To the Editor
The major causes of morbidity and mortality in patients with autoimmune diseases (AD) are cardiovascular (CV) disorders. [1] Cardiac involvement in autoimmune rheumatic diseases (ARD) is well established. However, its recognition depends on the clinical suspicion and skills of the patients' physicians–often their rheumatologist–and frequently occurs very late in the natural history of the disease. This results in major damage to the heart and vasculature and loss of the potential for early initiation of effective medical treatment. Recent data show that earlier treatment of CV disease favorably impacts survival and improves the quality of life in AD patients [1].
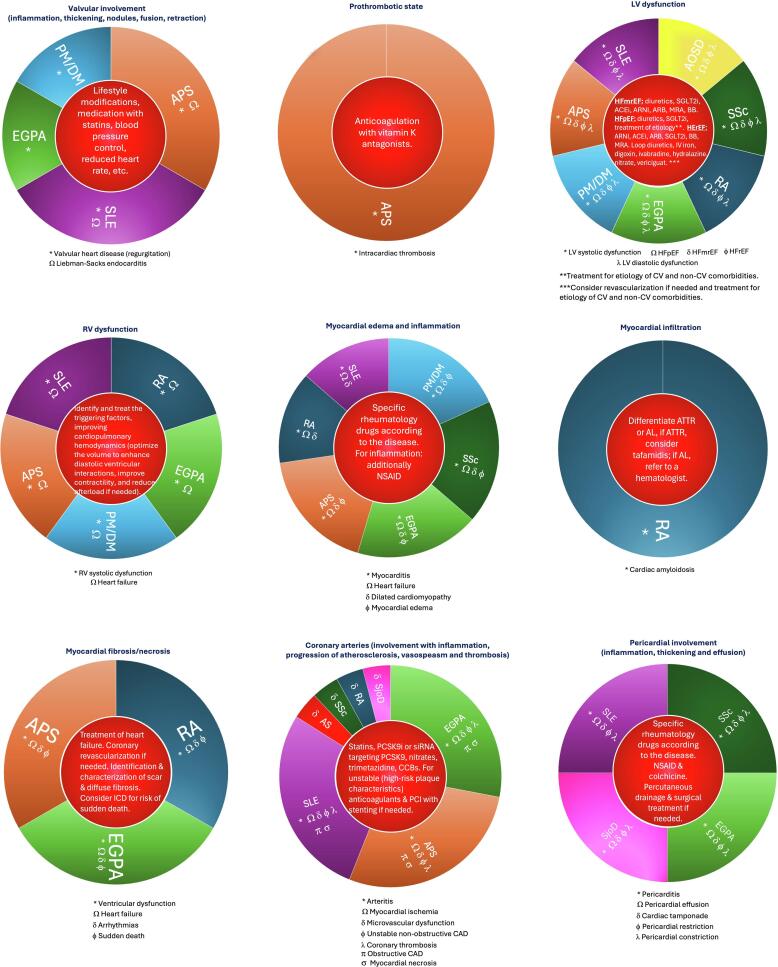
To better understand the extent of CV involvement, it is necessary to describe the pathophysiology underlying the damage in AD. Fig. 1 summarizes the main CV disease mechanisms for the most common AD. Each example reflects the relevant disease, the type of involvement, and the potential treatment for early intervention prior to the patient becoming symptomatic.
Fig. 1.
Summary of the main mechanisms involved in the most frequent diseases directly affecting the heart. The title of each circle corresponds to the pathologic mechanism; the bottom legends correspond to the cardiovascular consequence, and the inner circle corresponds to the potential early intervention. [1], [4], [5].
The myocardium and the coronary arteries are involved in almost all diseases to varying degrees. Cardiovascular magnetic resonance (CMR) is the gold standard for evaluating left and right ventricular function.[1], [2] This diagnostic technique offers greater value in this group of patients as the only noninvasive imaging modality that allows for tissue characterization and quantitative myocardial perfusion assessment. Unfortunately, it remains underused worldwide [3].
In a recent publication, Varghese et al. demonstrated that much of the CV morbidity and mortality in ARD is attributable to systemic inflammation that leads to myocarditis and coronary atherosclerosis [1]. CMR identifies subclinical cardiac involvement, allowing for early therapeutic interventions that improve clinical results and provide clear benefits to these patients [1], [2], [4].
Varghese et al. also reviewed the advances in parametric mapping and coronary blood flow (CBF) reserve measurements that aid in correctly diagnosing patients and allowing early therapeutic management and prognosis with risk stratification [1].
