Abstract
Importance
Early diagnosis of canine pancreatitis is challenging due to non-specific clinical signs. Currently, abdominal ultrasonography and measurement of canine pancreatic lipase (cPL) have been employed for the diagnosis of pancreatitis.
Objective
Many qualitative and quantitative commercial cPL tests have been developed and used in veterinary clinics. This study aimed to compare three different methodologies SNAP cPL, Spec cPL, and Vcheck cPL tests to assess the concordance of these assays.
Methods
Fifty serum samples were collected from 36 dogs with or without pancreatitis and subjected to SNAP cPL, Spec cPL, and Vcheck cPL tests. Agreement and correlation coefficients were calculated between the test results, and correlations were determined during the management of the patients.
Results
The results of the three cPL assays were strongly correlated in 47/50 serum samples (94%). Cohen’s kappa analysis between the Spec cPL and Vcheck cPL showed near perfect agreement (κ = 0.960, p < 0.001), SNAP cPL and Vcheck cPL (κ = 0.920, p < 0.001), and Spec cPL and SNAP cPL (κ = 0.880, p < 0.001). The correlation coefficients (r) between data from Spec cPL and Vcheck cPL tests was calculated by Spearman’s correlation test (r = 0.958, p < 0.001). Furthermore, the patterns of change in serum cPL concentrations determined using Spec cPL and Vcheck cPL were significantly consistent during the monitoring period in 11 patients.
Conclusions and Relevance
Our data illustrated that Spec cPL and Vcheck cPL tests are compatible for clinical use in the diagnosis and monitoring of canine pancreatitis.
Keywords: Canine pancreatitis, canine pancreatic lipase, SNAP cPL, Spec cPL, Vcheck cPL
INTRODUCTION
Pancreatitis is one of the most common disorders associated with pancreatic exocrine dysfunction in dogs [1]. For a proper diagnosis of pancreatitis, the patient’s clinical history, symptoms, abdominal ultrasonography, and laboratory test results should be collectively considered. Pancreatic biopsy is the gold standard for the diagnosis of pancreatitis [2]. However, tissue sampling procedure for biopsy is a major burden for both the examiner and patient. Thus, less-invasive diagnostic tests, including complete blood count (CBC), serum biochemistry profile, and abdominal ultrasonography have been utilized. However, these tests show low specificity in the diagnosis of canine pancreatitis [1].
Non-invasive serum biomarkers have been utilized for the diagnosis of canine pancreatitis [3,4,5]. Serum amylase and lipase activities have long been used; however, they are not specific for detecting spontaneous pancreatitis in dogs [6]. Serum trypsin-like immunoreactivity assay is the test of choice for exocrine pancreatic insufficiency but is not reliable for diagnosing canine pancreatitis [4,7]. Serum canine pancreatic lipase (cPL) is a lipase of pancreatic origin and gets elevated as a consequence of pancreatic acinar cell damage, suggesting a high specificity for exocrine pancreatic disorders [5]. Currently, serum cPL is a clinically useful biomarker in veterinary practice for diagnosing pancreatitis in dogs [8].
Different commercial cPL assays have been developed and used in veterinary fields [9]. SNAP cPL (IDEXX Laboratories Inc., USA) and Spec cPL (IDEXX Reference Laboratories, USA) are commonly used in veterinary clinics in South Korea. Both the assays were developed using dual monoclonal antibodies to capture and detect cPL [10,11]. The SNAP cPL kit is a point-of-care assay that can confirm the results in 10 min, but it only provides a qualitative, “normal” or “abnormal” result for pancreatitis. In contrast, the Spec cPL enzyme-linked immunosorbent assay (ELISA) provides quantitative data on pancreatic lipase levels; however, a relatively large amount of blood (1 mL of serum) is required for the assay. Moreover, it takes 2–3 days to obtain the results from the reference laboratory.
Recently, Vcheck cPL (Bionote Inc., Korea), a new commercial fluorescent immunoassay utilizing a specific anti-cPL antibody that binds to serum cPL was developed. This is a point-of-care assay that provides quantitative results using an in-house Vcheck analyzer (Bionote Inc.), which does not require sending the sample to a reference laboratory. Additionally, a very small amount of sample (25 μL of serum) is required for the assay.
This study compared the Vcheck cPL assay with other commercial cPL assays, such as SNAP cPL and Spec cPL. Samples from dogs with or without pancreatitis were subjected to the SNAP cPL, Spec cPL, and Vcheck cPL assays, and the results were compared to investigate the concordance and compatibility of these cPL measurement assays.
METHODS
Animals
Healthy client-owned dogs that visited the hospital for routine checkups, such as regular wellness tests, or routine physical examinations, were recruited as candidates for the control group. Dogs with clinical signs of anorexia, weakness, vomiting, abdominal pain, or other medical histories suggestive of pancreatitis were recruited for the test group. All dogs were recruited from March 2018 to September 2018 and owners signed consent for the use of leftover biological materials. This study was approved by the Institutional Animal Care and Use Committee (approval No. HACUC-2021003)
Data on history, signalments, clinical signs, CBC, serum chemistry including amylase and lipase, and C-reactive protein (CRP) using Vcheck Canine CRP (Bionote Inc.) were collected from all the candidates. Additionally, abdominal ultrasonography was performed, and the findings defining pancreatitis included hypoechoic areas within the pancreas, consistent with necrosis or fluid accumulation; hyperechoic areas of the pancreas, indicating the presence of pancreatic fibrosis; hyperechoic peripancreatic mesentery, suggesting necrosis of peripancreatic fat; an enlarged or irregular pancreas; dilation of the pancreatic or biliary duct; and abdominal effusion [12].
