Abstract
Adeno-associated virus (AAV) is inefficient at infecting differentiated airway epithelia because of a lack of receptors at the apical surface. We hypothesized that incorporation of AAV in a calcium phosphate coprecipitate would circumvent this barrier. Interestingly, coprecipitation of AAV type 2 improved gene transfer to differentiated human airway epithelia in vitro and to the mouse lung in vivo. These results suggest that delivery of AAV as a CaPi coprecipitate may significantly enhance its utility for gene transfer to the airway epithelia in vivo.
Adeno-associated viruses (AAVs) show promise as gene transfer vectors because they are capable of long-term gene expression in vivo, and since they do not encode viral genes, they do not elicit a cell-mediated immune response (4–7, 12, 17). Thus, AAV has some advantages over first-generation adenovirus vectors. However, AAV infection from the apical surfaces of well-differentiated airway epithelia is inefficient (2, 20). This inefficiency stems in large part from the lack of AAV receptors and coreceptors in the apical membrane. AAV is thought to infect cells by binding heparan sulfate proteoglycan (19). The virus may then interact with fibroblast growth factor receptor 1 and αvβ5 integrin to further stabilize binding and mediate endocytosis (14, 18). However, in human airway epithelia, these receptors and coreceptors are located on the basolateral membrane, where they are not accessible from the airway lumen (2).
It has recently been reported that a lack of receptors for adenovirus on the apical surfaces of well-differentiated human airway epithelia limits adenovirus-mediated gene transfer (13, 21, 24). In attempting to circumvent this barrier, it has been found that delivery of adenovirus as a calcium phosphate (CaPi) coprecipitate improves gene transfer to the airway epithelia in vitro and in vivo (3, 9, 10). Furthermore, members of our group observed that delivery of adenovirus as a CaPi coprecipitate improves gene transfer by increasing binding to the apical surface and that infection occurs independently of fiber knob-coxsackie adenovirus receptor and penton base-integrin interactions (22). Since AAV infection of human airway epithelia may also be limited by a lack of apical receptors, we hypothesized that delivery of AAV in a CaPi coprecipitate would increase gene transfer to well-differentiated airway epithelia.
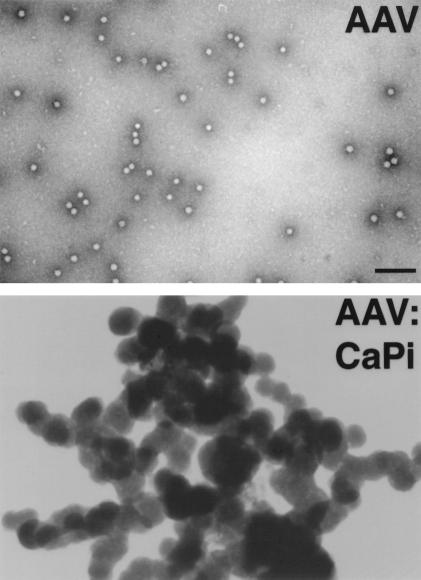
We tested our hypothesis in vitro by infecting well-differentiated human airway epithelia grown at the air-liquid interface. Airway epithelia cells were obtained from tracheae and bronchi of lungs removed for organ donation. Cells were cultured and maintained as previously described (8, 16, 23, 25). The culture medium consisted of a 1:1 mixture of Dulbecco's modified Eagle medium and Ham's F-12 medium, 5% Ultraser G (Biosepra SA, Villeneuve-la-Garenne, France), 100 U of penicillin per ml, 100 μg of streptomycin per ml, 2.5 μg of amphotericin B per ml, 1% nonessential amino acids, and 0.12 U of insulin per ml. When the epithelia are grown at the air-liquid interface, they