Abstract
Objective
This cross‐sectional study aimed to determine the epidemiology of olfactory and gustatory dysfunctions related to COVID‐19 in China.
Methods
This study was conducted by 45 tertiary Grade‐A hospitals in China. Online and offline questionnaire data were obtained from patients infected with COVID‐19 between December 28, 2022, and February 21, 2023. The collected information included basic demographics, medical history, smoking and drinking history, vaccination history, changes in olfactory and gustatory functions before and after infection, and other postinfection symptoms, as well as the duration and improvement status of olfactory and gustatory disorders.
Results
Complete questionnaires were obtained from 35,566 subjects. The overall incidence of olfactory and taste dysfunction was 67.75%. Being female or being a cigarette smoker increased the likelihood of developing olfactory and taste dysfunction. Having received four doses of the vaccine or having good oral health or being a alcohol drinker decreased the risk of such dysfunction. Before infection, the average olfactory and taste VAS scores were 8.41 and 8.51, respectively; after infection, they decreased to 3.69 and 4.29 and recovered to 5.83 and 6.55 by the time of the survey. The median duration of dysosmia and dysgeusia was 15 and 12 days, respectively, with 0.5% of patients having symptoms lasting for more than 28 days. The overall self‐reported improvement rate was 59.16%. Recovery was higher in males, never smokers, those who received two or three vaccine doses, and those that had never experienced dental health issues, or chronic accompanying symptoms.
Conclusions
The incidence of dysosmia and dysgeusia following infection with the SARS‐CoV‐2 virus is high in China. Incidence and prognosis are influenced by several factors, including sex, SARS‐CoV‐2 vaccination, history of head‐facial trauma, nasal and oral health status, smoking and drinking history, and the persistence of accompanying symptoms.
Keywords: epidemiologic studies, incidence, olfactory disorders, prognosis, SARS‐CoV‐2, taste disorders
INTRODUCTION
The WHO reported 649,753,806 cases of severe acute respiratory syndrome coronavirus (SARS‐CoV‐2) infection as of December 20, 2022, 1 which has had a considerable negative influence on the health of the world's population. SARS‐CoV‐2 symptoms vary, but the most common are fever and exhaustion, cough and sore throat, muscle and joint discomfort, and gastrointestinal issues. Additionally, neurological signs must not be disregarded. Researchers were interested when it was originally reported in June 2020 that six patients showed olfactory impairment. 2 Following SARS‐CoV‐2 infection, increases in the incidence of olfactory and gustatory abnormalities have since been documented worldwide. According to a meta‐analysis, patients with SARS‐CoV‐2 had an average prevalence of olfactory impairment ranging from 52.73% 3 to 85.61% 4 in European countries and the United States. The incidence of taste disorders ranged from 43.93% to 92.65%. 5 Although there are many influencing elements, the pathophysiology of the disease is still debatable.
In China, there are currently limited relevant epidemiological statistics. From the end of 2022 to the beginning of 2023, the Omicron strain of the virus was very prevalent in many parts of China. 6 Therefore, to establish a foundation for clinical diagnosis, treatment, and future research, we believed it imperative to conduct a national multicenter large‐sample survey to clarify the incidence, characteristics, prognosis, and related influencing factors of olfactory and taste disorders following infection with the SARS‐CoV‐2 Omicron strain in China. The editorial staff of the Chinese Journal of Otorhinolaryngology‐Head and Neck Surgery organized this multicenter clinical investigation.
METHODS
Questionnaire formation
The lead author took the initiative to draft the questionnaire, which was later reviewed and revised by a group of nine experts to collect more comprehensive information. The questionnaire underwent a pretest at the China‐Japan Friendship Hospital, where 16 individuals, including both COVID‐19 patients and healthy individuals, were surveyed. Based on the received feedback, the questionnaire text was revised to ensure readability and ultimately form the final 36 questions (Supporting Information S1: Annex 1). The developed questionnaire included basic demographic information of the patient, previous health status, history of smoking and drinking, vaccination history, perceived smell and taste function before and after infection, other accompanying symptoms after infection, duration of smell and taste disorders, and perceived improvement of the chemosensory dysfunction. Olfactory and taste visual analog scales (VASs) were used to assess the degree of the self‐reported olfactory and taste functions. The scale was 0–10 points, with 0 indicating olfactory or taste loss and 10 indicating normal function.
