of observed PTSD associations. Additionally, we observed an inverse association of PheRSresilience with multiple eye conditions. Previous studies revealed a higher PTSD prevalence in individuals with vision impairments (Bonsaksen et al., 2022). Also, a study of pupillometry in PTSD cases, trauma-exposed controls (PTSD resilient) and controls demonstrated an imbalance in the autonomous nervous system, revealing significant differences in pupil accommodation in the form of reduced parasympathetic activity (pupil construction) and increased sympathetic activity (pupil dilution) (McKinnon et al., 2020). Indeed, reviews have illustrated that stress could be both a cause and consequence of vision loss (Sabel et al., 2018). Interestingly, none of the eye conditions associated with PheRSresilience was associated with PheRSvulnerability (despite all being included), giving indications that the association is resilience-specific, distinguishing resilient individuals from PTSD cases with high trauma burden and PRS. Although speculative, the association might have a connection to already established and empirically validated PTSD treatment, namely eye movement desensitization and reprocessing therapy. For this approach, a key element is a stimulus of bilateral eye movement, usually by blinking lights, while the patients focus on the trauma memory. The therapy is effective in reducing PTSD and trauma symptoms, although the mechanism is not fully understood (Wilson et al., 2018). Our findings may support further analyses to understand whether there is a relationship between eye movement desensitization and reprocessing therapy and PTSD resilience.
In line with the broad impact of PTSD on human health (Hicks et al., 2023), PheRSvulnerability was associated with increased odds of several adverse health outcomes related to a wide range of medical domains. This contributes to the strength of the direct implication of PTSD pathogenesis on multiple somatic conditions. However, unexpectedly, PheRSvulnerability was also inversely related to several gastrointestinal outcomes. This appears to contradict the well-reported link between chronic stress and gastrointestinal disorders (Crawford et al., 2023; Sun et al., 2018; Yamasaki et al., 2017). However, previous studies did not control for trauma burden and genetic risk. The negative association between upper gastrointestinal disorders and PheRSvulnerability might be explained by distinct gastrointestinal changes specific for trauma-exposed controls and PTSD cases with high genetic risk.
Our study has several limitations. Firstly, our study focused on only five trauma subtypes retrospectively measured from UKB due to availability, which could induce ascertainment imprecision for who is defined as vulnerable and resilient. Additionally, a specific trauma subtype could only be reported once. Second, the number of reported potentially traumatic life events was set as a proxy measure for trauma severity. Given that the experienced trauma severity is highly subjective, this proxy measure might not necessarily fully capture the trauma severity. Third, we did not investigate the phenome before versus after trauma exposure, thus our analysis cannot elucidate the phecodes related to the traumatic event. Fourth, neither UKB nor AoU analyses identified phecodes related to mental health. This could be due to the fact that our discovery analysis included only one mental health phecode (i.e., phecode 316, “Substance addiction and disorders”). While this was associated with PheRSvulnerability and PheRSresilience in the expected direction (beta=0.563 and −0.501, respectively), UKB and AoU EHR have limited our ability to investigate the relationship of PTSD vulnerability and resilience with other psychiatric and behavioral outcomes. Further studies may need to use data sources more informative for mental health research. Fifth, the current results are based on participants of European descent and may not translate to individuals of diverse ancestral backgrounds. Because of possible biases due to genetic differences and health disparities affecting minority groups, we decided to not perform cross-ancestry PheRS analyses. More powerful datasets informative of diverse populations will be needed to translate our approach across ancestry groups. Sixth, while we limited our analysis to individuals of European descent, differences between UKB and AoU recruitment strategies and UK-US sociocultural factors likely limited the transferability of PheRSvulnerability and PheRSresilience and contributed to some of the unexpected results observed in AoU cohort.
In conclusion, this study contributes to dissecting the complexity of PTSD pathogenesis. Specifically, we demonstrated that PTSD resilience and vulnerability can be distinguished using information regarding trauma burden and polygenic risk and that EHR data can be informative to characterize the different patterns of physical health comorbidities.
Supplementary Material
Highlights.
