Graphical abstract
Keywords: Anthelmintic therapy, Nematicidal compounds, Rhabditis sp., Haemonchus contortus, Strongylidae sp., Heligmosomoides polygyrus/bakeri
Highlights
-
•
New thiosemicarbazide and 1,2,4-triazole derivatives, not described in the literature, were synthesized.
-
•
The anthelmintic activity was tested in vitro and in vivo on four pathogenic nematode species.
-
•
The in vivo studies showed no systemic toxicity of the tested compound (II-1).
-
•
The degree of inhibition of selected CYP450 enzymes depending on the concentration of the tested compound II-1 was assessed.
-
•
The in silico studies indicated tubulin and the SDH enzyme as potential molecular targets of the tested compound (II-1)
Abstract
Introduction
Intestinal parasitic infections are neglected diseases and, due to the increasing resistance of parasites to available drugs, they pose an increasing therapeutic challenge. Therefore, there is a great need for finding new compounds with antiparasitic activity. Objectives: In this work, new thiosemicarbazide and 1,2,4-triazole derivatives were synthesized and tested for their anthelmintic activity.
Methods
The synthesis was carried out by classical methods of organic chemistry. Anthelmintic activity tests were carried out in vitro (Rhabditis sp., Haemonchus contortus, Strongylidae sp.) in vivo (Heligmosomoides polygyrus/bakeri), and in silico analysis was performed.
Results
Quinoline-6-carboxylic acid derivative compounds were designed and synthesized. The highest activity in the screening tests in the Rhabditis model was demonstrated by compound II-1 with a methoxyphenyl substituent LC50 = 0.3 mg/mL. In the next stage of the research, compound II-1 was analyzed in the H. contortus model. The results showed that compound II-1 was active and had ovicidal (percentage of dead eggs > 45 %) and larvicidal (percentage of dead larvae > 75 %) properties. Studies in the Strongylidae sp. model confirmed the ovicidal activity of compound II-1 (percentage of dead eggs ≥ 55 %). In vivo studies conducted in the H. polygyrus/bakeri nematode model showed that the number of nematodes decreased by an average of 30 % under the influence of compound II-1. In silico studies have shown two possible modes of action of compound II-1, i.e. inhibition of tubulin polymerization and SDH. The test compound did not show any systemic toxic effects. Its influence on drug metabolism related to the activity of cytochrome CYP450 enzymes was also investigated.
Conclusion
The results obtained in the in vitro, in vivo, and in silico studies indicate that the test compound can be described as a HIT, which in the future may be used in the treatment of parasitic diseases in humans and animals.
Introduction
Parasitic infections of the gastrointestinal tract caused by nematodes are widespread in both humans and animals. Intestinal helminths are a considerable medical and economic challenge [1], [2], [3], [4], [5], [6]. People become infected with intestinal nematodes by ingesting infected eggs or, in some species, by larvae actively penetrating the human host skin. The most common parasitic diseases caused by intestinal nematodes include ascariasis, ancylostomiasis/necatoriasis, trichuriasis, and enterobiasis. Literature data indicate that there are approximately 800–1000 million cases of roundworm, 600–900 million cases of hookworm, 500 million cases of whipworm, and 200 million cases of pinworm infection worldwide [7], [8], [9], [10], [11], [12], [13], [14]. These nematodes can cause gastrointestinal symptoms, dehydration, and inhibition of psychophysical development in children [2], [15], [16]. A variety of agents are used in the treatment of intestinal nematodes, e.g. thiabendazole, mebendazole, and albendazole (benzimidazole derivatives), pyrantel, and ivermectin [17], [18], [19], [20]. In recent years, as a result of the abuse and inadequate use of drugs in the treatment of parasitic diseases in both humans and animals, an increase in the drug resistance of parasites to available drugs has been observed. Multidrug resistance means that the pool of effective substances used in the treatment of parasitic diseases is shrinking, which generates the need to search for new compounds with antiparasitic and anthelmintic properties [21], [22], [23], [24], [25], [26], [27], [28], [29]. In addition, due to the fact that parasitic diseases largely affect the population from poor regions of the world, drug companies do not want to invest in antiparasitic drugs for fear of low economic return [11], [30], [31]. Given the problems related to parasitic diseases and their treatment, scientists worldwide are now focusing on developing new compounds with antiparasitic activity [26], [31], [32], [33], [34], [35], [36]. The group of compounds with promising biological activity includes thiosemicarbazide and triazole derivatives.
Earlier studies on the antiparasitic activity of thiosemicarbazide and 1,2,4-triazole derivatives of Toxoplasma gondii indicated the activity of these compounds [37], [38]. The results published so far on the anthelmintic activity of thiosemicarbazide and derivatives of 1,2,4-triazole were obtained in research carried out mainly on non-pathogenic earthworms (Pheretima posthuma, Eudrilus eugeniae, Pentoscoplex corethrusus) [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49]. Literature data are collected in Table 1. There are very few studies of the anthelmintic activity of this class of compounds [31], [33]. In this work, we synthesized new compounds and focused our studies on testing anthelmintic activity in four different pathogenic nematode models. In vitro studies were carried out with the use of three nematode models: Rhabditis sp., Haemonchus contortus, and Strongylidae sp., while the Heligmosomoides polygyrus/bakeries nematodes were used in in vivo studies. Additionally, molecular docking (in silico) was performed to determine the possible mechanism of anthelmintic activity. The specific aim of this study was to analyze the anthelmintic activity of the newly synthesized quinoline-6-carboxylic acid thiosemicarbazide derivatives and their cyclic analogues 1,2,4-triazole-3-thione derivatives for their potential use in the treatment and control of parasitic diseases caused by nematodes in humans and animals. Quinoline was chosen because this heterocyclic amine is the skeleton of many drugs used in the treatment of protozoa and roundworm infections, including quinine, chloroquine and its derivatives, primaquine, pyrvinum, and oxamniquine.
Table 1.
Literature review of the anthelmintic activity of 1,2,4-triazole derivatives.
| Structure | Model | Activity | Ref |
|---|---|---|---|
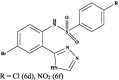 |
Pheretima posthuma | Time taken fordeath (min) in a dose of 50 µg/mL for 6d 40.03 ± 1.23 for 6f 38.83 ± 0.41 Piperazine citrate 49.17 ± 0.48 |
[39] |
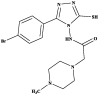 |
Pheretima posthuma | Time taken fordeath (min) in a dose of 10 mg 42.33 ± 2.52 Albendazole 32.67 ± 2.08 Piperazine 89.33 ± 0.58 |
[40] |
 |
Pheretima posthuma | Five-fold lower activity than that of Albendazole | [41] |
 |
Pheretima posthuma | Five-fold lower activity than that of Albendazole (concentration of 50 mg/mL) |
[41] |
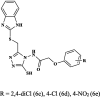 |
Pheretima posthuma | Time taken fordeath (min) in a dose of 1 mg/mL for 6c 0.4 ± 0.1 for 6d 1.28 ± 0.3 for 6e 1.07 ± 0.3 Albendazole 2.2 ± 0.03 Piperazine 2.5 ± 0.3 |
[42] |
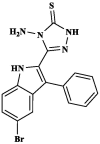 |
Pheretima posthuma | No value was given | [43] |
 |
Pheretima posthuma | Time taken fordeath (min) in a concentration of 0.2 % w/v 7.64 ± 0.16 min Albendazole 72.15 ± 0.37 |
[44] |
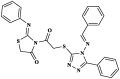 |
Pheretima posthuma | Time taken fordeath (min) in a concentration of 0.2 % w/v 8.35 ± 0.05 min Albendazole 72.15 ± 0.37 |
[44] |
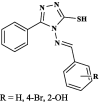 |
Pheretima posthuma | Time taken fordeath (min) for R = H 293 for R = 4-Br 231 for R = 2-OH 218 Albendazole 56 |
[45] |
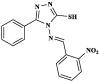 |
Pheretima posthuma | Four-fold lower activity than that of Albendazole in a concentration of 0.5 % w/v. | [46] |
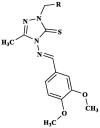 
|
Eudrilus eugeniae | Time taken fordeath (min) at a concentration of 2 % w/v for 6b 13.43 for 6d 11.58 for 6e 10.53 Albendazole 10.53 |
[47] |
 |
Pheretima pasthuma | Time taken fordeath (min) in a dose of 100 mg 23 ± 0.581 Albendazole 22 ± 2.5 |
[48] |
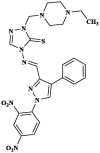 |
Pentoscoplex corethrusus | Time taken fordeath (min) in a dose of 100 mg 41.36 Albendazole 42.02 |
[49] |
Experimental
Molecular docking - methods
Anthelmintic activity - in silico
With the aim to achieve the theoretical base for development and further improvement of the new anthelmintic compounds, docking studies were carried out. In silico simulations were performed using AutoDock v.4.2.6.
Tubulin alpha-1B chain was chosen as the classic target for numerous anthelmintic substances [50]. In addition, succinate dehydrogenase was selected, as succinate dehydrogenase (SDH) inhibitors are highly effective against different types of the nematodes, including Caenorhabditis elegans [51]. In addition, some quinoline and triazole derivatives were reported as possible SDH inhibitors [52], [53]. The structure of the C. elegans tubulin was downloaded from the Protein Data Bank portal (PDB code 6E88). The experimental crystal structure of C. elegans SDH is not available in the Protein Data Bank; hence, its 3D structure was modeled using the Swiss-model web server. The C. elegans sequences of the subunits (UniProt entry Q09545) were retrieved from the UniProt database (uniprot.org). The most relevant generated SDH structure was selected based on the Global Model Quality Estimation (GMQE) and Qualitative Model Energy Analysis (QMEAN) values. Succinate dehydrogenase of Ascaris suum (PDB code 4YSX: sequence identity with SDH of C. elegans – 83.87 %) was used. The GMQE values are usually between 0 and 1, and the higher the number, the higher the reliability of the predicted structure, while a value below 4.0 shows the reliability of QMEAN. The GMQE and QMEAN values for the generated SDH structure were 0.75 and 0.80 ± 0.05, respectively. In addition, the obtained model was validated using the “WHAT IF” and “proQM” server [54], [55], [56]. The ProQM server predicts the quality of the model measured by the S-score between 0 and 1, where the 0-worst, 1-best. The S-score for the predicted SDH structure was 0.766, which allows a suggestion about the sufficient quality of the obtained homology structure. Owing to the absence of a co-crystallized ligand with the tubulin β chain and the SDH model obtained, we used the CB-dock portal [57] for detection of potential cavities for inhibitor binding. As a reference compound for estimating the inhibition potency of tubulin polymerization, we used colchicine, which is a potent inhibitor of the tubulin polymerization, and albendazole, i.e. a popular and commercially successful drug, which binds to the same pocket of tubulin [58]. For the SDH model, NN-23 and fluopyram were used as reference inhibitors [51], [59]. All standard procedures for preaparing proteins before the simulations were performed using AutoDock Tools v.1.5.6. Therefore, the structure of tubulin was cleaned from water molecules and other entities (ions, cofactors, ligands). Polar hydrogens were added, non-polar hydrogens were merged, and histidine protonation was modified manually. Kollman charges were calculated for the whole enzyme structure and spread over the residues. 3D structures of the compounds were made for further simulation using the AM1 semi-empirical quantum technique and HyperChem 7.5 software [60]. The 3D structure of all the reference ligands for SDH and tubulin-β were downloaded from the PubChem portal [61]. The Lamarckian genetic algorithm (LGA) [62] was applied in order to implement the docking simulations with all the docking parameters set to default, with an increase in the number of GA runs from 10 to 50 and the population size from 150 to 300. Visualizations and interpretations of the predicted ligand–protein complexes were performed using Discovery Studio Visualizer 21.1.0. Table 4S.