differentiate and develop a ciliated surface that is resistant to gene transfer with AAV (2). Differentiated epithelia were infected with recombinant AAV type 2 (rAAV2) which was produced by a CaPi cotransfection protocol and was purified through three rounds of isopycnic cesium chloride ultracentrifugation, as previously described (2). The proviral plasmid pCisAv.GFP3ori was used to generate rAAV2 (AV.GFP3ori) encoding the enhanced green fluorescent protein (eGFP) under the transcriptional control of the cytomegalovirus enhancer/promoter and the simian virus 40 polyadenylation signal. Recombinant viral stocks were heated at 58°C for 60 min to inactivate contaminating helper adenovirus. Typical yields were 109 particles/μl, determined on the basis of DNA slot blot hybridization assays against plasmid standards, and 106 transducing units/μl, determined by infection of 293 cells assayed for eGFP expression. The level of adenoviral contamination as based on a second reporter assay for the recombinant adenovirus used for propagation (Ad.CMV Alkphos) was less than 1 functional particle per 1010 DNA particles of rAAV2. Viral preparations were evaluated for contamination of wild-type AAV by immunocytochemical staining of AV.GFP3ori- and Ad.CMVLacZ-coinfected 293 cells with anti-Rep antibodies (American Research Products, Inc., Belmont, Mass.). All rAAV2 stocks demonstrated an absence of Rep immunoreactivity when 1010 rAAV2 particles were used for infection. Transfection with Rep- or Cap-encoding plasmids served as controls for antibody staining of Rep protein. The virus was dialyzed in phosphate-buffered saline (PBS) prior to use. CaPi coprecipitates were then formed by placing 1.5 × 1010 particles (1.5 × 107 IU) of AAV2 or 1.5 × 1010 copies of plasmid DNA in 1 ml of Eagle's modified essential medium, which contains 1.8 mM Ca2+ and 0.86 mM Pi. An aliquot of 2 M CaCl2 (Promega, Madison, Wis.) solution was then added to achieve a final Ca2+ concentration of 12 mM (3). The solutions were mixed by gentle vortexing and incubated at room temperature for 30 min prior to infection. The dose response for the incubation time was similar to that seen with adenovirus coprecipitates, with an optimum formation time of 30 min (data not shown) (3). Figure 1 shows representative electron micrographs of AAV alone and in a CaPi coprecipitate formed for 30 min. Samples were processed for transmission electron microscopy as previously described (3).
FIG. 1.
Electron micrographs of AAV and AAV-CaPi coprecipitates. Bar = 100 nm.
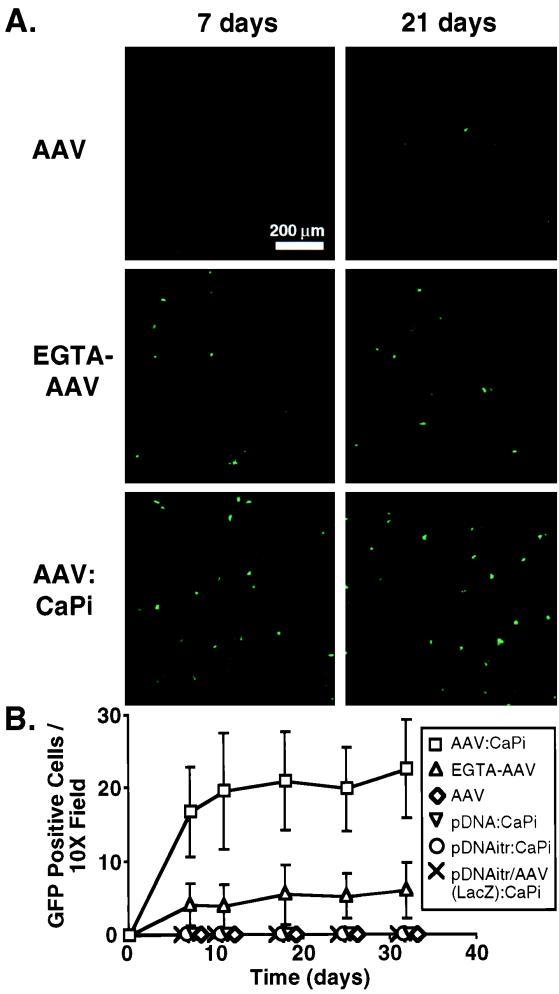
Human airway epithelia were incubated with 5 × 109 particles of AAV in a CaPi coprecipitate (AAV-CaPi) and compared to epithelia incubated with AAV alone. As additional controls, we applied 5 × 109 copies of plasmid DNA (both with and without inverted terminal repeats) in a CaPi coprecipitate and plasmid DNA with AAV in a DNA/AAV(LacZ)-CaPi coprecipitate. Following a 2-h incubation at 37°C, epithelia were washed twice with Eagle's modified essential medium to remove unbound virus and DNA. We then evaluated transgene expression by counting fluorescent