Abstract
Importance
The emergence and rapid increase in the incidence of multidrug-resistant (MDR) bacteria in pig farms has become a serious concern and reduced the choice of effective antibiotics.
Objective
This study analyzed the phylogenetics and diversity of antibiotic resistance genes (ARGs) and molecularly identified the source of ARGs in antibiotic-resistant Escherichia coli isolated from pig farms in Banten Province, Indonesia.
Methods
Forty-four antibiotic-resistant E. coli isolates from fecal samples from 44 pig farms in Banten Province, Indonesia, were used as samples. The samples were categorized into 14 clusters. Sequencing was performed using the Oxford Nanopore Technologies MinION platform, with barcoding before sequencing with Nanopore Rapid sequencing gDNA-barcoding (SQK-RBK110.96) according to manufacturing procedures. ARG detection was conducted using ResFinder, and the plasmid replicon was determined using PlasmidFinder.
Results
Three phylogenetic leaves of E. coli were identified in the pig farming cluster in Banten Province. The E. coli isolates exhibited potential resistance to nine classes of antibiotics. Fifty-one ARGs were identified across all isolates, with each cluster carrying a minimum of 10 ARGs. The ant(3'')-Ia and qnrS1 genes were present in all isolates. ARGs in the E. coli pig farming cluster originated mainly from plasmids, accounting for an average of 89.4%.
Conclusions and Relevance
The elevated potential for MDR events, coupled with the dominance of ARGs originating from plasmids, increases the risk of ARG spread among bacterial populations in animals, humans, and the environment.
Keywords: Antibiotic resistance, antibiotic resistance genes, Escherichia coli, multidrug-resistant, pig farm
INTRODUCTION
Antimicrobial resistance (AMR) is a critical and imminent threat to global public health. The swift rise of multidrug-resistant (MDR) pathogens, particularly those resilient to last-resort antibiotics, such as carbapenems, colistin, and tigecycline, raises serious concerns, narrowing the spectrum of effective antibiotic choices [1,2].
The extensive use of antimicrobials in livestock contributes significantly to the escalation of AMR on a global scale. Approximately 73% of all antimicrobials sold worldwide are deployed in livestock for growth promotion (Antibiotic Growth Promoter), increased production, and disease prevention [3]. In particular, a previous study indicated the highest prevalence of antibiotic-containing feed in pigs (55.4%) compared to chickens (42.2%), quail (18.9%), and ducks (9.2%) [4]. This widespread and unregulated use exerts selective pressure on bacteria, fostering the proliferation of resistant strains capable of spreading across human, animal, and environmental bacterial populations [5].
Certain antibiotic-resistant bacteria, particularly Escherichia coli [6], are of global concern. E. coli, a Gram-negative bacterium in the Enterobacteriaceae group, is a primary cause of foodborne infections and serves as a key reservoir for antibiotic resistance genes (ARGs). The propensity of E. coli for accumulating ARGs, predominantly through horizontal gene transfer facilitated by various mobile genetic elements (MGEs), particularly plasmids, underscores its essential role in disseminating multidrug resistance genes between diverse bacterial species [7]. Numerous studies highlight the role of E. coli in propagating critical ARGs, such as blaNDM-1, mcr, and tet(X3)/(X4), limiting the efficacy of antibiotics, including last-resort options (carbapenems, colistin, and tigecycline) and progressively diminishing the pool of effective treatments for human and animal health [1,2]. Furthermore, the emergence of extended-spectrum beta-lactamase (ESBL)-producing E. coli compounds poses a global health challenge [5], making E. coli a vital biomarker for monitoring AMR in livestock on farms and in hospitals [7].
The advent of whole genome sequencing (WGS) represents a groundbreaking advancement in molecular technology, offering extensive, high-resolution insights into pathogen subtypes. Leveraging WGS for the monitoring and surveillance of AMR is imperative because it provides critical information on the initial emergence and spread of resistance. The data highlight the need to develop effective policies to combat AMR. Sequencing data from AMR surveillance is integral for developing rapid diagnostic tools, complementing traditional phenotypic testing methods [8].
Oxford Nanopore Technologies (ONT) MinION is one of the platforms used for WGS on bacteria. The advantage of the ONT MinION platform is that its third-generation sequencing technology can generate long reads (up to 2.27 Mbp) that can span most repetitive sequences and provide the opportunity to link ARGs and their flanking regions, accurately identifying populations carrying ARGs. The ONT MinION platform can generate raw data in real-time, making it more accessible and efficient for genome assembly and complex structural detection [9]. ONT MinION produces long DNA/RNA sequences and is a portable, pocket-sized sequencing device that does not require PCR or chemical labeling during preparation [10].
Indonesia faces challenges in antibiotic use in the livestock industry, particularly in pig farming, coupled with inadequate waste treatment systems, posing a significant risk of spreading resistant bacteria to the broader environment and a severe threat to public health. Moreover, there is no comprehensive data on antimicrobial-resistant E. coli genotypes in pig farms, particularly in Banten Province. This study characterized the phylogenetic and ARG diversity and the molecular features of ARG-containing plasmids in antibiotic-resistant E. coli from pig farms in Banten Province. Research on molecular antibiotic resistance is crucial for predicting the resistance rates and is a foundational basis for controlling antibiotic resistance.
METHODS
Animal care
This research was exempt from ethical approval and Institutional Animal Care and Use Committee (IACUC) clearance because it did not involve the treatment of animals. Nevertheless, sample collection adhered to established protocols outlined in the Global Tricycle Surveillance ESBL E. coli from WHO, 2021 [11].
Sample collection and preparation
Forty-four antibiotic-resistant E. coli isolates obtained from fecal samples at 44 distinct pig farms in Banten Province, Indonesia, were used as samples. The samples were isolated and identified in a prior study [12,13]. These E. coli isolates were categorized into 14 clusters (pooling), determined by shared characteristics and proximity to the respective farm areas. E. coli was isolated and identified according to the protocols outlined in the Global Tricycle Surveillance ESBL E. coli from WHO, 2021 [11]. Antibiotic susceptibility testing of E. coli isolates used the Kirby–Bauer disk diffusion method on MHA media, referring to the Clinical and Laboratory Standards Institute (CLSI) 2018 [14].
DNA extraction and DNA quality control
E. coli DNA extraction was performed using a PowerWater DNA extraction kit (Qiagen, Germany) according to the manufacturer's specified procedures. The concentration of extracted DNA was then assessed using a Qubit Fluorometer (Thermo Fisher Scientific, USA).