Based on current data and the impact of cardiovascular involvement in ARD, researchers and clinicians worldwide are joining efforts with international societies such as the Society for Cardiovascular Magnetic Resonance (SCMR) to create special interest groups in Cardio-rheumatology. The European Association of Cardiovascular Imaging (EACVI) published a clinical consensus statement for CMR in ARD [5] to promote its use in diagnostic algorithms to benefit AD patients and to obtain additional scientific evidence to support its routine clinical application. However, the recommendations of those societies do not include the use of stress perfusion as part of the comprehensive CMR protocol as proposed by Varghese et al. [1] The authors highlighted that patients with AD manifest more coronary disease in the form of microvascular dysfunction than obstructive epicardial disease [1], [5], [8]. Microvascular dysfunction is a common feature in patients with systemic lupus erythematosus (SLE) [6] and systemic sclerosis (SSc), even in the absence of symptoms [7]. Rheumatoid arthritis (RA) patients with a coronary flow reserve (CFR) < 2 are at higher risk for CV events [8]. CFR correlates with disease activity and interleukin-6 levels, even in the absence of identifiable CV disease [9]. In Sjögren's disease and ankylosing spondylitis, there is an inverse correlation between CFR and all-cause mortality and MACE. [10] These data underscore the need to include stress CMR perfusion in the diagnostic imaging protocol [1].
These findings leave clinicians wondering how to incorporate CMR data into current clinical practice. They wish to know the clinical implications of microvascular dysfunction and myocardial fibrosis in their asymptomatic patients their asymptomatic patients. For example, when is the right time to begin medical treatment, and what are the most effective therapies?
Researchers are currently investigating these topics and how best to incorporate CMR as part of clinical trials and clinical guidelines.
The medical armamentarium to treat AD patients has grown dramatically in the past ten years. Obtaining scientific evidence supporting the earliest therapeutic interventions to modify prognosis and provide durable benefits to patients is crucial.
Newer parametric parameters obtained by myocardial T1 mapping pre and post-gadolinium contrast agent administration are key metrics in this exciting new frontier. Native T1 values and the extracellular volume represent a combination of inflammation and diffuse myocardial fibrosis and provide prognostic information in the acute phase of myocardial inflammation to predict those likely to progress to a dilated cardiomyopathy (DCM). [11], [12] The pattern and location of myocardial fibrosis also correlate with an increased risk of progression to DCM [12].
The native T2 value obtained by T2 mapping without contrast detects myocardial edema and/or inflammation in acute phases of AD with high diagnostic accuracy. [13] In terms of follow-up and the monitoring of medical response, CMR parametric techniques have demonstrated a critical role [1].
Several pathophysiological mechanisms are involved in CBF assessment in these diseases, including inflammation in the coronary arteries. Other factors can produce myocardial ischemia due to individual or combined mechanisms such as microvascular dysfunction, the presence of atherosclerosis producing unstable non-obstructive or obstructive coronary artery disease, coronary thrombosis, myocardial necrosis in small non-coronary distributions, or large myocardial infarctions in the territories of the epicardial coronary arteries. CMR provides a unique advantage in this scenario since it is the only imaging modality capable of delivering all necessary information within the same imaging acquisition. CMR allows discrimination of these unique mechanisms, permitting specific treatments to start earlier and providing vital prognostic information. It is crucial that attending physicians know the advantages of CMR and that the study be performed by expert personnel with knowledge of cardio-rheumatology. As mentioned by Varghese et al., the latest multi-societal chest pain guidelines recommend using CMR as a valid method of evaluating microvascular dysfunction as a non-ionizing radiation alternative to PET. [1], [14] Based on the supporting evidence, stress CMR perfusion should be included in clinical trials examining AD and be considered part of the recommended clinical protocols.
Several cases report that the use of drugs such as corticosteroids, disease-modifying antirheumatic drugs, anti-TNF-α agents, and others stop disease progression and can even reverse some structural changes of the heart, including myocardial edema, inflammation, and fibrosis when used early in the course of the disease. CMR accurately quantifies these findings with high reproducibility [1], [15].
The prognostic capability of CMR has also been demonstrated in several studies in the AD field, where all previously mentioned parameters have significant prognostic value [1].
Based on AD's broad, varied, and subtle cardiac and vascular involvement, it is important to recommend a baseline CMR even if the patient is asymptomatic and the routine electrocardiogram and echocardiogram are normal. The current European Society of Cardiology guidelines recommend a CMR protocol including stress perfusion for SLE and SSc patients [2]. However, those ADs are not alone in demonstrating CBF abnormalities [1]. In the future, the recommendation for stress perfusion testing may include all other ADs that could have coronary artery involvement.
