Abstract
Growth hormone (GH) is secreted by somatotropic cells of the anterior pituitary gland. The classical effects of GH comprise the stimulation of cell proliferation, tissue and body growth, lipolysis, and insulin resistance. The GH receptor (GHR) is expressed in numerous brain regions. Notably, a growing body of evidence indicates that GH-induced GHR signaling in specific neuronal populations regulates multiple physiological functions, including energy balance, glucose homeostasis, stress response, behavior, and several neurological/cognitive aspects. The importance of central GHR signaling is particularly evident when the organism is under metabolic stress, such as pregnancy, chronic food deprivation, hypoglycemia, and prolonged exercise. These particular situations are associated with elevated GH secretion. Thus, central GH action represents an internal signal that coordinates metabolic, neurological, neuroendocrine, and behavioral adaptations that are evolutionarily advantageous to increase the chances of survival. This review summarizes and discusses recent findings indicating that the brain is an important target of GH, and GHR signaling in different neuronal populations regulates essential physiological functions.
Keywords: central nervous system, food restriction, growth hormone, hypoglycemia, hypothalamus, metabolism
1. Introduction
Growth hormone (GH) is produced by somatotropic cells of the anterior pituitary gland. GH secretion is regulated by multiple factors, including neuropeptides released by hypothalamic neurons, like somatostatin (SST) and GH-releasing hormone (GHRH), the stomach-derived hormone ghrelin (from gh-releasing peptide), and metabolites like glucose, free fatty acids (FFAs), and amino acids [1, 2]. GH secretion presents a complex pattern characterized by pulses interspersed with periods of low circulating GH levels. GH secretion markedly increases during puberty [3] and shows a progressive decline with aging [1, 2]. The peak of GH secretion during puberty coincides with the timing of greatest body growth, highlighting the critical role of this hormone in somatic growth. Accordingly, defects in GH secretion or action during infancy and adolescence lead to reduced body growth, whereas GH oversecretion can cause gigantism. In adults, increased GH secretion does not cause a significant increase in height due to the closure of the epiphyseal plates of long bones. However, GH oversecretion in adults still causes the growth of some bones (e.g., in the head, hands, and feet) and fluid retention in several tissues, leading to the physical features of acromegaly [4].
GH's effects on body growth involve two mechanisms: a direct action on target tissues via activation of GH receptor (GHR) and an indirect mechanism through circulating insulin-like growth factor 1 (IGF-1) [5]. GHR signaling in the liver is necessary to induce IGF-1 production and is responsible for ~80% of circulating IGF-1. GHR ablation in hepatocytes causes a marked decrease in plasma IGF-1 [6]. Interestingly, liver-specific GHR knockout mice exhibit only a moderate reduction in body weight, demonstrating that a significant part of the effects of GH regulating body growth is independent of liver-derived IGF-1 [6].
Not only does GHR signaling plays a crucial role in regulating tissue and body growth, but GH action also produces significant metabolic effects. GH stimulates lipolysis in adipocytes, increases hepatic gluconeogenesis, and causes insulin resistance in multiple tissues [5, 7, 8]. In accordance with these effects, GH- or GHR-deficient humans and animals frequently present improved insulin sensitivity. So, they are protected from type 2 diabetes mellitus despite showing increased body adiposity [8]. On the other hand, patients with acromegaly exhibit a higher incidence of diabetes mellitus [4]. Since GHR expression is found in the liver, adipose tissue, and skeletal muscle, the diabetogenic effect of GH is thought to be mediated by a direct effect on these insulin-sensitive tissues [7]. The increased FFA flux and, consequently, the lipotoxicity caused by the lipolytic effect of GH is considered the main factor contributing to GH-induced insulin resistance [8].
The central nervous system (CNS) is also a target of GH action because GHR is expressed in multiple brain regions [9-12]. GH-responsive neurons are abundantly found in some hypothalamic and brainstem nuclei, amygdala, and hippocampus [9-12]. The importance of central GH action has only been studied in more detail recently (Figure 1). This review summarizes the newest findings on the physiological importance of GHR signaling in specific neuronal populations. These results were generated by analyzing genetically modified mice carrying GHR inactivation in specific neuronal populations. Thus, numerous neural-specific GHR knockout mouse models revealed that GH action in the brain regulates energy balance, glucose homeostasis, stress response, behavior, and several neurological/cognitive aspects. These findings indicate that the brain is an essential target of GH action to control a variety of homeostatic processes and present potential clinical implications that will be discussed below.
Figure 1.
Summary of the known effects caused by GH-induced GHR signaling in different tissues, particularly in the central nervous system. The brain actions of GH were grouped according to the region involved: hypothalamus, hippocampus, and amygdala. Created with BioRender.com (Agreement number: MP260AQD8T).
2. Neuroendocrine and cognitive effects of GH
Pioneer studies revealed that GH administration, either in hypophysectomized or sham-operated rats, affects hypothalamic gene expression of neuropeptides that control GH secretion [13], demonstrating that GH action in the brain controls pituitary GH secretion via a negative feedback loop [1, 2]. Thus, the first described function of GH in the brain is the regulation of its secretion via negative feedback on hypothalamic neurons. Since circulating IGF-1 levels are controlled by GH secretion [1, 2], changes in IGF-1 secretion also represent another cue informing the brain about the activity of the somatotropic axis. Accordingly, intracerebroventricular IGF-1 administration reduces GH secretion and body growth [14]. GH can also regulate the activity of other endocrine axes. GH deficiency or excess causes reproductive system alterations and decreases fertility [15, 16]. Recent evidence suggests that part of these effects can be mediated by the CNS since GHR ablation in specific neuronal populations affects the timing of puberty and hypothalamic expression of genes that control the hypothalamic-pituitary-gonadal axis [17, 18]. In addition, restraint stress-induced prolactin secretion is impaired in mice carrying ablation of GHR in dopaminergic neurons [19]. Although this effect was observed only in male mice [19], it suggests an interaction between GH and prolactin endocrine axes, especially in stress response.
In addition to its neuroendocrine effect in the hypothalamus, GH modulates synaptic function, neural plasticity, and glutamatergic neurotransmission in the hippocampus, positively affecting cognition and memory [20-24]. Brain-specific signal transducer and activator of transcription 5 (STAT5) knockout mice exhibit impairment in memory and learning, suggesting that STAT5 is a downstream effector of GH to modulate cognitive aspects [25]. There is also evidence that GH treatment can mitigate age-related cognitive impairment in old animals [26]. However, at least part of the neurological action of GH is mediated by IGF-1 because IGF-1 administration can mimic the effects observed by GH treatment [26, 27]. Furthermore, GH seems to possess neuroprotective effects [28-32]. Paradoxically, aged GH- or GHR-deficient mice exhibit better cognitive performance than age-matched wild-type mice and are protected against aging-induced neurodegeneration [33]. In line with these findings, GH oversecretion reduces cognitive performance in twelve-month-old mice, whereas transgenic expression of GHR antagonist improves learning in middle-aged mice [34]. The increased insulin sensitivity of GHR-deficient mice can partially explain the protection against aging-induced decline in cognition since insulin resistance is associated with cognitive decline [35, 36].
Another brain structure associated with GH action is the amygdala. Basolateral amygdala (BLA) stimulation induces GH secretion in anesthetized male rats [37]. Previous studies demonstrated that BLA neurons express GH [38]. Chronic stress or administration of a ghrelin receptor agonist increases GH expression in BLA of rats, suggesting a role of non-pituitary-derived GH [38]. Inhibition of GHR signaling in the amygdala decreases fear memory [38]. In contrast, virus-mediated GH overexpression in the BLA enhances fear memory and dendritic spine density in the amygdala of rats [39]. Notably, BLA does not contain GH-responsive neurons or express Ghr mRNA, whereas the central nucleus of the amygdala (CEA) exhibits a pronounced number of GH-responsive neurons in rodents [9, 10, 40]. Thus, BLA-derived GH may diffuse locally and act in a paracrine manner in nearby GHR-expressing neurons in the CEA. A recent study found that SST-expressing neurons in the CEA are highly responsive to GH [41]. GHR ablation in these neurons decreases fear memory in male and female mice [41]. Moreover, SSTΔGHR male mice exhibit increased anxiety-like behavior, but female knockouts show no alterations in anxiety [41]. These effects were associated with multiple and sex-dependent changes in the expression of factors (e.g., γ-aminobutyric acid and glutamate receptors, vesicular glutamate transporter 1, synaptophysin, and brain-derived neurotrophic factor) involved in synaptic plasticity and function in the amygdala [41]. Thus, GHR signaling in SST neurons, probably in the extended amygdala, modulates fear memory, and anxiety.