Chemistry - methods
The substances were purchased from Sigma-Aldrich (Munich, Germany) and POCH (Gliwice, Poland) and were used without further purification. The 1H and 13C NMR spectra were recorded in DMSO‑d6 on the Bruker Avance 300 or on the Bruker Avance 600 (Bruker BioSpin GmbH, Rheinstetten, Germany). The IR spectra were recorded in KBr discs using a Nicolet 6700 FTIR spectrometer (Thermo Scientific, Waltham, MA, USA). Chromatographic measurements (HRMS analysis) were performed using an LC/MS system consisting of a UHPLC chromatograph (UltiMate 3000, Dionex, Sunnyvale, CA, USA) connected with a linear trap quadrupole-Orbitrap mass spectrometer (LTQ-Orbitrap Velos from Thermo Fisher Scientific, San Jose, CA) equipped with an ESI source. In all analysis a Gemini C18 column (4.6 × 100 mm, 3 µm) (Phenomenex, USA) was used for chromatographic separation. During the chromatographic process isocratic elution was used. Mobile phase A (25 %) was 25 mM ammonium formate in water; mobile phase B (75 %) was 25 mM ammonium formate in acetonitrile. The mobile phase flow rate 0.5 mL/min by 30 min in each analysis.
In the course of each run, MS spectra in the range of 100–500 m/z were collected continuously.
The ESI was operated in positive polarity modes under the following specific conditions: spray voltage − 5.0 kV; sheath gas − 35 arbitrary units; auxiliary gas − 5 arbitrary units; sweep gas − 5 arbitrary units; and capillary temperature − 300 °C. Nitrogen (>99.98 %) was employed as a sheath, auxiliary, and sweep gas. The scan cycle used a full-scan event at the resolution of 140 000. The melting points were determined on a Stuart SMP50 device (Cole Parmer Ltd Stone, UK) and were uncorrected. The progress of the reactions was monitored by thin-layer chromatography TLC (aluminum sheet 60 F254 plates (Merck Co., Kenilworth, NJ, USA). A CHCl3/EtOH (10:1, v/v) mixture was used as the solvent system.
The UHPLC analysis for biological material was performed using the Ultimate 3000 series system (Dionex, Idstein, Germany) coupled with an Amazon SL ion trap mass spectrometer (Bruker Daltonik GmbH, Bremen, Germany). The separation was carried out with the Kinetex XB-C18 analytical column (150 mm × 2.1 mm × 1.9 µm), Phenomenex (Torrance, CA, USA). The column temperature was maintained at 25 °C. 0.1 % formic acid in deionized water and 0.1 % formic acid in acetonitrile (LC-MS grade) were used as mobile phases A and B, respectively. The flow of the mobile phase was 0.300 mL/min. The following gradient program was used for the separation of the analyzed compounds: 0 min – 25 %B, 15 min – 95 %B. The column was stabilized for 7 min between injections. 5-μl samples were analyzed. The ion trap mass spectrometer was equipped with an ESI interface. The parameters for the ESI source were set as follows: nebulizer pressure 45 psi, dry gas flow 9 L/min, dry temperature 134 °C, and capillary voltage 4.5 kV. The analysis was carried out using a scan from m/z 70–2,200. The compound was analyzed in the positive ion mode. The detection of the analyzed compounds was carried out by monitoring pseudomolecular ions [M + H]+ characteristic for each compound. Extracted ion chromatograms (EICs) were generated for the quantification of II-1 (EIC for m/z = 335.18).
Procedure for the synthesis of quinoline-6-carbohydrazide
0.01 mol of methyl quinoline-6-carboxylate was dissolved in 10 mL of anhydrous ethanol. Then, 1 mL of 80 % hydrazine hydrate was added. The mixture was refluxed for 6 h. The solution was then allowed stand to evaporate the solvent. The resulting precipitate was purified by crystallization from 96 % ethanol. Yield: 76 %. Creamy solid. (M.p. = 188-189˚C; according to the literature M.p. = 188˚C) [63].
Procedure for the synthesis of 1-(quinoline-6-carbonyl)-4-substituted thiosemicarbazide derivatives (I-1 – I-4)
A solution of 0.01 mol of quinoline-6-carbohydrazide and 0.01 mol of the appropriate isothiocyanate in 20 mL of anhydrous ethanol was heated under reflux for 30 min Next, the solution was cooled and the solid formed was filtered off, washed with water and diethyl ether, dried, and crystallized from 96 % ethanol.
4-(4-methoxyphenyl)-1-(quinoline-6-carbonyl)thiosemicarbazide I-1
Yield: 87 %. White solid. M.p.: 217–219 °C. 1H NMR 600 MHz, DMSO‑d6): 3.75 (s, 3H, CH3), 6.91 (d, 2H, ArH, J = 8.9 Hz), 7.31 (bs, 1H, ArH), 7.63 (dd, 1H, ArH, J = 8.3, 4.2 Hz), 8.13 (d, 1H, ArH, J = 8.8 Hz), 8.28 (dd, 1H, ArH, J = 8.9, 1.9 Hz), 8.51 (dd, 1H, ArH, J = 7.7, 1.8 Hz), 8.65 (s, 1H, ArH), 9.02 (dd, 1H, ArH, J = 4.2, 1.7 Hz), 9.75; 9.81; 10.79 (3 s, 3H, 3NH). 13C NMR (150 MHz, DMSO‑d6) 55.69, 113.72, 122.77, 127.44, 128.11, 128.59, 129.34, 129.45, 130.93, 132.54, 137.66, 149.32, 152.81, 157.29, 166.08, 181.89. IR (KBr, cm−1): 3385 (NH), 3095, 3018 (CH arom.), 2960 (CH aliph.), 1680 (C O), 1325 (C S). HRMS (ESI) calcd for C18H17N4O2S [M + H]+ 353.10722, found 353.10712.
4-(4-chlorophenyl)-1-(quinoline-6-carbonyl)thiosemicarbazide I-2
Yield: 85 %. Creamy solid. M.p.: 213–214 °C. 1H NMR (600 MHz, DMSO‑d6): 7.23 (d, 1H, ArH, J = 7.3 Hz), 7.37 (t, 1H, ArH, J = 8.0 Hz), 7.49 (ddd, 1H, ArH, J = 8.1, 2.1, 1.0 Hz), 7.64 (dd, 2H, ArH, J = 8.3, 4.2 Hz), 8.14 (d, 1H, ArH, J = 8.9 Hz), 8.27 (d, 1H, ArH, J = 8.0 Hz), 8.52 (dd, 1H, ArH, J = 8.1, 1.5 Hz), 8.64 (s, 1H, ArH), 9.02 (dd, 1H, ArH, J = 4.2, 1.7 Hz), 9.95, 10.01, 10.85 (3 s, 3H, 3NH). 13C NMR (150 MHz, DMSO‑d6): 122.81, 124.74, 125.29, 125.77, 127.46, 128.51, 129.45, 130.04, 130.77, 132.50, 137.68, 141.25, 149.35, 152.88, 166.05, 181.43. IR (KBr, cm−1): 3393 (NH), 3089, 3016 (CH arom.), 1684 (C O), 1335 (C S). HRMS (ESI) calcd for [M + H]+ C17H14ClN4OS 357.05769, found 357.05773.
4-(4-N,N-diethylaminephenyl)-1-(quinoline-6-carbonyl)thiosemicarbazide I-3
Yield: 75 %. Yellow solid. M.p.: 193–195 °C. 1H NMR (300 MHz, DMSO‑d6): 1.07 (t, 6H, 2CH3, J = 6.9 Hz), 3.28 (d, 4H, 2CH2, J = 6.9 Hz), 6.61 (d, 2H, ArH, J = 8.9 Hz), 7.12 (d, 2H, ArH, J = 8.3 Hz), 7.62 (dd, 1H, ArH, J = 8.3, 4.2 Hz), 8.10 (d, 1H, ArH, J = 8.9 Hz), 8.25 (d, 1H, ArH, J = 8.7 Hz), 8.49 (dd, 1H, ArH, J = 8.1, 1.5 Hz), 8.62 (s, 1H, ArH), 9.00 (dd, 1H, ArH, J = 4.3, 1.7 Hz), 9.55, 9.62, 10.72 (3 s, 3H, 3NH). 13C NMR (150 MHz, DMSO‑d6): 12.88, 44.22, 111.43, 122.74, 127.43, 128.60, 129.30, 129.40, 131.01, 137.64, 145.83, 149.31, 152.77, 166.05. IR (KBr, cm−1): 3403, 3360 (NH), 3085, 3023 (CH arom.), 2953 (CH aliph.), 1676 (C O), 1325 (C S). HRMS (ESI) calcd for [M + H]+ C21H24N5OS 394.17016, found 394.17003.
4-(4-nitrophenyl)-1-(quinoline-6-carbonyl)thiosemicarbazide I-4
Yield: 88 %. Yellow solid. M.p.: 225–226 °C. 1H NMR (300 MHz, DMSO‑d6): 7.64 (dd, 1H, ArH, J = 8.3, 4.2 Hz), 7.92 (d, 2H, ArH, J = 8.9 Hz), 8.13 (d, 1H, ArH, J = 8.9 Hz), 8.22 (d, 3H, ArH, J = 9.2 Hz), 8.53 (d, 1H, ArH, J = 9.6 Hz), 8.64 (s, 1H, ArH), 9.02 (dd, 1H, ArH, J = 4.2, 1.8 Hz), 10.26 (s, 2H, 2NH), 10.91 (s, 1H, NH). 13C NMR (150 MHz, DMSO‑d6): 122.84, 124.09, 125.40, 127.49, 128.49, 129.51, 130.64, 137.74, 143.94, 146.20, 149.34, 152.91, 166.10, 181.28. IR (KBr, cm−1): 3419, 3354 (NH), 3093, 3028 (CH arom.), 1680 (C O), 1323 (C S). HRMS (ESI) calcd for [M + H]+ C17H14N5O3S 368.08174, found 368.08171.
