Abstract
Human immunodeficiency virus type 1 (HIV-1) group N from Cameroon is phylogenetically close, in env, to the simian immunodeficiency virus (SIV) cpz-gab from Gabon and SIVcpz-US of unknown geographic origin. We screened 29 wild-born Cameroonian chimpanzees and found that three (Cam3, Cam4, and Cam5) were positive for HIV-1 by Western blotting. Mitochondrial DNA sequence analysis demonstrated that Cam3 and Cam5 belonged to Pan troglodytes troglodytes and that Cam4 belonged to P. t. vellerosus. Genetic analyses of the viruses together with serological data demonstrated that at least one of the two P. t. troglodytes chimpanzees (Cam5) was infected in the wild, and revealed a horizontal transmission between Cam3 and Cam4. These data confirm that P. t. troglodytes is a natural host for HIV-1-related viruses. Furthermore, they show that SIVcpz can be transmitted in captivity, from one chimpanzee subspecies to another. All three SIVcpz-cam viruses clustered with HIV-1 N in env. The full Cam3 SIVcpz genome sequence showed a very close phylogenetic relationship with SIVcpz-US, a virus identified in a P. t. troglodytes chimpanzee captured nearly 40 years earlier. Like SIVcpz-US, SIVcpz-cam3 was closely related to HIV-1 N in env, but not in pol, supporting the hypothesis that HIV-1 N results from a recombination event. SIVcpz from chimpanzees born in the wild in Cameroon are thus strongly related in env to HIV-1 N from Cameroon, demonstrating the geographic coincidence of these human and simian viruses and providing a further strong argument in favor of the origin of HIV-1 being in chimpanzees.
Human immunodeficiency virus type 1 (HIV-1) groups M, N, and O are considered to result from at least three independent introductions of simian immunodeficiency virus (SIV) cpz from chimpanzees into the human population (23). Four subspecies of chimpanzees are currently recognized: Pan troglodytes verus and P. t. vellerosus in West Africa, P. t. troglodytes in Central Africa, and P. t. schweinfurthii in East Africa (6, 17). The three SIVcpz viruses so far fully characterized originate either from P. t. troglodytes (SIVcpz-gab1 and SIVcpz-US) or from P. t. schweinfurthii (SIVcpz-ant) (4). SIVcpz-ant was isolated in former Zaire (19) and does not cluster within HIV-1 group M, N, or O (4). The closest relatives of HIV-1 have been found in P. t. troglodytes, and it has been suggested that this subspecies is the primary reservoir of HIV-1 (4). SIVcpz-gab1 was thus isolated from a wild P. t. troglodytes chimpanzee caught in Gabon (20), and is particularly related to HIV-1 N in env (23). The more recently characterized SIVcpz-US, from a P. t. troglodytes born in 1959 in an unknown Central African country, is even more closely related to HIV-1 N in env (4). It has been suggested that HIV-1 N arose through ancient recombination events between SIVcpz-US-related viruses and other SIVcpz strains in P. t. troglodytes, and subsequent transmission to humans (4). If this is so, SIVcpz viruses phylogenetically strongly related to HIV-1 group N might circulate in the same geographic area as group N viruses. All HIV-1 N viruses so far isolated originate from Cameroon (23). However, it is not known whether chimpanzees from Cameroon harbor SIVcpz viruses or whether these viruses are related to HIV-1. In addition, Cameroon is a major epidemiological center of group O viruses, but SIV clustering with group O (or group M) has not been identified yet. This prompted us to screen wild-born chimpanzees from Cameroon in order to look for HIV-1-related viruses in these apes.
The serological survey involved 29 wild-born chimpanzees that were rescued throughout Cameroon. All animals were screened with HIV-1 New Lav Blot kits (Sanofi-Pasteur, Paris, France). Three animals, Cam3, Cam4, and Cam5, were HIV-1-seropositive by Western blotting (data not shown). These chimpanzees were recovered in three distinct regions of Cameroon. Cam3 was found in 1992 in a village near the southern border with Gabon. Cam4 was rescued in 1992 in the southwestern province, near the border with Nigeria. Cam3 and Cam4 had estimated ages of 1 year and 2.5 years, respectively, at the time of rescue. They were housed together, first as pets and then, after November 1993, in the same enclosure at the Wildlife Rescue Center in the southwestern province. Cam3 died suddenly in 1998 with subacute pneumonia. Cam4 remained healthy, apart from episodes of fever and diarrhea. Cam5 was recovered in 1998 in the central province of Cameroon at an age of less than 1 year, and was housed at Yaoundé Zoo. The mother was killed by hunters. Cam5 rapidly died with diarrhea after arriving at the zoo, and all the samples were obtained postmortem. None of the clinical signs observed in the animals could be associated with an acquired immunodeficiency syndrome.