2.1. Potential clinical implications of cognitive and neurological effects of GH
The GH/IGF-1 axis is possibly involved in the cognitive alterations associated with aging and the development of neurodegenerative diseases like Alzheimer’s. On the one hand, normal GH/IGF-1 axis activity is favorable for fully developing cognitive functions such as memory [20-24, 26, 27]. For example, a recent study suggests that IGF-1 deficiency is behind Down syndrome's neurodegeneration [42]. Furthermore, since GH presents neuroprotective effects [28-32], this may help explain why young individuals, who naturally secrete more GH, have a greater neuroregeneration capacity than old individuals. However, GH also induces insulin resistance [7, 8]. Decreased insulin sensitivity is associated with cognitive decline and neurodegenerative diseases [35]. Importantly, brain insulin resistance in Alzheimer's patients is associated with IGF-1 resistance [34], probably contributing to the cognitive decline of this disease. Thus, the physiological decrease in GH secretion with aging may be beneficial in decreasing GH-induced insulin resistance and preventing the associated decline in cognitive function. The potential pros and cons of GH replacement therapy on cognitive aspects must be considered in this context. Additionally, there seems to be a Goldilocks effect both in young and older individuals, that is, too little or too much GH action at a particular age may lead to physiological problems.
Our recent findings revealed that GHR signaling is anxiolytic. However, it favors the development of fear memory, a key feature of post-traumatic stress disorder [41]. These results are in accordance with previous studies demonstrating a direct action of GH in the amygdala [38, 39]. These findings provide a mechanistic explanation of why GH-deficient individuals have a higher prevalence of anxiety and depression disorders [43]. Future studies need to investigate whether changes in the prevalence of mood disorders in the elderly are also associated with the GH/IGF-1 axis. Furthermore, some eating disorders, like anorexia and bulimia nervosa, are associated with a high prevalence of anxiety disorders [44]. Importantly, individuals with anorexia nervosa present a nutritionally acquired GH resistance [45]. Thus, it is currently unclear if there is an association between the loss of somatotroph axis activity and the development of anxiety in eating disorders. Finally, several physiological conditions associated with elevated GH secretion, including puberty [3], pregnancy [46, 47], and prolonged exercise [48], are known to cause alterations in mood and affect the prevalence of neuropsychiatric diseases. Therefore, the knowledge of how GH-responsive neurons regulate depression, anxiety, and other behavioral aspects will help explain how situations that alter GH secretion or local (brain) GH production can affect mood disorders.
3. GH regulates metabolism via hypothalamic neurons
3.1. Arcuate nucleus neurons mediate the metabolic effects of GH
3.1.1. GH-responsive neurons in the hypothalamus
The development of the in-situ hybridization technique allowed the identification of specific neuronal populations that express Ghr mRNA. Burton et al. [49] showed that Ghr mRNA is highly expressed in the periventricular nucleus (PV) and arcuate nucleus of the hypothalamus (ARH). This expression matches the GH-binding sites previously detected using in vitro autoradiographic analysis [50]. The PV contains the hypophysiotropic SST-expressing neurons that inhibit GH secretion, whereas GHRH neurons are found in the ARH, especially in its ventrolateral part [51]. Interestingly, while Ghr mRNA is expressed in approximately 70% of PVSST neurons [49], less than 10% of ARHGHRH express Ghr mRNA [52]. This result indicates that PVSST neurons are the major hypophysiotropic neuronal population that can sense variations in GH levels to control the activity of the somatotropic axis via negative feedback. Nonetheless, early-in-life GHR ablation in SST neurons causes only minor effects on GH secretion and body growth [53], suggesting the existence of additional cell populations involved in the control of GH secretion via negative feedback loops [54].
The presence of Ghr mRNA in non-GHRH neurons suggests that GH action on ARH neurons regulates different physiological functions besides somatotropic axis activity. ARH neurons regulate food intake, energy expenditure, substrate mobilization, and glucose homeostasis [55]. ARH neurons are specialized in receiving information on several hormones that control metabolism, including leptin, insulin, ghrelin, and glucagon-like peptide-1. The high sensitivity of ARH neurons to hormones is possible because of its proximity to the median eminence (ME). ME makes the interface between the hypothalamus and pituitary gland and contains fenestrated capillaries that allow the diffusion of molecules with the size of GH (22 kDa) [56]. Thus, blood GH can enter the brain through ME vessels and bind the GHR present in ARH neurons without the typical limitations imposed by the blood-brain barrier (BBB).
Two independent studies identified the neurons that produce the neuropeptide Y (NPY) as the major neuronal population in the ARH that expresses the Ghr mRNA [57, 58]. ARHNPY neurons are potent inducers of hunger [59], are activated by food deprivation [60] or ghrelin [61], and are inhibited by anorexigenic hormones like leptin [62]. Of note, ARHNPY neurons express other neurotransmitters, especially the inhibitory γ-aminobutyric acid and the agouti-related protein (AgRP) [63, 64].
3.1.2. GH activates ARHNPY/AgRP neurons and stimulates hunger
Since 95% of ARHNPY/AgRP neurons express the Ghr mRNA [57, 58], the activity of these cells is likely regulated by GH. Accordingly, 65% of ARHNPY neurons express the gene c-Fos, a marker of neuronal activation, after acute GH administration [65]. Using whole-cell patch clamp, another study demonstrated that approximately one-third of ARHNPY/AgRP neurons are directly depolarized by GH [60]. Thus, GH activates a neuronal population that induces hunger. These findings are in accordance with early reports showing that GH treatment in GH-deficient children stimulates appetite [66, 67]. GH overexpression in the CNS results in hyperphagia-induced obesity [68]. Increased food intake was also observed in fish overexpressing GH [69, 70]. In mice, an acute intracerebroventricular GH injection increases 24-hour food intake [60]. Together, the data show that central GHR signaling stimulates hunger.
Noteworthy, GH-deficient mice do not exhibit ghrelin-induced feeding response [71]. Mice carrying a deficiency of ghrelin receptors in somatotropic cells exhibit a blunted ghrelin effect on both GH secretion and feeding [72]. GHR ablation in the brain also prevents the ability of ghrelin to increase food intake [73]. Thus, central GHR signaling seems necessary for the ghrelin-induced orexigenic response. However, more studies are required to determine the mechanisms behind the interaction between GH and ghrelin to control food intake.
The specific neuronal population responsible for mediating the orexigenic action of GH has not yet been identified. However, ARHNPY/AgRP neurons are major candidates because GH oversecretion in fish and mice leads to hyperphagia and increased hypothalamic Agrp or Npy mRNA expression [68, 70]. Even acute GH administration can significantly increase Agrp and Npy mRNA expression in the mouse hypothalamus [60]. In humans, plasma AgRP level is higher in patients with active acromegaly than in healthy subjects [74]. Surgical or pharmacological acromegaly treatment leads to decreased plasma AgRP levels [74]. Additionally, plasma AgRP concentration positively correlates with circulating GH and IGF-1 levels [74]. Thus, GH seems to regulate the activity of AgRP neurons in different mammalian and non-mammalian species.
3.1.3. GHR signaling in ARHNPY/AgRP neurons is necessary for the metabolic adaptations to food deprivation or cold exposure
Numerous brain areas exhibit NPY-expressing neurons, but AgRP expression is exclusively found in the ARH [63]. Thus, the selectivity of AgRP expression has been used to produce cell-specific manipulations in ARHNPY/AgRP neurons [64]. To investigate the physiological importance of GHR signaling in ARHNPY/AgRP neurons, a mouse model carrying inactivation of Ghr gene, specifically in AgRP-expressing cells was generated. Ad libitum-fed AgRPΔGHR mice exhibit normal body weight, somatic growth, GH secretion, circulating IGF-1 levels, body adiposity, food intake, and energy expenditure [60, 75]. Prolonged food deprivation increases ghrelin and GH secretion [76]. Starved mice exhibit a change in the pattern of GH secretion, from pulses with large amplitude and small interpulse GH secretion to a predominantly tonic secretion [75]. GHR signaling in ARHNPY/AgRP neurons is unnecessary for this change [75]. However, AgRPΔGHR mice show reduced activation of ARHNPY/AgRP neurons during fasting or prolonged food restriction [60]. In addition, starvation-induced suppression of the thyroid axis, reproduction, and markers of thermogenesis in brown adipose tissue (BAT) was prevented in AgRPΔGHR mice [60]. Consequently, the progressive reduction in energy expenditure exhibited by food-deprived mice is partially prevented in AgRPΔGHR mice, leading to higher weight and fat loss [60]. Furthermore, ablation of the STAT5 genes in AgRP neurons partially reproduces the phenotype of AgRPΔGHR mice during food restriction, suggesting that GHR signaling activates STAT5 transcription factors to produce its metabolic effects [77]. Thus, GH secretion during prolonged food deprivation triggers neuroendocrine adaptations that ultimately suppress energy expenditure via activation of ARHNPY/AgRP neurons.
Treatment with a GHR antagonist (pegvisomant) can induce partial prevention in energy-saving adaptations during food restriction [60]. Noteworthy, the effects of pegvisomant in sustaining energy expenditure during food restriction are similar to those produced by leptin [60]. It is worth mentioning that pegvisomant is not detected in the cerebrospinal fluid, indicating that this drug does not cross the BBB [78]. However, since most ARHNPY/AgRP neurons are near the ME and outside the BBB [79], pegvisomant may block GH action in these cells, explaining its metabolic effects during food restriction [60].
