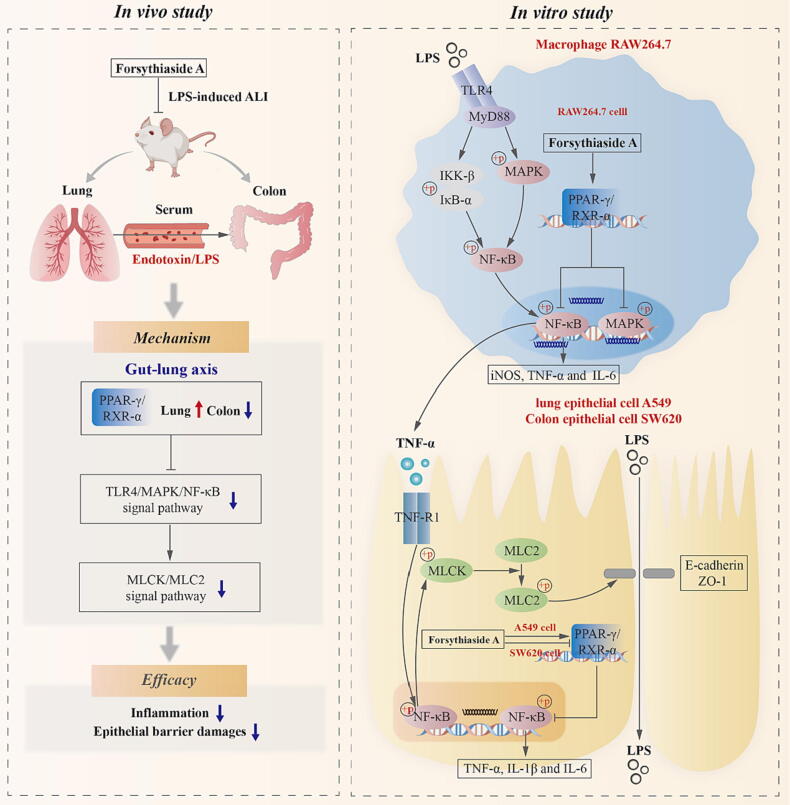
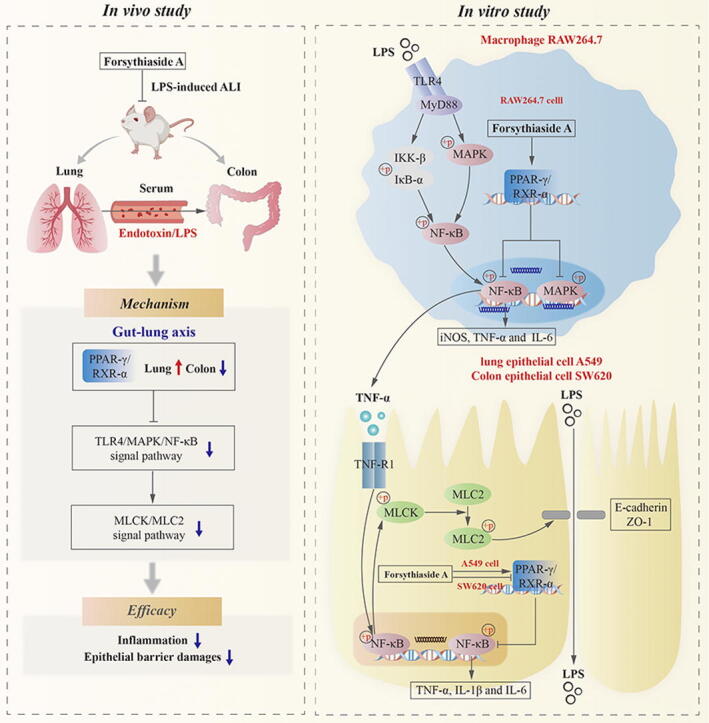
Graphical abstract
Keywords: Forsythiaside A, Acute lung injury, Gut-lung axis, LPS and TNF-α, TLR4/MAPK/NF-κB and NF-κB/MLCK/MLC2, PPAR-γ/RXR-α
Highlights
-
•
Forsythiaside A regulated PPAR-γ/RXR-α complex and inhibited TLR4/MAPK/NF-κB and MLCK/MLC2 signal pathways, thus inhibiting inflammation and epithelial barrier damages in the lungs and colons of LPS-induced ALI mice.
-
•
Forsythiaside A promoted the expression of PPAR-γ/RXR-α complex in lung tissues, whereas suppressed the overexpression of PPAR-γ/RXR-α complex in colon tissues.
-
•
Forsythiaside A regulated the activity of PPAR-γ/RXR-α complex to inhibit the activation of TLR4/MAPK/NF-κB and NF-κB/MLCK/MLC2 signal pathways induced by LPS and TNF-α, thereby suppressing inflammation and epithelial barrier damages in macrophages, as well as lung and colon epithelial cells.
-
•
Forsythiaside A had the cell-specific regulatory effects on the PPAR-γ/RXR-α complexes. It promoted the interactions between PPAR-γ/RXR-α proteins in LPS-induced macrophages RAW264.7 and TNF-α-induced lung epithelial cells A549, while inhibiting the interactions in TNF-α-induced colon epithelial cells SW620.
Abstract
Introduction
Acute lung injury (ALI) is a lung disease characterized by inflammation and still requires further drug development. Forsythiaside A as the active compound of Forsythiae Fructus has the therapeutic potential for ALI.
Objective
To investigate the mechanism of forsythiaside A in treating ALI through PPAR-γ and its conjugate RXR-α based on gut-lung axis.
Methods
This study constructed in vitro and in vivo injury models using LPS and TNF-α. Forsythiaside A was used for the drug treatment, and RXR-α inhibitor UVI3003 was used to interfere with PPAR-γ/RXR-α complexes in the cells. HE staining was used for histopathological examination. Serum endotoxin contents were determined using limulus lysate kit. IHC staining and Western blot were conducted to assess the protein expressions. ELISA was applied to examine the content of pro-inflammatory cytokines in the cell supernatants. The protein interactions were analyzed via CO-IP.
Results
In vivo results showed that forsythiaside A regulated PPAR-γ/RXR-α and inhibited TLR4/MAPK/NF-κB and MLCK/MLC2 signal pathways, thus inhibiting inflammation and epithelial barrier damages of lung and colon in ALI mice induced by intratracheal LPS. PPAR-γ/RXR-α were promoted by forsythiaside A in lungs, whereas inhibited by forsythiaside A in colons. Additionally, in vitro results showed that forsythiaside A suppressed inflammation and epithelial barrier damages in macrophages and lung/colon epithelial cells, by manipulating PPAR-γ/RXR-α to suppress the LPS- and TNF-α-induced activation of TLR4/MAPK/NF-κB and NF-κB/MLCK/MLC2 signal pathways. Moreover, further mechanism study indicated that forsythiaside A showed a cell-specific regulatory effect on PPAR-γ/RXR-α complex. Specifically, the PPAR-γ/RXR-α protein interactions were promoted by forsythiaside A in LPS-induced macrophages RAW264.7 and TNF-α-induced lung epithelial cells A549, but inhibited by forsythiaside A in TNF-α-induced colon epithelial cells SW620.
Conclusion
In the treatment of ALI, Forsythiaside A inhibited inflammation and epithelial barrier damages of lung and colon through its regulation on PPAR-γ/RXR-α complex.
Introduction
Acute lung injury (ALI) and acute respiratory distress syndrome (ARDS), are acknowledged as a type of lung disease distinguished by diffuse inflammatory cell infiltration, haemorrhage and pulmonary oedema [1], [2]. Direct and indirect factors that can induce ALI/ARDS include inhalation, pneumonia, sepsis, trauma, pancreatitis and transfusion, among others [3]. Many researches suggest that inflammation is critical to the pathogenesis of ALI/ARDS. Specifically, large numbers of immune cells aggregate at the sites of injury, initiating a series of inflammatory signaling transduction and releasing a plethora of pro-inflammatory cytokines. Subsequently, inflammation destroys the barrier function of alveolar epithelium and vascular endothelium, which increases the permeability of alveolar-capillary membrane. This causes protein-rich edema fluid to accumulate into the lung interstitium, ultimately leading to pulmonary oedema and tissue damage [4], [5]. Currently, in addition to mechanical ventilation, pharmacological intervention remains as the primary therapeutic approach for ALI/ARDS [6]. Corticosteroids are considered as the ideal drugs for treating ALI/ARDS due to their broad anti-inflammatory activity. However, the application of corticosteroids is limited by numerous adverse reactions, such as muscular weakness, damaged physical function, allergic reaction and neurocognitive dysfunction [7]. Therefore, there is a requirement for additional support in terms of treatment approaches and medication for ALI.
In current years, the emerging theory of gut-lung axis has uncovered the intimate connection between the intestines and lungs, with a focus on mucosal immunity. The mucosal sites of the intestines and lungs constitute the important barriers of the body to directly defend against the external environment, and have a bearing on the innate and adaptive immunities [8], [9], [10]. The gut-lung axis has elucidated how the intestines and lungs can influence each other's immune responses through the systemic circulation. For example, some studies have shown that patients and animal models with inflammatory bowel disease (IBD) exhibited the manifestations of pulmonary pathology [11], [12]. Conversely, patients afflicted with different lung diseases including asthma, chronic obstructive pulmonary disease (COPD) and Corona Virus Disease 2019 (COVID-19), also suffered from the symptoms of intestinal discomfort [13], [14]. Some studies have brought insight into the pathogenic mechanism between the gut and lung. During the time of lung and intestinal injuries, some pathogens can enter the bloodstream through the epithelial barrier and be recognized by immune cells from the gut and respiratory tract, triggering a cascade of local and distant inflammatory responses [8], [15]. The epithelial barrier is an important component for sustaining the structural stability and immunity homeostasis of intestines and lungs. Epithelial cells connect with each other through a series of protein complexes such as adherent junction proteins (for instance, E-cadherin) and tight junction proteins (for instance, ZO-1 and Claudin-1), forming an important barrier that resists external infections [16], [17], thus protecting local tissues and avoiding distant inflammatory injuries [18]. Inflammation is considered an important factor that damages epithelial barriers in both lung and intestine tissues. Pro-inflammatory cytokines consisting of IFN-γ, TNF-α, IL-1β and others can increase the cell permeability by reducing junction proteins. As a consequence, the barrier function of the lungs and intestines is compromised, leading to disruption and dysfunction [19], [20], [21], [22]. After damage to the epithelial barriers, bacterial toxins such as lipopolysaccharide (LPS) can enter the systemic circulation, exacerbating immune responses in distant tissues. For example, LPS derived from gut microbiota can be transported to the lungs where it activates the immune responses [23]. Lung infusion of LPS was able to disturb the stability of the gut epithelium and microbiome, increasing the infective risk from exogenous factors [24], [25]. These studies suggest that the epithelial barriers promote the stabilization of immunity function in the gut-lung axis but could be impaired by inflammation, further damaging the distant tissues. Therefore, inhibiting the development of inflammation and epithelial barrier damage may be an effective approach for treating lung diseases based on the gut-lung axis.
Forsythiae Fructus, derived from the dried fruit of herbal medicine Forsythia suspensa (Thunb.) Vahl, is being applied extensively for treating lung diseases [26]. Our previous work showed that Forsythiae Fructus extracts could regulate PPAR-γ/RXR-α complex activity to prevent pulmonary and colonic tissues from the inflammation and epithelial barrier damages, thus alleviating the LPS-induced ALI of mice [27]. However, it remains unclear which active substance of Forsythiae Fructus is responsible for treating ALI via the gut-lung axis. Previous researches have demonstrated that forsythiaside A, the main chemical compound of Forsythiae Fructus, had significant efficacy for LPS-induced ALI. For example, forsythiaside A could alleviate the inflammatory reactions in the ALI mice, with its mechanism of restraining the LPS-mediated activation of TLR4/NF-κB signal pathway [28]. Additionally, forsythiaside A was proven to alleviate LPS-induced ALI in mice and inflammation in macrophages RAW264.7 by up-regulating miR-124 [29]. Therefore, forsythiaside A holds great potential as the active substance of Forsythiae Fructus for pharmacological research, allowing for further investigation into its efficacy and mechanism in treating ALI grounded on the gut-lung axis.
Therefore, building upon the research foundation of the gut-lung axis, our study employed various biological techniques to study the efficacy and mechanism of forsythiaside A in an ALI animal model, focusing on the inflammation and epithelial barrier damages from lung and colon. At the cellular level, the pharmacological mechanism of forsythiaside A and its relationship with PPAR-γ, a key nuclear transcription factor with immunomodulatory activity in the lungs and colons were also verified [30], [31], [32]. Firstly, we constructed an ALI mouse model using intratracheal administration of bacterial LPS, to study the efficacy and mechanism of forsythiaside A in attenuating the inflammation and epithelial barrier damages from lung/colon tissues. Subsequently, we constructed the in vitro models of macrophages and lung/colon epithelial cells to validate the efficacy and mechanism of forsythiaside A.
Materials and methods
Chemicals and reagents
Forsythiaside A (Purity ≥ 98%) was obtained from Must Bio-technology (Chengdu, China). Bacterial LPS (Escherichia coli, O55:B5) and a selective RXR-α antagonist UVI3003 were purchased from Sigma (St Louis, MO, USA). Dexamethasone was purchased from Yuanye Bio-Technology (Shanghai, China). A limulus lysate kit for serum endotoxin testing was purchased from BIOENDO (Xiamen, China). Dulbecco’s modified Eagle medium (DMEM) was obtained from Gibco (USA). Ham's F-12K medium and fetal bovine serum (FBS) were provided by Procell (Wuhan, China). Bovine serum albumin (BSA), methyl thiazolyl tetrazolium (MTT) and enhanced chemiluminiscence (ECL) kit were purchased from Biosharp (Anhui, China). Dimethyl sulfoxide (DMSO) was provided by MP Biomedicals (California, USA). PBS, BCA Protein assay kit and 1% penicillin/streptomycin were provided by Servicebio (Wuhan, China). PMSF and protease/phosphatase inhibitors were purchased from Solarbio (Beijing, China). Trypsin (1:250) was purchased from Biofroxx (Germany).
Animals
Male ICR mice (SPF grade, 5–6 weeks old and weighing 20 ± 2 g) were purchased from Chengdu Dossy Experimental Animal Co., Ltd. (Chengdu, China) [No. SCXK (Chuan) 2020-030]. Prior to the experiment, the animals were fed with standard diet and water for at least one week. They were also acclimatized to a 12 h light/12 h dark cycle with a stable temperature (25 ± 2 °C) and humidity (50 ± 5%).
