Abstract
We established a plasmid-based system for generating infectious influenza virus-like particles entirely from cloned cDNAs. Human embryonic kidney cells (293T) were transfected with plasmids encoding the influenza A virus structural proteins and with a plasmid encoding an influenza virus-like viral RNA (vRNA) which contained an antisense copy of the cDNA for green fluorescence protein (GFP) flanked by an RNA polymerase I promoter and terminator. Intracellular transcription of the latter construct by RNA polymerase I generated GFP vRNA that was packaged into influenza virus-like particles. This system, which produced more than 104 infectious particles per ml of supernatant, would be useful in studies of influenza virus replication and particle formation. It might also benefit efforts in vaccine production and in the development of improved gene therapy vectors.
Influenza A viruses possess a genome of eight single-stranded negative-sense viral RNAs (vRNAs) that encode a total of 10 proteins. The influenza virus life cycle begins with binding of the hemagglutinin (HA) to sialic acid-containing receptors on the surface of the host cell (reviewed in reference 6), followed by receptor-mediated endocytosis. The low pH in late endosomes triggers a conformational shift in the HA, resulting in fusion of the viral and endosomal membranes and the consequent release of the matrix protein (M1) and ribonucleoprotein complexes (RNPs) into the cytosol of infected cells. RNPs consist of the nucleoprotein (NP), which encapsidates vRNA, and the viral polymerase complex, which is formed by the PA, PB1, and PB2 proteins. RNPs are transported into the nucleus, where transcription and replication take place. Newly synthesized RNPs are then exported from the nucleus and transported to the cellular membrane, where progeny virus particles are assembled. The neuraminidase (NA) protein plays a crucial role late in infection by removing sialic acid from sialyloligosaccharides, thus releasing newly assembled virions from the cell surface and preventing the self-aggregation of virus particles. Although virus assembly involves protein-protein and protein-vRNA interactions, the nature of these interactions is largely unknown.
A vaccinia virus-based system for the generation of influenza virus-like particles (VLPs) has been established (2, 7). In this system, an influenza virus-like vRNA carrying a reporter gene is transcribed in vitro and transfected into eukaryotic cells. All 10 influenza virus proteins are expressed from plasmids under the control of a T7 RNA polymerase promoter. When the transfected cells are infected with a recombinant vaccinia virus that expresses T7 RNA polymerase, they produce influenza VLPs containing the vRNA of an artificial reporter gene (7). However, vaccinia virus expresses more than 80 proteins, any of which could affect the influenza virus life cycle. We therefore sought to establish an efficient plasmid-driven system for the generation of infectious influenza VLPs containing a virus-like RNA segment.
Expression of the influenza virus proteins PB2, PB1, PA, and NP leads to replication and transcription of an artificial vRNA.
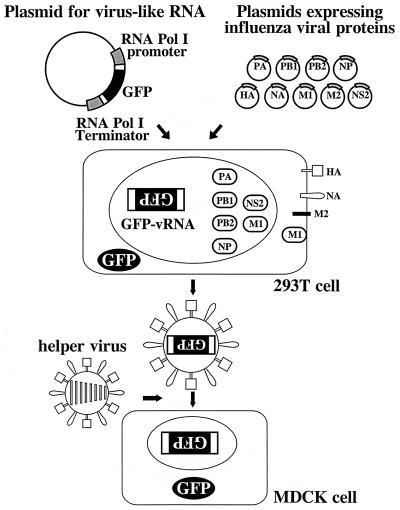
To generate influenza VLPs, we used the RNA polymerase I system for the intracellular synthesis of influenza virus RNAs (Fig. 1) (12). In this system, a cDNA carrying a reporter gene in antisense orientation is flanked by the 5′ and 3′ noncoding regions of an influenza virus RNA (Fig. 2). This cassette is inserted between an RNA polymerase I promoter and terminator. Transfection of such constructs into eukaryotic cells leads to transcription of the reporter gene by cellular RNA polymerase I, thereby generating influenza virus-like RNAs (12). Upon influenza virus infection, the artificial vRNAs are replicated and transcribed by the viral polymerase complex, resulting in the expression of the reporter gene (12).
FIG. 1.