The sera of Cam3, Cam4, and Cam5 were further tested with a peptide-based enzyme immunoassay (EIA) (15). All three showed strong cross-reactivity with SIVcpz-gab and HIV-1 N peptides (Table 1), a pattern resembling that observed for sera from HIV-1 N-infected patients (23). The Cam5 serum was also strongly reactive with an HIV-1 group M Env peptide, possibly on account of the young age at sampling (26).
TABLE 1.
Reactivity of the sera from three SIVcpz-infected chimpanzees of Cameroon with distinct V3 and gp41 reference peptidesa
| Peptide and virus or group | Serum reactivity
|
|||||
|---|---|---|---|---|---|---|
| Group M | Group O | Group N | Cam3 | Cam4 | Cam5 | |
| V3 | ||||||
| Group Mb | 1.54c | 0.26 | 0.23 | 0.31 | 0.17 | 0.56 |
| Group O | 0.11 | 1.09 | 0.13 | 0.11 | 0.10 | 0.11 |
| Group N | 0.11 | 0.13 | 0.76 | 0.70 | 0.37 | 0.55 |
| SIVcpz-gab | 0.11 | 0.12 | 0.56 | 0.51 | 0.30 | 0.58 |
| gp41 | ||||||
| Group Mb | 1.60 | 1.00 | 0.38 | 0.31 | 0.54 | 0.75 |
| Group O | 1.25 | 1.42 | 0.24 | 0.11 | 0.21 | 0.10 |
| Group N | 0.22 | 0.61 | 1.13 | 0.97 | 1.20 | 0.85 |
| SIVcpz-gab | 0.43 | 0.13 | 0.78 | 0.94 | 1.10 | 1.05 |
The assays were based on the EIA technique as previously described (16).
The peptide is based on an HIV-1 subtype A sequence.
Bold type indicates strong reactivity with the peptide.
Virus isolation was attempted by means of coculture with phytohemagglutinin-stimulated human peripheral blood mononuclear cells (PBMC) from HIV-negative blood donors (23). Virus production was detected after 9 days of culture in each case, confirming that all three chimpanzees were infected.
For Cam5 it was clear that the infection occurred in the wild since the sampling was performed immediately after its arrival at the zoo. Cam3 and Cam4 were, however, housed in the same enclosure for 5 years before being tested. We therefore sequenced both a highly conserved fragment (178 bp of the integrase region in pol) and a variable genomic region (1,332 bp in env) of SIVcpz-cam3 and SIVcpz-cam4, in order to determine their nucleotide diversity. SIVcpz-cam3 DNA was amplified from genomic DNA of 9-day PBMC cultures. Two long, overlapping fragments were separately amplified. The primers LSiGi-forward (positions 534 to 562 in SIVcpz-gab [23]) and ZRT6-reverse (5′-ACCTGCCATCTGTTTTCCATAATC-3′; positions 5099 to 5121) were used to amplify the 5′ half, and ZRT4 (5′-AAAAGAAAAGGGGGGATTGGGGGGTACA-3′; positions 4846 to 4873) and LSiGi-reverse (23) were used to amplify the 3′ half. Five microliters of the primary PCR product was submitted to a secondary PCR amplification with the following primer sets: LSiGi-forward and YRT1 (5′-GTATTITTATGGATTTTCIGGICCTATT-3′), LPBS (23) and ZRT6, ZRT4 and SK68 (5′-CCATAGTGCTTCCTGCTGC-3′), and ENV (5′-ATTCCIATACAITATTGTGCICCAGC-3′) and ZU3′ (5′-ACAGGCAGAAAGCAGCTGCTTATATGCA-3′). Cycling conditions using a long PCR procedure (Elongase; Gibco-BRL) comprised a hot start (5 min at 94°C), then 35 cycles of denaturation (94°C for 30 s), annealing (55°C for 40 s), and elongation (68°C for 8 min), followed by a final elongation step (68°C for 2 to 7 min). PCR products were purified and directly sequenced by using the ABI PRISM Big Dye Terminator Cycle Sequencing Ready Reaction kit with AmpliTaq FS DNA polymerase (Perkin-Elmer, Norwalk, Conn.) and an automated DNA sequencer (ABI-377A; Applied Biosystems). Serial SIVcpz-cam primers were synthesized to walk along the genomic fragments. For SIVcpz-cam4, genomic DNA from noncultured PBMC was used for PCR. The pol fragment was amplified with the Hpol primer set (3), and the env fragment was amplified by using ZRT6 sense and ZU3′ as the outer primers and V3SJ (5′-GCCAATTCCAATACATTACTGTGC-3′) and ENVas (5′-TATGTCAAACCAATTCCA-3′) as the inner primers.