PTSD vulnerability and resilience are associated with physical health outcomes
The inverse correlation between PTSD vulnerability and resilience is only partial
Trauma burden and polygenic risk can distinguish PTSD vulnerability and resilience
Aknowledgements
We want to thank all participants from the UK Biobank and the All of Us Research Program for their study participation. This study was funded by the University of Bergen for International Training Grant (SL); grants RF1MH132337 and R33DA047527 from the National Institutes of Health (RP), OneMind (RP), and MERIT grant I01CX001849 from the US Department of Veterans Affairs (JG) and the VA National Center for PTSD Research (CO). The All of Us Research Program is supported by the National Institutes of Health, Office of the Director: Regional Medical Centers: 1 OT2 OD026549; 1 OT2 OD026554; 1 OT2 OD026557; 1 OT2 OD026556; 1 OT2 OD026550; 1 OT2 OD 026552; 1 OT2 OD026553; 1 OT2 OD026548; 1 OT2 OD026551; 1 OT2 OD026555; IAA #: AOD 16037; Federally Qualified Health Centers: HHSN 263201600085U; Data and Research Center: 5 U2C OD023196; Biobank: 1 U24 OD023121; The Participant Center: U24 OD023176; Participant Technology Systems Center: 1 U24 OD023163; Communications and Engagement: 3 OT2 OD023205; 3 OT2 OD023206; and Community Partners: 1 OT2 OD025277; 3 OT2 OD025315; 1 OT2 OD025337; 1 OT2 OD025276. The funding sources were not involved in the study design or analysis at any stage.
Footnotes
Declaration of competing interest
Dr. Polimanti received a research grant from Alkermes for the present study. Drs. Polimanti and Gelernter received honorariums for editorial work in the journal Complex Psychiatry. The other authors have no conflicts of interest to declare.
Data availability
Data generated in the present study are included in the article and its supplemental material. Individual-level data from UK Biobank and All of Us Research Program are available upon application.
References
- Ben Assayag E, Tene O, Korczyn AD, Solomon Z, Bornstein NM, Shenhar-Tsarfaty S, Seyman E, Niry D, Molad J, & Hallevi H (2022). Posttraumatic Stress Symptoms After Stroke: The Effects of Anatomy and Coping Style. Stroke, 53(6), 1924–1933. 10.1161/strokeaha.121.036635 [DOI] [PubMed] [Google Scholar]
- Bonsaksen T, Brunes A, & Heir T (2022). Post-Traumatic Stress Disorder in People with Visual Impairment Compared with the General Population. Int J Environ Res Public Health, 19(2). 10.3390/ijerph19020619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bycroft C, Freeman C, Petkova D, Band G, Elliott LT, Sharp K, Motyer A, Vukcevic D, Delaneau O, O’Connell J, Cortes A, Welsh S, Young A, Effingham M, McVean G, Leslie S, Allen N, Donnelly P, & Marchini J (2018). The UK Biobank resource with deep phenotyping and genomic data. Nature, 562(7726), 203–209. 10.1038/s41586-018-0579-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi KW, Stein MB, Dunn EC, Koenen KC, & Smoller JW (2019). Genomics and psychological resilience: a research agenda. Mol Psychiatry, 24(12), 1770–1778. 10.1038/s41380-019-0457-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SW, & O’Reilly PF (2019). PRSice-2: Polygenic Risk Score software for biobank-scale data. Gigascience, 8(7). 