Analysis of Serum cPL Concentration
Three types of assays (SNAP cPL, Spec cPL, and Vcheck cPL) were used to measure serum cPL concentrations. The serum samples were stored at −80°C freezer before analysis, and were divided into three compartments before analysis. One compartment of 1 mL serum was shipped frozen to a commercial laboratory (IDEXX Reference Laboratories) for Spec cPL ELISA. SNAP cPL and Vcheck cPL were performed in-house, and all testing procedures were performed according to the respective manufacturers’ instructions.
For SNAP cPL measurement, three drops of the sample were thoroughly mixed with four drops of conjugate, and the entire mixture was poured into the sample well. The test result reflected in 10 min: if the color intensity of the sample spot was equal to or darker than the color intensity of the reference spot, the result was interpreted as abnormal. This abnormal interpretation may indicate > 200 ng/mL for Spec cPL [11]. If the color intensity of the sample spot was light, it was interpreted as normal.
Spec cPL results were provided by IDEXX Laboratories. The lower limit of detection was 30 ng/mL, and upper limit of detection was 2,000 ng/mL. According to the manufacturer’s instructions, Spec cPL concentration ≤ 200 ng/mL was interpreted as normal in this study. A concentration ≥ 400 ng/mL was consistent with pancreatitis, and concentrations between 201 and 399 ng/mL were equivocal for the diagnosis of pancreatitis in dogs.
For Vcheck cPL measurement, 25 μL of the serum was mixed with the buffer provided by the manufacturer, and 100 μL of the mixture was poured into the sample hole of the test device. Subsequently, Vcheck analyzer evaluated the density of the colored line and quantified the cPL concentration within 5 min. A Vcheck cPL result of < 200 ng/mL was within the reference interval i.e., normal; whereas a result of > 400 ng/mL was consistent with pancreatitis. Results ranging from 200–400 ng/mL were considered equivocal for the diagnosis of pancreatitis in dogs. The lower limit of detection was 50 ng/mL, and upper limit of detection was 2,000 ng/mL.
Clinical grouping
The candidates were retrospectively assigned to two groups (control and test) by a panel comprising two each of veterinary clinicians and radiologists. Results of CBC, serum chemistry, and abdominal ultrasonography were also used to define pancreatitis [1,13].
The final assignment of the test group was made after the analysis of serum cPL levels. Besides the abnormal clinical signs and above mentioned test results, the dog was diagnosed with pancreatitis and assigned to the test group if cPL levels of > 200 ng/mL were detected by Spec cPL. If the dog had clinical signs of pancreatitis and results of other tests but showed negative results in Spec cPL, it was subjected to further tests and a panel discussion for the final diagnosis. The panel was composed of four veterinarians who had Ph.D. degree in veterinary radiation or veterinary internal medicine, or had been working as a clinical veterinarian more than 10 years. Because of the relatively low sensitivity of abdominal ultrasonography in canine pancreatitis [1], the panel discussion was made based on the findings, including history, dietary or medications, clinical signs, predisposing factors such as hypertriglyceridemia, hypovolemia-induced ischemia, laboratory test results including Spec cPL analysis, in addition to supportive evidence of abdominal ultrasound examination [14]. A dog with history of steroid administration and/or hyperadrenocorticism was excluded because endogenous and exogenous corticosteroids can affect the level of cPL concentrations [15].
If renal, hepatic, or gastric disorders or systemic diseases were suspected, an additional diagnosis was made, and previously diagnosed diseases under management were recorded as concurrent diseases. Once a dog was assigned to the test group, it was allowed to visit the veterinary hospital again for follow-up and prognosis evaluation.
Statistical analysis
For statistical analysis, the results from SNAP cPL, Spec cPL, and Vcheck cPL tests were assigned to a score “0” when each test results were negative by SNAP cPL or below the threshold (< 200 ng/mL) by Spec cPL and Vcheck cPL. Further, a score “1” was assigned when each test result was positive by SNAP cPL or over the threshold (≥ 200 ng/mL) by Spec cPL and Vcheck cPL. The comparing continuous data obtained from the quantitative results from Spec cPL and Vcheck cPL. To represent the data on a graph, results of < 30 ng/mL or < 50 ng/mL were recorded as 30 ng/mL and 50 ng/mL in Spec cPL and Vcheck cPL, respectively. Additionally, results > 2,000 ng/mL in Spec cPL and Vcheck cPL were recorded as 2,000 ng/mL.
The normality of all the scores and data was analyzed using the Kolmogorov-Smirnov test. The scored results from SNAP cPL, Spec cPL and Vcheck cPL tests were assessed for agreement using Cohen’s kappa analysis. The strength of agreement was interpreted as follows: κ < 0.1, agreement equivalent to chance; 0.1–0.2, slight agreement; 0.21–0.4, fair agreement; 0.41–0.6, moderate agreement; 0.61–0.8, substantial agreement; 0.81–0.99, near perfect agreement and 1, perfect agreement [16]. The correlation between the quantitative data from Spec cPL and Vcheck cPL tests was determined by Spearman’s correlation test. The calculated correlation coefficients (r) were generated from 0–1, and higher r indicated greater agreement between the two tests. Statistical analysis and the generating graphs were performed using IBM SPSS (SPSS statistics 22; IBM SPSS Statistics Inc., USA).