We found very strong nucleotide identity between SIVcpz-cam3 and SIVcpz-cam4 (96.6 and 95.8% in pol and env, respectively), indicating that one of the animals had been infected by the other in captivity. The serum of Cam3 showed strong reactivity against all viral proteins in the Western blot assay, whereas the serum of Cam4 showed only weak reactivity, especially against the Pol and Env proteins (data not shown), a situation typically encountered in recent seroconvertors (10). These data suggested that it was Cam3 that was infected in the wild and that infected Cam4 in captivity. They demonstrate that captive chimpanzees can be infected by SIVcpz, and that they can transmit SIV to other animals in the colony.
As the chimpanzees' sera showed strong serological cross-reactivity with HIV-1 peptides, in particular group N but also group M peptides, we also compared the SIVcpz env sequences with those of HIV-1 M, N, and O. Previously described SIVcpz env sequences (4, 8, 27) were included in the comparative analysis. We used the same 1,332-bp env sequence used to study the relationship between SIVcpz-cam3 and SIVcpz-cam4, because it spans the regions coding for the V3 and gp41 peptides used in the EIA test. In addition, we determined the corresponding env sequence of the third chimpanzee (SIVcpz-cam5). The env fragment of SIVcpz-cam5 was amplified from genomic DNA of 9-day PBMC cultures with the same conditions as those used for SIVcpz-cam4. Amino acid sequences were aligned with the CLUSTAL W program to find the largest common overlap, and were then multiply aligned by the hidden Markov model in the SAM software package (9, 14). The shortest amino acid alignments with the fewest gaps were preferred and were corrected manually. The alignments were then backtranslated into codons by using the original nucleotide sequences. Phylogenetic analyses were based on both codon-based nucleotide and amino acid alignments. Trees were constructed by the neighbor-joining method (21) as implemented by the CLUSTAL W program (25). Multiple substitutions were corrected by the Kimura method (13). Tree topologies were also inferred by using the Fitch-Margoliash, maximum parsimony, and maximum likelihood methods with the FITCH, DNAPARS, and DNAML modules of the PHYLIP software package, respectively (2). Trees were also inferred by the maximum likelihood method implemented in PAUP4 (24). The empirical nucleotide frequencies were used, and transition-to-transversion ratios were set to 1.49, 1.5, and 2.2 for env, gag, and pol, respectively. Substitution rates were assumed to follow a gamma distribution with shape parameters estimated by maximum likelihood. The gamma distribution shape parameters were estimated to be 0.81, 0.55, and 0.46 for env, gag and pol, respectively. We used the Hasegawa-Kishino-Yano evolutionary model with rate heterogeneity (7). Estimating separate substitution rates for each position in the codon yielded significantly decreased likelihood values.
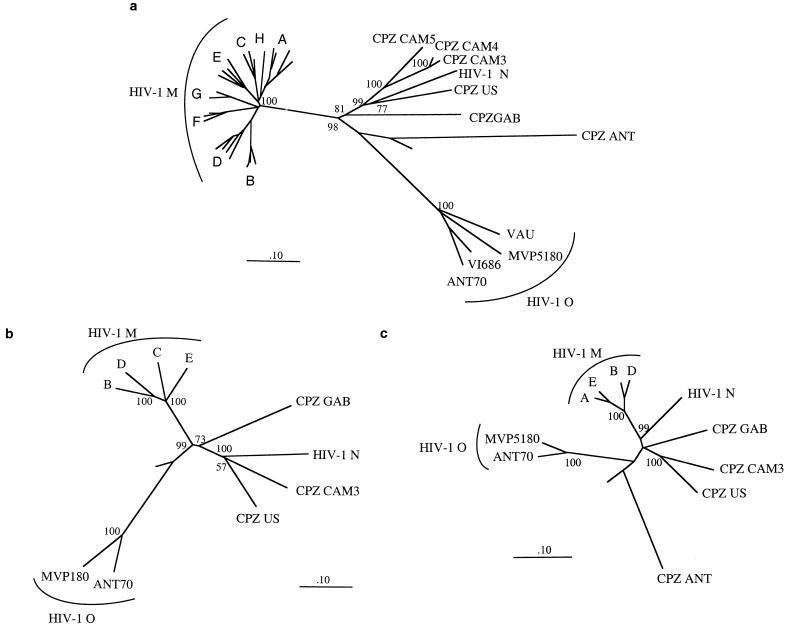
All three Cameroonian SIVcpz sequences clustered with SIVcpz-US and the HIV-1 N prototype strain YBF30 (Fig. 1a). The phylogenetic relationship with HIV-1 N was supported by a remarkably high bootstrap value (99%). None of the three Cameroonian sequences was clearly related to HIV-1 M or HIV-1 O. Interestingly, SIVcpz-cam3 and SIVcpz-cam5 were only slightly more closely related to each other than to SIVcpz-US, even though the latter had infected its host nearly 40 years earlier (4).