Another study demonstrated that AgRPΔGHR mice cannot adapt to variations in temperature, maintaining a lower core temperature when held at 10°C, 22°C, or 30°C compared to control mice [80]. AgRPΔGHR mice also exhibit defects in the activation of hypothalamic neurons and the expression of genes associated with thermogenesis in the BAT [80]. Furthermore, GHR signaling in ARHNPY/AgRP neurons is necessary for inducing alterations in the BAT gene expression when mice are exposed to thermoneutrality [80]. These findings reinforce the importance of GH action on ARHNPY/AgRP neurons to control aspects related to energy expenditure and thermogenesis.
3.1.4. Central GH action regulates glucoprivation- or pregnancy-induced hyperphagia
GH-induced activation of ARHNPY/AgRP neurons possibly stimulates hunger during food restriction, although additional studies are necessary to test this possibility. Glucoprivation causes a fast and transitory hyperphagia [81]. GHR ablation in AgRP or GABAergic neurons reduces glucoprivic hyperphagia [60, 82]. GHR ablation in pro-opiomelanocortin-expressing neurons, another ARH neuronal population that controls food intake [55], also reduces glucoprivation-induced hyperphagia [83]. Increased food intake is frequently observed in pregnancy [84]. Brain-specific GHR ablation, which primarily targets neurons, reduces food intake and body adiposity in pregnant mice [85]. In contrast, AgRPΔGHR mice exhibit normal food intake during pregnancy [85]. Thus, these studies indicate that different neuronal populations are likely involved in the effects of GH regulating food intake.
3.1.5. Neurotropic and neuroinflammatory effects of GH on the hypothalamus
Early-in-life metabolic insults can disturb the development of hypothalamic neurons that regulate metabolism [86]. Leptin or leptin receptor (LepR)-deficient mice exhibit a blunted development of the axonal projections of ARH neurons to important post-synaptic targets, like the paraventricular nucleus of the hypothalamus [87, 88]. Defects in the development of the neurocircuits that regulate metabolism may predispose individuals to obesity [86]. GH and IGF-1 can be considered neurotropic factors. Interestingly, GH but not IGF-1 deficiency reduces the density of the axonal projections from ARH neurons to different hypothalamic nuclei [89]. These effects depend on the GHR expression in ARHNPY/AgRP neurons since AgRPΔGHR mice have reduced density of AgRP axonal projections but normal pro-opiomelanocortin innervation [90].
GH-deficient mice exhibit reduced expression of inflammatory markers in the hypothalamus [89]. In contrast, GH oversecretion increases hypothalamic neuroinflammation [91]. These effects are mediated by GHR signaling since GHR ablation in neurons reduces the expression of pro-inflammatory markers in the hypothalamus [91]. In addition, the inactivation of GHR in hepatocytes increases GH secretion but drastically reduces circulating IGF-1 levels [91]. These mice show increased neuroinflammation in the hypothalamus, demonstrating the pro-inflammatory action of GH independently of IGF-1 secretion [91]. Importantly, hypothalamic neuroinflammation is tightly linked with metabolic diseases, such as obesity and diabetes mellitus [92]. Thus, GH secretion possibly affects the predisposition to metabolic diseases via its pro-inflammatory action on the hypothalamus.
3.2. Potential clinical implications of GH action regulating the activity of ARH neurons
The discovery that GH stimulates the activity of ARHNPY/AgRP neurons in rodents [60] and possibly humans [74] highlights the potential of this hormone to regulate hunger, energy expenditure, thermogenesis, and other metabolic aspects. These findings may explain variations in appetite under different physiological conditions. For example, increased appetite is observed in situations associated with high GH secretion, such as adolescence and pregnancy [3, 46, 47]. On the other hand, aging causes a decline in GH secretion and appetite [93]. These aspects must be considered when discussing possible hormone replacement therapy to treat GH-deficient individuals or prevent adverse effects of aging. Furthermore, the effects of GH-suppressing drugs, like pegvisomant or somatostatin receptor ligands, on feeding behavior and energy expenditure should be explored in future studies.
Dysfunctions in ARHNPY/AgRP neurons are a critical aspect observed in overnutrition, and it is part of the physiopathology of obesity, favoring the development of central leptin resistance [79, 94]. The potential role of GHR signaling in the development of obesity via alterations in the function of ARHNPY/AgRP neurons is unknown. However, GH induces neuroinflammation in the hypothalamus [89, 91], and hypothalamic inflammation predisposes animals to obesity [92, 94].
Weight loss decreases energy expenditure in humans [95] and rodents [96, 97], reducing the efficacy of obesity treatments and favoring weight regain. GHR signaling in ARHNPY/AgRP neurons exerts a robust modulation of adaptive responses that conserve energy during weight loss [60]. These effects are partially mediated by the STAT5 signaling pathway [77]. These findings reveal potential cellular pathways that can be manipulated to prevent the weight loss-induced reduction in energy expenditure and consequently increase the chances of long-term success in obesity treatment.
GH or GHR deficiency impairs the development of critical neuronal populations in the ARH that control metabolism independently of circulating IGF-1 levels [73, 89, 90]. Thus, alterations in GH secretion or action in crucial developmental periods may lead to long-term consequences on the neurocircuits that regulate food intake, energy expenditure, and body weight. The importance of adipokines, such as leptin and adiponectin, throughout development to affect the predisposition to metabolic diseases later in life is well-established [86]. Whether early-in-life dysfunctions in GHR signaling, especially in the CNS, can lead to metabolic programming is still unknown.
3.3. GH action in the brain prevents hypoglycemia and regulates insulin sensitivity
3.3.1. GHR signaling in hypothalamic neurons is necessary to induce a normal counter-regulatory response to hypoglycemia
Hypoglycemia induces a robust increase in GH secretion [98]. Spontaneous episodes of hypoglycemia in children are associated with paradoxically low serum GH levels [99]. Laron syndrome patients, characterized by GHR deficiency, frequently have juvenile hypoglycemia [100]. Juvenile hypoglycemia is also observed in GHR-deficient mice and pigs [100]. Thus, a blunted secretion/action of GH may reduce the ability of the organism to restore blood glucose to normal levels, especially in young individuals. GH increases blood glucose levels like counter-regulatory hormones like glucocorticoids, noradrenaline, and glucagon [101]. GH raises glycemia by stimulating hepatic glucose production and reducing glucose uptake [102]. It is currently thought that these effects are mediated by a direct action of GH on the liver, muscle, and adipose tissue and indirectly by inducing insulin resistance [8, 102].
The brain also plays an essential role in preventing hypoglycemia [101]. Glucose-sensing neurons distributed in different brain areas coordinate the counter-regulatory response (CRR) to avoid hypoglycemia and restore blood glucose to normal levels [101]. A significant number of GH-responsive neurons are found in the ventromedial nucleus of the hypothalamus (VMH) [9, 10], an area known to contain glucose-sensing neurons, and it is involved in the CRR [101, 103]. The physiological importance of GH action on VMH neurons was investigated in mice carrying ablation of GHR in steroidogenic factor 1 (SF1)-expressing neurons since SF1 expression in the brain is exclusively found in the VMH [104]. SF1ΔGHR mice exhibit no alterations in blood glucose levels, glucose tolerance, and insulin sensitivity. However, these mice present lower glycemia after insulin injection, suggesting defects in their ability to recover from insulin-induced hypoglycemia [104]. Furthermore, the CRR induced by 2-Deoxy-D-glucose (2DG) injection, which causes glucoprivation, is significantly attenuated in SF1ΔGHR mice, demonstrating defects in recovering from hypoglycemia [104]. The 2DG-induced secretion of counter-regulatory hormones is normal in SF1ΔGHR mice. In addition, pharmacological blockade of the sympathetic nervous system does not prevent the differences between the groups in the 2DG-induced CRR [104]. However, pharmacological inhibition of the parasympathetic nervous system restores the CRR induced by 2DG in SF1ΔGHR mice to levels found in control animals [104]. Finally, 2DG administration leads to an abnormally high activation of cholinergic neurons located at the dorsal motor nucleus of the vagus nerve (DMX), indicating that the absence of GH action in the VMH leads to a hyperactivation of the parasympathetic nervous system during the CRR. DMX neurons are also directly responsive to GH [105]. It is worth mentioning that the DMX is the primary source of vagal motor output to several abdominal organs, including the pancreas and liver. The parasympathetic nervous system inhibits endogenous glucose production. Thus, the hyperactivity of the parasympathetic nervous system shown by SF1ΔGHR mice during a CRR episode reduces hepatic glucose production when it is necessary to increase blood glucose levels to avoid hypoglycemia. These findings demonstrate that hypoglycemia-induced GH secretion modulates neurocircuits involved in the CRR, helping to recover blood glucose to normal levels.