RESULTS
Animals of the control group
Median age and weight of the 20 client-owned dogs assigned to the control group were 9.5 years and 4.8 Kg, respectively (Table 1). The control group was defined after Spec cPL analysis (Table 2). All dogs in the control group were normal in all the three cPL assays used in this study, except for one (patient No. C-10), a 14-year-old spayed female Cocker Spaniel that showed abnormal results on the SNAP cPL test, but normal results on the Spec cPL and Vcheck cPL assays (157 and 167 ng/mL, respectively). Additionally, no abnormal test results, signs of pancreatitis, or other diseases were observed in this group.
Table 1. Characteristics of the dogs in this study.
| Parameters | Control | Test | Total | |
|---|---|---|---|---|
| Number of dogs | 20 | 16 | 36 | |
| Sex | ||||
| Intact male | 2 | 1 | 3 | |
| Neutered male | 4 | 7 | 11 | |
| Intact female | 3 | 2 | 5 | |
| Spayed female | 11 | 6 | 17 | |
| Breed | ||||
| Beagle | 0 | 1 | 1 | |
| Boston Terrier | 0 | 1 | 1 | |
| Border Colie | 0 | 1 | 1 | |
| Cocker Spaniel | 2 | 1 | 3 | |
| Dachshund | 1 | 0 | 1 | |
| Chihuahua | 1 | 0 | 1 | |
| Jindo | 0 | 1 | 1 | |
| Maltese | 6 | 1 | 7 | |
| Miniature Schnauzer | 1 | 1 | 2 | |
| Miniature Poodle | 2 | 3 | 5 | |
| Miniature Pinscher | 0 | 1 | 1 | |
| Mixed | 2 | 1 | 3 | |
| Pomeranian | 1 | 1 | 2 | |
| Shih Tzu | 3 | 0 | 3 | |
| Yorkshire Terrier | 1 | 3 | 4 | |
| Age (yr) | ||||
| Min–Max | 1–14 | 5–17 | 1–17 | |
| Median | 9.5 | 10 | 10 | |
| Weight (kg) | ||||
| Min–Max | 1.8–13.2 | 1.2–24.8 | 1.2–24.8 | |
| Median | 4.8 | 5.6 | 5.1 | |
Table 2. Serum cPL analysis, abdominal ultrasonography, and final diagnosis of the control and test groups.
| Patient No. | Hospital visitationa | Serum cPL analysisb | Ultrasonographic findingsc | Final diagnosisd | |||
|---|---|---|---|---|---|---|---|
| Spec cPL (ng/mL) | SNAP cPL | Vcheck cPL (ng/mL) | |||||
| Control group | |||||||
| C-1 | 1st | < 30 | Normal | < 50 | Normal | None | |
| C-2 | 1st | 52 | Normal | < 50 | Normal | None | |
| C-3 | 1st | 50 | Normal | < 50 | Normal | None | |
| C-4 | 1st | < 30 | Normal | < 50 | Normal | None | |
| C-5 | 1st | 46 | Normal | < 50 | Normal | None | |
| C-6 | 1st | 51 | Normal | < 50 | Normal | None | |
| C-7 | 1st | 46 | Normal | < 50 | Normal | None | |
| C-8 | 1st | < 30 | Normal | < 50 | Normal | None | |
| C-9 | 1st | 74 | Normal | 86 | Normal | None | |
| C-10 | 1st | 157 | Abnormal | 167 | Normal | None | |
| C-11 | 1st | 62 | Normal | 70 | Normal | None | |
| C-12 | 1st | 37 | Normal | < 50 | Normal | None | |
| C-13 | 1st | 63 | Normal | 98 | Normal | None | |
| C-14 | 1st | 78 | Normal | 117 | Normal | None | |
| C-15 | 1st | 63 | Normal | 113 | Normal | None | |
| C-16 | 1st | 43 | Normal | 64 | Normal | None | |
| C-17 | 1st | < 30 | Normal | < 50 | Normal | None | |
| C-18 | 1st | < 30 | Normal | < 50 | Normal | None | |
| C-19 | 1st | < 30 | Normal | 58 | Normal | None | |
| C-20 | 1st | < 30 | Normal | < 50 | Normal | None | |
| Test group | |||||||
| P-1 | 1st | > 2,000 | Abnormal | > 2,000 | Hypoechoic areas within the pancreas, irregularity of the pancreas, abdominal effusion | Pancreatitis, peritonitis, gastroduodenitis, cystitis | |
| 2nd | 161 | Normal | 75 | ||||
| P-2 | 1st | > 2,000 | Abnormal | > 2,000 | Hypoechoic areas within the pancreas, hyperechoic peripancreatic mesentery | Pancreatitis, acute kidney injury | |
| 2nd | 168 | Abnormal | 179 | ||||
| 3rd | 63 | Normal | < 50 | ||||
| P-3 | 1st | 441 | Abnormal | 415 | No specific signs on the pancreas | Pancreatitis, diabetes mellitus | |
| 2nd | 41 | Normal | < 50 | ||||
| P-4 | 1st | 138 | Abnormal | 228 | Irregularity of the margin of the pancreas, hyperechoic peripancreatic mesentery | Pancreatitis, peritonitis, colitis | |
| 2nd | 51 | Normal | 118 | ||||
| P-5 | 1st | > 2,000 | Abnormal | > 2,000 | Hypoechoic areas within the pancreas, enlarged pancreas | Pancreatitis | |
| 2nd | > 2,000 | Abnormal | 1,787 | ||||
| P-6 | 1st | 288 | Abnormal | 292 | Hypoechoic areas within the pancreas | Pancreatitis, gastritis | |
| 2nd | 591 | Abnormal | 458 | ||||
| P-7 | 1st | 1,597 | Abnormal | 1,141 | Hypoechoic areas within the pancreas, hyperechoic peripancreatic mesentery, abdominal effusion | Pancreatitis, chronic kidney disease | |
| 2nd | 237 | Abnormal | 376 | ||||
| P-8 | 1st | 655 | Abnormal | 292 | Hyperechoic areas of the pancreas | Pancreatitis | |
| 2nd | 379 | Abnormal | 392 | ||||
| P-9 | 1st | 1,174 | Abnormal | 1,737 | Hypoechoic areas within the pancreas, enlarged pancreas | Pancreatitis, acute kidney injury | |