Survey methods
This was a cross‐sectional study. The Olfactory and Taste Survey Collaboration Group, composed of nasal experts organized by the Editorial Department of the Chinese Journal of Otolaryngology‐Head and Neck Surgery, were responsible for conducting the survey. This group was made up of members of otolaryngology‐head and neck surgery departments in 45 tertiary hospitals in 31 provinces, autonomous regions and municipalities. The questionnaire WeChat QR code was made and distributed on the Questionnaire Star platform, and the questionnaire was collected online and offline: the offline questionnaire was mainly completed by hospital outpatients and inpatients, and the online questionnaire was completed through public platforms such as the WeChat public number, official website, internal work group, and the community WeChat group of each survey center. Anyone who saw the advertisement could scan the QR code to participate in filling out the questionnaire. We did not specifically mention “smell loss” in our advertisements to avoid any potential bias or disproportionate recruitment based on participants' experiences with this symptom. The survey was carried out between December 28, 2022, and February 21, 2023.
Inclusion and exclusion criteria
The inclusion criteria were as follows: (1) patients diagnosed with SARS‐CoV‐2 (at least one positive SARS‐CoV‐2 nucleic acid or antigen test); (2) age ≥18 years; (3) patients who were fully aware of the purpose of this study and participated voluntarily; (4) patients who could complete the questionnaire independently or with the help of others. The sole exclusion criterion was incomplete survey data.
Statistical analysis
The collected data were statistically analyzed using SPSS 26.0. A t test was used for measurement data that followed a normal distribution. A χ 2 test was used for frequency data. Risk analysis was conducted using multivariate logistic regression analysis. Statistics were deemed significant at p < 0.05.
RESULTS
Basic data
The survey invited participants from 31 provinces, autonomous regions and municipalities in China (including Jiangsu, Anhui, Beijing, Fujian, Guangdong, Guangxi, Guizhou, Heilongjiang, Hebei, Henan, Hubei, Hunan, Jilin, Jiangxi, Liaoning, Inner Mongolia, Qinghai, Shandong, Shanxi, Shaanxi, Shanghai, Sichuan, Tianjin, Tibet, Xinjiang, Yunnan, Zhejiang, Chongqing, Gansu, Hainan, and Ningxia). The questionnaire was completed by 38,053 participants screened from 35,566 valid questionnaires on the basis of inclusion and exclusion criteria; 11,695 males and 23,871 females completed the questionnaire. The age range was 18–89 years, with an average of 38.37 years. This included 21,417 young individuals (18 to 40‐year‐old), 13,877 middle‐aged individuals (41 to 65‐year‐old), and 272 senior citizens (66 years of age and above).
Incidence of olfactory and taste disorders
There were 4203 people (11.82%) who reported only olfactory disorders, 4347 people (12.22%) who reported only taste disorders, and 15,546 people (43.71%) reporting both olfactory and taste disorders. Olfactory or taste disorder were the only symptoms in 235 patiens (0.66%). The overall incidence of olfactory and taste disorders was 67.75% (Figure 1A). Young people were more ple were more likely to have these disorders (χ 2 = 120.21, p < 0.001). The incidence of olfactory inversion was 7.88% (2803), and the incidence of olfactory hallucination was 8.51% (3027), accounting for 14.19% and 15.33% of patients with olfactory disorders, respectively. The incidence of gustatory hallucination was 28.59% (10168), accounting for 51.11% of patients with taste disorders. Most patients said that bitter and metallic tastes persisted in the mouth. Among the four basic flavors, patients were more likely to have abnormal perceptions of saltiness (χ 2 = 971.117, p < 0.001).
Figure 1.
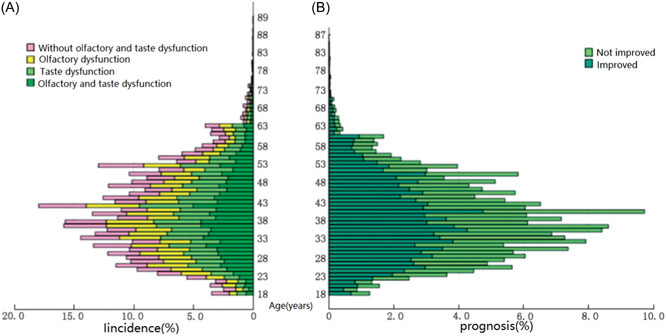
Comparison of incidence and prognosis of olfactory and taste disorders in patients infected with SARS‐CoV‐2 of all ages. (A) Incidence; (B) prognosis.