WGS
The purified DNA was prepared for sequencing on the Oxford Nanopore Technologies (ONT) MinION platform. Before sequencing, it was barcoded using a Nanopore Rapid sequencing gDNA-barcoding kit (SQK-RBK110.96) according to the manufacturer’s protocol.
Bioinformatic analysis
The quality of MinION fastq reads was assessed using FastQC. FastQC is a popular and straightforward tool for quality control checks on raw sequence data from high-throughput sequencing pipelines. The tool provides a modular set of analyses that can provide a quick impression of whether data has any problems before further analysis [15]. A mean read length and read quality greater than 1,000 and exceeding 8.0, respectively, met the criteria for successful QC [16]. The initial real-time analysis was conducted through the Oxford Nanopore EPI2ME web tool, followed by further analysis on Galaxy Europe, an online platform for constructing bioinformatic pipelines without command-line dependencies [17]. The ABRicate tool in Galaxy, which integrates data from the CARD database, ResFinder, NCBI, and PlasmidFinder, was used for ARG detection [18]. ARGs were identified using ResFinder, wherein assembled contigs were screened for various classes of antibiotics in the database. ResFinder is an open online resource for identifying ARGs in next-generation sequencing data and predicting the phenotypes from genotypes [19]. Plasmid replicons were determined using PlasmidFinder, a web tool for detecting and characterizing plasmid sequences in WGS data from Enterobacteriaceae [20]. Bioinformatics analysis was used to determine the proportions of multiple ARGs in bacterial samples. The phylogeny was constructed using RAxML (Randomized Axelerated Maximum Likelihood). RAxML is a fast, scalable, and user-friendly tool for maximum likelihood phylogenetic inference [21]. The final phylogenetic tree and heat map were generated using iTOL (Interactive Tree of Life). iTOL is an online tool for displaying, manipulating, and annotating phylogenetics and other trees [22].
RESULTS
Quality control of the sequencing results
Sequencing with ONT MinION yielded favorable results for further analysis. The average sequence length ranged from 1,839 to 4,502, with reads numbering from 20,244 to 57,632 and total bases spanning 57,415,877 to 248,034,237. The mean read quality consistently surpassed 8.0, ranging from 8.5 to 10.2 (Table 1). These quality control metrics met the requirements, indicating a robust dataset and a reliable foundation for subsequent in-depth analysis.
Table 1. Quality control of E. coli sequencing from fecal samples in pig farm clusters in Banten Province.
| Farm cluster | Quality control | |||
|---|---|---|---|---|
| Mean read length | Number of reads | Total bases | Mean read quality | |
| 1 | 2,950 | 34,207 | 100,919.39 | 9.0 |
| 2 | 3,519 | 20,244 | 71,252.89 | 8.5 |
| 3 | 1,839 | 34,039 | 62,629.45 | 9.3 |
| 4 | 4,502 | 35,847 | 161,405.93 | 8.8 |
| 5 | 4,319 | 57,418 | 248,034.24 | 10.2 |
| 6 | 4,398 | 32,598 | 143,384.19 | 10.1 |
| 7 | 4,371 | 52,101 | 227,738.50 | 10.0 |
| 8 | 2,118 | 53,950 | 114,308.53 | 8.7 |
| 9 | 3,147 | 48,080 | 151,307.77 | 9.7 |
| 10 | 2,082 | 39,805 | 82,900.50 | 8.7 |
| 11 | 3,217 | 57,632 | 185,449.88 | 9.8 |
| 12 | 2,083 | 27,559 | 57,415.88 | 8.7 |
| 13 | 3,649 | 51,634 | 188,451.33 | 9.3 |
| 14 | 2,299 | 35,354 | 81,311.64 | 8.8 |
Phylogenetic analysis and potential multidrug resistance
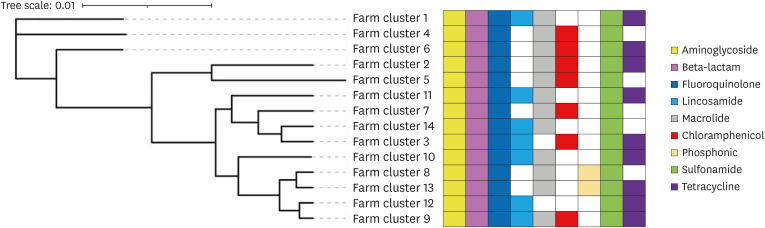
The results of the phylogenetic analysis conducted on E. coli within the pig farming cluster in Banten Province revealed the presence of three distinct phylogenetic branches. In particular, branches 1, 2, and 3 are associated with farm clusters 1, livestock cluster 4, and livestock clusters 6, 2, 5, 11, 7, 14, 3, 10, 8, 13, 12, and 9, respectively. Livestock clusters 8 and 13 exhibited a close genetic relationship, as did clusters 9 and 12, along with clusters 3 and 14 (Fig. 1).
Fig. 1. Phylogenetic tree and multidrug-resistant potential in E. coli pig farming clusters in Banten Province.
E. coli strains derived from fecal samples within these pig farming clusters exhibit a concerning potential for multidrug resistance. In particular, E. coli strains from fecal samples in Banten Province’s pig farming clusters showed the potential for resistance to nine antibiotic classes: aminoglycosides, beta-lactams, fluoroquinolones, lincosamides, macrolides, chloramphenicol, sulfonamides, tetracyclines, and phosphonics. Each livestock cluster, on average, exhibited the potential for resistance to at least six antibiotic classes. The aminoglycoside, beta-lactam, fluoroquinolone, and sulfonamide antibiotic groups consistently displayed resistance across all pig farming clusters. Among the livestock clusters, cluster 9 had the highest potential for resistance to E. coli, encompassing eight antibiotic classes (Fig. 1).
Diversity and distribution of ARGs
The genome sequence analysis of E. coli derived from fecal samples in pig farming clusters in Banten Province identified 51 distinct ARGs. Each livestock cluster harbors a minimum of 10 ARGs, with clusters 3 and 6 exhibiting the minimum and maximum, respectively, with 23 ARGs (Fig. 2). The distribution of ARGs across antibiotic classes included 15, 10, nine, eight, three, two, and one from the aminoglycoside group, sulfonamide group, tetracycline group, beta-lactam group, chloramphenicol group, macrolide and fluoroquinolone groups, and the lincosamide and phosphonic groups, respectively. In particular, the ant(3'')-Ia and qnrS1 genes are prevalent, being present in all pig farming clusters in Banten Province (Table 2).
Fig. 2. Distribution of ARGs in E. coli across each cluster of pig farms in Banten Province.