green cells per 10× microscopic field using indirect fluorescence microscopy (2). Fluorescent photomicrographs were obtained with a Bio-Rad MRC-1024 confocal microscope equipped with a Kr-Ar laser at a ×10 magnification. Figure 2 shows that gene transfer with AAV alone was minimal. In addition, no eGFP expression was observed with CaPi coprecipitates of eGFP plasmid DNA either with (pCisAv.GFP3ori) or without (pCMVeGFP [Clontech, Palo Alto, Calif.]) inverted terminal repeats. Moreover, we did not see eGFP expression with eGFP plasmid DNA delivered in a CaPi coprecipitate along with recombinant AAV expressing an irrelevant gene, lacZ. As a positive control, tight junctions were disrupted by incubating the epithelia with 6 mM EGTA for 20 min prior to vector application (2, 21). As previously reported (2), following pretreatment with EGTA, gene transfer with AAV alone increased 10-fold compared to that with AAV alone on intact epithelia. More importantly, when we infected intact epithelia with AAV-CaPi, gene transfer increased 100-fold. Thus, delivering AAV as a CaPi coprecipitate markedly enhanced AAV-mediated gene transfer to human airway epithelia in vitro.
FIG. 2.
Gene transfer to human airway epithelia with AAV-CaPi. Epithelia were incubated with AAV alone, plasmid DNA-CaPi both with and without inverted terminal repeats (itr), plasmid DNA/AAV(LacZ)-CaPi, AAV alone following EGTA pretreatment, or AAV-CaPi. All were then assayed for GFP expression. (A) Confocal photomicrographs of epithelia at 7 and 21 days after infection; (B) transgene expression as indicated by the number of GFP-positive cells at the indicated time after infection. Data are means ± standard deviations; n = 9.
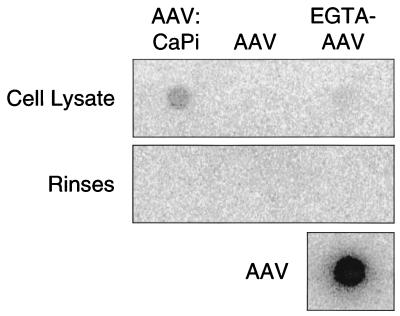
Because CaPi coprecipitation enhances adenovirus-mediated gene transfer by binding virus to the cell surface, we hypothesized that AAV-mediated gene transfer is enhanced by a similar mechanism. To test this hypothesis, we used a dot blot assay to probe for AAV viral DNA in epithelia treated with AAV alone, AAV alone following EGTA pretreatment, and AAV-CaPi. Epithelia were studied 24 h after infection. Prior to lysis, epithelia were rinsed six times with PBS at pH 5.0 in order to remove any free virus (a pH of 5 dissociates the CaPi coprecipitates [3]). Cells were then lysed with RNase-free H2O. Samples were prepared and applied to a nylon membrane (Ambion, Austin, Tex.). As a positive control, we blotted 5 × 109 particles of AAV (i.e., the same amount of virus used to infect the epithelia). Dot blots were probed with 32P-labeled pCisAv.GFP3ori and developed with a PhosphorImager (Molecular Dynamics, Sunnyvale, Calif.). As shown in Fig. 3, we did not detect AAV viral DNA in epithelia which were infected with AAV alone. When we infected EGTA-pretreated epithelia with AAV, a faint dot was present. However, when we infected epithelia with AAV-CaPi, we observed a 15-fold-greater hybridization than with AAV applied to EGTA-pretreated epithelia. These data suggest that enhanced gene transfer with AAV-CaPi is due to an increase in the amount of virus that infects cells.
FIG. 3.
