Abstract
Background
Low grip strength and gait speed are associated with mortality. However, investigation of the additional mortality risk explained by these measures, over and above other factors, is limited.
Aim
We examined whether grip strength and gait speed improve discriminative capacity for mortality over and above more readily obtainable clinical risk factors.
Methods
Participants from the Health, Aging and Body Composition Study, Osteoporotic Fractures in Men Study, and the Hertfordshire Cohort Study were analysed. Appendicular lean mass (ALM) was ascertained using DXA; muscle strength by grip dynamometry; and usual gait speed over 2.4–6 m. Verified deaths were recorded. Associations between sarcopenia components and mortality were examined using Cox regression with cohort as a random effect; discriminative capacity was assessed using Harrell’s Concordance Index (C-index).
Results
Mean (SD) age of participants (n = 8362) was 73.8(5.1) years; 5231(62.6%) died during a median follow-up time of 13.3 years. Grip strength (hazard ratio (95% CI) per SD decrease: 1.14 (1.10,1.19)) and gait speed (1.21 (1.17,1.26)), but not ALM index (1.01 (0.95,1.06)), were associated with mortality in mutually-adjusted models after accounting for age, sex, BMI, smoking status, alcohol consumption, physical activity, ethnicity, education, history of fractures and falls, femoral neck bone mineral density (BMD), self-rated health, cognitive function and number of comorbidities. However, a model containing only age and sex as exposures gave a C-index (95% CI) of 0.65(0.64,0.66), which only increased to 0.67(0.67,0.68) after inclusion of grip strength and gait speed.
Conclusions
Grip strength and gait speed may generate only modest adjunctive risk information for mortality compared with other more readily obtainable risk factors.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40520-024-02783-x.
Keywords: Epidemiology, Osteoporosis, Sarcopenia, Ageing, Mortality
Background
Sarcopenia is characterised by the excessive loss of muscle mass, strength and function with advancing age. Consequences of sarcopenia include increased risk of frailty and earlier mortality, significant loss of quality of life and considerable healthcare expenditure [1–4]. Sarcopenia has been recognised as a medical condition since 2016 according to the International Classification of Diseases [5].
Most sarcopenia definitions incorporate measures of grip strength, gait speed or lean mass. However, research over the last decade has demonstrated greater capacity of grip strength and gait speed to predict incident adverse health outcomes in comparison with measures of lean mass, particularly appendicular lean mass from dual-energy X-ray absorptiometry (DXA) [6]. Indeed, more recent sarcopenia definitions proposed by the 2019 European Working Group on Sarcopenia in Older People (EWGSOP2) [7] and the Sarcopenia Definitions and Outcomes Consortium (SDOC) [8] either place less importance on lean mass (EWGSOP2) or do not include this measure in the algorithm (SDOC).
Many studies have examined sarcopenia components in relation to risk of earlier mortality. However, to date, there is limited information on how much these measures might add, in terms of outcome prediction, to the risk information associated with clinical risk factors such as age, sex, BMI and smoking status, which are known to strongly influence mortality risk and can be easily ascertained from routine clinical data. We therefore aimed to quantify the additional predictive value of grip strength and gait speed, over and above other clinical risk factors in predicting mortality, using data from a multinational assembly of cohort studies comprising the Health, Aging and Body Composition (Health ABC) Study (USA), Osteoporotic Fractures in Men (MrOS) Study (USA) and the UK-based Hertfordshire Cohort Study (HCS).
Methods
Cohort studies
The Health ABC Study consists of 3075 US men and women (aged 70–79 years), who were recruited in 1997 to 1998 [9]. A random sample of White ethnicity and Black ethnicity Medicare beneficiaries from around Pittsburgh and Memphis was ascertained. Selected participants received a mailing and then a telephone eligibility screen. Participants reporting no difficulty in ascending 10 stairs or walking one quarter of a mile were eligible. The exclusion criteria were as follows: intending to move outside the area within three years; currently enrolled in a lifestyle intervention study; unable to communicate with the interviewer; clear cognitive impairment; having difficulties with activities of daily living or having a life-threatening illness; or requiring a walking aid. Institutional review boards at the University of Pittsburgh and the University of Tennessee approved the study. All participants provided written informed consent.
MrOS US comprises 5994 men (aged 65–100) who were enrolled from March 2000 to April 2002 at six sites [10, 11]. The following recruitment methods were utilised: targeted presentations; advertisements and features in seniors’ newspapers; participant and voter registration databases; and mailings from the Department of Motor Vehicles [12]. Only those without bilateral hip replacements and who could walk without assistance were eligible. Self-reported ethnicity was recorded. The study was approved by institutional review boards at each site. Written informed consent was provided by all participants.
The HCS consists of 2997 men and women born in Hertfordshire (UK) from 1931 to 1939 and who still lived there in 1998–2004, when they attended a baseline home interview and research clinic for a health assessment. Further details about this study have been published previously [13, 14]. A subset of HCS participants who underwent whole body DXA during a follow-up study in 2011 to 2012 (n = 346) were the basis of the HCS analysis in this manuscript. The Hertfordshire and Bedfordshire Local Research Ethics Committee provided approval for the baseline home interview and research clinic; all HCS follow-up studies also had ethical approval. All participants gave written informed consent.
Ascertainment of participant characteristics
Details on the ascertainment of participant information, including the procedures and measurement devices used, are provided in Table 1.
Table 1.
