Abstract
Cardiovascular disease is the leading cause of morbidity and mortality in patients with autoimmune rheumatic diseases. Much of this may be attributed to systemic inflammation resulting in coronary atherosclerosis and myocarditis. Cardiac magnetic resonance imaging is the gold standard for the evaluation of cardiac structure and function, including tissue characterization, which allows for detection of myocardial edema, inflammation, and fibrosis. Advances in parametric mapping and coronary flow reserve measurement techniques have the potential to change the diagnosis, risk stratification, and management of patients with autoimmune rheumatic diseases. We provide an overview of the current evidence and suggest potential future roles for the use of comprehensive cardiac magnetic resonance in patients with autoimmune rheumatic diseases in the field of cardio-rheumatology.
Keyword: Parametric mapping, Coronary flow reserve, Myocarditis, Autoimmune disorders, Rheumatic disease, Cardio Rheumatology
1. Introduction
Cardiovascular (CV) disease is a leading cause of morbidity and mortality in patients with autoimmune rheumatic disease (AIRD) [1,2]. Epidemiological studies have shown that patients with AIRD have a shorter lifespan compared to the general population [2], [3] Forty percent of all deaths in patients with rheumatoid arthritis (RA) are the result of cardiovascular disease [4], and patients with systemic lupus erythematosus (SLE) and systemic sclerosis (SSc) have a higher mortality rate than the general population [5]. It is hypothesized that the increased incidence of cardiovascular disease and associated mortality may be largely driven by myocardial inflammation promoting accelerated atherosclerosis [2], [6], [7].
Peripheral markers of inflammation or cardiac injury such as the erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), troponin, and pro-BNP lack sensitivity and specificity for detecting cardiac involvement whereas invasive endomyocardial biopsy (EMB), due to sampling error, can be inaccurate and is associated with a risk of complications [8], [9], [10].
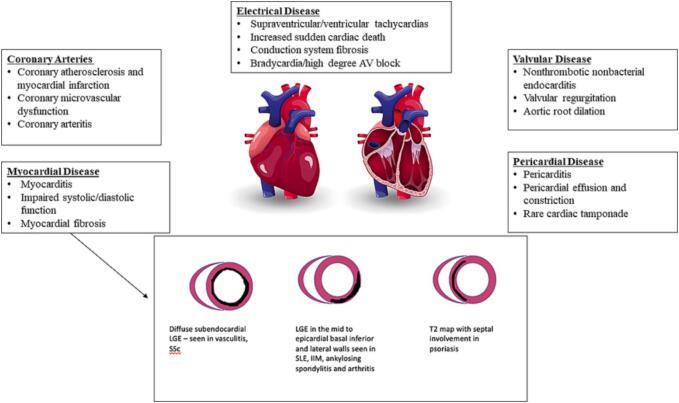
Cardiac magnetic resonance (CMR) is the gold standard for the assessment of cardiac structure and function and may be an ideal modality for the earlier detection of indolent AIRD-related cardiac disease [1], [2]. CMR is capable of phenotyping the broad array of potential cardiac involvement in AIRD, including valvular disease, myocardial and pericardial disease, epicardial and microvascular coronary artery disease, and myocardial fibrosis. (Central Illustration: Fig. 1) [2], [3].
Fig. 1.
Cardiovascular Pathology and CMR Patterns in AIRD (color).
Vasodilator stress imaging with CFR quantification can aid in the diagnosis of microvascular dysfunction [11]. Novel parametric mapping techniques allow for comprehensive tissue characterization. Thus, CMR has the potential to become a comprehensive diagnostic and prognostic tool in the management of patients with AIRD [12].
In this review of the current evidence for the role of CMR in various rheumatologic diseases, including systemic lupus erythematosus (SLE), systemic sclerosis (SSc), rheumatoid arthritis (RA), ANCA-associated vasculitis (AAV), Ankylosing Spondylitis (AS), Sjogren’s Disease (SjD), inflammatory myopathies (IIM), and psoriasis (PsO), we will attempt to answer four questions: 1) What are the CMR findings in each disease? 2) Do these findings correlate with disease activity? 3) How do these CMR findings translate into outcomes? 4) Do these CMR findings change with therapy? We will postulate how CMR findings could influence treatment decisions targeting systemic and myocardial inflammation with the goal of preventing overt clinical manifestations of CV disease in patients with AIRD (Supplement: Fig. 2).
1.1. Utility of cardiac magnetic resonance imaging (CMR)
Cardiac magnetic resonance (CMR) imaging is the gold standard for the evaluation of the cardiac structure, function, and tissue characterization, including inflammation, perfusion defects, and fibrosis [3]. Both ischemic and non-ischemic fibrosis may be seen in AIRD, though non-ischemic patterns are more commonly reported (see Fig. 1) [2], [3].
1.2. Parametric mapping
Parametric mapping provides a visual and quantitative measure of the T1 or T2 properties of a tissue that allows the evaluation of both focal and diffuse disease processes [12], [13]. This also allows for the creation of spatial maps where the T1, T2, and ECV values can be followed longitudinally over time [12], [13].