FIG. 1.
Phylogenetic relationship of SIVcpz-cam with other members of the HIV-1 and SIVcpz lineage. Nucleotide sequences for all viruses but SIVcpz from Cameroon were obtained from the HIV data bank (http://hiv-web.lanl.gov/). HIV-1 N is represented by the YBF30 strain (23). Sites with gaps or unidentified nucleotides were stripped from the alignment. The trees were rooted with HIV-2 Rod. Trees based on the codon-based nucleotide alignments and constructed by the neighbor-joining method are shown. The percentages of concordant branching during bootstrapping in 10,000 resamplings are displayed at the branch nodes. The branch lengths are drawn to scale. The bar indicates a genetic distance of 0.1 (10% of nucleotide divergence). The neighbor-joining trees were consistent with those inferred by the Fitch-Margoliash, the maximum parsimony, and the maximum likelihood methods. Only minor variations in branch lengths were seen for the maximum likelihood trees. No significant differences in the trees based on the amino acids of Pol and Env were observed. (a) Phylogenetic relationship of SIVcpz-cam3, SIVcpz-cam4, and SIVcpz-cam5 with other members of the HIV-1 and SIVcpz lineage in a partial (1,332-bp) env fragment (from C2 to the ectodomain of gp41); (b) SIVcpz-cam3 phylogenetic relationships with other HIV-1 and SIVcpz viruses in the whole env gene (2,164 nucleotides included); (c) SIVcpz-cam3 phylogenetic relationships with other HIV-1 and SIVcpz viruses in the pol gene.
We then analyzed the full-length genome (9,155 bp) of one Cameroonian SIVcpz virus (SIVcpz-cam3) by using the sequence approach described above. The SIVcpz-cam3 genome displayed the genomic organization typical of SIVcpz and HIV-1. Analysis of deduced amino acid sequences indicated that all the genes studied potentially encoded functional proteins. The highest nucleotide identity was always observed with SIVcpz-US (83% in pol and 76% in env). Phylogenetic analysis based on the entire gag, pol, and env genes confirmed the clustering of SIVcpz-cam3 and SIVcpz-US throughout their genomes (Fig. 1 and data not shown). These data demonstrate that viruses closely related to SIVcpz-US are currently circulating in wild-living chimpanzees in Cameroon.
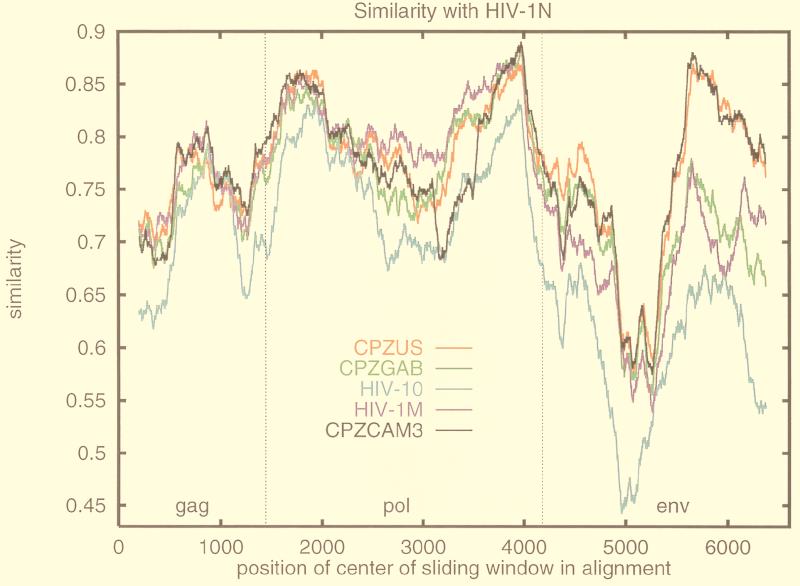
The nucleotide identities of SIVcpz-cam3 with HIV-1 N and SIVcpz-gab were also high (respectively, 79 and 80% in pol and 73 and 69% in env). We therefore scanned the HIV-1 N genome with the recombinant identification program (Fig. 2 and data not shown). Significant similarity, as indicated by sites with statistical confidence above 90%, was observed between HIV-1 N YBF30 and SIVcpz-cam3 in two short stretches in gag (99 sites in total), a 300-bp region in vif, a short region in 5′ env (V2, 130 bp), and a long fragment in 3′ env (1,210 bp). A long stretch of significant homology was therefore found only in env. The regions of higher identity are visualized in the similarity plot (Fig. 2). Phylogenetic analysis based on the entire env gene confirmed the clear clustering of SIVcpz-cam3 with HIV-1 N YBF30, with a highly significant bootstrap value (100%) (Fig. 1b). SIVcpz-cam3 and HIV-1 N YBF30 branched together with the same high bootstrap value (100%) in the trees based on the amino acid sequences of env (data not shown). The bootstrap value for gag was lower (82% with the codon-based nucleotide alignment and <50% with the amino acid alignment [data not shown]). No such clustering was observed for pol (Fig. 1c); indeed, HIV-1 N YBF30 rather formed an independent branch closely related to HIV-1 M, as previously reported (23). The clustering of HIV-1 N with group M viruses in pol is supported by highly significant bootstrap values in the trees based on both nucleotide alignment (99%, Fig. 1c) and amino acid sequence alignment (98% [data not shown]). The different branching orders of HIV-1 N observed according to the gene analyzed could be explained by ancestral recombination events as previously suggested (4, 12).