| 2nd | 357 | Abnormal | 320 | ||||
| P-10 | 1st | > 2,000 | Abnormal | > 2,000 | No specific signs on the pancreas | Pancreatitis, chronic kidney disease | |
| P-11 | 1st | > 2,000 | Abnormal | > 2,000 | Hypoechoic areas within the pancreas, hyperechoic peripancreatic mesentery | Pancreatitis, chronic kidney disease | |
| P-12 | 1st | 1,958 | Abnormal | > 2,000 | Hypoechoic areas within the pancreas, hyperechoic peripancreatic mesentery | Pancreatitis, chronic kidney disease, diabetes mellitus | |
| P-13 | 1st | 1,223 | Abnormal | 1,385 | Hyperechoic areas of the pancreas, hyperechoic peripancreatic mesentery | Pancreatitis, gastritis, colitis, peritonitis | |
| P-14 | 1st | 904 | Abnormal | 724 | Hyperechoic areas of the pancreas | Pancreatitis, chronic kidney disease | |
| 2nd | 399 | Abnormal | 463 | ||||
| 3rd | 451 | Abnormal | 619 | ||||
| P-15 | 1st | > 2,000 | Abnormal | > 2,000 | Hypoechoic areas within the pancreas, enlarged pancreas | Pancreatitis, inflammatory bowel disease | |
| 2nd | 1,870 | Abnormal | > 2,000 | ||||
| 3rd | 287 | Abnormal | 263 | ||||
| P-16 | 1st | > 2,000 | Abnormal | > 2,000 | No specific signs on the pancreas | Pancreatitis, diabetes mellitus | |
cPL, canine pancreatic lipase.
aDog had pancreatitis and was allowed to visit the veterinary hospital again for follow-up.
bFor the analysis of cPL in serum, three measurement tools (IDEXX Spec cPL, SNAP cPL, and Vcheck cPL) were used.
cUltrasonographic findings suggesting pancreatitis
dIncluding pancreatitis; other concurrent diseases such as renal, hepatic, and/or other systemic diseases were recorded.
Diagnosis of the test group
Sixteen dogs diagnosed with pancreatitis were assigned to the test group. Median age and weight of this group were 10 years and 5.6 kg, respectively (Table 1). Dogs in this group exhibited clinical signs, history, CBC, and serum analysis results suggestive of pancreatitis.
Thirteen dogs showed more than one ultrasonographic sign of pancreatitis (Table 2). In contrast, three dogs (patient No. P-3, P-10, and P-16) had no specific signs of pancreatitis on abdominal ultrasonography. However, these three dogs were included in the test group after the panel discussion, because they showed typical clinical signs of pancreatitis and also had abnormal results in all the SNAP cPL, Spec cPL and Vcheck cPL tests (Table 2).
The final diagnosis and assignment of the test groups were performed after Spec cPL analysis (Table 2). Abnormal levels of serum cPL was revealed in the Spec cPL test (> 200 ng/mL) in 15/16 dogs and were finally diagnosed with pancreatitis. Only one dog (patient No. P-4) showed a level within the reference interval by Spec cPL, but it was finally assigned to the test group after panel discussion, considering its history, clinical symptoms, abdominal ultrasonography, and other data.
All dogs except one in this group had more than one accompanying disease with pancreatitis (Table 2); the most frequent being chronic kidney disease (CKD), diagnosed in five dogs. Other urinary disorders such as acute kidney injury and cystitis, and gastrointestinal diseases such as peritonitis, gastritis, colitis, and inflammatory bowel disease were also identified. Frequently observed systemic disease was diabetes mellitus (DM) as diagnosed in three cases (patient No. P-3, P-12, and P-16).
Correlation between the three different cPL assays
At the initial visit of all 36 dogs, SNAP cPL, Spec cPL, and Vcheck cPL assays were performed. The dogs in the control group visited the hospital only once; however, some dogs in the test group visited more than once: eight dogs visited twice, and three dogs visited three times. Consequently, 50 samples were generated from 36 dogs, and 50 comparable data points were analyzed using the three cPL assays.
Most of the 50 test results of the three cPL assays were consistent (94%), with the exception of three samples. Three discordant samples were collected during the initial visit of patient No. C-10, second visit of patient No. P-2, and initial visit of patient No. P-4 (Table 2). Serum cPL analysis results at the initial visit of patient No. C-10 were described above. During the second visit of patient No. P-2: SNAP cPL was abnormal, but Spec cPL (168 ng/mL) and Vcheck cPL (179 ng/mL) levels were within the normal range. During the initial visit of patient No. P-4, SNAP cPL and Vcheck cPL (228 ng/mL) revealed abnormal results; however, Spec cPL results were within the normal range.