Influencing factors of SARS‐CoV‐2‐related olfactory and taste disorders
The incidence of olfactory and taste problems was correlated with sex, SARS‐CoV‐2 immunization, oral health status, smoking, and drinking history (Figure 2A). Those who were female or had a history of smoking were more likely to have olfactory and taste problems than those who had received four doses of the vaccine or had a history of alcohol consumption or had oral health problem. However, individuals with an alcohol consumption history or smoking history before illness had lower VAS scores for smell and taste than patients without such history (p < 0.05).
Figure 2.
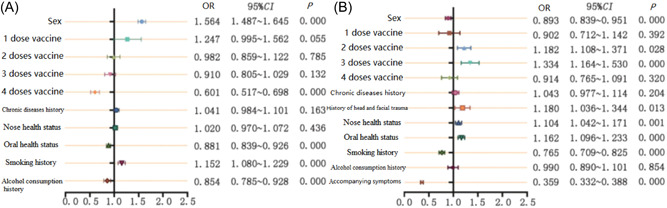
Influencing factors of the incidence and prognosis of SARS‐CoV‐2‐related olfactory and taste disorders. (A) Incidence factors; (B) prognostic factors.
The degree and duration of SARS‐CoV‐2‐related olfactory and taste disorders
Before infection, the average scores on the gustatory and olfactory VASs were 8.41 and 8.51, and these values dropped to 3.69 and 4.29, respectively, following infection. The values increased to 5.83 and 6.55 at the end of the survey (Figure 3). The median length of olfactory dysfunction was 15 days and 12 days for taste dysfunction, and 0.50% of patients had olfactory and taste dysfunction that lasted more than 28 days.
Figure 3.
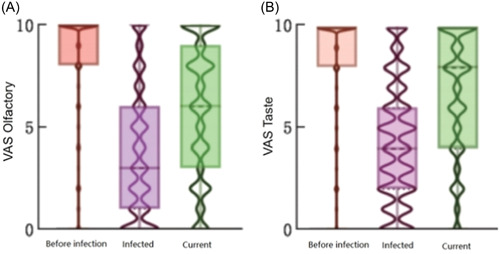
Comparison of VASs 24 096 patients with olfactory and taste disorders related to SARS‐CoV‐2. (A) olfaction; (B) taste.
Prognosis and influencing factors of SARS‐CoV‐2‐related olfactory and taste disorders
The overall self‐reported improvement rate of olfactory and taste disorders was 59.16% (Figure 1B). There were no significant differences in prognosis between age groups (χ 2 = 3.225, p = 0.199).
Fever (83.36%, 38.5–39°C), nasal congestion and runny nose (76.28%), dry mouth and sore throat (74.06%), and cough and expectoration (87.46%) were the most common symptoms in those SARS‐CoV‐2‐related olfactory and taste disorders sufferers.
By the end of the survey, 44.62% (4391) of patients had nasal congestion and runny nose, and 32.62% (3210) of patients had dry mouth and sore throat and still lacked olfactory and gustatory function. Only 24.96% (3558) of patients had nasal congestion and runny nose, and only 15.78% had dry mouth and sore throat.
The likelihood that olfactory taste function would return was higher among patients who were male, had received two or three doses of a SARS‐CoV‐2 vaccine, had never smoked, had no chronic accompanying symptoms, had experienced head or face trauma, and had nose and dental health issues (Figure 2B). However, compared to patients with prior health problems, those with a history of head and face injuries or nasal and dental health issues had lower olfactory and taste VAS scores before infection (p < 0.05).