3.3.2. Central GH action regulates systemic insulin sensitivity
Evidence shows that GHR signaling in specific neuronal populations can modulate insulin sensitivity in different tissues. For example, GHR signaling in LepR-expressing cells, which includes essential neuronal populations that control metabolism, regulates hepatic insulin sensitivity and peripheral lipid metabolism [106]. Another study showed that activation of GHR-expressing neurons in the ARH causes increases in glucose oxidation and, glucose uptake and insulin sensitivity in the muscle [12].
The diabetogenic effects of GH possibly explain the insulin resistance that naturally emerges in certain physiological situations associated with increased GH secretion, like puberty [107, 108] and late pregnancy [109]. While no study has demonstrated that GH is responsible for increasing insulin resistance in puberty, there is direct evidence that GH contributes to pregnancy-induced insulin resistance [85]. GHR ablation in LepR-expressing neurons or the entire brain causes a robust improvement in insulin tolerance and a reduction in circulating insulin levels in pregnant mice [85].
3.4. Potential clinical implications of GH action in the brain regulating glucose homeostasis
The brain controls glucose homeostasis by regulating systemic insulin sensitivity, defining the set point of circulating glucose levels, and modulating the secretion of hormones such as insulin, glucagon, and glucocorticoids. Most of these effects are mediated by changes in the sympathetic and parasympathetic nervous system activity [101]. Juvenile hypoglycemia is frequently associated with dysfunctions in the GH axis [99, 100]. The findings that GH-responsive neurons are involved in the CRR and the absence of GHR signaling in some of these neuronal populations blunts the CRR indicate a novel mechanism of action of GH to prevent hypoglycemia [100, 104].
It is undeniable that GH has a diabetogenic action [7, 8, 102, 110, 111]. The fact that central GH action regulates systemic insulin action [85] challenges the current paradigm postulating that GH-induced insulin resistance is exclusively mediated by an increase in FFA flux caused by the lipolytic effect of GH [7, 8, 102]. Future studies are necessary to identify the specific neural circuits involved in the central effects of GH regulating systemic insulin sensitivity. Importantly, this central mechanism does not discard the well-established insulin resistance caused by GH-induced lipotoxicity, but it adds a new mechanism to the already described ability of GH to regulate glucose homeostasis.
Gestational diabetes emerges when the increased insulin secretory capacity cannot compensate for the decrease in insulin sensitivity observed in pregnant individuals [109]. Previous studies indicate a 60% decrease in insulin sensitivity with normal pregnancy [109]. The usual management of gestational diabetes involves lifestyle modifications and insulin administration, if necessary. So, there is no specific drug to treat gestational diabetes. The finding that central GH action plays a significant role in inducing insulin resistance during pregnancy opens the possibility of partially blocking the action of GH, whose placental variant is highly secreted during pregnancy [47, 109], to treat severe cases of gestational diabetes. However, studies are needed to test the efficacy and safety of this possible treatment.
4. Miscellaneous effects of brain GH actions
4.1. Central GHR signaling regulates the adaptation capacity to aerobic exercise
Prolonged exercise induces GH secretion [48]. GH action during exercise provides important substrates to generate energy, either by stimulating hepatic glucose production or increasing FFAs [5, 7, 8]. Accordingly, GH-deficient patients frequently report decreased exercise capacity [112, 113]. To investigate the possible participation of central GHR signaling in regulating exercise tolerance, we generated mice carrying GHR ablation in VMH or LepR-expressing neurons [114]. VMHΔGHR and LepRΔGHR mice showed no alterations in their basal exercise performance tested on a treadmill or in voluntary wheel running [114]. However, after eight weeks of treadmill training, while LepRΔGHR mice exhibited an increased aerobic capacity compared to control animals, VMHΔGHR mice could not improve their exercise performance above baseline values [114]. The capacity to improve exercise performance in these mouse models was associated with different glycemic changes during exercise [114]. These findings suggest that central GHR signaling affects the adaptation capacity to aerobic exercise.
4.2. Central GH action regulates ventilatory response to hypoxia
Several brain areas that control breathing behaviors, including tyrosine hydroxylase-expressing cells in the rostroventrolateral medulla (C1 region) and locus coeruleus, contain GH-responsive neurons [54, 115]. Interestingly, acromegaly can lead to respiratory problems [4]. GH treatment improves respiratory parameters and decreases central apnea in Prader-Willi syndrome patients [116, 117]. The potential importance of central GHR signaling in modulating the respiratory activity of conscious unrestrained mice was investigated in brain-specific GHR knockout mice [115]. Control and brain-specific knockout GHR mice exhibit no differences in basal breathing and the hypercapnic ventilatory response [115]. However, tachypneic response to hypoxia is significantly attenuated in knockout mice [115]. These findings suggest a modest but significant effect of central GHR signaling modulating ventilatory response in stress situations, such as hypoxia.
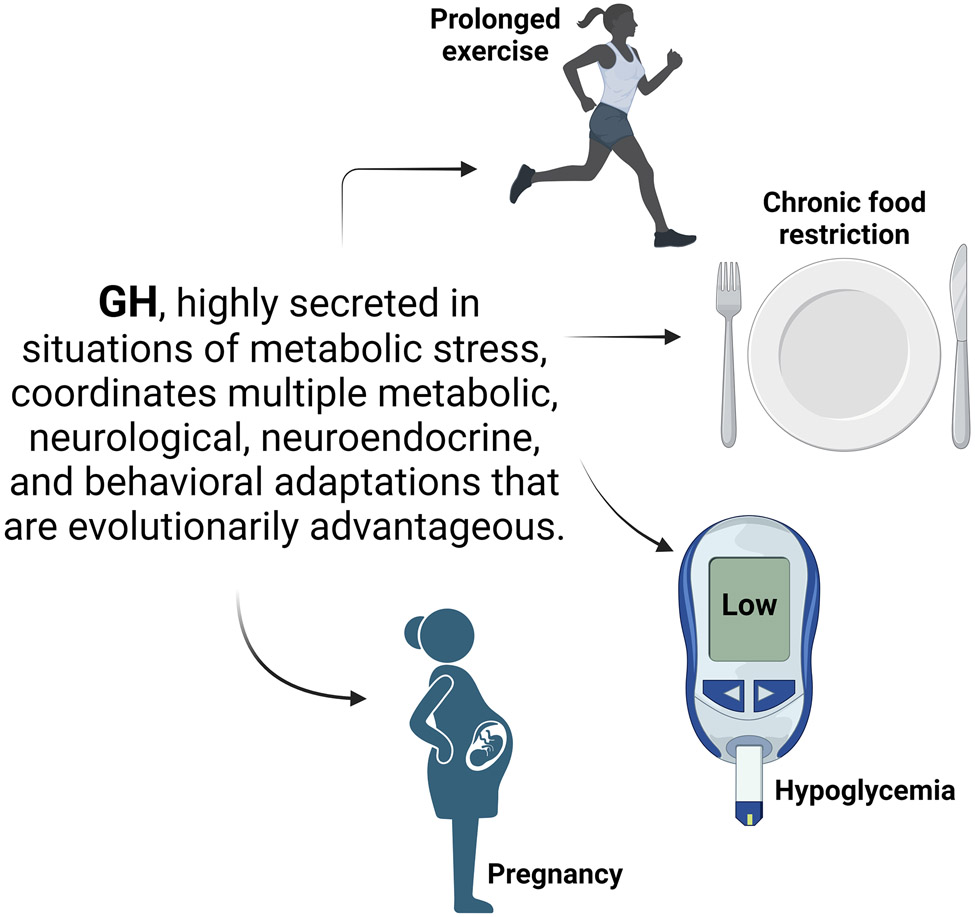
5. Concluding remarks
The present review summarized and discussed the recent findings indicating that GH can act in several neuronal populations to control distinct physiological aspects such as metabolism, neuroplasticity, stress response, behavior, mood, and cognition. As a major growth factor, GH presents developmental functions in the CNS, regulating synaptic function, axonal growth, and the formation of neural circuits. However, the most remarkable effects of central GH action are not associated with growth/development but involve physiological adjustments to restore homeostasis in situations of metabolic stress, such as puberty, pregnancy, chronic food deprivation, hypoglycemia, and prolonged exercise (Figure 2). Interestingly, all these conditions are characterized by elevated GH secretion. So, in these situations, the “growth function is left aside”, and GH may represent an internal signal that coordinates multiple metabolic, neurological, neuroendocrine, and behavioral adaptations that are evolutionarily advantageous and, therefore, increase the chances of survival [118]. For example, the GH-induced activation of ARHNPY/AgRP neurons likely stimulates hunger. Consequently, it increases energy availability in high metabolic demand situations (e.g., puberty, pregnancy, and exercise) or during insufficient supply of nutrients (e.g., hypoglycemia and food restriction). GH can act in different neuronal populations to prevent hypoglycemia and induce energy-saving adaptations, prolonging the time available to find food before dying of starvation. GH-induced insulin resistance is likely advantageous in metabolic stress situations because it saves glucose for processes that really depend on this nutrient (e.g., glucose-dependent cells or a growing fetus), increases hepatic gluconeogenesis, prevents hypoglycemia, and blocks the anti-lipolytic effect of insulin. It is essential to highlight that GH's central and peripheral metabolic actions are coordinated. Thus, GH action on adipose tissue increases the availability of substrates for gluconeogenesis (e.g., glycerol) and oxidation (e.g., FFAs). Increased levels of FFAs reduce glucose dependence and favor insulin resistance positively feeding back this cycle. Finally, the anxiolytic effect of GH seems evolutionarily advantageous when animals are under metabolic stress because it favors behavioral reactions that allow them to leave these potentially dangerous situations. However, GH action in the amygdala may be behind maladaptation to chronic stress, leading to excessive fear memory and the development of post-traumatic stress disorders. Taken together, the brain is an important target of GH, and GHR signaling in different brain areas regulates essential physiological functions.