LPS-induced ALI model and treatment
Forty-eight mice in total were distributed among six groups (with 8 mice per group): Control (CON), Model (MOD), Dexamethasone (DEX), as well as Low-, Medium- and High-doses of forsythiaside A (LFA, MFA and HFA). As per our previous report, all the mice excluding those in CON group underwent intratracheal instillation of 5 mg/kg of LPS (solved in normal saline, and the volume < 45 µL) [27]. In accordance with the relevant study, the daily doses of forsythiaside A for each mouse in LFA, MFA and HFA groups were set at 20, 40 and 80 mg/kg [33]. At specific points in time (1 h in advance of, 24 h and 48 h following LPS modeling), the mice in LFA, MFA and HFA groups were intragastrically administered with 20, 40 and 80 mg/kg of forsythiaside A (solved in 0.1% tween-80), respectively. The mice in DEX group were given with the intragastric administration of dexamethasone (the referenced dose: 5 mg/kg) [34]. Additionally, the mice in CON group were intratracheally instilled with the normal saline (equal volume) and intragastrically administered with the vehicle (0.1% tween-80). At the time that LPS modeling for 72 h, lung tissues, colon tissues and serum were then obtained for further study.
Ethics statement
All the research protocols were conducted in compliance with ethical guidelines and authorized by the Animal Experiment Ethics Committee in Chengdu University of Traditional Chinese Medicine (Approval NO. SYXK (Chuan) 2020-124).
Lung weight and lung index
Body weights of the mice were measured prior to their sacrifice. Lung weights were also measured after the lungs were removed from the mice. Lung index of each mouse was obtained based on the calculation equation: Lung index (mg/g) = lung weight/body weight.
Histopathological analysis
The right lungs and colon segments (2 cm) were immobilized in a 4% formaldehyde solution, followed by embedding in paraffin. Subsequently, the embedded specimens were sliced into sections of 4 µm thickness. Then, hematoxylin-eosin (HE) was prepared to stain the slices. The HE-staining tissue sections were finally scanned at 20 × magnification on the slide scanner NanoZoomer S60 (Hamamatsu, Japan), and the pathological images were obtained through the software NDP.view 2.7.52. Szapiel standard was used for the scoring of lung inflammation, which included the following four categories: 0-normal lung morphology, 1-tissuse inflammation damage < 20%, 2-tissuse inflammation damage 20–50%, and 3-tissuse inflammation damage > 50% [35].
Serum endotoxin assay
Referring to the manufacturer's instruction, this study measured the endotoxin/LPS levels in the serum of mice using a limulus lysate kit.
Immunohistochemistry (IHC) analysis
IHC-staining was performed to examine the expression of macrophage- and epithelial barrier-associated proteins in the lung and colon tissues. The tissue sections needed firstly to be dewaxed in xylene and rehydrated in the gradient alcohols. Subsequently, the slices were exposed to a 3% hydrogen peroxide solution and left to incubate at the room temperature for 5 min in order to suppress endogenous peroxidase activity. After sealing with 3% BSA for 30 min, the sections were left to incubate overnight with the corresponding antibodies including F4/80, E-cadherin, ZO-1 and Claudin-1. Subsequent to three washes with PBS, the sections were maintained at room temperature for 30 min during the incubation with the HRP-conjugated secondary antibody. Lastly, the sections were subjected to visualization by employing DAB as the chromogenic agent. The tissue sections were scanned at 20 × magnification on the slide scanner NanoZoomer S60 (Hamamatsu, Japan), which provided the images through the software NDP.view 2.7.52.
Cell lines and cultures
The murine macrophage cell line RAW264.7 was provided by National Collection of Authenticated Cell Cultures (Shanghai, China). The human alveolar epithelial cell line A549 and the human colonic epithelial cell line SW620 were provided by Procell (Wuhan, China). RAW264.7 and SW620 cells were cultured in DMEM medium added with 10% FBS and 1% penicillin/streptomycin, and then placed in 5% CO2 humidified air at 37 °C. A549 cells were cultured in Ham's F-12 K medium added with 10% FBS and 1% penicillin/streptomycin, and then placed in 5% CO2 humidified air at 37 °C.
Cell viability detection
The MTT method was employed to assess the cell viabilities of RAW264.7, A549, and SW620 cells. First, RAW264.7 (cell density: 5 × 104 cells/mL), A549 (cell density: 5 × 104 cells/mL) and SW620 (cell density: 1.5 × 105 cells/mL) cells were inoculated in 96-well plates and treated with forsythiaside A along with LPS or TNF-α for 24 h. After that, each well was supplemented with 20 μL solution of MTT (5 mg/mL) to be maintained at 37 °C for 4 h. Then, the supernatant was discarded and 100 μL DMSO was added into each well in the 96-well plate to dissolve the crystals. At last, the absorbance at 490 nm was measured for all the wells using the CYT5MFV microplate reader (Biotek, USA).
Cell morphological examination
To further investigate the influence of forsythiaside A on the cell morphologies of LPS-induced RAW264.7 cells and TNF-α-induced A549 and SW620 cells, we used an inverted microscope ECLIPSE Ts2 (Nikon, Japan). RAW264.7, A549 and SW620 cells in the phase of logarithmic growth were inoculated in 6-well plates at the cell densities of 5 × 104 cells/mL, 5 × 104 cells/mL and 1.5 × 105 cells/mL, respectively. RAW264.7, A549 and SW620 cells in CON groups were cultured in fresh DMEM or Ham's F-12 K medium. Cells in MOD groups were cultured in the fresh medium supplemented with LPS (1 μg/mL for RAW264.7 cells) or TNF-α (100 ng/mL for A549 cells and 50 ng/mL for SW620 cells). Cells in LFA, MFA and HFA groups were co-incubated with forsythiaside A at the Low-, Medium- and High-doses while being exposed to LPS or TNF-α. After 24 h of culture, the cellular morphologies were observed under the inverted microscope.
Enzyme-linked immunosorbent assay (ELISA)
The levels of pro-inflammatory cytokines TNF-α, IL-1β and IL-6 in the supernatants of RAW264.7 cells subjected to LPS, as well as the levels of IL-1β and IL-6 in the supernatants of A549 and SW620 cells exposed to TNF-α, were quantified using the MEIMIAN mouse or human ELISA kits (Jiangsu, China) as per the manufacturer's instructions.
Western blot (WB) analysis
Total proteins were obtained from tissues and cells using RIPA Lysis Buffer, which included 1% PMSF and 1% protease/phosphatase inhibitors. The BCA method was utilized to calculate the protein concentrations, which were subsequently equalized to a uniform level using the same buffer. Next, the protein samples were resolved on a 10% SDS-PAGE gel and transferred integrally into 0.2 μm PVDF membranes (Millipore, USA). The membranes were subjected to blocking with 5% BSA on a shaking platform at the room temperature for 2 h. Following that, the membranes were incubated with the corresponding antibodies at 4 °C overnight. Subsequent to three TBST washes, the membranes were incubated with Goat anti-rabbit IgG HRP at the room temperature for at least 1.5 h. Finally, ECL method was used to obtain the results of chemiluminescence in a Tanon 5200 Chemiluminescence Imaging system (Tanon, China). The relative protein expressions of WB bands were provided by the analysis of ImageJ software, which used GAPDH as the internal control. A detailed account of all the antibodies employed in this study can be found in Supplementary Table 1.
Co-immunoprecipitation (CO-IP) assay
A CO-IP Kit (Absin, China) was applied to detect the protein interactions between PPAR-γ and RXR-α in RAW264.7, A549 and SW620 cells exposed to LPS or TNF-α, as well as the effect of forsythiaside A on the protein interactions in these cells. Total cellular proteins were firstly obtained using IP lysis buffer, and the concentrations were calculated using BCA method. After that, the lysates were incubated with IP-graded primary antibody RXR-α on a shaking platform at 4 °C overnight, and then added with Protein A and Protein G agarose beads maintaining for 3 h. In the end, the protein samples were washed three times with the pre-cooling 1 × Wash buffer, followed by WB analysis.
The intervention of RXR-α inhibitor UVI3003
UVI3003, an RXR-α inhibitor, was used to downregulate RXR-α expression to intervene the interactions of PPAR-γ/RXR-α proteins in LPS- or TNF-α-induced RAW264.7, A549 and SW620 cells. This was done to verify whether the efficacy of forsythiaside A was related to PPAR-γ/RXR-α complex. Firstly, the appropriate concentrations of UVI3003 on different cells were first determined by assessing its effects on the protein activities of PPAR-γ and RXR-α. Subsequently, cells in HFA group were co-cultured with UVI3003 along with LPS (1 μg/mL, RAW264.7 cells) or TNF-a (100 ng/mL, A549 cells; 50 ng/mL, SW620 cells), and simultaneously treated with High-dose of forsythiaside A (HFA) for 24 h. Finally, the total cellular proteins were performed by WB analysis to examine the influences of UVI3003 intervention on the activity of PPAR-γ/P-PPAR-γ proteins, the expression of MAPK/NF-κB-related proteins, as well as inflammation/epithelial barrier indicators in the cells treated with forsythiaside A.
Statistical analysis
The Version 25 of SPSS software was employed for the data analysis. One-way analysis of variance (ANOVA) was applied to examine statistical differences among the groups. If the data failed to meet the assumptions of ANOVA analysis, Kruskal-Wallis test was further utilized for nonparametric statistical analysis. All the data were expressed as mean ± Standard Deviation (SD). The P-value of < 0.05 was recognized as statistically significant.
Results
Forsythiaside A inhibited lung inflammation in LPS-induced ALI mice
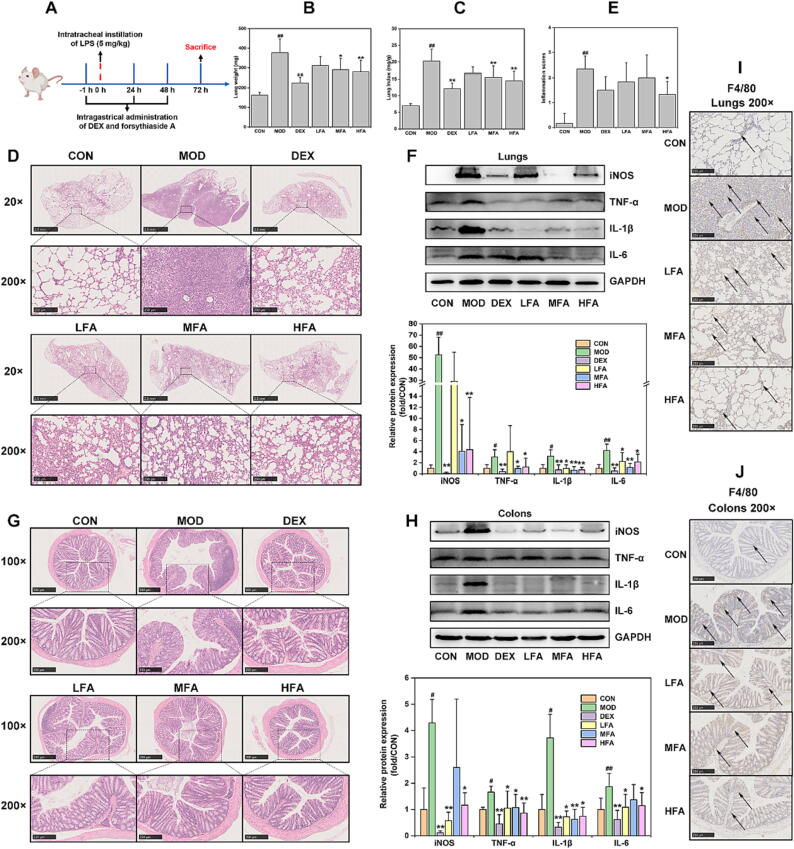
This study first constructed the LPS-induced mice model to evaluate the effectiveness of forsythiaside A on lung inflammation (Fig. 1A). Results from Fig. 1B and C indicated that LPS significantly increased lung weight and lung index in MOD group. Forsythiaside A (MFA and HFA) and DEX were able to significantly inhibit the increase in lung weight and lung index after LPS modeling. The HE-staining sections of lung tissues at 20 × and 200 × magnification revealed extensive inflammatory infiltration and severe destruction of alveolar structures in MOD group compared to that of CON group. The lung inflammation and alveolar structure damages were effectively improved by DEX treatment. In addition, forsythiaside A effectively alleviated the lung inflammation and alveolar structure damages caused by LPS, with higher doses of the drug demonstrating stronger therapeutic effects (Fig. 1D). The inflammation score based on HE-staining lung tissue sections showed that MOD group had a significantly higher score than CON group did, which could be significantly inhibited by High-dose of forsythiaside A (HFA) (Fig. 1E). Moreover, WB analysis was used in this study to further detect inflammation-related indicators in the lung tissues. The Fig. 1F results indicated that LPS significantly increased the protein expression of inflammatory indicators such as iNOS, TNF-α, IL-1β and IL-6 in the lung tissues of MOD group. However, both DEX and forsythiaside A (MFA and HFA) were effective in inhibiting the increase of these inflammatory indicators. Taken together, these results demonstrated that forsythiaside A could suppress the lung inflammatory reaction in LPS-induced ALI mice.
Fig. 1.
The effects of forsythiaside A on the inflammation of lung and colon in LPS-induced ALI mice. (A) Schematic diagram of DEX and forsythiaside A treatment in LPS-induced ALI mice; (B) Lung weight (mg, n = 8, per group); (C) Lung index (mg/g, n = 8, per group); (D) HE-staining representative images of lung tissues (20 × and 200 × magnifications); (E) Inflammation scores of lung tissues stained by HE (n = 6, per group); (F) WB bands and relative protein expressions of inflammatory indicators (iNOS, TNF-α, IL-1β and IL-6) in lung tissues (n = 6, per group); (G) HE-staining representative images of colonic tissues (100 × and 200 × magnifications); (H) WB bands and relative protein expressions of inflammatory indicators in colonic tissues (n = 6, per group); (I) IHC-staining images of macrophage-related protein F4/80 in lung tissues (200 × magnifications); (J) IHC-staining images of F4/80 in colon tissues (200 × magnifications). DEX referred to 5 mg/kg of dexamethasone, and LFA, MFA and HFA referred to 20, 40 and 80 mg/kg of forsythiaside A, respectively. Data were expressed as mean ± SD. *P < 0.05, **P < 0.01 versus MOD; #P < 0.05, ##P < 0.01 versus CON.