ARG, antibiotic resistance gene.
Table 2. ARGs profile of E. coli genomes in pig farm clusters in Banten Province.
| Antibiotic class | ARG | Pig farming cluster | ● | Percentage (%) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | ||||
| Aminoglycoside | aph(6)-Id | ● | x | x | x | x | x | x | x | ● | ● | ● | ● | x | ● | 6 | 42.9 |
| aph(6)-Id* | x | x | x | x | x | x | x | ● | x | x | x | x | ● | x | 2 | 14.3 | |
| aph(3'')-Ib | ● | x | x | x | x | x | x | x | ● | ● | ● | ● | x | ● | 6 | 42.9 | |
| aph(3'')-Ib* | x | x | x | x | x | x | x | ● | x | x | x | x | ● | x | 2 | 14.3 | |
| aph(3')-Ia* | x | x | x | x | x | x | x | ● | x | x | x | x | ● | x | 2 | 14.3 | |
| aac(3)-Iid | ● | x | x | x | x | x | x | x | ● | ● | ● | ● | x | ● | 6 | 42.9 | |
| ant(3'')-Ia* | x | x | x | x | x | x | x | x | x | x | x | x | ● | x | 1 | 7.1 | |
| ant(3'')-Ia | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | 14 | 100.0 | |
| ant(3'')-Ii | x | ● | x | ● | x | ● | ● | x | x | x | x | x | x | x | 4 | 28.6 | |
| aac(6')-Iid | x | ● | x | ● | x | ● | ● | x | x | x | x | x | x | x | 4 | 28.6 | |
| aadA2 | x | ● | ● | x | x | ● | ● | x | x | x | x | x | x | x | 4 | 28.6 | |
| aac(3)-Ib | x | x | ● | x | x | x | x | x | x | x | x | x | x | x | 1 | 7.1 | |
| aadA17 | x | x | x | x | x | x | x | x | x | ● | x | ● | x | x | 2 | 14.3 | |
| aadA12 | x | x | x | x | x | x | x | x | ● | x | x | x | x | x | 1 | 7.1 | |
| aadA5 | x | x | x | x | x | x | x | x | ● | x | x | x | x | x | 1 | 7.1 | |
| Beta-lactam | blaCTX-M-55 | ● | x | x | x | x | x | x | ● | ● | x | ● | ● | ● | ● | 7 | 50.0 |
| blaOXA-10 | x | ● | x | ● | ● | ● | ● | x | x | x | x | x | x | x | 5 | 35.7 | |
| blaTEM-1B | x | ● | ● | ● | ● | ● | ● | x | ● | x | x | x | x | x | 7 | 50.0 | |
| blaTEM-105 | x | x | x | ● | ● | ● | ● | x | x | x | x | x | x | x | 4 | 28.6 | |
| blaTEM-141 | x | x | x | ● | ● | ● | ● | x | x | x | x | x | x | x | 4 | 28.6 | |
| blaCTX-M-15 | x | x | x | ● | ● | ● | ● | x | x | ● | x | x | x | x | 5 | 35.7 | |
| blaTEM-122 | x | x | x | x | x | ● | x | x | x | x | x | x | x | x | 1 | 7.1 | |
| blaTEM1C | x | x | x | x | x | x | x | x | ● | x | x | x | x | x | 1 | 7.1 | |
| Fluoroquinolone | qnrS1 | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | 14 | 100.0 |
| qnrVC4 | x | ● | x | ● | ● | ● | ● | x | x | x | x | x | x | x | 5 | 35.7 | |
| Lincosamide | Inu (F) | ● | x | ● | x | x | x | x | x | ● | ● | ● | ● | x | ● | 7 | 50.0 |
| Macrolide | mef(B) | x | ● | x | ● | ● | ● | ● | x | x | x | x | x | x | x | 5 | 35.7 |
| mph (A) | ● | x | x | x | x | x | x | ● | ● | ● | ● | x | ● | ● | 7 | 50.0 | |
| Chloramphenicol | catA1 | x | x | x | x | x | x | x | x | ● | x | x | x | x | x | 1 | 7.1 |
| catB2 | x | ● | x | ● | ● | ● | ● | x | x | x | x | x | x | x | 5 | 35.7 | |
| cmlA1 | x | ● | ● | ● | ● | ● | ● | x | x | x | x | x | x | x | 6 | 42.9 | |
| Phosphonic | fosA4 | x | x | x | x | x | x | x | ● | x | x | x | x | ● | x | 2 | 14.3 |
| Sulfonamide | sul1 | x | ● | x | ● | ● | ● | ● | x | ● | x | x | x | x | x | 6 | 42.9 |
| sul2 | ● | ● | x | ● | ● | ● | ● | ● | ● | ● | ● | ● | x | ● | 12 | 85.7 | |
| sul2* | x | x | x | x | x | x | x | ● | x | x | x | x | ● | ● | 3 | 21.4 | |
| sul3 | x | ● | ● | ● | ● | ● | ● | x | x | x | x | x | x | x | 6 | 42.9 | |
| dfrA1 | x | x | x | ● | ● | ● | ● | x | x | x | x | x | x | x | 4 | 28.6 | |
| dfrA1* | x | x | x | x | x | x | x | x | x | ● | x | x | x | x | 1 | 7.1 | |
| dfrA12 | x | ● | x | ● | ● | ● | ● | x | x | x | x | x | x | x | 5 | 35.7 | |
| dfrA14 | ● | ● | x | ● | ● | ● | ● | ● | ● | ● | ● | x | ● | ● | 12 | 85.7 | |
| dfrA16 | x | ● | x | ● | ● | ● | ● | x | x | x | x | x | x | x | 5 | 35.7 | |
| dfrA17 | x | x | x | x | x | x | x | x | ● | x | x | x | x | x | 1 | 7.1 | |
| Tetracycline | tet(A) | x | x | x | x | x | ● | x | x | x | x | x | x | x | x | 1 | 7.1 |
| tetA* | x | x | x | x | x | x | x | x | x | x | x | ● | x | x | 1 | 7.1 | |
| tet(B) | x | x | x | x | x | x | x | x | ● | x | x | x | x | x | 1 | 7.1 | |
| tet(B)* | x | x | ● | x | x | x | x | x | x | x | ● | x | ● | x | 3 | 21.4 | |
| tet (M) | x | x | ● | x | x | x | x | x | x | x | x | x | x | x | 1 | 7.1 | |
| tetO | x | x | x | x | x | x | x | x | x | ● | x | x | x | x | 1 | 7.1 | |
| tetO(34) | ● | x | x | x | x | x | x | x | x | x | x | x | x | x | 1 | 7.1 | |
| tetX | x | ● | x | x | x | x | x | x | x | x | x | ● | x | x | 2 | 14.3 | |
| tetX* | x | ● | x | x | x | x | x | x | x | x | x | x | x | x | 1 | 7.1 | |
ARG, antibiotic resistance gene.