Figure 2.
Scheme illustrating GH's role in metabolic stress situations, like prolonged exercise, chronic food restriction, hypoglycemia, and pregnancy. In all these conditions, GH is highly secreted. Central and peripheral GH action coordinates several physiological responses that help to restore homeostasis and are evolutionarily advantageous. Created with BioRender.com (Agreement number: DV260AQLCU).
Acknowledgments
JDJ was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP/Brazil; grant number: 2020/01318-8) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq/Brazil; grant number: 303363/2019-3). JJK was supported, in part, by the State of Ohio’s Eminent Scholar Program that includes a gift from Milton and Lawrence Goll, National Institutes of Health grants NIH- R21AG081962, NIH -2R01AG059779, NIH -R01AR081804, NIH R25DK122952, the Edison Biotechnology Institute and Diabetes Institute at Ohio University.
Abbreviations
- 2DG
2-Deoxy-D-glucose
- AgRP
agouti-related protein
- ARH
arcuate nucleus of the hypothalamus
- BLA
basolateral amygdala
- BAT
brown adipose tissue
- BBB
blood-brain barrier
- CNS
central nervous system
- CEA
central nucleus of the amygdala
- CRR
counter-regulatory response
- DMX
dorsal motor nucleus of the vagus nerve
- FFAs
free fatty acids
- GH
growth hormone
- GHR
growth hormone receptor
- GHRH
growth hormone-resealing hormone
- IGF-1
insulin-like growth factor 1
- LepR
leptin receptor
- ME
median eminence
- NPY
neuropeptide Y
- PV
periventricular nucleus
- STAT5
signal transducer and activator of transcription 5
- SST
somatostatin
- SF1
steroidogenic factor 1
- VMH
ventromedial nucleus of the hypothalamus
Footnotes
Competing Interests and Funding
The authors declare they have no financial interests.
References
- [1].Steyn FJ, Tolle V, Chen C, Epelbaum J. Neuroendocrine Regulation of Growth Hormone Secretion. Compr Physiol 2016;6:687–735. [DOI] [PubMed] [Google Scholar]
- [2].Murray PG, Higham CE, Clayton PE. 60 years of neuroendocrinology: The hypothalamo-GH axis: the past 60 years. J Endocrinol 2015;226:T123–40. [DOI] [PubMed] [Google Scholar]
- [3].Ojeda SR, Jameson HE. Developmental patterns of plasma and pituitary growth hormone (GH) in the female rat. Endocrinology 1977;100:881–9. [DOI] [PubMed] [Google Scholar]
- [4].Pivonello R, Auriemma RS, Grasso LF, Pivonello C, Simeoli C, Patalano R, Galdiero M, Colao A. Complications of acromegaly: cardiovascular, respiratory and metabolic comorbidities. Pituitary 2017;20:46–62. [DOI] [PubMed] [Google Scholar]
- [5].Dehkhoda F, Lee CMM, Medina J, Brooks AJ. The Growth Hormone Receptor: Mechanism of Receptor Activation, Cell Signaling, and Physiological Aspects. Front Endocrinol (Lausanne) 2018;9:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].List EO, Berryman DE, Funk K, Jara A, Kelder B, Wang F, Stout MB, Zhi X, Sun L, White TA, LeBrasseur NK, Pirtskhalava T, Tchkonia T, Jensen EA, Zhang W, Masternak MM, Kirkland JL, Miller RA, Bartke A, Kopchick JJ. Liver-specific GH receptor gene-disrupted (LiGHRKO) mice have decreased endocrine IGF-I, increased local IGF-I, and altered body size, body composition, and adipokine profiles. Endocrinology 2014;155:1793–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Sharma R, Kopchick JJ, Puri V, Sharma VM. Effect of growth hormone on insulin signaling. Mol Cell Endocrinol 2020;518:111038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kopchick JJ, Berryman DE, Puri V, Lee KY, Jorgensen JOL. The effects of growth hormone on adipose tissue: old observations, new mechanisms. Nat Rev Endocrinol 2020;16:135–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Wasinski F, Klein MO, Bittencourt JC, Metzger M, Donato J Jr. Distribution of growth hormone-responsive cells in the brain of rats and mice. Brain Res 2021;1751:147189. [DOI] [PubMed] [Google Scholar]
- [10].Furigo IC, Metzger M, Teixeira PD, Soares CR, Donato J Jr. Distribution of growth hormone-responsive cells in the mouse brain. Brain Struct Funct 2017;222:341–63. [DOI] [PubMed] [Google Scholar]
- [11].Zhai Q, Lai Z, Roos P, Nyberg F. Characterization of growth hormone binding sites in rat brain. Acta Paediatr 1994;406:92–5. [DOI] [PubMed] [Google Scholar]
- [12].de Lima JBM, Debarba LK, Rupp AC, Qi N, Ubah C, Khan M, Didyuk O, Ayyar I, Koch M, Sandoval DA, Sadagurski M. ARC(GHR) Neurons Regulate Muscle Glucose Uptake. Cells 2021;10:1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Rogers KV, Vician L, Steiner RA, Clifton DK. The effect of hypophysectomy and growth hormone administration on pre-prosomatostatin messenger ribonucleic acid in the periventricular nucleus of the rat hypothalamus. Endocrinology 1988;122:586–91. [DOI] [PubMed] [Google Scholar]
- [14].Tannenbaum GS, Guyda HJ, Posner BI. Insulin-like growth factors: a role in growth hormone negative feedback and body weight regulation via brain. Science 1983;220:77–9. [DOI] [PubMed] [Google Scholar]
- [15].Brown-Borg HM. Hormonal control of aging in rodents: the somatotropic axis. Mol Cell Endocrinol 2009;299:64–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].de Paula DG, Bohlen TM, Zampieri TT, Mansano NS, Vieira HR, Gusmao DO, Wasinski F, Donato J Jr., Frazao R. Distinct effects of growth hormone deficiency and disruption of hypothalamic kisspeptin system on reproduction of male mice. Life Sci 2021;285:119970. [DOI] [PubMed] [Google Scholar]
- [17].Bohlen TM, Zampieri TT, Furigo IC, Teixeira PD, List EO, Kopchick J, Donato J Jr., Frazao R. Central growth hormone signaling is not required for the timing of puberty. J Endocrinol 2019;243:161–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Silveira MA, Zampieri TT, Furigo IC, Abdulkader F, Donato J Jr., Frazao R. Acute effects of somatomammotropin hormones on neuronal components of the hypothalamic-pituitary-gonadal axis. Brain Res 2019;1714:210–7. [DOI] [PubMed] [Google Scholar]
- [19].Wasinski F, Chaves FM, Pedroso JAB, Mansano NS, Camporez JP, Gusmao DO, List EO, Kopchick JJ, Frazao R, Szawka RE, Donato J Jr. Growth hormone receptor in dopaminergic neurones regulates stress-induced prolactin release in male mice. J Neuroendocrinol 2021;33:e12957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Le Greves M, Steensland P, Le Greves P, Nyberg F. Growth hormone induces age-dependent alteration in the expression of hippocampal growth hormone receptor and N-methyl-D-aspartate receptor subunits gene transcripts in male rats. Proc Natl Acad Sci U S A 2002;99:7119–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Mahmoud GS, Grover LM. Growth hormone enhances excitatory synaptic transmission in area CA1 of rat hippocampus. J Neurophysiol 2006;95:2962–74. [DOI] [PubMed] [Google Scholar]
- [22].Molina DP, Ariwodola OJ, Linville C, Sonntag WE, Weiner JL, Brunso-Bechtold JK, Adams MM. Growth hormone modulates hippocampal excitatory synaptic transmission and plasticity in old rats. Neurobiol Aging 2012;33:1938–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Nyberg F, Hallberg M. Growth hormone and cognitive function. Nat Rev Endocrinol 2013;9:357–65. [DOI] [PubMed] [Google Scholar]
- [24].Ramis M, Sarubbo F, Sola J, Aparicio S, Garau C, Miralles A, Esteban S. Cognitive improvement by acute growth hormone is mediated by NMDA and AMPA receptors and MEK pathway. Prog Neuropsychopharmacol Biol Psychiatry 2013;45:11–20. [DOI] [PubMed] [Google Scholar]
- [25].Furigo IC, Melo HM, Lyra ESNM, Ramos-Lobo AM, Teixeira PDS, Buonfiglio DC, Wasinski F, Lima ER, Higuti E, Peroni CN, Bartolini P, Soares CRJ, Metzger M, de Felice FG, Donato J Jr. Brain STAT5 signaling modulates learning and memory formation. Brain Struct Funct 2018;223:2229–41. [DOI] [PubMed] [Google Scholar]
