Direct oral anticoagulants (DOACs), widely used in clinical practice to reduce thrombotic risk, work by directly inhibiting factor Xa or thrombin. Recent research has revealed an ongoing clinical implication – their impact on protease-activated receptors (PARs) [1]. Factor Xa and thrombin activate PAR-1 and PAR-2, which can promote inflammatory and fibrotic responses. The present study aimed to assess PAR-1 and PAR-2 expressions on circulating neutrophils among atrial fibrillation (AF) patients treated with DOACs.
This study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki, and was approved by the Tohoku University Institutional Review Board (Approval Number: 2020-1-326). Informed consent was obtained from all patients. We enrolled 40 consecutive AF patients who were previously untreated with anticoagulants. Blood samples were collected both before and four weeks after the administration of DOACs, including dabigatran, rivaroxaban, apixaban, and edoxaban. We measured the expression of PAR-1 and PAR-2 in circulating neutrophiles, as well as prothrombin time (PT) and activated partial thromboplastin time (APTT). The assessment of PAR-1 and PAR-2 expression was conducted using Western blotting, employing the following antibodies; anti-PAR1/thrombin receptor antibody (ab183083), anti-PAR2 antibody [EPR13675] (ab180953), and anti-β-actin antibody [AC-15] (ab6276) for detecting PAR-1, PAR-2, and β-actin, respectively. β-actin served as the internal control (Supplementary File) [2].
For statistical analysis, continuous variables are presented as means ± standard deviation (SD). Group comparisons for multiple continuous variables were conducted using the Wilcoxon signed-rank test. Statistical significance was defined as a P-value of <0.05. All statistical analyses were carried out using JMP software, version 16.0 (SAS Institute, Cary, NC, USA).
Patient characteristics are presented in Table 1. The mean age was 58.3 ± 12.8 years, and 34 (85 %) were male. Among the enrolled patients, 25 (62 %) had paroxysmal AF, while 15 (38 %) had persistent AF. Dabigatran was administered to 10 (25 %) patients, rivaroxaban to 12 (30 %), apixaban to 9 (23 %), and edoxaban to 9 (23 %) (Table 1). Importantly, all patients enrolled in this study received DOAC treatment either at standard or reduced doses based on individual dosage reduction criteria.
Table 1.
Patient characteristics.
|
All (n = 40) |
Dabigatran (n = 10) |
Rivaroxaban (n = 12) |
Apixaban (n = 9) |
Edoxaban (n = 9) |
|
|---|---|---|---|---|---|
| Medication dose: n | 300 mg:5, 220 mg:5 | 15 mg:12 | 10 mg:9 | 60 mg:7, 30 mg:2 |
|
| Age, years | 58 ± 13 | 59 ± 12 | 51 ± 14 | 61 ± 13 | 63 ± 8 |
| Male, n (%) | 34 (85) | 10 (100) | 11 (92) | 7 (78) | 6 (67) |
| LVEF, % | 63 ± 11 | 57 ± 14 | 66 ± 7 | 59 ± 10 | 68 ± 5 |