Ascertainment of participant information within each cohort
| Participant characteristic | Osteoporotic Fractures in Men (MrOS) US Study | Health, Aging and Body Composition (Health ABC) Study | Hertfordshire Cohort Study (HCS) |
|---|---|---|---|
| Height | Measured using a Harpenden stadiometer | ||
| Weight | Measured using an electric scale or balance beam scale | Measured using a standard balance beam scale | Measured using a SECA floor scale, Chasmors Ltd, London, UK |
| Ascertained through researcher-administered questionnaires | |||
| Current smoker | Categorised as ‘current smoker’ or ‘never/previous smoker’ | ||
| High alcohol consumption | >7 drinks per week | >1 drink per day | >14 units per week |
| Physical activity | Assessed using the Physical Activity Scale for the Elderly [41] | Assessed over the past 7 days; approximate metabolic equivalent unit values were assigned to reported activities and intensity levels to derive caloric expenditure [42]. Total kilocalories expended per week was calculated as previously described [43]. | Assessed using the Longitudinal Aging Study Amsterdam Physical Activity Questionnaire [44] |
| Ethnicity | Ethnicity was self-reported and categorised as ‘White’ and ‘BAME’ for this analysis | White and black participants were recruited; black participants were categorised as ‘BAME’ | All participants were white |
| Left school early | Whether participants completed high school or not was ascertained from highest level of education attained | Whether participants completed Grade 12 or not was ascertained from highest level of education attained | Whether participants left school before 15 years of age or not was ascertained from age of leaving full-time education |
| Fall in previous year | Falls in previous 12 months were self-reported | ||
| Prior fracture | Fractures since age 50 years were self-reported | Fractures since age 45 years were self-reported | |
| Self-rated health (<good) | Self-rated health was ascertained from five multiple choice options and dichotomised as ‘good or better’ or ‘less than good’ | ||
| Low cognitive function | Obtained from the Modified Mini-Mental State Examination (3MS) [45] and categorised as <80 or ≥80 as in previous analyses of the MrOS US Study [46, 47] and the Health ABC Study [48, 49] | Obtained from the Mini-Mental State Examination (MMSE) [50] and categorised as <24 or ≥24 as in many previous studies [51] | |
| Number of comorbidities | Calculated from the number of the following doctor-diagnosed comorbidities that were self-reported: | ||
|
MrOS US • Heart disease (congestive heart failure, myocardial infarction, angina) • Lung disease (COPD) • Hypertension • Diabetes • Stroke • Arthritis • Osteoporosis • Thyroid disease • Parkinson's • Cancer |
Health ABC • Heart disease (congestive heart failure, myocardial infarction, angina) • Lung disease (COPD, asthma, pneumonia) • Hypertension • Diabetes • Stroke • Arthritis • Osteoporosis • Thyroid disease • Parkinson's • Cancer |
HCS • Heart disease (heart failure, myocardial infarction, angina) • Lung disease (COPD, asthma) • Hypertension • Diabetes • Stroke • Rheumatoid arthritis • Osteoporosis • Thyroid disease • Parkinson's • Cancer |
|
| Gait speed (m/s) | Calculated from the fastest time from two 6m gait speed tests. Participants were asked to walk at their usual pace. | Calculated from the fastest time from two 2.44m (8ft) gait speed tests. Participants were asked to walk at their usual pace. | |
| Grip strength (kg) | Assessed twice for each hand using a Jamar dynamometer; the highest measurement was used for analysis. Participants with recent arthritis/pain in their wrist or hand or who had undergone surgery of the upper extremity in the past 3 months did not have their grip strength assessed on that side. | Assessed three times for each hand using a Jamar dynamometer; the highest measurement was used for analysis | |
| ALM index (kg/m2) | ALM was ascertained from whole-body dual-energy X-ray absorptiometry scans (Hologic QDR 4500 [Hologic, Bedford, MA, USA]) | ALM was ascertained from whole-body dual-energy X-ray absorptiometry scans (Hologic QDR 4500A; Hologic, Bedford, MA, USA) | ALM was ascertained from whole-body dual-energy X-ray absorptiometry scans (Lunar Prodigy Advanced Scanner, GE Medical Systems, UK) |
| Femoral neck BMD (g/cm2) |
Ascertained by DXA using the same device as used in each cohort for measurement of ALM. T-scores were derived using US National Health and Nutrition Examination Survey (NHANES) III White female reference data from 20 to 29 year-olds [17], as recommended by the International Society for Clinical Densitometry (ISCD). |
||
| Mortality | Deaths were centrally adjudicated by physician review of death certificates and additional medical records | Deaths were determined from death certificates, hospital records and interviews with next of kin. All deaths were adjudicated by a central committee. | This cohort was flagged on the NHS Central Register for continuous notification of deaths |
BAME Black, Asian and minority ethnic; ALM Appendicular lean mass
Statistical methods
Summary statistics were used to describe participant characteristics. Cox regression models with cohort as the shared statistical frailty factor and mortality as the outcome were implemented; the shared frailties, assumed to be gamma-distributed latent random effects, reflect the fact that participants from the same cohort are likely to have more similar risks of mortality than participants from different cohorts. Different sets of exposures were defined as follows: Set 1: age and sex; Set 2: Set 1 + BMI, current smoker (yes/no), high alcohol consumption (yes/no), prior fracture since age 45 years (50 years in MrOS US Study) (yes/no), and femoral neck BMD T-score; Set 3: Set 2 + physical activity, BAME (Black, Asian and minority ethnic) ethnicity (yes/no), left school early (yes/no), fall in previous 12 months (yes/no), self-rated health of less than good (yes/no), low cognitive function (yes/no), and number of comorbidities. Set 2 comprised some of the key risk factors used in FRAX, the fracture risk assessment tool [15] and included BMD which is typically derived in the process used to ascertain ALM; relationships between lower BMD and increased mortality risk have also been reported in the literature, although this association may not be causal [16]. The following Cox models were then implemented: linear combinations of ALM index, grip strength and gait speed as exposures; Sets 1–3 as exposures; Sets 1–3 as exposures in addition to ALM index, grip strength and gait speed. For each model, the discriminative capacity according to Harrell’s Concordance Index (C-index) was examined as well as the strength of association between each sarcopenia component included in the model and risk of mortality. The C-index estimates the probability that for a randomly selected pair of participants, the participant with the higher predicted risk of the outcome experiences the outcome earlier. It ranges from 0 to 1 with 0.5 corresponding to the performance of a random classifier.
Cox models were then implemented to examine the relationship between each exposure and death with adjustment for age and sex; statistically significant exposures were then included in a single mutually-adjusted model. Each model was evaluated using the C-index. A minimal model, based on a small number of easily obtainable exposures, was then developed with the aim of achieving a similar C-index as the mutually-adjusted model.
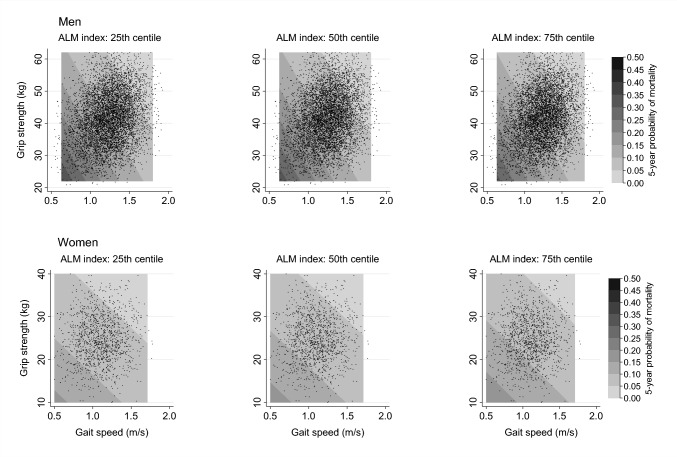
Contour plots were produced showing the 5-year probability of death according to grip strength and gait speed. These were estimated at 25th, 50th and 75th centiles (sex-specific) of ALM index and at the mean age (based on the entire sample of participants). Contour plots were estimated separately among men and women using logistic regression models with ALM index, grip strength, gait speed and age as linear terms.
Physical activity was assessed differently across cohorts so values were standardised within each cohort; femoral neck BMD T-scores were derived using US National Health and Nutrition Examination Survey (NHANES) III White female reference data from 20 to 29 year-olds [17]); and the remaining continuous measures were assessed on the same scale and, therefore, were standardised among the whole analysis sample. Analyses were conducted using Stata, release 17.0; men and women were pooled together in Cox models as sex-interactions regarding each sarcopenia component were not statistically significant (p > 0.05). The analytical sample comprised participants with complete data regarding all the variables used in the analysis.
Sensitivity analyses
Cox regression analyses were conducted separately within each cohort as a sensitivity analysis to check that findings were not affected by pooling data across cohorts. Furthermore, for participants who did not have their gait speed assessed over 6 m (gait speed was assessed over 8ft in HCS), analyses were repeated when gait speed values in this cohort were converted to those expected over 6 m using previously published equations [18, 19]. The results presented below are based on the raw gait speed values.
Results
Descriptive statistics
Participant characteristics of the entire sample (n = 8362) and stratified by both sex and cohort are presented in Table 2. Mean age of the analysis sample was 73.8 (5.1) years. Overall, 5231 (62.6%) participants died during follow-up and 897 (10.7%) died within the first 5-years of follow-up; median (lower quartile, upper quartile) follow-up time to death or until participants were censored was 13.3 (8.0, 17.0) years.
Table 2.