Effect of CaPi coprecipitation of AAV on virus association with human airway epithelia. Epithelia were treated with AAV alone, AAV following pretreatment with 6 mM EGTA to disrupt tight junctions, or AAV-CaPi. Samples were applied to a nylon membrane, and AAV was detected with a 32P-labeled probe. Also shown are rinses and application of 5 × 109 particles of AAV to the membrane.
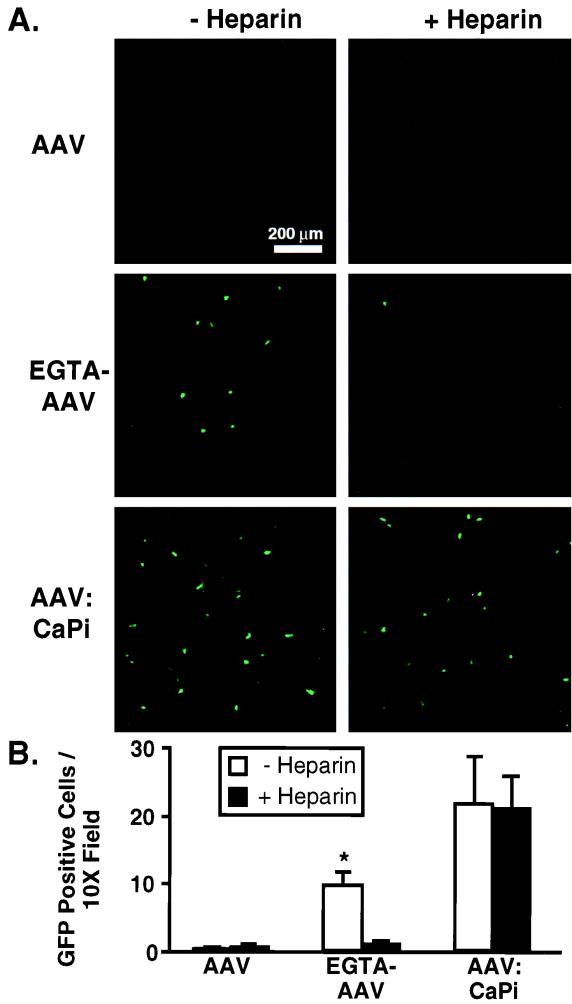
Since CaPi coprecipitation enhances infection from the apical surfaces of human airway epithelia and receptors for AAV are not present on the apical surfaces of these epithelia, we predicted that AAV-CaPi would not require an interaction with heparan sulfate proteoglycan for gene transfer. To test this prediction, we preincubated AAV or AAV-CaPi with 20 μg of soluble heparin sulfate (Sigma, St. Louis, Mo.) per ml for 30 min at room temperature and then infected the epithelia for 2 h at 37°C (1, 19). Figure 4 shows minimal gene transfer with AAV alone in the absence and presence of soluble heparin sulfate. However, when we delivered AAV alone to EGTA-pretreated epithelia, gene transfer was increased. This increase was inhibited by preincubation of AAV with soluble heparin sulfate, suggesting a receptor-dependent infection. In contrast, when we infected cells with AAV-CaPi, the increase in gene transfer was not blocked by soluble heparin sulfate. Thus, infection with AAV-CaPi did not require an interaction between AAV capsid proteins and cell surface heparan sulfate proteoglycan in order to enhance gene transfer.
FIG. 4.
Effect of heparin sulfate on AAV-CaPi gene transfer. Prior to infection, virus was incubated in the absence or presence of soluble heparin sulfate. Then human airway epithelia were treated with AAV alone, AAV following pretreatment with 6 mM EGTA to disrupt tight junctions, or AAV-CaPi. (A) Confocal photomicrographs of epithelia at 14 days after infection; (B) transgene expression as indicated by the number of GFP-positive cells at 14 days after infection. Data are means ± standard deviations; n = 5. The asterisk indicates a P value of <0.005.