- [26].Molina DP, Ariwodola OJ, Weiner JL, Brunso-Bechtold JK, Adams MM. Growth hormone and insulin-like growth factor-I alter hippocampal excitatory synaptic transmission in young and old rats. Age (Dordr) 2013;35:1575–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Le Greves M, Le Greves P, Nyberg F. Age-related effects of IGF-1 on the NMDA-, GH- and IGF-1-receptor mRNA transcripts in the rat hippocampus. Brain Res Bull 2005;65:369–74. [DOI] [PubMed] [Google Scholar]
- [28].Fleming T, Martinez-Moreno CG, Carranza M, Luna M, Harvey S, Aramburo C. Growth hormone promotes synaptogenesis and protects neuroretinal dendrites against kainic acid (KA) induced damage. Gen Comp Endocrinol 2018;265:111–20. [DOI] [PubMed] [Google Scholar]
- [29].Scheepens A, Sirimanne ES, Breier BH, Clark RG, Gluckman PD, Williams CE. Growth hormone as a neuronal rescue factor during recovery from CNS injury. Neuroscience 2001;104:677–87. [DOI] [PubMed] [Google Scholar]
- [30].Aberg ND, Brywe KG, Isgaard J. Aspects of growth hormone and insulin-like growth factor-I related to neuroprotection, regeneration, and functional plasticity in the adult brain. ScientificWorldJournal 2006;6:53–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Baltazar-Lara R, Zenil JM, Carranza M, Avila-Mendoza J, Martinez-Moreno CG, Aramburo C, Luna M. Growth Hormone (GH) Crosses the Blood-Brain Barrier (BBB) and Induces Neuroprotective Effects in the Embryonic Chicken Cerebellum after a Hypoxic Injury. Int J Mol Sci 2022;23:11546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Bianchi VE, Locatelli V, Rizzi L. Neurotrophic and Neuroregenerative Effects of GH/IGF1. Int J Mol Sci 2017;18:2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Hascup KN, Lynn MK, Fitzgerald PJ, Randall S, Kopchick JJ, Boger HA, Bartke A, Hascup ER. Enhanced Cognition and Hypoglutamatergic Signaling in a Growth Hormone Receptor Knockout Mouse Model of Successful Aging. J Gerontol A Biol Sci Med Sci 2017;72:329–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Basu A, McFarlane HG, Kopchick JJ. Spatial learning and memory in male mice with altered growth hormone action. Horm Behav 2017;93:18–30. [DOI] [PubMed] [Google Scholar]
- [35].Bomfim TR, Forny-Germano L, Sathler LB, Brito-Moreira J, Houzel JC, Decker H, Silverman MA, Kazi H, Melo HM, McClean PL, Holscher C, Arnold SE, Talbot K, Klein WL, Munoz DP, Ferreira ST, De Felice FG. An anti-diabetes agent protects the mouse brain from defective insulin signaling caused by Alzheimer's disease-associated Abeta oligomers. J Clin Invest 2012;122:1339–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Talbot K, Wang HY, Kazi H, Han LY, Bakshi KP, Stucky A, Fuino RL, Kawaguchi KR, Samoyedny AJ, Wilson RS, Arvanitakis Z, Schneider JA, Wolf BA, Bennett DA, Trojanowski JQ, Arnold SE. Demonstrated brain insulin resistance in Alzheimer's disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J Clin Invest 2012;122:1316–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Koibuchi N, Kakegawa T, Suzuki M. Electrical stimulation of the basolateral amygdala elicits only growth hormone secretion among six anterior pituitary hormones in the pentobarbital-anesthetized male rat. J Neuroendocrinol 1991;3:685–7. [DOI] [PubMed] [Google Scholar]
- [38].Meyer RM, Burgos-Robles A, Liu E, Correia SS, Goosens KA. A ghrelin-growth hormone axis drives stress-induced vulnerability to enhanced fear. Mol Psychiatry 2014;19:1284–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Gisabella B, Farah S, Peng X, Burgos-Robles A, Lim SH, Goosens KA. Growth hormone biases amygdala network activation after fear learning. Transl Psychiatry 2016;6:e960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Dos Santos WO, Gusmao DO, Wasinski F, List EO, Kopchick JJ, Donato J Jr. Effects of Growth Hormone Receptor Ablation in Corticotropin-Releasing Hormone Cells. Int J Mol Sci 2021;22:9908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Dos Santos WO, Juliano VAL, Chaves FM, Vieira HR, Frazao R, List EO, Kopchick JJ, Munhoz CD, Donato J Jr. Growth Hormone Action in Somatostatin Neurons Regulates Anxiety and Fear Memory. J Neurosci 2023;43:6816–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Araya P, Kinning KT, Coughlan C, Smith KP, Granrath RE, Enriquez-Estrada BA, Worek K, Sullivan KD, Rachubinski AL, Wolter-Warmerdam K, Hickey F, Galbraith MD, Potter H, Espinosa JM. IGF1 deficiency integrates stunted growth and neurodegeneration in Down syndrome. Cell Rep 2022;41:111883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Karachaliou FH, Karavanaki K, Simatou A, Tsintzou E, Skarakis NS, Kanaka-Gatenbein C. Association of growth hormone deficiency (GHD) with anxiety and depression: experimental data and evidence from GHD children and adolescents. Hormones (Athens) 2021;20:679–89. [DOI] [PubMed] [Google Scholar]
- [44].Kaye WH, Bulik CM, Thornton L, Barbarich N, Masters K. Comorbidity of anxiety disorders with anorexia and bulimia nervosa. Am J Psychiatry 2004;161:2215–21. [DOI] [PubMed] [Google Scholar]
- [45].Misra M, Klibanski A. Endocrine consequences of anorexia nervosa. Lancet Diabetes Endocrinol 2014;2:581–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Wasinski F, Teixeira PDS, List EO, Kopchick JJ, Donato J Jr. Growth hormone receptor contributes to the activation of STAT5 in the hypothalamus of pregnant mice. Neurosci Lett 2022;770:136402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Liao S, Vickers MH, Stanley JL, Baker PN, Perry JK. Human Placental Growth Hormone Variant in Pathological Pregnancies. Endocrinology 2018;159:2186–98. [DOI] [PubMed] [Google Scholar]
- [48].Nindl BC, Pierce JR, Rarick KR, Tuckow AP, Alemany JA, Sharp MA, Kellogg MD, Patton JF. Twenty-hour growth hormone secretory profiles after aerobic and resistance exercise. Med Sci Sports Exerc 2014;46:1917–27. [DOI] [PubMed] [Google Scholar]
- [49].Burton KA, Kabigting EB, Clifton DK, Steiner RA. Growth hormone receptor messenger ribonucleic acid distribution in the adult male rat brain and its colocalization in hypothalamic somatostatin neurons. Endocrinology 1992;131:958–63. [DOI] [PubMed] [Google Scholar]
- [50].Walsh RJ, Mangurian LP, Posner BI. The distribution of lactogen receptors in the mammalian hypothalamus: an in vitro autoradiographic analysis of the rabbit and rat. Brain Res 1990;530:1–11. [DOI] [PubMed] [Google Scholar]
- [51].Fodor M, Kordon C, Epelbaum J. Anatomy of the hypophysiotropic somatostatinergic and growth hormone-releasing hormone system minireview. Neurochem Res 2006;31:137–43. [DOI] [PubMed] [Google Scholar]
- [52].Burton KA, Kabigting EB, Steiner RA, Clifton DK. Identification of target cells for growth hormone's action in the arcuate nucleus. Am J Physiol 1995;269:E716–22. [DOI] [PubMed] [Google Scholar]
- [53].Chaves FM, Wasinski F, Tavares MR, Mansano NS, Frazao R, Gusmao DO, Quaresma PGF, Pedroso JAB, Elias CF, List EO, Kopchick JJ, Szawka RE, Donato J. Effects of the Isolated and Combined Ablation of Growth Hormone and IGF-1 Receptors in Somatostatin Neurons. Endocrinology 2022;163:bqac045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Wasinski F, Pedroso JAB, Dos Santos WO, Furigo IC, Garcia-Galiano D, Elias CF, List EO, Kopchick JJ, Szawka RE, Donato J Jr. Tyrosine Hydroxylase Neurons Regulate Growth Hormone Secretion via Short-Loop Negative Feedback. J Neurosci 2020;40:4309–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Andermann ML, Lowell BB. Toward a Wiring Diagram Understanding of Appetite Control. Neuron 2017;95:757–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Schaeffer M, Langlet F, Lafont C, Molino F, Hodson DJ, Roux T, Lamarque L, Verdie P, Bourrier E, Dehouck B, Baneres JL, Martinez J, Mery PF, Marie J, Trinquet E, Fehrentz JA, Prevot V, Mollard P. Rapid sensing of circulating ghrelin by hypothalamic appetite-modifying neurons. Proc Natl Acad Sci U S A 2013;110:1512–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Chan Y, Steiner R, Clifton D. Regulation of hypothalamic neuropeptide-Y neurons by growth hormone in the rat. Endocrinology 1996;137:1319–25. [DOI] [PubMed] [Google Scholar]