●, ARG detected; x, ARG undetected.
Plasmid profile
The prevalence of ARGs in E. coli from fecal samples within pig farming clusters in Banten Province is predominantly associated with plasmids rather than chromosomes. On average, 89.4% of the identified ARGs in the E. coli pig farming cluster were plasmid-borne, with values ranging from 43% to 97%. Clusters 4 and 7 exhibited the highest percentage of ARGs originating from plasmids, recording 97%. In contrast, ARGs sourced from chromosomes in E. coli isolates from pig farming clusters averaged only 10.6%, ranging from 3% to 57%. The highest percentage of chromosome-associated ARGs was observed in cluster 13 farms (Table 3).
Table 3. Percentage of plasmids carrying ARGs in E. coli from fecal samples of pig farm clusters in Banten Province.
| Farm cluster | Plasmid | Chromosome |
|---|---|---|
| 1 | 11 (91.7) | 1 (8.3) |
| 2 | 25 (92.6) | 2 (7.4) |
| 3 | 11 (84.6) | 2 (15.4) |
| 4 | 33 (97.0) | 1 (3.0) |
| 5 | 31 (94.0) | 2 (6.0) |
| 6 | 42 (95.5) | 2 (4.5) |
| 7 | 31 (97.0) | 1 (3.0) |
| 8 | 7 (54.0) | 6 (46.0) |
| 9 | 28 (96.5) | 1 (3.5) |
| 10 | 13 (86.7) | 2 (13.3) |
| 11 | 11 (77.0) | 2 (33.0) |
| 12 | 10 (91.0) | 1 (9.0) |
| 13 | 6 (43.0) | 8 (57.0) |
| 14 | 11 (92.0) | 1 (8.0) |
| Average | 89.4 | 10.6 |
Values are presented as number (%).
DISCUSSION
This research conducted a WGS analysis of E. coli isolates obtained from fecal samples in pig farming clusters in Banten Province, building upon prior investigations [12]. In particular, WGS research on E. coli derived from pig farm samples in Banten Province marked the pioneering effort in Indonesia, providing crucial data for developing preventive and control measures against antibiotic resistance. WGS applications offered comprehensive insights into the initial emergence and spread of antibiotic resistance, facilitating predictions regarding likely antibiotic resistance profiles and serving as a foundation for developing effective policies to control antibiotic resistance [8].
Phylogenetic analysis of E. coli in pig farming clusters in Banten Province revealed three distinct phylogenetic groups: Group 1 (cluster 1), Group 2 (cluster 4), and Group 3. Within phylogenetic Group 3, pig farming clusters were further categorized into two lineages (clades): the first clade (cluster 6) and the second clade (clusters 2, 5, 11, 7, 14, 3, 10, 8, 13, 12, and 9). The second clade was subsequently divided into two sub-clades: the first sub-clade (clusters 2 and 5) and the second sub-clade (clusters 11, 7, 14, 3, 10, 8, 13, 12, and 9). Close relationships were observed between clusters 9 and 12, clusters 8 and 13, and clusters 3 and 14. Interestingly, the study findings indicated that E. coli, which are closely related phylogenetically, do not always have similar distribution and resistance patterns (Fig. 1).
E. coli isolated from fecal samples in a pig farming cluster in Banten Province exhibited resistance to nine classes of antibiotics, including those crucial for human medicine. Dominant antibiotic resistance was observed in the aminoglycosides, beta-lactams, fluoroquinolones, sulfonamides, and macrolides groups in the pig farming cluster. Consistent with Peng et al. [23], E. coli isolated from pig farms in China displayed resistance to multiple antibiotic classes, including sulfonamides, tetracyclines, fluoroquinolones, macrolides, and beta-lactams. Similarly, Carhuaricra et al. [24] reported prevalent resistance in E. coli from fecal samples in pig farms in Lima, Peru, particularly to tetracyclines, sulfonamides, aminoglycosides, chloramphenicol, beta-lactams, and fluoroquinolones.
Even more concerning, all E. coli isolated from the fecal samples in the pig farm cluster showed multidrug resistance. Each livestock cluster exhibited resistance to at least six classes of antibiotics, with the highest achieving resistance to eight classes of antibiotics, as observed in cluster 9. The elevated prevalence of multidrug resistance in E. coli from pig farm samples has been reported consistently [23,25]. Zhang et al. [25] reported a rise in MDR E. coli in pig farms that they attributed to the excessive and uncontrolled use of antibiotics. This poses a serious risk because high levels of MDR E. coli in pig farms can disseminate into the environment, escalating the risk of transmission to humans and posing a significant threat to public health [26]. The heightened level of multidrug resistance raises serious concerns regarding the limited availability of effective antibiotics for treating bacterial diseases, diminishing the ability to combat infectious diseases in humans and animals [27].
Tetracycline antibiotics (oxytetracycline and tetracycline), beta-lactams (amoxicillin and penicillin), and sulfonamides (sulfadimethypyrimidine sodium, sulfadiazine sodium, sulfadimidine sodium, and sulfamerazine sodium) are commonly used in pig farming in Indonesia [28]. The majority of antibiotics in pig farms are used for treatment (55.21%), disease prevention (42.71%), and production enhancement (2.08%) [28]. Previous research also reported the use of sulfonamide antibiotics (sulfamonomethoxine and sulfamethoxazole), tetracyclines (chlortetracycline, doxycycline, and oxycycline), fluoroquinolones (enrofloxacin), macrolides (kitasamycin tartrate), and beta-lactams (cefotaxime, penicillin, and amoxicillin) in pig farming for growth promotion or disease treatment [23]. A previous study reported the highest percentage of feed containing antibiotics in pigs (55.4%) compared to chickens (42.2%), quail (18.9%), and ducks (9.2%). The most commonly added antibiotics in swine feed formulations were Bacitracin (24.8%), chlortetracycline (23.9%), and florfenicol (17.4%) [4].