DISCUSSION
This large‐scale cross‐sectional questionnaire survey examined the smell–taste disorders present in persons experiencing the SARS‐CoV‐2 Omicron virus in China. With a sample size of more than 35,000, it is the world's largest study of smell–taste disorders associated with SARS‐CoV‐2 infection. It found incidence rates of simple olfactory disorders, simple taste disorders, and mixed olfactory and taste disorders after SARS‐CoV‐2 to be 11.82%, 12.22%, and 43.71%, respectively. However, given evidence that many people confuse taste and smell, 7 , 8 these rates may be overestimated from what would be observed with quantitative testing. The prevalence of olfactory and gustatory disorders was 67.75% overall. According to reviews, the reported rates of SARS‐CoV‐2‐related olfactory abnormalities in adults range from 5% to 85%. 9 , 10 , 11 Early studies have shown that the prevalence of olfactory and taste problems following SARS‐CoV‐2 has been shown to be high in Europe and the United States (73.0% 12 and, 66.69% 13 in the United States, 72.6% 14 and 83.9% 15 in Canada, respectively) and low in China (5.1% 16 and 6.4%, 17 respectively). According to our study, the prevalence of olfactory and gustatory abnormalities following SARS‐CoV‐2 Omicron infection in China appears to be comparable to that in Europe and the United States. This study also revealed that the incidence was higher among women than among men, which is consistent with the findings of other research. 18
This study identified several contributing factors that were linked to the development of olfactory and taste problems following SARS‐CoV‐2 infection, including sex, SARS‐CoV‐2 vaccination status, oral health status, smoking history, and the history of alcohol consumption. Patients who had received four doses of the vaccine and had a history of alcohol consumption were less likely to develop olfactory and taste disorders; however, patients with a history of alcohol consumption had lower olfactory and taste VAS scores both before and after infection than patients without a history of alcohol consumption. Complete vaccination (four doses) was a protective factor, while incomplete vaccination (one dose) increased the likelihood of disorder development. 19 A study from China also showed that people who were unvaccinated or incompletely vaccinated were at higher risk of infection. 20 Foreign studies have also demonstrated that immunization can lower the likelihood of neurological symptoms (including olfactory and taste abnormalities), and people who receive two doses of the vaccine have a lower risk of developing the disease than those who receive one dose of the vaccine 21 and can recover more quickly even after symptom onset. 22 In our study, SARS‐CoV‐2‐related olfactory and taste impairments were not associated with previous nasal disorders, including allergic rhinitis. In earlier research, 23 alcohol consumption was viewed as a risk factor for SARS‐CoV‐2 infection, while smoking was seen as a protective factor. The results of this study are interesting in that they demonstrated that patients with a history of smoking and alcohol consumption had lower olfactory and taste scores before infection than those without a history of smoking and alcohol consumption, and the VAS scores decreased less after infection.
Only 0.66% of the patients in this study had olfactory and taste dysfunction as their only symptom. Fever (83.36%, 38.5–39°C), runny or stuffy nose (76.28%), dry mouth and sore throat (73.06%), and cough and expectoration (87.46%) were the predominant SARS‐CoV‐2 symptoms. While there were more reports of olfactory impairment as the initial and only symptom in Europe and the United States, 1 , 24 the proportion of cases among people with nasal congestion and runny nose was higher than that described in foreign studies. 25 , 26
The average olfactory and gustatory VAS scores before SARS‐CoV‐2 infection were 8.41 and 8.51, respectively, according to this study's findings on the severity, prognosis, and duration of olfactory and gustatory abnormalities. The scores dropped to 3.69 and 4.29, respectively, after SARS‐CoV‐2 infection. By the time the survey was over, the results had improved to 5.83 and 6.55, respectively. Of the affected cases, 59.16% returned to normal over time. It is hypothesized that following SARS‐CoV‐2 infection, the senses of taste and smell greatly decline and do not quickly recover. One finding that was in line with the literature was that 0.5% of patients said their olfactory and/or taste abnormalities lasted longer than 28 days. Early data indicated that olfactory impairment following SARS‐CoV‐2 infection has a high and quick recovery rate. 27 The majority of people can fully recover in 10 28 or 30 29 days. According to current reports, a substantial number of people with olfactory impairment recover within 2 weeks, with recovery rates ranging from 31.7% to 89%. 30 According to most research, 5% of patients have olfactory impairment that does not go away. 31
This study identified several variables that could influence the prognosis of olfactory and taste disorders following infection, including sex, SARS‐CoV‐2 vaccination, head and face trauma history, nasal and oral health status, smoking history, and the persistence of accompanying symptoms. This is in line with several reports from the literature: (1) women, those with nasal obstruction, and people with severe initial olfactory dysfunction had a poor prognosis for olfactory dysfunction in SARS‐CoV‐2, according to previous studies 32 ; (2) smokers had a poor prognosis, 33 although some studies claimed that there was no connection between smoking and prognosis 31 ; and (3) younger patients recovered their olfactory function more quickly, 34 , 35 but age was not associated with prognosis, according to a thorough analysis. 31
The limitations of this study were as follows: (1) since the survey was conducted both online and offline, online participants were unable to go to the hospital for relatively objective olfactory testing (e.g., T&T, Sniffin' Sticks, UPSIT), so the questionnaire was designed only with VAS scores. There may have been memory bias in the participants' evaluations of their own olfactory and taste function. (2) Due to the limitations of its design, the questionnaire failed to distinguish between people who spontaneously recovered from olfactory and taste disorders and those who recovered as a result of treatment. In addition, the type, manufacturer, and timing of the vaccine received by participants were not collected. (3) Because anyone who sees the advertisement can scan the QR code to participate in taking the questionnaire, it is unclear whether the participants participated in the survey through online or offline means. (4) Although we encouraged all types of subjects to complete the questionnaire by giving out bonuses, the fact that the questionnaires were released on public platforms may have skewed the sampling toward persons with olfactory and gustatory disorders. This may have resulted in an overestimate of the incidence rate of SARS‐CoV‐2‐related olfactory and taste disorders.