The native T1 (without contrast) is elevated in the presence of inflammation, edema, fibrosis, and/or infiltration and reduced in the presence of fat, lipids (as in Fabry’s disease), and iron overload [13]. T1 values are elevated across a range of chest pain syndromes, including acute myocardial infarction, myocarditis, and Takotsubo cardiomyopathy [12]. T1 mapping may also hold prognostic value in the acute phase of myocarditis to determine who is at risk for progression to dilated cardiomyopathy [12].
The myocardial ECV can be calculated with knowledge of the hemoglobin concentration and the pre- and post-contrast T1 relaxation times of the myocardium and blood pool [12], [13]. ECV carries important prognostic information and is a better predictor of mortality compared to LGE in diabetic patients [14] and has also been shown to identify myocardial involvement in various AIRDs [15], [16].
T2 mapping is sensitive to free water content in tissue and is considered particularly useful for detecting acute myocardial inflammation/edema [12], [13]. T2 mapping has superior diagnostic accuracy to conventional CMR techniques for the diagnosis of endomyocardial biopsy-proven myocarditis [12], [13].
1.3. Coronary flow reserve
The coronary flow reserve (CFR) is the ratio of the augmented coronary blood flow (CBF) with exercise, stress, or microcirculatory vasodilation compared to the CBF at rest [17]. The CFR provides a quantitative measure of the overall coronary vasculature because it provides information on blood flow in the epicardial vessels as well as the coronary microcirculation, which includes the distal small vessels and capillaries [11].
A recent meta-analysis of over 59,000 patients demonstrated that abnormal CFR was associated with a higher incidence of all-cause mortality [HR 3.78, 95 % confidence interval (CI) 2.39–––5.97] and major adverse cardiovascular events (MACE) [HR 3.42 2.92–––3.99)] across a range of pathology [17]. CFR can be measured with similar accuracy across a range of imaging modalities – including CMR – and can be useful to identify ischemia in the absence of visible perfusion abnormalities [11].
2. Literature review of the role of parametric mapping and CFR in patients with AIRD
2.1. What are the CMR findings in AIRD?
Autoimmune myocardial inflammation has been observed in many AIRDs including SLE, RA, spondyloarthropathies (SpA), SSc, and AAV [1], [2]. In fact, many small studies have shown that across a spectrum of AIRD, patients tend to have higher T1, T2, and ECV values compared to healthy controls, even in the absence of cardiovascular symptoms or focal LGE [1], [2], [3].
Patients with SLE have an increased incidence of CAD (8x higher risk than the general population), myocarditis (3–15 %), arrhythmias, pericarditis, vasculitis, and valvular heart disease (1) (Supplement: Fig. 3). Likewise, myocardial inflammation and fibrosis can cause cardiomyopathy and arrhythmias in SSc with cardiovascular disease accounting for 15 % of SSc deaths (1) (Supplement: Fig. 4). Among asymptomatic SLE patients with normal left ventricular systolic function by transthoracic echocardiography (TTE), CMR identified various pathologies, including elevated T1 and ECV, myocardial and pericardial LGE, and abnormal longitudinal strain [18]. Among SLE patients with atypical cardiac symptoms and a normal TTE, 27.5 % had abnormal CMR findings, including recent or prior myocarditis (18–22 %), prior myocardial infarction (40.9 %), and diffuse subendocardial fibrosis suggestive of vasculitis (18 %) [19]. Similarly, up to half of SSc patients without overt CV disease and normal ejection fraction by TTE had identifiable LGE as well as higher T1 and ECV on CMR compared to controls [16]. Given the degree of abnormal findings in asymptomatic patients, the European Society of Cardiovascular Imaging (ESC) suggests obtaining a baseline CMR in all SLE and SSc patients at the time of diagnosis (Supplement: Table 1) [1].
Identifying cardiac involvement in AAV is important as this typically portends a poor prognosis [2]. The presence of cardiomyopathy in patients with eosinophilic granulomatosis with polyangiitis (EGPA) is associated with a four-fold increase in mortality [15], [20], [21] CMR studies in EGPA patients without known cardiovascular disease have shown elevated global T1, T2, and ECV values independent of the presence of LGE [15], [21].
RA patients tend to have higher T1, T2, and ECV values than controls and a modest prevalence of LGE (18 %) [22], [23]. In AS, up to 21 % of patients have LGE in the mid to subepicardial regions of the basal inferior and inferolateral LV walls, a pattern typically seen in inflammatory cardiomyopathies [24]. Similarly, IIM patients tend to have LGE in the inferior and lateral distributions and demonstrate elevated T1, T2, and ECV compared to controls [25], [26], [27], [28], [29].
Patients with SjD without known cardiovascular disease have lower longitudinal and circumferential strain measurements suggesting subclinical manifestations of cardiovascular disease in asymptomatic SjD patients [30]. Patients with PsO with overt cardiovascular symptoms (including cardiomyopathy and ventricular arrhythmias), tend to have higher T2 values specifically in the septal wall [31].
In summary, comprehensive CMR with parametric mapping may detect subclinical cardiac inflammation, fibrosis, and dysfunction in many patients with AIRD, even when conventional imaging is unremarkable (Supplement: Fig. 5).