Procedure for the synthesis of 5-(quinolin-6-yl)-4-substituted-2,4-dihydro-3H-1,2,4-triazole-3-thione (II-1 – II-4)
The obtained thiosemicarbazide (I-1 – I-4) (0.01 mol) dissolved in 20 mL of 2 % aqueous NaOH was refluxed for 2 h. After cooling, the solution was neutralized with 3 N hydrochloric acid. The precipitate was filtered off and crystallized from 96 % ethanol.
4-(4-metoxyphenyl)-5-(quinolin-6-yl)-2,4-dihydro-3H-1,2,4-triazole-3-thione II-1
Yield: 95 %. White solid. M.p.: 205–207 °C. 1H NMR (600 MHz, DMSO‑d6): 3.79 (s, 3H, CH3), 7.03 (d, 2H, ArH, J = 9.1 Hz), 7.33 (d, 2H, ArH, J = 8.9 Hz), 7.57 (dd, 1H, ArH, J = 8.3, 4.2 Hz), 7.63 (dd, 1H, ArH, J = 8.9, 2.0 Hz), 7.96 (d, 1H, ArH, J = 8.9 Hz), 8.00 (d, 1H, ArH, J = 2.0 Hz), 8.28 (ddd, 1H, ArH, J = 8.5, 1.7, 0.7 Hz). 8.95 (dd, 1H. ArH, J = 4.2, 1.8 Hz), 14.23 (s, 1H, NH). 13C NMR (150 MHz, DMSO‑d6): 55.91, 115.03, 115.39, 123.05, 128.02, 129.25, 129.38, 129.74, 130.40, 138.97, 146.60, 150.54, 151.05, 151.62, 152.19, 160.12, 160.18, 169.51. IR (KBr, cm−1): 3408, 3323 (NH), 3085, 3026 (CH arom.), 2954 (CH aliph.), 1326 (C S). HRMS (ESI) calcd for [M + H]+ C18H15N4OS 335.09666, found 335.09653.
4-(4-chlorophenyl)-5-(quinolin-6-yl)-2,4-dihydro-3H-1,2,4-triazole-3-thione II-2
Yield: 90 %. Light yellow solid. M.p.: 189–190 °C. 1H NMR (600 MHz, DMSO‑d6): 7.48 (d, 2H, ArH, J = 8.7 Hz), 7.58 (d, 1H, ArH, J = 8.7 Hz), 7.59 (d, 1H, ArH, J = 8.3 Hz), 7.62 (dd, 1H, ArH, J = 8.8 Hz, 2.0 Hz), 7.99 (d, 1H. ArH, J = 8.0 Hz), 8.03 (d, 1H, ArH, J = 2.0 Hz), 8.33 (dd, 1H, ArH, J = 9.0, 1.2 Hz), 8.96 (dd, 1H, ArH, J = 4.2, 1.7 Hz), 14.30 (s, 1H, NH). 13C NMR (150 MHz, DMSO‑d6): 123.04, 124.44, 127.76, 128.39, 128.96, 129.60, 129.93, 130.30, 131.16, 133.73, 134.61, 138.31, 146.26, 147.07, 150.42, 152.12, 169.21. IR (KBr, cm−1): 3365 (NH), 3063, 3026 (CH arom.), 1324 (C S). HRMS (ESI) calcd for [M + H]+ C17H12ClN4S 339.04712, found 339.04717.
4-(4-N,N-diethylaminophenyl)-5-(quinolin-6-yl)-2,4-dihydro-3H-1,2,4-triazole-3-thione II-
Yield: 75 %. Yellow-brown solid. M.p.: 289–292 °C. 1H NMR (600 MHz, DMSO‑d6): 1.08 (t, 6H, 2CH3, J = 3.6 Hz), 3.34 (q, 4H, 2CH2, J = 3.6 Hz), 6.68 (d, 2H, ArH, J = 8.5 Hz), 7.12 (d, 2H, ArH, J = 9.0 Hz), 7.56 (dd, 1H, ArH, J = 8.3, 4.2 Hz), 7.66 (dd, 1H, ArH, J = 8.9, 2.0 Hz), 7.96 (d, 2H, ArH, J = 8.8 Hz), 8.01 (d, 1H, ArH, J = 2.0 Hz), 8.25 (ddd, 1H, ArH, J = 8.6, 1.7, 0.7 Hz), 8.94 (dd, 1H, ArH, J = 4.2 Hz, 1.7 Hz), 14.15 (s, 1H, NH). 13C NMR (150 MHz, DMSO‑d6): 12.55, 45.14, 112.75, 122.96, 124.84, 127.74, 128.83, 129.21, 129.37, 129.60, 129.86, 138.07, 147.10, 150.77, 152.01, 169.62. IR (KBr, cm−1): 3340 (NH), 3032 (CH arom.), 2965 (CH aliph), 1326 (C S). HRMS (ESI) calcd for [M + H]+ C21H22N5S 376.15959, found 376.15945.
4-(4-nitrophenyl)-5-(quinolin-6-yl)-2,4-dihydro-3H-1,2,4-triazole-3-thione II-4
Yield: 85 %. Brown solid. M.p.: 163–165 °C. 1H NMR (600 MHz, DMSO‑d6): 6.59 (d, 1H, ArH, J = 8.6 Hz), 6.97 (d, 1H, ArH, J = 8.7 Hz), 7.57 (dd, 1H, ArH, J = 8.3, 4.2 Hz), 7.63 (dd, 1H, ArH, J = 8.8, 2.0 Hz), 7.77 (d, 1H, ArH, J = 9.1 Hz), 7.98 (d, 2H, ArH, J = 8.7 Hz), 7.99 (d, 1H, ArH, J = 2.0 Hz), 8.35 (d, 2H, ArH, J = 9.1 Hz), 8.95 (dd, 1H, ArH, J = 4.1, 1.8 Hz), 14.19 (s, 1H, NH). 13C NMR (150 MHz, DMSO‑d6): 117.59, 122.97, 124.00, 125.07, 126.01, 127.73, 129.28, 129.49, 129.62, 130.87, 137.83, 140.29, 147.63, 148.10, 150.26, 152.43, 168.97. IR (KBr, cm−1): 3365 (NH), 3063, 3026 (CH arom.), 1324 (C S). HRMS (ESI) calcd for [M + H]+ C17H12N5O2S 350.07117, found 350.07129.
Biological evaluation - methods
Anthelmintic activity - in vitro (Rhabditis sp.)
The screening analysis of anthelmintic activity was carried out in accordance with the proprietary patent procedure No. PL 232918 in a model of nematodes of the genus Rhabditis sp. This model is similar to the nematode Caenorhabditis elegans model widely used in research [64], [65], [66]. Nematodes of the genus Rhabditis sp. can also be parasites of humans and animals. In humans, they have been detected, inter alia, in the outer ear, feces and urine [67], [68], [69]. In animals, mainly cattle, it can cause parasitic otitis [70], [71], [72]. The test compounds were added to the cultivation of Rhabditis sp. nematodes in five experimentally selected concentrations: 11.1 mg/mL, 5.5 mg/mL, 3.3 mg/mL, 1.1 mg/mL, and 0.2 mg/mL (larger dilutions were prepared if necessary). After 24 h of incubation, the nematodes were observed to assess their development, deformation, and damage. Additionally, in order to determine their viability, they were stained with methylene blue. The research was carried out in accordance with the previously described procedure [19], [32], [34].
Anthelmintic activity - in vitro (Haemonchus contortus)
The material for the research included eggs of Haemonchus contortus nematodes isolated from goat feces using the flotation-decantation method for isolating eggs [73]. The tested animals had not been dewormed for at least a year. The obtained eggs were suspended in distilled water in such a way that 1 mL of the obtained suspension contained about 100 eggs ( ± 10). Some of the eggs were examined immediately after isolation, and the remainder of the eggs were tested after incubation (48 h) and hatching of L1 larvae. The eggs were incubated in Petri dishes in appropriate dilutions of the test compounds: 11.1 mg/mL, 5.5 mg/mL, 3.3 mg/mL, 1.1 mg/mL, and 0.2 mg/mL. To confirm the correct incubation conditions, plates with the negative control (0.6 % NaCl) and the positive control (albendazole) in dilutions corresponding to the tested substance were also used (albendazole is one of the most commonly used active substances in nematicidal preparations for small ruminants). The plates were incubated at 24–26 °C. Subsequently, eggs at various stages of development and larvae were counted using a light microscope at a magnification of 100x. The analyses of the plates included determination of the percentage of eggs and the percentage of hatched larvae: dead and alive, active.
Anthelmintic activity - in vitro (Strongylidae sp.)
Fecal samples were collected from infected horses and subjected to macro and microscopic analysis. Microscopic examinations were performed using the modified sedimentation-flotation method. The research was conducted in accordance with the previously described procedure [74], [75], [76]. For further studies, samples with eggs of nematodes from the Strongylidae family were only used. The eggs were rinsed three times with physiological fluid and sedimented. Next, they were placed in a cultivation plate, with 100 eggs in each well. The test compound was added to the culture in the liquid medium in the form of a homogeneous suspension in the following concentrations: 11.1 mg/mL, 5.5 mg/mL, 3.3 mg/mL, 1.1 mg/mL, and 0.2 mg/mL as well as 0.6 %NaCl (negative control) and albendazole (positive control). The study included determination of the percentage of eggs (dead and alive) and the percentage of larvae (alive and moving abnormally).
Anthelmintic activity - in vivo (Heligmosomoides polygyrus/bakeri)
45 Mus musculus mice (strain C57BL/6) were used in the experiment. The mice were weighed and randomized into groups of 5 animals each. Five mice were assigned to the physiological control; they were not infected and were not administered any of the test compounds. On the first day of the experiment, 40 mice were infected with invasive L3 larvae of Heligmosomoides polygyrus/bakeri. 150 larvae of the nematode were administered per os to each mouse in 0.1 mL of water by means of an intragastric tube. On day 19 after infection, the state of infection was assessed by coproscopic examination. This study confirmed that all mice became infected, as there were nematode eggs in their feces. The viability of these eggs assessed by trypan blue staining and rhodamine 123 retention was 93 % and 92 %, respectively. On day 22 after infection, the test compound in the doses of 3 mg/kg and 6 mg/kg body weight was administered intragastrically in a volume of 0.1 mL; then, the dose was increased to 7.5 and 10 mg/kg body weight. Five mice received a suspension of the anthelmintic drug albendazole in a volume of 0.1 mL of 6 mg/kg body weight, followed by the administration of a commercial drug, Zentel, at a dose of 6 mg/kg body weight.
Ethics statement
All experiments involving animals were conducted according to the ethical policies and procedures approved by the Local Ethics Committee for Animal Experiments operating at the University of Life Sciences in Lublin, Resolution No. 63/2019.