AUTHOR CONTRIBUTIONS
Meng‐Fan Liu carried out research, collect data (data volume exceeds 2500), analyze data, write articles, revise articles. Rui‐Xia Ma, Xian‐Bao Cao, and Hua Zhang implemented research, collect data (data volume exceeds 1500), analyze data, and critically review the intellectual content of articles. Shui‐Hong Zhou, Wei‐Hong Jiang, Yan Jiang, Jing‐Wu Sun, Qin‐Tai Yang, Xue‐Zhong Li, Ya‐Nan Sun, Li Shi, Min Wang, Xi‐Cheng Song, Fu‐Quan Chen, Xiao‐Shu Zhang, Hong‐Quan Wei, Shao‐Qing Yu, Dong‐Dong Zhu, Luo Ba, Zhi‐Wei Cao, Xu‐Ping Xiao, Xin Wei, Zhi‐Hong Lin, Feng‐Hong Chen, Chun‐Guang Shan, Guang‐Ke Wang, Jing Ye, Shen‐Hong Qu, Chang‐Qing Zhao, Zhen‐Lin Wang, Hua‐Bin Li, Feng Liu, Xiao‐Bo Cui, Sheng‐Nan Ye, Zheng Liu, Yu Xu, Xiao Cai, Wei Huang, Ru‐Xin Zhang, Yu‐Lin Zhao, Guo‐Dong Yu, Guang‐Gang Shi, Mei‐Ping Lu, Yang Shen, Yu‐Tong Zhao, Jia‐Hong Pei, Shao‐Bing Xie, Long‐Gang Yu, Ye‐Hai Liu, and Shao‐Wei Gu conducted research, collect data, and critically review the intellectual content of articles. Lei Cheng implemented research, collect data (data volume exceeds 4800), and critically review the informative content of the article. Yu‐Cheng Yang implemented research, collect data (data volume exceeds 3400), analyze data, revise manuscripts, and critically review the intellectual content of articles. Jian‐Feng Liu done overall design, organization, and coordination of the implementation of research, critical review of the intellectual content of the article and determination of the final manuscript.
CONFLICT OF INTEREST STATEMENT
Professor Lei Cheng, Hua‐Bin Li, Jian‐Feng Liu, Zhen‐Lin Wang, Chang‐Qing Zhao and Dong‐Dong Zhu are the members of World Journal of Otorhinolaryngology ‐ Head and Neck Surgery editorial board and are not involved in the peer review process of this article. The other authors declare no conflict of interest.
ETHICS STATEMENT
N/A.
Supporting information
Supporting information.
ACKNOWLEDGMENTS
We would like to thank Professor Tao Liyuan from the Department of Epidemiology of Peking University Third Hospital, Professor Zhou Bing from Beijing Tongren Hospital affiliated to Capital Medical University, Professor Wang Dehui from Fudan University Eye, Ear, Nose and Throat Hospital, and Professor Guo Yufen from Gansu Provincial Health Commission. Natural Science Foundation of Beijing (7212090); National High Level Hospital Clinical Research Funding (2022‐NHLHCRF‐YGJE‐02).