2.2. Do the CMR findings correlate to disease activity and change with therapy?
Hinojar et al. studied SLE patients with suspected myocarditis, finding significantly higher parametric mapping values compared to controls (71 % of SLE patients had T1 values ≥ 5 SD above the mean) [32]. Those with biomarker evidence of myocardial injury (cardiac troponin) unsurprisingly had higher T1 and T2 values, and 71 % of SLE patients met Lake Louise’s criteria for the diagnosis of acute myocarditis. Fourteen patients received intensification of immunosuppression by Systemic Lupus Erythematosus Disease Activity Index (SLEDAI) scores, resulting in a greater reduction in native T1 and T2 values; this suggests that T1 and T2 may have a role in monitoring treatment response [32].
In SSc patients without overt cardiovascular symptoms and preserved ejection fraction by TTE, T1, and ECV values correlated with overall disease activity as assessed by the modified Rodnan skin score (mRSS) and Valentini disease activity index (VDAI scoring systems) [16].
In patients with RA, T1 and ECV values have modestly correlated with disease activity [33]. CMR has also demonstrated improvement in cardiac parameters after changing immunosuppression from DMARDs to tocilizumab [34], [35], [36]. Modification of therapy resulted in improvement of disease activity as evaluated by the ESR and Simple Disease Activity Index (SDAI), which correlated with improvement of LV strain measurements by CMR at 52 weeks [34], [35], [36].
Response to therapy has additionally been shown among patients with EGPA and cardiomyopathy, RA, AS, and PsA patients without known CV disease receiving anti-TNF therapy, and IIM patients without known CV disease [20], [37], [38]. Patients with EGPA and cardiomyopathy can experience resolution of LGE on CMR after cyclophosphamide and azathioprine, portending improved CV outcomes compared to patients without LGE resolution [20]. Ntusi et al. showed improvement in T1, T2, ECV, and strain values after the initiation of anti-TNF therapy in a cohort of RA, AS, or PsA patients [37]. IIM patients without known CV disease have also demonstrated improved T1, T2, and ECV values after the initiation of therapy [38].
The evidence suggests that CMR findings of cardiac inflammation and dysfunction may correspond to cardiac and systemic involvement in AIRD, and that CMR may be useful in monitoring the response to therapeutic interventions.
2.3. How do these CMR findings translate into outcomes?
There is increasing evidence for the role of CMR in prognostication and risk stratification of patients based on elevations in T1, ECV, and LGE.
The presence of LGE on CMR predicts adverse outcomes across a wide range of cardiac pathology [39]. In a ten year follow up study evaluating SLE patients, those with LGE at baseline had significantly higher rates of CAD, heart failure, pericarditis, myocarditis, and endocarditis [40]. In the French Vasculitis cohort, the presence of LGE predicted the development of cardiomyopathy, and the persistence of LGE despite therapy predicted more adverse cardiovascular events [20]. Elevated ECV is associated with adverse cardiac events among SSc patients without overt cardiac pathology [41], and LGE predicted ventricular arrhythmias in a multicenter trial using CMR for patients with SSc [42]. Table S2 summarizes key CMR studies in AIRD (Supplement: Table 2).
2.4. Stress CMR in AIRD
Many studies have shown that patients with AIRD may manifest coronary disease in the form of microvascular dysfunction rather than obstructive epicardial disease [1], [2]. Although many imaging and invasive modalities can provide information on microvascular dysfunction, stress CMR provides the advantage of evaluating epicardial ischemia (qualitative perfusion abnormalities) and microvascular coronary disease (with quantitative measures, such as CFR). Microvascular dysfunction imparts a poor prognosis across a range of pathology and therefore early detection may be crucial to improve patient outcomes [11].
Microvascular dysfunction, rather than epicardial coronary disease, is the more common manifestation of coronary disease in the SLE population [43]. The range of CFR in SLE has ranged from 1.8 to 3.4 with normal CFR among healthy adults typically being > 2.2 [44]. Symptomatic SLE patients tend to have more severe reductions in CFR when compared to symptomatic matched controls without SLE (1.91 ± 0.5 versus 2.4 ± 0.7, p < 0.0001) [44].
Reduction in CFR occurs in the majority (50–60 %) of patients with SSc even in the absence of symptoms and may serve as the basis for initiation of therapy with calcium channel blockers [45]. Montisci et al. and others have shown that patients with diffuse systemic sclerosis were more likely to have CFR < 2 and may indicate a subgroup at higher risk for cardiac events [46].
Impaired CFR may be detected in early RA patients (disease duration < 12 months) (2.5 ± 0.5 vs 3.5 ± 0.8; P < 0.01), and in those with longstanding, well-controlled RA when compared to controls (2.4 ± 0.5 vs. 2.7 ± 0.4, P = 0.002) [3], [43]. Up to 30 % of RA patients without CV disease have reduced CFR [47]. The CFR may correlate with disease activity as higher IL-6 levels have been associated with impaired CFR, and modification of therapy with anti-TNF as well as the IL-1 inhibitor anakinra is associated with an improvement in CFR [47], [48].
In Weber et al.’s study of patients with SjD, AS, and other AIRD, those with impaired CFR had increased mortality, and those with the lowest value of CFR (<1.65) had the highest rate of all-cause mortality (Hazard ratio 2.4 (1.05–––5.044), p = 0.038) and MACE (HR 3.6 (1.7–––7.6), p = 0.001) [44]. Importantly, this was independent of baseline ischemic heart disease or LV dysfunction as patients with obstructive CAD or LV dysfunction were excluded.