Forsythiaside A inhibited colon inflammation in LPS-induced ALI mice
To uncover the efficacy of forsythiaside A on colon inflammation of the ALI mice, this study was conducted on HE-staining pathological analysis and inflammation-related indicators detection in the colon tissues. The Fig. 1G results demonstrated that the colon mucosa in MOD group exhibited extensive inflammation infiltration and crypt destruction compared to CON group. In common with DEX, forsythiaside A improved the lesions and inflammation of colon tissues, with no significant effects among LFA, MFA and HFA. Besides, the results from Fig. 1H indicated that the proteins of inflammatory indicators (iNOS, TNF-α, IL-1β and IL-6) in the colon tissues significantly increased. However, DEX and forsythiaside A (especially HFA) exhibited significant suppression of the expression of these inflammatory indicators. Therefore, these results also indicated that forsythiaside A demonstrated effective anti-inflammatory properties in the colon tissues of LPS-induced ALI mice.
Forsythiaside A inhibited macrophage activation in the lung and colon tissues of LPS-induced ALI mice
Macrophages plays as the important immunity cells that can be provoked by LPS to produce multiple pro-inflammatory cytokines [36]. In this investigation, IHC-staining of the macrophage-related protein F4/80 was employed to assess the influence of forsythiaside A on the macrophage distribution in the lungs and colons of LPS-induced ALI mice. The results in Fig. 1I and J showed that macrophages were obviously activated in the lung and colon tissues from MOD group. Nevertheless, forsythiaside A could reduce the tissue distribution of macrophages in the lungs and colons, where macrophages were apparently fewer than that of MOD group. These results indicated that forsythiaside A had the ability to suppress lung and colonic inflammatory responses in LPS-induced ALI mice by inhibiting macrophages activation.
Forsythiaside A inhibited epithelial barrier damages in the lung and colon tissues of LPS-induced ALI mice
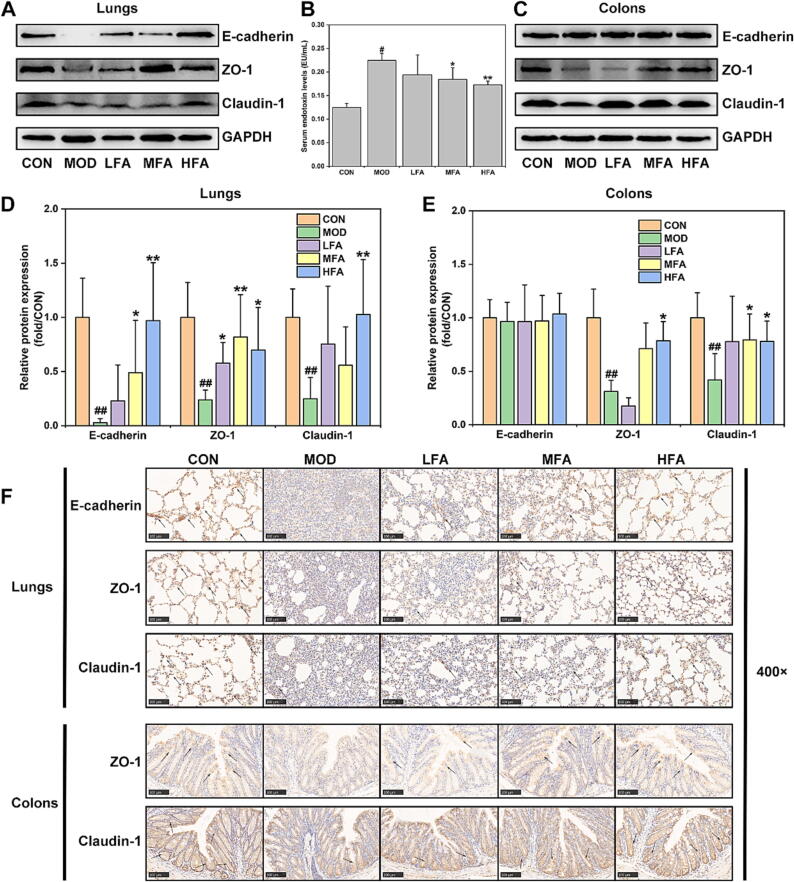
Since inflammation is an important factor in damaging epithelial barriers [19], [20], [21], [22], this study thus investigated the impacts of forsythiaside A on the lung/colon epithelial barriers after LPS-induced inflammation injury in ALI mice. As showed in Fig. 2A and D, the lung expression of epithelial barrier-related proteins (E-cadherin, ZO-1 and Claudin-1) was significantly down-regulated in MOD group rather than in CON group, but forsythiaside A could counteract this reduction. The results of Fig. 2C and E indicated that the colonic expression of ZO-1 and Claudin-1, but not E-cadherin, was significantly reduced in MOD group, while forsythiaside A could effectively reverse these effects. Particularly, high dose of forsythiaside A (HFA) could significantly inhibit the reduction of epithelial barrier proteins in both of lungs and colons. Additionally, the IHC-staining results in Fig. 2F provided additional evidence that forsythiaside A effectively inhibited the decreasing expression of E-cadherin, ZO-1 and Claudin-1 in lungs, as well as the depletion of ZO-1 and Claudin-1 in colons. Moreover, Fig. 2B confirmed that the serum level of endotoxin/LPS in MOD group was significantly increased, which could be significantly inhibited by MFA and HFA. Therefore, all these data suggested that forsythiaside A could suppress the reduction of lung and colonic epithelial barrier proteins and the increase of serum endotoxin/LPS level, thereby effectively inhibiting the lung and colonic epithelial barrier damages in LPS-induced ALI mice.
Fig. 2.
The effects of forsythiaside A on the lung and colonic epithelial barriers in LPS-induced ALI mice. (A) and (D) WB bands and relative protein expressions of epithelial barrier proteins in lung tissues; (B) Serum endotoxin levels (EU/mL); (C) and (E) WB bands and relative protein expressions of epithelial barrier proteins in colon tissues; (F) IHC-staining images of epithelial barrier proteins in lung and colon tissues (400 × magnifications). DEX referred to 5 mg/kg of dexamethasone, and LFA, MFA and HFA referred to 20, 40 and 80 mg/kg of forsythiaside A, respectively. Data were expressed as mean ± SD (n = 6, per group). *P < 0.05, **P < 0.01 versus MOD; #P < 0.05, ##P < 0.01 versus CON.
Forsythiaside A inhibited the activation of TLR4/MAPK/NF-κB and MLCK/MLC2 signal pathways in the lungs and colons of LPS-induced ALI mice
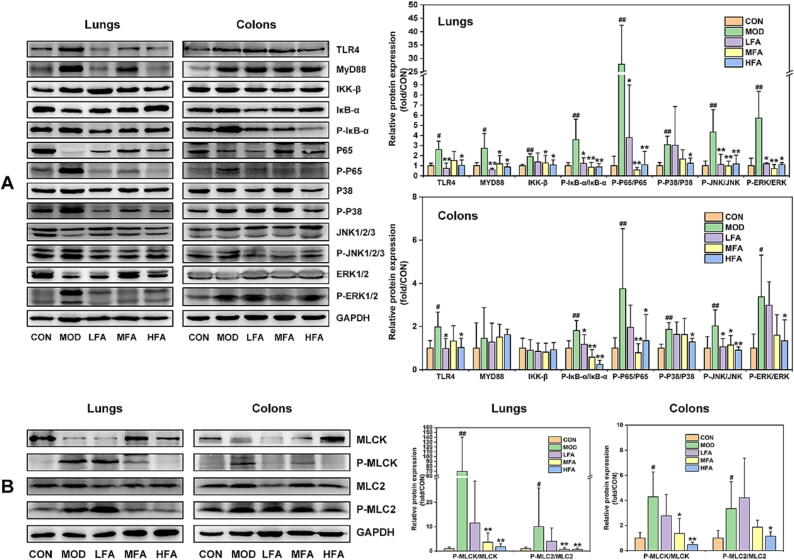
This study validated whether the efficacy of forsythiaside A in inhibiting lung and colon inflammation was in connection with the regulation of TLR4/MAPK/NF-κB signal pathway, which can be activated by LPS [37]. The results from Fig. 3A showed that LPS caused an up-regulation in the lung expression of TLR4/MAPK/NF-κB-related proteins (such as TLR4, MyD88, NF-κB(IKK-β, P-IκB-α/IκB-α and P-P65/P65) and MAPK (P-P38/P38, P-JNK/JNK and P-ERK/ERK)), as well as an increase in the colonic expression of TLR4/MAPK/NF-κB-related proteins excluding MyD88 and IKK-β in MOD group. However, forsythiaside A, especially in the high-dose group named HFA, significantly reduced TLR4/MAPK/NF-κB-related proteins in both of lung and colonic tissues.
Fig. 3.
The effects of forsythiaside A on TLR4/MAPK/NF-κB and MLCK/MLC2 signal pathways of lung and colon in LPS-induced ALI mice. (A) WB bands and relative protein expressions of TLR4/MAPK/NF-κB-related proteins in lung and colon tissues; (B) WB bands and relative protein expressions of MLCK/MLC2-related proteins in lung and colon tissues. DEX referred to 5 mg/kg of dexamethasone, and LFA, MFA and HFA referred to 20, 40 and 80 mg/kg of forsythiaside A, respectively. Data were expressed as mean ± SD (n = 6, per group). *P < 0.05, **P < 0.01 versus MOD; #P < 0.05, ##P < 0.01 versus CON.
Epithelial barrier damage can be caused due to the activation of MLCK/MLC signal pathway that can be activated by NF-κB [38]. This study already demonstrated that NF-κB was activated in both of lungs and colons, which might further initiate MLCK/MLC-mediated epithelial barrier damages. Therefore, we detected the protein expressions of MLCK/MLC2 signal pathway of lung and colon tissues in the ALI mice treated by forsythiaside A. The results from Fig. 3B revealed that compared to that of CON group, the expressions of P-MLCK/MLCK and P-MLC2/MLC2 were significantly elevated in the lung and colon tissues of MOD group. Nevertheless, treatment with forsythiaside A significantly suppressed the protein expressions of MLCK/MLC2 signal pathway. Therefore, these results validated that forsythiaside A could suppress TLR4/MAPK/NF-κB and MLCK/MLC2 signal pathways, thus inhibiting lung and colonic inflammation and epithelial barrier damages in LPS-induced ALI mice.
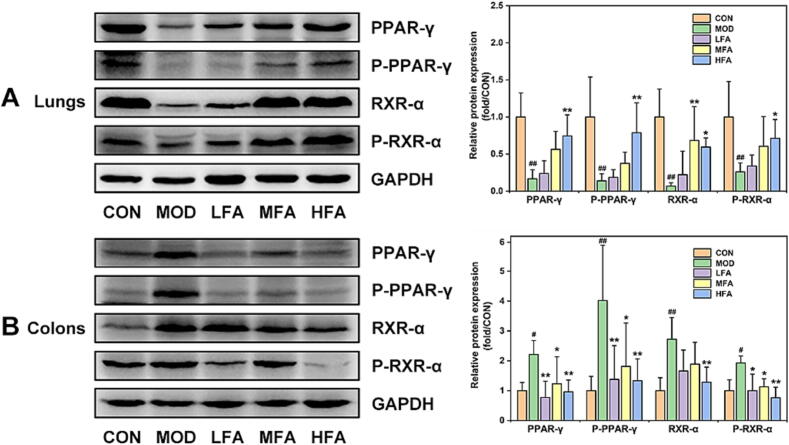
Forsythiaside A regulated the lung and colonic expressions of PPAR-γ/RXR-α complex in LPS-induced ALI mice
Based on previous research demonstrating that PPAR-γ can regulate both of MAPK and NF-κB signals to subside inflammation [39], [40], this study continued to focus on the effects of forsythiaside A on PPAR-γ and its conjugate RXR-α in the lung and colon tissues. The results demonstrated that intratracheal administration of LPS caused a significant reduction in the proteins and phosphorylated forms of PPAR-γ/RXR-α in lung tissues. In contrast, a significant increase was observed in the expression of PPAR-γ/RXR-α in colon tissues of MOD mice (Fig. 4). However, forsythiaside A was able to increase the lung expression of PPAR-γ/RXR-α complex, and inhibit the excessive expression and phosphorylation of PPAR-γ/RXR-α complex in colon tissues. Therefore, above these preliminarily proved that forsythiaside A increased the expression of PPAR-γ/RXR-α complex in the lungs while inhibiting its expression in the colons of ALI mice.
Fig. 4.
The effects of forsythiaside A on the lung and colonic expression of PPAR-γ/RXR-α complex in LPS-induced ALI mice. (A) and (B) WB bands and relative protein expressions of PPAR-γ/RXR-α in lung and colon tissues. DEX referred to 5 mg/kg of dexamethasone, and LFA, MFA and HFA referred to 20, 40 and 80 mg/kg of forsythiaside A, respectively. Data were expressed as mean ± SD (n = 6, per group). *P < 0.05, **P < 0.01 versus MOD; #P < 0.05, ##P < 0.01 versus CON.