Liu M‐F, Ma R‐X, Cao X‐B, et al. Incidence and prognosis of olfactory and gustatory dysfunctions related to SARS‐CoV‐2 Omicron strain infection in China: a national multicenter survey of 35,566 individuals. World J Otorhinolaryngol Head Neck Surg. 2024;10:113‐120. 10.1002/wjo2.167
Meng‐Fan Liu, Rui‐Xia Ma, Xian‐Bao Cao, and Hua Zhang are the first authors who contributed equally to this study. The Chinese version of this article was published in the Chinese Journal of Otorhinolaryngology Head and Neck Surgery.
[Correction added on 12 April 2024, after the first online publication: The article title was revised.]
Contributor Information
Yu‐Cheng Yang, Email: yychxh@163.com.
Lei Cheng, Email: chenglei@jsph.org.cn.
Jian‐Feng liu, Email: mmconfucius@163.com.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. World Health Organization . WHO Coronavirus (COVID‐19) Dashboard [EB/OL]. World Health Organization; 2022. https://covid19.who.int/?mapFilter=cases [Google Scholar]
- 2. Gane SB, Kelly C, Hopkins C. Isolated sudden onset anosmia in COVID‐19 infection. A novel syndrome? J Rhinol. 2020;58:299‐301. [DOI] [PubMed] [Google Scholar]
- 3. Tong JY, Wong A, Zhu D, Fastenberg JH, Tham T. The prevalence of olfactory and gustatory dysfunction in COVID‐19 patients: a systematic review and meta‐analysis. Otolaryngol Head Neck Surg. 2020;163:3‐11. [DOI] [PubMed] [Google Scholar]
- 4. Lechien JR, Chiesa‐Estomba CM, De Siati DR, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild‐to‐moderate forms of the coronavirus disease (COVID‐19): a multicenter European study. Eur Arch Otrhinolaryngol. 2020;277:2251‐2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bénézit F, Le Turnier P, Declerck C, et al. Utility of hyposmia and hypogeusia for the diagnosis of COVID‐19. Lancet Infect Dis. 2020;20:1014‐1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chinese Center for Disease Control and Prevention The Situation of the New Coronavirus Infection Epidemic in the Country [EB/OL]. Chinese Center for Disease Control and Prevention; 2023. https://www.chinacdc.cn/jkzt/crb/zl/szkb_11803/jszl_13141/202301/t20230125_263519.html [Google Scholar]
- 7. Burdach KJ, Doty RL. The effects of mouth movements, swallowing, and spitting on retronasal odor perception. Physiol Behav. 1987;41:353‐356. [DOI] [PubMed] [Google Scholar]
- 8. Hannum ME, Koch RJ, Ramirez VA, et al. Taste loss as a distinct symptom of COVID‐19: a systematic review and meta‐analysis. Chem Senses. 2023;48:bjad043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Qiu J, Yang X, Liu L, et al. Prevalence and prognosis of otorhinolaryngological symptoms in patients with COVID‐19: a systematic review and meta‐analysis. Eur Arch Otrhinolaryngol. 2022;279:49‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ahmad S, Sohail A, Shahid Chishti MA, Aemaz Ur Rehman M, Farooq H. How common are taste and smell abnormalities in COVID‐19? A systematic review and meta‐analysis. J Taibah Univ Med Sci. 2022;17:174‐185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Doty RL. Olfactory dysfunction in COVID‐19: pathology and long‐term implications for brain health. Trends Mol Med. 2022;28:781‐794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kaye R, Chang CWD, Kazahaya K, Brereton J, Denneny JC 3rd. COVID‐19 anosmia reporting tool: initial findings. Otolaryngol Head Neck Surg. 2020;163:132‐134. [DOI] [PubMed] [Google Scholar]
- 13. Callejón‐Leblic MA, Martín‐Jiménez DI, Moreno‐Luna R, et al. Analysis of prevalence and predictive factors of long‐lasting olfactory and gustatory dysfunction in COVID‐19 patients. Life. 2022;12:1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bussière N, Mei J, Lévesque‐Boissonneault C, et al. Chemosensory dysfunctions induced by COVID‐19 can persist up to 7 months: a study of over 700 healthcare workers. Chem Senses. 2021;46:bjab038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bussiere N, Mei J, Levesque‐Boissonneault C, et al. Persisting chemosensory impairments in 366 healthcare workers following COVID‐19: an 11‐month follow‐up. Chem Senses. 2022;47:bjac010. [DOI] [PubMed] [Google Scholar]