Stress CMR has been incorporated into the 2021 chest pain guidelines as a valid method of evaluating microvascular dysfunction, alongside invasive testing and PET imaging [49]. Given the promising findings noted above, future prospective studies evaluating the role of CFR via stress CMR in AIRD are necessary.
3. CMR in AIRD: Perspective and Suggestions
At our institution, a CMR is typically considered in the context of symptoms or subclinical findings in patients with AIRD (Supplement Fig. 6). The symptoms that may warrant workup with a stress CMR are cardiac chest pain, new heart failure symptoms, or arrhythmias. Per the 2021 AHA/ACC chest pain guidelines, a stress CMR can be considered as the first line diagnostic workup in patients presenting with stable chest pain with intermediate risk and no known coronary artery disease, chest pain with known non-obstructive CAD, suspected aortic syndrome, orsuspected myopericarditis [49]. In addition, stress CMR can be valuable in patients with persistent chest pain despite negative ischemic workup (negative TTE, SPECT or invasive coronary angiography) in order to evaluate for etiologies of ischemia with non-obstructive coronary arteries (INOCA)[49]. To evaluate for coronary microvascular dysfunction, we calculate the coronary flow reserve by assessing flow in the coronary sinus at rest and during vasodilator infusion (regadenoson) and consider an abnormal CFR when < 2 and with severe abnormalities defined by CFR < 1.5[50].
The evaluation of myopericarditis in AIRD is guided by parametric mapping though the clinical significance of borderline abnormalities in T1, T2, or ECV is unclear. Normative values for the myocardial T1 and T2 should be established on healthy patients on each cardiac magnet for ideal accuracy [51]. In our experience, elevated T1/T2 values up to 3 SD above the mean require interpretation in the clinical context, whereas values exceeding 3–5 SD above the mean likely represent pathology [51].
For subclinical findings (no cardiac symptoms) without other evidence of other cardiac dysfunction (abnormal troponin, BNP, EKG abnormalities), we recommend a baseline stress CMR for SLE and SSc patients, which is consistent with the ESC guidelines [1]. However, we also have a low threshold for a baseline CMR in patients with AAV due to the poor prognosis associated with cardiac involvement in AAV [15], [20], [21]. Otherwise, we reserve a CMR in patients with subclinical findings in patients with other cardiac abnormalities (elevated troponin levels, elevated BNP levels, EKG abnormalities such as RBBB, LBBB or AV block, mild to moderate LV dysfunction on TTE, new pericardial effusion or pericardial thickening or calcifications as noted by other imaging modalities, and aortopathies).
4. Future directions
CV manifestations of AIRD have been underdiagnosed and undertreated [2]. This may be because cardiac involvement in AIRD is usually silent and once it becomes clinically apparent, patients may have a poor prognosis. In addition, the relapsing and remitting nature of AIRD and the lack of availability of reliable diagnostic tools makes the evaluation of cardiac involvement in AIRD challenging. Larger, multicenter studies are necessary to study the potential role of parametric mapping and CFR in the evaluation of cardiac manifestations of AIRD [2], [3]. A National Institutes of Health (NIH) trial evaluating all patients with clinically suspected myocarditis (either idiopathic or in the context of AIRD) is underway (NCT04673409). This study will compare the diagnostic accuracy of novel and conventional MRI sequences and may provide insight into management of patients with AIRD.
The European Association of Cardiovascular Imaging suggests obtaining a baseline CMR in SSc and SLE patients at the time of diagnosis. In other AIRD, the EACI recommends using cardiovascular symptoms, the presence of abnormalities on physical exam, blood testing, echocardiography and/or EKG/24-hour Holter monitoring to determine if a patient would benefit from CMR [1] (Supplement: Table S1).
Table S3 summarizes key questions regarding the role of CMR for patients with AIRD (Supplement: Table 3).
5. Limitations
Several limitations prevent the widespread application of novel CMR techniques across the AIRD population [12]. The primary impediment is the limitation of access to technical expertise to perform and interpret quality CMR. CMR is not ubiquitous; it is primarily performed at tertiary care centers. The requirement for breath-holding, examination time, cost of CMR, the potential risk of gadolinium administration in the setting of renal dysfunction, and the presence of MRI-incompatible devices (such as ICD’s, pacemakers) are frequently cited limitations [2]. However, the 2021 consensus document by the American College of Radiology and the National Kidney Foundation has argued for the relaxation of the eGFR cut-off of 30 mL/min based on very low rates of nephrogenic systemic fibrosis with newer gadolinium agents [52]. In addition, opportunities exist for serial monitoring of non-contrast myocardial T1 and T2 values over time to chart disease activity and response to therapy that would permit longitudinal imaging surveillance, investigation, and an approach to reduce exam time, cost, and exposure to contrast agents. Finally, the “normal values” for parametric mapping are scanner-specific, and therefore need to be established for each scanner. Therefore, following a patient over time with parametric imaging requires that the patient be scanned with the same equipment and identical sequences/parameters.