| LAD, mm | 38 ± 5 | 39 ± 5 | 37 ± 6 | 37 ± 5 | 39 ± 5 |
| Persistent AF, n (%) | 15 (38) | 5 (50) | 2 (17) | 6 (67) | 2 (22) |
| Median CHADS2 Score | 0.5 ± 0.6 | 0.6 ± 0.7 | 0.3 ± 0.4 | 0.3 ± 0.5 | 0.9 ± 0.6 |
| CHF, n (%) | 1 (3) | 1 (10) | 0 | 0 | 0 |
| Hypertension, n (%) | 15 (38) | 3 (30) | 3 (25) | 3 (33) | 6 (67) |
| Age ≥ 75 years, n (%) | 2 (5) | 1 (10) | 0 | 0 | 1(11) |
| Diabetes mellitus, n (%) | 1 (3) | 1 (10) | 0 | 0 | 0 |
| Stroke/TIA, n (%) | 0 | 0 | 0 | 0 | 0 |
| History of MI | 0 | 0 | 0 | 0 | 0 |
| Smoking, n (%) | 16 (40) | 2 (20) | 4 (33) | 5 (56) | 4 (44) |
| Current smoker | 2 (5) | 0 | 1 (8) | 1 (11) | 0 |
| Ex-smoker | 14 (35) | 2 (20) | 3 (25) | 4 (44) | 5 (56) |
| Antiplatelet drug, n (%) | 0 | 0 | 0 | 0 | 0 |
| Statin, n (%) | 3 (8) | 1 (10) | 0 | 0 | 2 (22) |
LVEF, left ventricular ejection fraction; LAD, left atrial diameter; AF, atrial fibrillation; CHF, congestive heart failure; TIA, transient ischemic attack; MI, myocardial infarction
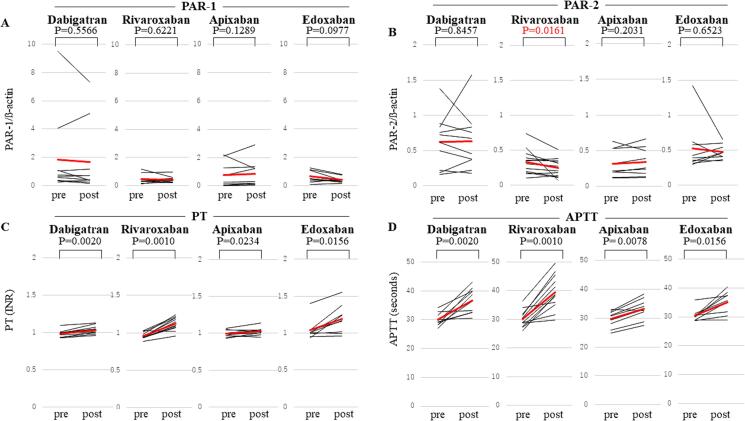
PAR-1 expression remained unchanged following administration of any DOACs (Fig. 1A). In contrast, rivaroxaban administration resulted in a significant decrease in PAR-2 expression, while other DOACs had no effect (Fig. 1B). Notably, all 4 DOACs significantly and similarly prolonged both PT and APTT (Fig. 1C and 1D).
Fig. 1.
Changes in PAR-1 and PAR-2 Expressions (Upper Panel), PT, and APTT (Lower Panel) following DOAC Administration. Panel A shows alterations in PAR-1 expression following the administration of DOACs (dabigatran, rivaroxaban, apixaban, and edoxaban). Similarly, panel B shows changes in PAR-2 expression, panel C shows PT, and panel D represents APTT. The black line represents individual data, and the red bold line indicates the average value. PAR, protease-activated receptors; PT, prothrombin time; INR, international normalized ratio; APTT, activated partial thromboplastin time; DOACs, direct oral anticoagulants. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
While DOACs are well-established for their role in inhibiting factor Xa and thrombin to suppress thrombotic conditions, emerging evidence suggests that their beneficial effects may extend beyond anticoagulation. The novel finding of the present study is that, despite the similar inhibitory effects on PT and APTT among the 4 DOACs, only rivaroxaban induced a significant reduction in PAR-2 expression.
Factor Xa and thrombin are known for their capacity to activate PARs, which can initiate processes associated with inflammation. Given the significant roles of PAR-1 and PAR-2 in the inflammation, targeting this pathway may present an intriguing potential strategy. DOACs with similar anticoagulant effects could also be characterized by different efficacy and safety profiles.