10.1093/gigascience/giz082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowdin N, Kobayashi I, & Mellman TA (2014). Theta frequency activity during rapid eye movement (REM) sleep is greater in people with resilience versus PTSD. Exp Brain Res, 232(5), 1479–1485. 10.1007/s00221-014-3857-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford DHG, Mellor R, Duenow P, & Connelly LB (2023). The impact of posttraumatic stress disorder on upper gastrointestinal investigations in Australian Defence Force veterans: a retrospective review. Internal Medicine Journal, 53(5), 841–844. 10.1111/imj.16088 [DOI] [PubMed] [Google Scholar]
- Denny JC, Rutter JL, Goldstein DB, Philippakis A, Smoller JW, Jenkins G, & Dishman E (2019). The “All of Us” Research Program. N Engl J Med, 381(7), 668–676. 10.1056/NEJMsr1809937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmondson D, & von Känel R (2017). Post-traumatic stress disorder and cardiovascular disease. Lancet Psychiatry, 4(4), 320–329. 10.1016/s2215-0366(16)30377-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman J, Hastie T, & Tibshirani R (2010). Regularization Paths for Generalized Linear Models via Coordinate Descent. J Stat Softw, 33(1), 1–22. [PMC free article] [PubMed] [Google Scholar]
- Ge T, Chen CY, Ni Y, Feng YA, & Smoller JW (2019). Polygenic prediction via Bayesian regression and continuous shrinkage priors. Nat Commun, 10(1), 1776. 10.1038/s41467-019-09718-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germain A, McKeon AB, & Campbell RL (2017). Sleep in PTSD: Conceptual model and novel directions in brain-based research and interventions. Curr Opin Psychol, 14, 84–89. 10.1016/j.copsyc.2016.12.004 [DOI] [PubMed] [Google Scholar]
- Hicks EM, Niarchou M, Goleva S, Kabir D, Ciarcia J, Smoller JW, Davis LK, Nievergelt CM, Koenen KC, Huckins LM, & Choi KW (2023). Comorbidity Profiles of Posttraumatic Stress Disorder Across the Medical Phenome. medRxiv. 10.1101/2023.08.25.23294572 [DOI] [Google Scholar]
- Hyppönen E, Mulugeta A, Zhou A, & Santhanakrishnan VK (2019). A data-driven approach for studying the role of body mass in multiple diseases: a phenome-wide registry-based case-control study in the UK Biobank. Lancet Digit Health, 1(3), e116–e126. 10.1016/s2589-7500(19)30028-7 [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, & Walters EE (2005). Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry, 62(6), 593–602. 10.1001/archpsyc.62.6.593 [DOI] [PubMed] [Google Scholar]
- Koenen KC, Ratanatharathorn A, Ng L, McLaughlin KA, Bromet EJ, Stein DJ, Karam EG, Meron Ruscio A, Benjet C, Scott K, Atwoli L, Petukhova M, Lim CCW, Aguilar-Gaxiola S, Al-Hamzawi A, Alonso J, Bunting B, Ciutan M, de Girolamo G, Degenhardt L, Gureje O, Haro JM, Huang Y, Kawakami N, Lee S, Navarro-Mateu F, Pennell BE, Piazza M, Sampson N, Ten Have M, Torres Y, Viana MC, Williams D, Xavier M, & Kessler RC (2017). Posttraumatic stress disorder in the World Mental Health Surveys. Psychol Med, 47(13), 2260–2274. 10.1017/s0033291717000708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebovitch DS, Johnson J,S, Dueñas HR, & Huckins LM. (2021). Phenotype Risk Scores: moving beyond ‘cases’ and ‘controls’ to classify psychiatric disease in hospital-based biobanks. medRxiv, 2021.2001.2025.21249615. 10.1101/2021.01.25.21249615 [DOI] [Google Scholar]