FIG. 2.
Similarity plot comparing the sequence relationships of HIV-1 N YBF30 with SIVcpz-cam3 and HIV-1 and SIVcpz reference sequences. The similarity plot was obtained by using the Recombinant Identification Program (http://hiv-web.lanl.gov) (22). The search was made in windows of 400 bp, with a threshold of 90% for statistical confidence; gaps were stripped. Similarity plots were obtained using a codon-based alignment of concatenated full-length gag, pol, and env nucleotide sequences. The background alignment included SIVcpz-cam3 (black), SIVcpz-US (red), SIVcpz-gab (green), HIV-1 group M subtype B HXB2 (purple), and HIV-1 group O ANT70 (blue). The x axis shows the nucleotide position along the alignment. The y axis indicates the similarity index between the viral nucleotide sequences (0.1 = 10% similarity).
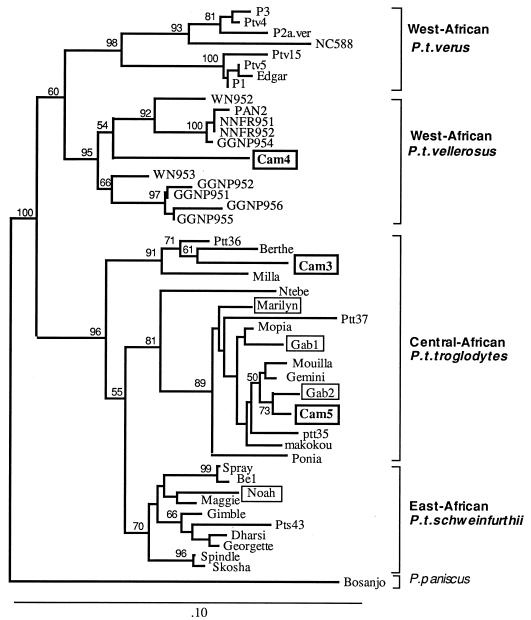
Since two distinct chimpanzee subspecies are present in Cameroon (6, 17), we determined the subspecies of Cam3, Cam4, and Cam5 by mitochondrial DNA (mtDNA) sequence analysis. DNA of the mitochondrial control region (D loop) was amplified from uncultured PBMC of Cam3 and Cam4. Previously described primers (1) were used for PCR amplification and sequence determination of the PCR product. For Cam5, only genomic DNA from a 9-day coculture of chimpanzee PBMC with human PBMC was available. We therefore designed primers that specifically amplify either human or chimpanzee mtDNA. For amplification of chimpanzee mtDNA we designed the primers mtcpzs (5′-CCTAAGTATTGGCTTATTCATT-3′) and mtcpzas (3′-CTATCTGAGGGGGGGCATCCG-3′). Human mtDNA was amplified as a control with the primers mthums (5′-CCCAAGTATTGACTCACCCATC-3′) and mthumas (5′-CTATCTGAGGGGGGTCATCCA-3′).
