Abstract
Daily agro-industrial waste, primarily cellulose, lignin, and hemicellulose, poses a significant environmental challenge. Harnessing lignocellulolytic enzymes, particularly endo-1,4-β-xylanases, for efficient saccharification is a cost-effective strategy, transforming biomass into high-value products. This study focuses on the cloning, expression, site-directed mutagenesis, purification, three-dimensional modeling, and characterization of the recombinant endo-1,4-β-xylanase (XlnA) from Aspergillus clavatus in Escherichia coli. This work includes evaluation of the stability at varied NaCl concentrations, determining kinetic constants, and presenting the heterologous expression of XlnAΔ36 using pET22b(+). The expression led to purified enzymes with robust stability across diverse pH levels, exceptional thermostability at 50 °C, and 96–100% relative stability after 24 h in 3.0 M NaCl. Three-dimensional modeling reveals a GH11 architecture with catalytic residues Glu 132 and 22. XlnAΔ36 demonstrates outstanding kinetic parameters compared to other endo-1,4-β-xylanases, indicating its potential for industrial enzymatic cocktails, enhancing saccharification. Moreover, its ability to yield high-value compounds, such as sugars, suggests a promising and ecologically positive alternative for the food and biotechnology industries.
Keywords: Aspergillus clavatus; Endo-1,4-beta-xylanase; NaCl stability; Site-directed mutagenesis
Introduction
Agro-industrial residues are one of the most abundant, and renewable, resources on the planet (Pasin et al. 2023). While there is significant interest in their potential for upgrading them to more valuable products such as prebiotics, in most cases they are underutilized by industry (Nordberg Karlsson et al. 2018; Pasin et al. 2022). Xylan is notable as the major hemicellulosic component of these residues and, in fact, it is the most abundant hemicellulose on Earth (Scarcella et al. 2021). Composed by a backbone chain of xylopyranose units linked by β-1,4 bonds, substituted by saccharide and phenolic side chains at various positions (Pasin et al. 2020a), this polysaccharide accounts for approximately one-third of the renewable organic carbon. The hydrolysis of xylan is performed by an assortment of xylanases, produced by a range of different microorganisms. Most of the main-chain acting endo-xylanases known to date have evolved from two main scaffolds: the (α/β)8 TIM barrel, found in three different glycoside hydrolase (GH) families of xylanases (GH5, GH10, and GH30), and the β-jelly roll (GH11) (Marinho et al. 2023). These are all retaining enzymes with a double-displacement mechanism and, depending on the enzyme family, xylans with a variety of substituents and substitution patterns can be hydrolyzed (Silva et al. 2023).
In order to obtain novel modes of xylan hydrolysis, and to generate products with interesting industrial characteristics, it will be necessary to either identify new endo-xylanases. Potentially this could be achieved by genome mining, but modification of known enzymes using site-directed mutagenesis or directed evolution, coupled with heterologous expression, also represents a viable strategy.
Here we report the use of the latter approach, with an endo-1,4-β-xylanase from Aspergillus clavatus. To date there have been a few studies of this enzyme (Pasin et al. 2020a, b; Segato et al. 2014; Squina et al. 2009); however, to our knowledge, there are no reports of its expression in a bacterial system. It is well established that expression in Escherichia coli has several advantages when compared to yeast and fungi as it is easy to cultivate, and has simple nutritional requirements and a short generation time (Sorensen and Mortensen 2005). Further, mutants are easily obtained using well-established methods and screening techniques. Here we describe the cloning of the A. clavatus xylanase, its mutagenesis and successful expression in E. coli, and determination of its kinetic parameters.
Materials and methods
A plasmid containing the codon-optimized synthetic XlnA gene (pUCIDT_xlnA_syn), as well as the primers used in PCR mutagenesis, were purchased from Integrated DNA Technologies (Coralville, USA). All sequencing was carried out by ACTG Inc. (Wheeling, IL, USA). A QuikChange mutagenesis kit was purchased from Agilent (Santa Clara, USA). E. coli Top10 (Invitrogen, Carlsbad, USA) was used as the host for the recombinant plasmids, while E. coli BL21 (DE3) (Novagen, Madison, USA) was used as the host for gene expression. E. coli was grown at 37 °C in Luria–Bertani (LB) medium containing 50 μg/mL ampicillin or kanamycin, as appropriate. Beechwood xylan was purchased from Sigma-Aldrich (São Paulo, Brazil). Other buffer/assay components were the highest grade commercially available.
Alignment of sequence and homology models
The Phyre2 web portal (http://www.sbg.bio.ic.ac.uk/phyre2/) was used to identify sequences homologous to XlnA and to construct homology models. Clustal Omega (Madeira et al. 2019) was used to align the sequence of XlnA with those of the templates for the top-scoring models identified by Phyre2. The structures of the templates were aligned using PyMOL (Schrödinger, Cambridge, USA).
Design of a codon-optimized gene for the XlnA from A. clavatus
Based on previous published results (Pasin et al. 2020a), the amino acid sequence of the putative GH11 endo-1,4-β-xylanase (XlnA) produced by A. clavatus NRRL 1 (NCBI Reference Sequence: XP_001273773.1) was used for the design of a synthetic A. clavatus xlnA gene. The codon optimization tool from Integrated DNA Technologies was used to optimize the gene for expression in E. coli. Concomitantly, additional restriction sites at the N- (Nde1) and C-termini (Xho1, Hind111) were added to facilitate later manipulation of this gene, now denoted xlnA_syn.
Construction of expression vectors for wtXlnA and its 6x-His tag variants
A plasmid midikit (Qiagen) was used to purify pUCIDT_xlnA_syn. The xlnA_syn gene was excised by digestion with Nde1 and Hind11, and subsequently ligated into the same sites in pET17 and pET28a (Novagen, Madison, USA). This provided pET17xlnA and pET28xlnAN-His which were used to express wtXlnA and wtXlnA containing an N-terminal 6x-His tag, respectively. To obtain a vector for expression of wtXlnA with a C-terminal 6x-His tag, a fragment was excised from pUCIDT_xlnA_syn by digestion with Nde1 and Xho1 and ligated into the same sites in pET17PNMT-His (Romero et al. 2004). The resultant vector was denoted pET17xlnAC-His.
Construction of expression vectors for N-terminal deletion variants
Using the QuikChange kit with forward (5′-CGAAACCGCGCTGCATATGTTTGCGCAACGCGCGG-3′) and reverse (5′-CCGCGCGTTGCGCAAACATATGCAGCGCGGTTTCG-3′) primers, a new Nde1 site was added to both pET17xlnAC-His and pET28xlnAN-His. After the fidelity of the mutagenesis was confirmed by sequencing, the plasmids were digested with Nde1 and religated to yield pET17xlnAC-HisΔ36 and pET28xlnAN-HisΔ36. The latter plasmid was subsequently digested with NcoI and HindIII and the xlnA gene fragment ligated into the same sites in pET22b to provide pET22xlnAN-HisΔ36.
Screening for expression of XlnA variants
The plasmids were transformed into E. coli BL21 (DE3), plated and grown overnight at 37 °C on LB medium supplemented with 50 μg/mL kanamycin (pET28 plasmids) or ampicillin (pET17 and pET22 plasmids). For an initial expression check, single colonies were used to inoculate 1 mL of Overnight Express (Millipore-Sigma, Billerica, USA) which was shaken overnight at 37 °C. A sample (250 μL) was subjected to heat denaturation and analyzed by SDS-PAGE (Laemmli 1970).