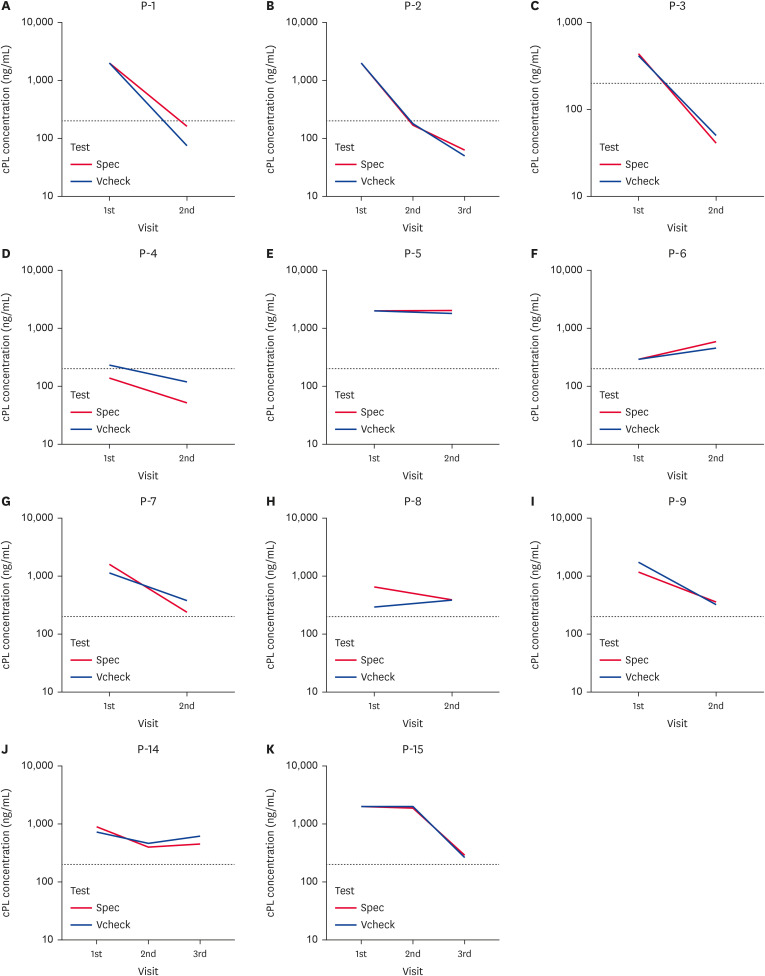
There was near perfect agreement between the Spec cPL and Vcheck cPL (κ = 0.960, p < 0.001), SNAP cPL and Vcheck cPL (κ = 0.920, p < 0.001), and Spec cPL and SNAP cPL (κ = 0.880, p < 0.001). Therefore, correlations between these three cPL assays were significantly high. The unique feature of the Spec cPL and Vcheck cPL tools is that they provide results in quantified concentrations (ng/mL). Fig. 1 shows the cPL concentrations measured from the 50 serum samples with Spec cPL and Vcheck cPL assays. The correlation coefficient of these two tests was significantly high (r = 0.958, p < 0.001). Furthermore, changes in serum cPL concentrations were observed in 11 dogs that visited the hospital more than once (Fig. 2). During treatment and management after the initial visit, the cPL concentration in dogs increased, decreased, or remained unchanged. These patterns were mostly consistent for the Spec cPL and Vcheck cPL.
Fig. 1. Comparison of the results from Spec cPL and Vcheck cPL.
Fifty serum samples from dogs with or without symptoms of pancreatitis were analyzed by the two quantitative analysis methods, Spec cPL and Vcheck cPL. The dotted line represents the threshold (200 ng/mL) of the Vcheck cPL and Spec cPL. Values obtained < 30 ng/mL or < 50 ng/mL were considered as 30 and 50 ng/mL in Spec cPL and Vcheck cPL, respectively. Further, values > 2,000 ng/mL in Spec cPL and Vcheck cPL were considered as 2,000 ng/mL.
cPL, canine pancreatic lipase.
Fig. 2. Serum cPL concentration changes in 11 dogs following hospital visits.
The dotted line represents the threshold (200 ng/mL) of the Vcheck cPL and Spec cPL. Values obtained < 30 ng/mL or < 50 ng/mL were considered as 30 and 50 ng/mL in Spec cPL and Vcheck cPL respectively. Further, values > 2,000 ng/mL in Spec cPL and Vcheck cPL were considered as 2,000 ng/mL. Patient No. of test group: (A) P-1, (B) P-2, (C) P-3, (D) P-4, (E) P-5, (F) P-6, (G) P-7, (H) P-8, (I) P-9, (J) P-14, and (K) P-15.
cPL, canine pancreatic lipase.