Biochemical and hematological examinations
The material for biochemical and hematological tests was blood collected without and with an anticoagulant (EDTA). The analysis of biochemical parameters was carried out using the ERBA XL 640 analyzer. The parameters of the liver (ALT - alanine aminotransferase, AST - aspartate aminotransferase), kidney (UREAV – urea, CREAV - creatinine), and pancreas (AMYV-amylase, LIPV–lipase) were examined. In turn, the MYTHIC 18 Cormay analyzer was used for the analysis of hematological parameters. Red blood cells (RBC - red blood cell, HGB - hemoglobin concentration, MCV - mean corpuscular volume, MCH - mean corpuscular hemoglobin, MCHC - mean corpuscular hemoglobin concentration, RDW-CV - red blood cell distribution width, RDW-SD - red blood cell distribution width standard deviation, HCT - hematocrit), white blood cells (WBC - white blood cells, BAS - basophils; NEU – neutrophils, EOS – eosinophils, LYM - lymphocytes; MON – monocytes), and platelet systems (PLT - platelet, MPV - mean platelet volume, PCT- plateletcrit) were examined.
Histopathological examinations
The material for histopathological examinations comprised samples of organs: the liver, kidney, intestine, and brain. The collected material was properly fixed for 24 h in 10 % formalin (pH – 7.2) and in a number of alcohol solutions, acetone, and xylene to prepare paraffin blocks in a tissue processor (Leica TP-20, Leica Biosystems). Histopathological slides stained with hematoxylin and eosin (H&E) were viewed under an optic microscope (Nikon Eclipse E-600, Zeiss).
Determination of CYP450 inhibition by II-1
The potential inhibitory effect of II-1 on the activity of selected cytochrome CYP450 isoforms was investigated using the Vivid CYP450 fluorescence screening assay (Thermo Fisher Scientific, Waltham, MA, USA). The experiments were performed according to the manufacturer's procedure. Vivid BOMCC substrate CYP2B6 blue, Vivid BOMCC substrate CYP3A4 blue, Vivid BOMCC substrate CYP3A5 blue, and Vivid EOMCC substrate CYP2C19 blue were used in the experiments. The endpoint test mode was used as the determination method in the experiments (reading after 10-min incubation). The potential inhibitory effect of II-1 on the activity of cytochrome CYP450 isoforms was investigated using the following final concentrations of the compound: 0.025 ng/mL, 0.05 ng/mL, 0.5 ng/mL, 5 ng/mL, 50 ng/mL, and 500 ng/mL. The test compound was dissolved in DMSO. The results are shown as the percent of control solvent activity. The tests were performed in duplicate. The data were plotted as the mean value ± standard error (SD) and analyzed using GraphPad v.5.01 (* statistically significant one-way ANOVA with p < 0.05). Compounds recommended by the manufacturer, i.e. miconazole (for CYP2C19 and CYP2B6) and ketoconazole (for CYP3A4 and CYP3A5), were used as positive controls (Sigma-Aldrich, St. Louis, MO, USA). The test was read using a BioTek Synergy H1 reader (BioTek Instruments, Inc., USA) according to the manufacturer's guidelines.
Statistical data analysis
The research results were subjected to statistical analysis using the STATISTICA v 13.3 and analyzed using GraphPad software v.5.01 and v 8.2.0. Statistical analysis was performed using ANOVA (RIR Tukey test).
Results and discussion
Selection of compounds for research
In search of new compounds with anthelmintic activity, we designed a series of compounds derived from quinoline-6-carboxylic acid. Three of them had aliphatic substituents: cyclopropyl (II-7), butyl (II-8), and pentyl (II-9) and six had a phenyl ring with different substituents in position 4 (II-1 – II-6) (Fig. 1).
Fig. 1.
Structure of designed compounds.
We designed the compounds with electron-donating and electron-withdrawing substituents to determine the influence of the nature of the substituent on their activity. All of the suggested compounds underwent in silico testing with regard to their affinities toward the selected proteins. The analysis of the binding energy (supp. Table 1S) revealed that compounds with aliphatic substituents did not show activity; therefore, they were not qualified for the study. Among the compounds with an electron-withdrawing halogen atom, the compound with the chlorine atom had the highest energy, hence the choice of this derivative for the synthesis.
Chemistry
Methyl quinoline 6-carboxylate was purchased and was used as a starting material. In the reaction with hydrazine hydrate, quinoline-6-carbohydrazide was obtained with good yield (76 %). The long time of reaction (6 h) is necessary. Next, in the reaction with 4-substituted phenylisothiocyanates, in anhydrous ethanol new thiosemicarbazide derivatives were obtained. Two of them have electron-donating substituents (methoxy and N,N-diethylamino groups) and the other two have electron-withdrawing substituents (the nitro group and chlorine atom). The progress of the reaction was checked by thin-layer chromatography. The alkaline cyclization of linear compounds using a 2 % solution of sodium hydroxide afforded the corresponding 5-(quinolin-6-yl)-4-substituted-2,4-dihydro-3H-1,2,4-triazole-3-thiones. The synthesis pathway yielding the new derivatives is depicted in Scheme 1.
Scheme 1.
Synthetic route for new derivatives I-1 – I-4 and II-1 – II-4. Conditions: (i) NH2NH2, C2H5OH reflux 6 h, (ii) C2H5OH reflux 30 min, (iii) NaOH reflux 2 h, HCl.
The synthesis was performed according to the procedures described in the literature for other types of thiosemicarbazides and 1,2,4-triazoles [77], [78], [79], [80], [81]. It is quite simple and the new derivatives of thiosemicarbazide and 1,2,4-triazole with a quinoline moiety were obtained in a good yield ranging from 75 to 95 %. This is extremely important when synthesizing compounds for biological research. The yields of the cyclization reaction were substantially higher than those of the formation of the thiosemicarbazides. only in one case, using 4-nitrophenyl isothiocyanate, the efficiency of the cyclization process was lower by 3 %. In the case of the presented reactions, it was possible to obtain compounds of high purity. This is primarily determined by the purity of the hydrazide. In the case of hydrazide contamination, subsequent reactions proceeded with low yields, and mixtures of compounds difficult to separate by flash chromatography were obtained. Complete structural elucidation was done by the IR, 1H, and 13C NMR spectra and HRMS. In the 1H NMR spectra of the thiosemicarbazides, the characteristic signals of proton linked to nitrogens were shown at 9.62–10.82 ppm as three (for compounds I-1 and I-3) or two singlets (for compounds I-2 and I-4). In the 1,2,4-triazoles, the signal of the NH proton was observed in the range of 14.15–14.31 ppm. Aromatic protons resonated at the typical range δ 6.58–9.03 ppm. In the IR spectra, characteristic absorption bands for the C O group of thiosemicarbazide derivatives were visible in the range of 1676–1684 cm−1, which were missing in the spectra of cyclic compounds. The absorption bands characteristic for the C S group were shown at 1323–1326 cm−1. In the 13C NMR spectra, carbon signals were observed at the expected values of the chemical shift (supp. Fig. 1S, Fig. 2S).
Biological evaluation
In vitro anthelmintic activity
Compounds selected in the in silico analysis were screened for their nematicidal activity. Eight compounds were tested during the experiments, including four new thiosemicarbazide derivatives and four new 1,2,4-triazole-3-thione derivatives. In the first stage of the research consisting in a screening analysis of the influence of the newly synthesized derivatives on the viability of free-living Rhabditis sp. nematodes, the LC50 concentration (lethal concentration for 50 % of nematodes) was determined for the test compounds. The results are presented in Table 2.
Table 2.
Structures of tested derivatives and determined concentrations of LC50.
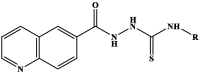 | |||
|---|---|---|---|
| Compound | R | LC50 (mg/mL) | LC50 (μM) |
| I-1 | 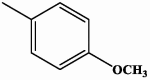 |
0.61 | 1.73 |
| I-2 | 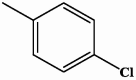 |
5.04 | 14.16 |
| I-3 | 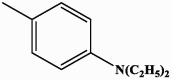 |
very low activity, not specified | very low activity, not specified |
| I-4 |  |
4.40 | 11.99 |
 | |||
| Compound | R | LC50 (mg/mL) | LC50 (μM) |
| II-1 | 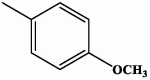 |
0.30 | 0.90 |
| II-2 | 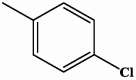 |
3.74 | 11.06 |
| II-3 |  |
4.20 | 11.20 |
| II-4 | 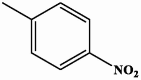 |
0.75 | 2.15 |
| Control | |||
| Albendazole | 19.78 | 74.55 | |
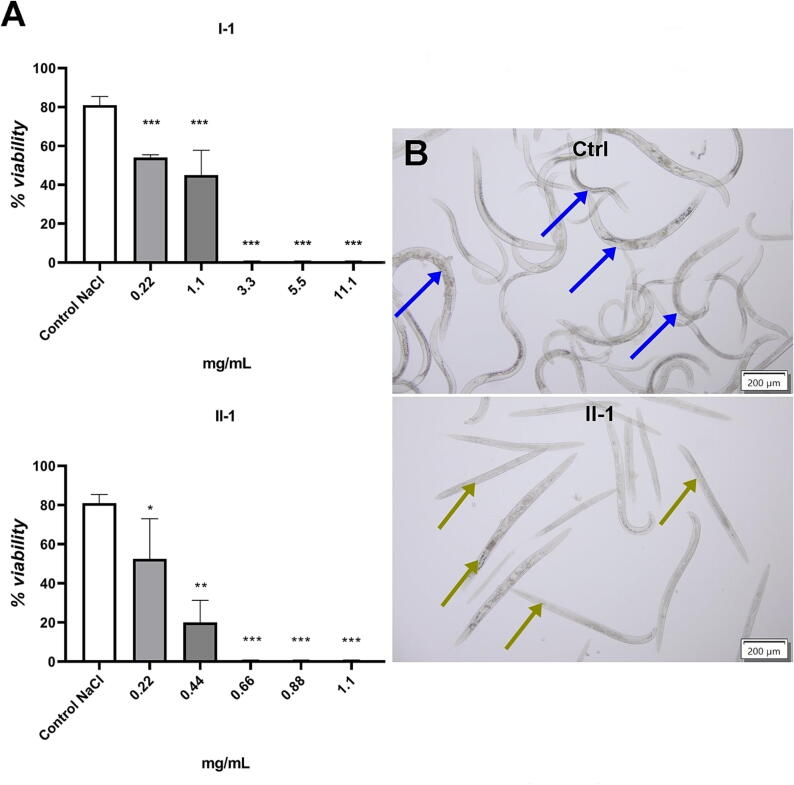
The test compounds differed in their anthelmintic activity. The highest anthelmintic activity was shown by compounds with the methoxyphenyl substituent: I-1 and II-1. The LC50 concentration of compound I-3 could not be determined, because the viability of the nematodes at the highest concentration of this compound was >50 %. The conducted analysis showed that the anthelmintic activity of most of the test compounds was higher than the activity of the currently used commercial anthelmintic drug - albendazole (LC50 = 19.78 mg/mL) (Fig. 2A and 2B). The linear compounds were less active than their cyclic analogs. The linear derivative of I-4 was observed to be six times less active than the cyclic derivative II-4 (compounds with a 4-nitrophenyl substituent). The most active compound II-1, a 1,2,4-triazole derivative with a 4-methoxyphenyl substituent, was twice as active as its linear precursor compound I-1. Among the cyclic derivatives, the lowest activity was shown by compound II-3. The low activity of compound II-3 may be related to the size of the substituent. The above statements will be verified in subsequent studies. Our research results demonstrated that the cyclic compounds were definitely more active. Compound II-1, which exhibited the highest anthelmintic activity among the test compounds, was selected for further studies.