Large-scale expression and purification of the XlnAN-HisΔ36 variant
For large-scale expression, an aliquot of a fresh overnight culture of pET22xlnAN-HisΔ36 in E. coli BL21(DE3) was used to inoculate 500 mL of autoinduction medium (Studier 2005) containing ampicillin (50 ug/mL). The culture was shaken at 25 °C, 250 rpm for 72 h before the cells were harvested by centrifugation and the supernatant (extracellular extract) stored at −80 °C. After the presence of the target protein was confirmed by SDS-PAGE, the supernatant was subjected to affinity chromatography on a His-Select Nickel Affinity Gel column, as previously described (Gopalakrishna et al. 2004). Fractions (5 mL) were collected and analyzed for xylanase activity. Active fractions were pooled and dialyzed against sodium citrate (50 mM, pH 6.0). After the purity was assessed using a 12% SDS-PAGE gel, the protein concentration (mg/mL) was determined by the Bradford method (Bradford 1976) using bovine serum albumin as standard. The purified protein was stored at −20 °C until use.
General method for determination of xylanolytic activity
A standard assay saw the enzyme (15 μL) incubated with 1% beechwood xylan, Sigma-Aldrich® (São Paulo, Brazil), (35 μL, final concentration 0.7%) in 0.1 M sodium citrate buffer, pH 5.0, at 50 °C. After 10 min, 50 μL of dinitrosalicylic acid (DNS) reagent (Miller 2002) was added, and the mixture boiled for 5 min before being cooled to room temperature. The absorbance of the samples was measured at 540 nm in a Packard SpectraCount™ plate reader (GNTLab Commercial Representation, Ribeirão Preto, SP, Brazil). Reducing sugars were quantified using a standard curve of 0.1 to 1.0 mg/mL xylose, and the extent of non-enzymatic hydrolysis was determined using controls with heat-inactivated enzyme (Pasin et al. 2017). When necessary, the enzyme concentration was adjusted to ensure linearity of product formation during the assay period. One enzyme unit (U) was defined as an amount of enzyme which releases 1 μmol of product per minute under tested conditions. Specific activity was expressed as U/mg total protein.
Effect of temperature and pH on XlnAΔ36 activity
The effect of pH on enzyme activity was determined with 1% beechwood xylan (Sigma-Aldrich®) over the pH range from 3 to 6 in sodium citrate buffer (0.1 M) and 6.5 to 8 in sodium phosphate buffer, using intervals of 0.5. For temperature, the activity was measured by carrying the standard assay at 30 °C. The temperature was increased in 5 °C increments until it reached 70 °C using a thermocycler (Eppendorf®, São Paulo, SP, Brazil).
Temperature and pH stability of XlnAΔ36 activity
The thermostability analysis was performed by incubating the enzyme without substrate, in a thermocycler (Eppendorf®, São Paulo, SP, Brazil) at 50 °C and 60 °C, for up to 12 h. After that, the enzyme was placed on ice and its activity subsequently determined using the standard assay.
The effect of pH on enzyme activity was measured over the range pH 3.0–8.0, in increments of 0.5 pH units. The activity was measured at 50 °C using a variation of the standard assay in which the buffer was replaced by 0.1 M sodium citrate in the range pH 3.0–6.0 and sodium phosphate in the range pH 6.5–8.0.
Effect of NaCl concentration on the activity and stability of XlnAΔ36
The halo-stability was examined by incubating XlnAΔ36 at 4 °C for 24 h in water or NaCl solutions at final incubation concentrations of 0–3.0 M. After the incubation, the residual activities were assayed at 50 °C in 0.1 M sodium citrate buffer, pH 6.0, containing 1% (w/v) beechwood xylan with residual NaCl concentration in the range 0–900 mM. The activities were expressed as percentages from the means of the two controls (Main and Parallel) activities. The main control was composed of the enzyme quickly used after being thawed. The parallel control was composed of the enzyme previously kept at 4 °C, for 24 h. Both the controls had activities measured through a standard enzymatic assay with NaCl concentrations in the range 0–900 mM. One-hundred percent enzymatic activity (mean of Main and Parallel control) corresponded to 1420 ± 10 U/mL. The values of standard deviations represent means from triplicate experiments (n = 3) carried out with three separate preparations of the pure enzyme and each experiment was performed with triplicate samples.
Determination of the kinetic constants for the expressed XlnAΔ36
Aiming to analyze the kinetic parameters, Km and Vmax, of the expressed xylanase, experiments were carried out with xylan beechwood (Sigma-Aldrich®) in 13 different concentrations (0.5–35 mg/mL) diluted in 0.1 M sodium citrate buffer, pH 6.0, for 5 min, at 50 °C, as previously reported (Pasin et al. 2017). The Km and Vmax values were calculated using the OriginPro 8.0 SRO software (OriginLab Corporation) and data were presented using the graphic representations of Michaelis et al. (2011). For the calculations of turnover number (kcat) and catalytic efficiency (kcat/Km), the molecular weight of the XlnA was used.
Results
Preliminary sequence analysis
The endo-1,4-β-xylanase from A. clavatus described in this work was first identified by Pasin et al. (2020a). Following tryptic digestion of the purified enzyme, mass spectrometry was used to obtain a partial amino acid sequence that corresponded to the putative GH11 xylanase (XlnA, EC:3.2.1.8) produced by A. clavatus NRRL 1 (NCBI Reference Sequence: XP_001273773.1). The enzyme comprised 229 amino acids with a calculated molecular mass and pI value of 25,003 Da and 5.85, respectively (http://protcalc.sourceforge.net/). SignalP v. 5.0 (http://www.cbs.dtu.dk/services/SignalP/) indicated that the XlnA sequence likely contained a signal peptide of 18 residues. Three N-glycosylation sites (N30, N100, and N106) were predicted using NetNGlyc 1.0 (http://www.cbs.dtu.dk/services/NetNGlyc/), while no O-glycosylation sites were predicted using NetOGlyc 4.0 (http://www.cbs.dtu.dk/services/NetOGlyc/). Among endo-1,4-β-xylanases with known three-dimensional structures, XlnA showed the highest identity (65.59%) with the thermostable endo-1,4-β-xylanase from Talaromyces cellulolyticus (PDB: 5HXV) (Pasin et al. 2020a).
Initial attempts at expression of XlnA in E. coli
Initially the codon optimization tool from Integrated DNA Technologies was used to design a gene suitable for expression of XlnA in E. coli. In addition to the XlnA coding sequence, the synthetic gene also contained a 5′ Nde1 restriction site, as well as restriction sites for Xho1 and Hind111 at the 3′ end. The last two sites added 2 amino acids, Leu-Glu as well as a stop codon. Ultimately, the synthesized gene comprised 711 bp and contained a 693 bp open reading frame (xlnA_syn) encoding a protein of 231 amino acids. Its sequence has been deposited as GenBank MW269568.