DISCUSSION
cPL is one of the most efficient biomarkers for the diagnosis of canine pancreatitis that could satisfy both specificity and sensitivity [8]. Commercial tools for measuring cPL, such as SNAP cPL and Spec cPL, are used in veterinary hospitals worldwide. Short turnaround time (TAT), specificity and sensitivity are critical points, when developing cPL-measuring methods because many canine pancreatitis patients are emergency cases and show poor prognosis unless quick and appropriate treatment is provided. SNAP cPL can check the results in approximately 10 min in-house, but this implies the possibility of reading errors because the results are revealed as color intensity. Spec cPL can compensate for these shortcomings because it presents the results in quantified concentration. However, the sample has to be sent to an external laboratory for testing and it takes several days to obtain the results. Recently, Vcheck cPL was developed, which requires approximately 5 min from sample loading to display the results presented in concentration form (ng/mL). In this study, the newly developed Vcheck cPL was compared to Spec cPL and SNAP cPL using clinical samples, with a focus on concordance.
Fifty samples from 36 dogs were tested with SNAP cPL, Spec cPL, and Vcheck cPL, and most of the results were consistent, except for three. In one case (patient No. P-4), 8 years spayed female Maltese with mucosal diarrhea, had normal Spec cPL (138 ng/mL), and abnormal Vcheck cPL (228 ng/mL) and SNAP cPL. Inflammatory changes were observed on abdominal ultrasonography of the pancreas and peripheral organs. Moreover, this patient underwent surgery for a skin fistula from which granulation tissues were generated close to the pancreas, colon, and right ureter. Particularly, irregularities of the pancreatic margin and a hyperechoic peripancreatic mesentery were observed on abdominal ultrasonography. Therefore, the patient was finally diagnosed with pancreatitis, colitis, and peritonitis after considering all the evaluated data, and was included in the test group after the panel discussion. Although the Spec cPL result was in the reference range, the dog could be diagnosed as pancreatitis based on the clinical and laboratory findings including abdominal ultrasonography. Analogously, the dog’s Vcheck cPL result was also in the equivocal range, so it was recommended to consider using other diagnostic criteria as well. Therefore, any cPL test should not be used alone for the diagnosis of canine pancreatitis, and the results of ultrasound or other clinical tests should be interpreted in an integrative manner.
In both discrepancy cases (patient No. C-10, P-2), abnormal results in SNAP cPL kits were obtained, while normal results were found in Spec cPL and Vcheck cPL. There are several reports on the discordance between the results of SNAP cPL and Spec cPL [11,17]. Most of the causes of this discrepancy were operational errors caused by visual reading of the SNAP cPL results; especially, near 200 ng/mL, where the color concentrations of the reference and sample spots are similar. Both discordant cases were observed between 150 and 200 ng/mL in the Spec cPL and Vcheck cPL tests.
As shown by the high rate of consistency between the cPL measurement methods in this study. Particularly the results of Spec cPL and Vcheck cPL were highly correlated each other. Moreover, during therapy, the increasing or decreasing patterns of serum cPL concentration in most patients were similar in the Spec cPL and Vcheck cPL results. Only in one patient, patient No. P-8, the cPL concentrations analyzed by Spec cPL decreased during therapy, whereas the Vcheck cPL results showed an increasing pattern (Fig. 2H). Despite the difference, all test results still indicated abnormalities in both methods, and the patient’s symptoms only showed a slight improvement in appetite during therapy without apparent relief. Therefore, in this study, all the quantified data generated by Spec cPL and Vcheck cPL provided similar information for monitoring treatment and establishing a subsequent treatment strategy. The high concordance of Spec cPL with Vcheck cPL may reduce the difficulty for a veterinarian who wants to replace the existing equipment with an alternative assay method.
A limitation of this study is that histological examination was not performed for the final diagnosis. Despite this, a suspicion of false-positive can be ruled out in most patients in the test group because they presented typical clinical signs, history, abnormal signs on abdominal ultrasonography, and elevated serum cPL concentrations in more than two assays tested in this study. Although three patients (patient No. P-3, P-10, and P-16) showed no abnormal signs on abdominal ultrasonography examinations; pancreatitis could not be ruled out because of the limited sensitivity of abdominal ultrasonography (43–89%) in dogs [1,12,18]. Nevertheless, the concurrent diseases of these three patients were reviewed because some non-pancreatic disorders affect elevated cPL levels [14,15,19,20,21]. Two of them had DM and the other had CKD, but pancreatitis was not ruled out from these dogs by panel discussion as described in this study. First, the two dogs had already been diagnosed with DM several months previously, and their symptoms were usually voracious appetite. On the day of the hospital visit, they showed classic signs of acute pancreatitis with abdominal pain, vomiting, and anorexia, in contrast to the usual symptoms of patients with DM only [22]. Another dog previously diagnosed with CKD was an outpatient under appropriate management. On the day that the dog showed pancreatitis-like symptoms, the dog was hospitalized and diagnosed with canine pancreatitis. Although it is difficult to differentiate the clinical symptoms of CKD from those of pancreatitis, there is no clinical relevance to the increase in serum lipase activity or serum cPL concentration in dogs with CKD [23]. Therefore, these history, clinical signs, and previous studies support the diagnosis of canine pancreatitis in these three cases.
False negative was observed in chronic pancreatitis, which showed no clinical signs or elevation of serum cPL levels because of pancreatic fibrosis and atrophy, which may not cause leakage of pancreatic enzymes from the pancreas [24]. Therefore, the sensitivity of cPL is usually lower in chronic pancreatitis than in acute pancreatitis, and histopathological examination of the pancreas is the gold standard for diagnosing chronic pancreatitis. The presence of permanent histopathological changes, such as fibrosis or atrophy is a representative sign of chronic pancreatitis [2,25]. Since histological examinations were not performed in this study, evidence of histological changes caused by chronic pancreatitis could only be inferred using ultrasonography. The accuracy of abdominal ultrasonography in the diagnosis of chronic pancreatitis is unknown; however, hyperechoic areas of the pancreas may indicate pancreatic fibrosis [26]. Hyperechoic signs of the pancreas were observed in three patients in the test group (patient No. P-8, P-13, and P-14) but not in the control group. These patients were not false-negative cases because of the high cPL concentrations measured in all the three assays.