6. Conclusion
In patients with AIRD, cardiac disease is often underdiagnosed and undertreated. Cardiac involvement in AIRD can be subclinical, and traditional non-invasive modalities have not been reliable tools in the evaluation of the myriad cardiac manifestations of AIRD, including myocardial inflammation and coronary microvascular dysfunction. Comprehensive CMR with parametric mapping may be a valuable tool in the management of patients with AIRD.
7. Ethics approval and consent to participate
The study has been performed in accordance with the Declaration of Helsinki.
8. Consent for publication
Not Applicable.
9. Availability of data and materials
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Author contributions
DC, SH, AG and BV designed the study and drafted the manuscript.
DC, JD, DL, DD, AP, and JW acquired data. KGD supported standardized CMR measurements and analysis. DC and JS supported statistical analysis. DC, SH, and JS substantially contributed to the manuscript and revised it critically for important intellectual content.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CRediT authorship contribution statement
Bibin Varghese: Writing – review & editing, Writing – original draft, Project administration, Methodology, Conceptualization. Andrew Gustafson: Writing – review & editing, Writing – original draft, Conceptualization. Erin Chew: Writing – review & editing, Conceptualization. Christopher Chew: Writing – review & editing. Tracy Frech: Writing – review & editing. Majd A. El-Harasis: Writing – review & editing. Anupam Kumar: Writing – review & editing. Benjamin Shoemaker: Writing – review & editing. Jonathan Chrispin: Writing – review & editing. Monica Mukherjee: Writing – review & editing, Conceptualization. Jeffrey M. Dendy: Writing – review & editing, Visualization. Sean G. Hughes: Writing – review & editing, Visualization, Supervision, Conceptualization. Daniel E. Clark: Writing – review & editing, Writing – original draft, Visualization, Supervision, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
None
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcha.2024.101381.
Contributor Information
Sean G. Hughes, Email: sean.g.hughes@vumc.org.
Daniel E. Clark, Email: danclark@stanford.edu.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Mavrogeni S., Pepe A., Nijveldt R., Ntusi N., Sierra-Galan L.M., Bratis K., et al. Cardiovascular magnetic resonance in autoimmune rheumatic diseases: a clinical consensus document by the european Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2022;23(9):e308–e322. doi: 10.1093/ehjci/jeac134. [DOI] [PubMed] [Google Scholar]
- 2.Mavrogeni S.I., Kitas G.D., Dimitroulas T., Sfikakis P.P., Seo P., Gabriel S., et al. Cardiovascular magnetic resonance in rheumatology: current status and recommendations for use. International Journal of Cardiology. 2016;217:135–148. doi: 10.1016/j.ijcard.2016.04.158. [DOI] [PubMed] [Google Scholar]
- 3.Mavrogeni S.I., Sfikakis P.P., Koutsogeorgopoulou L., Markousis-Mavrogenis G., Dimitroulas T., Kolovou G., et al. Cardiac tissue characterization and imaging in autoimmune rheumatic diseases. JACC: Cardiovascular Imaging. 2017;10(11):1387–1396. doi: 10.1016/j.jcmg.2017.08.017. [DOI] [PubMed] [Google Scholar]
- 4.Symmons D.P.M., Gabriel S.E. Epidemiology of CVD in rheumatic disease, with a focus on RA and SLE. Nat Rev Rheumatol. 2011;7(7):399–408. doi: 10.1038/nrrheum.2011.75. [DOI] [PubMed] [Google Scholar]
- 5.Bournia V.K., Fragoulis G.E., Mitrou P., Mathioudakis K., Tsolakidis A., Konstantonis G., et al. All-cause mortality in systemic rheumatic diseases under treatment compared with the general population, 2015–2019. RMD Open. 2021;7(3):e001694. doi: 10.1136/rmdopen-2021-001694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kerola A.M., Rollefstad S., Semb A.G. Atherosclerotic cardiovascular disease in rheumatoid arthritis: impact of inflammation and antirheumatic treatment. Eur Cardiol. 2021;16:e18. doi: 10.15420/ecr.2020.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lagan J., Schmitt M., Miller C.A. Clinical applications of multi-parametric CMR in myocarditis and systemic inflammatory diseases. Int J Cardiovasc Imaging. 2018;34(1):35–54. doi: 10.1007/s10554-017-1063-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caforio A.L.P., Pankuweit S., Arbustini E., Basso C., Gimeno-Blanes J., Felix S.B., et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the european Society of Cardiology Working Group on myocardial and pericardial diseases. European Heart Journal. 2013;34(33):2636–2648. doi: 10.1093/eurheartj/eht210. [DOI] [PubMed] [Google Scholar]