Schematic diagram of VLP generation strategy. Individual protein expression plasmids and a plasmid containing the RNA polymerase I promoter, a cDNA encoding the GFP reporter gene, and the RNA polymerase I terminator are transfected into 293T cells. Intracellular transcription by RNA polymerase I yields GFP vRNA of negative polarity, as indicated by inverted letters. Supernatants containing VLPs are harvested, mixed with influenza helper virus, and inoculated into MDCK cells.
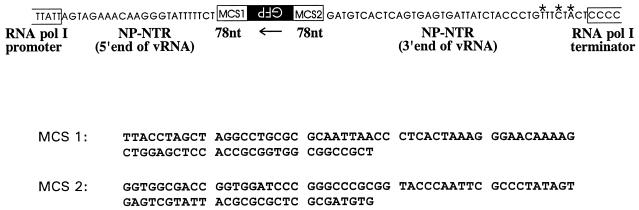
FIG. 2.
The pPolI-GFP plasmid for generating influenza virus-like RNA encoding the GFP protein. The GFP gene (derived from pEGFP-N1 [Clontech, Palo Alto, Calif.]) was inserted in the antisense orientation into pHL1844 (E. Hoffmann, Ph.D. thesis, Justus-Liebig University, Giessen, Germany), which contains the 5′ and 3′ noncoding regions of influenza A virus segment 5 (NP-NTRs). The gene was flanked by multiple cloning sites (MCS1 and MCS2) and by the human RNA polymerase I promoter and the mouse RNA polymerase I terminator. Asterisks indicate mutations introduced to upregulate promoter activity (10a).
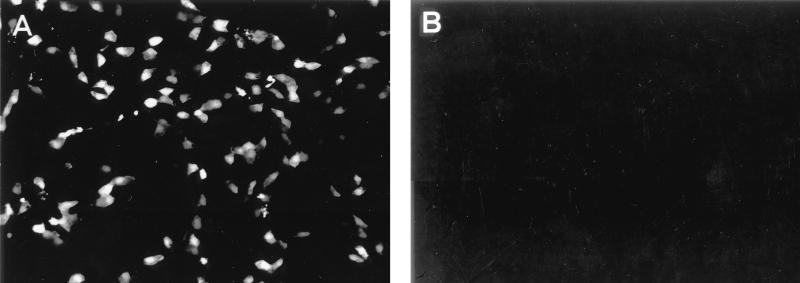
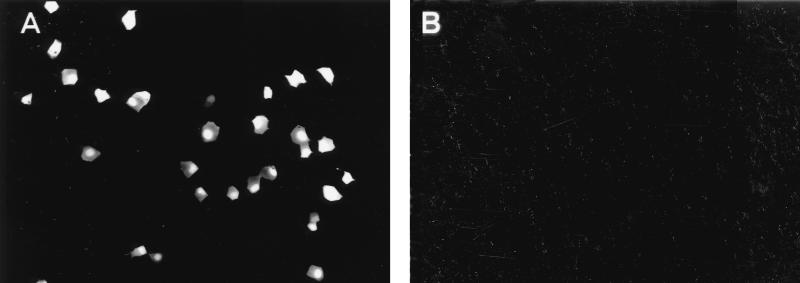
We first tested whether expression of the PB2, PB1, PA, and NP proteins leads to expression of the reporter gene carried by the RNA polymerase I-derived transcript. Plasmids (1 μg each) expressing the NP protein of A/WSN/33 (H1N1) virus under the control of the chicken β-actin promoter (pCAGGS-WSN-NP0/14) (10), the polymerase proteins of A/PR/8/34 virus under the control of the cytomegalovirus promoter [pcDNA762(PB2), pcDNA774(PB1), and pcDNA787(PA)] (14), and an RNA polymerase I reporter gene construct (pPolI-GFP) (Fig. 2) were transfected into human embryonic kidney (293T) cells as described previously (11). Forty-eight hours later, 30 to 40% of the cells were expressing green fluorescence protein (GFP) (Fig. 3). In contrast, GFP expression could not be detected in transfected cells lacking the polymerase or NP proteins. These results indicate that NP and the three influenza virus polymerase proteins had formed a functional complex that replicated and transcribed the RNA polymerase I-derived GFP vRNA.
FIG. 3.