Forsythiaside A inhibited LPS-induced inflammation in macrophages RAW264.7 by inhibiting TLR4/MAPK/NF-κB signal pathway
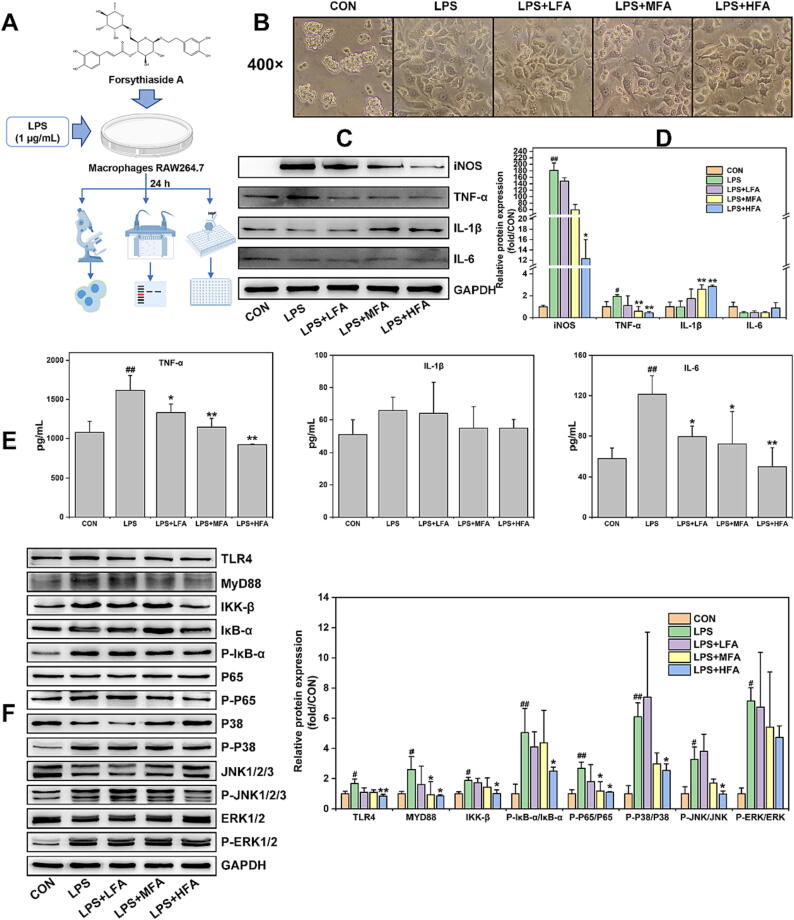
The inflammatory response induced by the activated macrophages can be due to LPS. LPS can activate TLR4 receptors to activate MAPK/NF-κB signals, ultimately inducing the release of pro-inflammatory cytokines of macrophages [36]. More importantly, macrophages as the important immune cells are reported widely distributed and existed in all tissues [41]. In this study, a widespread distribution of macrophages and a significant increase of inflammation indicators were observed in both of lung and colon tissues, indicating that macrophages were activated by LPS to cause tissue inflammation. However, the administration of forsythiaside A led to the inhibition of macrophage activation and pro-inflammatory indicators from lung and colon tissues. To validate the efficacy and mechanism of forsythiaside A, an in vitro inflammation model of LPS-induced macrophages RAW264.7 was constructed in this study (Fig. 5A).
Fig. 5.
The effects of forsythiaside A on the inflammation and TLR4/MAPK/NF-κB signal pathway in LPS-induced RAW264.7 cells. (A) Schematic diagram of forsythiaside A treatment in LPS-induced RAW264.7 cells; (B) Representative morphological images of LPS (1 μg/mL)-induced RAW264.7 cells with the treatment of forsythiaside A for 24 h; (C) and (D) WB bands and relative protein expressions of inflammatory indicators (iNOS, TNF-α, IL-1β and IL-6) in the cells; (E) Levels of TNF-α, IL-1β and IL-6 in the supernatants; (F) WB bands and relative protein expressions of TLR4/MAPK/NF-κB-related proteins in RAW264.7 cells with the induction of LPS and the treatment of forsythiaside A for 24 h. LFA, MFA and HFA referred to the Low-, Medium- and High-doses (50, 100 and 200 μM) of forsythiaside A, respectively. Data were expressed as mean ± SD (n = 3). *P < 0.05, **P < 0.01 versus LPS; #P < 0.05, ##P < 0.01 versus CON.
In this study, the MTT method was firstly utilized to determine the concentrations of forsythiaside A that affect the proliferation of RAW264.7 cells subjected to LPS (1 μg/mL). The LPS-caused inflammation model of RAW264.7 cells was referred to the relevant literature [42]. The MTT results of Fig. S1 showed that 50, 100 and 200 μM could be set as a Low-, Medium- and High-doses of forsythiaside A (LFA, MFA and HFA) for inhibiting LPS-induced the significant increase of cell viability of RAW264.7 cells. Then, the influence of forsythiaside A on the cellular morphology was examined under an inverted microscope. The results from Fig. 5B showed that LPS caused RAW264.7 cells in LPS group to stretch out the parapodium and show the fusiform or elongated morphology, indicating that LPS stimulation apparently change the cell morphology of macrophages. Meanwhile, there was no obvious change in the cell morphologies among LFA, MFA and HFA groups in contrast to that of LPS group. Furthermore, WB was employed in this study to assess the protein expressions of inflammatory indicators (iNOS, TNF-α, IL-1β, and IL-6) in the cells. ELISA was utilized to assess the levels of pro-inflammatory cytokines TNF-α, IL-1β, and IL-6 in the supernatants. As shown in Fig. 5C-E, LPS significantly increased the protein expression of iNOS and TNF-α in the cells as well as the levels of TNF-α and IL-6 in the supernatants, while having no significant effect on the expression of IL-1β and IL-6 in the cells and the level of IL-1β in the supernatants. However, forsythiaside A dose-dependently reduced the increase of these inflammatory indicators induced by LPS, but increased the expression of IL-1β in the RAW264.7 cells. As the activation of IL-1β in cells is also related to apoptosis and autophagy [43], this study did not further investigate the effects of forsythiaside A on IL-1β activity in RAW264.7 cells subjected to LPS. On the whole, these results demonstrated that forsythiaside A inhibited the inflammation response of macrophages RAW264.7 exposed to LPS.
Moreover, this study further examined whether the mechanism of forsythiaside A against the LPS-induced inflammation in RAW264.7 cells was in connection with the regulation of TLR4/MAPK/NF-κB signal pathway. Results from Fig. 5F indicated that LPS resulted in an elevated expression of TLR4/MAPK/NF-κB-associated proteins (TLR4, MyD88, NF-κB (IKK-β, P-IκB-α/IκB-α and P-P65/P65), and MAPK (P-P38/P38, P-JNK/JNK and P-ERK/ERK)) in RAW264.7 cells. However, forsythiaside A suppressed the LPS-mediated up-regulation of TLR4/MAPK/NF-κB-related proteins. These results thus proved that forsythiaside A could effectively inhibit the TLR4/MAPK/NF-κB signal pathway, leading to the decrease in LPS-induced inflammation of macrophages RAW264.7.
Forsythiaside A inhibited TNF-α-induced inflammation and epithelial barrier damages in lung and colon epithelial cells (A549 and SW620 cells) by inhibiting NF-κB/MLCK/MLC2 signal pathway
Inflammation is an important factor causing damage to the epithelial barrier. When inflammation occurs, it disrupts the connections between epithelial cells, causing increased epithelial permeability. This disorder can exacerbate mucosal inflammation and even trigger systemic inflammation [44], [45]. TNF-α, as a pivotal pro-inflammatory cytokine, exhibits the effects of damaging the epithelial barrier and increasing inflammation in the lungs and intestines. Recently, TNF-α has been frequently utilized to construct in vitro models of inflammation and epithelial barrier injury [21], [46], [47], [48]. This study found that the significant increase of TNF-α expression and the damage of epithelial barriers in lung and colon tissues of the ALI mice, while forsythiaside A could reverse such effects. However, it remained unclear whether forsythiaside A acted directly on lung and colon epithelial cells to inhibit pro-inflammatory cytokine-induced epithelial barrier damages. Therefore, this study constructed the in vitro models induced by TNF-α in lung and colon epithelial cells, with the objective of verifying the efficacy and mechanism of forsythiaside A against inflammation and epithelial barrier damages of the lungs and colons.
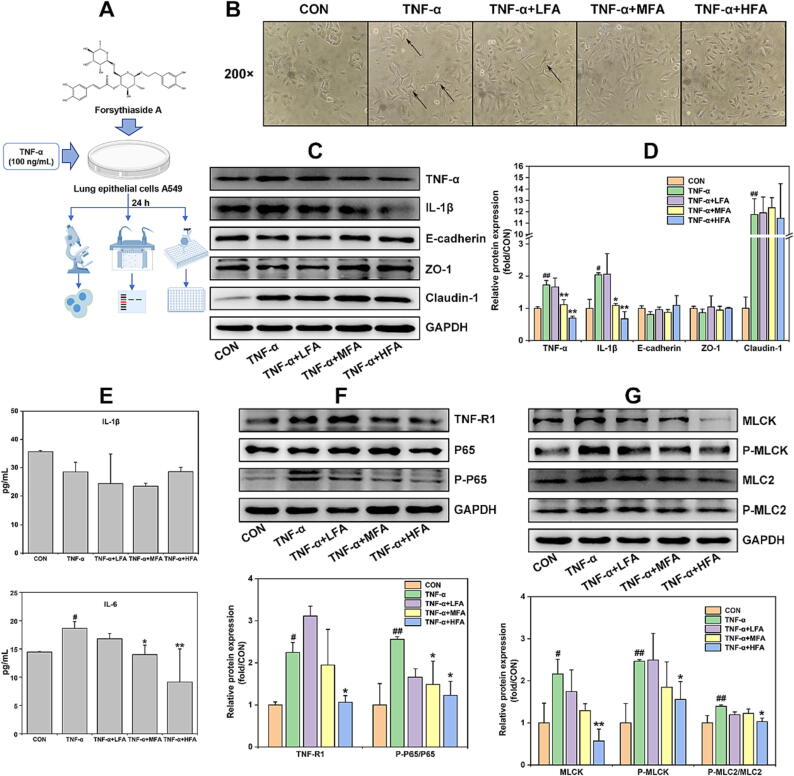
This study firstly verified whether forsythiaside A could inhibit TNF-α-induced inflammation and epithelial barrier damages in lung epithelial cells A549 (Fig. 6A). MTT and WB methods were used to determine the appropriate concentrations of forsythiaside A and the modeling concentration of TNF-α on the A549 cells. The MTT results from Fig. S2A indicated that forsythiaside A at concentrations of 200 μM and below could not significantly influence the cell viability of A549 cells. Additionally, the Fig. S2B and C results showed that TNF-α at the concentrations ≥ 100 ng/mL significantly increased the cell viability and protein expression of pro-inflammatory cytokines TNF-α and IL-1β instead of IL-6, but reduced the expression of epithelial barrier proteins E-cadherin and ZO-1 in A549 cells. Therefore, 50, 100 and 200 μM were set as Low-, Medium- and High-doses of forsythiaside A (LFA, MFA and HFA), and 100 ng/mL was set as the appropriate concentration of TNF-α to induce inflammation and epithelial barrier damages in A549 cells. The results from Fig. 6B showed that 100 ng/mL TNF-α caused some original spindle or cobblestone-shaped A549 cells to exhibit polygonal or long shuttle-shaped morphology. However, the administrations of forsythiaside A except for LFA significantly inhibited the TNF-α-induced changes of cellular morphology, presenting the cells with the similar morphology to that of CON group. Following this, this study used WB method to detect the effects of forsythiaside A on the expression of pro-inflammatory cytokines (TNF-α and IL-1β) and epithelial barrier proteins (E-cadherin, ZO-1 and Claudin-1) in the cells, and additionally used ELISA to examine the levels of IL-1β and IL-6 in the supernatants of A549 cells exposed to TNF-α. TNF-α was present as an inducing factor in the supernatants of A549 cells and would not be analyzed by ELISA, as observed in the same in vitro experiment with TNF-α induction of SW620 cells. The Fig. 6C–E results showed that TNF-α could significantly up-regulate the expression of TNF-α, IL-1β and Claudin-1 in the cells and the level of IL-6 rather than IL-1β in the supernatants, and down-regulate E-cadherin and ZO-1 expressions in the cells as well. Forsythiaside A treatment was found to inhibit the increase of TNF-α, IL-1β and IL-6 in the cells and supernatants, and increase the expression of E-cadherin and ZO-1 in the A549 cells induced by TNF-α. In accordance with these results, it can be inferred that forsythiaside A exhibited the ability to suppress TNF-α-induced inflammation and mitigate epithelial barrier damages in A549 cells.
Fig. 6.
The effects of forsythiaside A on the inflammation, epithelial barrier damages and NF-κB/MLCK/MLC2 signal pathway in TNF-α-induced A549 cells. (A) Schematic diagram of forsythiaside A treatment in TNF-α-induced A549 cells; (B) Representative morphological images of TNF-α (100 ng/mL)-induced A549 cells with the treatment of forsythiaside A for 24 h; (C) and (D) WB bands and relative protein expressions of pro-inflammatory cytokines (TNF-α, IL-1β and IL-6) and epithelial barrier proteins (E-cadherin, ZO-1 and Claudin-1) in the cells; (E) Levels of IL-1β and IL-6 in the supernatants; (F) and (G) WB bands and relative protein expressions of NF-κB/MLCK/MLC2 signal pathway including TNF-R1, NF-κB (P65 and P-P65), and MLCK/MLC2 (MLCK, P-MLCK, MLC2 and P-MLC2) in the cells. LFA, MFA and HFA referred to the Low-, Medium- and High-doses (50, 100 and 200 μM) of forsythiaside A, respectively. Data were expressed as mean ± SD (n = 3). *P < 0.05, **P < 0.01 versus TNF-α; #P < 0.05, ##P < 0.01 versus CON.