- Lee YC, Agnew-Blais J, Malspeis S, Keyes K, Costenbader K, Kubzansky LD, Roberts AL, Koenen KC, & Karlson EW (2016). Post-Traumatic Stress Disorder and Risk for Incident Rheumatoid Arthritis. Arthritis Care Res (Hoboken), 68(3), 292–298. 10.1002/acr.22683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinnon AI, Gray NS, & Snowden RJ (2020). Enhanced emotional response to both negative and positive images in post-traumatic stress disorder: Evidence from pupillometry. Biological Psychology, 154, 107922. 10.1016/j.biopsycho.2020.107922 [DOI] [PubMed] [Google Scholar]
- Minen MT, Begasse De Dhaem O, Kroon Van Diest A, Powers S, Schwedt TJ, Lipton R, & Silbersweig D (2016). Migraine and its psychiatric comorbidities. J Neurol Neurosurg Psychiatry, 87(7), 741–749. 10.1136/jnnp-2015-312233 [DOI] [PubMed] [Google Scholar]
- Ng QX, Soh AYS, Loke W, Venkatanarayanan N, Lim DY, & Yeo WS (2019). Systematic review with meta-analysis: The association between post-traumatic stress disorder and irritable bowel syndrome. J Gastroenterol Hepatol, 34(1), 68–73. 10.1111/jgh.14446 [DOI] [PubMed] [Google Scholar]
- Nievergelt CM, Maihofer AX, Atkinson EG, Chen CY, Choi KW, Coleman JRI, Daskalakis NP, Duncan LE, Polimanti R, Aaronson C, Amstadter AB, Andersen SB, Andreassen OA, Arbisi PA, Ashley-Koch AE, Austin SB, Avdibegovic E, Babic D, Bacanu SA, Baker DG, Batzler A, Beckham JC, Belangero S, Benjet C, Bergner C, Bierer LM, Biernacka JM, Bierut LJ, Bisson JI, Boks MP, Bolger EA, Brandolino A, Breen G, Bressan RA, Bryant RA, Bustamante AC, Bybjerg-Grauholm J, Baekvad-Hansen M, Borglum AD, Borte S, Cahn L, Calabrese JR, Caldas-de-Almeida JM, Chatzinakos C, Cheema S, Clouston SAP, Colodro-Conde L, Coombes BJ, Cruz-Fuentes CS, Dale AM, Dalvie S, Davis LK, Deckert J, Delahanty DL, Dennis MF, Desarnaud F, DiPietro CP, Disner SG, Docherty AR, Domschke K, Dyb G, Kulenovic AD, Edenberg HJ, Evans A, Fabbri C, Fani N, Farrer LA, Feder A, Feeny NC, Flory JD, Forbes D, Franz CE, Galea S, Garrett ME, Gelaye B, Gelernter J, Geuze E, Gillespie CF, Goleva SB, Gordon SD, Goci A, Grasser LR, Guindalini C, Haas M, Hagenaars S, Hauser MA, Heath AC, Hemmings SMJ, Hesselbrock V, Hickie IB, Hogan K, Hougaard DM, Huang H, Huckins LM, Hveem K, Jakovljevic M, Javanbakht A, Jenkins GD, Johnson J, Jones I, Jovanovic T, Karstoft KI, Kaufman ML, Kennedy JL, Kessler RC, Khan A, Kimbrel NA, King AP, Koen N, Kotov R, Kranzler HR, Krebs K, Kremen WS, Kuan PF, Lawford BR, Lebois LAM, Lehto K, Levey DF, Lewis C, Liberzon I, Linnstaedt SD, Logue MW, Lori A, Lu Y, Luft BJ, Lupton MK, Luykx JJ, Makotkine I, Maples-Keller JL, Marchese S, Marmar C, Martin NG, Martinez-Levy GA, McAloney K, McFarlane A, McLaughlin KA, McLean SA, Medland SE, Mehta D, Meyers J, Michopoulos V, Mikita EA, Milani L, Milberg W, Miller MW, Morey RA, Morris CP, Mors O, Mortensen PB, Mufford MS, Nelson EC, Nordentoft M, Norman SB, Nugent NR, O’Donnell M, Orcutt HK, Pan PM, Panizzon MS, Pathak GA, Peters ES, Peterson AL, Peverill M, Pietrzak RH, Polusny MA, Porjesz B, Powers A, Qin XJ, Ratanatharathorn A, Risbrough VB, Roberts AL, Rothbaum AO, Rothbaum BO, Roy-Byrne P, Ruggiero KJ, Rung A, Runz H, Rutten BPF, de Viteri SS, Salum GA, Sampson L, Sanchez SE, Santoro M, Seah C, Seedat S, Seng JS, Shabalin A, Sheerin CM, Silove D, Smith AK, Smoller JW, Sponheim SR, Stein DJ, Stensland S, Stevens JS, Sumner