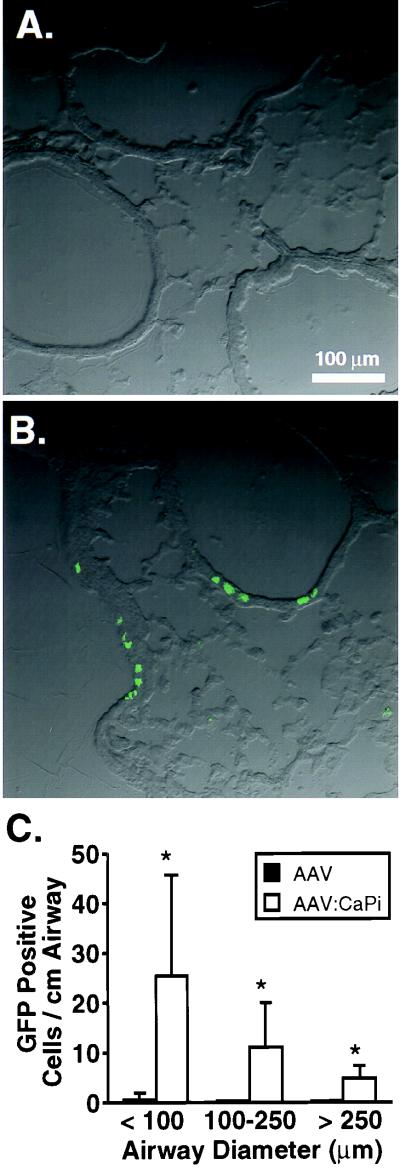
To determine whether CaPi coprecipitation would improve AAV-mediated gene transfer to airway epithelia in vivo, we infected six 6- to 8-week-old C57BL/6 mice (Jackson Laboratory, Bar Harbor, Maine) with 1.2 × 1010 particles of either AAV alone or AAV-CaPi by intranasal instillation of two 62.5-μl doses delivered under methoxyflurane anesthesia. Twenty-eight days after infection, animals were sacrificed. PBS (10 ml) was instilled into the right ventricle, and then the lungs and heart were removed intact. The trachea was intubated and inflated at 10 cm of H2O pressure with PBS, 4% paraformaldehyde, and then PBS again. Lungs were cryosectioned. Figure 5A and B show representative photomicrographs from lung sections of AAV- and AAV-CaPi-infected mice, respectively. Sections were analyzed by measuring the airway diameter and counting the number of eGFP-expressing cells per airway (Fig. 5C). We observed that gene transfer with AAV alone was minimal. In contrast, delivery of AAV as a CaPi coprecipitate significantly increased gene transfer to small, medium, and large airways, with a tendency toward infecting small airways. Based on an average width of airway cells of 4.9 μm, we calculated the percentage of cells in the small airways which expressed the transgene (3, 11). In small airways of AAV-infected mice, 0.02% ± 0.02% of cells expressed the transgene, whereas 0.25% ± 0.20% of cells expressed the transgene in AAV:CaPi-infected mice. Although these percentages are low, it is important to note that the dose of vector delivered was also low. Thus, delivery of AAV as a CaPi coprecipitate markedly enhanced AAV-mediated gene transfer in vivo, with particular propensity for the small airways.
FIG. 5.
AAV-CaPi gene transfer to mouse lung. Recombinant AAV was administered alone or as AAV-CaPi. Twenty-eight days later, lungs were fixed and cryosectioned. Confocal photomicrographs are from animals infected with AAV alone (A) or AAV-CaPi (B). (C) Transgene expression as indicated by the number of GFP-positive cells per centimeter of airway as a function of airway diameter. Data are means ± standard deviations; n = 15. The asterisk indicates a P value of <0.005.
Our data are consistent with earlier observations that apical delivery of AAV is inefficient at gene transfer (2, 7, 20). Our results with EGTA and heparin sulfate are also in agreement with a basolateral localization of AAV receptors. Recent reports have questioned the role of heparan sulfate proteoglycan and αv integrins as receptors for AAV (15). However, whatever the receptor is, its activity appears to be localized to the basolateral side. Our data show that by increasing binding to the apical surface, CaPi coprecipitation helps overcome this barrier. The enhancement in gene transfer is similar to that seen with adenovirus-CaPi and is more efficient than gene transfer with plasmid DNA-CaPi (9). Unlike plasmid DNA, AAV has viral proteins which may facilitate steps subsequent to binding. The 100-fold improvement in gene transfer when AAV was delivered as a CaPi coprecipitate supports this conclusion.