- [58].Kamegai J, Minami S, Sugihara H, Hasegawa O, Higuchi H, Wakabayashi I. Growth hormone receptor gene is expressed in neuropeptide Y neurons in hypothalamic arcuate nucleus of rats. Endocrinology 1996;137:2109–12. [DOI] [PubMed] [Google Scholar]
- [59].Krashes MJ, Koda S, Ye C, Rogan SC, Adams AC, Cusher DS, Maratos-Flier E, Roth BL, Lowell BB. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J Clin Invest 2011;121:1424–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Furigo IC, Teixeira PDS, de Souza GO, Couto GCL, Romero GG, Perello M, Frazao R, Elias LL, Metzger M, List EO, Kopchick JJ, Donato J Jr. Growth hormone regulates neuroendocrine responses to weight loss via AgRP neurons. Nat Commun 2019;10:662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Wang Q, Liu C, Uchida A, Chuang JC, Walker A, Liu T, Osborne-Lawrence S, Mason BL, Mosher C, Berglund ED, Elmquist JK, Zigman JM. Arcuate AgRP neurons mediate orexigenic and glucoregulatory actions of ghrelin. Mol Metab 2014;3:64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Schwartz MW, Seeley RJ, Campfield LA, Burn P, Baskin DG. Identification of targets of leptin action in rat hypothalamus. J Clin Invest 1996;98:1101–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Hahn TM, Breininger JF, Baskin DG, Schwartz MW. Coexpression of Agrp and NPY in fasting-activated hypothalamic neurons. Nat Neurosci 1998;1:271–2. [DOI] [PubMed] [Google Scholar]
- [64].Tong Q, Ye C-P, Jones JE, Elmquist JK, Lowell BB. Synaptic release of GABA by AgRP neurons is required for normal regulation of energy balance. Nat Neurosci 2008;11:998–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Kamegai J, Minami S, Sugihara H, Higuchi H, Wakabayashi I. Growth hormone induces expression of the c-fos gene on hypothalamic neuropeptide-Y and somatostatin neurons in hypophysectomized rats. Endocrinology 1994;135:2765–71. [DOI] [PubMed] [Google Scholar]
- [66].Nyberg F. Growth hormone in the brain: characteristics of specific brain targets for the hormone and their functional significance. Front Neuroendocrinol 2000;21:330–48. [DOI] [PubMed] [Google Scholar]
- [67].Blissett J, Harris G, Kirk J. Effect of growth hormone therapy on feeding problems and food intake in children with growth disorders. Acta Paediatr 2000;89:644–9. [DOI] [PubMed] [Google Scholar]
- [68].Bohlooly YM, Olsson B, Bruder CE, Linden D, Sjogren K, Bjursell M, Egecioglu E, Svensson L, Brodin P, Waterton JC, Isaksson OG, Sundler F, Ahren B, Ohlsson C, Oscarsson J, Tornell J. Growth hormone overexpression in the central nervous system results in hyperphagia-induced obesity associated with insulin resistance and dyslipidemia. Diabetes 2005;54:51–62. [DOI] [PubMed] [Google Scholar]
- [69].Zhong C, Song Y, Wang Y, Zhang T, Duan M, Li Y, Liao L, Zhu Z, Hu W. Increased food intake in growth hormone-transgenic common carp (Cyprinus carpio L.) may be mediated by upregulating Agouti-related protein (AgRP). Gen Comp Endocrinol 2013;192:81–8. [DOI] [PubMed] [Google Scholar]
- [70].Kim JH, Leggatt RA, Chan M, Volkoff H, Devlin RH. Effects of chronic growth hormone overexpression on appetite-regulating brain gene expression in coho salmon. Mol Cell Endocrinol 2015;413:178–88. [DOI] [PubMed] [Google Scholar]
- [71].Egecioglu E, Bjursell M, Ljungberg A, Dickson SL, Kopchick JJ, Bergstrom G, Svensson L, Oscarsson J, Tornell J, Bohlooly YM. Growth hormone receptor deficiency results in blunted ghrelin feeding response, obesity, and hypolipidemia in mice. Am J Physiol Endocrinol Metab 2006;290:E317–25. [DOI] [PubMed] [Google Scholar]
- [72].Gupta D, Patterson AM, Osborne-Lawrence S, Bookout AL, Varshney S, Shankar K, Singh O, Metzger NP, Richard CP, Wyler SC, Elmquist JK, Zigman JM. Disrupting the ghrelin-growth hormone axis limits ghrelin's orexigenic but not glucoregulatory actions. Mol Metab 2021;53:101258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Wasinski F, Barrile F, Pedroso JAB, Quaresma PGF, Dos Santos WO, List EO, Kopchick JJ, Perello M, Donato J. Ghrelin-induced Food Intake, but not GH Secretion, Requires the Expression of the GH Receptor in the Brain of Male Mice. Endocrinology 2021;162:bqab097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Freda PU, Reyes-Vidal C, Jin Z, Pugh M, Panigrahi SK, Bruce JN, Wardlaw SL. Plasma Agouti-Related Protein Levels in Acromegaly and Effects of Surgical or Pegvisomant Therapy. J Clin Endocrinol Metab 2019;104:5453–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].de Sousa ME, Gusmao DO, Dos Santos WO, Moriya HT, de Lima FF, List EO, Kopchick JJ, Donato J Jr. Fasting and prolonged food restriction differentially affect GH secretion independently of GH receptor signaling in AgRP neurons. J Neuroendocrinol 2023:e13254. [DOI] [PubMed] [Google Scholar]
- [76].Zhao TJ, Liang G, Li RL, Xie X, Sleeman MW, Murphy AJ, Valenzuela DM, Yancopoulos GD, Goldstein JL, Brown MS. Ghrelin O-acyltransferase (GOAT) is essential for growth hormone-mediated survival of calorie-restricted mice. Proc Natl Acad Sci U S A 2010;107:7467–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Furigo IC, Teixeira PD, Quaresma PGF, Mansano NS, Frazao R, Donato J. STAT5 ablation in AgRP neurons increases female adiposity and blunts food restriction adaptations. J Mol Endocrinol 2020;64:13–27. [DOI] [PubMed] [Google Scholar]
- [78].Veldhuis JD, Bidlingmaier M, Bailey J, Erickson D, Sandroni P. A pegylated growth hormone receptor antagonist, pegvisomant, does not enter the brain in humans. J Clin Endocrinol Metab 2010;95:3844–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Olofsson LE, Unger EK, Cheung CC, Xu AW. Modulation of AgRP-neuronal function by SOCS3 as an initiating event in diet-induced hypothalamic leptin resistance. Proc Natl Acad Sci U S A 2013;110:E697–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Stilgenbauer L, de Lima JBM, Debarba LK, Khan M, Koshko L, Kopchick JJ, Bartke A, Schneider A, Sadagurski M. Growth hormone receptor (GHR) in AgRP neurons regulates thermogenesis in a sex-specific manner. Geroscience 2023;45:1745–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Thompson CI, Zagon IS, McLaughlin PJ. Hypophagia follows the initial hyperphagia produced by 2-deoxy-D-glucose in rats. Physiol Behav 1979;23:187–90. [DOI] [PubMed] [Google Scholar]
- [82].Dos Santos WO, Wasinski F, Tavares MR, Campos AMP, Elias CF, List EO, Kopchick JJ, Szawka RE, Donato J. Ablation of Growth Hormone Receptor in GABAergic Neurons Leads to Increased Pulsatile Growth Hormone Secretion. Endocrinology 2022;163:bqac103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Quaresma PGF, Teixeira PDS, Furigo IC, Wasinski F, Couto GC, Frazao R, List EO, Kopchick JJ, Donato J Jr. Growth hormone/STAT5 signaling in proopiomelanocortin neurons regulates glucoprivic hyperphagia. Mol Cell Endocrinol 2019;498:110574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Zampieri TT, Ramos-Lobo AM, Furigo IC, Pedroso JA, Buonfiglio DC, Donato J Jr. SOCS3 deficiency in leptin receptor-expressing cells mitigates the development of pregnancy-induced metabolic changes. Mol Metab 2015;4:237–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Teixeira PDS, Couto GC, Furigo IC, List EO, Kopchick JJ, Donato J Jr. Central growth hormone action regulates metabolism during pregnancy. Am J Physiol Endocrinol Metab 2019;317:E925–E40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Donato J Jr. Programming of metabolism by adipokines during development. Nat Rev Endocrinol 2023;19:385–97. [DOI] [PubMed] [Google Scholar]