Phylogenetic analysis of mtDNA from the three source chimpanzees in our study showed that two animals (Cam3 and Cam5) belonged to P. t. troglodytes (Fig. 3). The third seropositive chimpanzee (Cam4) did not cluster with P. t. troglodytes but with the P. t. vellerosus lineage (Fig. 3). This classification of Cam4 based on the mtDNA sequence fits with the geographic origin of the chimpanzee (Southwest Cameroon). This is the first time that a West African chimpanzee has been shown to be infected by SIVcpz. Serological and genetic analysis suggest that Cam4 was infected by Cam3 in captivity, although we cannot totally exclude that the virus crossed from Cam4 to Cam3. It is the first documented case of cross-subspecies transmission of SIVcpz in captivity. As both apes were males, transmission might have occurred through biting, a common route of lentivirus transmission in animals (18), and not by sexual contact. These data also confirm that P. t. troglodytes is a natural host of HIV-1-related viruses, since Cam5, and maybe also Cam3, was infected in the wild (4). Both animals were captured at a very young age, as were all four other SIVcpz-infected chimpanzees reported in the literature (4, 19, 20). As two of the 29 infants tested here were apparently infected in the wild, the seroprevalence of SIVcpz in free-living adult chimpanzees may indeed be higher than previously assumed (4).
FIG. 3.
Phylogenetic tree of mtDNA (D loop) sequences. The trees constructed by the Fitch-Margoliash method are shown. The transition/transversion ratio was set to 10. For clarity, only bootstrap values over 50% are shown. Previously described chimpanzee mtDNA sequences were included (4, 6, 17). The three chimpanzees of this study together with the four other chimpanzees reported to carry SIVcpz (4, 11, 19, 20) are boxed. Brackets on the right indicate chimpanzee subspecies as previously proposed (6, 17).
An important implication of this study is that the area of HIV-1 N endemicity coincides with the natural habitat of chimpanzees carrying HIV-1 N-related SIVcpz, providing the most persuasive evidence that SIVcpz is the evolutionary ancestor of HIV-1.
The similarity between SIVcpz from P. t. troglodytes and HIV-1 N was, however, only seen in env. HIV-1 N therefore most likely resulted from an ancestral recombination event between viruses related to SIVcpz-cam/US and HIV-1 M (4). The recombination probably occurred in a chimpanzee (4), although one cannot entirely exclude the possibility that it occurred in a human. Only the discovery of such a recombinant virus in chimpanzees or of an HIV-1 N virus related to SIVcpz-cam/US throughout its genome can settle the matter. Additional HIV-1 N and SIVcpz sequences are needed to determine in which species and where exactly in the genome recombination took place.
HIV-1 groups M, N, and O are all circulating in Cameroon (16). It is therefore surprising that all Cameroonian SIVcpz sequences so far identified show a particular relationship with HIV-1 N, but not with HIV-1 M, as the latter has a considerably higher prevalence than N and O in Cameroon (16). It is also surprising that none is related to HIV-1 group O, as Cameroon is currently the epidemiological center of this group (16). The possibility that HIV-1 derives from species other than chimpanzees is unlikely, but it cannot be totally ruled out. Another explanation might be that group M- and group O-related viruses no longer exist in chimpanzees. Studies of wild-born chimpanzees are too limited to exclude, however, the presence of HIV-1 M- or O-related viruses in animals of other geographic regions or in subspecies distinct from P. t. troglodytes. The existence of divergent viruses (SIVcpz-gab and SIVcpz-cam/US) within chimpanzees of the same subspecies (P. t. troglodytes) but from distinct geographic origins, as well as the presence of the highly divergent SIVcpz-ant in another subspecies of East Africa (P. t. schweinfurthii), indicates indeed that chimpanzees in Eastern and Central Africa may harbor a broad spectrum of SIVcpz-type viruses. West African chimpanzees might harbor SIVcpz as well. Indeed, we report here on a case of cross-subspecies transmission between a Central and a West African chimpanzee in captivity. As both subspecies occur in Cameroon, it is easily conceivable that such transmissions occurred in the past between wild animals.
Although HIV-1 group M- and O-related viruses have not been identified yet in chimpanzees (or in any other nonhuman primate species), these African apes are clearly the best candidates for explaining the origin of HIV-1. HIV-2, as is generally agreed, originates from SIVsm present in a West African nonhuman primate, the sooty mangabey (5). The ancestors of SIVcpz and SIVsm, in contrast, are still totally unknown. The reservoir of SIVcpz and SIVsm (if it still exists) would be a further key to understanding the origin of these primate lentiviruses.
In conclusion, we demonstrate that SIVcpz and closely related HIV-1 viruses currently cocirculate in both wild chimpanzees and humans from the same geographic area, providing a further step towards the missing link between HIV-1 and related viruses in nonhuman primates.
Nucleotide sequence accession numbers.
SIVcpz-cam sequences have been deposited at the GenBank database under accession no. AF115393 to AF115395 and AF135498. Mitochondrial sequences of Cam3, Cam4, and Cam5 have been deposited at the GenBank database under accession no. AF13495 to AF135497.