The PA, PB1, PB2, and NP proteins of influenza A virus encapsidate GFP vRNA produced by RNA polymerase I, leading to GFP expression. 293T cells were transfected with plasmids expressing the PB2, PB1, PA, and NP proteins (A) or with all plasmids except the one expressing the NP protein (B), together with the RNA polymerase I-GFP gene plasmid for intracellular synthesis of reporter gene vRNA. Cells were fixed 48 h after transfection, and GFP expression was determined with a fluorescence microscope. Magnification, ×100.
Optimal vRNA transcription and replication.
To determine the amounts of plasmid DNA required for optimal reporter GFP expression, we modulated the expression of the polymerase proteins and NP. Previous studies had indicated that large amounts of PA reduce the extent of reporter gene expression in transcription-replication systems (7). We therefore reduced in a stepwise manner the expression of PA from the plasmid, identifying 0.1 μg of pcDNA787(PA) as the template amount yielding the strongest expression of GFP (data not shown). With NP, the major structural component of RNP complexes, large amounts of protein expression plasmid may be required. However, larger amounts of the plasmid did not appreciably affect the number of GFP-positive 293T cells (data not shown). In addition, various amounts of the PB2 and PB1 protein expression plasmids (ranging from 1.0 to 0.03 μg) did not affect GFP expression in 293T cells (data not shown). Hence, in all subsequent experiments, we used 0.1 μg of pcDNA787(PA) and 1.0 μg of pcDNA774(PB1), pcDNA762(PB2), and pCAGGS-WSN-NP0/14.
Formation of influenza VLPs from cloned cDNAs.
Previous studies with the vaccinia virus T7 RNA polymerase system showed that the formation of influenza VLPs requires nine influenza virus proteins: PB2, PB1, PA, HA, NA, NP, M1, M2, and NS2 (7). The NS1 protein, in contrast, is dispensable for particle formation (7). To establish an efficient plasmid-driven system for VLP generation, we generated cDNAs encoding the HA, NA, M1, M2, and NS2 genes and cloned them into the eukaryotic expression vector pCAGGS/MCS (controlled by the chicken β-actin promoter) (13), resulting in pEWSN-HA, pCAGGS-WNA15, pCAGGS-WSN-M1-2/1, pEP24c, and pCA-NS2, respectively. Expression of each protein was confirmed by Western blot analysis (data not shown).
To generate VLPs, we first transfected 106 293T cells with 1.0 μg of each protein expression plasmid [with the exception of pcDNA787(PA), for which we used 0.1 μg] and with 1 μg of the reporter gene construct pPolI-GFP. Culture supernatants were harvested 48 h after transfection and mixed with A/WSN/33 virus to provide the influenza virus proteins required for replication and transcription of GFP vRNA. The mixture was then inoculated into MDCK cells (Fig. 1). Ten hours after incubation, we detected GFP-positive MDCK cells, corresponding to ∼450 particles/ml of supernatant (Table 1). Thus, plasmid-driven expression of all influenza virus structural proteins resulted in the formation of infectious influenza VLPs containing GFP vRNA that could be delivered into subsequent cells.
TABLE 1.
Optimal amounts of plasmid DNA for the formation of infectious VLPsa
| Amt (μg) of plasmid DNA expressing:
|
Relative efficiency of VLP formationb | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PB2 | PB1 | PA | HA | NP | NA | M1 | M2 | NS2 | GFP vRNA | |
| 1.0 | 1.0 | 0.1 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1 |
| 1.0 | 1.0 | 0.1 | 1.0 | 1.0 | 1.0 | 1.0 | 0.1 | 1.0 | 1.0 | 28 |
| 1.0 | 1.0 | 0.1 | 1.0 | 1.0 | 1.0 | 1.0 | 0.03 | 1.0 | 1.0 | 17 |
| 1.0 | 1.0 | 0.1 | 1.0 | 1.0 | 1.0 | 1.0 | 0.1 | 1.0 | 1.0 | 28 |
| 1.0 | 1.0 | 0.1 | 1.0 | 1.0 | 1.0 | 1.0 | 0.1 | 0.3 | 1.0 | 24 |
| 1.0 | 1.0 | 0.1 | 1.0 | 1.0 | 1.0 | 1.0 | 0.1 | 0.1 | 1.0 | 11 |
| 1.0 | 1.0 | 0.1 | 1.0 | 1.0 | 1.0 | 1.0 | 0.1 | 1.0 | 1.0 | 28 |
| 1.0 | 1.0 | 0.1 | 1.0 | 1.0 | 1.0 | 2.0 | 0.1 | 1.0 | 1.0 | 220 |
293T cells were transfected with expression plasmids for all nine influenza virus structural proteins and with the RNA polymerase I-GFP gene plasmid. Forty-eight hours after transfection, VLP-containing supernatants were collected, mixed with A/WSN/33 helper virus, and inoculated into MDCK cells. The cells were fixed 10 h after infection, and GFP expression was determined with a fluorescence microscope. Only the amounts of the M1, M2, and NS2 plasmids were varied (bold type) to determine their optimal amounts for GFP expression in MDCK cells. Experiments were performed three times, and representative data are shown.