The synthesized gene was ligated into 3 expression vectors, pET17, pET28a, and pET17PNMT-His to provide pET17xlnA, pET28xlnA_synN-His, and pET17xlnA_synC-His, respectively. After transformation into E. coli BL21(DE3), it was expected that successful expression would provide wild-type (wt) XlnA as well as wtXlnA containing N- and C-terminal 6x-His tags. Unfortunately, whether expression was induced with IPTG or the culture was grown in autoinduction media, whole cell screening by SDS-PAGE showed only marginal (and variable) evidence of XlnA expression. Further, any enzyme that was produced did not survive cell lysis. Clearly an alternative approach was required.
In the initial study on XlnA, a homology model was prepared using the structure of the thermostable endo-1,4-beta-xylanase mutant from Talaromyces cellulolyticus (PDB id: 5HXV; 65.59% sequence identity) as the template (Pasin et al. 2020a). That model suggested XlnA had the typical β-jelly roll structure of glycosyl hydrolases belonging to the GH11 family but did not include the residues associated with the putative 18 residue XlnA signal peptide. Indeed, the sequence of the T. cellulolyticus enzyme contained only 190 residues, with essentially all the additional 29 residues of XlnA being found at the N-terminus. Given that it was not clear what role this signal peptide may play in heterologous expression, it was thought that truncation of the N-terminus may be appropriate. However, the optimal length of the deletion was still to be determined. One way to do this was to examine sequences and structures of other GH11 xylanases.
Sequence alignment and homology modeling
The Phyre2 web portal (Kelley et al. 2015) was used to identify X-ray structures with sequences homologous to XlnA that could be used as templates for homology modeling. The initial search provided more than 20 templates expected to produce models with 100% confidence. Of those, the majority had sequence identity in the region of 60–65%, but several had identity levels below 40%. According to Phyre, the optimal template was the structure of an environmentally isolated GH11 xylanase (PDB id: 2VUL) and, somewhat surprisingly, the list did not include 5HXV, the template used for the original XlnA homology model (Pasin et al. 2020a).
In the next step, Clustal Omega was used to align the sequence of XlnA with several of the templates identified by Phyre2 (Fig. 1). The alignments showed that, except for 5HXV (190 residues), all the sequences contained at least 215 residues, and that the additional residues were generally found at the N-terminus. Two xylanases, from Chaetomium thermophilum (1XNK) and Neocallimastix patriciarum (3WP6) contained ~ 25 additional residues at the C-terminus.
Fig. 1.
Clustal Omega alignment of sequences of XlnA and homologues from an environmentally isolated GH11 xylanase (2VUL), Triticum aestivum (1TE1), Chaetomium thermophilum (1XNK), Neocallimastix patriciarum (3WP6), and Talaromyces cellulolyticus CF-2612 (5HXV). Residues, not observed in the model, or the X-ray structure is in blue. The first fully conserved residue is highlighted in red
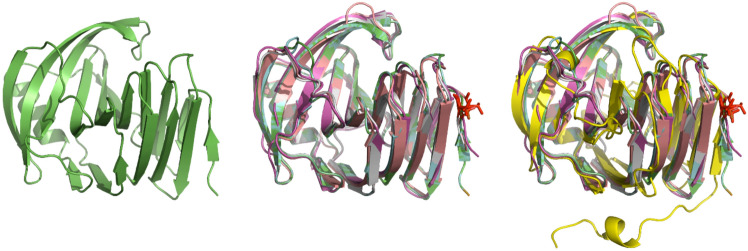
In addition, to obtain a broader perspective, the model of XlnA based on 2VUL (Fig. 2a) and the structures of the top 3 templates (PDB ids: 2VUL, 1TE1, and 1XNK) were aligned using PyMOL. For completeness, the structure of the template (5HXV) (Fig. 2b) and a template with a lower sequence identity (PDB id: 3WP6; 36% identity) were also included (Fig. 2c).
Fig. 2.
A Cartoon representation of the model of XlnA based on 2VUL. B Superposition of XlnA and closely related X-ray structures 2VUL (cyan), 1TE1 (pink), 1XNK (salmon), and 5HXV (gray). C Same view with the addition of the more distantly related 3WP6 (yellow) highlighting the extended N-terminal structure. The fully conserved N-terminal threonine residue is highlighted in red
Both structural and sequence alignments indicated that (1) there was considerable variation in N-terminal sequence and (2) in most cases the N-terminal residues were not observed in the X-ray structure. Further, the first region of structural similarity at the N-terminus contained an invariant threonine residue (Fig. 2) and at least two residues prior to the threonine were observable in all structures. Taken together this suggested that, instead of removing just the putative signal peptide, it may be more beneficial to remove many of the N-terminal residues that were not likely to be playing any significant structural role.
Cloning and expression of truncated XlnA variants
The 2VUL-based model of XlnA showed a defined structure from residue 38 (Fig. 2a). Given that there was a histidine at residue 36, to simplify the molecular biology it was decided to truncate XlnA by 34 residues and replace Glu36 with methionine. Further, it was noted that Glu39 was replaced by glutamine in many structures, so the final change was to make the E39Q replacement. Overall, the changes were expected to provide a truncated XlnA, denoted XlnAΔ36, with the modified N-terminal sequence of MPAQRAG. Accordingly, individual vectors were prepared to express the truncated xylanase with a 6x-His tag at the N- (pET28XlnAΔ36N-His) and the C-terminus (pET17XlnAΔ36C-His).
Both plasmids were transformed into E. coli BL21(DE3) and expression was induced either by growth in autoinduction medium (Studier 2005) or by the addition of IPTG. Culture samples were again screened using SDS-PAGE, and it was gratifying to see that both plasmids produced excellent expression levels. Unfortunately, the protein proved to be insoluble and to be refractory to refolding (data not shown). Lowering the induction temperature to 25 °C had no effect, nor did slowing down induction by taking advantage of the leaky expression from pET17 in the absence of inducing agent.
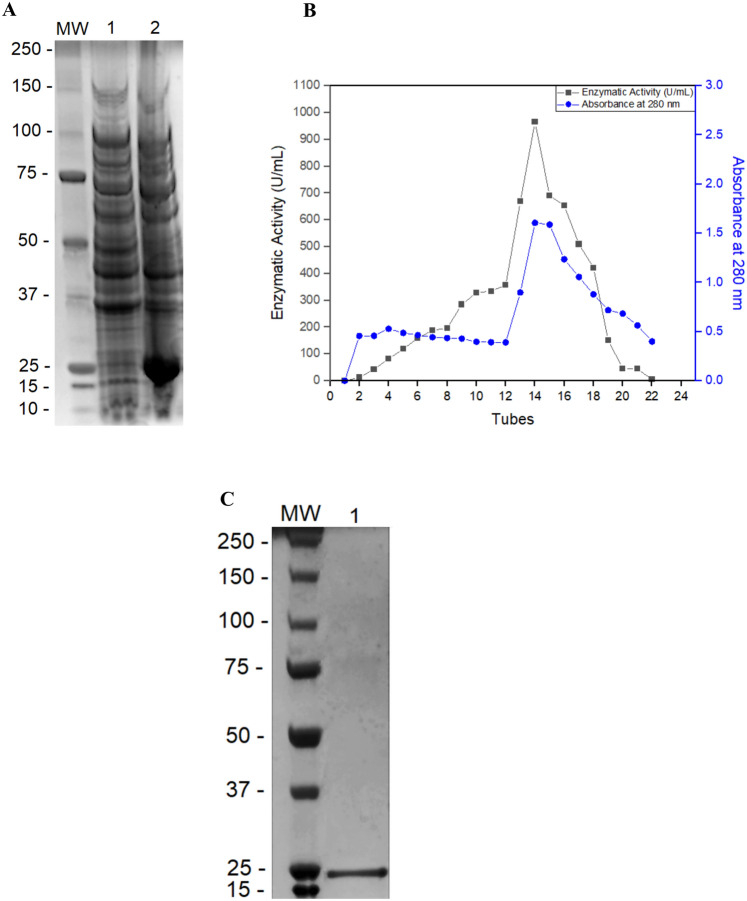
Periplasmic secretion has proved useful, particularly in the production of mammalian proteins which have otherwise formed inclusion bodies in E. coli (Dalton and Barton 2014; Lakkaraju et al. 2012). Therefore, the truncated XlnA gene containing the N-terminal 6x-His tag was excised from pET28XlnAΔ36N-His and ligated into the pET22b expression vector, in frame with the pelB secretion signal peptide. Test cultures were run as described previously but, unlike the previous experiments, xylanolytic activity was detected in the extracellular crude extract. SDS-PAGE analysis confirmed that this system provided excellent expression of the enzyme subsequently referred to as XlnAΔ36N (Fig. 3a).