Fig. 2.
Anthelmintic activity of the test compounds. A. The viability (%) of nematodes after 24-hour exposure to the concentrations of I-1 and II-1; (p < 0.05 *, p < 0.01 **, p < 0.001 ***); B. Example of Rhabditis sp. nematode culture, Ctrl − 0.6 % NaCl control - the blue arrow marks very mobile live nematodes, II-1 - test compound - the green arrow marks immobile dead nematodes. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
In the next stage of the research, compound II-1 was subjected to further analyses of anthelmintic activity using Haemonchus contortus nematodes (larvae and eggs). These nematodes are gastrointestinal parasites of mainly goats and sheep. As shown by the results of the experiment, the compound is active and exhibits ovicidal properties in a manner similar to that of the control containing albendazole. Eggs with dead larvae constituted the largest number of eggs and their percentage in the tested concentration variants was as follows: 0.2 mg/mL − 47.5 % ± 13.44 (Alb. 47 % ± 14.14), 1.1 mg/mL − 67 % (Alb. 56 % ± 5.66), 3.3 mg/mL − 63 % ± 15.56 (Alb. 60 % ± 5.66), 5.5 mg/mL − 74 % ± 5.66 (Alb. 60.5 % ± 2.12), and 11.1 mg/mL − 66 % ± 14.14 (Alb. 67.5 % ± 3.54) (Fig. 3A and 3B). Moreover, the tested derivative showed high larvicidal efficacy, which was comparable to that of albendazole. The percentage of dead larvae at each concentration was: 0.2 mg/mL − 76.5 % ± 4.76 (Alb. 84.33 % ± 8.14), 1.1 mg/mL – 77.33 % ± 5.65 (Alb. 86.33 % ± 4.93), 3.3 mg/mL − 83.17 % ± 4.17 (Alb. 91.33 % ± 0.58), 5.5 mg/mL − 88.17 % ± 4.88 (Alb. 94.67 % ± 0.58), and 11.1 mg/mL − 91.5 % ± 4.51 (Alb. 95.67 % ± 5.86). These results showed the highest effectiveness of compound II-1 at the stage of hatched larvae (Fig. 3C and 3D).
Fig. 3.
Anthelmintic activity of compound II-1 against Haemonchus contortus gastrointestinal nematodes. A. Eggs of H. contortus gastrointestinal nematodes isolated from goat feces (the blue arrow marks live eggs from the control, the green arrow marks a dead egg after treatment with compound II-1); B. determination of the percentage of eggs of H. contortus: 1. Eggs non-invasive (ENI), 2. Eggs with dead larvae (ED), 3. Eggs with live larvae (EA); C. L1 hatched larvae H. contortus (the blue arrow marks a live larva from the control; the green arrow marks a dead larva after treatment with compound II-1); D. Determination of the percentage of larvae H. contortus: (DL) dead and (AL) alive, p < 0.05 *, p < 0.01 **. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Subsequent studies were carried out in a model of nematodes from the Strongylidae family (larvae and eggs). These nematodes are parasites of mainly horses. The studies carried out on the nematodes from the Strongylidae family confirmed the ovicidal properties of the test compound. After the exposure of the nematode eggs to compound II-1, dead eggs constituted the highest percentage. The percentage of dead eggs in the individual concentration variants was 0.2 mg/mL − 55 % ± 16.43, 1.1 mg/mL – 70 % ± 17.03, 3.3 mg/mL – 77.5 ± 12.94, 5.5 mg/mL – 86.67 ± 12.52, and 11.1 mg/mL – 92.5 % ± 7.58 and was statistically significantly higher than in the control exposed to albendazole and 0.6 % NaCl. It was also observed that significantly fewer larvae hatched, and some of them moved in an incorrect way (larvae moving abnormally) (Fig. 4A and 4B).
Fig. 4.
Anthelmintic activity of compound II-1 against Strongylidae sp. nematodes. A. Eggs of Strongylidae sp. nematodes isolated from horse feces (the blue arrow indicates live eggs from the control; the green arrow indicates a dead egg after treatment with compound II-1); B. Determination of the percentage of eggs Strongylidae sp.: cleaved eggs (CE), eggs with larvae (EA), dead eggs (ED) and determination of the percentage of larvae of Strongylidae sp.: alive (AL) and larvae moving abnormally (DL) (C – Ctrl NaCl, CA – Ctrl albendazole), p < 0.05 *, p < 0.01 **, p < 0.001 ***. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
In vivo anthelmintic activity
The in vivo anthelmintic activity of compound II-1 was tested on the nematode Heligmosomoides polygyrus/bakeri parasitizing the small intestine of the experimental mice (Fig. 5A). The anthelmintic activity was monitored on the basis of the presence and number of nematode eggs in the feces of the infected mice. On the subsequent post-infection days, the presence of eggs was demonstrated by coproscopic examination. In the absence of a reaction of the nematode to the administered compound and albendazole, it was checked whether the parasite strain used showed drug resistance: the mice infected with the nematode and previously treated with albendazole were given a commercial drug Zentel at a dose of 6 mg/kg body weight. In the examination performed after 5 days of drug administration (38 days after infection), no eggs were found in the stool (Fig. 5B). However, on the day of euthanasia, nematode eggs were found in the Zentel-treated mice during coproscopic examinations, which may indicate that this strain is resistant to the drug administered and the tested derivative. In the next stage of the study, the anthelmintic activity of the tested derivative was monitored on the basis of the number of nematodes in the intestine of the mice (determined post-mortem). Both in the positive control group (Albendazole, Zentel) and in the test group (II-1), a reduction in the number of nematodes was observed compared to the control group infected with H. polygyrus/bakeri. The test compounds showed the anthelmintic effect measured by the number of nematodes. In vivo studies in the nematode model H. polygyrus/bakeri have shown that compound II-1 reduces the number of nematodes by an average of 30 % and was as follows in the individual concentration variants (mean): control NaCl (HP Ctrl) − 120.67, control albendazole (HP + Alb) – 53.00, II-1 3 mg/kg body weight (HP + II-1) – 79.67, and II-1 6 mg/kg body weight (HP + II-1′) – 80.00 (Fig. 5C). Although the effect of Zentel was better than that of compound II-1, the administration of this drug did not result in complete eradication of the parasite in the mice. This may be a result of the emergence of multidrug resistance in the tested nematode H. polygyrus/bakeri strain.
Fig. 5.
In vivo anthelmintic activity of compound II-1 against H. polygyrus/bakeri nematodes. A. Histopathological slide of the intestine of an infected mice - hematoxylin and eosin stain [H&E] (the nematode is marked with an arrow HP); B. Effect of compound II-1 and albendazole on egg production (EPG), C. Effect of compound II-1 and albendazole on changes in the number of nematodes (determined post mortem). (HP ctrl - H. polygyrus/bakeri-infected mice receiving no substances, HP + (Alb) – H. polygyrus/bakeri-infected mice receiving the nematicide drugs albendazole and Zentel in suspension, HP + (II-1) – H. polygyrus/bakeri-infected mice receiving the test compound in a lower concentration, HP + (II-1 ') – H. polygyrus/bakeri-infected mice receiving the test compound in a higher concentration.
Biochemical and hematological parameters
The results of the biochemical and hematological studies showed that the test derivative did not exert a large effect on blood parameters in the experimentally infected mice that received the test compound, compared to the controls (the Control Group - non-infected mice not given any medication, HP Group – infected mice not given any medication). The test derivative did not induce any significant changes in the levels of the biochemical and hematological parameters. The test results are presented in supp. Table 2S. The greatest changes were observed in the WBC and EOS parameters, which was a result of infecting the mice with the nematode H. polygyrus/bakeri.
Histopathological examinations
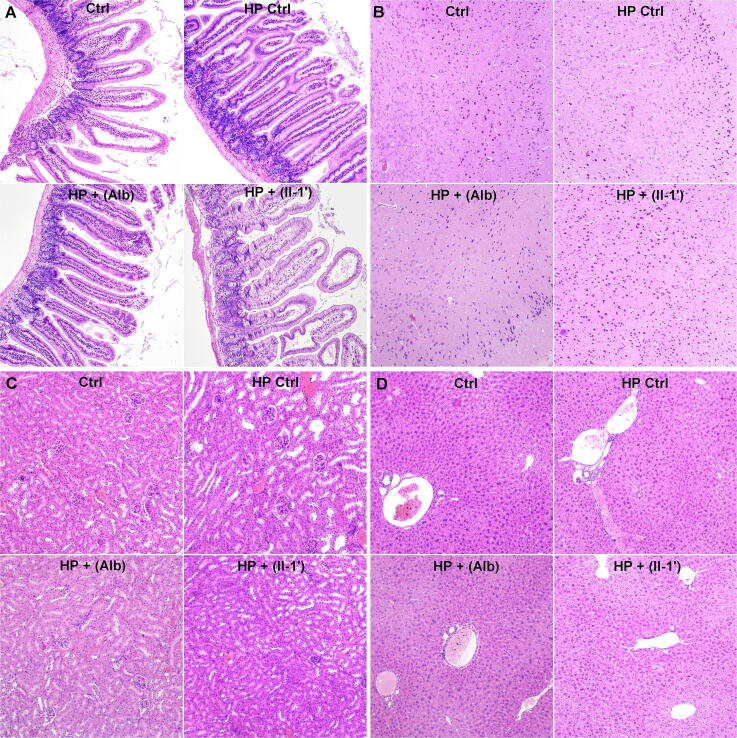
The results of histopathological examinations carried out in the experimentally infected mice receiving the test compound showed that the derivative did not cause significant changes in the structure and function of the intestine, brain, kidneys, and liver. No significant pathological features were found. Only slight parenchymal degeneration and hyperemia were observed, i.e. secondary changes resulting among others from euthanasia exerting no effect on the experiment (Fig. 6 and supp. Table 3S).
Fig. 6.
Histopathological preparations of organs taken from the mice - hematoxylin and eosin stain [H&E]. A. intestine, B. brain C. kidney, D. liver (Ctrl - non-infected mice not infected not given any medication HP crtl – H. polygyrus/bakeri-infected mice receiving no substances, HP + (Alb) – H. polygyrus/bakeri-infected mice receiving the nematicide drugs albendazole and zentel in suspension, HP + (II-1′) – H. polygyrus/bakeri-infected mice receiving the test compound in a higher concentration.
UHPLC-IT-MS analysis of mice serum
The peak for compound II-1 was observed at 5.6 min The calibration curves for each chemical were established by injections of different volumes of a mixture of standards prepared in methanol at 25 μg/mL. The detection limit of the method was approximately 0.05 ng/mL.