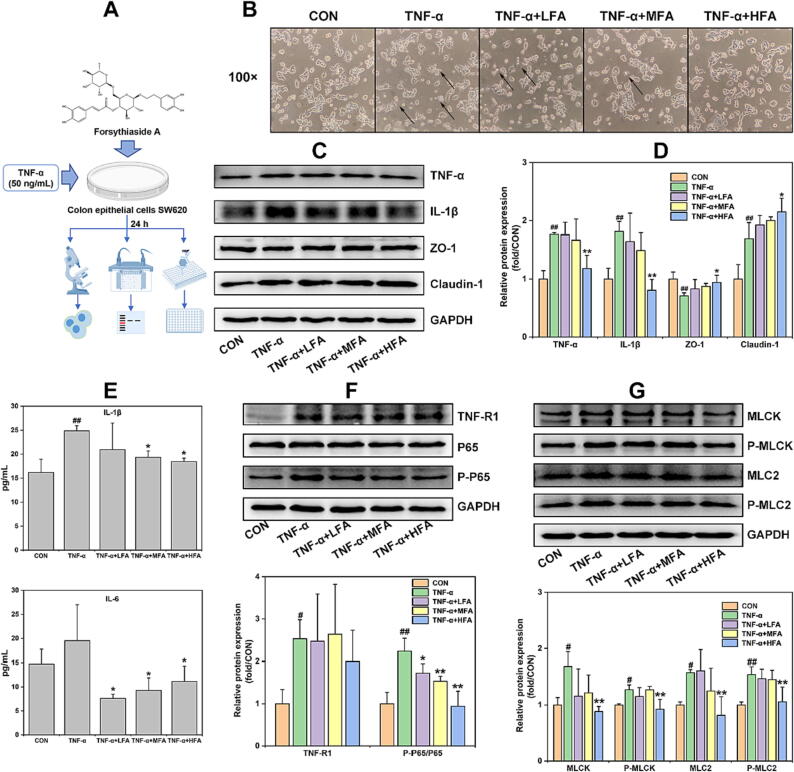
Afterwards, this study continued to examine the efficacy of forsythiaside A on the intestinal inflammation and epithelial barrier damages by constructing an in vitro model of colon epithelial cells SW620 induced by TNF-α (Fig. 7A). MTT and WB methods were used to ascertain the appropriate concentrations of forsythiaside A and the modeling concentration of TNF-α on SW620 cells. The results in Fig. S3 indicated that 25, 50 and 100 μM could be set as Low-, Medium- and High-doses of forsythiaside A (LFA, MFA and HFA), and 50 ng/mL could be set as the appropriate concentration of TNF-α for modeling the inflammation and epithelial damage in SW620 cells. The results in Fig. 7B indicated that 50 ng/mL TNF-α caused a significant decrease of cell density, and transformed the non-fimbriated globe morphology of many cells into a dispersed morphology compared with that of CON group. However, forsythiaside A (especially HFA) could significantly inhibit the changes of cell morphology and density induced by TNF-α. In addition, this study used WB to detect the influence of forsythiaside A on the expression of pro-inflammatory cytokines (TNF-α and IL-1β) and epithelial barrier proteins (ZO-1 and Claudin-1) in the cells, and used ELISA to examine the levels of IL-1β and IL-6 in the supernatants of SW620 cells exposed to TNF-α. The results in Fig. 7C–E showed that TNF-α could significantly increase the expression of TNF-α, IL-1β and Claudin-1 proteins in the cells and the level of IL-1β rather than IL-6 in the supernatants, and significantly reduce the expression of ZO-1 protein in SW620 cells. However, forsythiaside A (primarily HFA) significantly reduced TNF-α and IL-1β in the cells and supernatants, but increased ZO-1 and Claudin-1 in contrast to TNF-α group. Therefore, these results indicated that forsythiaside A could inhibit TNF-α-induced inflammation and epithelial barrier damage in colonic epithelial cell SW620.
Fig. 7.
The effects of forsythiaside A on the inflammation, epithelial barrier damages and NF-κB/MLCK/MLC2 signal pathway in TNF-α-induced SW620 cells. (A) Schematic diagram of forsythiaside A treatment in TNF-α-induced SW620 cells; (B) Representative morphological images of TNF-α (50 ng/mL)-induced SW620 cells with the treatment of forsythiaside A for 24 h; (C) and (D) WB bands and relative protein expressions of pro-inflammatory cytokines (TNF-α, IL-1β and IL-6) and epithelial barrier proteins (E-cadherin, ZO-1 and Claudin-1) in TNF-α (50 ng/mL)-induced SW620 cells with the treatment of forsythiaside A for 24 h; (E) Levels of IL-1β and IL-6 in the supernatants; (F) and (G) WB bands of NF-κB/MLCK/MLC2 signal pathway including TNF-R1, NF-κB (P65 and P-P65), and MLCK/MLC2 (MLCK, P-MLCK, MLC2 and P-MLC2) in the cells. LFA, MFA and HFA referred to the Low-, Medium- and High-doses (25, 50 and 100 μM) of forsythiaside A, respectively. Data were expressed as mean ± SD (n = 3). *P < 0.05, **P < 0.01 versus TNF-α; #P < 0.05, ##P < 0.01 versus CON.
TNF-α as the important pro-inflammatory cytokine needs to bind with the membrane receptor TNF-R1 and then to activate the NF-κB signal and its downstream MLCK/MLC signal pathway, resulting in cell inflammation and barrier damage [49], [50]. Therefore, this study further examined whether the in vitro pro-inflammatory cytokine TNF-α bonded to TNF-R1 to activate NF-κB/MLCK/MLC2 signal pathway in lung epithelial cells A549 and colon epithelial cells SW620, and whether this effect was regulated by forsythiaside A. The results from Fig. 6F and G showed that 100 ng/mL TNF-α could increase the expression of TNF-R1, P-P65/P65, MLCK, P-MLCK and P-MLC2/MLC2 in A549 cells. However, forsythiaside A, mainly at a concentration of HFA (200 µM), could significantly inhibit the TNF-α-induced overexpression of these NF-κB/MLCK/MLC2-related proteins in A549 cells. Furthermore, the results from Fig. 7F and G showed that 50 ng/mL TNF-α also increased the expression of TNF-R1, P-P65/P65, MLCK, P-MLCK, MLC2 and P-MLC2 in SW620 cells. However, forsythiaside A, mainly at a concentration of HFA (100 µM), could significantly decrease the expression level of NF-κB/MLCK/MLC2-related proteins except for TNF-R1 in TNF-α-induced SW620 cells. Therefore, these results proved that forsythiaside A could significantly inhibit the activation of NF-κB/MLCK/MLC2 signal pathway, effectively alleviating the inflammation and epithelial barrier damages caused by TNF-α in lung and colon epithelial cells.
Forsythiaside A regulated the activities of PPAR-γ/RXR-α complexes in RAW264.7, A549 and SW620 cells induced by LPS or TNF-α
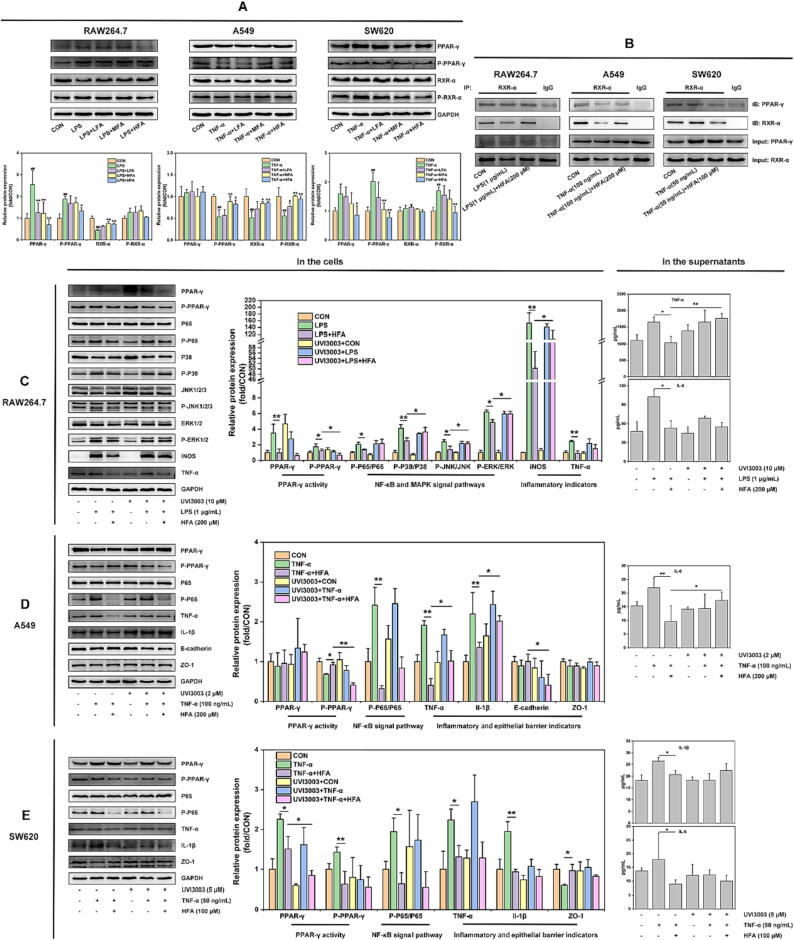
PPAR-γ has the function of regulating MAPK and NF-κB signal pathways to control inflammation [39], [40]. This study has demonstrated that forsythiaside A could inhibit the MAPK/NF-κB signal in LPS-induced macrophages (RAW264.7), and the NF-κB signal in TNF-α-induced lung/colon epithelial cells (A549 and SW620). It was unclear whether these effects were relevant to the activities of PPAR-γ and its binding protein RXR-α in the different cells. To this end, this study continued to use WB method to detect the influence of forsythiaside A on the protein activities of PPAR-γ/RXR-α, and took CO-IP method to detect the influence of forsythiaside A on the interactions between PPAR-γ and RXR-α proteins in RAW264.7, A549 and SW620 cells.
Our results showed that LPS and TNF-α exhibited different effects on the protein activities of PPAR-γ/RXR-α complexes in the cells, which could be inhibited by forsythiaside A treatment. The detailed results were shown in Fig. 8A: (Ⅰ) Macrophages RAW264.7: LPS caused a significant up-regulation of PPAR-γ and P-PPAR-γ proteins and a significant down-regulation of RXR-α protein, while having no significant effect on P-RXR-α protein in RAW264.7 cells. However, forsythiaside A (particularly at the high-dose of HFA) significantly inhibited these effects induced by LPS; (Ⅱ) Lung epithelial cells A549: TNF-α caused a significant down-regulation of P-PPAR-γ, RXR-α and P-RXR-α proteins, while having no significant effect on PPAR-γ protein in A549 cells. However, forsythiaside A significantly inhibited these effects induced by TNF-α; (Ⅲ) Colon epithelial cells SW620: TNF-α caused a significant up-regulation of PPAR-γ, P-PPAR-γ and P-RXR-α proteins, while having no apparent effect on the protein expression of RXR-α in SW620 cells. Compared to TNF-α group, HFA could significantly inhibit the excessive expression of PPAR-γ/RXR-α proteins in TNF-α-induced SW620 cells.
Fig. 8.
The regulation of forsythiaside A on PPAR-γ/RXR-α complexes and its effects on the inflammation and epithelial barrier damages in LPS-induced RAW264.7 cells and TNF-α-induced A549 and SW620 cells. (A) WB bands and relative protein expressions of PPAR-γ and RXR-α proteins in RAW264.7, A549 and SW620 cells with the treatment of forsythiaside A for 24 h. LFA, MFA and HFA in LPS (1 µg/mL)-induced RAW264.7 and TNF-α (100 ng/mL)-induced A549 cells referred to 50, 100 and 200 μM of forsythiaside A, respectively. In addition, LFA, MFA and HFA in TNF-α (50 ng/mL)-induced SW620 cells referred to 25, 50 and 100 μM of forsythiaside A, respectively. Data were expressed as mean ± SD (n = 3). *P < 0.05, **P < 0.01 versus LPS or TNF-α; #P < 0.05, ##P < 0.01 versus CON; (B) Subsequent CO-IP analysis was conducted to detect the changes of protein interactions between PPAR-γ and RXR-α in the different cells treated with forsythiaside A for 24 h. The CO-IP method was to use the IP-grade antibody of RXR-α to pull down the PPAR-γ/RXR-α complexes from the cells, followed by WB analysis using PPAR-γ/RXR-α antibodies. Proteins of Input: PPAR-γ and Input: RXR-α were set as the control; (C-E) RXR-α inhibitor UVI3003 was applied to study the influence of forsythiaside A on the inflammation and epithelial barrier damages of different cells through the regulation of PPAR-γ/RXR-α complex. LPS or TNF-α induced RAW264.7, A549 and SW620 cells interfered with or without UVI3003 were treated with or without with forsythiaside A (HFA) for 24 h, and were then analyzed by WB and ELISA. The cellular proteins analyzed by WB included PPAR-γ activity (PPAR-γ and P-PPAR-γ), NF-κB (P65 and P-P65) and MAPK (P38, P-P38, JNK1/2/3, P-JNK1/2/3, ERK1/2 and P-ERK1/2) signal pathways, inflammatory indicators (iNOS, TNF-α and IL-1β), epithelial barrier indicators (E-cadherin and ZO-1) and GAPDH. In addition, ELISA was used to detect the levels of pro-inflammatory cytokines (RAW264.7 cells: TNF-α and IL-6; A549 cells: IL-6; SW620 cells: IL-1β and IL-6) in the cell supernatants. Data were presented as means ± SD (n = 3), *P < 0.05 and **P < 0.01 vs. group.
To further uncover the mechanism by which forsythiaside A regulated the protein activities of PPAR-γ/RXR-α complexes in different cells, this study used CO-IP method to examine the effects of forsythiaside A on the protein interactions between PPAR-γ and RXR-α. The results in Fig. 8B indicated that: (Ⅰ) Macrophages RAW264.7: LPS reduced the protein interaction between PPAR-γ and RXR-α, while HFA (200 μM) increased the protein interaction in LPS-induced RAW264.7 cells; (Ⅱ) Lung epithelial cells A549: TNF-α reduced the protein interaction between PPAR-γ and RXR-α, while HFA (200 μM) increased the protein interaction in TNF-α-induced A549 cells; (Ⅲ) Colon epithelial cells SW620: TNF-α increased the protein interaction between PPAR-γ and RXR-α, while HFA (100 μM) reduced the protein interaction in TNF-α-induced SW620 cells. These results further demonstrated that forsythiaside A could regulate the activities of PPAR-γ/RXR-α complexes by regulating the interactions between PPAR-γ and RXR-α proteins in RAW264.7, A549 and SW620 cells cocultured with LPS or TNF-α. Specifically, the protein interactions between PPAR-γ and RXR-α were reduced in LPS-induced RAW264.7 cells and TNF-α-induced A549 cells, while were increased in TNF-α-induced SW620 cells, which all could be reversed by forsythiaside A treatment.
Forsythiaside A regulated PPAR-γ/RXR-α complex to inhibit the inflammation and epithelial barrier damages in RAW264.7, A549 and SW620 cells
This study already found that forsythiaside A regulated the protein interactions between PPAR-γ and RXR-α in RAW264.7, A549 cells and SW620 cells induced by LPS or TNF-α. Nevertheless, it remained unknown whether the observed inhibitory effects of forsythiaside A on inflammation and epithelial barrier damage in various cell types were attributed to its ability to regulate the PPAR-γ/RXR-α complexes. Therefore, to elucidate the efficacy and mechanism of forsythiaside A, this study used RXR-α inhibitor UVI3003 to interfere with the interaction between PPAR-γ and RXR-α proteins, and then evaluated the influence of forsythiaside A on PPAR-γ activity, MAPK and NF-κB signal pathways, and inflammation/epithelial barrier indicators in LPS or TNF-α-induced RAW264.7, A549 and SW620 cells.