Phylogenetic analysis of E. coli in the pig farming clusters in Banten Province revealed significant variations in antibiotic resistance levels and carried ARGs despite being the same breed and closely related. For example, livestock clusters 9 and 12, clusters 8 and 13, and clusters 3 and 14, although closely related, displayed substantial differences in antibiotic resistance. In particular, livestock cluster 9 exhibited resistance to macrolide and chloramphenicol antibiotics, while cluster 12 did not. Furthermore, the ARG diversity of cluster 9 included 18 ARGs. In contrast, cluster 12 carried 11 ARGs (Fig. 1). This variability may be influenced by the role of plasmids and other MGEs, which predominantly participate in carrying MDR genes and horizontally transferring genes between bacterial species and across unrelated species [7]. Consequently, the proliferation of bacteria carrying MDR genes extends beyond livestock to impact the environment and human populations.
The genome sequence analysis of E. coli in this study revealed a high diversity of ARGs across all pig farming clusters in Banten Province. Fifty-one ARGs from nine classes of antibiotics were identified in E. coli isolates, with each cluster carrying a minimum of 10 ARGs (cluster 3) and the highest count being 23 ARGs (cluster 6). Aminoglycoside class ARGs were the most frequently detected (15 ARGs), followed by sulfonamide class (10 ARGs), tetracycline class (9 ARGs), and beta-lactam class (8 ARGs) (Fig. 2).
Genes from the aminoglycoside class, such as ant(3'')-Ia (100%), aph(3'')-Ib (42.9%), aph(6)-Id (42.9%), and aac(3)-Iid (42.9%), were dominantly present in E. coli clusters from pig farms (Table 2). The same thing was also reported elsewhere [24,29]. In particular, the ant(3'')-Ia gene was found in all pig farming clusters, frequently associated with the cassettes gene in class 1 integrons, and is the most commonly found ARG in Gram-negative bacteria globally [7,30]. The aph(6)-Id and aph(3'')-Ib genes, which are associated with streptomycin resistance, are widespread in E. coli, and are often linked to unique mobile elements and globally distributed in pigs [31]. The ant(3'')-Ia, aph(3'')-Ib, aph(6)-Id, and aac(3)-Iid genes play a role in the enzymatic inactivation resistance mechanism, which can inactivate aminoglycosides by modifying the molecules so that they cannot reach or binding to the target site. The ant(3'')-Ia gene produces the nucleotidyltransferase enzyme. The aph(3'')-Ib and aph(6)-Id genes produce the phosphotransferase enzyme, while the aac(3)-Iid produces the acetyltransferase enzyme [7].
This study revealed ESBL-producing E. coli, which is a global health concern [24,29]. The predominant beta-lactam ARGs included blaCTX-M-55 (50% cluster) and blaTEM1B (50% cluster), followed by blaCTX-M-15 (35.7%) and blaOXA-10 (35.7%) (Table 2). Similar findings were reported in other studies, with blaCTX-M-55 dominating in pigs in Vietnam [32] and blaTEM1B being prevalent in pig farms in Peru [24]. The blaCTX-M gene in E. coli is distributed widely in livestock (pigs and poultry), meat, vegetables, and humans. The blaCTX-M gene has several variants including blaCTX-M-1, blaCTX-M-15, blaCTX-M-55, blaCTX-M-9, blaCTX-M-14, blaCTX-M-27, and blaCTX-M-65 [32,33].
From the fluoroquinolone class, two ARGs, qnrS1 (100%) and qnrVC4 (35.7%), were detected (Table 2). The qnrS1 gene, detected in all pig farming clusters, is the most commonly found gene in E. coli from pig populations [7,34]. The gene is associated with plasmid-mediated quinolone resistance (PMQR), protecting DNA from quinolone binding. These genes are often found on IncX1 and IncN plasmids, facilitating the spread of ARGs to various animal species and environments [35]. PMQR-mediated genes have been reported in the last two decades, resulting in an even more complex genetic backbone of fluoroquinolone resistance [7,34]. The high prevalence of qnrS1 poses challenges in controlling fluoroquinolone resistance, particularly considering the classification of fluoroquinolones as critically important antibiotics for human medicine [27]. Their use in livestock raises concerns about the cross-selection of genetic determinants of resistance to antimicrobials used in human medicine, posing a severe threat to public health [36].
The sul2 (85.7%) and dfrA14 (85.7%) genes were the most dominant genes detected from the sulfonamide class in this study (Table 2). The sul1, sul2, and sul3 genes are distributed widely in E. coli from various animal species worldwide, and sul2 is the most dominant ARG in pig farms. Several studies have reported that the sul1, sul2, and sul3 genes are often located on plasmids, including plasmids that also contain other ARGs (MDR) [31,37]. The sul2 gene is often associated with the streptomycin resistance gene strA-strB [7]. Similarly, sul1 is often found with other ARGs in gene cassettes in variable regions of class 1 integrons and MDR plasmids carrying ESBL genes [37]. The sul3 gene is also associated with the macrolide resistance gene mef(B) and class 1 integrons [7]. IncFII is the dominant type in sul2-carrying plasmids, while IncI1 is the most common type in sul1- and sul3-carrying plasmids [37]. The dfrA14 gene has been identified in E. coli from pigs in various countries worldwide [30]. Previous studies showed that the dfrA14 gene in E. coli originating from animals and food products from an animal origin is related to integrons. The functionally active dfrA14 gene from E. coli in food products of animal origin was found outside the integron but was inserted into the plasmid-borne strA gene [38].
Tetracycline, an antibiotic used extensively in animals, constitutes 37% of the total antimicrobial agent sales for animals in 25 European Union and European Economic Area countries [39]. The indiscriminate use of this antibiotic exerts selective pressure on bacteria, particularly E. coli, leading to the development of resistance to tetracycline antibiotics through various tetracycline resistance genes (tet genes). tetB* (21.4%) and tetX (14.3%) were predominant among the tet genes identified in this study. The tetA and tetB genes, prevalent in E. coli from animal samples, particularly pigs, are part of conjugative and nonconjugative transposons, such as Tn1721 (tetA) and Tn10 (tetB), integrated into plasmids. Single E. coli isolates often exhibit multiple tet genes resulting from various tet genes on plasmids or other MGEs acquired at different times and under different conditions. In addition, E. coli plasmid-borne tet genes can combine with other ARGs from diverse antibiotic classes, resulting in MDR plasmids [7]. MDR plasmids were reported in E. coli from various animal species in various countries, such as the T078 plasmid carrying the qnrS1, blaCTX-M-14, blaTEM-1, floR, and tetA genes in E. coli isolated from pigs in China [40].