JA, Teicher MH, Thompson WK, Tiwari AK, Trapido E, Uddin M, Ursano RJ, Valdimarsdottir U, Van Hooff M, Vermetten E, Vinkers CH, Voisey J, Wang Y, Wang Z, Waszczuk M, Weber H, Wendt FR, Werge T, Williams MA, Williamson DE, Winsvold BS, Winternitz S, Wolf C, Wolf EJ, Xia Y, Xiong Y, Yehuda R, Young KA, Young RM, Zai CC, Zai GC, Zervas M, Zhao H, Zoellner LA, Zwart JA, deRoon-Cassini T, van Rooij SJH, van den Heuvel LL, Study A, Estonian Biobank Research T, FinnGen I, Psychiatry HA-I, Stein MB, Ressler KJ, & Koenen KC (2024). Genome-wide association analyses identify 95 risk loci and provide insights into the neurobiology of post-traumatic stress disorder. Nat Genet. 10.1038/s41588-024-01707-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nievergelt CM, Maihofer AX, Klengel T, Atkinson EG, Chen CY, Choi KW, Coleman JRI, Dalvie S, Duncan LE, Gelernter J, Levey DF, Logue MW, Polimanti R, Provost AC, Ratanatharathorn A, Stein MB, Torres K, Aiello AE, Almli LM, Amstadter AB, Andersen SB, Andreassen OA, Arbisi PA, Ashley-Koch AE, Austin SB, Avdibegovic E, Babić D, Bækvad-Hansen M, Baker DG, Beckham JC, Bierut LJ, Bisson JI, Boks MP, Bolger EA, Børglum AD, Bradley B, Brashear M, Breen G, Bryant RA, Bustamante AC, Bybjerg-Grauholm J, Calabrese JR, Caldas-de-Almeida JM, Dale AM, Daly MJ, Daskalakis NP, Deckert J, Delahanty DL, Dennis MF, Disner SG, Domschke K, Dzubur-Kulenovic A, Erbes CR, Evans A, Farrer LA, Feeny NC, Flory JD, Forbes D, Franz CE, Galea S, Garrett ME, Gelaye B, Geuze E, Gillespie C, Uka AG, Gordon SD, Guffanti G, Hammamieh R, Harnal S, Hauser MA, Heath AC, Hemmings SMJ, Hougaard DM, Jakovljevic M, Jett M, Johnson EO, Jones I, Jovanovic T, Qin XJ, Junglen AG, Karstoft KI, Kaufman ML, Kessler RC, Khan A, Kimbrel NA, King AP, Koen N, Kranzler HR, Kremen WS, Lawford BR, Lebois LAM, Lewis CE, Linnstaedt SD, Lori A, Lugonja B, Luykx JJ, Lyons MJ, Maples-Keller J, Marmar C, Martin AR, Martin NG, Maurer D, Mavissakalian MR, McFarlane A, McGlinchey RE, McLaughlin KA, McLean SA, McLeay S, Mehta D, Milberg WP, Miller MW, Morey RA, Morris CP, Mors O, Mortensen PB, Neale BM, Nelson EC, Nordentoft M, Norman SB, O’Donnell M, Orcutt HK, Panizzon MS, Peters ES, Peterson AL, Peverill M, Pietrzak RH, Polusny MA, Rice JP, Ripke S, Risbrough VB, Roberts AL, Rothbaum AO, Rothbaum BO, Roy-Byrne P, Ruggiero K, Rung A, Rutten BPF, Saccone NL, Sanchez SE, Schijven D, Seedat S, Seligowski AV, Seng JS, Sheerin CM, Silove D, Smith AK, Smoller JW, Sponheim SR, Stein DJ, Stevens JS, Sumner JA, Teicher MH, Thompson WK, Trapido E, Uddin M, Ursano RJ, van den Heuvel LL, Van Hooff M, Vermetten E, Vinkers CH, Voisey J, Wang Y, Wang Z, Werge T, Williams MA, Williamson DE, Winternitz S, Wolf C, Wolf EJ, Wolff JD, Yehuda R, Young RM, Young KA, Zhao H, Zoellner LA, Liberzon I, Ressler KJ, Haas M, & Koenen KC (2019). International meta-analysis of PTSD genome-wide association studies identifies sex- and ancestry-specific genetic risk loci. Nat Commun, 10(1), 4558. 10.1038/s41467-019-12576-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak GA, Singh K, Choi KW, Fang Y, Kouakou MR, Lee YH, Zhou X, Fritsche LG, Wendt FR, Davis LK, & Polimanti R (2023). Genetic Liability to Posttraumatic Stress Disorder Symptoms and Its Association With Cardiometabolic and Respiratory Outcomes. JAMA Psychiatry. 