In conclusion, CMR is a powerful imaging modality capable of identifying early disease involvement in AD. It is invaluable in the assessment of the evolution of ARDs. It confirms the presence of multiple pathophysiological abnormalities, including microvascular dysfunction, active inflammation/myocardial edema, and the presence of focal or diffuse myocardial fibrosis. Comprehensive CMR evaluation of AD patients provides accurate disease staging and identifies patients who can benefit from early interventions. Future trials will determine the actual impact and optimization of these specific interventions.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Varghese B., Gustafson A., Chew E., Chew C., Frech T., El-Harasis M.A., et al. The role of comprehensive stress cardiac MRI in autoimmune rheumatic disease: a review. IJC Hear Vasc. 2024;101381 [Google Scholar]
- 2.Mavrogeni S., Pepe A., Nijveldt R., Ntusi N., Sierra-Galan L.M., Bratis K., et al. Cardiovascular magnetic resonance in autoimmune rheumatic diseases: a clinical consensus document by the European Association of Cardiovascular Imaging. Eur. Hear. J. Cardiovasc Imaging. 2022 Aug;23(9):e308–e322. doi: 10.1093/ehjci/jeac134. [DOI] [PubMed] [Google Scholar]
- 3.Sierra-Galan L.M., Estrada-Lopez E.E.S., Ferrari V.A., Raman S.V., Ferreira V.M., Raj V., et al. Worldwide variation in cardiovascular magnetic resonance practice models. J. Cardiovasc. Magn. Reson. 2023;25(1):38. doi: 10.1186/s12968-023-00948-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sierra-Galan L.M., Bhatia M., Alberto-Delgado A.L., Madrazo-Shiordia J., Salcido C., Santoyo B., et al. Cardiac magnetic resonance in rheumatology to detect cardiac involvement since early and pre-clinical stages of the autoimmune diseases: a narrative review. Front Cardiovasc Med. 2022;9 doi: 10.3389/fcvm.2022.870200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McDonagh T.A., Metra M., Adamo M., Gardner R.S., Baumbach A., Böhm M., et al. 2023 Focused Update of the 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: developed by the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) W. Eur. Heart J. 2023;44(37):3627–3639. doi: 10.1093/eurheartj/ehad195. [DOI] [PubMed] [Google Scholar]
- 6.Recio-Mayoral A., Mason J.C., Kaski J.C., Rubens M.B., Harari O.A., Camici P.G. Chronic inflammation and coronary microvascular dysfunction in patients without risk factors for coronary artery disease. Eur. Heart J. 2009 Aug;30(15):1837–1843. doi: 10.1093/eurheartj/ehp205. [DOI] [PubMed] [Google Scholar]
- 7.Zanatta E., Colombo C., D’Amico G., d’Humières T., Dal Lin C., Tona F. Inflammation and coronary microvascular dysfunction in autoimmune rheumatic diseases. Int. J. Mol. Sci. 2019 Nov;20(22) doi: 10.3390/ijms20225563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Montisci R., Vacca A., Garau P., Colonna P., Ruscazio M., Passiu G., et al. Detection of early impairment of coronary flow reserve in patients with systemic sclerosis. Ann. Rheum. Dis. 2003 Sep;62(9):890–893. doi: 10.1136/ard.62.9.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amigues I., Russo C., Giles J.T., Tugcu A., Weinberg R., Bokhari S., et al. Myocardial microvascular dysfunction in rheumatoid arthritis(quantitation by (13)N-ammonia positron emission tomography/computed tomography) Circ. Cardiovasc. Imaging. 2019 Dec;12(1):e007495. doi: 10.1161/CIRCIMAGING.117.007495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weber B.N., Stevens E., Perez-Chada L.M., Brown J.M., Divakaran S., Bay C., et al. Impaired coronary vasodilator reserve and adverse prognosis in patients with systemic inflammatory disorders. J. Am. Coll. Cardiol. Img. 2021 Nov;14(11):2212–2220. doi: 10.1016/j.jcmg.2020.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ntusi N.A.B., Piechnik S.K., Francis J.M., Ferreira V.M., Rai A.B.S., Matthews P.M., et al. Subclinical myocardial inflammation and diffuse fibrosis are common in systemic sclerosis–a clinical study using myocardial T1-mapping and extracellular volume quantification. J Cardiovasc Magn Reson off J Soc Cardiovasc Magn Reson. 2014 Mar;16(1):21. doi: 10.1186/1532-429X-16-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dunogué B., Terrier B., Cohen P., Marmursztejn J., Legmann P., Mouthon L., et al. Impact of cardiac magnetic resonance imaging on eosinophilic granulomatosis with polyangiitis outcomes: a long-term retrospective study on 42 patients. Autoimmun. Rev. 2015;14(9):774–780. doi: 10.1016/j.autrev.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 13.Nakou E., Patel R.K., Fontana M., Bucciarelli-Ducci C. Cardiovascular magnetic resonance parametric mapping techniques: clinical applications and limitations. Curr. Cardiol. Rep. 2021;23(12):185. doi: 10.1007/s11886-021-01607-y. [DOI] [PubMed] [Google Scholar]
- 14.Gulati M., Levy P.D., Mukherjee D., Amsterdam E., Bhatt D.L., Birtcher K.K., et al. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR guideline for the evaluation and diagnosis of chest pain: a report of the american college of cardiology/american heart association joint committee on clinical practice guidelines. J. Am. Col. Cardiol. U.S. 2021 doi: 10.1016/j.jacc.2021.07.053. [DOI] [PubMed] [Google Scholar]
- 15.Gulhane A., Ordovas K. Cardiac magnetic resonance assessment of cardiac involvement in autoimmune diseases. Front Cardiovasc Med. 2023;10:1215907. doi: 10.3389/fcvm.2023.1215907. [DOI] [PMC free article] [PubMed] [Google Scholar]