Participant characteristics stratified by cohort and sex
| Participant characteristic | All cohorts (n = 8362) | MrOS US | Health ABC | HCS | ||
|---|---|---|---|---|---|---|
| Men (n = 5550) | Men (n = 1264) | Women (n = 1277) | Men (n = 139) | Women (n = 132) | ||
| Age (years) | 73.8 (5.1) | 73.6 (5.9) | 74.2 (2.9) | 74.0 (2.8) | 75.2 (2.5) | 75.4 (2.5) |
| BMI (kg/m2) | 27.4 (4.1) | 27.4 (3.8) | 27.1 (3.9) | 27.7 (5.4) | 27.6 (3.7) | 28.0 (4.5) |
| Current smoker | 458 (5.5%) | 194 (3.5%) | 130 (10.3%) | 124 (9.7%) | 5 (3.6%) | 5 (3.8%) |
| High alcohol consumptiona | 1194 (14.3%) | 955 (17.2%) | 156 (12.3%) | 46 (3.6%) | 34 (24.5%) | 3 (2.3%) |
| Physical activityb | N/A | 142.4 (100.8, 186.3) | 5.5 (3.0, 8.9) | 4.5 (2.7, 7.3) | 193.6 (127.1, 285.7) | 206.4 (146.8, 283.6) |
| Ethnicity (BAME) | 1612 (19.3%) | 585 (10.5%) | 449 (35.5%) | 578 (45.3%) | 0 (0.0%) | 0 (0.0%) |
| Left school earlyc | 1003 (12.0%) | 352 (6.3%) | 333 (26.3%) | 272 (21.3%) | 24 (17.3%) | 22 (16.7%) |
| Fall in previous year | 1766 (21.1%) | 1165 (21.0%) | 226 (17.9%) | 308 (24.1%) | 34 (24.5%) | 33 (25.0%) |
| Fracture since age 45 yearsd | 1899 (22.7%) | 1267 (22.8%) | 209 (16.5%) | 360 (28.2%) | 29 (20.9%) | 34 (25.8%) |
| Self-rated health (< good) | 1177 (14.1%) | 758 (13.7%) | 193 (15.3%) | 179 (14.0%) | 22 (15.8%) | 25 (18.9%) |
| Low cognitive functione | 399 (4.8%) | 146 (2.6%) | 136 (10.8%) | 95 (7.4%) | 9 (6.5%) | 13 (9.8%) |
| Number of comorbiditiesf | 2.0 (1.0, 3.0) | 2.0 (1.0, 3.0) | 2.0 (1.0, 3.0) | 2.0 (1.0, 3.0) | 1.0 (1.0, 2.0) | 1.0 (0.0, 2.0) |
| Gait speed (m/s) | 1.22 (0.25) | 1.25 (0.24) | 1.24 (0.23) | 1.13 (0.22) | 0.82 (0.18) | 0.77 (0.18) |
| Grip strength (kg) | 38.6 (10.2) | 41.7 (8.5) | 40.9 (8.2) | 25.0 (5.7) | 37.4 (7.0) | 22.0 (6.3) |
| ALM index (kg/m2) | 7.7 (1.1) | 8.0 (0.9) | 8.0 (1.0) | 6.5 (1.1) | 8.1 (0.7) | 6.4 (0.7) |
| Femoral neck BMD (g/cm2) | 0.78 (0.14) | 0.79 (0.13) | 0.79 (0.14) | 0.70 (0.13) | 0.94 (0.13) | 0.83 (0.12) |
MrOS Osteoporotic Fractures in Men Study, Health ABC Health, Aging and Body Composition Study, HCS Hertfordshire Cohort Study, BAME: Black, Asian and minority ethnic, ALM Appendicular lean mass, BMD Bone mineral density
a MrOS US (> 7 drinks per week); Health ABC (> 1 drink per day); HCS (> 14 units per week)
b MrOS US (Physical Activity Scale for the Elderly score [possible range: 0–793]); Health ABC (Mcal/week); HCS (mins/day). Unable to present statistics for the entire cohort as units differ
c MrOS US (did not complete high school); Health ABC (did not complete Grade 12); HCS (left school before age 15 years)
d MrOS US (fracture since age 50 years)
e MrOS US and Health ABC (Modified Mini-Mental State Exam score < 80); HCS (Mini-Mental State Exam score < 24)
f Out of the following: heart disease, lung disease, hypertension, diabetes, stroke, arthritis, osteoporosis, thyroid disease, Parkinson’s and cancer
Associations between sarcopenia components and mortality
Associations between sarcopenia components and mortality risk are presented in Table 3; hazard ratios for all exposures in the models are included in Supplementary Table 1. Lower ALM index, grip strength and gait speed were associated with increased mortality risk in univariate analysis. However, when these factors were included as exposures simultaneously, only grip strength (hazard ratio (95% CI) per SD reduction: 1.18 (1.14,1.23)) and gait speed (1.45 (1.40,1.49)) were associated (p < 0.05) with death, with much weaker associations observed for ALM index (1.02 (0.99,1.06), p = 0.135). Associations for grip strength and gait speed were similar in mutually-adjusted analysis which also included ALM index and key clinical risk factors (age, sex, BMI, smoking status, alcohol consumption, fracture history and femoral neck BMD T-score) as covariates: grip strength (hazard ratio per SD reduction: 1.18 (1.13,1.23)) and gait speed (1.31 (1.26,1.35)). These estimates were only slightly attenuated (grip strength 1.14 (1.10,1.19), gait speed 1.21 (1.17,1.26)) in the fully-adjusted model that also included physical activity, ethnicity, education, fall history, self-rated health, cognitive function and number of comorbidities. P-values for sex-interactions regarding ALM index, grip strength and gait speed were 0.072, 0.054 and 0.505 respectively.
Table 3.
Mortality associations for sarcopenia components and discriminative capacity of models, depending on exposures included
| Exposures included | C-index (95% CI) | Associations for sarcopenia components (per SD lower level of component) | |||||
|---|---|---|---|---|---|---|---|
| ALM index (z-score) | Grip strength (z-score) | Gait speed (z-score) | |||||
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | ||
| ALM index | 0.53 (0.53,0.54) | 1.09 (1.06,1.12) | < 0.001 | – | – | – | – |
| Grip strength | 0.58 (0.57,0.58) | – | – | 1.31 (1.27,1.35) | < 0.001 | – | – |
| Gait speed | 0.61 (0.60,0.61) | – | – | – | – | 1.50 (1.46,1.55) | < 0.001 |
| ALM index, grip strength | 0.57 (0.57,0.58) | 0.97 (0.94,1.01) | 0.109 | 1.33 (1.28,1.37) | < 0.001 | – | – |
| ALM index, gait speed | 0.61 (0.60,0.62) | 1.09 (1.06,1.12) | < 0.001 | – | – | 1.51 (1.46,1.55) | < 0.001 |
| Grip strength, gait speed | 0.61 (0.61,0.62) | – | – | 1.20 (1.16,1.24) | < 0.001 | 1.44 (1.40,1.49) | < 0.001 |
| ALM index, grip strength, gait speed | 0.61 (0.61,0.62) | 1.02 (0.99,1.06) | 0.135 | 1.18 (1.14,1.23) | < 0.001 | 1.45 (1.40,1.49) | < 0.001 |
| ALM index, grip strength, gait speed, Set 1 | 0.67 (0.67,0.68) | 1.01 (0.98,1.05) | 0.483 | 1.18 (1.14,1.23) | < 0.001 | 1.32 (1.28,1.37) | < 0.001 |
| ALM index, grip strength, gait speed, Set 2 | 0.68 (0.67,0.69) | 1.03 (0.98,1.09) | 0.216 | 1.18 (1.13,1.23) | < 0.001 | 1.31 (1.26,1.35) | < 0.001 |
| ALM index, grip strength, gait speed, Set 3 | 0.70 (0.69,0.70) | 1.01 (0.95,1.06) | 0.817 | 1.14 (1.10,1.19) | < 0.001 | 1.21 (1.17,1.26) | < 0.001 |
| Set 1 | 0.65 (0.64,0.66) | – | – | – | – | – | – |
| Set 2 | 0.66 (0.65,0.67) | – | – | – | – | – | – |
| Set 3 | 0.69 (0.68,0.70) | – | – | – | – | – | – |
HR Hazard ratio, C-index Harrell’s Concordance Index
Exposures included in each adjustment set:
Set 1: Age, sex
Set 2: Set 1, BMI, current smoker (yes/no), high alcohol consumption (yes/no), fracture since age 45 years (50 years in MrOS US Study) (yes/no), femoral neck BMD T-score
Set 3: Set 2, physical activity, BAME ethnicity (yes/no), left school early (yes/no), fall in previous 12 months (yes/no), self-rated health of less than good (yes/no), low cognitive function (yes/no), number of comorbidities
The probability of mortality within 5-years of follow-up (at the mean age of the analysis sample) according to ALM index, grip strength and gait speed is presented in Fig. 1. The difference in this risk of mortality at varying levels of ALM index was much smaller than the difference according to varying levels of grip strength and gait speed, supporting the lack of association between ALM index and mortality described above.