Fig. 3.
A Electrophoresis of pET22xlnAN-HisΔ36 extract in (Line 1) LB medium and (Line 2) autoinduction medium, B Protein distribution ( )
and xylanase activity (■) after elution in His-Select nickel affinity chromatography, and C analysis of the enzyme purity by electrophoresis of the enzymatic pool obtained after the purification
)
and xylanase activity (■) after elution in His-Select nickel affinity chromatography, and C analysis of the enzyme purity by electrophoresis of the enzymatic pool obtained after the purification
Large-scale expression and purification of XlnAΔ36
In addition, the pelB secretion signal peptide, the N-terminal region of XlnAΔ36, also contained a 6x-His tag to facilitate purification. After the soluble extracts from a 500 mL culture were applied to a Ni2+-affinity column, any unbound proteins were removed by washing with buffer containing 25 mM imidazole. The remaining protein was eluted with buffer containing 250 mM imidazole at a flow rate of 1 mL/min. Fractions (5 mL) were collected during elution and subjected to enzymatic assay and protein quantification. A total of 22 tubes were obtained and only one peak of xylanase activity was observed (Fig. 3b). Finally, those fractions were pooled, and the SDS-PAGE analysis showed that, in a single chromatographic step, XlnAΔ36 had been purified to apparent homogeneity (Fig. 3c). The yield of the purification was 74%, with a 17.5-fold purification factor (Table 1).
Table 1.
Purification of extracellular endoxylanase expressed by E. coli
| Step | Volume (mL) | Protein (mg/mL) | Activity (U/mL) | Spec. activity (U/mg protein) | Yield (%) | Purification (fold) |
|---|---|---|---|---|---|---|
| Crude extract | 400 | 0.90 | 450 | 500 | 100 | 1 |
| Nickel column | 95 | 0.16 | 1400 | 8750 | 74 | 17.5 |
Effect of temperature and pH on XlnAΔ36 activity and stability
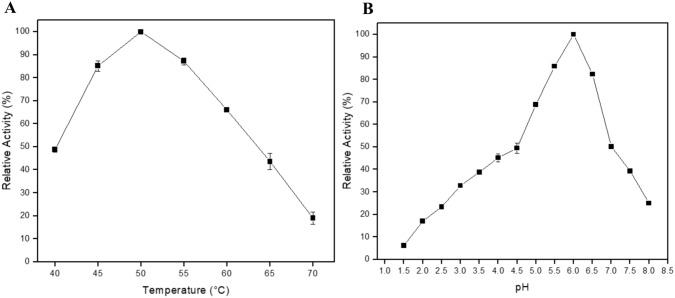
The maximum endo-1,4-β-xylanase activity was observed at 50 °C, with a specific activity of 1420 U/mL (100%). At temperatures between 45 and 55 °C, the enzyme kept more than 85% of its relative activity, while at 60 °C XlnAΔ36 retained more than 65% of its activity. However, further increases in temperature resulted in a decrease in activity, with only 40% relative activity being observed at 65 °C, and an even more rapid decrease to only 20% remaining by 70 °C (Fig. 4a).
Fig. 4.
Effect of A temperature and B pH on the expressed and purified endo-1,4-β-xylanase activity. It was used the sodium citrate buffer in the pH range of 3.0 to 6.0 and sodium phosphate buffer in the pH range of 6.5 to 8.0 for the pH experiment
The pH optimum for endo-1,4-β-xylanase activity was 6.0, where the enzyme showed 100% of its activity (1420 U/mL). In addition, the enzyme maintained high activity rates in the pH range 5.5–6.5, with a relative activity above 80%. In the pH 7.0, there was a sharp decrease in the xylanolytic activity, but the enzyme kept more than 50% of its relative activity. Between pH 7.5 and 8.0, the enzyme activity was 40% and 25% of the relative activity (Fig. 4b).
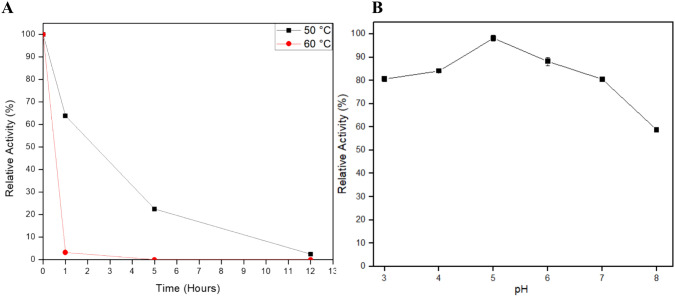
Using 1420 U/mL as the standard (100%), XlnAΔ36 was able to keep 65% of its relative activity at 50 °C after 1 h. After 5 h of experiment the endo-1,4-β-xylanase kept 22% of its activity, and after 12 h it presented 3% of relative activity. At 60 °C, the enzyme was not stable, reaching denaturation after 1 h of experiment (Fig. 5a).
Fig. 5.
Determination of the A temperature stability at (■) 50 °C and ( ) 60 °C
and B pH stability of the expressed and purified endo-1,4-β-xylanase. The enzymatic activity considered 100% was 1420 U/mL. The pH stability (residual activity) was measured after 24 h of incubation in different pH values
) 60 °C
and B pH stability of the expressed and purified endo-1,4-β-xylanase. The enzymatic activity considered 100% was 1420 U/mL. The pH stability (residual activity) was measured after 24 h of incubation in different pH values
During the stability tests with different pH, the enzyme showed high stability after 24 h, especially at acid pH, where it maintained a relative activity above 80% in the pH range 3.0–7.0. At pH 8.0, the enzyme did lose more activity, but still showed a relative activity of 58% (Fig. 5b).
Effect of different NaCl concentrations on the activity and stability of XlnAΔ36
The activity of XlnAΔ36 after a 24 h incubation in water or in 0–3.0 M NaCl presented the same effect, and it had a gradual decrease of activity as the salt concentration in the reaction increased (Fig. 6), reaching about 20% of relative activity in the final concentration of 0.9 M NaCl. It is important to note that the enzymatic activity remained almost constant after 24 h incubation of XlnAΔ36 at 4 °C in the presence of NaCl in the range 0–3.0 M with a relative activity between 96 and 100% (Fig. 6). Taken together, these results characterized XlnAΔ36 as a halotolerant enzyme.