- 9.Leone O., Veinot J.P., Angelini A., Baandrup U.T., Basso C., Berry G., et al. 2011 consensus statement on endomyocardial biopsy from the Association for European Cardiovascular Pathology and the Society for Cardiovascular Pathology. Cardiovascular Pathology. 2012;21(4):245–274. doi: 10.1016/j.carpath.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 10.Baughman K.L. Diagnosis of myocarditis: death of Dallas criteria. Circulation. 2006;113(4):593–595. doi: 10.1161/CIRCULATIONAHA.105.589663. [DOI] [PubMed] [Google Scholar]
- 11.Dewey M., Siebes M., Kachelrieß M., Kofoed K.F., Maurovich-Horvat P., Nikolaou K., et al. Clinical quantitative cardiac imaging for the assessment of myocardial ischaemia. Nat Rev Cardiol. 2020;17(7):427–450. doi: 10.1038/s41569-020-0341-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakou E., Patel R.K., Fontana M., Bucciarelli-Ducci C. Cardiovascular magnetic resonance parametric mapping techniques: clinical applications and limitations. Curr Cardiol Rep. 2021;23(12):185. doi: 10.1007/s11886-021-01607-y. [DOI] [PubMed] [Google Scholar]
- 13.Ferreira V.M., Piechnik S.K. CMR parametric mapping as a tool for myocardial tissue characterization. Korean Circ J. 2020;50(8):658–676. doi: 10.4070/kcj.2020.0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wong T.C., Piehler K.M., Kang I.A., Kadakkal A., Kellman P., Schwartzman D.S., et al. Myocardial extracellular volume fraction quantified by cardiovascular magnetic resonance is increased in diabetes and associated with mortality and incident heart failure admission. Eur Heart J. 2014;35(10):657–664. doi: 10.1093/eurheartj/eht193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greulich S., Mayr A., Kitterer D., Latus J., Henes J., Steubing H., et al. T1 and T2 mapping for evaluation of myocardial involvement in patients with ANCA-associated vasculitides. J Cardiovasc Magn Reson. 2017;19(1):6. doi: 10.1186/s12968-016-0315-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ntusi N.A., Piechnik S.K., Francis J.M., Ferreira V.M., Rai A.B., Matthews P.M., et al. Subclinical myocardial inflammation and diffuse fibrosis are common in systemic sclerosis – a clinical study using myocardial T1-mapping and extracellular volume quantification. J Cardiovasc Magn Reson. 2014;16(1):21. doi: 10.1186/1532-429X-16-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kelshiker MA, Seligman H, Howard JP, Rahman H, Foley M, Nowbar AN, et al. Coronary flow reserve and cardiovascular outcomes: a systematic review and meta-analysis. Eur Heart J. 2021 Nov 24;ehab775. [DOI] [PMC free article] [PubMed]
- 18.Puntmann V.O., D’Cruz D., Smith Z., Pastor A., Choong P., Voigt T., et al. Native myocardial T1 mapping by cardiovascular magnetic resonance imaging in subclinical cardiomyopathy in patients with systemic lupus erythematosus. circ. Cardiovascular Imaging. 2013;6(2):295–301. doi: 10.1161/CIRCIMAGING.112.000151. [DOI] [PubMed] [Google Scholar]
- 19.Mavrogeni S., Koutsogeorgopoulou L., Markousis-Mavrogenis G., Bounas A., Tektonidou M., Lliossis S.N.C., et al. Cardiovascular magnetic resonance detects silent heart disease missed by echocardiography in systemic lupus erythematosus. Lupus. 2018;27(4):564–571. doi: 10.1177/0961203317731533. [DOI] [PubMed] [Google Scholar]
- 20.Dunogué B., Terrier B., Cohen P., Marmursztejn J., Legmann P., Mouthon L., et al. Impact of cardiac magnetic resonance imaging on eosinophilic granulomatosis with polyangiitis outcomes: a long-term retrospective study on 42 patients. Autoimmunity Reviews. 2015;14(9):774–780. doi: 10.1016/j.autrev.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 21.Giollo A., Dumitru R.B., Swoboda P.P., Plein S., Greenwood J.P., Buch M.H., et al. Cardiac magnetic resonance imaging for the detection of myocardial involvement in granulomatosis with polyangiitis. Int J Cardiovasc Imaging. 2021;37(3):1053–1062. doi: 10.1007/s10554-020-02066-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greulich S., Mayr A., Kitterer D., Latus J., Henes J., Vecchio F., et al. Advanced myocardial tissue characterisation by a multi-component CMR protocol in patients with rheumatoid arthritis. Eur Radiol. 2017;27(11):4639–4649. doi: 10.1007/s00330-017-4838-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greulich S., Kitterer D., Kurmann R., Henes J., Latus J., Gloekler S., et al. Cardiac involvement in patients with rheumatic disorders: data of the RHEU-m(a)r study. Int. J. Cardiol. 2016;224:37–49. doi: 10.1016/j.ijcard.2016.08.298. [DOI] [PubMed] [Google Scholar]
- 24.Biesbroek P.S., Heslinga S.C., Konings T.C., van der Horst-Bruinsma I.E., Hofman M.B.M., van de Ven P.M., et al. Insights into cardiac involvement in ankylosing spondylitis from cardiovascular magnetic resonance. Heart. 2017;103(10):745–752. doi: 10.1136/heartjnl-2016-310667. [DOI] [PubMed] [Google Scholar]