Determination of CYP450 inhibition by II-1
An important issue in the development of new drugs is the determination of drug interactions to define the safety of a given drug in polytherapy. The main mechanisms responsible for the appearance of clinical symptoms of drug-drug interactions are the induction or inhibition of the activity of cytochrome CYP450 enzymes [82]. The effect of II-1 on drug metabolism related to the activity of cytochrome CYP450 enzymes was investigated using fluorescence assays. The degree of inhibition of CYP3A4 (Fig. 7A), CYP3A5 (Fig. 7B), CYP2B6 (Fig. 7C), and CYP2C19 (Fig. 7D) enzyme activity was checked depending on the concentration of the test compound. For the interpretation of the obtained results, it was assumed that if the concentration of compound II-1 in the serum of mice was not possible to determine using UHPLC, where the detection threshold was about 0.05 ng/mL, the concentration of the test compound in the body of the mice did not exceed the value of approximately 0.05 ng/mL. II-1 at a concentration of 0.05 ng/mL and below did not inhibit the activity of enzymes CYP3A4 (Fig. 7A), CYP3A5 (Fig. 7B), and CYP2C19 (Fig. 7D) statistically significantly, compared to the control (p > 0.05). II-1 at a concentration of 0.05 ng/mL inhibited the activity of the CYP2B6 enzyme statistically significantly, compared to the control (p < 0.05) (Fig. 7C). A statistically significant inhibitory effect of II-1 on the activity of cytochrome CYP 450 enzymes was observed at a concentration 10x higher (CYP3A4 and CYP2C19) or 100x higher (CYP3A5) than the expected concentration of the test compound in the serum of the mice. In the case of enzyme CYP2B6, the II-1 compound at a concentration of 0.025 ng/mL no longer had a statistically significant effect on the inhibition of its activity. The human CYP2B6 enzyme accounts for approximately 4 % of the hepatic CYP450 cytochrome. CYP2B6 is involved in the metabolism of analgesics (tramadol, diclofenac), antiplatelet agents (clopidogrel), anesthetics (ketamine, propofol), anxiolytics, and anticonvulsants (diazepam, clothiazepam, temazepam) [83], [84]. Due to the possible inhibition of the activity of this enzyme by II-1, the metabolism of these drugs may be reduced.
Fig. 7.
Screening of II-1 based on the concentration-dependent inhibition of CYP3A4 (A), CYP3A5 (B), CYP2B6 (C), and CYP2C19 (D) at 0.025, 0.05, 0.5, 5, 50, and 500 ng/mL. Ketoconazole at 10 µM or miconazole at 30 µM were included as positive inhibitors.
Molecular docking
In silico anthelmintic activity
Currently, benzimidazole derivatives are one of the groups of compounds commonly used in the treatment of parasitic diseases of the digestive system in humans and animals. Numerous scientific publications indicate that compounds from this group interact with tubulin [18], [19], [20]. Our studies confirmed the best potential anthelmintic activity of the II-1 derivative, compared to the other compounds. The docking studies demonstrated excellent affinities of II-1 to tubulin, which are comparable to those of colchicine and much higher than the energy of the albendazole-tubulin complex (supp. Table 1S). The molecule forms two strong hydrogen bonds with Asn204 (2.04 Å) and Gln245 (2.20 Å). The quinoline substituent is suited to the lipophilic cavity formed by Ile169, Ile229, Leu225, and Tyr222. In addition, Ile169 and Tyr222 interplay with 4-methoxyphenyl and 1,2,4-triazole cores through various types of hydrophobic interactions (Pi-Pi stacked, Alkyl, and Pi-Alkyl). Probably, the difference in the expression of the anthelmintic activity between II and 1 and the other compounds is the possibility to form a hydrogen bond by a 4-methoxyphenyl substituent, which stabilizes the II-1-tubulin complex. This purpose correlates with the fact that the second most active compound II-4 also possesses oxygen (—NO2 group) in the para position, which allows interaction with tubulin, similar to the methoxy substituent in II-1 (supp. Table 4S). The anthelmintic activity of II-1 may also be enforced by inhibition of SDH. The docking simulation revealed the influence of II-1 on SDH with binding energy close to that of the reported NN-23 inhibitor. II-1 interplays with SDH by only one hydrogen bond with Pro143 (2.10 Å). Additional binding energy is supported by hydrophobic interaction with the aromatic and aliphatic amino acids by quinoline and 4-methoxyphenyl substituents. The triazole ring also forms Pi-sigma and Pi-Alkyl bonds with Pro145 and Leu144, respectively. Nevertheless, II-1 demonstrated a nearly sixteen-fold lower affinity than fluopyram. However, the results of docking with SDH are rather controversial and need further experimental confirmation. Therefore, as shown by the in silico simulation, II-1 is a potent anthelmintic agent with possibly multiple mechanisms of action, with the prevalence of tubulin polymerization inhibition (supp. Fig. 3S).
Conclusions
The results of our studies showed that compound II-1 exhibited in vitro anthelmintic activity in all tested models (Rhabditis sp., Haemonchus contortus, Strongylidae sp.). Also, the in vivo studies indicated the anthelmintic activity of this compound measured by the reduction in the number of Heligmosomoides polygyrus/bakeri nematodes in the intestine of infected mice. Moreover, the in silico research suggested possible modes of action of the most active compound II-1 through the inhibition of tubulin polymerization and the SDH enzyme. Importantly, the tests of biochemical and hematological parameters as well as the histopathological tests showed no systemic toxicity of the analyzed compound. Based on the enzyme studies, it can be assumed that the use of II-1 in polytherapy with drugs metabolized by CYP3A4, CYP3A5, and CYP2C19 enzymes should not result in pharmacodynamic interactions increasing the concentration of concurrently administered drugs due to the inhibition of the activity of these enzymes by the test compound. Our research presents a very efficient synthesis of a new compound whose structure and activity have been patented by us (No. PL 236684). The test compound can be described as a HIT, which in the future has a great chance of becoming an anthelmintic drug for the treatment of parasitosis caused by intestinal nematodes in humans and animals.
Funding
The present research was funded as part of the “Innovation Incubator 2.0” project co-financed by the Smart Growth Operational Program 2014–2020, Action 4.4 (AB-K), project the National Science Center No. 2017/01/X/NZ6/01001 (PK), and the Statutory Fund of the Medical University of Lublin DS No. 43 (AB-K), DS No. 15 (MW).
CRediT authorship contribution statement
Przemysław Kołodziej: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Resources, Data curation, Writing – original draft, Writing – review & editing, Visualization, Project administration, Funding acquisition. Monika Wujec: Methodology, Validation, Writing – review & editing, Funding acquisition. Maria Doligalska: Methodology, Validation, Resources, Visualization. Anna Makuch-Kocka: Methodology, Validation, Writing – original draft. Dmytro Khylyuk: Methodology, Visualization. Jacek Bogucki: Formal analysis, Data curation, Visualization. Marta Demkowska-Kutrzepa: Methodology, Validation. Monika Roczeń-Karczmarz: Methodology, Validation. Maria Studzińska: Methodology, Validation. Krzysztof Tomczuk: Methodology, Validation. Marcin Kocki: Writing – original draft, Visualization. Patrycja Reszka-Kocka: Writing – original draft, Visualization. Sebastian Granica: Methodology, Validation. Rafał Typek: Methodology, Validation. Andrzej L. Dawidowicz: Methodology, Validation. Janusz Kocki: Writing – review & editing, Supervision. Anna Bogucka-Kocka: Conceptualization, Methodology, Validation, Resources, Writing – review & editing, Supervision, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Patent number PL 236684 covering the protection of compound II-1 was awarded a gold medal at the 13th International Inventions and Innovations Show INTARG® 2020 Online.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jare.2023.07.004.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Gupta R., Rayamajhee B., Sherchan S.P., Rai G., Mukhiya R.K., Khanal B., et al. Prevalence of intestinal parasitosis and associated risk factors among school children of Saptari district, Nepal: a cross-sectional study. Trop Med Health. 2020;48:73. doi: 10.1186/s41182-020-00261-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stepek G., Buttle D.J., Duce I.R., Behnke J.M. Human gastrointestinal nematode infections: are new control methods required? Int J Exp Pathol. 2006;87(5):325–341. doi: 10.1111/j.1365-2613.2006.00495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Charlier J., Höglund J., Morgan E.R., Geldhof P., Vercruysse J., Claerebout E. Biology and Epidemiology of Gastrointestinal Nematodes in Cattle. Vet Clin North Am Food Anim Pract. 2020;36(1):1–15. doi: 10.1016/j.cvfa.2019.11.001. PMID: 32029177. [DOI] [PubMed] [Google Scholar]
- 4.Feleke DG, Alemu Y, Bisetegn H, Mekonnen M, Yemanebrhane N. Intestinal parasitic infections and associated factors among street dwellers and prison inmates: A systematic review and meta-analysis. PLoS ONE 2021 5;16(8):e0255641. doi: 10.1371/journal.pone.0255641. PMID: 34352000; PMCID: PMC8341648. [DOI] [PMC free article] [PubMed]
- 5.Pehlivanoğlu B, Doğanavşargil B, Sezak M, Nalbantoğlu İ, Korkmaz M. Gastrointestinal Parasitosis: Histopathological Insights to Rare but Intriguing Lesions of the Gastrointestinal Tract. Turk Patoloji Derg. 2016;32(2):82–90. English. doi: 10.5146/tjpath.2015.01350. PMID: 27136106. [DOI] [PubMed]
- 6.https://www.cdc.gov/parasites/sth/index.html (accessed on 01.02.2022).
- 7.WHO online: https://www.who.int/news-room/fact-sheets/detail/soil-transmitted-helminth-infections (accessed on 06.10.2022).