Fig. 6.

Effect of NaCl on the (■) activity and ( )
stability of the expressed and purified endo-1,4-β-xylanase. The enzymatic activity considered 100% was 1420 U/mL. NaCl stability (residual activity) was determined after incubating the enzyme for 24 h at different salt concentrations
)
stability of the expressed and purified endo-1,4-β-xylanase. The enzymatic activity considered 100% was 1420 U/mL. NaCl stability (residual activity) was determined after incubating the enzyme for 24 h at different salt concentrations
Determination of the kinetic constants for the expressed XlnA
The expressed and purified XlnA showed a high specificity to beechwood xylan with a Km of 3.2 mg/mL and a Vmax of 12,600 U/mg. In addition, the kcat observed for the enzyme was 0.5 × 104 s−1 and the kcat/Km was 0.1 × 104 mL mg−1 s−1 (Table 2).
Table 2.
Kinetic parameters of expressed and purified XlnA
| Substrate | Km (mg/mL) | Vmax (U/mg) | kcat (s−1) | kcat/Km (mL mg−1 s−1) |
|---|---|---|---|---|
| Beechwood xylan | 3.2 | 12,600 | 0.5 × 104 | 0.1 × 104 |
Discussion
In our earlier paper, we showed that the xylanase, XlnA, produced by A. clavatus has significant catalytic potential, with a great capacity to hydrolyze waste and generate high concentrations of XOS (Pasin et al. 2020a; Polizeli et al. 2021). However, A. clavatus has been identified as a possible toxin producer, making the use of its extracts in industrial production problematic (Raduly et al. 2019). This work aimed at the heterologous expression of XlnA in E. coli, in the first instance to permit a more in-depth study of its properties and to carry out a more extensive examination of its potential for industrial use. The advancement of techniques that permit cloning and expression of such enzymes permits a range of choices for the expression host (Mital et al. 2021). As noted earlier, bacterial systems generally produce greater amounts of proteins than most eukaryotic systems, and certainly more than mammalian systems (Larentis et al. 2005). Of the available bacterial hosts, E. coli is generally the first choice in the biotechnology industry (Theisen 2017). Although it is not without drawbacks, it usually provides high levels of protein expression and grows rapidly on low-cost materials (Ferreira et al. 2018).
On that basis, E. coli was chosen as the host for our initial experiments and, for simplicity, a gene encoding the XlnA from A. clavatus was synthesized. This avoided having to extract the genomic DNA and synthesize cDNA, while permitting codon optimization for expression and the introduction of restriction sites to facilitate gene manipulation. The latter enabled transfer of the gene into vectors that enabled expression of the wt enzyme, as well as variants with 6x-His tags at either the N- or C-terminus.
Unfortunately, none of those variants were expressed at consistently detectable levels, so an alternative approach was required.
In their extensive review of the structures and sequences of GH11 xylanases, Paes et al. (2012) noted that a striking point of GH11 xylanase structure was the high overall conservation of the β-jelly-roll domain, with more than 60% of residues found in elements of secondary structure. The most common deviation from the typical architecture was the lack of the β-sheet at the N-terminal extremity. This suggested that removal of a few N-terminal residues of XlnA, which also included a putative signal peptide, should not be deleterious to XlnA function but may help in expression. A coupling of homology modeling with structural and sequence alignments identified 36 residues to be deleted in the first instance.
While variants of the truncated enzyme, XlnAΔ36, were successfully expressed with 6x-His tag at either of the termini, the protein was insoluble and could not be refolded. More often this problem is observed when expressing eukaryotic proteins in E. coli (Charalambous et al. 2009; Gai et al. 2013; Seetaraman Amritha et al. 2020). However, bacterial proteins are not immune from the issue, for example, the expression in E. coli of an endoxylanase from Bacillus subtilis was also problematic (Khasa et al. 2011). The use of the pET22b expression vector, which adds the pelB signal sequence for periplasmic localization proved to be successful, and XlnAΔ36, now containing the leader sequence, as well as the 6x-His tag was found to be exported into the culture medium, active and in good yield. At first glance, successful expression of XlnAΔ36 may not appear to be particularly remarkable. Indeed, other GH11 family xylanases such as that from Aspergillus niveus (Damásio et al. 2011), even another from A. clavatus (Squina et al. 2009), have been heterologously expressed. However, the significant difference is that those enzymes were both expressed in fungal system. In this work, not only was active enzyme produced in E. coli, with all the benefits that entails, but also the enzyme was secreted into the medium, making its production and purification likely to be more economically feasible.
Given the apparent diversity in the N-terminal sequences of GH11 xylanases, it seemed logical to focus initial attempts to purify and characterize the XlnA variant with an N-terminal, rather than C-terminal His tag. Accordingly, the plasmid encoding XlnAΔ36N-his was transformed into E. coli BL21(DE3). Following growth on autoinduction media and removal of cells by centrifugation, active enzyme was isolated from the supernatant and purified to apparent homogeneity using Ni2+-affinity chromatography.
The purified recombinant enzyme was found to possess excellent activity with optima at 50 °C and pH 6.0. Importantly, XlnAΔ36 had essentially the same activity, ~ 1400 U/mg, as the native enzyme isolated from A. clavatus. It was also encouraging to see both the temperature and pH profiles were very similar to those of the wt enzyme (Pasin et al. 2020a). It has been established that GH11 xylanases with optimal pH values around 3 tended to have an aspartate located adjacent to the catalytic glutamate, whereas those with higher pH optima typically had an adjacent asparagine (Liu et al. 2019). Consistent with those observations, XlnAΔ36 had both the higher pH optimum (pH 6.0), as well as an asparagine (Asn84) located within H-bonding distance of Glu216.
When assessing the properties of an enzyme for use as an industrial catalyst, it is important to know (1) the potential uses, (2) the properties required of an enzyme for each of those uses, and (3) the properties possessed by the enzyme under consideration. For a long time, xylanases have been used in food, feed, and paper industries and, more recently, production of sugars and other chemicals from agricultural and forest waste (Juturu and Wu 2012). Properties required for one industry do not necessarily transfer to another. Bio-bleaching of pulp and paper requires a thermostable alkaline xylanase (Kumar et al. 2016), whereas salt-tolerant xylanases can be used for wastewater treatment (Liu et al. 2013), and those stable at low or high pH values are suitable for saccharification of hemicellulosic biomass (Chakdar et al. 2016). Given those demands, the initial characterization of XlnAΔ36 was focused on the effect of temperature, pH, and salt on enzyme activity.