10.1001/jamapsychiatry.2023.4127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins JD, Wilkins SS, Kamran S, & Shuaib A (2021). Post-traumatic stress disorder and its association with stroke and stroke risk factors: A literature review. Neurobiol Stress, 14, 100332. 10.1016/j.ynstr.2021.100332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polopalli S, Yetukuri AR, Danduga R, & Kola PK (2022). A prognostic study on the effect of post-traumatic stress disorder on cerebral ischaemia reperfusion-induced stroke. World J Biol Psychiatry, 23(2), 136–150. 10.1080/15622975.2021.1935318 [DOI] [PubMed] [Google Scholar]
- Roberts AL, Agnew-Blais JC, Spiegelman D, Kubzansky LD, Mason SM, Galea S, Hu FB, Rich-Edwards JW, & Koenen KC (2015). Posttraumatic stress disorder and incidence of type 2 diabetes mellitus in a sample of women: a 22-year longitudinal study. JAMA Psychiatry, 72(3), 203–210. 10.1001/jamapsychiatry.2014.2632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosman L, Sico JJ, Lampert R, Gaffey AE, Ramsey CM, Dziura J, Chui PW, Cavanagh CE, Brandt C, Haskell S, & Burg MM (2019). Posttraumatic Stress Disorder and Risk for Stroke in Young and Middle-Aged Adults: A 13-Year Cohort Study. Stroke, 50(11), 2996–3003. 10.1161/strokeaha.119.026854 [DOI] [PubMed] [Google Scholar]
- Sabel BA, Wang J, Cárdenas-Morales L, Faiq M, & Heim C (2018). Mental stress as consequence and cause of vision loss: the dawn of psychosomatic ophthalmology for preventive and personalized medicine. Epma j, 9(2), 133–160. 10.1007/s13167-018-0136-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherrer JF, Salas J, Norman SB, Schnurr PP, Chard KM, Tuerk P, Schneider FD, van den Berk-Clark C, Cohen BE, Friedman MJ, & Lustman PJ (2019). Association Between Clinically Meaningful Posttraumatic Stress Disorder Improvement and Risk of Type 2 Diabetes. JAMA Psychiatry, 76(11), 1159–1166. 10.1001/jamapsychiatry.2019.2096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultebraucks K, Choi KW, Galatzer-Levy IR, & Bonanno GA (2021). Discriminating Heterogeneous Trajectories of Resilience and Depression After Major Life Stressors Using Polygenic Scores. JAMA Psychiatry, 78(7), 744–752. 10.1001/jamapsychiatry.2021.0228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Wang S, Qi M, Wu M, Zhang D, Yang Y, Zhao X, Jia J, Cao F, Su Y, & Zhang S (2018). Psychological distress in patients with chronic atrophic gastritis: the risk factors, protection factors, and cumulative effect. Psychol Health Med, 23(7), 797–803. 10.1080/13548506.2018.1428756 [DOI] [PubMed] [Google Scholar]
- UK Biobank. (2019). First Occurrence of Health Outcomes Defined by 3-character ICD10 code. https://biobank.ndph.ox.ac.uk/ukb/ukb/docs/first_occurrences_outcomes.pdf2019 (accessed 12 June 2023). [Google Scholar]
- Vaccarino V, Goldberg J, Magruder KM, Forsberg CW, Friedman MJ, Litz BT, Heagerty PJ, Huang GD, Gleason TC, & Smith NL (2014). Posttraumatic stress disorder and incidence of type-2 diabetes: a prospective twin study. J Psychiatr Res, 56, 158–164. 10.1016/j.jpsychires.2014.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W-Q, Bastarache LA, Carroll RJ, Marlo JE, Osterman TJ, Gamazon ER, Cox NJ, Roden DM, & Denny JC (2017). Evaluating phecodes, clinical classification software, and ICD-9-CM codes for phenome-wide association studies in the electronic health record. PLOS ONE, 12(7), e0175508. 