Determined by counting the number of GFP-positive cells in five microscopic fields. The sample containing 1 μg of each plasmid (which yielded ∼450 infectious VLPs/ml of supernatant) was chosen as the reference (value of 1).
Optimal assembly of influenza virus.
VLP formation was also studied in cells expressing different amounts of the RNA polymerase I reporter gene construct, as well as HA, NA, M1, M2, and NS2 plasmid DNAs. In experiments with pPolI-GFP, 1.0 μg of the plasmid DNA was highly efficient in generating VLPs, whereas the efficiency was significantly reduced for 2.0 or 3.0 μg (data not shown). Because the NS2 and M2 proteins are expressed in small amounts late in infection, we reasoned that relatively small amounts of the expression plasmids would be needed for optimal VLP formation. Reduction of the M2 expression construct from 1.0 to 0.1 μg resulted in more than a 10-fold increase in the number of GFP-positive MDCK cells (Table 1); further reduction to 0.03 μg did not increase the number of VLPs. For NS2, smaller amounts of plasmid (0.1 μg) were associated with less efficient formation of VLPs (Table 1).
The M1 protein is the major structural component of the virion. Thus, high levels of M1 expression are likely required for efficient formation of VLPs. This prediction was tested in experiments comparing VLP formation in cells transfected with 1.0 or 2.0 μg of M1 plasmid DNA. As shown in Table 1, larger amounts of plasmid resulted in more than a 10-fold increase in the number of GFP-positive MDCK cells. Comparison of two different amounts (1 and 2 μg) of plasmids expressing the HA and NA proteins did not reveal any appreciable differences in VLP formation (data not shown), leading to selection of 1 μg of each plasmid (pEWSN-HA and pCAGGS-WNA15) for use in subsequent experiments. Overall, these studies resulted in a >100-fold increase in the efficiency of VLP formation, ultimately leading to the production of more than 104 infectious influenza virus particles per ml of supernatant (Fig. 4).
FIG. 4.
Generation of infectious influenza VLPs. 293T cells were transfected with nine plasmids, each expressing a different viral structural protein (A), or with eight plasmids omitting the construct for NP (B), together with the RNA polymerase I-GFP gene plasmid. Forty-eight hours after transfection, supernatants were collected, mixed with A/WSN/33 helper virus, and inoculated into MDCK cells. Cells were fixed at 10 h after infection, and GFP expression in VLP-infected MDCK cells was determined with a fluorescence microscope. Magnification, ×100.
Authenticity of VLPs produced entirely from plasmids.
To verify that VLPs initiate infection in the same manner as authentic influenza viruses, we attempted to neutralize the VLPs with antibody to the A/WSN/33 HA. VLP-containing supernatants derived from plasmid-transfected 293T cells were incubated with a pool of anti-A/WSN/33 HA monoclonal antibodies or with a monoclonal antibody to the G protein of vesicular stomatitis virus (VSV) (negative control) for 1 h at room temperature. A/PR/8/34 helper virus, which is not neutralized by the pool of anti-A/WSN/33 HA monoclonal antibodies, was added to the mixture and inoculated into MDCK cells. Only the A/WSN/33 HA-specific monoclonal antibody neutralized the VLPs (data not shown), indicating that the HA mediates the attachment and entry of VLPs into cells.