- 25.Rosenbohm A., Buckert D., Gerischer N., Walcher T., Kassubek J., Rottbauer W., et al. Early diagnosis of cardiac involvement in idiopathic inflammatory myopathy by cardiac magnetic resonance tomography. J Neurol. 2015;262(4):949–956. doi: 10.1007/s00415-014-7623-1. [DOI] [PubMed] [Google Scholar]
- 26.Kersten J., Güleroglu A.M., Rosenbohm A., Buckert D., Ludolph A.C., Hackenbroch C., et al. Myocardial involvement and deformation abnormalities in idiopathic inflammatory myopathy assessed by CMR feature tracking. Int J Cardiovasc Imaging. 2021;37(2):597–603. doi: 10.1007/s10554-020-02020-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Y., Wang Q., Cao J., Li X., Lin L., Chen W., et al. Cardiovascular magnetic resonance mapping and strain assessment for the diagnosis of cardiac involvement in idiopathic inflammatory myopathy patients with preserved left ventricular ejection fraction. J Thorac Imaging. 2021;36(4):254–261. doi: 10.1097/RTI.0000000000000578. [DOI] [PubMed] [Google Scholar]
- 28.Zhao P., Huang L., Ran L., Tang D., Zhou X., Xia L. CMR T1 mapping and strain analysis in idiopathic inflammatory myopathy: evaluation in patients with negative late gadolinium enhancement and preserved ejection fraction. Eur Radiol. 2021;31(3):1206–1215. doi: 10.1007/s00330-020-07211-y. [DOI] [PubMed] [Google Scholar]
- 29.Yu L., Sun J., Sun J., Li J., Dong Y., Zhou X., et al. Early detection of myocardial involvement by T1 mapping of cardiac MRI in idiopathic inflammatory myopathy. J Magn Reson Imaging. 2018;48(2):415–422. doi: 10.1002/jmri.25945. [DOI] [PubMed] [Google Scholar]
- 30.Yokoe I., Kobayashi H., Nishiwaki A., Nagasawa Y., Kitamura N., Haraoka M., et al. Asymptomatic myocardial dysfunction was revealed by feature tracking cardiac magnetic resonance imaging in patients with primary sjögren’s syndrome. Int. J. Rheumatic Dis. 2021;24(12):1482–1490. doi: 10.1111/1756-185X.14227. [DOI] [PubMed] [Google Scholar]
- 31.Goldenberg M., Reynolds M., Smart S., Kaffenberger J., Raman S.V., Kaffenberger B.H. A retrospective study of myocardial abnormalities detected on cardiac magnetic resonance imaging among patients with psoriasis compared to inflammatory skin disease controls. J Eur Acad Dermatol Venereol. 2020;34(10):e606–e608. doi: 10.1111/jdv.16486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hinojar R., Foote L., Sangle S., Marber M., Mayr M., Carr-White G., et al. Native T1 and T2 mapping by CMR in lupus myocarditis: disease recognition and response to treatment. International Journal of Cardiology. 2016;222:717–726. doi: 10.1016/j.ijcard.2016.07.182. [DOI] [PubMed] [Google Scholar]
- 33.Ntusi N.A.B., Piechnik S.K., Francis J.M., Ferreira V.M., Matthews P.M., Robson M.D., et al. Diffuse myocardial fibrosis and inflammation in rheumatoid arthritis. JACC: Cardiovascular Imaging. 2015;8(5):526–536. doi: 10.1016/j.jcmg.2014.12.025. [DOI] [PubMed] [Google Scholar]
- 34.Kobayashi H., Kobayashi Y., Giles J.T., Yoneyama K., Nakajima Y., Takei M. Tocilizumab treatment increases left ventricular ejection fraction and decreases left ventricular mass index in patients with rheumatoid arthritis without cardiac symptoms: assessed using 3.0 tesla cardiac magnetic resonance imaging. J. Rheumatology. 2014;41(10):1916–1921. doi: 10.3899/jrheum.131540. [DOI] [PubMed] [Google Scholar]
- 35.Kobayashi Y., Kobayashi H., Giles J.T., Hirano M., Nakajima Y., Takei M. Association of tocilizumab treatment with changes in measures of regional left ventricular function in rheumatoid arthritis, as assessed by cardiac magnetic resonance imaging. Int. J. Rheumatic Dis. 2016;19(11):1169–1174. doi: 10.1111/1756-185X.12632. [DOI] [PubMed] [Google Scholar]
- 36.Kobayashi H., Kobayashi Y., Yokoe I., Akashi Y., Takei M., Giles J.T. Magnetic resonance imaging-detected myocardial inflammation and fibrosis in rheumatoid arthritis: associations with disease characteristics and N-terminal pro-brain natriuretic peptide levels. Arthritis Care Res. 2017;69(9):1304–1311. doi: 10.1002/acr.23138. [DOI] [PubMed] [Google Scholar]
- 37.Ntusi N.A.B., Francis J.M., Sever E., Liu A., Piechnik S.K., Ferreira V.M., et al. Anti-TNF modulation reduces myocardial inflammation and improves cardiovascular function in systemic rheumatic diseases. Int J Cardiol. 2018 Nov;1(270):253–259. doi: 10.1016/j.ijcard.2018.06.099. [DOI] [PubMed] [Google Scholar]