Fig. 1.
Probability of mortality within 5-years of follow-up according to ALM index, grip strength and gait speed. ALM Appendicular lean mass. Contour plots were estimated separately among men and women using logistic regression models with the following exposure variables: ALM index, grip strength, gait speed and age as linear terms. Contour plots were estimated at the mean age of the sex-pooled analysis sample
Discriminative capacity for mortality
Discriminative capacity of the models for mortality, assessed using the C-index, is shown in Table 3. When included in univariate models, C-indices (95% CI) were greater for gait speed (0.61 (0.60, 0.61)) compared to grip strength (0.58 (0.57, 0.58)) and ALM index (0.53 (0.53, 0.54)); a C-index of 0.61 (0.61, 0.62) was also achieved when these three sarcopenia components were included in the same model. Including age and sex as exposures, along with ALM index, grip strength and gait speed increased the C-index from 0.61 (0.61, 0.62) to 0.67 (0.67, 0.68); additionally including key clinical risk factors (BMI, smoking status, alcohol consumption, fracture history and femoral neck BMD T-score) increased this value to 0.68 (0.67,0.69); and also including physical activity, ethnicity, education, fall history, self-rated health, cognitive function and number of comorbidities increased the C-index to 0.70 (0.69, 0.70).
Although grip strength and gait speed remained independently associated with mortality in the fully-adjusted model, only minimal improvement in the discriminative capacity for death was observed with the inclusion of the three sarcopenia components as exposures over and above the covariates (Table 3). For example, the C-index of the model with age and sex as exposures increased only from 0.65 (0.64, 0.66) to 0.67 (0.67, 0.68) when ALM index, grip strength and gait speed were also included. Similarly, the model with age, sex, BMI, smoking status, alcohol consumption, fracture history and femoral neck BMD T-score as exposures had a C-index of 0.66 (0.65, 0.67); this only increased to 0.68 (0.67, 0.69) with the inclusion of the three sarcopenia components. Finally, the C-index of the full model (age, sex, BMI, smoking status, alcohol consumption, fracture history, femoral neck BMD T-score, physical activity, ethnicity, education, fall history, self-rated health, cognitive function and number of comorbidities as covariates) only increased from 0.69 (0.68, 0.70) to 0.70 (0.69, 0.70) with the inclusion of ALM index, grip strength and gait speed.
Age- and sex-adjusted associations and mutually-adjusted associations between exposures and mortality risk are presented in Table 4, along with the C-indices of the models fitted. The majority of the exposures considered were independently associated with mortality after adjustment for age and sex and in mutually-adjusted analysis. However, a minimal model, including only age, sex, smoking status, education, self-rated health and number of comorbidities as covariates, achieved a C-index of 0.69 (0.68, 0.69), close to that of the mutually-adjusted model (0.70 (0.69, 0.70)) which comprised 14 exposures. Even though the increases in the C-indices reported in this section were modest, they were all statistically significant (p < 0.01), probably due to the large number of participants included in the analysis. All the C-index values reported were significantly different from 0.50 (p < 0.001).
Table 4.
Mortality associations and discriminative capacity of individual exposures and combinations of exposures
| Exposure | Age and sex included in all models | Mutually-adjusted model | Minimal model | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Hazard ratio (95% CI) | P value | C-index (95% CI) | Hazard ratio (95% CI) | P value | C-index (95% CI) | Hazard ratio (95% CI) | P value | C-index (95% CI) | |
| ALM index | 1.01 (0.98,1.05) | 0.520 | 0.65 (0.64,0.66) | 0.70 (0.69,0.70) | 0.69 (0.68,0.69) | ||||
| Grip strength | 1.24 (1.20,1.29) | < 0.001 | 0.66 (0.65,0.67) | 1.15 (1.10,1.19) | < 0.001 | ||||
| Gait speed | 1.35 (1.30,1.39) | < 0.001 | 0.67 (0.66,0.68) | 1.21 (1.17,1.26) | < 0.001 | ||||
| Age | 1.79 (1.75,1.84) | < 0.001 | 0.65 (0.64,0.66) | 1.63 (1.58,1.68) | < 0.001 | 1.78 (1.73,1.83) | < 0.001 | ||
| Sex (female) | 0.70 (0.64,0.78) | < 0.001 | 0.65 (0.64,0.66) | 0.50 (0.44,0.56) | < 0.001 | 0.67 (0.61,0.74) | < 0.001 | ||
| BMI | 1.05 (1.02,1.08) | 0.002 | 0.65 (0.65,0.66) | 0.98 (0.95,1.01) | 0.156 | ||||
| Current smoker | 1.91 (1.71,2.13) | < 0.001 | 0.66 (0.65,0.67) | 1.81 (1.61,2.02) | < 0.001 | 1.93 (1.73,2.16) | < 0.001 | ||
| High alcohol consumption | 1.01 (0.94,1.09) | 0.761 | 0.65 (0.64,0.66) | ||||||
| Previous fracture | 1.09 (1.02,1.16) | 0.010 | 0.65 (0.64,0.66) | 1.03 (0.97,1.10) | 0.324 | ||||
| Femoral neck BMD T-score | 0.99 (0.97,1.02) | 0.615 | 0.65 (0.64,0.66) | ||||||
| Physical activity | 0.89 (0.86,0.91) | < 0.001 | 0.66 (0.65,0.66) | 0.96 (0.93,0.99) | 0.004 | ||||
| Ethnicity (BAME) | 1.14 (1.06,1.23) | < 0.001 | 0.65 (0.65,0.66) | 0.91 (0.84,0.99) | 0.025 | ||||
| Left school early | 1.33 (1.22,1.44) | < 0.001 | 0.66 (0.65,0.66) | 1.08 (0.99,1.18) | 0.082 | 1.18 (1.09,1.29) | < 0.001 | ||
| Fall in previous year | 1.11 (1.04,1.19) | 0.001 | 0.65 (0.65,0.66) | 1.00 (0.94,1.07) | 0.889 | ||||
| Self-rated health (< good) | 1.86 (1.73,2.00) | < 0.001 | 0.67 (0.66,0.67) | 1.39 (1.29,1.50) | < 0.001 | 1.54 (1.43,1.66) | < 0.001 | ||
| Low cognitive function | 1.71 (1.52,1.92) | < 0.001 | 0.66 (0.65,0.67) | 1.44 (1.27,1.64) | < 0.001 | ||||
| Number of comorbidities | 1.22 (1.20,1.25) | < 0.001 | 0.67 (0.66,0.68) | 1.16 (1.13,1.18) | < 0.001 | 1.19 (1.16,1.21) | < 0.001 | ||
Hazard ratios shown per SD lower level of ALM index, grip strength and gait speed; hazard ratios per SD higher level shown for other continuous exposures
C-index Harrell’s Concordance Index, MrOS Osteoporotic Fractures in Men Study, Health ABC Health, Aging and Body Composition Study, HCS Hertfordshire Cohort Study, BAME Black, Asian and minority ethnic, ALM Appendicular lean mass
High alcohol consumption: MrOS US (> 7 drinks per week); Health ABC (> 1 drink per day); HCS (> 14 units per week)
Physical activity: MrOS US (Physical Activity Scale for the Elderly score); Health ABC (Mcal/week); HCS (mins/day)
Left school early: MrOS US (did not complete high school); Health ABC (did not complete Grade 12); HCS (left school before age 15 years)
Previous fracture: MrOS US (fracture since age 50 years); fracture since age 45 for Health ABC and HCS
Low cognitive function: MrOS US and Health ABC (Modified Mini-Mental State Exam score < 80); HCS (Mini-Mental State Exam score < 24)
Number of comorbidities out of: heart disease, lung disease, hypertension, diabetes, stroke, arthritis, osteoporosis, thyroid disease, Parkinson's and cancer
Sensitivity analyses
Supplementary Tables 2–4 present results from the Cox regression analyses when conducted within each cohort. These results were broadly similar to those from the main analysis where cohorts were pooled together and a shared statistical frailty factor was included in Cox models to account for differences in the underlying mortality risk between cohorts.