Next, we identified the minimal set of proteins required for the formation of VLPs. Others have established that the three influenza virus polymerases and the NP are essential for the replication and transcription of vRNA (3). Therefore, we included each of these four proteins in our assay but consecutively omitted HA, NA, M1, M2, or NS2. Exclusion of either of the plasmids mentioned above did not affect the replication-transcription of GFP vRNA in transfected 293T cells (data not shown). Supernatants derived from transfected 293T cells that lacked the HA, NA, M1, or NS2 protein did not promote GFP expression in infected MDCK cells, indicating the absence of infectious VLPs. Infectious VLPs were detected with the omission of M2 but the number was low (>500-fold reduction compared to the full set of structural proteins). Thus, all influenza virus structural proteins are required for the efficient formation of infectious VLPs, in accord with data from studies of the vaccinia virus-based system (7).
VSV glycoprotein can replace the HA and NA proteins in the production of VLPs.
To test the utility of our plasmid-based system for virus assembly studies, we replaced the influenza virus HA and NA proteins with the VSV-G protein, which functions in receptor binding and fusion. When 293T cells were transfected with pPolI-GFP, with optimal amounts of the PB2, PB1, PA, NP, M1, M2, and NS2 expression constructs, and with 1 μg of the VSV-G construct (pCAGGS-VSV-G), substitution of the VSV-G protein for influenza virus glycoproteins did not adversely affect VLP formation (data not shown). In fact, we reproducibly found slightly higher numbers (∼1.2-fold) of GFP-positive cells when VSV-G, rather than the HA and NA, served as the viral glycoprotein. Thus, the VSV-G protein can be efficiently incorporated into virions and can function as well as the HA and NA in virus release and entry.
An efficient system for generating infectious influenza virus particles would be an asset in research with this virus and potentially in the production of vaccines and vectors for gene therapy. Moreover, this VLP system leads to the generation of influenza A viruses entirely from cloned cDNA (11). The VLP production strategy described here is highly efficient, both in the initial transfection of cells and in the yield of VLPs (>104 infectious particles/ml of supernatant). Moreover, it is driven entirely by plasmids expressing influenza virus proteins (i.e., in the absence of any other viral proteins), which greatly simplifies the interpretation of results. Another major advantage is the capability of studying the effects of lethal mutations in virion formation, the packaging of RNP complexes, the budding of virus replication, and the binding and fusion processes. In addition, we expect that our system would operate equally well with other viruses, e.g., paramyxoviruses and rhabdoviruses.
We demonstrated that the influenza virus HA and NA proteins can be functionally replaced by the VSV-G protein. Influenza viruses failed to incorporate VSV-G protein when provided by recombinant simian virus 40 (9). However, assays had not been available to replace the influenza virus glycoproteins by foreign glycoproteins. Therefore, the question of whether interactions of HA and/or NA with other viral proteins are essential for virus formation remained open. Although a novel virus has been generated upon expression of VSV-G protein as well as a Semliki Forest virus replicon in the same cell, the infectious titer of this new virus is at least 105-fold lower than that of normal VSV (15). Thus, the Semliki Forest virus replicon is not efficiently incorporated by VSV-G. By contrast, the infectious titers of VLPs with VSV-G, instead of HA and NA, were even higher than those of authentic influenza VLPs. Therefore, our finding suggests that neither HA nor NA is essential for the formation of VLPs. However, we cannot rule out a role for these glycoproteins in interactions with other viral proteins, which would affect the structure of virions and the efficiency of virion formation, as suggested by the elongated shapes of viruses expressing tailless HAs, NAs, or both (1, 4, 5, 8).
We emphasize the potential value of our plasmid-based system for therapeutic gene delivery. One can now generate VLPs that contain a vRNA encoding the proteins required for transcription and replication (i.e., the NP and the polymerases), as well as a vRNA encoding the protein of interest. These particles are infectious and would deliver a designated gene into target cells, where it would replicate and be transcribed. Because these particles do not contain a full complement of viral genes, they cannot produce infectious progeny viruses. This feature, together with the lack of integration of the viral genome into host chromosomes, would ensure the biological safety of gene delivery in human and nonhuman subjects. Finally, the availability of 15 HA and 9 NA subtypes and their variants would allow one to administer VLPs repeatedly, thereby overcoming immunoresistance to vector-generated proteins, which is one of the major obstacles faced with repeated use of other viral vectors, such as adenoviruses. We suggest that the greatest benefit of our plasmid-driven system would be realized in situations requiring only short-term expression of foreign proteins, as in cancer treatment.