- 16. Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77:683‐690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhang X, Jiang M, Wang T, et al. Clinical analysis and medical advice of COVID‐19 patients with nasal symptoms. Chin J Otorhinolaryngol‐Skull Base Surg. 2020;26:213‐215. [Google Scholar]
- 18. Speth MM, Singer‐Cornelius T, Oberle M, Gengler I, Brockmeier SJ, Sedaghat AR. Olfactory dysfunction and sinonasal symptomatology in COVID‐19: prevalence, severity, timing, and associated characteristics. Otolaryngol Head Neck Surg. 2020;163:114‐120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Al‐Aly Z, Bowe B, Xie Y. Long COVID after breakthrough SARS‐CoV‐2 infection. Nature Med. 2022;28:1461‐1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kang M, Xin H, Yuan J, et al. Transmission dynamics and epidemiological characteristics of SARS‐CoV‐2 Delta variant infections in Guangdong, China, May to June 2021. Euro Surveill. 2022;27:2100815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Antonelli M, Penfold RS, Merino J, et al. Risk factors and disease profile of post‐vaccination SARS‐CoV‐2 infection in UK users of the COVID Symptom Study app: a prospective, community‐based, nested, case‐control study. Lancet Infect Dis. 2022;22:43‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sagar P, Kumar R, Thakar A. Archetype of olfactory and gustatory dysfunction in breakthrough COVID‐19 illness. Indian J Otolaryngol Head Neck Surg. 2022;74:3220‐3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhong R, Zhang Q, Qiu Y, et al. Results of the adult COVID‐19 lifestyle matching study. Int J Public Health. 2022;67:1604329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hopkins C, Surda P, Kumar N. Presentation of new onset anosmia during the COVID‐19 pandemic. J Rhinol. 2020;58:295‐298. [DOI] [PubMed] [Google Scholar]
- 25. Eliezer M, Eloit C, Hautefort C. Olfactory loss of function as a possible symptom of COVID‐19‐Reply. JAMA Otolaryngol Head Neck Surg. 2020;146:874‐875. [DOI] [PubMed] [Google Scholar]
- 26. Kaye R, Chang CWD, Kazahaya K, Brereton J, Denneny JC 3rd. COVID‐19 anosmia reporting tool: initial findings. Otolaryngol Head Neck Surg. 2020;163:132‐134. [DOI] [PubMed] [Google Scholar]
- 27. Hopkins C, Surda P, Whitehead E, Kumar BN. Early recovery following new onset anosmia during the COVID‐19 pandemic—an observational cohort study. J Otolaryngol Head Neck Surg. 2020;49:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chiesa‐Estomba CM, Lechien JR, Radulesco T, et al. Patterns of smell recovery in 751 patients affected by the COVID‐19 outbreak. Eur J Neurol. 2020;27:2318‐2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. D'Ascanio L, Pandolfini M, Cingolani C, et al. Olfactory dysfunction in COVID‐19 patients: prevalence and prognosis for recovering sense of smell. Otolaryngol Head Neck Surg. 2021;164:82‐86. [DOI] [PubMed] [Google Scholar]
- 30. Karamali K, Elliott M, Hopkins C. COVID‐19 related olfactory dysfunction. Curr Opin Otolaryngol Head Neck Surg. 2022;30:19‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tan BKJ, Han R, Zhao JJ, et al. Prognosis and persistence of smell and taste dysfunction in patients with covid‐19: meta‐analysis with parametric cure modelling of recovery curves. BMJ. 2022;378:e069503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tan BKJ, Han R, Zhao JJ, et al. Prognosis and persistence of smell and taste dysfunction in patients with covid‐19: meta‐analysis with parametric cure modelling of recovery curves. BMJ. 2022;378:e069503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lucidi D, Molinari G, Silvestri M, et al. Patient‐reported olfactory recovery after SARS‐CoV‐2 infection: a 6‐month follow‐up study. Int Forum Allergy Rhinol. 2021;11:1249‐1252. [DOI] [PubMed] [Google Scholar]
- 34. Coelho DH, Reiter ER, Budd SG, Shin Y, Kons ZA, Costanzo RM. Predictors of smell recovery in a nationwide prospective cohort of patients with COVID‐19. Am J Otolaryngol. 2022;43:103239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jalessi M, Bagheri SH, Azad Z, et al. The outcome of olfactory impairment in patients with otherwise paucisymptomatic coronavirus disease 2019 during the pandemic. J Laryngol Otol. 2021;135:426‐435. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