Discussion
In this study, lower grip strength and gait speed were independently associated with mortality after accounting for a range of sociodemographic, lifestyle and clinical factors. In contrast, the association for ALM index was weaker in magnitude and not different from the null after consideration of other characteristics. However, in multivariate models, it was apparent that grip strength and gait speed did not substantially add predictive value for death over and above age and sex, and only minimally above models including clinical risk factors, educational factors and comorbidity burden. Thus, a model including only age, sex, smoking status, education, self-rated health and comorbidity burden as covariates achieved a similar predictive performance regarding mortality as a mutually-adjusted model comprising 14 individual exposures.
Many studies have examined grip strength, gait speed and lean mass measures in relation to risk of adverse health outcomes and have established stronger associations regarding grip strength and gait speed in comparison with measures of lean mass [6, 8, 20]. Indeed, the importance of physical performance as a predictor of mortality in older people has been reported previously [21]. However, research comparing the predictive or discriminative capacity of individual sarcopenia components in relation to mortality is limited. A study involving 645 US haemodialysis patients evaluated four lean mass indices, grip strength, and gait speed for their predictive accuracy for mortality [22]. The base Cox model, which included age, sex, ethnicity and comorbidities, had a C-index of 0.63. This increased proportionally by 5% to 0.66 with the addition of gait speed only, and by a further 3% to 0.68 with the addition of grip strength only. However, none of the lean mass indices achieved C-indices greater than 0.65 when individually added to the base model. This supports our study’s findings of higher C-indices for grip strength (0.58 (95% CI: 0.57, 0.58)) and gait speed (0.61 (0.60, 0.61)) as univariate exposures, compared to ALM index (0.53 (0.53, 0.54)). However, larger increases in C-indices were observed with the inclusion of grip strength and gait speed compared to our study, where the inclusion of all sarcopenia components only increased the C-index of the model with age and sex as exposures from 0.65 (0.64, 0.66) to 0.67 (0.67, 0.68). This could be due to the significantly younger average age of 56.7 years for the haemodialysis patients compared to 73.8 years in our study, leading to age having a higher discriminative capacity for mortality in our study and thus less improvement in predictive accuracy from additionally including the sarcopenia components. Differences in findings could also be due to differences in the study setting (haemodialysis patients versus community-dwelling older participants in our study).
There are several potential biological mechanisms which might underpin the observed associations between lower grip strength and gait speed and increased mortality risk. These may represent common underlying mechanisms for both exposure and outcome or more directly from the muscle measures to mortality. For example, underlying physiological processes such as age-related chronic inflammation (inflammaging), oxidative stress, accumulation of senescent cells, and endocrine dysfunction might be causally linked to declines in grip strength and gait speed, as well as increased mortality risk [23]. Furthermore, independent walking requires not only sufficient strength but also adequate motor control, balance, and coordination. It involves multiple anatomical systems, including the respiratory, cardiovascular, and nervous systems. Consequently, slower walking speed might indicate impairments in these systems, leading to a higher mortality risk [19].
As well as increasing risk of mortality, sarcopenia also has a significant impact on the health of older people more widely, affecting both quality of life and daily functionality. For example, in a recent systematic review and meta-analysis, investigating the impact of age-related sarcopenia on health-related quality of life using the Sarcopenia Quality of Life (SarQoL) questionnaire, individuals with sarcopenia had significantly lower health-related quality of life compared to those without sarcopenia [3]. Furthermore, in another systematic review and meta-analysis, sarcopenia was found to be associated with increased risk of fractures and falls, which can result in physical disability and loss of independence [24]. Reduced muscle strength and function are key components of sarcopenia and may limit the ability to perform daily activities such as walking, climbing stairs, and carrying groceries, thereby reducing individual autonomy and self-sufficiency. Indeed, sarcopenia was associated with increased risk of disability regarding basic activities of daily living (odds ratio: 1.58, 95%CI: 1.18–2.11) and instrumental activities of daily living (1.87, 95%CI: 1.40–2.51)) among participants, aged 60 years and older, from the China Health and Retirement Longitudinal Study [25].
There are several possible reasons why including additional exposures, especially sarcopenia components, provided limited improvement in mortality prediction in these cohorts over and above other factors. Socioeconomic disadvantage, poor health behaviours and greater comorbidity are risk factors for low grip strength and gait speed [26]. Therefore, sarcopenia components are likely to be correlated with these factors so the additional information on mortality risk provided by sarcopenia components may be limited if information on these other factors is already available. Similarly, exposures such as comorbidity burden and self-rated health are on the causal pathway from lifestyle factors to adverse health events and are known to be correlated with lifestyle factors [27, 28]. Therefore, improvements in discriminative capacity from the incorporation of lifestyle factors in a model already including comorbidity and self-rated health as exposures may be limited. Indeed, understanding the temporal and causal relationships between sarcopenia components, other participant characteristics and risk of mortality is a worthwhile topic for future research, but this would require longitudinal data ascertained over multiple time-points. Finally, participants of the Health ABC cohort had no mobility disability at baseline, and MrOS participants had to be able to walk without the assistance of another person. Therefore, measures such as grip strength and gait speed may have less variation in these two cohorts compared to in the general population of this age range, resulting in these measures having lower discriminative capacity regarding mortality. Furthermore, gait speed declines with age substantially [29], resulting in a strong correlation between older age and slower gait speed. Therefore, the improvement in discriminative capacity of gait speed regarding mortality over and above age may be minimal.