REFERENCES
- 1.García-Sastre A, Palese P. The cytoplasmic tail of the neuraminidase protein of influenza A virus does not play an important role in the packaging of this protein into viral envelopes. Virus Res. 1995;37:37–47. doi: 10.1016/0168-1702(95)00017-k. [DOI] [PubMed] [Google Scholar]
- 2.Gómez-Puertas P, Mena I, Castillo M, Vivo A, Perez-Pastrana E, Portela A. Efficient formation of influenza virus-like particles: dependence on the expression levels of viral proteins. J Gen Virol. 1999;80:1635–1645. doi: 10.1099/0022-1317-80-7-1635. [DOI] [PubMed] [Google Scholar]
- 3.Honda A, Uéda K, Nagata K, Ishihama A. RNA polymerase of influenza virus: role of NP in RNA chain elongation. J Biochem (Tokyo) 1988;104:1021–1026. doi: 10.1093/oxfordjournals.jbchem.a122569. [DOI] [PubMed] [Google Scholar]
- 4.Jin H, Leser G P, Lamb R A. The influenza virus hemagglutinin cytoplasmic tail is not essential for virus assembly or infectivity. EMBO J. 1994;13:5504–5515. doi: 10.1002/j.1460-2075.1994.tb06885.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jin H, Leser G P, Zhang J, Lamb R A. Influenza virus hemagglutinin and neuraminidase cytoplasmic tails control particle shape. EMBO J. 1997;16:1236–1247. doi: 10.1093/emboj/16.6.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lamb R A, Krug R M. Orthomyxoviridae: the viruses and their replication. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 1353–1395. [Google Scholar]
- 7.Mena I, Vivo A, Pérez E, Portela A. Rescue of a synthetic chloramphenicol acetyltransferase RNA into influenza virus-like particles obtained from recombinant plasmids. J Virol. 1996;70:5016–5024. doi: 10.1128/jvi.70.8.5016-5024.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mitnaul L J, Castrucci M R, Murti K G, Kawaoka Y. The cytoplasmic tail of influenza A virus neuraminidase (NA) affects NA incorporation into virions, virion morphology, and virulence in mice but is not essential for virus replication. J Virol. 1996;70:873–879. doi: 10.1128/jvi.70.2.873-879.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Naim H Y, Roth M G. Basis for selective incorporation of glycoproteins into the influenza virus envelope. J Virol. 1993;67:4831–4841. doi: 10.1128/jvi.67.8.4831-4841.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neumann G, Castrucci M R, Kawaoka Y. Nuclear import and export of influenza virus nucleoprotein. J Virol. 1997;71:9690–9700. doi: 10.1128/jvi.71.12.9690-9700.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10a.Neumann G, Hobam G. Mutational analysis of influenza virus promoter elements in vivo. J Gen Virol. 1995;76:1709–1717. doi: 10.1099/0022-1317-76-7-1709. [DOI] [PubMed] [Google Scholar]
- 11.Neumann G, Watanabe T, Ito H, Watanabe S, Goto H, Gao P, Hughes M, Perez D R, Donis R, Hoffmann E, Hobom G, Kawaoka Y. Generation of influenza A viruses entirely from cloned cDNAs. Proc Natl Acad Sci USA. 1999;96:9345–9350. doi: 10.1073/pnas.96.16.9345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neumann G, Zobel A, Hobom G. RNA polymerase I-mediated expression of influenza viral RNA molecules. Virology. 1994;202:477–479. doi: 10.1006/viro.1994.1365. [DOI] [PubMed] [Google Scholar]
- 13.Niwa H, Yamamura K, Miyazaki J. Efficient selection for high-expression transfectants by a novel eukaryotic vector. Gene. 1991;108:193–200. doi: 10.1016/0378-1119(91)90434-d. [DOI] [PubMed] [Google Scholar]
- 14.Perez D R, Donis R O. The matrix 1 protein of influenza A virus inhibits the transcriptase activity of a model influenza reporter genome in vivo. Virology. 1998;249:52–61. doi: 10.1006/viro.1998.9318. [DOI] [PubMed] [Google Scholar]
- 15.Rolls M M, Webster P, Balba N H, Rose J K. Novel infectious particles generated by expression of the vesicular stomatitis virus glycoprotein from a self-replicating RNA. Cell. 1994;79:497–506. doi: 10.1016/0092-8674(94)90258-5. [DOI] [PubMed] [Google Scholar]