This study firstly constructed the down-regulation system of RXR-α in RAW264.7, A549 and SW620 cells by using RXR-α inhibitor UVI3003 to determine the appropriate concentrations of UVI3003 in different cells. The WB analysis (Fig. S4) revealed the protein expressions of PPAR-γ and RXR-α in RAW264.7, A549, and SW620 cells following intervention with UVI3003 at various concentrations (0–20 μM). The appropriate concentrations of RXR-α inhibitor UVI3003 in RAW264.7, A549 and SW620 cells were thus set as 10, 2 and 5 μM, respectively.
Subsequently, this study used UVI3003 to incubate with RAW264.7, A549 and SW620 cells induced by LPS or TNF-α, and simultaneously treated each cell with HFA for 24 h to disclose the influence of forsythiaside A on the protein expressions of PPAR-γ activity, MAPK and NF-κB signal pathways, and inflammatory/epithelial barrier indicators in different cells. In the meantime, this study used ELISA to supplementally detect the effects of UVI3003 intervention on the suppressive effects of forsythiaside A on the pro-inflammatory cytokines that could be significantly increased by LPS or TNF-α in the supernatants. As our results of Fig. 5–7 showed, TNF-α and IL-6 in the supernatants of RAW264.7 cells, IL-6 in the supernatants of A549 cells, and IL-1β and IL-6 in the supernatants of SW620 cells, were all need to be detected due to their significant increase by the induction of LPS or TNF-α. The obtained results of Fig. 8C–E were listed as followed: (Ⅰ) Macrophages RAW264.7: UVI3003 inhibited the promoting effect of forsythiaside A on the protein interaction between PPAR-γ and RXR-α, thereby weakening the suppressive effects of forsythiaside A on MAPK/NF-κB pathway and inflammation in LPS-induced RAW264.7 cells; (Ⅱ) Lung epithelial cells A549: UVI3003 inhibited the promoting effect of forsythiaside A on the protein interaction between PPAR-γ and RXR-α, reducing the suppressive effects of forsythiaside A on NF-κB signal pathway, inflammation and epithelial barrier damages in TNF-α-induced A549 cells; (Ⅲ) Colon epithelial cells SW620: UVI3003 increased the inhibitive effect of forsythiaside A on the protein interaction between PPAR-γ and RXR-α, but did not affect the suppressive effects of forsythiaside A on NF-κB signal pathway, inflammation and epithelial barrier damages in TNF-α-induced SW620 cells. These results revealed that forsythiaside A promoted the protein interactions of PPAR-γ/RXR-α in RAW264.7 and A549 cells, while inhibited the protein interactions of PPAR-γ/RXR-α in SW620 cells. As a result of this, forsythiaside A effectively suppressed MAPK/NF-κB signal pathways, leading to the inhibition of inflammation and epithelial barrier damages triggered by LPS and TNF-α.
Discussion
In recent years, both Forsythiae Fructus and its compound forsythiaside A have been reported to have therapeutic effects on ALI, by regulating the molecular signals such as TLR4, MAPK, NF-κB, microRNA, and Nrf2 [28], [29], [51], [52]. Forsythiaside A can be regarded as a potential active substance of Forsythiae Fructus for treating ALI, but its pharmacological activity and underlying mechanism still need to be further studied. Our previous work showed that Forsythiae Fructus extracts could regulate the PPAR-γ/RXR-α complex to inhibit lung and colonic inflammation and epithelial barrier damages in ALI mice induced by LPS [27]. Therefore, this study further investigated the mechanism of forsythiaside A in treating ALI built upon the gut-lung axis, with the goal of developing the potential of forsythiaside A as the active compound of Forsythiae Fructus.
According to the research foundation of gut-lung axis, this study first focused on the efficacy of forsythiaside A in addressing lung inflammation and subsequent colonic inflammation in an ALI animal model. This study therefore established a murine ALI model provoked by intratracheal LPS introduction, and treated the mice with varying concentrations of forsythiaside A. The results in Fig. 1B–F demonstrated that forsythiaside A could effectively inhibit the lung pathological changes and inflammation caused by LPS, and reduce the expression of tissue inflammation indicators iNOS, TNF-α, IL-1β and IL-6. It could be concluded that forsythiaside A effectively alleviated the lung tissue damage and inflammatory response in the ALI mice. Additionally, to study the efficacy of forsythiaside A on the colonic inflammation in the ALI mice, this study evaluated the tissue pathology and inflammatory indicators expression in the colons. The results in Fig. 1G and H showed that compared with MOD group, forsythiaside A significantly improved the colonic pathological damage and tissue inflammation, and decreased the expression of these colonic inflammatory indicators. All these indicated that forsythiaside A could alleviate the inflammation in lung and colonic tissues of the mice with ALI induced by LPS.
The inflammatory indicator iNOS was found significantly increased in lungs and colons of the ALI mice. iNOS is an important enzyme that mediates inflammation and can be expressed by immune cells including macrophages. Activated macrophages can produce NO by activating iNOS to kill pathogens and promote the development of inflammation [53], [54]. As one of the effector cells involved in the inflammation reaction, macrophages can be stimulated by bacterial LPS to generate multiple pro-inflammatory cytokines (TNF-α, IL-1, IL-6, etc.) [36], [54]. Therefore, to further validate the anti-inflammatory effects of forsythiaside A, this study utilized IHC staining to detect the effects of forsythiaside A on the tissue distribution of macrophages in lungs and colons of the ALI mice. The results from Fig. 1I and J showed that compared with MOD group, forsythiaside A could reduce the extensive distribution of macrophages in the lung and colon tissues. Consequently, forsythiaside A could inhibit macrophage activation, thus alleviating the lung and colonic inflammation in the mice with ALI induced by LPS.
LPS is not only an important component of bacteria and but also a key factor that can activate systemic inflammation [55]. Currently, LPS has been recognized as the pathogenesis of endotoxemia or sepsis, causing severe inflammatory and epithelial barrier injuries to the lungs and intestines [56], [57]. Increasing evidences have shown that the inflammation caused by LPS is related to the specific recognition of cell surface TLR4 to LPS. TLR4, as a classic pattern recognition receptor localized on the cellular membrane, can activate the MAPK/NF-κB pathway through MyD88-dependent or independent pathways, and then increase the content of various pro-inflammatory cytokines [37], [58]. Additionally, previous reports have shown that MLCK/MLC signal activated by NF-κB is considered as the important pathway that leads to epithelial barrier loss [38]. Therefore, this study went on to focus on the efficacy of forsythiaside A on inflammation-caused epithelial barrier damages, and the mechanism of forsythiaside A on TLR4/MAPK/NF-κB and MLCK/MLC signal pathways in lung and colon tissues of ALI mice. The results indicated that forsythiaside A could promote the expression of lung and colonic epithelial barrier proteins such as E-cadherin, ZO-1 and Claudin-1, but inhibit the increase of serum endotoxin/LPS levels, thus protecting lung/colonic epithelial barriers damaged from inflammation in the ALI mice (Fig. 2). The in vivo mechanism results shown in Fig. 3 indicated that forsythiaside A could inhibit the activation of TLR4/MAPK/NF-κB and MLCK/MLC signal pathways in the tissues of lungs and colons. The above findings thus suggested that forsythiaside A could inhibit the tissue inflammation and epithelial barrier damages of lung and colon in the ALI mice, by suppressing TLR4/MAPK/NF-kB and MLCK/MLC signal pathways.
PPAR-γ is a ligand-activated nuclear receptor that needs to form a heterodimeric complex with RXR-α and then bind with PPAR response element (PPRE) to influence the transcriptional activity of targeted genes [59]. We focused on PPAR-γ as a potential mechanism by which forsythiaside A in treating ALI relying upon the gut-lung axis for the following reasons: (Ⅰ) It is known that PPAR-γ can be implicated in the immune activity of cells by regulating the MAPK/NF-κB signal pathways [39], [40]; (Ⅱ) Importantly, PPAR-γ can be abundantly expressed in diverse cell types, including macrophages and epithelial cells, to regulate lung and intestinal inflammation and immune responses [30], [31], [32]; (Ⅲ) In recent years, numerous agonists of PPAR-γ were proved to have the activity of attenuating the inflammatory injuries in lung and colon tissues. For instance, the PPAR-γ agonist rosiglitazone could treat endotoxemia-related inflammatory lung injury by activating PPAR-γ and inhibiting the nuclear expression of NF-κB [60]. J11-Cl could bind with the ligand-binding domain of PPAR-γ to alleviate colonic inflammation by increasing PPAR-γ transcriptional activity and inhibiting inflammatory signal pathways such as MAPK and NF-κB [61]. Therefore, PPAR-γ can be expected to develop into the potential target of drugs to inhibit inflammation from the lung and colon sites by intervening its downstream signal pathways. With that in mind, this study intended to investigate whether forsythiaside A could regulate the activity of PPAR-γ and its conjugate RXR-α, so as to suppress lung/colonic inflammation and epithelial barrier damages in the ALI mice by inhibiting MAPK and NF-κB signals. The results shown in Fig. 4 revealed that LPS significantly decreased the expression of PPAR-γ/RXR-α complex in lung tissues, but increased the expression of PPAR-γ/RXR-α complex in colon tissues of the ALI mice. Nevertheless, these effects could be inhibited by forsythiaside A treatment.
The opposite changes observed in the protein activities of PPAR-γ and RXR-α in the lungs and colons of ALI mice, and the opposing regulatory effects produced by forsythiaside A, suggest that caution needs to be exercised when interpreting our research results related to PPAR-γ activity. Some studies have shown that PPAR-γ exhibits cell-type specific effects on its immunomodulatory roles. For example, in the lungs, PPAR-γ was required for the anti-inflammatory activity in lung epithelial cells and alveolar macrophages, but could also act as a major transcriptional factor for pathogenic Th2 cells and play a vitally important role in the development of allergic inflammation [62], [63], [64]. In the intestines, the lack of PPAR-γ from mouse macrophages exacerbated the pathological manifestations of colitis, while PPAR-γ was up-regulated in the mouse colitis model and the in vitro inflammation model of macrophage RAW264.7 [65], [66]. Therefore, this study's focus on PPAR-γ activity cannot rely solely on in vivo data, but also requires further investigation on the activities of PPAR-γ and RXR-α at the cellular level. In this study, we used macrophages and lung/colon epithelial cells to construct in vitro models of inflammation and epithelial injury, and systematically investigated the efficacy of forsythiaside A in different cells. We then revealed the mechanism by which forsythiaside A inhibited inflammation and epithelial barrier damages through PPAR-γ/RXR-α complex.
Macrophages were widely distributed throughout multiple tissues of the whole body and become the important immunity cells involved in inflammatory response. Macrophages can stimulate the production of inflammatory indicators (iNOS, TNF-α, IL-1β and IL-6), by initiating MAPK and NF-κB signal pathways mediated by LPS-activated TLR4 signal [36], [54]. There is increasing evidence indicating that macrophage activation is a critical event in lung and intestinal injuries, and inhibiting macrophage activation can effectively alleviate tissue inflammatory injuries [67], [68]. Therefore, referring to relevant literature [42], this study constructed an in vitro inflammatory model of macrophages RAW264.7 by using 1 µg/mL LPS, and subsequently confirmed whether the mechanism of forsythiaside A was linked to the inhibition of TLR4/MAPK/NF-κB signal pathway. The results in Fig. 5 showed that forsythiaside A effectively suppressed TLR4-MAPK/NF-κB signal pathway in RAW264.7 cells, thereby attenuating the inflammation induced by macrophage activation resulting from LPS.
Inflammation is a key factor causing epithelial barrier injury. Several pro-inflammatory cytokines (IFN-γ, TNF-α, IL-1β, etc.) are proved to disrupt normal intercellular connections in epithelial cells, resulting in elevated cell permeability and dysfunction of barrier function [19], [20], [21], [22]. For example, TNF-α can bind to TNF-R1 receptor on the epithelial cells to activate NF-κB and its downstream MLCK/MLC, resulting in the epithelial barrier damages and inflammatory responses [38], [50], [69]. As a representative pro-inflammatory cytokine, TNF-α can be released not only from immunity cells including monocytes and macrophages, but also from lung and colonic epithelial cells [70], [71], [72]. TNF-α acts as an important factor in causing inflammation-induced epithelial barrier damages, and has been widely used in in vitro studies of TNF-α-induced epithelial cell models [21], [46], [47], [48]. According to these, this study constructed in vitro models of inflammation and epithelial damages using TNF-α to stimulate lung epithelial cells A549 and colon epithelial cells SW620, and then investigated whether forsythiaside A could suppress the cellular damages by suppressing NF-κB/MLCK/MLC2 signal pathway activated by the binding of TNF-α to TNF-R1. The results from Figs. 6 and 7 showed that forsythiaside A effectively suppressed NF-κB/MLCK/MLC2 signal pathway in A549 and SW620 cells, thereby improving the inflammatory and epithelial damages in lung and colon epithelial cells induced by TNF-α.
In the final part of this study, we attempted to address the critical issue about whether forsythiaside A involved PPAR-γ/RXR-α complexes in its efficacy of inhibiting inflammation and epithelial barrier damages in macrophages and lung/colon epithelial cells. Firstly, we used WB method to detect the influence of forsythiaside A on the protein activity of PPAR-γ/RXR-α complexes in the cells. The Fig. 8A results showed that LPS increased the expression of PPAR-γ and P-PPAR-γ, but decreased the expression of RXR-α in macrophages RAW264.7. In addition, TNF-α reduced the expression of P-PPAR-γ, RXR-α and P-RXR-α in lung epithelial cells A549, while increased the expression of PPAR-γ, P-PPAR-γ and P-RXR-α in colon epithelial cells SW620. However, forsythiaside A was able to reverse these effects induced by LPS and TNF-α. These results suggested that LPS and TNF-α exerted different regulatory effects on PPAR-γ/RXR-α protein activities in different cells, while forsythiaside A could act on these cells to inhibit such changes. In addition, this study continued to use CO-IP method to detect the effects of forsythiaside A on the protein interactions between PPAR-γ and RXR-α in RAW264.7, A549 and SW620 cells. The Fig. 8B results revealed that LPS reduced PPAR-γ/RXR-α protein interaction in RAW264.7 cells, and TNF-α reduced PPAR-γ/RXR-α protein interaction in A549 cells but increased PPAR-γ/RXR-α protein interaction in SW620 cells. However, forsythiaside A demonstrated effectiveness in inhibiting the effects of LPS and TNF-α on the PPAR-γ/RXR-α protein interactions in different cells. The results further demonstrated that LPS and TNF-α affected the activity of PPAR-γ/RXR-α complexes by altering the protein interactions, whereas forsythiaside A could act on these cells and then inhibit such changes.