- 38.Xu Y., Sun J., Wan K., Yu L., Wang J., Li W., et al. Multiparametric cardiovascular magnetic resonance characteristics and dynamic changes in myocardial and skeletal muscles in idiopathic inflammatory cardiomyopathy. J. Cardiovasc Magn. Reson. 2020;22(1):22. doi: 10.1186/s12968-020-00616-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kuruvilla S., Adenaw N., Katwal A.B., Lipinski M.J., Kramer C.M., Salerno M. Late gadolinium enhancement on cardiac magnetic resonance predicts adverse cardiovascular outcomes in nonischemic cardiomyopathy: a systematic review and meta-analysis. Circ Cardiovasc Imaging. 2014 Mar;7(2):250–258. doi: 10.1161/CIRCIMAGING.113.001144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Verma* R, Balaraju* V, Seneviratne M, Garsia R, Adelstein S, Puranik R, et al. A decade follow-up: On the prevalence, distribution and clinical correlates of myocardial fibrosis, as detected by cardiac magnetic resonance, in systemic lupus erythematosus. Lupus. 2020 Dec 1;29(14):1981–3. [DOI] [PubMed]
- 41.Dumitru R.B., Bissell L.A., Erhayiem B., Kidambi A., Dumitru A.M.H., Fent G., et al. Cardiovascular outcomes in systemic sclerosis with abnormal cardiovascular MRI and serum cardiac biomarkers. RMD Open. 2021;7(3):e001689. doi: 10.1136/rmdopen-2021-001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mavrogeni S., Gargani L., Pepe A., Monti L., Markousis-Mavrogenis G., De Santis M., et al. Cardiac magnetic resonance predicts ventricular arrhythmias in scleroderma: the scleroderma arrhythmia clinical utility study (SAnCtUS) Rheumatology. 2020;59(8):1938–1948. doi: 10.1093/rheumatology/kez494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Recio-Mayoral A., Mason J.C., Kaski J.C., Rubens M.B., Harari O.A., Camici P.G. Chronic inflammation and coronary microvascular dysfunction in patients without risk factors for coronary artery disease. European Heart Journal. 2009;30(15):1837–1843. doi: 10.1093/eurheartj/ehp205. [DOI] [PubMed] [Google Scholar]
- 44.Weber B.N., Stevens E., Perez-Chada L.M., Brown J.M., Divakaran S., Bay C., et al. Impaired coronary vasodilator reserve and adverse prognosis in patients with systemic inflammatory disorders. JACC Cardiovasc Imaging. 2021;14(11):2212–2220. doi: 10.1016/j.jcmg.2020.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zanatta E., Colombo C., D’Amico G., d’Humières T., Dal Lin C., Tona F. Inflammation and coronary microvascular dysfunction in autoimmune rheumatic diseases. Int J Mol Sci. 2019;20(22):E5563. doi: 10.3390/ijms20225563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Montisci R., Vacca A., Garau P., Colonna P., Ruscazio M., Passiu G., et al. Detection of early impairment of coronary flow reserve in patients with systemic sclerosis. Annals of the Rheumatic Diseases. 2003;62(9):890–893. doi: 10.1136/ard.62.9.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Amigues I., Russo C., Giles J.T., Tugcu A., Weinberg R., Bokhari S., et al. Myocardial microvascular dysfunction in rheumatoid ArthritisQuantitation by 13N-ammonia positron emission tomography/computed tomography. Circ Cardiovasc Imaging. 2019;12(1):e007495. doi: 10.1161/CIRCIMAGING.117.007495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ikonomidis I., Tzortzis S., Andreadou I., Paraskevaidis I., Katseli C., Katsimbri P., et al. Increased benefit of Interleukin-1 inhibition on vascular function, myocardial deformation, and twisting in patients with coronary artery disease and coexisting rheumatoid arthritis. circulation. Cardiovascular Imaging. 2014 Jul 1;7(4):619–628. doi: 10.1161/CIRCIMAGING.113.001193. [DOI] [PubMed] [Google Scholar]
- 49.Gulati M., Levy P.D., Mukherjee D., Amsterdam E., Bhatt D.L., Birtcher K.K., et al. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR guideline for the evaluation and diagnosis of CHEST pain: a report of the american college of cardiology/american heart association joint committee on clinical practice guidelines. Circulation. 2021;144(22):e368–e454. doi: 10.1161/CIR.0000000000001029. [DOI] [PubMed] [Google Scholar]
- 50.Knuuti J., Wijns W., Saraste A., Capodanno D., Barbato E., Funck-Brentano C., et al. 2019 ESC guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41(3):407–477. doi: 10.1093/eurheartj/ehz425. [DOI] [PubMed] [Google Scholar]
- 51.Messroghli D.R., Moon J.C., Ferreira V.M., Grosse-Wortmann L., He T., Kellman P., et al. Clinical recommendations for cardiovascular magnetic resonance mapping of T1, T2, T2* and extracellular volume: a consensus statement by the Society for cardiovascular magnetic resonance (SCMR) endorsed by the european association for cardiovascular imaging (EACVI) J Cardiovasc Magn Reson. 2017;19(1):75. doi: 10.1186/s12968-017-0389-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weinreb J.C., Rodby R.A., Yee J., Wang C.L., Fine D., McDonald R.J., et al. Use of intravenous gadolinium-based contrast media in patients with kidney disease: consensus statements from the american college of radiology and the national kidney foundation. Radiology. 2021;298(1):28–35. doi: 10.1148/radiol.2020202903. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.