Acknowledgments
We are grateful to P. Jenkins, L. Gadsby, A. Randall, and P. Gleason (Pandrillus association) for their collaboration at the Limbe Wildlife Rescue Center, to C. Mitchell at the Yaoundé Zoo, and to E. Nerrienet at the Centre Pasteur in Yaoundé, Cameroon. We thank J. Mfoupouendoun, E. Tina Abada, and S. Souquières for expert technical assistance, and C. Rapacki and H. H. Staerfeldt (Center for Biological Sequence Analysis, Technical University of Denmark) for their precious support. We are indebted to D. L. Swofford for providing the latest update of PAUP (version 4.062). We thank J. Felsenstein, E. Holmes, and D. L. Swofford for helpful discussions. We thank D. Young for checking the English.
This work was supported by the French National Agency for AIDS research (ANRS) and the Danish National Research Foundation. M.C.M.-T. was the recipient of a SIDAction fellowship, and A.A. had a fellowship from the French Ministry of Cooperation.
REFERENCES
- 1.Anderson S, Bankier A T, Barrell B G, de Bruijn M H L, Coulson A R, Drouin J, Eperon I C, Nierlich D P, Roe B A, Sanger F, Schreier P H, Smith A J H, Staden R, Young I G. Sequence and organization of the human mitochondrial genome. Nature. 1981;290:457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- 2.Felsenstein J. PHYLIP (Phylogeny inference package). Seattle: University of Washington; 1990. [Google Scholar]
- 3.Fransen K. Design and evaluation of new highly sensitive and specific primers for polymerase chain reaction detection of HIV-1 infected primary lymphocytes. Mol Cell Probes. 1994;8:317–322. doi: 10.1006/mcpr.1994.1043. [DOI] [PubMed] [Google Scholar]
- 4.Gao F, Bailes E, Robertson D L, Chen Y, Rodenburg C M, Michael S F, Cummins L B, Arthur I O, Peeters M, Shaw G, Sharp P M, Hahn B H. Origin of HIV-1 in the chimpanzee Pan troglodytes troglodytes. Nature. 1999;397:436–440. doi: 10.1038/17130. [DOI] [PubMed] [Google Scholar]
- 5.Gao F, Yue L, White A T, Pappas P G, Barchue J, Hanson A P, Greene B M, Sharp P M, Shaw G M, Hahn B H. Human infection by genetically diverse SIVsm-related HIV-2 in West Africa. Nature. 1992;358:495–499. doi: 10.1038/358495a0. [DOI] [PubMed] [Google Scholar]
- 6.Gonder M K, Oates J F, Disotell T, Forstner M R, Morales J C, Melnick D J. A new west African chimpanzee subspecies? Nature. 1997;388:337. doi: 10.1038/41005. [DOI] [PubMed] [Google Scholar]
- 7.Hasegawa M, Kishino H, Yano T. Dating of the human-ape splitting by a molecular clock of mitochondrial DNA. J Mol Evol. 1985;22:160–174. doi: 10.1007/BF02101694. [DOI] [PubMed] [Google Scholar]
- 8.Huet T, Chenyier R, Meyerhans A, Roelants G, Wain-Hobson S. Genetic organization of a chimpanzee lentivirus related to HIV-1. Nature. 1990;345:356–358. doi: 10.1038/345356a0. [DOI] [PubMed] [Google Scholar]
- 9.Hughey R. Parallel hardware for sequence comparison and alignment. Comput Appl Biosci. 1996;12:473–479. doi: 10.1093/bioinformatics/12.6.473. [DOI] [PubMed] [Google Scholar]
- 10.Janssen R S, Satten G A, Stramer S L, Rawal B D, O'Brien T R, Weiblen B J, Hecht F M, Jack N, Cleghorn F R, Kahn J O, Chesnay M A, Busch M P. New testing strategy to detect early HIV-1 infection for use in incidence estimates and for clinical and prevention purposes. JAMA. 1998;280:42–48. doi: 10.1001/jama.280.1.42. [DOI] [PubMed] [Google Scholar]
- 11.Janssens W. Phylogenetic analysis of a new chimpanzee lentivirus SIVcpz-gab2 from a wild-captured chimpanzee from Gabon. AIDS Res Hum Retrovir. 1994;10:1191–1192. doi: 10.1089/aid.1994.10.1191. [DOI] [PubMed] [Google Scholar]