Strengths of this study are that analyses were based on a large number of community-dwelling participants and that participants were recruited from cohorts where data were rigorously collected according to strict protocols. However, this study does have some limitations. The higher physical capability levels of the Health ABC and MrOS cohort, as discussed previously, may limit the generalizability of findings to the wider population of older people in this age group. Furthermore, the exclusion of participants with higher levels of disability from this study, such as nursing home residents or individuals with advanced disability, suggests that that the typical values of grip strength, gait speed and ALM index, and the risk of mortality in these studies are likely to differ compared to that of the general population in this age range. The generalizability of findings may also be limited by the fact that white men in the MrOS Study comprised 59% of the analysis sample; only 17% were women and 19% were BAME. This suggests that the findings of this study would be most applicable to older community-dwelling Caucasian men, who are able to walk unaided, and may be less generalisable to older women and older BAME individuals, and those in poorer health such as nursing home residents. Third, DXA lean mass is only a surrogate measure of muscle mass and also includes organ weight, water and other non-fat and non-bone soft tissue; other techniques such as the D3-Creatine (D3-Cr) dilution method, may provide a more direct and accurate assessment of muscle mass according to previous publications [30–32]. Computed tomography (CT) and magnetic resonance imaging (MRI) have also been shown to be useful in relating muscle measures to clinical outcomes. For example, adverse muscle composition (low muscle volume and high muscle fat infiltration), ascertained using MRI, was independently related to increased mortality risk in the UK Biobank [33], and CT-derived lower baseline muscle area was associated with increased risk of mortality in the Health ABC Study [34]. Fourth, functional status, arguably a more relevant outcome for sarcopenia than mortality, was not considered as an outcome in this study. This is because, unlike mortality, functional status was defined differently across the cohorts considered. Indeed, in a study of older outpatients admitted to a tertiary health centre, probable sarcopenia (low grip strength) was associated with subsequent deterioration in functional status [35]. However, this was only the case for population-specific thresholds, rather than the EWGSOP2 grip strength threshold. Fifth, the following limitations of Harrell’s Concordance Index in a survival analysis setting have been reported: it only depends on the ranks of the predicted probabilities; it can be insensitive to the addition of statistically and clinically significant exposures; it is strongly affected by the censoring distribution; and the ability to identify the difference in risk between any two subjects is often not of clinical interest [36]. However, the C-index was selected over other indices such as the Brier score due to its wide use in survival analysis, ease of interpretation, and focus on discriminative capacity. Furthermore, estimated 5-year cumulative incidence functions for mortality demonstrated minimal differences in mortality risk according to quartiles of grip strength and gait speed after controlling for the other risk factors considered (data not shown). This supports the lack of improvement in discriminative capacity of grip strength and gait speed, over and above other risk factors, even regarding short term mortality. Sixth, we cannot exclude the possibility that residual confounding could have contributed to the associations observed. For example, the inability to harmonize the required variables between cohorts meant that it was not possible to include mental health factors, such as measures of social isolation, loneliness and depression, as potential confounders in this study. Finally, some assessment protocols differed between cohorts, such as the distance used for gait speed tests that were 8ft in HCS and 6 m in the other cohorts. However, findings were similar when HCS gait speed values were transformed to those expected over 6 m using published equations [18, 19]. Furthermore, differences in the following between cohorts may have affected the analysis when the cohorts were pooled together: lack of calibration of DXA and grip strength devices between cohorts; methods used to ascertain exposures; and underlying risk of mortality. However, Cox models with cohort as the shared statistical frailty factor were implemented to account for differences in the underlying mortality risk between cohorts, and findings were broadly similar when replicated internally within each cohort.
Whilst a non-specific approach to predicting mortality risk may have limited clinical utility, insomuch as it does not point to any particular remedial intervention, greater risk of death is, de facto, likely to indicate poorer health, and thus identify individuals who may benefit from further clinical assessment. This might include specific physical measures relating to muscle strength and mobility, for which interventions such as exercise regimens might be appropriate [37–39]. However, in the first instance, given that the adjunctive risk information provided by such measurements appears to be minimal, any approach to high-level assessment of mortality risk may be most appropriately predicated on information likely to be already available in the primary and/or secondary care record rather than on further measures. Indeed, the modest set of clinical risk factors considered in Set 2 corresponds to input variables incorporated in the FRAX® Fracture Risk Assessment Tool [40], suggesting that this algorithm might be further evaluated in this context.
In conclusion, we have demonstrated that grip strength and gait speed are modest predictors of mortality during follow-up in three international cohorts, with minimal mortality association for DXA ALM index. Whilst lower grip strength and gait speed retained an association with greater mortality risk after adjustment for a wide range of covariates, the improvement in risk prediction gained through the addition of grip strength and gait speed to risk factor-based models was minimal. This suggests that approaches to clinical status based on mortality might most usefully incorporate existing measures likely to be readily available from the clinical record, rather than undertaking new assessments of muscle-related measures such as grip strength, gait speed and ALM index.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contributions
LDW conducted the statistical analysis and wrote the first draft of the manuscript; NCH and CC designed the study and made significant contributions to the content of the Introduction and Discussion; all authors made substantial contributions to the manuscript and approved the final version.
Funding
The Health, Aging and Body Composition Study was supported by National Institute on Aging (NIA) grants (R01AG027017, P30AG024827, T32AG021885, and K07AG033174); the Intramural Research program of the National Institutes of Health (N01AG62101, N01AG62103, N01AG62106, and R01AG028050); and a National Institute of Nursing Research grant (R01NR012459). The Osteoporotic Fractures in Men (MrOS) Study is supported by National Institutes of Health funding. The following institutes provide support: the National Institute on Aging (NIA), the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), the National Center for Advancing Translational Sciences (NCATS), and NIH Roadmap for Medical Research under the following grant numbers: U01 AG027810, U01 AG042124, U01 AG042139, U01 AG042140, U01 AG042143, U01 AG042145, U01 AG042168, U01 AR066160, R01 AG066671, and UL1 TR002369. The Hertfordshire Cohort Study was supported by the Medical Research Council University Unit Partnership grant number MRC_MC_UP_A620_1014. Roger Fielding’s participation was supported by the U.S. Department of Agriculture (USDA), under agreement No. 58–1950-4–003; any opinions, findings, conclusions, or recommendations expressed in this publication are those of the authors and do not necessarily reflect the view of the USDA. Cyrus Cooper, Elaine Dennison, Nicholas Harvey, Elizabeth Curtis and Leo Westbury are supported by the UK Medical Research Council [MC_PC_21003; MC_PC_21001], and the NIHR Southampton Biomedical Research Centre, Southampton, UK.
Data availability
The data used in this study cannot be shared due to consent restrictions.