The tetX gene, previously unreported in Indonesian pigs, was first identified in this study. The tetX gene, commonly with the tetX4 variant, is frequently associated with plasmids, facilitating the exchange of genetic information among bacteria. The plasmid-mediated tetX gene (tetX4 variant) has been found in animal-origin samples globally (pigs, ducks, geese, broilers, cows, freshwater fish, shrimp, and migratory birds), with pigs being the predominant source [41]. Studies from China revealed the emergence of tigecycline resistance mediated by the tetX4 plasmid in E. coli isolates. The highly transmissible IncQ1 plasmid carrying tetX4 can mobilize and stabilize in clinical and laboratory strains of Enterobacteriaceae bacteria. TetX4-positive E. coli strains, also containing mcr-1, are widespread in pigs, poultry, soil, and dust samples in China [42]. Plasmid-mediated tetX4 compromises the efficacy of all tetracycline group antibiotics, including tigecycline, which is a last-resort antibiotic for complicated bacterial infections caused by MDR Gram-negative and Gram-positive bacteria [43].
Chloramphenicol, an antibiotic prohibited in the Indonesian livestock industry according to Minister of Agriculture Regulation No. 14/PERMENTAN/PK.350/5/2017 [44] and Minister of Agriculture Decree No. 9736/PI.500/F/09/2020 [45], displayed resistance in seven pig farming clusters in this study. E. coli in these clusters carried three ARGs: cmlA1, catB2, and catA1. cmlA1 was the most prevalent (42.9%), followed by catB2 (35.7%) and catA1 (7.1%). Peng et al. [23] reported similar findings in China, where chloramphenicol-resistant E. coli (cmlA, floR) persisted in pig farms despite the antibiotic ban since 2002 [46], possibly because of the use of florfenicol, a related antibiotic, in pig farming [4,47]. AbuOun et al. [29] also reported the presence of catA1, catA6, cml, and floR genes in pig farms in the United Kingdom.
In pig farming clusters of Banten Province, the majority of ARGs in E. coli were plasmid-borne (89.4%) rather than chromosomal (10.6%). Clusters 4 and 7 exhibited the highest percentage of ARGs originating from plasmids (97%) (Table 3). Plasmids play a crucial role in the horizontal transfer of ARGs with conjugation between bacterial species, facilitating the spread of multidrug resistance genes [48]. The IncF plasmid type, prevalent in humans and animals, particularly in E. coli, is a major contributor to this dissemination, carrying resistance and virulence determinants [49]. This plasmid was identified most widely during the pandemic, carrying MDR genes, virulence of the E. coli O25:H4-ST131 clone, and blaCTX-M-15 gene [35]. IncHI1 and IncHI2 plasmids are also frequently associated with resistance to antibiotics, such as sulfonamides, aminoglycosides, tetracyclines, streptomycin, and cephalosporins [50]. These plasmids aid in the spread of specific ARGs and contribute to the selection and persistence of other ARGs [7].
The challenge in controlling antibiotic resistance lies in plasmid-borne ARGs, which contribute significantly to the spread of resistance determinants and undistinguishable plasmids identified in bacterial strains from very distant geographical areas, usually without any clear epidemiological link. Plasmids, common in natural bacteria, often carry multiple linked genetic determinants, simultaneously conferring resistance to several antibiotic classes. Furthermore, plasmids harbor virulence factors and addiction systems, enhancing their stability and persistence in bacterial hosts across diverse environmental conditions [35,48].
ACKNOWLEDGMENTS
The authors wish to thank the Head of the Tangerang Regency Agriculture and Food Security Service, along with the dedicated staff, for their invaluable support and the facilities provided throughout the research process. The authors also wish to express their appreciation to the Bogor Animal Product Quality Testing and Certification Center Laboratory and its staff for their assistance and the use of laboratory facilities, which contributed significantly to the successful execution of this research.
Footnotes
Funding: This research was supported by a Fundamental-Regular Research grant from the Ministry of Education, Culture, Research, and Technology under contract number 102/E5/PG.02.00.PL/2023.
Conflict of Interest: The authors declare no conflicts of interest.
- Conceptualization: Latif H, Basri C, Pazra DF.
- Data curation: Latif H, Basri C.
- Formal analysis: Latif H, Rahayu P.
- Funding acquisition: Latif H, Basri C.
- Investigation: Pazra DF.
- Methodology: Latif H, Rahayu P, Pazra DF.
- Project administration: Pazra DF.
- Validation: Latif H.
- Writing - original draft: Latif H, Pazra DF.
- Writing - review & editing: Basri C, Wibawan IWT.
References
- 1.Yao X, Doi Y, Zeng L, Lv L, Liu JH. Carbapenem-resistant and colistin-resistant Escherichia coli co-producing NDM-9 and MCR-1 . Lancet Infect Dis. 2016;16(3):288–289. doi: 10.1016/S1473-3099(16)00057-8. [DOI] [PubMed] [Google Scholar]
- 2.He T, Wang R, Liu D, Walsh TR, Zhang R, Lv Y, et al. Emergence of plasmid-mediated high-level tigecycline resistance genes in animals and humans. Nat Microbiol. 2019;4(9):1450–1456. doi: 10.1038/s41564-019-0445-2. [DOI] [PubMed] [Google Scholar]
- 3.Van Boeckel TP, Pires J, Silvester R, Zhao C, Song J, Criscuolo NG, et al. Global trends in antimicrobial resistance in animals in low- and middle-income countries. Science. 2019;365(6459):1–5. doi: 10.1126/science.aaw1944. [DOI] [PubMed] [Google Scholar]
- 4.Van Cuong N, Nhung NT, Nghia NH, Mai Hoa NT, Trung NV, Thwaites G, et al. Antimicrobial consumption in medicated feeds in vietnamese pig and poultry production. EcoHealth. 2016;13(3):490–498. doi: 10.1007/s10393-016-1130-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andersson DI, Hughes D. Microbiological effects of sublethal levels of antibiotics. Nat Rev Microbiol. 2014;12(7):465–478. doi: 10.1038/nrmicro3270. [DOI] [PubMed] [Google Scholar]
- 6.CDC Centers for Disease Control. Antibiotic resistance threats in the United States. US: Centers for Disease Control; 2019. pp. 73–74. [Google Scholar]