- [87].Bouret SG, Draper SJ, Simerly RB. Trophic Action of Leptin on Hypothalamic Neurons That Regulate Feeding. Science 2004;304:108–10. [DOI] [PubMed] [Google Scholar]
- [88].Ramos-Lobo AM, Teixeira PD, Furigo IC, Melo HM, de Lyra E. Silva NM, De Felice FG, Donato J Jr. Long-term consequences of the absence of leptin signaling in early life. Elife 2019;8:e40970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Sadagurski M, Landeryou T, Cady G, Kopchick JJ, List EO, Berryman DE, Bartke A, Miller RA. Growth hormone modulates hypothalamic inflammation in long-lived pituitary dwarf mice. Aging Cell 2015;14:1045–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Wasinski F, Furigo IC, Teixeira PDS, Ramos-Lobo AM, Peroni CN, Bartolini P, List EO, Kopchick JJ, Donato J Jr. Growth Hormone Receptor Deletion Reduces the Density of Axonal Projections from Hypothalamic Arcuate Nucleus Neurons. Neuroscience 2020;434:136–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Wasinski F, Tavares MR, Gusmao DO, List EO, Kopchick JJ, Alves GA, Frazao R, Donato J Jr. Central growth hormone action regulates neuroglial and proinflammatory markers in the hypothalamus of male mice. Neurosci Lett 2023;806:137236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Thaler JP, Choi SJ, Schwartz MW, Wisse BE. Hypothalamic inflammation and energy homeostasis: resolving the paradox. Front Neuroendocrinol 2010;31:79–84. [DOI] [PubMed] [Google Scholar]
- [93].Donini LM, Savina C, Cannella C. Eating habits and appetite control in the elderly: the anorexia of aging. Int Psychogeriatr 2003;15:73–87. [DOI] [PubMed] [Google Scholar]
- [94].Zhang X, Zhang G, Zhang H, Karin M, Bai H, Cai D. Hypothalamic IKKbeta/NF-kappaB and ER stress link overnutrition to energy imbalance and obesity. Cell 2008;135:61–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Leibel RL, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight. N Engl J Med 1995;332:621–8. [DOI] [PubMed] [Google Scholar]
- [96].Pedroso JAB, Wasinski F, Donato J Jr. Prolonged fasting induces long-lasting metabolic consequences in mice. J Nutr Biochem 2020;84:108457. [DOI] [PubMed] [Google Scholar]
- [97].Wang D, Townsend LK, DesOrmeaux GJ, Frangos SM, Batchuluun B, Dumont L, Kuhre RE, Ahmadi E, Hu S, Rebalka IA, Gautam J, Jabile MJT, Pileggi CA, Rehal S, Desjardins EM, Tsakiridis EE, Lally JSV, Juracic ES, Tupling AR, Gerstein HC, Pare G, Tsakiridis T, Harper ME, Hawke TJ, Speakman JR, Blondin DP, Holloway GP, Jorgensen SB, Steinberg GR. GDF15 promotes weight loss by enhancing energy expenditure in muscle. Nature 2023;619:143–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Roth J, Glick SM, Yalow RS, Berson SA. Hypoglycemia: a potent stimulus to secretion of growth hormone. Science 1963;140:987–8. [DOI] [PubMed] [Google Scholar]
- [99].Hussain K, Hindmarsh P, Aynsley-Green A. Spontaneous hypoglycemia in childhood is accompanied by paradoxically low serum growth hormone and appropriate cortisol counterregulatory hormonal responses. J Clin Endocrinol Metab 2003;88:3715–23. [DOI] [PubMed] [Google Scholar]
- [100].Hinrichs A, Renner S, Bidlingmaier M, Kopchick JJ, Wolf E. MECHANISMS IN ENDOCRINOLOGY: Transient juvenile hypoglycemia in growth hormone receptor deficiency - mechanistic insights from Laron syndrome and tailored animal models. Eur J Endocrinol 2021;185:R35–R47. [DOI] [PubMed] [Google Scholar]
- [101].Verberne AJ, Sabetghadam A, Korim WS. Neural pathways that control the glucose counterregulatory response. Front Neurosci 2014;8:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Moller N, Jorgensen JO. Effects of growth hormone on glucose, lipid, and protein metabolism in human subjects. Endocr Rev 2009;30:152–77. [DOI] [PubMed] [Google Scholar]
- [103].Tong Q, Ye C, McCrimmon RJ, Dhillon H, Choi B, Kramer MD, Yu J, Yang Z, Christiansen LM, Lee CE, Choi CS, Zigman JM, Shulman GI, Sherwin RS, Elmquist JK, Lowell BB. Synaptic glutamate release by ventromedial hypothalamic neurons is part of the neurocircuitry that prevents hypoglycemia. Cell Metab 2007;5:383–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Furigo IC, de Souza GO, Teixeira PDS, Guadagnini D, Frazao R, List EO, Kopchick JJ, Prada PO, Donato J Jr. Growth hormone enhances the recovery of hypoglycemia via ventromedial hypothalamic neurons. FASEB J 2019;33:11909–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Quaresma PGF, Teixeira PDS, Wasinski F, Campos AMP, List EO, Kopchick JJ, Donato J Jr. Cholinergic neurons in the hypothalamus and dorsal motor nucleus of the vagus are directly responsive to growth hormone. Life Sci 2020;259:118229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Cady G, Landeryou T, Garratt M, Kopchick JJ, Qi N, Garcia-Galiano D, Elias CF, Myers MG Jr., Miller RA, Sandoval DA, Sadagurski M. Hypothalamic growth hormone receptor (GHR) controls hepatic glucose production in nutrient-sensing leptin receptor (LepRb) expressing neurons. Mol Metab 2017;6:393–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Amiel SA, Sherwin RS, Simonson DC, Lauritano AA, Tamborlane WV. Impaired insulin action in puberty. A contributing factor to poor glycemic control in adolescents with diabetes. N Engl J Med 1986;315:215–9. [DOI] [PubMed] [Google Scholar]
- [108].Teixeira PDS, Tavares MR, Jose D. Temporal characterization of the insulin resistance during puberty in mice. Endocr Regul 2021;55:1–4. [DOI] [PubMed] [Google Scholar]
- [109].Catalano PM. Trying to understand gestational diabetes. Diabet Med 2014;31:273–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Zierler KL, Rabinowitz D. Roles of Insulin and Growth Hormone, Based on Studies of Forearm Metabolism in Man. Medicine (Baltimore) 1963;42:385–402. [DOI] [PubMed] [Google Scholar]
- [111].Houssay BA, Anderson E. Diabetogenic action of purified anterior pituitary hormones. Endocrinology 1949;45:627–9. [DOI] [PubMed] [Google Scholar]
- [112].Olczyk J, Kokoszko A, Lewinski A, Karbownik-Lewinska M. Quality of life and exercise capacity in obesity and growth hormone deficiency. Neuro Endocrinol Lett 2010;31:700–7. [PubMed] [Google Scholar]
- [113].Scarano E, Riccio E, Somma T, Arianna R, Romano F, Di Benedetto E, de Alteriis G, Colao A, Di Somma C. Impact of Long-Term Growth Hormone Replacement Therapy on Metabolic and Cardiovascular Parameters in Adult Growth Hormone Deficiency: Comparison Between Adult and Elderly Patients. Front Endocrinol (Lausanne) 2021;12:635983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Pedroso JAB, Dos Santos LBP, Furigo IC, Spagnol AR, Wasinski F, List EO, Kopchick JJ, Donato J Jr. Deletion of growth hormone receptor in hypothalamic neurons affects the adaptation capacity to aerobic exercise. Peptides 2021;135:170426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Silva TM, Wasinski F, Flor KC, List EO, Kopchick JJ, Takakura AC, Donato J Jr., Moreira TS. The effect of central growth hormone action on hypoxia ventilatory response in conscious mice. Brain Res 2022;1791:147995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Katz-Salamon M, Lindgren AC, Cohen G. The effect of growth hormone on sleep-related cardio-respiratory control in Prader-Willi syndrome. Acta Paediatr 2012;101:643–8. [DOI] [PubMed] [Google Scholar]
- [117].Berini J, Spica Russotto V, Castelnuovo P, Di Candia S, Gargantini L, Grugni G, Iughetti L, Nespoli L, Nosetti L, Padoan G, Pilotta A, Trifiro G, Chiumello G, Salvatoni A, Genetic Obesity Study Group of the Italian Society of Pediatric E, Diabetology. Growth hormone therapy and respiratory disorders: long-term follow-up in PWS children. J Clin Endocrinol Metab 2013;98:E1516–23. [DOI] [PubMed] [Google Scholar]
- [118].Tavares MR, Frazao R, Donato J. Understanding the role of growth hormone in situations of metabolic stress. J Endocrinol 2023;256:e220159. [DOI] [PubMed] [Google Scholar]