Acknowledgments
We thank Tom Moninger, Mike Seiler, Terry Grunst, Norma Anderson, Phil Karp, Pary Weber, Janice Launspach, Kathy Francois, and Theresa Mayhew for excellent assistance. We especially appreciated the generous help of the late Al Fasbender.
We appreciate the support of the University of Iowa Gene Transfer Vector Core (supported by the Roy J. Carver Charitable Trust, the National Heart, Lung, and Blood Institute, and the Cystic Fibrosis Foundation) and the University of Iowa In Vitro Cell Models Core. This work was supported by the National Heart, Lung, and Blood Institute (grant HL58340) (J.F.E.) and the Cystic Fibrosis Foundation (M.J.W.). M.J.W. is an Investigator of the Howard Hughes Medical Institute.
REFERENCES
- 1.Chiorini J A, Kim F, Yang L, Kotin R M. Cloning and characterization of adeno-associated virus type 5. J Virol. 1999;73:1309–1319. doi: 10.1128/jvi.73.2.1309-1319.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Duan D, Yue Y, Yan Z, McCray P B, Jr, Englehardt J F. Polarity influences the efficiency of recombinant adenoassociated virus infection in differentiated airway epithelia. Hum Gene Ther. 1998;9:2761–2776. doi: 10.1089/hum.1998.9.18-2761. [DOI] [PubMed] [Google Scholar]
- 3.Fasbender A, Lee J H, Walters R W, Moninger T O, Zabner J, Welsh M J. Incorporation of adenovirus in calcium phosphate precipitates enhances gene transfer to airway epithelia in vitro and in vivo. J Clin Investig. 1998;102:184–193. doi: 10.1172/JCI2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fisher K J, Jooss K, Alston J, Yang Y, Haecker S E, High K, Pathak R, Raper S E, Wilson J M. Recombinant adeno-associated virus for muscle directed gene therapy. Nat Med. 1997;3:306–312. doi: 10.1038/nm0397-306. [DOI] [PubMed] [Google Scholar]
- 5.Flotte T R, Afione S A, Solow R, Drumm M L, Markakis D, Guggino W B, Zeitlin P L, Carter B J. Expression of the cystic fibrosis transmembrane conductance regulator from a novel adeno-associated virus promoter. J Biol Chem. 1993;268:3781–3790. [PubMed] [Google Scholar]
- 6.Flotte T R, Carter B J. Adeno-associated virus vectors for gene therapy. Gene Ther. 1995;2:357–362. [PubMed] [Google Scholar]
- 7.Halbert C L, Standaert T A, Wilson C B, Miller A D. Successful readministration of adeno-associated virus vectors to the mouse lung requires transient immunosuppression during the initial exposure. J Virol. 1998;72:9795–9805. doi: 10.1128/jvi.72.12.9795-9805.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kondo M, Finkbeiner W E, Widdicombe J H. Simple technique for culture of highly differentiated cells from dog tracheal epithelium. Am J Physiol. 1991;261:L106–L117. doi: 10.1152/ajplung.1991.261.2.L106. [DOI] [PubMed] [Google Scholar]
- 9.Lee J H, Welsh M J. Enhancement of calcium phosphate-mediated transfection by inclusion of adenovirus in coprecipitates. Gene Ther. 1999;6:676–682. doi: 10.1038/sj.gt.3300857. [DOI] [PubMed] [Google Scholar]
- 10.Lee J H, Zabner J, Welsh M J. Delivery of an adenovirus vector in a calcium phosphate coprecipitate enhances the therapeutic index of gene transfer to airway epithelia. Hum Gene Ther. 1999;10:603–613. doi: 10.1089/10430349950018670. [DOI] [PubMed] [Google Scholar]
- 11.Mariassy A T. Epithelial cells of trachea and bronchi. In: Parent R A, editor. Comparative biology of the normal lung. Boca Raton, Fla: CRC Press; 1992. pp. 63–76. [Google Scholar]