- 7.Poirel L, Madec JY, Lupo A, Schink AK, Kieffer N, Nordmann P, et al. Antimicrobial resistance in Escherichia coli . Microbiol Spectr. 2018;6(4):1–27. doi: 10.1128/microbiolspec.arba-0026-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.WHO World Health Organization. GLASS Whole-Genome Sequencing for Surveillance of Antimicrobial Resistance. Geneva: World Health Organization; 2020. pp. 1–42. [Google Scholar]
- 9.Gong L, Wong CH, Cheng WC, Tjong H, Menghi F, Ngan CY, et al. Picky comprehensively detects high-resolution structural variants in nanopore long reads. Nat Methods. 2018;15(6):455–460. doi: 10.1038/s41592-018-0002-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feng Y, Zhang Y, Ying C, Wang D, Du C. Nanopore-based fourth-generation DNA sequencing technology. Genomics Proteomics Bioinformatics. 2015;13(1):4–16. doi: 10.1016/j.gpb.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.WHO World Health Organization. WHO Integrated Global Surveillance on ESBL-Producing E. coli Using a "One Health" Approach: Implementation and Opportunities. Geneva: World Health Organization; 2021. pp. 16–36. [Google Scholar]
- 12.Pazra DF, Latif H, Basri C, Wibawan IW, Rahayu P. Detection of tetracycline resistance genes and their diversity in Escherichia coli isolated from pig farm waste in Banten province, Indonesia. Vet World. 2023;16(9):1907–1916. doi: 10.14202/vetworld.2023.1907-1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pazra DF, Latif H, Basri C, Wibawan IW. Tetrasiklin Resistance in Escherichia coli Isolated from Pig Farm, Pig Slaughterhouse, and the Environment in Banten Province. J Kedokt Hewan. 2023;17(4):121–126. [Google Scholar]
- 14.CLSI Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing. 28th ed. Wayne: Clinical and Laboratory Standards Institute; 2018. pp. 30–37. [Google Scholar]
- 15.Andrews S. FastQC: A Quality Control Tool for High Throughput Sequence Data [Internet] England: Babraham Institute; [Updated 2024]. [Accessed 2024 April 2]. http://www.bioinformatics.babraham.ac.uk/projects/fastqc/ [Google Scholar]
- 16.Wang Y, Zhao Y, Bollas A, Wang Y, Au KF. Nanopore sequencing technology, bioinformatics and applications. Nat Biotechnol. 2021;39(11):1348–1365. doi: 10.1038/s41587-021-01108-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Community G Galaxy Community. The Galaxy platform for accessible, reproducible and collaborative biomedical analyses: 2022 update. Nucleic Acids Res. 2022;50(W1):W345–W351. doi: 10.1093/nar/gkac247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seemann T. ABRicate: Mass Screening of Contigs for Antibiotic Resistance Genes [Internet] California: GitHub; [Updated 2022]. [Accessed 2024 April 2]. https://github.com/tseemann/abricate . [Google Scholar]
- 19.Florensa AF, Kaas RS, Clausen PT, Aytan-Aktug D, Aarestrup FM. ResFinder - an open online resource for identification of antimicrobial resistance genes in next-generation sequencing data and prediction of phenotypes from genotypes. Microb Genom. 2022;8(1):000748. doi: 10.1099/mgen.0.000748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carattoli A, Hasman H. PlasmidFinder and in silico pmlst: identification and typing of plasmid replicons in whole-genome sequencing (WGS) Methods Mol Biol. 2020;2075:285–294. doi: 10.1007/978-1-4939-9877-7_20. [DOI] [PubMed] [Google Scholar]
- 21.Kozlov AM, Darriba D, Flouri T, Morel B, Stamatakis A. RAxML-NG: a fast, scalable and user-friendly tool for maximum likelihood phylogenetic inference. Bioinformatics. 2019;35(21):4453–4455. doi: 10.1093/bioinformatics/btz305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Letunic I, Bork P. Interactive Tree Of Life (iTOL) v5: an online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021;49(W1):W293–W296. doi: 10.1093/nar/gkab301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peng Z, Hu Z, Li Z, Zhang X, Jia C, Li T, et al. Antimicrobial resistance and population genomics of multidrug-resistant Escherichia coli in pig farms in mainland China. Nat Commun. 2022;13(1):1116. doi: 10.1038/s41467-022-28750-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carhuaricra D, Duran Gonzales CG, Rodríguez Cueva CL, Ignacion León Y, Silvestre Espejo T, Marcelo Monge G, et al. Occurrence and genomic characterization of mcr-1-harboring Escherichia coli isolates from chicken and pig farms in Lima, Peru. Antibiotics (Basel) 2022;11(12):1–10. doi: 10.3390/antibiotics11121781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang P, Shen Z, Zhang C, Song L, Wang B, Shang J, et al. Surveillance of antimicrobial resistance among Escherichia coli from chicken and swine, China, 2008-2015. Vet Microbiol. 2017;203(203):49–55. doi: 10.1016/j.vetmic.2017.02.008. [DOI] [PubMed] [Google Scholar]
- 26.Park J, Gasparrini AJ, Reck MR, Symister CT, Elliott JL, Vogel JP, et al. Plasticity, dynamics, and inhibition of emerging tetracycline resistance enzymes. Nat Chem Biol. 2017;13(7):730–736. doi: 10.1038/nchembio.2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.WHO World Health Organization. Critically Important Antimicrobials for Human Medicine. 6th Revision. Geneva: World Health Organization; 2018. pp. 1–45. [Google Scholar]
- 28.Kallau NH, Wibawan IW, Lukman DW, Sudarwanto MB. Analysis of relationship between knowledge and attitudes towards the practice of using antibiotics by pig farms in the city of Kupang, East Nusa Tenggara province. JSV. 2018;36(2):200–212. [Analisis Hubungan antara Pengetahuan dan Sikap terhadap Praktik Penggunaan Antibiotik oleh Peternakan Babi di Kota Kupang Provinsi Nusa Tenggara Timur] [Google Scholar]