As a starting point, the thermostability of the recombinant enzyme was found to be somewhat, albeit not greatly, lower than that observed for the wild-type enzyme (Pasin et al. 2020a). On the other hand, the wild-type enzyme also had an estimated carbohydrate of ~ 20% which may have contributed to its stability (Coutinho and Reilly 1997). For all that, after 1 h at 50 °C it still retained 65% activity (c.f. 80% for wt) which was better than that observed for a Bacillus tequilensis xylanase expressed in E. coli with only 50% active after the same time (Khandeparker et al. 2017). Stability at 60 °C was less impressive with > 90% of its activity being lost in an hour. Regardless, the improvement of thermal stability of xylanases using protein engineering is a rapidly evolving field (Hokanson et al. 2011; Kumar et al. 2018) and the fact that XlnAΔ36 can be expressed in soluble form makes it a viable candidate for future development. As future perspectives for this work, we intend to obtain an immobilized XlnAΔ36 in physically and chemically different supports/matrixes to improve the enzyme stability in higher temperatures and also scale up the enzyme production to large batches, which is much more feasible using the E. coli system of expression. The immobilizing experiment to obtain higher stable XlnAΔ36 is an approach that has been successfully used for other endo-xylanases in our laboratory before (Heinen et al. 2017) and it will be revisited for the XlnAΔ36.
Along similar lines, it was noted that, after 24 h at 4 °C the recombinant enzyme maintained over 80% of its activity across the pH range of 3.0 to 7.0 By contrast, after only 12 h an Aspergillus oryzae xylanase expressed in E. coli showed an average of 65% residual activity across the same pH range (Bhardwaj et al. 2020). Again, this is an area where directed evolution is playing an increasingly important role (Xiang et al. 2019) and, clearly, XlnAΔ36 offers itself as a prime candidate.
The purified recombinant enzyme showed a decrease in activity along with the increase of NaCl concentration in the reaction. However, at 0.45 M NaCl, the same concentration of sea water, over 70% of its activity, remained, a result similar to that observed for XlnA isolated from A. clavatus (Pasin et al. 2020a). By contrast, a xylanase from Colletotrichum graminicola had 90% of its relative activity in the presence of the same concentration of NaCl (Carli et al. 2016), albeit without the stability at 3.0 M NaCl observed in the present work. These data lead us to believe that XlnAΔ36 does not present halophilic characteristics, but it can be a halotolerant enzyme, as previously observed for the wild enzyme from A. clavatus (Pasin et al. 2020a), and proved by the experiments of stability in the presented study.
In general, it was possible to observe a lower activity of XlnAΔ36 in the presence of NaCl when compared to the pure wild enzyme obtained from A. clavatus (Pasin et al. 2020a). This result could be explained by the lack of glycosylation as previously discussed. In addition, another possible explanation would be the truncation done through site-directed mutagenesis of the N-terminal portion, which was composed of many small amino acids, such as alanine, proline, valine, serine, and threonine and, also, glutamate, an acidic amino acid (Pasin et al. 2020a). The presence of these amino acids in a protein results in an overall negative electrostatic potential. This fact is normally associated with an excellent activity of proteins in high concentrations of NaCl, because of the weakening of hydrophobicity or the strengthening of hydrophilic forces on the enzyme surface, which increases the water-binding capacity and prevents protein aggregation (Teo et al. 2019). Therefore, when these residues (Ala, Pro, Val—hydrophobic, Ser and Thr—uncharged polar, and Glu—negatively charged) were truncated, the protein lost some of the amino acids that played an important role in the turnover with high NaCl concentrations. In addition, during the expression, 7 histidine residues were added to the N-terminal portion to facilitate the purification of the enzyme, which confers a basic character generating a positive electrostatic potential and interfering in the enzyme interactions with NaCl (Teo et al. 2019). Even so, XlnAΔ36 proved to be very stable at different concentrations of NaCl, which makes this work even more interesting. Since the enzyme has undergone several modifications to be expressed in its active form by E. coli, it has maintained the halotolerance pattern previously observed. In addition, the halotolerance observed gives it important characteristics for using in the saccharification of agro-industrial residues.
The Km and Vmax values (3.2 mg/mL and 12,600 U/mg, respectively) showed that the XlnAΔ36 has a great specificity to beechwood xylan (Sigma-Aldrich®). It presented a 1.4 times smaller Km value, which represents an improvement in its specificity to the substrate. Additionally, it presented 6.7 times higher Vmax, when compared to the activity of wild XlnA produced by A. clavatus (Pasin et al. 2020a). In addition, kcat and kcat/Km (0.5 × 104 s−1 and 0.1 × 104 mL mg−1 s−1) were also much higher than those observed for wild XlnA (Pasin et al. 2020a). Similar results were observed by You et al. (2019), who studied a recombinant xylanase from Penicillium canescens expressed in Pichia pastoris after different site-directed mutagenesis experiments to replace some sequence fragments. With that, they obtained a mutant enzyme that presented a Km 1.3 times smaller, denoting greater specificity, a Vmax 5.2 times greater, and a kcat/Km 4 times greater when compared to the wild organism xylanase. According to Porter et al. (2016), the high catalytic efficiency and the excellent stability of xylanases are prerequisites for their successful application in biotechnological processes. However, wild-type enzymes generally do not have favorable enzymatic properties and high catalytic efficiency, which limits their application in some industries. Therefore, studies that try to improve the properties of these enzymes are important, and most of these studies aim at heterologous expression and replacement of modules or regions of the N-terminal portion, as it is commonly a more flexible region (Wang et al. 2014). This is consistent with that was observed and performed in this study in an effective, simple, and efficient way.
Conclusions
XlnAΔ36 showed improvement in catalytic efficiency, and it was produced by a well-established microorganism, which is industrially safe and economically viable, due to its ease of cultivation. These facts open avenues for the development of low-cost and environmentally friendly technologies aiming at the treatment of agro-industrial biomass followed by the production of compounds with high added value. Taking the results together, the use of XlnAΔ36 in industrial applications combined with commercial enzymatic cocktails or alone aiming at the hydrolysis of residues represents an important tool for several biotechnological process.
Acknowledgements
The authors thank Shivansh Mahajan for assistance in the gene cloning and expression and Mauricio de Oliveira for technical assistance.
Author contributions
T.M.P, R.C.L, M.J.M, and M.L.T.M.P conceived and designed the experiments. T.M.P performed the experiments. T.M.P, T.B.O, M.J.M, and M.L.T.M.P analyzed the data. T.M.P, R.C.L, T.B.O, M.J.M, and M.L.T.M.P wrote the paper. All authors have read and approved the final version of this manuscript.
Funding
This work was supported by grants from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES)/Brasil-financing Code 001, Programa de Doutorado Sanduíche no Exterior (PDSE/CAPES no. 88881.186934/2018-01), Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP)—Process numbers 2010/52322-3, 2018/07522-6, and 2014/50884-5, Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, process 563260/2010-6), and the National Institute of Science and Technology of the Bioethanol (465319/2014-9). T.M.P. was recipient of PDSE/CAPES Fellowship; R.C.L. was recipient of CAPES Fellowship; T.B.O. was recipient of FAPESP Fellowship (process 2017/09000-4); and M.L.T.M.P. is Research Fellow of CNPq (process 301963/2017–7).
Data availability
Data will be made available on request.
Declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
References
- Bhardwaj N, Verma VK, Chaturvedi V, Verma P. Cloning, expression and characterization of a thermo-alkali-stable xylanase from Aspergillus oryzae LC1 in Escherichia coli BL21(DE3) Protein Expr Purif. 2020;168:105551. doi: 10.1016/j.pep.2019.105551. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Carli S, Meleiro LP, Rosa JC, Moraes LAB, Jorge JA, Masui DC, et al. A novel thermostable and halotolerant xylanase from Colletotrichum graminicola. J Mol Catal B Enzym. 2016;133:S508–S517. doi: 10.1016/j.molcatb.2017.05.002. [DOI] [Google Scholar]
- Chakdar H, Kumar M, Pandiyan K, Singh A, Nanjappan K, Kashyap PL, et al. Bacterial xylanases: biology to biotechnology. 3 Biotech. 2016;6:150. doi: 10.1007/s13205-016-0457-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charalambous K, O’Reilly AO, Bullough PA, Wallace BA. Thermal and chemical unfolding and refolding of a eukaryotic sodium channel. Biochim Biophys Acta. 2009;1788:1279–1286. doi: 10.1016/j.bbamem.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutinho PM, Reilly PJ. Glucoamylase structural, functional, and evolutionary relationships. Proteins Struct Funct Genet. 1997;29:334–347. doi: 10.1002/(SICI)1097-0134(199711)29:3<334::AID-PROT7>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Dalton AC, Barton WA. Over-expression of secreted proteins from mammalian cell lines. Protein Sci. 2014;23:517–525. doi: 10.1002/pro.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damásio ARdL, Silva TM, Almeida FBdR, Squina FM, Ribeiro DA, Leme AFP, et al. Heterologous expression of an Aspergillus niveus xylanase GH11 in Aspergillus nidulans and its characterization and application. Process Biochem. 2011;46:1236–1242. doi: 10.1016/j.procbio.2011.01.027. [DOI] [Google Scholar]
- Ferreira RDG, Azzoni AR, Freitas S. Techno-economic analysis of the industrial production of a low-cost enzyme using E. coli: the case of recombinant beta-glucosidase. Biotechnol Biofuels. 2018;11:81. doi: 10.1186/s13068-018-1077-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gai Z, Nakamura A, Tanaka Y, Hirano N, Tanaka I, Yao M. Crystal structure analysis, overexpression and refolding behaviour of a DING protein with single mutation. J Synchrotron Radiat. 2013;20:854–858. doi: 10.1107/S0909049513020694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopalakrishna KN, Stewart BH, Kneen MM, Andricopulo AD, Kenyon GL, McLeish MJ. Mandelamide hydrolase from Pseudomonas putida: characterization of a new member of the amidase signature family. Biochemistry. 2004;43:7725–7735. doi: 10.1021/bi049907q. [DOI] [PubMed] [Google Scholar]
- Heinen PR, Pereira MG, Rechia CGV, Almeida PZ, Monteiro LMO, Pasin TM, et al. Immobilized endoxylanase of Aspergillus tamarii Kita: an interesting biological tool for production of xylooligosaccharides at high temperatures. Process Biochem. 2017;53:145–152. doi: 10.1016/j.procbio.2016.11.021. [DOI] [Google Scholar]
- Hokanson CA, Cappuccilli G, Odineca T, Bozic M, Behnke CA, Mendez M, et al. Engineering highly thermostable xylanase variants using an enhanced combinatorial library method. Protein Eng Des Sel. 2011;24:597–605. doi: 10.1093/protein/gzr028. [DOI] [PubMed] [Google Scholar]
- Juturu V, Wu JC. Microbial xylanases: engineering, production and industrial applications. Biotechnol Adv. 2012;30:1219–1227. doi: 10.1016/j.biotechadv.2011.11.006. [DOI] [PubMed] [Google Scholar]
- Kelley LA, Mezulis S, Yates CM, Wass MN, Sternberg MJ. The Phyre2 web portal for protein modeling, prediction and analysis. Nat Protoc. 2015;10:845–858. doi: 10.1038/nprot.2015.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandeparker R, Parab P, Amberkar U. Recombinant xylanase from Bacillus tequilensis BT21: biochemical characterisation and its application in the production of xylobiose from agricultural residues. Food Technol Biotechnol. 2017;55:164–172. doi: 10.17113/ftb.55.02.17.4896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khasa YP, Khushoo A, Tapryal S, Mukherjee KJ. Optimization of human granulocyte macrophage-colony stimulating factor (hGM-CSF) expression using asparaginase and xylanase gene’s signal sequences in Escherichia coli. Appl Biochem Biotechnol. 2011;165:523–537. doi: 10.1007/s12010-011-9272-5. [DOI] [PubMed] [Google Scholar]
- Kumar V, Marin-Navarro J, Shukla P. Thermostable microbial xylanases for pulp and paper industries: trends, applications and further perspectives. World J Microbiol Biotechnol. 2016;32:34. doi: 10.1007/s11274-015-2005-0. [DOI] [PubMed] [Google Scholar]
- Kumar V, Dangi AK, Shukla P. Engineering thermostable microbial xylanases toward its industrial applications. Mol Biotechnol. 2018;60:226–235. doi: 10.1007/s12033-018-0059-6. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lakkaraju AK, Thankappan R, Mary C, Garrison JL, Taunton J, Strub K. Efficient secretion of small proteins in mammalian cells relies on Sec62-dependent posttranslational translocation. Mol Biol Cell. 2012;23:2712–2722. doi: 10.1091/mbc.e12-03-0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larentis AL, Alves TLM, Martins OB. Cloning and expression of meta-cleavage enzyme (CarB) of carbazole degradation pathway from Pseudomonas stutzeri. Braz Arch Biol Technol. 2005;48:127–134. doi: 10.1590/S1516-89132005000400016. [DOI] [Google Scholar]
- Liu Z, Zhao X, Bai F. Production of xylanase by an alkaline-tolerant marine-derived Streptomyces viridochromogenes strain and improvement by ribosome engineering. Appl Microbiol Biotechnol. 2013;97:4361–4368. doi: 10.1007/s00253-012-4290-y. [DOI] [PubMed] [Google Scholar]
- Liu MQ, Li JY, Rehman AU, Xu X, Gu ZJ, Wu RC. Laboratory evolution of GH11 endoxylanase through DNA shuffling: effects of distal residue substitution on catalytic activity and active site architecture. Front Bioeng Biotechnol. 2019;7:350. doi: 10.3389/fbioe.2019.00350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madeira F, Park YM, Lee J, Buso N, Gur T, Madhusoodanan N, et al. The EMBL-EBI search and sequence analysis tools APIs in 2019. Nucleic Acids Res. 2019;47:W636–W641. doi: 10.1093/nar/gkz268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinho GO, Nogueira EA, Pasin TM, Oliveira TBd, Roa JPB, Nelson DL, et al. An environmentally safe production of xylanases by Fusarium sp. EA 1.3.1 using agroindustrial residues: biochemical characterization and potential applications. Asian J Biochem Genet Mol Biol. 2023;14:11–26. doi: 10.9734/ajbgmb/2023/v14i4319. [DOI] [Google Scholar]
- Michaelis L, Menten ML, Johnson KA, Goody RS. The original Michaelis constant: translation of the 1913 Michaelis-Menten paper. Biochemistry. 2011;50:8264–8269. doi: 10.1021/bi201284u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GL. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem. 2002;31:426–428. doi: 10.1021/ac60147a030. [DOI] [Google Scholar]