10.1371/journal.pone.0175508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendt FR, Pathak GA, Deak JD, De Angelis F, Koller D, Cabrera-Mendoza B, Lebovitch DS, Levey DF, Stein MB, Kranzler HR, Koenen KC, Gelernter J, Huckins LM, & Polimanti R (2022). Using phenotype risk scores to enhance gene discovery for generalized anxiety disorder and posttraumatic stress disorder. Mol Psychiatry, 27(4), 2206–2215. 10.1038/s41380-022-01469-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson G, Farrell D, Barron I, Hutchins J, Whybrow D, & Kiernan MD (2018). The Use of Eye-Movement Desensitization Reprocessing (EMDR) Therapy in Treating Post-traumatic Stress Disorder-A Systematic Narrative Review. Front Psychol, 9, 923. 10.3389/fpsyg.2018.00923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu P, Gifford A, Meng X, Li X, Campbell H, Varley T, Zhao J, Carroll R, Bastarache L, Denny JC, Theodoratou E, & Wei W-Q (2019). Mapping ICD-10 and ICD-10-CM Codes to Phecodes: Workflow Development and Initial Evaluation. JMIR Med Inform, 7(4), e14325. 10.2196/14325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Johnson JS, Signer R, Birgegård A, Jordan J, Kennedy MA, Landén M, Maguire SL, Martin NG, Mortensen PB, Petersen LV, Thornton LM, Bulik CM, & Huckins LM (2022). Exploring the clinical and genetic associations of adult weight trajectories using electronic health records in a racially diverse biobank: a phenome-wide and polygenic risk study. Lancet Digit Health, 4(8), e604–e614. 10.1016/s2589-7500(22)00099-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki T, Tomita T, Takimoto M, Kondo T, Tozawa K, Ohda Y, Oshima T, Fukui H, Watari J, & Miwa H (2017). Intravenous Corticotropin-releasing Hormone Administration Increases Esophageal Electrical Sensitivity in Healthy Individuals. J Neurogastroenterol Motil, 23(4), 526–532. 10.5056/jnm17067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarei MR, Shabani M, Chamani G, Abareghi F, Razavinasab M, & Nazeri M (2016). Migraine patients have a higher prevalence of PTSD symptoms in comparison to chronic tension-type headache and healthy subjects: a case-control study. Acta Odontol Scand, 74(8), 633–635. 10.1080/00016357.2016.1232435 [DOI] [PubMed] [Google Scholar]
- Zheng NS, Feng Q, Kerchberger VE, Zhao J, Edwards TL, Cox NJ, Stein CM, Roden DM, Denny JC, & Wei WQ (2020). PheMap: a multi-resource knowledge base for high-throughput phenotyping within electronic health records. J Am Med Inform Assoc, 27(11), 1675–1687. 10.1093/jamia/ocaa104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheutlin AB, Dennis J, Karlsson Linnér R, Moscati A, Restrepo N, Straub P, Ruderfer D, Castro VM, Chen CY, Ge T, Huckins LM, Charney A, Kirchner HL, Stahl EA, Chabris CF, Davis LK, & Smoller JW (2019). Penetrance and Pleiotropy of Polygenic Risk Scores for Schizophrenia in 106,160 Patients Across Four Health Care Systems. Am J Psychiatry, 176(10), 846–855. 10.1176/appi.ajp.2019.18091085 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data generated in the present study are included in the article and its supplemental material. Individual-level data from UK Biobank and All of Us Research Program are available upon application.