- 29.AbuOun M, O’Connor HM, Stubberfield EJ, Nunez-Garcia J, Sayers E, Crook DW, et al. Characterizing antimicrobial resistant Escherichia coli and associated risk factors in a cross-sectional study of pig farms in Great Britain. Front Microbiol. 2020;11(861):861. doi: 10.3389/fmicb.2020.00861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marchant M, Vinué L, Torres C, Moreno MA. Change of integrons over time in Escherichia coli isolates recovered from healthy pigs and chickens. Vet Microbiol. 2013;163(1-2):124–132. doi: 10.1016/j.vetmic.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 31.Zhang WJ, Xu XR, Schwarz S, Wang XM, Dai L, Zheng HJ, et al. Characterization of the IncA/C plasmid pSCEC2 from Escherichia coli of swine origin that harbours the multiresistance gene cfr . J Antimicrob Chemother. 2014;69(2):385–389. doi: 10.1093/jac/dkt355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hounmanou YM, Bortolaia V, Dang ST, Truong D, Olsen JE, Dalsgaard A. ESBL and AmpC b-Lactamase encoding genes in E. coli from pig and pig farm workers in Vietnam and their association with mobile genetic elements. Front Microbiol. 2021;12(629139):629139. doi: 10.3389/fmicb.2021.629139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zurfluh K, Nüesch-Inderbinen M, Morach M, Zihler Berner A, Hächler H, Stephan R. Extended-spectrum-β-lactamase-producing Enterobacteriaceae isolated from vegetables imported from the Dominican Republic, India, Thailand, and Vietnam. Appl Environ Microbiol. 2015;81(9):3115–3120. doi: 10.1128/AEM.00258-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jacoby GA, Strahilevitz J, Hooper DC. Plasmid-mediated quinolone resistance. Microbiol Spectr. 2014;2(5):1–42. doi: 10.1128/microbiolspec.PLAS-0006-2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carattoli A. Plasmids and the spread of resistance. Int J Med Microbiol. 2013;303(6-7):298–304. doi: 10.1016/j.ijmm.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 36.Burow E, Grobbel M, Tenhagen BA, Simoneit C, Ladwig M, Szabó I, et al. Antimicrobial susceptibility in faecal Escherichia coli from pigs after enrofloxacin administration in an experimental environment. Berl Munch Tierarztl Wochenschr. 2018;131(5-6):170–181. [Google Scholar]
- 37.Wu S, Dalsgaard A, Hammerum AM, Porsbo LJ, Jensen LB. Prevalence and characterization of plasmids carrying sulfonamide resistance genes among Escherichia coli from pigs, pig carcasses and human. Acta Vet Scand. 2010;52(1):47. doi: 10.1186/1751-0147-52-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kikuvi GM, Schwarz S, Ombui JN, Mitema ES, Kehrenberg C. Streptomycin and chloramphenicol resistance genes in Escherichia coli isolates from cattle, pigs, and chicken in Kenya. Microb Drug Resist. 2007;13(1):62–68. doi: 10.1089/mdr.2006.9998. [DOI] [PubMed] [Google Scholar]
- 39.Grave K, Torren-Edo J, Muller A, Greko C, Moulin G, Mackay D, et al. ESVAC Group. Variations in the sales and sales patterns of veterinary antimicrobial agents in 25 European countries. J Antimicrob Chemother. 2014;69(8):2284–2291. doi: 10.1093/jac/dku106. [DOI] [PubMed] [Google Scholar]
- 40.Huang SY, Zhu XQ, Wang Y, Liu HB, Dai L, He JK, et al. Co-carriage of qnrS1, floR, and bla(CTX-M-14) on a multidrug-resistant plasmid in Escherichia coli isolated from pigs. Foodborne Pathog Dis. 2012;9(10):896–901. doi: 10.1089/fpd.2012.1131. [DOI] [PubMed] [Google Scholar]
- 41.Zhang S, Wen J, Wang Y, Wang M, Jia R, Chen S, et al. Dissemination and prevalence of plasmid-mediated high-level tigecycline resistance gene tet (X4) Front Microbiol. 2022;13(13):969769. doi: 10.3389/fmicb.2022.969769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun J, Chen C, Cui CY, Zhang Y, Liu X, Cui ZH, et al. Plasmid-encoded tet(X) genes that confer high-level tigecycline resistance in Escherichia coli . Nat Microbiol. 2019;4(9):1457–1464. doi: 10.1038/s41564-019-0496-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.He F, Shi Q, Fu Y, Xu J, Yu Y, Du X. Tigecycline resistance caused by rpsJ evolution in a 59-year-old male patient infected with KPC-producing Klebsiella pneumoniae during tigecycline treatment. Infect Genet Evol. 2018;66(66):188–191. doi: 10.1016/j.meegid.2018.09.025. [DOI] [PubMed] [Google Scholar]
- 44.Republic of Indonesia. Regulation of the Ministry of Agriculture of the Republic of Indonesia Number 14/Permentan/PK.350/5/2017 concerning Classification of Veterinary Medicines [Peraturan Menteri Pertanian Republik Indonesia Nomor 14/Permentan/PK.350/5/2017 tentang Klasifikasi Obat Hewan] 2017. https://www.fao.org/faolex/results/details/en/c/LEX-FAOC169455/
- 45.Republic of Indonesia. Decree of the Ministry of Agriculture of the Republic of Indonesia Number 9736/PI.500/F/09/2020 concerning Amendments to Attachment III to Regulation of the Ministry of Agriculture of the Republic of Indonesia Number 14/Permentan/PK.350/5/2017 concerning Classification of Veterinary Medicines [Keputusan Menteri Pertanian Republik Indonesia Nomor 9736/PI.500/F/09/2020 tentang Perubahan Lampiran III Peraturan Menteri Pertanian Republik Indonesia Nomor 14/Permentan/PK.350/5/2017 tentang Klasifikasi Obat Hewan] 2020. [Google Scholar]
- 46.MOA. Announcement of the Ministry of Agriculture and Rural People's Republic of China No. 193 [Internet] China: MOA; [Updated 2002]. [Accessed 2023 October 23]. http://www.moa.gov.cn/ztzl/ncpzxzz/flfg/200709/t20070919_893091.htm . [Google Scholar]
- 47.Yang H, Paruch L, Chen X, van Eerde A, Skomedal H, Wang Y, et al. Antibiotic application and resistance in swine production in China: current situation and future perspectives. Front Vet Sci. 2019;6(136):136. doi: 10.3389/fvets.2019.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Partridge SR, Kwong SM, Firth N, Jensen SO. Mobile genetic elements associated with antimicrobial resistance. Clin Microbiol Rev. 2018;31(4):1–61. doi: 10.1128/CMR.00088-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rozwandowicz M, Brouwer MS, Fischer J, Wagenaar JA, Gonzalez-Zorn B, Guerra B, et al. Plasmids carrying antimicrobial resistance genes in Enterobacteriaceae. J Antimicrob Chemother. 2018;73(5):1121–1137. doi: 10.1093/jac/dkx488. [DOI] [PubMed] [Google Scholar]
- 50.Egervärn M, Börjesson S, Byfors S, Finn M, Kaipe C, Englund S, et al. Escherichia coli with extended-spectrum beta-lactamases or transferable AmpC beta-lactamases and Salmonella on meat imported into Sweden. Int J Food Microbiol. 2014;171(171):8–14. doi: 10.1016/j.ijfoodmicro.2013.11.005. [DOI] [PubMed] [Google Scholar]