- Mital S, Christie G, Dikicioglu D. Recombinant expression of insoluble enzymes in Escherichia coli: a systematic review of experimental design and its manufacturing implications. Microb Cell Fact. 2021;20:208. doi: 10.1186/s12934-021-01698-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordberg Karlsson E, Schmitz E, Linares-Pasten JA, Adlercreutz P. Endo-xylanases as tools for production of substituted xylooligosaccharides with prebiotic properties. Appl Microbiol Biotechnol. 2018;102:9081–9088. doi: 10.1007/s00253-018-9343-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paes G, Berrin JG, Beaugrand J. GH11 xylanases: structure/function/properties relationships and applications. Biotechnol Adv. 2012;30:564–592. doi: 10.1016/j.biotechadv.2011.10.003. [DOI] [PubMed] [Google Scholar]
- Pasin TM, Benassi VM, Heinen PR, Damasio ARL, Cereia M, Jorge JA, et al. Purification and functional properties of a novel glucoamylase activated by manganese and lead produced by Aspergillus japonicus. Int J Biol Macromol. 2017;102:779–788. doi: 10.1016/j.ijbiomac.2017.04.016. [DOI] [PubMed] [Google Scholar]
- Pasin TM, Salgado JCS, Scarcella ASA, de Oliveira TB, de Lucas RC, Cereia M, et al. A halotolerant endo-1,4-beta-xylanase from Aspergillus clavatus with potential application for agroindustrial residues saccharification. Appl Biochem Biotechnol. 2020;191:1111–1126. doi: 10.1007/s12010-020-03232-x. [DOI] [PubMed] [Google Scholar]
- Pasin TM, Scarcella ASA, de Oliveira TB, Lucas RC, Cereia M, Betini JHA, et al. Paper industry wastes as carbon sources for Aspergillus species cultivation and production of an enzymatic cocktail for biotechnological applications. Ind Biotechnol. 2020;16:56–60. doi: 10.1089/ind.2020.29201.tmp. [DOI] [Google Scholar]
- Pasin TM, Moreira EA, Benassi VM, Spencer PVD, Peres NTA, Cereia M, et al. Effects of ultraviolet exposure on the tropical fungi Aspergillus carbonarius and Aspergillus japonicus: survival, amylase production, and thermostability. Trop Conserv Sci. 2022;15:1–7. doi: 10.1177/19400829221092638. [DOI] [Google Scholar]
- Pasin TM, Betini JHA, de Lucas RC, Polizeli M. Biochemical characterization of an acid-thermostable glucoamylase from Aspergillus japonicus with potential application in the paper bio-deinking. Biotechnol Prog. 2023;40:e3384. doi: 10.1002/btpr.3384. [DOI] [PubMed] [Google Scholar]
- Polizeli MdLTM, Cereia M, Oliveira TBd, Lucas RCd, Scarcella ASdA, Pasin TM. An eco-friendly production of a novel and highly active endo-14-beta-xylanase from Aspergillus clavatus. Asian J Biochem Genet Mol Biol. 2021;9:20–33. [Google Scholar]
- Porter JL, Rusli RA, Ollis DL. Directed evolution of enzymes for industrial biocatalysis. ChemBioChem. 2016;17:197–203. doi: 10.1002/cbic.201500280. [DOI] [PubMed] [Google Scholar]
- Raduly Z, Szabo L, Madar A, Pocsi I, Csernoch L. Toxicological and medical aspects of Aspergillus-derived mycotoxins entering the feed and food chain. Front Microbiol. 2019;10:2908. doi: 10.3389/fmicb.2019.02908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero FA, Vodonick SM, Criscione KR, McLeish MJ, Grunewald GL. Inhibitors of phenylethanolamine N-methyltransferase that are predicted to penetrate the blood-brain barrier: design, synthesis, and evaluation of 3-fluoromethyl-7-(N-substituted aminosulfonyl)-1,2,3,4-tetrahydroisoquinolines that possess low affinity toward the alpha2-adrenoceptor. J Med Chem. 2004;47:4483–4493. doi: 10.1021/jm0400653. [DOI] [PubMed] [Google Scholar]
- Scarcella ASdA, Pasin TM, de Oliveira TB, de Lucas RC, Ferreira-Nozawa MS, Freitas ENd, et al. Saccharification of different sugarcane bagasse varieties by enzymatic cocktails produced by Mycothermus thermophilus and Trichoderma reesei RP698 cultures in agro-industrial residues. Energy. 2021;226:120360. doi: 10.1016/j.energy.2021.120360. [DOI] [Google Scholar]
- Seetaraman Amritha TM, Mahajan S, Subramaniam K, Chandramohan Y, Dhanasekaran A. Cloning, expression and purification of recombinant dermatopontin in Escherichia coli. PLoS ONE. 2020;15:e0242798. doi: 10.1371/journal.pone.0242798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segato F, Damasio AR, de Lucas RC, Squina FM, Prade RA. Genomics review of holocellulose deconstruction by aspergilli. Microbiol Mol Biol Rev. 2014;78:588–613. doi: 10.1128/MMBR.00019-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva MT, Lopes PHS, Pasin TM, Nelson DL, Benassi VM. Produção de beta-D-frutofuranosidases por Aspergillus sp. M2.4 e caracterização bioquímica. J Eng Exact Sci. 2023;9:15943–16001. doi: 10.18540/jcecvl9iss5pp15943-01e. [DOI] [Google Scholar]
- Sorensen HP, Mortensen KK. Advanced genetic strategies for recombinant protein expression in Escherichia coli. J Biotechnol. 2005;115:113–128. doi: 10.1016/j.jbiotec.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Squina FM, Mort AJ, Decker SR, Prade RA. Xylan decomposition by Aspergillus clavatus endo-xylanase. Protein Expr Purif. 2009;68:65–71. doi: 10.1016/j.pep.2009.06.014. [DOI] [PubMed] [Google Scholar]
- Studier FW. Protein production by auto-induction in high density shaking cultures. Protein Expr Purif. 2005;41:207–234. doi: 10.1016/j.pep.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Teo SC, Liew KJ, Shamsir MS, Chong CS, Bruce NC, Chan KG, et al. Characterizing a halo-tolerant GH10 xylanase from Roseithermus sacchariphilus strain RA and its CBM-truncated variant. Int J Mol Sci. 2019;20:2284. doi: 10.3390/ijms20092284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theisen M, Liao JC. Industrial biotechnology: Escherichia coli as a host. In: Wittmann C, Liao J, editors. Industrial biotechnology. Weinheim: Wiley-VCH; 2017. pp. 149–181. [Google Scholar]
- Wang K, Luo H, Bai Y, Shi P, Huang H, Xue X, et al. A thermophilic endo-1,4-beta-glucanase from Talaromyces emersonii CBS394.64 with broad substrate specificity and great application potentials. Appl Microbiol Biotechnol. 2014;98:7051–7060. doi: 10.1007/s00253-014-5680-0. [DOI] [PubMed] [Google Scholar]
- Xiang L, Lu Y, Wang H, Wang M, Zhang G. Improving the specific activity and pH stability of xylanase XynHBN188A by directed evolution. Bioresour Bioprocess. 2019;6:2463–2477. doi: 10.1186/s40643-019-0262-8. [DOI] [Google Scholar]
- You S, Xie C, Ma R, Huang HQ, Herman RA, Su XY, et al. Improvement in catalytic activity and thermostability of a GH10 xylanase and its synergistic degradation of biomass with cellulase. Biotechnol Biofuels. 2019;12:278. doi: 10.1186/s13068-019-1620-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.