- 12.Jin M J, Hui H, Robertson D L, Müller M C, Barré-Sinoussi F, Hirsch V M, Allan J S, Shaw G M, Sharp P M, Hahn B H. Mosaic genome structure of simian immunodeficiency virus from West African monkeys. EMBO J. 1994;13:2935–2947. doi: 10.1002/j.1460-2075.1994.tb06588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kimura M. A simple method for estimating evolutionary rates of base substitution through comparative studies of nucleotide sequences. J Mol Evol. 1980;16:111–120. doi: 10.1007/BF01731581. [DOI] [PubMed] [Google Scholar]
- 14.Krogh A, Mian I S, Haussler D. A hidden Markov model that finds genes in E. coli DNA. Nucleic Acids Res. 1994;22:4768–4778. doi: 10.1093/nar/22.22.4768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mauclère P, Damon F, Apetrei C, Loussert-Ajaka I, Souquières S, Buzelay L, Dalbon P, Jolivet M, Mony Lobe M, Brun-Vézinet F, Simon F, Barin F. Synthetic peptide ELISAs for detection of and discrimination between group M and group O HIV type 1 infections. AIDS Res Hum Retrovir. 1997;13:987–993. doi: 10.1089/aid.1997.13.987. [DOI] [PubMed] [Google Scholar]
- 16.Mauclère P, Loussert-Ajaka I, Damon F, Fagot P, Souquières S, Lobe M M, Keou F X M, Barré-Sinoussi F, Saragosti S, Brun-Vézinet F, Simon F. Serological and virological characterization of HIV-1 group O infection in Cameroon. AIDS. 1997;11:445–453. doi: 10.1097/00002030-199704000-00007. [DOI] [PubMed] [Google Scholar]
- 17.Morin P A. Kin selection, social structure, gene flow, and the evolution of chimpanzees. Science. 1994;265:1193–1201. doi: 10.1126/science.7915048. [DOI] [PubMed] [Google Scholar]
- 18.Nérrienet E, Amouretti X, Müller-Trutwin M C, Poaty-Mavoungou V, Bedjebaga I, Thi Nguyen H, Dubreuil G, Corbet S, Wickings E J, Barré-Sinoussi F, Georges A J, Georges-Courbot M-C. Phylogenetic analysis of SIV and STLV type I in mandrills (mandrillus sphinx): indications that intracolony transmissions are predominantly the result of male-to-male aggressive contacts. AIDS Res Hum Retrovir. 1998;14:785–796. doi: 10.1089/aid.1998.14.785. [DOI] [PubMed] [Google Scholar]
- 19.Peeters M, Fransen K, Delaporte E, van den Haesevelde M, Gershy-Damet G, Kestens L, van der Groen G, Piot P. Isolation and characterization of a new chimpanzee lentivirus (simian immunodeficiency virus isolate cpz-ant) from a wild captured chimpanzee. AIDS. 1992;6:447–451. doi: 10.1097/00002030-199205000-00002. [DOI] [PubMed] [Google Scholar]
- 20.Peeters M, Honore C, Huet T, Bedjabaga L, Ossari S, Bussi P, Cooper R W, Delaporte E. Isolation and partial characterization of an HIV-related virus occurring naturally in chimpanzees in Gabon. AIDS. 1989;3:625–630. doi: 10.1097/00002030-198910000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 22.Siepel A C, Korber B. Scanning the database for recombinant HIV-1 genomes. Los Alamos, N.Mex: Los Alamos National Laboratory; 1995. [Google Scholar]
- 23.Simon F, Mauclère P, Roques P, Loussert-Ajaka I, Müller-Trutwin M C, Saragosti S, Georges-Courbot M-C, Barré-Sinoussi F, Brun-Vézinet F. Identification of a new human immunodeficiency virus type 1 distinct from group M and group O. Nat Med. 1998;4:1032–1037. doi: 10.1038/2017. [DOI] [PubMed] [Google Scholar]
- 24.Swofford D L. Phylogenetic analysis using parsimony (PAUP). Champaign: Illinois Natural History Survey; 1984. [Google Scholar]
- 25.Thompson J D, Higgins D G, Gibson T J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Turbica I, Simon F, Besnier J M, LeJeune B, Choutet P, Goudeau A, Barin F. Temporal development and prognostic value of antibody response to the major neutralizing epitopes of gp120 during HIV-1 infection. J Med Virol. 1997;52:309–315. [PubMed] [Google Scholar]
- 27.Vanden Haesevelde M M, Peeters M, Jannes G, Janssens W, van der Groen G, Sharp P M, Saman E. Sequence analysis of a highly divergent HIV-1-related lentivirus isolated from a wild captured chimpanzee. Virology. 1996;221:346–350. doi: 10.1006/viro.1996.0384. [DOI] [PubMed] [Google Scholar]