To further verify whether forsythiaside A inhibited inflammation and epithelial barrier damages by regulating PPAR-γ/RXR-α complexes, UVI3003 as the RXR-α inhibitor was utilized in this study to interfere with the protein interaction between PPAR-γ and RXR-α in various cells. Furthermore, the influence of forsythiaside A on PPAR-γ activity, MAPK and NF-κB signal pathways, and inflammatory and epithelial barrier indicators were also examined. The results in Fig. 8C-E showed that forsythiaside A promoted PPAR-γ/RXR-α protein interactions in LPS-induced RAW264.7 cells and TNF-α-induced A549 cells, but inhibited PPAR-γ/RXR-α protein interactions in TNF-α-induced SW620 cells. These effects ultimately prompted that forsythiaside A inhibited inflammation and epithelial barrier damages in macrophages and lung/colonic epithelial cells by inhibiting MAPK and NF-κB signal pathways.
This study, in its inaugural attempt, studied the efficacy and mechanism of forsythiaside A on the inflammation and epithelial barrier damages for treating ALI mice based on gut-lung axis. In addition, this study also used in vitro models of macrophage and lung/colon epithelial cell to reveal the inhibitory mechanism of forsythiaside A on inflammation and epithelial injuries, which was linked with the PPAR-γ/RXR-α protein interactions (Fig. 9). This study showed that forsythiaside A effectively promoted the lung expression of PPAR-γ/RXR-α complexes, whereas reduced the colonic expression of PPAR-γ/RXR-α complexes in the ALI mice induced by LPS. At the cellular level, forsythiaside A effectively inhibited the reduction of PPAR-γ/RXR-α protein interactions in LPS-induced macrophages RAW264.7 and TNF-α-induced lung epithelial cells A549, while inhibited the increase of PPAR-γ/RXR-α protein interactions in TNF-α-induced colon epithelial cells SW620, thereby suppressing inflammation and epithelial barrier damages. All these suggested that forsythiaside A has the cell type-specific regulatory effects on PPAR-γ activity in the cells involved in inflammation and epithelial barrier damages.
Fig. 9.
The efficacy and mechanism of forsythiaside A on the lung and colonic inflammation and epithelial barrier damages in the treatment of ALI.
In fact, the immunoregulatory activity of PPAR-γ has been controversial till now and is considered to have cell type-specific effects [62], [63], [64], [65], [66]. Many studies have already revealed that PPAR-γ has the immunoregulatory roles in the macrophages and lung/colon epithelial cells, which helps to analyze the influence of forsythiaside A on PPAR-γ activities in different cell types. For example, previous research has shown that lung epithelial cells A549 could rely on PPAR-γ activation to inhibit inflammation mediated by NF-κB pathway [64]. This finding is consistent with the our results, demonstrating that forsythiaside A up-regulated the expression level of PPAR-γ/RXR-α to inhibit lung inflammation in the ALI mice and TNF-α-induced A549 cells. However, increased PPAR-γ phosphorylation in macrophages RAW264.7 can lead to the reduction of PPAR-γ activity. This is because LPS-induced activation of MAPK can promote PPAR-γ phosphorylated at the Ser112 site, which weakens the transcriptional and immunoregulatory functions of PPAR-γ produced in RAW264.7 cells [73]. This may explain why this study found that LPS significantly elevated the expression of PPAR-γ and P-PPAR-γ, but caused a reduction in PPAR-γ/RXR-α protein interactions in RAW264.7 cells. However, forsythiaside A could suppress the overexpression of PPAR-γ and P-PPAR-γ, and inhibit cell inflammation by promoting PPAR-γ/RXR-α protein interactions in LPS-induced RAW264.7 cells. Furthermore, this study also found the overexpression of PPAR-γ and P-PPAR-γ in colon tissues from LPS-induced ALI mice, which was in concert with some previous researches. These findings have shown that PPAR-γ expression increased in mouse models of colitis, which was thought to be due to the excessive phosphorylation of PPAR-γ in activated macrophages. These studies indicated that phosphorylation of PPAR-γ led to a reduction in PPAR-γ activity [66], [73]. However, this study found that forsythiaside A could inhibit the overexpression of PPAR-γ/P-PPAR-γ and alleviated inflammation and epithelial barrier damages by inhibiting PPAR-γ/RXR-α protein interactions in TNF-α-induced SW620 cells. The overexpression of P-PPAR-γ in the intestines may be related to the decrease of transactivating activity of PPAR-γ. This is because AMPK-mediated PPAR-γ phosphorylation and SIRT1 activation can inhibit the transactivating activity of PPAR-γ, which may further affect the regulatory role of PPAR-γ in intestinal inflammation [74]. In brief, this study provided another possible explanation for the inhibition of forsythiaside A on PPAR-γ/RXR-α complex in colon tissues of the ALI mice, which might be caused by the inhibition of protein and phosphorylation of PPAR-γ in colonic epithelial cells induced by inflammation.
Conclusion
The main results in this study can be concluded as following: (Ⅰ) Forsythiaside A, an active compound of Forsythiae Fructus, could regulate the activity of PPAR-γ/RXR-α complex and inhibit the TLR4/MAPK/NF-κB and MLCK/MLC2 signal pathways, suppressing lung and colonic inflammation and epithelial barrier damages in the ALI mice induced by LPS. Forsythiaside A also promoted the expression of PPAR-γ/RXR-α complex in lung tissues, whereas suppressed the overexpression of PPAR-γ/RXR-α complex in colon tissues; (Ⅱ) Forsythiaside A could regulate PPAR-γ/RXR-α complex to inhibit the LPS- and TNF-α-induced activation of TLR4/MAPK/NF-κB and NF-κB/MLCK/MLC2 signal pathways, thereby suppressing inflammation and epithelial barrier damage in macrophages and lung/colon epithelial cells as well; (Ⅲ) Forsythiaside A had the cell-specific regulatory effects on PPAR-γ/RXR-α complexes in different cells. Specifically, it promoted the protein interactions of PPAR-γ/RXR-α in LPS-induced macrophages RAW264.7 and TNF-α-induced lung epithelial cells A549, while inhibited the protein interaction in TNF-α-induced colon epithelial cells SW620. To sum up, during the treatment of ALI, forsythiaside A inhibited the inflammation and mitigated epithelial barrier injuries in lung and colon through its regulation on PAR-γ/RXR-α complex that exerted the cellular-specific effects.
CRediT authorship contribution statement
Jing Wang: Investigation, Data curation, Visualization, Writing - original draft. Xinyan Xue: Investigation, Visualization, Writing - original draft. Xingtao Zhao: Investigation, Data curation. Lin Luo: Investigation, Data curation. Juan Liu: Investigation, Data curation. Shu Dai: Investigation, Visualization, Methodology. Fang Zhang: Investigation, Visualization, Methodology. Rui Wu: Visualization, Investigation, Methodology. Yanfang Liu: Investigation, Visualization, Methodology. Cheng Peng: Funding acquisition, Supervision, Writing - review & editing. Yunxia Li: Funding acquisition, Supervision, Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by National Natural Science Foundation of China (NO: 81891012, 81630101 and U19A2010), Sichuan Province Science and Technology Support Program (NO: 2021JDRC0041, 22ZYZYTS0071 and 2022ZYD0088) and Sichuan TCM Science and Technology Industry Innovation Team (NO. 2022C001). This work was also supported by Innovation Team and Talents Cultivation Program of National Administration of Traditional Chinese Medicine (NO: ZYYCXTD-D-202209).
Compliance with Ethics Requirements
All Institutional and National Guidelines for the care and use of animals were followed.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jare.2023.08.006.
Contributor Information
Cheng Peng, Email: cdtcmpengcheng@126.com.
Yunxia Li, Email: lyxtgyxcdutcm@163.com.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Wheeler A.P., Bernard G.R. Acute lung injury and the acute respiratory distress syndrome: a clinical review. Lancet. 2007;369(9572):1553–1564. doi: 10.1016/S0140-6736(07)60604-7. [DOI] [PubMed] [Google Scholar]
- 2.Johnson E.R., Matthay M.A. Acute lung injury: epidemiology, pathogenesis, and treatment. J Aerosol Med Pulm Drug Deliv. 2010;23(4):243–252. doi: 10.1089/jamp.2009.0775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bos L.D.J., Ware L.B. Acute respiratory distress syndrome: causes, pathophysiology, and phenotypes. Lancet. 2022;400(10358):1145–1156. doi: 10.1016/S0140-6736(22)01485-4. [DOI] [PubMed] [Google Scholar]
- 4.Mowery N.T., Terzian W.T.H., Nelson A.C. Acute lung injury. Curr Probl Surg. 2020;57(5) doi: 10.1016/j.cpsurg.2020.100777. [DOI] [PubMed] [Google Scholar]
- 5.Matthay M.A., Zemans R.L., Zimmerman G.A., Arabi Y.M., Beitler J.R., Mercat A., et al. Acute respiratory distress syndrome. Nat Rev Dis Primers. 2019;5(1) doi: 10.1038/s41572-019-0069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.He Y.-Q., Zhou C.-C., Yu L.-Y., Wang L., Deng J.-L., Tao Y.-L., et al. Natural product derived phytochemicals in managing acute lung injury by multiple mechanisms. Pharmacol Res. 2021;163 doi: 10.1016/j.phrs.2020.105224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mokra D., Mikolka P., Kosutova P., Mokry J. Corticosteroids in acute lung injury: the dilemma continues. Int J Mol Sci. 2019;20 doi: 10.3390/ijms20194765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Enaud R., Prevel R., Ciarlo E., Beaufils F., Wieërs G., Guery B., et al. The gut-lung axis in health and respiratory diseases: a place for inter-organ and inter-kingdom crosstalks. Front Cell Infect Microbiol. 2020;10:9. doi: 10.3389/fcimb.2020.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Melo-González F., Sepúlveda-Alfaro J., Schultz B.M., Suazo I.D., Boone D.L., Kalergis A.M., et al. Distal consequences of mucosal infections in intestinal and lung inflammation. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.877533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anand S., Mande S.S. Diet, microbiota and gut-lung connection. Front Microbiol. 2018;9:2147. doi: 10.3389/fmicb.2018.02147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Massart A., Hunt D.P. Pulmonary manifestations of inflammatory bowel disease. Am J Med. 2020;133(1):39–43. doi: 10.1016/j.amjmed.2019.07.007. [DOI] [PubMed] [Google Scholar]
- 12.Wen Y., Xiao H., Liu Y., Yang Y., Wang Y., Xu S., et al. Polysaccharides from Dendrobium officinale ameliorate colitis-induced lung injury via inhibiting inflammation and oxidative stress. Chem Biol Interact. 2021;347 doi: 10.1016/j.cbi.2021.109615. [DOI] [PubMed] [Google Scholar]
- 13.Chunxi L.i., Haiyue L., Yanxia L., Jianbing P., Jin S.u. The gut microbiota and respiratory diseases: new evidence. J Immunol Res. 2020;2020:1–12. doi: 10.1155/2020/2340670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carvalho A., Alqusairi R., Adams A., Paul M., Kothari N., Peters S., et al. SARS-CoV-2 gastrointestinal infection causing hemorrhagic colitis: implications for detection and transmission of COVID-19 disease. Am J Gastroenterol. 2020;115(6):942–946. doi: 10.14309/ajg.0000000000000667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma Y., Yang X., Chatterjee V., Wu M.H., Yuan S.Y. The gut-lung axis in systemic inflammation. Role of mesenteric lymph as a conduit. Am J Respir Cell Mol Biol. 2021;64(1):19–28. doi: 10.1165/rcmb.2020-0196TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Celebi Sözener Z., Cevhertas L., Nadeau K., Akdis M., Akdis C.A. Environmental factors in epithelial barrier dysfunction. J Allergy Clin Immunol. 2020;145(6):1517–1528. doi: 10.1016/j.jaci.2020.04.024. [DOI] [PubMed] [Google Scholar]
- 17.Akdis C.A. Does the epithelial barrier hypothesis explain the increase in allergy, autoimmunity and other chronic conditions? Nat Rev Immunol. 2021;21(11):739–751. doi: 10.1038/s41577-021-00538-7. [DOI] [PubMed] [Google Scholar]
- 18.Dang A.T., Marsland B.J. Microbes, metabolites, and the gut-lung axis. Mucosal Immunol. 2019;12(4):843–850. doi: 10.1038/s41385-019-0160-6. [DOI] [PubMed] [Google Scholar]
- 19.Semin I., Ninnemann J., Bondareva M., Gimaev I., Kruglov A.A. Interplay between microbiota, toll-like receptors and cytokines for the maintenance of epithelial barrier integrity. Front Med (Lausanne) 2021;8 doi: 10.3389/fmed.2021.644333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han X.u., Lee A., Huang S., Gao J., Spence J.R., Owyang C. Lactobacillus rhamnosus GG prevents epithelial barrier dysfunction induced by interferon-gamma and fecal supernatants from irritable bowel syndrome patients in human intestinal enteroids and colonoids. Gut Microbes. 2019;10(1):59–76. doi: 10.1080/19490976.2018.1479625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sim T., Harith H., Tham C., Md Hashim N., Shaari K., Sulaiman M., et al. The protective effects of a synthetic geranyl acetophenone in a cellular model of TNF-α-induced pulmonary epithelial barrier dysfunction. Molecules. 2018;23(6):1355. doi: 10.3390/molecules23061355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaminsky L.W., Al-Sadi R., Ma T.Y. IL-1β and the intestinal epithelial tight junction barrier. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.767456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Q., Tian X., Maruyama D., Arjomandi M., Prakash A. Lung immune tone via gut-lung axis: gut-derived LPS and short-chain fatty acids' immunometabolic regulation of lung IL-1β, FFAR2, and FFAR3 expression. Am J Physiol Lung Cell Mol Physiol. 2021;321(1):L65–L78. doi: 10.1152/ajplung.00421.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sze MA, Tsuruta M, Yang SW, Oh Y, Man SF, Hogg JC, et al. Changes in the bacterial microbiota in gut, blood, and lungs following acute LPS instillation into mice lungs. PLoS One. 2014;9:e111228. [DOI] [PMC free article] [PubMed]
- 25.Xu Y., Zhu J., Feng B., Lin F., Zhou J., Liu J., et al. Immunosuppressive effect of mesenchymal stem cells on lung and gut CD8(+) T cells in lipopolysaccharide-induced acute lung injury in mice. Cell Prolif. 2021;54(5):e13028. doi: 10.1111/cpr.13028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China: China Med. Sci. Press; 2020. p. 177-8.