Rivaroxaban, an active factor X (FXa) inhibitor, has been demonstrated to exert protective effects in various diseases. Pretreatment with rivaroxaban reduced intracerebral hemorrhage volume in a rat cerebral infarction model compared to warfarin [3]. In murine pressure-overload heart models, rivaroxaban administration attenuated cardiac inhomogeneous interstitial fibrosis and macrophage infiltration [4]. Additionally, in a murine myocardial infarction model, rivaroxaban treatment improved survival curves and attenuated heart failure [5]. Rivaroxaban also showed protective effects against angiotensin II-induced murine renal damage, including glomerular hypertrophy, mesangial matrix expansion, podocyte foot process effacement, and thickened glomerular basement membrane [6]. Inhibition of FXa by rivaroxaban has been reported to decrease the functional expression of PAR-2 in human smooth muscle cells (SMCs), thereby mitigating vascular remodeling such as SMC proliferation and migration [7].
Considering the central role of the inflammatory response via the PAR-2 pathway in the aforementioned pathological conditions, rivaroxaban-mediated PAR-2 inhibition could represent a novel therapeutic strategy for human inflammatory diseases. Here, we present the first successful demonstration of rivaroxaban's inhibitory effect on PAR-2 in circulating neutrophils, a key player in inflammatory conditions. Previous studies have suggested that dabigatran, a direct thrombin inhibitor, may increase platelet reactivity by upregulating the density of thrombin receptors, specifically PAR-1 and PAR-4 [8]. While our findings may appear inconsistent with these results, it is important to note that our analysis focused on PAR expression in circulating neutrophils, which could contribute to these differing outcomes.
Indeed, previous studies reported the effectiveness of rivaroxaban, even at a reduced dose of 2.5 mg twice daily, in reducing thrombotic events and all-cause mortality when combined with dual antiplatelet therapy in patients with acute coronary syndrome [9]. In stable coronary artery disease (CAD) patients, the addition of low-dose rivaroxaban to acetylsalicylic acid has shown promise in reducing thrombotic risk [10]. Notably, in patients with AF and stable CAD, monotherapy with rivaroxaban has demonstrated non-inferior efficacy and superior safety compared to combination therapy with rivaroxaban and a single antiplatelet agent, as evidenced in the AFIRE trial [11].
Several limitations should be mentioned. First, although the inhibitory effects of rivaroxaban were evident, the sample size was small in a single center study. Second, since PAR-1 expression remained unchanged, further studies are warranted.
In conclusion, the present study demonstrated the novel inhibitory effect of rivaroxaban on PAR-2 expression, suggesting its anti-inflammatory effect.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcha.2024.101387.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Supplementary Fig. 1.
References
- 1.Spronk H.M., de Jong A.M., Crijns H.J., Schotten U., Van Gelder I.C., Ten Cate H. Pleiotropic effects of factor Xa and thrombin: what to expect from novel anticoagulants. Cardiovasc. Res. 2014;101:344–351. doi: 10.1093/cvr/cvt343. [DOI] [PubMed] [Google Scholar]
- 2.Rashid M., Tawara S., Fukumoto Y., Seto M., Yano K., Shimokawa H. Importance of Rac1 signaling pathway inhibition in the pleiotropic effects of HMG-CoA reductase inhibitors. Circ. J. 2009;73:361–370. doi: 10.1253/circj.cj-08-0817. [DOI] [PubMed] [Google Scholar]