- 12.Muzyczka N. Use of adeno-associated virus as a general transduction vector for mammalian cells. Curr Top Microbiol Immunol. 1992;158:97–129. doi: 10.1007/978-3-642-75608-5_5. [DOI] [PubMed] [Google Scholar]
- 13.Pickles R J, McCarty D, Matsui H, Hart P J, Randell S H, Boucher R C. Limited entry of adenovirus vectors into well-differentiated airway epithelium is responsible for inefficient gene transfer. J Virol. 1998;72:6014–6023. doi: 10.1128/jvi.72.7.6014-6023.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qing K, Mah C, Hansen J, Zhou S, Dwarki V, Srivastava A. Human fibroblast growth factor receptor 1 is a co-receptor for infection by adeno-associated virus 2. Nat Med. 1999;5:71–77. doi: 10.1038/4758. [DOI] [PubMed] [Google Scholar]
- 15.Qui J, Mizukami H, Brown K E. Adeno-associated virus 2 co-receptors? Nat Med. 1999;5:467–468. doi: 10.1038/8328. [DOI] [PubMed] [Google Scholar]
- 16.Smith J J, Travis S M, Greenberg E P, Welsh M J. Cystic fibrosis airway epithelia fail to kill bacteria because of abnormal airway surface fluid. Cell. 1996;85:229–236. doi: 10.1016/s0092-8674(00)81099-5. [DOI] [PubMed] [Google Scholar]
- 17.Snyder R O, Miao C H, Patijn G A, Spratt S K, Danos O, Nagy D, Gown A M, Winther B, Meuse L, Cohen L K, Thompson A R, Kay M A. Persistent and therapeutic concentrations of human factor IX in mice after hepatic gene transfer of recombinant AAV vectors. Nat Genet. 1997;16:270–276. doi: 10.1038/ng0797-270. [DOI] [PubMed] [Google Scholar]
- 18.Summerford C, Bartlett J S, Samulski R J. αvβ5 integrin: a co-receptor for adeno-associated virus type 2 infection. Nat Med. 1999;5:78–82. doi: 10.1038/4768. [DOI] [PubMed] [Google Scholar]
- 19.Summerford C, Samulski R J. Membrane-associated heparan sulfate proteoglycan is a receptor for adeno-associated virus type 2 virions. J Virol. 1998;72:1438–1445. doi: 10.1128/jvi.72.2.1438-1445.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Teramoto S, Bartlett J S, McCarty D, Xiao X, Samulski R J, Boucher R C. Factors influencing adeno-associated virus-mediated gene transfer to human cystic fibrosis airway epithelial cells: comparison with adenovirus vectors. J Virol. 1998;72:8904–8912. doi: 10.1128/jvi.72.11.8904-8912.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walters R W, Grunst T, Bergelson J M, Finberg R W, Welsh M J, Zabner J. Basolateral localization of fiber receptors limits adenovirus infection from the apical surface of airway epithelia. J Biol Chem. 1999;274:10219–10226. doi: 10.1074/jbc.274.15.10219. [DOI] [PubMed] [Google Scholar]
- 22.Walters, R. W., and M. J. Welsh. Mechanism by which calcium phosphate coprecipitation enhances adenovirus mediated gene transfer. Gene Ther., in press. [DOI] [PubMed]
- 23.Yamaya M, Finkbeiner W E, Chun S Y, Widdicombe J H. Differentiated structure and function of cultures from human tracheal epithelium. Am J Physiol. 1992;262:L713–L724. doi: 10.1152/ajplung.1992.262.6.L713. [DOI] [PubMed] [Google Scholar]
- 24.Zabner J, Freimuth P, Puga A, Fabrega A, Welsh M J. Lack of high affinity fiber receptor activity explains the resistance of ciliated airway epithelia to adenovirus infection. J Clin Investig. 1997;100:1144–1149. doi: 10.1172/JCI119625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zabner J, Zeiher B G, Friedman E, Welsh M J. Adenovirus-mediated gene transfer to ciliated airway epithelia requires prolonged incubation time. J Virol. 1996;70:6994–7003. doi: 10.1128/jvi.70.10.6994-7003.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]