Declarations
Conflict of interests
Elaine Dennison has received lecture fees and honoraria from UCB, Pfizer, Lilly and Viatris outside of the submitted work. Nicholas Harvey reports consultancy, lecture fees and honoraria (outside the submitted work) from Alliance for Better Bone Health, AMGEN, MSD, Eli Lilly, Servier, Shire, UCB, Kyowa Kirin, Consilient Healthcare, Radius Health, Theramex and Internis Pharma. Roger Fielding reports grants from National Institutes of Health (National Institute on Aging) and the USDA, during the conduct of the study; grants, personal fees and other from Axcella Health, other from Inside Tracker, grants and personal fees from Biophytis, grants and personal fees from Astellas, personal fees from Pfizer, Reneo, Cytokinetics, Embion, Glaxo Smith Kline and Amazentis, and grants and personal fees from Nestle' outside the submitted work. John Kanis and Eugene McCloskey are directors of Osteoporosis Research Ltd that develops and maintains FRAX. Peggy Cawthon declares personal fees from MyoCorps and owns stock in MyoCorps. The remaining authors declare that they have no conflicts of interest.
Informed consent
All participants provided informed consent prior to their inclusion in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Shaw SC, Dennison EM, Cooper C. Epidemiology of sarcopenia: determinants throughout the lifecourse. Calcif Tissue Int. 2017;101:229–247. doi: 10.1007/s00223-017-0277-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beaudart C, Reginster J-Y, Amuthavalli Thiyagarajan J, Bautmans I, Bauer J, Burlet N, et al. Measuring health-related quality of life in sarcopenia: summary of the SarQoL psychometric properties. Aging Clin Exp Res. 2023;35:1581–1593. doi: 10.1007/s40520-023-02438-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beaudart C, Tilquin N, Abramowicz P, Baptista F, Peng DJ, de Souza OF, et al. Quality of life in sarcopenia measured with the SarQoL questionnaire: A meta-analysis of individual patient data. Maturitas. 2024;180:107902. doi: 10.1016/j.maturitas.2023.107902. [DOI] [PubMed] [Google Scholar]
- 4.Pinedo-Villanueva R, Westbury LD, Syddall HE, Sanchez-Santos MT, Dennison EM, Robinson SM, Cooper C. Health Care Costs Associated With Muscle Weakness: A UK Population-Based Estimate. Calcif Tissue Int. 2019;104:137–144. doi: 10.1007/s00223-018-0478-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cao L, Morley JE. Sarcopenia Is Recognized as an Independent Condition by an International Classification of Disease, Tenth Revision, Clinical Modification (ICD-10-CM) Code. J Am Med Dir Assoc. 2016;17:675–677. doi: 10.1016/j.jamda.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 6.Harvey NC, Kanis JA, Liu E, Johansson H, Lorentzon M, McCloskey E. Appendicular lean mass and fracture risk assessment: implications for FRAX® and sarcopenia. Osteoporos Int. 2019;30:537–539. doi: 10.1007/s00198-019-04904-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48:16–31. doi: 10.1093/ageing/afy169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhasin S, Travison TG, Manini TM, Patel S, Pencina KM, Fielding RA, et al. Sarcopenia Definition: The Position Statements of the Sarcopenia Definition and Outcomes Consortium. J Am Geriatr Soc. 2020;68:1410–1418. doi: 10.1111/jgs.16372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Houston DK, Nicklas BJ, Ding J, Harris TB, Tylavsky FA, Newman AB, et al. Dietary protein intake is associated with lean mass change in older, community-dwelling adults: the Health, Aging, and Body Composition (Health ABC) Study. Am J Clin Nutr. 2008;87:150–155. doi: 10.1093/ajcn/87.1.150. [DOI] [PubMed] [Google Scholar]
- 10.Orwoll E, Blank JB, Barrett-Connor E, Cauley J, Cummings S, Ensrud K, et al. Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study—a large observational study of the determinants of fracture in older men. Contemp Clin Trials. 2005;26:569–585. doi: 10.1016/j.cct.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 11.San Francisco Coordinating Center. MrOS Online. The Osteoporotic Fractures in Men (MrOS) Study; A multi-center observational study of 5,994 men. 2024. https://mrosonline.ucsf.edu/ (Accessed 04/03/2024).
- 12.Blank JB, Cawthon PM, Carrion-Petersen ML, Harper L, Johnson JP, Mitson E, Delay RR. Overview of recruitment for the osteoporotic fractures in men study (MrOS) Contemp Clin Trials. 2005;26:557–568. doi: 10.1016/j.cct.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 13.Syddall HE, Sayer AA, Dennison EM, Martin HJ, Barker DJP, Cooper C. Cohort Profile: The Hertfordshire Cohort Study. Int J Epidemiol. 2005;34:1234–1242. doi: 10.1093/ije/dyi127. [DOI] [PubMed] [Google Scholar]
- 14.Syddall HE, Simmonds SJ, Carter SA, Robinson SM, Dennison EM, Cooper C. The Hertfordshire Cohort Study: an overview. F1000Res. 2019;8:7457. doi: 10.12688/f1000research.17457.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kanis JA, Johnell O, Oden A, Johansson H, McCloskey E. FRAX™ and the assessment of fracture probability in men and women from the UK. Osteoporos Int. 2008;19:385–397. doi: 10.1007/s00198-007-0543-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qu X, Huang X, Jin F, Wang H, Hao Y, Tang T, Dai K. Bone mineral density and all-cause, cardiovascular and stroke mortality: a meta-analysis of prospective cohort studies. Int J Cardiol. 2013;166:385–393. doi: 10.1016/j.ijcard.2011.10.114. [DOI] [PubMed] [Google Scholar]
- 17.Looker AC, Borrud LG, Hughes JP, Fan B, Shepherd JA, Melton LJ., 3rd Lumbar spine and proximal femur bone mineral density, bone mineral content, and bone area: United States, 2005–2008. Vital Health Stat. 2012;11:1–132. [PubMed] [Google Scholar]
- 18.Guralnik JM, Ferrucci L, Pieper CF, Leveille SG, Markides KS, Ostir GV, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000;55:M221–M231. doi: 10.1093/gerona/55.4.M221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Studenski S, Perera S, Patel K, Rosano C, Faulkner K, Inzitari M, et al. Gait speed and survival in older adults. JAMA. 2011;305:50–58. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harvey NC, Odén A, Orwoll E, Lapidus J, Kwok T, Karlsson MK, et al. Measures of Physical Performance and Muscle Strength as Predictors of Fracture Risk Independent of FRAX, Falls, and aBMD: A Meta-Analysis of the Osteoporotic Fractures in Men (MrOS) Study. J Bone Miner Res. 2018;33:2150–2157. doi: 10.1002/jbmr.3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Demonceau C, Buckinx F, Reginster J-Y, Bruyère O. Assessment of risk factors associated with long-term mortality in nursing homes: result from the SENIOR cohort. Aging Clin Exp Res. 2023;35:2997–3005. doi: 10.1007/s40520-023-02579-5. [DOI] [PubMed] [Google Scholar]
- 22.Kittiskulnam P, Chertow GM, Carrero JJ, Delgado C, Kaysen GA, Johansen KL. Sarcopenia and its individual criteria are associated, in part, with mortality among patients on hemodialysis. Kidney Int. 2017;92:238–247. doi: 10.1016/j.kint.2017.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cooper R, Kuh D, Hardy R. Objectively measured physical capability levels and mortality: systematic review and meta-analysis. BMJ. 2010;341:4467. doi: 10.1136/bmj.c4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yeung SS, Reijnierse EM, Pham VK, Trappenburg MC, Lim WK, Meskers CG, Maier AB. Sarcopenia and its association with falls and fractures in older adults: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle. 2019;10:485–500. doi: 10.1002/jcsm.12411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou H, Ding X, Luo M. The association between sarcopenia and functional disability in older adults. J Nutr Health Aging. 2024;28:100016. doi: 10.1016/j.jnha.2023.100016. [DOI] [PubMed] [Google Scholar]