- 8.Else K.J., Keiser J., Holland C.V., Grencis R.K., Sattelle D.B., Fujiwara R.T., et al. Whipworm and roundworm infections. Nat Rev Dis Primers. 2020;6(1):44. doi: 10.1038/s41572-020-0171-3. PMID: 32467581. [DOI] [PubMed] [Google Scholar]
- 9.Eyayu T., Kiros T., Workineh L., Sema M., Damtie S., Hailemichael W., et al. Prevalence of intestinal parasitic infections and associated factors among patients attending at Sanja Primary Hospital, Northwest Ethiopia: An institutional-based cross-sectional study. PLoS One. 2021;16(2):e0247075. doi: 10.1371/journal.pone.0247075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Norhayati M., Fatmah M.S., Yusof S., Edariah A.B. Intestinal parasitic infections in man: a review. Med J Malaysia. 2003;58(2):296–305. quiz 306. PMID: 14569755. [PubMed] [Google Scholar]
- 11.Husen E.A., Tafesse G., Hajare S.T., Chauhan N.M., Sharma R.J., Upadhye V.J. Cross-Sectional Study on Assessment of Frequency of Intestinal Helminth Infections and Its Related Risk Factors among School Children from Adola Town. Ethiopia Biomed Res Int. 2022;2022:5908938. doi: 10.1155/2022/5908938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hotez P.J., Brindley P.J., Bethony J.M., King C.H., Pearce E.J., Jacobson J. Helminth infections: the great neglected tropical diseases. J Clin Invest. 2008;118(4):1311–1321. doi: 10.1172/JCI34261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fan C.K., Chuang T.W., Huang Y.C., Yin A.W., Chou C.M., Hsu Y.T., et al. Enterobius vermicularis infection: prevalence and risk factors among preschool children in kindergarten in the capital area, Republic of the Marshall Islands. BMC Infect Dis. 2019;19(1):536. doi: 10.1186/s12879-019-4159-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abate A., Kibret B., Bekalu E., Abera S., Teklu T., Yalew A., et al. Cross-Sectional Study on the Prevalence of Intestinal Parasites and Associated Risk Factors in Teda Health Centre. Northwest Ethiopia ISRN Parasitol. 2013;2013 doi: 10.5402/2013/757451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Braseth A.L., Elliott D.E., Ince M.N. Parasitic Infections of the Gastrointestinal Track and Liver. Gastroenterol Clin North Am. 2021;50(2):361–381. doi: 10.1016/j.gtc.2021.02.011. PMID: 34024446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noyer C.M., Brandt L.J. Parasitic infections of the gastrointestinal tract. Curr Gastroenterol Rep. 1999;1(4):282–291. doi: 10.1007/s11894-999-0111-6. PMID: 10980962. [DOI] [PubMed] [Google Scholar]
- 17.Tadege B., Mekonnen Z., Dana D., Tiruneh A., Sharew B., Dereje E., et al. Assessment of the nail contamination with soil-transmitted helminths in schoolchildren in Jimma Town, Ethiopia. PLoS One. 2022;17(6):e0268792. doi: 10.1371/journal.pone.0268792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kappagoda S., Singh U., Blackburn B.G. Antiparasitic therapy. Mayo Clin Proc. 2011;86(6):561–583. doi: 10.4065/mcp.2011.0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tejchman W., Kołodziej P., Kalinowska-Tłuścik J., Nitek W., Żuchowski G., Bogucka-Kocka A., et al. Discovery of Cinnamylidene Derivative of Rhodanine with High Anthelmintic Activity against Rhabditis sp. Molecules. 2022;27(7):2155. doi: 10.3390/molecules27072155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holden-Dye L., Walker R.J. Anthelmintic drugs. WormBook. 2007:1–13. doi: 10.1895/wormbook.1.143.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Geerts S., Gryseels B. Anthelmintic resistance in human helminths: a review. Trop Med Int Health. 2001;6(11):915–921. doi: 10.1046/j.1365-3156.2001.00774.x. PMID: 11703846. [DOI] [PubMed] [Google Scholar]
- 22.Wolstenholme A.J., Fairweather I., Prichard R., von Samson-Himmelstjerna G., Sangster N.C. Drug resistance in veterinary helminths. Trends Parasitol. 2004;20(10):469–476. doi: 10.1016/j.pt.2004.07.010. PMID: 15363440. [DOI] [PubMed] [Google Scholar]
- 23.Partridge F.A., Forman R., Bataille C.J.R., Wynne G.M., Nick M., Russell A.J., et al. Anthelmintic drug discovery: target identification, screening methods and the role of open science. Beilstein J Org Chem. 2020;16:1203–1224. doi: 10.3762/bjoc.16.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahuir-Baraja A.E., Cibot F., Llobat L., Garijo M.M. Anthelmintic resistance: is a solution possible? Exp Parasitol. 2021;230 doi: 10.1016/j.exppara.2021.108169. PMID: 34627787. [DOI] [PubMed] [Google Scholar]
- 25.Rose Vineer H., Morgan E.R., Hertzberg H., Bartley D.J., Bosco A., Charlier J., et al. Increasing importance of anthelmintic resistance in European livestock: creation and meta-analysis of an open database. Parasite. 2020;27:69. doi: 10.1051/parasite/2020062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Le T.G., Kundu A., Ghoshal A., Nguyen N.H., Preston S., Jiao Y., et al. Novel 1-Methyl-1 H-pyrazole-5-carboxamide Derivatives with Potent Anthelmintic Activity. J Med Chem. 2019;62(7):3367–3380. doi: 10.1021/acs.jmedchem.8b01790. PMID: 30875218. [DOI] [PubMed] [Google Scholar]
- 27.Geary T.G., Chibale K., Abegaz B., Andrae-Marobela K., Ubalijoro E. A new approach for anthelmintic discovery for humans. Trends Parasitol. 2012;28(5):176–181. doi: 10.1016/j.pt.2012.02.006. PMID: 22424638. [DOI] [PubMed] [Google Scholar]
- 28.Kelleher A.C., Good B., de Waal T., Keane O.M. Anthelmintic resistance among gastrointestinal nematodes of cattle on dairy calf to beef farms in Ireland. Ir Vet J. 2020;73:12. doi: 10.1186/s13620-020-00167-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.El Ridi R.A., Tallima H.A. Novel therapeutic and prevention approaches for schistosomiasis: review. J Adv Res. 2013;4(5):467–478. doi: 10.1016/j.jare.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pink R., Hudson A., Mouriès M.A., Bendig M. Opportunities and challenges in antiparasitic drug discovery. Nat Rev Drug Discov. 2005;4(9):727–740. doi: 10.1038/nrd1824. PMID: 16138106. [DOI] [PubMed] [Google Scholar]
- 31.Paprocka R., Kołodziej P., Wiese-Szadkowska M., Helmin-Basa A., Bogucka-Kocka A. Evaluation of Anthelmintic and Anti-Inflammatory Activity of 1,2,4-Triazole Derivatives. Molecules. 2022;27(14):4488. doi: 10.3390/molecules27144488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bogucka-Kocka A., Kołodziej P., Makuch-Kocka A., Różycka D., Rykowski S., Nekvinda J., et al. Nematicidal activity of naphthalimide-boron cluster conjugates. Chem Commun (Camb) 2022;58(15):2528–2531. doi: 10.1039/d1cc07075d. PMID: 35098961. [DOI] [PubMed] [Google Scholar]
- 33.Dziduch K., Kołodziej P., Paneth A., Bogucka-Kocka A., Wujec M. Synthesis and Anthelmintic Activity of New Thiosemicarbazide Derivatives-A Preliminary Study. Molecules. 2020;25(12):2770. doi: 10.3390/molecules25122770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dziduch K., Greniuk D., Wujec M. The Current Directions of Searching for Antiparasitic Drugs. Molecules. 2022;27(5):1534. doi: 10.3390/molecules27051534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Le T.G., Kundu A., Ghoshal A., Nguyen N.H., Preston S., Jiao Y., et al. Optimization of Novel 1-Methyl-1 H-Pyrazole-5-carboxamides Leads to High Potency Larval Development Inhibitors of the Barber's Pole Worm. J Med Chem. 2018;61(23):10875–10894. doi: 10.1021/acs.jmedchem.8b01544. PMID: 30403349. [DOI] [PubMed] [Google Scholar]
- 36.Piplani M., Rajak H., Sharma P.C. Synthesis and characterization of N-Mannich based prodrugs of ciprofloxacin and norfloxacin: In vitro anthelmintic and cytotoxic evaluation. J Adv Res. 2017;8(4):463–470. doi: 10.1016/j.jare.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Węglińska L., Bekier A., Trotsko N., Kaproń B., Plech T., Dzitko K., et al. Inhibition of Toxoplasma gondii by 1,2,4-triazole-based compounds: marked improvement in selectivity relative to the standard therapy pyrimethamine and sulfadiazine. J Enzyme Inhib Med Chem. 2022;37(1):2621–2634. doi: 10.1080/14756366.2022.2112576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bekier A., Węglińska L., Paneth A., Paneth P., Dzitko K. 4-Arylthiosemicarbazide derivatives as a new class of tyrosinase inhibitors and anti-Toxoplasma gondii agents. J Enzyme Inhib Med Chem. 2021;36(1):1145–1164. doi: 10.1080/14756366.2021.1931164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khan G., Sreenivasa S., Govindaiah S., Chondramohan V., Shetty R.P. Synthesis, biological screening, in silico study and fingerprint applications of novel 1, 2, 4-triazole derivatives. J Heterocycl Chem. 2020;57(4):2010–2023. doi: 10.1002/jhet.3929. [DOI] [Google Scholar]
- 40.Gupta J.K., Mishra P. Antimicrobial and anthelmintic activities of some newly synthesized triazoles. Asian J Pharm Clin Res. 2017;10(6):139–145. doi: 10.22159/ajpcr.2017.v10i6.17800. [DOI] [Google Scholar]
- 41.Panda S., Nayak S. Antibacterial, antioxidant and anthelmintic studies of inclusion complexes of some 4-arylidenamino-5-phenyl-4H-1,2,4-triazole-3-thiols. Supramolecular Chem. 2015;27(10):679–689. doi: 10.1080/10610278.2015.1075533. [DOI] [Google Scholar]
- 42.Kumar P.S., Sahoo J. Anthelmintic evaluation of some novel synthesized 1,2,4-triazole moiety clubbed with benzimidazole ring. Oriental J Chem. 2014;30(1):211–217. doi: 10.13005/ojc/300125. [DOI] [Google Scholar]
- 43.Patil R.D., Biradar J.S. Synthesis and biological activities of fused biheterocycles containing indole nucleus: reactions of 3-(5’-substituted-3’-phenylindol-2’-yl)-4-amino-4,5-dihydro-s-triazol-3-thiones. Ind J Chem. 2000;39(12):929–935. [Google Scholar]
- 44.Sharma P.C., Kumar D., Gorsi R., Sharma A., Rajak H. Synthesis and biological evaluation of clubbed triazole-thiazolidinone derivatives. Bull Pharm Res. 2014;4(2):72–80. [Google Scholar]
- 45.Nayak S., Panda S. Impact of inclusion complex formation on antibacterial, antioxidant and anthelmintic activities of some 4-arylidenamino-5-phenyl-4H-1,2,4-triazole-3-thiols. Indian J Chem. 2016;55B:1144–1150. [Google Scholar]
- 46.Nayak S., Badatya M., Swai S.K., Garnaik B. Studies on inclusion complexes of some 4- arylidenamino-5-phenyl-4h-1, 2, 4-triazole-3-thiols. World Journal of Pharmacy and Pharmaceutical Sciences. 2016;5:2233–2248. [Google Scholar]
- 47.Jayappa M.K.D., Dasappa J.P., Chandrashekar K.R., Sheik S., Chaluvaiah K., Naik P. Synthesis, antimicrobial and anthelmintic activity studies of some novel triazole Schiff and Mannich bases. Der Pharma Chemica. 2017;9:163–171. [Google Scholar]
- 48.Sahoo B.M., Nagamounika K., Banik B.K. Microwave-assisted synthesis of Schiff’s bases of 1,2,4-triazole derivatives and their anthelmintic activity. J Indian Chem Soc. 2018;95:1289–1294. [Google Scholar]
- 49.Rahiman AM, Kalluraya B, Yogesh SH, Manju N, Asma and Suresha MG. Regioselective reaction: synthesis of 1, 2, 4-triazole based Mannich bases and their biological activity. Heterocycl Lett 2019;9:279–88.