Another important factor in chronic pancreatitis is that the cPL concentration often increases, even in dogs with mild or no clinical signs [27]. In such cases, it is appropriate to establish long-term outpatient treatment plans rather than intensive treatment through hospitalization. Therefore, veterinarians should avoid establishing treatment plans based solely on the cPL measurement result but should consider other factors such as history, clinical signs, CRP level, abdominal ultrasonography, and other blood test results. In the control group, patient No. C-10 had a relatively elevated cPL level but remained within the normal range. This patient had no specific abnormalities in serum chemistry and no abnormal signs of abdominal issues in the ultrasonography; hence, pancreatitis was ruled out. Therefore, it is difficult to suggest a false-negative diagnosis in this study.
To summarize, this study compared three serum cPL measurement assays: SNAP cPL, Spec cPL, and Vcheck cPL; moreover, these three tools demonstrated good correlations. Notably, our data illustrated that both Spec cPL and Vcheck cPL provide valuable diagnostic information for pancreatitis in dogs, making them suitable for clinical applications in veterinary hospitals.
ACKNOWLEDGMENTS
We would like to thank Ms. Young Min Kim for technical assistance.
Footnotes
Funding: This research was funded by Bionote Inc.
Conflict of Interest: Jungho Kim and Soungjin Ji are the Director and Associate Director of the R&D department of Bionote Inc., respectively. The remaining authors declare no conflicts of interest.
- Conceptualization: Kim Y.
- Data curation: Kim JK, Kim SE, Lee G.
- Formal analysis: Hwang SY.
- Funding acquisition: Kim Y.
- Methodology: Ji S, Kim J.
- Project administration: Kim JK.
- Software: Ji S, Kim J.
- Supervision: Kim Y.
- Validation: Ji S.
- Writing - original draft: Hwang SY.
- Writing - review & editing: Kim JK, Kim Y.
References
- 1.Xenoulis PG. Diagnosis of pancreatitis in dogs and cats. J Small Anim Pract. 2015;56(1):13–26. doi: 10.1111/jsap.12274. [DOI] [PubMed] [Google Scholar]
- 2.Newman S, Steiner J, Woosley K, Barton L, Ruaux C, Williams D. Localization of pancreatic inflammation and necrosis in dogs. J Vet Intern Med. 2004;18(4):488–493. doi: 10.1892/0891-6640(2004)18<488:lopian>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 3.Brobst D, Ferguson AB, Carter JM. Evaluation of serum amylase and lipase activity in experimentally induced pancreatitis in the dog. J Am Vet Med Assoc. 1970;157(11):1697–1702. [PubMed] [Google Scholar]
- 4.Archer FJ, Kerr ME, Houston DM. Evaluation of three pancreas specific protein assays, TLI(trypsin-like immunoreactivity), PASP (pancreas specific protein) and CA 19-9 (glycoprotein) for use in the diagnosis of canine pancreatitis. Zentralbl Veterinarmed A. 1997;44(2):109–113. doi: 10.1111/j.1439-0442.1997.tb01092.x. [DOI] [PubMed] [Google Scholar]
- 5.Steiner JM, Williams DA. Development and validation of a radioimmunoassay for the measurement of canine pancreatic lipase immunoreactivity in serum of dogs. Am J Vet Res. 2003;64(10):1237–1241. doi: 10.2460/ajvr.2003.64.1237. [DOI] [PubMed] [Google Scholar]
- 6.Simpson KW, Simpson JW, Lake S, Morton DB, Batt RM. Effect of pancreatectomy on plasma activities of amylase, isoamylase, lipase and trypsin-like immunoreactivity in dogs. Res Vet Sci. 1991;51(1):78–82. doi: 10.1016/0034-5288(91)90035-m. [DOI] [PubMed] [Google Scholar]
- 7.Wiberg ME, Nurmi AK, Westermarck E. Serum trypsinlike immunoreactivity measurement for the diagnosis of subclinical exocrine pancreatic insufficiency. J Vet Intern Med. 1999;13(5):426–432. doi: 10.1892/0891-6640(1999)013<0426:stimft>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 8.Steiner JM, Newman S, Xenoulis P, Woosley K, Suchodolski J, Williams D, et al. Sensitivity of serum markers for pancreatitis in dogs with macroscopic evidence of pancreatitis. Vet Ther. 2008;9(4):263–273. [PubMed] [Google Scholar]
- 9.Cridge H, MacLeod AG, Pachtinger GE, Mackin AJ, Sullivant AM, Thomason JM, et al. Evaluation of SNAP cPL, Spec cPL, VetScan cPL rapid test, and precision PSL assays for the diagnosis of clinical pancreatitis in dogs. J Vet Intern Med. 2018;32(2):658–664. doi: 10.1111/jvim.15039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huth SP, Relford R, Steiner JM, Strong-Townsend MI, Williams DA. Analytical validation of an ELISA for measurement of canine pancreas-specific lipase. Vet Clin Pathol. 2010;39(3):346–353. doi: 10.1111/j.1939-165X.2010.00245.x. [DOI] [PubMed] [Google Scholar]