- 26.Curtis E, Litwic A, Cooper C, Dennison E. Determinants of Muscle and Bone Aging. J Cell Physiol. 2015;230:2618–2625. doi: 10.1002/jcp.25001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feenstra M, van Munster BC, Vroomen JLM, de Rooij SE, Smidt N. Trajectories of self-rated health in an older general population and their determinants: the Lifelines Cohort Study. BMJ Open. 2020;10:e035012. doi: 10.1136/bmjopen-2019-035012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dhalwani NN, Zaccardi F, O’Donovan G, Carter P, Hamer M, Yates T, et al. Association Between Lifestyle Factors and the Incidence of Multimorbidity in an Older English Population. J Gerontol A Biol Sci Med Sci. 2017;72:528–534. doi: 10.1093/gerona/glw146. [DOI] [PubMed] [Google Scholar]
- 29.Westbury LD, Syddall HE, Fuggle NR, Dennison EM, Cauley JA, Shiroma EJ, et al. Long-term rates of change in musculoskeletal aging and body composition: findings from the Health, Aging and Body Composition Study. Calcif Tissue Int. 2020;106:616–624. doi: 10.1007/s00223-020-00679-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cawthon PM, Blackwell T, Cummings SR, Orwoll ES, Duchowny KA, Kado DM, et al. Muscle Mass Assessed by the D3-Creatine Dilution Method and Incident Self-reported Disability and Mortality in a Prospective Observational Study of Community-Dwelling Older Men. J Gerontol A Biol Sci Med Sci. 2021;76:123–130. doi: 10.1093/gerona/glaa111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Evans WJ, Hellerstein M, Orwoll E, Cummings S, Cawthon PM. D3-Creatine dilution and the importance of accuracy in the assessment of skeletal muscle mass. J Cachexia Sarcopenia Muscle. 2019;10:14–21. doi: 10.1002/jcsm.12390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cawthon PM, Orwoll ES, Peters KE, Ensrud KE, Cauley JA, Kado DM, et al. Strong relation between muscle mass determined by D3-creatine dilution, physical performance, and incidence of falls and mobility limitations in a prospective cohort of older men. J Gerontol A Biol Sci Med Sci. 2019;74:844–852. doi: 10.1093/gerona/gly129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Linge J, Petersson M, Forsgren MF, Sanyal AJ, Dahlqvist LO. Adverse muscle composition predicts all-cause mortality in the UK Biobank imaging study. J Cachexia Sarcopenia Muscle. 2021;12:1513–1526. doi: 10.1002/jcsm.12834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Farsijani S, Xue L, Boudreau RM, Santanasto AJ, Kritchevsky SB, Newman AB. Body composition by computed tomography vs dual-energy x-ray absorptiometry: long-term prediction of all-cause mortality in the Health ABC Cohort. J Gerontol A Biol Sci Med Sci. 2021;76:2256–2264. doi: 10.1093/gerona/glab105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bahat G, Bozkurt ME, Ozkok S, Kilic C, Karan MA. The longitudinal associations of sarcopenia definitions with functional deterioration: a comparative study. Aging Clin Exp Res. 2023;35:2089–2099. doi: 10.1007/s40520-023-02498-5. [DOI] [PubMed] [Google Scholar]
- 36.Hartman N, Kim S, He K, Kalbfleisch JD. Pitfalls of the concordance index for survival outcomes. Stat Med. 2023;42:2179–2190. doi: 10.1002/sim.9717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Daly RM, Dalla Via J, Duckham RL, Fraser SF. Exercise for the prevention of osteoporosis in postmenopausal women: an evidence-based guide to the optimal prescription. Braz J Phys Ther. 2019;23:170–180. doi: 10.1016/j.bjpt.2018.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cruz-Jentoft AJ, Sayer AA. Sarcopenia. Lancet. 2019;393:2636–2646. doi: 10.1016/S0140-6736(19)31138-9. [DOI] [PubMed] [Google Scholar]
- 39.Burley CV, Casey A-N, Jones MD, Wright KE, Parmenter BJ. Nonpharmacological approaches for pain and symptoms of depression in people with osteoarthritis: systematic review and meta-analyses. Sci Rep. 2023;13:15449. doi: 10.1038/s41598-023-41709-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schini M, Johansson H, Harvey N, Lorentzon M, Kanis J, McCloskey E. An overview of the use of the fracture risk assessment tool (FRAX) in osteoporosis. J Endocrinol Invest. 2023;47:501–511. doi: 10.1007/2Fs40618-023-02219-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol. 1993;46:153–162. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- 42.Ainsworth BE, Haskell WL, Leon AS, Jacobs DR, Jr, Montoye HJ, Sallis JF, Paffenbarger R., Jr Compendium of physical activities: classification of energy costs of human physical activities. Med Sci Sports Exerc. 1993;25:71–80. doi: 10.1249/00005768-199301000-00011. [DOI] [PubMed] [Google Scholar]
- 43.Visser M, Simonsick EM, Colbert LH, Brach J, Rubin SM, Kritchevsky SB. Type and intensity of activity and risk of mobility limitation: the mediating role of muscle parameters. J Am Geriatr Soc. 2005;53:762–770. doi: 10.1111/j.1532-5415.2005.53257.x. [DOI] [PubMed] [Google Scholar]
- 44.Stel VS, Smit JH, Pluijm SMF, Visser M, Deeg DJH, Lips P. Comparison of the LASA Physical Activity Questionnaire with a 7-day diary and pedometer. J Clin Epidemiol. 2004;57:252–258. doi: 10.1016/j.jclinepi.2003.07.008. [DOI] [PubMed] [Google Scholar]
- 45.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48:314–318. [PubMed] [Google Scholar]
- 46.Koo BB, Blackwell T, Lee HB, Stone KL, Louis ED, Redline S. Restless Legs Syndrome & Depression: Effect Mediation by Disturbed Sleep and Periodic Limb Movements. Am J Geriatr Psychiatry. 2016;24:1105–1116. doi: 10.1016/2Fj.jagp.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bauer SR, Scherzer R, Suskind AM, Cawthon P, Ensrud KE, Ricke WA, et al. Co-occurrence of lower urinary tract symptoms and frailty among community-dwelling older men. J Am Geriatr Soc. 2020;68:2805–2813. doi: 10.1111/jgs.16766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wright RM, Roumani YF, Boudreau R, Newman AB, Ruby CM, Studenski SA, et al. Effect of central nervous system medication use on decline in cognition in community-dwelling older adults: findings from the Health, Aging And Body Composition Study. J Am Geriatr Soc. 2009;57:243–250. doi: 10.1111/j.1532-5415.2008.02127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Swenor BK, Wang J, Varadaraj V, Rosano C, Yaffe K, Albert M, Simonsick EM. Vision Impairment and Cognitive Outcomes in Older Adults: The Health ABC Study. J Gerontol A Biol Sci Med Sci. 2019;74:1454–1460. doi: 10.1093/gerona/gly244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Folstein MF, Folstein SE, McHugh PR. Mini-mental state. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975 doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 51.Anbar R, Sultan SR, Al Saikhan L, Alkharaiji M, Chaturvedi N, Hardy R, et al. Is carotid artery atherosclerosis associated with poor cognitive function assessed using the Mini-Mental State Examination? A systematic review and meta-analysis. BMJ Open. 2022;12:e055131. doi: 10.1136/bmjopen-2021-055131. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used in this study cannot be shared due to consent restrictions.