- 27.Wang J., Luo L., Zhao X., Xue X., Liao L.i., Deng Y., et al. Forsythiae Fructuse extracts alleviates LPS-induced acute lung injury in mice by regulating PPAR-γ/RXR-α in lungs and colons. J Ethnopharmacol. 2022;293 doi: 10.1016/j.jep.2022.115322. [DOI] [PubMed] [Google Scholar]
- 28.Zhou L, Yang H, Ai YS, Xie Y, Fu YJ. Protective effect of forsythiaside A on acute lung injure induced by lipopolysaccharide in mice. Chinese J Cell Mol Immunol 2014;30:151-4+9. [PubMed]
- 29.Lu Z, Yang H, Cao H, Huo C, Chen Y, Liu D, et al. Forsythoside A protects against lipopolysaccharide-induced acute lung injury through up-regulating microRNA-124. Clin Sci. 2020;134:2549-63. [DOI] [PubMed]
- 30.Abdalla H.B., Napimoga M.H., Lopes A.H., de Macedo Maganin A.G., Cunha T.M., Van Dyke T.E., et al. Activation of PPAR-γ induces macrophage polarization and reduces neutrophil migration mediated by heme oxygenase 1. Int Immunopharmacol. 2020;84 doi: 10.1016/j.intimp.2020.106565. [DOI] [PubMed] [Google Scholar]
- 31.Cho R.-L., Yang C.-C., Tseng H.-C., Hsiao L.-D., Lin C.-C., Yang C.-M. Haem oxygenase-1 up-regulation by rosiglitazone via ROS-dependent Nrf2-antioxidant response elements axis or PPARγ attenuates LPS-mediated lung inflammation. Br J Pharmacol. 2018;175(20):3928–3946. doi: 10.1111/bph.14465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Venkataraman B., Almarzooqi S., Raj V., Dudeja P.K., Bhongade B.A., Patil R.B., et al. α-Bisabolol mitigates colon inflammation by stimulating colon PPAR-γ transcription factor. In vivo and in vitro study. PPAR Res. 2022;2022:1–22. doi: 10.1155/2022/5498115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tong C., Chen T., Chen Z., Wang H., Wang X., Liu F., et al. Forsythiaside a plays an anti-inflammatory role in LPS-induced mastitis in a mouse model by modulating the MAPK and NF-κB signaling pathways. Res Vet Sci. 2021;136:390–395. doi: 10.1016/j.rvsc.2021.03.020. [DOI] [PubMed] [Google Scholar]
- 34.de Oliveira M.T.P., de Sá C.D., Tenório de Souza É., Stanisçuaski Guterres S., Pohlmann A.R., Silva P.M.R., et al. Orally delivered resveratrol-loaded lipid-core nanocapsules ameliorate LPS-induced acute lung injury via the ERK and PI3K/Akt pathways. Int J Nanomed. 2019;14:5215–5228. doi: 10.2147/IJN.S200666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Szapiel S.V., Elson N.A., Fulmer J.D., Hunninghake G.W., Crystal R.G. Bleomycin-induced interstitial pulmonary disease in the nude, athymic mouse. Am Rev Respir Dis. 1979;120:893–899. doi: 10.1164/arrd.1979.120.4.893. [DOI] [PubMed] [Google Scholar]
- 36.Ostareck D.H., Ostareck-Lederer A. RNA-binding proteins in the control of LPS-induced macrophage response. Front Genet. 2019;10:31. doi: 10.3389/fgene.2019.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fitzgerald K.A., Kagan J.C. Toll-like receptors and the control of immunity. Cell. 2020;180(6):1044–1066. doi: 10.1016/j.cell.2020.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang J., Zhao H., Lv K.e., Zhao W., Zhang N., Yang F., et al. Pterostilbene ameliorates DSS-induced intestinal epithelial barrier loss in mice via suppression of the NF-κB-mediated MLCK-MLC signaling pathway. J Agric Food Chem. 2021;69(13):3871–3878. doi: 10.1021/acs.jafc.1c00274. [DOI] [PubMed] [Google Scholar]
- 39.Hernandez-Quiles M., Broekema M.F., Kalkhoven E. PPARgamma in metabolism, immunity, and cancer: unified and diverse mechanisms of action. Front Endocrinol (Lausanne) 2021;12 doi: 10.3389/fendo.2021.624112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carvalho M.V., Gonçalves-de-Albuquerque C.F., Silva A.R. PPAR gamma: from definition to molecular targets and therapy of lung diseases. Int J Mol Sci. 2021;22:805. doi: 10.3390/ijms22020805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mosser D.M., Hamidzadeh K., Goncalves R. Macrophages and the maintenance of homeostasis. Cell Mol Immunol. 2021;18(3):579–587. doi: 10.1038/s41423-020-00541-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang M., Tian X., Wang Y.a., Wang D., Li W., Chen L., et al. Immunomodulating activity of the polysaccharide TLH-3 from tricholomalobayense in RAW264.7 macrophages. Int J Biol Macromol. 2018;107:2679–2685. doi: 10.1016/j.ijbiomac.2017.10.165. [DOI] [PubMed] [Google Scholar]
- 43.Tan S., Chen S. The mechanism and effect of autophagy, apoptosis, and pyroptosis on the progression of silicosis. Int J Mol Sci. 2021;22(15):8110. doi: 10.3390/ijms22158110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ivanov A.I., Parkos C.A., Nusrat A. Cytoskeletal regulation of epithelial barrier function during inflammation. Am J Pathol. 2010;177(2):512–524. doi: 10.2353/ajpath.2010.100168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mitamura Y., Ogulur I., Pat Y., Rinaldi A.O., Ardicli O., Cevhertas L., et al. Dysregulation of the epithelial barrier by environmental and other exogenous factors. Contact Dermatitis. 2021;85(6):615–626. doi: 10.1111/cod.13959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen Y.-C., Sung H.-C., Chuang T.-Y., Lai T.-C., Lee T.-L., Lee C.-W., et al. Vitamin D(3) decreases TNF-α-induced inflammation in lung epithelial cells through a reduction in mitochondrial fission and mitophagy. Cell Biol Toxicol. 2022;38(3):427–450. doi: 10.1007/s10565-021-09629-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li Y., Tian X., Li S., Chang L., Sun P., Lu Y., et al. Total polysaccharides of adlay bran (Coix lachryma-jobi L.) improve TNF-α induced epithelial barrier dysfunction in Caco-2 cells via inhibition of the inflammatory response. Food Funct. 2019;10(5):2906–2913. doi: 10.1039/c9fo00590k. [DOI] [PubMed] [Google Scholar]
- 48.Sivaraman K., Shanthi C. Purified fish skin collagen hydrolysate attenuates TNF-α induced barrier dysfunction in-vitro and DSS induced colitis in-vivo model. Int J Biol Macromol. 2022;222:448–461. doi: 10.1016/j.ijbiomac.2022.09.122. [DOI] [PubMed] [Google Scholar]
- 49.He F., Peng J., Deng X.-l., Yang L.-F., Camara A.D., Omran A., et al. Mechanisms of tumor necrosis factor-alpha-induced leaks in intestine epithelial barrier. Cytokine. 2012;59(2):264–272. doi: 10.1016/j.cyto.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 50.Ting A.T., Bertrand M.J.M. More to life than NF-κB in TNFR1 signaling. Trends Immunol. 2016;37(8):535–545. doi: 10.1016/j.it.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang R., Zhao G.L., Han L. The protective effect of forsythiaside A on the lung tissue of mice with acute lung injury mediated by lipopolysaccharide. Guiding J Tradit Chinese Med Pharmacol. 2020;26:10–13. [Google Scholar]
- 52.Xue B.Q., Zhu Y.H., Yu H.Y., Lv T.T., Cui R.Q., Li K., et al. Study on the mechanism of Fructus Forsythiae in the treatment of acute lung injury based on UPLC-Q-TOF-MS/MS and network pharmacology. Chinese J Hospital Pharm. 2022;42:2208–2215. [Google Scholar]
- 53.Xue Q., Yan Y., Zhang R., Xiong H. Regulation of iNOS on immune cells and its role in diseases. Int J Mol Sci. 2018;19(12):3805. doi: 10.3390/ijms19123805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pérez S., Rius-Pérez S. Macrophage polarization and reprogramming in acute inflammation: a redox perspective. Antioxidants (Basel) 2022;11:1394. doi: 10.3390/antiox11071394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brooks D., Barr L.C., Wiscombe S., McAuley D.F., Simpson A.J., Rostron A.J. Human lipopolysaccharide models provide mechanistic and therapeutic insights into systemic and pulmonary inflammation. Eur Respir J. 2020;56(1):1901298. doi: 10.1183/13993003.01298-2019. [DOI] [PubMed] [Google Scholar]
- 56.Li T, Zhang J, Feng J, Li Q, Wu L, Ye Q, et al. Resveratrol reduces acute lung injury in a LPS‑induced sepsis mouse model via activation of Sirt1. Mol Med Rep. 2013;7:1889-95. [DOI] [PubMed]
- 57.Candelli M., Franza L., Pignataro G., Ojetti V., Covino M., Piccioni A., et al. Interaction between Lipopolysaccharide and gut microbiota in inflammatory bowel diseases. Int J Mol Sci. 2021;22(12):6242. doi: 10.3390/ijms22126242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ciesielska A., Matyjek M., Kwiatkowska K. TLR4 and CD14 trafficking and its influence on LPS-induced pro-inflammatory signaling. Cell Mol Life Sci. 2021;78(4):1233–1261. doi: 10.1007/s00018-020-03656-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chandra V., Huang P., Hamuro Y., Raghuram S., Wang Y., Burris T.P., et al. Structure of the intact PPAR-gamma-RXR- nuclear receptor complex on DNA. Nature. 2008;456:350–356. doi: 10.1038/nature07413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu D., Zeng B.X., Zhang S.H., Yao S.L. Rosiglitazone, an agonist of peroxisome proliferator-activated receptor gamma, reduces pulmonary inflammatory response in a rat model of endotoxemia. Inflamm Res. 2005;54:464–470. doi: 10.1007/s00011-005-1379-0. [DOI] [PubMed] [Google Scholar]
- 61.Choo J., Lee Y., Yan X.-J., Noh T.H., Kim S.J., Son S., et al. A novel peroxisome proliferator-activated receptor (PPAR)γ agonist 2-hydroxyethyl 5-chloro-4,5-didehydrojasmonate exerts anti-inflammatory effects in colitis. J Biol Chem. 2015;290(42):25609–25619. doi: 10.1074/jbc.M115.673046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nobs SP, Kopf M. PPAR-γ in innate and adaptive lung immunity. J Leukoc Biol. 2018;104:737-41. [DOI] [PubMed]
- 63.Malur A, Mccoy AJ, Arce S, Barna BP, Kavuru MS, Malur AG, et al. Deletion of PPARγ in alveolar macrophages is associated with a Th-1 pulmonary inflammatory response. J Immunol 2009;182:5816-22. [DOI] [PubMed]
- 64.Neri T., Armani C., Pegoli A., Cordazzo C., Carmazzi Y., Brunelleschi S., et al. Role of NF-kappaB and PPAR-gamma in lung inflammation induced by monocyte-derived microparticles. Eur Respir J. 2011;37:1494–1502. doi: 10.1183/09031936.00023310. [DOI] [PubMed] [Google Scholar]
- 65.Hontecillas R., Horne W.T., Climent M., Guri A.J., Evans C., Zhang Y., et al. Immunoregulatory mechanisms of macrophage PPAR-γ in mice with experimental inflammatory bowel disease. Mucosal Immunol. 2011;4(3):304–313. doi: 10.1038/mi.2010.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shi L., Lin Q., Yang T., Nie Y., Li X., Liu B.o., et al. Oral administration of Lentinus edodes β-glucans ameliorates DSS-induced ulcerative colitis in mice via MAPK-Elk-1 and MAPK-PPARγ pathways. Food Funct. 2016;7(11):4614–4627. doi: 10.1039/c6fo01043a. [DOI] [PubMed] [Google Scholar]
- 67.Ye C., Li H., Bao M., Zhuo R., Jiang G., Wang W. Alveolar macrophage - derived exosomes modulate severity and outcome of acute lung injury. Aging (Albany NY) 2020;12(7):6120–6128. doi: 10.18632/aging.103010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jones G.-R., Bain C.C., Fenton T.M., Kelly A., Brown S.L., Ivens A.C., et al. Dynamics of colon monocyte and macrophage activation during colitis. Front Immunol. 2018;9 doi: 10.3389/fimmu.2018.02764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.He W.Q., Wang J., Sheng J.Y., Zha J.M., Graham W.V., Turner J.R. Contributions of myosin light chain kinase to regulation of epithelial paracellular permeability and mucosal homeostasis. Int J Mol Sci. 2020;21:993. doi: 10.3390/ijms21030993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dömling A., Li X. TNF-α: The shape of small molecules to come? Drug Discov Today. 2022;27(1):3–7. doi: 10.1016/j.drudis.2021.06.018. [DOI] [PubMed] [Google Scholar]
- 71.Yang L., Xie X., Tu Z., Fu J., Xu D., Zhou Y. The signal pathways and treatment of cytokine storm in COVID-19. Signal Transduct Target Ther. 2021;6:255. doi: 10.1038/s41392-021-00679-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Andrews C., McLean M.H., Durum S.K. Cytokine tuning of intestinal epithelial function. Front Immunol. 2018;9:1270. doi: 10.3389/fimmu.2018.01270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shi L., Lin Q., Li X., Nie Y., Sun S., Deng X., et al. Alliin, a garlic organosulfur compound, ameliorates gut inflammation through MAPK-NF-κB/AP-1/STAT-1 inactivation and PPAR-γ activation. Mol Nutr Food Res. 2017;61(9):1601013. doi: 10.1002/mnfr.201601013. [DOI] [PubMed] [Google Scholar]
- 74.Yuan G., Chen X., Li D. Modulation of peroxisome proliferator-activated receptor gamma (PPAR γ) by conjugated fatty acid in obesity and inflammatory bowel disease. J Agric Food Chem. 2015;63(7):1883–1895. doi: 10.1021/jf505050c. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.