- 3.Morihara R., Yamashita T., Kono S., Shang J., Nakano Y., Sato K., Hishikawa N., Ohta Y., Heitmeier S., Perzborn E., Abe K. Reduction of intracerebral hemorrhage by rivaroxaban after tPA thrombolysis is associated with downregulation of PAR-1 and PAR-2. J. Neurosci. Res. 2017;95:1818–1828. doi: 10.1002/jnr.24013. [DOI] [PubMed] [Google Scholar]
- 4.Kondo H., Abe I., Fukui A., Saito S., Miyoshi M., Aoki K., Shinohara T., Teshima Y., Yufu K., Takahashi N. Possible role of rivaroxaban in attenuating pressure-overload-induced atrial fibrosis and fibrillation. J. Cardiol. 2018;71:310–319. doi: 10.1016/j.jjcc.2017.08.007. [DOI] [PubMed] [Google Scholar]
- 5.Liu J., Nishida M., Inui H., Chang J., Zhu Y., Kanno K., Matsuda H., Sairyo M., Okada T., Nakaoka H., Ohama T., Masuda D., Koseki M., Yamashita S., Sakata Y. Rivaroxaban suppresses the progression of ischemic cardiomyopathy in a murine model of diet-induced myocardial infarction. J. Atheroscler. Thromb. 2019;26:915–930. doi: 10.5551/jat.48405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ichikawa H., Shimada M., Narita M., Narita I., Kimura Y., Tanaka M., Osanai T., Okumura K., Tomita H. Rivaroxaban, a direct factor Xa inhibitor, ameliorates hypertensive renal damage through inhibition of the inflammatory response mediated by protease-activated receptor pathway. J. Am. Heart Assoc. 2019;8:e012195. doi: 10.1161/JAHA.119.012195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jobi K., Rauch B.H., Dangwal S., Freidel K., Doller A., Eberhardt W., Fischer J.W., Schror K., Rosenkranz A.C. Redox regulation of human protease-activated receptor-2 by activated factor X. Free Radic. Biol. Med. 2011;51:1758–1764. doi: 10.1016/j.freeradbiomed.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 8.Achilles A., Mohring A., Dannenberg L., Grandoch M., Hohlfeld T., Fischer J.W., Levkau B., Kelm M., Zeus T., Polzin A. Dabigatran enhances platelet reactivity and platelet thrombin receptor expression in patients with atrial fibrillation. J. Thromb. Haemost. 2017;15:473–476. doi: 10.1111/jth.13595. [DOI] [PubMed] [Google Scholar]
- 9.Mega J.L., Braunwald E., Mohanavelu S., Burton P., Poulter R., Misselwitz F., Hricak V., Barnathan E.S., Bordes P., Witkowski A., Markov V., Oppenheimer L., Gibson C.M., group AA-Ts Rivaroxaban versus placebo in patients with acute coronary syndromes (ATLAS ACS-TIMI 46): a randomised, double-blind, phase II trial. Lancet. 2009;374:29–38. doi: 10.1016/S0140-6736(09)60738-8. [DOI] [PubMed] [Google Scholar]
- 10.Eikelboom J.W., Connolly S.J., Bosch J., Dagenais G.R., Hart R.G., Shestakovska O., Diaz R., Alings M., Lonn E.M., Anand S.S., Widimsky P., Hori M., Avezum A., Piegas L.S., Branch K.R.H., Probstfield J., Bhatt D.L., Zhu J., Liang Y., Maggioni A.P., Lopez-Jaramillo P., O'Donnell M., Kakkar A.K., Fox K.A.A., Parkhomenko A.N., Ertl G., Stork S., Keltai M., Ryden L., Pogosova N., Dans A.L., Lanas F., Commerford P.J., Torp-Pedersen C., Guzik T.J., Verhamme P.B., Vinereanu D., Kim J.H., Tonkin A.M., Lewis B.S., Felix C., Yusoff K., Steg P.G., Metsarinne K.P., Cook Bruns N., Misselwitz F., Chen E., Leong D., Yusuf S., Investigators C. Rivaroxaban with or without aspirin in stable cardiovascular disease. N. Engl. J. Med. 2017;377:1319–1330. doi: 10.1056/NEJMoa1709118. [DOI] [PubMed] [Google Scholar]
- 11.Yasuda S., Kaikita K., Akao M., Ako J., Matoba T., Nakamura M., Miyauchi K., Hagiwara N., Kimura K., Hirayama A., Matsui K., Ogawa H., for the AFIRE Investigators Antithrombotic therapy for atrial fibrillation with stable coronary disease. N. Engl. J. Med. 2019;381:1103–1113. doi: 10.1056/NEJMoa1904143. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.