- 50.Abongwa M., Martin R.J., Robertson A.P. A brief review on the mode of action of antinematodal drugs. Acta Vet (Beogr) 2017;67(2):137–152. doi: 10.1515/acve-2017-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schleker A.S.S., Rist M., Matera C., Damijonaitis A., Collienne U., Matsuoka K., et al. Mode of action of fluopyram in plant-parasitic nematodes. Sci Rep. 2022;12(1):11954. doi: 10.1038/s41598-022-15782-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cheng W., Yan Y., Xiao T., Zhang G., Zhang T., Lu T., et al. Design, synthesis and inhibitory activity of novel 2, 3-dihydroquinolin-4(1H)-one derivatives as potential succinate dehydrogenase inhibitors. Eur J Med Chem. 2021;15(214) doi: 10.1016/j.ejmech.2021.113246. PMID: 33582385. [DOI] [PubMed] [Google Scholar]
- 53.Wang J., Lu T., Xiao T., Cheng W., Jiang W., Yan Y., et al. Novel quinolin-2(1H)-one analogues as potential fungicides targeting succinate dehydrogenase: design, synthesis, inhibitory evaluation and molecular modeling. Pest Manag Sci. 2022 Dec 23 doi: 10.1002/ps.7332. PMID: 36562216. [DOI] [PubMed] [Google Scholar]
- 54.https://swift.cmbi.umcn.nl/servers/html/index.html (accessed on 12.12.2022).
- 55.http://www.bioinfo.ifm.liu.se/ProQM/index.php?about=proqm (accessed on 12.12.2022).
- 56.Ray A., Lindahl E., Wallner B. Model quality assessment for membrane proteins. Bioinformatics. 2010;26(24):3067–3074. doi: 10.1093/bioinformatics/btq581. PMID: 20947525. [DOI] [PubMed] [Google Scholar]
- 57.http://clab.labshare.cn/cb-dock/php/index.php (accessed on 12.12.2022).
- 58.Wang Y., Zhang H., Gigant B., Yu Y., Wu Y., Chen X., et al. Structures of a diverse set of colchicine binding site inhibitors in complex with tubulin provide a rationale for drug discovery. FEBS J. 2016;283(1):102–111. doi: 10.1111/febs.13555. PMID: 26462166. [DOI] [PubMed] [Google Scholar]
- 59.Inaoka D.K., Shiba T., Sato D., Balogun E.O., Sasaki T., Nagahama M., et al. Structural Insights into the Molecular Design of Flutolanil Derivatives Targeted for Fumarate Respiration of Parasite Mitochondria. Int J Mol Sci. 2015;16(7):15287–15308. doi: 10.3390/ijms160715287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dewar M.J.S., Zoebisch E.G., Healy E.F., Stewart J.J.P. Development and use of quantum mechanical molecular models. 76. AM1: a new general purpose quantum mechanical molecular model. J Am Chem Soc. 1985;107(13):3902–3909. [Google Scholar]
- 61.https://pubchem.ncbi.nlm.nih.gov (accessed on 12.12.2022).
- 62.Morris G.M., Goodsell D.S., Halliday R.S., Huey R., Hart W.E., Belew R.K., et al. Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J Comput Chem. 1998;19(14):1639–1662. [Google Scholar]
- 63.Cook A.H., Heilbron I.M., Steger L. New therapeutic agents of the quinoline series. Part IV Lutidylquinolines J Chem Soc. 1943:413. [Google Scholar]
- 64.Hahnel S.R., Dilks C.M., Heisler I., Andersen E.C., Kulke D. Caenorhabditis elegans in anthelmintic research - Old model, new perspectives. Int J Parasitol Drugs Drug Resist. 2020;14:237–248. doi: 10.1016/j.ijpddr.2020.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kiontke K., Fitch D.H. The phylogenetic relationships of Caenorhabditis and other rhabditids. WormBook. 2005;1–11 doi: 10.1895/wormbook.1.11.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ziaja-Sołtys M., Kołodziej P., Stefaniuk D., Matuszewska A., Jaszek M., Bogucka-Kocka A. Low-Molecular-Weight Secondary Metabolites from Fungi: Cerrena unicolor as a New Proposal of an Effective Preparation against Rhabditis Nematodes. Molecules. 2022;27(5):1660. doi: 10.3390/molecules27051660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fadaei Tehrani M., Sharifdini M., Zahabiun F., Latifi R., Kia E.B. Molecular characterization of human isolates of Strongyloides stercoralis and Rhabditis spp. based on mitochondrial cytochrome c oxidase subunit 1 (cox1) BMC Infect Dis. 2019;19(1):776 doi: 10.1186/s12879-019-4407-3. PMID: 31488073; PMCID: PMC6727369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Teschner M., Würfel W., Sedlacek L., Suerbaum S., Tappe D., Hornef M.W. Outer ear canal infection with Rhabditis sp. nematodes in a human. J Clin Microbiol. 2014;52(5):1793–1795. doi: 10.1128/JCM.00115-14. PMID: 24599974; PMCID: PMC3993619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yang S., Wang J., Shi D., Cui L., Qiao R., Li S. Polyparasitism of Rhabditis Axei and Enterobius Vermicularis in a Child from Beijing, China. Clin Lab. 2018;1;64(10):1773–1776 doi: 10.7754/Clin.Lab.2018.180540. PMID: 30336527. [DOI] [PubMed] [Google Scholar]
- 70.Duarte E.R., Melo M.M., Hamdan J.S. Epidemiological aspects of bovine parasitic otitis caused by Rhabditis spp. and/or Raillietia spp. in the state of Minas Gerais, Brazil. Vet Parasitol. 2001;101(1):45–52. doi: 10.1016/s0304-4017(01)00492-7. PMID: 11587832. [DOI] [PubMed] [Google Scholar]
- 71.Sobral S.A., Ferraz C.M., Souza R.I.L., Queiroz L.M., Reinó N., Junior O.L.F., et al. Association between Duddingtonia flagrans, dimethylsulfoxide and ivermectin for the control of Rhabditis spp. in cattle. Trop Anim Health Prod. 2022;54(4):198 doi: 10.1007/s11250-022-03197-5. PMID: 35666291. [DOI] [PubMed] [Google Scholar]
- 72.Sobral S.A., Ferreira B.S., Senna C.C., Ferraz C.M., Moreira T.F., Fidelis Junior O.L., et al. Rhabditis spp., in the Espírito Santo, State of Brazil and evaluation of biological control. Rev Bras Parasitol Vet. 2019;28(2):333–337. doi: 10.1590/S1984-29612019020. PMID: 31188945. [DOI] [PubMed] [Google Scholar]
- 73.Demkowska-Kutrzepa M., Szczepaniak K., Dudko P., Roczeń-Karczmarz M., Studzińska M., Żyła S., et al. Determining the occurrence of the Uncinaria stenocephala and Ancylostoma caninum nematode invasion in dogs in Poland, with special emphasis on the Lublin region. Med Weter. 2018;74(8):526–531. [Google Scholar]
- 74.Gundłach J.L., Sadzikowski A.B., Tomczuk K. Diagnosis of tapeworm infestation in horses. Medycyna Wet. 2003;59(6):532–535. [Google Scholar]
- 75.Tomczuk K., Kostro K., Szczepaniak K.O., Grzybek M., Studzińska M., Demkowska-Kutrzepa M., et al. Comparison of the sensitivity of coprological methods in detecting Anoplocephala perfoliata invasions. Parasitol Res. 2014;113(6):2401–2406. doi: 10.1007/s00436-014-3919-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tomczuk K., Kostro K., Grzybek M., Szczepaniak K., Studzińska M., Demkowska-Kutrzepa M., et al. Seasonal changes of diagnostic potential in the detection of Anoplocephala perfoliata equine infections in the climate of Central Europe. Parasitol Res. 2015;114(2):767–772. doi: 10.1007/s00436-014-4279-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cheptea C., Sunel V., Desbrieres J., Popa M. Synthesis and Antimicrobial Activity of New Derivatives of 1,3,4-Thiadiazoles and 1,2,4-Triazoles with 5-Nitroindazole as Support. J Heterocycl Chem. 2013;50:366–372. doi: 10.1002/jhet.1738. [DOI] [Google Scholar]
- 78.Thomas K.D., Vasudeva Adhikari A., Telkar S., Chowdhury I.H., Mahmood R., Pal N.K., et al. Design, synthesis and docking studies of new quinoline-3-carbohydrazide derivatives as antitubercular agents. Eur J Med Chem. 2011;46(11):5283–5292. doi: 10.1016/j.ejmech.2011.07.033. [DOI] [PubMed] [Google Scholar]
- 79.Pintilie O., Profire L., Sunel V., Popa M., Pui A. Synthesis and antimicrobial activity of some new 1,3,4-thiadiazole and 1,2,4-triazole compounds having a D. L-methionine moiety Molecules. 2007;12:103–113. doi: 10.3390/12010103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Plech T., Wujec M., Siwek A., Kosikowska U., Malm A. Synthesis and antimicrobial activity of thiosemicarbazides, s-triazoles and their Mannich bases bearing 3-chlorophenyl moiety. Eur J Med Chem. 2011;46:241–248. doi: 10.1016/j.ejmech.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 81.Pitucha M., Woś M., Miazga-Karska M., Kloimek K., Mirosłąw B., Pachuta-Stec A., et al. Synthesis, antibacterial and antiproliferative potential of some new 1-pyridinecarbonyl-4-substituted thiosemicarbazide derivatives. Med Chem Res. 2016;25:1667–1677. doi: 10.1007/s00044-016-1599-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Koukouritaki S.B., Manro J.R., Marsh S.A., Stevens J.C., Rettie A.E., McCarver D.G., et al. Developmental expression of human hepatic CYP2C9 and CYP2C19. J Pharmacol Exp Ther. 2004;308(3):965–974. doi: 10.1124/jpet.103.060137. PMID: 14634042. [DOI] [PubMed] [Google Scholar]
- 83.Mangó K., Kiss Á.F., Fekete F., Erdős R., Monostory K. CYP2B6 allelic variants and non-genetic factors influence CYP2B6 enzyme function. Sci Rep. 2022;12(1):2984. doi: 10.1038/s41598-022-07022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Langmia I.M., Just K.S., Yamoune S., Brockmöller J., Masimirembwa C., Stingl J.C. CYP2B6 Functional Variability in Drug Metabolism and Exposure Across Populations-Implication for Drug Safety, Dosing, and Individualized Therapy. Front Genet. 2021;12 doi: 10.3389/fgene.2021.692234. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.