- 11.Beall MJ, Cahill R, Pigeon K, Hanscom J, Huth SP. Performance validation and method comparison of an in-clinic enzyme-linked immunosorbent assay for the detection of canine pancreatic lipase. J Vet Diagn Invest. 2011;23(1):115–119. doi: 10.1177/104063871102300120. [DOI] [PubMed] [Google Scholar]
- 12.Hess RS, Saunders HM, Van Winkle TJ, Shofer FS, Washabau RJ. Clinical, clinicopathologic, radiographic, and ultrasonographic abnormalities in dogs with fatal acute pancreatitis: 70 cases (1986-1995) J Am Vet Med Assoc. 1998;213(5):665–670. [PubMed] [Google Scholar]
- 13.McCord K, Morley PS, Armstrong J, Simpson K, Rishniw M, Forman MA, et al. A multi-institutional study evaluating the diagnostic utility of the spec cPL™ and SNAP® cPL™ in clinical acute pancreatitis in 84 dogs. J Vet Intern Med. 2012;26(4):888–896. doi: 10.1111/j.1939-1676.2012.00951.x. [DOI] [PubMed] [Google Scholar]
- 14.Israeli I, Steiner J, Segev G, Kass PH, Suchodolski JS, Sattasathuchana P, et al. Serum pepsinogen-A, canine pancreatic lipase immunoreactivity, and C-reactive protein as prognostic markers in dogs with gastric dilatation-volvulus. J Vet Intern Med. 2012;26(4):920–928. doi: 10.1111/j.1939-1676.2012.00940.x. [DOI] [PubMed] [Google Scholar]
- 15.Mawby DI, Whittemore JC, Fecteau KA. Canine pancreatic-specific lipase concentrations in clinically healthy dogs and dogs with naturally occurring hyperadrenocorticism. J Vet Intern Med. 2014;28(4):1244–1250. doi: 10.1111/jvim.12376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–174. [PubMed] [Google Scholar]
- 17.Haworth MD, Hosgood G, Swindells KL, Mansfield CS. Diagnostic accuracy of the SNAP and Spec canine pancreatic lipase tests for pancreatitis in dogs presenting with clinical signs of acute abdominal disease. J Vet Emerg Crit Care. 2014;24(2):135–143. doi: 10.1111/vec.12158. [DOI] [PubMed] [Google Scholar]
- 18.Cridge H, Sullivant AM, Wills RW, Lee AM. Association between abdominal ultrasound findings, the specific canine pancreatic lipase assay, clinical severity indices, and clinical diagnosis in dogs with pancreatitis. J Vet Intern Med. 2020;34(2):636–643. doi: 10.1111/jvim.15693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bolton TA, Cook A, Steiner JM, Fosgate GT. Pancreatic lipase immunoreactivity in serum of dogs with diabetic ketoacidosis. J Vet Intern Med. 2016;30(4):958–963. doi: 10.1111/jvim.13955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Verkest KR, Fleeman LM, Morton JM, Groen SJ, Suchodolski JS, Steiner JM, et al. Association of postprandial serum triglyceride concentration and serum canine pancreatic lipase immunoreactivity in overweight and obese dogs. J Vet Intern Med. 2012;26(1):46–53. doi: 10.1111/j.1939-1676.2011.00844.x. [DOI] [PubMed] [Google Scholar]
- 21.Hulsebosch SE, Palm CA, Segev G, Cowgill LD, Kass PH, Marks SL. Evaluation of canine pancreas-specific lipase activity, lipase activity, and trypsin-like immunoreactivity in an experimental model of acute kidney injury in dogs. J Vet Intern Med. 2016;30(1):192–199. doi: 10.1111/jvim.13806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davison LJ. Diabetes mellitus and pancreatitis--cause or effect? J Small Anim Pract. 2015;56(1):50–59. doi: 10.1111/jsap.12295. [DOI] [PubMed] [Google Scholar]
- 23.Steiner JM, Finco DR, Williams DA. Serum lipase activity and canine pancreatic lipase immunoreactivity (cPLI) concentration in dogs with experimentally induced chronic renal failure. Vet Res. 2010;3(3):58–63. [Google Scholar]
- 24.Neilson-Carley SC, Robertson JE, Newman SJ, Kutchmarick D, Relford R, Woosley K, et al. Specificity of a canine pancreas-specific lipase assay for diagnosing pancreatitis in dogs without clinical or histologic evidence of the disease. Am J Vet Res. 2011;72(3):302–307. doi: 10.2460/ajvr.72.3.302. [DOI] [PubMed] [Google Scholar]
- 25.Bostrom BM, Xenoulis PG, Newman SJ, Pool RR, Fosgate GT, Steiner JM. Chronic pancreatitis in dogs: a retrospective study of clinical, clinicopathological, and histopathological findings in 61 cases. Vet J. 2013;195(1):73–79. doi: 10.1016/j.tvjl.2012.06.034. [DOI] [PubMed] [Google Scholar]
- 26.Hecht S, Henry G. Sonographic evaluation of the normal and abnormal pancreas. Clin Tech Small Anim Pract. 2007;22(3):115–121. doi: 10.1053/j.ctsap.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 27.Xenoulis PG, Suchodolski JS, Steiner JM. Chronic pancreatitis in dogs and cats. Compend Contin Educ Vet. 2008;30(3):166–180. [PubMed] [Google Scholar]